CHAPTER 48 Anterior Lumbar Interbody Fusion
Low back pain has long been noted to be one of the most disabling conditions in the United States and the Western world. Disability from back pain has been reported to cost approximately $100 billion annually.1 Lumbar disc degeneration is often divided into three basic categories: internal disc derangement, degenerative disc disease (DDD), and motion segment instability. Internal disc derangement2 encompasses annular tears and dark disc disease, DDD describes isolated disc resorption and spondylosis, and motion segment instability involves listhesis and scoliotic changes. This description of lumbar disc degeneration, although oversimplified, encompasses a dynamic process with overlapping findings at individual and adjacent levels. DDD, although controversial in its exact role in patients with back pain, has been shown to be a pain source generator.3,4 Removal of the pain generator, the intervertebral disc, is a viable and logical approach for selected patients.
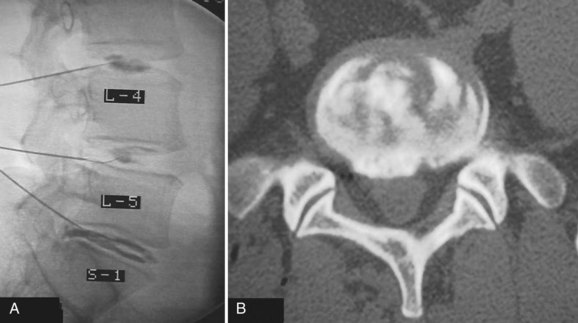
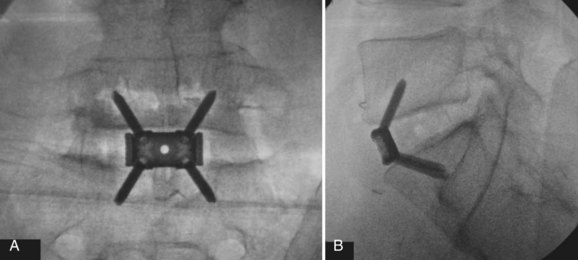
Determining the ideal candidate for surgical management of DDD can be more challenging than performing the procedure itself because of the unclear relationship between patient symptoms, diagnostic studies, and surgical outcomes. The patient should have failed conservative management, including oral medication, lifestyle modification, and active rehabilitation, before surgical intervention. To maximize the predictive value of lumbar interbody fusion for the treatment of lumbar disc degeneration, the patient’s history should be consistent with mechanical back pain, and radiographic studies should show degeneration at discrete levels. Discography, as discussed in an earlier chapter, is at this point the only diagnostic test in the authors’ opinion that can isolate discogenic pain and should reproduce concordant pain and abnormal disc morphology (Fig. 48–1). Patients with a significant behavioral component to their pain should be considered poor surgical candidates. Preoperative psychological screening can be helpful in the evaluation of these patients.5
Anterior lumbar interbody fusion (ALIF), as an option for treating DDD, has had a tumultuous history with increasing and decreasing interest and success over the years. ALIF as a procedure favors load transmission through the anterior column, recreates lordosis, restores disc height, and tensions lateral and posterior annular or ligamentous fibers (Fig. 48–2).6 Direct anterior exposure allows complete removal of disc material, increasing the fusion rate,7 while avoiding trauma to the posterior musculature, making it an attractive option for the treatment of DDD.
ALIF as a treatment for low back pain was initially performed with varied success. In 1972, Stauffer and Coventry8 reported 36% good results in 77 patients with lumbar disc rupture. In 1988, Inoue and colleagues9 reviewed 350 cases of ALIF for disc herniation and found 73% of patients had relief of back pain. Blumenthal and colleagues10 reported on 34 patients in 1988 with 73% fusion rate and 74% clinically satisfactory results. Loguidice and colleagues11 found an overall fusion rate of 80% radiographically using various combinations of autograft and allograft interbody spacers. Of 85 patients, 74% responded that the surgery helped.
