CHAPTER 4 Applied anatomy and physiology of the gastrointestinal tract (GIT)
Overview
The gastrointestinal (GI) tract, also known as the alimentary canal, commences at the buccal cavity of the mouth and terminates at the anus. It can be divided into an upper GI tract (consisting of mouth, pharynx, esophagus and stomach) and a lower GI tract (small and large intestines). The three primary functions of the GI tract are the ingestion of food and water, the digestion of food and absorption of nutrients and the expulsion of waste matter. These primary functions are carried out in conjunction with the accessory digestive organs such as the salivary glands, pancreas, liver and gall bladder. Further detailed exploration of the accessory abdominal organs is outside the remit of this book, but suitable references are suggested at the end of the chapter.
Embryology of the GI tract
As the newly implanted embryo reaches the fourth week of gestation it begins to fold ventrally (anteriorly) in two directions. The head and tail end of the embryo curl towards each other and the sides of the embryo begin to fold ventrally towards each other. A constriction results between the embryo and the greater part of the yolk sac, trapping a small part of the yolk sac within the embryo (Figure 4.1A). The constriction corresponds to the future umbilicus. The trapped part of the yolk sac becomes the primitive digestive tube (Lewis, 2000).
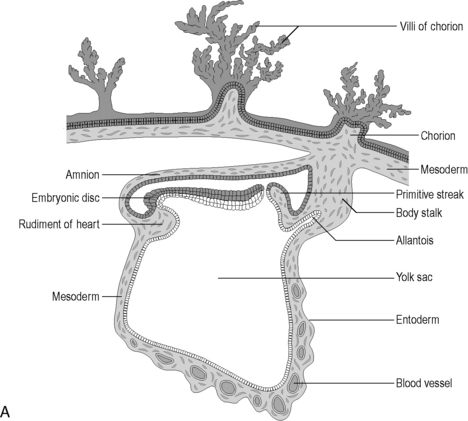
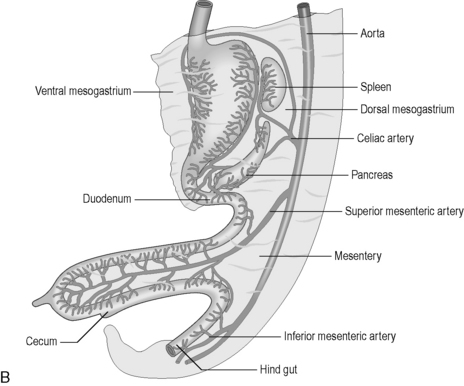
Figure 4.1 (A) The development of the primitive digestive tube. The embryonic disk curves ventrally trapping some of the yolk sac within it. This forms the primitive digestive tube. (B) The embryological gut and its blood supply.
(From an original drawing in Gray’s Anatomy).
Further anterior growth of the cranial end of the embryo results in folding of the buccopharyngeal membrane and this causes a diverticulum of the digestive yolk sac to form – this becomes the foregut. Another diverticulum also extends from the caudal end, becoming the hindgut. The remaining primitive digestive tube is known as the midgut. For the first four weeks of gestation, a wide communication exists between the digestive tube and the main yolk sac, but this gradually narrows to form the vitelline duct. This usually regresses but, where it remains after birth, it is known as a Meckel’s diverticulum, found in approx 2% of individuals (Lewis, 2000). The diverticulum is a 5 cm long blind-ended pouch projecting from the ileum in the affected adult, approximately 1 metre from the ileocecal valve (Lewis, 2000).
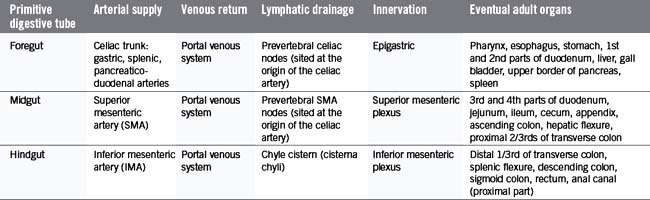
The primitive gut is well vascularized, receiving blood from the aorta via the celiac trunk (foregut), the superior mesenteric artery (midgut) and the inferior mesenteric artery (hindgut) (Figure 4.1B).
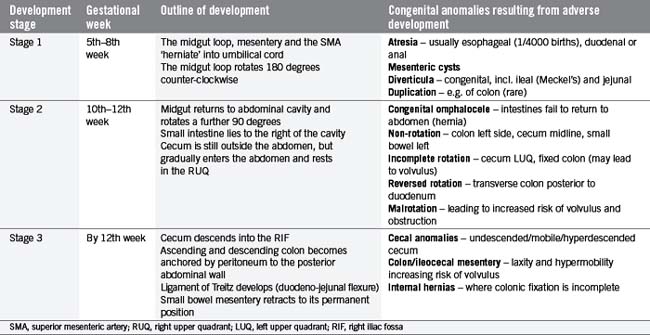
In the fifth gestational week, the primitive gut grows rapidly and reorganizes into the permanent GI tract structures (Table 4.1). This progression is traditionally divided into three stages:
Table 4.2 outlines the three stages and considers some congenital anomalies that can be seen as a result of developmental problems during these three stages. In particular, midgut rotational abnormalities are relatively common and can be seen in Figure 4.2.
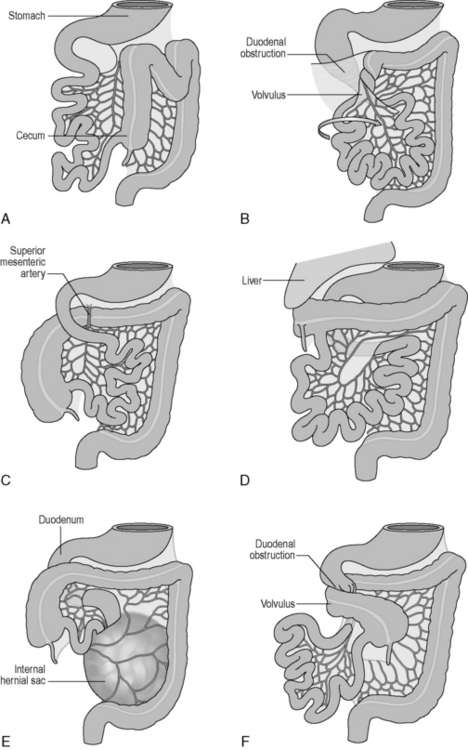
Figure 4.2 Abnormalities of midgut rotation. (A) Nonrotation; (B) Mixed rotation and volvulus (twisting) of the intestines; (C) Reversed rotation; (D) Subhepatic cecum; (E) Paraduodenal hernia; (F) Midgut volvulus.
