CHAPTER 60 Aortic and Mitral Valvular Disease
AORTIC STENOSIS
Definition
Aortic stenosis is obstruction to left ventricular outflow at or near the level of the aortic valve. The stenosis can be subvalvular, supravalvular, or valvular. The valvular stenosis is by far the most frequently encountered type in adults, most commonly secondary to calcification of tricuspid or congenitally bicuspid aortic leaflets.1
Prevalence
Aortic stenosis has become the most common valvular disease in the Western world, largely because of the increased life expectancy of the population. The prevalence of aortic stenosis in the population older than 65 years is 2% to 7%; aortic sclerosis, the precursor to aortic stenosis in which there is valve thickening but no stenosis, is present in approximately 25% of this age group.2,3 Whereas aortic stenosis is seen in patients with both tricuspid and bicuspid aortic valves, the patients with bicuspid aortic valves will become symptomatic and present for valve replacement an average of one to two decades earlier in life.4 Nonatherosclerotic causes of aortic stenosis, such as rheumatic and congenital, are rare in the developed world.2
Etiology and Pathophysiology
Aortic stenosis should be considered part of a disease continuum in which initially non–hemodynamically significant valve thickening and calcification eventually progress to heavily calcified, immobile leaflets causing obstruction of left ventricular outflow. The early, asymptomatic portion of this continuum may last several decades; the symptomatic phase is often short and rapidly progressive, with a 2-year survival rate of 50% after onset of symptoms.4 At that point, only aortic valve replacement can reverse the poor prognosis and offer long-term survival.
Aortic stenosis is associated with several risk factors, the main ones being increased age, male gender, hypertension, smoking, diabetes mellitus, and elevated serum low-density lipoprotein and elevated lipoprotein levels.1,4 Aortic stenosis is an active disease process that follows a pattern similar to that seen in vascular atherosclerosis—inflammation followed by lipid accumulation and dystrophic calcification.3 The aortic calcifications tend to accumulate preferentially at the edges of the valve leaflets, which influences valvular function.
Manifestations of Disease
Clinical Presentation
Aortic stenosis in asymptomatic patients is usually identified incidentally during the cardiac auscultation portion of the physical examination, when note is made of a late systolic murmur or a normally split second heart sound. Symptomatic patients can present with angina, syncope, dyspnea on exertion, and eventually symptoms of heart failure.1 The patient’s symptoms are associated with the degree of stenosis at the level of the aortic valve and with the degree of resulting left ventricular dysfunction. As the stenosis becomes more significant, with a higher pressure gradient across the valve, the left ventricle responds to the systolic pressure overload with concentric hypertrophy. Whereas this increased wall thickness is the expected, appropriate adaptation to increased pressure, it in turn causes a diastolic dysfunction that reduces cardiac output. In addition, hypertrophy may also cause reduced or imbalanced distribution of coronary blood flow, thereby increasing the risk of subendocardial ischemia and worsening the symptoms of heart failure.1
Imaging Indications
The grading of aortic stenosis is based on several values—the valvular orifice area, the pressure gradient across the valve, and the stenotic jet velocity—all of which require imaging for measurement. Transthoracic echocardiography is the imaging modality of choice and therefore the gold standard for grading of aortic stenosis severity. The severity of symptoms does not always correlate with the degree of stenosis, and some patients with moderate stenosis become symptomatic, whereas others with severe stenosis by imaging criteria remain without symptoms.1 The importance of this is underlined by the fact that the timing of therapy depends more on the presence of symptoms than on the grade of aortic stenosis.2
Imaging Techniques
Radiography
The most prominent finding on chest radiographs in adults with aortic stenosis is enlargement of the ascending aorta, although extent of dilation does not directly correlate with severity of stenosis (Fig. 60-1).5 Conversely, the degree of aortic valve calcification does correlate with disease severity. Other, less specific findings, such as pulmonary venous congestion and pulmonary edema, can occur late in the disease process as a result of left ventricular dysfunction.5
Echocardiography
As previously noted, echocardiography is the gold standard for evaluation of aortic stenosis. It is the modality of choice for both initial diagnosis and assessment of disease severity as well as for re-evaluation and monitoring of both asymptomatic patients and those in whom symptoms have appeared or are progressing.1 In general, asymptomatic patients with mild to moderate stenosis are monitored every 1 to 2 years; those with moderate to severe stenosis are evaluated every 6 to 12 months.4
In addition to evaluating the valve orifice size and velocity of the stenotic jet, echocardiography can be used to assess the thickness of the left ventricular wall as well as left ventricular size and function. By use of the modified Bernoulli equation (ΔP = 4V2), the pressure gradient across the valve is calculated by measuring the peak velocity of the flow jet. Whereas a normal aortic valve has a peak jet velocity of 0.9 to 1.8 m/sec, a peak pressure gradient of less than 25 mm Hg, and a valve orifice area of 2.0 to 3.5 cm2, a severely stenotic valve will have a peak velocity of more than 4.0 m/sec, a peak gradient of more than 64 mm Hg, and a valve area of less than 0.5 cm2.4
Computed Tomography
Although echocardiography will likely continue to be the first-line modality for diagnosis, grading, and monitoring of aortic stenosis, ECG-gated multislice CT can add more information in patients in whom clinical symptoms for some reason do not match the echocardiographic findings or in patients who are technically challenging.6 The main advantage of CT over transthoracic echocardiography, other than being faster, is the more reliably accurate measurement of valve orifice area by planimetry, which in CT is not limited by hemodynamic factors, such as low cardiac output, as it is in transthoracic echocardiography (Figs. 60-2 and 60-3).6 In addition, CT can give a reproducible assessment and quantification of valve calcification, a major component of stenosis that correlates with its severity (Figs. 60-4 and 60-5).7 Newer multidetector CT scanners do not require pretreatment with β blockers for rate control if the heart rate is below 85 beats/min and allow a dynamic display of valve motion throughout the full cardiac cycle.8 The main disadvantages of CT, in addition to the relatively higher cost and lower availability, are radiation and the need for intravenous administration of contrast material.
