CHAPTER 17 Approach to Chondral Damage in the Patellofemoral Joint
Chondral defects of the patellofemoral joint are the most common type of articular cartilage defect found in the knee.1 They also comprise a significant portion of most isolated grade IV articular cartilage defects in the knee joint. These lesions may be found incidentally but also may be a common source of pain and discomfort in the knee.2 Trochlear articular cartilage defects can be symptomatic. The approach to this joint can be complex and may require a combination of arthroscopic and open surgical techniques, up to and including arthroplasty, which is beyond the scope of this chapter.3,4 I shall primarily discuss arthroscopic techniques for chondral defects.
ANATOMY AND PATHOANATOMY
The patellofemoral joint of the knee is a complex articulation, with multiple facets on the patella and a complex, saddle-shaped trochlear groove.5–7 The articular cartilage on the patella itself is the thickest articular cartilage in the body, measuring up to 7 mm thick on the medial facet. Chondral defects on the patella may be have several causes, including degeneration, direct trauma, malalignment, and patellar instability.8–10 The mechanics of the patellofemoral joint are complex and are determined by a number of factors, including neuromuscular coordination, geometry of the trochlea, overall alignment of the extensor mechanism, including the quadriceps tendon, trochlear groove, and tibial tubercle, and soft tissue restraints such as the medial patellofemoral ligament.3,11–13 These restraints help guide patellofemoral motion and often determine whether articular cartilage damage occurs and is symptomatic. For example, excessive patellar malalignment with a laterally tilted and subluxated patella can contribute to excessive articular cartilage wear on the lateral facet of the patella.14,15 Direct trauma to the patella, such as from a dashboard-type injury, in which a flexed knee contacts the dashboard, may result in a central articular cartilage damage to the patella or trochlea.16–19 In addition, many central articular cartilage defects are noted in young athletic individuals. These often have a linear fissure in the center of the trochlea. This reflects the significant forces placed on the patellofemoral joint during activities of daily living and other forms of physical exertion. For example, the joint reaction forces on the patellofemoral joint during stair climbing are up to two to four times body weight, and jumping can create forces up to six to eight times body weight.20–25 These loads can result in failure of the underlying articular cartilage and subchondral bone.
PATIENT EVALUATION
History and Physical Examination
Patients with symptomatic articular cartilage lesions of the patellofemoral joint generally complain of anterior knee pain, particularly when ascending or descending stairs or after sitting.4 Occasionally, patients may complain of crepitus or a symptomatic click as the knee goes into flexion or extension. Elements of the history to obtain from the patient also include known or suspected rheumatologic disease, history of trauma to the knee from a direct blow, history of patellar instability, activity level of the patient, recent changes in activity level, timing of the injury, and whether the pain is acute or chronic. In patients who have already sought previous treatment, it is important to identify the nature of treatment, including physical therapy, bracing, and taping and any previous surgery. Complaints of recurrent or consistent effusions should also be identified. Knee pain diagrams often provide relevant clinical information.26
Physical examination should combine static and dynamic evaluation. The presence or absence of any effusion should be noted and the knee should be carefully palpated and evaluated for ligamentous and patellar instability. At 30 degrees of knee flexion, the patella will typically translate laterally approximately 9 mm and have a relatively firm end point because of the check rein of the medial patellofemoral ligament.27 The patella should also translate medially 7 mm and the lateral patellar retinaculum should allow the patella to be tilted to a neutral position, where the anterior surface of the patella is parallel to the ground while the patient is supine. Quadriceps development, particularly that of the vastus medialis obliquus muscle, should be assessed.4 Extensor alignment including femoral version, Q angle, and tibial rotation can provide insights into patellar tracking.
Dynamic examination of the knee should be performed as well. Abnormalities of gait should be identified.28 Evaluation of knee stability and identification of pain or crepitus while doing a step-up or step-down maneuver may help elicit specific complaints. A single leg squat test may also elicit pain and will allow evaluation of relative knee valgus or internal rotation, which can increase patellofemoral forces.
