Anticholinergic (parasympatholytic) bronchodilators
After reading this chapter, the reader will be able to:
1. Define terms that pertain to anticholinergic bronchodilators
2. Differentiate between parasympathomimetic and parasympatholytic
3. Differentiate between cholinergic and anticholinergic
4. Differentiate between muscarinic and antimuscarinic
5. List all available anticholinergic agents used in respiratory therapy
6. Discuss the indication for anticholinergic agents
7. Explain the mode of action for anticholinergic agents
8. Identify the route of administration available for anticholinergic agents
9. Discuss adverse effects for anticholinergic agents
10. Discuss the clinical application for anticholinergic agents
Anticholinergic bronchodilator
Agent that blocks parasympathetic nervous fibers, which allow relaxation of smooth muscle in the airway.
Same as anticholinergic bronchodilator—agent that blocks the effect of acetylcholine at the cholinergic site.
Agent that produces the effect of acetylcholine.
Same as cholinergic—agent that produces the effect of acetylcholine or an agent that mimics acetylcholine.
Blocking parasympathetic nervous fibers.
Producing effects similar to the parasympathetic nervous system.
Specific anticholinergic (parasympatholytic) agents
Parasympatholytic (anticholinergic, or antimuscarinic) agents that are given by aerosol include ipratropium, a combination of ipratropium and albuterol, and tiotropium. Table 7-1 provides dosage and administration information for each agent.
TABLE 7-1
Inhaled Anticholinergic Bronchodilator Agents*
| DRUG | BRAND NAME | ADULT DOSAGE | TIME COURSE (ONSET, PEAK, DURATION) |
| Ipratropium bromide | Atrovent HFA |
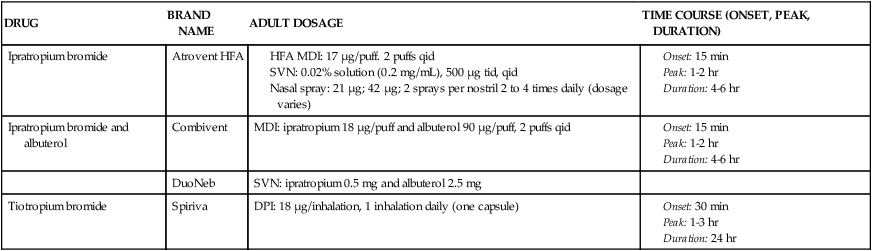
*A holding chamber is recommended with MDI administration to prevent accidental eye exposure.
Atropine sulfate had been administered as a nebulized solution, using either the injectable solution or, preferably, solutions marketed for aerosolization; however, this agent is no longer aerosolized. Duration of bronchodilation and the incidence of side effects are dose dependent. Dosages for children based on dose-response curves had been given as 0.05 mg/kg three or four times daily.1 Dosages for adults are based on a schedule of 0.025 mg/kg three or four times daily.2 Although greater bronchodilation and duration were seen with dosage schedules of 0.05 or 0.1 mg/kg for adults, the side effects of dry mouth, blurred vision, and tachycardia became unacceptable. Because it is a tertiary ammonium compound and not fully ionized, atropine is readily absorbed from the gastrointestinal tract and respiratory mucosa. Systemic side effects (which are discussed subsequently) were seen in doses required for effective bronchodilation when given as an inhaled aerosol. The drug is not recommended for inhalation as a bronchodilator because of its widespread distribution in the body and the availability of the approved agents ipratropium and tiotropium.
Ipratropium bromide (Atrovent) is a nonselective antagonist of M1, M2, and M3 receptors (for a discussion of muscarinic receptors, see Chapter 5). Ipratropium is currently available in two formulations for bronchodilator use: as a hydrofluoroalkane-propelled metered dose inhaler (HFA MDI) with 17 μg/puff and as a nebulizer solution of 0.02% concentration in a 2.5-mL vial, giving a 500-μg dose per treatment. One other option for the delivery of ipratropium is the soft-mist, propellant-free Respimat inhaler. This formulation at the time of this edition is unavailable in the United States; however, it may soon become available. This agent is an N-isopropyl derivative of atropine. As a quaternary ammonium derivative of atropine, ipratropium is fully ionized and does not distribute well across lipid membranes, limiting its distribution more to the lung when inhaled. Ipratropium is approved specifically for the maintenance treatment of airflow obstruction in COPD.
