FIGURE 202-1 Acid-fast bacillus smear showing M. tuberculosis bacilli. (Courtesy of the Centers for Disease Control and Prevention, Atlanta.)
EPIDEMIOLOGY
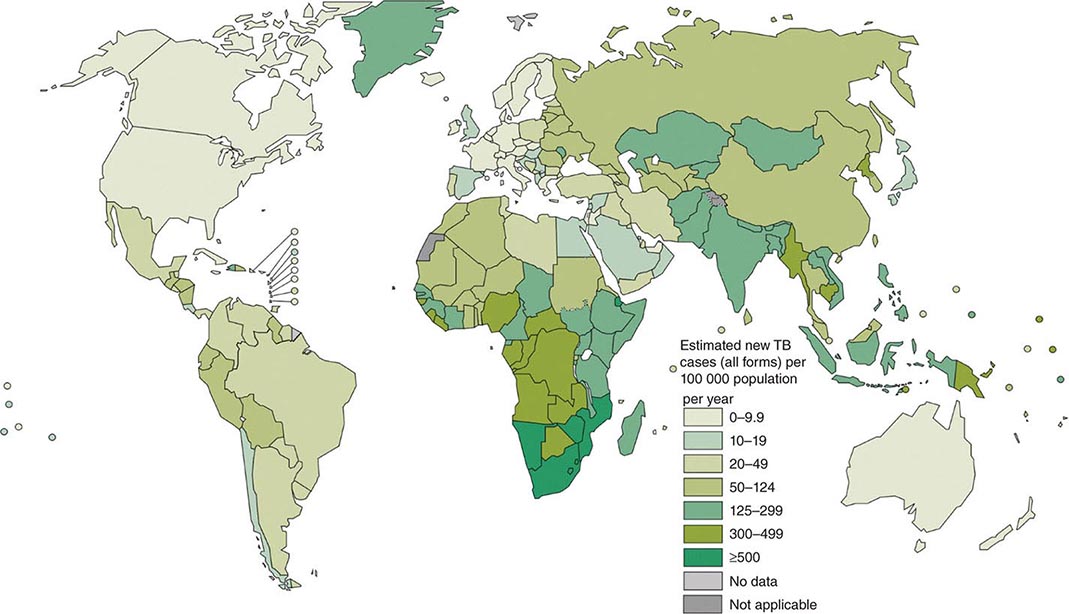
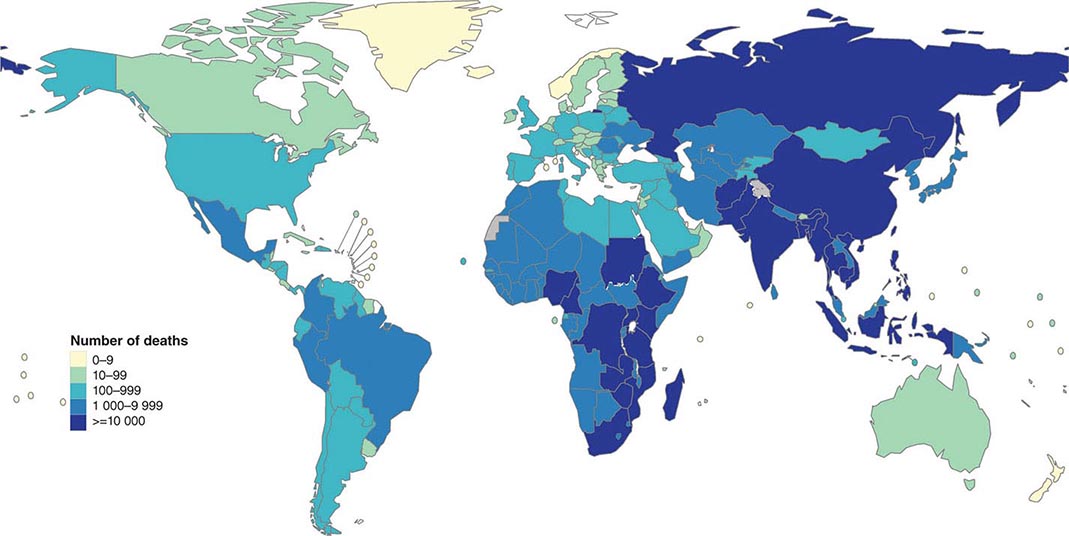
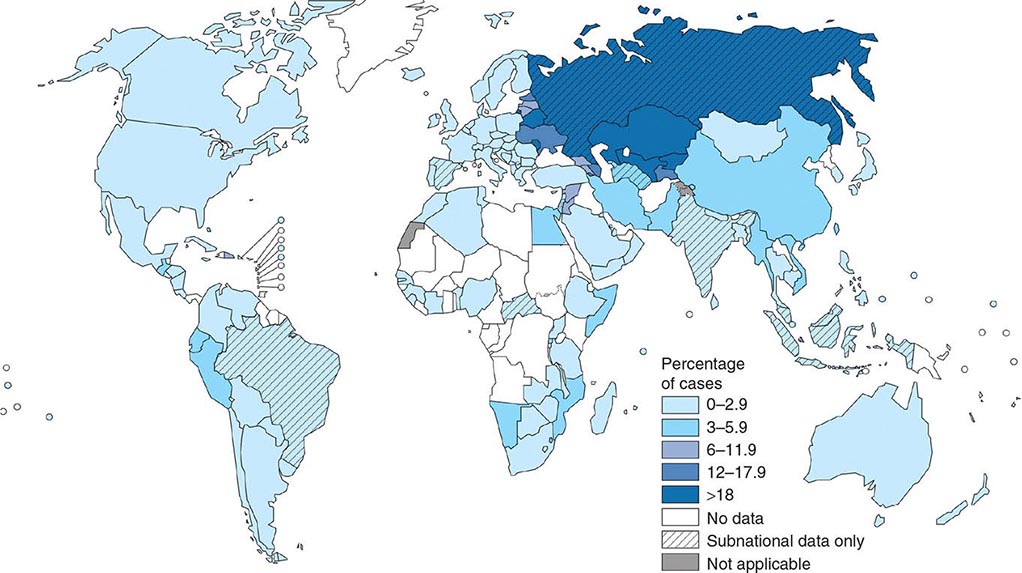
![]() More than 5.7 million new cases of TB (all forms, both pulmonary and extrapulmonary) were reported to the World Health Organization (WHO) in 2013; 95% of cases were reported from developing countries. However, because of insufficient case detection and incomplete notification, reported cases may represent only about two-thirds of the total estimated cases. The WHO estimated that 9 million (range, 8.6–9.4 million) new cases of TB occurred worldwide in 2013, 95% of them in developing countries of Asia (5 million), Africa (2.6 million), the Middle East (0.7 million), and Latin America (0.3 million). It is further estimated that 1.49 million (range, 1.32–1.67 million) deaths from TB, including 0.36 million among people living with HIV infection, occurred in 2013, 96% of them in developing countries. Estimates of TB incidence rates (per 100,000 population) and numbers of TB-related deaths in 2013 are depicted in Figs. 202-2 and 202-3, respectively. During the late 1980s and early 1990s, numbers of reported cases of TB increased in industrialized countries. These increases were related largely to immigration from countries with a high incidence of TB; the spread of the HIV epidemic; social problems, such as increased urban poverty, homelessness, and drug abuse; and dismantling of TB services. During the past few years, numbers of reported cases have begun to decline again or have stabilized in most industrialized nations. In the United States, with the re-establishment of stronger control programs, the decline resumed in 1993 and has since been maintained. In 2013, 9582 cases of TB (3.0 cases/100,000 population) were reported to the Centers for Disease Control and Prevention (CDC).
More than 5.7 million new cases of TB (all forms, both pulmonary and extrapulmonary) were reported to the World Health Organization (WHO) in 2013; 95% of cases were reported from developing countries. However, because of insufficient case detection and incomplete notification, reported cases may represent only about two-thirds of the total estimated cases. The WHO estimated that 9 million (range, 8.6–9.4 million) new cases of TB occurred worldwide in 2013, 95% of them in developing countries of Asia (5 million), Africa (2.6 million), the Middle East (0.7 million), and Latin America (0.3 million). It is further estimated that 1.49 million (range, 1.32–1.67 million) deaths from TB, including 0.36 million among people living with HIV infection, occurred in 2013, 96% of them in developing countries. Estimates of TB incidence rates (per 100,000 population) and numbers of TB-related deaths in 2013 are depicted in Figs. 202-2 and 202-3, respectively. During the late 1980s and early 1990s, numbers of reported cases of TB increased in industrialized countries. These increases were related largely to immigration from countries with a high incidence of TB; the spread of the HIV epidemic; social problems, such as increased urban poverty, homelessness, and drug abuse; and dismantling of TB services. During the past few years, numbers of reported cases have begun to decline again or have stabilized in most industrialized nations. In the United States, with the re-establishment of stronger control programs, the decline resumed in 1993 and has since been maintained. In 2013, 9582 cases of TB (3.0 cases/100,000 population) were reported to the Centers for Disease Control and Prevention (CDC).
FIGURE 202-2 Estimated tuberculosis (TB) incidence rates (per 100,000 population) in 2013. The designations used and the presentation of material on this map do not imply the expression of any opinion whatsoever on the part of the World Health Organization (WHO) concerning the legal status of any country, territory, city, or area or of its authorities or concerning the delimitation of its frontiers or boundaries. Dotted, dashed, and white lines represent approximate border lines for which there may not yet be full agreement. (Courtesy of the Global TB Programme, WHO; with permission.)
FIGURE 202-3 Estimated numbers of tuberculosis-related deaths in 2013. (See disclaimer in Fig. 202-2. Courtesy of the Global TB Programme, WHO; with permission.)
In the United States, TB is uncommon among young adults of European descent, who have only rarely been exposed to M. tuberculosis infection during recent decades. In contrast, because of a high risk of transmission in the past, the prevalence of latent M. tuberculosis infection (LTBI) is relatively high among elderly whites. In general, adults ≥65 years of age have the highest incidence rate per capita (4.9 cases/100,000 population in 2013) and children <14 years of age the lowest (0.8 case/100,000 population). Blacks account for the highest proportion of cases (37%; 1257 cases in 2013) among U.S.-born persons. TB in the United States is also a disease of adult members of the HIV-infected population, the foreign-born population (64.6% of all cases in 2013), and disadvantaged/marginalized populations. Of the 6193 cases reported among foreign-born persons in 2013, 37% occurred in persons from the Americas and 32% occurred in persons born in the Western Pacific region. Overall, the highest rates per capita were among Asian Americans (18.7 cases/100,000 population). A total of 536 deaths were caused by TB in the United States in 2011. In Canada in 2013, 1638 TB cases were reported (4.7 cases/100,000 population); 70% (1145) of these cases occurred in foreign-born persons and 19% (309 cases) occurred in members of the Canadian aboriginal peoples, whose per capita rate is disproportionately high (23.4 cases/100,000 population) with a peak in the Nunavut territory of 143 cases/100,000 population—a rate similar to that in many highly endemic countries. Similarly, in Europe, TB has reemerged as an important public health problem, mainly as a result of cases among immigrants from high-incidence countries and among marginalized populations, often in large urban settings like London; in 2013, 41% of all cases reported from the United Kingdom occurred in London, and the rate per capita (36 cases/100,000 population) was similar to that in some middle-income countries. In most Western European countries, there are more cases annually among foreign-born than native populations.
Recent data on global trends indicate that in 2013 the TB incidence was stable or falling in most regions; this trend began in the early 2000s and appears to have continued, with an average annual decline of 2% globally. This global decrease is explained largely by the simultaneous reduction in TB incidence in sub-Saharan Africa, where rates had risen steeply since the 1980s as a result of the HIV epidemic and the lack of capacity of health systems and services to deal with the problem effectively, and in Eastern Europe, where incidence increased rapidly during the 1990s because of a deterioration in socioeconomic conditions and the health care infrastructure (although, after peaking in 2001, incidence in Eastern Europe has since declined slowly).
Of the estimated 9 million new cases of TB in 2013, 13% (1.1 million) were associated with HIV infection, and 78% of these HIV-associated cases occurred in Africa. An estimated 0.36 million persons with HIV-associated TB died in 2013. Furthermore, an estimated 480,000 cases (range, 350,000–610,000) of multidrug-resistant TB (MDR-TB)—a form of the disease caused by bacilli resistant at least to isoniazid and rifampin—occurred in 2013. Only 28% of these cases were diagnosed because of a lack of culture and drug-susceptibility testing capacity in most settings worldwide. The countries of the former Soviet Union have reported the highest proportions of MDR disease among new TB cases (up to 35–40% in some regions of Russia and Belarus). Overall, 60% of all MDR-TB cases occur in China, India, the Russian Federation, Pakistan, and Ukraine. Since 2006, 100 countries, including the United States, have reported cases of extensively drug-resistant TB (XDR-TB), in which MDR-TB is compounded by additional resistance to the most powerful second-line anti-TB drugs (fluoroquinolones and at least one of the injectable drugs amikacin, kanamycin, and capreomycin). Up to 10% of the MDR-TB cases worldwide may actually be XDR-TB, but the vast majority of XDR-TB cases remain undiagnosed because reliable methods for drug susceptibility testing are lacking and laboratory capacity is limited. Lately, cases deemed resistant to all anti-TB drugs have been reported from countries such as India, Italy, and Iran; however, this information must be interpreted with caution because drug susceptibility testing for several second-line drugs is neither accurate nor reproducible.
FROM EXPOSURE TO INFECTION
M. tuberculosis is most commonly transmitted from a person with infectious pulmonary TB by droplet nuclei, which are aerosolized by coughing, sneezing, or speaking. The tiny droplets dry rapidly; the smallest (<5–10 μm in diameter) may remain suspended in the air for several hours and may reach the terminal air passages when inhaled. There may be as many as 3000 infectious nuclei per cough. Other routes of transmission of tubercle bacilli (e.g., through the skin or the placenta) are uncommon and of no epidemiologic significance. The probability of contact with a person who has an infectious form of TB, the intimacy and duration of that contact, the degree of infectiousness of the case, and the shared environment in which the contact takes place are all important determinants of the likelihood of transmission. Several studies of close-contact situations have clearly demonstrated that TB patients whose sputum contains AFB visible by microscopy (sputum smear–positive cases) are the most likely to transmit the infection. The most infectious patients have cavitary pulmonary disease or, much less commonly, laryngeal TB and produce sputum containing as many as 105–107 AFB/mL. Patients with sputum smear–negative/culture-positive TB are less infectious, although they have been responsible for up to 20% of transmission in some studies in the United States. Those with culture-negative pulmonary TB and extrapulmonary TB are essentially noninfectious. Because persons with both HIV infection and TB are less likely to have cavitations, they may be less infectious than persons without HIV co-infection. Crowding in poorly ventilated rooms is one of the most important factors in the transmission of tubercle bacilli because it increases the intensity of contact with a case.
The risk of acquiring M. tuberculosis infection is determined mainly by exogenous factors. Because of delays in seeking care and in making a diagnosis, it is generally estimated that, in high-prevalence settings, up to 20 contacts may be infected by each AFB-positive case before the index case is diagnosed.
FROM INFECTION TO DISEASE
Unlike the risk of acquiring infection with M. tuberculosis, the risk of developing disease after being infected depends largely on endogenous factors, such as the individual’s innate immunologic and nonimmunologic defenses and the level at which the individual’s cell-mediated immunity (CMI) is functioning. Clinical illness directly following infection is classified as primary TB and is common among children in the first few years of life and among immunocompromised persons. Although primary TB may be severe and disseminated, it generally is not associated with high-level transmissibility. When infection is acquired later in life, the chance is greater that the mature immune system will contain it at least temporarily. Bacilli, however, may persist for years before reactivating to produce secondary (or postprimary) TB, which, because of frequent cavitation, is more often infectious than is primary disease. Overall, it is estimated that up to 10% of infected persons will eventually develop active TB in their lifetime—half of them during the first 18 months after infection. The risk is much higher among HIV-infected persons. Reinfection of a previously infected individual, which is common in areas with high rates of TB transmission, may also favor the development of disease. At the height of the TB resurgence in the United States in the early 1990s, molecular typing and comparison of strains of M. tuberculosis suggested that up to one-third of cases of active TB in some inner-city communities were due to recent transmission rather than to reactivation of old latent infection. Age is an important determinant of the risk of disease after infection. Among infected persons, the incidence of TB is highest during late adolescence and early adulthood; the reasons are unclear. The incidence among women peaks at 25–34 years of age. In this age group, rates among women may be higher than those among men, whereas at older ages the opposite is true. The risk increases in the elderly, possibly because of waning immunity and comorbidity.
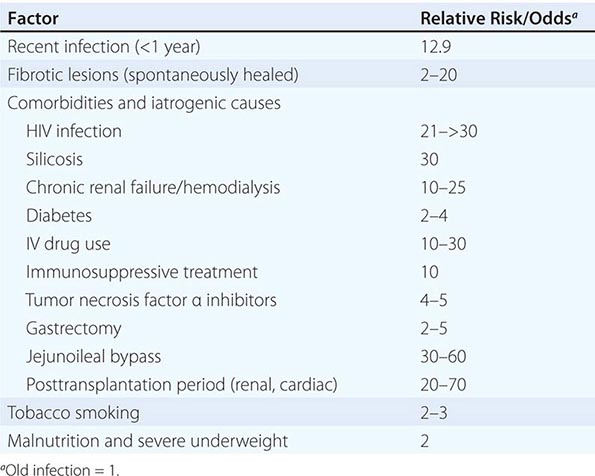
A variety of diseases and conditions favor the development of active TB (Table 202-1). In absolute terms, the most potent risk factor for TB among infected individuals is clearly HIV co-infection, which suppresses cellular immunity. The risk that LTBI will proceed to active disease is directly related to the patient’s degree of immunosuppression. In a study of HIV-infected, tuberculin skin test (TST)–positive persons, this risk varied from 2.6 to 13.3 cases/100 person-years and increased as the CD4+ T cell count decreased.
|
RISK FACTORS FOR ACTIVE TUBERCULOSIS IN PERSONS WHO HAVE BEEN INFECTED WITH TUBERCLE BACILLI |
NATURAL HISTORY OF DISEASE
Studies conducted in various countries before the advent of chemotherapy showed that untreated TB is often fatal. About one-third of patients died within 1 year after diagnosis, and more than 50% died within 5 years. The 5-year mortality rate among sputum smear–positive cases was 65%. Of the survivors at 5 years, ~60% had undergone spontaneous remission, while the remainder were still excreting tubercle bacilli. With effective, timely, and proper chemotherapy, patients have a very high chance of being cured. However, improper use of anti-TB drugs, while reducing mortality rates, may also result in large numbers of chronic infectious cases, often with drug-resistant bacilli.
PATHOGENESIS AND IMMUNITY
INFECTION AND MACROPHAGE INVASION
The interaction of M. tuberculosis with the human host begins when droplet nuclei containing viable microorganisms propelled into the air by infectious patients are inhaled by a close bystander. Although the majority of inhaled bacilli are trapped in the upper airways and expelled by ciliated mucosal cells, a fraction (usually <10%) reach the alveoli, a unique immunoregulatory environment. There, alveolar macrophages that have not yet been activated (prototypic alternatively activated macrophages) phagocytose the bacilli. Adhesion of mycobacteria to macrophages results largely from binding of the bacterial cell wall to a variety of macrophage cell-surface molecules, including complement receptors, the mannose receptor, the immunoglobulin GFcγ receptor, and type A scavenger receptors. Phagocytosis is enhanced by complement activation leading to opsonization of bacilli with C3 activation products such as C3b and C3bi. (Bacilli are resistant to complement-mediated lysis.) Binding of certain receptors, such as the mannose receptor, regulates postphagocytic events such as phagosome–lysosome fusion and inflammatory cytokine production. After a phagosome forms, the survival of M. tuberculosis within it seems to depend in part on reduced acidification due to lack of assembly of a complete vesicular proton-adenosine triphosphatase. A complex series of events is generated by the bacterial cell-wall lipoglycan lipoarabinomannan (ManLAM). ManLAM inhibits the intracellular increase of Ca2+. Thus, the Ca2+/calmodulin pathway (leading to phagosome–lysosome fusion) is impaired, and the bacilli survive within the phagosomes. The M. tuberculosis phagosome has been found to inhibit the production of phosphatidylinositol 3-phosphate (PI3P). Normally, PI3P earmarks phagosomes for membrane sorting and maturation, including phagolysosome formation, which would destroy the bacteria. Bacterial factors have also been found to block the host defense of autophagy, in which the cell sequesters the phagosome in a double-membrane vesicle (autophagosome) that is destined to fuse with lysosomes. If the bacilli are successful in arresting phagosome maturation, then replication begins and the macrophage eventually ruptures and releases its bacillary contents. Other uninfected phagocytic cells are then recruited to continue the infection cycle by ingesting dying macrophages and their bacillary content, thus in turn becoming infected themselves and expanding the infection.
VIRULENCE OF TUBERCLE BACILLI
![]() M. tuberculosis must be viewed as a complex formed by a multitude of strains that differ in virulence and are capable of producing a variety of manifestations of disease. Since the elucidation of the M. tuberculosis genome in 1998, large mutant collections have been generated, and many bacterial genes that contribute to M. tuberculosis virulence have been found. Different patterns of virulence defects have been defined in various animal models—predominantly mice but also guinea pigs, rabbits, and nonhuman primates. The katG gene encodes for a catalase/peroxidase enzyme that protects against oxidative stress and is required for isoniazid activation and subsequent bactericidal activity. Region of difference 1 (RD1) is a 9.5-kb locus that encodes two key small protein antigens—early secretory antigen-6 (ESAT-6) and culture filtrate protein-10 (CFP-10)—as well as a putative secretion apparatus that may facilitate their egress; the absence of this locus in the vaccine strain M. bovis bacille Calmette-Guérin (BCG) has been shown to be a key attenuating mutation. The validity of a recent observation in M. marinum needs to be confirmed in M. tuberculosis; in M. marinum, a mutation in the RD1 virulence locus encoding the ESX1 secretion system impairs the capacity of apoptotic macrophages to recruit uninfected cells for further rounds of infection. The results are less replication and fewer new granulomas. Mutants lacking key enzymes of bacterial biosynthesis become auxotrophic for the missing substrate and often are totally unable to proliferate in animals; these include the leuCD and panCD mutants, which require leucine and pantothenic acid, respectively. The isocitrate lyase gene icl1 encodes a key step in the glyoxylate shunt that facilitates bacterial growth on fatty acid substrates; this gene is required for long-term persistence of M. tuberculosis infection in mice with chronic TB. M. tuberculosis mutants in regulatory genes such as sigma factor C and sigma factor H (sigC and sigH) are associated with normal bacterial growth in mice, but they fail to elicit full tissue pathology. Finally, the mycobacterial protein CarD (expressed by the carD gene) seems essential for the control of rRNA transcription that is required for replication and persistence in the host cell. Its loss exposes mycobacteria to oxidative stress, starvation, DNA damage, and ultimately sensitivity to killing by a variety of host mutagens and defensive mechanisms.
M. tuberculosis must be viewed as a complex formed by a multitude of strains that differ in virulence and are capable of producing a variety of manifestations of disease. Since the elucidation of the M. tuberculosis genome in 1998, large mutant collections have been generated, and many bacterial genes that contribute to M. tuberculosis virulence have been found. Different patterns of virulence defects have been defined in various animal models—predominantly mice but also guinea pigs, rabbits, and nonhuman primates. The katG gene encodes for a catalase/peroxidase enzyme that protects against oxidative stress and is required for isoniazid activation and subsequent bactericidal activity. Region of difference 1 (RD1) is a 9.5-kb locus that encodes two key small protein antigens—early secretory antigen-6 (ESAT-6) and culture filtrate protein-10 (CFP-10)—as well as a putative secretion apparatus that may facilitate their egress; the absence of this locus in the vaccine strain M. bovis bacille Calmette-Guérin (BCG) has been shown to be a key attenuating mutation. The validity of a recent observation in M. marinum needs to be confirmed in M. tuberculosis; in M. marinum, a mutation in the RD1 virulence locus encoding the ESX1 secretion system impairs the capacity of apoptotic macrophages to recruit uninfected cells for further rounds of infection. The results are less replication and fewer new granulomas. Mutants lacking key enzymes of bacterial biosynthesis become auxotrophic for the missing substrate and often are totally unable to proliferate in animals; these include the leuCD and panCD mutants, which require leucine and pantothenic acid, respectively. The isocitrate lyase gene icl1 encodes a key step in the glyoxylate shunt that facilitates bacterial growth on fatty acid substrates; this gene is required for long-term persistence of M. tuberculosis infection in mice with chronic TB. M. tuberculosis mutants in regulatory genes such as sigma factor C and sigma factor H (sigC and sigH) are associated with normal bacterial growth in mice, but they fail to elicit full tissue pathology. Finally, the mycobacterial protein CarD (expressed by the carD gene) seems essential for the control of rRNA transcription that is required for replication and persistence in the host cell. Its loss exposes mycobacteria to oxidative stress, starvation, DNA damage, and ultimately sensitivity to killing by a variety of host mutagens and defensive mechanisms.
INNATE RESISTANCE TO INFECTION
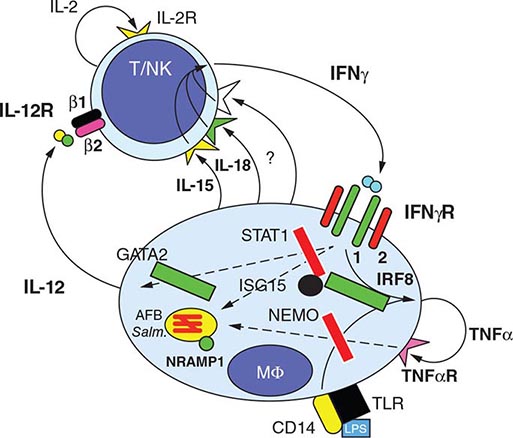
![]() Several observations suggest that genetic factors play a key role in innate nonimmune resistance to infection with M. tuberculosis and the development of disease. The existence of this resistance, which is polygenic in nature, is suggested by the differing degrees of susceptibility to TB in different populations. In mice, a gene called Nramp1 (natural resistance–associated macrophage protein 1) plays a regulatory role in resistance/susceptibility to mycobacteria. The human homologue NRAMP1, which maps to chromosome 2q, may play a role in determining susceptibility to TB, as is suggested by a study among West Africans. Studies of mouse genetics identified a novel host resistance gene, ipr1, which is encoded within the sst1 locus; ipr1 encodes an interferon (IFN)–inducible nuclear protein that interacts with other nuclear proteins in macrophages primed with IFNs or infected by M. tuberculosis. In addition, polymorphisms in multiple genes, such as those encoding for various major histocompatibility complex (MHC) alleles, IFN-γ, T cell growth factor β, interleukin (IL) 10, mannose-binding protein, IFN-γ receptor, Toll-like receptor 2, vitamin D receptor, and IL-1, have been associated with susceptibility to TB.
Several observations suggest that genetic factors play a key role in innate nonimmune resistance to infection with M. tuberculosis and the development of disease. The existence of this resistance, which is polygenic in nature, is suggested by the differing degrees of susceptibility to TB in different populations. In mice, a gene called Nramp1 (natural resistance–associated macrophage protein 1) plays a regulatory role in resistance/susceptibility to mycobacteria. The human homologue NRAMP1, which maps to chromosome 2q, may play a role in determining susceptibility to TB, as is suggested by a study among West Africans. Studies of mouse genetics identified a novel host resistance gene, ipr1, which is encoded within the sst1 locus; ipr1 encodes an interferon (IFN)–inducible nuclear protein that interacts with other nuclear proteins in macrophages primed with IFNs or infected by M. tuberculosis. In addition, polymorphisms in multiple genes, such as those encoding for various major histocompatibility complex (MHC) alleles, IFN-γ, T cell growth factor β, interleukin (IL) 10, mannose-binding protein, IFN-γ receptor, Toll-like receptor 2, vitamin D receptor, and IL-1, have been associated with susceptibility to TB.
THE HOST RESPONSE, GRANULOMA FORMATION, AND “LATENCY”
In the initial stage of host–bacterium interaction, prior to the onset of an acquired CMI response, M. tuberculosis disseminates widely through the lymph vessels, spreading to other sites in the lungs and other organs, and undergoes a period of extensive growth within naïve unactivated macrophages; additional naïve macrophages are recruited to the early granuloma. Studies suggest that M. tuberculosis uses specific virulence mechanisms to subvert host cellular signaling and to elicit an early regulated proinflammatory response that promotes granuloma expansion and bacterial growth during this key early phase. A study of M. marinum infection in zebrafish has delineated one molecular mechanism by which mycobacteria induce granuloma formation. The mycobacterial protein ESAT-6 induces secretion of matrix metalloproteinase 9 (MMP9) by nearby epithelial cells that are in contact with infected macrophages. MMP9 in turn stimulates recruitment of naïve macrophages, thus inducing granuloma maturation and bacterial growth. Disruption of MMP9 function results in reduced bacterial growth. Another study has shown that M. tuberculosis–derived cyclic AMP is secreted from the phagosome into host macrophages, subverting the cell’s signal transduction pathways and stimulating an elevation in the secretion of tumor necrosis factor α (TNF-α) as well as further proinflammatory cell recruitment. Ultimately, the chemoattractants and bacterial products released during the repeated rounds of cell lysis and infection of newly arriving macrophages enable dendritic cells to access bacilli; these cells migrate to the draining lymph nodes and present mycobacterial antigens to T lymphocytes. At this point, the development of CMI and humoral immunity begins. These initial stages of infection are usually asymptomatic.
About 2–4 weeks after infection, two host responses to M. tuberculosis develop: a macrophage-activating CMI response and a tissue-damaging response. The macrophage-activating response is a T cell–mediated phenomenon resulting in the activation of macrophages that are capable of killing and digesting tubercle bacilli. The tissue-damaging response is the result of a delayed-type hypersensitivity (DTH) reaction to various bacillary antigens; it destroys unactivated macrophages that contain multiplying bacilli but also causes caseous necrosis of the involved tissues (see below). Although both of these responses can inhibit mycobacterial growth, it is the balance between the two that determines the forms of TB that will develop subsequently. With the development of specific immunity and the accumulation of large numbers of activated macrophages at the site of the primary lesion, granulomatous lesions (tubercles) are formed. These lesions consist of accumulations of lymphocytes and activated macrophages that evolve toward epithelioid and giant cell morphologies. Initially, the tissue-damaging response can limit mycobacterial growth within macrophages. As stated above, this response, mediated by various bacterial products, not only destroys macrophages but also produces early solid necrosis in the center of the tubercle. Although M. tuberculosis can survive, its growth is inhibited within this necrotic environment by low oxygen tension and low pH. At this point, some lesions may heal by fibrosis, with subsequent calcification, whereas inflammation and necrosis occur in other lesions. Some observations have challenged the traditional view that any encounter between mycobacteria and macrophages results in chronic infection. It is possible that an immune response capable of eradicating early infection may sometimes develop as a consequence, for instance, of disabling mutations in mycobacterial genomes rendering their replication ineffective. Individual granulomas that are formed during this phase of infection can vary in size and cell composition; some can contain the spread of mycobacteria, while others cannot. LTBI ensues as a result of this dynamic balance between the microorganism and the host. According to recent developments, latency may not be an accurate term because bacilli may remain active during this “latent” stage, forming biofilms in necrotic areas within which they temporarily hide. Thus, the term persister is probably more accurate to indicate the behavior of the bacilli in this phase. It is important to recognize that latent infection and disease represent not a binary state but rather a continuum along which infection will eventually move in the direction of full containment or disease. The ability to predict, through systemic biomarkers, which affected individuals will progress toward disease would be of immense value in devising prophylactic interventions.
