CHAPTER 108 Antibiotic-Associated Diarrhea, Pseudomembranous Enterocolitis, and Clostridium difficile-Associated Diarrhea and Colitis
ANTIBIOTIC-ASSOCIATED DIARRHEA
ETIOLOGY
Diarrhea is a common side effect of antibiotic use and can result from a variety of mechanisms.1 The most common type of diarrhea, often called simple antibiotic-associated diarrhea (AAD), is believed to result from a disturbance of the normal colonic microflora, leading to alterations in bacterial degradation of nonabsorbed carbohydrates and bile salts. Colonic bacteria normally ferment the complex carbohydrates in dietary fiber and other carbohydrates that are not absorbed in the small intestine, and the fermentation products are then metabolized and absorbed in the colon. Disruption of this process by antibiotic therapy is believed to cause osmotic diarrhea. Some, but not all, bacteria can deconjugate bile salts, and unconjugated bile salts are known to stimulate fluid secretion by the colonic mucosa; another mechanism for AAD may be reduced bacterial degradation of bile salts within the colonic lumen. Other mechanisms that can account for AAD include stimulation of intestinal motility through the motilin-like effect of erythromycin, an allergic reaction, or infection with microorganisms other than Clostridium difficile, including Clostridium perfringens type A, Staphylococcus aureus, and Salmonella enterica.2–4
The genotype of C. perfringens that causes AAD appears to be distinct from those that induce food poisoning.3,5 Type A strains isolated from patients with AAD carry the C. perfringens enterotoxin (CPE) gene on a plasmid, whereas those that cause food poisoning have a chromosomal CPE gene. S. aureus was identified as a cause of severe AAD and enterocolitis before C. difficile-associated diarrhea was identified.2,6 Since the advent of sensitive and specific testing for C. difficile, however, very few cases of S. aureus AAD have been confirmed, and the true role played by this pathogen in AAD is unclear. Antibiotic-associated infection with Klebsiella oxytoca has been described in patients with right-sided hemorrhagic colitis. This rare pathogen releases several potent toxins, and it appears to colonize the bowel after the indigenous flora has been altered by exposure to antibiotics.7
AAD complicates 2% to 5% of antibiotic treatment courses, but the incidence varies depending on the antibiotic used; it is more common, for example, during therapy with ampicillin (5%-10%), amoxicillin-clavulanate (10%-25%), or cefixime (15%-20%) and less common during therapy with fluoroquinolones (1%-2%) or trimethoprim-sulfamethoxazole (<1%).8–9
Most cases of AAD are mild and self-limited. Pseudomembranous colitis is absent, and significant complications are rare. C. difficile infection accounts for less than 10% of AAD cases but is an important pathogen to identify because it often requires specific antimicrobial therapy and can lead to life-threatening complications, as discussed in the following section. A comparison between the clinical features of AAD caused by C. difficile and AAD from other causes is presented in Table 108-1.
Table 108-1 Differences between Antibiotic-Associated Diarrhea from Clostridium difficile Infection and from Other Causes
| CHARACTERISTIC | AAD FROM C. DIFFICILE INFECTION | AAD FROM OTHER CAUSES |
|---|---|---|
| Most commonly implicated antibiotics | Clindamycin, cephalosporins, penicillins, fluoroquinolones | Clindamycin, cephalosporins, ampicillin, or amoxicillin-clavulanate |
| History | Usually no history of antibiotic intolerance | History of diarrhea with antibiotic therapy is common |
| Clinical Features | ||
| Diarrhea | May be florid; evidence of colitis with cramps, fever, and fecal leukocytes is common | Usually moderate in severity (nuisance diarrhea) without evidence of colitis |
| Findings on CT or colonoscopy | Evidence of colitis is common; pseudomembranes often are present | Usually normal |
| Complications | Hypoalbuminemia, anasarca, toxic megacolon; relapse can occur after treatment with metronidazole or vancomycin | Usually none except occasional cases of volume depletion |
| Results of assay for C. difficile toxin | Positive | Negative |
| Epidemiologic pattern | May be epidemic or endemic in hospitals or long-term care facilities | Sporadic |
| Treatment | ||
| Withdrawal of implicated antibiotic | Condition can resolve but often persists or progresses | Condition usually resolves |
| Antiperistaltic agents | Contraindicated | Often useful |
| Oral metronidazole or vancomycin | Prompt response | Not indicated |
AAD, antibiotic-associated diarrhea; CT, computed tomography.
From Bartlett JG. Clinical practice: Antibiotic-associated diarrhea. N Engl J Med 2002; 346:334.
TREATMENT
Because AAD is believed to result from an alteration of the normal colonic microflora, a variety of probiotic agents has been evaluated for its treatment and prevention. In a double-blind controlled clinical trial, oral capsules containing viable Saccharomyces boulardii, a nonpathogenic yeast, were coadministered with antibiotics; this combination treatment reduced the incidence of AAD in hospitalized patients from 22% in the placebo group to 9.5% in the S. boulardii group (P = 0.04).10 Another randomized, placebo-controlled trial, however, failed to demonstrate a beneficial effect for S. boulardii in an elderly population of antibiotic recipients.11 Lactobacillus species, in particular Lactobacillus rhamnosus GG, also have been studied in clinical trials of AAD. In one study, Lactobacillus GG was effective in reducing the incidence of AAD to 5% in children being treated for respiratory tract infections compared with a 16% incidence in the placebo group12; other clinical trials of Lactobacillus GG have yielded negative results.13
A meta-analysis examined the results of randomized, double-blind, placebo-controlled trials of probiotic therapy for AAD published between 1966 and 2000.14 Nine studies were analyzed, including four using S. boulardii and four using Lactobacillus GG. The combined odds ratio for AAD in the probiotic-treated groups was 0.37 compared with placebo (95% confidence interval [CI]: 0.26-0.53; P < 0.001). For S. boulardii, the odds ratio in favor of active treatment over placebo was 0.39 (95% CI: 0.25-0.62, P < 0.001) and for lactobacilli the odds ratio was 0.34 (95% CI: 0.19-0.61, P < 0.01). A second meta-analysis yielded similar results.15 Thus, the weight of published evidence suggests that probiotic agents such as S. boulardii and lactobacilli, when used in combination with antibiotics, reduce the risk for AAD. Such therapy may be especially advantageous in patients with a history of susceptibility to AAD.
PSEUDOMEMBRANOUS ENTEROCOLITIS
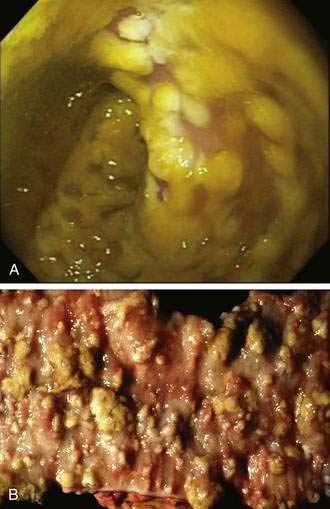
A case report by Finney published in 1893 is considered to be the first description in the medical literature of pseudomembranous enterocolitis.9,16 In that instance, fatal pseudomembranous inflammation of the small intestine followed surgery in a debilitated young woman with gastric outlet obstruction caused by peptic ulcer disease. The presence of an inflammatory pseudomembrane overlying the intestinal mucosa characterizes pseudomembranous colitis (when the colon alone is involved) or pseudomembranous enterocolitis (when the small intestine also is involved).9 The pseudomembrane comprises inflammatory and cellular debris and forms distinctive patches of yellow or gray exudate that obscure the underlying mucosa. In early lesions, a 1- to 2-mm area of punctate ulceration may be visible. Classic pseudomembranes consist of ovoid plaques of 2 to 10 mm in diameter separated by areas of normal or hyperemic mucosa. Histologically, pseudomembranes can be seen to emanate from central areas of epithelial ulceration to form the mucosal plaques. In more-severe cases, the areas of ulceration and the overlying pseudomembranes can coalesce to cover large areas of mucosa.
Risk factors for the development of pseudomembranous enterocolitis in the absence of C. difficile infection include intestinal surgery, intestinal ischemia, and other enteric infections. During the 1940s to the 1970s, most reported cases of pseudomembranous enterocolitis occurred following abdominal or pelvic surgery.17,18 Bartlett has identified numerous descriptions of pseudomembranous enterocolitis in the medical literature associated with a wide variety of other intestinal disorders including Shigella infection, Crohn’s disease, neonatal necrotizing enterocolitis, intestinal obstruction, Hirschsprung’s disease, and colonic carcinoma.9 Intestinal ischemia can result in histologic changes similar to those observed in severe C. difficile colitis, although well-defined characteristic patchy pseudomembranes usually are not seen. Severe systemic insults including shock, advanced renal failure, spinal fracture, extensive burns, heavy metal poisoning, and hemolytic-uremic syndrome also have been associated with pseudomembranous enterocolitis. A potential common etiologic factor shared by many of these disorders is hypoperfusion of the intestinal mucosa leading to ischemic necrosis and ulceration.
Other infectious agents have been implicated as causes of pseudomembranous colitis in the absence of C. difficile infection, most notably S. aureus.2,3,6 Before C. difficile was identified as the most common cause of pseudomembranous colitis, S. aureus often was identified in stool cultures of patients with postoperative pseudomembranous enterocolitis, and oral vancomycin proved to be an effective therapy.6 In retrospect, it is difficult to ascertain whether the efficacy of vancomycin reflected its activity against staphylococcal infection or against unrecognized infection with C. difficile. Currently, 2% to 3% of patients with antibiotic-associated pseudomembranous colitis have negative tests for C. difficile and its toxins in stool specimens despite use of the most sensitive available assays; it remains unclear what proportion of these patients have false-negative tests for C. difficile or instead are infected with an as-yet-unidentified infectious agent.
CLOSTRIDIUM DIFFICILE-ASSOCIATED DIARRHEA AND COLITIS
C. difficile, an anaerobic, Gram-positive, spore-forming, toxigenic bacillus, was first isolated in 1935 from the fecal flora of healthy neonates.19 The organism passed into obscurity until 1978, when the association between toxins released by this organism and antibiotic-induced pseudomembranous colitis first was reported.20,21 Since that time, the incidence of C. difficile infection has increased dramatically, and the organism is now recognized as the primary cause of nosocomial infectious diarrhea in developed countries.22–27
The reported incidence of C. difficile-associated diarrhea has risen steadily over the past decade. For example, in the United States, the rate of nosocomial C. difficile infection per 100,000 population rose from 31 in 1996 to 61 in 200028 to 84 in 2005 (personal communication, L. Clifford McDonald, Centers for Disease Control and Prevention, Atlanta, Ga.). Similarly, C. difficile as a primary or contributing cause of death in the United Kingdom rose from 1000 cases in 2000 to 6500 cases in 2006.29 The reported incidence of community-acquired C. difficile AAD is substantially lower, ranging from 8 to 12 cases per 100,000 person-years.30,31
C. difficile infection also appears to be accompanied by greater morbidity and mortality in the past decade, owing in part to the emergence of increasingly virulent strains. One such strain, designated NAP1/BI, has a mutation in a bacterial gene called txcD, which allows the organism to produce more toxins. The mutant strain also produces a third toxin (called binary toxin) and is resistant to fluoroquinolones, making it more prevalent in patients receiving this class of antibiotics.32
PATHOGENESIS AND EPIDEMIOLOGY
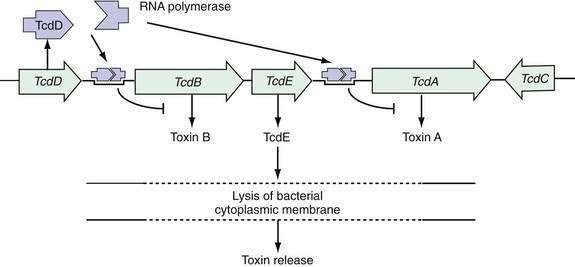
The pathogenesis of C. difficile infection requires the following conditions: alteration of the normal colonic microflora by antibiotics or, rarely, chemotherapeutic agents; oral ingestion of C. difficile or its spores with resultant colonization of the large intestine; release of toxins A and B into the colonic lumen; binding and internalization of toxins by colonocytes; and subsequent colonic damage (colitis). Several host factors, particularly the immune response to C. difficile toxins, determine whether a patient remains an asymptomatic carrier or develops colitis (Fig. 108-1).33
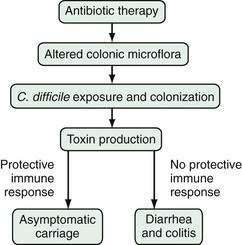
Figure 108-1. Pathogenesis of Clostridium difficile-associated diarrhea and colitis.
(From Kyne L, Farrell R, Kelly CP. Clostridium difficile. Gastroenterol Clin North Am 2001; 30:753.)
