Appendicitis
Appendicitis is one of the most common surgical emergencies in children. Over 70,000 cases are seen in the USA each year.1,2 The lifetime risk of appendicitis is 9% in boys and 7% in girls. Unfortunately, there is a lack of general consensus regarding its diagnosis and management.3
Pathophysiology
The spectrum of appendicitis ranges from simple inflammation to gross perforation. This concept was initially described by van Zwalenberg in 1905 and confirmed in an experimental model by Wangensteen in 1939.4,5 Obstruction of the lumen can occur from multiple causes including fecal material (fecalith), lymphoid hyperplasia, foreign body, or parasites. Fecaliths are present in roughly 20% of children with acute appendicitis and 30–40% of children with perforated appendicitis.6,7 Fecaliths and appendicitis are more common in developed countries with low-fiber diets compared to developing countries with high-fiber diets.8 Hyperplasia of the lymphoid tissue near the base of the appendix is also a common cause of appendiceal obstruction in children. Interestingly, the incidence of appendicitis closely resembles the amount of appendiceal lymphoid follicles present.9 Organisms such as Yersenia, Salmonella, and Shigella can cause a local or generalized reaction of the lymphoid tissue leading to obstruction. In similar fashion, parasitic infestations from Entamoeba, Strongyloides, Enterobius, Schistosoma, or Ascaris species and viral infections such as mumps virus, coxsackie virus B, cytomegalovirus, and adenovirus can lead to luminal obstruction secondary to lymphoid hyperplasia.10–18 In children with cystic fibrosis, obstruction may be due to abnormal production of mucus leading to painful distention with or without inflammation.19 Appendicitis in neonates is rare and warrants evaluation for cystic fibrosis and Hirschsprung disease.20 It is difficult to distinguish neonatal appendicitis from necrotizing enterocolitis confined to the appendix.21
Following obstruction, the appendix becomes distended from the accumulation of mucus and proliferation of bacteria. As intraluminal pressure increases, lymphatic and venous drainage are impaired resulting in local edema. A further increase in pressure will limit arterial inflow, thus jeopardizing tissue integrity and ultimately leading to tissue necrosis and perforation. Although the natural history of untreated appendicitis is usually perforation and abscess, not all patients will progress in this fashion. Resolution of untreated appendicitis has been described and may be the mechanism behind the clinical phenomenon of relapsing or chronic appendicitis.22,23
Historically, appendicitis has been considered a somewhat time-sensitive condition such that a significant delay in treatment may lead to an increased risk of perforation. It is for this reason that young children have a higher appendiceal perforation rate compared to older children.24 Younger children have less ability to understand or articulate their developing symptoms. As a result, perforation rates have been reported to be as high as 82% in children younger than 5 years and nearly 100% in 1-year olds.25
Age is not the only factor accounting for delays in treatment and therefore higher perforation rates. One of the biggest concerns contributing to this delay is the lack of access to health care. It follows that patients with poor access to health care will have higher perforation rates. Indeed, children with no insurance or public insurance have higher rates of appendiceal perforation compared to children with private insurance.26–29 Minorities also have higher perforation rates compared to whites.26–29 Encouragingly, settings in which patients have equal access to health care or a well-established primary care network eliminate these racial, ethnic, and socioeconomic differences.30,31
Clinical Presentation
Fever, tachycardia, and leukocytosis develop as a consequence of systemic inflammatory mediators released by ischemic tissues, white blood cells, and bacteria. The inflamed appendix then irritates the overlying peritoneum, typically by direct contact. This leads to focal peritonitis and localized right lower quadrant pain. This process explains the typical migrating pain from the umbilicus to the right lower quadrant. Any movement of the peritoneum will lead to an exacerbation of the pain. Thus, children will often demonstrate voluntary guarding of the right lower quadrant during the exam. Furthermore, children will usually resist walking and jumping due to the increased pain associated with such movement.
Laboratory studies often show a mild leukocytosis. A markedly elevated leukocyte count suggests perforation or another diagnosis. Patients with appendicitis will have higher leukocyte counts compared to patients without appendicitis.32 However a broad range of sensitivity (52–96%) exists, which limits the usefulness of this laboratory value alone. A left-shifted differential count may be a better diagnostic indicator, but a wide range in sensitivity (39–96%) also can lead to misinterpretation.33–35 Other inflammatory markers including C-reactive protein (CRP), procalcitonin, and D-lactate have also been investigated. Of these markers, only CRP has been shown to be useful. A value greater than 3 mg/dL has been associated with the definitive diagnosis of appendicitis when compared to children with abdominal pain from a different etiology.32 The combination of elevated leukocyte count and CRP level has the highest correlation of definitively diagnosing appendicitis.32,36 Although normal values of both leukocyte count and CRP make the diagnosis of appendicitis less likely, the clinical signs and symptoms should be carefully considered as appendicitis cannot be excluded based on normal laboratory values. A urine analysis is typically obtained and is usually free of bacteria, but a few or moderate number of red or white blood cells may be found as the inflammatory process of the appendix may locally affect the bladder or ureter.
The typical presentation of appendicitis as described previously is found in roughly 50% of patients.37 Children with appendicitis often present with wide deviations from this classic picture making for a challenging diagnosis. In patients with an atypical presentation of appendicitis, clinical scoring systems have been used to aid in making the diagnosis.38,39 Accuracy of these scoring systems has been inconsistent which limits their usefulness over clinical judgment.40–42 They have, however been shown to decrease the use of computed tomography (CT) scans.43 Recent studies have stratified patients into risk categories based on history, physical examination, and laboratory studies to determine which patients should have surgical consultation (high risk), additional imaging studies (medium risk), or be discharged (low risk).38–42 This is the most applicable use of a scoring system or clinical pathway at the present time.
Imaging Studies
Misdiagnosing appendicitis can lead to significant delays in treatment. Children are often diagnosed with gastroenteritis and parents are reassured that their child will improve, which may delay them from seeking further care. Epidemiologic data have shown the risk of a missed diagnosis in children to be higher in hospitals with a volume of less than one pediatric appendectomy per week.44 Historically, negative appendectomy rates of 10% to 20% were not only considered appropriate but advisable to minimize the number of patients with a missed diagnosis and to decrease perforation rates. Some authors have questioned this philosophy, citing the risk and expense of an avoidable operation.45 Appropriate use of diagnostic imaging can minimize both the negative appendectomy and perforation rates. Currently, the negative appendectomy rate from high-volume children’s hospitals is 3–4%.46–48 Despite the increased use of imaging studies, correctly diagnosing children less than 5 years of age continues to be challenging with negative appendectomy rates ranging from 13–17%.48
Ultrasonography (US) offers the advantages of being an efficient bedside technique that is noninvasive, requires no contrast, and emits no radiation. Thus, ultrasound should be the first imaging study utilized in patients with atypical presentations of appendicitis. Common ultrasound findings include a fluid-filled, noncompressible appendix, a diameter greater than 6 mm (Fig. 42-1), appendicolith, periappendiceal or pericecal fluid, and increased periappendiceal echogenicity caused by inflammation.49,50 Most studies demonstrate a sensitivity greater than 85% and specificity greater than 90%.51,52 However, ultrasound is operator dependent and results of published studies may not be similar to results obtained in many clinical settings. Patient factors such as bowel gas pattern, obesity, and guarding or movement can affect the accuracy. False-positive results may be due to a large appendix or another tubular structure being mistaken for the appendix. When a normal appendix is identified, it is a reliable study to rule out appendicitis. Unfortunately, only 10–50% of children with normal appendices can be identified.52–54 When a normal appendix is not seen, there is still a risk of appendicitis despite an otherwise normal ultrasound study.55 Graded compression ultrasound places pressure on the transducer to displace bowel loops and identify the appendix. The pressure is felt adequate if the psoas muscle and the iliac vessels are identified, which assure the range of view is posterior to the appendix. Furthermore, data from a large series employing upward graded compression, posterior manual compression, left oblique lateral decubitus position, and a low frequency convex transducer demonstrated that nearly all appendices could be identified with over 98% accuracy for correctly diagnosing appendicitis.56 Contrast-enhanced power Doppler ultrasound imaging demonstrated similar accuracy in a small study.57
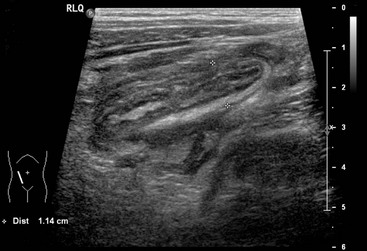
FIGURE 42-1 This longitudinal view of an ultrasound in a patient with acute appendicitis shows an enlarged appendix measuring 11 mm. in diameter.
