CHAPTER 116 Appendicitis
HISTORICAL PERSPECTIVE
The first anatomic mention of the appendix was made by Leonardo da Vinci in the early 15th century. The first clearly recognizable case report of appendicitis was recorded in 1711 by the German surgeon Lorenz Heister,1 but it was not until 25 years later that the first inflamed appendix was removed by Claudius Amyand, a Sergeant Surgeon to Queen Ann, King George I, and King George II. Amyand operated on an 11-year-old boy with a perforated appendix within a scrotal hernia that he was able to excise and repair, respectively.2 Throughout the 18th and 19th centuries, the prevailing medical opinion was that acute abdominal pain and right lower quadrant inflammation was a consequence of inflammation of the cecum or its surrounding tissues. The modern description of the pathophysiology of appendicitis and the role of the appendix in acute abdominal syndromes dates to 1886, the year Reginald Fitz presented a paper to the Massachusetts Medical Society in which he coined the term appendicitis and espoused early surgical intervention as its appropriate treatment.1
The first now-customary appendectomy for classic acute appendicitis actually had been performed by Lawson Tait in 1880, but it was not reported until Charles McBurney, one of the great contributors to our understanding of appendicitis, published his recommendation for early laparotomy for the treatment of appendicitis in 1889.4 It is in this paper that what subsequently became known as McBurney’s point is described as the point of “maximum tenderness, one half to two inches inside the right anterior spinous process of the ilium on a line drawn from the umbilicus.”2,3
Almost a century later, the first laparoscopic approach to appendectomy was described by Kurt Semm,4 and with development of natural orifice transluminal endoscopic surgery (NOTES), the first successful transvaginal appendectomy was reported by Sanntiago Horgan and Mark A. Talamini in early 2009.5
EPIDEMIOLOGY
Appendicitis is the most common acute abdominal emergency seen in developed countries. The crude incidence rate of appendicitis in the United States for all age groups is 11/10,000 persons per year,6 and similar rates are noted in other developed countries. Inexplicably, the rates of appendicitis are as much as 10 times lower in many less-developed African countries.7 The incidence rate of the disease peaks between 15 and 19 years of age at 48.1/10,000 population per year and falls to about 5/10,000 population per year by age 45 years, after which it remains constant.6 Men are at greater risk than women, with a case ratio in most series of 1.4 : 1. The lifetime risk of appendicitis has been estimated at 8.6% in men and 6.7% in women.6
Approximately 250,000 appendectomies are performed each year in the United States; data from most developed countries suggest that the incidence of appendicitis is decreasing. Between 1989 and 2000, a 15% decrease in the overall incidence of appendicitis was noted in an English study8; similar trends have been noted in Canada and Greece.9,10 One paper, however, suggests that, at least in the United States, the number of appendectomies performed for acute appendicitis has been increasing since 1995.11 Regardless of the direction of the epidemiologic trend, appendicitis remains the most common indication for emergency abdominal surgery.
ANATOMY AND EMBRYOLOGY
The vermiform appendix and the cecum are best thought of as a single anatomic unit. Developmentally part of the midgut, the appendix and cecum form between the 8th and 12th weeks of gestation as a bud arising from the midgut loop, before the ascending colon has become delineated. Congenital malformations of the appendix such as agenesis and duplication are very rare. With an average length of 9 cm,12 the origin of the appendix varies and the appendix may assume any of the positions of a clock hand, with the center considered the appendiceal origin.
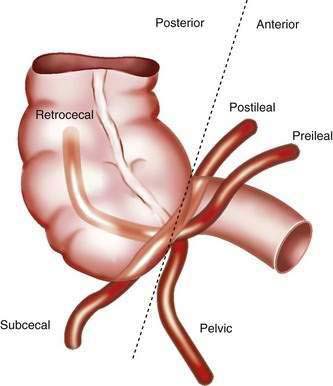
Although the right colon is fixed in the retroperitoneum, the appendix and cecum have a more variable position within the abdomen. The position of the appendix depends upon a number of factors: the degree of cecal descent and peritoneal fixation, the configuration of the cecum, appendiceal length, associated adhesions, and the habitus of the person.13 Typically, the location of the appendix is described as retrocecal, pelvic, subcecal, or para-ileal (Fig. 116-1). The position of the appendix has important clinical implications: For example, the classic progression of symptoms requires irritation of the parietal peritoneum by a mobile appendix; as many as 60% of people have a retrocecal or pelvic-positioned appendix, resulting in an atypical clinical presentation of acute appendicitis.
The classic surface anatomy of the appendix was described by McBurney in 1889, and as mentioned earlier, McBurney’s point is located at the junction of the lateral and middle thirds of a line drawn from the right anterior superior iliac spine to the umbilicus.4 Classically, this surface marking has been important in both the diagnosis and treatment of acute appendicitis; however, investigators have shown that the appendix is located within 5 cm of McBurney’s point in less than 50% of the cases.14 This anatomic finding helps explain why pain or tenderness located at McBurney’s point is not found in all cases of appendicitis.
PATHOLOGY
Acute appendicitis is classified as acute, gangrenous, or perforated. The earliest gross findings of acute appendicitis are injection of the serosal blood vessels and edema of the appendiceal wall. In more-advanced cases, the serosal surface appears dull and is covered by fibrinopurulent exudates. Over time, focal areas of gangrene develop, marked by greenish and black discoloration of the wall; with perforation, focal necrosis of the appendiceal wall develops and adjacent abscesses form.15
Microscopically, each of these forms of appendicitis has distinctive characteristics. In acute or suppurative appendicitis, a neutrophilic infiltrate involves the muscularis propria layer circumferentially, accompanied by acute inflammation and ulceration of the mucosa, edema and microabscesses in the appendicular wall, and vascular thrombosis. The hallmarks of gangrenous appendicitis are transmural inflammation of the appendix with focal areas of mural necrosis. Vascular thrombosis is more prominent in gangrenous than in suppurative appendicitis. The presence of mucosal inflammation alone (“catarrhal” inflammation) is more characteristic of infectious enteritis or colitis and is not considered evidence of acute appendicitis; for the microscopic diagnosis of appendicitis to be made, inflammation must extend to the muscularis propria.15
PATHOGENESIS
Despite more than 100 years of study, there still is no single explanation for all cases of appendicitis. The classic hypothesis is that obstruction of the appendiceal lumen by either a fecalith or lymphoid hyperplasia produces an increase in intraluminal pressure, which in turn results in venous hypertension, ischemia of the appendiceal wall, and subsequent bacterial invasion of the appendix with necrosis and perforation. Experimental evidence in animal models exists to support this hypothesis of the etiology of acute appendicitis.16 This hypothesis, however, does not explain all cases of appendicitis. Careful review of pathologic series shows that luminal obstruction is found in a minority of cases. Fecaliths are present in only 8% to 44% of cases of acute appendicitis, with most series at the lower end of the range,15,17 and lymphoid hyperplasia is more common in noninflamed appendices than in acute appendicitis.18 Other causes of luminal obstruction such as foreign bodies, tumors, and fibrous bands are uncommon. Direct measurement of intraluminal pressure at appendectomy for appendicitis reveals an elevated pressure in only a minority of cases.19
An alternative hypothesis for the etiology of appendicitis is based on the concept that either bacterial or viral enteric infection leads to mucosal ulceration of the appendix and subsequent bacterial invasion from the normal colonic flora. The finding that up to 75% of cases of appendicitis demonstrate well-defined superficial mucosal ulceration supports this theory. Furthermore, mucosal ulceration is a more consistent finding than dilatation of the appendix or fecaliths and is found earlier in the course of appendicitis.20 One report found human cytomegalovirus (HCMV) early antigen expression in 64% of cases with acute appendicitis and no HCMV antigens in normal appendices, suggesting that in some cases CMV, infection might produce mucosal ulcerations leading to acute appendicitis.21
Additional support for the role of infection in the etiology of appendicitis is found in two lines of epidemiologic evidence. The first is based in the hygiene theory of appendicitis advocated by Barker in the mid 1980s.22 According to this hypothesis, changes in sanitation tied to the Industrial Revolution resulted in a decrease in enteric infections in infants, with subsequent decreased immunity to the same infections in childhood and young adulthood. Acquisition of these infections later in life was believed to predispose people to appendicitis, explaining the rise in the incidence rates of appendicitis in the first half of the 20th century. The decrease in the overall rate of enteric infections during the last half of the 20th century explains the overall decline in appendicitis. The second line of epidemiologic evidence supporting the role of infection in the etiology of appendicitis is the seasonal variance in incidence of appendicitis and the occurrence of temporal and spatial clusters of appendicitis, both hallmarks of infectious diseases.23 It is important to recognize, however, that no specific infectious agent has been linked with all cases of appendicitis, suggesting that infection is not the complete story.