Magnetic Resonance
Qualitative assessment of aortic valve stenosis can be performed by steady-state free precession (SSFP) cine MR because of the excellent temporal resolution, which allows highly accurate evaluation of valve motion.7 A signal void due to spin dephasing representing the stenotic jet is identified projecting from the valve toward the proximal aorta. Adequate quantitative measurements are not possible with the SSFP MR technique. For quantification of aortic stenosis, phase contrast MR imaging is typically used. With this technique, the peak flow of the jet in the ascending aorta can be measured, and as with echocardiography, the pressure gradient can be calculated by the modified Bernoulli equation (ΔP = 4V2) (Fig. 60-6). A disadvantage of MR in evaluation of aortic stenosis is the poor visualization of leaflet calcification, a major factor in the disease process.
Treatment Options
Medical
The key elements of medical management of asymptomatic patients with aortic stenosis include advising against strenuous exercise in cases of moderate to severe stenosis; antibiotic prophylaxis against endocarditis for dental or other interventional procedures; antihypertensive therapy; and close monitoring both of the severity of stenosis and for appearance of symptoms.1 The last item is of great importance, both because disease progression tends to be unpredictable and as surgical therapy, namely, aortic valve replacement, is usually indicated once symptoms appear or are thought to be imminent. Recognition of the onset of symptoms can be especially difficult in patients with other comorbidities, and special attention needs to be paid to any change in tolerance of strenuous activity or appearance of chest pain in either rest or stress.1
Surgical
As the combined risk of aortic valve replacement (up to 10% mortality in the elderly or in those with severe comorbidities) and complications associated with having a prosthetic valve (2% to 3% per year) is much greater than the risk of sudden cardiac death in the asymptomatic patient, even those with severe stenosis by imaging, surgical therapy is usually not indicated until symptoms appear.4 The exception to this rule of thumb is if there is significant stenosis-related left ventricular dysfunction or if there has been a substantial increase in peak aortic jet velocity (>0.3 m/sec within 1 year), indicating imminent onset of symptoms.2
In terms of surgical techniques, open aortic valve replacement is the conventional surgical therapy, with either a bioprosthesis or a mechanical valve (Fig. 60-7). The mechanical valves have a longer lifespan, but they require permanent anticoagulation and are therefore usually used in younger patients. Aortic balloon valvotomy can sometimes be used to relieve stenosis, although it is usually reserved for young patients without valve calcifications. In older adults, it is used only in cases of palliation in poor surgical candidates or as a temporary bridge to valve replacement in clinically unstable patients.4
AORTIC REGURGITATION
Prevalence
Trace or mild chronic aortic regurgitation is relatively common, affecting approximately 13% of men and 8.5% of women.9 Moderate or severe chronic aortic regurgitation is rare. The main predictors for chronic aortic regurgitation are increased age and male gender.
Etiology and Pathophysiology
Chronic aortic regurgitation is most commonly a result of atherosclerotic degeneration of a normal, tricuspid aortic valve or a congenital abnormality, namely, a bicuspid valve. Aortic valve leaflet structure and closure can also be affected by the diameter of the adjacent aorta, and thus diseases that cause dilation of the aortic root or annulus can also lead to regurgitation.1 These include aortic dissection (Stanford A or DeBakey type I), ascending aortic aneurysm, Marfan syndrome, and Ehlers-Danlos syndrome. Acute aortic regurgitation is most commonly a result of bacterial endocarditis, acute dissection, or severe thoracic trauma and usually has a poor prognosis.9
As chronic aortic regurgitation progresses from mild to severe, the initial minimal volume overload at end-diastole becomes a significant volume overload with concomitant pressure overload secondary to systolic hypertension. There is progressive dilation of the left ventricle as well as dilation of the aortic root, which in turn exacerbates the regurgitation.9 This progression parallels the path from a compensated state to a decompensated state and simultaneous development of symptoms.