The profile of clinical effect for ipratropium differs from that of inhaled β-adrenergic agonists. The onset of bronchodilation begins within minutes but proceeds more slowly to a peak effect 1 to 2 hours after inhalation. β agonists can peak between 20 minutes and 30 minutes depending on the agent. In asthma, the duration of bronchodilator effect is about the same for ipratropium as for β agonists. In COPD, the duration is longer by 1 to 2 hours.3
Ipratropium bromide (Atrovent nasal spray) is also available for treatment of rhinopathies and rhinorrhea, including nonallergic perennial rhinitis, viral infectious rhinitis (colds), and allergic rhinitis, if intranasal corticosteroids fail to control symptoms.4 The nasal spray is available in two strengths, with a 0.03% solution delivering 21 μg/spray and the 0.06% solution delivering 42 μg/spray. The 0.03% strength is given as two sprays per nostril two or three times daily, and the 0.06% strength is given as two sprays per nostril three or four times daily. Optimal dosage varies. Intranasal ipratropium has been shown to reduce significantly the volume of nasal secretions and symptoms in patients with allergic rhinitis and in patients with nonallergic rhinitis.4 Side effects with the nasal spray are largely local and have included nasal dryness, itching, and epistaxis in a few patients. Dry mouth and dry throat have also occurred. Systemic symptoms such as blurred vision or urinary hesitancy are rare.
Ipratropium and albuterol (Combivent) is a combination MDI product, with the usual doses of each agent (18 μg/puff of ipratropium, 90 μg/puff of albuterol). The combination therapy has been shown to be more effective in stable COPD than either agent alone.5 Another agent, DuoNeb, is available as a combination of ipratropium (0.5 mg) and albuterol base (2.5 mg). Another option for the delivery of Combivent is the soft-mist, propellant-free, Respimat inhaler. As mentioned earlier, Respimat inhaler at the time of this edition is unavailable in the United States. The chlorofluorocarbon (CFC) version of Combivent will be removed from the market on December 31, 2013.
Glycopyrrolate is a quaternary ammonium derivative of atropine that, similar to ipratropium, does not distribute well across lipid membranes in the body. It is usually administered parenterally as an antimuscarinic agent during reversal of neuromuscular blockade, as an alternative to atropine, with fewer ocular or central nervous system (CNS) side effects. The injectable solution has been nebulized in a 1-mg dose for bronchodilation. Gal and colleagues6 reported a comparison of glycopyrrolate with atropine and established dose-response curves. Glycopyrrolate has an onset of action of approximately 15 to 30 minutes, a peak effect at 0.5 to 1 hour, and a duration of approximately 6 hours. Although the injectable formulation of glycopyrrolate is used as a less expensive alternative to the Atrovent brand of ipratropium, it is not approved for inhalation. More recently in new research of asthma and COPD, glycopyrrolate as an inhaled agent currently under investigation (NVA-237) has found favor. In a small study, Hansel and coworkers7 found superiority over ipratropium in the protection of bronchospasm from methacholine.
Tiotropium bromide (Spiriva), a muscarinic receptor antagonist, is a long-acting bronchodilator. It is a quaternary ammonium compound structurally related to ipratropium. Similar to ipratropium, tiotropium is poorly absorbed after inhalation. Inhalation of a single dose gives a peak plasma level within 5 minutes, with a rapid decline to very low levels within 1 hour.8,9 Tiotropium exhibits receptor subtype selectivity for M1 and M3 receptors. The drug binds to all three muscarinic receptors (M1, M2, and M3) but dissociates much more slowly than ipratropium from the M1 and M3 receptors. This results in a selectivity of action on M1 and M3 receptors. Atropine and ipratropium block all three types of muscarinic receptors. The M2 receptor is an autoreceptor inhibiting further release of acetylcholine, so that blockade can increase acetylcholine release and may offset the bronchodilating effect of atropine or ipratropium.8 In patients with COPD, tiotropium gives a bronchodilating effect for up to 24 hours with adequate dose. The effect of tiotropium can be seen for 32 hours; however, it dips between 16 hours and 24 hours owing to circadian rhythm. The drug also gives a prolonged, dose-dependent protection against inhaled methacholine challenge.10
Several studies have examined the bronchodilating effect of various doses of tiotropium compared with placebo and ipratropium.10–12 A single dose of 18 μg inhaled once daily from a dry powder inhaler (DPI), the HandiHaler,13 provided significant bronchodilation for up to 24 hours, with a low side-effect profile. An increase of 15% from baseline forced expiratory volume in 1 second (FEV1) occurred 30 minutes after inhalation, with a peak effect at about 3 hours. Improvement in FEV1 was greater 3 hours after inhalation for tiotropium than for ipratropium. After a dose of tiotropium, the trough, or lowest, value for FEV1 remained above that of ipratropium because of the prolonged action of tiotropium. Ipratropium had a more rapid onset of action than tiotropium, but after the initial dosing this difference loses relevance because tiotropium maintains a higher level of baseline bronchodilation. In a meta-analysis, Barr and associates14 found that tiotropium reduces COPD exacerbations and hospitalizations, improves quality-of-life symptoms, and may slow the decline in a patient’s FEV1.