Alteration of the Colonic Microflora
Alteration of the resident colonic microflora, as a consequence of antimicrobial therapy, occurs in nearly all patients who develop C. difficile infection. The protective barrier provided by the intestinal microflora often is referred to as colonization resistance; its impairment by antibiotics and subsequent infection with C. difficile can be demonstrated in animal models.34,35 C. difficile also can colonize the intestines of germ-free mice but is eliminated after these animals are inoculated with fecal flora from normal mice, clearly confirming the importance of the normal flora in preventing colonization.34 Colonization resistance can be demonstrated in vitro by growth inhibition of C. difficile by fecal extracts from healthy adults but not by sterilized extracts.36
Human neonates and infants have poor colonization resistance because they have not yet developed a stable complex colonic microflora.37,38 Colonization rates with C. difficile of 25% to 80% have been reported in healthy infants and children up to 24 months of age, who, despite large concentrations of toxins in the feces, rarely develop C. difficile-associated diarrhea.39 Absence of toxin receptor expression on the immature colonic epithelium has been suggested as a mechanism to explain the carrier state in infants and children.40
Almost all antimicrobial agents can predispose to C. difficile diarrhea and colitis, including vancomycin and metronidazole,41,42 but the precise risks associated with individual agents are difficult to establish.43–45 The frequency of association of specific antibiotics is related to their frequency of use, their route of administration, and their effect on the colonic microflora.43,45 Antibiotics commonly associated with C. difficile infection and diarrhea include clindamycin, cephalosporins, ampicillin, amoxicillin, and (more recently) the fluoroquinolones (Table 108-2).32,44,46–48 Cancer chemotherapy agents that possess antibacterial properties and bowel preparation regimens (e.g., before colonoscopy or colonic surgery) rarely can result in sufficient disturbance of the intestinal microflora to allow subsequent colonization with C. difficile.49 One report indicated that healthy adults without known exposure to antibiotics or other modifiers of the colonic microflora also occasionally develop C. diffile colitis.30
Table 108-2 Antimicrobial Agents That Predispose to Clostridium difficile-Associated Diarrhea and Colitis
Adapted from Kelly CP, LaMont JT. Treatment of Clostridium difficile diarrhea and colitis. In: Wolfe MM, editor. Gastrointestinal Pharmacotherapy. Philadelphia: WB Saunders; 1993. p 199; and Bouza E, Burillo A, Muñoz P. Antimicrobial therapy of Clostridium difficile–associated diarrhea. Med Clin North Am 2006; 90:1141-63.
Hospital Epidemiology of Clostridium difficile Infection
Chronic intestinal carriage rates of C. difficile in healthy adults are low (0% to 3% in American and European populations) and might represent intestinal transit without true colonization.27,50,51 It also is unclear whether carriage is a temporary or permanent state.42
In contrast, hospital inpatients treated with antibiotics have reported colonization rates of 10% to 21%.23,27,52–55 The hospital environment is a major source of C. difficile infection; not only infected stool, but also environmental surfaces, soiled bedding, bedpans, toilet seats, and the hands and stethoscopes of health care workers are potential sources of nosocomial C. difficile infection.23,54 In one study, C. difficile was acquired on average in 3.2 days by patients who shared a room with an infected roommate compared with 18.9 days by patients in single rooms or with roommates whose stool cultures were culture negative for C. difficile.52 In the same study, C. difficile was cultured from the hands of 59% of hospital workers caring for patients with positive C. difficile cultures. The organism also was frequently cultured from bedrails, toilets, floors, call buttons, and other surfaces in the rooms of infected patients.
Asymptomatic carriers rarely develop C. difficile-associated diarrhea, but they serve as an important reservoir of nosocomial infection.55,56 In one study, 29% of environmental cultures taken from the hospital rooms of symptom-free carriers were positive for C. difficile, compared with only 8% of cultures from rooms of patients who were culture-negative for C. difficile.52
In antibiotic-treated animals, the infective dose of toxigenic C. difficile may be as low as two organisms.21 If human susceptibility is similar, control of C. difficile infection in hospitals will continue to be a major challenge because up to 109 organisms per gram are excreted in liquid feces.57,58 Highly resistant spores of C. difficile can persist for many months in the hospital environment and can result in infection if ingested by a susceptible host.57
Although it is not possible to eradicate C. difficile and its spores from the hospital environment, certain control measures have been recommended to reduce the prevalence of C. difficile-associated diarrhea (Table 108-3).59,60 Infected inpatients should be bedded in private rooms whenever possible to reduce patient-to-patient spread. Strict enteric precautions and regular hand washing after patient contact should be observed, because C. difficile can be cultured from the hands of health care workers after as many as 60% of contacts with infected patients.52 The use of alcohol-based hand gels is not as effective as washing with soap and running water in removing C. difficile spores.60 A controlled trial of using vinyl disposable gloves during patient contact also reduced the transmission of infection.61 After discharge of infected patients, surface environmental disinfection is best performed using a cleaning agent (e.g., hypochlorite solution) containing at least 5000 ppm available chlorine.60
Table 108-3 Practice Guidelines for the Prevention of Clostridium difficile Diarrhea
From Fekety R. Guidelines for the diagnosis and management of Clostridium difficile-associated diarrhea and colitis. American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol 1997; 92:739.
Hospital outbreaks of C. difficile-associated diarrhea are common and likely result from the close approximation of susceptible persons (elderly and infirm patients) who are taking antibiotics and who are then exposed to the pathogen either in the hospital environment or through person-to-person spread. Some recent reports suggest that hospital and community outbreaks are related to the emergence of mutated hypervirulent strains, which are highly toxigenic and resistant to numerous antibiotics including fluoroquinolines.32,48 Prophylactic therapy with metronidazole or vancomycin is not effective as a disease control measure,62 and C. difficile diarrhea is prevented best by avoiding the unnecessary use of broad-spectrum antibiotics, especially in hospitalized patients, and by careful attention to hand hygiene.
In the future, increasing individual and herd immunity to C. difficile by vaccination or by passive immunotherapy may become a viable approach to reducing the prevalence of this common nosocomial disease.59,63–65 Prophylactic measures such as the use of bacterial and yeast probiotics or toxin binders in high-risk hospital patients also warrant further investigation.66–69
Clostridium difficile Toxins
Pathogenic strains of C. difficile produce two structurally similar protein exotoxins, toxin A and toxin B, which are the only known virulence factors. The genes encoding toxin A and toxin B reside in a 19.6-kb chromosomal region, the C. difficile pathogenicity locus, which contains the genes encoding toxin A (tcdA) and B (tcdB) as well as two putative regulatory genes (tcdC and tcdD, also called tcdR) (Fig. 108-2).70–75 The tcdD gene product appears to up-regulate toxin transcription by complexing with RNA polymerase that binds to the toxin promoter regions. The tcdC gene is transcribed in the opposite direction to tcdA, tcdB, and tcdD, and its gene product appears to decrease toxin production.70–75 Mutations of tcdC are associated with increased virulence that may be related to increased toxin production.76,77 The fifth gene of the pathogenicity locus, tcdE, encodes a protein of undetermined function, although some data support the theory that it acts to lyse bacterial cell walls, thereby releasing toxins A and B.78
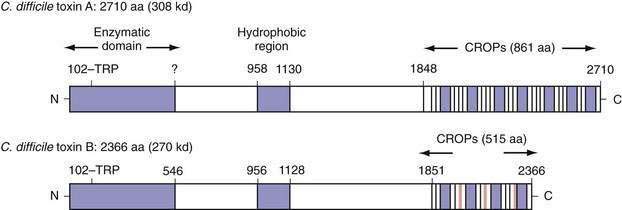
Toxins A (308 kd) and B (220 kd) are members of the large clostridial cytotoxin family, share a number of structural features, and are 49% identical at the amino acid level.79–81 Both toxins carry an N-terminal enzymatic domain that mediates their toxic effects on mammalian cells, a central hydrophobic region that might act as a transmembrane domain to facilitate entry into the cytoplasm, and a C-terminal domain consisting of a series of repeated sequences that mediate toxin binding (Fig. 108-3). Both toxins function as uridine diphosphate glucose (UDP-glucose) hydrolases and glucosyltransferases, a requirement for their cellular toxic effects.
Following internalization into the host cell cytoplasm, the toxins catalyze the transfer and covalent attachment of a glucose residue from UDP-glucose to a conserved threonine amino acid on small (20 to 25 kd) guanosine triphosphate–binding rho proteins. Rho proteins are part of the Ras superfamily, are expressed in all eukaryotic cells, and act as intracellular signaling molecules to regulate cytoskeletal organization and gene expression. The rho proteins, RhoA, Cdc42, and Rac, are substrates for both toxins A and B, whereas Rap is a substrate for toxin A only.74,82–84 Glucosylation of rho proteins by the toxins leads to disordered cell signaling, disorganization of the cytoskeleton, disruption of protein synthesis, cell rounding, and cell death.74,85 Both toxins also activate nuclear factor κB (NF-κB), mitogen-activated protein (MAP) kinases, and cyclooxygenase (COX)-2 in target cells, leading to the release of proinflammatory cytokines including interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-8.85,86 These cellular proinflammatory effects contribute to the marked intestinal inflammatory response evident in C. difficile-associated diarrhea and pseudomembranous colitis.
Toxin A initially was thought to be the only enterotoxin based on studies in animals85,87,88 whereas toxin B, an extremely potent cytotoxin, had minimal enterotoxic activity in animals. This suggested that toxin B did not contribute to diarrhea and colitis in humans,87,89–91 although studies on human colon show that, in fact, toxin B is 10 times more potent than toxin A in inducing in vitro colon injury.92,93 Furthermore, toxin A−/toxin B+ strains of C. difficile have been isolated from patients with diarrhea and pseudomembranous colitis,94–97 confirming that toxin B is a major virulence factor in human disease.
The Immune Response to Clostridium difficile
Serum IgG and IgA antibodies against C. difficile toxins are found in more than 50% of healthy children and adults.98–102 Mucosal IgA antitoxin antibodies also are detectable in more than 50% of human colonic secretions and might inhibit receptor binding of toxin A.100,102 Immunization against C. difficile toxins protects animals from C. difficile colitis but does not protect against colonization—a situation that may be similar to the asymptomatic carrier state in humans.53,103
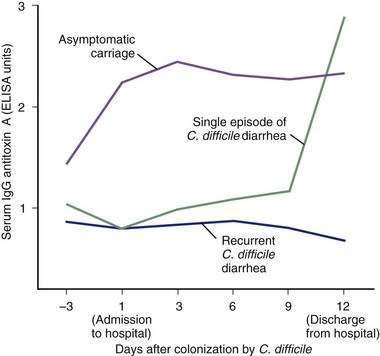
High serum IgG antitoxin A antibody concentrations are associated with protection against C. difficile-associated diarrhea and colitis.63–65 Recurrent C. difficile diarrhea has been associated with low serum antitoxin antibody concentrations in children and in adults.98,102,104,105 In one study, adult inpatients with C. difficile diarrhea and a low level of serum IgG against toxin A had a 48-fold greater risk of recurrent disease after successful treatment compared with patients who had high antitoxin concentrations (Fig. 108-4).106 High serum IgG antitoxin A concentrations also have been identified in asymptomatic carriers of toxigenic C. difficile.
In a prospective study of nosocomially acquired C. difficile, the 51% of infected patients who were asymptomatic carriers had serum IgG antitoxin A concentrations that were three times higher than those in patients with diarrhea (see Fig. 108-4).53 The immune response to toxin B has not yet been clearly correlated with specific clinical outcomes. Nonetheless, toxin B is both pathogenic and immunogenic in humans, and antibody responses to toxin B might contribute to immune protection against C. difficile-associated diarrhea.63–65
Other Risk Factors for Clostridium difficile Infection
In addition to antimicrobial therapy, increasing age and increased comorbidity are important risk factors for C. difficile infection.107 In England and Wales, 75% of reported C. difficile infections between 1992 and 1996 occurred in patients aged 65 years or older.50 Data from the United States also demonstrate that age is an independent risk factor for this infection.108,109 The elderly particularly are predisposed to infection with C. difficile because of increased nosocomial exposure and frequent courses of antibiotics and a reduced ability of their polymorphonuclear leukocytes to phagocytose these organisms.110 In one study of antibiotic recipients, patients with severe underlying disease at the time of hospital admission were eight times more likely to develop C. difficile infection compared with patients who were less severely ill.53 Other reported risk factors for C. difficile infection include the use of a nasogastric tube, gastrointestinal procedures, intensive care unit stay, and length of hospital stay.43 The strengths of the associations of these risk factors with C. difficile vary from study to study. These factors often are markers of disease severity, older age, or both, and the significance of their association with C. difficile can decline or be lost after controlling for these confounding variables.53,107,109
The role of acid suppression in C. difficile infection is unclear. In theory, reduction of gastric acid could allow a greater number of viable spores to reach the colon; however, because spores are generally acid resistant, the importance of this effect is unclear. Some studies have shown an increased risk of C. difficile infection with acid suppression,109 but others have not confirmed this after adjusting for confounding variables.32,111
Patients undergoing cytotoxic chemotherapy for malignancy are at risk for C. difficile-associated diarrhea infection because of frequent antibiotic use, nosocomial exposure to C. difficile, and severe comorbidity.111,112 Even in the absence of antibiotic use, antineoplastic chemotherapy, especially with methotrexate, predisposes to C. difficile infection, reflecting the ability of these drugs to alter the colonic microflora and reduce C. difficile colonization resistance.49 C. difficile-associated diarrhea also has been reported in patients undergoing immunosuppressive therapy in the setting of solid organ or bone marrow transplantation.113,114
Patients with human immunodeficiency virus (HIV) infection also are at risk for C. difficile-associated diarrhea because of multiple risk factors, including frequent prophylactic and therapeutic antibiotic use, hospitalization, and immunocompromise.115–118 C. difficile colitis behaves the same in patients with acquired immunodeficiency syndrome (AIDS) as it does in control groups118 and testing for C. difficile should be a routine part of the diagnostic evaluation in patients with HIV infection, diarrhea, and a history of current or recent antibiotic treatment.