When ultrasound is unable to exclude or confirm appendicitis, additional imaging or observation with serial examinations is warranted. In order to avoid hospitalization for observation, many physicians obtain a CT scan. The findings of an enlarged appendix (>6 mm), appendiceal wall thickening (>1 mm), periappendiceal fat stranding, and appendiceal wall enhancement are useful diagnostic criteria (Fig. 42-2).58,59 For the most part, the sensitivity and specificity of CT are around 95%.60–66 These values are significantly lower in diagnosing perforated appendicitis.67 The perceived improved diagnostic accuracy of CT has led to a dramatic increase in the number of CT scans performed in children even though there is not good evidence that supports its routine use for the diagnosis of appendicitis.68–71
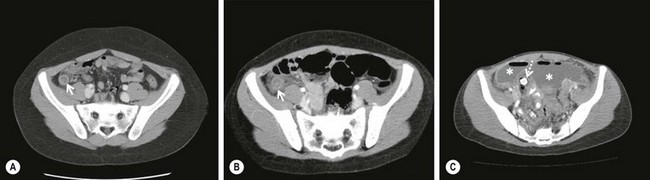
FIGURE 42-2 These three CT scans show differing presentations for appendicitis. (A) The appendix (arrow) is enlarged and has a thickened wall. There are no inflammatory changes such as periappendiceal fat stranding seen on this study. (B) The appendix (arrow) is enlarged and there is free fluid and inflammatory changes medially indicating likely perforation. (C) The patient presented with a one week history of pain and the appendix has perforated with the development of two abscesses (asterisks). In addition, a fecalith is seen medially (dotted arrow). This patient was initially managed nonoperatively with drainage of the abscesses and intravenous antibiotics. She underwent laparoscopic interval appendectomy 10 weeks following the initial admission.
There are, however, several concerns with CT. Some protocols require a delay in the emergency department for contrast administration, and younger children may require sedation. Recently, the ease of rapid helical CT has led to an estimated 200% increase in pediatric CT scans, significantly increasing radiation exposure in young patients.72 This has become a growing concern because although no direct connection between CT scan and malignancy has been made, lifetime radiation exposure has been linked to an increased risk of malignancy.73 It has been estimated that a complete abdominal CT scan is equivalent to 25.7 months of natural background radiation exposure.74 Developing tissues are more sensitive to the effects of radiation as evidenced by an increased risk of radiation-induced malignancy in patients exposed at a younger age.73,75 The risk of a fatal radiation-induced malignancy is estimated at 0.18% for a 1-year-old child. In other words, one death due to malignancy would result from an abdominal CT scan done on 555 1-year-old patients, whereas about twice as many 15-year-olds would need to be scanned to equal that risk. Although this estimate seems significant, it represents only a 0.35% increase in overall risk compared to the risk of cancer mortality with natural background radiation.76 Use of a staged imaging protocol, performing CT scan only if ultrasound findings are equivocal, has shown a reduction in the number of CT scans performed and therefore overall radiation exposure without sacrificing diagnostic sensitivity and specificity.77 In addition, international guidelines on radiation protection have implemented the ALARA principle (as low as reasonably achievable), thus decreasing radiation exposure in children by 30-50%.72,75,77 Although the overall increase in risk may be miniscule, it is important to attempt to limit radiation exposure when evaluating children with acute appendicitis.
Magnetic resonance imaging (MRI) is an intriguing nonradiation alternative to CT and is extremely accurate in diagnosing appendicitis.78 The current version of this technology makes it impractical for widespread application, but future generations of scanners could allow it to be the preferred diagnostic imaging modality.
Differential Diagnosis
Acute appendicitis can mimic virtually any intra-abdominal process and should be high on the differential diagnosis in children with abdominal pain.79 Causes of acute right lower quadrant pain that are often indistinguishable from appendicitis include tubo-ovarian pathologic processes, Crohn disease, mesenteric adenitis, cecal diverticulitis, Meckel diverticulitis, constipation, viral gastroenteritis, and regional bacterial enteritis (Yersinia and Campylobacter in particular). Lower abdominal pain or vague nonfocal pain can result from a urinary tract infection, kidney stone, ureteropelvic junction obstruction, uterine pathologic process, right lower lobe pneumonia, sigmoid diverticulitis, cholecystitis, pancreatitis, gastroenteritis, vasculitis, bowel obstruction, and malignancy (lymphoma). The most common diagnosis made in the presence of missed appendicitis is reported to be gastroenteritis.80 Although many of these conditions may seem easily distinguishable, they each possess a spectrum of presentation that overlaps with appendicitis.
Treatment
The treatment of appendicitis begins with intravenous fluids and broad-spectrum antibiotics to provide coverage of enteric organisms. Management after initiating antimicrobial therapy depends on the severity of inflammation and the discussion must therefore be separated into uncomplicated (nonperforated) and complicated (perforated appendicitis). This distinction, however, is not always clear. Diagnostic imaging may help but cannot accurately diagnose perforation and many patients will not undergo preoperative imaging.61 Even intraoperative assessment showed high rates of discordance when compared to histologic evaluation of gangrenous and/or ruptured appendicitis.81 Surgeons polled with photographs showed extreme incongruence on which patients had perforation,82 and a survey of American Pediatric Surgical Association members revealed that most surgeons base their practice patterns on individual preferences.3 For this reason, the literature focusing on perforated appendicitis must be viewed with caution.
In reality, appendicitis presents as a spectrum of disease and it is important to distinguish which patients are at higher risk of complications. The data comparing outcomes of nonperforated versus perforated appendicitis is extensive, but most studies fail to use a strict definition of perforation. One prospective study showed that defining perforation as a visible hole in the appendix or a fecalith in the abdomen effectively identified those with greater risk of developing intra-abdominal abscesses (Fig. 42-3).83 In addition, outcomes in gangrenous appendicitis are similar to acute appendicitis and many patients may actually be over-treated.84 Thus, in the following discussion, the management of uncomplicated appendicitis will include acute, suppurative, and gangrenous appendicitis whereas complicated appendicitis will be synonymous with perforated appendicitis.
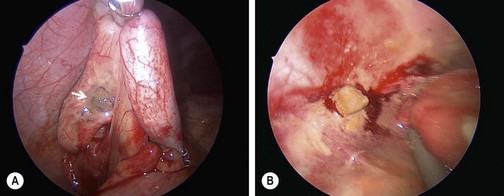
FIGURE 42-3 These two images depict the definition of perforation used in a prospective randomized trial.103 This definition of either (A) a hole in the appendix (arrow) or (B) stool in the abdomen was subsequently validated.83 An objective definition of perforation allows surgeons to compare outcomes data more accurately about perforated appendicitis.
Uncomplicated Appendicitis
After intravenous fluids and administration of broad-spectrum antibiotics, the current standard of care for uncomplicated appendicitis is appendectomy. Prophylactic antimicrobial agents should be given for 24 hours or less. In fact, a single preoperative dose of antibiotics has shown to decrease the risk of wound infection and abscess.85,86 Following appendectomy, patients are typically discharged within 24 hours. Additional postoperative antibiotics for acute appendicitis are not necessary or recommended.85,87 However, it may be reasonable to administer additional antibiotics for patients with suppurative or gangrenous appendicitis during the first 24 hours after appendectomy or longer based on the patient’s clinical status.