(Reproduced from Moore KL and Persaud TVN, Before we are Born: Essentials of Embryology and Birth Defects, 7th edn. Saunders, Philadelphia, 2007.)
Histology of the GI tract
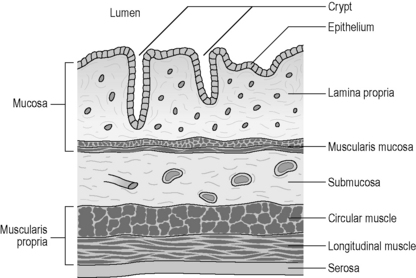
The GI tract displays a uniform histology throughout its length, with subtle differences between regions corresponding to functional specialization. The GI tract is divided into four concentric layers surrounding the lumen as shown in Figure 4.3 (mucosa, submucosa, muscularis externa and serosa or adventitia).
The innermost layer is known as the mucosa, which surrounds the lumen of the GI tract. It has both a protective function (from injury and infection), as well as a digestive function (chemical breakdown, absorption and secretion). The mucosa can be further subdivided into three layers. The epithelium is the innermost layer, in contact with the ingested material. It is supported structurally and nutritionally by the lamina propria, a layer of loose irregular connective tissue, well supplied by blood capillaries and lymphatic tissue. The outermost subdivision of the mucosa is a thin layer of smooth muscle known as the muscularis mucosae. This smooth muscle throws the mucosa into small folds, increasing the absorptive surface area and encouraging the turbulence of the fluid contents (chyme). The mucosal layer is highly specialized within each organ of the GI tract, reflecting the different functions and chemical environments (Table 4.3).
| GI tract organ | Cellular composition of mucosa | Mucosal features |
|---|---|---|
| Mouth | Stratified squamous epithelium for protection | The epithelium is partially keratinized on gums and hard palate. A muscularis mucosae is not present |
| Pharynx |
The muscularis propria (externa) generally consists of two smooth muscle layers; however, in the esophagus, striated (skeletal) muscle replaces the smooth muscle in the proximal third. Throughout the rest of the GI tract, the inner circular muscle layer has smooth muscle fibers lying in a concentric fashion around the circumference of the GI tract, while the outer longitudinal muscle layer has fibers orientated parallel to the direction of the GI tract. (Note that in the stomach there is also an additional innermost oblique layer of muscle fibers, mainly limited to the cardiac end of the stomach). In between the two layers lies a second enteric nerve plexus, known as the myenteric (or Auerbach’s) plexus. This plexus is responsible for coordinating motility (movement) of the GI tract, including peristalsis, whereby coordinated contractions of the two muscle layers assist in propelling the food bolus along the lumen.
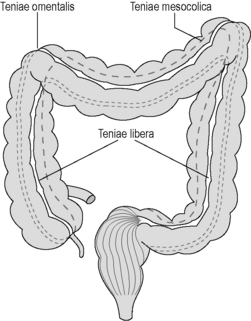
In the colon, the longitudinal muscle layer is incomplete, being gathered into three 1 cm thick bands called the teniae coli, with only a thin layer of muscle in between. These bands (the teniae omentalis, libera and mesocolica) converge on the base of the appendix, run the full length of the colon, fanning out into a continuous layer surrounding the rectum (Figure 4.4). Contraction of the teniae coli gathers up the colon in a concertina effect, the resulting sacculations being known as haustral pouches or haustrations. These are clearly defined and fixed in the proximal colon, but require active contraction in the distal colon, where the teniae are thinner (see Chapter 16).
Oral cavity
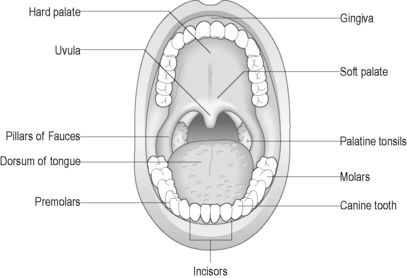
The oral cavity (mouth) is designed to support chewing, swallowing and speech. Two rows of teeth are embedded within the maxilla above and the mandible below and are surrounded by the gums (gingivae). The functions of the mouth are also facilitated by the hard and soft palate above, the floor of the mouth below, the cheeks laterally, the tongue, and the upper and lower lips externally (Figure 4.5). Two separate sets of teeth are grown in humans: the primary dentition (deciduous teeth) develops during early childhood and consists of 20 teeth. The secondary dentition (permanent teeth) gradually replaces the deciduous set with 32 adult teeth. These teeth are adapted for different functions, including cutting (incisors), tearing (canines), crushing (premolars) and grinding (molars).
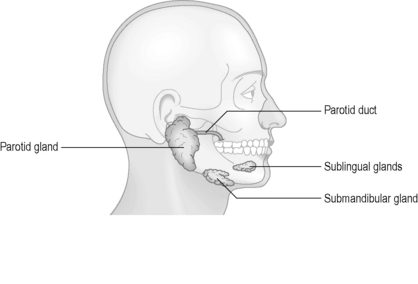
Several other openings within the oral cavity are associated with the salivary ducts. Three pairs of salivary glands secrete saliva into the oral cavity in response to food stimuli (Figure 4.6). Saliva is composed of primarily water (approximately 98%), in combination with ions, salivary amylase (which begins the breakdown of starch to sugars), lysozymes (antibacterial), as well as trace quantities of urea (a waste product). The saliva lubricates the food and helps to form it into a bolus suitable for swallowing.