Patients with inflammatory bowel disease (IBD) are at increased risk for C. difficile infection.119–122 Infection with a broad range of enteric pathogens including C. difficile, Campylobacter, and Salmonella species can precipitate or mimic disease relapse in IBD. C. difficile is the most commonly identified specific pathogen in IBD patients in North America and Europe, however, and is present in as many as 5% to 19% of patients with relapse in some case series.119–122 Some IBD patients with C. difficile infection do not have a history of recent antibiotic use, suggesting that IBD itself might impair colonization resistance. The possibility of enteric infection with C. difficile or other pathogens should be considered in patients with an increase in IBD disease activity. If C. difficile infection is identified, antimicrobial therapy with metronidazole or vancomycin is indicated in combination with other IBD therapies. In one study, IBD inpatients with coexisting C. difficile infection were more likely to have severe disease and to require colectomy than similar patients without coexisting infection.122
CLINICAL FEATURES
Clinical manifestations of C. difficile infection range from asymptomatic carriage to mild or moderate diarrhea to life-threatening pseudomembranous colitis. Asymptomatic carriage of C. difficile is common in hospitalized patients. Several large epidemiologic studies indicate that 10% to 21% of hospital inpatients receiving antibiotics in high-risk units are carriers.28,52,53,55,123 Although most of the C. difficile isolates from carriers are toxin producing, the carriers do not develop symptomatic disease, perhaps as a result of protective immunity.28,55,104
In patients who develop diarrhea with C. difficile, symptoms usually begin soon after colonization. The incubation period is usually less than a week, with a median time of onset of approximately two days.28,52,53,124 Colonization can occur during or after antibiotic treatment. Olson and associates26 reported that 96% of patients with symptomatic C. difficile infection had received antibiotics within 14 days of the onset of diarrhea and that all had received an antibiotic within the previous three months.
C. difficile diarrhea typically is associated with the frequent passage of loose or watery bowel movements. Mucus or occult blood may be present, but melena or hematochezia is uncommon and, if present, should suggest the presence of IBD, colon cancer, or another source of bleeding. Some patients present with fever, leukocytosis, and cramping abdominal pain.125 Because C. difficile is not an invasive pathogen, extraintestinal manifestations of C. difficile infection such as septic arthritis, bacteremia, or splenic abscess are extremely rare.126–129 An oligoarticular, asymmetrical, nondeforming large-joint arthropathy, similar to that seen in other infectious colitides, sometimes is seen.130
Patients with more severe disease can develop a colonic ileus or toxic dilatation and present with minimal or even no diarrhea.125 In the absence of diarrhea, the only clues to the diagnosis may be high fever, moderate or marked (e.g., leukemoid) polymorphonuclear leukocytosis, lower or diffuse abdominal pain, tenderness, and distention.
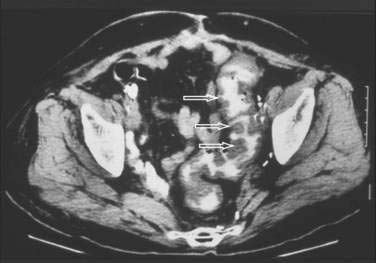
Abdominal plain films might reveal a dilated colon (more than 7 cm in its greatest diameter), toxic megacolon, or small bowel ileus with air-fluid levels mimicking intestinal obstruction or ischemia. In such cases, a computed tomographic scan of the abdomen may reveal nonspecific features common to ischemic, infectious, and inflammatory colitides (Fig. 108-5).131 Radiologic features of pseudomembranous colitis include mucosal edema, a thickened colonic wall, pancolitis, and pericolonic inflammation with or without ascites, usually without any small bowel involvement other than ileus.132 Flexible sigmoidoscopy or colonoscopy is sometimes indicated to identify pseudomembranous colitis (see later) when the diagnosis remains unclear after initial evaluation.
Complications of severe C. difficile colitis include dehydration, hypoalbuminemia, electrolyte disturbances, renal failure, hypotension, toxic megacolon, systemic inflammatory response syndrome, bowel perforation, and death.59,125
DIAGNOSIS
The diagnosis of C. difficile diarrhea or colitis is based on a history of recent or current antimicrobial therapy, development of diarrhea or other evidence of acute colitis, and demonstration of infection by toxigenic C. difficile, usually by detection of toxin A or B, or both, in a stool sample.48,59
Tests for Clostridium difficile Infection
The diagnosis of C. difficile diarrhea should be considered in any patient with acute diarrhea who has received antibiotics within the previous three months and especially in anyone whose diarrhea began 72 hours or more after hospitalization. Approximately 40% of patients with C. difficile diarrhea at tertiary referral centers are symptomatic on admission to the hospital, and most have had a recent prior hospitalization.52,53,133 Although a history of recent antibiotic use is common, it is not a requirement for diagnosis.30
Testing of solid or formed stools for C. difficile toxin is not recommended because only patients with diarrhea require treatment.52,53,59,62,123 Treatment of asymptomatic carriers with antimicrobial agents against C. difficile is not recommended because it might only prolong the carrier state beyond the usual two to six weeks.62 Follow-up stool testing is not indicated in an asymptomatic patient, even in patients discharged to chronic care facilities, because asymptomatic carriage is already highly prevalent in these facilities. Stool carriage of C. difficile can persist for up to six weeks after cessation of symptoms and does not require therapy.134 Because asymptomatic carriers can act as hidden reservoirs for C. difficile infection, especially in hospitals and nursing homes, universal precautions should be followed for all patients to reduce the likelihood of patient-to-patient spread of nosocomial infectious disease.
If C. difficile diarrhea is suspected, a freshly passed stool sample should be submitted immediately to the laboratory in a clean watertight container to be tested for the presence of fecal toxin A or B. Anaerobic storage or the use of transport media is not necessary, but storage at ambient temperatures can result in denaturation of fecal toxin; samples should therefore be tested immediately or refrigerated or frozen, pending later testing.54,135
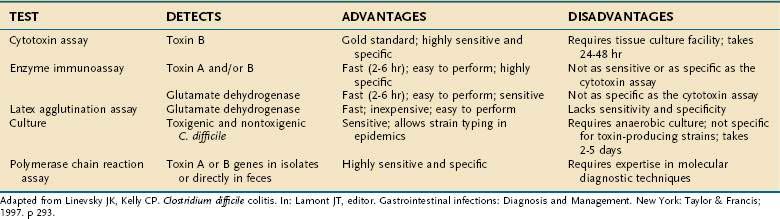
A variety of laboratory tests are available to diagnose infection with toxigenic C. difficile, but enzyme immunoassays (EIAs) to detect toxin antigens in stool currently are used most commonly (Table 108-4). These tests have the advantages of being relatively inexpensive, quick (2 to 12 hours), and highly specific, although their relatively low sensitivity (about 90%) leads to some false-negative results. The tissue culture cytotoxicity assay is more sensitive and has greater diagnostic accuracy, but it is more costly and time consuming (24 to 72 hours).
Tissue Culture Cytotoxicity Assay
The current gold standard test to identify C. difficile toxins in stool is the tissue culture cytotoxicity assay.136–138 Toxins A and B inactivate rho proteins, causing a disintegration of the actin cytoskeleton and a characteristic rounding of cells in tissue culture. A suspension of diarrheal stool diluted in phosphate-buffered saline is centrifuged, filtered, and then added to a monolayer of cultured cells, such as fibroblasts or Chinese hamster ovary cells. The monolayer is examined at 24 hours and again at 48 hours for cell rounding. The specificity of a positive result is established by preincubating an aliquot of the patient’s stool specimen with specific neutralizing antitoxin antibody; stool that gives a positive result when tested alone and becomes negative after incubation with antitoxin antibody is a true positive. False-positive results can be seen with pathogens other than C. difficile and with non–C. difficile enterotoxins.
The cytotoxicity assay is highly sensitive (67% to 100%) and specific (85% to 100%) if it is performed under optimal conditions; however, sensitivity may be reduced by inactivation of toxins during transport and storage, by the age and type of cell line used, and by dilution of the stool.54,135,139–141 Therefore, a negative cytotoxicity test does not completely exclude C. difficile as the cause of diarrhea. Disadvantages of the cytotoxicity assay are that it is relatively expensive, requires technical expertise and a cell culture facility, and takes 24 to 72 hours for the test to be completed.
Enzyme-Linked Immunoassays
Commercially available EIAs are widely used to detect toxin A or toxins A and B of C. difficile in stool specimens.136–138142 Toxin is detected by its interaction with either a monoclonal antibody or polyclonal antiserum that specifically recognizes toxin epitopes. EIAs are easier to perform than is the cytotoxicity test, are relatively inexpensive, and are fast; results may be available within two to six hours. Although they have high specificity (75% to 100%) for toxins, their main drawback is that they are less sensitive than the cytotoxicity test (63% to 99%). In addition, some EIA kits detect only toxin A, in which case diarrhea due to a toxin A−/B+ strain of C. difficile has a falsely negative test result.96 For this reason, commercial kits that detect both toxins A and B have a slight advantage over those that detect toxin A alone.142
Distinct from tests for C. difficile toxins A and B, immunoassays also have been used to detect C. difficile common antigen (glutamate dehydrogenase, GDH) in stool. The initial latex agglutination assay method lacked diagnostic accuracy and is not recommended. More recent EIAs for fecal GDH have shown improved sensitivity (85% to 95%) and specificity (89% to 99%), are rapid, and are not expensive. These changes have led to the use of EIA for GDH as an initial screening test, with confirmation of positive results using another test such as the tissue culture cytotoxicity assay.143
Clostridium difficile Culture
Culture of stool for C. difficile is sensitive (89% to 100%) but is not specific for toxin-producing strains of the bacterium. Therefore, cultured isolates then must be tested in vitro for toxin production to improve test specificity, but this is costly and time consuming. One advantage of culturing C. difficile is that it permits strain typing of individual isolates, and therefore it is useful in tracking hospital outbreaks for epidemiologic studies.56,57,75–77
Polymerase Chain Reaction for Detection of Toxin Genes
Polymerase chain reaction (PCR), using specific primers based on the genes for toxins A and B, can detect toxigenic C. difficile in clinical isolates.144–152 PCR is highly sensitive (100%) and specific (96.7% to 100%), and PCR methods for detecting toxin genes directly in feces have been described.147–152 Using a nested PCR assay on stool samples, Alonso and colleagues152 reported 99% concordance with the cytotoxicity assay and a sensitivity and specificity of 96.3% and 100%, respectively. Application of PCR methods in the clinical laboratory might help to overcome some of the limitations of the currently used EIA and tissue culture assays.
Sigmoidoscopy and Colonoscopy
Neither sigmoidoscopy nor colonoscopy is required for diagnosis in most patients with C. difficile diarrhea.59 Endoscopy is helpful, however, when the diagnosis is in doubt or when disease severity demands rapid diagnosis. Sigmoidoscopy may be normal in patients with mild diarrhea or might demonstrate nonspecific colitis in moderate cases. The finding of colonic pseudomembranes in a patient with AAD is virtually pathognomonic for C. difficile colitis (Fig. 108-6).152,153 Pseudomembranes appear as yellow, gray, or white plaques 2 to 5 mm in diameter, and in some areas they can coalesce to cover large portions of the mucosal surface. Sigmoidoscopy might not be sufficient to identify all patients with pseudomembranous colitis, because approximately 15% to 20% only have pseudomembranes in the more proximal areas of the colon.154 Other nonspecific endoscopic findings include erythema, edema, friability, small ulcerations, and erosions.
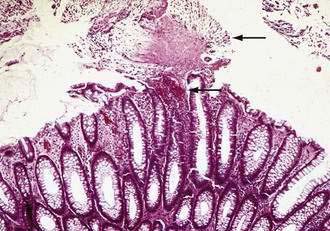
In mild disease, colonic mucosal biopsies may be normal or demonstrate only mild and nonspecific acute inflammatory changes with neutrophil infiltration. In more severe cases, colonic histology shows focal ulceration of the mucosa associated with the eruption of inflammatory cells and necrotic debris that covers the area of ulceration, the so-called summit or volcano lesion (Fig. 108-7).152,155
Miscellaneous Laboratory Tests
Many patients with acute C. difficile diarrhea develop a polymorphonuclear leukocytosis with a left shift. Occasionally a leukemoid reaction with an extremely high white blood cell count of more than 50,000 cells/mm3 is seen. A peripheral white blood cell count of greater than 15,000 cells/mm3 is associated with negative clinical outcomes and a count of greater than 25,000 cells/mm3 is associated with an increased mortality risk.156 Decreased serum albumin and elevated creatinine levels also are markers of severe disease. Patients with protein-losing colopathy and severe hypoalbuminemia can also develop peripheral edema, ascites, or anasarca.
TREATMENT
Mild to Moderately Severe Clostridium difficile Diarrhea and Colitis
The first step in the management of C. difficile diarrhea and colitis is to discontinue the precipitating antibiotics if possible (Table 108-5).59 Diarrhea resolves in approximately 15% to 25% of patients without specific anti-C. difficile therapy.26,157 Conservative therapy alone, however, is not appropriate in patients who are severely ill or who have multiple other active medical problems. In patients with active infections elsewhere (e.g., pneumonia and urinary tract infection) and in whom antibiotic therapy must be continued, the antibiotic regimen should be switched, if possible, to agents with a relatively low likelihood of exacerbating C. difficile diarrhea, for example, parenteral aminoglycosides, trimethoprim, or erythromycin (see Table 108-2).46 Antimotility agents such as diphenoxylate plus atropine (Lomotil), loperamide (Imodium), or narcotics often are avoided because of concern for impaired toxin clearance or precipitation of ileus and toxic dilatation, but the data supporting this practice are limited and contradictory.59,158–159 Treatment of asymptomatic carriers with antimicrobial agents against C. difficile is not recommended because it can prolong the carrier state.62
Table 108-5 Treatment of Clostridium difficile Diarrhea and Colitis
Adapted from Fekety R. Guidelines for the diagnosis and management of Clostridium difficile-associated diarrhea and colitis. American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol 1997; 92:739.
