119 Antimicrobials in Chemotherapy Strategy
Infections are frequently suspected or documented in critically ill patients. Patients are often admitted to the intensive care unit (ICU) for treatment of community-acquired or hospital-acquired infections, whereas many other patients require treatment for nosocomial infections acquired during their ICU stay. Although patients in ICUs represent only 8% to 15% of hospital admissions in the United States,1 these patients suffer a disproportionately high rate of infectious complications and are exposed to very high rates of antimicrobial use.1–3 The importance of antimicrobial drugs in the modern management of critically ill patients with a variety of bacterial, fungal, and viral infections can scarcely be understated. However, despite the availability of improved diagnostic techniques and a wide variety of potent, highly effective antimicrobials, the prevention and appropriate treatment of infections in ICU patients remain a formidable challenge to the clinician.
 Antimicrobial Resistance in the ICU
Antimicrobial Resistance in the ICU
The continuing emergence of antimicrobial resistance in ICUs is a major factor in the appropriate selection and use of antimicrobials in the critical care setting. It has been estimated that 50% to 60% of all nosocomial infections occurring each year in the United States are caused by antimicrobial-resistant strains of bacteria.3,4 The overall incidence of infections due to antibiotic-resistant pathogens, changes in the epidemiology of infections caused by specific pathogens, and increasing resistance to even the most potent broad-spectrum agents make the selection of appropriate antimicrobial therapy extremely challenging in many institutions.1–4 The difficulties in selecting antimicrobial therapy are particularly acute in ICUs because of the higher prevalence of antimicrobial resistance in these areas compared with other non-ICU settings.1,5–8
A number of factors are associated with high rates of antimicrobial resistance in the ICU. Chief among these is the heavy use of antimicrobial agents in critically ill patients. A number of studies have identified a close association between antimicrobial use and the subsequent development of antibiotic resistance.9–19 Whereas use of antibiotics is associated with the emergence of resistance during therapy, previous exposure to antibiotics is also a well-established risk factor for antimicrobial resistance.1–38 The higher severity of illness found among ICU patients is also related to several other risk factors for antimicrobial resistance, including the presence of invasive devices such as endotracheal tubes and intravascular and urinary catheters, prolonged length of hospital stay, immune suppression, and malnutrition.1,2,4,5,8–19 The increasing prevalence of antimicrobial-resistant pathogens among residents in long-term care facilities is also an increasingly important source for resistant bacteria in ICUs.1–5,8,20 Finally, antimicrobial-resistant pathogens are easily cross-transmitted among patients in ICUs, owing to poor adherence of hospital personnel to appropriate infection prevention techniques, contamination of equipment, and frequent overcrowding of patients.1–58 All of these various factors combine to make ICUs the epicenter of antimicrobial resistance in hospitalized patients.1,5
Increased antimicrobial resistance has been observed among both gram-positive and gram-negative bacteria as well as among certain fungi, particularly Candida species. Table 119-1 summarizes important trends in increasing resistance in the United States among selected pathogens and drug classes.1,3,4,21 Much of the changing epidemiology of infection in the ICU has centered around the emergence of gram-positive organisms as predominant pathogens in the critically ill patient. Surveillance programs such as the National Healthcare Safety Network [NHSN], which incorporates the former National Nosocomial Infection Surveillance (NNIS) System sponsored by the Centers for Disease Control and Prevention, have repeatedly documented impressive increases in antimicrobial resistance among pathogens such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and multidrug-resistant Streptococcus pneumoniae.1–320
TABLE 119-1 Trends in Antimicrobial Resistance Among Selected Nosocomial Pathogens from ICU Patients in the United States, 1998-2002 and 2006-2007
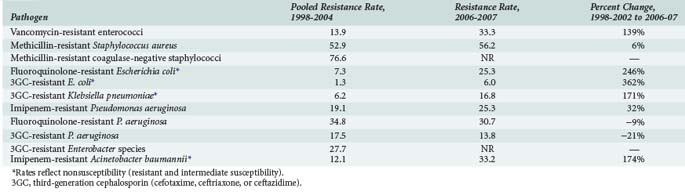
Rates of MRSA and methicillin-resistant coagulase-negative staphylococci have continued to steadily increase over the past decade and are most commonly associated with central catheter-associated bloodstream and wound infections,1–3,5,7,8 whereas MRSA has also been increasingly documented as a frequent pathogen in ventilator-associated pneumonias as well as skin/soft tissue and other infections.1,21–23 Although MRSA has been traditionally regarded as a hospital-acquired pathogen, this bacteria has also emerged as a common cause of community-acquired infections1,24,25; approximately 30% to 40% of all MRSA isolates found in hospitals are now actually community acquired.26 The increase in methicillin resistance among staphylococci has led to a heavy reliance on vancomycin as a drug of choice for infections due to these pathogens and is perhaps related to the dramatic increase in the number of infections caused by VRE among ICU patients. High-level penicillin resistance among S. pneumoniae is approximately 20% to 30% in most geographic areas.27–29 Additionally, penicillin-resistant pneumococci tend to be multidrug resistant; 25% to 30% of S. pneumoniae have decreased susceptibility to macrolide antibiotics, and rates of resistance to several other drug classes including sulfonamides, tetracyclines, and cephalosporins have also increased.27–29 Although the prevalence of fluoroquinolone resistance among S. pneumoniae is still very low (<1%),27–29 there is still significant concern regarding excessive use of fluoroquinolones and the potential for significant resistance in the future.27–30
Antimicrobial resistance continues to be a problem of major importance among gram-negative bacilli. Of particular concern is the rapid spread of resistance mediated by extended-spectrum β-lactamases (ESBLs) among organisms such as Escherichia coli and Klebsiella pneumoniae. Organisms that produce ESBLs are usually resistant to multiple antimicrobials, including third- (e.g., ceftriaxone, ceftazidime) and fourth-generation (e.g., cefepime) cephalosporins and aztreonam, and are also associated with high rates of resistance to aminoglycosides and fluoroquinolones.31–34 The increase in ESBL-mediated resistance is reflected in rates of E. coli and K. pneumoniae resistance to third-generation cephalosporins, as shown in Table 119-1. Antimicrobial resistance among Pseudomonas aeruginosa is also alarming in that nearly all major drug classes are currently being affected; nearly 10% of P. aeruginosa isolates are now resistant to multiple drug classes, including cephalosporins, carbapenems, aminoglycosides, and/or fluoroquinolones.* Multidrug resistance is also very common (approximately 30% of isolates) among strains of Acinetobacter baumanii.4,35 Fluoroquinolone resistance is being increasingly reported among organisms such as E. coli that were previously considered to be extremely susceptible to this class of drugs.1–436 Antimicrobial resistance among gram-negative organisms such as P. aeruginosa has been of great concern in the ICU setting for many years, but increasing resistance among previously susceptible organisms and the involvement of multiple drug classes clearly indicates that the problem continues to grow worse. An additional troubling development in recent years is the detection of K. pneumoniae carbapenemase (KPC) enzymes which, as the name implies, confer resistance to a broad range of β-lactam-type antibiotics including the carbapenems.37 The increase in ESBL-producing strains and other multidrug-resistant pathogens has led to a heavy reliance on the carbapenems for treatment of gram-negative infections. Although KPC-producing strains are still relatively uncommon, their rapid spread through many geographic areas has led to serious concerns regarding the loss of carbapenems as reliable agents for empirical or “definitive” (i.e., based on culture and susceptibility information) treatment of many infections in ICU patients.37
Candida albicans is now the fourth most common pathogen associated with nosocomial infections in critically ill patients in the United States. While C. albicans is associated with approximately 7% of all nosocomial infections, it is the second most common cause of nosocomial urinary tract infections (15% of infections), the third most common cause of central line–associated bloodstream infections (6% of infections), and fourth most common cause of all nosocomial bloodstream infections.1,2 Resistance to antifungal agents among Candida species is now a significant problem in many hospitals, with fluconazole resistance being reported in up to 10% of C. albicans isolates from bloodstream infections.38–40 Because susceptibility testing for Candida species is not routinely performed in most hospitals, the true scope of resistance among C. albicans and other strains is not well characterized and may in fact be higher than currently assumed. It is well documented, however, that the relative frequency of fungal infections with Candida glabrata, Candida krusei, and other strains with decreased susceptibility to azole antifungals is increasing among certain populations, such as the critically ill and patients with hematologic malignancies.38–40 The increased proportion of non-albicans strains of Candida is particularly problematic because it has often led to the use of non-azole type agents such as the echinocandins for empirical therapy of patients at high risk for Candida infections.38–40