The pharynx
The pharynx is a complex anatomical structure serving as a gateway to both the digestive and respiratory passageways. During respiration, the pharynx provides a patent airway from the nose and mouth through to the larynx. During swallowing (deglutition) it provides a passageway from the mouth to the esophagus, while ensuring that the bolus cannot enter the airway. The pharynx also has important functions in relation to speech, choking, vomiting and yawning (Rubesin, 1999).
The pharynx lies posteriorly to the nasal cavities, the oral cavity and the larynx. Laterally lie the muscles of the neck, the lateral portions of the hyoid bone and thyroid cartilage and the carotid sheath (Rubesin, 2000).
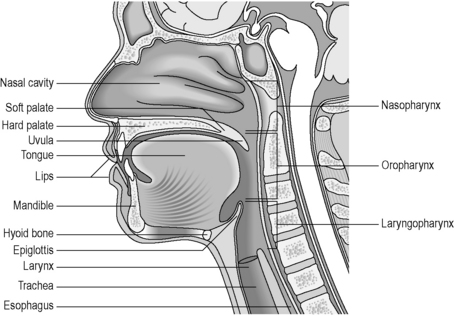
In humans, the pharynx can be divided into three parts: the nasopharynx, the oropharynx and the laryngopharynx (hypopharynx) (Figure 4.7). The nasopharynx, a permanently patent respiratory tract structure lying behind the nasal cavity, is normally excluded from the digestive tract by the soft palate. During swallowing, the bilateral pharyngo-tympanic (Eustachian) tubes, which connect the middle ears to the nasopharynx, open up to allow equilibrium of air pressures on either side of the tympanic membrane. During respiration, this passageway is closed. Within the posterior walls of the nasopharynx lie the pharyngeal tonsils, or adenoids, which are most prominent during childhood. These can create a nodular appearance on radiological examination and, if enlarged, can interfere with speech and respiration.
The oropharynx extends from the soft palate to the level of the hyoid bone, although this is a seemingly arbitrary description, as the hyoid bone and soft palate position changes during speech, respiration and swallowing (Rubesin, 1999). The oropharynx communicates anteriorly with the oral cavity, with the anterior wall made up of the base of the tongue and the valleculae. The valleculae are paired cup-shaped spaces sitting behind the tongue, separating the tongue from the epiglottis, a leaf-shaped cartilage which is essential in closing off the respiratory passages during swallowing. The valleculae are not permanent structures, disappearing on swallowing as the epiglottis inverts. The aryepiglottic folds form the upper lateral margins of the epiglottis. The lateral wall of the oropharynx is made up of the palatine tonsils, tonsillar fossa and faucial pillars.
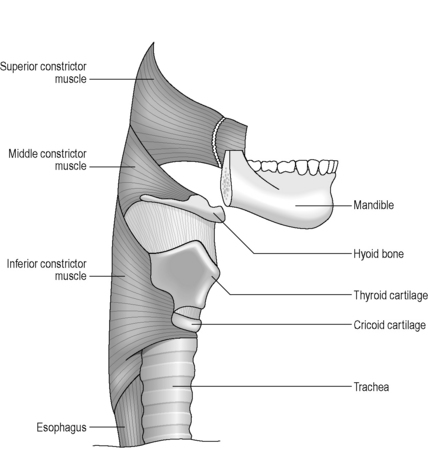
The shape of the pharynx is determined by the underlying muscles as well as the indentation of cartilages as described above. The pharynx is divided into two muscle layers: an inner longitudinal layer and an outer circular layer. The outermost layer forms a ring of constricting muscles, which are incomplete anteriorly. These constrictor muscles are divided into superior, middle and inferior bands, which serve to push the food bolus sequentially through the pharynx (Figure 4.8). The internal layer of longitudinal muscle is closely associated with major folds of mucosa, resulting in the appearance of longitudinal striations on the lateral and posterior walls on contrast studies. Transverse patterns along the anterior wall result from redundant mucosa overlying the arytenoid and cricoid cartilages (Rubesin, 1999). Killian’s dehiscence is a triangular area in the wall of the pharynx, lying in the midline between the inferior constrictor muscle and the cricopharyngeus muscle. It is of clinical significance as it represents a potentially weak spot where a pulsion diverticulum (Zenker’s diverticulum) is more likely to occur.
Pharyngeal function depends on the interplay of the intrinsic muscles of the pharynx and larynx, along with the extrinsic muscles of the pharynx, arising from the base of skull, neck, tongue, mandible and hyoid bone. The mechanism of swallowing, known as deglutition, depends on a complex sequence of muscular contraction coordinated by six cranial nerves and three cervical nerves (Jones, 2000), enabling passage of the food bolus into the esophagus, while protecting the airway. In preparation for swallowing, the pharynx is drawn upwards and sideways, so increasing its transverse diameter. Anterior and superior movement of the tongue and larynx open the pharynx anteroposteriorly. When the food bolus is passed into the pharynx, the elevator muscles relax, the pharynx descends and the constrictor muscles begin to contract sequentially, so conveying the bolus into the esophagus. The process of deglutition is commonly divided into an oral (voluntary) stage, followed by involuntary pharyngeal and esophageal phases. These phases will be discussed in detail in Chapter 7.
