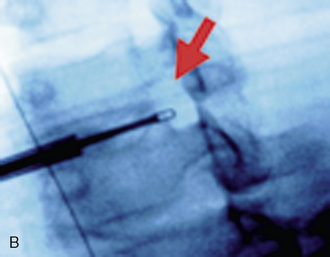
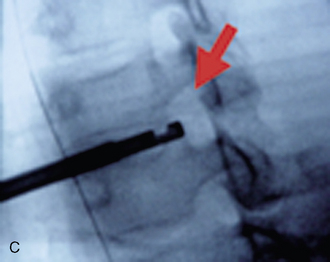
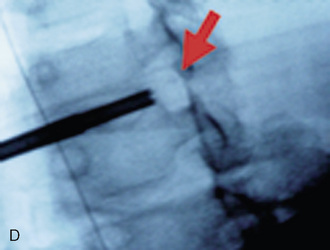
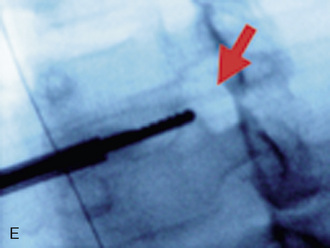
Chapter 22 Anterior endoscopic cervical microdecompression of disc and foramen
The standard treatment for cervical disc protrusions and foraminal stenosis ha s been anterior cervical microdecompression of the disc and foramen with or without bony fusion [1–5]. These open operations are associated with significant local morbidity [6,7], such as graft collapse, graft extrusion, hardware failure, nonfusion with resultant instability, infections, esophageal perforation with infection, and permanent pain, peripheral nerve injury, or infection at the graft donor site. Anterior cervical fusion (ACF) is associated with a 15% or greater chance of junctional disc herniation or adjacent segment disease at interspaces adjacent to fused levels [8,9].
The evolution of spinal surgery is trending toward less invasive techniques [9–16]. Advancements in microinstrumentation, fiberoptics, improved fluoroscopic imaging, and high-resolution digital video imaging endoscopy, along with the accumulation of experience in percutaneous lumbar discectomy [17–20] and spinal laser applications [20–23], have facilitated the development of anterior endoscopic cervical microdecompression (AECM) and foraminal decompression [9,14,19]. AECM, as minimally invasive surgery, does not affect the stability of adjacent vertebral segments [8–10]. Although ACF is often an unattractive treatment for patients with multiple-level disc symptoms, AECM can be safely utilized for treatment of such patients.
Treatment objectives
The primary objective of AECM is to perform decompression of the herniated cervical disc and foraminal disc. It is a minimally invasive outpatient procedure that aims to reduce tissue trauma with much less morbidity than open cervical spinal surgery [9,10,12]. There is no graft donor site to cause secondary problems, and the period of convalescence and the costs of the procedure are significantly less than those of traditional open operations.
Indications
The indications for AECM are as follows [9,12,19]:
 Physical findings of sensory loss, muscle weakness, and/or decreased reflexes in the upper extremities that correlate with the level of involvement
Physical findings of sensory loss, muscle weakness, and/or decreased reflexes in the upper extremities that correlate with the level of involvement Magnetic resonance imaging (the imaging study of choice) or computed tomography findings of disc herniation consistent with the dermatome of clinical symptoms
Magnetic resonance imaging (the imaging study of choice) or computed tomography findings of disc herniation consistent with the dermatome of clinical symptomsContraindications
Advantages
The advantages of AECM in comparison with open procedures are as follows [8,9,12,19,22]:
Instrumentation
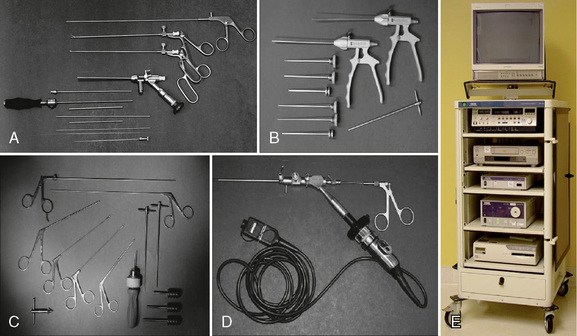
The following instruments and equipment are needed for AECM (Fig. 22-1) [9,12]:
 Endoscopic tower equipped with digital video monitor, DVT/VHS recorder, light source, photo printer, and tri-chip digital camera system.