A decrease in dietary fiber intake (the fiber hypothesis) also has been proposed as a cause of appendicitis. According to this hypothesis, decreased dietary fiber causes firm stool and an increased enteric transit time, resulting in more fecaliths and more appendicitis. This hypothesis was felt to explain both the rise in appendicitis rates in the early 20th century and the marked differences in appendicitis rates between more-developed Western countries and less-developed African countries. Doubt has been cast upon this hypothesis, however, for several reasons. First, although dietary fiber ingestion has been falling in urban Africans, appendicitis rates have not risen markedly.24 Second, rates of appendicitis in the Western world have fallen without changes in dietary fiber intake. Finally, a prospective series from Africa demonstrated continued high fiber intake even in patients with appendicitis.25
CLINICAL FEATURES
A detailed history and careful physical examination remain cornerstones of the diagnosis of acute appendicitis. Although no single item of the history, in isolation, allows the diagnosis to be made reliably, combination of the classic symptoms and the typical progression of symptoms coupled with right lower quadrant tenderness allows good diagnostic accuracy. In the classic presentation of acute appendicitis, patients first note vague, poorly localized epigastric or periumbilical discomfort, which typically is not severe and often is attributed to “gastric upset.” Patients commonly report feeling that a bowel movement should make the pain better, a sensation known as the downward urge.26
Diarrhea sometimes is seen early on with appendicitis, but this is not common. Within 4 to 12 hours of the onset of pain, most patients also note nausea, anorexia, vomiting, or some combination of these three symptoms. The nausea usually is mild to moderate, and most patients have only a few episodes of emesis. If vomiting is the major symptom, the diagnosis of appendicitis should be questioned. Likewise, emesis that occurs before the onset of pain should suggest other diagnoses.27 Many patients report a mild fever or chills; high fevers or significant rigors are uncommon. The patient’s abdominal pain typically increases in intensity, and a characteristic shift in the pain to the right lower quadrant occurs over 12 to 24 hours. The character of the pain becomes achy and more localized. Localization of the pain to the right lower quadrant is a valuable finding when present and occurs in more than 80% of patients with appendicitis.27
On physical examination, most patients appear slightly ill. Tachycardia is uncommon with simple appendicitis, but it may be seen with complicated appendicitis. Most patients with simple appendicitis have a temperature less than 100.5°F; temperature greater than 100.5°F is most often associated with perforated or gangrenous appendicitis.17 Patients with appendicitis, like other patients with peritonitis, tend to lie still rather than move about. Right lower quadrant tenderness and rigidity, both voluntary and involuntary, are common findings. Localized right lower quadrant tenderness is an important finding when present, but its absence does not rule out appendicitis. A variety of methods exist to elicit localized right lower quadrant peritonitis, including the cough sign (the presence of point tenderness with a cough), percussion tenderness, and formal elicitation of rebound tenderness. Although all of these techniques are reasonably sensitive, one small study showed rebound tenderness to be the most accurate predictor of the localized peritonitis associated with appendicitis.28
Additional findings that may be helpful in diagnosing appendicitis include the psoas sign, the obturator sign, Rovsing’s sign, and rectal tenderness. The psoas sign is sought by having a supine patient actively flex the right hip against resistance, or by the examiner flexing and extending the patient’s right hip with the patient in the left lateral decubitus position. Pain with either of these maneuvers is thought to result from irritation of the underlying psoas muscle by an inflamed retroperitoneal appendix. The obturator sign is elicited by internally and externally rotating the flexed right hip. Pain is thought to arise when the inflamed pelvic appendix irritates the adjacent obturator internus muscle. Rovsing’s sign is the finding of right lower quadrant pain during palpation of the left side of the abdomen or when left-sided rebound tenderness is elicited. All of these findings are valuable when present, but their absence does not exclude appendicitis.27
Appendicitis in infants and young children remains a difficult diagnostic challenge because of difficulties in obtaining an accurate history. In young patients, the characteristic history of pain is difficult to elicit, and nonspecific findings of vomiting, lethargy, and irritability tend to predominate. Physical examination is difficult to perform because of poor patient cooperation and because localized right lower quadrant tenderness is found in less than 50% of patients.29 In addition, the characteristic laboratory findings often are not present. Leukopenia is as common as leukocytosis in young infants.30 As a result, errors in diagnosis are common, and the frequency of complicated appendicitis is as high as 40% to 70%.31
The diagnosis of appendicitis in elderly patients also may be a challenge. In the elderly, the classic pattern of pain migration, right lower quadrant tenderness, fever, and leukocytosis are observed in only 15% to 30% of cases.30,32 Older patients also tend to present to medical attention in a delayed time frame relative to younger patients. For all of these reasons, the complication and perforation rates can be as high as 63% in patients older than 50 years.33
The presentation of appendicitis during pregnancy also is associated with an atypical clinical presentation, particularly in the later stages of pregnancy. In one series, only 57% of pregnant women with appendicitis had the classic progression of pain.34 Nausea and vomiting tend to be more common in pregnant women with appendicitis, but they also are common occurrences during normal pregnancy. Fever and leukocytosis are less commonly seen in pregnant woman than in other patient groups, and the value of leukocytosis is obscured by the physiologic leukocytosis of pregnancy. Although right-sided abdominal pain and tenderness are found in more than 90% of pregnant women with appendicitis, pain is located in the right lower quadrant only 75% of the time.34
Immunocompromised patients in general, and patients with AIDS in particular, represent a challenging group in which to diagnose appendicitis. Abdominal pain is reported in 12% to 45% of AIDS patients with appendicitis. The range of diagnoses responsible for this pain is significantly greater than in patients without HIV and includes opportunistic infections and malignancies, although in most cases, the pain is related to a diagnosis not associated with HIV.35 Research suggests that appendicitis occurs more often in HIV-infected patients than in HIV-negative patients, with as much as a four-fold increase in incidence.36 Although patients with AIDS usually present with the classic symptoms of appendicitis, there often is a history of chronic abdominal pain. Diarrhea also is a more common presenting symptom of appendicitis in HIV-positive patients, and leukocytosis is relatively uncommon. Declining CD4 counts are associated with delays in presentation to medical attention and increased perforation rates.37 Despite the challenges of diagnosing appendicitis in patients with HIV, the surgical outcomes with appropriate treatment are quite good; the largest series to date had no mortalities and a 13% complication rate, which is comparable to outcomes in patients without HIV.37
DIAGNOSIS
Diagnosis of appendicitis remains a significant clinical challenge because of the many different entities that manifest with acute abdominal pain and the relatively nonspecific initial presentation of the disease. Because the natural history of appendicitis is a time-dependent progression to perforation, there is some urgency in making a prompt and accurate diagnosis. Not all causes of acute abdominal pain, however, require surgical intervention, and a negative appendectomy carries some risks for the patient, including adhesion formation, infection, and postoperative disability. Table 116-1 illustrates common diagnoses that can mimic acute appendicitis. Compounding this diagnostic challenge, there is no single symptom, finding, or laboratory test that is completely sensitive or specific for appendicitis.27
| DIAGNOSIS | FINDINGS THAT HELP DIFFERENTIATE FROM APPENDICITIS |
|---|---|
| Bacterial or viral enteritis | Nausea, vomiting, and diarrhea are severe; pain usually develops after vomiting |
| Mesenteric adenitis | Duration of symptoms is longer; fever is uncommon; RLQ physical findings are less marked; WBC count is usually normal |
| Pyelonephritis | Pain is more likely to be felt in the right flank; high fever and rigors are common; marked pyuria or bacteriuria and urinary symptoms are present; abdominal rigidity is less marked |
| Renal colic | Pain radiates to the right groin; significant hematuria; character of the pain is clearly colic |
| Acute pancreatitis | Pain and vomiting are more severe; tenderness is less well localized; serum amylase and lipase levels are elevated |
| Crohn’s disease | History of similar attacks; diarrhea is more common; palpable mass is more common; extraintestinal manifestations may occur |
| Cholecystitis | History of prior attacks is common; pain and tenderness are greater; radiation of pain is to the right shoulder; nausea is more marked; liver biochemical tests are more likely to be abnormal |
| Meckel’s diverticulitis | Very difficult to distinguish preoperatively from appendicitis |
| Cecal diverticulitis | Difficult to distinguish preoperatively from appendicitis; symptoms are milder and of longer duration; CT scan is helpful |
| Sigmoid diverticulitis | Usually occurs in older patients; changes in bowel habits are more common; radiation of pain is to the suprapubic area, not RLQ; fever and WBC count are higher |
| Small bowel obstruction | History of abdominal surgery; colicky pain; vomiting and distention are more marked; RLQ localization is uncommon |
| Ectopic pregnancy | History of menstrual irregularities; characteristic progression of symptoms is absent; syncope; positive pregnancy test |
| Ruptured ovarian cyst | Occurs in the middle of the menstrual cycle; pain is of sudden onset; nausea and vomiting are less common; WBC count is normal |
| Ovarian torsion | Vomiting is more marked and occurs at the same time as the pain; progression of symptoms is absent; abdominal or pelvic mass often is palpable |
| Acute salpingitis or tubo-ovarian abscess | Longer duration of symptoms; pain begins in the lower abdomen; often there is a history of STDs, vaginal discharge, and marked cervical tenderness often are present |
CT, computed tomography; RLQ, right lower quadrant; STD, sexually transmitted disease; WBC, white blood cell.
