Chapter 25 Appropriate Use of Nuclear Cardiology
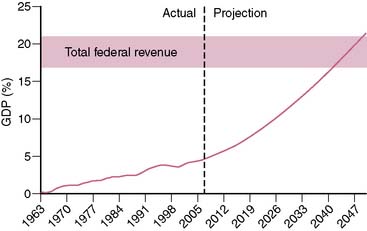
Spending on health care in the United States has been increasing at an alarming rate. Over the past 40 years, the annual increase in federal health care spending has outpaced the annual growth of the gross domestic product (GDP) by an average of 2.5%.1 Currently, U.S. health care spending exceeds $2 trillion and accounts for 16% of the GDP.2 The Congressional Budget Office projects that if current trends were to persist over the next 40 years, federal spending on Medicare and Medicaid (ignoring state spending on Medicaid) would approach 20% of the GDP (Fig. 25-1). To appreciate the significance of this projection, it is important to realize that total federal revenue over the past 60 years has ranged between 17.0% and 20.9% of the GDP and has never in the history of the United States exceeded 20.9%.3 In other words, the projected federal spending on Medicare and Medicaid alone by the midpoint of this century would equal (as a percentage of the GDP) what has historically been spent on all components of the federal budget (social security, defense, education, interest on the national debt, agriculture, etc.).
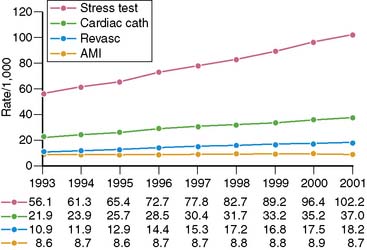
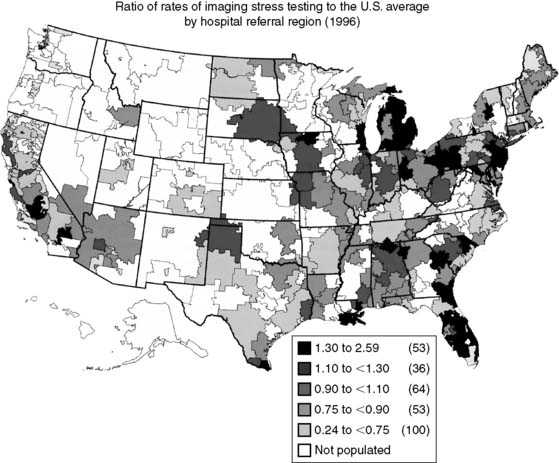
Spending on cardiovascular diseases accounts for a substantial proportion of total health care spending. In 2007, the estimated direct and indirect costs for cardiovascular diseases were nearly $432 billion.4 The rate of growth in cardiac imaging services has recently been especially steep. Stress imaging, which as defined by Medicare incorporates both stress single-photon emission computed tomography (SPECT) and stress echocardiography, grew at a rate of 6% per year between 1993 and 2001.5 This rate of growth for stress imaging far exceeded the rate of growth in acute myocardial infarction, cardiac catheterizations, or coronary artery revascularization procedures during this time period (Fig. 25-2). Published data further demonstrate that the use of stress imaging is highly heterogenous across the country, with rates of imaging varying by more than 10-fold between different geographic regions (Fig. 25-3).6
In response to these concerns about the use of cardiovascular imaging, the American College of Cardiology Foundation (ACCF) initiated a process to address the appropriateness of different cardiovascular imaging procedures.7 Examining the appropriateness of medical resource use is not a novel concept.8–10 The American College of Radiology (ACR) published appropriateness criteria for radiologic imaging procedures in 1995.11 The ACR criteria address a variety of imaging procedures across multiple medical and surgical disciplines. They have not been widely applied to the practice of clinical cardiology. The ACCF appropriateness criteria focus on single cardiovascular imaging procedures. In October 2005 the ACCF, in collaboration with the American Society of Nuclear Cardiology (ASNC), published the first set of appropriateness criteria, which addressed SPECT myocardial perfusion imaging (SPECT MPI).12 Since then, appropriateness criteria have been published for cardiac computed tomography and cardiac magnetic resonance imaging,13 transthoracic and transesophageal echocardiography,14 stress echocardiography,15 and coronary revascularization.16
