Chapter 8 Aphasia and Anosognosia
Handedness
Although the left hemisphere is dominant in approximately 95% of all people (and the rest of this chapter assumes it is always the case), sometimes neurologists must establish dominance with certainty. For example, when neurosurgeons must resect a portion of the dominant temporal lobe because of a tumor or intractable partial complex epilepsy (see Chapter 10), they can resect only a limited, carefully mapped area. Resecting too large or the wrong area might be complicated by devastating aphasia or memory impairment.
Aphasia
The Perisylvian Language Arc
Impulses conveying speech, music, and simple sounds travel from the ears along the acoustic (eighth cranial) nerves into the brainstem, where they synapse in the medial geniculate body. Then postsynaptic, crossed and uncrossed brainstem tracts bring the impulses to the primary auditory cortex, Heschl’s gyri, in each temporal lobe (see Fig. 4-16). Most music and some other sounds remain based in the nondominant hemisphere. In contrast, the brain transmits language impulses to Wernicke’s area, which is situated in the dominant temporal lobe. From there, they travel in the arcuate fasciculus, coursing posteriorly through the temporal and parietal lobes, and then looping anteriorly to the frontal lobe’s Broca’s area. This vital language center – located immediately anterior to the motor center for the right face, larynx, pharynx, and arm (Fig. 8-1) – receives processed, integrated language impulses, converts them to speech, and activates the adjacent motor cortex. A horseshoe-shaped region of cerebral cortex, which surrounds the sylvian fissure, the perisylvian language arc, contains Wernicke’s area, the arcuate fasciculus, and Broca’s area.
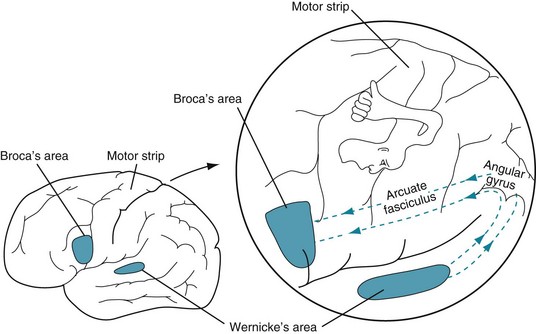
FIGURE 8-1 In the standard model of language function, the left cerebral hemisphere contains Wernicke’s area in the temporal lobe and Broca’s area in the frontal lobe. The arcuate fasciculus, the “language superhighway,” which connects these areas, curves posteriorly from the temporal lobe to the parietal lobe. It then passes through the angular gyrus and anteriorly to the frontal lobe. These structures surrounding the sylvian fissure, which comprise the perisylvian language arc, form the central processing unit of the language system. Note the proximity of Broca’s area to the motor strip’s representation of the face, throat, and upper extremity.
Using the perisylvian language arc model, researchers have established normal and abnormal language patterns. For example, when normal people repeat aloud what they hear, auditory impulses go first to Wernicke’s area, then pass around the arcuate fasciculus, and finally arrive in Broca’s area for speech production (Fig. 8-2, A). Reading aloud is a complicated variation of repeating aloud because reading requires both hemispheres and a learned system of decoding symbols, i.e., having been taught to read. As people read, their geniculocalcarine pathway transmits visual impulses from the lateral geniculate bodies to the calcarine (visual) cortex in both the left and right occipital lobes (see Fig. 4-1). Impulses from the left visual field go to the right occipital cortex. Then those impulses must travel through the posterior corpus callosum to reach the left (dominant) cerebral hemisphere. The impulses that have crossed from the right visual cortex merge with those already in the left hemisphere’s parietal lobe. Decoded, coherent language information then travels from the left parietal lobe via the arcuate fasciculus to Broca’s area for articulation (Fig. 8-2, B).
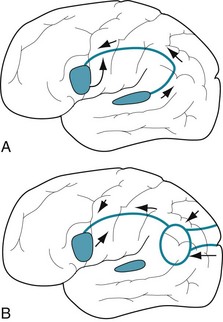
FIGURE 8-2 A, When people repeat aloud, language information arrives in Wernicke’s area, located adjacent to Heschl’s gyrus (see Fig. 4-16), and then travels through the parietal lobe in the arcuate fasciculus to Broca’s area. This area innervates the adjacent cerebral cortex for the tongue, lips, larynx, and pharynx. B, When people read aloud, visual impulses are received by the left and right occipital visual cortex regions. Both regions send impulses to a left parietal lobe association region (the oval), which converts text to language. Impulses from the left visual field, which are initially received in the right cortex, must first pass through the posterior corpus callosum to reach the language centers (see Fig. 8-4).
Clinical Evaluation
The most useful classification of the aphasias, nonfluent/fluent, rests on the quantity of the patient’s verbal output (Table 8-1A). It suffices for clinical evaluations and roughly correlates with imaging studies. Aphasia aficionados subdivide nonfluent aphasia and fluent aphasia each into four categories based primarily on the language examination showing the presence or absence of the patient’s ability to comprehend, repeat, and name objects. When repetition ability remains intact in either nonfluent or fluent category, neurologists add the designation transcortical.