MACROPHAGE-ACTIVATING RESPONSE
CMI is critical at this early stage. In the majority of infected individuals, local macrophages are activated when bacillary antigens processed by macrophages stimulate T lymphocytes to release a variety of lymphokines. These activated macrophages aggregate around the lesion’s center and effectively neutralize tubercle bacilli without causing further tissue destruction. In the central part of the lesion, the necrotic material resembles soft cheese (caseous necrosis)—a phenomenon that may also be observed in other conditions, such as neoplasms. Even when healing takes place, viable bacilli may remain dormant within macrophages or in the necrotic material for many years. These “healed” lesions in the lung parenchyma and hilar lymph nodes may later undergo calcification.
DELAYED-TYPE HYPERSENSITIVITY
In a minority of cases, the macrophage-activating response is weak, and mycobacterial growth can be inhibited only by intensified DTH reactions, which lead to lung tissue destruction. The lesion tends to enlarge further, and the surrounding tissue is progressively damaged. At the center of the lesion, the caseous material liquefies. Bronchial walls and blood vessels are invaded and destroyed, and cavities are formed. The liquefied caseous material, containing large numbers of bacilli, is drained through bronchi. Within the cavity, tubercle bacilli multiply, spill into the airways, and are discharged into the environment through expiratory maneuvers such as coughing and talking. In the early stages of infection, bacilli are usually transported by macrophages to regional lymph nodes, from which they gain access to the central venous return; from there they reseed the lungs and may also disseminate beyond the pulmonary vasculature throughout the body via the systemic circulation. The resulting extrapulmonary lesions may undergo the same evolution as those in the lungs, although most tend to heal. In young children with poor natural immunity, hematogenous dissemination may result in highly fatal miliary TB or tuberculous meningitis.
ROLE OF MACROPHAGES AND MONOCYTES
While CMI confers partial protection against M. tuberculosis, humoral immunity plays a less well-defined role in protection (although evidence is accumulating on the existence of antibodies to lipoarabinomannan, which may prevent dissemination of infection in children). In the case of CMI, two types of cells are essential: macrophages, which directly phagocytose tubercle bacilli, and T cells (mainly CD4+ T lymphocytes), which induce protection through the production of cytokines, especially IFN-γ. After infection with M. tuberculosis, alveolar macrophages secrete various cytokines responsible for a number of events (e.g., the formation of granulomas) as well as systemic effects (e.g., fever and weight loss). However, alternatively activated alveolar macrophages may be particularly susceptible to M. tuberculosis growth early on, given their more limited proinflammatory and bactericidal activity, which is related in part to being bathed in surfactant. New monocytes and macrophages attracted to the site are key components of the immune response. Their primary mechanism is probably related to production of oxidants (such as reactive oxygen intermediates or nitric oxide) that have antimycobacterial activity and increase the synthesis of cytokines such as TNF-α and IL-1, which in turn regulate the release of reactive oxygen intermediates and reactive nitrogen intermediates. In addition, macrophages can undergo apoptosis—a defensive mechanism to prevent release of cytokines and bacilli via their sequestration in the apoptotic cell. Recent work also describes the involvement of neutrophils in the host response, although the timing of their appearance and their effectiveness remain uncertain.
ROLE OF T LYMPHOCYTES
Alveolar macrophages, monocytes, and dendritic cells are also critical in processing and presenting antigens to T lymphocytes, primarily CD4+ and CD8+ T cells; the result is the activation and proliferation of CD4+ T lymphocytes, which are crucial to the host’s defense against M. tuberculosis. Qualitative and quantitative defects of CD4+ T cells explain the inability of HIV-infected individuals to contain mycobacterial proliferation. Activated CD4+ T lymphocytes can differentiate into cytokine-producing TH1 or TH2 cells. TH1 cells produce IFN-γ—an activator of macrophages and monocytes—and IL-2. TH2 cells produce IL-4, IL-5, IL-10, and IL-13 and may also promote humoral immunity. The interplay of these various cytokines and their cross-regulation determine the host’s response. The role of cytokines in promoting intracellular killing of mycobacteria, however, has not been entirely elucidated. IFN-γ may induce the generation of reactive nitrogen intermediates and regulate genes involved in bactericidal effects. TNF-α also seems to be important. Observations made originally in transgenic knockout mice and more recently in humans suggest that other T cell subsets, especially CD8+ T cells, may play an important role. CD8+ T cells have been associated with protective activities via cytotoxic responses and lysis of infected cells as well as with production of IFN-γ and TNF-α. Finally, natural killer cells act as co-regulators of CD8+ T cell lytic activities, and γδ T cells are increasingly thought to be involved in protective responses in humans.
MYCOBACTERIAL LIPIDS AND PROTEINS
Lipids have been involved in mycobacterial recognition by the innate immune system, and lipoproteins (such as 19-kDa lipoprotein) have been proven to trigger potent signals through Toll-like receptors present in blood dendritic cells. M. tuberculosis possesses various protein antigens. Some are present in the cytoplasm and cell wall; others are secreted. That the latter are more important in eliciting a T lymphocyte response is suggested by experiments documenting the appearance of protective immunity in animals after immunization with live, protein-secreting mycobacteria. Among the antigens that may play a protective role are the 30-kDa (or 85B) and ESAT-6 antigens. Protective immunity is probably the result of reactivity to many different mycobacterial antigens. These antigens are being incorporated into newly designed vaccines on various platforms.
SKIN TEST REACTIVITY
Coincident with the appearance of immunity, DTH to M. tuberculosis develops. This reactivity is the basis of the TST, which is used primarily for the detection of M. tuberculosis infection in persons without symptoms. The cellular mechanisms responsible for TST reactivity are related mainly to previously sensitized CD4+ T lymphocytes, which are attracted to the skin-test site. There, they proliferate and produce cytokines. Although DTH is associated with protective immunity (TST-positive persons are less susceptible to a new M. tuberculosis infection than TST-negative persons), it by no means guarantees protection against reactivation. In fact, cases of active TB are often accompanied by strongly positive skin-test reactions. There is also evidence of reinfection with a new strain of M. tuberculosis in patients previously treated for active disease. This evidence underscores the fact that previous latent or active TB may not confer fully protective immunity.
CLINICAL MANIFESTATIONS
TB is classified as pulmonary, extrapulmonary, or both. Depending on several factors linked to different populations and bacterial strains, extrapulmonary TB may occur in 10–40% of patients. Furthermore, up to two-thirds of HIV-infected patients with TB may have both pulmonary and extrapulmonary TB or extrapulmonary TB alone.
PULMONARY TB
Pulmonary TB is conventionally categorized as primary or postprimary (adult-type, secondary). This distinction has been challenged by molecular evidence from TB-endemic areas indicating that a large percentage of cases of adult pulmonary TB result from recent infection (either primary infection or reinfection) and not from reactivation.
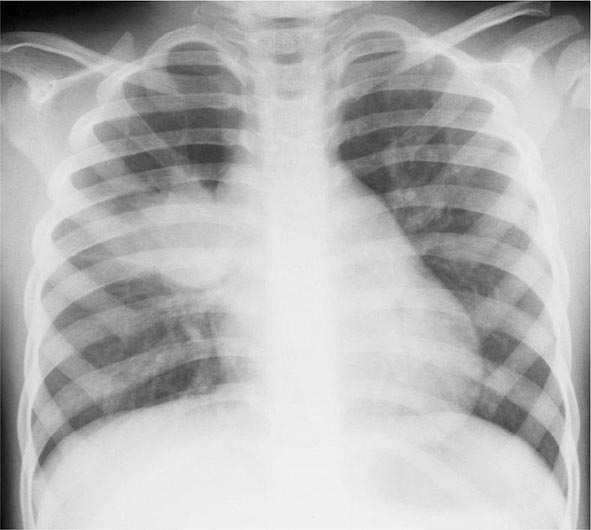
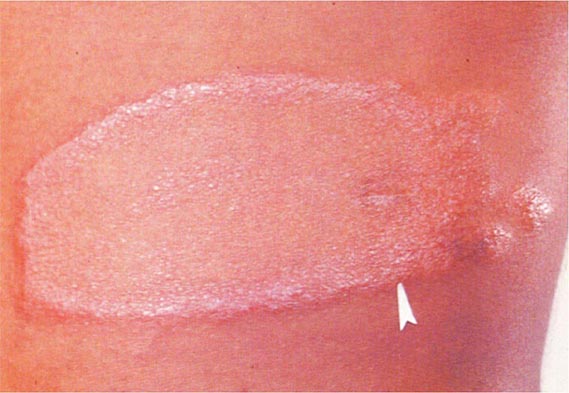
Primary Disease Primary pulmonary TB occurs soon after the initial infection with tubercle bacilli. It may be asymptomatic or may present with fever and occasionally pleuritic chest pain. In areas of high TB transmission, this form of disease is often seen in children. Because most inspired air is distributed to the middle and lower lung zones, these areas are most commonly involved in primary TB. The lesion forming after initial infection (Ghon focus) is usually peripheral and accompanied by transient hilar or paratracheal lymphadenopathy, which may or may not be visible on standard chest radiography (Fig. 202-4). Some patients develop erythema nodosum on the legs (see Fig. 25e-40) or phlyctenular conjunctivitis. In the majority of cases, the lesion heals spontaneously and becomes evident only as a small calcified nodule. Pleural reaction overlying a subpleural focus is also common. The Ghon focus, with or without overlying pleural reaction, thickening, and regional lymphadenopathy, is referred to as the Ghon complex.
FIGURE 202-4 Chest radiograph showing right hilar lymph node enlargement with infiltration into the surrounding lung tissue in a child with primary tuberculosis. (Courtesy of Prof. Robert Gie, Department of Paediatrics and Child Health, Stellenbosch University, South Africa; with permission.)
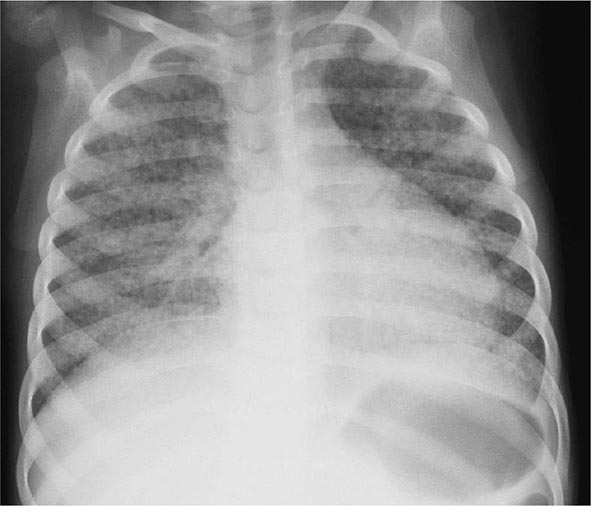
In young children with immature CMI and in persons with impaired immunity (e.g., those with malnutrition or HIV infection), primary pulmonary TB may progress rapidly to clinical illness. The initial lesion increases in size and can evolve in different ways. Pleural effusion, which is found in up to two-thirds of cases, results from the penetration of bacilli into the pleural space from an adjacent subpleural focus. In severe cases, the primary site rapidly enlarges, its central portion undergoes necrosis, and cavitation develops (progressive primary TB). TB in young children is almost invariably accompanied by hilar or paratracheal lymphadenopathy due to the spread of bacilli from the lung parenchyma through lymphatic vessels. Enlarged lymph nodes may compress bronchi, causing total obstruction with distal collapse, partial obstruction with large-airway wheezing, or a ball-valve effect with segmental/lobar hyperinflation. Lymph nodes may also rupture into the airway with development of pneumonia, often including areas of necrosis and cavitation, distal to the obstruction. Bronchiectasis (Chap. 312) may develop in any segment/lobe damaged by progressive caseating pneumonia. Occult hematogenous dissemination commonly follows primary infection. However, in the absence of a sufficient acquired immune response, which usually contains the infection, disseminated or miliary disease may result (Fig. 202-5). Small granulomatous lesions develop in multiple organs and may cause locally progressive disease or result in tuberculous meningitis; this is a particular concern in very young children and immunocompromised persons (e.g., patients with HIV infection).
FIGURE 202-5 Chest radiograph showing bilateral miliary (millet-sized) infiltrates in a child. (Courtesy of Prof. Robert Gie, Department of Paediatrics and Child Health, Stellenbosch University, South Africa; with permission.)
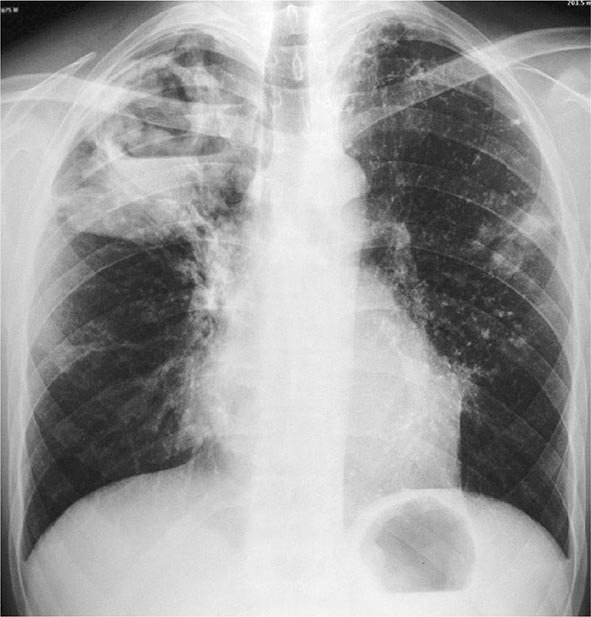
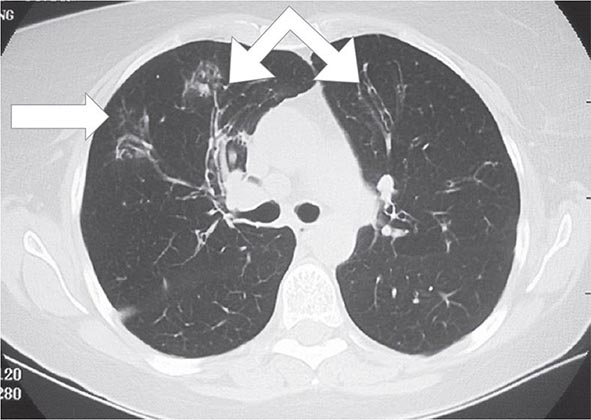
Postprimary (Adult-Type) Disease Also referred to as reactivation or secondary TB, postprimary TB is probably most accurately termed adult-type TB because it may result from endogenous reactivation of distant LTBI or recent infection (primary infection or reinfection). It is usually localized to the apical and posterior segments of the upper lobes, where the substantially higher mean oxygen tension (compared with that in the lower zones) favors mycobacterial growth. The superior segments of the lower lobes are also more frequently involved. The extent of lung parenchymal involvement varies greatly, from small infiltrates to extensive cavitary disease. With cavity formation, liquefied necrotic contents are ultimately discharged into the airways and may undergo bronchogenic spread, resulting in satellite lesions within the lungs that may in turn undergo cavitation (Figs. 202-6 and 202-7). Massive involvement of pulmonary segments or lobes, with coalescence of lesions, produces caseating pneumonia. While up to one-third of untreated patients reportedly succumb to severe pulmonary TB within a few months after onset (the classic “galloping consumption” of the past), others may undergo a process of spontaneous remission or proceed along a chronic, progressively debilitating course (“consumption” or phthisis). Under these circumstances, some pulmonary lesions become fibrotic and may later calcify, but cavities persist in other parts of the lungs. Individuals with such chronic disease continue to discharge tubercle bacilli into the environment. Most patients respond to treatment, with defervescence, decreasing cough, weight gain, and a general improvement in well-being within several weeks.
FIGURE 202-6 Chest radiograph showing a right-upper-lobe infiltrate and a cavity with an air-fluid level in a patient with active tuberculosis. (Courtesy of Dr. Andrea Gori, Department of Infectious Diseases, S. Paolo University Hospital, Milan, Italy; with permission.)
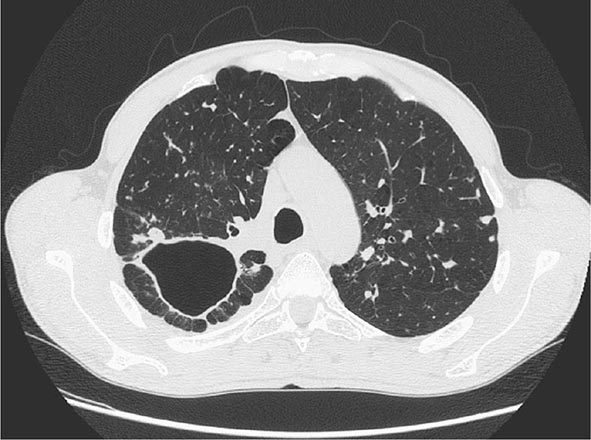
FIGURE 202-7 CT scan showing a large cavity in the right lung of a patient with active tuberculosis. (Courtesy of Dr. Elisa Busi Rizzi, National Institute for Infectious Diseases, Spallanzani Hospital, Rome, Italy; with permission.)
Early in the course of disease, symptoms and signs are often nonspecific and insidious, consisting mainly of diurnal fever and night sweats due to defervescence, weight loss, anorexia, general malaise, and weakness. However, in up to 90% of cases, cough eventually develops—often initially nonproductive and limited to the morning and subsequently accompanied by the production of purulent sputum, sometimes with blood streaking. Hemoptysis develops in 20–30% of cases, and massive hemoptysis may ensue as a consequence of the erosion of a blood vessel in the wall of a cavity. Hemoptysis, however, may also result from rupture of a dilated vessel in a cavity (Rasmussen’s aneurysm) or from aspergilloma formation in an old cavity. Pleuritic chest pain sometimes develops in patients with subpleural parenchymal lesions or pleural disease. Extensive disease may produce dyspnea and, in rare instances, adult respiratory distress syndrome. Physical findings are of limited use in pulmonary TB. Many patients have no abnormalities detectable by chest examination, whereas others have detectable rales in the involved areas during inspiration, especially after coughing. Occasionally, rhonchi due to partial bronchial obstruction and classic amphoric breath sounds in areas with large cavities may be heard. Systemic features include fever (often low-grade and intermittent) in up to 80% of cases and wasting. Absence of fever, however, does not exclude TB. In some cases, pallor and finger clubbing develop. The most common hematologic findings are mild anemia, leukocytosis, and thrombocytosis with a slightly elevated erythrocyte sedimentation rate and/or C-reactive protein level. None of these findings is consistent or sufficiently accurate for diagnostic purposes. Hyponatremia due to the syndrome of inappropriate secretion of antidiuretic hormone has also been reported.
EXTRAPULMONARY TB
In order of frequency, the extrapulmonary sites most commonly involved in TB are the lymph nodes, pleura, genitourinary tract, bones and joints, meninges, peritoneum, and pericardium. However, virtually all organ systems may be affected. As a result of hematogenous dissemination in HIV-infected individuals, extrapulmonary TB is seen more commonly today than in the past in settings of high HIV prevalence.
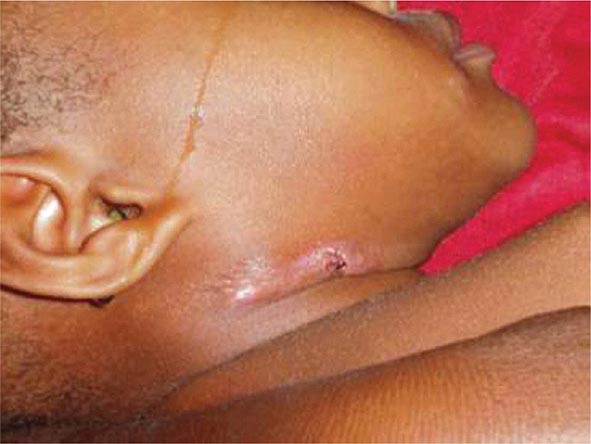
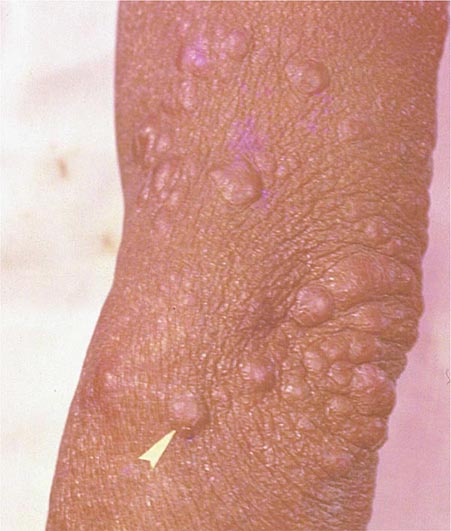
Lymph Node TB (Tuberculous Lymphadenitis) The most common presentation of extrapulmonary TB in both HIV-seronegative and HIV-infected patients (35% of cases worldwide and more than 40% of cases in the United States in recent series), lymph node disease is particularly frequent among HIV-infected patients and among children (Fig. 202-8). In the United States, besides children, women (particularly non-Caucasians) seem to be especially susceptible. Once caused mainly by M. bovis, tuberculous lymphadenitis today is due largely to M. tuberculosis. Lymph node TB presents as painless swelling of the lymph nodes, most commonly at posterior cervical and supraclavicular sites (a condition historically referred to as scrofula). Lymph nodes are usually discrete in early disease but develop into a matted nontender mass over time and may result in a fistulous tract draining caseous material. Associated pulmonary disease is present in fewer than 50% of cases, and systemic symptoms are uncommon except in HIV-infected patients. The diagnosis is established by fine-needle aspiration biopsy (with a yield of up to 80%) or surgical excision biopsy. Bacteriologic confirmation is achieved in the vast majority of cases, granulomatous lesions with or without visible AFBs are typically seen, and cultures are positive in 70–80% of cases. Among HIV-infected patients, granulomas are less well organized and are frequently absent entirely, but bacterial loads are heavier than in HIV-seronegative patients, with higher yields from microscopy and culture. Differential diagnosis includes a variety of infectious conditions, neoplastic diseases such as lymphomas or metastatic carcinomas, and rare disorders like Kikuchi’s disease (necrotizing histiocytic lymphadenitis), Kimura’s disease, and Castleman’s disease.
FIGURE 202-8 Tuberculous lymphadenitis affecting the cervical lymph nodes in a 2-year-old child from Malawi. (Courtesy of Prof. S. Graham, Centre for International Child Health, University of Melbourne, Australia; with permission.)
Pleural TB Involvement of the pleura accounts for ~20% of extrapulmonary cases in the United States and elsewhere. Isolated pleural effusion usually reflects recent primary infection, and the collection of fluid in the pleural space represents a hypersensitivity response to mycobacterial antigens. Pleural disease may also result from contiguous parenchymal spread, as in many cases of pleurisy accompanying postprimary disease. Depending on the extent of reactivity, the effusion may be small, remain unnoticed, and resolve spontaneously or may be sufficiently large to cause symptoms such as fever, pleuritic chest pain, and dyspnea. Physical findings are those of pleural effusion: dullness to percussion and absence of breath sounds. A chest radiograph reveals the effusion and, in up to one-third of cases, also shows a parenchymal lesion. Thoracentesis is required to ascertain the nature of the effusion and to differentiate it from manifestations of other etiologies. The fluid is straw colored and at times hemorrhagic; it is an exudate with a protein concentration >50% of that in serum (usually ~4–6 g/dL), a normal to low glucose concentration, a pH of ~7.3 (occasionally <7.2), and detectable white blood cells (usually 500–6000/μL). Neutrophils may predominate in the early stage, but lymphocyte predominance is the typical finding later. Mesothelial cells are generally rare or absent. AFB are rarely seen on direct smear, and cultures often may be falsely negative for M. tuberculosis; positive cultures are more common among postprimary cases. Determination of the pleural concentration of adenosine deaminase (ADA) may be a useful screening test, and TB may be excluded if the value is very low. Lysozyme is also present in the pleural effusion. Measurement of IFN-γ, either directly or through stimulation of sensitized T cells with mycobacterial antigens, can be helpful. Needle biopsy of the pleura is often required for diagnosis and is recommended over pleural fluid; it reveals granulomas and/or yields a positive culture in up to 80% of cases. Pleural biopsy can yield a positive result in ~75% of cases when real-time automated nucleic acid amplification is used (the Xpert® MTB/RIF assay [Cepheid, Sunnyvale, CA]; see “Nucleic Acid Amplification Technology,” below), although pleural fluid testing with this assay is not recommended because of low sensitivity. This form of pleural TB responds rapidly to chemotherapy and may resolve spontaneously. Concurrent glucocorticoid administration may reduce the duration of fever and/or chest pain but is not of proven benefit.
Tuberculous empyema is a less common complication of pulmonary TB. It is usually the result of the rupture of a cavity, with spillage of a large number of organisms into the pleural space. This process may create a bronchopleural fistula with evident air in the pleural space. A chest radiograph shows hydropneumothorax with an air-fluid level. The pleural fluid is purulent and thick and contains large numbers of lymphocytes. Acid-fast smears and mycobacterial cultures are often positive. Surgical drainage is usually required as an adjunct to chemotherapy. Tuberculous empyema may result in severe pleural fibrosis and restrictive lung disease. Removal of the thickened visceral pleura (decortication) is occasionally necessary to improve lung function.
TB of the Upper Airways Nearly always a complication of advanced cavitary pulmonary TB, TB of the upper airways may involve the larynx, pharynx, and epiglottis. Symptoms include hoarseness, dysphonia, and dysphagia in addition to chronic productive cough. Findings depend on the site of involvement, and ulcerations may be seen on laryngoscopy. Acid-fast smear of the sputum is often positive, but biopsy may be necessary in some cases to establish the diagnosis. Carcinoma of the larynx may have similar features but is usually painless.
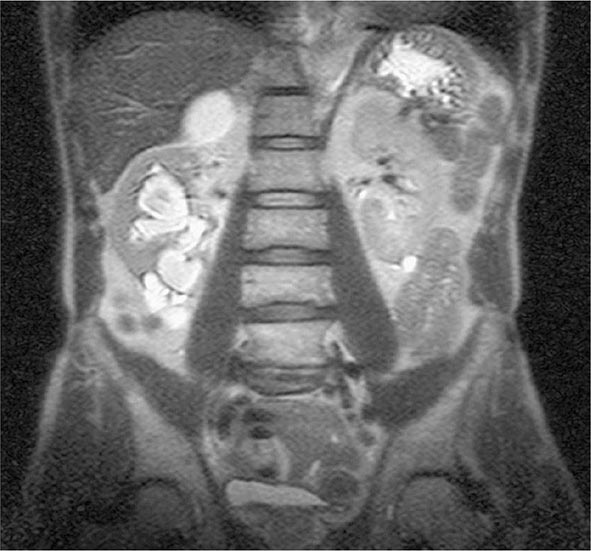
Genitourinary TB Genitourinary TB, which accounts for ~10–15% of all extrapulmonary cases in the United States and elsewhere, may involve any portion of the genitourinary tract. Local symptoms predominate, and up to 75% of patients have chest radiographic abnormalities suggesting previous or concomitant pulmonary disease. Urinary frequency, dysuria, nocturia, hematuria, and flank or abdominal pain are common presentations. However, patients may be asymptomatic and their disease discovered only after severe destructive lesions of the kidneys have developed. Urinalysis gives abnormal results in 90% of cases, revealing pyuria and hematuria. The documentation of culture-negative pyuria in acidic urine should raise the suspicion of TB. IV pyelography, abdominal computed tomography (CT), or magnetic resonance imaging (MRI) (Fig. 202-9) may show deformities and obstructions; calcifications and ureteral strictures are suggestive findings. Culture of three morning urine specimens yields a definitive diagnosis in nearly 90% of cases. Severe ureteral strictures may lead to hydronephrosis and renal damage. Genital TB is diagnosed more commonly in female than in male patients. In female patients, it affects the fallopian tubes and the endometrium and may cause infertility, pelvic pain, and menstrual abnormalities. Diagnosis requires biopsy or culture of specimens obtained by dilation and curettage. In male patients, genital TB preferentially affects the epididymis, producing a slightly tender mass that may drain externally through a fistulous tract; orchitis and prostatitis may also develop. In almost half of cases of genitourinary TB, urinary tract disease is also present. Genitourinary TB responds well to chemotherapy.
FIGURE 202-9 MRI of culture-confirmed renal tuberculosis. T2-weighted coronary plane: coronal sections showing several renal lesions in both the cortical and the medullary tissues of the right kidney. (Courtesy of Dr. Alberto Matteelli, Department of Infectious Diseases, University of Brescia, Italy; with permission.)
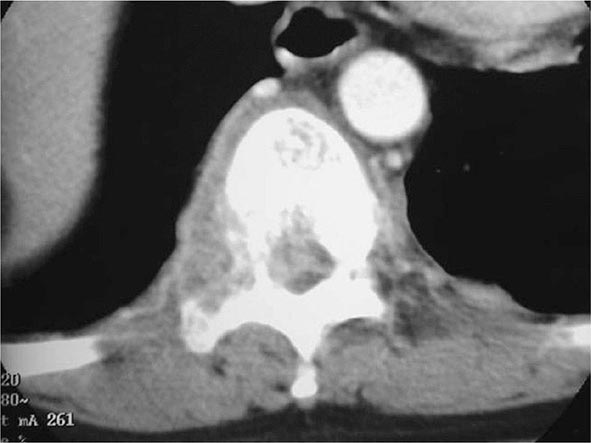
Skeletal TB In the United States, TB of the bones and joints is responsible for ~10% of extrapulmonary cases. In bone and joint disease, pathogenesis is related to reactivation of hematogenous foci or to spread from adjacent paravertebral lymph nodes. Weight-bearing joints (the spine in 40% of cases, the hips in 13%, and the knees in 10%) are most commonly affected. Spinal TB (Pott’s disease or tuberculous spondylitis; Fig. 202-10) often involves two or more adjacent vertebral bodies. Whereas the upper thoracic spine is the most common site of spinal TB in children, the lower thoracic and upper lumbar vertebrae are usually affected in adults. From the anterior superior or inferior angle of the vertebral body, the lesion slowly reaches the adjacent body, later affecting the intervertebral disk. With advanced disease, collapse of vertebral bodies results in kyphosis (gibbus). A paravertebral “cold” abscess may also form. In the upper spine, this abscess may track to and penetrate the chest wall, presenting as a soft tissue mass; in the lower spine, it may reach the inguinal ligaments or present as a psoas abscess. CT or MRI reveals the characteristic lesion and suggests its etiology. The differential diagnosis includes tumors and other infections. Pyogenic bacterial osteomyelitis, in particular, involves the disk very early and produces rapid sclerosis. Aspiration of the abscess or bone biopsy confirms the tuberculous etiology, as cultures are usually positive and histologic findings highly typical. A catastrophic complication of Pott’s disease is paraplegia, which is usually due to an abscess or a lesion compressing the spinal cord. Paraparesis due to a large abscess is a medical emergency and requires rapid drainage. TB of the hip joints, usually involving the head of the femur, causes pain; TB of the knee produces pain and swelling. If the disease goes unrecognized, the joints may be destroyed. Diagnosis requires examination of the synovial fluid, which is thick in appearance, with a high protein concentration and a variable cell count. Although synovial fluid culture is positive in a high percentage of cases, synovial biopsy and tissue culture may be necessary to establish the diagnosis. Skeletal TB responds to chemotherapy, but severe cases may require surgery.