Manifestations of Disease
Imaging Indications
Although transthoracic echocardiography is the most important and widely used diagnostic imaging modality in evaluation of aortic regurgitation, it has inherent limitations, the most prominent being operator dependence, interobserver variability, and susceptibility to a patient’s morphologic characteristics.7 During the last few years, both MDCT and MRI have been increasingly applied to the evaluation of aortic regurgitation.
Imaging Techniques
Radiography
Aortic regurgitation is commonly associated with aortic root disease and eventually, if untreated, causes left ventricular dilation due to the high end-diastolic volume (Fig. 60-8). Findings on plain chest radiography therefore correspond to the severity of the regurgitation; the two most typical findings are cardiomegaly and ascending aortic dilation.5 Pulmonary venous congestion and edema do not usually occur until late in the natural progression of the disease or in the setting of acute severe regurgitation, such as can be seen with bacterial endocarditis (see Fig. 60-5).5
Echocardiography
Transthoracic echocardiography allows evaluation of morphology of both valve leaflets and the aortic root as well as characterization of left ventricular size and function. Doppler color flow mapping can be used to identify the presence of regurgitation initially and to gauge its severity.9
Computed Tomography
MDCT can quantify the left ventricular volume and function as well as the aortic root diameter, useful for following the course of aortic regurgitation. The main advantage of MDCT over echocardiography and MR, especially with the advent of the 64-slice scanner, is the excellent spatial resolution, which, as in aortic stenosis, allows better evaluation of valvular anatomy and morphology (see Fig. 60-4).7 Specifically, planimetric measurement of the regurgitant orifice area can be performed, enabling direct quantification of the area between the cusps that remains open during diastole. The severity of aortic regurgitation corresponds directly to the size of the orifice, and as the regurgitant orifice area increases, so too does the severity of the symptoms. A study has shown a high degree of sensitivity in differentiating between mild, moderate, and severe aortic regurgitation on the basis of the regurgitant orifice area, as determined independently by transthoracic echocardiography.10 Whereas both echocardiography and MR often measure the valvular dysfunction indirectly, by the regurgitant jet and the left ventricular volume measurements, MDCT can provide direct high-resolution visualization of the valve.11 The main disadvantage of CT with respect to echocardiography is radiation exposure to the patient (mean of 11 mSv), which is especially important in aortic regurgitation given the high rate of repeated studies needed to closely monitor the disease.10
Magnetic Resonance
Cardiac MR can be used to identify aortic regurgitation initially and to monitor its effects on left ventricular volume and function. As in aortic stenosis, SSFP cine MR is used to identify the signal void that represents the regurgitant flow jet, which in aortic regurgitation projects from the closed aortic valve back into the left ventricle during diastole (Fig. 60-9).5 In addition, actual quantification of the regurgitant volume can be performed by comparing the right and left ventricular volumes and subtracting the left ventricular volume from the right, assuming only one valve is regurgitant.5 During diastole, there is often observation of anterior motion of the anterior mitral leaflet as a result of turbulent regurgitant flow.7 Last, cine MR allows quantification of left ventricular volume and function, as these two parameters are crucial for treatment planning.
