CHAPTER 19 Anterior Exposure to Lumbosacral Spine
Anatomy and Techniques
Anatomy
Vascular Anatomy: Variability, Bifurcations, Iliolumbar Vein, Segmental Vessels, and Middle Sacral Vessels
Surgical Approach
Preoperative Considerations
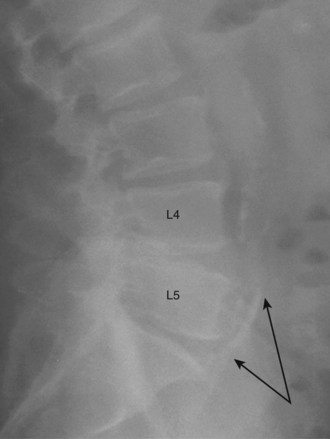
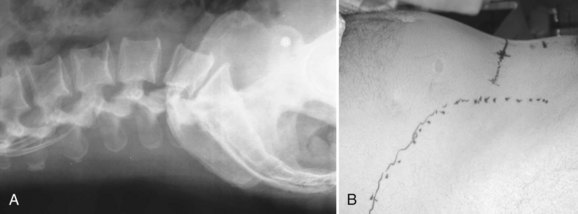
Communication is the mainstay to a successful partnership between the spine surgeon and access surgeon and the supporting surgical team. Before the day of surgery, relevant radiologic imaging will have been obtained and reviewed to determine the surgical plan. Any additional imaging should be pursued at that time to take into account disease processes that the patient may have. Examining plain films for signs of arterial calcifications, which may provide indirect evidence of vascular disease (Fig. 19–1), is particularly useful for the access surgeon. Additionally, the plain film should be evaluated for osteophytes and spondylolisthesis, which can exaggerate misleading anatomic bony features or be an indicator of potential inflammatory changes. A thorough vascular examination should be repeated at the time of surgery. Pulse oximetry on the patient’s left lower extremity is a useful adjunct for monitoring arterial flow distal to the area of dissection. Positioning of the patient is important to facilitate intraoperative placement of personnel, fixed retraction, and fluoroscopy machines.
Open Approaches
Retroperitoneal
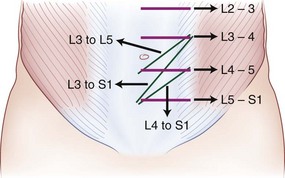
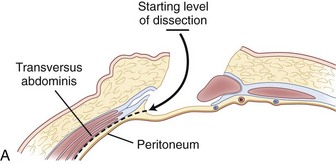
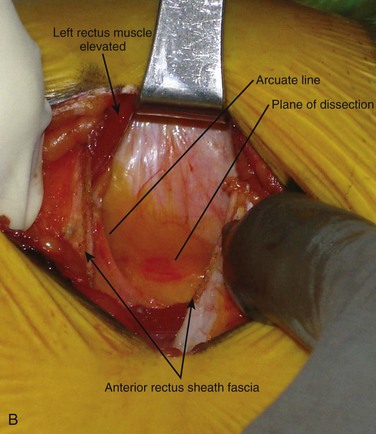
The retroperitoneal approach can proceed from various incisions, including vertical midline, paramedian, oblique, and transverse. Extensive lateral incisions should be avoided because this may denervate medially situated rectus muscle. The incision needs to take into account the spinal level and the number of lumbar levels to be exposed. An infraumbilical transverse incision can accommodate most approaches to the L4-5 and L5-S1 disc levels, whereas a more obliquely oriented incision is favored for access to disc levels above L4 (Figs. 19-2 and 19-3). This incision allows for access to the L2-3 disc level and possibly L1-2 disc level in patients with a favorable body habitus.