The esophagus
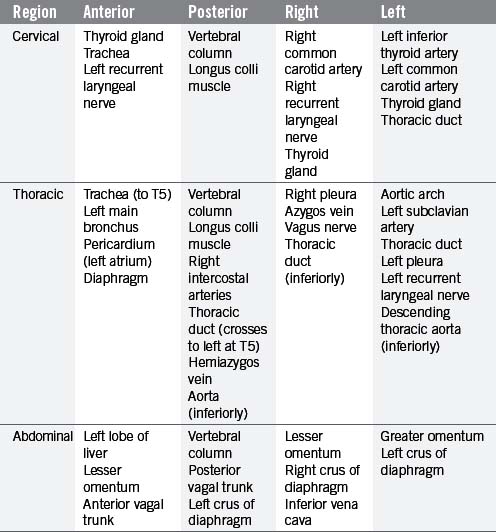
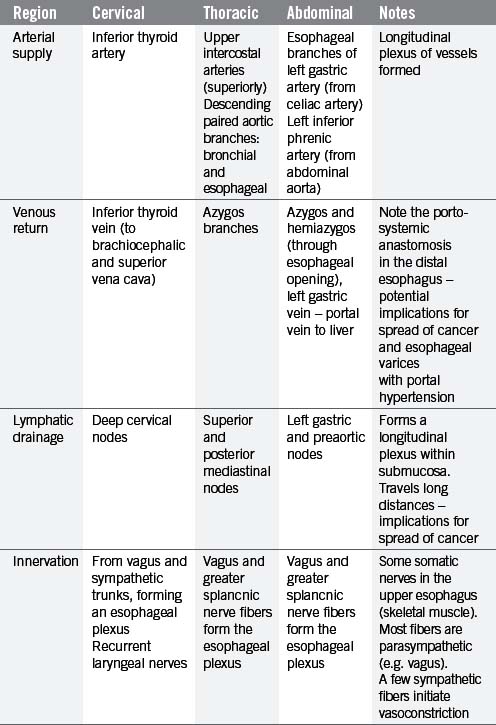
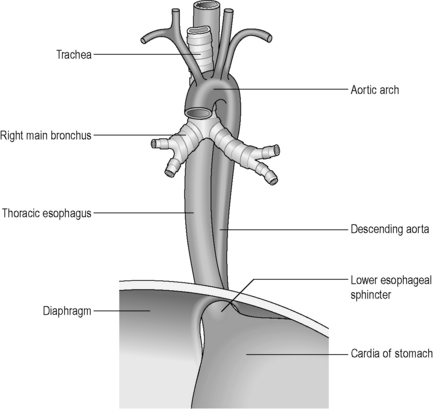
The esophagus is a muscular tube (approximately 23 cm long), commencing at the base of the laryngopharynx (CV6) and descending through the superior and posterior mediastinum of the thoracic cavity immediately anterior to the vertebral column. It passes through the diaphragm to enter the abdominal cavity, connecting with the stomach at the cardiac orifice (TV11). The esophagus can therefore be divided into arbitrary divisions known as the cervical, thoracic and abdominal portions (see Table 4.4 of relations), although in radiological reports the esophagus is often delineated into upper, middle and lower thirds. As the esophagus traverses through these regions of the body, the associated anatomical relations, blood supply, venous return, innervation and lymphatic drainage will change accordingly (Tables 4.4 and 4.5). While the course of the esophagus is predominantly vertical, it also follows the curve of the vertebral column anteroposteriorly, and changes course laterally at several vertebral levels (Figure 4.9). A number of anatomical and physiological indentations occur along its course (Table 4.6).
| Level of constriction | Anatomical impression |
|---|---|
| CV6 | Inferior constrictor muscle impression |
| TV4 | Crossing of the aortic arch |
| TV5–6 | Crossing of the left main bronchus |
| TV10 | Passage through the diaphragm at the esophageal hiatus |
The esophagus has four coats: an external fibrous coat (adventitia), a muscular coat, a submucosal coat and an internal mucous coat. The muscular coat comprises an outer longitudinal coat and inner circular muscles, continuous with the inferior pharyngeal constrictor muscles and the circular muscles of the stomach. The muscle fibers are striated in the upper esophagus and smooth in the lower two thirds. The submucosal coat contains numerous glands secreting mucus, protecting and lubricating the epithelium and aiding the passage of the food bolus. The mucosa is lined by stratified squamous epithelium and is orientated in longitudinal folds which disappear on distension (Lewis, 2000).
The passage of a food bolus from the pharynx into the esophagus is regulated by the upper esophageal sphincter. This sphincter is formed primarily by contraction of the cricopharyngeal muscle (the horizontal portion of the inferior constrictor muscle). The lower esophageal sphincter is not a true muscular sphincter mechanism, but is defined as a high-pressure zone measuring 2–4 cm in length in the esophago-gastric region (Ott, 2000).
At rest, the esophageal body is in a collapsed state, with the upper and lower esophageal sphincters closed to prevent retrograde flow of ingested food contents. When swallowing is initiated, the esophagus propels the food bolus from the pharynx into the stomach, aided by peristalsis, as well as gravity with upright posture. Peristalsis is the rhythmic contraction of smooth muscles, creating a pressure wave, thus propelling the food bolus along the GI tract. Peristalsis is normally initiated by circular muscles, which contract behind the food bolus to prevent retrograde flow. This is followed by longitudinal muscle contraction, driving the bolus forwards. The primary peristaltic wave is initiated when the food bolus enters the pharynx. The upper esophageal sphincter relaxes almost immediately, with relaxation of the lower esophageal sphincter occurring several seconds later. This wave moves through the esophagus in approximately 6–8 seconds (Ott, 2000). Once the food bolus reaches the stomach, the lower esophageal sphincter returns to its resting tone to help prevent refluxing of stomach contents. If a food bolus gets stuck, or moves slower than the primary wave, the distension stimulates local stretch receptors in the esophagus, which initiate a secondary peristaltic wave. This secondary wave works behind the bolus to drive it forwards and continues until the bolus enters the stomach. In contrast to these peristaltic waves, occasionally tertiary or non-peristaltic waves can be seen. These waves occur spontaneously or during swallowing, and while they may be non-specific, they may also be associated with structural or motility disorders of the esophagus (Ott, 2000).
The stomach
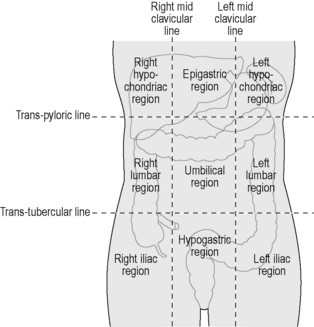
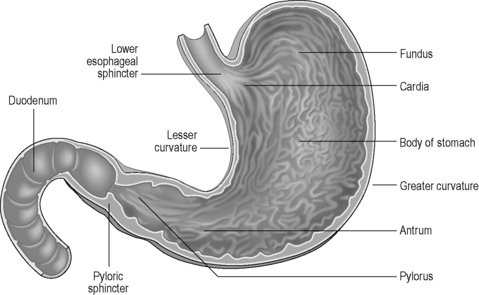
The stomach lies within the epigastric, umbilical and left hypochondriac regions of the abdomen (Figure 4.10) and is the most dilated part of the GI tract. Its shape and position varies from person to person and within the phase of digestion, but is classically said to represent a ‘J’ shape. The stomach presents two openings, two curvatures and two surfaces for description purposes (Figure 4.11). The esophagus communicates with the stomach via the cardiac orifice, situated to the left of the midline at approximately TV10. The left border of the esophagus is continuous with the greater curvature of the stomach, while the right border is continuous with the lesser curvature. This right esophageal border meets the stomach at a sharp angle, known as the cardiac incisura. The inferior opening of the stomach is the pyloric orifice, communicating with the duodenum, lying to the right of the midline at the level of the upper border of LV1.
