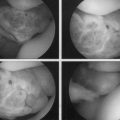
CHAPTER 20 Anterior Cruciate Ligament Repair
Anterior cruciate ligament (ACL) repair is not a new idea, having first been reported by the Mayo Robinson in 1885.1 Prior to 1970, little emphasis was given to repairing the ACL. Most techniques centered on repairing extra-articular structures with the idea that a competent ACL was not required for normal knee function. Conservative treatment of ACL tears resulted in poor healing rates and a high incidence of instability.2,3 Primary repair alone did not improve results.4–6 Reasons for this lack of healing potential were thought to be lack of blood clot formation, insufficient blood supply, differences in intrinsic cell migration, impaired growth factor ability, and the effect of synovial fluid on cell morphology.7,8
In the 1970s, Marshall and colleagues9 increased interest in repairing ACL tears; their technique used primary suture repair. Good results with the repair of proximal tears were obtained. Two randomized studies2,3 have shown no difference in functional outcomes between repair and conservative treatment with about one third in each group having residual instability. Subsequent focus centered on reconstruction of the torn ACL rather than repair.10 With better understanding of ACL anatomy, including ligamentous function and insertion footprint anatomy, interest in repair of the ACL has resurfaced. Recent basic science research11–15 looking at growth factors, gene therapy, and tissue-friendly scaffolds has shown promise for future progress in ACL repair.
ANATOMY
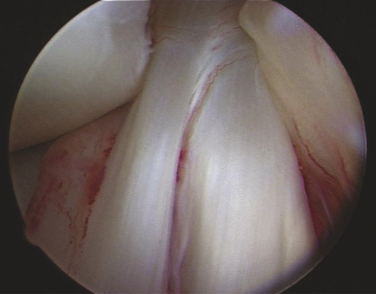
The ACL has been described anatomically in two bundles, one anterior medial (AM) and one posterior lateral (PL; Fig. 20-1). Whereas the primary function of the larger anterior medial bundle is to resist anterior translation of the tibia on the femur, the posterior lateral bundle also resists abnormal anterior lateral rotation of the tibia on the femur.
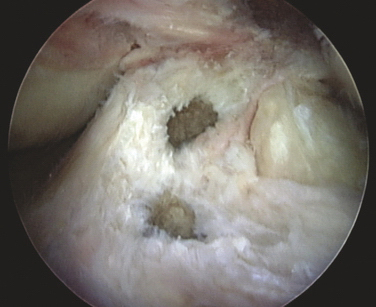
This attention to multiple ligamentous fiber attachment sites has led to the concept of ACL femoral and tibial footprints (Fig. 20-2). A goal of preserving or reconstructing attachment sites corresponding to the footprints should more closely restore the natural function of the ACL.
The ACL arises from a fossa in front of and lateral to the anterior spine in a footprint that averages 11 mm medial to lateral and 17 mm front to back.16 Its average length is 38 mm, with a width of 10 mm in its midpoint. It inserts on the posterior medial surface of the lateral femoral condyle. Its blood supply is via the middle geniculate artery and it is innervated by branches of the tibial nerve.
PATIENT EVALUATION
History and Physical Examination
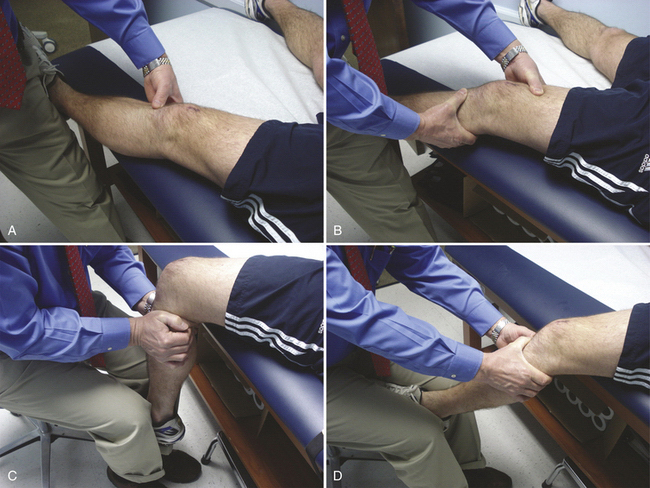
Have the patient lie supine, close to the edge of the examination table. Rest the heel of the patient’s injured extremity just off the edge of the table, with the calf fully supported on the table (Fig. 20-3A). This position will allow about 10 degrees of knee flexion and usually the thigh muscles will relax. Accessing the presence of even a small effusion is easy by gently pushing side to side on each side of the patella. Performing a Lachman test is also enhanced by this position, along with medial and lateral stability (see Fig. 20-3B).