LABORATORY STUDIES
Laboratory findings in acute appendicitis include a variety of markers of acute inflammation. An elevated white blood cell count (WBC) in the range of 11,000 to 17,000/mm3 is seen in approximately 80% of patients, but the specificity of this finding for acute appendicitis versus other causes of acute abdominal pain is poor.38 An elevated proportion of granulocytes in the total white count or an elevated total neutrophil count (left shift) also is seen in the vast majority of patients with appendicitis, but is not specific for appendicitis.38 C-reactive protein (CRP), an acute phase reactant synthesized by the liver, is thought to rise within 12 hours of the development of an acute inflammatory process. Although CRP is elevated in 50% to 90% of cases of appendicitis, CRP is nonspecific when cutoff values of 5 to 25 mg/L are used.39 A urinalysis often is obtained in patients with acute appendicitis to exclude urinary tract infections, but mild abnormalities, either pyuria or hematuria, are present in about 50% of cases of appendicitis.40
The value of laboratory investigations in diagnosing acute appendicitis has been a matter of some debate. In patients with a classic presentation by history and physical examination, many authors think that little additional information is obtained from laboratory studies. When all cases of appendicitis are considered, however, adding laboratory studies such as WBC, left shift, and CRP has been shown to improve diagnostic accuracy.41 When clinical findings are compared with inflammatory markers, inflammatory markers are stronger predictors of appendicitis than individual history or physical findings. Direct comparison of WBC and CRP suggests that total WBC or total granulocyte count is more sensitive and accurate than CRP for detecting acute appendicitis.39,41 The diagnostic performance of inflammatory markers is even better in identifying patients with perforated appendicitis.
All patients with suspected acute appendicitis should have a CBC. A pregnancy test should be obtained in women of childbearing age. The value of other laboratory tests such as amylase, liver biochemical tests, or urinalysis lies in helping to exclude other diagnoses that can mimic acute appendicitis (see Table 116-1).
IMAGING STUDIES
Traditionally, there has been little role for routine imaging studies in patients with suspected acute appendicitis. As is stated in the classic text Cope’s Early Diagnosis of the Acute Abdomen, “Over reliance on laboratory tests and radiological evaluations will very often mislead the clinician, especially if the history and physical examination are less than diligent and complete.”28,27 In 50% to 60% of cases, the diagnosis of appendicitis requires no imaging studies and can be made on clinical grounds alone.42,43 When diagnosis is less certain, a variety of imaging tests has been used to help confirm or exclude the diagnosis of acute appendicitis: plain abdominal films, abdominal ultrasound, radionuclide scans, and abdominal and pelvic computed tomography (CT).
Plain Abdominal Films
Plain films of the abdomen often are the initial imaging test for patients with acute abdominal pain. Findings on plain films of the abdomen consistent with appendicitis include a radiopaque right lower quadrant coprolith; focal right lower quadrant ileus or a sentinel loop; loss of the right psoas shadow; and a right lower quadrant soft tissue mass. All of these findings are suggestive of but not definitive for appendicitis. In a prospective study in which plain abdominal films were ordered on all patients with suspected appendicitis, the films changed clinical management in only 6% of cases.44
Ultrasonography
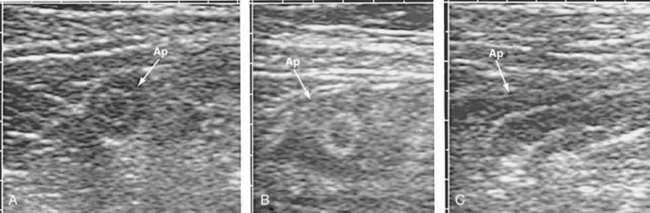
The ultrasound characteristics of appendicitis are well defined. Using a 5 or 7.5 MHz transducer, the technique of graded compression is used to displace the mobile loops of bowel in the right lower quadrant of the abdomen. The diagnosis of appendicitis can be made with confidence if a 7-mm or thicker noncompressible blind-ended loop of bowel is identified (Fig. 116-2). A shadowing appendicolith, pericecal inflammation, or a localized pericecal fluid collection all suggest appendicitis.45
Appendicitis is excluded during ultrasound study by demonstration of a normal appendix. A normal appendix, however, is demonstrated in less than 50% of cases even by experienced sonographers, thus reducing the value of a “negative” ultrasound study.46
The reported sensitivity of ultrasound in the diagnosis of appendicitis in adults is 86% and its specificity is 81% in a collected review.47 Ultrasound appears to be more sensitive and specific in children than in adults, with sensitivity and specificity greater than 90% in most series and detection of a normal appendix in up to 90% of cases.48,49
There are some important limitations to the usefulness of ultrasound in the diagnosis of appendicitis. All ultrasound-based techniques are operator-dependent. The excellent results just mentioned were achieved in dedicated trials performed by interested and experienced ultrasonographers. In one multicenter trial focused on diagnosis of the acute abdomen, the real world sensitivity of ultrasound fell to 55%.50 Ultrasound also is less sensitive in patients with a body mass index (BMI) greater than 25 and in those with perforated appendicitis.51 Finally, ultrasound is more useful in confirming than in excluding the diagnosis of appendicitis, reducing its clinical utility in patients with a low pretest probability of appendicitis.
Radionuclide Scanning
Radionuclide scanning has been advocated when the diagnosis of appendicitis is uncertain. Two major techniques are used: either HMPAO (99mTc-hexamethylpropyleneamine oxime) labeling of the patient’s leukocytes, or 99Tc-labeled antigranulocyte antibodies. In both of these techniques, an accumulation of the radionuclide in the right lower quadrant is considered positive for appendicitis. The reported sensitivity of radionuclide scanning is 91% to 94%, and specificities are in the 82% to 94% range.52 Limitations of these techniques remain their lack of availability in all hospitals, the relatively long time required to perform them, and operator dependence in their interpretation.
Computed Tomography
Abdominal CT scans are considered the imaging study of choice in nonclassic cases of appendicitis. With the development of rapid helical and multidetector CT scanners, CT imaging is used increasingly to evaluate patients with acute abdominal pain. CT has long been considered valuable in making the diagnosis of appendiceal abscess, and CT-based therapy of these abscesses has become common.53 Since the 1990s, a number of authors have advocated broadening the use of CT scans to assist in the diagnosis of atypical appendicitis. A wide variety of techniques has been used for appendiceal protocol CT scans, which differ in terms of the amount of the abdomen scanned, the thickness of the individual cuts, and the types of contrast used. Several conclusions have emerged from these studies: thin (5 mm) cuts are better than thick (10 mm) cuts,54 and enteric contrast improves accuracy.
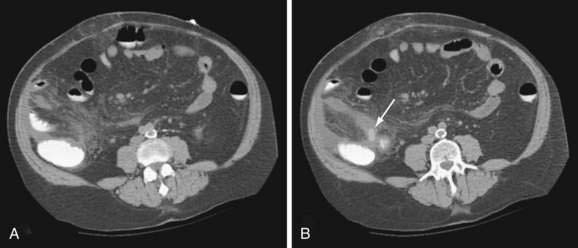
CT findings consistent with appendicitis include an inflamed, distended (more than 6 mm) appendix that fails to fill with contrast or air (Fig. 116-3), often accompanied by an appendicolith or appendiceal wall thickening; periappendiceal inflammation, cecal apical thickening, and pericecal fluid collections are associated findings in appendicitis.55 Identification of a normal appendix or the finding of alternative intra-abdominal pathology constitutes a negative study.
The performance of CT scanning for appendicitis has been impressive, with sensitivity rates of 94% and specificity rates of 95% in one collected review of multiple studies.47 The best results occur when enteric contrast is administered both by mouth and by rectum and contrast opacification of the cecum occurs. Limitations of CT scanning for appendicitis include the time required for enteric contrast to fill the bowel, decreased sensitivity in patients with low body fat, allergic reactions to intravenous contrast agents, exposure to ionizing radiation, and cost.