FIGURE 202-10 CT scan demonstrating destruction of the right pedicle of T10 due to Pott’s disease. The patient, a 70-year-old Asian woman, presented with back pain and weight loss and had biopsy-proven tuberculosis. (Courtesy of Charles L. Daley, MD, University of California, San Francisco; with permission.)
Tuberculous Meningitis and Tuberculoma TB of the central nervous system accounts for ~5% of extrapulmonary cases in the United States. It is seen most often in young children but also develops in adults, especially those infected with HIV. Tuberculous meningitis results from the hematogenous spread of primary or postprimary pulmonary TB or from the rupture of a subependymal tubercle into the subarachnoid space. In more than half of cases, evidence of old pulmonary lesions or a miliary pattern is found on chest radiography. The disease often presents subtly as headache and slight mental changes after a prodrome of weeks of low-grade fever, malaise, anorexia, and irritability. If not recognized, tuberculous meningitis may evolve acutely with severe headache, confusion, lethargy, altered sensorium, and neck rigidity. Typically, the disease evolves over 1–2 weeks, a course longer than that of bacterial meningitis. Because meningeal involvement is pronounced at the base of the brain, paresis of cranial nerves (ocular nerves in particular) is a frequent finding, and the involvement of cerebral arteries may produce focal ischemia. The ultimate evolution is toward coma, with hydrocephalus and intracranial hypertension.
Lumbar puncture is the cornerstone of diagnosis. In general, examination of cerebrospinal fluid (CSF) reveals a high leukocyte count (up to 1000/μL), usually with a predominance of lymphocytes but sometimes with a predominance of neutrophils in the early stage; a protein content of 1–8 g/L (100–800 mg/dL); and a low glucose concentration. However, any of these three parameters can be within the normal range. AFBs are infrequently seen on direct smear of CSF sediment, and repeated lumbar punctures increase the yield. Culture of CSF is diagnostic in up to 80% of cases and remains the gold standard. Real-time automated nucleic acid amplification (the Xpert MTB/RIF assay; see “Nucleic Acid Amplification Technology,” below) has a sensitivity of up to 80% and is the preferred initial diagnostic option. Treatment should be initiated immediately upon a positive Xpert MTB/RIF result. A negative result does not exclude a diagnosis of TB and requires further diagnostic workup. Imaging studies (CT and MRI) may show hydrocephalus and abnormal enhancement of basal cisterns or ependyma. If unrecognized, tuberculous meningitis is uniformly fatal. This disease responds to chemotherapy; however, neurologic sequelae are documented in 25% of treated cases, in most of which the diagnosis has been delayed. Clinical trials have demonstrated that patients given adjunctive glucocorticoids may experience faster resolution of CSF abnormalities and elevated CSF pressure. In one study, adjunctive dexamethasone significantly enhanced the chances of survival among persons >14 years of age but did not reduce the frequency of neurologic sequelae. The dexamethasone schedule was (1) 0.4 mg/kg per day given IV with tapering by 0.1 mg/kg per week until the fourth week, when 0.1 mg/kg per day was administered; followed by (2) 4 mg/d given by mouth with tapering by 1 mg per week until the fourth week, when 1 mg/d was administered.
Tuberculoma, an uncommon manifestation of central nervous system TB, presents as one or more space-occupying lesions and usually causes seizures and focal signs. CT or MRI reveals contrast-enhanced ring lesions, but biopsy is necessary to establish the diagnosis.
Gastrointestinal TB Gastrointestinal TB is uncommon, making up 3.5% of extrapulmonary cases in the United States. Various pathogenetic mechanisms are involved: swallowing of sputum with direct seeding, hematogenous spread, or (largely in developing areas) ingestion of milk from cows affected by bovine TB. Although any portion of the gastrointestinal tract may be affected, the terminal ileum and the cecum are the sites most commonly involved. Abdominal pain (at times similar to that associated with appendicitis) and swelling, obstruction, hematochezia, and a palpable mass in the abdomen are common findings at presentation. Fever, weight loss, anorexia, and night sweats are also common. With intestinal-wall involvement, ulcerations and fistulae may simulate Crohn’s disease; the differential diagnosis of this entity is always difficult. Anal fistulae should prompt an evaluation for rectal TB. Because surgery is required in most cases, the diagnosis can be established by histologic examination and culture of specimens obtained intraoperatively.
Tuberculous peritonitis follows either the direct spread of tubercle bacilli from ruptured lymph nodes and intraabdominal organs (e.g., genital TB in women) or hematogenous seeding. Nonspecific abdominal pain, fever, and ascites should raise the suspicion of tuberculous peritonitis. The coexistence of cirrhosis (Chap. 363) in patients with tuberculous peritonitis complicates the diagnosis. In tuberculous peritonitis, paracentesis reveals an exudative fluid with a high protein content and leukocytosis that is usually lymphocytic (although neutrophils occasionally predominate). The yield of direct smear and culture is relatively low; culture of a large volume of ascitic fluid can increase the yield, but peritoneal biopsy (with a specimen best obtained by laparoscopy) is often needed to establish the diagnosis.
Pericardial TB (Tuberculous Pericarditis) Due either to direct extension from adjacent mediastinal or hilar lymph nodes or to hematogenous spread, pericardial TB has often been a disease of the elderly in countries with low TB prevalence. However, it also develops frequently in HIV-infected patients. Case–fatality rates are as high as 40% in some series. The onset may be subacute, although an acute presentation, with dyspnea, fever, dull retrosternal pain, and a pericardial friction rub, is possible. An effusion eventually develops in many cases; cardiovascular symptoms and signs of cardiac tamponade may ultimately appear (Chap. 288). In the presence of effusion, TB must be suspected if the patient belongs to a high-risk population (HIV-infected, originating in a high-prevalence country); if there is evidence of previous TB in other organs; or if echocardiography, CT, or MRI shows effusion and thickness across the pericardial space. A definitive diagnosis can be obtained by pericardiocentesis under echocardiographic guidance. The pericardial fluid must be submitted for biochemical, cytologic, and microbiologic evaluation. The effusion is exudative in nature, with a high count of lymphocytes and monocytes. Hemorrhagic effusion is common. Direct smear examination is very rarely positive. Culture of pericardial fluid reveals M. tuberculosis in up to two-thirds of cases, whereas pericardial biopsy has a higher yield. High levels of ADA, lysozyme, and IFN-γ may suggest a tuberculous etiology.
Without treatment, pericardial TB is usually fatal. Even with treatment, complications may develop, including chronic constrictive pericarditis with thickening of the pericardium, fibrosis, and sometimes calcification, which may be visible on a chest radiograph. Systematic reviews and meta-analyses show that adjunctive glucocorticoid treatment remains controversial, with no conclusive evidence of benefits for all principal outcomes of pericarditis—i.e., no significant impact on resolution of effusion, no significant difference in functional status after treatment, and no significant reduction in the frequency of development of constriction or death. However, in HIV-infected patients, glucocorticoids do improve functional status after treatment.
Caused by direct extension from the pericardium or by retrograde lymphatic extension from affected mediastinal lymph nodes, tuberculous myocarditis is an extremely rare disease. Usually it is fatal and is diagnosed postmortem.
Miliary or Disseminated TB Miliary TB is due to hematogenous spread of tubercle bacilli. Although in children it is often the consequence of primary infection, in adults it may be due to either recent infection or reactivation of old disseminated foci. The lesions are usually yellowish granulomas 1–2 mm in diameter that resemble millet seeds (thus the term miliary, coined by nineteenth-century pathologists). Clinical manifestations are nonspecific and protean, depending on the predominant site of involvement. Fever, night sweats, anorexia, weakness, and weight loss are presenting symptoms in the majority of cases. At times, patients have a cough and other respiratory symptoms due to pulmonary involvement as well as abdominal symptoms. Physical findings include hepatomegaly, splenomegaly, and lymphadenopathy. Eye examination may reveal choroidal tubercles, which are pathognomonic of miliary TB, in up to 30% of cases. Meningismus occurs in fewer than 10% of cases.
A high index of suspicion is required for the diagnosis of miliary TB. Frequently, chest radiography (Fig. 202-5) reveals a miliary reticulonodular pattern (more easily seen on underpenetrated film), although no radiographic abnormality may be evident early in the course and among HIV-infected patients. Other radiologic findings include large infiltrates, interstitial infiltrates (especially in HIV-infected patients), and pleural effusion. Sputum-smear microscopy is negative in most cases. Various hematologic abnormalities may be seen, including anemia with leukopenia, lymphopenia, neutrophilic leukocytosis and leukemoid reactions, and polycythemia. Disseminated intravascular coagulation has been reported. Elevation of alkaline phosphatase levels and other abnormal values in liver function tests are detected in patients with severe hepatic involvement. The TST may be negative in up to half of cases, but reactivity may be restored during chemotherapy. Bronchoalveolar lavage and transbronchial biopsy are more likely to provide bacteriologic confirmation, and granulomas are evident in liver or bone-marrow biopsy specimens from many patients. If it goes unrecognized, miliary TB is lethal; with proper early treatment, however, it is amenable to cure. Glucocorticoid therapy has not proved beneficial.
A rare presentation seen in the elderly, cryptic miliary TB has a chronic course characterized by mild intermittent fever, anemia, and—ultimately—meningeal involvement preceding death. An acute septicemic form, nonreactive miliary TB, occurs very rarely and is due to massive hematogenous dissemination of tubercle bacilli. Pancytopenia is common in this form of disease, which is rapidly fatal. At postmortem examination, multiple necrotic but nongranulomatous (“nonreactive”) lesions are detected.
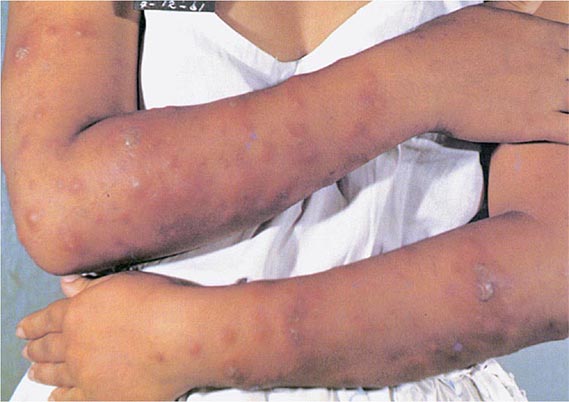
Less Common Extrapulmonary Forms TB may cause chorioretinitis, uveitis, panophthalmitis, and painful hypersensitivity-related phlyctenular conjunctivitis. Tuberculous otitis is rare and presents as hearing loss, otorrhea, and tympanic membrane perforation. In the nasopharynx, TB may simulate granulomatosis with polyangiitis. Cutaneous manifestations of TB include primary infection due to direct inoculation, abscesses and chronic ulcers, scrofuloderma, lupus vulgaris (a smoldering disease with nodules, plaques, and fissures), miliary lesions, and erythema nodosum. Tuberculous mastitis results from retrograde lymphatic spread, often from the axillary lymph nodes. Adrenal TB is a manifestation of disseminated disease presenting rarely as adrenal insufficiency. Finally, congenital TB results from transplacental spread of tubercle bacilli to the fetus or from ingestion of contaminated amniotic fluid. This rare disease affects the liver, spleen, lymph nodes, and various other organs.
Post-TB Complications TB may cause persisting pulmonary damage in patients whose infection has been considered cured on clinical grounds. Chronic impairment of lung functions, bronchiectasis, aspergillomas, and chronic pulmonary aspergillosis (CPA) have been associated with TB. CPA may manifest as simple aspergilloma (fungal ball) or chronic cavitary aspergillosis. Early studies revealed that, especially in the presence of large residual cavities, Aspergillus fumigatus may colonize the lesion and produce symptoms such as respiratory impairment, hemoptysis, persistent fatigue, and weight loss, often resulting in the erroneous diagnosis of TB recurrence. The detection of Aspergillus precipitins (IgG) in the blood suggests CPA, as do radiographic abnormalities such as thickening of the cavitary walls or the presence of a fungal ball inside the cavity. Treatment is difficult. Recent preliminary studies on the use of itraconazole for 6 months suggest that treatment with this agent may be superior to conservative treatment in improving radiologic and clinical manifestations of CPA. Surgical removal of lesions is risky.
HIV-Associated TB (See also Chap. 226) TB is one of the most common diseases among HIV-infected persons worldwide and a major cause of death in this population; more specifically, it is responsible for an estimated 24% of all HIV-related mortality. In certain urban settings in some African countries, the rate of HIV infection among TB patients reaches 70–80%. A person with a positive TST who acquires HIV infection has a 3–13% annual risk of developing active TB. A new TB infection acquired by an HIV-infected individual may evolve to active disease in a matter of weeks rather than months or years. TB can appear at any stage of HIV infection, and its presentation varies with the stage. When CMI is only partially compromised, pulmonary TB presents in a typical manner (Figs. 202-6 and 202-7), with upper-lobe infiltrates and cavitation and without significant lymphadenopathy or pleural effusion. In late stages of HIV infection, when the CD4+ T cell count is <200/μL, a primary TB–like pattern, with diffuse interstitial and subtle infiltrates, little or no cavitation, pleural effusion, and intrathoracic lymphadenopathy, is more common. However, these forms are becoming less common because of the expanded use of antiretroviral treatment (ART). Overall, sputum smears are less frequently positive among TB patients with HIV infection than among those without; thus, the diagnosis of TB may be difficult, especially in view of the variety of HIV-related pulmonary conditions mimicking TB. Extrapulmonary TB is common among HIV-infected patients. In various series, extrapulmonary TB—alone or in association with pulmonary disease—has been documented in 40–60% of all cases in HIV-co-infected individuals. The most common forms are lymphatic, disseminated, pleural, and pericardial. Mycobacteremia and meningitis are also common, particularly in advanced HIV disease. The diagnosis of TB in HIV-infected patients may be complicated not only by the increased frequency of sputum-smear negativity (up to 40% in culture-proven pulmonary cases) but also by atypical radiographic findings, a lack of classic granuloma formation in the late stages, and a negative TST. The Xpert MTB/RIF assay (see “Nucleic Acid Amplification Technology,” below) is the preferred initial diagnostic option, and therapy should be started on the basis of a positive result because treatment delays may be fatal. A negative Xpert MTB/RIF result does not exclude a diagnosis of TB, and culture remains the gold standard.
Exacerbations in systemic (lymphadenopathy) or respiratory symptoms, signs, and laboratory or radiographic manifestations of TB—termed the immune reconstitution inflammatory syndrome (IRIS) or TB immune reconstitution disease (TB-IRD)—have been associated with the administration of ART and occur in ~10% of HIV-infected TB patients. Usually developing 1–3 months after initiation of ART, IRIS is more common among patients with advanced immunosuppression and extrapulmonary TB. “Unmasking IRIS” may also develop after the initiation of ART in patients with undiagnosed subclinical TB. The earlier ART is started and the lower the baseline CD4+ T cell count, the greater the risk of IRIS. Death due to IRIS is relatively infrequent and occurs mainly among patients who have a high preexisting mortality risk. The presumed pathogenesis of IRIS consists of an immune response that is elicited by antigens released as bacilli are killed during effective chemotherapy and that is temporally associated with improving immune function. There is no diagnostic test for IRIS, and its confirmation relies heavily upon case definitions incorporating clinical and laboratory data; a variety of case definitions have been suggested. The first priority in the management of a possible case of IRIS is to ensure that the clinical syndrome does not represent a failure of TB treatment or the development of another infection. Mild paradoxical reactions can be managed with symptom-based treatment. Glucocorticoids have been used for more severe reactions, and prednisolone given for 4 weeks at a low dosage (1.5 mg/kg per day for 2 weeks and half that dose for the remaining 2 weeks) has reduced the need for hospitalization and therapeutic procedures and hastened alleviation of symptoms, as reflected by Karnofsky performance scores, quality-of-life assessments, radiographic response, and C-reactive protein levels. The effectiveness of glucocorticoids in alleviating the symptoms of IRIS is probably linked to suppression of proinflammatory cytokine concentrations, as these medications reduce serum concentrations of IL-6, IL-10, IL-12p40, TNF-α, IFN-γ, and IFN-γ-inducible protein 10 (IP-10). Recommendations for the prevention and treatment of TB in HIV-infected individuals are provided below.
DIAGNOSIS
The key to the diagnosis of TB remains a high index of suspicion. Diagnosis is not difficult in persons belonging to high-risk populations who present with typical symptoms and a classic chest radiograph showing upper-lobe infiltrates with cavities (Fig. 202-6). On the other hand, the diagnosis can easily be missed in an elderly nursing-home resident or a teenager with a focal infiltrate. Often, the diagnosis is first entertained when the chest radiograph of a patient being evaluated for respiratory symptoms is abnormal. If the patient has no complicating medical conditions that cause immunosuppression, the chest radiograph may show typical upper-lobe infiltrates with cavitation (Fig. 202-6). The longer the delay between the onset of symptoms and the diagnosis, the more likely is the finding of cavitary disease. In contrast, immunosuppressed patients, including those with HIV infection, may have “atypical” findings on chest radiography—e.g., lower-zone infiltrates without cavity formation.
The several approaches to the diagnosis of TB require, above all, a well-organized laboratory network with an appropriate distribution of tasks at different levels of the health care system. At the peripheral and community levels, screening and referral are the principal tasks—besides clinical assessment and radiography—that can be accomplished through AFB microscopy and/or real-time automated nucleic acid amplification technology (the Xpert MTB/RIF assay; see below). At a secondary level (e.g., a traditional district hospital in a high-incidence setting), additional technology can be adopted, including rapid culture and drug susceptibility testing.
AFB MICROSCOPY
A presumptive diagnosis is commonly based on the finding of AFB on microscopic examination of a diagnostic specimen, such as a smear of expectorated sputum or of tissue (e.g., a lymph node biopsy). Although inexpensive, AFB microscopy has relatively low sensitivity (40–60%) in culture-confirmed cases of pulmonary TB. The traditional method—light microscopy of specimens stained with Ziehl-Neelsen basic fuchsin dyes—is nevertheless satisfactory, although time-consuming. Most modern laboratories processing large numbers of diagnostic specimens use auramine–rhodamine staining and fluorescence microscopy; this approach is more sensitive than the Ziehl-Neelsen method. However, it is expensive because it requires high-cost mercury vapor light sources and a dark room. Less expensive light-emitting diode (LED) fluorescence microscopes are now available. They are as sensitive as—or more sensitive than—traditional fluorescence microscopes. As a result, conventional light and fluorescence microscopes are being replaced with this more recent technology, especially in developing countries. For patients with suspected pulmonary TB, it has been recommended that two or three sputum specimens, preferably collected early in the morning, should be submitted to the laboratory for AFB smear and mycobacterial culture. Two specimens collected on the same visit may be as effective as three. If tissue is obtained, it is critical that the portion of the specimen intended for culture not be put in formaldehyde. The use of AFB microscopy in examining urine or gastric lavage fluid is limited by the presence of commensal mycobacteria that can cause false-positive results.
NUCLEIC ACID AMPLIFICATION TECHNOLOGY
Several test systems based on amplification of mycobacterial nucleic acid have become available in the past few years. These tests are most useful for the rapid confirmation of TB in persons with AFB-positive specimens, but some also have utility for the diagnosis of AFB-negative pulmonary and extrapulmonary TB. One system that permits rapid diagnosis of TB with high specificity and sensitivity (approaching that of culture) is the fully automated, real-time nucleic acid amplification technology known as the Xpert MTB/RIF assay. Xpert MTB/RIF can simultaneously detect TB and rifampin resistance in <2 h and has minimal biosafety and training requirements. Therefore, it can be housed in nonconventional laboratory settings. The WHO recommends its use worldwide as the initial diagnostic test in adults and children presumed to have MDR-TB or HIV-associated TB. Taking into account the availability of resources, the test may also be used in any adult or child presumed to have TB or as a follow-up test after microscopy in adults presumed to have TB but not at risk of MDR-TB or HIV-associated TB. Xpert MTB/RIF should be the initial test applied to CSF from patients in whom TB meningitis is suspected as well as a replacement test (over conventional microscopy, culture, and histopathology) for selected nonrespiratory specimens—obtained by gastric lavage, fine-needle aspiration, or pleural or other biopsies—from patients in whom extrapulmonary TB is suspected. This test has a sensitivity of 98% among AFB-positive cases and ~70% among AFB-negative specimens. Other tests, such as those based on manual amplification platforms, have not yet been deemed satisfactory for introduction into clinical practice as replacements for existing tests.
MYCOBACTERIAL CULTURE
Definitive diagnosis depends on the isolation and identification of M. tuberculosis from a clinical specimen or the identification of specific DNA sequences in a nucleic acid amplification test. Specimens may be inoculated onto egg- or agar-based medium (e.g., Löwenstein-Jensen or Middlebrook 7H10) and incubated at 37°C (under 5% CO2 for Middlebrook medium). Because most species of mycobacteria, including M. tuberculosis, grow slowly, 4–8 weeks may be required before growth is detected. Although M. tuberculosis may be identified presumptively on the basis of growth time and colony pigmentation and morphology, a variety of biochemical tests have traditionally been used to speciate mycobacterial isolates. In modern, well-equipped laboratories, liquid culture for isolation and species identification by molecular methods or high-pressure liquid chromatography of mycolic acids has replaced isolation on solid media and identification by biochemical tests. A widely used technology is the mycobacterial growth indicator tube (BBL™ MGIT™; BD, Franklin Lakes, NJ), which uses a fluorescent compound sensitive to the presence of oxygen dissolved in the liquid medium. The appearance of fluorescence detected by fluorometric technology indicates active growth of mycobacteria. A low-cost, rapid immunochromatographic lateral-flow assay based on detection of MTP64 antigen may also be used for species identification of the M. tuberculosis complex in culture isolates. These new methods, which are also being introduced in low-income countries, have decreased the time required for bacteriologic confirmation of TB to 2–3 weeks.
DRUG SUSCEPTIBILITY TESTING
Any initial isolate of M. tuberculosis should be tested for susceptibility to isoniazid and rifampin in order to detect drug resistance and/or MDR-TB, particularly if one or more risk factors for drug resistance are identified or if the patient either fails to respond to initial therapy or has a relapse after the completion of treatment (see “Treatment Failure and Relapse,” below). In addition, expanded susceptibility testing for second-line anti-TB drugs (especially the fluoroquinolones and the injectable drugs) is mandatory when MDR-TB is found. Susceptibility testing may be conducted directly (with the clinical specimen) or indirectly (with mycobacterial cultures) on solid or liquid medium. Results are obtained rapidly by direct susceptibility testing on liquid medium, with an average reporting time of 3 weeks. With indirect testing on solid medium, results may be unavailable for ≥8 weeks. Highly reliable genotypic methods for the rapid identification of genetic mutations in gene regions known to be associated with resistance to rifampin (such as those in rpoB) and isoniazid (such as those in katG and inhA) have been developed and are being widely implemented for screening of patients at increased risk of drug-resistant TB. Apart from the Xpert MTB/RIF assay, which, as mentioned above, detects rifampin resistance, the most widely used are molecular line probe assays. After extraction of DNA from M. tuberculosis isolates or from clinical specimens, the resistance gene regions are amplified by polymerase chain reaction (PCR), and labeled and probe-hybridized PCR products are detected by colorimetric development. This assay reveals the presence of M. tuberculosis as well as mutations in target resistance gene regions. A similar approach has been developed for second-line anti-TB drugs such as the fluoroquinolones, the aminoglycosides kanamycin and amikacin, and capreomycin, but the diagnostic accuracy of the current technology is not yet sufficient to recommend its use in clinical practice. Finally, a few noncommercial, inexpensive culture and drug-susceptibility testing methods (e.g., microscopically observed drug susceptibility, or MODS; nitrate reductase assays; and colorimetric redox indicator assays) may be useful in resource-limited settings. Their use is restricted to national reference laboratories with proven proficiency and adequate external quality control as an interim solution while genotypic or automated liquid culture technology is introduced.
RADIOGRAPHIC PROCEDURES
As noted above, the initial suspicion of pulmonary TB is often based on abnormal chest radiographic findings in a patient with respiratory symptoms. Although the “classic” picture is that of upper-lobe disease with infiltrates and cavities (Fig. 202-6), virtually any radiographic pattern—from a normal film or a solitary pulmonary nodule to diffuse alveolar infiltrates in a patient with adult respiratory distress syndrome—may be seen. In the era of AIDS, no radiographic pattern can be considered pathognomonic. CT (Fig. 202-7) may be useful in interpreting questionable findings on plain chest radiography and may be helpful in diagnosing some forms of extrapulmonary TB (e.g., Pott’s disease; Fig. 202-10). MRI is useful in the diagnosis of intracranial TB.
ADDITIONAL DIAGNOSTIC PROCEDURES
Other diagnostic tests may be used when pulmonary TB is suspected. Sputum induction by ultrasonic nebulization of hypertonic saline may be useful for patients who cannot produce a sputum specimen spontaneously. Frequently, patients with radiographic abnormalities that are consistent with other diagnoses (e.g., bronchogenic carcinoma) undergo fiberoptic bronchoscopy with bronchial brushings and endobronchial or transbronchial biopsy of the lesion. Bronchoalveolar lavage of a lung segment containing an abnormality may also be performed. In all cases, it is essential that specimens be submitted for AFB smear, mycobacterial culture, and molecular testing with the Xpert MTB/RIF assay. For the diagnosis of primary pulmonary TB in children, who often do not expectorate sputum, induced sputum specimens and specimens from early-morning gastric lavage may yield positive cultures and/or Xpert MTB/RIF assay results.
Invasive diagnostic procedures are indicated for patients with suspected extrapulmonary TB. In addition to testing of specimens from involved sites (e.g., CSF for tuberculous meningitis, pleural fluid and biopsy samples for pleural disease), biopsy and culture of bone marrow and liver tissue have a good diagnostic yield in disseminated (miliary) TB, particularly in HIV-infected patients, who also have a high frequency of positive blood cultures. In some cases, culture or Xpert MTB/RIF assay results are negative but a clinical diagnosis of TB is supported by consistent epidemiologic evidence (e.g., a history of close contact with an infectious patient) and a compatible clinical and radiographic response to treatment. In the United States and other industrialized countries with low rates of TB, some patients with limited abnormalities on chest radiographs and sputum positive for AFB are infected with nontuberculous mycobacteria, most commonly organisms of the M. avium complex or M. kansasii (Chap. 204). Factors favoring the diagnosis of nontuberculous mycobacterial disease over TB include an absence of risk factors for TB and the presence of underlying chronic pulmonary disease.
Patients with HIV-associated TB pose several diagnostic problems (see “HIV-Associated TB,” above). Moreover, HIV-infected patients with sputum culture–positive, AFB-positive TB may present with a normal chest radiograph. The Xpert MTB/RIF assay is the preferred rapid diagnostic test in this population of patients because of simplicity of use and a sensitivity of ~60% among AFB-negative culture-positive cases and of 97% among AFB-positive cases. With the advent of ART, the occurrence of disseminated M. avium complex disease that can be confused with TB has become much less common.
SEROLOGIC AND OTHER DIAGNOSTIC TESTS FOR ACTIVE TB
A number of serologic tests based on detection of antibodies to a variety of mycobacterial antigens are marketed in developing countries but not in the United States. Careful independent assessments of these tests suggest that they are not useful as diagnostic aids—especially in persons with a low probability of TB—because of their low sensitivity and specificity and their poor reproducibility. After a rigorous evaluation of the tests, the WHO issued a “negative” recommendation in 2011 in order to prevent their abuse in the private sector of many resource-limited countries. Various methods aimed at detection of mycobacterial antigens in diagnostic specimens are being investigated but are limited at present by low sensitivity. Determinations of ADA and IFN-γ levels in pleural fluid may be useful adjunctive tests in the diagnosis of pleural TB; their utility in the diagnosis of other forms of extrapulmonary TB (e.g., pericardial, peritoneal, and meningeal) is less clear.
DIAGNOSIS OF LATENT M. TUBERCULOSIS INFECTION
Tuberculin Skin Testing In 1891, Robert Koch discovered that components of M. tuberculosis in a concentrated liquid culture medium, subsequently named “old tuberculin,” were capable of eliciting a skin reaction when injected subcutaneously into patients with TB. In 1932, Seibert and Munday purified this product by ammonium sulfate precipitation to produce an active protein fraction known as tuberculin purified protein derivative (PPD). In 1941, PPD-S, developed by Seibert and Glenn, was chosen as the international standard. Later, the WHO and UNICEF sponsored large-scale production of a master batch of PPD (RT23) and made it available for general use. The greatest limitation of PPD is its lack of mycobacterial species specificity, a property due to the large number of proteins in this product that are highly conserved in the various species. In addition, subjectivity of the skin-reaction interpretation, deterioration of the product, and batch-to-batch variations limit the usefulness of PPD.
The skin test with tuberculin PPD (TST) is most widely used in screening for LTBI. It probably measures the response to antigenic stimulation by T cells that reside in the skin rather than the response of recirculating memory T cells. The test is of limited value in the diagnosis of active TB because of its relatively low sensitivity and specificity and its inability to discriminate between LTBI and active disease. False-negative reactions are common in immunosuppressed patients and in those with overwhelming TB. False-positive reactions may be caused by infections with nontuberculous mycobacteria (Chap. 204) and by BCG vaccination. Repeated TST can produce larger reaction sizes due to either boosting or true conversion. The “boosting phenomenon” is a spurious TST conversion resulting from boosting of reactivity on subsequent TST 1–5 weeks after the initial test. Distinguishing boosting from true conversion is difficult yet important and can be based on clinical and epidemiologic considerations. For instance, true conversions are likely after BCG vaccination in a previously TST-negative person or in a close contact of an infectious patient.
IFN-γ Release Assays Two in vitro assays that measure T cell release of IFN-γ in response to stimulation with the highly TB-specific antigens ESAT-6 and CFP-10 are available. The T-SPOT®.TB test (Oxford Immunotec, Oxford, United Kingdom) is an enzyme-linked immunospot (ELISpot) assay, and the QuantiFERON®-TB Gold test (Qiagen GmbH, Hilden, Germany) is a whole-blood enzyme-linked immunosorbent assay (ELISA) for measurement of IFN-γ. The QuantiFERON®-TB Gold In-Tube assay, which facilitates blood collection and initial incubation, also contains another specific antigen, TB7.7. These tests likely measure the response of recirculating memory T cells—normally part of a reservoir in the spleen, bone marrow, and lymph nodes—to persisting bacilli producing antigenic signals.
In settings or population groups with low TB and HIV burdens, IFN-γ release assays (IGRAs) have previously been reported to be more specific than the TST as a result of less cross-reactivity due to BCG vaccination and sensitization by nontuberculous mycobacteria. Recent studies, however, suggest that IGRAs may not perform well in serial testing (e.g., among health care workers) and that interpretation of test results is dependent on cutoff values used to define positivity. Potential advantages of IGRAs include logistical convenience, the need for fewer patient visits to complete testing, and the avoidance of somewhat subjective measurements such as skin induration. However, IGRAs require that blood be drawn from the individual and then delivered to the laboratory in a timely fashion. IGRAs also require that testing be performed in a laboratory setting. These requirements pose challenges similar to those faced with the TST, including cold-chain requirements and batch-to-batch variations. Because of higher specificity and other potential advantages, IGRAs have usually replaced the TST for LTBI diagnosis in low-incidence, high-income settings. However, in high-incidence TB and HIV settings and population groups, there is limited and inconclusive evidence about the performance and usefulness of IGRAs. In view of higher costs and increased technical requirements, the WHO does not recommend the replacement of the TST by IGRAs in low- and middle-income countries.