Infections caused by antimicrobial-resistant bacteria have been demonstrated to be associated with higher mortality rates, longer length of ICU and hospital stays, and higher medical costs.41–44 Antimicrobial-resistant strains of bacteria have been demonstrated to express virulence factors that may be different from those expressed by antimicrobial-susceptible strains; this may explain some of the increased mortality associated with these infections.6,7,37,44 However, increased mortality associated with infections caused by resistant bacteria may also be explained by the increased likelihood that patients will receive inadequate antimicrobial treatment. Inadequate antimicrobial therapy, defined as the use of drugs with poor in vitro activity against the infecting pathogen and/or improper dosing of drugs, has been demonstrated in numerous studies to be significantly associated with increased mortality and other measures of poor patient outcomes.45–54 Treatment with inadequate antimicrobial therapy is particularly problematic during the initial empirical treatment of infections when specific pathogens and antibiotic susceptibility information are not yet known.45,47,51,53,54 It is logical to assume that selection of adequate empirical therapy becomes more difficult as the organisms become more resistant to antimicrobial therapy, and it has in fact been demonstrated in clinical studies that most inadequate treatment of nosocomial infections in the ICU is related to the presence of pathogens that are resistant to the selected antibiotics.46,48,51–53 Furthermore, it has been shown in patients with nosocomial pneumonia that changing to more appropriate antibiotics when culture and susceptibility results became available (typically 48–72 hours after initiating therapy) did not significantly lower mortality rates compared with patients who received inadequate antibiotics for the entire duration of therapy.45 The importance of antimicrobial resistance in terms of antimicrobial selection and patient outcomes is thus difficult to overstate.
 Strategies to Reduce Antimicrobial Resistance
Strategies to Reduce Antimicrobial Resistance
Various strategies have been recommended to decrease problems of resistance through improved use of antimicrobials. These strategies include the use of antimicrobial protocols and guidelines, hospital formulary-based antimicrobial restrictions, scheduled antimicrobial rotation or “cycling,” improved techniques for detection and/or diagnosis of infections, improved dosing of antimicrobials based on pharmacokinetic and pharmacodynamic concepts, use of combination antimicrobial therapy, decreased duration of antimicrobial therapy, and early involvement of infectious diseases specialists in the management of infected patients.55 All of these various strategies fall within the realm of “antimicrobial stewardship,” a process for collectively improving the overall use of antimicrobials through many different means.56
Among these various strategies, the roles of antimicrobial restrictions and antimicrobial cycling are two particularly controversial issues. Hospital formulary–driven restriction of specific drugs or drug classes is a common method of controlling antimicrobial use within an institution. Formulary-based restrictions have historically been used to control drug costs; they may also reduce rates of adverse effects of high-risk agents.56 Antimicrobial restrictions are also used in an attempt to either decrease overall emergence of antimicrobial resistance within an institution or to control acute outbreaks of resistance affecting specific drugs and pathogens.56–59 The effectiveness of antimicrobial restrictions in reducing overall levels of resistance has not been consistently demonstrated. Indeed, it can be argued that antimicrobial restrictions cause intense selective pressure from a small number of agents and may actually promote the emergence of resistance rather than preventing it.60 Antibiotic restrictions that are instituted in response to specific outbreaks of antibiotic-resistant infections, together with appropriate infection control measures, have been shown to successfully manage specific resistance problems.56–59 However, it has also been shown that restriction of a drug in response to a resistance issue may in turn cause other resistance problems affecting other drugs.60 This phenomenon is sometimes referred to as “squeezing the balloon” because the enforcement of antimicrobial restrictions leads to new selective pressures that may effectively solve the original problem but cause the development of new resistance issues.61 A classic example involved restriction of ceftazidime and increased use of imipenem in response to an outbreak of ceftazidime-resistant K. pneumoniae. Although ceftazidime resistance among K. pneumoniae isolates was effectively decreased by 44%, the rates of imipenem-resistant P. aeruginosa significantly increased by 69%.60 Although antimicrobial restrictions may be effective in reducing drug costs and limiting specific outbreaks of resistant infections, the emphasis must clearly be on appropriate and rational drug use rather than relying on such restrictions to overcome resistance problems.
Antibiotic cycling, in which a specific drug or an entire antibiotic class is periodically withdrawn from clinical use and replaced with a different drug or class, has been investigated as a means of decreasing resistance by limiting narrow selective pressures and exposing organisms to a wide variety of different antimicrobials over time.62–65 Although initial studies were promising and demonstrated reduced antimicrobial resistance as well as decreased incidence of certain nosocomial infections and reduced patient mortality,62–65 these studies have not been consistent in the overall effectiveness of the antibiotic cycling strategy. In addition, a number of important questions concerning antibiotic cycling have not been adequately addressed by previous studies. These questions include which specific agents or classes are most appropriate to cycle, whether agents or classes of drugs should be cycled in a specific order, how often to change drugs within the scheduled cycle, and whether the potential effectiveness of antimicrobial cycling is maintained over long periods of time.62–65 Further research is clearly needed to answer these and other relevant questions, and cycling is currently not widely accepted as an effective means of improving infection-related patient outcomes and reducing resistance.
 Principles of Appropriate Antimicrobial Use
Principles of Appropriate Antimicrobial Use
Whereas many of the issues regarding antimicrobial use in critically ill patients are currently centered on issues related to antimicrobial resistance, adherence to basic principles of appropriate drug use is still crucial in overall optimization of drug therapy (Table 119-2).
TABLE 119-2 Basic Principles of Appropriate Antimicrobial Use in Critically Ill Patients
Diagnostic Issues
Establishing a definitive diagnosis of infection is paramount to the appropriate selection and use of antimicrobials. Once infection is suspected in the ICU patient, a comprehensive workup must be performed to identify the site of infection. The microbial causes of various ICU infections are reasonably predictable once the actual site of infection is known; appropriate drug selection thus properly begins with identification of a known or suspected site of infection. Unfortunately, the site of infection is often unable to be identified with any certainty; studies in septic patients have shown that no source of infection is identified in up to 30% to 40% of patients.53,66 Modern ICU practitioners have access to a wide range of invasive and noninvasive diagnostic techniques, and these should be employed when appropriate. However, the institution of antimicrobial therapy should not be unnecessarily delayed for the sake of performing exhaustive diagnostic tests. Although not yet in common use, polymerase chain reaction (PCR) and other molecular-based laboratory methods may offer the potential to improve detection of causative pathogens and facilitate the early initiation of appropriate antimicrobial therapy.67,68
Gram stain of appropriate specimens from potential sites of infection should also be utilized to help determine appropriate empirical or antimicrobial therapy. Although the yield of useful information from Gram stains is usually not high in critically ill patients, performing this test is nevertheless of value for those patients in whom causative pathogens are identified.45,67,68 Gram stains from specimens obtained from certain sites such as the respiratory tract and wounds should be interpreted with caution, owing to high rates of colonization with nonpathogenic organisms, particularly in patients who have already been hospitalized for several days. Studies have clearly demonstrated the high frequency and rapid time course of microbial colonization of ICU patients.69–71 Classic studies demonstrated that rates of colonization of the oropharynx and bronchi of critically ill patients with gram-negative organisms reached 45% and 65% within 5 days after ICU admission, respectively, and over 90% at both sites by day 10.72 These patients also become highly colonized with gram-positive cocci and particularly yeast soon after ICU admission.
