172 Anticonvulsants
 Anticonvulsants: General ICU Concerns
Anticonvulsants: General ICU Concerns
Protein Binding
Drugs such as phenytoin, carbamazepine, and valproic acid are extensively protein bound, but only the unbound drug in the plasma is biologically active. Critically ill patients are often catabolic and have abnormally low circulating protein levels; thus, the concentration of unbound drug can be greater than anticipated despite a total serum (or plasma) drug level that is within the normal target range for the medication.1 Patients with hepatic and/or renal dysfunction are prone to discordance between total and unbound (free) serum levels. Routine monitoring of free drug levels is expensive but warranted in these patients. Unfortunately, most hospital laboratories routinely offer unbound serum levels for only one commonly used anticonvulsant, phenytoin.
Metabolic Derangements
Hyponatremia has been reported in patients who have been treated with carbamazepine, oxcarbazepine, and (rarely) other anticonvulsants. Anticonvulsant-induced hyponatremia has been attributed to the syndrome of inappropriate antidiuretic hormone (SIADH) (Table 172-1). Selected subgroups of patients are more at risk for anticonvulsant-induced hyponatremia, including elderly persons, menstruating women, patients who require administration of large fluid volumes, patients with renal failure, postoperative patients, and patients who are concurrently receiving other medications associated with hyponatremia.2
| Barbiturates | Haloperidol |
| Carbamazepine | Chlorpropamide |
| Oxcarbazepine | Thioridazine |
| Thiazides | Imipramine |
| Vincristine | MAO inhibitors |
| Cyclophosphamide | Bromocriptine |
| General anesthetics | Oxytocin |
| Nicotine | Acetamides |
| Clofibrate | Tolbutamide |
| Nonsteroidal antiinflammatory drugs |
Adapted from Asconape J. Some common issues in the use of antiepileptic drugs. Semin Neurol 2002;22:27.
Drug Fever
Development of a fever coincident with initiation of an anticonvulsant in the ICU setting complicates patient management and is a serious potential concern. Drug fever is a particularly common occurrence with the two agents, phenytoin and fosphenytoin, but can occur with other anticonvulsants as well.1 Peripheral eosinophilia supports the diagnosis. However, it is frequently the case that the diagnosis of drug-induced fever is firmly established only when hyperthermia resolves after an alternative anticonvulsant is substituted for the original agent.
Alteration In Neurologic Examination
The toxic side effects of phenytoin or carbamazepine can promote development of ataxia. Valproic acid can induce tremors. Carbamazepine toxicity can present in a biphasic fashion (i.e., acutely and subacutely) as a consequence of increasing levels of a toxic metabolite.1
Renal Disease
Clearance of anticonvulsants can be significantly reduced when the glomerular filtration rate (GFR) falls below 10 mL/min. The clearance of phenobarbital and carbamazepine are not greatly affected by low GFR, but the clearance of phenytoin and valproic acid can be affected by changes in renal function. The higher protein binding exhibited by these latter agents makes measurement of the free levels of these drugs a better guide for dosage adjustments.1 Hemodialysis does not affect circulating phenytoin levels to a large extent, but renal replacement therapy can markedly affect serum levels of phenobarbital.