Aclidinium bromide is a novel, long-acting, inhaled muscarinic antagonist currently being developed by Forest Laboratories as a maintenance treatment for COPD.15 This agent has shown potent antagonism of all muscarinic receptors, dissociating slowly at M3 and a shorter time at M2, indicating the potential to provide sustained bronchodilation that is similar in action to tiotropium.16 Aclidinium is rapidly hydrolyzed in human plasma, in contrast to ipratropium and tiotropium.16,17 This rapid hydrolysis results in very low and transient systemic exposure, suggesting a reduced potential for systemic side effects.16,17
Early clinical studies in healthy subjects have confirmed the low systemic bioavailability and favorable safety profile of single and multiple doses of aclidinium.18,19 In a subsequent study, which included patients with moderate to severe COPD, aclidinium displayed long-lasting bronchodilation and was well tolerated.20
Clinical pharmacology
Structure-activity relationships
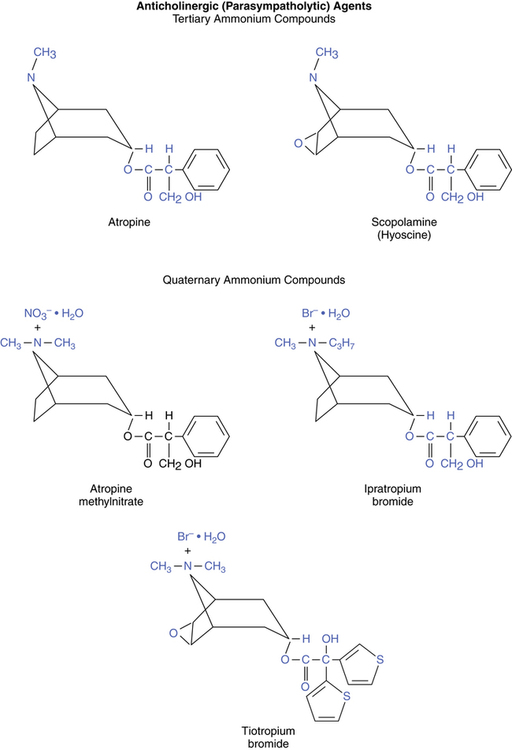
Chemical structures of the two naturally occurring belladonna alkaloids, atropine and scopolamine (also called hyoscine), are illustrated in Figure 7-1. Atropine, including its sulfate (atropine sulfate), and scopolamine are both tertiary ammonium compounds that differ from each other only by an oxygen bridging the carbon-6 and carbon-7 positions. Quaternary ammonium derivatives of atropine include atropine, ipratropium, and tiotropium. Another quaternary atropine derivative, which has been administered experimentally as a bronchodilator by aerosol, is glycopyrrolate (Robinul) (not shown in Figure 7-1).
Pharmacologic effects of anticholinergic (antimuscarinic) agents
The general effects of cholinergic (muscarinic) stimulation and the corresponding effects produced by anticholinergic (antimuscarinic) action are listed in Table 7-2. Specific effects differ for tertiary and quaternary ammonium compounds because of their absorption differences as previously outlined for their structure-activity relationships. These effects and their differences are summarized in Table 7-3 and discussed subsequently.
TABLE 7-2
| CHOLINERGIC EFFECT | ANTICHOLINERGIC EFFECT |
| Decreased heart rate | Increased heart rate |
| Miosis (contraction of iris, eye) | Mydriasis (pupil dilation) |
| Contraction (thickening) of lens, eye | Cycloplegia (lens flattened) |
| Salivation | Drying of upper airway |
| Lacrimation | Inhibition of tear formation |
| Urination | Urinary retention |
| Defecation | Antidiarrheal or constipation |
| Secretion of mucus | Mucociliary slowing |
| Bronchoconstriction | Inhibition of constriction |
TABLE 7-3
Pharmacologic Effects of Tertiary versus Quaternary Anticholinergic Agents Given by Inhaled Aerosol
| TERTIARY (ATROPINE) | QUATERNARY (IPRATROPIUM AND TIOTROPIUM) | |
| Respiratory tract | Bronchodilation | Bronchodilation |
| Decreased mucociliary clearance | Little or no change in mucociliary clearance | |
| Blockage of hypersecretion | Blockage of nasal hypersecretion | |
| CNS | Altered CNS function (dose related) | No effect |
| Eye | Mydriasis | Usually no effect* |
| Cycloplegia | ||
| Increased intraocular pressure | ||
| Cardiac | Minor slowing of heart rate (small dose); increased heart rate (larger dose) | No effect |
| Gastrointestinal | Dry mouth, dysphagia; slows motility | Dry mouth |
| Genitourinary | Urinary retention | Usually no effect† |
*Assumes aerosol is not sprayed into eye; use with caution in glaucoma.