Endoscopic tower equipped with digital video monitor, DVT/VHS recorder, light source, photo printer, and tri-chip digital camera system. Cervical endoscopic discectomy set (Karl Storz, Tuttlingen, Germany), including 4-mm, 0-degree endoscope.
Cervical endoscopic discectomy set (Karl Storz, Tuttlingen, Germany), including 4-mm, 0-degree endoscope. Cervical discectomy sets (2.5 and 3.5 mm) (Blackstone Medical, Inc., Springfield, MA) with short cervical discectomes.
Cervical discectomy sets (2.5 and 3.5 mm) (Blackstone Medical, Inc., Springfield, MA) with short cervical discectomes. More aggressively toothed trephines used for spurs and spondylitic ridges at the anterior and posterior disc space.
More aggressively toothed trephines used for spurs and spondylitic ridges at the anterior and posterior disc space. Holmium:yttrium-aluminum-garnet (Ho:YAG) laser generator (Trimedyne, Inc., Irvine, CA) with right-angle (side-firing) probe (Fig. 22-2).
Holmium:yttrium-aluminum-garnet (Ho:YAG) laser generator (Trimedyne, Inc., Irvine, CA) with right-angle (side-firing) probe (Fig. 22-2).Procedure
Patient Positioning
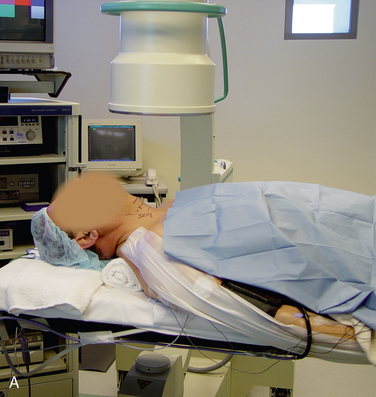
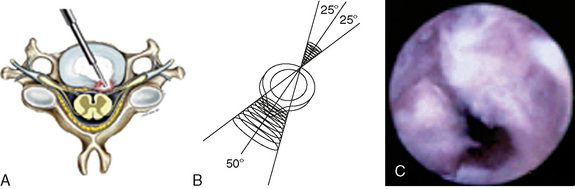
Figure 22-3 illustrates patient positioning for AECM [9,12]:
Fluoroscopy and Neurophysiologic Monitoring
Figure 22-3 illustrates the monitoring equipment used for AECM [9,12]:
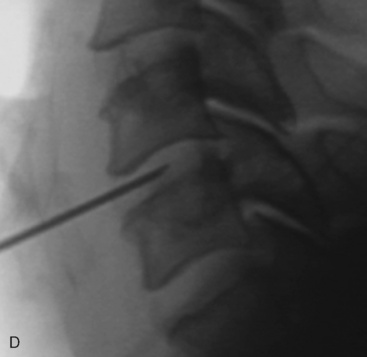
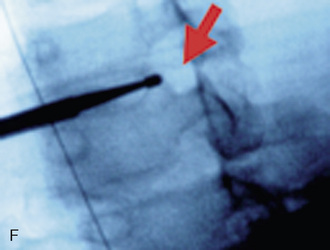
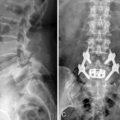
 Digital C-arm fluoroscopy (see Fig. 22-3) is used in the anteroposterior and lateral planes to control the placement of all instruments throughout the operation.