Recent data in adults suggests that administration of antibiotics without appendectomy may be sufficient to treat uncomplicated appendicitis. Multiple prospective randomized trials in adults have demonstrated similar outcomes from acute appendicitis treated with antibiotics alone with success rates ranging from 44% to 85%.88–92 Adults managed nonoperatively demonstrated fewer complications and less pain, although recurrence rates were high, ranging from 14% to 37%.93 There have been no prospective, randomized trials in children comparing antibiotics alone to appendectomy. Regardless of treatment modality, once antibiotics have been initiated, appendectomy is no longer considered to be an emergency and may even be considered somewhat elective.88,89,93–98 Until there is enough prospective randomized data in pediatric patients proving the efficacy of primary antibiotic treatment, appendectomy remains the standard of care for uncomplicated appendicitis. However, the need for operation may not be as urgent as previously thought.
Complicated Appendicitis
Patients with perforated appendicitis should receive postoperative antibiotics until clinical resolution has occurred. The antibiotic regimen employed for perforated appendicitis has traditionally been triple antibiotic therapy (ampicillin, gentamicin, and clindamycin or metronidazole). However recently there has been a shift towards more simple antibiotic regimens. Single agent therapy with piperacillin/tazobactam or cefotaxime, or double agent therapy with ceftriaxone and metronidazole, has been shown to be as efficacious as triple antibiotic therapy but is more cost effective.99–103 Several authors have highlighted a decrease in antibiotic expense with once daily dosing of ceftriaxone compared with multi-dose monotherapeutic agents. In addition, a prospective randomized study confirmed that single daily dosing of ceftriaxone and metronidazole is equal to and more cost effective than traditional triple antibiotic therapy in the treatment of perforated appendicitis.103 Therefore, current best evidence suggests once-a-day dosing with ceftriaxone at 50 mg/kg/day and metronidazole at 30 mg/kg/day provides the simplest and least expensive regimen.
Although the length of antibiotic course for perforated appendicitis is not yet standardized, current findings from multiple systematic reviews recommend continuation of antibiotics until resolution of clinical symptoms.85,102 This includes normalization of leukocyte count and differential, full return of gastrointestinal function, resolution of fever, and normalization of physical exam. In addition, if the duration of intravenous antibiotic therapy is less than 5 days, patients can be discharged safely on oral antibiotics to complete a 7-day course.104 A patient who is clinically well by postoperative day three is unlikely to develop an abscess.105 However, if a patient’s clinical symptoms have not resolved, it should raise the suspicion of an intra-abdominal abscess and intravenous antibiotics should be continued.
After initial intravenous fluid administration and antibiotics, the management of complicated appendicitis can be separated into nonoperative and operative treatment. Choice of treatment depends on identification of patients at high risk for treatment failure. It is also important to consider that many patients will not be diagnosed with perforated appendicitis preoperatively. Risk factors for failure of nonoperative management include greater than 15% band forms on the white blood cell differential count, disease that extends beyond the right lower quadrant, absence of a well-defined abscess, or presence of an appendicolith on imaging.106–108 Conversely, a large meta-analysis comparing appendectomy versus conservative treatment for complicated appendicitis as defined by abscess or phlegmon on presentation demonstrated higher rates of overall complications, wound infections, and intra-abdominal abscesses in those who had immediate appendectomy.109
The concept of managing complicated appendicitis with antibiotics alone is to decrease the significant local and regional inflammation that may make an immediate operation very difficult and potentially more dangerous. Once treated, most surgeons will perform interval appendectomy after six to ten weeks. However, some advocate that the interval appendectomy is not necessary as recurrence rates are low, ranging from 8–14%.110,111 One problem with these studies is the relatively short length of follow-up. A recent systematic review found a 20.5% overall risk of recurrent appendicitis with a range of 0–42%. However, nearly all studies were retrospective and thus only included patients specifically selected for nonoperative management.112 Some studies showed high rates of pathologic findings in interval appendectomy specimens.113,114 Although there is a lack of long-term data to accurately predict the rates of recurrence in both adults and children, some studies suggest that most recurrences will occur within three years and the majority within one to six months.110–112 For these reasons, most pediatric surgeons perform interval appendectomy in patients with complicated appendicitis who were initially managed nonoperatively.3
The majority of patients who present with a well-formed abscess on initial imaging are managed nonoperatively (see Fig. 42-2C). Historically, immediate appendectomy in this patient population was difficult, required a larger incision, and had a high morbidity. Primary treatment of the abscess with antibiotics alone, or antibiotics and percutaneous drainage with or without drain placement for larger fluid collections, is a widely accepted treatment strategy. Interval appendectomy is then performed after the inflammation has subsided.3,115–119 Although treatment with percutaneous drainage and interval appendectomy has inherent risk of complications, success rates have been reported to be as high as 88%.107 A recent pilot randomized trial comparing initial laparoscopic appendectomy versus antibiotics, percutaneous drainage and subsequent interval laparoscopic appendectomy in patients presenting with perforated appendicitis and abscess demonstrated no difference in the rate of recurrent abscess, length of hospital stay, or hospital charges.120 Patients undergoing immediate appendectomy had longer operations and a longer time to return of bowel function. Alternately, patients who had interval appendectomies had more CT scans. Quality of life surveys at presentation, 2 weeks, and 12 weeks in both groups from this study showed that families experience significant parenting distress related to disruption in the child’s quality of life until the appendectomy is performed.121
The majority of patients with complicated appendicitis can be safely managed with appendectomy. Specifically, patients with a phlegmonous mass, appendicolith, or absence of a well-formed abscess on imaging have a higher risk of failure of nonoperative management.107,122 These patients can safely and reliably undergo immediate laparoscopic appendectomy.123,124 In patients with perforated appendicitis without abscess, a recent prospective randomized trial demonstrated lower rates of adverse events, shorter length of hospitalization, and earlier return to normal activity with early appendectomy.122 In addition, mean total hospital charges and resource use were significantly higher in patients undergoing interval appendectomy, likely due to the increased number of adverse events.125
Operative Technique
First described in 1893 by McBurney, the traditional method of appendectomy was a muscle-splitting, right lower quadrant incision.126 The cecum is delivered through the incision, the mesoappendix is divided, and the appendix is ligated at its base. In the laparoscopic approach, both surgeon and first assistant stand on the patient’s left facing a video monitor positioned on the right (Fig. 42-4A).127 The patient is positioned supine on the operating table, and the abdomen is prepped widely. After insertion of a 10–12 mm umbilical cannula, pneumoperitoneum is established. Two 5 mm ports are then placed, one in the left lower quadrant and one in the left suprapubic area (Fig 42-4B). A 5 mm 30° or 45° telescope is introduced through the left lower quadrant port, and the other two ports are the working ports. This allows effective triangulation of instruments to maximize utility in a small space, a core principle of endoscopic surgery. Diagnostic laparoscopy is initially performed. If present, abscesses are opened and purulent fluid is aspirated from the pelvis, perihepatic space and paracolic gutters. The appendix is located by following the taenia of the cecum inferiorly. After grasping the appendix and retracting it inferiorly, a window is created in the mesoappendix close to the cecum. The endoscopic stapler is inserted through the 12 mm umbilical cannula and used to divide the appendix and mesoappendix (Fig. 42-5). The appendix is usually divided first, followed by division of the mesoappendix. On occasion, however, it may be more expedient to ligate the mesoappendix first. If the appendix can be delivered through the cannula, an endoscopic bag is not used. However, if the appendix is too large for the cannula, an endoscopic bag is employed to avoid dragging the appendix through the umbilical incision. Drains are not routinely utilized for advanced disease.
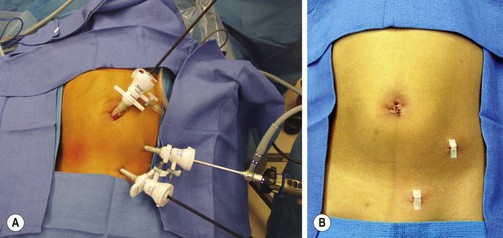
FIGURE 42-4 (A) Port positions for a laparoscopic appendectomy. Typically three cannulas are used, with the endoscopic stapler introduced through the 12 mm umbilical port. The appendix is removed through this site as well. (B) Postoperative appearance.
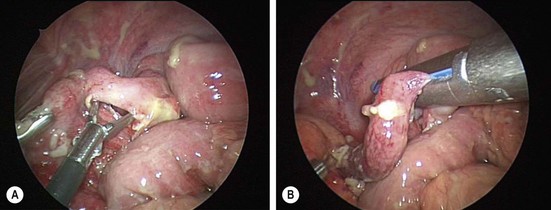
FIGURE 42-5 (A) Initially, a window is made in the mesoappendix. (B) Usually, the appendix is ligated and divided with the stapler first, followed by ligation/division of the mesoappendix.