Many antimicrobial agents show activity against C. difficile in vitro,160–166 but resistance to cephalosporins is so widespread that cefoxitin is used in selective media to culture C. difficile.167 Clindamycin resistance is seen in some clinical isolates and has been associated with nosocomial outbreaks.168 There is increasing evidence of fluoroquinolone resistance among nosocomial C. difficile isolates, and the epidemic NAP1/BI strain that has caused several outbreaks shows high-level fluoroquinolone resistance.32,169 Fortunately, resistance to metronidazole is rare, and resistance to vancomycin is essentially nonexistent. In one study of 186 clinical isolates, all were sensitive to both metronidazole and vancomycin, with minimum inhibiting concentrations (MICs) of 0.5 to 4 mg/mL.167 In another series from Spain, 6% of 415 isolates showed intermediate sensitivity to metronidazole (MIC > 16 mg/mL),170 but this partial resistance pattern was not clonal and was not sustained in serial culture. These findings suggest an acquired tolerance rather than genetically determined metronidazole resistance.
Many antimicrobial agents, such as ampicillin or amoxicillin, which have in vitro activity against C. difficile, are common causes of C. difficilea-ssociated diarrhea in clinical practice.161,171 This observation illustrates the fact that in vitro sensitivity testing alone is a poor predictor of therapeutic efficacy in this disease. The preclinical efficacy of therapeutic agents for C. difficile-associated diarrhea has been assessed in clindamycin-exposed Syrian hamsters.66,162,165,172,173 These animals develop a fulminant and fatal cecitis when exposed to toxigenic C. difficile after administration of clindamycin. Historically, this animal model has provided a reliable indication of the effectiveness of therapeutic agents for C. difficile infection and diarrhea.
Specific antibiotic therapy to eradicate C. difficile is required in patients with severe symptoms or in those whose symptoms persist despite discontinuation of antibiotic treatment. The most effective antimicrobials for the treatment of C. difficile diarrhea are metronidazole (250 to 500 mg three or four times a day for 10 to 14 days) and vancomycin (125 to 500 mg four times a day for 10 to 14 days) (Table 108-6).174 Bacitracin, teicoplanin, and fusidic acid also have been used to treat acute infection but have few if any advantages over metronidazole or vancomycin. In a systematic review, none of these alternative antibiotics were superior in terms of response rates.175 The advantages and disadvantages of specific therapeutic agents are discussed in the following sections.
Table 108-6 Comparison of Metronidazole and Vancomycin for the Treatment of Clostridium difficile Diarrhea
| VARIABLE | METRONIDAZOLE | VANCOMYCIN |
|---|---|---|
| Dose | 250-500 mg | 125-500 mg |
| Frequency | Three or four times daily | Four times daily |
| Duration | 10-14 days | 10-14 days |
| Route | Oral or intravenous | Oral only |
| Response rate | 87% | 97% |
| Disadvantages | Systemic side effects; rare resistant strains of C. difficile | Can encourage proliferation of nosocomial VRE |
VRE, vancomycin resistant enterococci.
Adapted from Kelly CP, LaMont JT. Treatment of Clostridium difficile diarrhea and colitis. In: Wolfe MM, editor, Therapy of digestive disorders. Philadelphia: WB Saunders; 2000. p 513; Aslam S, Hamill RJ, Musher DM. Treatment of Clostridium difficile-associated disease: old therapies and new strategies. Lancet Infect Dis 2005; 5:549-57; and Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302-7.
Metronidazole
Metronidazole generally is recommended as the drug of first choice for mild to moderately severe C. difficile diarrhea and colitis.48,59,176 It is inexpensive ($0.50 per 250-mg tablet) and is usually effective. Several clinical studies before 2000 indicated that metronidazole therapy resulted in the resolution of diarrhea and colitis in more than 95% of patients treated.26,160,175 For example, in a prospective, randomized trial of acute C. difficile infection, metronidazole (250 mg four times a day for 10 days) was as effective as vancomycin (500 mg four times daily for 10 days) in terms of response and recurrence rates.157 Studies published between 2004 and 2007, however, report an average failure rate of 19% for metronidazole (range, 7% to 38%) compared with only 4% for vancomycin (range, 3% to 6%).48,177,178 In one trial, subjects with acute C. difficile infection were stratified according to disease severity and then randomized to receive either metronidazole 250 mg or vancomycin 125 mg each given four times per day. In mild disease, both treatments yielded similar response rates (90% and 98%, P = 0.36). In severe disease however, metronidazole was less efficacious (76% versus 97%, P = 0.02).178 Thus oral vancomycin should be used as the first-line agent in severe disease.
Metronidazole, unlike vancomycin, is well absorbed in the upper intestine following oral administration. Fecal concentrations are low or absent in healthy persons or asymptomatic carriers of C. difficile, but higher fecal concentrations are observed in patients with C. difficile colitis because metronidazole is secreted through the inflamed intestinal mucosa.179 Intravenous metronidazole (500 mg four times per day) may be used in patients who cannot tolerate oral medication, because it accumulates in bactericidal levels within the inflamed colon.179
Oral metronidazole therapy usually is well tolerated but can be associated with systemic side effects.179 In one report of more than 600 patients receiving metronidazole for C. difficile diarrhea, only 1% experienced significant side effects.26 Adverse effects include nausea, a metallic taste, a disulfiram-like reaction with alcohol, and a peripheral sensory neuropathy with prolonged therapy. Metronidazole can potentiate the action of warfarin, resulting in prolongation of the prothrombin time.
Enigmatically, metronidazole has been identified as the antibiotic agent responsible for causing some cases of C. difficile diarrhea, demonstrating the importance of reduced colonization resistance in the pathophysiology of C. difficile-associated diarrhea.180–182
Vancomycin
Vancomycin was introduced for treating C. difficile-associated diarrhea and colitis in 1978,183 and its pharmacokinetic properties make it an ideal agent for treating this infection. When given orally, vancomycin is neither absorbed nor metabolized significantly, and as a result, high concentrations in the colonic lumen are achieved. The efficacy of vancomycin in treating C. difficile colitis has been demonstrated in controlled trials.51,157,177,178,183,184 Improvement in diarrhea usually is evident within 72 hours of initiating therapy, and complete resolution of symptoms occurs in most patients (96% overall) by the end of a 10-day treatment course.26,175,177
Fekety and coworkers185 demonstrated that vancomycin at a dose of 125 mg four times a day is as effective as vancomycin 500 mg four times a day. The lower dose is recommended for patients with mild to moderate colitis, and the higher dose is recommended for critically ill patients (i.e., those with ileus, colonic dilatation, or fulminant pseudomembranous colitis). Vancomycin may be administered by mouth, nasogastric tube, or even by enema,26,59 but it should not be given intravenously to treat C. difficile infection because effective colonic luminal concentrations are not obtained following parenteral administration.186,187
Oral vancomycin is not absorbed appreciably, and as a result, systemic side effects are rare. Despite its many advantages, oral vancomycin now is considered a second-line agent for the treatment of mild to moderately severe C. difficile infection because of its higher cost (a 10-day course can cost as much as $800) and concerns regarding the spread of vancomycin-resistant enterococci.176 Vancomycin therapy is recommended, however, for patients with severe infection and for patients who fail to respond to metronidazole, are intolerant of metronidazole, are pregnant, or are younger than 10 years.59,176
Other Antimicrobial Agents
Bacitracin (25,000 units four times daily for seven to 10 days) is less effective than metronidazole or vancomycin for treating C. difficile diarrhea, with an overall response rate of only 80% and a relapse rate of 30%.184,188–190 In randomized therapeutic trials, teicoplanin, 100 mg twice a day for 10 days, was as effective as vancomycin for treating C. difficile diarrhea.191,192 Teicoplanin, however, is relatively expensive and is not available for oral administration in the United States. Fusidic acid has been tested in a limited number of patients but appears to be less effective than metronidazole or vancomycin and is associated with a relapse rate of approximately 28%.192,193 Treatment with colestipol, an ion exchange resin that binds toxins (10 g four times daily), is associated with a low response rate (36%) and is not recommended as primary therapy.51
Severe Pseudomembranous Colitis
Severe or fulminant pseudomembranous colitis occurs in only a minority of patients with C. difficile infection but is associated with a mortality rate of up to 65%.129,194,195 Many patients who develop fulminant C. difficile disease already have substantial comorbid disease and often are critically ill.196,197 Diarrhea may be minimal or absent because of ileus, and patients can present with abdominal pain, peritoneal signs, colonic dilatation, leukocytosis, and a clinical picture of progressive sepsis.88,157,198 Prompt diagnosis and aggressive therapy are necessary to avoid substantial morbidity and mortality.
The first step is to discontinue precipitating antibiotics if possible and start therapy with high-dose oral vancomycin (500 mg four times per day), although there are no data to demonstrate that this higher dose is more effective than the standard dose of 125 mg four times per day.59 Intravenous metronidazole should be given if oral medication is not tolerated well. Intravenous vancomycin is not recommended, for the reasons mentioned earlier. In the presence of ileus, vancomycin (500 mg every six hours) may be administered via nasogastric tube with intermittent clamping of the tube.26 Intracecal infusion of vancomycin has been reported but is not recommended because of the risks associated with placement of a narrow-bore tube over a guidewire at colonoscopy in patients with severe, active colitis.199
For critically ill patients, a combination of antibiotics administered by various routes may be indicated. Six of eight patients with severe ileus were treated successfully using a combination of vancomycin administered by nasogastric tube, intravenous metronidazole, and vancomycin-retention enemas (vancomycin 500 mg in 100 mL of normal saline administered every six hours via a Foley catheter inserted into the rectum). Patients treated with this regimen responded within 5 to 17 days.26
Passive immunization with pooled human immunoglobulin has been used empirically in patients with severe colitis who were not responsive to metronidazole or vancomycin. As discussed earlier, patients with severe or prolonged C. difficile diarrhea have low serum and fecal concentrations of antibody against C. difficile toxins.53,98,102,104–106 Intravenous infusion of normal pooled human immunoglobulin (400 mg/kg body weight) increases serum IgG antitoxin concentrations and has been used to treat patients with severe C. difficile colitis, although its efficacy has not been tested in controlled trials.105,200–202
Emergency surgery sometimes is required in patients with severe colitis not responding to medical therapy and in whom bowel perforation is impending or has already occurred.132,195,197,198,202 A surgical consultation should be sought immediately in patients with fulminant disease. The operation of choice usually is a subtotal colectomy with temporary ileostomy. Surgical intervention in this setting is associated with a high perioperative mortality rate, making the decision to operate difficult. Grundfest-Bronitowski and associates197 reported an overall mortality rate of 42% in a series of patients undergoing surgery for fulminant, severe C. difficile infection. In another series of five patients with toxic megacolon, subtotal colectomy and ileostomy were successful in only one patient.203
Recurrent Clostridium difficile Diarrhea
One of the most difficult clinical problems in treating patients with C. difficile infection is the high incidence of recurrences.59,204,205 Multiple episodes of recurrent C. difficile-associated diarrhea are not uncommon, and some patients have experienced more than 10 bouts of recurrence. Approximately 20% of patients successfully treated with vancomycin or metronidazole relapse after completing their initial antibiotic therapy.69,106,175,205,206
The clinical features of recurrence are similar to the initial attack, with watery diarrhea, cramping abdominal pain, or fever occurring 2 to 14 days after discontinuing therapy. Late recurrences are less common but can occur more than two months after stopping antibiotic treatment. The diagnosis of recurrent C. difficile-associated diarrhea is best confirmed by stool toxin assay whenever possible or in certain instances by colonoscopy and biopsy (see later). In patients with typical symptoms of recurrence, therapy can be reinstituted while awaiting stool assay results (Table 108-7). Prompt therapy is especially important in patients whose initial attack of C. difficile diarrhea was severe, because they are more likely to suffer from severe and recurrent disease, possibly because of their inadequate immune response to C. difficile toxins.65,106
Table 108-7 Approach to Management of Recurrent Clostridium difficile Colitis
* Because cholestyramine binds vancomycin, oral doses of these two agents must be separated by two to three hours, making this regimen difficult to implement.
Adapted from Linevsky JK, Kelly CP. Clostridium difficile colitis. In: Lamont JT, editor. Gastrointestinal Infections: Diagnosis and management. New York: Taylor & Francis; 1997. p 293.
Some patients with persistent symptoms following successful therapy of C. difficile infection develop diarrhea as a result of postinfection irritable bowel syndrome.207 Frequent watery diarrhea and cramping lower abdominal pain may be partially responsive to antibiotic therapy. Patients with post-C. difficile irritable bowel syndrome have normal colonoscopy and biopsy, and their stools are negative for toxin. Other diarrheal conditions that require differentiation from recurrent C. difficile infection include IBD, microscopic colitis, celiac disease, and food (e.g., lactose) intolerance.