The approach the ACCF has followed to examine the appropriateness of a cardiovascular imaging modality is based on a method originally developed by the RAND Corporation, in collaboration with researchers from the University of California, Los Angeles (UCLA), to examine the appropriateness of medical and surgical procedures.17 Although the ACCF methodology is based on the RAND/UCLA approach, it is important to note that the RAND/UCLA method focuses on the therapeutic benefit of invasive procedures, whereas cardiovascular imaging has different goals. Another major difference relates to the number of clinical scenarios considered. The RAND/UCLA document addresses literally hundreds of clinical scenarios. The ACCF approach limits the number of situations to fewer, more broadly defined clinical scenarios where imaging is most commonly applied. The ACCF method follows the precedent set by the ACR and uses the modified Delphi method to develop appropriateness criteria. With this approach, members of an expert panel assign ratings for imaging to various clinical indications, initially without any interaction with other panel members. The panel then meets to discuss the assigned ratings, and members again assign ratings after this panel interaction. The ACCF approach differs from the ACR approach by not trying to achieve consensus of panel members and by using fewer rounds of ratings.7
The definition of an appropriate imaging study selected by the ACCF is:7
It is important to note that the “negative consequences” listed in this formal definition do not directly mention cost of the procedure. However, the ACCF methodology document does acknowledge the importance of cost: “Therefore, to identify the true risks of imaging, both inherent risks and downstream effects, including costs, must be considered. As such, if the imaging study provides little incremental information for an indication over standard clinical judgment and care, then cost considerations should contribute to deeming the procedure inappropriate.”7
For each imaging modality being rated for appropriateness, the ACCF method follows 4 steps:
It is important to distinguish between appropriateness criteria and clinical guidelines. ACCF/American Heart Association (AHA) guidelines are based on comprehensive reviews of the medical literature (evidence-based) and employ a level-of-evidence rating scheme to assign indications to diagnostic procedures and therapies. Conversely, the appropriateness criteria are based on consideration of benefit versus risk of performing the imaging modality. Panel members are encouraged to consider the published literature when assigning a rating for a given indication, but there is no requirement that the rating be based on the literature. The emphasis is on a less formal approach of whether it is “reasonable” to consider performing the imaging study.7
The technical panel for SPECT MPI appropriateness criteria was composed of 12 physicians, 58% of whom were specialists in nuclear cardiology. This panel rated 52 clinical indications for SPECT imaging. Most (49) of these indications addressed stress SPECT for detection or risk assessment of coronary artery disease, one indication addressed assessment of viability, and two indications addressed evaluation of ventricular function (Tables 25-1 through 25-9).12 Of the 52 indications, 27 were rated appropriate, 12 uncertain, and 13 inappropriate.
Table 25-1 Detection of Coronary Artery Disease: Symptomatic
| Indication | Appropriateness Criteria (Median Score) | |
|---|---|---|
| Evaluation of Chest Pain Syndrome | ||
| 1. | I (2.0) | |
| 2. | U* (6.5) | |
| 3. | A (7.0) | |
| 4. | A (9.0) | |
| 5. | A (8.0) | |
| 6. | A (9.0) | |
| Acute Chest Pain (in Reference to Rest Perfusion Imaging) | ||
| 7. | A (9.0) | |
| 8. | I (1.0) | |
| New-Onset/Diagnosed Heart Failure With Chest Pain Syndrome | ||
| 9. | A (8.0) | |
A, appropriate; I, inappropriate; U, uncertain.
* Median scores of 3.5 and 6.5 were rounded to the middle (U).