Drug Interactions
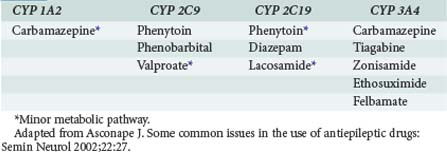
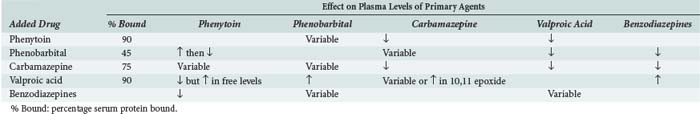
Many anticonvulsants can affect metabolism and or protein binding of other agents. Phenytoin, carbamazepine, and phenobarbital are all potent inducers of the hepatic P450 enzyme systems (Tables 172-2 and 172-3), and treatment with these anticonvulsants can affect the circulating concentrations of other medications (Tables 172-4 and 172-5) including concomitantly administered anticonvulsant drugs (see Table 172-5). Phenytoin can reduce the plasma concentrations of carbamazepine and valproic acid, whereas interaction with phenobarbital is variable. Phenytoin decreases the effectiveness of warfarin and theophylline. Valproic acid inhibits the metabolism of phenobarbital and carbamazepine (including its 10,11-epoxide metabolite), which can result in increased serum levels. Carbamazepine increases the hepatic metabolism of diazepam and valproic acid. Phenobarbital results in decreased circulating levels of warfarin, theophylline, and cimetidine.3 Cimetidine, amiodarone, isoniazid (INH), and chlorpromazine all decrease hepatic metabolism of many drugs including phenytoin (Table 172-6). Drugs that commonly decrease circulating phenytoin levels include digoxin, cyclosporine, corticosteroids, warfarin, and theophylline. Aluminum hydroxide, magnesium hydroxide, and calcium-containing antacids decrease the absorption of enterally administered phenytoin. Some of the newer anticonvulsants such as levetiracetam and lacosamide are excreted via the kidneys for the most part, and their circulating levels are unaffected by hepatic metabolism. In addition, drug-drug interactions are not a major concern with these newer agents, and they do not affect the levels of other anticonvulsants.
TABLE 172-3 Anticonvulsant Induction of Hepatic Metabolic Enzymes
| Inducers | Inhibitors | No or Minimal Effect |
|---|---|---|
| Carbamazepine | Valproate | Gabapentin |
| Phenytoin | Felbamate | Lamotrigine |
| Phenobarbital | Topiramate | |
| Primidone | Tiagabine | |
| Oxcarbazepine | ||
| Levetiracetam | ||
| Zonisamide |
Adapted from Asconape J. Some common issues in the use of antiepileptic drugs: Semin Neurol 2002;22:27.
TABLE 172-6 Common Drug Interactions of Anticonvulsants
| Phenytoin and Carbamazepine | ||
|---|---|---|
| Added Drug | Phenytoin | Carbamazepine |
| Salicylates | ↑ | |
| Erythromycin | ↑↑ | |
| Chloramphenicol | ↑ | |
| Trimethoprim | ↑ | |
| Isoniazid | ↑ | ↑ |
| Propoxyphene | ↑ | ↑ |
| Amiodarone | ↑ | |
| Diltiazem, verapamil | ↑ | |
| Cimetidine | ↑ | ↑ |
| Ethanol | ↓ | |
| Rifampin | ↓ | |
| Digitoxin | ↓ | |
| Cyclosporine | ↓ | |
| Warfarin | ↓ | |
| Theophylline | ↓ | |
| Glucocorticoids | ↓ | |
↓, decrease in plasma levels; ↑, increase in plasma levels.
Idiosyncratic Reactions
Hypersensitivity reactions are common with phenytoin and carbamazepine and can be manifested by fever, rash, and/or eosinophilia.1 Drugs associated with a high risk for the development of rash include phenytoin, phenobarbital, primidone, lamotrigine, carbamazepine, oxcarbazepine, and zonisamide4 (Table 172-7). Transient leukopenia and thrombocytopenia are commonly seen with carbamazepine and valproate. Other less common drug-related effects include hepatic failure, pancreatitis (valproic acid), agranulocytosis, aplastic anemia, megaloblastic anemia (phenytoin), Stevens-Johnson syndrome, and lupus-like syndromes. Although rare, severe hepatic dysfunction secondary to formation of a toxic metabolite can occur with valproic acid therapy. This potentially fatal reaction most often occurs in children younger than 2 years of age who are also receiving aspirin and other drugs for control of seizures.
| High Risk | Low Risk |
|---|---|
| Phenytoin | Valproate |
| Phenobarbital | Topiramate |
| Primidone | Gabapentin |
| Carbamazepine | Tiagabine |
| Oxcarbazepine | Levetiracetam |
| Lamotrigine | Lacosamide |
| Zonisamide |
Data from Asconape J. Some common issues in the use of antiepileptic drugs: Semin Neurol 2002;22:27.