Bacteriologic typing studies demonstrate that symptomatic recurrence can result from reinfection with either the same strain that caused the initial episode or a different strain of C. difficile.208,209 Resistance to metronidazole or to vancomycin is seldom if ever an important factor in recurrence. For example, Bartlett and colleagues were unable to demonstrate in vitro vancomycin resistance in 23 isolates of C. difficile from relapsing patients.210 In some patients, C. difficile can be cultured from the stools during successful vancomycin therapy, and these patients may be more likely to relapse than those in whom eradication of the pathogen occurs during therapy208; however, C. difficile can also be cultured from the stools during and after successful antibiotic treatment in patients who do not relapse.134
Culture positivity during symptomatic improvement might reflect the persistence of antibiotic-resistant spores. In one study, 18 of 22 patients with recurrence were noted to have colonic diverticula, leading to the speculation that spores might survive in diverticula where they escape the normal cleansing action of diarrhea and might not be exposed to the high luminal concentration of antibiotics204; however, reinfection by bacterial spores through the usual fecal-oral route is a more likely mechanism of recurrence.208,209 In a study of 569 patients who had a positive C. difficile assay, 135 of whom had diverticulosis and 434 who did not, there was a non–statistically significant trend to a higher rate of recurrence in those with diverticulosis (15.6% vs. 12.0%).211
Conservative Therapy
In a report of 20 patients with clindamycin-associated pseudomembranous colitis, published before the discovery of vancomycin as effective therapy, all patients eventually recovered when clindamycin was stopped.212 An important advantage to this form of management is that recurrence of diarrhea or colitis does not occur, probably because stopping all antimicrobial agents allows restoration of the colonic microflora and C. difficile colonization resistance. Many patients with mild symptoms of recurrence can be managed conservatively without specific antibiotic treatment, thereby avoiding subsequent recurrences; this approach might not be appropriate for elderly or infirm patients and is not advised for those with moderate or severe symptoms.
Repeat Treatment with Vancomycin or Metronidazole
Patients with recurrence typically are treated with a second course of the same antibiotic used to treat the initial attack, but treatment is usually for 14 days; this has a success rate of about 40%. Patients with one recurrence have a substantial risk of further recurrences, and in two independent studies, patients with one or more previous recurrences had a subsequent recurrence rate of greater than 50% following standard therapy with metronidazole or vancomycin.69,106
Prolonged or Tapering and Pulsed Vancomycin Therapy
Tedesco and colleagues204 treated 22 patients who had multiple recurrences of C. difficile colitis using tapering doses of vancomycin for a three-week period, followed by every-other-day therapy for one week and every third day for an additional week. All patients responded symptomatically and remained well during a mean follow-up period of six months.
Although data from randomized, controlled trials are not available, one subsequent study that examined various physician-selected antibiotic regimens to treat recurrent C. difficile infection found that regimens incorporating prolonged or pulsed-dose oral vancomycin were the most effective.213 Overall, 73 of 163 patients (45%) treated for recurrent C. difficile infection had a subsequent recurrence. Of all the regimens used, only those incorporating prolonged-dose vancomycin (9 of 29 recurred [31%], P = 0.01) or pulsed-dose vancomycin (one of seven recurred [14%], P = 0.02) showed significantly lower recurrence rates. The mechanism whereby this treatment approach is effective is unknown and might simply reflect prolonged treatment.
Toxin production by C. difficile does not occur during the early exponential growth phase of the bacterium but rather in the subsequent stationary phase.72,75 Hence, after active C. difficile toxin-induced diarrhea and colitis have been controlled by treatment with vancomycin, a period of 24 to 72 hours is needed for the bacteria to reinitiate production and release of toxin. Thus, pulsed dosing might prevent toxin production and release while also facilitating restoration of resistance to colonization.
Binding Resins
Binding resins, which bind to toxins in the bowel lumen, have been proposed as a possible alternative to antimicrobial therapy. Clinical studies have been performed using colestipol, cholestyramine, and tolevamer. For colestipol, the symptomatic response in patients with acute C. difficile colitis was a disappointing 36%, compared with a placebo response rate of 22%.51,175 Cholestyramine therapy yielded a somewhat better overall response rate of 68%,51,175,214 but this still compares poorly with response rates of more than 95% with vancomycin or metronidazole. Therefore, binding resins are not used as primary therapy for C. difficile colitis but may be beneficial in treating recurrence as an adjunctive agent (see below).
Tedesco treated 11 patients who had relapsing C. difficile colitis with tapering doses of vancomycin plus colestipol 5 g every 12 hours.215 Because anion-exchange resins bind vancomycin and other drugs, they must be taken at least two or three hours apart from the vancomycin, making such combination therapy cumbersome.
Tolevamer is a soluble anionic polymer specifically developed to bind C. difficile toxins. In preclinical studies, tolevamer strongly inhibited the cytotoxicity and enterotoxicity of C. difficile toxins and was superior to metronidazole in protecting hamsters from death caused by C. difficile cecitis.66 In a phase II human clinical trial, results with tolevamer were similar to those of vancomycin when used as primary treatment for mild or moderately severe infection.67 In two larger phase III studies, however, response rates with tolevamer were substantially lower than with either vancomycin or metronidazole.216 Interestingly, recurrence rates after successful tolevamer treatment were substantially lower than after antibiotic treatment with metronidazole or vancomycin, suggesting its potential use for primary or secondary disease prevention.67
Probiotic Therapy
In contrast to treatment with antimicrobial agents that further delay recolonization by normal colonic bacteria, probiotic agents are an attractive therapeutic option for recurrent C. difficile infection, because restoration of colonization resistance can lead to permanent eradication of C. difficile from the colon. Bacteriotherapy has been reported in patients with recurrent infection using enemas of fresh feces from a healthy relative, administration of fecal filtrates via a nasogastric tube, or by rectal infusions of a mixture of 10 different aerobic and anaerobic bacteria.217–219 The defined bacterial mixture led to bowel colonization with Bacteroides species, as well as prompt elimination of C. difficile, suggesting that Bacteroides may be one of the organisms that normally protects against pathogenic colonization with C. difficile.
Another probiotic therapy for C. difficile diarrhea is the oral administration of a nontoxigenic strain of C. difficile that was reported to be effective in two patients with relapsing C. difficile diarrhea.220 Preclinical studies are under way to characterize a nontoxigenic strain of C. difficile that may be suitable for administration as a prophylactic agent to prevent infection with toxigenic C. difficile in hospital patients who are receiving antibiotics.68
Lactobacillus species have been used widely as probiotics. In an open-label study, Lactobacillus strain GG was reported to be effective in preventing diarrhea in patients with recurrent C. difficile colitis.221 A subsequent controlled clinical trial, however, did not demonstrate that Lactobacillus GG was effective in protecting against AAD in hospital patients.222 Another placebo-controlled study of hospital patients receiving antibiotics used a probiotic drink mixture containing Lactobacillus casei, Lactobacillus bulgaricus, and Streptococcus thermophilus (DanActive) and found that simple AAD was reduced from 34% to 12% (placebo versus active; P = 0.007), and C. difficile infection was reduced from 17% to 0% (placebo versus active; P = 0.001).223 These dramatic positive results need to be confirmed in additional, multicenter studies.
The yeast S. boulardii is used widely as a probiotic agent in continental Europe and is now available in the United States without prescription.10,224 In a double-blind, controlled clinical trial, coadministration of oral capsules containing viable S. boulardii with antibiotics significantly reduced the incidence of AAD in hospitalized patients (from 22% on placebo to 9.5% in the S. boulardii group; P = 0.04).10 In that study, however, few patients had C. difficile-associated diarrhea. A second randomized, placebo-controlled trial examined the efficacy of S. boulardii in combination with either vancomycin or metronidazole in patients with C. difficile diarrhea.69 Diarrhea recurrence rates were similar in subjects treated during their first episode of C. difficile diarrhea (19% in the S. boulardii group vs. 24% in the placebo group; P = 0.86). In contrast, patients with a history of recurrent C. difficile diarrhea who received S. boulardii had fewer recurrences than the placebo group (35% and 65%, respectively; P = 0.04). In a subsequent study, S. boulardii (500 mg twice daily for 28 days) only reduced relapse rates (from 50% to 17%; P = 0.05) in patients treated with high-dose vancomycin (500 mg four times a day for 10 days) but not in patients on other antibiotic regimens.225 These controlled clinical trials indicate that S. boulardii is safe and effective in some patients with a history of recurrent C. difficile-associated diarrhea, but its protective effects are not uniform. S. boulardii should not be administered to immunocompromised patients because of the risk of fungemia.
Bacteriotherapy
It has long been recognized that alteration of the bacterial flora of the colon by antibiotics is the major predisposing cause of C. difficile infection, and that restoration of the normal flora eventually eliminates this pathogen. Based on this knowledge, measures to hasten reconstitution of the normal fecal bacteria are an attractive and logical mode of treatment. Therapy with microorganisms including stool transplantation and colonization with nontoxigenic strains of C. difficile have been reported. Several reports have documented efficacy of stool transplants from healthy relatives and intimate contacts delivered by colonoscopy or through a nasogastric tube in recurrent 217,225 and acute226 C. difficile infection. However, concern for the inadvertent spread of pathogenic microorganisms from donor to host as well as low patient and physician palatability for the procedure has limited the widespread acceptance of this approach. Oral administration of nontoxigenic strains of C. difficile to compete with and limit the growth of toxin-producing strains also has been reported to lead to remission of recurrent C. difficile infection in two patients.220
Immunization Against Clostridium difficile Toxins
As described earlier, there is considerable evidence that some persons have protective immunity against C. difficile-associated diarrhea and that protection is associated with higher antitoxin antibody concentrations in serum, intestinal secretions, or both.53,64,65,102,104–106 Leung and coworkers reported on six children with multiple relapses of C. difficile-associated diarrhea who had low concentrations of serum IgG antibody to toxin A.105 Five of these children were treated with intravenous immune globulin at a dose of 400 mg/kg, which contained high-titer IgG antitoxin A. Symptoms resolved following treatment. Similar results have been reported by other investigators.201
A C. difficile vaccine has been produced containing inactivated toxoid A and B. In early clinical trials, this vaccine was immunogenic,227,228 and in a small case series, vaccination was associated with resolution of recurrent C. difficile diarrhea in three subjects.229 Further studies are needed to determine whether passive or active immunization against C. difficile and its toxins can be effective in treating patients with refractory or recurrent disease. If effective, these therapeutic approaches also may be useful in preventing C. difficile-associated diarrhea in high-risk persons, such as elderly and infirm patients receiving antibiotic therapy in the hospital.
Overall Approach
The management of a first episode of recurrent C. difficile-associated diarrhea does not differ greatly from treatment of an initial episode (Table 108-7).131 Stool samples should be obtained to reconfirm infection with toxigenic C. difficile. Patients with mild symptoms of recurrence may be able to be managed conservatively without additional antibiotic treatment, just like patients with a primary episode. If symptoms persist or are severe, a 14-day course of metronidazole or vancomycin should be administered. If a second recurrence occurs, other treatment approaches should be considered.
Tedesco and associates proposed a tapering and pulsed antibiotic regimen that is well tolerated and often successful.204,212 In two recent case series, treatment with oral rifaximin 600 mg to 1200 mg daily for two to four weeks after completion of a standard course of vancomycin was effective in preventing recurrence in 12 of 14 patients with multiple previous recurrences.230,231 If this method fails, a wide range of other approaches have been described, some of which are summarized in Table 108-7.
Aslam S, Hamill RJ, Musher DM. Treatment of Clostridium difficile–associated disease: Old therapies and new strategies. Lancet Infect Dis. 2005;5:549-57. (Ref 177.)
Bartlett JG, Chang TW, Gurwith M, et al. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978;298:531-4. (Ref 20.)
Fekety R. Guidelines for the diagnosis and management of Clostridium difficile–associated diarrhea and colitis. American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 1997;92:739-50. (Ref 59.)
Kelly CP, LaMont JT. Clostridium difficile: more difficult than ever. N Engl J Med. 2008;359:1932-40. (Ref 48.)
Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med. 2000;342:390-7. (Ref 53.)
Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357:189-93. (Ref 106.)
McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996 to 2003. Emerg Infect Dis. 2006;12:409-15. (Ref 28.)
McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433-41. (Ref 169.)
McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320:204-10. (Ref 52.)
Mylonaki M, Langmead L, Pantes A, et al. Enteric infection in relapse of inflammatory bowel disease: Importance of microbiological examination of stool. Eur J Gastroenterol Hepatol. 2004;16:775-8. (Ref 121.)
Pepin J, Valiquette L, Alary ME, et al. Clostridium difficile–associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004;171:466-72. (Ref 156.)
Tedesco FJ, Gordon D, Fortson WC. Approach to patients with multiple recurrences of antibiotic-associated pseudo-membranous colitis. Am J Gastroenterol. 1985;80:867-8. (Ref 204.)
Viscidi R, Laughon BE, Yolken R, et al. Serum antibody response to toxins A and B of Clostridium difficile. J Infect Dis. 1983;148:93-100. (Ref 101.)
Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079-84. (Ref 76.)
Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile–associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-7. (Ref 178.)
1. Hogenauer C, Hammer HF, Krejs GJ, Reisinger EC. Mechanisms and management of antibiotic-associated diarrhea. Clin Infect Dis. 1998;27:702-10.
2. Altemeier WA, Hummel RP, Hill EO. Staphylococcal enterocolitis following antibiotic therapy. Ann Surg. 1963;157:847-58.
3. Borriello SP, Larson HE, Welch AR, et al. Enterotoxigenic Clostridium perfringens: a possible cause of antibiotic-associated diarrhoea. Lancet. 1984;1:305-7.
4. Olsen SJ, DeBess EE, McGivern TE, et al. A nosocomial outbreak of fluoroquinolone-resistant salmonella infection. N Engl J Med. 2001;344:1572-9.
5. Sparks SG, Carman RJ, Sarker MR, McClane BA. Genotyping of enterotoxigenic Clostridium perfringens fecal isolates associated with antibiotic-associated diarrhea and food poisoning in North America. J Clin Microbiol. 2001;39:883-8.