Diagnostic Imaging
Radiography
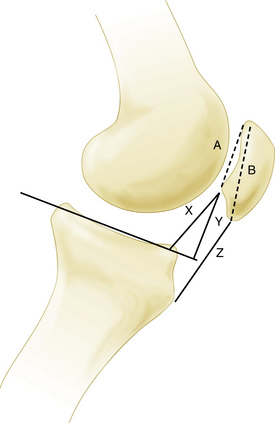
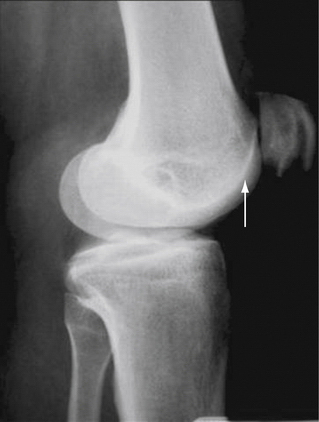
A true lateral view with overlapping femoral condyles, with less than 5% displacement, can provide valuable information regarding the relative patellar height. This can be assessed using various ratios, including the Insall-Salvati index and Blackburne-Peel method (Fig. 17-1)—the articular surface length compared with the distance from its inferior margin to the tibial plateau. This technique has been demonstrated to be the most reliable. A true lateral view can also yield information about the degree of trochlear dysplasia. The trochlear groove can be identified on the lateral x-rays, on which a dense white line of the trochlea should not intersect vvthe bone of the lateral femoral condyle (Fig. 17-2). A sunrise or Merchant view at 45 degrees of flexion can give indicate patellar tracking within the trochlea and the presence of lateral patellar tilt or subluxation. A number of measurements have been described; however, I agree with Merchant that “(f)or most patients, it is quicker and just as good to eyeball the film rather than spend time drawing lines and calculating measurements.”29
Magnetic Resonance Imaging
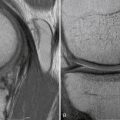
Magnetic resonance imaging (MRI) is currently the best modality to image articular cartilage defects.30,31 Appropriate articular cartilage sequences and thin cuts often reveal articular cartilage damage, such as chondromalacia, chondral flaps, or delamination. In addition, it can also identify areas of underlying subchondral bony edema. MRI is also useful to assess abnormalities of soft tissue that may be contributing to underlying chondral damage or patellofemoral pain, such as synovitis, lesions of the medial patellofemoral ligament, tendinopathy, and articular cartilage loose bodies. MRI can also be used to perform measurements of tibial tubercle to trochlear groove distance. This can indicate conditions that could lead to patellar instability and subsequent articular cartilage damage.
TREATMENT
Conservative Management
In most cases, initial management should consist of physical therapy for quadriceps strengthening, with or without patellar bracing, relative rest, and appropriate analgesic medications, including acetaminophen or nonsteroidal anti-inflammatory drugs (NSAIDs). Patients with symptomatic patellofemoral pain may have been previously shown to have substantial weakness of quadriceps muscle activity and, specifically, weakness of the vastus medialis obliquus.32
Arthroscopic Technique
Arthroscopic surgical techniques for the patellofemoral joint vary from simple débridement to microfracture procedures and osteochondral autograft transplantation. In addition, in rare cases, arthroscopic lateral release or medial plication can assist in the realignment of soft tissues affecting the patellofemoral joint and patellar tracking. However, isolated lateral release has extremely limited application and may result in medial instability.4,33
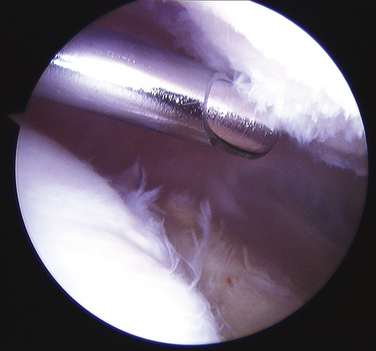
Arthroscopic chondroplasty should consist of débridement of unstable articular cartilage flaps to a stable margin. These flaps can be a source of pain and discomfort. Patients may experience a click at a specific degree of knee flexion or significant crepitus. Such delaminations often occur at the calcified cartilage layer and can easily progress. I recommend the use of mechanical débridement2,34 with an arthroscopic shaver to remove these flaps rather than electrocautery or radiofrequency devices, because there is a concern for adjacent articular cartilage damage caused by the heat produced by the electrocautery.35,36 Technical recommendations for the mechanical débridement of grade II or III chondromalacia include the use of a small-radius (3.5- to 4.5-mm), high-speed, smooth-edged shaver. Arthroscopic portal placement may need to be adjusted distally to allow instruments access to the undersurface of the patella. Typically, in cases in which there is significant grade III or crabmeat-type chondromalacia, the noncutting part of the shaver blade is placed at the base of the articular cartilage surface so that the fronds can be sharply amputated at their base (Fig. 17-3). The irregular strands and rough surface can lead to symptomatic crepitus and recurrent effusions in the joint. If there are full-thickness articular cartilage flaps, it is sometimes easier to amputate them sharply at their base by using an arthroscopic Beaver blade. Occasionally, it is useful to use a curved shaver to access the undersurface of the patella.