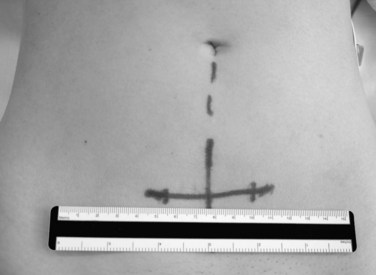
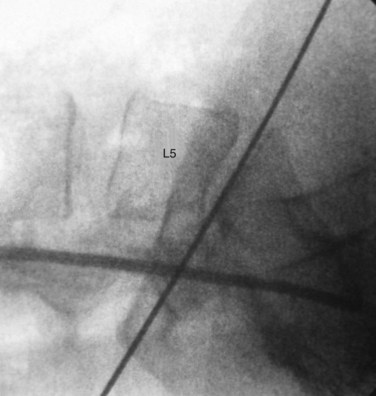
Lateral fluoroscopy can be used to assess the level of the incision (Fig. 19–4). This imaging is done in conjunction with a radiopaque probe or rod to determine the angle of the approach and level, which can be marked on the patient’s abdomen. Fluoroscopy can be especially important in obese patients because there may be no other anatomic landmarks palpable to guide the placement of the abdominal incision (Fig. 19–5). In most nonobese patients, palpation by an experienced access surgeon can be used to locate the sacral prominence to identify the L5-S1 disc space. A transverse approach to the L4-5 level should be directed at approximately the level of the superior anterior iliac spine, although fluoroscopic confirmation should be used if there is any question. All of the landmarks can be confirmed by fluoroscopic guidance, and fluoroscopy is especially important in patients who have distorted spine anatomy, had prior spine surgery, or are obese.
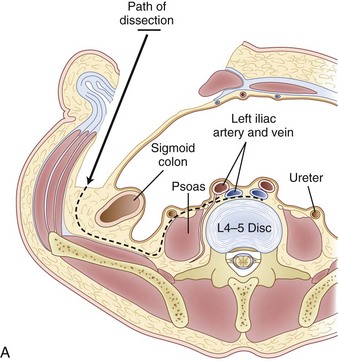
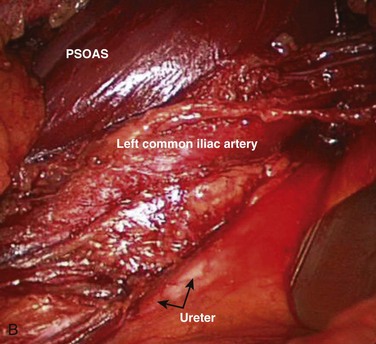
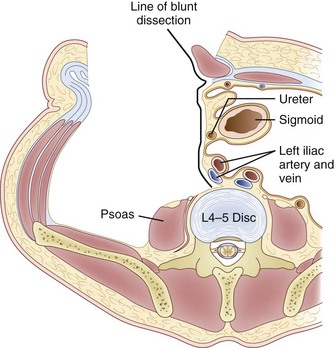
Inspection and gentle finger dissection can be used to identify the inferior edge of the posterior rectus sheath. This provides the landmark to begin blunt separation of the peritoneal cavity laterally to start the exposure into the retroperitoneal space (Fig. 19–6). Standing on the patient’s right side allows the access surgeon to take advantage of the natural motion of his or her hand to roll the peritoneum in a medial direction, although many surgeons proceed with the approach from the left side to maintain direct visualization of the vasculature (Fig. 19–7). The left ureter easily rolls up as part of this maneuver. The landmark to feel for is the bulge of the psoas muscle and the pulse of the common iliac artery. A common mistake is to persist in aggressive dissection lateral to the psoas muscle thinking that the planes may be adhesed. In almost all virgin retroperitoneal spaces, the blunt dissection should be able to be accomplished easily and without use of force (Fig. 19–8).
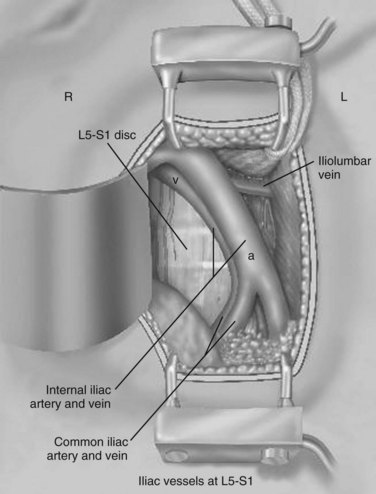
The L5-S1 disc space can be mobilized with the use of Kittner dissectors. The middle sacral vessels can be mobilized and divided between ligatures or small clips. The surgeon should be judicious in the use of clips because they may become dislodged or caught during dissection of the spine. Blunt dissection generally can be used to expose the entire face of the L5-S1 disc level without significant need for major vascular mobilization (Fig. 19–9). Care should be taken during the mobilization to elevate the superior hypogastric plexus with the peritoneum. This plexus typically feels like a fibrous band within the peritoneal fat and typically elevates with the peritoneal packet.