6. Khan MY, Hall WH. Staphylococcal enterocolitis: Treatment with oral vancomycin. Ann Intern Med. 1966;65:1-8.
7. Högenauer C, Langner C, Beubler E, et al. Klebsiella oxytoca as a causative organism of antibiotic-associated hemorrhagic colitis. N Engl J Med. 2006;355:2418-26.
8. Wistrom J, Norrby SR, Myhre EB, et al. Frequency of antibiotic-associated diarrhoea in 2462 antibiotic-treated hospitalized patients: A prospective study. J Antimicrob Chemother. 2001;47:43-50.
9. Bartlett JG. Pseudomembranous enterocolitis and antibiotic-associated diarrhea. In: Feldman M, Friedman LS, Sleisenger MH, editors. Sleisenger and Fordtran’s gastrointestinal and liver disease. 7th ed. Philadelphia: WB Saunders; 2002:1914-31.
10. Surawicz CM, Elmer GW, Speelman P, et al. Prevention of antibiotic-associated diarrhea by Saccharomyces boulardii: A prospective study. Gastroenterology. 1989;96:981-8.
11. Lewis SJ, Potts LF, Barry RE. The lack of therapeutic effect of Saccharomyces boulardii in the prevention of antibiotic-related diarrhoea in elderly patients. J Infect. 1998;36:171-4.
12. Arvola T, Laiho K, Torkkeli S, et al. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: A randomized study. Pediatrics. 1999;104:e64.
13. Thomas MR, Litin SC, Osmon DR, et al. Lack of effect of Lactobacillus GG on antibiotic-associated diarrhea: A randomized, placebo-controlled trial. Mayo Clin Proc. 2001;76:883-9.
14. D’Souza AL, Rajkumar C, Cooke J, Bulpitt CJ. Probiotics in prevention of antibiotic associated diarrhoea: meta-analysis. BMJ. 2002;324:1361.
15. Cremonini F, Di Caro S, Nista EC, et al. Meta-analysis: the effect of probiotic administration on antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2002;16:1461-7.
16. Finney JMT. Gastroenterostomy for cicatrizing ulcer of the pylorus. Bull Johns Hopkins Hosp. 1893;4:53.
17. Wakefield RD, Sommers SD. Fatal membranous staphylococcal enteritis in surgical patients. Ann Surg. 1953;138:249.
18. Dixon CF, Weismann RE. Acute pseudomembranous enteritis or enterocolitis: A complication following intestinal surgery. Surg Clin North Am. 1948;28:99.
19. Hall IC, O’Toole E. Intestinal flora in newborn infants: with a description of a new pathogenic anaerobe, Bacillus difficilis. Am J Dis Child. 1935;49:390-402.
20. Bartlett JG, Chang TW, Gurwith M, et al. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978;298:531-4.
21. Larson HE, Price AB, Honour P, Borriello SP. Clostridium difficile and the aetiology of pseudomembranous colitis. Lancet. 1978;1:1063-6.
22. Guerrant RL, Hughes JM, Lima NL, Crane J. Diarrhea in developed and developing countries: magnitude, special settings, and etiologies. Rev Infect Dis. 1990;12(Suppl 1):S41-50.
23. McFarland LV. Epidemiology of infectious and iatrogenic nosocomial diarrhea in a cohort of general medicine patients. Am J Infect Control. 1995;23:295-305.
24. Alfa MJ, Du T, Beda G. Survey of incidence of Clostridium difficile infection in Canadian hospitals and diagnostic approaches. J Clin Microbiol. 1998;36:2076-80.
25. Lai KK, Melvin ZS, Menard MJ, et al. Clostridium difficile–associated diarrhea: epidemiology, risk factors, and infection control. Infect Control Hosp Epidemiol. 1997;18:628-32.
26. Olson MM, Shanholtzer CJ, Lee JTJr, Gerding DN. Ten years of prospective Clostridium difficile–associated disease surveillance and treatment at the Minneapolis VA Medical Center, 1982-1991. Infect Control Hosp Epidemiol. 1994;15:371-81.
27. Samore MH, DeGirolami PC, Tlucko A, et al. Clostridium difficile colonization and diarrhea at a tertiary care hospital. Clin Infect Dis. 1994;18:181-7.
28. McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996 to 2003. Emerg Infect Dis. 2006;12:409-15.
29. United Kingdom National Statistics. www.statistics.gov.uk
30. Centers for Disease Control and Prevention (CDC). Severe Clostridium difficile–associated disease in populations previously at low risk. MMWR Morb Mortal Wkly Rep. 2005;54:1201-5.
31. Levy DG, Stergachis A, McFarland LV, et al. Antibiotics and Clostridium difficile diarrhea in the ambulatory care setting. Clin Ther. 2000;22:91-102.
32. Pepin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile–associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41:1254-60.
33. Kyne L, Farrell R, Kelly CP. Clostridium difficile. Gastroenterol Clin North Am. 2001;30:753-77.
34. Onderdonk AB, Cisneros RL, Bartlett JG. Clostridium difficile in gnotobiotic mice. Infect Immun. 1980;28:277-82.
35. Wilson KH, Silva J, Fekety FR. Suppression of Clostridium difficile by normal hamster cecal flora and prevention of antibiotic-associated cecitis. Infect Immun. 1981;34:626-8.
36. Borriello SP, Barclay FE. An in vitro model of colonisation resistance to Clostridium difficile infection. J Med Microbiol. 1986;21:299-309.
37. Borriello SP. 12th C. L. Oakley Lecture: Pathogenesis of Clostridium difficile infection of the gut. J Med Microbiol. 1990;33:207-15.
38. Larson HE, Barclay FE, Honour P, Hill ID. Epidemiology of Clostridium difficile in infants. J Infect Dis. 1982;146:727-33.
39. Kelly CP, LaMont JT. Clostridium difficile infection. Annu Rev Med. 1998;49:375-90.
40. Eglow R, Pothoulakis C, Itzkowitz S, et al. Diminished Clostridium difficile toxin A sensitivity in newborn rabbit ileum is associated with decreased toxin A receptor. J Clin Invest. 1992;90:822-9.
41. Hecht JR, Olinger EJ. Clostridium difficile colitis secondary to intravenous vancomycin. Dig Dis Sci. 1989;34:148-9.
42. Saginur R, Hawley CR, Bartlett JG. Colitis associated with metronidazole therapy. J Infect Dis. 1980;141:772-4.
43. Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect. 1998;40:1-15.
44. Gorbach SL. Antibiotics and Clostridium difficile. N Engl J Med. 1999;341:1690-1.
45. Ambrose N. The effects of single doses of antibiotics on faecal flora with a reference to their mode of excretion. J Drug Dev. 1989;1:233-41.
46. Kelly CP, LaMont JT. Treatment of Clostridium difficile diarrhea and colitis. In: Wolfe MM, editor. Gastrointestinal Pharmacotherapy. Philadelphia: WB Saunders; 1993:199-212.
47. Bouza E, Burillo A, Muñoz P. Antimicrobial therapy of Clostridium difficile–associated diarrhea. Med Clin North Am. 2006;90:1141-63.
48. Kelly CP, LaMont JT. Clostridium difficile: more difficult than ever. N Engl J Med. 2008;359:1932-40.
49. Anand A, Glatt AE. Clostridium difficile infection associated with antineoplastic chemotherapy: a review. Clin Infect Dis. 1993;17:109-13.
50. Djuretic T, Wall PG, Brazier JS. Clostridium difficile: an update on its epidemiology and role in hospital outbreaks in England and Wales. J Hosp Infect. 1999;41:213-18.
51. Mogg GA, Arabi Y, Youngs D, et al. Therapeutic trials of antibiotic-associated colitis. Scand J Infect Dis Suppl. 1980;22:41-5.
52. McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320:204-10.
53. Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med. 2000;342:390-7.
54. Gerding DN, Johnson S, Peterson LR, et al. Clostridium difficile–associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16:459-77.
55. Shim JK, Johnson S, Samore MH, et al. Primary symptomless colonisation by Clostridium difficile and decreased risk of subsequent diarrhoea. Lancet. 1998;351:633-6.
56. Clabots CR, Johnson S, Olson MM, et al. Acquisition of Clostridium difficile by hospitalized patients: Evidence for colonized new admissions as a source of infection. J Infect Dis. 1992;166:561-7.
57. Fekety R, Kim KH, Brown D, et al. Epidemiology of antibiotic-associated colitis: isolation of Clostridium difficile from the hospital environment. Am J Med. 1981;70:906-8.
58. Hoffman P. Clostridium difficile in hospitals. Curr Opin Infect Dis. 1994;7:471-4.
59. Fekety R. Guidelines for the diagnosis and management of Clostridium difficile–associated diarrhea and colitis. American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 1997;92:739-50.
60. Wilcox MH, Fawley WN. Hospital disinfectants and spore formation by Clostridium difficile. Lancet. 2000;356:1324.
61. Johnson S, Gerding DN, Olson MM, et al. Prospective, controlled study of vinyl glove use to interrupt Clostridium difficile nosocomial transmission. Am J Med. 1990;88:137-40.
62. Johnson S, Homann SR, Bettin KM, et al. Treatment of asymptomatic Clostridium difficile carriers (fecal excretors) with vancomycin or metronidazole: a randomized, placebo-controlled trial. Ann Intern Med. 1992;117:297-302.
63. Kelly CP. Immune response to Clostridium difficile infection. Eur J Gastroenterol Hepatol. 1996;8:1048-53.
64. Giannasca PJ, Warny M. Active and passive immunization against Clostridium difficile diarrhea and colitis. Vaccine. 2004;22:848-56.
65. Wilcox M, Minton J. Role of antibody response in outcome of antibiotic-associated diarrhoea. Lancet. 2001;357:158-9.
66. Kurtz CB, Cannon EP, Brezzani A, et al. GT160-246, a toxin binding polymer for treatment of Clostridium difficile colitis. Antimicrob Agents Chemother. 2001;45:2340-7.
67. Louie TJ, Peppe J, Watt CK, et al. Tolevamer Study Investigator Group. Tolevamer, a novel nonantibiotic polymer, compared with vancomycin in the treatment of mild to moderately severe Clostridium difficile–associated diarrhea. Clin Infect Dis. 2006;43:411-20.
68. Sambol SP, Merrigan MM, Tang JK, et al. Colonization for the prevention of Clostridium difficile disease in hamsters. J Infect Dis. 2002;186:1781-9.
69. McFarland LV, Surawicz CM, Greenberg RN, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA. 1994;271:1913-18.
70. Hammond GA, Johnson JL. The toxigenic element of Clostridium difficile strain VPI 10463. Microb Pathol. 1995;19:203-13.
71. Mani N, Dupuy B. Regulation of toxin synthesis in Clostridium difficile by an alternative RNA polymerase sigma factor. Proc Natl Acad Sci U S A. 2001;98:5844-9.
72. Dupuy B, Sonenshein AL. Regulated transcription of Clostridium difficile toxin genes. Mol Microbiol. 1998;27:107-20.
73. Hammond GA, Lyerly DM, Johnson JL. Transcriptional analysis of the toxigenic element of Clostridium difficile. Microb Pathog. 1997;22:143-54.
74. Warny M, Kelly CP. Pathogenicity of Clostridium difficile toxins. In: Hecht G, editor. Microbial Pathogenesis and the Intestinal Epithelial Cell. Washington, DC: ASM Press; 2003:503-24.
75. Moncrief JS, Barroso LA, Wilkins TD. Positive regulation of Clostridium difficile toxins. Infect Immun. 1997;65:1105-8.
76. Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079-84.
77. MacCannell DR, Louie TJ, Gregson DB, et al. Molecular analysis of Clostridium difficile PCR ribotype 027 isolates from Eastern and Western Canada. J Clin Microbiol. 2006;44:2147-52.
78. Tan KS, Wee BY, Song KP. Evidence for holin function of tcdE gene in the pathogenicity of Clostridium difficile. J Med Microbiol. 2001;50:613-19.
79. Barroso LA, Wang SZ, Phelps CJ, et al. Nucleotide sequence of Clostridium difficile toxin B gene. Nucleic Acids Res. 1990;18:4004.
80. Dove CH, Wang SZ, Price SB, et al. Molecular characterization of the Clostridium difficile toxin A gene. Infect Immun. 1990;58:480-8.
81. von Eichel-Streiber C, Laufenberg-Feldmann R, Sartingen S, et al. Cloning of Clostridium difficile toxin B gene and demonstration of high N-terminal homology between toxin A and B. Med Microbiol Immunol. 1990;179:271-9.
82. Chaves-Olarte E, Weidmann M, von Eichel-Streiber C, Thelestam M. Toxin A and B from Clostridium difficile differ with respect to enzymatic potencies, cellular substrate specificities and surface binding to cultured cells. J Clin Invest. 1997;100:1734-41.
83. Just I, Selzer J, Wilm M, et al. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 1995;375:500-3.
84. Just I, Wilm M, Selzer J, et al. The enterotoxin from Clostridium difficile (ToxA) monoglucosylates the Rho proteins. J Biol Chem. 1995;270:13932-6.
85. Pothoulakis C. Pathogenesis of Clostridium difficile–associated diarrhoea. Eur J Gastroenterol Hepatol. 1996;8:1041-7.
86. Warny M, Keates AC, Keates S, et al. p38 MAP kinase activation by Clostridium difficile toxin A mediates monocyte necrosis, IL-8 production, and enteritis. J Clin Invest. 2000;105:1147-56.