†Use with caution in prostatic enlargement or urinary retention.
Tertiary ammonium compounds
Respiratory tract effects.
Atropine sulfate, a prototype tertiary compound, inhibits and reduces mucociliary clearance, as shown by Groth and associates.21 Atropine seems to block hypersecretion stimulated by cholinergic agonists in both the lower airway and the nose (upper airway) more than basal secretion.22 Atropine relaxes airway smooth muscle, the basis for its use in asthma.
Central nervous system effects.
Tertiary compounds cross the blood-brain barrier and produce dose-related effects. Small doses of 0.5 to 1.0 mg can cause effects that include restlessness, irritability, drowsiness, fatigue, or, alternatively, mild excitement. Increased doses can cause disorientation, hallucinations, or coma. Inhaled atropine has been reported to cause an acute psychotic reaction.23,24
Gastrointestinal effects.
Anticholinergic agents generally cause dryness of the mouth as a result of inhibition of salivary gland secretions, and atropine is used for this effect to reduce upper airway secretions before surgery and anesthesia or when reversing neuromuscular blockade (see Chapter 18). Larger doses can cause dysphagia. Gastrointestinal motility is slowed, an effect that is the basis for the inclusion of atropine in Lomotil, an antidiarrheal. Inhibition of gastrointestinal motility and emptying has been noted with normal doses of atropine given by aerosol to asthmatics.25
Quaternary ammonium compounds
Cardiac effects.
Ipratropium has minimal effects on heart rate or blood pressure when given by inhaled aerosol. However, several meta-analyses have suggested that ipratropium and tiotropium may cause an increase in cardiovascular events. When other meta-analyses were conducted and reexamined, no incidence of cardiovascular involvement from inhaled anticholinergics was found. Currently, no information has been conclusive in showing that these agents have any adverse effects on the cardiovascular system.26
Gastrointestinal effects.
In most patients, inhaled ipratropium has little effect on gastrointestinal motility. A portion of the aerosol dose is swallowed, however, allowing exposure of the gastrointestinal tract to the drug. There has been a report of meconium ileus in an adult with cystic fibrosis receiving nebulized ipratropium.27 Use of a reservoir device with MDI administration can reduce oropharyngeal impaction and the amount of swallowed drug.
Mode of action
In Chapter 5, the autonomic innervation of the airway is outlined; this consists of the traditional sympathetic and parasympathetic branches and nonadrenergic, noncholinergic (NANC) inhibitory and excitatory branches. The sympathetic branch does not actually extend its fibers beyond the peribronchial ganglia or plexuses to the airway, although adrenergic receptors are present throughout the airway, especially in the periphery. Parasympathetic nerves enter the lung at the hila, deriving from the vagus, and travel along the airways. Parasympathetic postganglionic fibers terminate on or near the airway epithelium, submucosal mucous glands, smooth muscle, and probably mast cells. Parasympathetic innervation and muscarinic receptors are concentrated in the larger airways, although they are present from the trachea to the respiratory bronchioles.
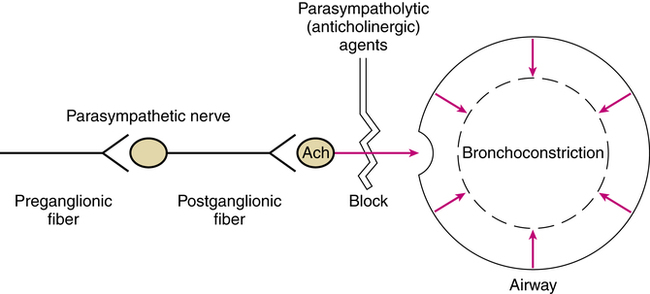
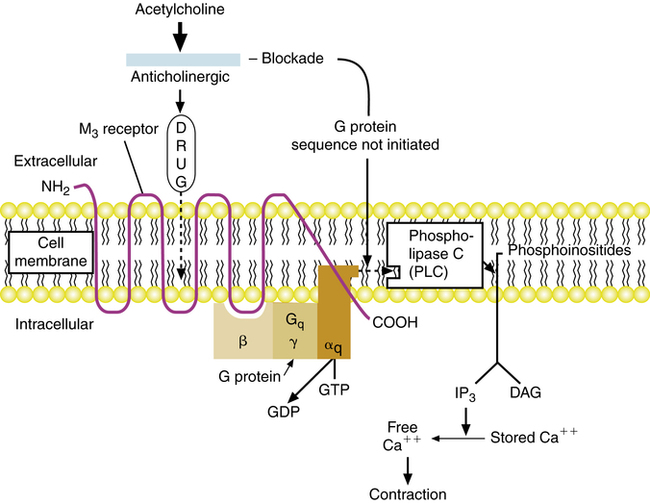
Cholinergic stimulation of muscarinic receptors on airway smooth muscle and submucosal glands causes contraction and release of mucus. Anticholinergic agents such as atropine or ipratropium are antimuscarinic; they competitively block the action of acetylcholine at parasympathetic postganglionic effector cell receptors. Because of this action, anticholinergic agents block cholinergic-induced bronchoconstriction, as shown in Figure 7-2.