A number of national guidelines on the use of IGRAs for LTBI testing have been issued. In the United States, an IGRA is preferred to the TST for most persons over the age of 5 years who are being screened for LTBI. However, for those at high risk of progression to active TB (e.g., HIV-infected persons), either test—or, to optimize sensitivity, both tests—may be used. Because of the paucity of data on the use of IGRAs in children, the TST is preferred for LTBI testing of children under age 5. In Canada and some European countries, a two-step approach for those with positive TSTs—i.e., initial TST followed by an IGRA—is recommended. However, a TST may boost an IGRA response if the interval between the two tests exceeds 3 days. Similar to the TST, current IGRAs have only modest predictive value for incident active TB, are not useful in identifying patients with the highest risk of progression toward disease, and cannot be used for diagnosis of active TB.
|
TREATMENT |
TUBERCULOSIS |
The two aims of TB treatment are (1) to prevent morbidity and death by curing TB while preventing the emergence of drug resistance and (2) to interrupt transmission by rendering patients noninfectious. Chemotherapy for TB became possible with the discovery of streptomycin in 1943. Randomized clinical trials clearly indicated that the administration of streptomycin to patients with chronic TB reduced mortality rates and led to cure in the majority of cases. However, monotherapy with streptomycin eventually was associated with the development of resistance to this drug and the resulting failure of treatment. With the introduction into clinical practice of para-aminosalicylic acid (PAS) and isoniazid, it became axiomatic in the early 1950s that cure of TB required the concomitant administration of at least two agents to which the organism was susceptible. Furthermore, early clinical trials demonstrated that a long period of treatment—i.e., 12–24 months—was required to prevent recurrence. The introduction of rifampin (rifampicin) in the early 1970s heralded the era of effective short-course chemotherapy, with a treatment duration of <12 months. The discovery that pyrazinamide, which was first used in the 1950s, augmented the potency of isoniazid/rifampin regimens led to the use of a 6-month course of this triple-drug regimen as standard therapy.
DRUGS
Four major drugs are considered first-line agents for the treatment of TB: isoniazid, rifampin, pyrazinamide, and ethambutol (Table 202-2). These drugs are well absorbed after oral administration, with peak serum levels at 2–4 h and nearly complete elimination within 24 h. These agents are recommended on the basis of their bactericidal activity (i.e., their ability to rapidly reduce the number of viable organisms and render patients noninfectious), their sterilizing activity (i.e., their ability to kill all bacilli and thus sterilize the affected tissues, measured in terms of the ability to prevent relapses), and their low rate of induction of drug resistance by selection of mutant bacilli. Two additional rifamycins, rifapentine and rifabutin, are also available in the United States; however, the level of cross-resistance with rifampin is high. For a detailed discussion of the drugs used for the treatment of TB, see Chap. 205e.
|
RECOMMENDED DOSAGEa FOR INITIAL TREATMENT OF TUBERCULOSIS IN ADULTSb |
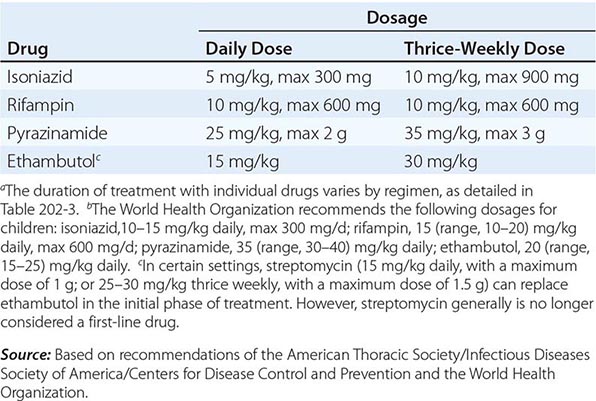
Because of a lower degree of efficacy and a higher degree of intolerability and toxicity, six classes of second-line drugs are generally used only for the treatment of patients with TB resistant to first-line drugs: (1) the fluoroquinolone antibiotics; (2) the injectable aminoglycosides kanamycin, amikacin, and streptomycin; (3) the injectable polypeptide capreomycin; and the oral agents (4) ethionamide and prothionamide, (5) cycloserine and terizidone (therizidone), and (6) PAS. Streptomycin, formerly a first-line agent, is now rarely used for drug-resistant TB because resistance levels worldwide are high and it is more toxic than the other drugs in the same class; however, the level of cross-resistance with the other injectables is low. Of the quinolones, later-generation agents such as levofloxacin and moxifloxacin are preferred. Gatifloxacin (no longer marketed in several countries, including the United States, because of previously observed dysglycemia) has recently been tested in a 4-month regimen that produced no detectable major side effects; thus, this drug could be reconsidered as a good alternative. Other drugs (referred to by the WHO as “group 5”) whose efficacy is not clearly defined are used in the treatment of patients with TB resistant to most of the first- and second-line agents; these drugs include clofazimine, linezolid, amoxicillin/clavulanic acid, clarithromycin, and carbapenems such as imipenem/cilastatin and meropenem. Today amithiozone (thiacetazone) is used very rarely because it has been associated with severe and at times fatal skin reactions among HIV-infected patients. Two novel drugs belonging to two new antibiotic classes—the diarylquinoline bedaquiline and the nitroimidazole delamanid—have recently been approved for use in severe cases of MDR-TB by stringent regulatory authorities (the U.S. Food and Drug Administration [FDA] and the European Medicine Agency [EMA] in the case of bedaquiline; the EMA and the Pharmaceuticals and Medical Devices Agency of Japan in the case of delamanid).
REGIMENS
Standard short-course regimens are divided into an initial, or bactericidal, phase and a continuation, or sterilizing, phase. During the initial phase, the majority of the tubercle bacilli are killed, symptoms resolve, and usually the patient becomes noninfectious. The continuation phase is required to eliminate persisting mycobacteria and prevent relapse. The treatment regimen of choice for virtually all forms of drug-susceptible TB in adults consists of a 2-month initial (or intensive) phase of isoniazid, rifampin, pyrazinamide, and ethambutol followed by a 4-month continuation phase of isoniazid and rifampin (Table 202-3). This regimen can cure TB in more than 90% of patients. In children, most forms of TB in the absence of HIV infection or suspected isoniazid resistance can be safely treated without ethambutol in the intensive phase. Treatment should be given daily throughout the course. However, daily treatment during the intensive phase and intermittently (three times weekly) during the continuation phase is an alternative for patients who can be directly supervised and properly supported. A fully supervised, three-times-weekly regimen throughout the course also can be offered in the absence of HIV infection, although the risk of acquired drug resistance is higher than that among patients treated daily for the full course. In addition, if the infecting strain is resistant to isoniazid, the risks of both acquired resistance and treatment failure are higher with three-times-weekly intensive therapy than with daily treatment in the intensive phase. HIV-infected patients should always receive their initial-phase regimen daily (see below). A continuation phase of once-weekly rifapentine and isoniazid has been shown to be equally effective for HIV-seronegative patients with noncavitary pulmonary TB who have negative sputum cultures at 2 months. Patients with cavitary pulmonary TB and delayed sputum-culture conversion (i.e., those who remain culture-positive at 2 months) should be tested immediately for drug-resistant TB, and a change of regimen should be considered. To prevent isoniazid-related neuropathy, pyridoxine (10–25 mg/d) should be added to the regimen given to persons at high risk of vitamin B6 deficiency (e.g., alcoholics; malnourished persons; pregnant and lactating women; and patients with conditions such as chronic renal failure, diabetes, and HIV infection, which are also associated with neuropathy). A full course of therapy (completion of treatment) is defined more accurately by the total number of doses taken than by the duration of treatment, although the course should not include interruptions of longer than 4 weeks. Specific recommendations on the required number of doses for each of the various treatment regimens have been published jointly by the American Thoracic Society, the Infectious Diseases Society of America, and the CDC. In some developing countries where the ability to ensure adherence to treatment is limited, a continuation-phase regimen of daily isoniazid and ethambutol for 6 months was used in the past. However, this regimen is associated with a higher rate of relapse, failure, and death, especially among HIV-infected patients, and is no longer recommended by the WHO.
|
RECOMMENDED ANTITUBERCULOSIS TREATMENT REGIMENS |
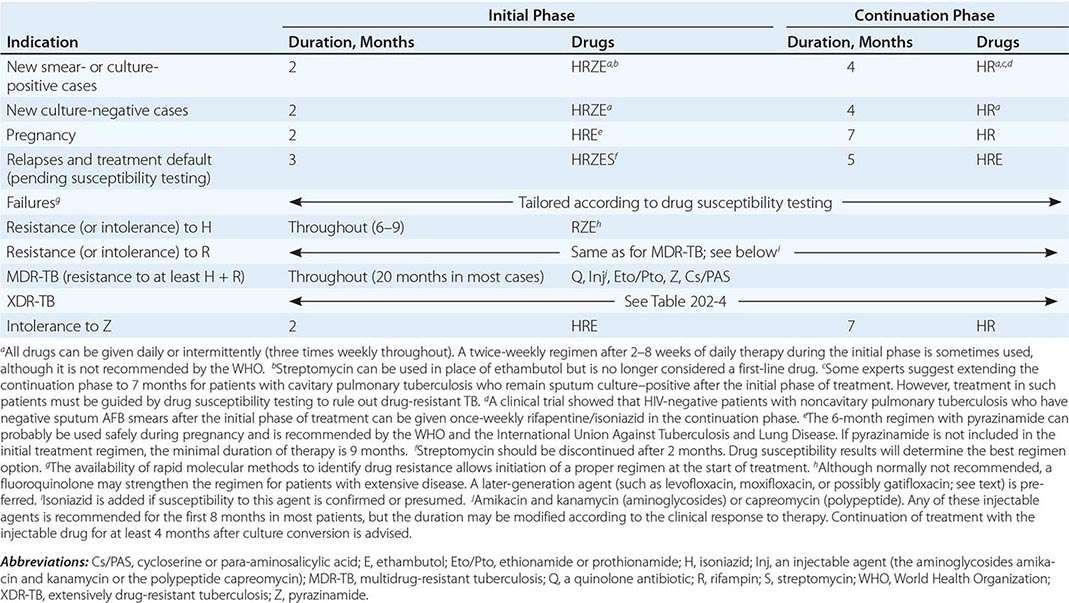
Lack of adherence to treatment is recognized worldwide as the most important impediment to cure. Moreover, the tubercle bacilli infecting patients who do not fully adhere to the prescribed regimen are likely to become drug resistant. Both patient- and provider-related factors may affect adherence. Patient-related factors include a lack of belief that the illness is significant and/or that treatment will have a beneficial effect; the existence of concomitant medical conditions (notably alcohol or substance abuse); lack of social support; fear of stigma and discrimination associated with TB; and poverty, with attendant joblessness and homelessness. Provider-related factors that may promote adherence include the support, education, and encouragement of patients and the offering of convenient clinic hours. In addition to specific measures promoting adherence, two other strategic approaches are used: direct supervision of treatment with support to the patient, consisting of incentives and enablers such as meals, travel vouchers, cash transfers, and grants to replace income loss; and provision of fixed-drug-combination products that reduce the number of tablets the patient needs to swallow. Because it is difficult to predict which patients will adhere to the recommended treatment for a disease that has important public as well as individual health implications, all patients should have their therapy directly supervised, especially during the initial phase, with proper social support including education, psychosocial counseling, and material sustainment. In an increasing number of countries, personnel to supervise therapy are usually available through TB control programs of local public health departments and from members of the community who are accepted by the patient to undertake that role and who have been properly educated by health workers. Direct supervision with patient support usually increases the proportion of patients completing treatment in all settings and greatly lessens the chances of failure, relapse, and acquired drug resistance. Fixed-drug-combination products (e.g., isoniazid/rifampin, isoniazid/rifampin/pyrazinamide, and isoniazid/rifampin/pyrazinamide/ethambutol) are available and are strongly recommended as a means of minimizing the likelihood of prescription error and of the development of drug resistance as the result of monotherapy. In some formulations of these combination products, the bioavailability of rifampin has been found to be substandard. Stringent regulatory authorities ensure that combination products are of good quality; however, this type of quality assurance is not always operative in low-income countries. Alternative regimens for patients who exhibit drug intolerance or adverse reactions are listed in Table 202-3. However, severe side effects prompting discontinuation of any of the first-line drugs and use of these alternative regimens are uncommon. The fluoroquinolones moxifloxacin and gatifloxacin have been tested as 4-month treatment-shortening regimens for drug-susceptible TB. Recently published results from these clinical trials failed to show that a 4-month regimen substituting gatifloxacin for ethambutol or moxifloxacin for either ethambutol or isoniazid is noninferior to the standard 6-month regimen. Thus, currently there is no 4-month regimen available for TB treatment.
MONITORING TREATMENT RESPONSE AND DRUG TOXICITY
Bacteriologic evaluation through culture and/or smear microscopy is essential in monitoring the response to treatment for TB. In addition, the patient’s weight should be monitored regularly and the drug dosage adjusted with any significant weight change. Patients with pulmonary disease should have their sputum examined monthly until cultures become negative to allow early detection of treatment failure. With the recommended regimen, more than 80% of patients will have negative sputum cultures at the end of the second month of treatment. By the end of the third month, the sputum of virtually all patients should be culture negative. In some patients, especially those with extensive cavitary disease and large numbers of organisms, AFB smear conversion may lag behind culture conversion. This phenomenon is presumably due to the expectoration and microscopic visualization of dead bacilli. As noted above, patients with cavitary disease in whom sputum culture conversion does not occur by 2 months require immediate testing for drug resistance. When a patient’s sputum cultures remain positive at ≥3 months, treatment failure and drug resistance or poor adherence to the regimen are likely, and testing of drug resistance should guide the choice of the best treatment option (see below). A sputum specimen should be collected by the end of treatment to document cure. If mycobacterial cultures are not practical, then monitoring by AFB smear examination should be undertaken at 2, 5, and 6 months. Smears that are positive after 3 months of treatment when the patient is known to be adherent are indicative of treatment failure and possible drug resistance. Therefore, if not done at the start of treatment, drug susceptibility testing is mandatory at this stage. Bacteriologic monitoring of patients with extrapulmonary TB is more difficult and often is not feasible. In these cases, the response to treatment must be assessed clinically and radiographically.
Monitoring of the response during chemotherapy by nucleic acid amplification technology has not been shown to be suitable. Thus Xpert MTB/RIF should not be used to monitor treatment. Likewise, serial chest radiographs are not recommended because radiographic changes may lag behind bacteriologic response and are not highly sensitive. After the completion of treatment, neither sputum examination nor chest radiography is recommended for routine follow-up purposes. However, a chest radiograph obtained at the end of treatment may be useful for comparative purposes should the patient develop symptoms of recurrent TB months or years later. Patients should be instructed to report promptly for medical assessment if they develop any such symptoms. In addition, an end-of-treatment chest radiograph may reveal earlier the post-TB complications described above.
During treatment, patients should be monitored for drug toxicity. The most common adverse reaction of significance is hepatitis. Patients should be carefully educated about the signs and symptoms of drug-induced hepatitis (e.g., dark urine, loss of appetite) and should be instructed to discontinue treatment promptly and see their health care provider should these symptoms occur. Although biochemical monitoring is not routinely recommended, all adult patients should undergo baseline assessment of liver function (e.g., measurement of serum levels of hepatic aminotransferases and bilirubin). Older patients, those with concomitant diseases, those with a history of hepatic disease (especially hepatitis C), and those using alcohol daily should be monitored especially closely (i.e., monthly), with repeated measurements of aminotransferases, during the initial phase of treatment. Up to 20% of patients have small increases in aspartate aminotransferase (up to three times the upper limit of normal) that are not accompanied by symptoms and are of no consequence. For patients with symptomatic hepatitis and those with marked (five- to sixfold) elevations in serum levels of aspartate aminotransferase, treatment should be stopped and drugs reintroduced one at a time after liver function has returned to normal. Hypersensitivity reactions usually require the discontinuation of all drugs and rechallenge to determine which agent is the culprit. Because of the variety of regimens available, it usually is not necessary—although it is possible—to desensitize patients. Hyperuricemia and arthralgia caused by pyrazinamide can usually be managed by the administration of acetylsalicylic acid; however, pyrazinamide treatment should be stopped if the patient develops gouty arthritis. Individuals who develop autoimmune thrombocytopenia secondary to rifampin therapy should not receive the drug thereafter. Similarly, the occurrence of optic neuritis with ethambutol is an indication for permanent discontinuation of this drug. Other common manifestations of drug intolerance, such as pruritus and gastrointestinal upset, can generally be managed without the interruption of therapy.
TREATMENT FAILURE AND RELAPSE
As stated above, treatment failure should be suspected when a patient’s sputum smears and/or cultures remain positive after 3 months of treatment. In the management of such patients, it is imperative that the current isolate be urgently tested for susceptibility to first- and second-line agents. Initial molecular testing for rifampin resistance should be done if the technology is available. When the results of susceptibility testing are based on molecular methods and are expected to become available within a few days, changes in the regimen can be postponed until that time. However, if the patient’s clinical condition is deteriorating, an earlier change in regimen may be indicated. A cardinal rule in the latter situation is always to add more than one drug at a time to a failing regimen: at least two and preferably three drugs that have never been used and to which the bacilli are likely to be susceptible should be added. The patient may continue to take isoniazid and rifampin along with these new agents pending the results of susceptibility tests.
Patients who experience a recurrence after apparently successful treatment (relapse) are less likely to harbor drug-resistant strains (see below) than are patients in whom treatment has failed. Acquired resistance is uncommon among strains from patients in whom relapse follows the completion of a standard short-course regimen. However, pending the results of susceptibility testing, it is prudent to begin the treatment of all patients whose infections have relapsed with a standard regimen containing all four first-line drugs plus streptomycin. In less affluent countries and other settings where facilities for culture and drug susceptibility testing are not yet routinely available and where the prevalence of MDR-TB is low, the WHO recommends that a standard regimen with all four first-line drugs plus streptomycin be used in all instances of relapse and treatment default. Patients with treatment failure and those relapsing or defaulting with a high likelihood of MDR-TB should receive a regimen that includes second-line agents and is based on their history of anti-TB treatment and the drug resistance patterns in the population (Table 202-3). Once drug susceptibility test results are available, the regimen can be adjusted accordingly.
DRUG-RESISTANT TB
Strains of M. tuberculosis resistant to individual drugs arise by spontaneous point mutations in the mycobacterial genome that occur at low but predictable rates (10–7–10–10 for the key drugs). Resistance to rifampin is associated with mutations in the rpoB gene in 95% of cases; that to isoniazid with mutations mainly in the katG (50–95% of cases) and inhA (up to 45%) genes; that to pyrazinamide in the pncA gene (up to 98%); that to ethambutol in the embB gene (50–65%); that to the fluoroquinolones in the gyrA–gyrB genes (75–95%); and that to the aminoglycosides mainly in the rrs gene (up to 80%). Because there is no cross-resistance among the commonly used drugs, the probability that a strain will be resistant to two drugs is the product of the probabilities of resistance to each drug and thus is low. The development of drug-resistant TB is almost invariably the result of monotherapy—i.e., the failure of the health care provider to prescribe at least two drugs to which tubercle bacilli are susceptible or of the patient to take properly prescribed therapy. In addition, the use of drugs of substandard quality may cause the emergence of drug resistance. Drug-resistant TB may be either primary or acquired. Primary drug resistance is that which develops in a patient infected from the start by a drug-resistant strain. Acquired resistance is that which develops during treatment with an inappropriate regimen. In North America, Western Europe, most of Latin America, and the Persian Gulf States, rates of primary resistance are generally low and isoniazid resistance is most common. In the United States, although rates of primary isoniazid resistance have been stable at ~7–8%, the rate of primary MDR-TB has declined from 2.5% in 1993 to 1% since 2000. As described above, MDR-TB is an increasingly serious problem in some regions, especially in the states of the former Soviet Union and some countries of Asia (Fig. 202-11). Even more serious is the recently described occurrence of XDR-TB due to MDR strains that are also resistant to any fluoroquinolones and to any of three second-line injectable agents (amikacin, kanamycin, and capreomycin). Creation of drug-resistant TB can be prevented by adherence to the principles of sound treatment: inclusion of at least two quality-assured, bactericidal drugs to which the organism is susceptible; use of fixed-drug-combination products; supervision of treatment with patient support; and verification that patients complete the prescribed course. Transmission of drug-resistant strains can be prevented by implementation of respiratory infection-control measures (see below).
FIGURE 202-11 Percentage of new tuberculosis cases with multidrug resistance in all countries surveyed by the World Health Organization (WHO) Global Drug Resistance Surveillance Project during 1994–2013. (See disclaimer in Fig. 202-2. Courtesy of the Global TB Programme, WHO; with permission.)
Although the 6-month regimen described in Table 202-3 is generally effective for patients with initial isoniazid-resistant disease, it is prudent to include at least ethambutol and possibly pyrazinamide for the full 6 months and to consider extending the treatment course to 9 months. In such cases, isoniazid probably does not contribute to a successful outcome and could be omitted. In case of documented resistance to both isoniazid and ethambutol, a 9- to 12-month regimen of rifampin, pyrazinamide, and a fluoroquinolone can be used. Any patients whose isolates exhibit resistance to rifampin should be managed as if they had MDR-TB (see below), with the addition of isoniazid if susceptibility to this agent is confirmed via rapid testing or is presumed. MDR-TB, in which bacilli are resistant to (at least) isoniazid and rifampin, is more difficult to manage than is disease caused by drug-susceptible organisms because these two bactericidal drugs are the most potent agents available and because associated resistance to other first-line drugs as well (e.g., ethambutol) is not uncommon. For treatment of MDR-TB, the WHO recommends that in most patients five drugs be used in the initial phase of at least 8 months: a later-generation fluoroquinolone, an injectable agent (the aminoglycosides amikacin or kanamycin or the polypeptide capreomycin), ethionamide (or prothionamide), either cycloserine or PAS, and pyrazinamide. Ethambutol can be added (Table 202-3). Although the optimal duration of treatment is not known, a course of at least 20 months is recommended for previously untreated patients, including the initial phase with an injectable agent, which is usually discontinued at 4 months after culture conversion.
In late 2012, the FDA granted accelerated approval of bedaquiline, a diarylquinoline antibiotic. This new drug, when given for the first 24 weeks (400 mg daily for 2 weeks followed by 200 mg thrice weekly for 22 weeks), has been shown to increase the efficacy of the WHO standard regimen for MDR-TB with faster sputum conversion. Bedaquiline should be used with caution in people >65 years of age and in HIV-infected patients; its use is not advised in children and pregnant women. In early 2014, the European Medical Agency granted accelerated approval of another new agent, the nitroimidazole compound delamanid. Data from a phase 2B clinical trial in which delamanid was added to the WHO-recommended standard MDR-TB regimen have shown increased culture conversion at 2 months. Pending phase 3 trial results and in view of potential side effects of both new drugs (including QT interval prolongation in both cases and hepatotoxicity in the case of bedaquiline), the WHO recommends limiting the use of bedaquiline and delamanid to cases of MDR-TB when an effective WHO-recommended standard MDR-TB regimen cannot be designed because of known resistance, intolerance, or nonavailability of any second-line drugs in the regimen. Patients treated with bedaquiline or delamanid should be counseled, should give informed consent, and should be closely monitored during treatment. In particular, patients with cardiac anomalies such as prolonged QT interval or a history of ventricular arrhythmias should not be given these drugs. Currently, there is no information about simultaneous use of these two agents; therefore, combining them is not recommended.
Finally, a shorter (9-month) regimen consisting of gatifloxacin or moxifloxacin, clofazimine, ethambutol, and pyrazinamide given throughout the treatment period and supplemented by prothionamide, kanamycin, and high-dose isoniazid during an intensive phase of at least 4 months is reportedly effective for MDR-TB in certain settings. Further investigations are necessary to elucidate the role of this shorter regimen in MDR-TB treatment.
Patients with XDR-TB have fewer treatment options and a much poorer prognosis. However, observational studies have shown that aggressive management of cases comprising early drug-susceptibility testing, rational combination of at least five drugs, readjustment of the regimen, strict directly observed therapy, monthly bacteriologic monitoring, and intensive patient support may result in cure and avert death. Table 202-4 summarizes the management of patients with XDR-TB. Some recently published studies regarding the use of linezolid in patients with XDR-TB suggest that, although it carries a high level of toxicity, this drug increases culture conversion.
|
MANAGEMENT GUIDELINES FOR PATIENTS WITH DOCUMENTED OR STRONGLY SUSPECTED EXTENSIVELY DRUG-RESISTANT TUBERCULOSIS (XDR-TB) |
aThis recommendation is made because, although the reproducibility and reliability of susceptibility testing with injectable agents are good, few data are available on the correlation of clinical efficacy with test results. Options with XDR-TB are very limited, and some strains may be affected in vivo by an injectable agent even though they test resistant in vitro.bGatifloxacin (no longer marketed in several countries, including the United States, because of previously observed dysglycemia) has recently been tested in a 4-month regimen that produced no detectable major side effects; thus, this drug could be reconsidered as a good alternative.cThe number of drugs added is based on how many oral bacteriostatic drugs (see point 4 above) are believed to be effective: the advice is to add one drug if there is confidence in all three bacteriostatic drugs; two if there is confidence in only two bacteriostatic drugs; and three or more if there is confidence in only one bacteriostatic drug or none.
For patients with localized disease and sufficient pulmonary reserve, lobectomy or pneumonectomy may be considered. Because the management of patients with MDR- and XDR-TB is complicated by both social and medical factors, care of these patients is ideally provided in specialized centers or, in their absence, in the context of programs with adequate resources and capacity, including community support.
HIV-ASSOCIATED TB
Several observational studies and randomized controlled trials have shown that treatment of HIV-associated TB with anti-TB drugs and simultaneous use of ART are associated with significant reductions in mortality risk and AIDS-related events. Evidence from randomized controlled trials shows that early initiation of ART during anti-TB treatment is associated with a 34–68% reduction in mortality rates, with especially good results in patients with CD4+ T cell counts of <50/μL. Therefore, the main aim in the management of HIV-associated TB is to initiate anti-TB treatment and to immediately consider initiating or continuing ART. All HIV-infected TB patients, regardless of CD4+ T cell count, are candidates for ART, which optimally is initiated as soon as possible after the diagnosis of TB and within the first 8 weeks of anti-TB therapy. However, ART should be started within the first 2 weeks of TB treatment for patients with CD4+ T cell counts of <50/μL. In general, the standard 6-month daily regimen is equally efficacious in HIV-negative and HIV-positive patients for treatment of drug-susceptible TB. As for any other adult living with HIV (Chap. 226), first-line ART for TB patients should consist of two nucleoside reverse transcriptase inhibitors (NRTIs) plus a nonnucleoside reverse transcriptase inhibitor (NNRTI). Although TB treatment modalities are similar to those in HIV-negative patients, adverse drug effects may be more pronounced in HIV-infected patients. In this regard, three important considerations are relevant: an increased frequency of paradoxical reactions, interactions between ART components and rifamycins, and development of rifampin monoresistance with intermittent treatment. IRIS—i.e., the exacerbation of symptoms and signs of TB—has been described above. Rifampin, a potent inducer of enzymes of the cytochrome P450 system, lowers serum levels of many HIV protease inhibitors and some NNRTIs—essential drugs used in ART. In such cases, rifabutin, which has much less enzyme-inducing activity, has been used in place of rifampin. However, dosage adjustments for rifabutin and protease inhibitors are still being assessed. Several clinical trials have found that patients with HIV-associated TB whose degree of immunosuppression is advanced (e.g., CD4+ T cell counts of <100/μL) are prone to treatment failure and relapse with rifampin-resistant organisms when treated with “highly intermittent” (i.e., once- or twice-weekly) rifamycin-containing regimens. Consequently, it is recommended that all TB patients who are infected with HIV receive a rifampin-containing regimen on a daily basis. Because recommendations are frequently updated, consultation of the following websites is advised: www.who.int/hiv, www.who.int/tb, www.cdc.gov/hiv, and www.cdc.gov/tb.
SPECIAL CLINICAL SITUATIONS
Although comparative clinical trials of treatment for extrapulmonary TB are limited, the available evidence indicates that most forms of disease can be treated with the 6-month regimen recommended for patients with pulmonary disease. The WHO and the American Academy of Pediatrics recommend that children with bone and joint TB, tuberculous meningitis, or miliary TB receive up to 12 months of treatment. Treatment for TB may be complicated by underlying medical problems that require special consideration. As a rule, patients with chronic renal failure should not receive aminoglycosides and should receive ethambutol only if serum drug levels can be monitored. Isoniazid, rifampin, and pyrazinamide may be given in the usual doses in cases of mild to moderate renal failure, but the dosages of isoniazid and pyrazinamide should be reduced for all patients with severe renal failure except those undergoing hemodialysis. Patients with hepatic disease pose a special problem because of the hepatotoxicity of isoniazid, rifampin, and pyrazinamide. Patients with severe hepatic disease may be treated with ethambutol, streptomycin, and possibly another drug (e.g., a fluoroquinolone); if required, isoniazid and rifampin may be administered under close supervision. The use of pyrazinamide by patients with liver failure should be avoided. Silicotuberculosis necessitates the extension of therapy by at least 2 months.
The regimen of choice for pregnant women (Table 202-3) is 9 months of treatment with isoniazid and rifampin supplemented by ethambutol for the first 2 months. Although the WHO has recommended routine use of pyrazinamide for pregnant women, this drug has not been recommended in the United States because of insufficient data documenting its safety in pregnancy. Streptomycin is contraindicated because it is known to cause eighth-cranial-nerve damage in the fetus. Treatment for TB is not a contraindication to breast-feeding; most of the drugs administered will be present in small quantities in breast milk, albeit at concentrations far too low to provide any therapeutic or prophylactic benefit to the child.
Medical consultation on difficult-to-manage cases is provided by the U.S. CDC Regional Training and Medical Consultation Centers (www.cdc.gov/tb/education/rtmc/).
PREVENTION
The best way to prevent TB is to diagnose and isolate infectious cases rapidly and to administer appropriate treatment until patients are rendered noninfectious (usually 2–4 weeks after the start of proper treatment) and the disease is cured. Additional strategies include BCG vaccination and treatment of persons with LTBI who are at high risk of developing active disease.
BCG VACCINATION
BCG was derived from an attenuated strain of M. bovis and was first administered to humans in 1921. Many BCG vaccines are available worldwide; all are derived from the original strain, but the vaccines vary in efficacy, ranging from 80% to nil in randomized, placebo-controlled trials. A similar range of efficacy was found in recent observational studies (case–control, historic cohort, and cross-sectional) in areas where infants are vaccinated at birth. These studies and a meta-analysis also found higher rates of efficacy in the protection of infants and young children from serious disseminated forms of childhood TB, such as tuberculous meningitis and miliary TB. BCG vaccine is safe and rarely causes serious complications. The local tissue response begins 2–3 weeks after vaccination, with scar formation and healing within 3 months. Side effects—most commonly, ulceration at the vaccination site and regional lymphadenitis—occur in 1–10% of vaccinated persons. Some vaccine strains have caused osteomyelitis in ~1 case per million doses administered. Disseminated BCG infection (“BCGitis”) and death have occurred in 1–10 cases per 10 million doses administered, although this problem is restricted almost exclusively to persons with impaired immunity, such as children with severe combined immunodeficiency syndrome or adults with HIV infection. BCG vaccination induces TST reactivity, which tends to wane with time. The presence or size of TST reactions after vaccination does not predict the degree of protection afforded.
BCG vaccine is recommended for routine use at birth in countries with high TB prevalence. However, because of the low risk of transmission of TB in the United States and other high-income countries, the unreliable protection afforded by BCG, and its impact on the TST, the vaccine is not recommended for general use. HIV-infected adults and children should not receive BCG vaccine. Moreover, infants whose HIV status is unknown but who have signs and symptoms consistent with HIV infection or who are born to HIV-infected mothers should not receive BCG.