An additional method of quantification is phase contrast MR, in which a plane perpendicular to the blood flow is chosen at the level of the ascending aorta and main pulmonary artery. This method compares the stroke volumes during systole and can also identify and quantify the retrograde aortic flow during diastole.5
Treatment Options
Medical
Treatment options differ according to where the patient is along the spectrum of disease. On one end, the asymptomatic patients with normal systolic function and left ventricular size are usually monitored for development of symptoms or progressive left ventricular dilation or dysfunction. At the other end of the spectrum, symptomatic patients with chronic severe aortic regurgitation have a mortality rate that is greater than 10% per year and thus need to undergo surgical intervention unless it is contraindicated by severe comorbidities.9 The key question in terms of treatment is at which point an asymptomatic or minimally symptomatic patient should undergo surgery to prevent irreversible left ventricular function. Multiple studies during the past decade have pointed to better outcomes in patients with left ventricular ejection fractions above 55% or an end-systolic left ventricular diameter of less than 55 mm.9 This guideline, termed the 55 rule, can be used to select patients for surgery.9 Medical treatment, namely, the use of antihypertensives to reduce systolic hypertension and to improve left ventricular function, is limited to two groups of patients: those with severe chronic aortic regurgitation who are not surgical candidates; and those who are asymptomatic and in whom surgery might be delayed even longer.1
Surgical
As discussed before, aortic valve replacement or repair is usually reserved for symptomatic patients with severe aortic regurgitation or asymptomatic patients with left ventricular dysfunction. In addition, patients with severe aortic regurgitation undergoing planned coronary bypass or other aortic surgery should also undergo valve repair or replacement. Because regurgitation is associated with aortic root dilation in many patients, simultaneous aortic root reconstruction is performed as well.1
MITRAL STENOSIS
Definition
Mitral stenosis is defined as obstruction of the left ventricular outflow tract at the level of the mitral valve due to either a structural abnormality of the valve itself or a space-occupying lesion, such as a mass or a thrombus, that effectively prevents proper filling of the left ventricle during diastole.1
Prevalence and Epidemiology
With the dramatic decrease in incidence of rheumatic fever in the developed world, mitral stenosis, which is most commonly caused by rheumatic carditis, has become a rare entity in the United States, and most patients today who present with mitral stenosis in this country have in fact emigrated at some point from developing countries.12 In 2004, approximately 1500 patients in the United States underwent balloon mitral valvotomies, the most common interventional therapy for mitral stenosis, which gives a rough estimate of the prevalence of the severe form of the disease.12
Apart from rheumatic fever, mitral stenosis can be caused by congenital anomalies, severe mitral annular calcifications, left atrial myxomas, carcinoid syndrome, and obstructive left atrial thrombus. Although rates of rheumatic fever are equal between the genders, there is a female predominance of nearly 2 : 1 in presentation of isolated mitral stenosis, a discrepancy thought to be associated with the autoimmune nature of the process in the heart after infection with group A streptococcus.1
Etiology and Pathophysiology
The rheumatic process causes inflammation of the heart valves, which leads to thickening and calcification of the valve leaflets as well as to fusion of the valve commissure. This results in progressive narrowing of the valve area, which normally is between 4 and 5 cm2. Symptoms of mitral stenosis do not usually develop until the valve area has decreased to less than 2.5 cm2, at which point the free flow of blood between the left atrium and left ventricle is impeded and a pressure gradient develops across the valve.1 As the pressure gradient increases during diastole, it is transmitted in a retrograde fashion, in turn leading to left atrial enlargement, pulmonary venous hypertension, pulmonary arterial hypertension, and eventually, in severe cases, pulmonary regurgitation and right ventricular dilation. With worsening stenosis, there is also flow restriction causing decreased left ventricular output. The dilation of the left atrium can alter its electrophysiologic properties and can lead to atrial fibrillation, which in turn increases the risk of stroke and other arterial embolization.12 In most patients with untreated mitral stenosis, mortality is a result of progressive pulmonary and systemic congestion; the majority of the remaining patients die of either systemic or pulmonary embolism.1
Manifestations of Disease
Clinical Presentation
As in aortic stenosis or regurgitation, mild disease is often asymptomatic. In many patients, initial symptomatic presentation with mitral stenosis is directly attributable to the increased pressure gradient and lowered left ventricular output, resulting in nonspecific symptoms such as fatigue and dyspnea.1 In others, the symptoms during initial presentation are due to atrial fibrillation. An increase in heart rate causes a shortened diastole, which, given the hemodynamic obstruction at the level of the mitral valve, in turn leads to decreased left ventricular filling. Therefore, the initial symptoms and clinical manifestations occur during exercise, stress, or pregnancy.1
Imaging Indications
As in the evaluation of aortic valve disease, the diagnostic gold standard in identification, evaluation, grading, and monitoring of mitral stenosis remains echocardiography.12 With technologic advances in both MDCT and MRI, these modalities have also been applied to the evaluation of mitral stenosis.