Since the introduction of laparoscopic appendectomy 25 years ago, there has been an abundance of data comparing open and endoscopic techniques. Initial advantages of the open approach seemed to be a shorter length of operation and fewer postoperative intra-abdominal abscesses.128–130 However, as expected, there were higher rates of wound infections, presumably due to contamination of the incision when delivering an infected appendix through the wound (Fig. 42-6). Advantages of the laparoscopic approach are multiple. It allows better visualization of the entire abdomen, which is especially beneficial in obese patients who would otherwise require a larger incision, and fertile females who may have other intra-abdominal pathology.131–133 Laparoscopy also allows lysis of interloop abscesses and aspiration of purulent fluid, and it facilitates dissection in obese patients in whom open appendectomy would be challenging. Use of laparoscopy has been associated with a higher negative appendectomy rate.134 However, this discrepancy may be explained by the increased use of diagnostic laparoscopy in patients whose diagnosis is not clear, specifically teenage females who may have gynecologic findings. Open appendectomy may be easier in younger patients due to lack of space in the peritoneal cavity relative to the size of the laparoscopic instruments.
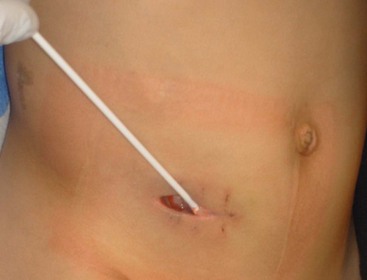
FIGURE 42-6 This child underwent open appendectomy through a right lower abdominal incision and developed a wound infection which is being treated. The significant reduction in the incidence of wound infections is one of the benefits of the laparoscopic approach, especially for perforated appendicitis.
The use of laparoscopy has increased from about 20% in 1998 to 70% in 2007.135–137 In the past decade, there have been multiple prospective randomized trials, large retrospective studies, and meta-analyses comparing outcomes in open versus the laparoscopic approach. While early studies found increased operating times for laparoscopy, more recent studies have shown no difference in length of operation.138–142 A few studies actually demonstrated shorter operating times with laparoscopy.139–140 Laparoscopic appendectomy is also associated with shorter hospitalization, fewer postoperative outpatient visits, decreased time off work, and earlier return to routine activity.134–136,138,142–144 One study suggested increased inpatient costs but lower outpatient costs for the laparoscopic approach, while a large national database review found increased costs in uncomplicated appendicitis but similar costs for complicated appendicitis.137,142 A prospective randomized double-blind study demonstrated that patients who underwent laparoscopic appendectomy had an improved quality of life at two weeks.145
Overall complication rates are less than 3% for uncomplicated appendicitis and 16% to 18% for complicated appendicitis with many studies showing the same if not lower rates after laparoscopy.131,135,137,138,144 One of the more common complications following appendectomy is an intra-abdominal abscess. Rates of postoperative abscess are estimated to be less than 1% in uncomplicated appendicitis and 1–15% in complicated appendicitis. However, in many of these studies, there is not uniform definition of perforation. When a uniform definition is used, rates as high as 20% have been reported.83,105,120,137 One group found an increase in abscess rate from 19% to 46% when comparing non-obese to obese patients.146 A decade ago, a few groups reported a higher incidence of postoperative abscesses in patients who had a laparoscopic appendectomy.128–130 However, there is now an abundance of level 1, 2, and 3 evidence showing no difference in rates of intra-abdominal abscesses.136–138,144,147–155 In fact, the most recent national database review evaluated 212,958 pediatric patients and found a higher rate of postoperative abscess in patients who underwent open appendectomy for both uncomplicated and complicated appendicitis.137 This discrepancy may be due to increasing surgeon experience with laparoscopy, more advanced endoscopic equipment, or possibly using an endobag for removal of the perforated appendix.156
Regardless of whether laparoscopic or open appendectomy is performed, culture of the fluid has not been shown to be helpful at the time of the initial operation.157,158 One study demonstrated that children whose antibiotic treatment was based on the cultures did somewhat worse than those whose fluid was not cultured.158 Peritoneal lavage with either saline or antibiotic solution has also not been shown to decrease the incidence of abdominal abscesses.159 Similarly, the use of drains has not proved useful except in cases of walled-off abscess cavities.160,161
With respect to wound infections, this complication occurs in less than 1% of patients with uncomplicated appendicitis. In contrast, patients with complicated appendicitis may have up to 16% incidence of wound infection.144 Most recent studies have found that laparoscopy has a lower rate of surgical site infections.24,135–138,143,144 The use of laparoscopy has also demonstrated a nearly fourfold decrease in postoperative bowel obstructions.162,163 Other less common postoperative complications include urinary tract infection and pneumonia.
Recently, the use of single-site laparoscopic surgical techniques have been reported.164–171 In single-incision laparoscopic appendectomy (SILA), a single transumbilical incision is made and a 5 mm or 12 mm port is placed. One or two additional ports are placed through the same incision using multi-port devices or separate fascial incisions. Subsequent dissection and appendectomy are identical to the traditional three-port procedure. Advantages of this technique are thought to be shorter length of hospitalization, better cosmesis, and lower hospital costs. Technically the procedure can be more challenging as close approximation of instruments limits range of motion and narrows the visual field.172 Theoretically a larger fascial incision may result in increased postoperative pain and higher rates of incisional hernias although preliminary evidence is limited. Hybrid procedures such as laparoscopic-assisted single-port appendectomy (SPA) and transumbilical laparoscopic-assisted appendectomy (TULAA) are other described techniques that combine a laparoscopic single-incision approach for dissection followed by extracorporeal removal of the appendix through the umbilicus as in the traditional open procedure (Fig. 42-7). Early retrospective reviews have shown no difference in postoperative complication rates and similar or even decreased hospital costs when compared to open and other laparoscopic techniques.167,168,170 A recent prospective randomized trial comparing single site to traditional three-port appendectomy in patients with nonperforated appendicitis demonstrated no difference in postoperative wound infection or abscess rates, length of hospital stay, or hospital charges.164 This particular study found a longer operative time (in minutes) for the single-incision approach, but this was not clinically relevant.
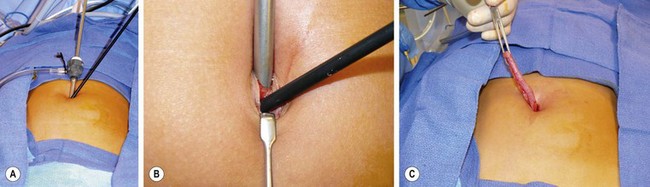
FIGURE 42-7 This 10-year-old underwent a transumbilical laparoscopic-assisted appendectomy. (A) A 5 mm reusable cannula was introduced in the cephalad aspect of the umbilical fascia followed by insertion of a 5 mm grasping forceps inferior to the cannula for mobilization of the cecum and appendix..(B) Close-up view of the separate fascial incisions for introduction of the cannula and instrument. Note the fascial bridge between the instrument and the cannula. This bridge prevents escape of CO2 around the instruments. (C) Following mobilization of the cecum and appendix, an extracorporeal appendectomy was then performed.
References
1. Sivit, CJ, Siegel, MJ, Applegate, KE, et al. When appendicitis is suspected in children. RadioGraphics. 2001; 21:247–262.
2. Wagner, JM, McKinney, WP, Carpenter, JL. Does this patient have appendicitis? JAMA. 1996; 276:1589–1594.
3. Chen, C, Botelho, C, Cooper, A, et al. Current practice patterns in the treatment of perforated appendicitis in children. J Am Coll Surg. 2003; 196:212–221.
4. van Zwalenburg, C. The relation of mechanical distention to the etiology of appendicitis. Ann Surg. 1905; 41:437–450.
5. Wangensteen, OH, Dennis, C. Experimental proof of obstructive origin of appendicitis. Ann Surg. 1939; 110:629–647.
6. Curran, TJ, Meunchow, SK. The treatment of complicated appendicitis in children using peritoneal drainage: Results from a public hospital. J Pediatr Surg. 1993; 28:204–208.