Over the past decade, renewed research and development efforts have been made toward a new TB vaccine. In mid-2014, 16 candidates were in clinical trials and 12 were being field tested. The first new vaccine, for which results of a clinical trial became available in early 2013, is MVA85A/AERAS-485; unfortunately, this viral-vectored vaccine did not show clinical benefit as a booster to BCG.
|
TREATMENT |
LATENT TUBERCULOSIS INFECTION |
It is estimated that about 2 billion people, or nearly one-third of the human population, have been infected with M. tuberculosis. Although only a small fraction of these infections will progress toward active disease, new active cases will continue to emerge from this pool of “latently” infected individuals. Unfortunately, there is no diagnostic test at present that can predict which individuals with LTBI will develop active TB. Treatment of selected persons with LTBI aims at preventing active disease. This intervention (also called preventive chemotherapy or chemoprophylaxis) is based on the results of a large number of randomized, placebo-controlled clinical trials demonstrating that a 6- to 9-month course of isoniazid reduces the risk of active TB in infected people by up to 90%. Analysis of available data indicates that the optimal duration of treatment is ~9 months. In the absence of reinfection, the protective effect is believed to be lifelong. Clinical trials have shown that isoniazid reduces rates of TB among TST-positive persons with HIV infection. Studies in HIV-infected patients have also demonstrated the effectiveness of shorter courses of rifampin-based treatment.
Candidates for treatment of LTBI are listed in Table 202-5. They can be identified by TST or IGRA of persons in defined high-risk groups. For skin testing, 5 tuberculin units of polysorbate-stabilized PPD should be injected intradermally into the volar surface of the forearm (i.e., the Mantoux method). Multipuncture tests are not recommended. Reactions are read at 48–72 h as the transverse diameter (in millimeters) of induration; the diameter of erythema is not considered. In some persons, TST reactivity wanes with time but can be recalled by a second skin test administered ≥1 week after the first (i.e., two-step testing). For persons periodically undergoing the TST, such as health care workers and individuals admitted to long-term-care institutions, initial two-step testing may preclude subsequent misclassification of those who have boosted reactions as TST converters. The cutoff for a positive TST (and thus for treatment) is related both to the probability that the reaction represents true infection and to the likelihood that the individual, if truly infected, will develop TB. Table 202-5 suggests possible cutoff by risk group. Thus, positive reactions for persons with HIV infection, recent close contacts of infectious cases, organ transplant recipients, previously untreated persons whose chest radiograph shows fibrotic lesions consistent with old TB, and persons receiving drugs that suppress the immune system are defined as an area of induration ≥5 mm in diameter. A 10-mm cutoff is used to define positive reactions in most other at-risk persons. For persons with a very low risk of developing TB if infected, a cutoff of 15 mm is used. (Except for employment purposes where longitudinal screening is anticipated, the TST is not indicated for these low-risk persons.) A positive IGRA is based on the manufacturers’ recommendations; however, good clinical practice requires that epidemiologic and clinical factors also guide the decision to implement treatment for LTBI and that active TB be definitively excluded before the initiation of chemoprophylaxis.
|
TUBERCULIN REACTION SIZE AND TREATMENT OF LATENT MYCOBACTERIUM TUBERCULOSIS INFECTION |
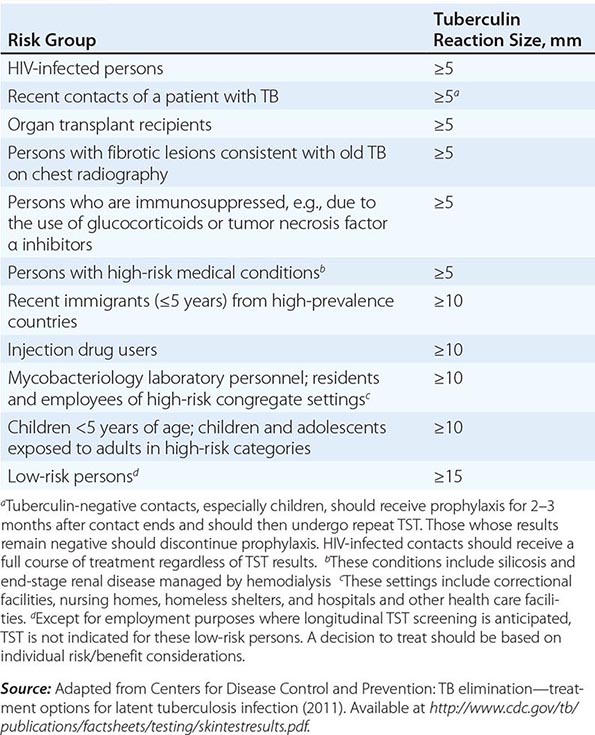
Some TST- and IGRA-negative individuals are also candidates for treatment. Once an appropriate clinical evaluation has excluded active TB, infants and children who have come into contact with infectious cases should be treated for presumed LTBI. HIV-infected persons who have been exposed to an infectious TB patient should receive treatment regardless of the TST result. Any HIV-infected candidate for LTBI treatment must be screened carefully to exclude active TB, which would necessitate full treatment. The use of a clinical algorithm based on four symptoms (current cough, fever, weight loss, and night sweats) helps to define which HIV-infected person is a candidate for LTBI treatment. The absence of all four symptoms tends to exclude active TB. The presence of one of the four symptoms, on the other hand, warrants further investigation for active TB before treatment of LTBI is started. Although administering a TST is prudent, this test is not an absolute requirement—given the logistical challenges—among people living with HIV in high-TB-incidence and low-resource settings.
Before treatment of LTBI begins, it is mandatory to carefully exclude active TB. Several regimens can be used to treat LTBI. The most widely used is that based on isoniazid alone at a daily dose of 5 mg/kg (up to 300 mg/d) for 9 months. On the basis of cost-benefit analyses and concerns about feasibility, a 6-month period of treatment is currently recommended by the WHO, especially in highly TB-endemic countries. Isoniazid can be administered intermittently (twice weekly) at a dose of 15 mg/kg (up to 900 mg) but only as directly observed therapy. An alternative regimen for adults is 4 months of daily rifampin. A 3-month regimen of daily isoniazid and rifampin is used in some countries (e.g., the United Kingdom) for both adults and children who are known not to have HIV infection. A previously recommended regimen of 2 months of rifampin and pyrazinamide has been associated with serious or even fatal hepatotoxicity and now is generally not recommended. The rifampin-containing regimens should be considered for persons who are likely to have been infected with an isoniazid-resistant strain. A recent clinical trial showed that a regimen of isoniazid (900 mg) and rifapentine (900 mg) given once weekly for 12 weeks is as effective as the standard 9-month isoniazid regimen. This regimen was associated with higher treatment completion (82% vs 69%) and less hepatotoxicity (0.4% vs 2.7%) than isoniazid alone, although the rate of permanent discontinuation due to an adverse event was higher (4.9% vs 3.7%).
Currently, the isoniazid–rifapentine regimen is not recommended for children <2 years of age, people living with HIV infection who are receiving ART, or pregnant women. Rifampin and rifapentine are contraindicated in HIV-infected individuals receiving protease inhibitors and most NNRTIs. (Efavirenz is the safest agent in this class of antiretrovirals for simultaneous administration with a rifamycin.) Clinical trials to assess the efficacy of long-term isoniazid administration (i.e., for at least 3 years) among people living with HIV in high-TB-transmission settings have shown that this regimen can be more effective than 9 months of isoniazid and is therefore recommended under those circumstances. Isoniazid should not be given to persons with active liver disease. All persons at increased risk of hepatotoxicity (e.g., those abusing alcohol daily and those with a history of liver disease) should undergo baseline and then monthly assessment of liver function. All patients should be carefully educated about hepatitis and instructed to discontinue use of the drug immediately should any symptoms develop. Moreover, patients should be seen and questioned monthly during therapy about adverse reactions and should be given no more than a 1-month supply of drug at each visit. Treatment of LTBI among persons likely to have been infected by a multidrug-resistant strain is a challenge because no regimens have yet been tested in clinical trials. Close observation for early signs of disease is one option; consultation with a TB expert is advised.
It may be more difficult to ensure compliance when treating persons with LTBI than when treating those with active TB. If family members of active cases are being treated, compliance and monitoring may be easier. When feasible, supervised therapy may increase the likelihood of completion. As in active cases, the provision of incentives may also be helpful.
PRINCIPLES OF TB CONTROL
The highest priority in any TB control program is the prompt detection of cases and the provision of short-course chemotherapy to all TB patients under proper case-management conditions, including directly observed therapy. In addition, screening of high-risk groups, including immigrants from high-prevalence countries, migratory workers, prisoners, homeless individuals, substance abusers, and HIV-seropositive persons, is recommended. TST-positive high-risk persons should be treated for LTBI as described above. Contact investigation is an important component of efficient TB control. In the United States and other countries worldwide, a great deal of attention has been given to the transmission of TB (particularly in association with HIV infection) in institutional settings such as hospitals, homeless shelters, and prisons. Measures to limit such transmission include respiratory isolation of persons with suspected TB until they are proven to be noninfectious (at least by sputum AFB smear negativity), proper ventilation in rooms of patients with infectious TB, use of ultraviolet irradiation in areas of increased risk of TB transmission, and periodic screening of personnel who may come into contact with known or unsuspected cases of TB. In the past, radiographic surveys, especially those conducted with portable equipment and miniature films, were advocated for case finding. Today, however, the prevalence of TB in industrialized countries is sufficiently low that “mass miniature radiography” is not cost-effective.
In high-prevalence countries, most TB control programs have made remarkable progress in reducing morbidity and mortality since the mid-1990s by adopting and implementing the strategy promoted by the WHO. Between 2000 and 2013, 37 million lives were saved, and since 1995, 61 million TB cases have been successfully treated. The essential elements of good TB care and control (the DOTS strategy) are (1) political commitment with increased and sustained financing; (2) case detection through quality-assured bacteriology (starting with examination of sputum from patients with cough of >2–3 weeks’ duration); (3) administration of standardized short-course chemotherapy, with direct supervision and patient support; (4) an effective drug supply and management system; and (5) a monitoring and evaluation system, with impact measurement (including assessment of treatment outcomes—e.g., cure, completion of treatment without bacteriologic proof of cure, death, treatment failure, and default—in all cases registered and notified). In 2006, the WHO indicated that, although these essential elements remain the fundamental components of any control strategy, additional steps must be undertaken to reach the 2015 international TB control targets set within the United Nations Millennium Development Goals. Thus, a new “Stop TB Strategy” with six components has been promoted since 2006: (1) Pursue high-quality DOTS expansion and enhancement. (2) Address HIV-associated TB, MDR-TB, and the needs of poor and vulnerable populations. (3) Contribute to health system strengthening. (4) Engage all care providers. (5) Empower people with TB and their communities. (6) Enable and promote research. As part of the fourth component, evidence-based International Standards for Tuberculosis Care—focused on diagnosis, treatment, and public health responsibilities—have been introduced for wide adoption by medical and professional societies, academic institutions, and all practitioners worldwide (http://www.who.int/tb/publications/ISTC_3rdEd.pdf?ua=1).
Care and control of HIV-associated TB are particularly challenging in developing countries because existing interventions require collaboration between HIV/AIDS and TB programs as well as standard services. While TB programs must test every patient for HIV in order to provide access to trimethoprim-sulfamethoxazole prophylaxis against common infections and ART, HIV/AIDS programs must regularly screen persons living with HIV/AIDS for active TB, provide treatment for LTBI, and ensure infection control in settings where people living with HIV congregate.
Early and active case detection is considered an important intervention not only among persons living with HIV/AIDS but also among other vulnerable populations, as it reduces transmission in a community and provides early effective care. For TB control efforts to succeed and for elimination to become a realistic target, programs must optimize their performance and include additional interventions as described. Moreover, the approach to TB control and care needs to become holistic and engage beyond dedicated programs. Therefore, the WHO’s “End TB” strategy has been designed and builds on three pillars for the post-2015 era of increased efforts by governments and national programs worldwide: (1) integrated, patient-centered care and prevention; (2) bold policies and supportive systems; and (3) intensified research and innovation. The first pillar incorporates all technological innovations, such as early diagnostic approaches (including universal drug-susceptibility testing and systematic screening of identified, setting-specific, high-risk groups); well-designed treatment regimens for all forms of TB; proper management of HIV-associated TB and other comorbidities; and preventive treatment of persons at high risk. The second pillar is fundamental and is normally beyond the control of dedicated programs, relying on policies forged by the highest-level health and governmental authorities: availability of adequate and well-identified human and financial resources; engagement of civil society organizations and all relevant public and private providers to facilitate care and prevention of all patients; a policy of universal health coverage (which implies avoidance of catastrophic expenditures caused by TB among the poorest); regulatory frameworks for case notifications, vital registration, quality and rational use of medicines, and infection control; social protection mechanisms; poverty alleviation strategies; and interventions on the broader determinants of TB. Finally, the third pillar of the new strategy emphasizes intensification of engagement in research and development of new tools and interventions as well as optimization of implementation and rapid adoption of new tools in endemic countries. In the end, besides specific clinical care and control interventions as described in this chapter, elimination of TB ultimately will require control and attenuation of the multitude of risk factors (e.g., HIV, smoking, and diabetes) and socioeconomic determinants (e.g., extreme poverty, inadequate living conditions and bad housing, alcoholism, malnutrition, and indoor air pollution) with clearly implemented policies within the health sector and other sectors linked to human development and welfare.
ACKNOWLEDGMENT
The contributions of Richard J. O’Brien to this chapter in previous editions are gratefully acknowledged.
203 |
Leprosy |
Leprosy, first described in ancient Indian texts from the sixth century B.C., is a nonfatal, chronic infectious disease caused by Mycobacterium leprae, the clinical manifestations of which are largely confined to the skin, peripheral nervous system, upper respiratory tract, eyes, and testes. The unique tropism of M. leprae for peripheral nerves (from large nerve trunks to microscopic dermal nerves) and certain immunologically mediated reactional states are the major causes of morbidity in leprosy. The propensity of the disease, when untreated, to result in characteristic deformities and the recognition in most cultures that the disease is communicable from person to person have resulted historically in a profound social stigma. Today, with early diagnosis and the institution of appropriate and effective antimicrobial therapy, patients can lead productive lives in the community, and deformities and other visible manifestations can largely be prevented.
ETIOLOGY
M. leprae is an obligate intracellular bacillus (0.3–1 μm wide and 1–8 μm long) that is confined to humans, armadillos in certain locales, and sphagnum moss. The organism is acid-fast, indistinguishable microscopically from other mycobacteria, and ideally detected in tissue sections by a modified Fite stain. Strain variability has been documented in this organism. M. leprae produces no known toxins and is well adapted to penetrate and reside within macrophages, yet it may survive outside the body for months. In untreated patients, only ~1% of M. leprae organisms are viable. The morphologic index (MI), a measure of the number of acid-fast bacilli (AFB) in skin scrapings that stain uniformly bright, correlates with viability. The bacteriologic index (BI), a logarithmic-scaled measure of the density of M. leprae in the dermis, may be as high as 4–6+ in untreated patients and falls by 1 unit per year during effective antimicrobial therapy; the rate of decrease is independent of the relative potency of therapy. A rising MI or BI suggests relapse and perhaps—if the patient is being treated—drug resistance. Drug resistance can be confirmed or excluded in the mouse model of leprosy, and resistance to dapsone and rifampin can be documented by the recognition of mutant genes. However, the availability of these technologies is extremely limited.
![]() As a result of reductive evolution, almost half of the M. leprae genome contains nonfunctional genes; only 1605 genes encode for proteins, and 1439 genes are shared with Mycobacterium tuberculosis. In contrast, M. tuberculosis uses 91% of its genome to encode for 4000 proteins. Among the lost genes in M. leprae are those for catabolic and respiratory pathways; transport systems; purine, methionine, and glutamine synthesis; and nitrogen regulation. The genome of M. leprae provides a metabolic rationale for its obligate intracellular existence and reliance on host biochemical support, a template for targets of drug development, and ultimately a pathway to cultivation. The finding of strain variability among M. leprae isolates has provided a powerful tool with which to address anew the organism’s epidemiology and pathobiology and to determine whether relapse represents reactivation or reinfection. The bacterium’s complex cell wall contains large amounts of an M. leprae–specific phenolic glycolipid (PGL-1), which is detected in serologic tests. The unique trisaccharide of M. leprae binds to the basal lamina of Schwann cells; this interaction is probably relevant to the fact that M. leprae is the only bacterium to invade peripheral nerves.
As a result of reductive evolution, almost half of the M. leprae genome contains nonfunctional genes; only 1605 genes encode for proteins, and 1439 genes are shared with Mycobacterium tuberculosis. In contrast, M. tuberculosis uses 91% of its genome to encode for 4000 proteins. Among the lost genes in M. leprae are those for catabolic and respiratory pathways; transport systems; purine, methionine, and glutamine synthesis; and nitrogen regulation. The genome of M. leprae provides a metabolic rationale for its obligate intracellular existence and reliance on host biochemical support, a template for targets of drug development, and ultimately a pathway to cultivation. The finding of strain variability among M. leprae isolates has provided a powerful tool with which to address anew the organism’s epidemiology and pathobiology and to determine whether relapse represents reactivation or reinfection. The bacterium’s complex cell wall contains large amounts of an M. leprae–specific phenolic glycolipid (PGL-1), which is detected in serologic tests. The unique trisaccharide of M. leprae binds to the basal lamina of Schwann cells; this interaction is probably relevant to the fact that M. leprae is the only bacterium to invade peripheral nerves.
Although it was the first bacterium to be etiologically associated with human disease, M. leprae remains one of the few bacterial species that still has not been cultivated on artificial medium or tissue culture. The multiplication of M. leprae in mouse footpads (albeit limited, with a doubling time of ~2 weeks) has provided a means to evaluate antimicrobial agents, monitor clinical trials, and screen vaccines. M. leprae grows best in cooler tissues (the skin, peripheral nerves, anterior chamber of the eye, upper respiratory tract, and testes), sparing warmer areas of the skin (the axilla, groin, scalp, and midline of the back).
EPIDEMIOLOGY
![]() Demographics Leprosy is almost exclusively a disease of the developing world, affecting areas of Asia, Africa, Latin America, and the Pacific. While Africa has the highest disease prevalence, Asia has the most cases. More than 80% of the world’s cases occur in a few countries: India, China, Myanmar, Indonesia, Brazil, Nigeria, Madagascar, and Nepal. Within endemic locales, the distribution of leprosy is quite uneven, with areas of high prevalence bordering on areas with little or no disease. In Brazil the majority of cases occur in the Amazon basin and two western states, while in Mexico leprosy is mostly confined to the Pacific coast. Except as imported cases, leprosy is largely absent from the United States, Canada, and northwestern Europe. In the United States, ~4000 persons have leprosy and 100–200 new cases are reported annually, most of them in California, Texas, New York, and Hawaii among immigrants from Mexico, Southeast Asia, the Philippines, and the Caribbean.
Demographics Leprosy is almost exclusively a disease of the developing world, affecting areas of Asia, Africa, Latin America, and the Pacific. While Africa has the highest disease prevalence, Asia has the most cases. More than 80% of the world’s cases occur in a few countries: India, China, Myanmar, Indonesia, Brazil, Nigeria, Madagascar, and Nepal. Within endemic locales, the distribution of leprosy is quite uneven, with areas of high prevalence bordering on areas with little or no disease. In Brazil the majority of cases occur in the Amazon basin and two western states, while in Mexico leprosy is mostly confined to the Pacific coast. Except as imported cases, leprosy is largely absent from the United States, Canada, and northwestern Europe. In the United States, ~4000 persons have leprosy and 100–200 new cases are reported annually, most of them in California, Texas, New York, and Hawaii among immigrants from Mexico, Southeast Asia, the Philippines, and the Caribbean.
![]() The comparative genomics of single-nucleotide polymorphisms support the likelihood that four distinct strains exist, having originated in East Africa or Central Asia. A mutation spread to Europe and subsequently underwent two separate mutations that were then followed by spread to West Africa and the Americas.
The comparative genomics of single-nucleotide polymorphisms support the likelihood that four distinct strains exist, having originated in East Africa or Central Asia. A mutation spread to Europe and subsequently underwent two separate mutations that were then followed by spread to West Africa and the Americas.
The global prevalence of leprosy is difficult to assess, given that many of the locales with high prevalence lack a significant medical or public health infrastructure. Estimates range from 0.6 to 8 million affected individuals. The lower estimate includes only persons who have not completed chemotherapy, excluding those who may be physically or psychologically damaged from leprosy and who may yet relapse or develop immune-mediated reactions. The higher figure includes patients whose infections probably are already cured and many who have no leprosy-related deformity or disability. Although the figures on the worldwide prevalence of leprosy are debatable, incidence is not falling; there are still an estimated 500,000 new cases annually.
Leprosy is associated with poverty and rural residence. It appears not to be associated with AIDS, perhaps because of leprosy’s long incubation period. Most individuals appear to be naturally immune to leprosy and do not develop disease manifestations after exposure. The time of peak onset is in the second and third decades of life.
![]() The most severe lepromatous form of leprosy is twice as common among men as among women and is rarely encountered in children. The frequency of the polar forms of leprosy in different countries varies widely and may in part be genetically determined; certain human leukocyte antigen (HLA) associations are known for both polar forms of leprosy (see below). Furthermore, variations in immunoregulatory genes are associated with an increased susceptibility to leprosy, particularly the multibacillary form. In India and Africa, 90% of cases are tuberculoid; in Southeast Asia, 50% are tuberculoid and 50% lepromatous; and in Mexico, 90% are lepromatous. (For definitions of disease types, see Table 203-1 and “Clinical, Histologic, and Immunologic Spectrum,” below.)
The most severe lepromatous form of leprosy is twice as common among men as among women and is rarely encountered in children. The frequency of the polar forms of leprosy in different countries varies widely and may in part be genetically determined; certain human leukocyte antigen (HLA) associations are known for both polar forms of leprosy (see below). Furthermore, variations in immunoregulatory genes are associated with an increased susceptibility to leprosy, particularly the multibacillary form. In India and Africa, 90% of cases are tuberculoid; in Southeast Asia, 50% are tuberculoid and 50% lepromatous; and in Mexico, 90% are lepromatous. (For definitions of disease types, see Table 203-1 and “Clinical, Histologic, and Immunologic Spectrum,” below.)
|
CLINICAL, BACTERIOLOGIC, PATHOLOGIC, AND IMMUNOLOGIC SPECTRUM OF LEPROSY |
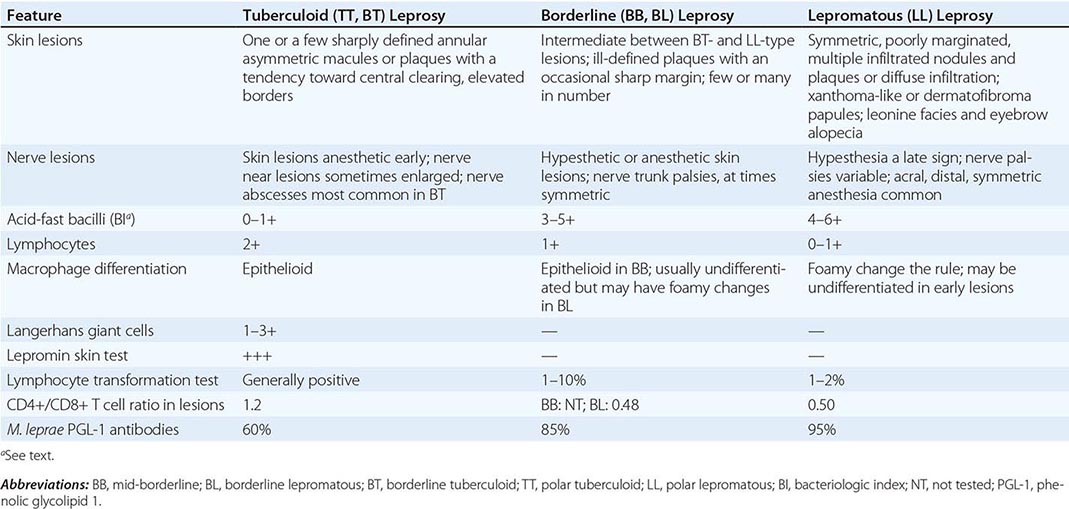
Transmission The route of transmission of leprosy remains uncertain, and transmission routes may in fact be multiple. Nasal droplet infection, contact with infected soil, and even insect vectors have been considered the prime candidates. Aerosolized M. leprae can cause infection in immunosuppressed mice, and a sneeze from an untreated lepromatous patient may contain >1010 AFB. Furthermore, both IgA antibody to M. leprae and genes of M. leprae—demonstrable by polymerase chain reaction (PCR)—have been found in the nose of individuals from endemic areas who have no signs of leprosy and in 19% of occupational contacts of lepromatous patients. Several lines of evidence implicate soil transmission. (1) In endemic countries such as India, leprosy is primarily a rural and not an urban disease. (2) M. leprae products reside in soil in endemic locales. (3) Direct dermal inoculation (e.g., during tattooing) may transmit M. leprae, and common sites of leprosy in children are the buttocks and thighs, suggesting that microinoculation of infected soil may transmit the disease. Evidence for insect vectors of leprosy includes the demonstration that bedbugs and mosquitoes in the vicinity of leprosaria regularly harbor M. leprae and that experimentally infected mosquitoes can transmit the infection to mice. Skin-to-skin contact generally is not considered an important route of transmission.
In endemic countries, ~50% of leprosy patients have a history of intimate contact with an infected person (often a household member), while, for unknown reasons, leprosy patients in nonendemic locales can identify such contact only 10% of the time. Moreover, household contact with an infected lepromatous case carries an eventual risk of disease acquisition of ~10% in endemic areas as opposed to only 1% in nonendemic locales. Contact with a tuberculoid case carries a very low risk. Physicians and nurses caring for leprosy patients and the co-workers of these patients are not at risk for leprosy.
![]() Although multilocus variable-number short-nucleotide tandem-repeat (VNTR) analyses have generally demonstrated considerable variability among isolates, highly similar and even identical VNTR results have been obtained with isolates from a limited number of families with multiple cases. Moreover, VNTR results have been similar for isolates within certain geographic locales and divergent for isolates within others. These findings suggest that genomic analyses may prove useful in the future for defining M. leprae transmission patterns.
Although multilocus variable-number short-nucleotide tandem-repeat (VNTR) analyses have generally demonstrated considerable variability among isolates, highly similar and even identical VNTR results have been obtained with isolates from a limited number of families with multiple cases. Moreover, VNTR results have been similar for isolates within certain geographic locales and divergent for isolates within others. These findings suggest that genomic analyses may prove useful in the future for defining M. leprae transmission patterns.
M. leprae causes disease primarily in humans. However, in Texas and Louisiana, 15% of nine-banded armadillos are infected, and armadillo contact occasionally results in human disease. Armadillos develop disseminated infection after IV inoculation of live M. leprae.
CLINICAL, HISTOLOGIC, AND IMMUNOLOGIC SPECTRUM
The incubation period prior to manifestation of clinical disease can vary between 2 and 40 years, although it is generally 5–7 years in duration. This long incubation period is probably, at least in part, a consequence of the extremely long doubling time for M. leprae (14 days in mice versus in vitro doubling times of 1 day and 20 min for M. tuberculosis and Escherichia coli, respectively). Leprosy presents as a spectrum of clinical manifestations that have bacteriologic, pathologic, and immunologic counterparts. The spectrum from polar tuberculoid (TT) to borderline tuberculoid (BT) to mid-borderline (BB, which is rarely encountered) to borderline lepromatous (BL) to polar lepromatous (LL) disease is associated with an evolution from asymmetric localized macules and plaques to nodular and indurated symmetric generalized skin manifestations, an increasing bacterial load, and loss of M. leprae–specific cellular immunity (Table 203-1). Distinguishing dermatopathologic characteristics include the number of lymphocytes, giant cells, and AFB as well as the nature of epithelioid cell differentiation. Where a patient presents on the clinical spectrum largely determines prognosis, complications, reactional states, and the intensity of antimicrobial therapy required.
![]() Tuberculoid Leprosy At the less severe end of the spectrum is tuberculoid leprosy, which encompasses TT and BT disease. In general, these forms of leprosy result in symptoms confined to the skin and peripheral nerves. TT leprosy is the most common form of the disease encountered in India and Africa but is virtually absent in Southeast Asia, where BT leprosy is frequent.
Tuberculoid Leprosy At the less severe end of the spectrum is tuberculoid leprosy, which encompasses TT and BT disease. In general, these forms of leprosy result in symptoms confined to the skin and peripheral nerves. TT leprosy is the most common form of the disease encountered in India and Africa but is virtually absent in Southeast Asia, where BT leprosy is frequent.
The skin lesions of tuberculoid leprosy consist of one or a few hypopigmented macules or plaques (Fig. 203-1) that are sharply demarcated and hypesthetic, often have erythematous or raised borders, and are devoid of the normal skin organs (sweat glands and hair follicles) and thus are dry, scaly, and anhidrotic. AFB are generally absent or few in number. Tuberculoid leprosy patients may have asymmetric enlargement of one or a few peripheral nerves. Indeed, leprosy and certain rare hereditary neuropathies are the only human diseases associated with peripheral-nerve enlargement. Although any peripheral nerve may be enlarged (including small digital and supraclavicular nerves), those most commonly affected are the ulnar, posterior auricular, peroneal, and posterior tibial nerves, with associated hypesthesia and myopathy.
FIGURE 203-1 Tuberculoid (TT) leprosy: a well-defined, hypopigmented, anesthetic macule with anhidrosis and a raised granular margin (arrowhead).
In tuberculoid leprosy, T cells breach the perineurium, and destruction of Schwann cells and axons may be evident, resulting in fibrosis of the epineurium, replacement of the endoneurium with epithelial granulomas, and occasionally caseous necrosis. Such invasion and destruction of nerves in the dermis by T cells are pathognomonic for leprosy.
Circulating lymphocytes from patients with tuberculoid leprosy readily recognize M. leprae and its constituent proteins, patients have positive lepromin skin tests (see “Diagnosis,” below), and—owing to a type 1 cytokine pattern in tuberculoid tissues—strong T cell and macrophage activation results in a localized infection. In tuberculoid leprosy tissue, there is a 2:1 predominance of helper CD4+ over CD8+ T lymphocytes. Tuberculoid tissues are rich in the mRNAs of the proinflammatory TH1 family of cytokines: interleukin (IL) 2, interferon γ (IFN-γ), and IL-12; in contrast, IL-4, IL-5, and IL-10 mRNAs are scarce.
Lepromatous Leprosy Lepromatous leprosy patients present with symmetrically distributed skin nodules (Fig. 203-2), raised plaques, or diffuse dermal infiltration, which, when on the face, results in leonine facies. Late manifestations include loss of eyebrows (initially the lateral margins only) and eyelashes, pendulous earlobes, and dry scaling skin, particularly on the feet. In LL leprosy, bacilli are numerous in the skin (as many as 109/g), where they are often found in large clumps (globi), and in peripheral nerves, where they initially invade Schwann cells, resulting in foamy degenerative myelination and axonal degeneration and later in Wallerian degeneration. In addition, bacilli are plentiful in circulating blood and in all organ systems except the lungs and the central nervous system. Nevertheless, patients are afebrile, and there is no evidence of major organ system dysfunction.
FIGURE 203-2 Lepromatous (LL) leprosy: advanced nodular lesions.