Clinicians must keep in mind that there are numerous sources of fever in critically ill patients that are not associated with infection (Table 119-3). The occurrence of new fever in an ICU patient should prompt a thorough evaluation of noninfectious sources for the fever before initiation of antimicrobial therapy. Patients who have been started on antimicrobial therapy and have persistent fever despite the resolution of other signs and symptoms of infection should also be evaluated for noninfectious sources of fever.
TABLE 119-3 Noninfectious Sources of Fever in Critically III Patients
| Hemorrhage |
Selection of Empirical Drug Therapy
Initial selection of adequate drug therapy is of vital importance in optimizing outcomes of antimicrobial use in critically ill patients. Selection of inadequate therapy has been demonstrated in numerous clinical studies to be associated with increased patient mortality,45–54 and the risk of inadequate therapy is often directly related to rates of antimicrobial resistance in certain pathogens.46,48,51–53 A number of factors are therefore important to consider when choosing initial empirical therapy. These considerations should include suspected site(s) of infection and corresponding potential pathogens, rates of resistance of these pathogens to potentially used drugs, a patient’s prior exposure to antimicrobial therapy that may potentially increase the likelihood of antimicrobial resistance, and the results of any pertinent prior diagnostic tests. A reasonable understanding of the pharmacology, pharmacokinetics, pharmacodynamics, potential toxicities, potential drug interactions, and appropriate dosing of individual antimicrobials is also important in the selection of a specific agent once the type of drug to be used has been decided on. These drug-specific considerations are discussed in more detail later in this chapter. In general, empirical antimicrobial regimens for critically ill patients should be aggressive, that is, sufficiently broad spectrum in pharmacologic activity to cover the most likely (rather than all possible) pathogens, initiated promptly, and given in relatively high doses when the presence of any significant renal or hepatic dysfunction is considered.
Clinicians should be familiar with patterns and rates of resistance of key pathogens involved in both community-acquired and nosocomial infections. Resistance rates for pathogens occurring in community-acquired infections may be very different from those same types of pathogens causing nosocomial infections.1 For example, E. coli causing community-acquired urinary tract infections may have a rate of resistance to ciprofloxacin of 1% to 2%, whereas E. coli associated with nosocomial urinary tract infections may display resistance to ciprofloxacin in greater than 10% to 15% of strains.73,74 Likewise, S. aureus associated with community-acquired infections is usually susceptible to methicillin, whereas the rate of MRSA is now 60% to 70% in many hospitals in the United States.1,3 Information concerning rates of antimicrobial resistance in the outside community is often not as readily available as information concerning institutional susceptibilities, but ICU practitioners should nevertheless be familiar with resistance rates in both settings in order to choose appropriate antibiotics. Although antibiograms summarizing drug susceptibilities of key pathogens are available in most institutions, clinicians should recognize that published susceptibilities often do not differentiate between ICU and non-ICU isolates. It is well recognized that resistance rates are often much higher among isolates obtained from patients in ICUs where antimicrobial use is heaviest and more risk factors for resistance (e.g., higher severity of illness, invasive devices, immune suppression) are present.1,2,4,5,8–19 It is also known that susceptibilities often differ markedly among different types of ICUs (e.g., medical, surgical, burn, trauma) owing to patients with varying risk factors and potential differences in the types and amounts of antimicrobials used in each of these areas.2,3 When such information is available, ICU practitioners must be aware of any important differences between unit-specific drug susceptibilities and resistance rates for the institution as a whole. Appropriate use of such information can lead to more effective drug selection and enhance the provision of adequate drug therapy.75
Combination Therapy
Combinations of drugs are often recommended and used in both empirical and definitive antimicrobial regimens as a means of increasing the spectrum of pharmacologic activity, providing potentially additive or synergistic activity against selected organisms such as P. aeruginosa, improving clinical efficacy, and minimizing the potential for emergence of resistance during therapy.76–83 Combination regimens are also associated with the potential disadvantages of increased drug-related toxicities and increased drug costs. Although combination therapy is considered standard practice for certain specific infections, such as some types of endocarditis,84 the efficacy of combination therapy has not been well proven in respect to its presumed advantages. Whereas combinations of drugs may increase the overall spectrum of activity compared with the same drugs used alone, single agents such as carbapenems (imipenem/cilastatin and meropenem) and piperacillin/tazobactam provide very broad ranges of pharmacologic activity that includes gram-negative (including P. aeruginosa), gram-positive, and anaerobic bacteria. A number of older studies concerning the treatment of sepsis showed that monotherapy with ceftazidime, cefepime, or carbapenems is similar in efficacy to combination regimens (77%–93% and 76%–94% clinical response rates, respectively), with no differences in the development of resistance during therapy.79 However, a more recent study found that because of the current spectrum of pathogens and frequent antimicrobial resistance encountered in contemporary ICU practice, the use of combination therapy was associated with significantly higher rates of adequate antibiotic therapy and improved survival in gram-negative sepsis.80 Recent studies have also shown that the use of combination regimens for empirical treatment of ventilator-associated pneumonia may lead to improved patient outcomes in those patients proven to be infected specifically with multidrug-resistant pathogens such as P. aeruginosa and Acinetobacter.81,82 Although it is most appropriate to use antimicrobials with a narrow spectrum of activity whenever possible, monotherapy during empirical treatment may not be feasible in many institutions in which high rates of antimicrobial resistance are present among common pathogens such P. aeruginosa and S. aureus. As previously discussed, the selection of adequate empirical antimicrobial regimens is becoming more difficult as bacteria become more resistant; the routine use of monotherapy regimens is very difficult in many institutions from this standpoint. In institutions with high antibiotic resistance rates and/or high rates of infection with multidrug-resistant organisms, the best strategy seems to be use of combination regimens for empirical therapy until pathogen susceptibilities are known, followed by rapid narrowing or “de-escalation” of therapy to a suitable monotherapy regimen when possible.