Next, while seated, gently abduct the hip and flex the knee off the side of the bed to 90 degrees. Keeping the thigh on the table, stabilize the foot between your knees (see Fig. 20-3C). Grasping the proximal tibia with both hands allows a gentle posterior drawer test to be performed.
Bring the knee to 25 degrees of flexion and, with both hands, a quick jerk forward will usually detect the presence or absence of an end point. This maneuver is especially helpful with large patients in whom a routine Lachman test is difficult to perform simply because of the size of the leg or the presence of muscle spasm (see Fig. 20-3D).
Diagnostic Imaging
Every examination of the knee should include at least four high-quality x-rays—standing anteroposterior (AP) at 0- and 30-degree, lateral, and sunrise patellar views. Subtle bony injuries such as articular and Segond fractures17 can be identified. A rim fracture of the patella or lateral femoral condoyle can be helpful in differentiating between a patella dislocation and acute ACL tear, both of which may present with hemarthrosis and muscular spasm.
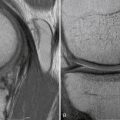
Magnetic resonance imaging (MRI) can be helpful in evaluating ACL tears, especially in diagnosing associated injuries to meniscal and articular surfaces. The posterior cruciate ligament (PCL) is usually distinct on MRI scan because of its saggital orientation, but the ACL can be more indistinct on routine MRI because of its more oblique orientation. An experienced MRI technician may be helpful in obtaining cuts more in line with ACL orientation and increasing the sensitivity of MRI for diagnosing an ACL tear.
TREATMENT
Conservative Treatment
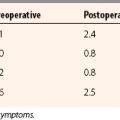
Nonoperative treatment of ACL tears may be appropriate in older sedentary patients or patients with conditions nonconducive to surgery. The ACL can heal in up to one third of cases,18,19 with a third having detectable but not debilitating instability and a third having significant functional instability. Results of patients with ACL repair versus nonoperative treatment have shown better objective stability in the surgical groups but no significant difference in patient satisfaction.20
One study21 of nonoperative treatment for acute ACL tears with MRI-proven normal menisci, with a goal of early return to sport in an ACL brace, has shown that only 72% returned to their sport at an average of 5.7 weeks postinjury. Patients estimated only an 80% return to their preinjury ability and, at ACL reconstruction, 57% had sustained meniscal tears.
Arthroscopic Technique
Arthroscopic reconstruction of the ACL is a well-proven technique in which the entire ACL is replaced by a tissue graft. Careful attention to surgical detail can achieve clinical success in 90% of patients. A drawback to this technique is that it does not reestablish the complex function of the ACL fibers within its footprints. Recent efforts at double-bundle22 reconstruction are an effort to reconstruct the functional anatomy of the ACL more closely.
With the evolution of understanding the functional anatomy of the ACL, recent interest in ACL repair and preservation of as much of the normal ligament and bundles as possible has increased.23–27 With proper patient selection, repair of the ACL can produce good stability, with predictable results.28 Repair alone or repair of a midsubstance tear is not advisable. The two indications for repair are when the ligament is torn near its proximal attachment or when only one bundle is torn. In the case of proximal tears, the ligament can be repaired with multiple sutures to drill holes in the bone and can be augmented with a soft tissue graft.