TABLE 8-1A Salient Features of the Nonfluent and Fluent Aphasias
| Feature | Nonfluent | Fluent |
|---|---|---|
| Other terms | Expressive | Receptive |
| Motor | Sensory | |
| Broca’s | Wernicke’s | |
| Spontaneous Speech | Nonverbal | Verbal |
| Content | Paucity of words, mostly nouns and verbs | Complete sentences with normal syntax |
| Articulation | Dysarthric, slow, stuttering | Good |
| Errors | Telegraphic speech | Paraphasic errors, circumlocutions, tangentialities, clang associations |
| Associated deficits | Right hemiparesis (arm, face > leg) | Hemianopsia, hemisensory loss |
| Localization of lesion | Frontal lobe | Temporal or parietal lobe |
| Occasionally diffuse |
Clinicians usually detect aphasia in a patient during the introductory conversation, history taking, or mental status examination. They perform a standard series of simple verbal tests to identify and classify the aphasia. The tests systematically evaluate three basic language functions: comprehension, naming, and repetition (Box 8-1). Depending on the clinical situation, the examiner may request increasingly difficult levels of comprehension, more uncommon objects, or more complicated phrases. The examiner may also perform the same testing with written requests and responses; however, with one notable exception, alexia without agraphia (see later), defects in written communication parallel those in verbal communication.
Nonfluent Aphasia
Characteristics
The four major subdivisions of nonfluent aphasia are the following (Table 8-1B):
• Broca’s aphasia: commonly occurring, classic nonfluent aphasia with comprehension intact and repetition lost.
• Transcortical motor aphasia: an aphasia similar to Broca’s except that repetition remains intact.
• Mixed transcortical or isolation aphasia: a unique aphasia with loss of comprehension except that repetition remains intact.
• Global aphasia: a devastating aphasia with loss of both comprehension and repetition (see later).
| Comprehension | Repetition | |
|---|---|---|
| Broca’s | Intact | Lost |
| Transcortical motor | Intact | Intact |
| Mixed transcortical | Lost | Intact |
| (isolation) | ||
| Global | Lost | Lost |
Localization and Etiology
Lesions responsible for nonfluent aphasias usually encompass, surround, or sit near Broca’s area (Fig. 8-3, A). The etiology is usually a middle cerebral artery stroke or other discrete structural lesion. Their location, not their pathology, produces the aphasia. Whatever the etiology, these lesions tend to be so extensive that they damage neighboring structures, such as the motor cortex and posterior sensory cortex. Moreover, because they are usually spherical or conical, rather than superficial two-dimensional lesions, they damage underlying white-matter tracts, including the visual pathway. Diffuse cerebral injuries, such as anoxia, metabolic disturbances or Alzheimer disease, rarely cause nonfluent aphasia.
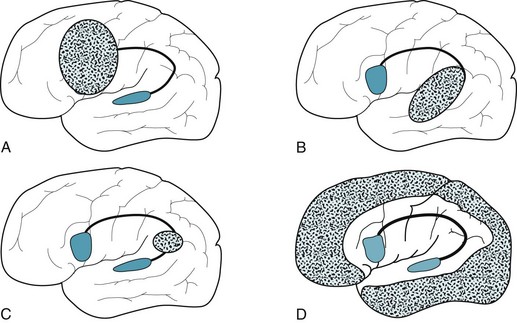
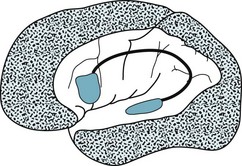
FIGURE 8-3 A, Lesions that cause nonfluent aphasia are typically located in the frontal lobe and encompass Broca’s area and the adjacent cortex motor strip. B, Those causing fluent aphasia are in the temporoparietal region. They may even consist of diffuse injury, such as from neurodegenerative illnesses, and encompass Wernicke’s areas and more posterior regions. However, they usually spare the motor strip. C, Those causing conduction aphasia, which are relatively small, interrupt the arcuate fasciculus in the parietal or posterior temporal lobe. D, Those causing mixed transcortical (isolation) aphasia involve the watershed region, which encircles the perisylvian language arc.
Mixed Transcortical or Isolation Aphasia
Damage to the entire remaining cortex usually causes cognitive impairment, usually to the point of dementia. It also usually causes decorticate posture (see Fig. 11-5).
The etiology of this aphasia usually stems from the loss of the precarious blood supply of the cerebral cortex. While major branches of left middle cerebral artery perfuse the perisylvian arc, only thin, fragile, distal branches of middle, anterior, and posterior cerebral arteries perfuse its border with the surrounding cortex (the watershed area). When these vessels deliver insufficient cerebral blood flow to this portion of the cortex, it suffers a watershed infarction (Fig. 8-3, D). Thus, cardiac or respiratory arrest, suicide attempts using carbon monoxide, and other hypotensive or hypoxic episodes cause isolation aphasia. When Alzheimer disease strikes cerebral areas other than the language and motor regions, which is uncommon, it too can cause isolation aphasia.