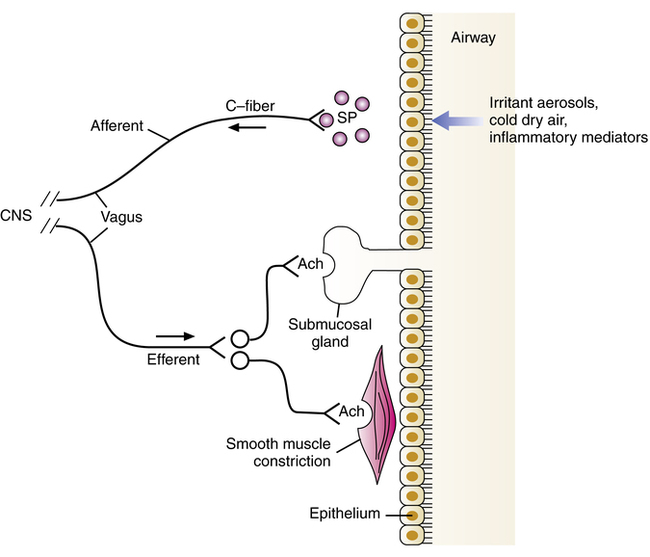
Vagally mediated reflex bronchoconstriction
A portion of the bronchoconstriction seen in COPD may be due to a mechanism of vagally mediated reflex innervation of airway smooth muscle (Figure 7-3). Sensory C-fiber nerves respond to various stimuli, such as irritant aerosols (hypotonic or hypertonic), cold air and high airflow rates, cigarette smoke, noxious fumes, and mediators of inflammation such as histamine. When activated, they produce an afferent nerve impulse to the CNS, which results in a reflex cholinergic efferent impulse, to cause constriction of airway smooth muscle and release of secretion from mucous glands as well as cough.
Changes in the airway may also sensitize the subepithelial cough receptors, making them more responsive to lower thresholds of stimulation. This sensitization is often seen during colds that involve lung congestion. Lung inflation during a deep breath stimulates the cough receptors, resulting not only in coughing but also increased bronchomotor tone. It has been suggested that greater bronchial reactivity in asthmatic patients or patients with COPD may be caused by mucosal edema and deformation of airway tissue, which increases the sensitivity of these receptors in response to irritants. Several reviews of cholinergic mechanisms of airway obstruction have been published.29–33
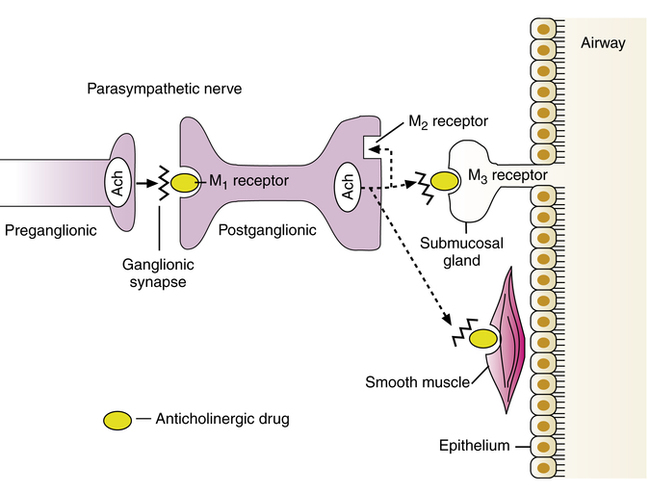
Muscarinic receptor subtypes
Anticholinergic agents cause bronchodilation by blocking muscarinic receptor subtypes: M1 receptors at the parasympathetic ganglia, which facilitate cholinergic neurotransmission and bronchoconstriction, and M3 receptors on airway smooth muscle, which cause bronchoconstriction. Muscarinic receptor subtypes are reviewed in Chapter 5 and are illustrated for the lung in Figure 7-4. M1 receptors on the postganglionic parasympathetic neuron facilitate cholinergic nerve transmission, leading to release of acetylcholine. Acetylcholine stimulates M3 receptor subtypes on airway smooth muscle and submucosal glands, causing contraction of smooth muscle and exocytosis of secretion from the mucous gland. The M2 receptor subtype at cholinergic nerve endings inhibits further acetylcholine release from the postganglionic neuron.