7. Stringel, G. Appendicitis in children: A systematic approach for a low incidence of complications. Am J Surg. 1987; 154:631–635.
8. Jones, BA, Demetriades, D, Segal, I. The prevalence of appendiceal fecoliths in patients with and without appendicitis: A comparative study from Canada and South Africa. Ann Surg. 1985; 202:80–82.
9. Burkitt, DP. The aetiology of appendicitis. Br J Surg. 1971; 58:695–699.
10. Attwood, SE, Mealy, K, Cafferkey, MT, et al. Yersinia infection and acute abdominal pain. Lancet. 1987; 1:529–533.
11. Rabau, MY, Avigad, I, Wolfstein, I. Rubella and acute appendicitis. Pediatrics. 1980; 66:813.
12. Rodgers, B, Karn, G. Yersinia enterocolitis. J Pediatr Surg. 1975; 10:497–499.
13. Sanders, DY, Cort, CR, Stubbs, AJ. Shigellosis associated with appendicitis. J Pediatr Surg. 1972; 7:315–317.
14. Adebamowo, CA, Akang, EE, Ladipo, JK, et al. Schistosomiasis of the appendix. Br J Surg. 1991; 78:1219–1221.
15. Nadler, S, Cappell, MS, Bhatt, B, et al. Appendiceal infection by Entamoeba histolytica and Strongyloides stercoralis presenting like acute appendicitis. Dig Dis Sci. 1990; 35:603–608.
16. Schnell, VL, Yandell, R, Van Zandt, S, et al. Enterobius vermicularis salpingitis: A distant episode from precipitating appendicitis. Obstet Gynecol. 1992; 80:553–555.
17. Kwong, MS, Dinner, M. Neonatal appendicitis masquerading as necrotizing enterocolitis. J Pediatr. 1980; 96:917–918.
18. Valerdiz-Casasola, S, Pardo-Mindan, FJ. Cytomegalovirus infection of the appendix in patient with the acquired immunodeficiency syndrome. Gastroenterology. 1991; 101:247.
19. Coughlin, JP, Gauderer, MW, Stern, RC, et al. The spectrum of appendiceal disease in cystic fibrosis. J Pediatr Surg. 1990; 25:835–839.
20. Martin, LW, Perrin, EV. Neonatal perforation of the appendix in association with Hirschsprung’s disease. Ann Surg. 1967; 166:799–802.
21. Stiefel, D, Stallmach, T, Sacher, P. Acute appendicitis in neonates: Complication or morbus sui generis? Pediatr Surg Int. 1998; 14:122–123.
22. Heller, MB, Skolnick, LM. Ultrasound documentation of spontaneously resolving appendicitis. Am J Emerg Med. 1993; 11:51–53.
23. Mattei, P, Sola, JE, Yeo, CJ. Chronic and recurrent appendicitis are uncommon entities often misdiagnosed. J Am Coll Surg. 1994; 178:385–389.
24. Lee, SL, Stark, R, Yaghoubian, A, et al. Does age affect the outcomes and management of pediatric appendicitis? J Pediatr Surg. 2011; 46:2342–2345.
25. Nance, ML, Adamson, WT, Hedrick, HL. Appendicitis in the young child: A continuing diagnostic challenge. Pediatr Emerg Care. 2000; 16:160–162.
26. Jablonski, KA, Guagliardo, MF. Pediatric appendicitis rupture rate: A national indicator of disparities in healthcare access. Popul Health Metr. 2005; 3:4–9.
27. Ponsky, TA, Huang, ZJ, Kittle, K, et al. Hospital- and patient-level characteristics and the risk of appendiceal rupture and negative appendectomy in children. JAMA. 2004; 292:1977–1982.
28. Gadmonski, A, Jenkins, P. Ruptured appendicitis among children as an indicator of access to care. Health Serv Res. 2001; 36:129–142.
29. Guagliardo, MF, Teach, SJ, Huang, ZJ, et al. Racial and ethnic disparities in pediatric appendicitis rupture rate. Acad Emerg Med. 2003; 10:1218–1227.
30. Lee, SL, Shekherdimian, S, Chiu, VY, et al. Perforated appendicitis in children: Equal access to care eliminates racial and socioeconomic disparities. J Pediatr Surg. 2010; 45:1203–1207.
31. Nwomeh, BC, Chisolm, DJ, Caniano, DA, et al. Racial and socioeconomic disparity in perforated appendicitis among children: Where is the problem? Pediatrics. 2006; 117:870–875.
32. Kwan, KY, Nager, AL. Diagnosing pediatric appendicitis: Usefulness of laboratory markers. Am J Emerg Med. 2010; 28:1009–1015.
33. Bolton, JP, Craven, ER, Croft, RJ, et al. An assessment of the value of the white-cell count in the management of suspected acute appendicitis. Br J Surg. 1975; 62:906–908.
34. Doraiswany, NV. Leucocyte counts in the diagnosis and prognosis of acute appendicitis in children. Br J Surg. 1979; 66:782–784.
35. Hoffman, J, Rasmussen, OO. Aids in the diagnosis of acute appendicitis. Br J Surg. 1989; 76:774–779.
36. Stefanutti, G, Ghirado, V, Gamba, P. Inflammatory markers for acute appendicitis in children: Are they helpful? J Pediatr Surg. 2007; 42:773–776.
37. Rothrock, SG, Skeoch, G, Rush, JJ, et al. Clinical features of misdiagnosed appendicitis in children. Ann Emerg Med. 1991; 20:45–50.
38. Alvarado, A. A practical score for the early diagnosis of acute appendicitis. Ann Emerg Med. 1986; 15:557–564.
39. Samuel, M. Pediatric appendicitis score. J Pediatr Surg. 2002; 37:877–881.
40. Macklin, CP, Radcliffe, GS, Merei, JM, et al. A prospective evaluation of the modified Alvarado score for acute appendicitis in children. Ann R Coll Surg Engl. 1997; 79:203–205.
41. McKay, R, Shepherd, J. The use of the clinical scoring system by Alvarado in the decision to perform computed tomography for acute appendicitis in the ED. Am J Emerg Med. 2007; 25:489–493.
42. Escriba, A, Gamell, AM, Fernandez, Y, et al. Prospective validation of two systems of classification for the diagnosis of acute appendicitis. Pediatr Emer Care. 2011; 27:165–169.
43. Rezak, A, Hussain, MA, Abbas, A, et al. Decreased use of computed tomography with a modified clinical scoring system in diagnosis of pediatric acute appendicitis. Arch Surg. 2011; 146:64–67.
44. Smink, DS, Finkelstein, JA, Kleinman, K, et al. The effect of hospital volume of pediatric appendectomies on the misdiagnosis of appendicitis in children. Pediatrics. 2004; 113:18–23.
45. Flum, DR, Koepsell, T. The clinical and economic correlates of misdiagnosed appendicitis: Nationwide analysis. Arch Surg. 2002; 137:799–804.
46. Newman, K, Ponsky, T, Kittle, K, et al. Appendicitis 2000: Variability in practice, outcomes, and resource utilization at thirty pediatric hospitals. J Pediatr Surg. 2003; 38:372–379.
47. Smink, DS, Finkelstein, JA, Garcia Peña, BM, et al. Diagnosis of acute appendicitis in children using a clinical practice guideline. J Pediatr Surg. 2004; 39:458–463.
48. Bachur, RG, Hennelly, K, Callahan, MJ, et al. Diagnostic imaging and negative appendectomy rates in children: Effects of age and gender. Pediatrics. 2012; 129:877–884.
49. Hayden, CK, Jr., Kuchelmeister, J, Lipscomb, TS. Sonography of acute appendicitis in childhood: Perforation versus nonperforation. J Ultrasound Med. 1992; 11:209–216.
50. Hahn, HB, Hoepner, FU, Kalle, T, et al. Sonography of acute appendicitis in children: 7 years’ experience. Pediatr Radiol. 1998; 28:147–151.