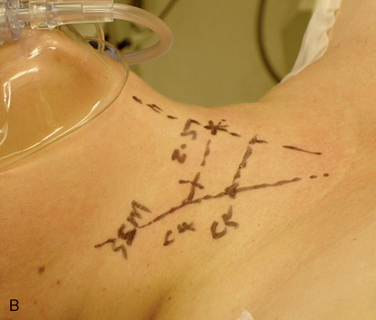
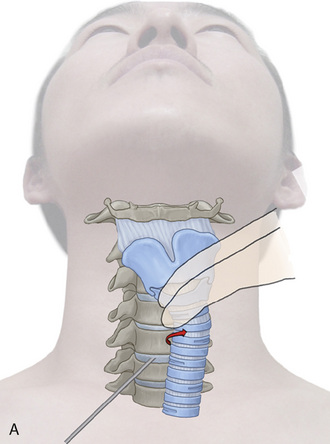
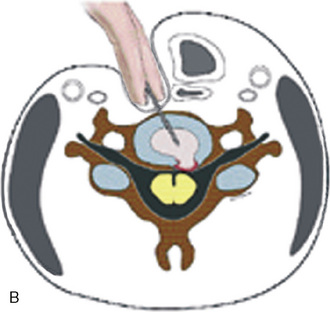
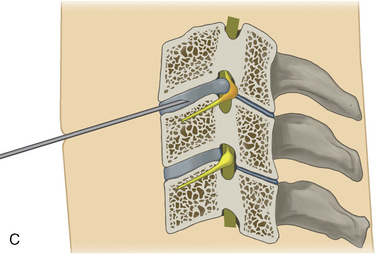
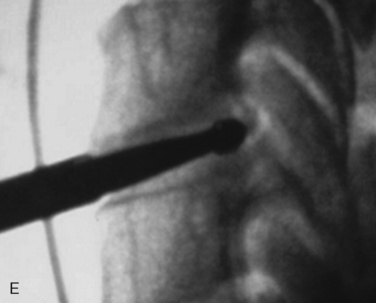
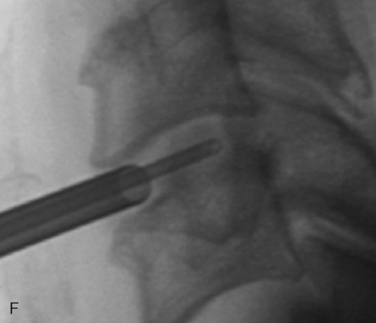
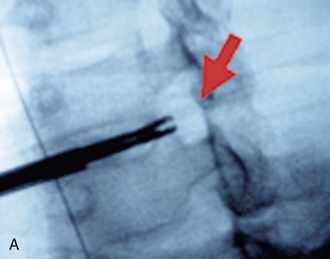
Digital C-arm fluoroscopy (see Fig. 22-3) is used in the anteroposterior and lateral planes to control the placement of all instruments throughout the operation.Localization and Point of Entry
Surgical Technique
The surgical technique for AECM is as follows [8,9,12,19,22]:
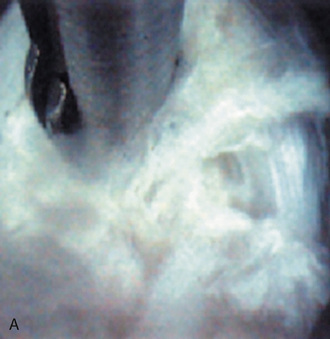
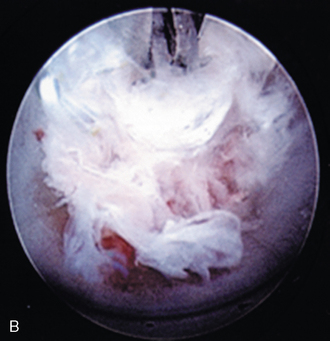
Figure 22–6 Endoscopic views of AECM with forceps removing disc material (A) and removed disc material (B).