Fluent Aphasia
The four major subdivisions of fluent aphasia are the following (Table 8-1C):
• Wernicke’s aphasia: commonly occurring, classic fluent aphasia with loss of comprehension, naming, and repetition.
• Transcortical sensory aphasia: an aphasia similar to Wernicke’s except with repetition intact.
• Conduction aphasia: an aphasia restricted to inability to repeat.
• Anomic aphasia: an aphasia restricted to inability to name.
| Comprehension | Repetition | |
|---|---|---|
| Wernicke’s | Lost | Lost |
| Transcortical sensory | Lost | Intact |
| Conduction | Intact | Lost |
| Anomic | Intact | Intact |
Localization and Etiology
Discrete structural lesions, such as small strokes, in the temporoparietal region are the usual cause of Wernicke aphasia and fluent aphasias in general (Figs 8-3, B and 20-16). In addition, neurodegenerative illnesses, particularly Alzheimer disease and frontotemporal dementia (see Chapter 7), often cause fluent aphasia among other symptoms.
Conduction Aphasia
The most frequent cause of conduction aphasia is an embolic stroke in the parietal or posterior temporal lobe (Fig. 8-3, C). Infarctions that cause conduction aphasia are usually so small that they cause little or no physical deficit. At worst, patients show right lower facial weakness.
Comorbid Depression
Psychiatrists face obstacles when assessing mood in aphasic patients because they tend to appear apathetic, cannot freely communicate, and offer potentially misleading facial expressions. Aphasic patients who apparently show symptoms of depression may actually be manifesting underlying dementia or pseudobulbar palsy. For example, following one or more strokes, patients may have aphasia along with dementia or poststroke depression (see Chapter 11). In another example, lesions damaging both frontal lobes reduce patients to a paucity or absence of speech with little emotion (abulia), responsiveness, or voluntary movement (akinesia). Here too, extensive frontal lobe damage invariably produces neurologic-based depressive symptoms (see frontal lobe disorders, Chapter 7).
Mental Abnormalities with Language Impairment
Other Disorders
Children with autism spectrum disorders may demonstrate language impairment – not only in their verbal expression, but also in their facial and bodily communication, such as failure to point. In many cases, nonsensical repetitions (stereotypies), idiosyncrasies, and echolalia overwhelm their speech. These children often fail to appreciate the nuance and affective components of language. In girls with Rett syndrome, language begins to regress after several years of normal development (see Chapter 13). Despite the possibility of a neurologic condition being responsible, when language regression appears in children, particularly boys and those younger than 3 years, they face a high probability of having an autistic disorder.
Mutism and other apparent language abnormalities are frequently manifestations of psychogenic disturbances (see Chapter 3). In these cases, apparent language impairment is usually inconsistent and amenable to suggestion. Acquired stuttering also often indicates a psychogenic impairment. For example, a patient with psychogenic aphasia might stutter and seem to be at a loss for words, but communicate normally by writing. An amobarbital infusion during an interview might reveal perfectly intact language function.
Disorders Related to Aphasia
Alexia and Agraphia
In the exception, alexia without agraphia (Fig. 8-4), patients demonstrate little or no impairment in comprehending speech or expressing themselves verbally or by writing; however, they simply cannot read. For example, patients can transcribe another person’s dictation and write their own thoughts, but then be unable to read their own handwriting. Alexia without agraphia, which should really be called “alexia with graphia,” results from a destructive lesion encompassing the dominant (left) occipital lobe and adjacent posterior corpus callosum. Aside from having a right homonymous hemianopsia, patients lack physical deficits.
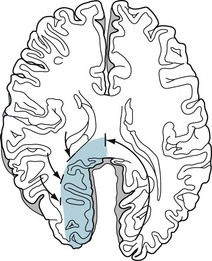
FIGURE 8-4 Lesions that damage the left occipital lobe and the posterior corpus callosum cause alexia without agraphia. Patients are unable to see anything in their right visual field because of the left occipital cortex damage. Left visual images still reach the right cortex, but they cannot be transmitted to the left cerebral language centers because the critical posterior corpus callosum is damaged. With obstacles in both information routes (the right visual field cut and transfer of information to the dominant hemisphere), patients cannot comprehend written material presented to either visual field. In contrast, they can still write full sentences from memory, imagination, or dictation because these forms of information still reach the language centers.
Gerstmann Syndrome
Gerstmann syndrome, a classic disorder, consists of four neuropsychologic disturbances: acalculia (impaired arithmetic skills), finger agnosia (inability to identify fingers), left/right confusion, and agraphia. When all four elements occur, neurologists usually attribute the syndrome to a lesion in the angular gyrus of the dominant parietal lobe (see Fig. 8-1).
Apraxia
Although apraxia can be readily differentiated from simple paresis, it is often comorbid with aphasia or dementia. In fact, apraxia often appears as a symptom of Alzheimer disease and other cortical dementias (see Chapter 7).