Overall Approach
What constitutes the best imaging study has not been conclusively determined for patients in whom a diagnosis of appendicitis cannot be made confidently after clinical history, physical examination, and review of laboratory findings. Based on current evidence, however, it would appear that CT scanning is more sensitive, more specific, and less operator-dependent than ultrasound in adults.56 In pregnant women and in very thin patients, especially in institutions with experienced ultrasonographers, abdominal ultrasound is probably an alternative first imaging study in atypical cases of appendicitis. In pediatric patients, when the diagnosis of appendicitis cannot be made confidently after evaluation by a pediatric surgeon, ultrasound should be the first imaging test selected. This recommendation is based on the increased sensitivity of ultrasound in children and on the theoretical 10-fold increase in lifetime cancer risk engendered by exposure of children to ionizing radiation.57 In patients of any age, the initial step in evaluating patients with suspected acute appendicitis should be evaluation of the patient by an experienced surgeon, because this diagnostic evaluation is at least as accurate as any imaging study.58
CLINICAL SCORING SYSTEMS AND COMPUTER-AIDED DIAGNOSIS
Based upon data suggesting that examiner experience improves diagnostic accuracy in acute appendicitis, a variety of scoring systems has been devised since the 1990s to aid in the diagnosis of appendicitis. Most of these scoring systems assign numerical weights to findings from history, physical examination, and laboratory values in an attempt to predict the probability of appendicitis. More than 10 different scoring systems have been published, all of which purport to reduce errors in diagnosis and negative appendectomy rates. In an examination of the performance of multiple, published scoring systems on a single, well defined patient data set, the ability of all scoring systems to predict appendicitis was disappointing.59
The ability of scoring systems to perform well when applied to patient populations other than the population for which they had been developed remains a problem with these systems; other studies have reported similar results looking at individual scores.60 At this point there is no universally applicable scoring system for the diagnosis of acute appendicitis.
LAPAROSCOPY
Diagnostic laparoscopy has been proposed to assist in diagnosing equivocal cases of acute appendicitis. Inserting a laparoscope into the abdomen allows direct inspection of the appendix without appendectomy, if the appendix is found to be normal. The appeal of this approach is greatest in woman of childbearing age in whom gynecologic causes of acute abdominal pain can cloud diagnosis and who often are amenable to laparoscopic treatment. Two prospective studies of diagnostic laparoscopy in cases of possible appendicitis revealed gynecologic causes of pain in 48% to 73% of women with a normal appendix.61,62 Because there is some, albeit weak, evidence to suggest appendectomy might predispose women to tubal infertility,63 avoidance of unnecessary appendectomies is desirable in women of childbearing age. Diagnostic laparoscopy has been used in two prospective series to nearly eliminate negative appendectomies in women of childbearing age.61,62
Despite these promising results, some cautionary notes must be sounded. Most studies of diagnostic laparoscopy report examinations performed under general anesthesia, making this a resource-intensive test compared with radiologic imaging studies. Although diagnostic laparoscopy can be performed under local anesthesia, inherent technical constraints reduce its success rate. For example, gynecologic pelvic laparoscopy performed under local anesthesia fails to obtain complete visualization of the pelvis in up to 15% of cases64; this incomplete examination rate compares poorly with CT scanning. Currently, diagnostic laparoscopy cannot be recommended over appendiceal protocol CT scanning as an initial test, but it probably should be used as a supplement to CT or ultrasound evaluations in which the results are equivocal.
DIAGNOSTIC ACCURACY
The concept of diagnostic accuracy refers to the fact that not all patients with a preoperative diagnosis of appendicitis are found to have acute appendicitis at operation. Because of the time-dependent risk of appendiceal perforation with its resultant increase in complications, it is important to make the diagnosis of appendicitis as quickly as possible.65 As a result, treatment decisions often are made in the presence of incomplete clinical information. An appendectomy is termed negative when a normal appendix is found at exploration for acute appendicitis.
Traditionally, an inverse relationship has been found between the frequency of negative appendectomies and the frequency of perforation at operation. Studies have shown that an increased diagnostic accuracy at operation carries an increased perforation rate,66 a tradeoff believed to be a consequence of the increased time required to confirm the etiology of acute abdominal pain in the absence of any specific test for appendicitis. In the interests of avoiding complications, standard teaching has been to accept a certain negative appendectomy rate to improve patient outcomes. Without diagnostic imaging, a negative appendectomy rate of 10% to 30% with a perforation rate of 10% to 25% is felt to represent a “good” balance6,17,42; in these series, the negative appendectomy rate was higher in women than men.
In recent years, the use of imaging studies has improved the diagnostic accuracy for appendicitis without concomitant increases in perforation risk. In several series in which CT scanning was used selectively or universally in cases of presumed appendicitis, negative appendectomy rates have been reduced to 2% to 8% without an increase in perforation rates.42,43,67–70 This improvement in diagnostic accuracy has been observed in all patient groups, but most notably in women and children. These results suggest that with diagnostic imaging, it is possible to increase our diagnostic certainty without exposing patients to an increased risk of perforation. Whether a policy of increased use of imaging studies in the diagnosis of appendicitis will prove to be cost-effective is not yet clear, but early data suggest it might be, if enough negative explorations can be avoided.71
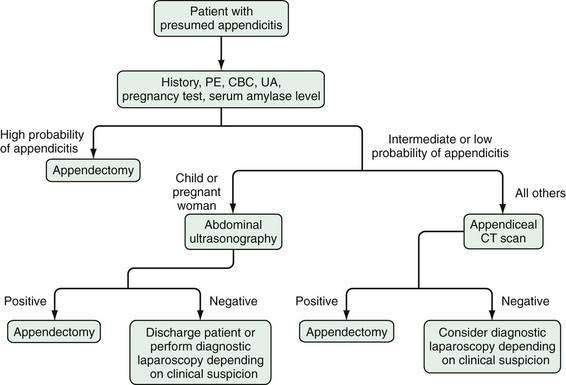
As a result of all of these recent diagnostic modalities, a new approach is emerging to the patient with acute abdominal pain and suspected appendicitis (Fig. 116-4). The goal of this new approach relies on imaging techniques and laparoscopy to eliminate in-hospital observation as a tool for improving diagnostic accuracy, thereby reducing the time required to increase diagnostic certainty and decreasing the likelihood of time-dependent complications.
A strong incentive exists for avoiding negative appendectomies beyond diagnostic pride. Complication rates between 5% and 15% have been reported with the removal of a normal appendix, the majority of which are infectious, including wound, pulmonary, and urinary tract infections. A 1.3% risk of small bowel obstruction is reported in the series with the longest follow-up.72 Of patients found to have a normal appendix at operation, about 12% are found to have another surgical disease. An additional 18% to 20% have intra-abdominal findings that can explain their symptoms but are nonsurgical, the most common causes being ileitis or ileocolitis, mesenteric adenitis, or right ovarian cystic disease.73 An additional advantage of using CT scan in cases of atypical appendicitis is that many of these diagnoses can be made at CT scan.42 In as many as 60% of patients with negative appendectomy, no diagnosis can be made, even on subsequent evaluation.
COMPLICATIONS
The major complication of untreated appendicitis is perforation, with resultant peritonitis, abscess, and portal pylephlebitis. Overall, the perforation rate in most series is between 10% and 30%, but the rate of perforation varies widely with age; perforation is most common at the extremes of age. Perforation rates as high as 90% have been reported in children younger than two years,29 and patients older than 70 years have perforation rates between 50% and 70%.33,74 Patients between the ages of 10 and 30 years have the lowest perforation rates, generally between 10% and 20%.
The risk of perforation appears to increase as the duration of illness increases, particularly after 24 hours. Perforation of the appendix is a consequence of delay in diagnosis, and several studies have shown that patients with perforation have symptoms that average 30 hours longer than do patients with simple appendicitis.75 Much of this delay appears to be a result of delays in presentation to medical attention rather than delays in medical decision-making, but patients with perforation often have atypical presentations of their appendicitis.
Patients with perforation are more likely to have significant fever, leukocytosis, and physical findings of peritonitis than are patients with uncomplicated appendicitis. Although perforation often can be predicted preoperatively based on the presence of these findings, not all patients with these findings have perforation.76 Free perforation into the peritoneal cavity results in findings of diffuse peritonitis and can be associated with free intraperitoneal air on abdominal plain films. Patients with generalized peritonitis from appendicitis are difficult to distinguish preoperatively from patients with other causes of diffuse peritonitis.
The most severe complication of appendiceal perforation is septic thrombophlebitis of the portal vein, also known as portal pylephlebitis. Although more common early in the 20th century, there are still cases of this disease today, although diverticular disease is now the most common cause. This rare complication should be considered in a patient with appendicitis who presents with high fever and mild jaundice. Treatment of pylephlebitis is control of the inciting infection and long-term (four to six weeks) antibiotic therapy. The major organisms causing pylephlebitis are Gram-negative enteric aerobes and anaerobes. Even with aggressive therapy, the incidence of hepatic abscesses following pylephlebitis is 50%, and mortality rates are 30% to 50%.77 A long-term complication of pylephlebitis is portal vein thrombosis with cavernous transformation of the portal vein and esophagogastric varices.