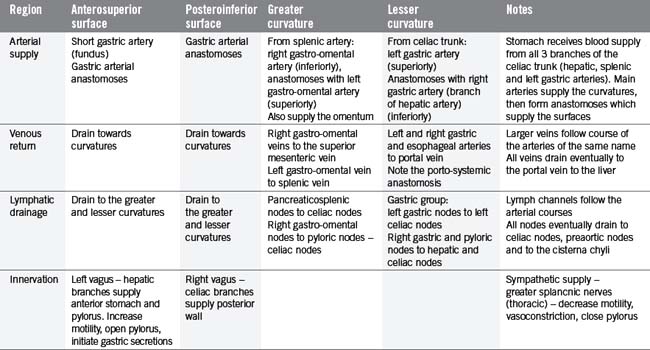
The stomach can be subdivided into a left portion or body, a superior portion or fundus and a right portion or antrum, as shown in Figure 4.11. The circulation, innervation and lymphatic drainage of these subdivisions is demonstrated in Table 4.7. When erect the fundus is often distended with gas.
The wall of the stomach consists of four coats as outlined previously. The serous coat is derived from peritoneum, completely enveloping the stomach except at the curvatures where the omenta attach – this creates a small space through which nutrient vessels and nerves pass (Lewis, 2000). The muscular coat consists of an outer longitudinal layer, with fibers continuous with those of the esophagus and distally with the duodenum and pyloric sphincter. The circular fibers are continuous with the esophageal muscles and are most abundant within the pylorus, forming the sphincter. The oblique fibers lie internal to the circular layer, mainly limited to the cardiac region. The submucosa is loose connective tissue, connecting muscular with mucosal layers. The mucosal layer is thick and is thrown into numerous longitudinally-orientated bands called rugae. These bands are most visible towards the pylorus and greater curvature and disappear upon distension. There is a sharp change from stratified epithelium in the distal esophagus to columnar epithelium with mucos-secreting goblet cells in the stomach.
A network of shallow grooves divides the mucosa up into gastric areas approximately 1–5 mm in diameter (areae gastricae). This surface pattern can be seen in barium studies as a faint honeycomb appearance in approximately 70% of patients (Levine and Laufer, 1999). Small shallow depressions on the mucosal surface are the openings of the gastric pits, into which several gastric glands spill their contents. Mucus-secreting cells secrete alkaline mucus which sticks to the columnar epithelium, creating a 1 mm thick protective barrier. Secretion of hydrochloric acid by parietal cells creates a hostile environment within the stomach lumen of around pH 1–4, and this, coupled with the secretion of gastric enzymes which begin the digestion of proteins, put significant stresses upon the stomach mucosa. A number of factors combine to help to reduce the chances of autodigestion of the stomach lining (Table 4.8), which would result in ulceration and potential perforation.
Table 4.8 Factors which assist in preventing stomach autodigestion
| Factor | Action |
|---|---|
| Mucus-secreting cells line the epithelium with alkaline mucous | Provide a physical and chemical barrier to attack by HCL |
| Pepsin produced in an inactive form (pepsinogen) | To prevent digestion of the protein based cell walls in gastric glands |
| Pepsinogen and hydrochloric acid produced and secreted separately | Pepsinogen only converted to active form pepsin in presence of HCL – will only occur once released from gastric glands into the lumen |
| Gastric mucosa has a high mitotic index (cell turnover) | Rapid replacement of damaged cells |
| Rich blood supply to gastric lining | Repair damage and high cell turnover |
HCL, hydrochloric acid
The function of the stomach is to receive the food bolus from the esophagus and begin the breakdown of large food molecules into smaller components that will eventually be absorbed in the small intestine. Strong mechanical activity called mixing waves help to churn the stomach contents, reducing food particles in size and helping to mix them with the chemical secretions from gastric juice. Up to 3 litres of gastric juice can be secreted per day. While the low pH environment stops the action of salivary amylase, it also promotes the conversion of the inactive pepsinogen to the enzyme pepsin. Pepsin begins the process of protein digestion, converting proteins to polypeptides. Other secretions include intrinsic factor from the parietal cells, which work to promote the absorption of vitamin B12 (vital to prevent pernicious anemia) from the small intestine. In infants, an enzyme known as rennin is also secreted, which emulsifies milk so that other enzymes can break it down further.
The small intestine
The small intestine is a convoluted tube, reported to have a mean length of 6.38 metres (Herlinger, 2001), which gradually diminishes in size from its commencement at the pyloric sphincter of the stomach to its termination at the ileocecal valve. The small intestine resides within the central and lower parts of the abdomen, bordered superiorly and laterally by the large intestine, while the greater omentum lies anteriorly. The small intestine is suspended from the vertebral column by a fold of peritoneum called the mesentery of the small intestine.