![]() Found almost exclusively in western Mexico and the Caribbean is a form of lepromatous leprosy without visible skin lesions but with diffuse dermal infiltration and a demonstrably thickened dermis, termed diffuse lepromatosis.
Found almost exclusively in western Mexico and the Caribbean is a form of lepromatous leprosy without visible skin lesions but with diffuse dermal infiltration and a demonstrably thickened dermis, termed diffuse lepromatosis.
In lepromatous leprosy, nerve enlargement and damage tend to be symmetric, result from actual bacillary invasion, and are more insidious but ultimately more extensive than in tuberculoid leprosy. Patients with LL leprosy have acral, distal, symmetric peripheral neuropathy and a tendency toward symmetric nerve-trunk enlargement. They may also have signs and symptoms related to involvement of the upper respiratory tract, the anterior chamber of the eye, and the testes.
In untreated LL patients, lymphocytes regularly fail to recognize either M. leprae or its protein constituents, and lepromin skin tests are negative (see “Diagnosis,” below). This loss of protective cellular immunity appears to be antigen-specific, as patients are not unusually susceptible to opportunistic infections, cancer, or AIDS and maintain delayed-type hypersensitivity to Candida, Trichophyton, mumps virus, tetanus toxoid, and even purified protein derivative of tuberculin. At times, M. leprae–specific anergy is reversible with effective chemotherapy. In LL tissues, there is a 2:1 ratio of CD8+ to CD4+ T lymphocytes. LL patients have a predominant TH2 response and hyperglobulinemia, and LL tissues demonstrate a TH2 cytokine profile, being rich in mRNAs for IL-4, IL-5, and IL-10 and poor in those for IL-2, IFN-γ, and IL-12. It appears that cytokines mediate a protective tissue response in leprosy, as injection of IFN-γ or IL-2 into lepromatous lesions causes a loss of AFB and histopathologic conversion toward a tuberculoid pattern. Macrophages of lepromatous leprosy patients appear to be functionally intact; circulating monocytes exhibit normal microbicidal function and responsiveness to IFN-γ.
Reactional States Lepra reactions comprise several common immunologically mediated inflammatory states that cause considerable morbidity. Some of these reactions precede diagnosis and the institution of effective antimicrobial therapy; indeed, these reactions may precipitate presentation for medical attention and diagnosis. Other reactions follow the initiation of appropriate chemotherapy; these reactions may cause patients to perceive that their leprosy is worsening and to lose confidence in conventional therapy. Only by warning patients of the potential for these reactions and describing their manifestations can physicians treating leprosy patients ensure continued credibility.
TYPE 1 LEPRA REACTIONS (DOWNGRADING AND REVERSAL REACTIONS) Type 1 lepra reactions occur in almost half of patients with borderline forms of leprosy but not in patients with pure lepromatous disease. Manifestations include classic signs of inflammation within previously involved macules, papules, and plaques and, on occasion, the appearance of new skin lesions, neuritis, and (less commonly) fever—generally low-grade. The nerve trunk most frequently involved in this process is the ulnar nerve at the elbow, which may be painful and exquisitely tender. If patients with affected nerves are not treated promptly with glucocorticoids (see below), irreversible nerve damage may result in as little as 24 h. The most dramatic manifestation is footdrop, which occurs when the peroneal nerve is involved.
When type 1 lepra reactions precede the initiation of appropriate antimicrobial therapy, they are termed downgrading reactions, and the case becomes histologically more lepromatous; when they occur after the initiation of therapy, they are termed reversal reactions, and the case becomes more tuberculoid. Reversal reactions often occur in the first months or years after the initiation of therapy but may also develop several years thereafter.
Edema is the most characteristic microscopic feature of type 1 lepra lesions, whose diagnosis is primarily clinical. Reversal reactions are typified by a TH1 cytokine profile, with an influx of CD4+ T helper cells and increased levels of IFN-γ and IL-2. In addition, type 1 reactions are associated with large numbers of T cells bearing γ/δ receptors—a unique feature of leprosy.
TYPE 2 LEPRA REACTIONS: ERYTHEMA NODOSUM LEPROSUM Erythema nodosum leprosum (ENL) (Fig. 203-3) occurs exclusively in patients near the lepromatous end of the leprosy spectrum (BL/LL), affecting nearly 50% of this group. Although ENL may precede leprosy diagnosis and the initiation of therapy (sometimes, in fact, prompting the diagnosis), in 90% of cases it follows the institution of chemotherapy, generally within 2 years. The most common features of ENL are crops of painful erythematous papules that resolve spontaneously in a few days to a week but may recur; malaise; and fever that can be profound. However, patients may also experience symptoms of neuritis, lymphadenitis, uveitis, orchitis, and glomerulonephritis and may develop anemia, leukocytosis, and abnormal liver function tests (particularly increased aminotransferase levels). Individual patients may have either a single bout of ENL or chronic recurrent manifestations. Bouts may be either mild or severe and generalized; in rare instances, ENL results in death. Skin biopsy of ENL papules reveals vasculitis or panniculitis, sometimes with many lymphocytes but characteristically with polymorphonuclear leukocytes as well.
FIGURE 203-3 Moderately severe skin lesions of erythema nodosum leprosum, some with pustulation and ulceration.
Elevated levels of circulating tumor necrosis factor (TNF) have been demonstrated in ENL; thus, TNF may play a central role in the pathobiology of this syndrome. ENL is thought to be a consequence of immune complex deposition, given its TH2 cytokine profile and its high levels of IL-6 and IL-8. However, in ENL tissue, the presence of HLA-DR framework antigen of epidermal cells—considered a marker for a delayed-type hypersensitivity response—and evidence of higher levels of IL-2 and IFN-γ than are usually seen in polar lepromatous disease suggest an alternative mechanism.
LUCIO’S PHENOMENON Lucio’s phenomenon is an unusual reaction seen exclusively in patients from the Caribbean and Mexico who have the diffuse lepromatosis form of lepromatous leprosy, most often those who are untreated. Patients with this reaction develop recurrent crops of large, sharply marginated, ulcerative lesions—particularly on the lower extremities—that may be generalized and, when so, are frequently fatal as a result of secondary infection and consequent septic bacteremia. Histologically, the lesions are characterized by ischemic necrosis of the epidermis and superficial dermis, heavy parasitism of endothelial cells with AFB, and endothelial proliferation and thrombus formation in the larger vessels of the deeper dermis. Like ENL, Lucio’s phenomenon is probably mediated by immune complexes.
![]() Complications • THE EXTREMITIES Complications of the extremities in leprosy patients are primarily a consequence of neuropathy leading to insensitivity and myopathy. Insensitivity affects fine touch, pain, and heat receptors but generally spares position and vibration appreciation. The most commonly affected nerve trunk is the ulnar nerve at the elbow, whose involvement results in clawing of the fourth and fifth fingers, loss of dorsal interosseous musculature in the affected hand, and loss of sensation in these distributions. Median nerve involvement in leprosy impairs thumb opposition and grasp; radial nerve dysfunction, although rare in leprosy, leads to wristdrop. Tendon transfers can restore hand function but should not be performed until 6 months after the initiation of antimicrobial therapy and the conclusion of episodes of acute neuritis.
Complications • THE EXTREMITIES Complications of the extremities in leprosy patients are primarily a consequence of neuropathy leading to insensitivity and myopathy. Insensitivity affects fine touch, pain, and heat receptors but generally spares position and vibration appreciation. The most commonly affected nerve trunk is the ulnar nerve at the elbow, whose involvement results in clawing of the fourth and fifth fingers, loss of dorsal interosseous musculature in the affected hand, and loss of sensation in these distributions. Median nerve involvement in leprosy impairs thumb opposition and grasp; radial nerve dysfunction, although rare in leprosy, leads to wristdrop. Tendon transfers can restore hand function but should not be performed until 6 months after the initiation of antimicrobial therapy and the conclusion of episodes of acute neuritis.
Plantar ulceration, particularly at the metatarsal heads, is probably the most common complication of leprous neuropathy. Therapy requires careful debridement; administration of appropriate antibiotics; avoidance of weight-bearing until ulcerations are healed, with slowly progressive ambulation thereafter; and wearing of special shoes to prevent recurrence.
Footdrop as a result of peroneal nerve palsy should be treated with a simple nonmetallic brace in the shoe or with surgical correction attained by tendon transfers. Although uncommon, Charcot’s joints, particularly of the foot and ankle, may result from leprosy.
The loss of distal digits in leprosy is a consequence of insensitivity, trauma, secondary infection, and—in lepromatous disease—a poorly understood and sometimes profound osteolytic process. Conscientious protection of the extremities during cooking and work and the early institution of therapy have substantially reduced the frequency and severity of distal digit loss in recent times.
THE NOSE In lepromatous leprosy, bacillary invasion of the nasal mucosa can result in chronic nasal congestion and epistaxis. Saline nose drops may relieve these symptoms. Long-untreated LL leprosy may further result in destruction of the nasal cartilage, with consequent saddle-nose deformity or anosmia (more common in the preantibiotic era than at present). Nasal reconstructive procedures can ameliorate significant cosmetic defects.
THE EYE Owing to cranial nerve palsies, lagophthalmos and corneal insensitivity may complicate leprosy, resulting in trauma, secondary infection, and (without treatment) corneal ulcerations and opacities. For patients with these conditions, eyedrops during the day and ointments at night provide some protection from such consequences. Furthermore, in LL leprosy, the anterior chamber of the eye is invaded by bacilli, and ENL may result in uveitis, with consequent cataracts and glaucoma. Thus leprosy is a major cause of blindness in the developing world. Slit-lamp evaluation of LL patients often reveals “corneal beading,” representing globi of M. leprae.
THE TESTES M. leprae invades the testes, while ENL may cause orchitis. Thus males with lepromatous leprosy often manifest mild to severe testicular dysfunction, with an elevation of luteinizing and follicle-stimulating hormones, decreased testosterone, and aspermia or hypospermia in 85% of LL patients but in only 25% of BL patients. LL patients may become impotent and infertile. Impotence is sometimes responsive to testosterone replacement.
AMYLOIDOSIS Secondary amyloidosis is a complication of LL leprosy and ENL that is encountered infrequently in the antibiotic era. This complication may result in abnormalities of hepatic and particularly renal function.
NERVE ABSCESSES Patients with various forms of leprosy, but particularly those with the BT form, may develop abscesses of nerves (most commonly the ulnar), with a cellulitic appearance of adjacent skin. In such conditions, the affected nerve is swollen and exquisitely tender. Although glucocorticoids may reduce signs of inflammation, rapid surgical decompression is necessary to prevent irreversible sequelae.
DIAGNOSIS
Leprosy most commonly presents with both characteristic skin lesions and skin histopathology. Thus the disease should be suspected when a patient from an endemic area has suggestive skin lesions or peripheral neuropathy. The diagnosis should be confirmed by histopathology. In tuberculoid leprosy, lesional areas—preferably the advancing edge—must be biopsied because normal-appearing skin does not have pathologic features. In lepromatous leprosy, nodules, plaques, and indurated areas are optimal biopsy sites, but biopsies of normal-appearing skin also are generally diagnostic. Lepromatous leprosy is associated with diffuse hyperglobulinemia, which may result in false-positive serologic tests (e.g., Venereal Disease Research Laboratory, rheumatoid arthritis, and antinuclear antibody tests) and therefore may cause diagnostic confusion. On occasion, tuberculoid lesions may not (1) appear typical, (2) be hypesthetic, and (3) contain granulomas (instead containing only nonspecific lymphocytic infiltrates). In such instances, two of these three characteristics are considered sufficient for a diagnosis. It is preferable to overdiagnose leprosy rather than to allow a patient to remain untreated.
IgM antibodies to PGL-1 are found in 95% of patients with untreated lepromatous leprosy; the titer decreases with effective therapy. However, in tuberculoid leprosy—the form of disease most often associated with diagnostic uncertainty owing to the absence or paucity of AFB—patients have significant antibodies to PGL-1 only 60% of the time; moreover, in endemic locales, exposed individuals without clinical leprosy may harbor antibodies to PGL-1. Thus PGL-1 serology is of little diagnostic utility in tuberculoid leprosy. Heat-killed M. leprae (lepromin) has been used as a skin test reagent. It generally elicits a reaction in tuberculoid leprosy patients, may do so in individuals without leprosy, and gives negative results in lepromatous leprosy patients; consequently, it is likewise of little diagnostic value. Unfortunately, PCR of the skin for M. leprae, although positive in LL and BL disease, yields negative results in 50% of tuberculoid cases, again offering little diagnostic assistance.
DIFFERENTIAL DIAGNOSIS
Included in the differential diagnosis of lesions that resemble leprosy are sarcoidosis, leishmaniasis, lupus vulgaris, dermatofibroma, histiocytoma, lymphoma, syphilis, yaws, granuloma annulare, and various other disorders causing hypopigmentation (notably pityriasis alba, tinea, and vitiligo). Sarcoidosis may result in perineural inflammation, but actual granuloma formation within dermal nerves is pathognomonic for leprosy. In lepromatous leprosy, sputum specimens may be loaded with AFB—a finding that can be incorrectly interpreted as representing pulmonary tuberculosis.
|
TREATMENT |
LEPROSY |
ANTIMICROBIAL THERAPY
Active Agents Established agents used to treat leprosy include dapsone (50–100 mg/d), clofazimine (50–100 mg/d, 100 mg three times weekly, or 300 mg monthly), and rifampin (600 mg daily or monthly; see “Choice of Regimens,” below). Of these drugs, only rifampin is bactericidal. The sulfones (folate antagonists), the foremost of which is dapsone, were the first antimicrobial agents found to be effective for the treatment of leprosy and are still the mainstays of therapy. With sulfone treatment, skin lesions resolve and numbers of viable bacilli in the skin are reduced. Although primarily bacteriostatic, dapsone monotherapy results in a resistance-related relapse rate of only 2.5%; after ≥18 years of therapy and subsequent discontinuation, only another 10% of patients relapse, developing new, usually asymptomatic, shiny, “histoid” nodules. Dapsone is generally safe and inexpensive. Individuals with glucose-6-phosphate dehydrogenase deficiency who are treated with dapsone may develop severe hemolysis; those without this deficiency also have reduced red cell survival and a hemoglobin decrease averaging 1 g/dL. Dapsone’s usefulness is limited occasionally by allergic dermatitis and rarely by the sulfone syndrome (including high fever, anemia, exfoliative dermatitis, and a mononucleosis-type blood picture). It must be remembered that rifampin induces microsomal enzymes, necessitating increased doses of medications such as glucocorticoids and oral birth control regimens. Clofazimine is often cosmetically unacceptable to light-skinned leprosy patients because it causes a red-black skin discoloration that accumulates, particularly in lesional areas, and makes the patient’s diagnosis obvious to members of the community.
Other antimicrobial agents active against M. leprae in animal models and at the usual daily doses used in clinical trials include ethionamide/prothionamide; the aminoglycosides streptomycin, kanamycin, and amikacin (but not gentamicin or tobramycin); minocycline; clarithromycin; and several fluoroquinolones, particularly ofloxacin. Next to rifampin, minocycline, clarithromycin, and ofloxacin appear to be most bactericidal for M. leprae, but these drugs have not been used extensively in leprosy control programs. Most recently, rifapentine and moxifloxacin have been found to be especially potent against M. leprae in mice. In a clinical trial in lepromatous leprosy, moxifloxacin was profoundly bactericidal, matched in potency only by rifampin.
Choice of Regimens Antimicrobial therapy for leprosy must be individualized, depending on the clinical/pathologic form of the disease encountered. Tuberculoid leprosy, which is associated with a low bacterial burden and a protective cellular immune response, is the easiest form to treat and can be cured reliably with a finite course of chemotherapy. In contrast, lepromatous leprosy may have a higher bacillary load than any other human bacterial disease, and the absence of a salutary T cell repertoire requires prolonged or even lifelong chemotherapy. Hence, careful classification of disease prior to therapy is important.
In developed countries, clinical experience with leprosy classification is limited; fortunately, however, the resources needed for skin biopsy are highly accessible, and those for pathologic interpretation are readily available. In developing countries, clinical expertise is greater but is now waning substantially as the care of leprosy patients is integrated into general health services. In addition, access to dermatopathology services is often limited. In such instances, skin smears may prove useful, but in many locales access to the resources needed for their preparation and interpretation also may be unavailable. Use of skin smears is no longer encouraged by the World Health Organization (WHO) and is often replaced by mere counting of lesions, which, together with a lack of capacity for histopathologic assessment, may negatively affect decisions about chemotherapy, increase the potential for reactions, and worsen the ultimate prognosis. A reasoned approach to the treatment of leprosy is confounded by these and several other issues:
1. Even without therapy, TT leprosy may heal spontaneously, and prolonged dapsone monotherapy (even for LL leprosy) is generally curative in 80% of cases.
2. In tuberculoid disease, it is common for no bacilli to be found in the skin prior to therapy, and thus there is no objective measure of therapeutic success. Furthermore, despite adequate treatment, TT and particularly BT lesions often resolve minimally or incompletely, while relapse and late type 1 lepra reactions can be difficult to distinguish.
3. LL leprosy patients commonly harbor viable persistent M. leprae organisms after prolonged intensive therapy; the propensity of these organisms to initiate clinical relapse is unclear. Because relapse in LL patients after discontinuation of rifampin-containing regimens usually begins only after 7–10 years, follow-up over the very long term is necessary to assess ultimate clinical outcomes.
4. Even though primary dapsone resistance is exceedingly rare and multidrug therapy is generally recommended (at least for lepromatous leprosy), there is a paucity of information from experimental animals and clinical trials on the optimal combination of antimicrobial agents, dosing schedule, and duration of therapy.
In 1982, the WHO made recommendations for leprosy chemotherapy administered in control programs. These recommendations came on the heels of the demonstration of the relative success of long-term dapsone monotherapy and in the context of concerns about dapsone resistance. Other complicating considerations included the limited resources available for leprosy care in the very areas where it is most prevalent and the frustration and discouragement of patients and program managers with the previous requirement for lifelong therapy for many leprosy patients. Thus, for the first time, the WHO advocated a finite duration of therapy for all forms of leprosy and—given the prohibitive cost of daily rifampin treatment in developing countries—encouraged the monthly administration of this agent as part of a multidrug regimen. Over the ensuing years, the WHO recommendations have been broadly implemented, and the duration of therapy required, particularly for lepromatous leprosy, has been progressively shortened. For treatment purposes, the WHO classifies patients as paucibacillary or multibacillary. Previously, patients without demonstrable AFB in the dermis were classified as paucibacillary and those with AFB as multibacillary. Currently, in light of the perceived unreliability of skin smears in the field, patients are classified as multibacillary if they have six or more skin lesions and as paucibacillary if they have fewer. (Unfortunately, this classification method has been found wanting, as some patients near the lepromatous pole have only one or a few skin lesions.) The WHO recommends that paucibacillary adults be treated with 100 mg of dapsone daily and 600 mg of rifampin monthly (supervised) for 6 months (Table 203-2). For patients with single-lesion paucibacillary leprosy, the WHO recommends as an alternative a single dose of rifampin (600 mg), ofloxacin (400 mg), and minocycline (100 mg). Multibacillary adults should be treated with 100 mg of dapsone plus 50 mg of clofazimine daily (unsupervised) and with 600 mg of rifampin plus 300 mg of clofazimine monthly (supervised). Originally, the WHO recommended that lepromatous patients be treated for 2 years or until smears became negative (generally in ~5 years); subsequently, the acceptable course was reduced to 1 year—a change that remains especially controversial in the absence of supporting clinical trials.
|
ANTIMICROBIAL REGIMENS RECOMMENDED FOR THE TREATMENT OF LEPROSY IN ADULTS |
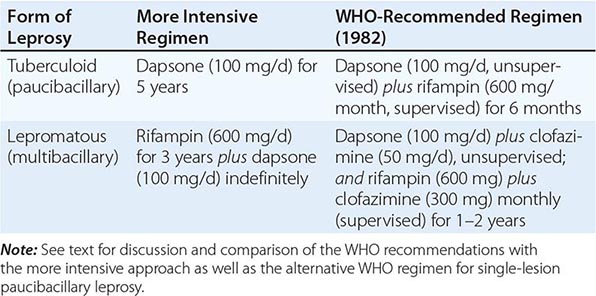
Several factors have caused many authorities to question the WHO recommendations and to favor a more intensive approach. Among these factors are—for multibacillary patients—a high (double-digit) relapse rate in several locales (reaching 20–40% in one locale, with the rate directly related to the initial bacterial burden) and—for paucibacillary patients—demonstrable lesional activity for years in fully half of patients after the completion of therapy. The more intensive approach (Table 203-2) calls for tuberculoid leprosy to be treated with dapsone (100 mg/d) for 5 years and for lepromatous leprosy to be treated with rifampin (600 mg/d) for 3 years and with dapsone (100 mg/d) throughout life.
With effective antimicrobial therapy, new skin lesions and signs and symptoms of peripheral neuropathy cease appearing. Nodules and plaques of lepromatous leprosy noticeably flatten in 1–2 months and resolve in 1 year or a few years, while tuberculoid skin lesions may disappear, ameliorate, or remain relatively unchanged. Although the peripheral neuropathy of leprosy may improve somewhat in the first few months of therapy, rarely is it significantly alleviated by treatment.
Despite the drawbacks of the WHO’s recommendations for multidrug therapy, these regimens have been used almost exclusively worldwide. Although two of the three recommended drugs (dapsone and clofazimine) are only bacteriostatic against M. leprae and bactericidal agents have been identified since the WHO formulated its recommendations, significant studies employing the available alternatives in newly designed regimens have not been initiated. Given the recent findings that moxifloxacin, like rifampin, is profoundly bactericidal in leprosy patients and that short-course chemotherapy for tuberculosis is possible only when two or more bactericidal agents are used, a moxifloxacin/rifamycin-based regimen including either minocycline or clarithromycin appears promising; such a regimen may prove to be more reliably curative than WHO-recommended multidrug therapy for lepromatous leprosy and may allow a considerably shorter course of treatment.
THERAPY FOR REACTIONS
Type 1 Type 1 lepra reactions are best treated with glucocorticoids (e.g., prednisone, initially at doses of 40–60 mg/d). As inflammation subsides, the glucocorticoid dose can be tapered, but steroid therapy must be continued for at least 3–6 months lest recurrence supervene. Because of the myriad toxicities of prolonged glucocorticoid therapy, the indications for its initiation are strictly limited to lesions whose intense inflammation poses a threat of ulceration; lesions at cosmetically important sites, such as the face; and cases in which neuritis is present. Mild to moderate lepra reactions that do not meet these criteria should be tolerated and glucocorticoid treatment withheld. Thalidomide is ineffective against type 1 lepra reactions. Clofazimine (200–300 mg/d) is of questionable benefit but in any event is far less efficacious than glucocorticoids.
Type 2 Treatment of ENL must be individualized. If ENL is mild (i.e., if it occurs without fever or other organ involvement and with occasional crops of only a few skin papules), it may be treated with antipyretics alone. However, in cases with many skin lesions, fever, malaise, and other tissue involvement, brief courses (1–2 weeks) of glucocorticoid treatment (initially 40–60 mg/d) are often effective. With or without therapy, individual inflamed papules last for <1 week. Successful therapy is defined by the cessation of skin lesion development and the disappearance of other systemic signs and symptoms. If, despite two courses of glucocorticoid therapy, ENL appears to be recurring and persisting, treatment with thalidomide (100–300 mg nightly) should be initiated, with the dose depending on the initial severity of the reaction. Because even a single dose of thalidomide administered early in pregnancy may result in severe birth defects, including phocomelia, the use of this drug in the United States for the treatment of fertile female patients is tightly regulated and requires informed consent, prior pregnancy testing, and maintenance of birth control measures. Although the mechanism of thalidomide’s dramatic action against ENL is not entirely clear, the drug’s efficacy is probably attributable to its reduction of TNF levels and IgM synthesis and its slowing of polymorphonuclear leukocyte migration. After the reaction is controlled, lower doses of thalidomide (50–200 mg nightly) are effective in preventing relapses of ENL. Clofazimine in high doses (300 mg nightly) has some efficacy against ENL, but its use permits only a modest reduction of the glucocorticoid dose necessary for ENL control.
Lucio’s Phenomenon Neither glucocorticoids nor thalidomide is effective against this syndrome. Optimal wound care and therapy for bacteremia are indicated. Ulcers tend to be chronic and heal poorly. In severe cases, exchange transfusion may prove useful.
PREVENTION AND CONTROL
Vaccination at birth with bacille Calmette-Guérin (BCG) has proved variably effective in preventing leprosy: the results have ranged from total inefficacy to 80% efficacy. The addition of heat-killed M. leprae to BCG does not increase the effectiveness of the vaccine. Because whole mycobacteria contain large amounts of lipids and carbohydrates that have proved in vitro to be immunosuppressive for lymphocytes and macrophages, M. leprae proteins may prove to be superior vaccines. Data from a mouse model support this possibility.
Chemoprophylaxis with dapsone may reduce the number of tuberculoid leprosy cases but not the number of lepromatous cases and hence is not recommended, even for household contacts. In addition, single-dose rifampin prophylaxis is of doubtful efficacy. Because leprosy transmission appears to require close prolonged household contact, hospitalized patients need not be isolated.
In 1992, the WHO—on the basis of that organization’s treatment recommendations—launched a landmark campaign to eliminate leprosy as a public health problem by the year 2000 (goal, <1 case per 10,000 population). The campaign mobilized and energized nongovernmental organizations and national health services to treat leprosy with multiple drugs and to clean up outdated registries. In these respects, the effort has proved hugely successful, with >6 million patients completing therapy. However, the target of leprosy elimination has not yet been reached. In fact, the success of the WHO campaign in reducing the number of cases worldwide has been largely attributable to the redefinition of what constitutes a case of leprosy. Formerly calculated by disease prevalence, the count is now limited to cases not yet treated with multiple drugs. Worldwide, the annual incidence of leprosy has not fallen. Furthermore, after the completion of therapy, when a patient is no longer considered to represent a “case,” half of all patients continue to manifest disease activity for years; relapse rates (at least for multibacillary patients) are unacceptably high; disabilities and deformities go unchecked; and the social stigma of the disease persists.
During most of the twentieth century, nongovernmental organizations, particularly Christian missionaries, provided a medical infrastructure devoted to the care and treatment of leprosy patients—the envy of those with other medical priorities in the developing world. With the public perception that leprosy is near eradication, resources for patient care are rapidly being diverted, and the burden of patient care is being transferred to nonexistent or overloaded national health services and to health workers who lack the tools and skills needed for the disease’s diagnosis and classification and for the selection of nuanced therapy (particularly in cases of reactional neuritis). Thus the prerequisites for a salutary outcome increasingly go unmet.
204 |
Nontuberculous Mycobacterial Infections |
Several terms—nontuberculous mycobacteria (NTM), atypical mycobacteria, mycobacteria other than tuberculosis, and environmental mycobacteria—all refer to mycobacteria other than Mycobacterium tuberculosis, its close relatives (M. bovis, M. caprae, M. africanum, M. pinnipedii, M. canetti), and M. leprae. The number of identified species of NTM is growing and will continue to do so because of the use of DNA sequence typing for speciation. The number of known species currently exceeds 150. NTM are highly adaptable and can inhabit hostile environments, including industrial solvents.
EPIDEMIOLOGY
NTM are ubiquitous in soil and water. Specific organisms have recurring niches, such as M. simiae in certain aquifers, M. fortuitum in pedicure baths, and M. immunogenum in metalworking fluids. Most NTM cause disease in humans only rarely unless some aspect of host defense is impaired, as in bronchiectasis, or breached, as by inoculation (e.g., liposuction, trauma). There are few instances of human-to-human transmission of NTM, which occurs almost exclusively in cystic fibrosis. Because infections due to NTM are rarely reported to health agencies and because their identification is sometimes problematic, reliable data on incidence and prevalence are lacking. Disseminated disease denotes significant immune dysfunction (e.g., advanced HIV infection), whereas pulmonary disease, which is much more common, is highly associated with pulmonary epithelial defects but not with systemic immunodeficiency.
In the United States, the incidence and prevalence of pulmonary infection with NTM, mostly in association with bronchiectasis (Chap. 312), have for many years been several-fold higher than the corresponding figures for tuberculosis, and rates of the former are increasing among the elderly. Among patients with cystic fibrosis, who often have bronchiectasis, rates of clinical infection with NTM range from 3% to 15%, with even higher rates among older patients. Although NTM may be recovered from the sputa of many individuals, it is critical to differentiate active disease from commensal harboring of the organisms. A scheme to help with the proper diagnosis of pulmonary infection caused by NTM has been developed by the American Thoracic Society and is widely used. The bulk of nontuberculous mycobacterial disease in North America is due to M. kansasii, organisms of the M. avium complex (MAC), and M. abscessus.
![]() In Europe, Asia, and Australia, the distribution of NTM in clinical specimens is roughly similar to that in North America, with MAC species and rapidly growing organisms such as M. abscessus encountered frequently. M. xenopi and M. malmoense are especially prominent in northern Europe. M. ulcerans causes the distinct clinical entity Buruli ulcer, which occurs throughout tropical zones, especially in western Africa. M. marinum is a common cause of cutaneous and tendon infections in coastal regions and among individuals exposed to fish tanks or swimming pools.
In Europe, Asia, and Australia, the distribution of NTM in clinical specimens is roughly similar to that in North America, with MAC species and rapidly growing organisms such as M. abscessus encountered frequently. M. xenopi and M. malmoense are especially prominent in northern Europe. M. ulcerans causes the distinct clinical entity Buruli ulcer, which occurs throughout tropical zones, especially in western Africa. M. marinum is a common cause of cutaneous and tendon infections in coastal regions and among individuals exposed to fish tanks or swimming pools.
The true international epidemiology of infections due to NTM is hard to determine because the isolation of these organisms often is not reported and speciation often is not performed for M. tuberculosis and NTM. The increasing ease of identification and speciation of these organisms is likely to have a major impact on the description of their international epidemiology in the next few years.
PATHOBIOLOGY
Because exposure to NTM is essentially universal and disease is rare, it can be assumed that normal host defenses against these organisms must be strong and that otherwise healthy individuals in whom significant disease develops are highly likely to have specific susceptibility factors that permit NTM to become established, multiply, and cause disease. At the advent of HIV infection, CD4+ T lymphocytes were recognized as key effector cells against NTM; the development of disseminated MAC disease was highly correlated with a decline in CD4+ T lymphocyte numbers. Such a decrease has also been implicated in disseminated MAC infection in patients with idiopathic CD4+ T lymphocytopenia. Potent inhibitors of tumor necrosis factor α (TNF-α), such as infliximab, adalimumab, certolizumab, golimumab, and etanercept, can neutralize this critical cytokine. The occasional result is severe mycobacterial or fungal infection; these associations indicate that TNF-α is a crucial element in mycobacterial control. However, in cases without the above risk factors, much of the genetic basis of susceptibility to disseminated infection with NTM is accounted for by specific mutations in the interferon γ (IFN-γ)/interleukin 12 (IL-12) synthesis and response pathways.