A microfracture procedure for chondral defects of the patellofemoral joint is typically less successful than microfracture involving the femoral condyles.37 The lesion is typically prepared by débriding unstable articular cartilage edges until a very sharply defined margin of the defect is created. The calcified cartilage layer in the bed of the defect must be removed. This is sometimes easier to perform by using sharp curettes or an arthroscopic Beaver blade to scrape away the calcified cartilage layer. Multiple perforations are then made in the bone, starting at the periphery and separated by approximately 3 to 4 mm. This can be done with a sharp awl or K wire or drill bit. Particularly on the patella, drilling rather than an awl may be preferred because the underlying subchondral bone is thick, and it can be difficult to stabilize both the patella and awl enough to allow adequate perforation of the underlying bone. If an awl is chosen, a 90-degree angle may be helpful to avoid skiving.38 Drilling can be performed by using accessory nontraditional portals located more posteriorly than the standard medial inferior lateral or proximal superior lateral portals (Fig. 17-4).
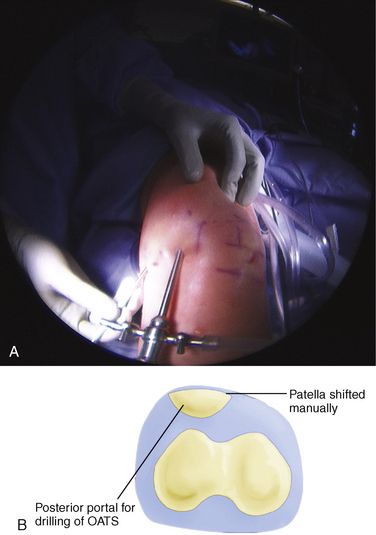
FIGURE 17-4 A, B, Posterior portal for perpendicular patellar drilling for the osteochondral allograft procedure.
Osteochondral Autograft Transplantation
Osteochondral autograft transplantation has been demonstrated in some studies to be effective in the patellofemoral joint.8,10,39,40 With relatively larger defects. there is difficulty in reproducing the saddle-shaped geometry of the trochlear articulation. In addition, the articular cartilage thickness of the patella is typically not replicated by the use of osteochondral plugs harvested from the femoral condyle. Despite this, the results for osteochondral autograft transplantation for this area are relatively good, and is my preferred technique for significant articular cartilage lesions of the patellofemoral joint, in particular for those involving damage to or cystic changes of the subchondral bone.
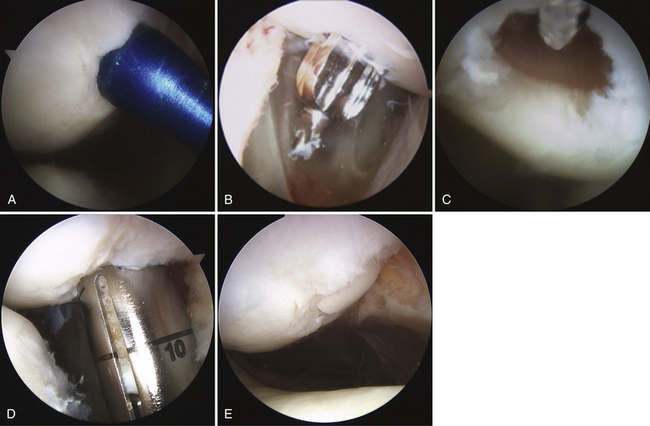
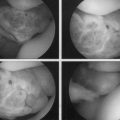
Typically, the area of the defect is débrided and the margins of the defect identified. After appropriate identification of the perpendicular orientation to the articular cartilage defect, the sizing tools are placed arthroscopically through the appropriate portal to determine the circumference of the defect (Fig. 17-5A) and the number of appropriate osteochondral autograft transplant plugs that will be needed to fill in the defect. In the case of articular cartilage defects on the patella it may be extremely difficult to use standard arthroscopic portals to achieve a perpendicular location to the patella. The spinal needle can be used to identify the appropriate trajectory and orientation of a portal. I have used a more posterior nonstandard portal.