TREATMENT
Treatment of acute appendicitis is and remains appendectomy, despite the advent of sophisticated diagnostic and therapeutic modalities. Little has changed since Fitz and McBurney advocated early operative treatment of appendicitis in the late 19th century. Appendectomy is recommended, even though some cases of appendicitis resolve spontaneously. Although small studies have demonstrated that the vast majority of cases will improve with intravenous antibiotics alone, more than 35% of these patients have a relapse in one year.78 We currently lack the ability to identify prospectively self-limited cases, however, and to wait for resolution places patients at risk for perforation with its resultant life-threatening complications. Thus, appendectomy is a surgical urgency, and not a true emergency. Patients with appendicitis should be given appropriate intravenous fluids to correct intravascular volume depletion and electrolyte imbalances, and intravenous antibiotics to decrease wound infection rates; they should be taken to the operating room when they are stable. Brief periods of time may be taken to optimize the patient’s concomitant medical conditions before operation, but long delays increase the rate of perforation and compromise outcome.
Two standard operative approaches exist for performing an appendectomy, either open appendectomy or laparoscopic appendectomy. Open appendectomy is performed though a muscle-splitting right lower quadrant incision; either an oblique or a transverse skin incision may be used. The appendix is identified and removed even if it is found to be normal. If normal, it is removed primarily to prevent future diagnostic confusion, and an exploration is carried out to identify other intra-abdominal causes of the patient’s symptoms. If other surgical pathology is found at exploration, the initial incision may be extended or a separate incision performed to address the problem. In advanced cases with severe inflammation, cecectomy may be required.79 Any abscesses are drained, and the abdomen is irrigated and closed.
The other common approach to appendectomy is the laparoscopic appendectomy. First described by Semm in 1983,4 this procedure has been the subject of considerable study since that time. The technique of laparoscopic appendectomy has become standardized, and typically it is performed via a three-trocar technique. After gaining access to the abdomen, the appendix and then the entire abdomen are inspected. If the appendix is inflamed, an appendectomy is performed. If other intra-abdominal surgical pathology is found, it can be treated laparoscopically, or an appropriate open surgical procedure can be performed.
It remains controversial whether laparoscopic appendectomy is superior to open appendectomy. There have now been more than 20 randomized, controlled trials and five recent meta-analyses, including an analysis by the Cochrane collaboration, comparing the two procedures80; all of these studies have remarkably similar conclusions. Both procedures are safe and effective in the treatment of nonperforated appendicitis. After laparoscopic appendectomy, however, patients require less pain medication and return to normal activity about one week sooner than after open appendectomy. The wound infection rate is 50% lower than after open appendectomy, but there may be an increased rate of intra-abdominal abscess formation. The hospital course after laparoscopic appendectomy is 0.7 days shorter, and patients resume a normal diet at about the same time as after open appendectomy. Laparoscopic appendectomy takes more time to perform, and is associated with higher equipment costs.
At this point, it is not possible to say that one procedure is superior to another for all patients; however, for some patient groups, especially young women, employed patients who need to return to work as soon as possible, and those with an uncertain diagnosis, laparoscopic appendectomy is preferable.80 Additionally, many consider the laparoscopic appendectomy the procedure of choice for the treatment of acute appendicitis in the morbidly obese population because it has been associated with shorter length of stay and lower morbidity.81,82
An exception to the statement that all patients with appendicitis require urgent appendectomy is the patient with perforation and a palpable right lower quadrant mass. These patients usually have extensive periappendiceal inflammation or abscess formation. In patients with a palpable mass who do not have diffuse peritonitis or toxicity, initial management can be operative or nonoperative. Although data are limited, there is a suggestion that early operative intervention may be associated with a higher complication rate.53 With initial nonoperative management, patients are placed on bowel rest and given intravenous fluids and antibiotics, and a CT scan of the abdomen is obtained. If a single abscess 3 cm or larger is discovered, percutaneous drainage of the abscess under CT guidance is performed. If multiple abscesses are found or the patient does not improve within 24 to 48 hours of conservative therapy, operative drainage is performed. Success rates of 88% to 95% have been reported with initial nonoperative management.83,84 Following resolution of the acute illness in older patients in whom a perforated cecal cancer is a possibility, colonoscopy, barium enema, or virtual colonography should be performed, because the incidence of appendiceal or cecal cancer in patients older than 60 years who present with acute appendicitis can exceed 20%.85 Some authors recommend interval appendectomy when the acute inflammation has resolved (6 to 12 weeks later), but the role of interval appendectomy remains controversial because the rate of recurrent appendicitis is less than 20% at one year.86
Natural orifice transluminal endoscopic surgery (NOTES) is an emerging field in minimally invasive surgery that is driving the development of new technology and techniques for procedures such as transluminal appendectomy. Although mostly investigational, NOTES procedures are performed using a transgastric, transcolonic, or transvaginal access point to the peritoneum. In 2004, Kalloo and his colleagues were able to demonstrate that natural orifices provide a port of entry via the gastrointestinal tract to the peritoneal cavity. This approach requires the creation of a per-oral transgastric perforation into the peritoneal cavity using conventional endoscopes.87 Theoretically, this approach could reduce postoperative abdominal wall pain, wound infection, hernia formation, and adhesions. A number of case reports describing the removal of the appendix using a NOTES technique have been reported in registries established by the Natural Orifice Surgery Consortium for Assessment and Research (NOSCAR) or other organized groups such as EuroNOTES and the Brazilian Registry. In the United States, Sanntiago Horgan and Mark A. Talamini were the first to report the successful removal of an inflamed appendix through a patient’s vagina.5 With a number of rigorous laboratory and clinical research studies under way, it is hoped that as data are accumulated and instrumentation improves, NOTES may play an integral role in the future of abdominal surgery, including transluminal appendectomy.
OUTCOMES
The modern treatment of simple acute appendicitis is associated with excellent outcomes. Factors responsible for these outcomes are advances in anesthesia, antibiotics, intravenous fluids, and blood products. The mortality rate from acute appendicitis in one large series was 0.08% with a complication rate of 5%.17 Older series have reported mortality rates of 0.2% with a complication rate of 6%.6 Patients typically are hospitalized for 24 to 48 hours after open appendectomy and 24 to 36 hours after laparoscopic appendectomy. Patients usually return to full activity two weeks after laparoscopic appendectomy and three weeks after open appendectomy.88
Morbidity and mortality attributable to appendicitis increase markedly with complicated appendicitis and in particular with perforation. Mortality rates of 1% to 4% and complication rates of 12% to 25% have been reported for perforated appendicitis.83 In patients older than 70 years, in whom perforation and significant medical comorbidity are common, mortality has been reported to be as high as 32%.33 Death in these circumstances usually is attributable to uncontrolled gram-negative sepsis or peritonitis, and patients with perforated appendicitis often have a stormy postoperative course, with intra-abdominal abscesses and need for operative or percutaneous abscess drainage. Wound infection and dehiscence also are common in patients who have had open appendectomy, but these often promptly respond to wound drainage and antibiotics. These complications are minimized when a laparoscopic approach is chosen.
SPECIAL TOPICS
THE APPENDIX AND ULCERATIVE COLITIS
A number of epidemiologic studies suggest that appendectomy protects against the development of ulcerative colitis (UC),89 particularly when performed for appendicitis; a similar relationship is not seen with Crohn’s disease. A meta-analysis of 17 case-control studies suggests that the relative risk of developing UC after appendectomy is about 0.3 times that of controls.90 Although these data come from case-control studies and questions can be raised about the appropriateness of the controls, this conclusion has been supported in one of the two large cohort studies performed.91 Some researchers have suggested that appendectomy also attenuates the course of active UC.92,93 Several investigators have also reported the improvement of UC after appendectomy, especially in young patients.94 In a mouse model of autoimmune colitis similar to UC, removal of the appendix early in life prompted significant attenuation of colonic inflammation.95 Although these findings are far from conclusive, they provide potential insights into both UC and the potential normal function of the appendix.
CROHN’S DISEASE OF THE APPENDIX
Although the appendix is often involved in patients with Crohn’s disease of the ileum or colon, isolated Crohn’s disease of the appendix is quite rare.96 Crohn’s appendicitis is difficult to distinguish from acute appendicitis preoperatively, although patients with Crohn’s appendicitis commonly have a longer history of pain. The treatment of appendiceal Crohn’s disease is appendectomy, which can be accomplished with a low rate of postoperative fistula formation.97 The clinical course of Crohn’s disease isolated to the appendix appears to be much more benign than that of typical Crohn’s disease. Because isolated Crohn’s disease of the appendix is quite rare, any patient found to have Crohn’s appendicitis should undergo evaluation of the remainder of the gastrointestinal tract for evidence of Crohn’s disease.
RECURRENT AND CHRONIC APPENDICITIS
Recurrent appendicitis is the clinical scenario in which a patient with pathologically confirmed acute appendicitis relates one or more prior episodes with identical symptoms, which resolved without surgical intervention. This diagnosis remains somewhat controversial but has been documented in clinical series.98 The diagnosis of recurrent appendicitis presupposes that some cases of appendicitis can resolve without medical intervention. Series of such cases exist in the radiologic literature, where patients with imaging findings consistent with appendicitis had rapid resolution of their symptoms without treatment. The percentage of cases of appendicitis that resolve spontaneously is unknown, but it is estimated at 6% to 8%. In small series of patients with spontaneous resolution of appendicitis, the recurrence rate is approximately 40%.99 No prospective means of identifying spontaneously resolving appendicitis have been identified, and therefore all cases of appendicitis should be treated surgically. The existence of recurrent appendicitis serves as a reminder not to discount the diagnosis of appendicitis in patients just because of prior episodes of similar abdominal pain.