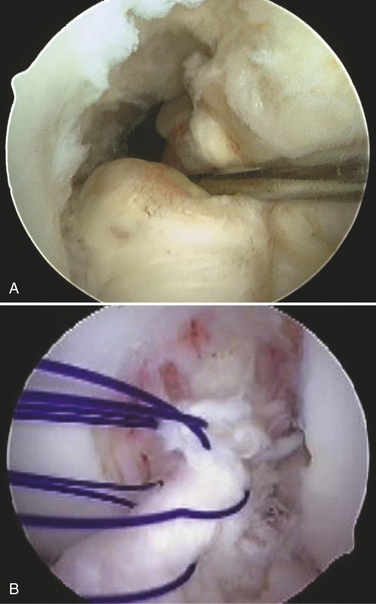
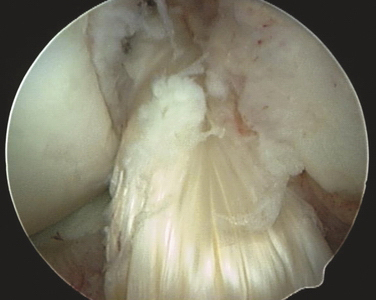
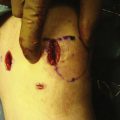
After careful examination under anesthesia, routine arthroscopy is carried out through standard portals. The ACL is visualized and probed to assess its potential for repair (Fig. 20-4A). Only ACL tears in the very proximal portion of the ligament should be considered for repair. All other pathology should be corrected prior to proceeding with the ACL repair. Four or five simple sutures are then placed in the ligament with an intra-articular suture device, spacing each suture up and down the unattached ligament as much as possible (see Fig. 20-4B). The two ends of each suture are separated into two groups, one medial and one lateral. Each suture should have one limb in each group.
Pull the two groups of sutures with sufficient tension to bring the ligament back up to its femoral attachment (Fig. 20-5). With the ligament pulled tight, a standard ACL guide is placed in the tibial footprint just at the posterior aspect of the ligament and a 6- to 8-mm tunnel is reamed from outside-in into the posterior aspect of the tibial footprint. Care must be taken at this point not to disrupt the intact ACL fibers with the reamer as it pierces the tibial cortex; slowly advancing the reamer tip or reaming the last 1 to 2 mm with a hand reamer helps prevent damage to the intact ACL fibers. The graft is then passed through the tibial tunnel, out the femoral tunnel using a commercial suture passer, and brought down the femoral tunnel from outside-in and out the tibial tunnel. The tibial side is secured with an interference screw in the tibial tunnel. Tension is applied to the graft with the knee at 30 degrees of flexion and the two suture bundles are tied together over the femoral condyle. Final fixation is accomplished by placing an outside-in interference screw in the femoral tunnel.
Repair of the Posterolateral Bundle
PEARLS& PITFALLS
PEARLS
CONCLUSIONS
Repairing proximal and one-bundle ACL tears using a soft tissue graft has been successful. Broader footprint attachments of the ACL results from this salvage of ACL tissue. Future developments, such as stimulation of cell migration, addition of growth factors, introducing gene therapy, and using scaffolds, will further refine the concept of ACL repair.
1. Mayo Robison AW. Ruptured crucial ligaments and their repair by operation. Ann Surg. 1903;37:716-718.
2. Andersson C, Odensten M, Good L, Gillquist J. Surgical or non-surgical treatment of acute rupture of the anterior cruciate ligament. A randomized study with long-term follow-up. J Bone Joint Surg Am. 1989;71:965-974.
3. Sanberg R, Balkfors B, Nilsson B, Westlin N. Operative versus non-operative treatment of recent injuries to the ligaments of the knee. A prospective randomized study. J Bone Joint Surg Am. 1987;69:1120-1126.
4. Sherman MF, Lieber L, Bonamo JR, et al. The long-term follow-up of primary anterior cruciate ligament repair: defining a rationale for augmentation. Am J Sports Med. 1991;19:243-255.
5. Feagon JA, Curl WW. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med. 1976;4:95-100.
6. Strand T, Molster A, Hordvik M, Krukhaung Y. Long-term follow-up after primary repair of the anterior cruciate ligament: clinical and radiological evaluation 15-23 years post operatively. Arch Orthop Trauma Surg. 2005;125:217-221.
7. Frank CB, Jackson DW. Current concepts review. The science of reconstruction of the anterior cruciate ligament. J. Bone Joint Surg Am. 1997;79:1556-1576.