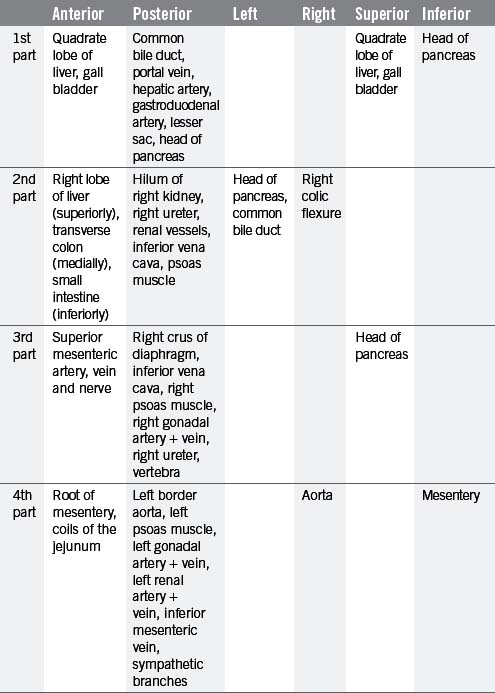
The small intestine can be divided into three parts known as the duodenum, jejunum and ileum. The duodenum is the shortest, widest and most fixed part of the small intestine, forming a 25 cm C-shaped curve around the head of the pancreas (Figure 4.12). From the diagram it can be seen that the duodenum can be subdivided into 1st part (superior), 2nd part (descending), 3rd part (transverse) and the 4th part (ascending), uniting with the jejunum at the abrupt duodenojejunal flexure. The 1st part of the duodenum is relatively mobile, being surrounded by peritoneum; however, the inferior portions of the duodenum are fixed in position. The ascending portion and the duodenojejunal flexure are suspended from the left crus of the diaphragm by the ligament of Treitz. The relations of each part of the duodenum are seen in Table 4.9.
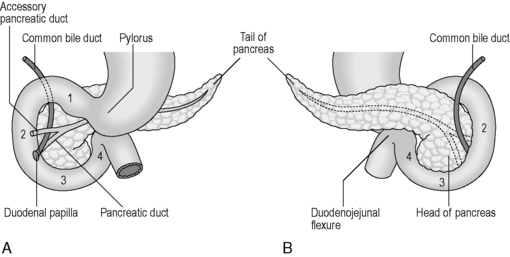
Figure 4.12 Parts of the duodenum and their relationship with the pancreas. (A) Anterior view; (B) posterior view.
The majority of the duodenum is only partially covered by peritoneum, thus resulting in it lying within the retroperitoneal cavity. The 2nd part houses within its medial wall the opening of the common bile duct and pancreatic duct at the duodenal ampulla (ampulla of Vater), situated approximately 7–10 cm below the pylorus (Lewis, 2000). The accessory pancreatic duct sometimes pierces the mucosa 2 cm above and in front of the ampulla.
The jejunum and ileum have a number of similar features, making it difficult to state categorically where the transition from one to another occurs. However, some subtle mucosal changes are seen as one moves from the proximal to distal ends of the small intestine and many of these may be identified radiologically and endoscopically. Table 4.10 outlines these distinguishing features, including the presence or absence of circular folds and single and aggregated lymphatic nodules (known as Peyer’s patches) and the presence and type of intestinal villi.
| Feature | Jejunum | Ileum |
|---|---|---|
| Abdominal position | Left upper/middle abdominal portions | Right middle and lower abdominal portions |
| Length | Proximal 2/5th (2.5 meters) | Distal 3/5th (3.8 meters) |
| Caliber | Wider, normally up to 3.5 cm | Gradually narrows, normally up to 2.5 cm |
| Bowel wall thickness | Wider – 2 mm | Thinner – 1 mm |
| Vascularization | More vascular, with deeper color as a result | Slightly less vascular, lighter in color |
| Presence of circular folds | Large and thick | Small and absent in distal ileum |
| Presence of intestinal villi | Very long villi and most numerous | Short villi and sparse (especially in terminal ileum) |
| Presence of lymphatic patches (solitary aggregated nodules) | No Peyer’s patches in upper part, few in lower part. Some single nodules | Peyer’s patches larger and more numerous. Abundant single nodules |
| Presence of intestinal glands | Numerous | Numerous |
Adapted from Herlinger (2001). Note that variations exist in measurements related to the type of procedure (e.g. barium study or cross-sectional imaging).
The intestinal villi are highly vascular processes projecting from the mucous membrane of the intestinal wall. They are largest and most numerous in the duodenum and jejunum, becoming gradually smaller and more sparse within the ileum. The villi create a huge surface area for absorption of the products of digestion, with blood capillaries and lacteals (fat transport) in close approximation with the intestinal fluids (chyle) within the intestine. Intestinal glands, commonly known as the crypts of Lieberkuhn, are found throughout all of the small intestine in large numbers. They secrete a range of enzymes which continue the process of breaking down complex structures into simpler molecules which are suitable for absorption via the intestinal villi.
The large intestine
The large intestine is approximately 1.5 meters long from cecum to anus. It lies peripheral to the loops of the jejunum and ileum, being generally more fixed and of greater caliber than the small intestine. With the exception of the appendix and anal canal, the different parts of the large intestine feature the same histology. In most patients, the mucosal surface appears smooth and featureless (Laufer, 2000). The mucosal layer possesses columnar epithelium in conjunction with intestinal glands or crypts, which often group together around shallow grooves. This may, in some patients, result in a geometric surface pattern similar to that found in the stomach and known as the areae colonicae or innominate grooves. These grooves frequently fill with mucus from goblet cells (Reeders and Rosenbusch, 1994). The columnar epithelium absorbs water and water-soluble substances, such as electrolytes, very readily, with over 90% of water being absorbed in the colon. The large intestine displays sacculations known as haustra, resulting from contraction of the muscularis mucosa and the concertina effect created by the teniae coli. The teniae coli are three thickened bands of longitudinal muscle, arising from the base of the appendix and travelling the length of the colon (see Figure 4.4). They fan out at the rectosigmoid junction to cover the rectum in a complete band. The haustra are believed to cause turbulence of passing chyme and slow the passage of the digested matter.