Chronic appendicitis is diagnosed when pathologic findings of fibrosis and chronic inflammation are found with a clinical syndrome consistent with appendicitis. Many of these patients report previous episodes of pain and relief of their symptoms after appendectomy.100 This is not a common problem, and caution should be used in applying this diagnosis to patients with poorly characterized chronic abdominal pain, because many of these patients are unlikely to improve with appendectomy.
DIVERTICULITIS OF THE APPENDIX
Diverticula of the appendix are uncommon, with a reported incidence in appendectomy specimens between 0.004% and 2.1%.101 Two forms of diverticula exist: congenital and acquired. True congenital diverticula are quite rare, but acquired diverticula are found in 1% to 2% of appendectomy specimens.102 Although the etiology of acquired appendiceal diverticula is unclear, they are thought to be pulsion diverticula, like colonic diverticula. Appendiceal diverticula typically are diagnosed incidentally on barium enema or CT scan or at surgical exploration.103 Acute inflammation of appendiceal diverticula (diverticulitis) produces a clinical picture that mimics acute appendicitis, making diverticulitis of the appendix a difficult preoperative diagnosis. Appendiceal diverticulitis, however, typically occurs in patients in the fourth decade of life rather than in the first or second decades, and it tends to manifest with a more insidious course, with many days of pain before presentation.104 CT scan can readily make the diagnosis. Appendiceal diverticulitis is more likely to be complicated by perforation than the usual case of appendicitis, making surgery, rather than nonoperative management, the treatment of choice.
EPITHELIAL MALIGNANCIES OF THE APPENDIX
Tumors of the appendix are rare and are found in approximately 1% of appendix specimens submitted for pathologic examination. The vast majority of appendiceal tumors are carcinoid, but this tumor is a rare cause of appendicitis because it usually arises from the tip of the appendix, not the base. (see Chapter 31). The incidence of epithelial malignancies of the appendix has been estimated to be 0.12 per 1 million persons per year.105
There are two types of epithelial malignancies: mucinous adenocarcinoma or cystadenocarcinoma of the appendix and colonic type (non-mucinous) adenocarcinoma of the appendix. Mucin-producing tumors are roughly twice as common as non–mucin-producing tumors.106 Non–mucin-producing tumors of the appendix typically manifest with a clinical picture indistinguishable from that of acute appendicitis, with acute right lower quadrant pain and tenderness. On CT scan, findings of a soft tissue mass or an appendix more than 15 mm in diameter should raise the suspicion of an appendiceal cancer.107 In contrast, less than one third of mucinous appendiceal adenocarcinomas manifest as acute appendicitis. More commonly these lesions are found incidentally on imaging studies as a cystic right lower quadrant mass or in a patient with increasing abdominal girth secondary to pseudomyxoma peritonei.
The optimal treatment of all adenocarcinomas of the appendix is right hemicolectomy, either as a primary operation or as a secondary operation after adenocarcinoma of the appendix is noted on pathologic examination of an appendectomy specimen. Additionally, patients with appendiceal adenocarcinoma have a significant risk of synchronous and metachronous neoplasms, which often originate from the gastrointestinal tract.105 Overall survival of patients with adenocarcinoma of the appendix is roughly 60% at five years and is a function of tumor stage at presentation.
Appendiceal lymphoma is extremely uncommon, and primary lymphoma of the appendix accounts for 1% to 3% of all gastrointestinal lymphomas (see Chapter 29).108 Patients with appendiceal lymphoma usually present with acute appendicitis and an appendiceal diameter more than 2.5 cm, with surrounding soft-tissue thickening. Management of appendiceal lymphoma is appendectomy; right hemicolectomy is indicated only if there is extension of tumor beyond the appendix onto the mesentery or cecum.109
Appendiceal mucoceles are uncommon entities arising from a variety of different pathologic processes, of which only a small subset are associated with development of pseudomyxoma peritonei. Hence, the pathologic diagnosis determines further management.110 Perforation of a mucocele results in intraperitoneal dissemination of mucoid material, which can be acellular or can contain cells with varying degrees of dysplasia; cellular spread to the peritoneal surfaces leads to pseudomyxoma peritonei. These tumors usually are less aggressive than colorectal cancer, however, and they rarely manifest with lymph node or liver metastasis.111 The combination of surgery and complete cytoreduction should be followed by intraperitoneal rather than intravenous chemotherapy because the mainstay goal is prevention of locoregional recurrence, not prevention of systemic spread of disease.
INCIDENTAL OR PROPHYLACTIC APPENDECTOMY
The lifetime risk of appendicitis at birth is about one in 12, and declines to one in 35 by age 35 years. The greatest risk of appendicitis in a given year occurs over the second decade of life with a risk of about 0.25% per year.6 Although appendicitis is the most common cause of emergent abdominal surgery, given the low lifetime risk of appendicitis, elective prophylactic appendectomy cannot be recommended. Incidental appendectomy, the removal of a normal appendix at the time of other abdominal surgery was, at one time, the leading cause of appendectomy in women. In light of the falling incidence of appendicitis, enthusiasm for incidental appendectomy has declined. In operations where it will not add morbidity, however, a case may exist for incidental appendectomy in patients younger than 30 years. In older patients, the low residual lifetime risk of appendicitis makes incidental appendectomy difficult to defend.
Addiss DG, Shaffer N, Fowler BS, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol. 1990;132:910-25. (Ref 6.)
Andersson RE. Meta-analysis of the clinical and laboratory diagnosis of appendicitis. Br J Surg. 2004;91:28-37. (Ref 41.)
Carr NJ. The pathology of acute appendicitis. Ann Diagn Pathol. 2000;4:46-58. (Ref 15.)
Ditillo MF, Dziura JD, Rabinovici R. Is it safe to delay appendectomy in adults with acute appendicitis? Ann Surg. 2006;244:656-60. (Ref 65.)
Golden RL, Reginald H. Fitz, appendicitis, and the Osler connection—a discursive review. Surgery. 1995;118:504-9. (Ref 1.)
Rao PM, Rhea JT, Rattner DW, et al. Introduction of appendiceal CT: impact on negative appendectomy and appendiceal perforation rates. Ann Surg. 1999;229:344-9. (Ref 42.)
Rothrock SG, Pagane J. Acute appendicitis in children: emergency department diagnosis and management. Ann Emerg Med. 2000;36:39-51. (Ref 29.)
Sauerland S, Lefering R, Neugebauer EA. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev 2004:CD001546. (Ref 80.)
Schumpelick V, Dreuw B, Ophoff K, Prescher A. Appendix and cecum. Embryology, anatomy, and surgical applications. Surg Clin North Am. 2000;80:295-318. (Ref 12.)
Terasawa T, Blackmore CC, Bent S, Kohlwes RJ. Systematic review: computed tomography and ultrasonography to detect acute appendicitis in adults and adolescents. Ann Intern Med. 2004;141:537-46. (Ref 47.)
van Randen A, Bipat S, Zwinderman AH, et al. Acute appendicitis: meta-analysis of diagnostic performance of CT and graded compression US related to prevalence of disease. Radiology. 2008;249:97-106. (Ref 56.)
Wagner JM, McKinney WP, Carpenter JL. Does this patient have appendicitis? JAMA. 1996;276:1589-94. (Ref 27.)
1. Golden RL, Reginald H. Fitz, appendicitis, and the Osler connection—a discursive review. Surgery. 1995;118:504-9.
2. McBurney C. Experience with early operative interference in cases of diseases of the vermiform appendix. N Y Med J. 1889;21:676.
3. McBurney CIV. The incision made in the abdominal wall in cases of appendicitis, with a description of a new method of operating. Ann Surg. 1894;20:38-43.
4. Semm K. Endoscopic appendectomy. Endoscopy. 1983;15:59-64.
5. Sharples T. The no incision appendectomy. Time 2008 April 3 [cited 2009 June 3]. Available from http://www.time.com/time/health/article/0,8599,1727656,00.html?cnn=yes
6. Addiss DG, Shaffer N, Fowler BS, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol. 1990;132:910-25.
7. Walker AR, Segal I. Appendicitis: an African perspective. J R Soc Med. 1995;88:616-17.
8. Kang JY, Hoare J, Majeed A, et al. Decline in admission rates for acute appendicitis in England. Br J Surg. 2003;90:1586-92.
9. Papadopoulos AA, Polymeros D, Kateri M, et al. Dramatic decline of acute appendicitis in Greece over 30 years: index of improvement of socioeconomic conditions or diagnostic aids? Dig Dis. 2008;26:80-4.