It is important to divide the vessel securely when there appears to be tethering because excessive traction on this structure can cause tearing with associated significant blood loss. Additional mobilization of the segmental vessels immediately adjacent and superior to the L4-5 region is sometimes necessary (Fig. 19–10). Pulse oximetry is important when mobilizing the L4-5 disc space or higher. Before retraction or mobilization, the oxygen saturation and waveform of a left lower extremity pulse oximeter should be evaluated. When retraction has been applied, if the saturation diminishes to zero, the surgeon has approximately 45 to 50 minutes before the retraction should be released to allow for resumption of unimpeded blood flow. The waveform should normalize over a brief period before replacement of the retractors. This can be repeated with periodic release of the retractors every 30 minutes or so after that. If there is not a return to baseline over a few minutes, vascular examination with ultrasound should be performed to minimize the risk of a missed injury or thrombosis.
Laparoscopic or Robotic Approach
Laparoscopic or robotic minimally invasive approaches have been used over the last 10 years as more attention has been given to overall patient recovery and length of stay. The immediacy of improvement with minimally invasive spine approaches has not followed the same pathway as for other general surgical procedures. The improvements in length of stay and cosmesis are sometimes seen but usually at a tradeoff of prolonged operative times and increased complication rates. These approaches are technically more complicated and are not in uniform or widespread use and have generally been superseded by the mini-open approach.1–3
Complications
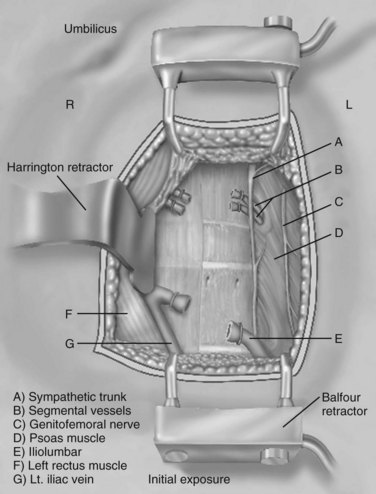
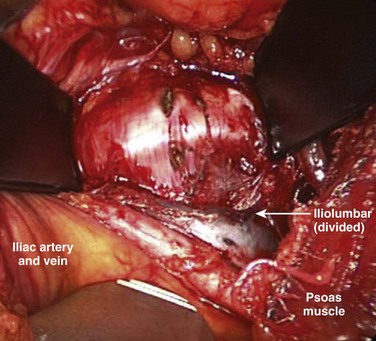
When the primary retractors are set to hold the peritoneum, the vascular dissection can begin (either under direct vision or with laparoscopic visualization). In noninflamed tissue, gentle vascular mobilization can be accomplished with the use of peanut dissectors and a vein retractor. Clearly identified venous branches should be divided between ligatures to minimize avulsion injuries. The left iliolumbar vein can be easily avulsed during the exposure of the L4-5 disc space if this is not controlled during vascular mobilization (Fig. 19–11). The variable location of this vein may account for part of the significantly higher rate of vascular injury at this level.4 This vascular injury can lead to significant bleeding, which may be difficult to control if the distal end retracts. Small (<1 mm) venous branches can be cauterized to decrease the risk of larger venous avulsion injury. Most small venous tears can be controlled with mild direct pressure with or without topical thrombotic agents. Venous injuries that are on an exposed surface or in the angle of retraction should be controlled with a small monofilament suture on a taper needle if necessary. This suture decreases the risk of propagation into a large venous injury; however, it may increase the risk of postoperative thrombosis at the site of repair.
The arterial and venous structures are at risk during the period of instrumentation. Vessels that are under tension are more susceptible to extensive sharp injury than when in situ. Both structures can be traumatized by instrumentation against fixed field retraction to cause a scissoring or pinching effect. The ureter is also at risk for this type of injury. Arterial bruising can propagate a small vascular wall hematoma into a thrombotic event secondary to direct or indirect trauma. Thrombosis of the left common iliac artery is the most common arterial complication.5–7 “Snap” injuries can also occur where the artery is quickly moved from one position to another. In patients who have palpable atherosclerotic plaque disease, there may be an increased risk of thromboembolic events. It is important to perform a full vascular mobilization of the left common and external iliac arteries to decrease the potential risk of injury secondary to stretch or angulation. This injury can give rise to the formation of a plaque lip, which could give rise to a mural thrombosis, which could then propagate. It is important to check frequently the pulse examination of the distal extent of the exposed left external and internal iliac arteries and to use continuous pulse oximetry to minimize potential rare arterial injuries.