Imaging Techniques
Radiography
Although understandably limited in utility, plain chest radiography can be a useful tool in initial evaluation of mitral stenosis by roughly assessing its severity, namely, by identifying pulmonary venous hypertension and the extent of left atrial enlargement (Fig. 60-10).5 The presence of pulmonary edema can also be noted. In addition, signs of concomitant tricuspid or aortic valvular disease can also be identified by evaluating for right-sided heart enlargement or ascending aortic prominence, respectively.5
Echocardiography
As noted before, two-dimensional echocardiography is the first-line modality for evaluation of mitral stenosis, given its ability to accurately assess mitral annular structure and function, valve leaflets, chordae tendineae, papillary muscles, and left ventricular size and function.13 As in other cardiac valvular disease, velocity of blood flow in the stenotic jet is used to quantify the gradient pressure by the modified Bernoulli equation, and mean pressure gradient across the valve and the valve orifice area are used to estimate severity of the disease (Fig. 60-11B).13
Computed Tomography
As in the case of aortic valve evaluation, the distinct advantage of MDCT in mitral valve imaging is the excellent spatial resolution it provides.7 This again allows better characterization of valve anatomy (Fig. 60-12). Evaluation of the valve area by planimetric measurement allows more accurate staging of the stenosis, and close morphologic assessment can help in deciding the type of therapy and its timing. Specifically, the mobility of the leaflets and the presence or absence of commissural calcifications and subvalvular or valvular thickening help assess the suitability of one of the main interventional therapies.1
Magnetic Resonance
Just as with aortic valvular disease, the advantage of MR is in its temporal resolution. Cine MR imaging is used to identify the flow jet signal void, which in the case of mitral stenosis is seen projecting into the left ventricle during diastole. With phase contrast MR, the peak pressure gradient across the mitral valve can be calculated by measuring the peak velocity of the stenotic jet during diastole and then applying the modified Bernoulli equation. New valve tracking algorithms are currently on the way to account for the significant through-plane motion of the mitral valve during the cardiac cycle, which can affect the phase contrast MR measurements. Whereas there is good correlation between MR and echocardiography in assessing the pressure gradient, MR tends to underestimate the value because of a lower sampling rate.5
Treatment Options
Medical
Because mitral stenosis is almost entirely a surgical disease, medical therapy is mostly limited to antibiotic prophylaxis against bacterial endocarditis and to rate control and anticoagulation in cases of atrial fibrillation.1 Keeping a steady sinus rhythm is key in preventing a shortening of the diastolic left ventricular filling period. Patients are also advised to avoid physical exertion, which has the same effect by raising the heart rate.1
Surgical
Balloon Mitral Valvotomy
The least invasive of the procedures, this involves percutaneous placement of a balloon across the mitral valve and inflating it to “fracture” the fused leaflets at the commissures, allowing better opening of the valve during diastole. Balloon mitral valvotomy is most successful in patients with good leaf mobility, limited valve calcification, and mild leaflet thickening. The main contraindication to the procedure is a thrombus in the left atrium.12
Open Commissurotomy
Whereas open surgical commissurotomy is more invasive than balloon valvotomy, it enables a direct visual inspection of the valve apparatus and allows a more exact separation of the commissures and debulking of the valvular calcifications.1 Permitting conservation of the native valve, this procedure can also include simultaneous amputation of the left atrial appendage, which results in a decreased risk of thrombus development. As in balloon mitral valvotomy, suitability of the procedure depends on valvular morphology.12
Mitral Valve Replacement
In the presence of severe fibrosis, valvular calcification, and subvalvular disease, the native valve cannot be conserved and replacement is necessary. Because of the inherent intraoperative risk, the usual presence of comorbidities, and the potential short- and long-term complications of having a prosthetic valve, mitral valve replacement is usually performed as the last resort, often after failed attempts at balloon valvotomy or even open commissurotomy. The type of prosthesis depends on the patient’s age, the risk of long-term anticoagulation, and the patient’s preference.12
MITRAL REGURGITATION
Etiology and Pathophysiology
Mitral valve prolapse, in which myxomatous degeneration of the mitral leaflets leads to their ballooning and “billowing” into the left atrium during systole, is quite common, affecting 2.3% of the population, although only a small percentage of these develop significant regurgitation.14 Secondary mitral regurgitation is seen in approximately 30% of patients with coronary artery disease, although again, the severity is usually mild.15 Nevertheless, ischemic mitral regurgitation, even if it is mild, worsens prognosis after myocardial infarction.16 Dilated cardiomyopathy results in a change in the size and shape of the left atrium and ventricle and can also cause dilation of the mitral annulus, all of which lead to poor coaptation of the mitral leaflets.
In acute mitral regurgitation, there is a sudden volume overload on both the left atrium and left ventricle. In the absence of adaptive left ventricular hypertrophy, the cardiac output decreases. In addition, the left atrium is unable to handle the regurgitant volume, and the overload is transferred to the pulmonary veins, resulting in pulmonary vascular congestion.1 In severe cases, both cardiogenic shock and pulmonary edema are evident. In chronic mitral regurgitation, the patients remain asymptomatic for years, during which there evolves compensatory left ventricular enlargement, which results in increased left ventricular end-diastolic volume that can maintain the increased stroke volume needed to sustain the cardiac output, although eventually there is left ventricular dysfunction as well.1
Manifestations of Disease
Clinical Presentation
As previously noted, most patients with chronic mitral regurgitation, including many with severe disease, remain asymptomatic. Some who do present clinically do so with associated entities, such as infective endocarditis and atrial fibrillation, that point to the underlying valvular dysfunction. On physical examination, the classic sign is an apical holosystolic murmur radiating to the axilla.1 This is then usually confirmed on echocardiography. Patients who eventually progress to symptomatic disease present with the expected findings of left ventricular dysfunction and pulmonary venous congestion, namely, dyspnea, exercise intolerance, and fatigue.