M3 receptors are G protein–linked receptors (introduced in Chapter 2 and again in Chapter 5). Table 7-4 lists the various muscarinic receptor subtypes and their G proteins, along with their effector enzymes. Stimulation of the M3 receptor subtype activates a Gq protein, which activates phospholipase C (PLC). PLC causes the breakdown of phosphoinositides into inositol triphosphate (IP3) and diacylglycerol (DAG); this ultimately leads to an increase in the cytoplasmic concentration of free calcium and smooth muscle contraction or gland exocytosis. As shown in Figure 7-5, the competitive blockade of M3 receptors by anticholinergic agents prevents this sequence. The blockade of M1 receptor subtypes by anticholinergic agents also inhibits nerve transmission by acetylcholine at the ganglionic synapse. Both ipratropium and tiotropium also block the M2 receptor. This receptor inhibits continued release of acetylcholine. As a result, blockade of the M2 receptor can enhance acetylcholine release, possibly counteracting the bronchodilator effect of M3 receptor blockade. As noted in the discussion of ipratropium and tiotropium, tiotropium has selective affinity for M1 and M3 receptors because it dissociates much more rapidly from the M2 receptor and remains bound to the M1 and M3 subtypes.
TABLE 7-4
Muscarinic Receptor Subtypes: G Proteins and Effector Systems
| RECEPTOR | G PROTEIN | EFFECTOR |
| M1 | Gq | Phospholipase C |
| M2 | Gi | Adenylyl cyclase (decreases) |
| M3 | Gq | Phospholipase C |
| M4 | Gi | Adenylyl cyclase (decreases) |
| M5 | Gq | Phospholipase C leads to an increase |
The use of agents such as ipratropium for allergic and nonallergic rhinitis is based on the parasympathetic control of submucosal glands in the nasal mucosa. Acetylcholine stimulates muscarinic receptors in the nose, where approximately 55% are M3 receptors and the rest are M1 receptors.34 M2 receptors were not identified in human nasal mucosa in an autoradiographic study by Okayama and associates.35 Blockade of muscarinic M1 and M3 receptors on submucosal nasal glands by ipratropium given as a nasal spray prevents gland secretion and rhinitis.
Adverse effects
It has been stated that the safety profile of quaternary ammonium antimuscarinic bronchodilators (e.g., ipratropium and tiotropium) is superior to that of β agonists, particularly with regard to cardiovascular effects.9 Changes in electrocardiogram, blood pressure, or heart rate are not usually seen. There is no worsening of ventilation-perfusion abnormalities in COPD, which would otherwise cause an increase in hypoxemia. A tolerance to bronchodilation and loss of bronchial protection has not been observed. The lack of these effects is due to the poor absorption and systemic distribution of quaternary compounds such as ipratropium. Cugell36 provided a detailed review of the clinical pharmacology and toxicology of ipratropium.
The side effects seen with the MDI and small volume nebulizer (SVN) formulations of ipratropium, the agent with the most clinical experience, are primarily related to the local, topical delivery to the upper and lower airway with inhalation. Similar side effects would be expected with other antimuscarinic agents such as tiotropium. The most common side effect seen with this class of bronchodilator is dry mouth. Possible side effects are listed in Box 7-1. The SVN solution has also been associated with additional side effects in a few patients, including pharyngitis, dyspnea, flulike symptoms, bronchitis, and upper respiratory infection. The amount of drug in the nebulizer dose is more than 10 times greater than in the MDI dose (500 μg versus 40 μg). If the patient receives approximately 10% of an inhaled aerosol to the lung, a much larger dose is given with an SVN. The orally swallowed portion would be proportionately higher also. Systemic side effects such as tachycardia, palpitations, urinary hesitancy, constipation, blurred vision, and increased ocular pressure are less likely with quaternary agents such as ipratropium or tiotropium than with tertiary agents such as atropine. Although ipratropium is not contraindicated in subjects with prostatic hypertrophy, urinary retention, or glaucoma, the drug should be used with caution and adequate evaluation for possible systemic side effects in these subjects.
Clinical application
Anticholinergic (antimuscarinic) aerosols have been investigated for use with asthma and with COPD. Table 7-5 compares the general effects seen with anticholinergic bronchodilators and β-adrenergic bronchodilators.