Management Of Anticonvulsant Toxicity
Management of patients suffering from severe toxicity requires comprehensive supportive therapy including airway management, hemodynamic support, and oral administration of activated charcoal. Charcoal has been especially useful for managing cases of acute valproate acid intoxication.5 In cases of valproic acid or carbamazepine poisoning, concurrent hemoperfusion and hemodialysis to enhance elimination of the anticonvulsant can be useful when patients are hemodynamically unstable and the clinical condition is worsening despite aggressive supportive care.6
 Specific Anticonvulsant Properties by Class
Specific Anticonvulsant Properties by Class
Benzodiazepines
For immediate therapy, benzodiazepines are still considered first-line treatment for most seizures. These drugs are highly lipophilic, are potent γ-aminobutyric acid (GABA)-activated agonists, and serve to improve local inhibition of signal transmission. The most commonly used benzodiazepines in the ICU are diazepam, lorazepam, and midazolam. In the case of hepatic failure, oxazepam may be preferred because it is the only benzodiazepine not metabolized by the liver.7
Diazepam
Dosing
Pharmacokinetics
Midazolam
When a short-acting benzodiazepine is needed, most clinicians now employ midazolam instead of diazepam. Midazolam is highly lipophilic, and the onset of its effects occur very rapidly following IV administration.13 Midazolam is marketed as a water-soluble prodrug. Following IV administration, the drug is transformed into a lipophilic compound by virtue of rapid closure of the diazepine ring. Thus the drug is less irritating to veins than diazepam.
Dosing
Pharmacokinetics
Lorazepam
Lorazepam is the least lipid-soluble agent among the three commonly used benzodiazepines. As a consequence, the pharmacologic effects of lorazepam are delayed in onset and prolonged in duration.15 Lorazepam is ideally suited for acute therapy, together with longer prophylaxis against recurrence of seizures. In a 5-year randomized double-blind multicenter trial of four IV regimens for the treatment of generalized status epilepticus, Treiman et al. found that treatment with lorazepam (0.1 mg/kg) was successful in 64.9% of patients and significantly superior to phenytoin (P = 0.002) in a pairwise comparison.16 It is important to note that lorazepam’s longer duration of action can adversely impact the neurologic examination for several hours, potentially complicating medical management.
Dosing
Pharmacokinetics
Phenytoin
Phenytoin has been and remains the drug most commonly used in the ICU for prophylaxis against seizures. Several reasons for the continued popularity of phenytoin include its ease of administration, its availability in formulations suitable for either IV or enteral administration, its relative safety (severe toxic reactions are uncommon), and its efficacy against many seizure syndromes that occur in the ICU setting, including status epilepticus. Temkin et al. reported that prophylactic administration of phenytoin decreased the incidence of seizures during the first week following traumatic head injury by 73% compared to placebo.18 In light of its non-GABA-agonist action, phenytoin is not particularly effective against most drug-induced convulsions, especially those triggered by β-lactam antibiotics. Phenytoin is indicated for use against generalized tonic/clonic seizures and focal and complex-partial seizures. Phenytoin also is indicated for prevention of seizures following head trauma or elective neurosurgical procedures.
Dosing
Pharmacokinetics
As a known inducing agent for hepatic metabolism, phenytoin increases the clearance of corticosteroids and many anticonvulsants (barbiturates, carbamazepine, ethosuximide, felbamate, lamotrigine, tiagabine, topiramate, and zonisamide).3 Thus, anticonvulsant polypharmacy can be frustrated by the addition of phenytoin. However, phenytoin does not affect gabapentin or levetiracetam levels. As would be expected, circulating levels of phenytoin can be decreased by concomitant use of other “hepatic enzyme inducers” (e.g., barbiturates, carbamazepine, chronic ethanol, dexamethasone, rifampin). Because it can precipitate acute attacks, use of phenytoin should be avoided if possible in patients with hepatic forms of porphyria.