The defect site is then prepared. This can be done by using a perpendicularly placed tubular chisel or a drilling system to create the bony socket for the osteochondral plug. Particularly for patellar defects, it is recommended that a drill system be used because the bone of the patella is extremely hard and the patella has minimal stability. This can be done by one of two techniques. A guide pin is placed perpendicular to the surface, followed by appropriate reaming over the guide pin. The second method is to use a specially designed drill guide with a flat tip and a retrograde drilling technique. With this method, a retro drill pin is passed anterior to posterior through the patella; the cutter tip is flipped and the drill bit attached within the joint to drill a socket in a retrograde fashion (see Fig. 17-5B and C). The patella typically has to be subluxated manually, either medially or laterally, so that instruments will not damage the adjacent femoral articular cartilage.
After the appropriate socket has been created, a calibrated measuring rod is placed within the socket to provide more precise measurement of the socket in the various quadrants. The donor plug is then appropriately rotated to the correct orientation to minimize any incongruities and gently delivered into the prepared socket using a press-fit technique (see Fig. 17-5D).
It is critical to have congruency of the articular surface. If perfect congruency cannot be achieved, it is preferable to have the donor cartilage slightly countersunk with respect to the adjacent articular cartilage (see Fig. 17-5E).41
PEARLS& PITFALLS
PEARLS
PITFALLS
Postoperative Rehabilitation
Following arthroscopic chondroplasty of the patella, patients may undergo immediate weight bearing. If a simple débridement has been performed, the knee can go through a full range of motion, with normal weight bearing. However, if a microfracture or osteochondral autograft transplant has been performed, weight bearing with the knee in a flexed position may unduly load the treated areas. Weight bearing can be permitted with the knee in full extension. However, weight bearing while the knee is flexed should be strictly limited during the first 4 to 6 weeks following the procedure.38 Continuous passive motion may enhance the articular cartilage repair and there are some limited data suggesting that the use of hyaluronic acid may also enhance the articular cartilage repair.
1. Curl WW, Krome J, Gordon ES, et al. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13:456-460.
2. Levy AS, Lohnes J, Sculley S, et al. Chondral delamination of the knee in soccer players. Am J Sports Med. 1996;24:634-639.
3. Fulkerson JP. The effects of medialization and anteromedialization of the tibial tubercle on patellofemoral mechanics and kinematics. Am J Sports Med. 2007;35:147.
4. Fulkerson JP. Diagnosis and treatment of patients with patellofemoral pain. Am J Sports Med. 2002;30:447-456.
5. Feller JA, Amis AA, Andrish JT, et al. Surgical biomechanics of the patellofemoral joint. Arthroscopy. 2007;23:542-553.
6. Senavongse W, Amis AA. The effects of articular, retinacular, or muscular deficiencies on patellofemoral joint stability. J Bone Joint Surg Br. 2005;87:577-582.
7. Shih YF, Bull AM, Amis AA. The cartilaginous and osseous geometry of the femoral trochlear groove. Knee Surg Sports Traumatol Arthrosc. 2004;12:300-306.
8. Jakob RP, Franz T, Gautier E, Mainil-Varlet P. Autologous osteochondral grafting in the knee: indication, results, reflections. Clin Orthop Relat Res. 2002;(401):170-184.
9. Nomura E, Inoue M, Kurimura M. Chondral and osteochondral injuries associated with acute patellar dislocation. Arthroscopy. 2003;19:717-721.
10. Nho SJ, Foo LF, Green DM, et al. Magnetic resonance imaging and clinical evaluation of patellar resurfacing with press-fit osteochondral autograft plugs. Am J Sports Med. 2008;36:1101-1109.
11. Amis AA. Current concepts on anatomy and biomechanics of patellar stability. Sports Med Arthrosc. 2007;15:48-56.
12. Farahmand F, Naghi Tahmasbi M, Amis AA. The contribution of the medial retinaculum and quadriceps muscles to patellar lateral stability—an in-vitro study. Knee. 2004;11:89-94.
13. Merican AM, Kondo E, Amis AA. The effect on patellofemoral joint stability of selective cutting of lateral retinacular and capsular structures. J Biomech. 2009;42:291-296.
14. Hunter DJ, Zhang YQ, Niu JB, et al. Patella malalignment, pain and patellofemoral progression: the Health ABC Study. Osteoarthritis Cartilage. 2007;15:1120-1127.
15. Kalichman L, Zhu Y, Zhang Y, et al. The association between patella alignment and knee pain and function: an MRI study in persons with symptomatic knee osteoarthritis. Osteoarthritis Cartilage. 2007;15:1235-1240.
16. Atkinson PJ, Haut RC. Injuries produced by blunt trauma to the human patellofemoral joint vary with flexion angle of the knee. J Orthop Res. 2001;19:827-833.