8. Murray MM. Effect of the intra-articular environment on healing of the ruptured anterior cruciate ligament. J Bone Joint Surg Am. 2001.
9. Marshall JL, Warren RF, Wickiewicz TL. Primary surgical treatment of anterior cruciate ligament lesions. Am J Sports Med. 1982;10:103-107.
10. Drogset JO, Grontvedt T, Robak OR, et al. A sixteen-year follow-up of three operative techniques for the treatment of acute ruptures of the anterior cruciate ligament. J Bone Joint Surg Am. 2006;88:944-952.
11. Konda E, Yasuda K, Yamanaka M, et al. Effects of administration of exogenous growth factors on biomechanical properties of the elongation-type anterior cruciate ligament injury with partial laceration. Am J Sports Med. 2005;33:188-196.
12. Pascher A, Steiner AF, Palmer GD, et al. Enhanced repair of the anterior cruciate ligament by in situ gene transfer: evaluation in an in vitro model. Mol Ther. 2004;10(2):327-336.
13. Splindler KP, Murray MM, Devin C, et al. The central ACL defect as a model for failure of intra-articular healing. J Orthop Res. 2006;24:401-406.
14. Murray MM, Forsythe B, Chen F, et al. The effect of thrombin on ACL fibroblast interactions with collagen hydrogels. J Orthop Res. 2006;24:508-515.
15. Murray MM, Splinder KP, Devin C, et al. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res. 2006;24:820-830.
16. Arnoczky SP. Anatomy of the anterior cruciate ligament. Clin Orthop Relat Res. 1983;(172):19-25.
17. Segond P. Recherches cliniques et experimental les sur les epanchiements sanquin du genou paretorse. Prog Med. 1879;7:297-299.
18. Petrigliano FA, McAllister DR, Wu BM. Tissue engineering for anterior cruciate ligament reconstruction: a review of current strategies. Arthroscopy. 2006;24(4):441-451.
19. Meunier A, Odensten M, Good L. Long-term results after primary repair or non-surgical treatment of anterior cruciate ligament rupture: a randomized study with a 15-year follow-up. Scand J Med Sci Sports. 2007;17:230-237.
20. Meuffels DE, Favejee M, Vissers M, et al. Ten-year follow-up study comparing conservative versus operative treatment of anterior cruciate ligament ruptures. A matched-pair analysis of high level athletes. Br J Sports Med. 2009;43:347-351.
21. Shelton WR, Barrett GR, Dukes A. Early season anterior cruciate ligament tears; a treatment dile mma. Am J Sports Med. 1997;25(5):656-658.
22. Fu FH, Irrgang JJ, Bost JE. Re: outcome of single-bundle versus double-bundle reconstruction of the anterior cruciate ligament: a meta-analysis. Am J Sports Med. 2009;37:421-422.
23. Steiner ME, Murray MM, Rodeo SA. Strategies to improve anterior cruciate ligament healing and graft placement. Am J Sports Med. 2008;36:176-189.
24. Seitz H, Menth-Chiarai WA, Lang S, Nau T. Histological evaluation of the healing potential of the anterior cruciate ligament by means of augmented and non-augmented repair: an in vitro animal study. Knee Surg Sports Traumatol Arthosc. 2008;16:1087-1093.
25. Fleming BC, Carey JL, Spindler KP, Murray MM. Can suture repair of ACL transaction restore normal anteroposterior laxity of the knee? An ex vivo study. J Orthop Res. 2008;26:1500-1505.
26. Altman GH, Horan RL, Weitzel P, Richmond JC. The use of long-term bioresorbable scaffolds for anterior cruciate ligament repair. J Am Acad Orthop Surg. 2008;16:177-187.
27. Murray MM, Splindler KP, Abreu E, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:81-91.
28. Buda R, Ferruzzi A, Vannini F, et al. Augmentation technique with semitendinosus and graciliis tendons in chronic partial lesions of the ACL: clinical and arthrometric analysis. Knee Surg Sports Traumatol Arthrosc. 2006;14:1101-1107.