In contrast, inhibitors of the hepatic enzymes, CYP28/C9 (e.g., amiodarone, cimetidine, fluvoxamine, some nonsteroidal antiinflammatory drugs, metronidazole, ritonavir, sulfonamides, troglitazone, valproic acid) and CYP2C19 (e.g., felbamate, fluconazole, fluoxetine, fluvoxamine, omeprazole) can increase circulating phenytoin levels.4
Fosphenytoin
Fosphenytoin (Cerebyx) is a phosphate ester prodrug of phenytoin. It is highly water soluble. When administered parenterally (IV or IM), fosphenytoin is rapidly metabolized into phenytoin. It can be infused up to three times faster than phenytoin (i.e., maximal rate of infusion, 150 mg/min).22 The times to peak effect are similar for phenytoin and fosphenytoin, because enzymatic conversion of the prodrug occurs rapidly. Kugler et al. suggested that fosphenytoin and phenytoin are likely to control status epilepticus with similar rapidity.23 The benefits of fosphenytoin compared to phenytoin are faster safe rate of administration and lower likelihood for certain adverse effects (e.g., hypotension, phlebitis, and soft-tissue injury from extravasation). Although fosphenytoin is more expensive than phenytoin, the costs associated with treating complications from the use of IV phenytoin can be substantially greater; accordingly, fosphenytoin may be advantageous on a pharmaco-economic basis.20
Dosing
Although a different drug from phenytoin when initially administered, the dosage of fosphenytoin is always described in phenytoin equivalents (PE). Because fosphenytoin is water soluble, it can be administered safely IM, whereas phenytoin cannot.24
Pharmacokinetics
Absorption: the rise in serum concentration of fosphenytoin may be faster compared to phenytoin when administered IV, because of the higher maximal recommended infusion rate for the prodrug (150 mg/min versus 50 mg/min, respectively). However, owing to the necessary biotransformation (conversion to phenytoin after IV administration is approximately 15 minutes), the resulting time-to-peak serum levels of phenytoin are similar for the two agents.22 Bioavailability of each approaches 100%.
Carbamazepine
Dosing
Pharmacokinetics
Valproic Acid
Valproic acid is indicated as monotherapy and as adjunctive therapy in the treatment of almost all seizures types, including complex partial seizures, absence seizures, generalized tonic/clonic seizures, myoclonic seizures, and other partial seizures. In two European studies, IV valproate was shown to be effective for the treatment of refractory status epilepticus.29,30 Because of the recent availability of an IV formulation, valproic acid is now used relatively commonly in the ICU setting and as a treatment for acute seizures including status epilepticus.
Dosing
Pharmacokinetics
Acute valproic acid intoxication induces mild to moderate lethargy at lower doses and coma or fatal cerebral edema at higher, more toxic doses.35 In contrast to either phenytoin or carbamazepine, nystagmus, dysarthria, and ataxia are rarely noted following valproic acid overdose. Valproic acid can increase serum ammonia levels through interaction with carnitine. In the management of valproic acid intoxication, naloxone occasionally is effective for reversing symptoms.
Propofol
Dosing
Pharmacokinetics
A “propofol infusion syndrome” has been described, and common clinical features can include hyperkalemia, hepatomegaly, lipemia, metabolic acidosis, myocardial failure, and rhabdomyolysis.39 This syndrome was initially described in children who were cared for in an ICU for prolonged periods, using high doses of propofol for sedation.40–42 Propofol-induced lactic acidosis and myocardial dysfunction also can occur in adults.39 Administration of propofol in the ICU should be restricted to doses ≤ 5 mg/kg/h, and infusion of propofol for the purpose of sedating critically ill adults should be limited to 48 hours, especially if high (general anesthesia level) doses are being used.
Phenobarbital
Phenobarbital remains a mainstay of anticonvulsant therapy. As a potent GABA agonist, phenobarbital is an effective anticonvulsant against a broad range of seizure types, The drug is used most commonly to treat or prevent generalized motor seizures. A favorable feature is its relative lack of serious toxic effects. Additional desirable characteristics of the drug which recommend it for use in the ICU include its broad efficacy, its availability for IV administration, ability to titrate the dose of the drug to burst suppression on the EEG,43–45 and ease of transition to PO dosing if desired. Its chief negative attributes include its long half-life and its tendency to induce hepatic enzyme expression.