17. Buckwalter JA, Martin JA, Olmstead M, et al. Osteochondral repair of primate knee femoral and patellar articular surfaces: implications for preventing post-traumatic osteoarthritis. Iowa Orthop J. 2003;23:66-74.
18. Gobbi A, Kon E, Berruto M, et al. Patellofemoral full-thickness chondral defects treated with Hyalograft-C: a clinical, arthroscopic, histologic review. Am J Sports Med. 2006;34:1763-1773.
19. Hjelle K, Solheim E, Strand T, et al. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;8:730-734.
20. Crossley KM, Cowan SM, Bennell KL, McConnell J. Knee flexion during stair ambulation is altered in individuals with patellofemoral pain. J Orthop Res. 2004;22:267-274.
21. Hungerford DS, Barry M. Biomechanics of the patellofemoral joint. Clin Orthop Relat Res. 1979;(144):9-15.
22. Mason JJ, Leszko F, Johnson T, Komistek RD. Patellofemoral joint forces. J Biomech. 2008;41:2337-2348.
23. Powers CM, Ward SR, Chen YJ, et al. Effect of bracing on patellofemoral joint stress while ascending and descending stairs. Clin J Sports Med. 2004;14:206-214.
24. Powers CM, Ward SR, Chen YJ, et al. The effect of bracing on patellofemoral joint stress during free and fast walking. Am J Sports Med. 2004;32:224-231.
25. Reilly DT, Martens M. Experimental analysis of the quadriceps muscle force and patellofemoral joint reaction force for various activities. Acta Orthop Scand. 1972;43:126-137.
26. Post WR, Fulkerson J. Knee pain diagrams: correlation with physical examination findings in patients with anterior knee pain. Arthroscopy. 1994;10:618-623.
27. Fithian DC, Mishra DK, Balen PF, et al. Instrumented measurement of patellar mobility. Am J Sports Med. 1995;23:607-615.
28. Heino Brechter J, Powers CM. Patellofemoral stress during walking in persons with and without patellofemoral pain. Med Sci Sports Exerc. 2002;34:1582-1593.
29. Merchant AC. Patellofemoral imaging. Clin Orthop Relat Res. 2001;(389):15-21.
30. Potter HG, Linklater JM, Allen AA, et al. Magnetic resonance imaging of articular cartilage in the knee. An evaluation with use of fast-spin-echo imaging. J Bone Joint Surg Am. 1998;80:1276-1284.
31. Shindle MK, Foo LF, Kelly BT, et al. Magnetic resonance imaging of cartilage in the athlete: current techniques and spectrum of disease. J Bone Joint Surg Am. 2006;88(suppl 4):27-46.
32. Makhsous M, Lin F, Koh JL, et al. In vivo and noninvasive load sharing among the vasti in patellar malalignment. Med Sci Sports Exerc. 2004;36:1768-1775.
33. Fithian DC, Paxton EW, Post WR, et al. Lateral retinacular release: a survey of the International Patellofemoral Study Group. Arthroscopy. 2004;20:463-468.
34. Federico DJ, Reider B. Results of isolated patellar debridement for patellofemoral pain in patients with normal patellar alignment. Am J Sports Med. 1997;25:663-669.
35. Edwards RB 3rd, Lu Y, Uthamanthil RK, Bogdanske JJ, et al. Comparison of mechanical debridement and radiofrequency energy for chondroplasty in an in vivo equine model of partial thickness cartilage injury. Osteoarthritis Cartilage. 2007;15:169-178.
36. Ryan A, Bertone AL, Kaeding CC, et al. The effects of radiofrequency energy treatment on chondrocytes and matrix of fibrillated articular cartilage. Am J Sports Med. 2003;31:386-391.
37. Mithoefer K, Williams RJ 3rd, Warren RF, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005;87:1911-1920.
38. Mithoefer K, Williams RJ 3rd, Warren RF, et al. Chondral resurfacing of articular cartilage defects in the knee with the microfracture technique. Surgical technique. J Bone Joint Surg Am. 2006;88(suppl 1):294-304.
39. Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85(suppl 2):25-32.
40. Gudas R, Kalesinskas RJ, Kimtys V, et al. A prospective randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint in young athletes. Arthroscopy. 2005;21:1066-1075.
41. Koh JL, Wirsing K, Lautenschlager E, Zhang LO. The effect of graft height mismatch on contact pressure following osteochondral grafting: a biomechanical study. Am J Sports Med. 2004;32:317-320.