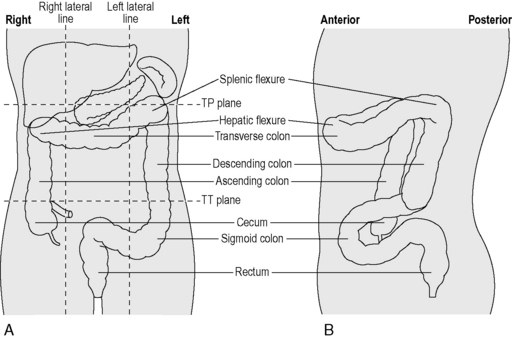
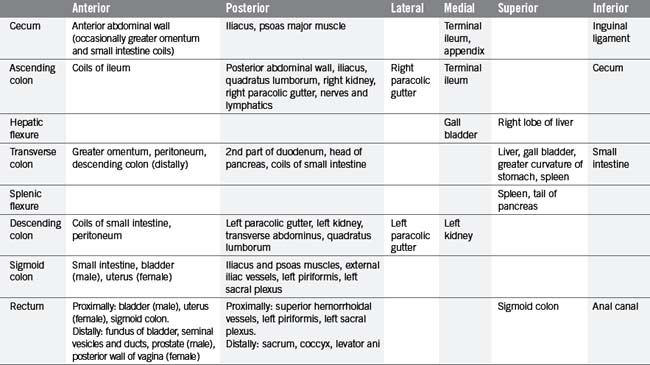
From the right iliac fossa, the cecum extends as the ascending colon (Figure 4.13) before it turns abruptly to the left, beneath the liver, at the hepatic flexure. Crossing the abdomen, the transverse colon reaches the left hypochondriac region where, at the splenic flexure, it continues downwards as the descending colon, then forming small bends known as the sigmoid colon. The sigmoid colon passes along the posterior wall of the pelvis where, at the level of the sacral prominentary the rectosigmoid junction merges into the rectum. The rectum is a dilated part of the intestine, connecting with the anal canal and anus. The main relations of each part of the large intestine are identified in Table 4.11.
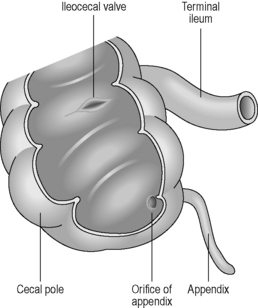
Situated within the right iliac fossa, the cecum is a dilated pouch extending superiorly to the upper border of the ileocecal valve, where it continues as the ascending colon (Figure 4.14). The cecum is approximately 6.25 cm in length and 7.5 cm in width, although its size can vary (Lewis, 2000). It may also vary considerably in both shape and position, due to a complete covering of peritoneum in many cases (Lewis, 2000). The apex of the cecum in 90% of individuals is pushed to the left toward the ileocecal junction and gives rise to the vermiform appendix (Lewis, 2000). The appendix is a long narrow blind-ended tube, varying between 2 and 20 cm in length and 0.5–1 cm in width (Reeders and Rosenbusch, 1994), occupying variable intraperitoneal and retroperitoneal positions (see Figure 4.14). Histologically, the mucosa and submucosa of the appendix demonstrate numerous lymph follicles, particularly in children. Over time the appendiceal lumen obliterates with fibrous tissue in many adults (Reeders and Rosenbusch, 1994; Balthazar, 2000).
The terminal ileum opens into the posteromedial border of the large intestine, where the cecum and colon meet. The opening is guarded by the ileocecal valve, comprising two horizontal lips of mucosa and circular muscle fibers projecting into the lumen of the large intestine (see Figure 4.14). This valve is thought to act as a sphincter, preventing contents of the ileum from passing too quickly into the cecum. It is also thought to assist in the prevention of reflux of cecal contents back into the small intestine (Zuidema and Yeo, 2002).
The ascending colon is continuous with the cecum, though is of smaller caliber. It is normally fixed to the posterior abdominal wall by a covering of peritoneum on its anterior and lateral surfaces. Lateral to the ascending colon lies the right paracolic gutter. The shallow hepatic flexure leads into the transverse colon. Normally completely invested in peritoneum, it is suspended from the lower border of the pancreas by the transverse mesocolon. The transverse colon forms an arch passing anteriorly and inferiorly, resulting in the mid transverse colon normally being a more anterior structure than the ascending and descending colon and the flexures (Figure 4.13).
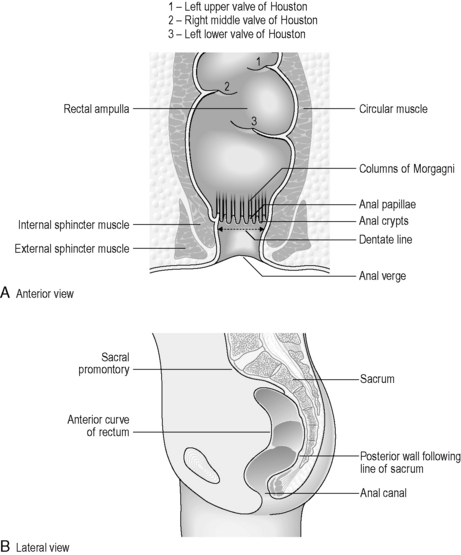
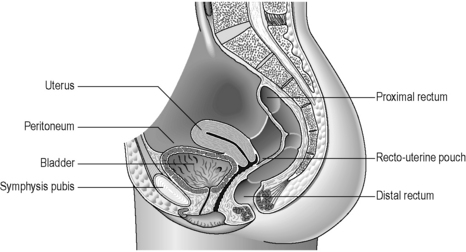
The rectum lies within the sacrococcygeal curve and presents two anteroposterior curves along its length. The upper curve has its convexity posteriorly, while the lower curve (into the anal canal) has its convexity anteriorly. Smaller lateral curves are also noted (Figure 4.15). Rectal length is approximately 12 cm, but the width expands from a similar caliber to the sigmoid colon at its commencement, to a dilated rectal ampulla distally. The main features of the rectum can be seen in Figure 4.15. The rectum lacks any haustration, but normally displays three permanent semilunar transverse folds. Two folds are situated to the left of the rectal wall and the middle fold is situated to the right. The middle fold is the largest and may be referred to as the valve of Houston. These folds are 12 mm in width (Lewis, 2000) and are thought to help to slow the transit (Reeders and Rosenbusch, 1994) and support the weight of fecal matter while in the rectum (Lewis, 2000). The peritoneum covers the anterior and lateral sides of the upper third of the rectum and only the anterior wall of the middle third. At this level, the peritoneum forms the recto-uterine recess (females) or rectovesical recess (males), also known as the pouch of Douglas. The lower third is not covered with peritoneum (sub-peritoneal), which reflects over the seminal vesicles in the male and the posterior vaginal wall in the female (Figure 4.16).