In assessing patients for apraxia, the examiner usually first tests their buccofacial (lips, face, tongue) and limb movements when they make gestures or perform “symbolic acts” (Table 8-2). Next, the examiner asks them to perform imagined actions (pantomime), first on pretend objects and then on actual ones. After seeing the examiner perform an action, patients with apraxia typically can copy it. For example, a patient with apraxia might not be able to follow the request “Please, pretend to salute an officer,” but after the examiner demonstrates the salute, the patient will duplicate it. Similarly, when patients with apraxia are handed an actual object, which gives them a cue, they can often perform the object’s intended action. For example, a patient with apraxia might not be able to pretend to use a comb, but when presented with one, the patient will use it appropriately.
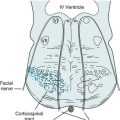
Despite its complexity, neurologists designate several clinically useful categories of apraxia. Ideomotor apraxia, the most common category, consists basically of the inability to convert an idea into an action. For example, patients with ideomotor apraxia cannot pantomime despite possessing a clear understanding and retaining the physical ability to comply. Clinicians might envision ideomotor apraxia as the result of a disconnection between cognitive or language regions and motor regions (Fig. 8-5). Almost invariably, a left-sided frontal or parietal lobe lesion gives rise to the apraxia. Thus, ideomotor apraxia often coexists with aphasia, particularly nonfluent aphasia, and inability of the right hand to pantomime.
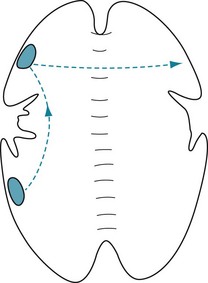
FIGURE 8-5 In a schematic transaxial view, requests for normal movements travel to Wernicke’s area in the dominant (left) posterior temporal lobe. They then travel anteriorly to the motor regions and, through the anterior corpus callosum, to the contralateral motor strip. Interruptions of the path within the left cerebral hemisphere result in ideomotor apraxia of both arms, causing bilateral limb apraxia. Lesions in the anterior corpus callosum interrupt only those impulses destined to control the left arm and leg, causing unilateral left arm and leg ideomotor apraxia.
In other sections, this book covers several other apraxias: Construction and dressing apraxias, which are typically manifestations of nondominant-hemisphere lesions (see later), and gait apraxia, which is a hallmark of normal-pressure hydrocephalus (see Fig. 7-10).
Nondominant-Hemisphere Syndromes
Hemi-Inattention
Patients with nondominant-hemisphere injury most prominently display hemi-inattention (hemispatial neglect). They typically ignore visual, tactile, and other sensory stimuli that originate from their left side (Fig. 8-6). For example, they disregard, fail to perceive, or misinterpret objects in their left visual field (Fig. 8-7). Sometimes men with this condition leave the left side of their face unshaven. In contrast to patients with homonymous hemianopsia, who usually develop some awareness and thus make compensatory movements to keep objects in the preserved visual field, those with hemi-inattention remain oblivious to their situation and make no normal exploratory eye or limb movements.
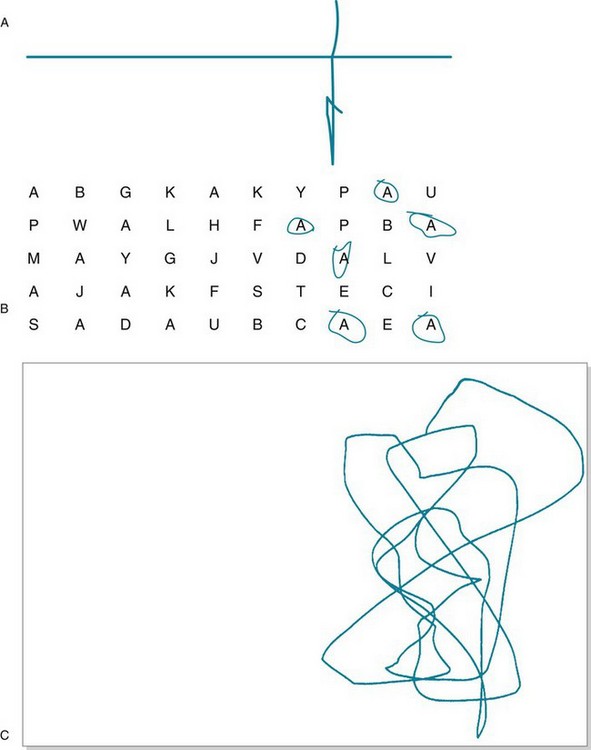
FIGURE 8-6 Simple bedside tests for hemi-inattention. A, The examiner has asked the patient, who has just sustained a right middle cerebral infarction, to bisect a horizontal line. The patient, neglecting a portion of the line’s left side, draws the vertical line off-center to the right and bisects only the line’s perceived segment. B, Then the examiner asks the patient to circle all the “A”s on the page. Again neglecting the left side of the page, the patient circles only those on the right. C, An examiner has asked a 4-year-old boy, recovering from right parietal trauma, to draw a man. Because of a combination of hemi-inattention and constructional apraxia (see later), he merely scribbles on the right side of the paper.