51. Yacoe, ME, Jeffrey, RB. Sonography of appendicitis and diverticulitis. Radiol Clin North Am. 1994; 32:899–912.
52. Trout, AT, Sanchez, R, Ladino-Torres, MF, et al. A critical evaluation of ultrasound for the diagnosis of pediatric acute appendicitis in a real-life setting: How can we improve the diagnostic value of sonography? Pediatr Radiol. 2012.
53. Weyant, MJ, Eachempati, SR, Maluccio, MA, et al. Is imaging necessary for the diagnosis of acute appendicitis. Adv Surg. 2003; 37:327–345.
54. Sivit, CJ, Applegate, KE. Imaging of acute appendicitis in children. Semin Ultrasound CT MR. 2003; 24:74–82.
55. Jaremko, JL, Crockett, A, Rucker, D, et al. Incidence and significance of inconclusive results in ultrasound for appendicitis in children and teenagers. Can Assoc Radiol. 2011; 62:197–202.
56. Chen, SC, Chen, KM, Wang, SM, et al. Abdominal sonography screening of clinically diagnosed or suspected appendicitis before surgery. World J Surg. 1998; 22:449–452.
57. Horton, MD, Counter, SF, Florence, MG, et al. A prospectivetrial of computed tomography and ultrasonography for diagnosing appendicitis in the atypical patient. Am J Surg. 2000; 179:379–381.
58. Gwynn, LK. Appendiceal enlargement as a criterion for clinical diagnosis of acute appendicitis: Is it reliable and valid? J Emerg Med. 2002; 23:9–14.
59. Choi, D, Park, H, Lee, YR, et al. The most useful findings for diagnosing acute appendicitis on contrast-enhanced helical CT. Acta Radiol. 2003; 44:574–582.
60. Lowe, LH, Penney, MW, Stein, SM, et al. Unenhanced limited CT of the abdomen in the diagnosis of appendicitis in children: Comparison with sonography. AJR Am J Roentgenol. 2001; 176:31–35.
61. Peña, BM, Taylor, GA, Fishman, SJ, et al. Costs and effectiveness of ultrasonography and limited computed tomography for diagnosing appendicitis in children. Pediatrics. 2000; 106:672–676.
62. Garcia Peña, BM, Mandl, KD, Kraus, SJ, et al. Ultrasonography and limited computed tomography in the diagnosis and management of appendicitis in children. JAMA. 1999; 282:1041–1046.
63. Pickuth, D, Spielmann, RP. Unenhanced spiral CT for evaluating acute appendicitis in daily routine: A prospective study. Hepatogastroenterology. 2001; 48:140–142.
64. Funaki, B, Grosskreutz, SR, Funaki, CN. Using unenhanced helical CT with enteric contrast material for suspected appendicitis in patients treated at a community hospital. AJR Am J Roentgenol. 1998; 171:997–1001.
65. Weltman, DI, Yu, J, Krumenacker, J, Jr., et al. Diagnosis of acute appendicitis: Comparison of 5- and 10-mm CT sections in the same patient. Radiology. 2000; 216:172–177.
66. Jacobs, JE, Birnbaum, BA, Macari, M, et al. Acute appendicitis: Comparison of helical CT diagnosis focused technique with oral contrast material versus nonfocused technique with oral and intravenous contrast material. Radiology. 2001; 220:683–690.
67. Fraser, JD, Aguayo, P, Sharp, SW, et al. Accuracy of computed tomography in predicting appendiceal perforation. J Pediatr Surg. 2010; 45:231–235.
68. Brenner, DJ. Estimating cancer risks from pediatric CT: Going from the qualitative to the quantitative. Pediatr Radiol. 2002; 32:228–233.
69. Garcia Pena, BM, Cook, EF, Mandl, KD. Selective imaging strategies for the diagnosis of appendicitis in children. Pediatrics. 2004; 113:24–28.
70. Pena, BM, Taylor, GA, Fishman, SJ, et al. Effect of an imaging protocol on clinical outcomes among pediatric patients with appendicitis. Pediatrics. 2002; 110:1088–1093.
71. Martin, AE, Vollman, D, Adler, B, et al. CT scans may not reduce the negative appendectomy rate in children. J Pediatr Surg. 2004; 39:886–890.
72. Linton, OW, Mettler, FA, Jr. National Council on Radiation Protection and Measurements. National conference on dose reduction in CT, with an emphasis on pediatric patients. AJR Am J Roentgenol. 2003; 181:321–329.
73. Brody, AS, Frush, DP, Huda, W, et al. Radiation risk to children from computed tomography. Pediatrics. 2007; 120:677–682.
74. Brennan, GD. Pediatric appendicitis: Pathophysiology and appropriate use of diagnostic imaging. CJEM. 2006; 8:425–432.
75. Ware, DE, Huda, W, Mergo, PJ, et al. Radiation effective doses to patients undergoing abdominal CT examinations. Radiology. 1999; 210:645–650.
76. Brenner, DJ, Elliston, CD, Hall, EJ, et al. Estimated risks of radiation-induced fatal cancer from pediatric CT. Br J Radiol. 2008; 81:362–378.
77. Krishnamoorthi, R, Ramarajan, N, Wang, N, et al. Effectiveness of a staged ultrasound and CT protocol for the diagnosis of pediatric appendicitis: Reducing radiation exposure in the age of ALARA. Radiology. 2011; 259:231–239.
78. Horman, M, Paya, K, Eibenberger, K, et al. MR imaging in children with nonperforated acute appendicitis: Value of unenhanced MR imaging in sonographically selected cases. AJR Am J Roentgenol. 1998; 171:467–470.
79. Cope, Z. Appendicitis and the differential diagnosis of acute appendicitis. In: Silen W, ed. Cope’s Early Diagnosis of the Acute Abdomen. New York: Oxford University Press, 1991.
80. Cappendijk, VC, Hazebroek, FW. The impact of diagnostic delay on the course of acute appendicitis. Arch Dis Child. 2000; 83:64–66.
81. Bliss, D, McKee, J, Cho, D, et al. Discordance of the pediatric surgeon’s intraoperative assessment of pediatric appendicitis with the pathologists report. J Pediatr Surg. 2010; 45:1398–1403.
82. Ponsky, T, Hafi, M, Heiss, K, et al. Interobserver variation in the assessment of appendiceal perforation. J Laparoendosc Adv Surg Tech A. 2009; 19(Suppl. 1):S15–S18.
83. St Peter, SD, Sharp, SW, Holcomb, GW, et al. An evidence-based definition for perforated appendicitis derived from a prospective randomized trial. J Pediatr Surg. 2008; 43:2242–2245.
84. Emil, S, Gaied, F, Lo, A, et al. Gangrenous appendicitis in children: A prospective evaluation of definition, bacteriology, histopathology, and outcomes. J Surg Res. 2012.
85. Nadler, EP, Gaines, BA. Therapeutic Agents Committee of the Surgical Infection Society: The Surgical Infection Society guidelines on antimicrobial therapy for children with appendicitis. Surg Infect (Larchmt). 2008; 9:75–83.
86. Andersen, BR, Kallehave, FL, Andersen, HK. Antibiotics versus placebo for prevention of postoperative complications after appendicectomy. Cochrane Database of Systematic Reviews. 2005.
87. Mui, LM, Ng, CS, Wong, SK, et al. Optimum duration of prophylactic antibiotics in acute non-perforated appendicitis. Aust NZ J Surg. 2005; 75:425–428.
88. Eriksson, S, Granström, L. Randomized controlled trial of appendicectomy versus antibiotic therapy for acute appendicitis. Br J Surg. 1995; 82:166–169.
89. Struyd, J, Eriksson, S, Nilsson, I, et al. Appendectomy versus antibiotic treatment in acute appendicitis. A prospective multicenter randomized controlled trial. World J Surg. 2006; 30:1033–1037.
90. Hansson, J, Körner, U, Khorram-Manesh, A, et al. Randomized clinical trial of antibiotic therapy versus appendicectomy as primary treatment of acute appendicitis in unselected patients. Br J Surg. 2009; 96:473–481.
91. Vons, C, Barry, C, Maitre, S, et al. Amoxicillin plus clavulanic acid versus appendectomy for treatment of acute uncomplicated appendicitis: An open-label, non-inferiority, randomised controlled trial. Lancet. 2011; 377(9777):1573–1579.