TABLE 7-5
Comparison of Effects for Anticholinergic and β-Adrenergic Bronchodilators
| ANTICHOLINERGIC | β AGONIST | |
| Onset | Slightly slower | Faster |
| Time to peak effect | Slower | Faster |
| Duration | Longer | Shorter |
| Tremor | None | Yes |
| Decrease in Pao2 | None | Yes |
| Tolerance | None | Yes |
| Site of action | Larger, central airways | Central and peripheral airways |
Use in chronic obstructive pulmonary disease
Antimuscarinic agents were found to be more potent bronchodilators than β-adrenergic agents in bronchitis/emphysema, and this is likely to be their primary clinical application. This difference is illustrated in Figure 7-6 with data from Tashkin and colleagues.37 In that 90-day, multicenter study, the investigation compared 40 μg of ipratropium with 1.5 mg of metaproterenol, both given by MDI, in a population of patients with COPD. Explanations for the superiority of anticholinergic action in COPD are debated but may relate to the complicated, inflammatory, noncholinergic pathways seen in asthma, especially owing to stimuli mentioned previously in the section on vagally mediated reflex bronchoconstriction. Conversely, the pathology of COPD may reveal the reason for the superior effect of anticholinergic over β-adrenergic drugs. Ipratropium has been approved by the FDA specifically for use in the treatment of COPD, although the drug is also prescribed for treatment of asthma.
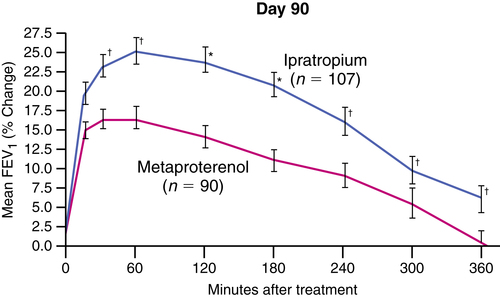
Rennard and associates38 analyzed data from the clinical trials of ipratropium compared with a β agonist for FDA approval. Their analysis showed that use of ipratropium over the 90-day interval tested was associated with improved baseline lung function and response to acute bronchodilator use. Subjects using β agonists over the same period had little change in baseline lung function and a small decrease in airway response to acute bronchodilator treatment.
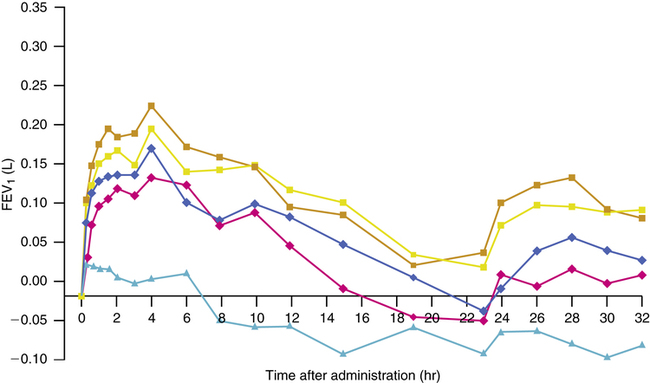
Tiotropium, an antimuscarinic bronchodilator, offers a prolonged duration of action of 24 hours with a single daily inhalation. In dose-ranging trials, an inhaled dose of 18 μg once per day has been found to give significant bronchodilation in patients with COPD with few side effects.10,12 Figure 7-7 illustrates the effect on FEV1 with single inhaled doses of tiotropium of various strengths compared with placebo.9 There is also prolonged dose-dependent protection against inhaled methacholine challenge. Both the bronchodilating and the bronchoprotective effect can be compared with the 6-hour effect of ipratropium. Perhaps one of the more important effects of a long-acting drug such as tiotropium is the elevation in baseline, predose FEV1. In contrast to ipratropium, lung function is maintained more consistently at a higher level throughout the day with tiotropium. This effect may be significant in relation to quality of life and reduction of breathlessness in patients with COPD. Beeh and colleagues39 reported that an inhaled dose of 18 μg once per day improved lung function and reduced exacerbations in patients with COPD of different severities. Adams and associates40 also found improvement in lung function and dyspnea in patients with COPD. The prolonged effect may also be useful in controlling nocturnal asthma symptoms, where cholinergic mechanisms seem to increase airway tone.41
Current COPD guidelines do not dictate the use of any one specific bronchodilator.42,43 It is noted, however, that the use of a short-term β2 agonist and an anticholinergic, such as ipratropium, improves the FEV1 in patients with COPD.43 The use of a long-term anticholinergic, such as tiotropium, improves the health of patients with COPD.43 The use of a single agent or combination is dictated by the patient’s response.