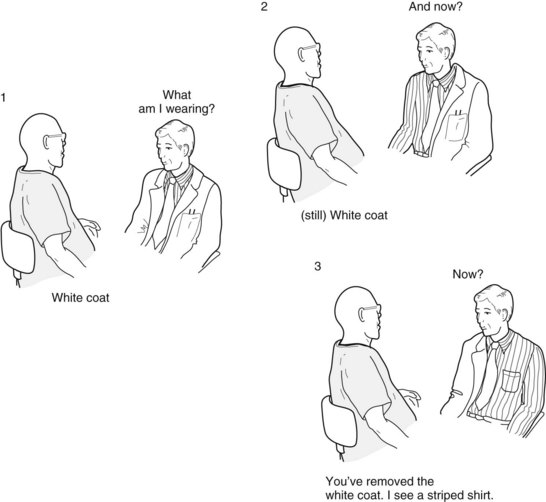
FIGURE 8-7 In a classic demonstration of left hemi–inattention, the “white coat test,” the patient neglects the left-sided stimulation and perceives only the examiner’s clothing in his right visual field. Even if the patient’s problem were simply a left homonymous hemianopsia, he still would have explored and discovered, with his intact right visual field, that the examiner was half dressed.
Constructional Apraxia
Another frequently occurring manifestation of nondominant-hemisphere injury is constructional apraxia, which is a visuospatial perceptual impairment. Patients with this disorder cannot organize visual information and integrate it with fine motor skills. For example, they cannot copy simple figures or arrange matchsticks in patterns (Fig. 8-8). However, in contrast to the other nondominant symptoms, clinicians cannot attribute constructional apraxia exclusively to a nondominant lesion. It also appears in tests of patients with mental retardation, diffuse cerebral dysfunction, and executive impairment from frontal lobe disease.
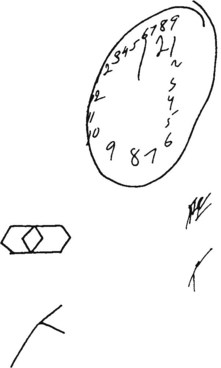
FIGURE 8-8 When asked to draw a clock, a patient with constructional apraxia drew an incomplete circle, repeated (perseverated) the numerals, and placed them unevenly. When attempting to copy the top left figure, the patient only repeated several lines. The patient also misplaced and rotated the position of the bottom left figure (see Fig. 2-8). Such abnormalities are not peculiar to nondominant-hemisphere injuries: They often appear in Bender-Gestalt and Wechsler Adult Intelligence Scale testing.
Disconnection Syndromes
Injuries that sever communication pathways, but spare the actual area, cause uncommon but interesting phenomena, the disconnection syndromes. Neurologists predicted their existence before verifying them in actual patients, much as physicists have predicted certain subatomic particles before demonstrating them. This chapter has previously discussed several disconnection syndromes: (1) alexia without agraphia; (2) conduction aphasia; and (3) ideomotor apraxia with its varieties, buccofacial and limb apraxia. Subsequent chapters will present other disconnection syndromes, including the medial longitudinal fasciculus syndrome, also known as intranuclear ophthalmoplegia (see Chapters 12 and 15).
The anterior cerebral artery syndrome results from an occlusion of both anterior cerebral arteries that causes an infarction of both frontal lobes and the anterior corpus callosum. In this syndrome, information cannot pass between the left hemisphere language centers and the right hemisphere motor centers. Although the patient’s left arm and leg will have normal spontaneous movement, those limbs fail to follow an examiner’s verbal or written requests to move them. In other words, the patient will have unilateral (left-sided) limb apraxia (see Fig. 8-5).
Split-Brain Syndrome
The most important disconnection syndrome referable to the corpus callosum is the split–brain syndrome. Now rare, this disorder previously most often resulted from a longitudinal surgical division of the corpus callosum (commissurotomy) performed by neurosurgeons in an effort to control intractable epilepsy (see Chapter 10). The commissurotomy almost completely isolated each cerebral hemisphere.
In cases of the split-brain syndrome, examiners may present certain information to only a single, isolated hemisphere. For example, examiners can show pictures, writing, and other visual information in one of the patient’s visual fields to present information to only the contralateral hemisphere (Fig. 8-9). Likewise, by having a blindfolded patient touch objects with the one hand, examiners can present tactile information to only the contralateral hemisphere. However, auditory information cannot be presented exclusively – only predominantly – to one hemisphere. (Because pathways are duplicated in the brainstem [see Fig. 4-16], sounds presented to one ear travel, after the medial geniculate synapse, to both hemispheres, but predominantly to the contralateral one.)