Table 22.1 Laser Settings for Cervical Laser Thermodiscoplasty*
| Stage | Watts | Joules |
|---|---|---|
| First | 8 | 300 |
| Second | 5 | 200 |
* Nonablative levels of laser energy are used—at 10 Hz for 5 seconds on and 5 seconds off.
Postoperative managment
Postoperatively, patients are ambulatory within 1 hour and are discharged subsequently. Patients may shower the following day. A soft cervical collar is used for 2 or 3 days or as needed. Ice packs are helpful; mild analgesics and muscle relaxants are required at times. Progressive neck exercise begins on the second postoperative day. Patients are allowed to return to work in 1 to 2 weeks, provided that heavy labor and prolonged sitting are not involved [8,9,12,19,22].
Complications
The complications of AECM, as well as their prevention or treatment, are as follows [12]:
Neural injury is extremely rare with minimally invasive approaches. No spinal cord injuries have been reported. Nerve root and spinal cord injury, though possible, can be avoided with continuous intraoperative EMG neurophysiologic monitoring and direct endoscopic visualization. Neural complications of ACF, including hypoglossal, spinal accessory, phrenic, auricular and cutaneous nerves, can be prevented by careful technique. Recurrent laryngeal nerve injury, though a recognized complication of ACF, is extremely rare with AECM. One case of postoperative hiccough and one of postoperative hoarseness have occurred transiently out of 1200 cases of AECM [12].
Sympathetic nerve injury is extremely rare but can occur from injury to cervical sympathetic and stellate ganglions. One case of incidental transient Horner syndrome or oculosympathetic dysfunction lasting 1 day following AECM was noted [12].
Dural tears which is one of the complications of ACF have not been reported after AECM.
Conclusion
CASE STUDY 22.1
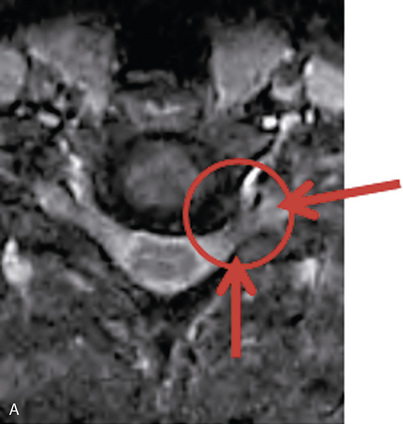
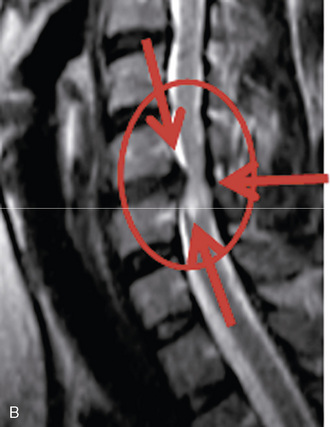
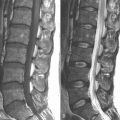
A 35-year-old professional musician with increasing and intractable neck and upper extremity pain and numbness of the fingers was unable to perform because of his condition. Magnetic resonance imaging showed a large left foraminal herniated C5-C6 disc compressing the C6 nerve root (Fig. 22-9). AECM and left C5-C6 foraminal decompressive discectomy and foraminoplasty provided immediate relief of all the patient’s symptoms.
1 Ascher P.W. Application of the laser in neurosurgery. Lasers Surg Med. 1986;2:91-97.
2 Bailey R.W., Badgley C.E. Stabilization of the cervical spine by anterior fusion. J Bone Joint Surg Am. 1958;40A:607-624.
3 Cloward R.B. The anterior approach for removal of ruptured cervical discs. J Neurosurg. 1958;15:602-617.
4 Robinson R.A., Smith G.W. Anterolateral cervical disc removal and interbody fusion for cervical disc syndrome. Bull Johns Hopkins Hosp. 1955;96:223-224.