Aside from considerations regarding empirical antimicrobial regimens, combination regimens are appropriately used in the treatment of mixed infections caused by aerobic and anaerobic bacteria, gram-negative and gram-positive bacteria, and/or bacteria and fungi. In these situations it is often more appropriate to select two or more agents with focused activity against known pathogens rather than treat with an excessively broad-spectrum single agent. Combination regimens are also often recommended in the treatment of systemic infections caused by certain gram-negative organisms such as P. aeruginosa, Acinetobacter species, Enterobacter species, and Serratia marcescens, as well as severe staphylococcal and enterococcal infections to achieve the potential benefits of antibiotic synergy, improved efficacy, and decreased resistance.79 Although some studies indicate that combination regimens for gram-negative pathogens such as P. aeruginosa are no more efficacious than monotherapy with newer agents such as cefepime and the carbapenems,79,81–83 use of combination regimens will likely remain controversial and based largely on clinical preference. The use of combination regimens is, however, often recommended for critically ill patients with neutropenia or other conditions that cause them to be severely immunocompromised.85,86
Drug Dosage and Administration
Pharmacokinetic Considerations
Pharmacokinetic properties that should be specifically considered in critically ill patients include distribution to various tissues and fluids, and routes of metabolism and excretion.87 The ability of a drug to penetrate to the site of infection in sufficient quantities to have activity against a pathogen is crucial for achieving clinical and microbiologic efficacy. Although the distributional characteristics of antimicrobials are often only specifically considered in the treatment of central nervous system or bone infections, good penetration to tissues and fluids present at the site of infection is a necessary consideration when selecting agents for any infection in ICU patients. Routes of drug metabolism and elimination are also important pharmacokinetic properties because of the prevalence of acute and chronic organ failures in most critically ill populations. Severe organ dysfunction, particularly of the liver or kidneys, should prompt clinicians to select agents that do not rely on that organ for metabolism or excretion from the body to avoid excessive drug accumulation and increased potential for unacceptable drug toxicities. Clinicians should also be mindful of the fact that some common antimicrobials (e.g., ceftriaxone, ciprofloxacin) are dependent on both the liver and kidneys for metabolism and excretion, and their use may be particularly problematic in patients with dysfunction of both of these organ systems. Practitioners must be familiar with the pharmacokinetic properties of commonly used antimicrobials to use them in the most efficacious and safe manner.87
Pharmacodynamic Considerations
Pharmacodynamics is the discipline that attempts to define and apply the relationships between concentrations of a drug and its pharmacologic effects (both desirable and undesirable).88–90 Although both the pharmacologic activity of an agent and its pharmacokinetic disposition are important considerations in drug selection and dosing, it is the combination of these two properties that is critical to achieving optimal outcomes during treatment of infections. The pharmacologic activities of antibacterial drugs are commonly defined by their minimal inhibitory concentration (MIC) as determined by in vitro testing. The MIC is the minimal concentration required to inhibit the growth of a target organism; highly active agents are associated with low MICs—that is, only low concentrations are required to inhibit bacterial growth, whereas agents with poor activity are associated with high MICs for the organism in question. It is logical that even extremely active agents with very low MICs will not be efficacious against a pathogen if the drug does not reach the site of infection in sufficient quantity; likewise, agents with relatively poor activity and higher MICs may be just as clinically efficacious if they are able to achieve high drug concentrations at the site of infection. Pharmacodynamic considerations combine MIC-defined pharmacologic activity and pharmacokinetic properties of a drug to make predictions regarding the drug’s probable efficacy in the treatment of a given type of infection. Models of infection have allowed antibacterial drugs to be broadly classified into two major categories: concentration-dependent agents and time-dependent (concentration-independent) agents.88–90
Concentration-dependent agents, particularly aminoglycosides and fluoroquinolones, exert bactericidal activities when drug concentrations are well above the MIC of the organism; the higher the ratio of drug concentration at the site of infection to the MIC, the more rapid and/or complete the bacterial killing becomes. Previous studies have established that important pharmacodynamic predictors of clinical efficacy of concentration-dependent agents include the ratio of maximum serum concentration divided by the MIC (Cmax/MIC) and the ratio of the 24-hour area under the serum concentration-versus-time curve divided by the MIC (AUC0-24/MIC).88–94 Although the ratios required to achieve maximal effects are not exactly known, in vitro and in vivo studies indicate that Cmax/MIC ratios of at least 10 to 12 and, for the fluoroquinolones, AUC0-24/MIC ratios of 30 to 50 for gram-positive and 125 to 250 for gram-negative organisms are required for optimal clinical and microbiologic outcomes as well as for the prevention of antimicrobial resistance.88–94 Both Cmax/MIC and AUC0-24/MIC ratios appear to be important determinants of clinical and microbiologic outcomes, although it is less clear which of these parameters is most predictive of drug efficacy because they are closely linked by the pharmacokinetic properties of the drugs.
Time-dependent killing agents only exert antimicrobial effects when their concentrations at the site of infection are higher than the MIC of the pathogen; the so-called time above MIC (T>MIC) thus becomes the pharmacodynamic parameter of interest for these drugs.88–90 Important time-dependent agents common in ICU practice include the penicillins, cephalosporins, carbapenems, clindamycin, and the macrolides. Studies indicate that T>MIC should be at least 40% to 50% of the dosing interval, although it has also been suggested that achieving T>MIC for 100% of the dosing interval may be desirable for optimal outcome.88–90 These studies have also suggested that both the AUC0-24/MIC as well as the T>MIC are important predictors of clinical efficacy and the risk of the development of microbial resistance.88–90
Because patients in the ICU are frequently infected with serious nosocomial pathogens that display decreased susceptibilities to antimicrobials and are prone to developing resistance with inadequate therapy, failure to properly dose antimicrobial agents predisposes patients to clinical and microbiologic failure. The appropriate consideration of pharmacodynamic principles in the treatment of infection in critically ill patients enables clinicians to select dosing regimens that will maximize the potential effectiveness of the specific agent. Thus, aminoglycosides and fluoroquinolones (concentration-dependent drugs) should be used in relatively high doses that facilitate their distribution into infected tissues and achieve concentrations many-fold higher than the MIC of pathogens. Direct application of these pharmacodynamic principles has resulted in the common use of extended-interval dosing (also referred to as once-daily or single-daily dosing) of aminoglycosides, in which these drugs are administered in single doses of 6 to 9 mg/kg rather than smaller divided doses,91,92,95–97 as well as the use of increased daily doses of fluoroquinolones (ciprofloxacin and levofloxacin) for severe infections such as nosocomial pneumonia and complicated skin and skin structure infections.98 Likewise, β-lactam antibiotics such as the penicillins and cephalosporins are best given as several smaller divided doses administered intermittently throughout the day, or even as a continuous infusion of drug to maintain high concentrations of drug over long periods of time. Thus, β-lactams are usually administered every 4 to 12 hours depending on achievable serum concentrations and the serum half-life of the specific agent.
The severity of infections encountered in the ICU population and the need for adequate Cmax/MIC and AUC0-24/MIC ratios are important considerations in severely ill patients. However, there is still much to learn regarding the direct application of pharmacodynamic principles to the routine care of critically ill patients. Although it is assumed that serum concentrations of most drugs are related to their concentrations in various tissues, the use of serum Cmax/MIC and AUC0-24/MIC ratios does not always accurately predict tissue concentrations of drugs. A particularly important limitation of pharmacodynamic principles in the routine care of ICU patients is that they have not been thoroughly clinically validated in critically ill populations. Numerous studies have demonstrated that the pharmacokinetics of antimicrobials are often significantly altered in critical illness and that there is a high degree of interpatient (and even intrapatient) variability in this population.91,99–104 Distribution of antimicrobials to infected tissues may also be affected by hemodynamic instability and regional or local changes in perfusion of various organs and tissues. The difficult combination of severe illness, pharmacokinetic variability, and life-threatening infections involving potentially drug-resistant pathogens makes the ICU population a difficult one in which to optimize drug therapy through appropriate application of pharmacodynamic principles. However, it is also only through the use of these principles that optimization of antimicrobial therapy is likely to be achieved in any consistent manner.