87. Sullivan NM, Pellett S, Wilkins TD. Purification and characterization of toxins A and B of Clostridium difficile. Infect Immun. 1982;35:1032-40.
88. Triadafilopoulos G, Pothoulakis C, O’Brien MJ, LaMont JT. Differential effects of Clostridium difficile toxins A and B on rabbit ileum. Gastroenterology. 1987;93:273-9.
89. Lyerly DM, Saum KE, MacDonald DK, Wilkins TD. Effects of Clostridium difficile toxins given intragastrically to animals. Infect Immun. 1985;47:349-52.
90. Mitchell TJ, Ketley JM, Haslam SC, et al. Effect of toxin A and B of Clostridium difficile on rabbit ileum and colon. Gut. 1986;27:78-85.
91. Pothoulakis C, Barone LM, Ely R, et al. Purification and properties of Clostridium difficile cytotoxin B. J Biol Chem. 1986;261:1316-21.
92. Riegler M, Sedivy R, Pothoulakis C, et al. Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J Clin Invest. 1995;95:2004-11.
93. Savidge TC, Pan WH, Newman P, et al. Clostridium difficile toxin B is an inflammatory enterotoxin in human intestine. Gastroenterology. 2003;125:413-20.
94. Kato H, Kato N, Katow S, et al. Deletions in the repeating sequences of the toxin A gene of toxin A-negative, toxin B-positive Clostridium difficile strains. FEMS Microbiol Lett. 1999;175:197-203.
95. Kato H, Kato N, Watanabe K, et al. Identification of toxin A-negative, toxin B-positive Clostridium difficile by PCR. J Clin Microbiol. 1998;36:2178-82.
96. Limaye AP, Turgeon DK, Cookson BT, Fritsche TR. Pseudomembranous colitis caused by a toxin A−B+ strain of Clostridium difficile. J Clin Microbiol. 2000;38:1696-7.
97. Lyerly DM, Barroso LA, Wilkins TD, et al. Characterization of a toxin A–negative, toxin B–positive strain of Clostridium difficile. Infect Immun. 1992;60:4633-9.
98. Aronsson B, Granstrom M, Mollby R, Nord CE. Serum antibody response to Clostridium difficile toxins in patients with Clostridium difficile diarrhoea. Infection. 1985;13:97-101.
99. Johnson S, Gerding DN, Janoff EN. Systemic and mucosal antibody responses to toxin A in patients infected with Clostridium difficile. J Infect Dis. 1992;166:1287-94.
100. Kelly CP, Pothoulakis C, Orellana J, LaMont JT. Human colonic aspirates containing immunoglobulin A antibody to Clostridium difficile toxin A inhibit toxin A–receptor binding. Gastroenterology. 1992;102:35-40.
101. Viscidi R, Laughon BE, Yolken R, et al. Serum antibody response to toxins A and B of Clostridium difficile. J Infect Dis. 1983;148:93-100.
102. Warny M, Vaerman JP, Avesani V, Delmee M. Human antibody response to Clostridium difficile toxin A in relation to clinical course of infection. Infect Immun. 1994;62:384-9.
103. Kim PH, Iaconis JP, Rolfe RD. Immunization of adult hamsters against Clostridium difficile–associated ileocecitis and transfer of protection to infant hamsters. Infect Immun. 1987;55:2984-92.
104. Bacon AEIII, Fekety R. Immunoglobulin G directed against toxins A and B of Clostridium difficile in the general population and patients with antibiotic-associated diarrhea. Diagn Microbiol Infect Dis. 1994;18:205-9.
105. Leung DY, Kelly CP, Boguniewicz M, et al. Treatment with intravenously administered gamma globulin of chronic relapsing colitis induced by Clostridium difficile toxin. J Pediatr. 1991;118:633-7.
106. Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357:189-93.
107. Brandt LJ, Kosche KA, Greenwald DA, et al. Clostridium difficile–associated diarrhea in the elderly. Am J Gastroenterol. 1999;94:3263.
108. Brown E, Talbot GH, Axelrod P, et al. Risk factors for Clostridium difficile toxin-associated diarrhea. Infect Control Hosp Epidemiol. 1990;11:283-90.
109. McFarland LV, Surawicz CM, Stamm WE. Risk factors for Clostridium difficile carriage and C. difficile–associated diarrhea in a cohort of hospitalized patients. J Infect Dis. 1990;162:678-84.
110. Bassaris HP, Lianou FE, Legakis NJ, et al. Interaction between Clostridium difficile and polymorphonuclear leukocytes from the elderly and postoperative cancer patients: phagocytosis and bacterial function. Med Microbiol Immunol. 1984;173:49.
111. Kyne L, Sougioultzis S, McFarland LV, Kelly CP. Underlying disease severity as a major risk factor for nosocomial Clostridium difficile diarrhea. Infect Control Hosp Epidemiol. 2002;23:653-9.
112. Blot E, Escande MC, Besson D, et al. Outbreak of Clostridium difficile–related diarrhoea in an adult oncology unit: risk factors and microbiological characteristics. J Hosp Infect. 2003;53:187-92.
113. Keven K, Basu A, Re L, et al. Clostridium difficile colitis in patients after kidney and pancreas-kidney transplantation. Transpl Infect Dis. 2004;6:10-4.
114. Tomblyn M, Gordon L, Singhal S, et al. Rarity of toxigenic Clostridium difficile infections after hematopoietic stem cell transplantation: implications for symptomatic management of diarrhea. Bone Marrow Transplant. 2002;30:517-19.
115. Pulvirenti JJ, Mehra T, Hafiz I, et al. Epidemiology and outcome of Clostridium difficile infection and diarrhea in HIV infected inpatients. Diagn Microbiol Infect Dis. 2002;44:325-30.
116. Barbut F, Meynard JL, Guiguet M, et al. Clostridium difficile–associated diarrhea in HIV-infected patients: Epidemiology and risk factors. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:176-81.
117. Tumbarello M, Tacconelli E, Leone F, et al. Clostridium difficile–associated diarrhoea in patients with human immunodeficiency virus infection: A case-control study. Eur J Gastroenterol Hepatol. 1995;7:259-63.
118. Lu SS, Schwartz JM, Simon DM, Brandt LJ. Clostridium difficile–associated diarrhea in patients with HIV positivity and AIDS: A prospective controlled study. Am J Gastroenterol. 1994;89:1226-9.
119. LaMont JT, Trnka YM. Therapeutic implications of Clostridium difficile toxin during relapse of chronic inflammatory bowel disease. Lancet. 1980;1:381-3.
120. Meyer AM, Ramzan NN, Loftus EVJr, et al. The diagnostic yield of stool pathogen studies during relapses of inflammatory bowel disease. J Clin Gastroenterol. 2004;38:772-5.
121. Mylonaki M, Langmead L, Pantes A, et al. Enteric infection in relapse of inflammatory bowel disease: importance of microbiological examination of stool. Eur J Gastroenterol Hepatol. 2004;16:775-8.
122. Nguyen GC, Kaplan GG, Harris ML, Brant SR. A national survey of the prevalence and impact of Clostridium difficile infection among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103:1443-50.
123. Gerding DN, Olson MM, Peterson LR, et al. Clostridium difficile–associated diarrhea and colitis in adults. A prospective case-controlled epidemiologic study. Arch Intern Med. 1986;146:95-100.
124. Johnson S, Clabots CR, Linn FV, et al. Nosocomial Clostridium difficile colonisation and disease. Lancet. 1990;336:97-100.
125. Triadafilopoulos G, Hallstone AE. Acute abdomen as the first presentation of pseudomembranous colitis. Gastroenterology. 1991;101:685-91.
126. Feldman RJ, Kallich M, Weinstein MP. Bacteremia due to Clostridium difficile: case report and review of extraintestinal C. difficile infections. Clin Infect Dis. 1995;20:1560-2.
127. Pron B, Merckx J, Touzet P, et al. Chronic septic arthritis and osteomyelitis in a prosthetic knee joint due to Clostridium difficile. Eur J Clin Microbiol Infect Dis. 1995;14:599-601.
128. Studemeister AE, Beilke MA, Kirmani N. Splenic abscess due to Clostridium difficile and Pseudomonas paucimobilis. Am J Gastroenterol. 1987;82:389-90.
129. Kyne L, Merry C, O’Connell B, et al. Factors associated with prolonged symptoms and severe disease due to Clostridium difficile. Age Ageing. 1999;28:107-13.
130. Wolf LE, Gorbach SL, Granowitz EV, Extraintestinal. Clostridium difficile: ten years’ experience at a tertiary-care hospital. Mayo Clin Proc. 1998;73:943-7.
131. Linevsky JK, Kelly CP. Clostridium difficile colitis. In: LaMont JT, editor. Gastrointestinal Infections: Diagnosis and Management. New York: Marcel Dekker; 1997:293-325.
132. Cleary RK. Clostridium difficile–associated diarrhea and colitis: clinical manifestations, diagnosis, and treatment. Dis Colon Rectum. 1998;41:1435-49.
133. Kyne L, Merry C, O’Connell B, et al. Community-acquired Clostridium difficile infection. J Infect. 1998;36:287-8.
134. Issack MI, Elliott TS. Clostridium difficile carriage after infection. Lancet. 1990;335:610-11.
135. Brazier JS. The diagnosis of Clostridium difficile–associated disease. J Antimicrob Chemother. 1998;41(Suppl C):29-40.
136. Barbut F, Kajzer C, Planas N, Petit JC. Comparison of three enzyme immunoassays, a cytotoxicity assay, and toxigenic culture for diagnosis of Clostridium difficile–associated diarrhea. J Clin Microbiol. 1993;31:963-7.
137. Merz CS, Kramer C, Forman M, et al. Comparison of four commercially available rapid enzyme immunoassays with cytotoxin assay for detection of Clostridium difficile toxin(s) from stool specimens. J Clin Microbiol. 1994;32:1142-7.
138. Whittier S, Shapiro DS, Kelly WF, et al. Evaluation of four commercially available enzyme immunoassays for laboratory diagnosis of Clostridium difficile–associated diseases. J Clin Microbiol. 1993;31:2861-5.
139. Peterson LR, Kelly PJ. The role of the clinical microbiology laboratory in the management of Clostridium difficile–associated diarrhea. Infect Dis Clin North Am. 1993;7:277-93.
140. Tichota-Lee J, Jaqua-Stewart MJ, Benfield D, et al. Effect of age on the sensitivity of cell cultures to Clostridium difficile toxin. Diagn Microbiol Infect Dis. 1987;8:203-14.
141. Walker RC, Ruane PJ, Rosenblatt JE, et al. Comparison of culture, cytotoxicity assays, and enzyme-linked immunosorbent assay for toxin A and toxin B in the diagnosis of Clostridium difficile–related enteric disease. Diagn Microbiol Infect Dis. 1986;5:61-9.
142. Lyerly DM, Neville LM, Evans DT, et al. Multicenter evaluation of the Clostridium difficile TOX A/B TEST. J Clin Microbiol. 1998;36:184-90.
143. Ticehurst JR, Aird DZ, Dam LM, et al. Effective detection of toxigenic Clostridium difficile by a two-step algorithm including tests for antigen and cytotoxin. J Clin Microbiol. 2006;44:1145-9.
144. Alonso R, Munoz C, Pelaez T, et al. Rapid detection of toxigenic Clostridium difficile strains by a nested PCR of the toxin B gene. Clin Microbiol Infect. 1997;3:145-7.
145. Kato N, Ou CY, Kato H, et al. Identification of toxigenic Clostridium difficile by the polymerase chain reaction. J Clin Microbiol. 1991;29:33-7.
146. Wren B, Clayton C, Tabaqchali S. Rapid identification of toxigenic Clostridium difficile by polymerase chain reaction. Lancet. 1990;335:423.
147. Boondeekhun HS, Gurtler V, Odd ML, et al. Detection of Clostridium difficile enterotoxin gene in clinical specimens by the polymerase chain reaction. J Med Microbiol. 1993;38:384-7.
148. Green GA, Riot B, Monteil H. Evaluation of an oligonucleotide probe and an immunological test for direct detection of toxigenic Clostridium difficile in stool samples. Eur J Clin Microbiol Infect Dis. 1994;13:576.
149. Gumerlock PH, Tang YJ, Meyers FJ, Silva JJr. Use of the polymerase chain reaction for the specific and direct detection of Clostridium difficile in human feces. Rev Infect Dis. 1991;13:1053-60.
150. Kato N, Ou CY, Kato H, et al. Detection of toxigenic Clostridium difficile in stool specimens by the polymerase chain reaction. J Infect Dis. 1993;167:455-8.
151. Alonso R, Munoz C, Gros S, et al. Rapid detection of toxigenic Clostridium difficile from stool samples by a nested PCR of toxin B gene. J Hosp Infect. 1999;41:145-9.
152. Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. N Engl J Med. 1994;330:257-62.
153. Kwon JH, Kelly CP. Clostridium difficile and antibiotic-associated diarrhea. In: Bayless RM, Diehl AM, editors. Advanced Therapy in Gastroenterology and Liver Disease. 5th ed. Hamilton, Ontario: BC Decker; 2005:302.
154. Tedesco FJ, Corless JK, Brownstein RE. Rectal sparing in antibiotic-associated pseudomembranous colitis: a prospective study. Gastroenterology. 1982;83:1259.
155. Price AB, Davies DR. Pseudomembranous colitis. J Clin Pathol. 1977;30:1.
156. Pepin J, Valiquette L, Alary ME, et al. Clostridium difficile–associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004;171:466-72.
157. Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium difficile–associated diarrhoea and colitis. Lancet. 1983;2:1043-6.