Few nerve structures are directly at risk during lumbosacral exposure. The hypogastric nerve is a potential source of injury, however, with significant clinical consequences, especially in men. The expected rate of retrograde ejaculation should be less than 1%, but the reported rate is 0 to 5.9%.8 The incidence of retrograde ejaculation is increased 10-fold with a transperitoneal approach versus a retroperitoneal approach.9 Expectant management shows resolution of the problem in 50% to 80% of patients over 6 to 12 months; however, it is best avoided by a retroperitoneal approach and careful dissection. Sympathetic injury can lead to a hyperthermic response in the corresponding left lower extremity with subsequent feeling of increased warmth and mild edema. This condition also resolves over 6 to 12 months, but permanent autoregulatory dysfunction can occur in rare cases. Additional nerve injuries are rare and are usually related to compression injuries of the lateral abdominal wall somatic nerves causing temporary dermal hypoesthesias.
Infectious complications, although rare, can be devastating and educational. Superficial wound infections can be treated with local drainage and antibiotic therapy and rarely lead to deep infection. Deep space infection is rare and occurs in less than 1% of patients.10 Presentation of deep infection would lead to concern over a possible missed injury with subsequent field contamination, although this can be supported further by culture results. Late presentation may occur years after the initial operation.
Conclusion
Acknowledgments
The authors extend special thanks to Sal Brau.
1 Kaiser MG, Haid RWJr, Subach BR, et al. Comparison of the mini-open versus laparoscopic approach for anterior lumbar interbody fusion: A retrospective review. Neurosurgery. 2002;51:97-103.
2 Brau SA, Delamarter RB, Schiffman ML, et al. Vascular injury during anterior lumbar surgery. Spine J. 2004;4:409-412.
3 Sasso RC, Kenneth Burkus J, LeHuec JC. Retrograde ejaculation after anterior lumbar interbody fusion: Transperitoneal versus retroperitoneal exposure. Spine. 2003;28:1023-1026.
4 Faciszewski T, Winter RB, Lonstein JE, et al. The surgical and medical perioperative complications of anterior spinal fusion surgery in the thoracic and lumbar spine in adults: A review of 1223 procedures. Spine. 1995;20:1592-1599.
1 Chung SK, Lee SH, Lim SR, et al. Comparative study of laparoscopic L5-S1 fusion versus open mini-ALIF, with a minimum 2-year follow up. Eur Spine J. 2003;12:613-617.
2 Kaiser MG, Haid RWJr, Subach BR, et al. Comparison of the mini-open versus laparoscopic approach for anterior lumbar interbody fusion: A retrospective review. Neurosurgery. 2002;51:97-103.
3 Zdeblick TA, David SM. A prospective comparison of surgical approach for anterior L4-L5 fusion: Laparoscopic versus mini anterior lumbar interbody fusion. Spine. 2000;25:2682-2687.
4 Hamdan AD, Malek JY, Schermerhorn ML, et al. Vascular injury during anterior exposure of the spine. J Vasc Surg. 2008;48:650-654.
5 Oskouian RJJr, Johnson JP. Vascular complications in anterior thoracolumbar spinal reconstruction. J Neurosurg. 2002;96:1-5.
6 Kulkarni SS, Lowery GL, Ross RE, et al. Arterial complications following anterior lumbar interbody fusion: Report of eight cases. Eur Spine J. 2003;12:55-56.
7 Brau SA, Delamarter RB, Schiffman ML, et al. Vascular injury during anterior lumbar surgery. Spine J. 2004;4:409-412.
8 Tiusanen H, Seitsalo S, Osterman K, et al. Retrograde ejaculation after anterior interbody fusion. Eur Spine J. 1995;4:339-342.
9 Sasso RC, Kenneth Burkus J, LeHuec JC. Retrograde ejaculation after anterior lumbar interbody fusion: Transperitoneal versus retroperitoneal exposure. Spine. 2003;28:1023-1026.
10 Faciszewski T, Winter RB, Lonstein JE, et al. The surgical and medical perioperative complications of anterior spinal fusion surgery in the thoracic and lumbar spine in adults: A review of 1223 procedures. Spine. 1995;20:1592-1599.