Anatomy and Approach
Developed in the 1990s, laparoscopic ALIF achieved early success with its pioneers.12 Laparoscopic ALIF was reported to be less invasive with less blood loss and faster recovery; however, later reports contradicted these findings, noting no identifiable advantages, added technical challenge, and increased specific complications such as retrograde ejaculation.13,14
Indications for Interbody Fusions
Axial back pain caused by the degenerative process of the disc and spine as a whole is currently the main indication for ALIF. By correctly identifying discogenic pain via radiographs, magnetic resonance imaging (MRI), and provocative discography, one can remove the pathologic disc and stabilize the motion segment with an interbody graft. Using an ALIF procedure, patients with associated radicular leg pain secondary to foraminal narrowing can be indirectly decompressed by restoring the foraminal height, elongating redundant posterior and lateral anulus (if not removed), and realigning overlapping incongruent facet joints and more directly decompressed by removing compressive nuclear material.15,16 Patients with a herniated nucleus pulposus can undergo direct decompression and discectomy via an anterior exposure. Interbody implants placed through an anterior approach can be used alone or in conjunction with posterior fusion techniques for cases requiring a more robust construct, as discussed later in this chapter.
Interbody Implants and Graft Material
The race between temporary mechanical support and biology has been run since the origins of orthopaedic care. In the spine, the goal of any interbody device is to provide anterior column mechanical support while a bony fusion develops. A single question remains to be answered: How rigid does a construct need to be to provide early stability without negatively affecting the spine when fusion is present? Creating an unnecessarily stiff construct may lead to stress shielding,17 additional surgery, and implantation of costly implants; however, too little stiffness leads to biomechanical failure or pseudarthrosis. Pilliar and colleagues18 showed that small micromotion of 28 µm does not affect bone ingrowth into porous-surfaced implants and large micromotion greater than 150 µm can produce a fibrous interface. Nevertheless, the current consensus seems to be that adequate stabilization must greatly increase the stiffness above the native segment. In vitro studies do not fully recreate in vivo experiences, and individual patient factors, such as bone quality, size, and load demand, are variable and dynamic, complicating the goal.
The first lumbar interbody fusion was reported by Burns19 in 1933, using a tibial peg to treat spondylolisthesis. Interbody fusion for DDD was described by Harmon20 in 1963 and then by Crock2 and Stauffer and Coventry.8 Early days of interbody grafting incorporated the use of bicortical or tricortical spacers harvested from iliac crest. When used alone, this graft is associated with significant rates of mechanical failure, loss of correction, and pseudarthrosis.21,22 Sterile allograft (i.e., bone dowels or tricortical grafts) have often been used because they are stronger than equivalent fresh, autologous bone and eliminate the need for autologous harvesting.23
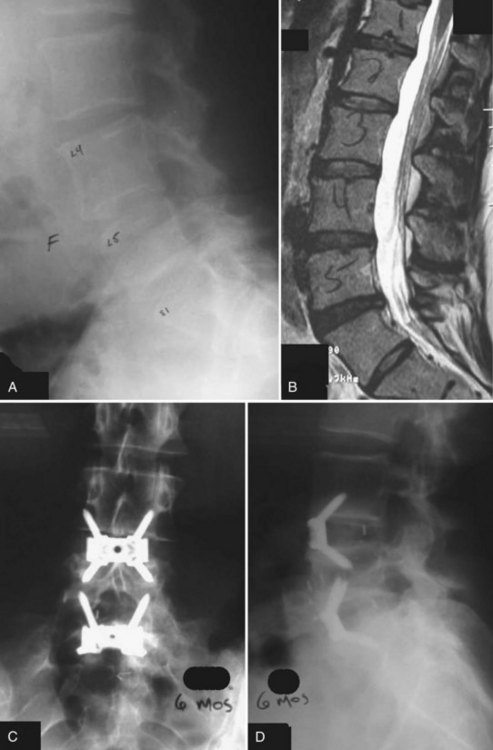
Femoral ring allograft (FRA), used frequently, obviates the need for cortical autograft and provides a strut with significant compressive strength,22,23 incorporates with host bone, and provides a medium easier than metal to evaluate graft incorporation (Fig. 48–3). Previously, allograft rings were fashioned by surgeons on back operating room tables. Now precision machined grafts provide surgeons the option to trial size and implant the most appropriately sized graft to fit the patient’s anatomic needs and obtain the most stable construct with the allograft reaching the dense peripheral ring of subchondral bone. To augment fusion, allograft or autograft cancellous bone can be placed in the center of the cortical ring. FRA as a stand-alone intervertebral spacer has been shown to have a high rate of pseudarthrosis and subsidence.24,25 Anterior or posterior augmentation has been recommended.