5 Robertson J.T. Anterior removal of cervical disc without fusion. Clin Neurosurg. 1973;20:259-261.
6 McCulloch J., Young P. Essentials of Spinal Microsurgery. Philadelphia: Lippincott-Raven, 1998;209-215.
7 Shea M., Takeuchi T.Y., Wittenberg R.H., et al. A comparison of the effects of automated percutaneous diskectomy and conventional diskectomy on intradiscal pressure, disc geometry, and stiffness. J Spinal Disord. 1994;7:317-325.
8 Chiu J., Clifford T., Princenthal R. Junctional disc herniation in post spinal fusion treated with endoscopic spine surgery. Surg Technol Int. 2005;14:305-315.
9 Chiu J., Clifford T., Sison R. Anterior endoscopic cervical microdiscectomy. In: Savitz M., Chiu J., Rauschning W., Yeung A., editors. The Practice of Minimally Invasive Spinal Technique. 2005 ed. New City, NY: AAMISS Press; 2005:409-414.
10 Lee S.H. Comparison of percutaneous endoscopic discectomy to open anterior discectomy for cervical herniations. J Minim Invasive Spinal Techn. 2001;1:17-19.
11 Hijikata S. Percutaneous nucleotomy: A new concept of technique and 12 years experience. Clin Orthop Relat Res. 1989;238:9-23.
12 Chiu J. Anterior endoscopic cervical microdiscectomy. In: Kim D., Fessler R., Regan J., editors. Endoscopic Spine Surgery and Instrumentation. New York: Thieme; 2004:48-58.
13 Onik G., Mooney V., Maroon J.C., et al. Automated percutaneous discectomy: A prospective multi-institutional study. Neurosurgery. 1990;26:228-233.
14 Chiu J. Digital technology assisted minimally invasive spinal surgery (MISS) for spinal motion preservation. In: Lemke H.U., Vannier M.N., Invamura R.D., editors. Computer Assisted Radiology and Surgery. London: Elsevier; 2004:461-466.
15 Krause D., Drape J.L., Jambon F., et al. Cervical nucleolysis: Indications, technique, results: 190 patients. J Neuroradiol. 1993;20:42-59.
16 Kambin P. Posterolateral percutaneous lumbar discectomy and decompression: Arthroscopic microdiscectomy. In: Kambin P., editor. Arthroscopic Microdiscectomy: Minimal Intervention in Spinal Surgery. Baltimore: Urban & Schwarzenberg; 1991:67-100.
17 Mayer H.M., Brock M. Percutaneous endoscopic discectomy: Surgical technique and preliminary results compared to microsurgical discectomy. J Neurosurg. 1993;78:21-25.
18 Schreiber A., Suezawa Y., Leu H.J. Does percutaneous nucleotomy with discoscopy replace conventional discectomy? Eight years of experience and results in treatment of herniated lumbar disc. Clin Orthop Relat Res. 1989;238:35-42.
19 Chiu J.C., Hansraj K.K., Akiyama C., Greenspan M. Percutaneous (endoscopic) decompressive discectomy for non-extruded cervical herniated nucleus pulposus. Surg Technol Int. 1997;6:405-411.
20 Chiu J. Evolving transforaminal endoscopic microdecompression for herniated lumbar discs and spinal stenosis. Surg Technol Int. 2004;13:276-286.
21 Yonezawa T., Onomura T., Kosaka R., et al. The system and procedures of percutaneous intradiscal laser nucleotomy. Spine. 1990;15:1175-1185.
22 Chiu J., Clifford T., Greenspan M. Percutaneous microdecompressive endoscopic cervical discectomy with laser thermodiskoplasty. Mt Sinai J Med. 2000;67:278-282.
23 Chiu J., Savitz M. Multicenter study of percutaneous endoscopic discectomy. In: Savitz M., Chiu J., Rauschning W., Yeung A., editors. The Practice of Minimally Invasive Spinal Technique. 2005 ed. New City, NY: AAMISS Press; 2005:622-626.