158. Novak E, Lee JG, Seckman CE, et al. Unfavorable effect of atropine-diphenoxylate (Lomotil) therapy in lincomycin-caused diarrhea. JAMA. 1976;235:1451-4.
159. Wilcox MH, Howe R. Diarrhoea caused by Clostridium difficile: response time for treatment with metronidazole and vancomycin. J Antimicrob Chemother. 1995;36:673-9.
160. Peterson LR, Gerding DN. Antimicrobial agents. In: Rambaud JC, Ducluzeau R, editors. Clostridium difficile–Associated Intestinal Diseases. Paris: Springer-Verlag; 1990:115-27.
161. Bartlett JG, Taylor NS, Chang T, Dzink J. Clinical and laboratory observations in Clostridium difficile colitis. Am J Clin Nutr. 1980;33:2521-6.
162. Bartlett JG, Chang TW, Onderdonk AB. Comparison of five regimens for treatment of experimental clindamycin-associated colitis. J Infect Dis. 1978;138:81-6.
163. Clabots CR, Shanholtzer CJ, Peterson LR, Gerding DN. In vitro activity of efrotomycin, ciprofloxacin, and six other antimicrobials against Clostridium difficile. Diagn Microbiol Infect Dis. 1987;6:49-52.
164. Dzink J, Bartlett JG. In vitro susceptibility of Clostridium difficile isolates from patients with antibiotic-associated diarrhea or colitis. Antimicrob Agents Chemother. 1980;17:695-8.
165. Fekety R, Silva J, Toshniwal R, et al. Antibiotic-associated colitis: Effects of antibiotics on Clostridium difficile and the disease in hamsters. Rev Infect Dis. 1979;1:386-97.
166. Young GP, Ward PB, Bayley N, et al. Antibiotic-associated colitis due to Clostridium difficile: double-blind comparison of vancomycin with bacitracin. Gastroenterology. 1985;89:1038-45.
167. Drummond LJ, McCoubrey J, Smith DG, et al. Changes in sensitivity patterns to selected antibiotics in Clostridium difficile in geriatric inpatients over an 18-month period. J Med Microbiol. 2003;52:259-63.
168. Johnson S, Samore MH, Farrow KA, et al. Epidemics of diarrhea caused by a clindamycin-resistant strain of Clostridium difficile in four hospitals. N Engl J Med. 1999;341:1645-51.
169. McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433-41.
170. Pelaez T, Alcala L, Alonso R, et al. Reassessment of Clostridium difficile susceptibility to metronidazole and vancomycin. Antimicrob Agents Chemother. 2002;46:1647-50.
171. Fekety R, Silva J, Armstrong J, et al. Treatment of antibiotic-associated enterocolitis with vancomycin. Rev Infect Dis. 1981;3(Suppl):S273-81.
172. Bartlett JG, Onderdonk AB, Cisneros RL, Kasper DL. Clindamycin-associated colitis due to a toxin-producing species of Clostridium in hamsters. J Infect Dis. 1977;136:701-5.
173. Browne RA, Fekety RJr, Silva JJr, et al. The protective effect of vancomycin on clindamycin-induced colitis in hamsters. Johns Hopkins Med J. 1977;141:183-92.
174. Kelly CP, LaMont JT. Treatment of Clostridium difficile diarrhea and colitis. In: Wolfe MM, editor. Therapy of Digestive Disorders. Philadelphia: WB Saunders; 2000:513-22.
175. Zimmerman MJ, Bak A, Sutherland LR. Review article: Treatment of Clostridium difficile infection. Aliment Pharmacol Ther. 1997;11:1003-12.
176. Hospital Infection Control Practices Advisory Committee. Recommendations for preventing the spread of vancomycin resistance: recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). Am J Infect Control. 1995;23:87-94.
177. Aslam S, Hamill RJ, Musher DM. Treatment of Clostridium difficile–associated disease: old therapies and new strategies. Lancet Infect Dis. 2005;5:549-57.
178. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile–associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-7.
179. Bolton RP, Culshaw MA. Faecal metronidazole concentrations during oral and intravenous therapy for antibiotic associated colitis due to Clostridium difficile. Gut. 1986;27:1169-72.
180. Bingley PJ, Harding GM. Clostridium difficile colitis following treatment with metronidazole and vancomycin. Postgrad Med J. 1987;63:993-4.
181. Saginur R, Hawley CR, Bartlett JG. Colitis associated with metronidazole therapy. J Infect Dis. 1980;141:772-4.
182. Thomson G, Clark AH, Hare K, Spilg WG. Pseudomembranous colitis after treatment with metronidazole. BMJ. 1981;282:864-5.
183. Keighley MR, Burdon DW, Arabi Y, et al. Randomised controlled trial of vancomycin for pseudomembranous colitis and postoperative diarrhoea. BMJ. 1978;2:1667-9.
184. Young GP, Ward PB, Bayley N, et al. Antibiotic-associated colitis due to Clostridium difficile: double-blind comparison of vancomycin with bacitracin. Gastroenterology. 1985;89:1038-45.
185. Fekety R, Silva J, Kauffman C, et al. Treatment of antibiotic-associated Clostridium difficile colitis with oral vancomycin: Comparison of two dosage regimens. Am J Med. 1989;86:15-19.
186. Kleinfeld DI, Sharpe RJ, Donta ST. Parenteral therapy for antibiotic-associated pseudomembranous colitis. J Infect Dis. 1988;157:389.
187. Oliva SL, Guglielmo BJ, Jacobs R, Pons VG. Failure of intravenous vancomycin and intravenous metronidazole to prevent or treat antibiotic-associated pseudomembranous colitis. J Infect Dis. 1989;159:1154-5.
188. Chang TW, Gorbach SL, Bartlett JG, Saginur R. Bacitracin treatment of antibiotic-associated colitis and diarrhea caused by Clostridium difficile toxin. Gastroenterology. 1980;78:1584-6.
189. Dudley MN, McLaughlin JC, Carrington G, et al. Oral bacitracin versus vancomycin therapy for Clostridium difficile–induced diarrhea: a randomized double-blind trial. Arch Intern Med. 1986;146:1101-4.
190. Tedesco FJ. Bacitracin therapy in antibiotic-associated pseudomembranous colitis. Dig Dis Sci. 1980;25:783-4.
191. de Lalla F, Nicolin R, Rinaldi E, et al. Prospective study of oral teicoplanin versus oral vancomycin for therapy of pseudomembranous colitis and Clostridium difficile–associated diarrhea. Antimicrob Agents Chemother. 1992;36:2192-6.
192. Wenisch C, Parschalk B, Hasenhundl M, et al. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile–associated diarrhea. Clin Infect Dis. 1996;22:813-18.
193. Cronberg S, Castor B, Thoren A. Fusidic acid for the treatment of antibiotic-associated colitis induced by Clostridium difficile. Infection. 1984;12:276-9.
194. Jobe BA, Grasley A, Deveney KE, et al. Clostridium difficile colitis: an increasing hospital-acquired illness. Am J Surg. 1995;169:480-3.
195. Rubin MS, Bodenstein LE, Kent KC. Severe Clostridium difficile colitis. Dis Colon Rectum. 1995;38:350-4.
196. Anand A, Bashey B, Mir T, Glatt AE. Epidemiology, clinical manifestations, and outcome of Clostridium difficile–associated diarrhea. Am J Gastroenterol. 1994;89:519-23.
197. Grundfest-Broniatowski S, Quader M, Alexander F, et al. Clostridium difficile colitis in the critically ill. Dis Colon Rectum. 1996;39:619-23.
198. Morris JB, Zollinger RMJr, Stellato TA. Role of surgery in antibiotic-induced pseudomembranous enterocolitis. Am J Surg. 1990;160:535-9.
199. Pasic M, Jost R, Carrel T, et al. Intracolonic vancomycin for pseudomembranous colitis. N Engl J Med. 1993;329:583.
200. Salcedo J, Keates S, Pothoulakis C, et al. Intravenous immunoglobulin therapy for severe Clostridium difficile colitis. Gut. 1997;41:366-70.
201. Wilcox MH. Descriptive study of intravenous immunoglobulin for the treatment of recurrent Clostridium difficile diarrhoea. J Antimicrob Chemother. 2004;53:882-4.
202. McPherson S, Rees CJ, Ellis R, et al. Intravenous immunoglobulin for the treatment of severe, refractory, and recurrent Clostridium difficile diarrhea. Dis Colon Rectum. 2006;49(5):640-5.
203. Synnott K, Mealy K, Merry C, et al. Timing of surgery for fulminating pseudomembranous colitis. Br J Surg. 1998;85:229-31.
204. Tedesco FJ, Gordon D, Fortson WC. Approach to patients with multiple recurrences of antibiotic-associated pseudo-membranous colitis. Am J Gastroenterol. 1985;80:867-8.
205. Kyne L, Kelly CP. Recurrent Clostridium difficile diarrhoea. Gut. 2001;49:152-3.
206. McFarland LV, Surawicz CM, Rubin M, et al. Recurrent Clostridium difficile disease: epidemiology and clinical characteristics. Infect Control Hosp Epidemiol. 1999;20:43-50.
207. Grinspin AM, Bernheim OE, Yousefzadeh E, et al. Clostridium difficile as a causative agent of post-infection irritable bowel syndrome. Gastroenterol. 2008;134:A-91.
208. Walters BA, Roberts R, Stafford R, Seneviratne E. Relapse of antibiotic-associated colitis: Endogenous persistence of Clostridium difficile during vancomycin therapy. Gut. 1983;24:206-12.
209. Wilcox MH, Fawley WN, Settle CD, Davidson A. Recurrence of symptoms in Clostridium difficile infection: relapse or reinfection? J Hosp Infect. 1998;38:93-100.
210. Bartlett JG, Tedesco FJ, Shull S, et al. Symptomatic relapse after oral vancomycin therapy of antibiotic-associated pseudomembranous colitis. Gastroenterology. 1980;78:431-4.
211. Thanjan AJ, Southern W, Anand N, et al. Is Clostridium difficile infection more difficult to eradicate in patients with diverticulosis? Am J Gastroenterology. 2008;103:S195. (A504)
212. Tedesco FJ, Barton RW, Alpers DH. Clindamycin-associated colitis: a prospective study. Ann Intern Med. 1974;81:429-33.
213. McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97:1769-75.
214. Kreutzer EW, Milligan FD. Treatment of antibiotic-associated pseudomembranous colitis with cholestyramine resin. Johns Hopkins Med J. 1978;143:67-72.
215. Tedesco FJ. Treatment of recurrent antibiotic-associated pseudomembranous colitis. Am J Gastroenterol. 1982;77:220-1.
216. Louie T, Gerdsom M, Grimard D, et al. Results of a phase III trial comparing tolevamer, vancomycin and metronidazole in patients with Clostridium difficile–associated diarrhea (CDI). 47th Annual ICAAC Meeting September 17-20, 2007, Chicago, Ill. [Abstract].
217. Tvede M, Rask-Madsen J. Bacteriotherapy for chronic relapsing Clostridium difficile diarrhoea in six patients. Lancet. 1989;1:1156-60.
218. Persky SE, Brandt LJ. Treatment of recurrent Clostridium difficile–associated diarrhea by administration of donated stool directly through a colonoscope. Am J Gastroenterol. 2000;95:3283-5.
219. Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: Case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003;36:580-5.
220. Seal D, Borriello SP, Barclay F, et al. Treatment of relapsing Clostridium difficile diarrhoea by administration of a non-toxigenic strain. Eur J Clin Microbiol. 1987;6:51-3.
221. Gorbach SL, Chang TW, Goldin B. Successful treatment of relapsing Clostridium difficile colitis with Lactobacillus GG. Lancet. 1987;2:1519.
222. Thomas MR, Litin SC, Osmon DR, et al. Lack of effect of Lactobacillus GG on antibiotic-associated diarrhea: a randomized, placebo-controlled trial. Mayo Clin Proc. 2001;76:883-9.
223. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80.
224. Elmer GW, Surawicz CM, McFarland LV. Biotherapeutic agents: a neglected modality for the treatment and prevention of selected intestinal and vaginal infections. JAMA. 1996;275:870-6.
225. Surawicz CM, McFarland LV, Greenberg RN, et al. The search for a better treatment for recurrent Clostridium difficile disease: use of high-dose vancomycin combined with Saccharomyces boulardii. Clin Infect Dis. 2000;31:1012-17.
226. You DM, Franzos MA, Holman RP. Successful treatment of fulminant Clostridium difficile infection with fecal bacteriotherapy. Ann Intern Med. 2008;148:632-3.
227. Kotloff KL, Wasserman SS, Losonsky GA, et al. Safety and immunogenicity of increasing doses of a Clostridium difficile toxoid vaccine administered to healthy adults. Infect Immun. 2001;69:988-95.
228. Aboudola S, Kotloff KL, Kyne L, et al. Clostridium difficile vaccine and serum immunoglobulin G antibody response to toxin A. Infect Immun. 2003;71:1608-10.
229. Sougioultzis S, Kyne L, Drudy D, et al. Clostridium difficile toxoid vaccine in recurrent C. difficile–associated diarrhea. Gastroenterology. 2005;128:764-70.
230. Johnson S, Schriever C, Galang M, et al. Interruption of recurrent Clostridium difficile–associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis. 2007;44:846-8.
231. Garey KW, Jiang ZD, Bellard A, Dupont HL. Rifaximin in treatment of recurrent Clostridium difficile–associated diarrhea: an uncontrolled pilot study. J Clin Gastroenterol. 2008 Apr 1. [epub ahead of print]