![]() Mycobacteria are typically phagocytosed by macrophages, which respond with the production of IL-12, a heterodimer composed of IL-12p35 and IL-12p40 moieties that together make up IL-12p70. IL-12 activates T lymphocytes and natural killer cells through binding to its receptor (composed of IL-12Rβ1 and IL-12Rβ2/IL-23R), with consequent phosphorylation of STAT4. IL-12 stimulation of STAT4 leads to secretion of IFN-γ, which activates neutrophils and macrophages to produce reactive oxidants, to increase expression of the major histocompatibility complex and Fc receptors, and to concentrate certain antibiotics intracellularly. Signaling by IFN-γ through its receptor (composed of IFN-γR1 and IFN-γR2) leads to phosphorylation of STAT1, which in turn regulates IFN-γ-responsive genes, such as those coding for IL-12 and TNF-α. TNF-α signals through its own receptor via a downstream complex containing the nuclear factor κB (NF-κB) essential modulator (NEMO). Therefore, the positive feedback loop between IFN-γ and IL-12/IL-23 drives the immune response to mycobacteria and other intracellular infections. These genes are known to be the critical ones in the pathway of mycobacterial control: specific Mendelian mutations have been identified in IFN-γR1, IFN-γR2, STAT1, GATA2, ISG15, IRF8, IL-12A, IL-12Rβ1, IL-12Rβ2, CYBB, and NEMO (Fig. 204-1). Despite the identification of genes associated with disseminated disease, only ~70% of cases of disseminated nontuberculous mycobacterial infections that are not associated with HIV infection have a genetic diagnosis; the implication is that more mycobacterial susceptibility genes and pathways remain to be identified.
Mycobacteria are typically phagocytosed by macrophages, which respond with the production of IL-12, a heterodimer composed of IL-12p35 and IL-12p40 moieties that together make up IL-12p70. IL-12 activates T lymphocytes and natural killer cells through binding to its receptor (composed of IL-12Rβ1 and IL-12Rβ2/IL-23R), with consequent phosphorylation of STAT4. IL-12 stimulation of STAT4 leads to secretion of IFN-γ, which activates neutrophils and macrophages to produce reactive oxidants, to increase expression of the major histocompatibility complex and Fc receptors, and to concentrate certain antibiotics intracellularly. Signaling by IFN-γ through its receptor (composed of IFN-γR1 and IFN-γR2) leads to phosphorylation of STAT1, which in turn regulates IFN-γ-responsive genes, such as those coding for IL-12 and TNF-α. TNF-α signals through its own receptor via a downstream complex containing the nuclear factor κB (NF-κB) essential modulator (NEMO). Therefore, the positive feedback loop between IFN-γ and IL-12/IL-23 drives the immune response to mycobacteria and other intracellular infections. These genes are known to be the critical ones in the pathway of mycobacterial control: specific Mendelian mutations have been identified in IFN-γR1, IFN-γR2, STAT1, GATA2, ISG15, IRF8, IL-12A, IL-12Rβ1, IL-12Rβ2, CYBB, and NEMO (Fig. 204-1). Despite the identification of genes associated with disseminated disease, only ~70% of cases of disseminated nontuberculous mycobacterial infections that are not associated with HIV infection have a genetic diagnosis; the implication is that more mycobacterial susceptibility genes and pathways remain to be identified.
FIGURE 204-1 Cytokine interactions of infected macrophages (Mφ) with T and natural killer (NK) lymphocytes. Infection of macrophages by mycobacteria (AFB) leads to the release of heterodimeric interleukin 12 (IL-12). IL-12 acts on its receptor complex (IL-12R), with consequent STAT4 activation and production of homodimeric interferon γ (IFNγ). Through its receptor (IFNγR), IFNγ activates STAT1, stimulating the production of tumor necrosis factor α (TNFα) and leading to the killing of intracellular organisms such as mycobacteria, salmonellae (Salm), and some fungi. Homotrimeric TNFα acts through its receptor (TNFαR) and requires nuclear factor κB essential modulator (NEMO) to activate nuclear factor κB, which also contributes to the killing of intracellular bacteria. Both IFNγ and TNFα lead to upregulation of IL-12. TNFα-blocking antibodies work either by blocking the ligand (infliximab, adalimumab, certolizumab, golimumab) or by providing soluble receptor (etanercept). Mutations in IFNγR1, IFNγR2, IL-12p40, IL-12Rβ1, IL-12Rβ2, STAT1, GATA2, ISG15, IRF8, CYBB, and NEMO have been associated with a predisposition to mycobacterial infections. Other cytokines, such as IL-15 and IL-18, also contribute to IFNγ production. Signaling through the Toll-like receptor (TLR) complex and CD14 also upregulates TNFα production. LPS, lipopolysaccharide; NRAMP1, natural resistance-associated macrophage protein 1.
In contrast to the recognized genes and mechanisms associated with disseminated nontuberculous mycobacterial infection, the best-recognized underlying condition for pulmonary infection with NTM is bronchiectasis (Chap. 312). Most of the well-characterized forms of bronchiectasis, including cystic fibrosis, primary ciliary dyskinesia, STAT3-deficient hyper-IgE syndrome, and idiopathic bronchiectasis, have high rates of association with nontuberculous mycobacterial infection. The precise mechanism by which bronchiectasis predisposes to locally destructive but not systemic involvement is unknown.
Unlike disseminated or pulmonary infection, “hot-tub lung” represents pulmonary hypersensitivity to NTM—most commonly MAC organisms—growing in underchlorinated, often indoor hot tubs.
CLINICAL MANIFESTATIONS
Disseminated Disease Disseminated MAC or M. kansasii infections in patients with advanced HIV infection are now uncommon in North America because of effective antimycobacterial prophylaxis and improved treatment of HIV infection. When such mycobacterial disease was common, the portal of entry was the bowel, with spread to bone marrow and the bloodstream. Surprisingly, disseminated infections with rapidly growing NTM (e.g., M. abscessus, M. fortuitum) are very rare in HIV-infected patients, even those with very advanced HIV infection. Because these organisms are of low intrinsic virulence and disseminate only in conjunction with impaired immunity, disseminated disease can be indolent and progressive over weeks to months. Typical manifestations of malaise, fever, and weight loss are often accompanied by organomegaly, lymphadenopathy, and anemia. Because special cultures or stains are required to identify the organisms, the most critical step in diagnosis is to suspect infection with NTM. Blood cultures may be negative, but involved organs typically have significant organism burdens, sometimes with a grossly impaired granulomatous response. In a child, disseminated involvement (i.e., involvement of two or more organs) without an underlying iatrogenic cause should prompt an investigation of the IFN-γ/IL-12 pathway. Recessive mutations in IFN–γR1 and IFN–γR2 typically lead to severe infection with NTM. In contrast, dominant negative mutations in IFN–γR1, which lead to overaccumulation of a defective interfering mutant receptor on the cell surface, inhibit normal IFN-γ signaling and thus lead to nontuberculous mycobacterial osteomyelitis. Dominant negative mutations in STAT1 and recessive mutations in IL-12Rβ1 can produce variable phenotypes consistent with their residual capacities for IFN-γ synthesis and response. Male patients who have disseminated nontuberculous mycobacterial infections along with conical, peg, or missing teeth and an abnormal hair pattern should be evaluated for defects in the pathway that activates NF-κB through NEMO. These patients may have associated immune globulin defects as well. Patients with myelodysplasia and mycobacterial disease should be investigated for GATA2 deficiency. A recently recognized group of patients that often develops disseminated infections with rapidly growing NTM (predominantly M. abscessus) as well as other opportunistic infections has high-titer neutralizing autoantibodies to IFN-γ. Thus far, this syndrome has been reported most frequently in East Asian female patients.
IV catheters can become infected with NTM, usually as a consequence of contaminated water. M. abscessus and M. fortuitum sometimes infect deep indwelling lines as well as fluids used in eye surgery, subcutaneous injections, and local anesthetics. Infected catheters should be removed.
Pulmonary Disease Lung disease is by far the most common form of nontuberculous mycobacterial infection in North America and the rest of the industrialized world. The clinical presentation typically consists of months or years of throat clearing, nagging cough, and slowly progressive fatigue. Patients will often have seen physicians multiple times and received symptom-based or transient therapy before the diagnosis is entertained and samples are sent for mycobacterial stains and cultures. Because not all patients can produce sputum, bronchoscopy may be required for diagnosis. The typical lag between onset of symptoms and diagnosis is ~5 years in older women. Predisposing factors include underlying lung diseases such as bronchiectasis (Chap. 312), pneumoconiosis (Chap. 311), chronic obstructive pulmonary disease (Chap. 314), primary ciliary dyskinesia (Chap. 312), α1 antitrypsin deficiency (Chap. 367e), and cystic fibrosis (Chap. 313). Bronchiectasis and nontuberculous mycobacterial infection often coexist and progress in tandem. This situation makes causality difficult to determine in a given index case, but bronchiectasis is certainly among the most critical predisposing factors that are exacerbated by infection.
MAC organisms are the most common causes of pulmonary nontuberculous mycobacterial infection in North America, but rates vary somewhat by region. MAC infection most commonly develops during the sixth or seventh decade of life in women who have had months or years of nagging intermittent cough and fatigue, with or without sputum production or chest pain. The constellation of pulmonary disease due to NTM in a tall and thin woman who may have chest wall abnormalities is often referred to as Lady Windermere syndrome, after an Oscar Wilde character of the same name. In fact, pulmonary MAC infection does afflict older nonsmoking white women more than men, with onset at ~60 years. Patients tend to be taller and thinner than the general population, with high rates of scoliosis, mitral valve prolapse, and pectus anomalies. Whereas male smokers with upper-lobe cavitary disease tend to carry the same single strain of MAC indefinitely, nonsmoking females with nodular bronchiectasis tend to carry several strains of MAC simultaneously, with changes over the course of their disease.
M. kansasii can cause a clinical syndrome that strongly resembles tuberculosis, consisting of hemoptysis, chest pain, and cavitary lung disease. The rapidly growing NTM, such as M. abscessus, have been associated with esophageal motility disorders such as achalasia. Patients with pulmonary alveolar proteinosis are prone to pulmonary nontuberculous mycobacterial and Nocardia infections; the underlying mechanism may be inhibition of alveolar macrophage function due to the autoantibodies to granulocyte-macrophage colony-stimulating factor found in these patients.
Cervical Lymph Nodes The most common form of nontuberculous mycobacterial infection among young children in North America is isolated cervical lymphadenopathy, caused most frequently by MAC organisms but also by other NTM. The cervical swelling is typically firm and relatively painless, with a paucity of systemic signs. Because the differential diagnosis of painless adenopathy includes malignancy, many children have infection with NTM diagnosed inadvertently at biopsy; cultures and special stains may not have been requested because mycobacterial disease was not ranked high in the differential. Local fistulae usually resolve completely with resection and/or antibiotic therapy. Likewise, the entity of isolated pediatric intrathoracic nontuberculous mycobacterial infection, which is probably related to cervical lymph node infection, is usually mistaken for cancer. In neither isolated cervical nor isolated intrathoracic infections with NTM have children with underlying immune defects been identified, nor do the affected children go on to develop other opportunistic infections.
Skin and Soft Tissue Disease Cutaneous involvement with NTM usually requires a break in the skin for introduction of the bacteria. Pedicure bath–associated infection with M. fortuitum is more likely if skin abrasion (e.g., during leg shaving) has occurred just before the pedicure. Outbreaks of skin infection are often caused by rapidly growing NTM (especially M. abscessus, M. fortuitum, and M. chelonae) acquired via skin contamination from surgical instruments (especially in cosmetic surgery), injections, and other procedures. These infections are typically accompanied by painful, erythematous, draining subcutaneous nodules, usually without associated fever or systemic symptoms.
M. marinum lives in many water sources and can be acquired from fish tanks, swimming pools, barnacles, and fish scales. This organism typically causes papules or ulcers (“fish-tank granuloma”), but the infection can progress to tendinitis with significant impairment of manual dexterity. Lesions appear days to weeks after inoculation of organisms by a typically minor trauma (e.g., incurred during the cleaning of boats or the handling of fish). Tender nodules due to M. marinum can advance up the arm in a pattern also seen with Sporothrix schenckii (sporotricoid spread). The typical carpal tendon involvement may be the first presenting manifestation and may lead to surgical exploration or steroid injection. The index of suspicion for M. marinum infections must be high to ensure that proper specimens obtained during procedures are sent for culture.
M. ulcerans, another waterborne skin pathogen, is found mainly in the tropics, especially in tropical areas of Africa. Infection follows skin trauma or insect bites that allow admission to contaminated water. The skin lesions are typically painless, clean ulcers that slough and can cause osteomyelitis. The toxin mycolactone accounts for the modest host inflammatory response and the painless ulcerations.
DIAGNOSIS
NTM can be detected on acid-fast or fluorochrome smears of sputum or other body fluids. When the organism burden is high, the organisms may appear as gram-positive beaded rods, but this finding is unreliable. (In contrast, nocardiae may appear as gram-positive and beaded but filamentous bacteria.) Again, the requisite and most sensitive step in the diagnosis of any mycobacterial disease is to think of including it in the differential. In almost all laboratories, mycobacterial sample processing, staining, and culture are conducted separately from routine bacteriologic tests; thus many infections go undiagnosed because of the physician’s failure to request the appropriate test. In addition, mycobacteria usually require separate blood culture media. NTM are broadly differentiated into rapidly growing (<7 days) and slowly growing (≥7 days) forms. Because M. tuberculosis typically takes ≥2 weeks to grow, many laboratories refuse to consider culture results final until 6 weeks have elapsed. Newer techniques using liquid culture media permit more rapid isolation of mycobacteria from specimens than is possible with traditional media. Species more readily detected with incubation at 30°C include M. marinum, M. haemophilum, and M. ulcerans. M. haemophilum prefers iron supplementation or blood, whereas M. genavense requires supplemented medium with the additive mycobactin J. Bacterial formation of pigment in light conditions (photochromogenicity) or dark conditions (scotochromogenicity) or a lack of bacterial pigment formation (nonchromogenicity) has been used to help categorize NTM. In contrast to NTM colonies, M. tuberculosis colonies are beige, rough, dry, and flat. Current identification schemes can reliably use biochemical, nucleic acid, or cell wall composition, as assessed by high-performance liquid chromatography or mass spectrometry, for speciation. With the remarkable decline in U.S. cases of tuberculosis over recent decades, NTM have become the mycobacteria most commonly isolated from humans in North America. However, not all isolations of NTM, especially from the lung, reflect pathology and require treatment. Whereas identification of an organism in a blood or organ biopsy specimen in a compatible clinical setting is diagnostic, the American Thoracic Society recommends that pulmonary infection due to NTM be diagnosed only when disease is clearly demonstrable—i.e., in an appropriate clinical and radiographic setting (nodules, bronchiectasis, cavities) and with repeated isolation of NTM from expectorated sputum or recovery of NTM from bronchoscopy or biopsy specimens. Given the large number of species of NTM and the importance of accurate diagnosis for the implementation of proper therapy, identification of these organisms is ideally taken to the species level.
The purified protein derivative (PPD) of tuberculin is delivered intradermally to evoke a memory T cell response to mycobacterial antigens. This test is variously referred to as the PPD test, the tuberculin skin test, and the Mantoux test, among other designations. Unfortunately, the cutaneous immune response to these tuberculosis-derived filtrate proteins does not differentiate well between infection with NTM and that with M. tuberculosis. Because intermediate reactions (~10 mm) to PPD in latent tuberculosis and nontuberculous mycobacterial infections can overlap significantly, the progressive decline in active tuberculosis in the United States means that NTM probably account for increasing proportions of PPD reactivity. In addition, bacille Calmette-Guérin (BCG) can cause some degree of cross-reactivity, posing problems of interpretation for patients who have received BCG vaccine. Assays to measure the elaboration of IFN-γ in response to the relatively tuberculosis-specific proteins ESAT6 and CFP10 form the basis for IFN-γ-release assays (IGRAs). These assays can be performed with whole blood or on membranes. It is important to note that M. marinum, M. kansasii, and M. szulgai also have ESAT6 and CFP10 and may cause false-positive reactions in IGRAs. Despite cross-reactivity with NTM, large PPD reactions (>15 mm) most commonly signify tuberculosis.
Isolation of NTM from blood specimens is clear evidence of disease. Whereas rapidly growing mycobacteria may proliferate in routine blood culture media, slow-growing NTM typically do not; thus it is imperative to suspect the diagnosis and to use the correct bottles for cultures. Isolation of NTM from a biopsy specimen constitutes strong evidence for infection, but cases of laboratory contamination do occur. Identification of organisms on stained sections of biopsy material confirms the authenticity of the culture. Certain NTM require lower incubation temperatures (M. genavense) or special additives (M. haemophilum) for growth. Some NTM (e.g., M. tilburgii) remain noncultivable but can be identified molecularly in clinical samples.
The radiographic appearance of nontuberculous mycobacterial disease in the lung depends on the underlying disease, the severity of the infection, and the imaging modality used. The advent and increase in the use of computed tomography (CT) has allowed the identification of characteristic changes that are highly consistent with nontuberculous mycobacterial infection, such as the “tree-in-bud” pattern of bronchiolar inflammation (Fig. 204-2). Involvement of the lingual and right-middle lobes is commonly seen on chest CT but is difficult to appreciate on plain film. Severe bronchiectasis and cavity formation are common in more advanced disease. Isolation of NTM from respiratory samples can be confusing. M. gordonae is often recovered from respiratory samples but is not usually seen on smear and is almost never a pathogen. Patients with bronchiectasis occasionally have NTM recovered from sputum culture with a negative smear. The American Thoracic Society has developed guidelines for the diagnosis of infection with MAC, M. abscessus, and M. kansasii. A positive diagnosis requires the growth of NTM from two of three sputum samples, regardless of smear findings; a positive bronchoscopic alveolar sample, regardless of smear findings; or a pulmonary parenchyma biopsy sample with granulomatous inflammation or mycobacteria found on section and NTM found on culture. These guidelines probably apply to other NTM as well.
FIGURE 204-2 Chest computed tomography of a patient with pulmonary Mycobacterium avium complex infection. Arrows indicate the “tree-in-bud” pattern of bronchiolar inflammation (peripheral right lung) and bronchiectasis (central right and left lungs).
Although many laboratories use DNA probes to identify M. tuberculosis, MAC, M. gordonae, and M. kansasii, speciation of NTM helps determine the antimycobacterial therapy to be used. Only testing of MAC organisms for susceptibility to clarithromycin and of M. kansasii for susceptibility to rifampin is indicated; few data support other in vitro susceptibility tests, attractive though they appear. MAC isolates that have not been exposed to macrolides are almost always susceptible. NTM that have persisted beyond a course of antimicrobial therapy are often tested for antibiotic susceptibility, but the value and meaning of these tests are undetermined.
PREVENTION
Prophylaxis of MAC disease in patients infected with HIV is started when the CD4+ T lymphocyte count falls to <50/μL. Azithromycin (1200 mg weekly), clarithromycin (1000 mg daily), or rifabutin (300 mg daily) is effective. Macrolide prophylaxis in immunodeficient patients who are susceptible to NTM (e.g., those with defects in the IFN-γ/IL-12 axis) has not been prospectively validated but seems prudent.
|
TREATMENT |
NONTUBERCULOUS MYCOBACTERIA |
NTM cause chronic infections that evolve relatively slowly over a period of weeks to years. Therefore, it is rarely necessary to initiate treatment on an emergent basis before the diagnosis is clear and the infecting species is known. Treatment of NTM is complex, often poorly tolerated, and potentially toxic. Just as in tuberculosis, inadequate single-drug therapy is almost always associated with the emergence of antimicrobial resistance and relapse.
MAC infection often requires multidrug therapy, the foundation of which is a macrolide (clarithromycin or azithromycin), ethambutol, and a rifamycin (rifampin or rifabutin). For disseminated nontuberculous mycobacterial disease in HIV-infected patients, the use of rifamycins poses special problems—i.e., rifamycin interactions with protease inhibitors. For pulmonary MAC disease, thrice-weekly administration of a macrolide, a rifamycin, and ethambutol has been successful. Therapy is prolonged, generally continuing for 12 months after culture conversion; typically, a course lasts for at least 18 months. Other drugs with activity against MAC organisms include IV and aerosolized aminoglycosides, fluoroquinolones, and clofazimine. In elderly patients, rifabutin can exert significant toxicity. However, with only modest efforts, most antimycobacterial regimens are well tolerated by most patients. Resection of cavitary lesions or severely bronchiectatic segments has been advocated for some patients, especially those with macrolide-resistant infections. The success of therapy for pulmonary MAC infections depends on whether disease is nodular or cavitary and on whether it is early or advanced, ranging from 20% to 80%.
M. kansasii lung disease is similar to tuberculosis in many ways and is also effectively treated with isoniazid (300 mg/d), rifampin (600 mg/d), and ethambutol (15 mg/kg per day). Other drugs with very high-level activity against M. kansasii include clarithromycin, fluoroquinolones, and aminoglycosides. Treatment should continue until cultures have been negative for at least 1 year. In most instances, M. kansasii infection is easily cured.
Rapidly growing mycobacteria pose special therapeutic problems. Extrapulmonary disease in an immunocompetent host is usually due to inoculation (e.g., via surgery, injections, or trauma) or to line infection and is often treated successfully with a macrolide and another drug (with the choice based on in vitro susceptibility), along with removal of the offending focus. In contrast, pulmonary disease, especially that caused by M. abscessus, is extremely difficult to cure. Repeated courses of treatment are usually effective in reducing the infectious burden and symptoms. Therapy generally includes a macrolide along with an IV-administered agent such as amikacin, a carbapenem, cefoxitin, or tigecycline. Other oral agents (used according to in vitro susceptibility testing and tolerance) include fluoroquinolones, doxycycline, and linezolid. Because nontuberculous mycobacterial infections are chronic, care must be taken in the long-term use of drugs with neurotoxicities, such as linezolid and ethambutol. Prophylactic pyridoxine has been suggested in these cases. Durations of therapy for M. abscessus lung disease are difficult to predict because so many cases are chronic and require intermittent therapy. Expert consultation and management are strongly recommended.
Once recognized, M. marinum infection is highly responsive to antimicrobial therapy and is cured relatively easily with any combination of a macrolide, ethambutol, and a rifamycin. Therapy should be continued for 1–2 months after clinical resolution of isolated soft tissue disease; tendon and bone involvement may require longer courses in light of clinical evolution. Other drugs with activity against M. marinum include sulfonamides, trimethoprim-sulfamethoxazole, doxycycline, and minocycline.
Treatment of the other NTM is less well defined, but macrolides and aminoglycosides are usually effective, with other agents added as indicated. Expert consultation is strongly encouraged for difficult or unusual infections due to NTM.
PROGNOSIS
The outcomes of nontuberculous mycobacterial infections are closely tied to the underlying condition (e.g., IFN-γ/IL-12 pathway defect, cystic fibrosis) and can range from recovery to death. With no or inadequate treatment, symptoms and signs can be debilitating, including persistent cough, fever, anorexia, and severe lung destruction. With treatment, patients typically regain strength and energy. The optimal duration of therapy when NTM persist in sputum is unknown, but treatment in this situation can be prolonged.
GLOBAL CONSIDERATIONS
![]() In many countries, pulmonary tuberculosis is diagnosed by smear alone, which is also the method used for monitoring of response and relapse. However, examination of mycobacteria from the affected patients shows that a significant proportion of isolates are actually NTM. Overall, as rates of tuberculosis decline, the proportion of positive smears caused by NTM will increase. Advances in speciation will distinguish tuberculosis from nontuberculous mycobacterial infections and thereby affect rates of assumed relapse and resistance, leading to more targeted and appropriate therapy.
In many countries, pulmonary tuberculosis is diagnosed by smear alone, which is also the method used for monitoring of response and relapse. However, examination of mycobacteria from the affected patients shows that a significant proportion of isolates are actually NTM. Overall, as rates of tuberculosis decline, the proportion of positive smears caused by NTM will increase. Advances in speciation will distinguish tuberculosis from nontuberculous mycobacterial infections and thereby affect rates of assumed relapse and resistance, leading to more targeted and appropriate therapy.
205e |
Antimycobacterial Agents |
![]() Agents used for the treatment of mycobacterial infections, including tuberculosis (TB), leprosy, and infections due to nontuberculous mycobacteria (NTM), are administered in multiple-drug regimens for prolonged courses. Currently, more than 160 species of mycobacteria have been identified, the majority of which do not cause disease in humans. While the incidence of disease caused by Mycobacterium tuberculosis has been declining in the United States, TB remains a leading cause of morbidity and mortality in developing countries—particularly in sub-Saharan Africa, where the HIV epidemic rages. Effective drug regimens are not all that is needed; without a well-organized infrastructure for diagnosis and treatment of TB, therapeutic and control efforts are severely hampered (Chaps. 2 and 13e). Infections with NTM have gained in clinical prominence in the United States and other developed countries. These largely environmental organisms often establish infection in immunocompromised patients or in persons with structural lung disease.
Agents used for the treatment of mycobacterial infections, including tuberculosis (TB), leprosy, and infections due to nontuberculous mycobacteria (NTM), are administered in multiple-drug regimens for prolonged courses. Currently, more than 160 species of mycobacteria have been identified, the majority of which do not cause disease in humans. While the incidence of disease caused by Mycobacterium tuberculosis has been declining in the United States, TB remains a leading cause of morbidity and mortality in developing countries—particularly in sub-Saharan Africa, where the HIV epidemic rages. Effective drug regimens are not all that is needed; without a well-organized infrastructure for diagnosis and treatment of TB, therapeutic and control efforts are severely hampered (Chaps. 2 and 13e). Infections with NTM have gained in clinical prominence in the United States and other developed countries. These largely environmental organisms often establish infection in immunocompromised patients or in persons with structural lung disease.
TUBERCULOSIS
GENERAL PRINCIPLES
The earliest recorded human case of TB dates back 9000 years. Early treatment modalities, such as bloodletting, were replaced by sanatorium regimens in the late nineteenth century. The discovery of streptomycin in 1943 launched the era of antibiotic treatment for TB. Over subsequent decades, the discovery of additional agents and the use of multiple-drug regimens allowed progressive shortening of the treatment course from years to as little as 6 months with the regimen for drug-susceptible TB. Latent TB infection (LTBI) and active TB disease are diagnosed by history, physical examination, radiographic imaging, tuberculin skin test, interferon γ release assays, acid-fast staining, mycobacterial cultures, and/or new molecular diagnostics. LTBI is treated with isoniazid (optimally given daily or twice weekly for 9 months), rifampin (daily for 4 months), or isoniazid plus rifapentine (weekly for 3 months) (Table 205e-1).
|
REGIMENS FOR THE TREATMENT OF LATENT TUBERCULOSIS INFECTION IN ADULTS |
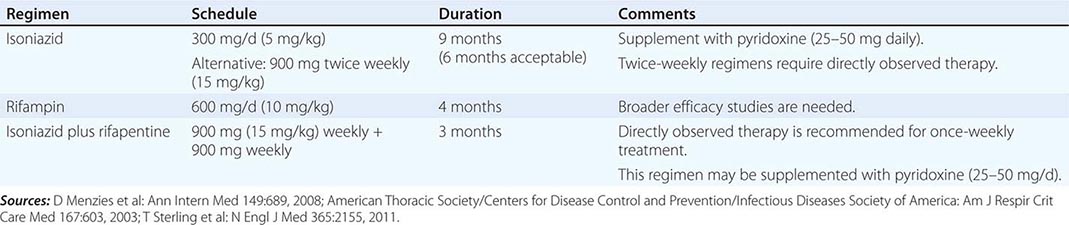
For active or suspected TB disease, clinical factors, including HIV co-infection, symptom duration, radiographic appearance, and public health concerns about TB transmission, drive diagnostic testing and treatment initiation. Multiple-drug regimens are used for the treatment of TB disease (Table 205e-2). Initially, an intensive phase consisting of four drugs—isoniazid, rifampin, pyrazinamide, and ethambutol—given for 2 months is followed by a continuation phase of isoniazid and rifampin for 4 months, for a total treatment duration of 6 months. The continuation phase is extended to 7 months (for a total treatment duration of 9 months) if the 2-month course of pyrazinamide is not completed or, for patients with cavitary pulmonary TB, if sputum cultures remain positive beyond 2 months of treatment (delayed culture conversion).
|
SIMPLIFIED APPROACH TO TREATMENT OF ACTIVE TUBERCULOSIS (TB) IN ADULTS |
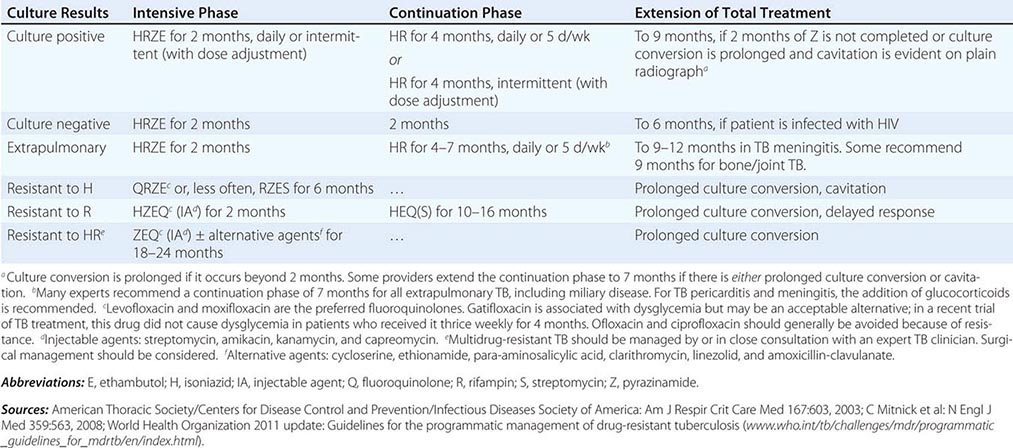
Treatment of TB in individuals co-infected with HIV poses significant challenges, but some progress is being made. Recent data show improved survival when antiretroviral therapy (ART) is initiated early during TB treatment. Interactions of rifampin with protease inhibitors or non-nucleoside reverse transcriptase inhibitors are significant and require close monitoring and dose adjustments. The TB immune reconstitution inflammatory syndrome (IRIS) may appear as early as 1 week after initiation of ART and manifests as paradoxical worsening or unmasking of existing TB infection. Conservative management consists of continued administration of ART and TB medications. However, severe or debilitating IRIS has been anecdotally treated with varying doses of glucocorticoids. Intermittent therapy in patients co-infected with HIV and M. tuberculosis has been associated with low plasma levels of several key TB drugs and with higher rates of treatment failure or relapse; therefore, intermittent twice-weekly therapy for TB in HIV-co-infected individuals is not recommended.