92. Wilms, IM, de Hoog, DE, de Visser, DC, et al. Appendectomy versus antibiotic treatment for acute appendicitis. Cochrane Database Syst Rev. (11):2011.
93. Varadhan, KK, Neal, KR, Lobo, DN. Safety and efficacy of antibiotics compared with appendicectomy for treatment of uncomplicated acute appendicitis: Meta-analysis of randomized controlled trials. BMJ. 2012; 344:e2156.
94. Liu, K, Ahanchi, S, Pisaneschi, M, et al. Can acute appendicitis be treated by antibiotics alone? Am Surg. 2007; 73:1161–1165.
95. Friedell, ML, Perez-Izquierdo, M. Is there a role for interval appendectomy in the management of acute appendicitis? Am Surg. 2000; 66:1158–1162.
96. Surana, R, Quinn, F, Puri, P. Is it necessary to perform appendectomy in the middle of the night in children? BMJ. 1993; 306:1168.
97. Yardeni, D, Hirschl, RB, Drongowski, RA, et al. Delayed versus immediate surgery in acute appendicitis: Do we need to operate during the night? J Pediatr Surg. 2004; 39:464–469.
98. Stahlfeld, K, Hower, J, Homitsky, S, et al. Is acute appendicitis a surgical emergency? Am Surg. 2007; 73:626–629.
99. Results of the North American trial of piperacillin/tazobactam compared with clindamycin and gentamicin in the treatment of severe intra-abdominal infections. Investigators of the Piperacillin/Tazobactam Intra-abdominal Infection Study Group. Eur J Surg Suppl. 1994; 573:61–66.
100. Nadler, EP, Reblock, KK, Ford, HR, et al. Monotherapy versus multi-drug therapy for the treatment of perforated appendicitis in children. Surg Infect (Larchmt). 2003; 4:327–333.
101. Maltezou, HC, Nikolaidis, P, Lebesii, E, et al. Piperacillin/tazobactam versus cefotaxime plus metronidazole for treatment of children with intra-abdominal infections requiring surgery. Eur J Clin Microbiol Infect Dis. 2001; 20:643–646.
102. Lee, SL, Islam, S, Cassidy, LD, et al. Antibiotics and appendicitis in the pediatric population: An American Pediatric Surgical Association outcomes and clinical trials committee systematic review. J Pediatr Surg. 2010; 45:2181–2185.
103. St Peter, SD, Tsao, K, Spilde, TL, et al. Single daily dosing of ceftriaxone and metronidazole vs. standard triple antibiotic regimen for perforated appendicitis in children: A prospective randomized trial. J Pediatr Surg. 2008; 43:981–985.
104. Fraser, JD, Aguayo, P, Leys, CM, et al. A complete course of intravenous antibiotics vs a combination of intravenous and oral antibiotics for perforated appendicitis in children: A prospective, randomized trial. J Pediatr Surg. 2010; 45:1198–1202.
105. Henry, MC, Walker, A, Silverman, BL, et al. Risk factors for the development of abdominal abscess following operation for perforated appendicitis in children: A multicenter case-control study. Arch Surg. 2007; 142:236–241.
106. Kogut, KA, Blakely, ML, Schropp, KP, et al. The association of elevated percent bands on admission with failure and complications of interval appendectomy. J Pediatr Surg. 2001; 36:165–168.
107. Aprahamian, CJ, Barnhart, DC, Bledsoe, SE, et al. Failure in the nonoperative management of pediatric ruptured appendicitis: predictors and consequences. J Pediatr Surg. 2007; 42:934–938.
108. Levin, T, Whyte, C, Borzykowski, R, et al. Nonoperative management of perforated appendicitis in children: Can CT predict outcome? Pediatr Radiol. 2007; 37:251–255.
109. Simillis, C, Symeonides, P, Shorthouse, AJ, et al. A meta-analysis comparing conservative treatment versus acute appendectomy for complicated appendicitis (abscess or phlegmon). Surgery. 2010; 147:818–829.
110. Ein, SH, Shandling, B. Is interval appendectomy necessary after rupture of an appendiceal mass? J Pediatr Surg. 1996; 31:849–850.
111. Puapong, D, Lee, SL, Haigh, PI, et al. Routine interval appendectomy in children is not indicated. J Pediatr Surg. 2007; 42:1500–1503.
112. Hall, NJ, Jones, CE, Eaton, S, et al. Is interval appendectomy justified after successful nonoperative treatment of an appendix mass in children? A systematic review. J Pediatr Surg. 2011; 46:767–771.
113. Gahukamble, DB, Gahukamble, LD. Surgical and pathological basis for interval appendectomy after resolution of appendicular mass in children. J Pediatr Surg. 2000; 35:424–427.
114. Mazziotti, MV, Marley, EF, Winthrop, AL, et al. Histopathologic analysis of interval appendectomy specimens: Support for the role of interval appendectomy. J Pediatr Surg. 1997; 32:806–809.
115. Janik, JS, Ein, SH, Shandling, B, et al. Nonsurgical management of appendiceal mass in late presenting children. J Pediatr Surg. 1980; 15:574–576.
116. Muehlstedt, SG, Pham, TQ, Schmeling, DJ. The management of pediatric appendicitis: A survey of North American Pediatric Surgeons. J Pediatr Surg. 2004; 39:875–879.
117. Morrow, SE, Newman, KD. Current management of appendicitis. Semin Pediatr Surg. 2007; 16:34–40.
118. Owen, A, Moore, O, Marven, S, et al. Interval laparoscopic appendectomy in children. J Laparoendosc Adv Surg Tech. 2006; 16:308–311.
119. Weiner, DZ, Katz, A, Hirschl, RB, et al. Interval appendectomy in perforated appendicitis. Pediatr Surg Int. 1995; 10:82–85.
120. St Peter, SD, Aguayo, P, Fraser, JD, et al. Initial laparoscopic appendectomy versus initial nonoperative management and interval appendectomy for perforated appendicitis with abscess: A prospective, randomized trial. J Pediatr Surg. 2010; 45:236–240.
121. Schurman, JV, Cushing, CC, Garey, CL, et al. Quality of life assessment between laparoscopic appendectomy at presentation and interval appendectomy for perforated appendicitis with abscess: Analysis of a prospective, randomized trial. J Pediatr Surg. 2011; 46:1121–1125.
122. Blakely, ML, Williams, R, Dassinger, MS, et al. Early vs. interval appendectomy for children with perforated appendicitis. Arch Surg. 2011; 146:660–665.
123. Goh, BK, Chui, CH, Yap, TL, et al. Is early laparoscopic appendectomy feasible in children with acute appendicitis presenting with an appendiceal mass? A prospective study. J Pediatr Surg. 2005; 40:1134–1137.
124. Senapathi, PS, Bhattacharya, D, Ammori, BJ. Early laparoscopic appendectomy for appendicular mass. Surg Endosc. 2002; 16:1783–1785.
125. Myers, AL, Williams, RF, Giles, K, et al. Hospital cost analysis of a prospective randomized trial of early vs interval appendectomy for perforated appendicitis in children. J Am Coll Surg. 2012; 214:427–434.
126. McBurney, C. The incision made in the abdominal wall in cases of appendicitis, with a description of a new method of operating. Ann Surg. 1894; 20:38–43.
127. Semm, K. Endoscopic appendectomy. Endoscopy. 1983; 15:54–64.
128. Lintula, H, Kokki, H, Vanamo, K, et al. Laparoscopy in children with complicated appendicitis. J Pediatr Surg. 2002; 37:1317–1320.
129. Horwitz, JR, Custer, MD, May, BH, et al. Should laparoscopic appendectomy be avoided for complicated appendicitis in children? J Pediatr Surg. 1997; 32:1601–1603.
130. Eypasch, E, Sauerland, S, Lefering, R, et al. Laparoscopic versus open appendectomy: Between evidence and common sense. Dig Surg. 2002; 19:518–522.
131. Nataraja, RM, Teague, WJ, Galea, J, et al. Comparison of intraabdominal abscess formation after laparoscopic and open appendectomies in children. J Pediatr Surg. 2012; 47:317–321.