Dosing
Pharmacokinetics
Newer Anticonvulsants
Several newer anticonvulsants have been introduced into the market during the past 15 years. However, the lack of available IV preparations severely limits their use in treating seizures in the ICU. The agents typically used are initiated when enteral therapy is suitable. Some studies have demonstrated that the oral preparations of some of these agents can still be of some benefit. Topiramate tablets, for example, have been administered when crushed to a powder and mixed with water and administered via nasogastric tube and have been shown to be effective in refractory status epilepticus. Agents like gabapentin, lamotrigine, topiramate, and vigabatrin are often considered more suitable for adjunctive therapy than for monotherapy. Lamotrigine is the only agent approved for monotherapy, but gabapentin and oxcarbazepine also soon may have such an indication.8,49
Gabapentin and vigabatrin are excreted unchanged in the urine and are useful for treating patients with hepatic failure. In patients with renal failure, vigabatrin, gabapentin, and topiramate should be used cautiously and in reduced dosages. The pharmacokinetics of tiagabine are not affected by either renal or hepatic dysfunction. The possibility of drug interaction is important to know as well. Combination therapy with lamotrigine and carbamazepine can increase the risk of carbamazepine-induced toxic effects. Other anticonvulsant drugs have little effect on gabapentin; it also has no substantial influence on the pharmacokinetics and serum concentrations of other seizure medications.8,49
Levetiracetam (Keppra)
Dosing
Pharmacokinetics
Lacosamide (Vimpat)
Lacosamide (previously known as harkoseride) is indicated as adjunctive treatment for partial-onset seizures. It comes in both a PO and IV formulation and is hence an alternative agent for those patients unable to take oral preparations.50
Dosing
Pharmacokinetics
Gabapentin (Neurontin)
Dosing
Pharmacokinetics
Dreifuss FE. Toxic effects of drugs used in the ICU. Anticonvulsant agents. Crit Care Clin. 1991;7:521-532.
Cramer JA, Fisher R, Ben-Menachem E, et al. New antiepileptic drugs: comparison of key clinical trials. Epilepsia. 1999;40:590-600.
Treiman DM, Meyers PD, Walton NY, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med. 1998;339:792-798.
Mirski MA, Williams MA, Hanley DF. Prolonged pentobarbital and phenobarbitone coma for refractory generalized status epilepticus. Crit Care Med. 1995;23:400-404.
Varelas P, Mirski MA. Seizures in the ICU. J Neurosurg Anesthesiol. 2001;13:163-175.
Asconape J. Some common issues in the use of antiepileptic drugs. Semin Neurol. 2002;22:27-39.
1 Dreifuss FE. Toxic effects of drugs used in the ICU. Anticonvulsant agents. Crit Care Clin. 1991;7:521.
2 Wasserstein A. Antiepileptic drug-induced hyponatremia: a reference guide. Medical Education Resources, Inc.; July 2001.
3 Leppik IE, Wolff DL. Antiepileptic medication interactions. Neurol Clin. 1993;11:905.
4 Asconape J. Some common issues in the use of antiepileptic drugs. Semin Neurol. 2002;22:27.
5 Farrar HC, Herold DA, Reed MD. Acute valproic acid intoxication: enhanced drug clearance with oral-activated charcoal. Crit Care Med.. 1993;21:299.
6 Fernandez MC, Walter FG, Kloster JC, et al. Hemodialysis and hemoperfusion for treatment of valproic acid and gabapentin poisoning. Vet Hum Toxicol.. 1996;38:438.
7 Willmore LJ. New antiepileptic drugs; basic science and clinical use in children and adults. Epilepsia. 1999;40(Suppl 5):S1.
8 Klotz U, Avant GR, Hoyumpa A, et al. The effects of age and liver disease on the disposition and elimination of diazepam in adult man. J Clin Invest. 1975;55:347.
9 Pomara N, Stanley B, Block R, et al. Increased sensitivity of the elderly to the central depressant effects of diazepam. J Clin Psychiatry. 1985;46:185.