Table 25-2 Detection of Coronary Artery Disease: Asymptomatic (Without Chest Pain Syndrome)
| Indication | Appropriateness Criteria (Median Score) | |
|---|---|---|
| Asymptomatic | ||
| 10. | I (1.0) | |
| 11. | U (5.5) | |
| New-Onset or Diagnosed Heart Failure or LV Systolic Dysfunction Without Chest Pain Syndrome | ||
| 12. | A (7.5) | |
| Valvular Heart Disease Without Chest Pain Syndrome | ||
| 13. | U (5.5) | |
| New-Onset Atrial Fibrillation | ||
| 14. | U*(3.5) | |
| 15. | A (8.0) | |
| Ventricular Tachycardia | ||
| 16. | A (9.0) | |
A, appropriate; I, inappropriate; U, uncertain.
* Median scores of 3.5 and 6.5 are rounded to the middle (U).
Table 25-3 Risk Assessment: General and Specific Patient Populations
| Indication | Appropriateness Criteria (Median Score) | |
|---|---|---|
| Asymptomatic | ||
| 17. | I (1.0) | |
| 18. | U (4.0) | |
| 19. | A (8.0) | |
| 20. | A (7.5) | |
A, appropriate; I, inappropriate; U, uncertain.
Table 25-4 Risk Assessment with Prior Test Results
| Indication | Appropriateness Criteria (Median Score) | |
|---|---|---|
| 21. | I (3.0) | |
| 22. | A (7.0) | |
| 23. | I (2.5) | |
| 24. | A (7.5) | |
| 25. | A (9.0) | |
| 26. | U* (6.5) | |
| 27. | A (7.5) | |
| 28. | I (1.5) | |
| 29. | A (9.0) | |
| Duke Treadmill Score | ||
| 30. | A (9.0) | |
A, appropriate; I, inappropriate; U, uncertain.
* Median scores of 3.5 and 6.5 are rounded to the middle (U).
Table 25-5 Risk Assessment: Preoperative Evaluation for Non-Cardiac Surgery
| Indication | Appropriateness Criteria (Median Score) | |
|---|---|---|
| Low-Risk Surgery | ||
| 31. | I (1.0) | |
| Intermediate-Risk Surgery | ||
| 32. | I (3.0) | |
| 33. | A (8.0) | |
| High-Risk Surgery | ||
| 34. | U (4.0) | |
| 35. | A (8.0) | |
| 36. | I (3.0) | |
A, appropriate; I, inappropriate; U, uncertain.
Table 25-6 Risk Assessment: Following Acute Coronary Syndrome
| Indication | Appropriateness Criteria (Median Score) | |
|---|---|---|
| STEMI—Hemodynamically Stable | ||
| 37. | A (8.0) | |
| STEMI—Hemodynamically Unstable, Signs of Cardiogenic Shock, or Mechanical Complications | ||
| 38. | I (1.0) | |
| UA/NSTEMI—No Recurrent Ischemia OR No Signs of HF | ||
| 39. | A (8.5) | |
| ACS—Asymptomatic Post Revascularization (PCI or CABG) | ||
| 40. | I (1.0) | |
A, appropriate; I, inappropriate; U, uncertain.
Table 25-7 Risk Assessment: Post-Revascularization (PCI or CABG)
| Indication | Appropriateness Criteria (Median Score) | |
|---|---|---|
| Symptomatic | ||
| 41. | A (8.0) | |
| Asymptomatic | ||
| 42. | U (6.0) | |
| 43. | U (4.5) | |
| 44. | A (7.5) | |
| 45. | A (7.5) | |
| 46. | U* (6.5) | |
| 47. | I (3.0) | |
| 48. | U* (6.5) | |
| 49. | U (5.5) | |
A, appropriate; I, inappropriate; U, uncertain.
* Median scores of 3.5 and 6.5 are rounded to the middle (U).
Table 25-8 Assessment of Viability/Ischemia
| Indication | Appropriateness Criteria (Median Score) | |
|---|---|---|
| 50. | A (8.5) | |
A, appropriate; I, inappropriate; U, uncertain.
Table 25-9 Evaluation of Ventricular Function
| Indication | Appropriateness Criteria (Median Score) | |
|---|---|---|
| Evaluation of Left Ventricular Function | ||
| 51. | A (9.0) | |
| Use of Potentially Cardiotoxic Therapy (e.g., doxorubicin) | ||
| 52. | A (9.0) | |
A, appropriate; I, inappropriate; U, uncertain.