Route of Administration
For initial therapy for serious infections, antimicrobials should generally be administered by the intravenous route to avoid any problems of drug absorption related to gut malperfusion and to ensure rapid, adequate serum and tissue concentrations. However, although drugs are usually given intravenously at the initiation of therapy, drugs with good oral bioavailability may be effectively switched to oral formulations once patients are stable and responding to therapy. A number of drugs including levofloxacin, linezolid, and fluconazole have oral bioavailabilities approaching 100%; such agents may be administered orally without any apparent loss of therapeutic efficacy and with substantial cost savings.99,105 Oral antibiotics are an option for many hospitalized patients, including those in the ICU, and should be considered when patients have responded favorably to initial parenteral regimens and are able to take oral medications.99,105
Adverse Effects and Toxicities
Critically ill patients have higher rates of adverse effects from drugs compared to the general population of non-ICU patients. This is attributable to several factors, including the frequent presence of renal and/or hepatic dysfunction that may lead to excessive accumulation and excessively high concentrations of drugs, administration of many concurrent medications that may have overlapping adverse effect profiles or additive toxicities, and underlying illness that makes the patients more predisposed to adverse effects such as central nervous system or renal toxicities. Clinicians must carefully evaluate patients for any predisposing conditions potentially associated with increased risk of drug toxicities, and either use high-risk antimicrobials with caution or avoid them altogether. A common example of this concept is the use of aminoglycosides. The overall incidence of aminoglycoside-induced nephrotoxicity is approximately 10% or less, compared with rates of 16% to 36% in the critically ill.91,92,95,106 Although they may be effectively used in ICU patients, aminoglycosides must be carefully dosed and monitored to decrease the risk of toxicities. Alternatively, many clinicians would choose an agent such as a fluoroquinolone that may be used as part of combination regimens as alternatives to aminoglycosides and do not have the risk of nephrotoxicity. Clinicians must be familiar with the safety profiles of the various antimicrobials they commonly use and apply appropriate benefit-versus-risk considerations when selecting agents for a specific patient. The use of multidisciplinary teams in the ICU has also been associated with a substantially decreased incidence of adverse effects in critically ill patients.107–110
Drug Interactions
Patients in ICUs are often managed with large numbers of drugs. With polypharmacy being the rule rather than the exception, clinicians must be alert to the potential for adverse drug interactions.111 Drug interactions involving delayed or decreased absorption of orally administered agents and metabolic interactions involving inhibition of hepatic enzyme systems (e.g., azole antifungals, macrolides) are among the most common types of interactions likely to be seen in this population and should be avoided whenever possible. Drug–disease state interactions involving antimicrobials and increased risk of adverse effects should also be considered and prospectively monitored.111
Duration of Antimicrobial Therapy
The appropriate duration of antimicrobial therapy for most infectious processes has been poorly studied. Beyond community-acquired urinary tract infections, endocarditis, and a handful of other infections, the appropriate duration of treatment for most infections remains incompletely defined. This is particularly true in critically ill patients wherein relatively few studies have specifically examined the appropriate duration of antibiotic therapy for various infections. The general tendency has been to treat severe infections for long periods of time on the assumption that long courses of antimicrobials are required to provide good clinical efficacy, reduce the probability of treatment failure or relapse, and prevent the emergence of resistance due to the incomplete eradication of pathogens. However, long durations of therapy may themselves contribute to the development of resistance by subjecting endogenous or colonizing bacterial flora to unnecessary antimicrobial exposure. Long durations of treatment may also increase the risk of drug-related toxicities and add unnecessary treatment costs. Available studies, although few, have shown that shorter courses of antimicrobial therapy (e.g., 8 days versus 15 days for ventilator-associated pneumonia) are equal or superior in efficacy to longer courses and may be associated with a decreased incidence of superinfections and decreased antimicrobial use, drug costs, adverse effects, and antimicrobial resistance.112–114 Despite the potential advantages of shorter treatment durations, the decision to discontinue antimicrobial use in seriously ill patients is often very difficult to make on clinical grounds. Clinical response to antimicrobial therapy may be masked by underlying illnesses or concurrent drugs, and critically ill patients may not always manifest an association between successful treatment of an infection and rapid improvement in clinical signs and symptoms.115 Until additional research is able to better define optimal treatment durations for specific types of infections in ICU populations, the decision to discontinue therapy will largely rest on the clinical judgment of the ICU practitioner. Nevertheless, clinicians must remain cognizant of the desirability of limiting antimicrobial treatment durations and seek to de-escalate (i.e., reduce the number of drugs used in treatment or discontinue antibiotics altogether) whenever appropriate.
Monitoring Response to Antimicrobials
Clinicians should be mindful that failure of patients to promptly respond to antimicrobial therapy does not necessarily imply that the patient is receiving inadequate therapy. Critically ill patients are often slow to respond to therapy because of severity of the infection, concomitant disease states, immunosuppression, advanced age, and a number of other patient-specific factors.115 Thus, patients who are not clearly showing signs of clinical improvement within 24 to 48 hours after initiating antibiotics may merely require additional time to respond and do not necessarily require the modification of antimicrobial regimens. In addition, many noninfectious sources of fever are present in ICU patients and may confound assessment of a patient’s response to therapy. The finding of a persistent fever while other clinical signs and symptoms are improving should thus prompt clinicians to carefully assess patients for other noninfectious sources of fever or failure to respond. Finally, it must be recognized that not every patient treated in the ICU will recover from their infection, and failure to respond does not mean that antimicrobial therapy is inadequate. Whether failure to respond to therapy is in fact related to inadequate drug therapy can only be discerned through careful patient monitoring and assessment. However, even with the most conscientious ongoing assessment, this is often a very difficult distinction to make. Appropriate management of patients who are initially unresponsive to antimicrobial therapy is one of the most challenging dilemmas in the treatment of infections in the ICU.
 Protocols and Guidelines for Use of Antimicrobials
Protocols and Guidelines for Use of Antimicrobials
The use of prescribing guidelines and protocols, often electronically based or embedded into computerized clinical care systems (e.g., computerized physician order entry, or CPOE), has been shown to effectively improve overall antimicrobial appropriateness,116–118 decrease the incidence of adverse drug effects,119 avoid unnecessary antimicrobial use,118,120,121 reduce or stabilize bacterial resistance rates,61,121 reduce drug costs,117,120,121 and improve mortality and other outcomes.122–124 The use of guidelines for the treatment of ventilator-associated pneumonia in ICU patients has also been associated with increased initial administration of adequate antimicrobial therapy and decreased durations of antibiotic therapy.116,117 Although using clinical guidelines and protocols has been demonstrated to produce a number of favorable results, the implementation of such tools is often difficult because they are perceived as being too restrictive on clinical decision making by individual practitioners. Properly prepared guidelines are multidisciplinary in their preparation and implementation, involve key physicians in their development to make them practical and promote support from other practitioners, and are tailored to the individual institution. Although numerous established guidelines are available in the literature and elsewhere, they must be adapted to each institution and based on specific needs and practice patterns. Guidelines and protocols must also involve intensive education of all affected parties, physicians and non-physicians alike; this education must precede implementation and must also be ongoing to optimize guideline use. Finally, practitioners involved in use of the guidelines must be regularly updated regarding benefits already achieved and areas for continued improvement. Guidelines and protocols that are based on these principles are more likely to be successful and achieve the potential benefits associated with their use.
Key Points
Chastre J, Wolff M, Fagon J-Y, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290:2588-2598.
Kollef MH, Sherman G, Ward S, et al. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115:462-474.
Luna CM, Vujacich P, Niederman MS, et al. Impact of BAL data on the therapy and outcome of ventilator-associated pneumonia. Chest. 1997;111:676-685.
Heyland DK, Dodek P, Muscedere J, et al. Randomized trial of combination versus monotherapy for the empiric treatment of suspected ventilator-associated pneumonia. Crit Care Med. 2008;36:737-744.
U.S. Department of Public Health and Human Services, Public Health Service. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992-June 2003, issued August 2003. Am J Infect Control. 2003;31:481-498.
Craig WA. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1-12.
Pea F, Viale P, Furlanut M. Antimicrobial therapy in critically ill patients: a review of pathophysiological conditions responsible for altered disposition and pharmacokinetic variability. Clin Pharmacokinet. 2005;44:1009-1034.
1 Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect Control Hosp Epidemiol. 2008;29:996-1011.
2 Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1-12.
3 Kallan AJ, Hidron AI, Patel J, et al. Multidrug resistance among gram-negative pathogens that caused healthcare-associated infections reported to the National Healthcare Safety Network, 2006-2008. Infect Control Hosp Epidemiol. 2010;31:528-531.
