Chapter 16 Antiinflammatory Drugs
Corticosteroids
Pharmacodynamics
Cellular, Tissue, and Systemic Effects
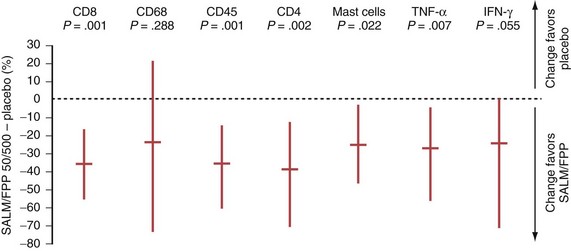
In COPD, however, even high-dose inhaled corticosteroids have little effect on airway inflammation, although they are effective when combined with a long-acting β2-agonist (LABA) (Figure 16-1).
Molecular Mechanisms
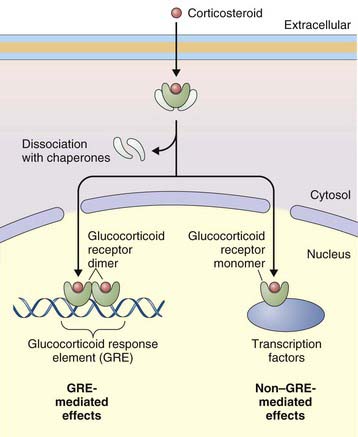
The primary effect of corticosteroids appears to be at the genetic level, activating transcription of antiinflammatory genes and repressing proinflammatory genes. They act primarily by binding to intracellular glucocorticoid receptors, which in turn regulate gene expression through glucocorticoid response elements (GREs) (Figure 16-2). Once inside the nucleus, glucocorticoid receptors dimerize and bind to GREs in the promoter regions of steroid-responsive genes, altering gene transcription and initiating a cascade of antiinflammatory effects downstream. Mediators affected by corticosteroids include cytokines, adhesion molecules, and chemokines. Nuclear glucocorticoid receptor monomers also interact with transcription factors such as nuclear factor (NF)-κB, to suppress expression of a number of proinflammatory genes. Corticosteroids can also decrease protein synthesis by decreasing messenger RNA (mRNA) stability.
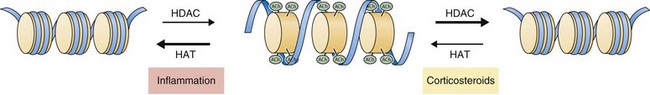
Recent work has focused on the role of corticosteroids in regulating gene expression through effects on histone acetylation and chromatin compaction. Inflammatory signals cause chromatin unwinding by histone acetyltransferase activity. Corticosteroids can directly inhibit histone acetyltransferases and recruit histone deacetylases (HDACs) to their site of action, seemingly independent of glucocorticoid receptor binding to GREs. Corticosteroids interact with specific HDACs (such as HDAC2) that target specific histone proteins (e.g., histone H4), regulating expression of particular regions of the genome. The net effect is to decrease histone acetylation, promoting chromatin compaction and downregulation of inflammatory gene expression (Figure 16-3).
Pharmacokinetics
Inhaled
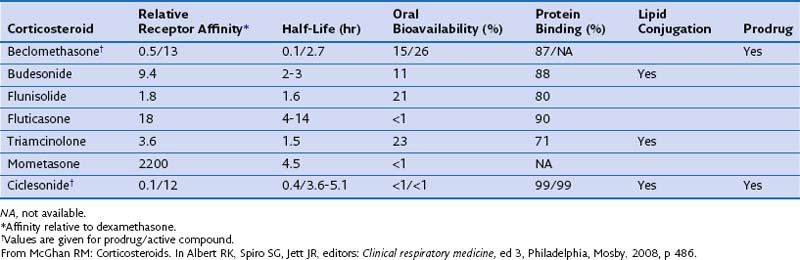
The pharmacokinetic characteristics of inhaled steroidal agents are more complex than those of systemic steroids. These drugs have undergone extensive development to improve activity in the lungs while decreasing systemic activity. An “ideal” inhalational agent of this class would have a small particle size (to allow access to small airways of the lung), low oral bioavailability, long residence in the lung (through slow absorption and/or lipid conjugation), delivery of a lung-activated prodrug, high receptor affinity, and high binding to proteins in circulating blood. Table 16-1 reviews many of these properties for seven available ICSs.
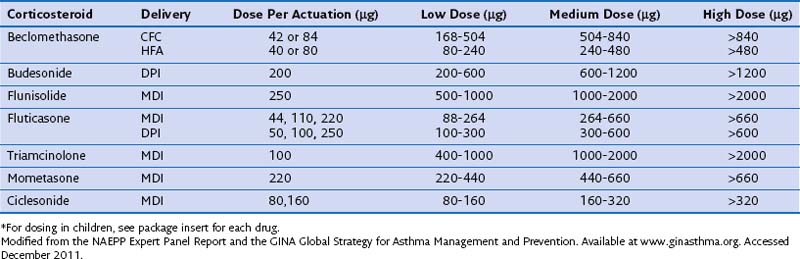
Direct comparison of the pharmacologic activity of various inhaled agents is difficult but nevertheless important, because management of airway diseases requires the ability to titrate the potency of delivered drug to achieve the desired clinical effect. The National Asthma Education and Prevention Program (NAEPP) Expert Panel Report, the Global Initiative for Asthma (GINA), and the Global Strategy for Asthma Management and Prevention have integrated a large amount of clinical and pharmacokinetic data to classify equivalent doses of a variety of different ICSs (Table 16-2). In general, a dose-response relationship without increased systemic effects is typical in the low to medium dose range.