In its initial document, the ACCF stated: “Both validation and evaluation of the proposed appropriateness ratings by modality are essential steps.”7 Since the ACCF appropriateness criteria ratings concept is only in its infancy, there are little published data that examines the application of the criteria in clinical practice.
We undertook a project to evaluate the appropriateness of stress SPECT MPI in our institution.18 We retrospectively applied the criteria listed in the ACCF/ASNC document for stress SPECT MPI (see Tables 25-1 through 25-7) to 284 patients referred to our laboratory over a 2-week period between May 1 to 15, 2005. Since we were interested in studying the appropriateness of cardiovascular imaging in general terms, we also applied the same stress SPECT MPI criteria to 298 patients who underwent stress echocardiography at our institution during this same time period. For this initial project, we intentionally selected a timeframe before the publication of the ACCF appropriateness criteria for individual imaging techniques to establish a baseline for the use of stress imaging in our institution. Our goal in future projects will be to assess the impact of publication of the ACCF appropriateness criteria documents on our practice.
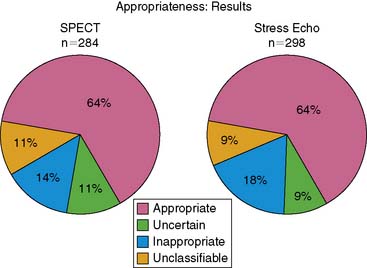
The major finding of our study was that 64% of stress SPECT MPI studies were appropriate, 11% were uncertain, and 14% were inappropriate. Eleven percent of the studies were unclassifiable. Results were similar for stress echocardiography (Fig. 25-4). The vast majority (88%) of inappropriate studies were performed for one of four indications: asymptomatic patients with low-risk Framingham score referred for screening for coronary artery disease and (indication #10 in Table 25-2 of the ACCF/ASNC SPECT MPI document); preoperative evaluation for intermediate-risk noncardiac surgery with normal exercise tolerance (indication #32 in Table 25-5); symptomatic patients with chest pain who had a low pretest probability for coronary artery disease and an interpretable ECG, and were able to exercise (indication #1 in Table 25-1); and preoperative evaluation for low-risk noncardiac surgery (indication #31 in Table 25-5).
Performing this project highlighted many difficulties that arise related to the application of the ACCF appropriateness criteria to clinical databases.18 The Mayo Clinic nuclear cardiology and stress echocardiography laboratories both have electronic databases. Because we had no previous experience applying the appropriate criteria to our databases, we first performed a pilot project applying these criteria to a single day of tests from both laboratories (50 patients). Five physicians and 2 experienced cardiovascular nurse abstractors independently attempted to apply the appropriateness criteria to these 50 patients. We discovered that a number of “assumptions” were necessary to apply the appropriateness criteria in a standardized manner. Some examples of these assumptions included assigning a hierarchical ranking to the appropriateness tables (since some patients met more than one test indication), assuming that certain surgical procedures not covered by the ACC/AHA preoperative guidelines would be low risk (e.g., endoscopic inguinal hernia repair), and interpreting the symptom of “dyspnea” as being equivalent to “atypical angina” for purposes of determining a patient’s pretest probability of coronary artery disease. (The ACCF/ASNC SPECT MPI appropriateness criteria document indicates that an appropriateness rating can be assigned to patients being evaluated for dyspnea, but the published criteria for determining pretest probability of disease address only address chest pain and not dyspnea.) Once we completed the pilot project, the actual appropriateness ratings for the 284 SPECT MPI patients and the 298 stress echocardiography patients were assigned by the two experienced nurse abstractors, each blinded to the other’s ratings and neither of whom was affiliated with either laboratory. Differences between the two nurses’ ratings were resolved by consensus of two physicians. The overall level of agreement between the two nurses was only modest (kappa = 0.56). This finding illustrates the lack of consistency that is likely to occur when applying these ratings in clinical practice.