10 Varelas PN, Mirski MA. Seizures in the adult intensive care unit. J Neurosurg Anesthesiol.. 2001;13:163.
11 Allikmets E, et al. Long-term use of benzodiazepines: abrupt withdrawal versus withdrawal under nifedipine cover. Pharmacol Toxicol. 1995;76(Suppl. 3):Abstr 8.
12 Votey SR, Bosse GM, Bayer MJ, et al. Flumazenil: A new benzodiazepine antagonist. Ann Emerg Med. 1991;20:181.
13 Allonen H, Ziegler G, Klotz U. Midazolam kinetics. Clin Pharmacol Ther. 1981;30:653.
14 Kanto J, Aaltonen L, Himberg JJ, et al. Midazolam as an intravenous induction agent in the elderly: a clinical and pharmacokinetic study. Anesth Analg. 1986;65:15.
15 Ameer B, Greenblatt DJ. Lorazepam: a review of its clinical pharmacological properties and therapeutic uses. Drugs. 1981;21:162.
16 David M, Treiman, et al. A comparison of four treatments for generalized convulsive status epilepticus. New Engl J Med. 1998;339:792.
17 Laine GA, Hossain SM, Solis RT, et al. Polyethylene glycol nephrotoxicity secondary to prolonged high-dose intravenous lorazepam. Ann Pharmacother. 1995;29:1110.
18 Temkin NR, Dikmen SS, Wilensky AJ, Keihm J, Chabal S, Winn HR. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures [see comments]. N Engl J Med. 1990;323:497.
19 Markowsky SJ, Skaar DJ, Christie JM, Eyer SD, Ehresman DJ. Phenytoin protein binding and dosage requirements during acute and convalescent phases following brain injury. Ann Pharmacother. 1996;30:443.
20 Obrein TJ, Cascino GD, So EL, Hanna DR. Incidence and clinical consequence of the purple glove syndrome in patients receiving intravenous phenytoin. Neurology. 1998;51:1034.
21 Wolfram Schummer, Claudia Schummer, Christoph Kuwert. Toxic epidermal necrolysis after phenytoin usage in a brain trauma patient. J Neurosurg Anesthesiol. 2002;14:229.
22 Boucher BA, Feler CA, Dean JC, et al. The safety, tolerability, and pharmacokinetics of fosphenytoin after intramuscular and intravenous administration in neurosurgery patients. Pharmacotherapy. 1996;16:638.
23 Kugler AR, Knapp LE, Eldon MA. Attainment of therapeutic phenytoin concentrations following administration of loading doses of fosphenytoin: A metaanalysis. Neurology. 1996;46:A176.
24 Wilder BJ, Campbell K, Ramsay RE, et al. Safety and tolerance of multiple doses of intramuscular fosphenytoin substituted for oral phenytoin in epilepsy or neurosurgery. Arch Neurol. 1996;53:764.
25 Jamerson BD, Dukes GE, Brouwer KL, et al. Venous irritation related to intravenous administration of phenytoin versus fosphenytoin. Pharmacotherapy. 1994;14:47.
26 Liu H, Delgado MR. Influence of sex, age, weight, and carbamazepine dose on serum concentrations, concentration ratios, and level/dose ratios of carbamazepine and its metabolites. Ther Drug Monit. 1994;16:469.
27 Tomson T, Bertilsson L. Kinetics, metabolism and effects of carbamazepine-10,11-epoxide in man. Epilepsy Res Suppl. 1991;3:177.
28 Apfelbaum JD, Caravati EM, Kerns WP2nd, et al. Cardiovascular effects of carbamazepine toxicity. Ann Emerg Med. 1995;25:631.
29 Giroud M, Gras D, Escousse A, et al. Use of injectable valproic acid in status epilepticus. A pilot study. Drug Invest. 1993;5:154.
30 Price DJ. Intravenous valproate: experience in neurosurgery. Fourth Int Symp Sodium Valproate and Epilepsy. Roy Soc Med Int Congr Sympt Ser. 1989;152:197.
31 Dreifuss FE, Santilli N, Langer DH, et al. Valproic acid hepatic fatalities: a retrospective review. Neurology. 1987;37:379.