Holte and colleagues26 reported in 1994 on the use of FRA with and without supplemental posterior fixation. These investigators achieved fusion rates of 96% in some cases, depending on the number of levels fused. Sarwat and colleagues27 reviewed 43 patients undergoing combined anterior-posterior fusions using FRA packed with cancellous allograft with supplemental posterior fixation; 100% of the one-level fusions and 93% of the two-level fusions were radiographically deemed solid fusions.
The addition of posterior fusion, instrumentation, and cortically lined grafts added to the mechanical stability of these constructs, but surgical time and complications led surgeons to seek other options. Transforaminal lumbar interbody fusion and posterior lumbar interbody fusion allowed surgeons to place interbody grafts from posterior approaches, providing anterior column support and negating anterior exposures. Posterior based surgery has been shown, however, to have increased operative time, patient morbidity, and complications compared with anterior based surgery.28,29 Also, patient anatomy and the degree of pathology can make achieving an adequate discectomy and ideal graft size and position and placement a challenge. These factors have contributed to less restoration of disc height, less than complete fusion bed preparation, and less mechanical stability at the time of implantation than with ALIF.
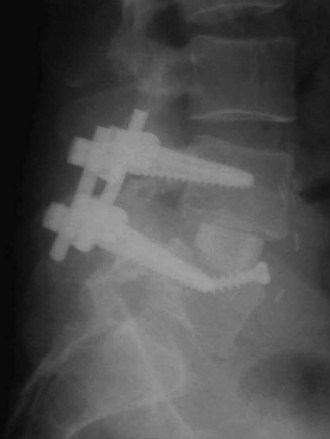
Horizontal threaded cylinder cages, typically made from titanium, were brought on the market with huge fanfare but have fallen out of favor. These were originally developed to treat wobbler syndrome, which is a chronic cervical instability causing myelopathy in thoroughbred horses.30 The U.S. Food and Drug Administration (FDA) investigational device exemption (IDE) studies for the BAK (Zimmer, Warsaw, IN) and Ray TFC (Stryker, Kalamazoo, MI) indicated that threaded cages used as stand-alone devices without supplemental posterior fixation performed well (Fig. 48–4).31,32 Ray32 reported his results during the FDA IDE clinical trial using the Ray titanium fusion cages in 236 cases showing 91% fusion rate and 80% average clinical improvement.
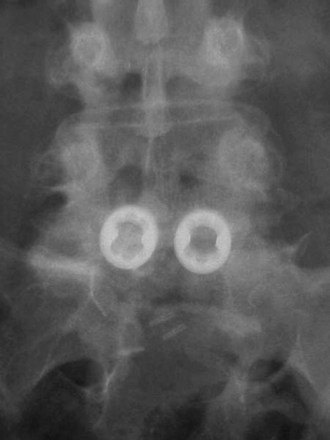
FIGURE 48–4 Anteroposterior radiograph of threaded fusion cages at 1 year, with bone growth across cages.
Kuslich and colleagues31 reported on 247 ALIF patients with 24-month follow-up; they observed a fusion rate of 98% among single-level procedures and 80% among two-level procedures. They reported clinical pain improvement from a mean of 5 to 2.9 at 2 years, on a scale of 0 to 6. The functional scores improved from 20.9 preoperative mean to 15.2 at 2-year follow-up, on a 7 to 32 point scale evaluating sitting, walking, and other activities of daily living and recreational activities. Tran and colleagues33 reported that only 5.2% of their group underwent additional posterior surgery at the same level for unresolved or new-onset pain, supporting the use of stand-alone cages. Preparation of the disc space for threaded cages violates the peripheral subchondral ring of bone, theoretically increasing the risk of subsidence. Although approved for stand-alone interbody fusion, threaded cages were later questioned for their amount of resistance to motion in the unstable spine; some authors adopted a 360-degree approach.34,35
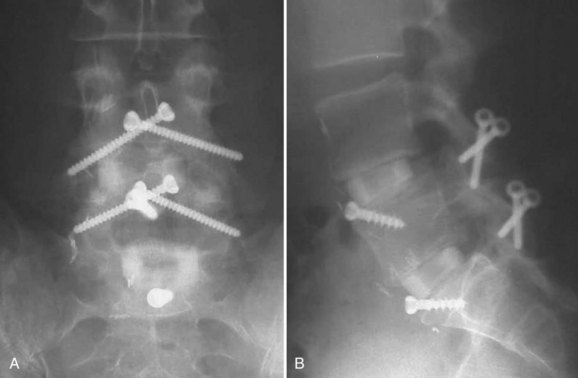
The advent of more stable anterior column support allowed surgeons to use less invasive posterior surgical options with stronger biomechanical constructs than with an associated posterolateral fusion.6 Translaminar screws, facet screws, and percutaneous pedicle screw constructs (termed 270-degree fusions) provided surgeons the additive effect of immediate mechanical strength and long-term fusion stability without necessitating significant posterior fusion bed creation, associated soft tissue devitalization, and patient morbidity (Fig. 48–5).36–38 Ferrara and colleagues39 compared pedicle and translaminar facet screws and found both to be reliable constructs with similar properties after 180,000 cycles and recommended that surgeon preference and patient-specific needs could drive the choice. In 2001, Schofferman and colleagues40 prospectively compared the addition of posterior spinal fusion in patients who had ALIF with pedicle screw instrumentation (360 degrees vs. 270 degrees). These investigators found that posterolateral fusion was associated with greater blood loss, operating room time, and cost, with no significant improvement in the arthrodesis rate; in addition, 68% of posterolateral fusions failed to show radiographic fusion.