To date, the only other publication that addresses the application of the ACCF/ASNC SPECT MPI appropriateness criteria is a study from the University of Chicago nuclear cardiology laboratory involving 1209 patients who underwent stress SPECT MPI.19 These authors found that a higher percentage (97%) of patients referred for SPECT MPI could be assigned to one of the indications in the ACCF/ASNC SPECT MPI document. In the patients who could be assigned to an indication, a higher percentage of patients were rated appropriate in this study compared to our study. The ratings results for the study by Mehta et al. were 80% appropriate, 7% uncertain, and 13% inappropriate. These authors also found that a small number (3) of similar indications accounted for the large majority (85%) of inappropriate tests: symptomatic patients with a low pretest probability of coronary artery disease, preoperative evaluation for low-risk surgery, and preoperative evaluation for intermediate-risk surgery with low to intermediate clinical predictors and normal exercise tolerance. Additional analyses undertaken by these authors included examination of the prevalence of normal SPECT MPI according to rating categories and the impact of the specialty of the ordering physician on rating categories. Predictably, the percentage of normal images was significantly smaller in the appropriate (45%) category versus the uncertain (53%) or inappropriate (68%) categories. Anesthesiologists were more likely to order SPECT MPI for inappropriate indications, compared to other physician specialties.
Differences in study methodology may explain some of the differences in results between the study by Mehta et al.19 and our study.18 The assignment of appropriateness category ratings by Mehta and colleagues was performed by a single individual affiliated with the University of Chicago nuclear cardiology laboratory. This individual understandably may have been biased toward rating studies as appropriate.20 Additionally, the chart review in the Mehta et al. study was performed after the publication of the ACCF/ASNC SPECT MPI appropriateness criteria document. Conceivably, awareness of this document may have influenced test ordering at the University of Chicago.
These two studies18,19 share common features, including application of retrospective chart review and similar practice settings (academic medical centers located in the Midwest). Use of SPECT MPI might be substantially different in private practice settings or in other geographic regions. Both the Rochester, Minnesota, and Chicago Medicare referral regions have reported rates of stress imaging that are far below those reported for multiple regions in Michigan, New Jersey, and Florida.6 The results of these two studies should therefore not be interpreted to reflect the use of SPECT MPI on a national basis.
Currently there are only a limited number of studies that have applied appropriateness criteria to other imaging modalities.21,22 Following our initial study,18 we examined the application of the stress echocardiography appropriateness criteria15 to the same 298 patients who underwent stress echocardiography in a subsequent study.23 When stress SPECT MPI was applied to the stress echocardiography patients, 64% of studies were rated appropriate, 9% uncertain, 18% inappropriate, and 9% unclassifiable (see Fig. 25-4). Application of the stress echocardiography appropriateness criteria to the stress echocardiography patients resulted in significantly fewer studies (54%) rated as appropriate and more studies (19%) rated as unclassifiable, with similar percentages rated as uncertain (8%) or inappropriate (19%) (P < 0.001 for overall difference compared to the ratings using the SPECT MPI criteria). These differences suggest that future revisions of appropriateness criteria documents for stress imaging should attempt to reduce the differences that currently exist in these criteria for the different imaging modalities.
The appropriateness criteria document for coronary revascularization16 does not address the appropriate use of SPECT MPI but does require that the presence of ischemia or the presence of intermediate-risk or high-risk findings on noninvasive imaging be considered to assign a rating of appropriateness to many revascularization scenarios. For SPECT imaging specifically, intermediate-risk findings include mild to moderate resting left ventricular dysfunction (left ventricular ejection fraction 35% to 49%) or stress-induced moderate perfusion defect without left ventricular dilation or increased (thallium-201) lung uptake. There are several high-risk SPECT findings: severe resting left ventricular dysfunction (ejection fraction < 35%); severe exercise left ventricular dysfunction (exercise ejection fraction < 35%); stress-induced large perfusion defect (particularly if anterior); stress-induced multiple perfusion defects of moderate size; large fixed perfusion defect with left ventricular dilation or increased lung uptake; and stress-induced moderate perfusion defect with left ventricular dilation or increased lung uptake. Thus, the findings from SPECT MPI can play a major role in assigning a revascularization procedure to an appropriateness rating.