Imaging Indications
As with all cardiac valvular disease, echocardiography is the modality of choice for diagnosis, grading, and monitoring of mitral regurgitation. Clinicians will use the echocardiographic grading or severity, in conjunction with clinical signs and symptoms, to make decisions about the necessity and timing of surgical intervention.17 Both cardiac MR and MDCT serve as adjunct imaging tools in case the transthoracic echocardiographic examination is technically difficult or if additional anatomic information is needed for surgical planning.
Imaging Techniques
Radiography
With severe acute mitral regurgitation, the characteristic finding on chest radiography is pulmonary edema in the setting of a normal heart size.16 With long-standing chronic mitral regurgitation, there is enlargement of both the left atrium and ventricle due to chronic volume overload.5
Echocardiography
The main goals of echocardiography in mitral regurgitation are to establish an etiology and mechanism for the regurgitation, to grade its severity, and to determine the need for surgical intervention. Compared with evaluation of mitral stenosis, the size and velocity of the regurgitant jet in mitral regurgitation do not correlate well with the clinical severity of the disease. A more important factor is the size of the left ventricle and its systolic function, as measured by the end-systolic diameter and ejection fraction, respectively (see Fig. 60-11A).13
Treatment Options
Medical
As most patients incidentally diagnosed with mitral regurgitation never become symptomatic, the goals of nonsurgical treatment are to monitor the severity of the disease and to prevent associated complications.14 In mild to moderate mitral regurgitation without left ventricular dysfunction, echocardiography does not need to be performed on a regular basis, and patients are instructed to report any change in exercise tolerance. In moderate to severe mitral regurgitation, echocardiography should be performed on a yearly basis, unless there is a change in physical examination findings or symptoms appear. In addition, for all patients with known mitral regurgitation, antibiotic prophylaxis to prevent endocarditis should be administered before any invasive procedure, including dental work. In patients with atrial fibrillation, rate control and anticoagulation therapy is needed.
Surgical
There is consensus for performing mitral valve repair or replacement for patients with one of three entities: acute mitral regurgitation, symptomatic chronic mitral regurgitation, or asymptomatic chronic mitral regurgitation with a certain level of left ventricular dysfunction as measured by ejection fraction (<60%) and end-systolic left ventricular diameter (>45 mm).18 In general, surgery in asymptomatic patients should be timed before the development of irreversible systolic dysfunction.14 Surgical treatment of secondary mitral regurgitation, as in ischemia or dilated cardiomyopathy, is controversial because the problem lies not within the valve itself but rather with the supporting structures.18 As with mitral stenosis, the choice between repair and replacement depends on the state of the valve leaflets. Repair, if possible, is obviously preferred, given the advantage of avoiding long-term anticoagulation and preserving the continuity of the papillary muscles and mitral annulus. On average, operative mortality for repair is 1% to 2%, compared with 5% to 10% with replacement.18
1 American College of Cardiology/American Heart Association Task Force on Practice GuidelinesSociety of Cardiovascular AnesthesiologistsSociety for Cardiovascular Angiography and InterventionsSociety of Thoracic SurgeonsBonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84-e231.
2 Baumgartner H. Aortic stenosis: medical and surgical management. Heart. 2005;91:1483-1488.
3 Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316-3326.
4 Cowell SJ, Newby DE, Boon NA, et al. Calcific aortic stenosis: same old story? Age Ageing. 2004;33:538-544.
5 Webb WR, Higgins CB. Thoracic Imaging: Pulmonary and Cardiovascular Radiology. Philadelphia: Lippincott Williams & Wilkins; 2004.
6 Bouvier E, Logeart D, Sablayrolles JL, et al. Diagnosis of aortic valvular stenosis by multislice cardiac computed tomography. Eur Heart J. 2006;27:3033-3038.
7 Vogel-Claussen J, Pannu H, Spevak PJ, et al. Cardiac valve assessment with MR imaging and 64-section multi-detector row CT. Radiographics. 2006;26:1769-1784.
8 Feuchtner GM, Muller S, Bonatti J, et al. Sixty-four slice CT evaluation of aortic stenosis using planimetry of the aortic valve area. AJR Am J Roentgenol. 2007;189:197-203.
9 Bekeredjian R, Grayburn PA. Valvular heart disease: aortic regurgitation. Circulation. 2005;112:125-134.
10 Alkadhi H, Desbiolles L, Husmann L, et al. Aortic regurgitation: assessment with 64-section CT. Radiology. 2007;245:111-121.
11 Gilkeson RC, Markowitz AH, Balgude A, et al. MDCT evaluation of aortic valvular disease. AJR Am J Roentgenol. 2006;186:350-360.