Previously, stand-alone anterior interbody grafts were fraught with reported complications, such as migration, subsidence, and pseudarthrosis. As interbody graft technology has progressed, there has been renewed interest, however, in stand-alone anterior interbody fusion, single-approach procedures. Biomechanical studies have reported that stand-alone anterior interbody grafts without fixation are weakest in sheer strength, rotation, and extension.41 The addition of a blocking screw or plate for FRA and synthetic spacers such as polyetheretherketone (PEEK) has minimized the occurrence of anterior migration. Currently available in PEEK, metal carbon fiber, and machined allograft and other various materials and combinations, interbody synthetic grafts have been optimized compared with their predecessors. Cage design from cylindric to a box shape has led to better matching of the endplate geometry and has been shown to decrease motion at the intervertebral segment.42 Grant and colleagues,43 performing a human cadaveric biomechanical investigation, showed that lumbar endplate density and thickness increased toward the periphery, with the strongest being posterolateral just in front of the pedicles.
With the addition of anterior intersegmental instrumentation, many authors are performing stand-alone anterior interbody fusions. In 1958, Humphries and Hawk44 first reported the use of an anterior plate to stabilize the motion segment after ALIF. Potential danger to the great vessels and limited use of the ALIF technique contributed to the early demise of this technique, however. Tzermiadianos and colleagues45 showed marked in vitro reduction in range of motion using interbody graft with the ATB (Synthes Spine, West Chester, PA); they posited that the ATB may be a biomechanically sound construct, especially compared with additional posterior surgery and its morbidity. Gerber and colleagues46 noted slightly better motion segment immobilization using pedicle screws versus an anterior plate in cadaveric spines with double-threaded metallic cages.
Many studies have shown the stability-enhancing effect of integrated anterior instrumentation and a biomechanical argument for stand-alone ALIF (Fig. 48–6). Kuzhupilly and colleagues37 looked at stand-alone FRA versus industrial FRA with integrated crossed cancellous screws into the adjacent vertebral bodies. They found significant improvement in extension stability only. Le Huec and colleagues47 tested an anterolateral threaded cage connected to an anterolateral cage and found significant increase in stiffness in all loading directions. No statistical significance was noted whether or not the plate was attached to the cage.
Schleicher and colleagues48 compared the SynFix-LR (Synthes GmbH, Solothurn, Switzerland) and the STALIF (Centinel Spine, Minneapolis, MN), two stand-alone ALIF cages, and found statistically significant increase in stiffness over the native in vitro segment, with the SynFix-LR, a locking four-screw implant, showing a higher stabilizing effect in lateral bending than the STALIF. Flexion-extension finite element analysis revealed that the cage bears most of the force in flexion, and the screws and the screw-plate interface take most of the stress in extension. Cain and colleagues49 showed the SynFix-LR had equal stiffness versus 270-degree and 360-degree constructs. The test device showed a higher ability to withstand axial torque compared with standard pedicle screw instrumentation. At the present time, a few products have FDA approval for stand-alone interbody use, with many more devices seeking approval.