32 Kerrick JM, Wolff DL, Graves NM. Predicting unbound phenytoin concentrations in patients receiving valproic acid: a comparison of two prediction methods. Ann Pharmacother. 1995;29:470.
33 Evans RJ, Miranda RN, Jordan J, et al. Fatal acute pancreatitis caused by valproic acid. Am J Forensic Med Pathol. 1995;16:62.
34 Tohen M, Castillo J, Baldessarini RJ, et al. Blood dyscrasias with carbamazepine and valproate: a pharmacoepidemiological study of 2,228 patients at risk. Am J Psychiatry. 1995;152:413.
35 Alberto G, Erickson T, Popiel R, et al. Central nervous system manifestation of a valproic acid overdose responsive to naloxone. Ann Emerg Med. 1989;18:889.
36 Stecker MM, Kramer TH, Raps EC, et al. Treatment of refractory status epilepticus with propofol: clinical and pharmacological findings. Epilepsia. 1998;39:18.
37 Langley MS, Heel RC. Propofol: a review of its pharmacodynamic and pharmacokinetic properties and use as an intravenous anaesthetic. Drugs. 1988;35:334.
38 Bennett SN, McNeil MM, Bland LA, et al. Postoperative infections traced to contamination of an intravenous anesthetic, propofol. N Engl J Med. 1995;333:147.
39 Kang TM. Propofol infusion syndrome in critically ill patients. Ann Pharmacother. 2002;36:1453.
40 Bray RJ. Fatal myocardial failure associated with a propofol infusion in a child. Anaesthesia. 1995;50:94.
41 Mirenda J. Prolonged propofol sedation in the critical care unit. Crit Care Med. 1995;23:1304.
42 Strickland RA, Murray MJ. Fatal metabolic acidosis in a pediatric patient receiving an infusion of propofol in the intensive care unit: is there a relationship. Crit Care Med. 1995;23:405.
43 Mirski MA, Williams MA, Hanley DF. Prolonged pentobarbital and phenobarbitone coma for refractory generalized status epilepticus. Crit Care Med. 1995;23:400.
44 Mirski MA. Rapid treatment of status epilepticus with low dose phenobarbital. Crit Care Report. 1989;1:150.
45 Lowenstein DH, Aminoroff MJ, Simon RP. Barbiturate anesthesia in the treatment of status epilepticus: clinical experience with 14 patients. Neurology. 1988;38:395.
46 Amitai Y, Degani Y. Treatment of phenobarbital poisoning with multiple dose of activated charcoal in an infant. J Emerg Med. 1990;8:449.
47 Jacobsen D, Wiik-Larsen E, Dahl T, et al. pharmacokinetic evaluation of haemoperfusion in phenobarbital poisoning. Eur J Clin Pharmacol. 1984;26:109.
48 Lin JL, Jeng LB, Critical. Acutely poisoned patients treated with continuous arteriovenous hemoperfusion in the emergency department. Ann Emerg Med. 1995;25:75.
49 Cramer JA, Fisher R, Ben-Menachem E, et al. New antiepileptic drugs: comparison of key clinical trials. Epilepsia. 1999;40:590.
50 Beydoun A, D’Souza J, Hebert D, Doty P. American University of Beirut Medical Center, Beirut. Lacosamide: pharmacology, mechanisms of action and pooled efficacy and safety data in partial-onset seizures. Expert Rev Neurother. 2009;9:33.
51 Schiltmeyer B, Cawello W, Kropeit D, Hammes W, Horstman R. Pharmacokinetics of the new antiepileptic drug SPM 927 in human subjects with different age and gender. Epilepsia. 2004;45(Suppl. 7):313.
52 Ben-Menachem E, Biton V, Jatuzis D, Abou-Khalil B, Doty P, Rudd GD. Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures. Epilepsia. 2007;48:1308.
53 Thomas D, Scharfenecker U, Nickel B, Doty P, Cawello W, Horstmann R. Lacosamide has low potential for drug–drug interaction. Presented at: 8th European Congress on Epileptology. Berlin, Germany, 21-25 September 2008.