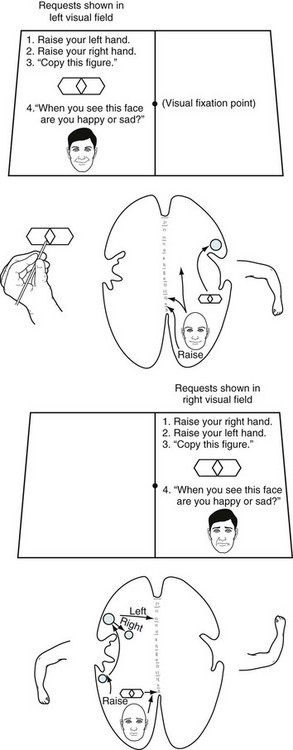
FIGURE 8-9 After a commissurotomy, patients typically have the split-brain syndrome. Each hemisphere can be tested individually by showing requests, objects, and pictures in the contralateral visual field. Top, Objects and written requests shown in the left visual field are perceived by the right visual field. Since connections to the ipsilateral motor area are intact, the left hand can copy figures. However, since the right hemisphere is unable to transmit information through the corpus callosum to the dominant left cerebral hemisphere, which governs language function, patients cannot read the requests or describe the objects. Although patients cannot speak of the feelings evoked by emotionally laden pictures shown in their left visual field, they have sympathetic, nonverbal responses. Bottom, Written requests and objects shown in the right visual field are perceived by the left hemisphere. Patients can read those written requests, copy those objects with the right hand, and comply with the requests; however, because the language areas cannot send information through the corpus callosum, the left hand cannot comply. When patients describe emotions portrayed in a picture, their language lacks affect, derived from the nondominant hemisphere.
Benton AL. Gerstmann’s syndrome. Arch Neurol. 1992;49:445–447.
Besson M, Schon D. Comparison between language and music. Ann NY Acad Sci. 2001;930:232–358.
Brust JC. Music and the neurologist. Ann NY Acad Sci. 2001;930:143–152.
Buxbaum LJ, Ferraro MK, Veramonti T, et al. Hemispatial neglect. Neurology. 2004;62:749–756.
Croquelois A, Bogousslavsky J. Stroke aphasia. Cerebrovasc Dis. 2011;31:392–399.
Faber R, Abrams R, Taylor MA, et al. Comparison of schizophrenic patients with formal thought disorder and neurologically impaired patients with aphasia. Am J Psychiatry. 1983;140:1348–1351.
Feinberg TE, Schindler RJ, Flanagan NG, et al. Two alien hand syndromes. Neurology. 1992;42:19–24.
Greenberg VD. Freud and his Aphasia Book: Language and the Sources of Psychoanalysis. Ithaca, NY: Cornell University Press; 1998.
Greener J, Enderby P, Whurr R. Speech and language therapy for aphasia following stroke. Cochrane Database Syst Rev. 2, 2000. CD000425
Hemenway D. Bimanual dexterity in baseball players. N Engl J Med. 1983;309:1587.
Hickok G, Bellugi U, Klima ES. Sign language in the brain. Sci Am. 2001;June:59–65.
Hills AE. Aphasia: Progress in the last quarter of a century. Neurology. 2007;69:200–213.
Kikkert MA, Ribbers GM, Koudstaal PJ. Alien hand syndrome in stroke: A report of 2 cases and review of the literature. Arch Phys Med Rehabil. 2006;87:728–732.
Laurent-Vannier A, Pradat-Diehl P, Chevignard M, et al. Spatial and motor neglect in children. Neurology. 2003;60:202–207.
Motley MT. Slips of the tongue. Sci Am. 1985;253:116–125.
Orfei MD, Robinson RG, Prigatano GP, et al. Anosognosia for hemiplegia after stroke is a multifaceted phenomenon: A systemic review of the literature. Brain. 2007;130:3075–3090.
Portal JM, Romano PE. Patterns of eye–hand dominance in baseball players. N Engl J Med. 1988;319:655.
Shinnar S, Rapin I, Arnold S, et al. Language regression in childhood. Pediatr Neurol. 2001;24:183–189.
Vocat R, Staub F, Stroppni T, et al. Anosognosia for hemiplegia: A clinical-anatomic prospective study. Brain. 2010;133:3578–3597.
Chapter 8 Questions and Answers
This man has anomic aphasia, which is a variety of fluent aphasia. In this disorder, language impairment is restricted to the improper identification of objects (a naming impairment or deficit). The patient knows the function and category of the objects and other cognitive functions remain intact. Anomic aphasia and other fluent aphasias may originate in Alzheimer disease or other neurodegenerative illness, but usually they originate in small structural lesions. In this case, the mitral stenosis and headache indicate that the origin was probably a small embolic cerebrovascular accident (see Chapter 11).
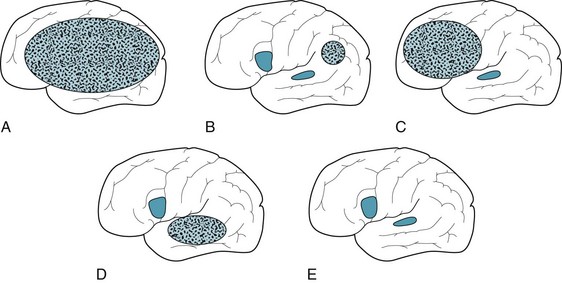
6–10. Match the lesions that are pictured schematically with those expected in cases 1–5.
case l, drawing A; case 2, drawing C; case 3, drawing D; case 4, drawing B or D; case 5, drawing E.
11–26. Match the lesion with the expected associated finding(s).