Most current implants allow the incorporation of graft material to augment the fusion process. Historically, the “gold standard” has been autogenous morcellized bone graft; however, to minimize patient morbidity, allograft and biologic materials have been developed. Multiple studies have evaluated allograft efficacy in fusion patients as extenders and stand-alone with good results.50 The addition of the recombinant human bone morphogenetic protein (rhBMP-2) InFUSE (Medtronic, Memphis, TN) has been shown to promote osteoinduction and to stimulate early incorporation of grafts (Fig. 48–7).51,52 Burkus and colleagues52 reported 100% fusion rate with stand-alone interbody fusion using threaded cylinder allograft dowels with rhBMP compared with autograft. Pradhan and colleagues53 showed, however, that the use of rhBMP-2 with stand-alone FRA can lead to an aggressive early osteoclastic response causing graft and endplate osteolysis with potential subsidence risk. These authors believed augmenting with intersegmental instrumentation can support the FRA with bone morphogenetic protein during this mechanically vulnerable time.
Circumferential Fusion
Circumferential fusion is often indicated in patients with significant instability (i.e., trauma, postlaminectomy), significant bone loss (i.e., trauma, osteomyelitis), high risk for nonunion (i.e., multilevel, tobacco users), or osteoporosis.54,55 To increase fusion rates, decrease subsidence risk, and maximize construct stiffness, many surgeons adopted posterior instrumentation, including pedicle screw–based systems, translaminar screws, or facet fixation. There is no question that the addition of posterior instrumentation provides greater construct rigidity; however, the ideal amount of micromotion to stimulate fusion while structurally stabilizing the motion segment until fusion occurs has yet to be determined. Pedicle screw–based instrumentation has been shown to decrease the subsidence rate associated with interbody allografts and cylindric cages.56 The disadvantages of posterior supplementation include increased operative time, increased blood loss, increased cost, and potentially a more difficult recovery for patients. Factors for and against posterior instrumentation are present in all cases and must be weighed carefully to optimize patient outcome and minimize morbidity.
Postoperative Management
Evaluation of fusion progression can be difficult in the presence of an interbody graft. Evaluation of fusions that is 100% reliable is available only with histologic examination, which is rarely possible or done. Radiolucent and nonferromagnetic implants such as carbon fiber and polymer synthetics have been developed to allow better visualization of surrounding bone and soft tissues on plain radiographs, MRI, and computed tomography (CT). Cizek and Boyd57 evaluated cadaveric implant models radiographically and found neither CT nor radiographic interpretation reliable. Evidence of a fusion can be evaluated by the following radiographic features: (1) no motion on flexion-extension films (<5 degrees has been accepted as fused in the literature and by the FDA, which the authors believe is too lenient); (2) anterior or posterior bridging bone across the disc space, termed sentinel sign58; (3) bridging trabecular bone across the intervertebral space and endplates; and (4) the absence of “a windshield wiping halo,” or lucency around instrumentation indicating implant motion.
Complications
Vascular injury to the common iliac vessels with L5-S1 exposure is most commonly seen. Clear identification of the disc space before discectomy must be obtained and maintained during the operation. In particular, the common iliac vein, being compressible and dorsal to the artery, can be mistaken for soft tissue during the approach. The iliolumbar vein, also termed the ascending lumbar vein, is at risk during approaches to the L4-5 interspace and should be controlled as dictated by the amount of exposure necessary. Some surgeons believe ligation of this vessel should be obtained in 100% of exposures to minimize the risk of tearing during retraction. Arterial thrombosis from aggressive retraction or injury or both has also been reported.59,60
Retrograde ejaculation as result of hypogastric plexus injury has been reported to range from 8%61 to 0.4%.62 In one of the largest single ALIF trials to date, Kuslich and colleagues31 reported a 4% rate in 591 patients. Loguidice and colleagues11 in 58 patients and Brau63 in 686 exposures each noted only 1 case of retrograde ejaculation. The preaortic (prevertebral) sympathetic plexus runs along the anterolateral edge of the vertebral bodies, adjacent to the psoas, then traverses over the aortic bifurcation and common iliac vessels forming the hypogastric plexus. Blunt dissection to mobilize the more cephalad prevertebral plexus before the hypogastric plexus can aid the exposure. Aggressive electrocautery should be minimized during the approach in this area and during the disc space preparation. Male patients should be counseled on this potential adverse event and advised that there also is a chance of spontaneous recovery. If the patient is concerned, he can predonate and store sperm.
Conclusion
Key Points
1 Burkus JK, Gornet MF, Dickman CA, et al. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15:337-349.
2 Brau S. Mini-open approach to the spine for anterior lumbar interbody fusion: Description of the procedure, results and complications. Spine (Phila Pa 1976). 2002;2:216-223.