Side Effects
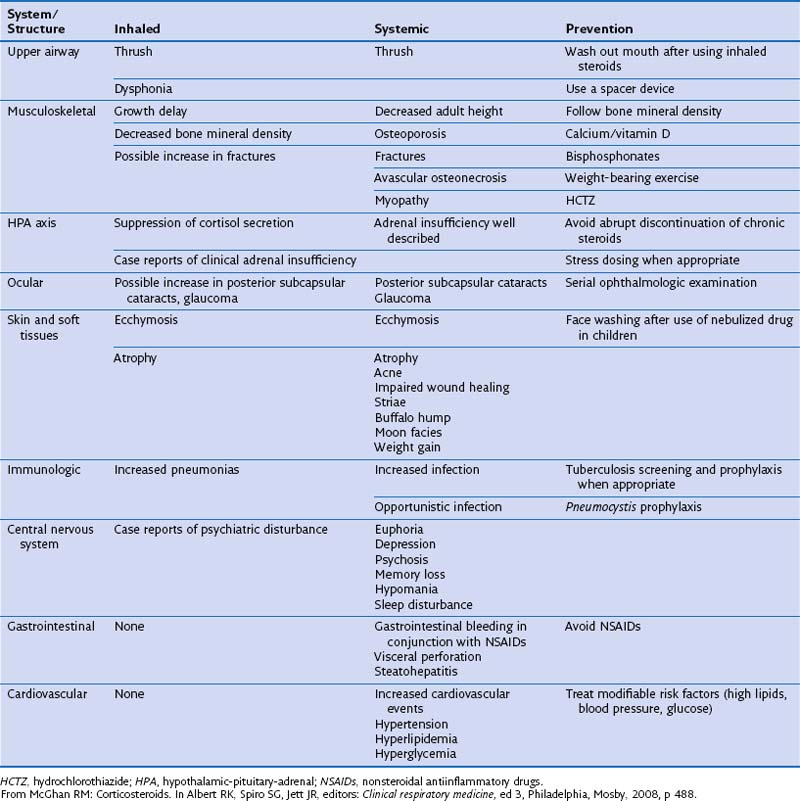
Generally, side effects are more common with systemic steroids than with inhaled agents (Table 16-3). Tolerability can be improved further by reducing dosage or treatment duration where possible, and with improvements in formulation as described above. Long-term use of systemic steroids can be associated with weight gain, increased susceptibility to infection secondary to immunosuppression, growth retardation in children, and osteoporosis. All forms of corticosteroids can hinder growth in children, although growth retardation associated with inhaled steroids is transient and of no long-term significance. The Childhood Asthma Management Program study monitored growth in 1041 children treated with budesonide, nedocromil, or placebo for 4 to 6 years and found a 1.1-cm lag in height gained in patients taking budesonide compared with placebo. This lag was experienced primarily during the first year of the study.
Phosphodiesterase 4 Inhibitors
PDE4 is one of 11 PDEs in the phosphodiesterase enzyme superfamily. It is expressed in many cell types, notably in inflammatory cells such as neutrophils, and in airway smooth muscle cells, where it regulates inflammation by means of the second messenger cyclic adenosine monophosphate (cAMP). PDE4 was identified as a potential target for antiinflammatory therapies many years ago, and several PDE4 inhibitors have been developed specifically as treatments for respiratory disease. Most of these agents have failed in clinical testing owing to a high incidence of gastrointestinal side effects, although compounds with greater substrate potency and specificity have increased efficacy at low doses (Table 16-4).
Table 16-4 Relative Potency and Suggested Dosage of Selected Phosphodiesterase 4 (PDE4) Inhibitors and Theophylline for Chronic Obstructive Pulmonary Disease
| PDE4 Inhibition: IC50 (nM)* | Dosage | |
|---|---|---|
| Roflumilast | 0.8 | 0.5 mg once daily |
| Cilomilast | 120 | 15 mg twice daily |
| Rolipram | 1100 | — |
| Theophylline | >10,000 | 100-600 mg daily |
* Potency expressed as half-maximal inhibition concentration for PDE4 activity.
Modified from Wang D, Cui X: Evaluation of PDE4 inhibition for COPD, Int J COPD 1:373–379, 2006.