4 Lockhart SR, Abramson MA, Beekmann SE, et al. Antimicrobial resistance among gram-negative bacilli causing infections in intensive care unit patients in the United States between 1993 and 2004. J Clin Microbiol. 2007;45:3352-3359.
5 Kollef MH, Fraser VJ. Antibiotic resistance in the intensive care unit. Ann Intern Med. 2001;134:298-314.
6 Paterson DL. Impact of antibiotic resistance in gram-negative bacilli on empirical and definitive antibiotic therapy. Clin Infect Dis. 2008;47(suppl 1):S14-S20.
7 Hawkey PM, Jones AM. The changing epidemiology of resistance. J Antimicrob Chemother. 2009;64(Suppl 1):i3-10.
8 Aly NYA, Al-Mousa HH, Al Asar ESM. Nosocomial infections in a medical-surgical intensive care unit. Med Princ Pract. 2008;17:373-377.
9 Harris AD, Smith D, Johnson JA, et al. Risk factors for imipenem-resistant Pseudomonas aeruginosa among hospitalized patients. Clin Infect Dis. 2002;34:340-345.
10 Lautenbach E, LaRosa LA, Marr AM, et al. Changes in the prevalence of vancomycin-resistant enterococci in response to formulary interventions: impact of progressive restrictions on use of vancomycin and third-generation cephalosporins. Clin Infect Dis. 2003;36:440-446.
11 Paramythiotou E, Lucet J-C, Timsit J-F, et al. Acquisition of multidrug-resistant Pseudomonas aeruginosa in patients in intensive care units: role of antibiotics with antipseudomonal activity. Clin Infect Dis. 2004;38:670-677.
12 Lee S-O, Kim NJ, Choi S-H, et al. Risk factors for acquisition of imipenem-resistant Acinetobacter baumannii: a case-control study. Antimicrob Agents Chemother. 2004;48:224-228.
13 Marshall C, Wolfe R, Kossman T, et al. Risk factors for acquisition of methicillin-resistant Staphylococcus aureus (MRSA) by trauma patients in the intensive care unit. J Hosp Infect. 2004;57:245-252.
14 Dinubile MJ, Friedland I, Chan CY, et al. Bowel colonization with resistant gram-negative bacilli after antimicrobial therapy of intra-abdominal infections: observations from two randomized comparative clinical trials of ertapenem therapy. Eur J Clin Microbiol Infect Dis. 2005;24:443-449.
15 Georges B, Conil JM, Dubouix A, et al. Risk of emergence of Pseudomonas aeruginosa resistance to beta-lactam antibiotics in intensive care units. Crit Care Med. 2006;34:1636-1641.
16 Neuhauser MM, Weinstein RA, Rydman R, et al. Antibiotic resistance among gram-negative bacilli in US intensive care units: Implications for fluoroquinolone use. JAMA. 2003;289:885-888.
17 Ortega M, Marco F, Soriano A, et al. Analysis of 4758 Escherichia coli bacteraemia episodes: predictive factors for isolation of an antibiotic-resistant strain and their impact on outcome. J Antimicrob Chemother. 2009;63:568-574.
18 Hsu L-Y, Tan T-Y, Tam VH, et al. Surveillance and correlation of antibiotic prescription and resistance of gram-negative bacteria in Singaporean hospitals. Antimicrob Agents Chemother. 2010;54:1173-1178.
19 Aloush V, Navon-Venezia S, Seigman-Igra Y, et al. Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrob Agents Chemother. 2006;50:43-48.
20 Daum RS. Skin and soft tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2007;357:380-390.
21 National Nosocomial Infections Surveillance (NNIS). System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470-485.
22 Combes A, Luyt C-E, Fagon J-Y, et al. Impact of methicillin resistance on outcome of Staphylococcus aureus ventilator-associated pneumonia. Am J Resp Crit Care Med. 2004;170:786-792.
23 Wunderink RG, Rello J, Cammarata SK, et al. Linezolid vs vancomycin: Analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest. 2003;124:1789-1797.
24 Chambers HF. The changing epidemiology of Staphylococcus aureus?. Emerg Infect Dis. 2001;7:178-182.
25 Bukharie H, Abdelhadi M, Saeed I, et al. Emergence of methicillin-resistant Staphylococcus aureus as a community pathogen. Diagn Microbiol Infect Dis. 2001;40:1-4.
26 Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: A meta-analysis of prevalence and risk factors. Clin Infect Dis. 2003;36:131-139.
27 Karlowsky JA, Thornsberry C, Jones ME, et al. Factors associated with relative rates of antimicrobial resistance among Streptococcus pneumoniae in the United States: Results from the TRUST surveillance program (1998-2000). Clin Infect Dis. 2003;36:963-970.
28 Thornsberry C, Sahm DF, Kelly LJ, et al. Regional trends in antimicrobial resistance among clinical isolates of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the United States: Results from the TRUST surveillance program, 1999-2000. Clin Infect Dis. 2002;34(Suppl 1):S4-16.
29 Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27-S72.
30 Goldstein EJC, Garabedian-Ruffalo SM. Widespread use of fluoroquinolones versus emerging resistance in pneumococci. Clin Infect Dis. 2002;35:1505-1511.
31 Paterson DL, Ko W-C, Von Gottberg A, et al. Antibiotic therapy for Klebsiella pneumonia: implications of production of extended-spectrum β-lactamases. Clin Infect Dis. 2003;39:31-37.
32 Lewis JSII, Herrera M, Wickes B, et al. First report of the emergence of CTX-M-type extended spectrum β-lactamases (ESBLs) as the predominant ESBL isolated in a U.S. health care system. Antimicrob Agents Chemother. 2007;51:4015-4021.
33 Baudry PJ, Nichol K, DeCorby M, et al. Comparison of antimicrobial resistance profiles among extended-spectrum β-lactamase-producing and acquired AmpC β-lactamase-producing Escherichia coli from Canadian intensive care units. Antimicrob Agents Chemother. 2008;52:1846-1849.
34 Paterson DL, Mulazimoglu L, Casellas JM, et al. Epidemiology of ciprofloxacin resistance and its relationship to extended-spectrum beta-lactamase production in Klebsiella pneumoniae isolates causing bacteremia. Clin Infect Dis. 2000;30:473-478.
35 Karlowsky JA, Draghi DC, Jones ME, et al. Surveillance for antimicrobial susceptibility among clinical isolates of Pseudomonas aeruginosa and Acinetobacter baumannii from hospitalized patients in the United States, 1998 to 2001. Antimicrob Agents Chemother. 2003;47:1681-1688.
36 Zervos MJ, Hershberger E, Nicolau DP, et al. Relationship between fluoroquinolone use and changes in susceptibility to fluoroquinolones of selected pathogens in 10 United States teaching hospitals, 1991-2000. Clin Infect Dis. 2003;37:1643-1648.
37 Souli M, Galani I, Antoniadou A, et al. An outbreak of infection due to β-lactamase Klebsiella pneumoniae carbapenemase 2-producing K. pneumoniae in a Greek university hospital: molecular characterization, epidemiology, and outcomes. Clin Infect Dis. 2010;50:364-373.
38 Bassetti M, Righi E, Costa A, et al. Epidemiological trends in nosocomial candidemia in intensive care. BMC Infect Dis. 2006;6:21-26.
39 Perlroth J, Cho B, Spellberg B. Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med Mycology. 2007;45:321-346.
40 Vazquez JA. Invasive fungal infections in the intensive care unit. Semin Resp Crit Care Med. 2010;31:79-86.
41 Song X, Srinivasan A, Plaut D, et al. Effect of nosocomial vancomycin-resistant enterococcal bacteremia on mortality, length of stay, and costs. Infect Control Hosp Epidemiol. 2003;24:251-256.