11. Paresis of one recurrent laryngeal nerve
14. Dominant hemisphere temporal lobe lesion
15. Lateral medullary syndrome
17. Dominant hemisphere angular gyrus lesion
18. Dominant hemisphere parietal lobe lesion
19. Nondominant hemisphere parietal lobe lesion
20. Bilateral frontal lobe tumor
21. Bilateral anterior cerebral artery infarction
24. Periaqueductal hemorrhagic necrosis (Wernicke’s encephalopathy)
27. Emergency medical services workers revived a 34-year-old man after he attempted suicide by sitting in a garaged car with the motor running. During the next week he only sat in bed and looked out of the window. He displayed no emotion and did not respond to requests. Although he was otherwise mute, he seemed to repeat in intricate detail whatever he was asked and, depending on the show, whatever was spoken on television. Physicians found brisk deep tendon reflexes and bilateral palmomental reflexes, but only equivocal plantar reflexes.
The patient was probably exposed to excessive carbon monoxide and had resultant cerebral anoxia. As in many cases of survival following cardiac arrest or strangulation, cerebral anoxia creates irreparable cerebral cortex damage. When patients permanently lose all consciousness, cognitive ability, and voluntary motor function, neurologists judge them to be in the persistent vegetative state (see Chapter 11).
c. In the Wada test, infusion of barbiturates directly into the carotid artery produces aphasia. This test can determine if a cerebral hemisphere is dominant before removal of an epilepsy scar focus. PET scans are difficult to perform because they require short-lived cyclotron-generated substrates and their spatial resolution is insufficiently detailed. Neither the CT nor MRI will reliably determine the dominant hemisphere. Functional MRI (fMRI, see Chapter 20), a noninvasive test, will probably replace the Wada test in locating the language regions, i.e., correlating anatomy and function.
33. A 45-year-old airplane pilot reported that when she awoke earlier in the morning, for approximately 5 minutes she had “expressive aphasia,” by which she meant that she was unable to speak or gesture, but she understood most of the news on the radio. During that time, she had no other symptoms. Which of the following conditions are reasonable explanations for her episode?
e. Each of those conditions is a reasonable explanation. She might have had a partial seizure originating in the left frontal lobe that led to transient, postictal aphasia and right hemiparesis. A TIA in the distribution of the left carotid artery might also have caused aphasia with or without right hemiparesis. Hemiplegic migraines, which can cause speech or language impairment, can occasionally affect adults. An episode of sleep paralysis, such as hypnopompic cataplexy, may cause brief quadriparesis and mutism. The possible etiologies of transient aphasia and transient hemiparesis are similar (see Hemiplegic migraines, Chapter 9, and Carotid artery TIAs, Chapter 11).
34. A 70-year-old newspaper reporter suddenly developed inability to read. Although he can write his name and most sentences that are dictated to him, he cannot read aloud or copy written material. His speech is fluent and contains no paraphasic errors. He can see objects only in his left visual field. He remains articulate and has no paresis. What is this man’s difficulty, and where is the responsible lesion(s)?
a. He clearly has alexia, as demonstrated by his inability to read, and a right homonymous hemianopsia. He does not have agraphia because he can transcribe dictation and write words from memory. Nor does he have aphasia. Thus, he has the syndrome of alexia without agraphia. A lesion encompassing the left occipital lobe and posterior corpus callosum causes alexia without agraphia (see Fig. 8-4). Because memory and auditory circuits, as well as the corticospinal system, are intact, he can write words that he hears or remembers. The etiology of alexia without agraphia is usually an infarction of the left posterior cerebral artery or infiltrating brain tumor, such as a glioblastoma.
35. A 68-year-old taxi cab driver had an accident because he drifted into oncoming traffic. The emergency room physician found that he has a left homonymous hemianopsia and a mild left hemiparesis, which he fails to appreciate. Which neuropsychologic problem describes his denying his left arm weakness?
b. Words and sound heard in the right ear are transmitted to both the right and, more so, left cerebral cortex. The dominant hemisphere planum temporale, which is integral to language function, has greater surface area than its counterpart. Each hemisphere’s Heschl’s gyrus, which processes the auditory qualities of sound, is symmetric (see Fig. 4-16).
50. In nonfluent aphasia, why is the arm typically more paretic than the leg?
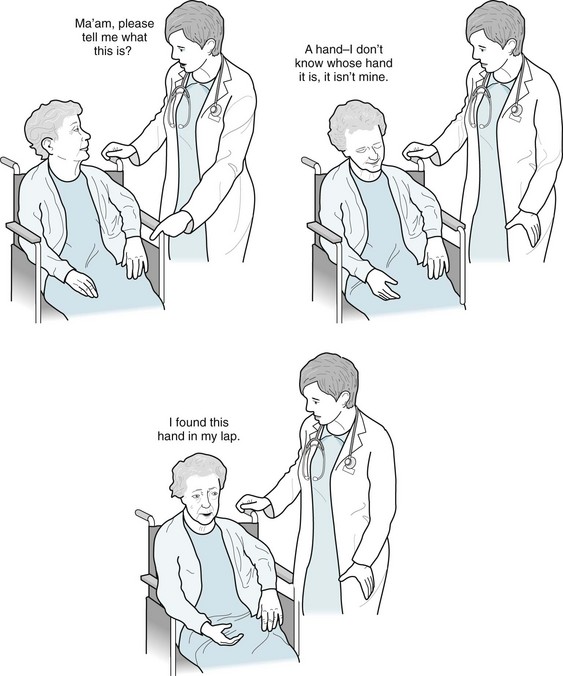
d, e, and f. The perception of one’s hand seems to be particularly vulnerable. Persistent burning pain in a paretic hand may be a manifestation of the thalamic pain syndrome (see Chapter 14). The misperception that a paralyzed hand is normal constitutes anosognosia. Attraction to another person’s hand is not a neurologic condition. Persistent burning pain in the hand of someone with hemiparesis is usually a deafferentation pain syndrome resulting from damage to the contralateral thalamus.