10. Al Omran M, Mamdani M, McLeod RS. Epidemiologic features of acute appendicitis in Ontario, Canada. Can J Surg. 2003;46:263-8.
11. Livingston EH, Woodward WA, Sarosi GA, Haley RW. Disconnect between incidence of nonperforated and perforated appendicitis: implications for pathophysiology and management. Ann Surg. 2007;245:886-92.
12. Schumpelick V, Dreuw B, Ophoff K, Prescher A. Appendix and cecum. Embryology, anatomy, and surgical applications. Surg Clin North Am. 2000;80:295-318.
13. Beneventano TC, Schein CJ, Jacobson HG. The roentgen aspects of some appendiceal abnormalities. Am J Roentgenol Radium Ther Nucl Med. 1966;96:344-60.
14. Ramsden WH, Mannion RA, Simpkins KC, deDombal FT. Is the appendix where you think it is—and if not does it matter? Clin Radiol. 1993;47:100-3.
15. Carr NJ. The pathology of acute appendicitis. Ann Diagn Pathol. 2000;4:46-58.
16. Pieper R, Kager L, Tidefeldt U. Obstruction of appendix vermiformis causing acute appendicitis. An experimental study in the rabbit. Acta Chir Scand. 1982;148:63-72.
17. Hale DA, Molloy M, Pearl RH, et al. Appendectomy: a contemporary appraisal. Ann Surg. 1997;225:252-61.
18. Chang AR. An analysis of the pathology of 3003 appendices. Aust N Z J Surg. 1981;51:169-78.
19. Arnbjornsson E, Bengmark S. Obstruction of the appendix lumen in relation to pathogenesis of acute appendicitis. Acta Chir Scand. 1983;149:789-91.
20. Sisson RG, Ahlvin RC, Harlow MC. Superficial mucosal ulceration and the pathogenesis of acute appendicitis. Am J Surg. 1971;122:378-80.
21. Dzabic M, Bostrom L, Rahbar A. High prevalence of an active cytomegalovirus infection in the appendix of immunocompetent patients with acute appendicitis. Inflamm Bowel Dis. 2008;14:236-41.
22. Barker DJ. Rise and fall of Western diseases. Nature. 1989;338:371-2.
23. Gallerani M, Boari B, Anania G, et al. Seasonal variation in onset of acute appendicitis. Clin Ter. 2006;157:123-7.
24. Walker AR, Segal I. Effects of transition on bowel diseases in sub-Saharan Africans. Eur J Gastroenterol Hepatol. 1997;9:207-10.
25. Naaeder SB, Archampong EQ. Acute appendicitis and dietary fibre intake. West Afr J Med. 1998;17:264-7.
26. Silen W. Cope’s early diagnosis of the acute abdomen. New York: Oxford University Press; 1991.
27. Wagner JM, McKinney WP, Carpenter JL. Does this patient have appendicitis? JAMA. 1996;276:1589-94.
28. Golledge J, Toms AP, Franklin IJ, et al. Assessment of peritonism in appendicitis. Ann R Coll Surg Engl. 1996;78:11-14.
29. Rothrock SG, Pagane J. Acute appendicitis in children: emergency department diagnosis and management. Ann Emerg Med. 2000;36:39-51.
30. Paajanen H, Mansikka A, Laato M, et al. Are serum inflammatory markers age dependent in acute appendicitis? J Am Coll Surg. 1997;184:303-8.
31. Nance ML, Adamson WT, Hedrick HL. Appendicitis in the young child: a continuing diagnostic challenge. Pediatr Emerg Care. 2000;16:160-2.
32. Paranjape C, Dalia S, Pan J, Horattas M. Appendicitis in the elderly: a change in the laparoscopic era. Surg Endosc. 2007;21:777-81.
33. Franz MG, Norman J, Fabri PJ. Increased morbidity of appendicitis with advancing age. Am Surg. 1995;61:40-4.
34. Andersen B, Nielsen TF. Appendicitis in pregnancy: diagnosis, management and complications. Acta Obstet Gynecol Scand. 1999;78:758-62.
35. Saltzman DJ, Williams RA, Gelfand DV, Wilson SE. The surgeon and AIDS: twenty years later. Arch Surg. 2005;140:961-7.
36. Crum-Cianflone N, Weekes J, Bavaro M. Appendicitis in HIV-infected patients during the era of highly active antiretroviral therapy. HIV Med. 2008;9:421-6.
37. Flum DR, Steinberg SD, Sarkis AY, Wallack MK. Appendicitis in patients with acquired immunodeficiency syndrome. J Am Coll Surg. 1997;184:481-6.
38. Andersson RE, Hugander AP, Ghazi SH, et al. Diagnostic value of disease history, clinical presentation, and inflammatory parameters of appendicitis. World J Surg. 1999;23:133-40.
39. Hallan S, Asberg A. The accuracy of C-reactive protein in diagnosing acute appendicitis—a meta-analysis. Scand J Clin Lab Invest. 1997;57:373-80.
40. Puskar D, Bedalov G, Fridrih S, et al. Urinalysis, ultrasound analysis, and renal dynamic scintigraphy in acute appendicitis. Urology. 1995;45:108-12.
41. Andersson RE. Meta-analysis of the clinical and laboratory diagnosis of appendicitis. Br J Surg. 2004;91:28-37.
42. Rao PM, Rhea JT, Rattner DW, et al. Introduction of appendiceal CT: impact on negative appendectomy and appendiceal perforation rates. Ann Surg. 1999;229:344-9.
43. Antevil JL, Rivera L, Langenberg BJ, et al. Computed tomography–based clinical diagnostic pathway for acute appendicitis: prospective validation. J Am Coll Surg. 2006;203:849-56.
44. Boleslawski E, Panis Y, Benoist S, et al. Plain abdominal radiography as a routine procedure for acute abdominal pain of the right lower quadrant: prospective evaluation. World J Surg. 1999;23:262-4.
45. Birnbaum BA, Jeffrey RBJr. CT and sonographic evaluation of acute right lower quadrant abdominal pain. AJR Am J Roentgenol. 1998;170:361-71.
46. Yabunaka K, Katsuda T, Sanada S, Fukutomi T. Sonographic appearance of the normal appendix in adults. J Ultrasound Med. 2007;26:37-43.
47. Terasawa T, Blackmore CC, Bent S, Kohlwes RJ. Systematic review: computed tomography and ultrasonography to detect acute appendicitis in adults and adolescents. Ann Intern Med. 2004;141:537-46.
48. Peletti AB, Baldisserotto M. Optimizing US examination to detect the normal and abnormal appendix in children. Pediatr Radiol. 2006;36:1171-6.
49. Guillerman RP, Brody AS, Kraus SJ. Evidence-based guidelines for pediatric imaging: the example of the child with possible appendicitis. Pediatr Ann. 2002;31:629-40.
50. Franke C, Bohner H, Yang Q, et al. Ultrasonography for diagnosis of acute appendicitis: results of a prospective multicenter trial. Acute Abdominal Pain Study Group. World J Surg. 1999;23:141-6.
51. Josephson T, Styrud J, Eriksson S. Ultrasonography in acute appendicitis. Body mass index as selection factor for US examination. Acta Radiol. 2000;41:486-8.
52. Barron B, Hanna C, Passalaqua AM, et al. Rapid diagnostic imaging of acute, nonclassic appendicitis by leukoscintigraphy with sulesomab, a technetium 99m–labeled antigranulocyte antibody Fab fragment. LeukoScan Appendicitis Clinical Trial Group. Surgery. 1999;125:288-96.
53. Andersson RE, Petzold MG. Nonsurgical treatment of appendiceal abscess or phlegmon: A systematic review and meta-analysis. Ann Surg. 2007;246:741-8.
54. Weltman DI, Yu J, Krumenacker JJr, et al. Diagnosis of acute appendicitis: Comparison of 5- and 10-mm CT sections in the same patient. Radiology. 2000;216:172-7.
55. Rao PM, Rhea JT, Novelline RA. Helical CT of appendicitis and diverticulitis. Radiol Clin North Am. 1999;37:895-910.
56. van Randen A, Bipat S, Zwinderman AH, et al. Acute appendicitis: meta-analysis of diagnostic performance of CT and graded compression US related to prevalence of disease. Radiology. 2008;249:97-106.
57. Hall EJ. Lessons we have learned from our children: cancer risks from diagnostic radiology. Pediatr Radiol. 2002;32:700-6.
58. Kosloske AM, Love CL, Rohrer JE, et al. The diagnosis of appendicitis in children: outcomes of a strategy based on pediatric surgical evaluation. Pediatrics. 2004;113:29-34.
59. Ohmann C, Yang Q, Franke C. Diagnostic scores for acute appendicitis. Abdominal Pain Study Group. Eur J Surg. 1995;161:273-81.
60. Jahn H, Mathiesen FK, Neckelmann K, et al. Comparison of clinical judgment and diagnostic ultrasonography in the diagnosis of acute appendicitis: experience with a score-aided diagnosis. Eur J Surg. 1997;163:433-43.