3 Cain CM, Schleicher P, Gerlach R, et al. A new stand-alone anterior lumbar interbody fusion device: Biomechanical comparison with established fixation techniques. Spine (Phila Pa 1976). 2005;30:2631-2636.
4 Schofferman J, Slosar P, Reynolds J, et al. A prospective randomized comparison of 270 degrees fusions to 360 degrees fusions (circumferential fusions). Spine (Phila Pa 1976). 2001;26:E207-E212.
5 Stauffer RN, Coventry MB. Anterior interbody lumbar spine fusion: Analysis of Mayo Clinic series. J Bone Joint Surg Am. 1972;54:756-768.
1 Katz JN. Lumbar disc disorders and low-back pain: Socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(Suppl 2):21-24.
2 Crock HV. A reappraisal of intervertebral disc lesions. Med J Aust. 1970;16:983-989.
3 Freemont AJ, Peacock TE, Gourille P, et al. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178-181.
4 Weinstein J, Claverie W, Gibson S. The pain of discography. Spine (Phila Pa 1976). 1988;13:1344-1348.
5 Block AR, Ohnmeiss DD, Guyer RD, et al. The use of presurgical psychological screening to predict the outcome of spine surgery. Spine J. 2001;1:274-282.
6 Evans JH. Biomechanics of lumbar fusion. Clin Orthop Relat Res. 1985;193:38-46.
7 McAfee PC, Lee GA, Fedder IL, et al. Anterior BAK instrumentation and fusion: Complete versus partial discectomy. Clin Orthop Relat Res. 2002;394:55-63.
8 Stauffer RN, Coventry MB. Anterior interbody lumbar spine fusion: Analysis of Mayo Clinic series. J Bone Joint Surg Am. 1972;54:756-768.
9 Inoue S, Watanabe T, Goto S, et al. Degenerative spondylolisthesis: Pathophysiology and results of anterior interbody fusion. Clin Orthop Relat Res. 1988;227:90-98.
10 Blumenthal SL, Baker J, Dossett A, et al. The role of anterior lumbar fusion for internal disc disruption. Spine (Phila Pa 1976). 1988;13:566-569.
11 Loguidice VA, Johnson RG, Guyer RD, et al. Anterior lumbar interbody fusion. Spine (Phila Pa 1976). 1988;13:366-369.
12 Regan JJ, Aronoff RJ, Ohnmeiss DD, et al. Laparoscopic approach to L4-L5 for interbody fusion using BAK cages: Experience in the first 58 cases. Spine (Phila Pa 1976). 1999;24:2171-2174.
13 Zdeblick TA, David SM. A prospective comparison of surgical approach for anterior L4-L5 fusion: Laparoscopic versus mini anterior lumbar interbody fusion. Spine (Phila Pa 1976). 2000;25:2682-2687.
14 Escobar E, Transfeldt E, Garvey T, et al. Video-assisted versus open lumbar spine fusion surgery: A comparison of four techniques and complications in 135 patients. Spine (Phila Pa 1976). 2003;28:729-732.
15 Chen D, Fay LA, Lok J, et al. Increasing neuroforaminal volume by anterior interbody distraction in degenerative lumbar spine. Spine (Phila Pa 1976). 1995;20:74-79.
16 Sandu HS, Turner S, Kabo JM, et al. Distractive properties of a threaded interbody fusion device: An in vivo model. Spine (Phila Pa 1976). 1996;21:1201-1210.
17 Craven TG, Carson WL, Asher MA, et al. The effects of implant stiffness on the bypassed bone mineral density and facet fusion stiffness of the canine spine. Spine (Phila Pa 1976). 1994;19:1664-1673.
18 Pilliar RM, Lee JM, Maniatopoulous C. Observations on the effect of movement on bone ingrowth into porous-surfaced implants. Clin Orthop Relat Res. 1986;208:108-113.
19 Burns BH. An operation for spondylolisthesis. Lancet. 1933;1:1233-1239.
20 Harmon PH. Anterior excision and vertebral body fusion operation for intervertebral disk syndromes of the lower lumbar spine: Three-to five-year results in 244 cases. Clin Orthop Relat Res. 1963;26:107-127.
21 Siff TE Kanaric E, Noble PC, et al. Femoral ring versus fibular strut allografts in anterior lumbar interbody arthrodesis: A biomechanical analysis. Spine (Phila Pa 1976). 1999;24:659-665.