42 Engemann JJ, Carmeli Y, Cosgrove SE, et al. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin Infect Dis. 2003;36:592-598.
43 Cosgrove SE, Kaye KS, Eliopoulous GM, et al. Health and economic outcomes of the emergence of third-generation cephalosporin resistance in Enterobacter species. Arch Intern Med. 2002;162:185-190.
44 Roberts RR, Hota B, Ahmed I, et al. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Antimicrob Agents Chemother. 2009;49:1175-1184.
45 Luna CM, Vujacich P, Niederman MS, et al. Impact of BAL data on the therapy and outcome of ventilator-associated pneumonia. Chest. 1997;111:676-685.
46 Kollef MH, Sherman G, Ward S, et al. Inadequate antimicrobial treatment of infections: A risk factor for hospital mortality among critically ill patients. Chest. 1999;115:462-474.
47 Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother. 2005;49:3640-3645.
48 Shorr AF, Micek ST, Kollef MH. Inappropriate therapy for methicillin-resistant Staphylococcus aureus: resource utilization and cost implication. Crit Care Med. 2008;36:2335-2340.
49 Ortega M, Marco F, Soriano A, et al. Candida spp. Bloodstream infection: influence of antifungal treatment on outcome. J Antimicrob Chemother. 2010;65:562-568.
50 Arnold HM, Micek ST, Shorr AF, et al. Hospital resource utilization and costs of inappropriate treatment of candidemia. Pharmacother. 2010;30:361-368.
51 Alvarez-Lerma F. ICU-Acquired Pneumonia Study Group. Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. Intensive Care Med. 1996;22:387-394.
52 Ibrahim EH, Ward S, Sherman G, Kollef MH. A comparative analysis of patients with early-onset vs late-onset nosocomial pneumonia in the ICU setting. Chest. 2000;117:1434-1442.
53 Garnacho-Montero J, Garcia-Garmendia JL, Barrero-Almodovar A, et al. Impact of adequate empiric antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Crit Care Med. 2003;31:2742-2751.
54 MacArthur RD, Miller M, Albertson T, et al. Adequacy of early empiric antibiotic treatment and survival in severe sepsis: Experience from the MONARCS trial. Clin Infect Dis. 2004;38:284-288.
55 Byl B, Clevenbergh P, Jacobs F, et al. Impact of infectious diseases specialists and microbiological data on the appropriateness of antimicrobial therapy for bacteremia. Clin Infect Dis. 1999;29:60-66.
56 Owens RCJr. Antimicrobial stewardship: application in the intensive care unit. Infect Dis Clin N Am. 2009;23:683-702.
57 Rice LB, Eckstein EC, DeVente J, Shlaes DM. Ceftazidime-resistant Klebsiella pneumoniae isolates recovered at the Cleveland Department of Veterans Affairs Medical Center. Clin Infect Dis. 1996;23:118-124.
58 Giamarellou H, Antoniadou A. The effect of monitoring of antibiotic use on decreasing antibiotic resistance in the hospital. Ciba Found Symp. 1997;207:76-86.
59 Climo MW, Israel DS, Wong ES, et al. Hospital-wide restriction of clindamycin: Effect on the incidence of Clostridium difficile–associated infection and cost. Ann Intern Med. 1998;128:989-995.
60 Rahal JJ, Urban C, Horn D, et al. Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella. JAMA. 1998;280:1233-1237.
61 Burke JP. Antibiotic resistance—squeezing the balloon? JAMA. 1998;280:1270-1271.
62 Gruson D, Hilbert G, Vargas F, et al. Rotation and restricted use of antibiotics in a medical intensive care unit: Impact on the incidence of ventilator-associated pneumonia caused by antibiotic-resistant gram-negative bacteria. Am J Respir Crit Care Med. 2000;162:837-843.
63 Raymond DP, Pellitier SJ, Crabtree TD, et al. Impact of a rotating empiric antibiotic schedule on infectious mortality in an intensive care unit. Crit Care Med. 2001;29:1101-1108.
64 Gruson D, Hilbert G, Vargas F, et al. Strategy of antibiotic rotation: Long-term effect on incidence and susceptibilities of Gram-negative bacilli responsible for ventilator-associated pneumonia. Crit Care Med. 2003;31:1908-1914.
65 Hughes MG, Evans HL, Chong TW, et al. Effect of an intensive care unit rotating empiric antibiotic schedule on the development of hospital-acquired infections on the non–intensive care unit ward. Crit Care Med. 2004;32:53-60.
66 Bochud P-Y, Glauser MP, Calandra T. Antibiotics in sepsis. Intensive Care Med. 2001;27(Suppl 1):S33-S48.
67 Bloos F, Hinder F, Becker K, et al. A multicenter trial to compare blood culture with polymerase chain reaction in severe human sepsis. Intensive Care Med. 2010;36:241-247.
68 Lehmann LE, Hunfeld K-P, Steinbrucker M, et al. Improved detection of blood stream pathogens by real-time PCR in severe sepsis. Intensive Care Med. 2010;36:49-56.
69 Durairaj L, Mohamad Z, Launspach JL, et al. Patterns and density of early tracheal colonization in intensive care unit patients. J Crit Care. 2009;24:114-121.
70 Oostdijk EAN, de Smet AMGA, Blok BEM, et al. Ecological effects of selective decontamination on resistant gram-negative bacterial colonization. Am J Resp Crit Care Med. 2010;181:452-457.
71 Nijssen S, Fluit A, van de Vijer D, et al. Effects of reducing beta-lactam antibiotic pressure on intestinal colonization of antibiotic-resistant gram-negative bacteria. Intensive Care Med. 2010;36:512-519.
72 Kerver AJ, Rommes JH, Mevissen-Verhage EA, et al. Colonization and infection in surgical intensive care patients—a prospective study. Intensive Care Med. 1987;13:347-351.
73 Gupta K, Sahm DF, Mayfield D, Stamm WE. Antimicrobial resistance among uropathogens that cause community-acquired urinary tract infections in women: A nationwide analysis. Clin Infect Dis. 2001;33:89-94.
74 Wagenlehner FM, Niemetz A, Dalhoff A, Naber KG. Spectrum and antibiotic resistance of uropathogens from hospitalized patients with urinary tract infections:1994-2000. Int J Antimicrob Agents. 2002;19:557-564.
75 Beardsley JR, Williamson JC, Johnson JW, et al. Using local microbiologic data to develop institution-specific guidelines for the treatment of hospital-acquired pneumonia. Chest. 2006;130:787-793.
76 Bouza E, Muñoz P. Monotherapy versus combination therapy for bacterial infections. Med Clin North Am. 2000;84:1358-1389.
77 Burgess DS, Nathisuwan S. Cefepime, piperacillin/tazobactam, gentamicin, ciprofloxacin, and levofloxacin alone and in combination against Pseudomonas aeruginosa. Diagn Microbiol Infect Dis. 2002;44:35-41.
78 Fish DN, Choi MK, Jung R. Synergic activity of cephalosporins plus fluoroquinolones against Pseudomonas aeruginosa with resistance to one or both drugs. J Antimicrob Chemother. 2002;50:1045-1049.
79 Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis. 2004;4:519-527.
80 Micek ST, Welch EC, Khan J, et al. Empiric combination antibiotic is associated with improved outcome against sepsis due top gram-negative bacteria: a retrospective analysis. Antimicrob Agents Chemother. 2010;54:1742-1748.
81 Garnacho-Montero J, Sa-Borges M, Sole-Violan J, et al. Optimal management therapy for Pseudomonas aeruginosa ventilator-associated pneumonia: an observational, multicenter study comparing monotherapy with combination antibiotic therapy. Crit Care Med. 2007;35:1888-1895.