Adherence to medications is critical in achieving a cure with antimycobacterial therapy. Consequently, directly observed therapy (DOT) by trained staff, either in the clinic or at home, is recommended to ensure adherence. In addition, monthly dispensing of TB medicines is recommended because monthly clinical monitoring for hepatotoxicity due to these medications is essential for all patients. Discontinuation of suspected offending agents at the onset of hepatitis symptoms reduces the risk of progression to fatal hepatitis. Clinical monitoring includes at least monthly assessment for symptoms (nausea, vomiting, abdominal discomfort, and unexplained fatigue) and signs (jaundice, dark urine, light stools, diffuse pruritus) of hepatotoxicity, although the latter represent comparatively late manifestations (Table 205e-3). The presence of such symptoms and signs mandates provisional discontinuation of potentially hepatotoxic agents. Biochemical testing of at least serum alanine aminotransferase and total bilirubin levels and exclusion of other causes of these abnormalities are also indicated during treatment for those at risk for hepatotoxicity (Table 205e-3). For patients with active TB, monthly mycobacterial cultures of sputum are recommended until it is certain that the organisms have been cleared and the patient has responded to therapy or until no sputum is available for culture.
|
MONITORING AND CLINICAL MANAGEMENT OF TUBERCULOSIS TREATMENT IN ADULTSa |
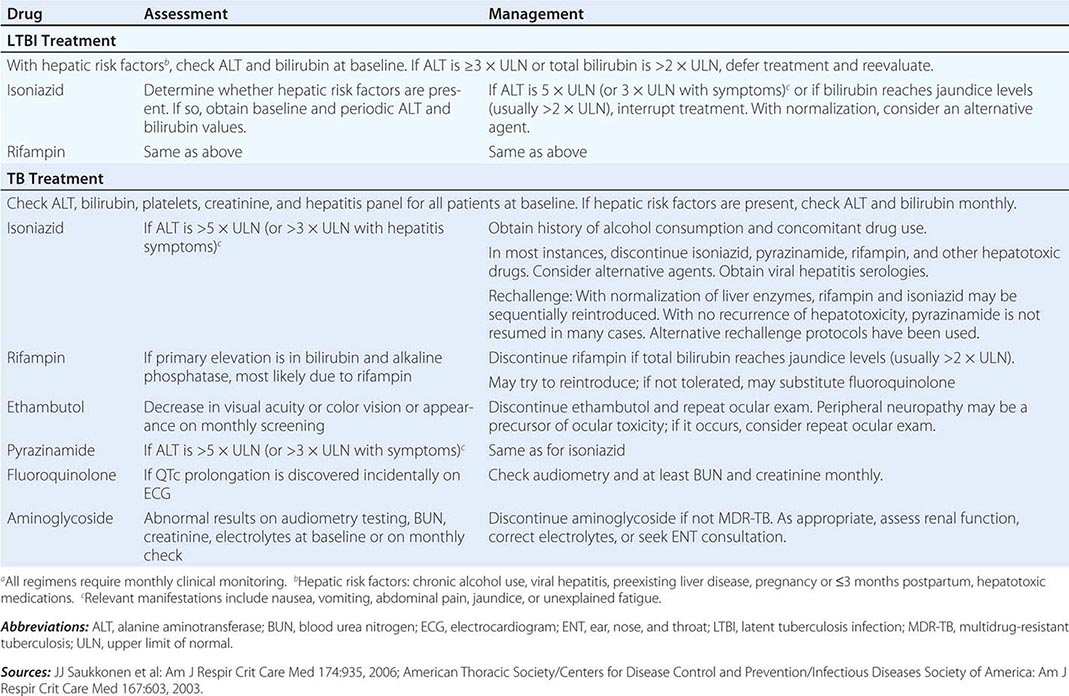
If significant clinical improvement does not occur or the patient’s condition deteriorates over the course of therapy, possibilities include treatment failure due to nonadherence, poor medication absorption, or the development of resistance. For patients co-infected with HIV and M. tuberculosis, IRIS, which is a diagnosis of exclusion, should also be a consideration. Drug susceptibility testing should be repeated at this point. If resistance is documented or strongly suspected, at least two efficacious drugs to which the isolate is susceptible or which the patient has not already taken should be added to the therapeutic regimen.
Multidrug-resistant TB (MDR-TB) is defined as disease caused by a strain of M. tuberculosis that is resistant to both isoniazid and rifampin—the most efficacious of the first-line TB drugs. The risk of MDR-TB is elevated in patients presenting from geographic areas in which ≥5% of incident cases are MDR-TB and in patients previously treated for TB. Treatment regimens for MDR-TB generally include a late-generation fluoroquinolone and an injectable second-line agent (such as capreomycin, amikacin, or kanamycin). Regimens of at least five drugs are recommended for the treatment of MDR-TB. Both standardized and optimized/customized regimens are in use around the world. Extensively drug-resistant TB (XDR-TB) is defined as MDR-TB with additional resistance to any fluoroquinolone and at least one of the second-line injectable agents. Treatment of XDR-TB is individualized on the basis of complete phenotypic and, if possible, genotypic antimicrobial susceptibility testing. Therapeutic regimens for either MDR-TB or XDR-TB should be constructed with input from experienced clinicians who should continue the management of the disease.
FIRST-LINE ANTITUBERCULOSIS DRUGS
The following discussion of individual anti-TB agents focuses on treatment of TB in adults, unless otherwise noted. Several agents are being actively investigated during the current remarkable period of drug development for TB treatment.
Isoniazid Isoniazid is a critical drug for treatment of both TB disease and LTBI. Isoniazid has excellent bactericidal activity against both intracellular M. tuberculosis and extracellular, actively dividing organisms. This drug is bacteriostatic against slowly dividing organisms. In treatment of LTBI, isoniazid is considered the first-line agent because it is generally well tolerated, has well-established efficacy, and is inexpensive. In this setting, the drug is taken daily or intermittently (i.e., twice weekly) as DOT for 9 months. The 9-month course is more efficacious than the 6-month course (75–90% vs ≤65%), but extension of treatment to 12 months is not likely to provide further protection. A 6-month course of daily or intermittent isoniazid is considered second-line, but acceptable, therapy. A recent large open-label, multicenter, randomized, controlled trial showed that weekly DOT with isoniazid and rifapentine, administered over 3 months, was not inferior to daily isoniazid given for 9 months and had a higher treatment completion rate than the single-drug regimen.
For treatment of TB disease, isoniazid is used in combination with other agents to ensure killing of both actively dividing M. tuberculosis and slowly growing “persister” organisms. Unless the organism is resistant, the standard regimen includes isoniazid, rifampin, ethambutol, and pyrazinamide (Table 205e-2). Isoniazid is often given together with 25–50 mg of pyridoxine daily to prevent drug-related peripheral neuropathy.
MECHANISM OF ACTION Isoniazid is a prodrug activated by the mycobacterial KatG catalase-peroxidase; isoniazid is coupled with reduced nicotinamide adenine dinucleotide (NADH). The resulting isonicotinic acyl–NADH complex blocks the mycobacterial ketoenoylreductase known as InhA, binding to its substrate and inhibiting fatty acid synthase and ultimately mycolic acid synthesis. Mycolic acids are essential components of the mycobacterial cell wall. KatG activation of isoniazid also results in the release of free radicals that have antimycobacterial activity, including nitric oxide.
The minimal inhibitory concentrations (MICs) of isoniazid for wild-type (untreated) susceptible strains are <0.1 μg/mL for M. tuberculosis and 0.5–2 μg/mL for Mycobacterium kansasii.
PHARMACOLOGY Isoniazid is the hydrazide of isonicotinic acid, a small, water-soluble molecule. The usual adult oral daily dose of 300 mg results in peak serum levels of 3–5 μg/mL within 30 min to 2 h after ingestion—well in excess of the MICs for most susceptible strains of M. tuberculosis. Both oral and IM preparations of isoniazid reach effective levels in the body, although antacids and high-carbohydrate meals may interfere with oral absorption. Isoniazid diffuses well throughout the body, reaching therapeutic concentrations in body cavities and fluids, with concentrations in cerebrospinal fluid (CSF) comparable to those in serum.
Isoniazid is metabolized in the liver via acetylation by N-acetyltransferase 2 (NAT2) and hydrolysis. Both fast- and slow-acetylation phenotypes occur; patients who are “fast acetylators” may have lower serum levels of isoniazid, whereas “slow acetylators” may have higher levels and experience more toxicity. Satisfactory isoniazid levels are attained in the majority of homozygous fast NAT2 acetylators given a dose of 6 mg/kg and in the majority of homozygous slow acetylators given only 3 mg/kg. Genotyping is increasingly being used to characterize isoniazid-related pharmacogenomic responses.
Isoniazid’s interactions with other drugs are due primarily to its inhibition of the cytochrome P450 system. Among the drugs with significant isoniazid interactions are warfarin, carbamazepine, benzodiazepines, acetaminophen, clopidogrel, maraviroc, dronedarone, salmeterol, tamoxifen, eplerenone, and phenytoin.
DOSING The recommended daily dose for the treatment of TB in the United States is 5 mg/kg for adults and 10–20 mg/kg for children, with a maximal daily dose of 300 mg for both. For intermittent therapy in adults (usually twice per week), the dose is 15 mg/kg, with a maximal daily dose of 900 mg. Isoniazid does not require dosage adjustment in patients with renal disease. When the 12-dose, 3-month weekly LTBI regimen is used, the dose of isoniazid is 15 mg/kg, with a maximal dose of 900 mg, and the drug is coadministered with rifapentine.
RESISTANCE Although isoniazid, along with rifampin, is the mainstay of TB treatment regimens, ~7% of clinical M. tuberculosis isolates in the United States are resistant. Rates of primary isoniazid resistance among untreated patients are significantly higher in many populations born outside the United States. Five separate pathways for isoniazid resistance have been elucidated. Most strains have amino acid changes in either the catalase-peroxidase gene (katG) or the mycobacterial ketoenoylreductase gene (inhA). Less frequently, alterations in kasA, the gene for an enzyme involved in mycolic acid elongation, and loss of NADH dehydrogenase 2 activity confer isoniazid resistance. In 20–30% of isoniazid-resistant M. tuberculosis isolates, increased expression of efflux pump genes, such as efpA, mmpL7, mmr, p55, and the Tap-like gene Rv1258c, has been implicated as the underlying mechanism of resistance.
ADVERSE EFFECTS Although isoniazid is generally well tolerated, drug-induced liver injury and peripheral neuropathy are significant adverse effects associated with this agent. Isoniazid may cause asymptomatic transient elevation of aminotransferase levels (often termed hepatic adaptation) in up to 20% of recipients. Other adverse reactions include rash (2%), fever (1.2%), anemia, acne, arthritic symptoms, a systemic lupus erythematosus–like syndrome, optic atrophy, seizures, and psychiatric symptoms. Symptomatic hepatitis occurs in fewer than 0.1% of persons treated with isoniazid alone for LTBI, and fulminant hepatitis with hepatic failure occurs in fewer than 0.01%. Isoniazid-associated hepatitis is idiosyncratic, but its incidence increases with age, with daily alcohol consumption, and in women who are within 3 months postpartum.
In patients who have liver disorders or HIV infection, who are pregnant or in the 3-month postpartum period, who have a history of liver disease (e.g., hepatitis B or C, alcoholic hepatitis, or cirrhosis), who use alcohol regularly, who have multiple medical problems, or who have other risk factors for chronic liver disease, the risks and benefits of treatment for LTBI should be weighed. If treatment is undertaken, these patients should have serum concentrations of alanine aminotransferase (ALT) determined at baseline. Routine baseline hepatic ALT testing based solely on an age of >35 years is optional and depends on individual concerns. Monthly biochemical monitoring during isoniazid treatment is indicated for patients whose baseline liver function tests yield abnormal results and for persons at risk for hepatic disease, including the groups just mentioned. Guidelines recommend that isoniazid be discontinued in the presence of hepatitis symptoms or jaundice and an ALT level three times the upper limit of normal or in the absence of symptoms with an ALT level five times the upper limit of normal (Table 205e-3).
Peripheral neuropathy associated with isoniazid occurs in up to 2% of patients given 5 mg/kg. Isoniazid appears to interfere with pyridoxine (vitamin B6) metabolism. The risk of isoniazid-related neurotoxicity is greatest for patients with preexisting disorders that also pose a risk of neuropathy, such as HIV infection; for those with diabetes mellitus, alcohol abuse, or malnutrition; and for those simultaneously receiving other potentially neuropathic medications, such as stavudine. These patients should be given prophylactic pyridoxine (25–50 mg/d).
Rifampin Rifampin is a semisynthetic derivative of Amycolatopsis rifamycinica (formerly known as Streptomyces mediterranei). The most active antimycobacterial agent available, rifampin is the keystone of first-line treatment for TB. Introduced in 1968, this drug eventually permitted dramatic shortening of the TB treatment course. Rifampin has both sterilizing and bactericidal activity against dividing and nondividing M. tuberculosis. The drug is also active against an array of other organisms, including some gram-positive and gram-negative bacteria, Legionella, M. kansasii, and Mycobacterium marinum.
Rifampin, administered for 4 months, is also an alternative agent to isoniazid for the treatment of LTBI, although efficacy data are scant at this time. A 3-month course of rifampin alone has been found to be similar in efficacy to a 6-month course of isoniazid. Although the efficacy of the 4-month regimen of rifampin is under study, comparison of this regimen with 9 months of isoniazid in randomized safety and tolerability studies suggests fewer adverse events, including hepatotoxicity; less treatment interruption; a higher completion rate; and greater cost-effectiveness.
MECHANISM OF ACTION Rifampin exerts both intracellular and extracellular bactericidal activity. Like other rifamycins, rifampin specifically binds to and inhibits mycobacterial DNA-dependent RNA polymerase, blocking RNA synthesis. Susceptible strains of M. tuberculosis as well as M. kansasii and M. marinum are inhibited by rifampin concentrations of 1 μg/mL.
PHARMACOLOGY Rifampin is a fat-soluble, complex macrocyclic molecule readily absorbed after oral administration. Serum levels of 10–20 μg/mL are achieved 2.5 h after the usual adult oral dose of 10 mg/kg (given without food). Rifampin has a half-life of 1.5–5 h. The drug distributes well throughout most body tissues, including CSF. Rifampin turns body fluids such as urine, saliva, sputum, and tears a reddish-orange color—an effect that offers a simple means of assessing patients’ adherence to this medication. Rifampin is excreted primarily through the bile and enters the enterohepatic circulation; <30% of a dose is renally excreted.
As a potent inducer of the hepatic cytochrome P450 system, rifampin can decrease the half-life of some drugs, such as digoxin, warfarin, phenytoin, prednisone, cyclosporine, methadone, oral contraceptives, clarithromycin, azole antifungal agents, quinidine, antiretroviral protease inhibitors, and non-nucleoside reverse transcriptase inhibitors. The Centers for Disease Control and Prevention has issued guidelines for the management of drug interactions during treatment of HIV and M. tuberculosis co-infection (www.cdc.gov/tb/publications/guidelines/TB_HIV_Drugs/default.htm).
DOSING The daily dosage of rifampin is 10 mg/kg for adults and 10–20 mg/kg for children, with a maximum of 600 mg/d for both. The drug is given once daily, twice weekly, or three times weekly. No adjustments of dose or frequency are necessary in patients with renal insufficiency.
RESISTANCE Resistance to rifampin in M. tuberculosis, Mycobacterium leprae, and other organisms is the consequence of spontaneous, mostly missense point mutations in a core region of the bacterial gene coding for the β subunit of RNA polymerase (rpoB). RNA polymerase altered in this manner is no longer subject to inhibition by rifampin. Most rapidly and slowly growing NTM harbor intrinsic resistance to rifampin, for which the mechanism has yet to be determined.
ADVERSE EFFECTS Adverse events associated with rifampin are infrequent and generally mild. Hepatotoxicity due to rifampin alone is uncommon in the absence of preexisting liver disease and often consists of isolated hyperbilirubinemia rather than aminotransferase elevation. Other adverse reactions include rash, pruritus, gastrointestinal symptoms, and pancytopenia. Rarely, a hypersensitivity reaction may occur with intermittent therapy, manifesting as fever, chills, malaise, rash, and—in some instances—renal and hepatic failure.
Ethambutol Ethambutol is a bacteriostatic antimycobacterial agent first synthesized in 1961. A component of the standard first-line regimen, ethambutol provides synergy with the other drugs in the regimen and is generally well tolerated. Susceptible species include M. tuberculosis, M. marinum, M. kansasii, and organisms of the Mycobacterium avium complex (MAC); however, among first-line drugs, ethambutol is the least potent against M. tuberculosis. This agent is also used in combination with other agents in the continuation phase of treatment when patients cannot tolerate isoniazid or rifampin or are infected with organisms resistant to either of the latter drugs.
MECHANISM OF ACTION Ethambutol is bacteriostatic against M. tuberculosis. Its primary mechanism of action is the inhibition of the arabinosyltransferases involved in cell wall synthesis, which probably inhibits the formation of arabinogalactan and lipoarabinomannan. The MIC of ethambutol for susceptible strains of M. tuberculosis is 0.5–2 μg/mL.
PHARMACOLOGY AND DOSING From a single dose of ethambutol, 75–80% is absorbed within 2–4 h of administration. Serum levels peak at 2–4 μg/mL after the standard adult daily dose of 15 mg/kg. Ethambutol is well distributed throughout the body except in the CSF; a dosage of 25 mg/kg is necessary for attainment of a CSF level half of that in serum. For intermittent therapy, the dosage is 50 mg/kg twice weekly. To prevent toxicity, the dosage must be lowered and the frequency of administration reduced for patients with renal insufficiency.
ADVERSE EFFECTS Ethambutol is usually well tolerated and has no significant interactions with other drugs. Optic neuritis, the most serious adverse effect reported, typically presents as reduced visual acuity, central scotoma, and loss of the ability to see green (or, less commonly, red). The cause of this neuritis is unknown, but it may be due to an effect of ethambutol on the amacrine and bipolar cells of the retina. Symptoms typically develop several months after initiation of therapy, but ocular toxicity soon after initiation of ethambutol has been described. The risk of ocular toxicity is dose dependent, occurring in 1–5% of patients, and can be increased by renal insufficiency. The routine use of ethambutol in younger children is not recommended because monitoring for visual complications can be difficult. If drug-resistant TB is suspected, ethambutol can be used for treatment of children.
All patients starting therapy with ethambutol should have a baseline test for visual acuity, visual fields, and color vision and should undergo an examination of the optic fundus. Visual acuity and color vision should be monitored monthly or less often as needed. Cessation of ethambutol in response to early symptoms of ocular toxicity usually results in reversal of the deficit within several months. Recovery of all visual function may take up to 1 year. In the elderly and in patients whose symptoms are not recognized early, deficits may be permanent. Some experts think that supplementation with hydroxocobalamin (vitamin B12) is beneficial for patients with ethambutol-related ocular toxicity. Other adverse effects of ethambutol are rare. Peripheral sensory neuropathy occurs in rare instances.
RESISTANCE Ethambutol resistance in M. tuberculosis and NTM is associated primarily with missense mutations in the embB gene that encodes for arabinosyltransferase. Mutations have been found in resistant strains at codon 306 in 50–70% of cases. Mutations at embB306 can cause significantly increased MICs of ethambutol, resulting in clinical resistance.
Pyrazinamide A nicotinamide analog, pyrazinamide is an important bactericidal drug used in the initial phase of TB treatment. Its administration for the first 2 months of therapy with rifampin and isoniazid allows treatment duration to be shortened from 9 months to 6 months and decreases rates of relapse.
MECHANISM OF ACTION Pyrazinamide’s antimycobacterial activity is essentially limited to M. tuberculosis. The drug is more active against slowly replicating organisms than against actively replicating organisms. Pyrazinamide is a prodrug that is converted by the mycobacterial pyrimidase to the active form, pyrazinoic acid (POA). This agent is active only in acidic environments (pH <6.0), as are found within phagocytes or granulomas. The exact mechanism of action of POA is unclear, but fatty acid synthetase I may be the primary target in M. tuberculosis. Susceptible strains of M. tuberculosis are inhibited by pyrazinamide concentrations of 16–50 μg/mL at pH 5.5.
PHARMACOLOGY AND DOSING Pyrazinamide is well absorbed after oral administration, with peak serum concentrations of 20–60 μg/mL at 1–2 h after ingestion of the recommended adult daily dose of 15–30 mg/kg (maximum, 2 g/d). It distributes well to various body compartments, including CSF, and is an important component of treatment for tuberculous meningitis. The serum half-life of the drug is 9–11 h with normal renal and hepatic function. Pyrazinamide is metabolized in the liver to POA, 5-hydroxypyrazinamide, and 5-hydroxy-POA. A high proportion of pyrazinamide and its metabolites (~70%) is excreted in the urine. The dosage must be adjusted according to the level of renal function in patients with reduced creatinine clearance.
ADVERSE EFFECTS At the higher dosages used previously, hepatotoxicity was seen in as many as 15% of patients treated with pyrazinamide. However, at the currently recommended dosages, hepatotoxicity now occurs less commonly when this drug is administered with isoniazid and rifampin during the treatment of TB. Older age, active liver disease, HIV infection, and low albumin levels may increase the risk of hepatotoxicity. The use of pyrazinamide with rifampin for the treatment of LTBI is no longer recommended because of unacceptable rates of hepatotoxicity and death in this setting. Hyperuricemia is a common adverse effect of pyrazinamide therapy that usually can be managed conservatively. Clinical gout is rare.
![]() Although pyrazinamide is recommended by international TB organizations for routine use in pregnancy, it is not recommended in the United States because of inadequate teratogenicity data.
Although pyrazinamide is recommended by international TB organizations for routine use in pregnancy, it is not recommended in the United States because of inadequate teratogenicity data.
RESISTANCE The basis of pyrazinamide resistance in M. tuberculosis is a mutation in the pncA gene coding for pyrazinamidase, the enzyme that converts the prodrug to active POA. Resistance to pyrazinamide is associated with loss of pyrazinamidase activity, which prevents conversion of pyrazinamide to POA. Of pyrazinamide-resistant M. tuberculosis isolates, 72–98% have mutations in pncA. Conventional methods of testing for susceptibility to pyrazinamide may produce both false-negative and false-positive results because the high-acidity environment required for the drug’s activation also inhibits the growth of M. tuberculosis. There is some controversy as to the clinical significance of in vitro pyrazinamide resistance.
OTHER FIRST-LINE DRUGS
Rifabutin Rifabutin, a semisynthetic derivative of rifamycin S, inhibits mycobacterial DNA-dependent RNA polymerase. Rifabutin is recommended in place of rifampin for the treatment of HIV-co-infected individuals who are taking protease inhibitors or non-nucleoside reverse transcriptase inhibitors, particularly nevirapine. Rifabutin’s effect on hepatic enzyme induction is less pronounced than that of rifampin. Protease inhibitors may cause significant increases in rifabutin levels through inhibition of hepatic metabolism. Rifabutin is more active in vitro than rifampin against MAC organisms and other NTM, but its clinical superiority has not been established.
PHARMACOLOGY Like rifampin, rifabutin is lipophilic and is absorbed rapidly after oral administration, reaching peak serum levels 2–4 h after ingestion. Rifabutin distributes best to tissues, reaching levels 5–10 times higher than those in plasma. Unlike rifampin, rifabutin and its metabolites are partially cleared by the hepatic microsomal system. Rifabutin’s slow clearance results in a mean serum half-life of 45 h—much longer than the 3- to 5-h half-life of rifampin. Clarithromycin (but not azithromycin) and fluconazole appear to increase rifabutin levels by inhibiting hepatic metabolism.
ADVERSE EFFECTS Rifabutin is generally well tolerated, with adverse effects occurring at higher doses. The most common adverse events are gastrointestinal; other reactions include rash, headache, asthenia, chest pain, myalgia, and insomnia. Less common adverse reactions include fever, chills, a flulike syndrome, anterior uveitis, hepatitis, Clostridium difficile–associated diarrhea, a diffuse polymyalgia syndrome, and yellow skin discoloration (“pseudo-jaundice”). Laboratory abnormalities include neutropenia, leukopenia, thrombocytopenia, and increased levels of liver enzymes. Approximately 80% of patients who develop rifampin-related adverse events are able to complete TB treatment with rifabutin.
RESISTANCE Similar to rifampin resistance, resistance to rifabutin is mediated by some mutations in rpoB.
Rifapentine Rifapentine is a semisynthetic cyclopentyl rifamycin, sharing a mechanism of action with rifampin. Rifapentine is lipophilic and has a prolonged half-life that permits weekly or twice-weekly dosing. Therefore, this drug is the subject of intensive clinical investigation aimed at determining optimal dosing and frequency of administration. Currently, rifapentine is an alternative to rifampin in the continuation phase of treatment for noncavitary drug-susceptible pulmonary TB in HIV-seronegative patients who have negative sputum smears at completion of the initial phase of treatment. When administered in these specific circumstances, rifapentine (10 mg/kg, up to 600 mg) is given once weekly with isoniazid. Because of higher rates of relapse, this regimen is not recommended for patients with TB disease and HIV co-infection. A large randomized controlled trial recently demonstrated that, for latent TB, a 12-dose (3-month) regimen of weekly DOT with a weight-based dose of isoniazid and rifapentine was noninferior to daily isoniazid for 9 months. Although the rate of permanent drug discontinuation due to adverse events was higher with rifapentine/isoniazid, this regimen had a higher treatment completion rate than daily isoniazid in this study. The efficacy of this combination regimen in HIV-infected individuals not receiving ART and in children <12 years of age is under study. The regimen is not recommended for pregnant women, for persons with hypersensitivity reactions to isoniazid or rifampin, or for HIV-infected individuals taking ART.
PHARMACOLOGY Rifapentine’s absorption is improved when the drug is taken with food. After oral administration, rifapentine reaches peak serum concentrations in 5–6 h and achieves a steady state in 10 days. The half-life of rifapentine and its active metabolite, 25-desacetyl rifapentine, is ~13 h. The administered dose is excreted via the liver (70%).
ADVERSE EFFECTS The adverse-effects profile of rifapentine is similar to that of other rifamycins. Rifapentine is teratogenic in animal models and is relatively contraindicated in pregnancy.
RESISTANCE Rifapentine resistance is mediated by mutations in rpoB. Mutations that cause resistance to rifampin also cause resistance to rifapentine.
![]() Streptomycin Streptomycin was the first antimycobacterial agent used for the treatment of TB. Derived from Streptomyces griseus, streptomycin is bactericidal against dividing M. tuberculosis organisms but has only low-level early bactericidal activity. This drug is administered only by the IM and IV routes. In developed nations, streptomycin is used infrequently because of its toxicity, the inconvenience of injections, and drug resistance. In developing countries, however, streptomycin is used because of its low cost.
Streptomycin Streptomycin was the first antimycobacterial agent used for the treatment of TB. Derived from Streptomyces griseus, streptomycin is bactericidal against dividing M. tuberculosis organisms but has only low-level early bactericidal activity. This drug is administered only by the IM and IV routes. In developed nations, streptomycin is used infrequently because of its toxicity, the inconvenience of injections, and drug resistance. In developing countries, however, streptomycin is used because of its low cost.
MECHANISM OF ACTION Streptomycin inhibits protein synthesis by binding at a site on the 30S mycobacterial ribosome.
PHARMACOLOGY AND DOSING Serum levels of streptomycin peak at 25–45 μg/mL after a 1-g dose. This agent penetrates poorly into the CSF, reaching levels that are only 20% of serum levels. The usual daily dose of streptomycin (given IM either daily or 5 days per week) is 15 mg/kg for adults and 20–40 mg/kg for children, with a maximum of 1 g/d for both. For patients ≥60 years of age, 10 mg/kg is the recommended daily dose, with a maximum of 750 mg/d. Because streptomycin is eliminated almost exclusively by the kidneys, its use in patients with renal impairment should be avoided or implemented with caution, with lower doses and less frequent administration.
ADVERSE EFFECTS Adverse reactions occur frequently with streptomycin (10–20% of patients). Ototoxicity (primarily vestibulotoxicity), neuropathy, and renal toxicity are the most common and the most serious. Renal toxicity, usually manifested as nonoliguric renal failure, is less common with streptomycin than with other frequently used aminoglycosides, such as gentamicin. Manifestations of vestibular toxicity include loss of balance, vertigo, and tinnitus. Patients receiving streptomycin must be monitored carefully for these adverse effects, undergoing audiometry at baseline and monthly thereafter.
RESISTANCE Spontaneous mutations conferring resistance to streptomycin are relatively common, occurring in 1 in 106 organisms. In the two-thirds of streptomycin-resistant M. tuberculosis strains exhibiting high-level resistance, mutations have been identified in one of two genes: a 16S rRNA gene (rrs) or the gene encoding ribosomal protein S12 (rpsL). Both targets are believed to be involved in streptomycin ribosomal binding. However, low-level resistance, which is seen in about one-third of resistant isolates, has no associated resistance mutation. A gene (gidB) that confers low-level resistance to streptomycin has recently been identified. Strains of M. tuberculosis resistant to streptomycin generally are not cross-resistant to capreomycin or amikacin. Streptomycin is not used for the treatment of MDR-TB or XDR-TB because of (1) the high prevalence of streptomycin resistance among strains resistant to isoniazid and (2) the unreliability of drug susceptibility testing.
SECOND-LINE ANTITUBERCULOSIS DRUGS
Second-line antituberculosis agents are indicated for treatment of drug-resistant TB, for patients who are intolerant or allergic to first-line agents, and when first-line supplemental agents are unavailable.
Fluoroquinolones Fluoroquinolones inhibit mycobacterial DNA gyrase and topoisomerase IV, preventing cell replication and protein synthesis, and are bactericidal. The later-generation fluoroquinolones levofloxacin and moxifloxacin are the most active against M. tuberculosis and are recommended for the treatment of MDR-TB. They are also being investigated for their potential to shorten the course of treatment for TB. In a recent trial, gatifloxacin, which had been withdrawn from the market because of significant dysglycemia, was assessed for treatment shortening; although its inclusion in the TB treatment regimen did not shorten the duration of therapy from 6 to 4 months, the drug did not cause dysglycemia in TB patients who took it thrice weekly for 4 months. Ciprofloxacin and ofloxacin are no longer recommended for the treatment of TB because of poor efficacy. Despite the documented resistance of the infecting strains to these and other early-generation fluoroquinolones, use of a later-generation fluoroquinolone in patients with XDR-TB has been associated with favorable outcomes. Fluoroquinolones are also considered safe alternatives for patients who develop treatment-limiting adverse effects due to first-line agents. Levofloxacin and moxifloxacin have both been used effectively in the treatment of MDR-TB. The optimal dose of levofloxacin for this indication is being actively studied, but doses of at least 750 mg are commonly used.
The fluoroquinolones are well absorbed orally, reach high serum levels, and distribute well into body tissues and fluids. Their absorption is decreased by co-ingestion with products containing multivalent cations, such as antacids. Adverse effects are relatively infrequent (0.5–10% of patients) and include gastrointestinal intolerance, rashes, dizziness, and headache. Most studies of fluoroquinolone side effects have been based on relatively short-term administration for bacterial infections, but trials have now shown the relative safety and tolerability of fluoroquinolones administered for months during TB treatment in adults. Although the potential to prolong the QTc interval, leading to cardiac arrhythmias, has been a source of concern with fluoroquinolones, cessation of treatment due to this adverse effect is rare. There is increasing interest in the use of fluoroquinolones in children, which has traditionally been avoided because of the risks of tendon rupture and cartilage damage, because the benefits in treatment of drug-resistant TB may outweigh the risks.
Mycobacterial resistance can develop rapidly when a fluoroquinolone is inadvertently administered alone. Empirical fluoroquinolone therapy for presumed community-acquired pneumonia is associated with increased fluoroquinolone resistance in M. tuberculosis. Mutations in the genes encoding for DNA gyrase (gyrA and gyrB) are implicated in the majority of cases—but not all cases—of clinical resistance to fluoroquinolones.
Injectable Agents • CAPREOMYCIN Capreomycin, a cyclic peptide antibiotic derived from Streptomyces capreolus, is an important first-choice second-line agent used for treatment of MDR-TB, particularly when additional resistance to aminoglycosides is documented. Capreomycin is administered by the IM route; an inhaled preparation is under study. A dose of 15 mg/kg per day is given five to seven times per week (maximal daily dose, 1 g) and results in peak blood levels of 20–40 μg/mL. The dosage may be reduced to 1 g two or three times per week 2–4 months after mycobacterial cultures become negative. For individuals ≥60 years of age, the dose should be reduced to 10 mg/kg per day (maximal daily dose, 750 mg). For patients with renal insufficiency, the drug should be given intermittently and at lower dosage (12–15 mg/kg two or three times per week). A minimal duration of 3 months is recommended for MDR-TB treatment. Penetration of capreomycin into the CSF is believed to be poor.