12 Carabello BA. Modern management of mitral stenosis. Circulation. 2005;112:432-437.
13 Mora S, Wu K. Echocardiography in cardiac surgery. In: Yuh D, Vricella L, Baumgartner W, editors. The Johns Hopkins Manual of Cardiothoracic Surgery. New York: McGraw-Hill Medical; 2007:943.
14 Keeffe BG, Otto CM. Mitral regurgitation. Minerva Cardioangiol. 2003;51:29-39.
15 Levine RA, Schwammenthal E. Ischemic mitral regurgitation on the threshold of a solution: from paradoxes to unifying concepts. Circulation. 2005;112:745-758.
16 Filsoufi F, Salzberg SP, Adams DH. Current management of ischemic mitral regurgitation. Mt Sinai J Med. 2005;72:105-115.
17 Irvine T, Li XK, Sahn DJ, et al. Assessment of mitral regurgitation. Heart. 2002;88(Suppl 4):iv11-iv19.
18 Otto CM. Timing of surgery in mitral regurgitation. Heart. 2003;89:100-105.

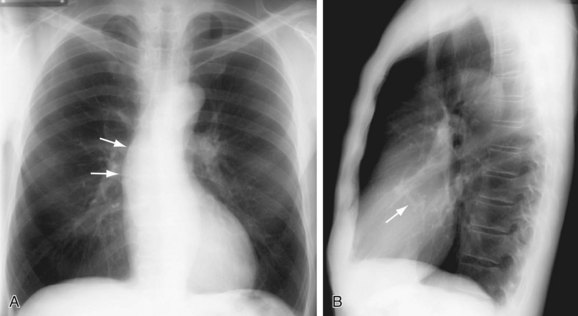
 FIGURE 60-1
FIGURE 60-1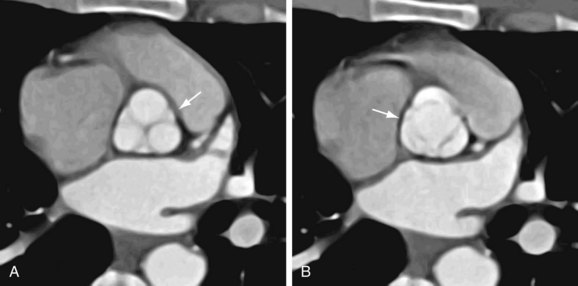
 FIGURE 60-2
FIGURE 60-2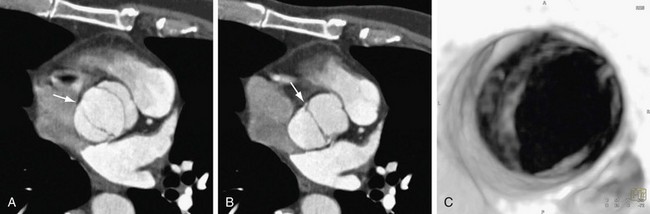
 FIGURE 60-3
FIGURE 60-3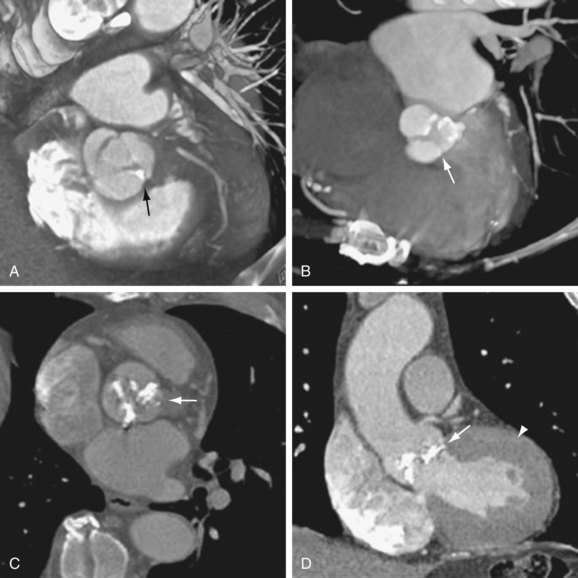
 FIGURE 60-4
FIGURE 60-4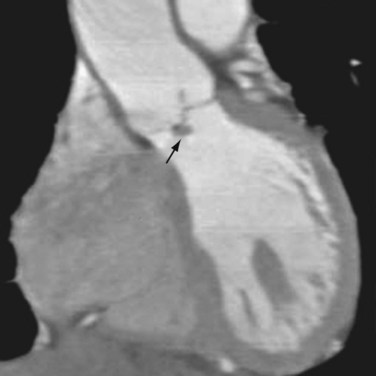
 FIGURE 60-5
FIGURE 60-5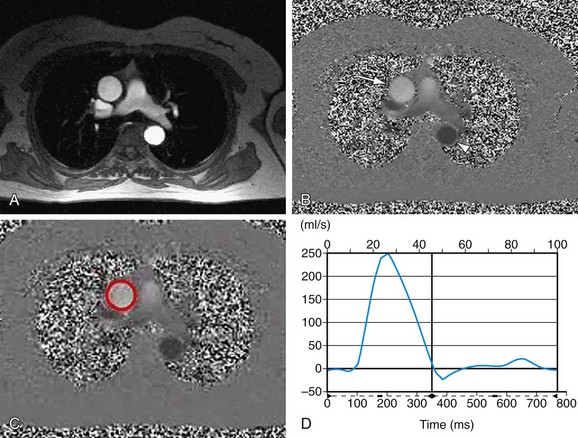
 FIGURE 60-6
FIGURE 60-6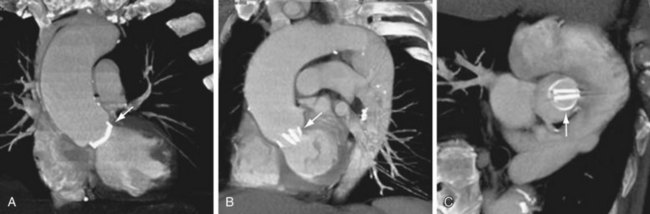
 FIGURE 60-7
FIGURE 60-7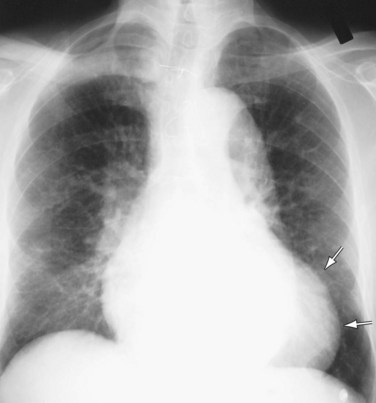
 FIGURE 60-8
FIGURE 60-8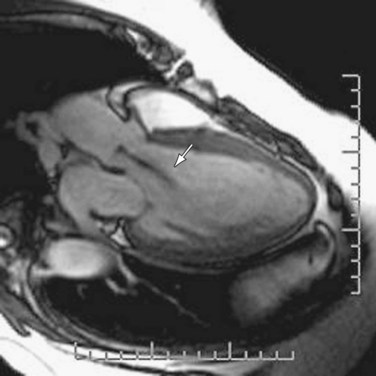
 FIGURE 60-9
FIGURE 60-9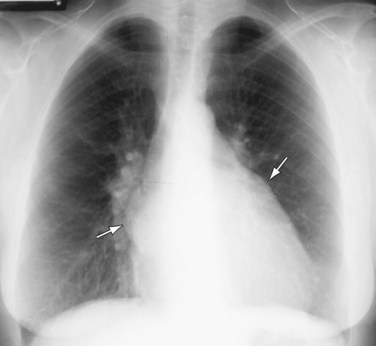
 FIGURE 60-10
FIGURE 60-10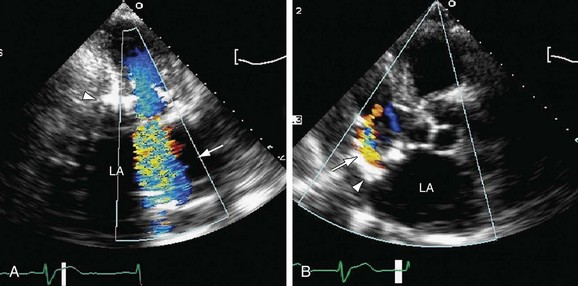
 FIGURE 60-11
FIGURE 60-11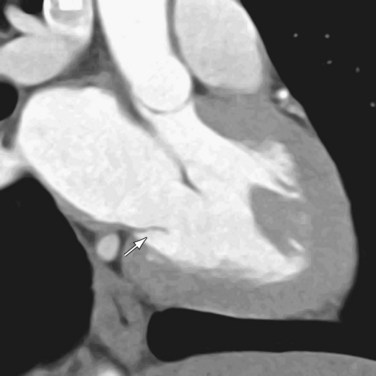
 FIGURE 60-12
FIGURE 60-12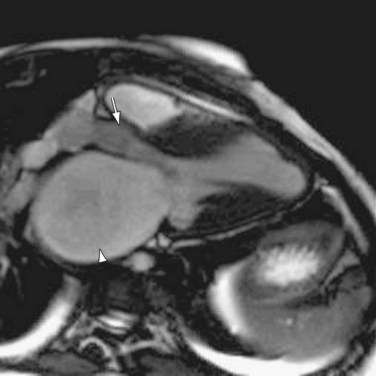
 FIGURE 60-13
FIGURE 60-13