The ACCF appropriateness criteria initiative is intended to be a dynamic rather than a static process.7 Once the appropriateness criteria documents are published, the ACCF anticipates that there will be many different groups applying these criteria to many different practices. The ACCF designed this process to include periodic reassessment of the individual documents, with revisions based on feedback from users. For instance, new indications may need to be added that were not addressed in the original document, and the rating category assigned to an individual indication may need to be altered based on experience or new published evidence. The ASNC Quality Assurance Committee has already published suggestions for revisions of the ACCF/ASNC SPECT MPI appropriateness criteria.24
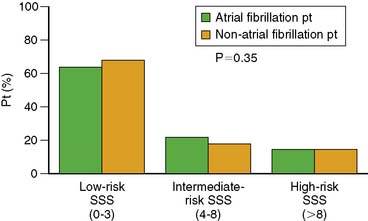
Our laboratory has already published new evidence on the subject of screening for coronary artery disease in asymptomatic patients with new-onset atrial fibrillation.25 The ACCF/ASNC criteria created separate indications for such patients based on clinical risk. Askew et al.25 found that the distribution of summed stress scores in such patients was similar to asymptomatic patients without atrial fibrillation who were matched for age and gender (Fig. 25-5). There was no evidence that the identification of coronary artery disease was more likely in such patients. Moreover, clinical risk factors did not predict an increased prevalence of high-risk summed stress scores in such patients, as implied by the appropriateness ratings of “appropriate” for high-clinical-risk patients and “uncertain” for low-clinical-risk patients.
Precisely how the ACCF appropriateness criteria documents12–16 will be applied remains to be determined. The ACCF anticipates that clinicians, researchers, and payers will use these documents. A SPECT MPI appropriateness criteria decision support application has been developed to facilitate applying the criteria in the clinical setting. This tool can be downloaded from several different internet sites:
Clinicians can apply these documents to examine the appropriateness of test ordering in their institution. The ACCF hopes that indications rated as “uncertain” will stimulate research that will help assign these indications to a more certain rating category. Payers are expected to apply these documents to decide which imaging studies merit reimbursement. The ACCF has launched an initiative with United Health Care to test the appropriateness criteria at the point of service in 10 centers across the country to examine the impact on physician-ordering test patterns. A common goal between clinicians and payers should be to decrease and ultimately eliminate imaging studies being performed for inappropriate indications. Achievement of this goal will be an important step toward the goal of enhancing the efficiency of the health care system and containing escalating health care costs. Hopefully, they will be considered by the National Quality Forum during its ongoing project to develop “voluntary consensus standards for outpatient imaging efficiency.”26
1. Orszag P.R.: Health care and the budget: issues and challenges for reform, 2007. CBO Testimony before the Committee on the Budge United States Senate
2. Orszag P.R., Ellis P. The challenge of rising health care costs—a view from the Congressional Budget Office. N Engl J Med. 2007;357:1793-1795.
3. Gibbons R.J. Finding value in imaging: what is appropriate? J Nucl Cardiol. 2008;15:178-185.
4. Rosamond W., Flegal K., Friday G., et al. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69-e171.
5. Lucas F.L., DeLorenzo M.A., Siewers A.E., et al. Temporal trends in the utilization of diagnostic testing and treatments for cardiovascular disease in the United States. Circulation. 2006;113:374-379.
6. Wennberg J.E., Birkmeyer J.D., Birkmeyer N.J.O., et al. The Dartmouth Atlas of Cardiovascular Health Care. Chicago: American Hospital Association Press, 1999.
7. Patel M.R., Spertus J.A., Brindis R.G., et al. ACCF proposed method for evaluating the appropriateness of cardiovascular imaging. J Am Coll Cardiol. 2005;46:1606-1613.
8. Winslow C.M., Solomon D.H., Chassin M.R., et al. The appropriateness of carotid endarterectomy. N Engl J Med. 1988;318:721-727.