Use in asthma
Anticholinergic (antimuscarinic) agents such as ipratropium do not have a labeled indication for asthma in the United States. Current asthma guidelines state that ipratropium may have some additive benefit when given with inhaled β agonists.44,45 Antimuscarinic bronchodilators are not clearly superior to β-adrenergic agents in treating asthma. Antimuscarinic and β-adrenergic agents have an approximately equal effect on flow rates in many patients. These agents may be especially useful in the following applications when prescribed for asthmatic patients:46
• Nocturnal asthma, in which the slightly longer duration of action may protect against nocturnal deterioration of flow rates47
• Psychogenic asthma, which may be mediated through vagal parasympathetic fibers
• Asthmatic patients with glaucoma, angina, or hypertension who require treatment with β-blocking agents
• As an alternative to theophylline in patients with notable side effects from that drug
• Acute, severe episodes of asthma not responding well to β agonists
A large, randomized controlled study by Qureshi and colleagues48 in 434 children 2 to 8 years old with acute moderate to severe asthma found that the overall rate of hospitalization was reduced with addition of inhaled ipratropium to nebulized albuterol. However, the most striking effect on admission was seen in children with severe asthma (peak expiratory flow less than 50% predicted). A meta-analysis of the addition of anticholinergic bronchodilators to β agonists in children and adolescents, conducted by Plotnick and Ducharme,49 concluded that adding multiple doses of anticholinergic (antimuscarinic) bronchodilators to β2 agonists was safe, improved lung function, and may avoid hospital admission in 1 of 11 treated patients. Multiple doses should be preferred to single doses of antimuscarinic agents.
Combination therapy: β-adrenergic and anticholinergic agents in chronic obstructive pulmonary disease
• Complementarity of sites of action exists, with anticholinergic effect seen in the more central airways and β-agonist effect in the smaller, more peripheral airways.
• Mechanisms of action from anticholinergic and β-adrenergic agents are separate and complementary.
Additive effect of β agonists and anticholinergic agents
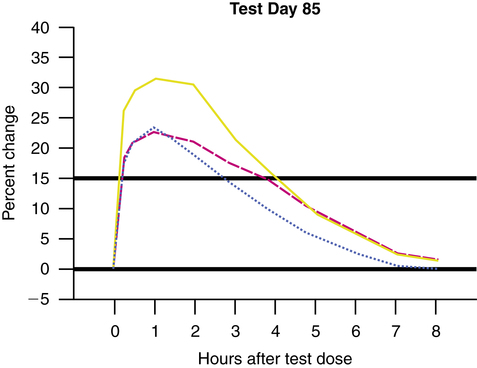
Conflicting results have been found regarding the question of whether the bronchodilator effect of β agonists is increased by adding an anticholinergic agent, in either COPD or asthma.50–54 Many of the studies performed with combined anticholinergic and β-agonist bronchodilator therapy have small sample sizes and poor statistical power. Before the approval of combined albuterol and ipratropium (Combivent), a large, well-controlled study was conducted over 85 days with 462 patients at 24 centers.5 Patients had stable COPD. The study showed superior efficacy of the combination therapy of ipratropium and albuterol compared with either agent alone. Figure 7-8 shows a comparison from this study of ipratropium plus albuterol with albuterol or ipratropium alone on the percentage change in FEV1. The mean peak increases in FEV1 were 31% to 33% for combined drug therapy compared with 24% to 25% for ipratropium alone and 24% to 27% for albuterol alone. Flow rates were significantly better on all test days. Symptom scores did not differ among the three groups, however. As a large, well-designed study, these results support combination anticholinergic and β-agonist therapy in COPD.
Sequence of administration
Most discussion and clinical use of β2 agonists and anticholinergics have centered on agents with shorter duration. Other combination agents have included long-acting β2 agonists and inhaled corticosteroids. The use of long-acting β2 agonists and long-acting anticholinergics may be as effective as shorter acting agents. In a study of tiotropium and formoterol, it was found that lung function improved with the combination compared with the agents given as monotherapy in patients with stable COPD.55 Another study of moderate to severe COPD patients found that receiving a combination of tiotropium and formoterol increased forced vital capacity (FVC) and FEV1 more than delivering the agents alone.56
Two of the largest, long-term drug trials for COPD—Towards a Revolution in COPD Health (TORCH) and Understanding Potential Long-Term Impacts on Function with Tiotropium (UPLIFT)—have provided insight into the prescribing practice of medications used to treat COPD. Miravitlles and Anzueto57 reviewed both studies. The main conclusion concerning combination agents is that long-term agents, tiotropium and salmeterol, with the addition of an inhaled corticosteroid improve lung function and may reduce the progression of COPD. The use of these agents is being termed, “triple therapy” suggesting improvement when all three are used in combination.58–60