Pharmacodynamics
Molecular Mechanisms
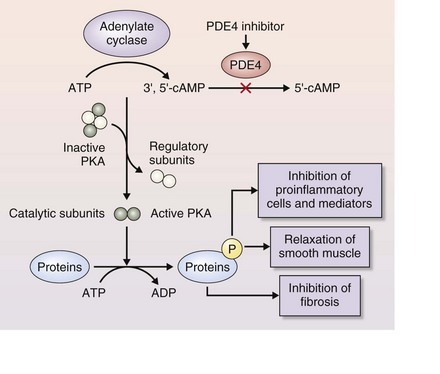
Roflumilast and other PDE4 inhibitors bind directly to PDE4 to block its activity, thereby reducing inflammation (Figure 16-4). PDE4 is expressed in many cells involved in the inflammatory response that underlies COPD.
Pharmacokinetics
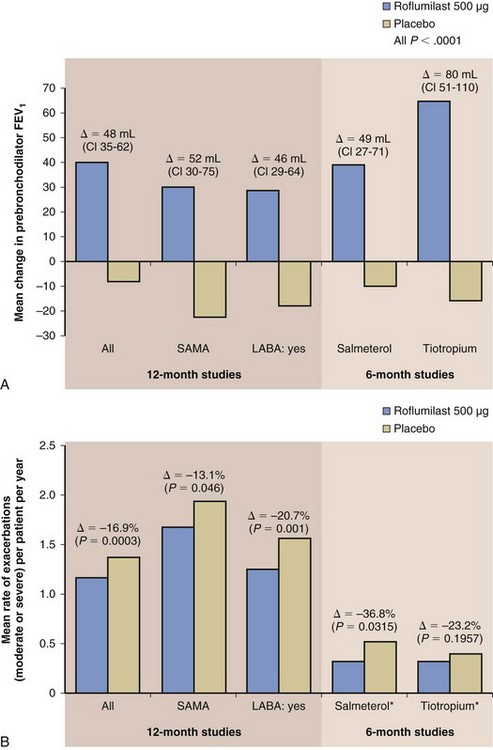
Phase III clinical testing has shown that roflumilast is most effective in a subpopulation of patients with COPD who are at increased risk for COPD exacerbations. The target subgroup includes patients with severe COPD (i.e., with a postbronchodilator forced expiratory volume in 1 second [FEV1] less than 50%), symptoms of chronic bronchitis (which is linked to an increased risk of exacerbations), and a history of more than one exacerbation in the past year. These patients are most likely to suffer from repeated exacerbations (the “frequent exacerbator” phenotype) and therefore benefit the most from the clinical effects of roflumilast—that is, in reducing exacerbation frequency. Clinical studies have shown that roflumilast significantly improves lung function and reduces exacerbations when added to most COPD maintenance therapy regimens—including a LABA, a long-acting muscarinic antagonist (LAMA), or inhaled steroid—in this patient subgroup as described (Figure 16-5).
Pipeline Products
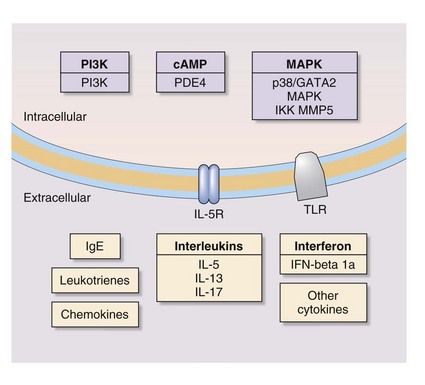
In addition, stem cell therapy is an emerging area of research on treatment of respiratory disease. Figure 16-6 depicts novel molecular targets for COPD and asthma drugs.
Acknowledgments
This chapter was based in part on Chapter 37 on corticosteroids, by Ryan McGhan, in the third edition of this book. Sarah Nelson provided editorial assistance with preparation of the chapter.
Allen DB, Bielory L, Derendorf H, et al. Inhaled corticosteroids: past lessons and future issues. J Allergy Clin Immunol. 2003;112:S1–40.
Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol. 2011;163:29–43.
Calverley PM, Rabe KF, Goehring UM, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomized clinical trials. Lancet. 2009;374:685–694.
Derendorf H, Nave R, Drollman A, et al. Relevance of pharmacokinetics and pharmacodynamics of inhaled corticosteroids to asthma. Eur Respir J. 2006;28:1042–1050.
Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomized clinical trials. Lancet. 2009;374:695–703.
Hatzelmann A, Morcillo EJ, Lungarella G, et al. The preclinical pharmacology of roflumilast—a selective oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2010;23:235–256.
Leung DY, Bloom JW. Update on glucocorticoid action and resistance. J Allergy Clin Immunol. 2003;111:3–22.
Rabe KF. Roflumilast for the treatment of chronic obstructive pulmonary disease. Expert Rev Respir Med. 2010;4:543–555.
Rodrigo GJ. Rapid effects of inhaled corticosteroids in acute asthma: an evidence-based evaluation. Chest. 2006;130:1301–1311.
Sin DD, Man SF. Corticosteroids and adrenoceptor agonists: the compl[e]ments for combination therapy in chronic airways diseases. Eur J Pharmacol. 2006;533:28–35.