9. Winslow C.M., Kosecoff J.B., Chassin M., et al. The appropriateness of performing coronary artery bypass surgery. JAMA. 1988;260:505-509.
10. Chassin M.R., Kosecoff J., Park R.E., et al. Does inappropriate use explain geographic variations in the use of health care services? A study of three procedures. JAMA. 1987;258:2533-2537.
11. American College of Radiology. Appropriateness Criteria. Reston, VA: American College of Radiology, 1995.
12. Brindis R.G., Douglas P.S., Hendel R.C., et al. ACCF/ASNC appropriateness criteria for single-photon emission computed tomography myocardial perfusion imaging (SPECT MPI): a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group and the American Society of Nuclear Cardiology endorsed by the American Heart Association. J Am Coll Cardiol. 2005;46:1587-1605.
13. Hendel R.C., Patel M.R., Kramer C.M., et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48:1475-1497.
14. Douglas P.S., Khandheria B., Stainback R.F., et al. ACCF/ASE/ACEP/ASNC/SCAI/SCCT/SCMR 2007 appropriateness criteria for transthoracic and transesophageal echocardiography: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American Society of Echocardiography, American College of Emergency Physicians, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and the Society for Cardiovascular Magnetic Resonance endorsed by the American College of Chest Physicians and the Society of Critical Care Medicine. J Am Coll Cardiol. 2007;50:187-204.
15. Douglas P.S., Khandheria B., Stainback R.F., et al. ACCF/ASE/ACEP/AHA/ASNC/SCAI/SCCT/SCMR 2008 appropriateness criteria for stress echocardiography. J Am Coll Cardiol. 2008;51:1127-1147.
16. Patel M.R., Dehmer G.J., Hirshfeld J.W., et al. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 appropriateness criteria for coronary revascularization. J Am Coll Cardiol. 2009;53:530-553.
17. Fitch K., Bernstein S.J., Aguilar M.S., et al. The RAND/UCLA Appropriateness Method User’s Manual. Santa Monica: The RAND Corporation, 2001.
18. Gibbons R.J., Miller T.D., Hodge D.O., et al. Application of appropriateness criteria to stress single-photon emission computed tomography sestamibi studies and stress echocardiograms in an academic medical center. J Am Coll Cardiol. 2008;51:283-290.
19. Mehta R., Agarwal R., Chandra S.W., et al. Evaluation of the ACCF/ASNC appropriateness criteria for SPECT myocardial perfusion imaging. J Nucl Cardiol. 2008;15:337-344.
20. Miller T.D., Hendel R.C. An appropriate challenge for the nuclear cardiology community. J Nucl Cardiol. 2008;15:305-307.
21. Ayyad A.M., Cole J., Syed A., et al. Temporal trends in utilization of cardiac computed tomography. J Cardiovasc Comput Tomogr. 2009;3:16-21.
22. Ward R.P., Mansour I.N., Lemieux N., et al. Prospective evaluation of the clinical application of the American College of Cardiology Foundation/American Society of Echocardiography appropriateness criteria for transthoracic echocardiography. JACC Cardiovasc Imaging. 2008;1:663-671.
23. McCully R.B., Pellikka P.A., Hodge D.O., et al. Applicability of appropriateness criteria for stress imaging: Similarities and differences between stress echocardiography and single-photon emission computed tomography myocardial perfusion imaging (SPECT MPI) criteria. Circ Cardiovasc Imaging. 2009;2:213-218.
24. Ward R.P., Al-Mallah M.H., Grossman G.B., et al. American Society of Nuclear Cardiology review of the ACCF/ASNC appropriateness criteria for single-photon emission computed tomography myocardial perfusion imaging (SPECT MPI). J Nucl Cardiol. 2007;14:e26-e38.
25. Askew J.W., Miller T.D., Hodge D.O., et al. The value of myocardial perfusion single-photon emission computed tomography in screening asymptomatic patients with atrial fibrillation for coronary artery disease. J Am Coll Cardiol. 2007;50:1080-1085.
26. http://www.qualityforum.org/projects/ongoing Last accessed April 26, 2008