132. Varela, JE, Hinojosa, MW, Nguyen, NT. Laparoscopy should be the approach of choice for acute appendicitis in the morbidly obese. Am J Surg. 2008; 196:218–222.
133. Corneille, MG, Steigelman, MB, Myers, JG, et al. Laparoscopic appendectomy is superior to open appendectomy in obese patients. Am J Surg. 2007; 194:877–880.
134. Esposito, C, Borzi, P, Valla, JS, et al. Laparoscopic versus open appendectomy in children: A retrospective comparative study of 2,332 cases. World J Surg. 2007; 31:750–755.
135. Jen, HC, Shew, SB. Laparoscopic versus open appendectomy in children: Outcomes comparison based on a statewide analysis. J Surg Res. 2010; 161:13–17.
136. Lee, SL, Yaghoubian, A, Kaji, A. Laparoscopic versus open appendectomy in children: Outcomes comparison based on age, sex, and perforation status. Arch Surg. 2011; 146:1118–1121.
137. Masoomi, H, Mills, S, Dolich, MO, et al. Comparison of outcomes of laparoscopic versus open appendectomy in children: Data from the nationwide inpatient sample (NIS), 2006–2008. World J Surg. 2012; 36:573–578.
138. Aziz, O, Athanasiou, T, Tekkis, PP, et al. Laparoscopic versus open appendectomy in children: A meta-analysis. Ann Surg. 2006; 243:17–27.
139. Yau, KK, Siu, WT, Tang, CN, et al. Laparoscopic versus open appendectomy for complicated appendicitis. J Am Coll Surg. 2007; 205:60–65.
140. Olmi, S, Magnone, S, Bertolini, A, et al. Laparoscopic versus open appendectomy in acute appendicitis: A randomized prospective study. Surg Endosc. 2005; 19:1193–1195.
141. Moberg, AC, Berndsen, F, Palmquist, I, et al. Randomized clinical trial of laparoscopic versus open appendicectomy for confirmed appendicitis. Br J Surg. 2005; 92:298–304.
142. Carbonell, AM, Burns, JM, Lincourt, AE, et al. Outcomes of laparoscopic versus open appendectomy. Am Surg. 2004; 70:759–765.
143. Sauerland, S, Jaschinski, T, Neugebauer, EA. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Databse Syst Rev. 2010.
144. Taqi, E, Hadher, SA, Ryckman, J, et al. Outcome of laparoscopic appendectomy for perforated appendicitis in children. J Pediatr Surg. 2008; 43:893–895.
145. Katkhouda, N, Mason, RJ, Towfigh, S, et al. Laparoscopic versus open appendectomy: A prospective randomized double-blind study. Ann Surg. 2005; 242:439–448.
146. Garey, CL, Laituri, CA, Little, DC, et al. Outcomes of perforated appendicitis in obese and non-obese children. J Pediatr Surg. 2011; 46:2346–2348.
147. Menezes, M, Das, L, Alagtal, M, et al. Laparoscopic appendectomy is recommended for the treatment of complicated appendicitis in children. Pediatr Surg Int. 2008; 24:303–305.
148. Paterson, HM, Qadan, M, de Luca, SM, et al. Changing trends in surgery for acute appendicitis. Br J Surg. 2008; 95:363–368.
149. Khan, MN, Fayyad, T, Cecil, TD, et al. Laparoscopic versus open appendectomy: The risk of postoperative infectious complications. JSLS. 2007; 11:363–367.
150. Lin, HF, Wu, JM, Tseng, LM, et al. Laparoscopic versus open appendectomy for perforated appendicitis. J Gastrointest Surg. 2006; 10:906–910.
151. Ikeda, H, Ishimaru, Y, Takayasu, H, et al. Laparoscopic versus open appendectomy in children with uncomplicated and complicated appendicitis. J Pediatr Surg. 2004; 39:1680–1685.
152. Guller, U, Hervey, S, Purves, H, et al. Laparoscopic versus open appendectomy: Outcomes comparison based on a large administrative database. Ann Surg. 2004; 239:43–52.
153. Marzouk, M, Khater, M, Elsadek, M, et al. Laparoscopic versus open appendectomy: A prospective comparative study of 227 patients. Surg Endosc. 2003; 17:721–724.
154. Nadler, EP, Reblock, KK, Qureshi, FG, et al. Laparoscopic appendectomy in children with perforated appendicitis. J Laparoendosc Adv Surg Tech. 2006; 16:159–163.
155. Fraser, JD, Aguayo, P, Sharp, SW, et al. Physiologic predictors of postoperative abscess in children with perforated appendicitis: Subset analysis from a prospective randomized trial. Surgery. 2010; 147:729–732.
156. Yagmurlu, A, Vernon, A, Barnhart, DC, et al. Laparoscopic appendectomy for perforated appendicitis: A comparison with open appendectomy. Surg Endosc. 2006; 20:1051–1054.
157. Bilik, R, Burnweit, C, Shandling, B. Is abdominal cavity culture of any value in appendicitis? Am J Surg. 1998; 175:267–270.
158. Kokoska, ER, Silen, ML, Tracy, TF, et al. The impact of intraoperative culture on treatment and outcome in children with perforated appendicitis. J Pediatr Surg. 1999; 34:749–753.
159. Sherman, JO, Luck, SR, Borger, JA. Irrigation of the peritoneal cavity for appendicitis in children: A double-blind study. J Pediatr Surg. 1976; 11:371–374.
160. Kokoska, ER, Silen, ML, Tracy, TF, et al. Perforated appendicitis in children: Risk factors for the development of complications. Surgery. 1998; 124:619–625.
161. David, IB, Buck, JR, Filler, RM. Rational use of antibiotics for perforated appendicitis in childhood. J Pediatr Surg. 1982; 17:494–500.
162. Kaselas, C, Molinaro, F, Lacreuse, I, et al. Postoperative bowel obstruction after laparoscopic appendectomy in children: A 15-year experience. J Pediatr Surg. 2009; 44:1581–1585.
163. Tsao, KJ, St Peter, SD, Valusek, PA, et al. Adhesive small bowel obstruction after appendectomy in children: Comparison between the laparoscopic and open approach. J Pediatr Surg. 2007; 42:939–942.
164. St Peter, SD, Adibe, OO, Juang, D, et al. Single incision versus standard 3-port laparoscopic appendectomy: A prospective randomized trial. Ann Surg. 2011; 254:586–590.
165. Langness, SM, Hill, SJ, Wulkan, ML. Single-site laparoscopic appendectomy: A comparison to traditional laparoscopic technique in children. Am Surg. 2011; 77:961–964.
166. Muensterer, OJ, Puga Nouges, C, Adibe, OO, et al. Appendectomy using single-incision pediatric endosurgery for acute and perforated appendicitis. Surg Endosc. 2010; 24:3201–3204.
167. Sesia, SB, Haecker, FM, Kubiak, R, et al. Laparoscopy-assisted single-port appendectomy in children: Is the postoperative complication rate different? J Laparoendosc Adv Surg Tech. 2010; 20:867–871.
168. Shekherdimian, S, DeUgarte, D. Transumbilical laparoscopic-assisted appendectomy: An extracorporeal single-incision alternative to conventional laparoscopic techniques. Am Surg. 2011; 77:557–560.
169. Guana, R, Gesmundo, R, Maiullari, E, et al. Treatment of acute appendicitis with one-port transumbilical laparoscopic appendectomy: A six-year, single-centre experience. Afr J Paediatr Surg. 2010; 7:169–173.
170. Stanfill, AB, Matinsky, DK, Kalvakuri, K, et al. Transumbilical laparoscopically assisted appendectomy: An alternative minimally invasive technique in pediatric patients. J Laparoendosc Adv Surg Tech. 2010; 20:873–876.
171. Ohno, Y, Morimura, T, Hayashi, S. Transumbilical laparoscopically assisted appendectomy in children: The results of a single-port, single-channel procedure. Surg Endosc. 2012; 26:523–527.
172. Garey, CL, Laituri, CA, Ostlie, DJ, et al. A review of single site minimally invasive surgery in infants and children. Pediatr Surg Int. 2010; 26:451–456.