61. Larsson PG, Henriksson G, Olsson M, et al. Laparoscopy reduces unnecessary appendicectomies and improves diagnosis in fertile women. A randomized study. Surg Endosc. 2001;15:200-2.
62. Van Den Broek WT, Bijnen AB, van Eerten PV, et al. Selective use of diagnostic laparoscopy in patients with suspected appendicitis. Surg Endosc. 2000;14:938-41.
63. Coste J, Job-Spira N, Fernandez H, et al. Risk factors for ectopic pregnancy: A case-control study in France, with special focus on infectious factors. Am J Epidemiol. 1991;133:839-49.
64. Zupi E, Marconi D, Sbracia M, et al. Is local anesthesia an affordable alternative to general anesthesia for minilaparoscopy? J Am Assoc Gynecol Laparosc. 2000;7:111-14.
65. Ditillo MF, Dziura JD, Rabinovici R. Is it safe to delay appendectomy in adults with acute appendicitis? Ann Surg. 2006;244:656-60.
66. Wen SW, Naylor CD. Diagnostic accuracy and short-term surgical outcomes in cases of suspected acute appendicitis. CMAJ. 1995;152:1617-26.
67. Guss DA, Behling CA, Munassi D. Impact of abdominal helical computed tomography on the rate of negative appendicitis. J Emerg Med. 2008;34:7-11.
68. Rhea JT, Halpern EF, Ptak T, et al. The status of appendiceal CT in an urban medical center 5 years after its introduction: experience with 753 patients. AJR Am J Roentgenol. 2005;184:1802-8.
69. Tsao K, St Peter SD, Valusek PA, et al. Management of pediatric acute appendicitis in the computed tomographic era. J Surg Res. 2008;147:221-4.
70. Pena BM, Taylor GA, Fishman SJ, Mandl KD. Effect of an imaging protocol on clinical outcomes among pediatric patients with appendicitis. Pediatrics. 2002;110:1088-93.
71. Rao PM, Rhea JT, Novelline RA, et al. Effect of computed tomography of the appendix on treatment of patients and use of hospital resources. N Engl J Med. 1998;338:141-6.
72. Andersson RE. Small bowel obstruction after appendicectomy. Br J Surg. 2001;88:1387-91.
73. Lau WY, Fan ST, Yiu TF, et al. Negative findings at appendectomy. Am J Surg. 1984;148:375-8.
74. Flum DR, Morris A, Koepsell T, Dellinger EP. Has misdiagnosis of appendicitis decreased over time? A population-based analysis. JAMA. 2001;286:1748-53.
75. Temple CL, Huchcroft SA, Temple WJ. The natural history of appendicitis in adults. A prospective study. Ann Surg. 1995;221:278-81.
76. Oliak D, Yamini D, Udani VM, et al. Can perforated appendicitis be diagnosed preoperatively based on admission factors? J Gastrointest Surg. 2000;4:470-4.
77. Plemmons RM, Dooley DP, Longfield RN. Septic thrombophlebitis of the portal vein (pylephlebitis): diagnosis and management in the modern era. Clin Infect Dis. 1995;21:1114-20.
78. Eriksson S, Granstrom L. Randomized controlled trial of appendicectomy versus antibiotic therapy for acute appendicitis. Br J Surg. 1995;82:166-9.
79. Sarkar R, Bennion RS, Schmit PJ, Thompson JE. Emergent ileocecectomy for infection and inflammation. Am Surg. 1997;63:874-7.
80. Sauerland S, Lefering R, Neugebauer EA. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev 2004:CD001546.
81. Varela JE, Hinojosa MW, Nguyen NT. Laparoscopy should be the approach of choice for acute appendicitis in the morbidly obese. Am J Surg. 2008;196:218-22.
82. Corneille MG, Steigelman MB, Myers JG, et al. Laparoscopic appendectomy is superior to open appendectomy in obese patients. Am J Surg. 2007;194:877-80.
83. Margenthaler JA, Longo WE, Virgo KS, et al. Risk factors for adverse outcomes after the surgical treatment of appendicitis in adults. Ann Surg. 2003;238:59-66.
84. Oliak D, Yamini D, Udani VM, et al. Initial nonoperative management for periappendiceal abscess. Dis Colon Rectum. 2001;44:936-41.
85. Todd RD, Sarosi GA, Nwariaku F, Anthony T. Incidence and predictors of appendiceal tumors in elderly males presenting with signs and symptoms of acute appendicitis. Am J Surg. 2004;188:500-4.
86. Deakin DE, Ahmed I. Interval appendicectomy after resolution of adult inflammatory appendix mass—is it necessary? Surgeon. 2007;5:45-50.
87. Kalloo AN, Singh VK, Jagannath SB, et al. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc. 2004;60:114-17.
88. Hellberg A, Rudberg C, Kullman E, et al. Prospective randomized multicentre study of laparoscopic versus open appendicectomy. Br J Surg. 1999;86:48-53.
89. Hallas J, Gaist D, Sorensen HT. Does appendectomy reduce the risk of ulcerative colitis? Epidemiology. 2004;15:173-8.
90. Koutroubakis IE, Vlachonikolis IG, Kouroumalis EA. Role of appendicitis and appendectomy in the pathogenesis of ulcerative colitis: a critical review. Inflamm Bowel Dis. 2002;8:277-86.
91. Andersson RE, Olaison G, Tysk C, Ekbom A. Appendectomy and protection against ulcerative colitis. N Engl J Med. 2001;344:808-14.
92. Radford-Smith GL, Edwards JE, Purdie DM, et al. Protective role of appendicectomy on onset and severity of ulcerative colitis and Crohn’s disease. Gut. 2002;51:808-13.
93. Cosnes J, Carbonnel F, Beaugerie L, et al. Effects of appendicectomy on the course of ulcerative colitis. Gut. 2002;51:803-7.
94. Matsushita M, Uchida K, Okazaki K. Role of the appendix in the pathogenesis of ulcerative colitis. Inflammopharmacology. 2007;15:154-7.
95. Mizoguchi A, Mizoguchi E, Chiba C, et al. Role of appendix in the development of inflammatory bowel disease in TCR-α mutant mice. J Exp Med. 1996;184:707-15.
96. Richards ML, Aberger FJ, Landercasper J. Granulomatous appendicitis: Crohn’s disease, atypical Crohn’s or not Crohn’s at all? J Am Coll Surg. 1997;185:13-17.
97. Prieto-Nieto I, Perez-Robledo JP, Hardisson D, et al. Crohn’s disease limited to the appendix. Am J Surg. 2001;182:531-3.
98. Barber MD, McLaren J, Rainey JB. Recurrent appendicitis. Br J Surg. 1997;84:110-12.
99. Cobben LP, de Van Otterloo AM, Puylaert JB. Spontaneously resolving appendicitis: frequency and natural history in 60 patients. Radiology. 2000;215:349-52.
100. Mattei P, Sola JE, Yeo CJ. Chronic and recurrent appendicitis are uncommon entities often misdiagnosed. J Am Coll Surg. 1994;178:385-9.
101. Friedlich M, Malik N, Lecompte M, Ayroud Y. Diverticulitis of the appendix. Can J Surg. 2004;47:146-7.
102. Phillips BJ, Perry CW. Appendiceal diverticulitis. Mayo Clin Proc. 1999;74:890-2.
103. Chiou YY, Pitman MB, Hahn PF, et al. Rare benign and malignant appendiceal lesions: spectrum of computed tomography findings with pathologic correlation. J Comput Assist Tomogr. 2003;27:297-306.
104. Place RJ, Simmang CL, Huber PJJr. Appendiceal diverticulitis. South Med J. 2000;93:76-9.
105. McCusker ME, Cote TR, Clegg LX, Sobin LH. Primary malignant neoplasms of the appendix: a population-based study from the surveillance, epidemiology and end-results program, 1973-1998. Cancer. 2002;94:3307-12.
106. Ito H, Osteen RT, Bleday R, et al. Appendiceal adenocarcinoma: long-term outcomes after surgical therapy. Dis Colon Rectum. 2004;47:474-80.
107. Pickhardt PJ, Levy AD, Rohrmann CAJr, Kende AI. Primary neoplasms of the appendix manifesting as acute appendicitis: CT findings with pathologic comparison. Radiology. 2002;224:775-81.
108. Crump M, Gospodarowicz M, Shepherd FA. Lymphoma of the gastrointestinal tract. Semin Oncol. 1999;26:324-37.
109. Pickhardt PJ, Levy AD, Rohrmann CAJr, et al. Non-Hodgkin’s lymphoma of the appendix: clinical and CT findings with pathologic correlation. AJR Am J Roentgenol. 2002;178:1123-7.
110. Pai RK, Longacre TA. Appendiceal mucinous tumors and pseudomyxoma peritonei: histologic features, diagnostic problems, and proposed classification. Adv Anat Pathol. 2005;12:291-311.
111. Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006;7:69-76.