55. Which conclusion has stemmed from studies of patients who have undergone a commissurotomy?
56. As part of a research project, a 60-year-old man who has undergone a commissurotomy placed his hands in a closed box containing many objects. A researcher put a set of keys in his left hand. By voice and gesture, the researcher asked the man to identify the keys. Which would be his most accurate response?
57. A psychiatrist is called to see a 74-year-old woman at a nursing home because she constantly reports intruders in her nursing home room. On multiple occasions, security guards found no evidence of an intrusion. Her family had placed her in the nursing home following partial recovery from a right-sided parietal stroke. Its residual deficits were a mild left hemiparesis and hemisensory impairment but no dementia. The psychiatrist finds that the woman has a tenuous relationship to her hand, which moves freely and, without her knowledge, pulls at her clothing. The woman does not deny that she had a stroke, but disclaims the hand at the end of her arm. “I am not moving it. Who is?” she finally asks the psychiatrist. Which is the most likely description for her perception?
61. When the emergency room resident asked a 68-year-old retired US Navy captain why he had come there, he responded, “Get lost. Need assistance.” When asked his location, the captain replied, “Sick bay.” He complied with requests to pick up his left hand and then to stick out his tongue. He did not even begin to respond to requests to repeat phrases. He could not name the month and year, or spell the word “world” backwards. All of his speech was dysarthric but comprehensible. Which of the following conditions does the examination indicate?
63. One week after surviving a cardiac arrest, a 50-year-old retired baseball player remained mute and apathetic. He tended to assume a fetal position. Although his pupils reacted to light and he had roving eye movements, he failed to establish eye contact with objects held in front of him, his examiners, or family members. A neuro-ophthalmologist could not demonstrate opticokinetic nystagmus, which is a normal function. Whether or not examiners spoke directly to him, he repeated long phrases. He failed to respond to questions and requests, but instead would often repeat the sentence. His vital signs, general medical function, and routine laboratory tests were normal. The MRI showed only changes in intensities indicative of diffuse cerebral ischemia, except around the perisylvian area, which appeared normal. Which is the most likely location of the responsible lesion?
66. The children of a 60-year-old man have brought their father for a psychiatric evaluation because during the previous year he has become loud and unrestrained, and he has made unwise investments. Although he has no history of depressive symptoms, they have suggested a diagnosis of bipolar disorder. His children also report that his own father – their paternal grandfather – had developed similar behavior in his late 50s and died several years later of Alzheimer disease.
67. The brother of an aging retired salesman has developed a slovenly appearance. He remains well spoken, oriented, and has retained his good mood, memory, and judgment. The physician confirms the brother’s description and notes that the patient is unshaven, his belt does not go through the loops on his pants, and the buttons on his shirt are not aligned. The neurologic examination detects a left homonymous hemianopsia and a mild left hemiparesis. Which is the most precise description of his slovenly appearance?
68. An intern summons a psychiatry consultant to assess the decisional capacity of a 78-year-old woman who insists on leaving the hospital against advice, despite having had a series of strokes that caused blindness but no paresis. The woman, who has walked to the nursing station in her hospital gown, has remained oblivious to explanations of her condition. She states that she is not blind. Not only does she insist on leaving, she wants to drive home. Although she cannot find it, she states she still has her driver’s license and that is “all I need.” She also states that she does not have Alzheimer disease because she knows the date and place. Which is the best diagnosis?
69. In the middle of the night, the intern calls again because another patient insists on leaving. The patient, a 72-year-old retired priest, had been hospitalized against his will for left hemiparesis. Earlier in the day, he had refused rehabilitation and further testing. Although unable to walk, he demands his clothing so that he may return home. He had become agitated, argumentative, and hostile. Moreover, he even threatened physical violence. Aside from neurologic and psychiatric disorders, his general medical evaluation showed no abnormality. Which would be the best management?
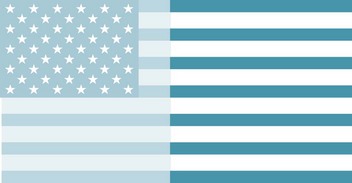
72. Neurologists have been caring for an elderly man who sustained a nondominant hemisphere stroke that caused left-sided hemiparesis and sensory loss. He has been uncooperative with hospital routine and insisting on discharge to his apartment. When they show him an American flag, he reports seeing only striped lines.