22 Janssen ME, Nguyen C, Beckham R, et al. Biologic cages. Eur Spine J. 2000;9(Suppl 1):S102-S109.
23 Summers BN, Eisenstein SN. Donor site pain from the ilium: A complication of lumbar spine fusion. J Bone Joint Surg Br. 1989;71:677-680.
24 Flynn JC, Hogue MA. Anterior fusion of the lumbar spine: End-result study with long-term follow-up. J Bone Joint Surg Am. 1979;61:1143-1150.
25 Kumar A, Kozak JA, Doherty BJ, et al. Interspace distraction and graft subsidence after anterior lumbar fusion with femoral strut allograft. Spine (Phila Pa 1976). 1993;18:2393-2400.
26 Holte DC, O’Brien JP, Renton P. Anterior lumbar fusion using a hybrid interbody graft: A preliminary radiographic report. Eur Spine J. 1994;3:32-38.
27 Sarwat AM, O’Brien JP, Renton P, et al. The use of allograft (and avoidance of autograft) in anterior lumbar interbody fusion: A critical analysis. Eur Spine J. 2001;10:237-241.
28 Fritzell P, Hagg O, Wessberg P, et al. Chronic low back pain and fusion: A comparison of three surgical techniques: A prospective multicenter randomized study from the Swedish Lumbar Spine Study Group. Spine (Phila Pa 1976). 2002;27:1131-1141.
29 Scaduto AA, Gamradt SC, Yu WD, et al. Perioperative complications of threaded cylindrical lumbar interbody fusion devices: Anterior versus posterior approach. J Spinal Disord Tech. 2003;16:502-507.
30 DeBowes RM, Grant BD, Bagby GW, et al. Cervical vertebral interbody fusion in the horse: A comparative study of bovine xenografts and autografts supported by stainless steel baskets. Am J Vet Res. 1984;45:191-199.
31 Kuslich SD, Ulstrom CL, Griffith SL, et al. The Bagby and Kuslich method of lumbar interbody fusion: History, techniques, and 2-year follow-up results of a United States prospective, multicenter trial. Spine (Phila Pa 1976). 1998;23:1267-1278.
32 Ray CD. Threaded titanium cages for lumbar interbody fusions. Spine (Phila Pa 1976). 1997;22:667-679.
33 Tran V, Ohnmeiss DD, Blumenthal SL, et al. Analysis of reoperations when using cages as stand-alone devices: Minimum three year follow-up study. Presented at Meeting of the Americas. New York. 2002.
34 Oxland TR, Lund T. Biomechanics of stand-alone cages and cages in combination with posterior fixation: A literature review. Eur Spine J. 2000;9(Suppl 1):S95-S101.
35 Cagli SJ, Crawford NR, Sonntag VK, et al. Biomechanics of grade I degenerative lumbar spondylolisthesis. Part 2: Treatment with threaded interbody cages/dowels and pedicle screws. J Neurosurg. 2001;94(1 Suppl):51-60.
36 Rathonyi GC, Oxland TR, Gerich U, et al. The role of supplemental translaminar screws in anterior lumbar interbody fixation: A biomechanical study. Eur Spine J. 1998;7:400-407.
37 Kuzhupilly RR, Lieberman IH, McLain RF, et al. In vitro stability of FRA spacers with integrated crossed screws for anterior lumbar interbody fusion. Spine (Phila Pa 1976). 2002;27(9):923-928.
38 Volkman T, Horton WC, Hutton WC. Transfacet screws with lumbar interbody reconstruction: Biomechanical study of motion segment stiffness. J Spinal Disord. 1996;9:425-432.
39 Ferrara LA, Secor JL, Jin BH, et al. A biomechanical comparison of facet and pedicle screw fixation: Effects of short-term and long-term repetitive cycling. Spine (Phila Pa 1976). 2003;28:1226-1234.
40 Schofferman J, Slosar P, Reynolds J, et al. A prospective randomized comparison of 270 degrees fusions to 360 degrees fusions (circumferential fusions). Spine (Phila Pa 1976). 2001;26:E207-E212.
41 Pizen T, Matthis D, Steudel WI. The effect of posterior instrumentation following PLIF with BAK cages is most pronounced in weak bone. Acta Neurochir (Wien). 2002;144:121-128.
42 Tsantrizos A, Andreou A, Aebi M, et al. Biomechanical stability of five stand-alone anterior lumbar interbody fusion constructs. Eur Spine J. 2000;9:14-22.