82 Heyland DK, Dodek P, Muscedere J, et al. Randomized trial of combination versus monotherapy for the empiric treatment of suspected ventilator-associated pneumonia. Crit Care Med. 2008;36:737-744.
83 Chamot E, Boffi El Amari E, et al. Effectiveness of combination antimicrobial therapy for Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother. 2003;47:2756-2764. 86
84 Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications. Circulation. 2005;111:394-434.
85 Mouton JW. Combination therapy as a tool to prevent emergence of bacterial resistance. Infection. 1999;27(Suppl 2):S24-S28.
86 Bouza E, Muñoz P. Monotherapy versus combination therapy for bacterial infections. Med Clin N Am. 2000;84:1358-1389.
87 Roberts JA, Lipman J. Antibacterial dosing in intensive care. Pharmacokinetics, degree of disease and pharmacodynamics of sepsis. Clin Pharmacokinet. 2006;45:755-773.
88 Craig WA. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1-12.
89 DeRyke CA, Lee SY, Kuti JL, Nicolau DP. Optimising dosing strategies of antibacterials utilising pharmacodynamic principles: impact on the development of resistance. Drugs. 2006;66:1-14.
90 Ambrose PG, Bhavnani SM, Rubina CM, et al. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it’s not just for mice anymore. Clin Infect Dis. 2007;44:79-86.
91 Fish DN. Extended-interval dosing of aminoglycoside antibiotics in critically ill patients. J Pharm Pract. 2002;15:285-295.
92 Drusano GL, Ambrose PG, Bhavnani SM, et al. Back to the future: using aminoglycosides again and how to dose them optimally. Clin Infect Dis. 2007;45:753-760.
93 Wright DH, Brown GH, Peterson ML, Rotschafer JC. Application of fluoroquinolone pharmacodynamics. J Antimicrob Chemother. 2000;46:669-683.
94 Ambrose PG, Grasela DM, Grasela TH, et al. Pharmacodynamics of fluoroquinolones against Streptococcus pneumoniae in patients with community-acquired respiratory tract infections. Antimicrob Agents Chemother. 2001;45:2793-2797.
95 Rea RS, Capitano B. Optimizing use of aminoglycosides in the critically ill. Semin Resp Crit Care Med. 2007;28:596-603.
96 Buijk SE, Mouton JW, Gyssens IC, et al. Experience with a once-daily dosing program of aminoglycosides in critically ill patients. Intensive Care Med. 2002;28:936-942.
97 Mueller EW, Boucher BA. The use of extended-interval aminoglycoside dosing strategies for the treatment of moderate-to-severe infections encountered in the critically ill surgical patients. Surg Infect. 2009;10:563-570. 95
98 West M, Boulanger BR, Fogarty C, et al. Levofloxacin compared with imipenem/cilastatin followed by ciprofloxacin in adult patients with nosocomial pneumonia: A multicenter, prospective, randomized, open-label study. Clin Ther. 2003;25:485-506.
99 Pea F, Viale P, Furlanut M. Antimicrobial therapy in critically ill patients: a review of pathophysiological conditions responsible for altered disposition and pharmacokinetic variability. Clin Pharmacokinet. 2005;44:1009-1034.
100 Boucher BA, Wood GC, Swanson JM. Pharmacokinetic changes in critical illness. Crit Care Clin. 2006;22:255-271.
101 Rebuck JA, Fish DN, Abraham E. Pharmacokinetics of intravenous and oral levofloxacin in critically ill patients in a medical intensive care unit. Pharmacotherapy. 2002;22:1216-1225.
102 Georges B, Concil J-M, Seguin T, Ruiz S, Minville V, Cougot P, et al. Population pharmacokinetics of ceftazidime in intensive care unit patients: influence of glomerular filtration rate, mechanical ventilation, and reason for admission. Antimicrob Agents Chemother. 2009;53:4483-4489.
103 Belzberg H, Zhu J, Cornwell EEIII, Murray JA, Sava J, Salim A, et al. Imipenem levels are not predictable in the critically ill patient. J Trauma. 2004;56:111-117.
104 Conil J-M, Georges B, de Lussy A, Khachman D, Seguin T, Ruiz S, et al. Ciprofloxacin use in critically ill patients: pharmacokinetic and pharmacodynamic approaches. Int J Antimicrob Agents. 2008;32:505-510.
105 Ramirez JA, Vargas S, Ritter GW, et al. Early switch from intravenous to oral antibiotics and early hospital discharge: A prospective observational study of 200 consecutive patients with community-acquired pneumonia. Arch Intern Med. 1999;159:2449-2454.
106 Oliveira JFP, Silva CA, Barbieri CD, et al. Prevalence and risk factors for aminoglycoside nephrotoxicity in intensive care units. Antimicrob Agents Chemother. 2009;53:2887-2891.
107 Leape LL, Cullen DJ, Clapp MD, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;282:267-270.
108 Kopp BJ, Mrsan M, Erstad BL, et al. Cost implications of and potential adverse effects prevented by interventions of a critical care pharmacist. Am J Health Syst Pharm. 2007;64:2483-2487.
109 MacLaren R, Bond CA, Martin SJ, et al. Clinical and economic outcomes of involving pharmacists in the direct care of critically ill patients with infections. Crit Care Med. 2008;36:3184-3189.
110 Weant KA, Armistead JA, Ladha AM, et al. Cost effectiveness of a clinical pharmacist on a neurosurgical team. Neurosurg. 2009;65:946-951.
111 Pea F, Furlanut M. Pharmacokinetic aspects of treating infections in the intensive care unit: focus on drug interactions. Clin Pharmacokinet. 2001;40:833-868.
112 Singh N, Rogers P, Atwood C, et al. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit: A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162:505-511.
113 Chastre J, Wolff M, Fagon J-Y, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: A randomized trial. JAMA. 2003;290:2588-2598.
114 Dunbar LM, Wunderink RG, Habib MP, et al. High-dose, short-course levofloxacin for community-acquired pneumonia: A new treatment paradigm. Clin Infect Dis. 2003;37:752-760.
115 Dennesen PJW, van der Ven AJAM, Kessels A, et al. Resolution of infectious parameters after antimicrobial therapy in patients with ventilator-associated pneumonia. Am J Respir Crit Care Med. 2001;163:1371-1375.
116 Ibrahim EH, Ward S, Sherman G, et al. Experience with a clinical guideline for the treatment of ventilator-associated pneumonia. Crit Care Med. 2001;29:1109-1115.
117 Thuong M, Shortgen F, Zazempa V, et al. Appropriate use of restricted antimicrobial agents in hospitals: The importance of empirical therapy and assisted re-evaluation. J Antimicrob Chemother. 2000;46:501-508.
118 Samore MH, Bateman K, Alder SC, et al. Clinical decision support and appropriateness of antimicrobial prescribing: a randomized trial. JAMA. 2005;294:2305-2314.
119 Classen DC, Pestotnik SL, Evans RS, et al. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1997;277:301-306.
120 Ruttimann S, Keck B, Hartmeier C, et al. Long-term antibiotic cost savings from a comprehensive intervention program in a medical department of a university-affiliated teaching hospital. Clin Infect Dis. 2004;38:348-356.
121 Pestotnik SL, Classen DC, Evans RS, et al. Implementing antibiotic prescribing guidelines through computer-assisted decision support: clinical and financial outcomes. Arch Intern Med. 1996;124:884-890.
122 Nguyen HB, Corbett SW, Steele R, et al. Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Crit Care Med. 2007;35:1105-1112.
123 Thiel SW, Asghar MF, Micek ST, et al. Hospital-wide impact of a standardized order set for the management of severe sepsis. Crit Care Med. 2009;37:819-824.
124 Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med. 2010;36:222-231.