The anal canal measures approximately 2.5–4 cm in length (Lewis, 2000) and is directed postero-inferiorly from the anorectal junction to the anus. In the upper half of the lumen are found between 6 and 10 vertical vascular folds known as the anal columns (of Morgagni), separated by grooves known as anal sinuses. These fuse distally in a transverse saw-toothed margin called the dentate line (see Figure 4.15). Above the dentate line, the mucosa is covered with columnar epithelium, but below the line this changes into keratinized epithelium, similar to skin tissue. Anal closure is the result of a double sphincter mechanism. The internal sphincter is formed from a thickening of circular intestinal muscle fibers, while surrounding this the external sphincter is formed from striated (skeletal) muscle, which is under voluntary control. The levator ani is the major muscle of the perineum, supporting the rectum in a muscular sling. In particular, one part of the levator ani, the puborectal sling, is important in maintaining rectal continence.
The large intestine is supplied by three arterial systems: the superior mesenteric artery (SMA) and the inferior mesenteric artery (IMA), both major branches of the abdominal aorta, and the internal iliac arteries. The branches of these major arteries supply different parts of the intestine, although the exact nature of the supply is variable. However, the branches of the SMA will supply the right colon from the cecum to the mid transverse colon in the majority of cases (Reeders and Rosenbusch, 1994) and the IMA branches supply the left colon from the distal transverse colon through to the mid rectum. Branches of the external iliac and internal iliac arteries supply the mid rectum through to the anal canal. However, the IMA and internal iliac branches form an extensive intramural and extramural network around the anorectum.
The circulation noted above can form many anastomotic connections, which may serve as a collateral circulation in the event of a blockage (arterial occlusion). The intermesenteric arcade (of Riolan) is formed by a branch of the left colic artery (IMA) anastomosing with the middle colic artery (SMA) near to the splenic flexure. This vascular arc would only be noticed arteriographically if an occlusion is present on one side (Reeders and Rosenbusch, 1994). The marginal colonic artery (of Drummond) is a paracolic anastomosis along the mesenteric (centrally facing) border of the colon. It connects the five arteries of the SMA and may extend from the cecum to the sigmoid colon. The marginal artery gives rise to the vasa recta, which pierce the colon perpendicularly to supply the wall with blood. The Griffith’s point is the site of a critical anastomosis between the marginal artery and other vessels at the site of the splenic flexure (Figure 4.17). This anastomosis is said to be absent in 43% of individuals (Reeders and Rosenbusch, 1994), resulting in a site that may have low resistance to ischemic events.
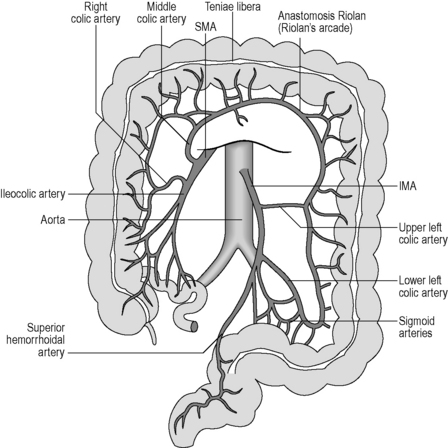
Figure 4.17 Major arteries supplying the large intestine. The colon is shown with the transverse portion raised so that the arteries may be seen more clearly. Note the Riolan anastomosis between branches of the superior mesenteric artery (SMA) and the inferior mesenteric artery (IMA). This is a potential area of reduced blood supply.
Balthazar E.J.. Diseases of the appendix. Gore R.M., Levine M.S., editors. Textbook of gastrointestinal radiology, vol. 1. Philadelphia: WB Saunders, 2000. second ed.
Herlinger H. Anatomy of the small intestine. In Maglinte D., Herlinger H., Bernbaum B., editors: Clinical imaging of the small intestine, second ed., New York: Springer-Verlag, 2001.
Jones B.. Functional abnormalities of the pharynx. Gore R.M., Levine M.S., editors. Textbook of gastrointestinal radiology, vol. 1. Philadelphia: WB Saunders, 2000. second ed.
Laufer I.. Barium studies of the colon. Gore R.M., Levine M.S., editors. Textbook of gastrointestinal radiology, vol. 1. Philadelphia: WB Saunders, 2000. second ed.
Levine M.S., Laufer I. Stomach. In Levine M.S., Rubesin S.E., Laufer I., editors: Double contrast gastrointestinal radiology, third ed., Philadelphia: WB Saunders, 1999.
(Gray, H., Ed.).]. Lewis W.H., editor. Gray’s anatomy of the human body. Philadelphia: Lea & Febiger, 2000. 1918; revised and re-edited by Warren H Lewis for Bartleby.com, 2000. < www.bartleby.com/107/ > (accessed 10.12.07)
Moore K.L., Persaud T.V.N. Before we are born: essentials of embryology and birth defects, seventh ed. Philadelphia: WB Saunders, 2007.
Ott D.J.. Motility disorders of the oesophagus. Gore R.M., Levine M.S., editors. Textbook of gastrointestinal radiology, vol. 1. Philadelphia: WB Saunders, 2000. second ed.
Reeders W.A.J., Rosenbusch G. Clinical radiology and endoscopy of the colon. New York: Thieme Medical Publishers, 1994.
Rubesin S.E. Pharynx. In Levine M.S., Rubesin S.E., Laufer I., editors: Double contrast gastrointestinal radiology, third ed., Philadelphia: WB Saunders, 1999.
Rubesin S.E.. Pharynx: normal anatomy and examination techniques. Gore R.M., Levine M.S., editors. Textbook of gastrointestinal radiology, vol. 1. Philadelphia: WB Saunders, 2000. second ed.
Stradling S. Gray’s Anatomy [(Editor-in-Chief). The anatomical basis of clinical practice. Churchill Livingstone, Edinburgh, 2005. 39th ed.