43 Grant JP, Oxland TR, Dvorak MF. Mapping the structural properties of the lumbosacral vertebral endplates. Spine (Phila Pa 1976). 2001;26:889-896.
44 Humphries AW, Hawk WA. Anterior fusion of the lumbar spine using an internal fixative device. Surg Forum. 1958;9:770-773.
45 Tzermiadianos MN, Mekhail A, Voronov LI, et al. Enhancing the stability of anterior lumbar interbody fusion: A biomechanical comparison of anterior plate versus posterior transpedicular instrumentation. Spine (Phila Pa 1976). 2008;33:E38-E43.
46 Gerber M, Crawford NR, Chamberlain RH, et al. Biomechanical assessment of anterior lumbar interbody fusion with an anterior lumbosacral fixation screw-plate: Comparison to stand-alone anterior lumbar interbody fusion and anterior lumbar interbody fusion with pedicle screws in an unstable human cadaver model. Spine (Phila Pa 1976). 2006;31:762-768.
47 Le Huec J, Liu M, Skalli W, et al. Lumbar lateral interbody cage with plate augmentation: In vitro biomechanical analysis. Eur Spine J. 2002;11:130-136.
48 Schleicher P, Gerlach R, Schar B, et al. Biomechanical comparison of two different concepts for stand alone anterior lumbar interbody fusion. Eur Spine J. 2008;17:1757-1765.
49 Cain CM, Schleicher P, Gerlach R, et al. A new stand-alone anterior lumbar interbody fusion device: Biomechanical comparison with established fixation techniques. Spine (Phila Pa 1976). 2005;30:2631-2636.
50 Hashimoto T, Shigenobu K, Kanayama M, et al. Clinical results of single-level posterior lumbar interbody fusion using the Brantigan I/F carbon cage filled with a mixture of local morselized bone and bioactive ceramic granules. Spine (Phila Pa 1976). 2002;27:258-262.
51 Boden SD, Zdeblick TA, Sandhu HS, et al. The use of rhBMP-2 in interbody fusion cages: Definitive evidence of osteoinduction in humans: A preliminary report. Spine (Phila Pa 1976). 2000;25:376-381.
52 Burkus JK, Gornet MF, Dickman CA, et al. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15:337-349.
53 Pradhan BB, Bae HW, Kropf MA, et al. Kyphoplasty reduction of osteoporotic vertebral compression fractures: Correction of local kyphosis versus overall sagittal alignment. Spine (Phila Pa 1976). 2006;31:435-441.
54 O’Brien JP, Dawson MH, Heard CW. Simultaneous combined anterior and posterior fusion: A surgical solution for failed spinal surgery with a brief review of the first 150 patients. Clin Orthop. 1986;203:191-195.
55 Enker P, Steffe AD. Interbody fusion and instrumentation. Clin Orthop. 1994;300:90-101.
56 Shetty AP, Osti OL, Abraham G, et al. Cylindrical threaded cages for lumbar degenerative disc disease: A prospective long term radiological study. Presented at International Society for the Study of the Lumbar Spine, Adelaide, Australia. 2000.
57 Cizek GR, Boyd LM. Imaging pitfalls of interbody spinal implants. Spine (Phila Pa 1976). 2000;25:2633-2636.
58 McAfee PC. Interbody fusion cages in reconstructive operations on the spine. J Bone Joint Surg Am. 1999;81:859-880.
59 Rajaraman V, Vingan R, Roth P, et al. Visceral and vascular complications resulting from anterior lumbar interbody fusion. J Neurosurg. 1999;91(1 Suppl):60-64.
60 Hackenberg L, Liljenqvist U, Halm H, et al. Occlusion of the left common iliac artery and consecutive thromboembolism of the left popliteal artery following anterior lumbar interbody fusion. J Spinal Disord. 2001;14:365-368.
61 Christensen FB, Bunger CE. Retrograde ejaculation after retroperitoneal lower lumbar interbody fusion. Int Orthop. 1997;21:176-180.
62 Flynn JC, Price CT. Sexual complications of anterior fusion of the lumbar spine. Spine (Phila Pa 1976). 1984;9:489-492.
63 Brau S. Mini-open approach to the spine for anterior lumbar interbody fusion: Description of the procedure, results and complications. Spine (Phila Pa 1976). 2002;2:216-223.