Chapter 7 Anterior Cruciate Ligament Primary and Revision Reconstruction
Diagnosis, Operative Techniques, and Clinical Outcomes
INDICATIONS
Anterior Cruciate Ligament Primary Reconstruction in Acute and Chronic Ruptures
Patients presenting with acute complete ruptures of the anterior cruciate ligament (ACL; >5 mm of increased anterior tibial translation and positive pivot shift test) are treated with rehabilitation until pain and swelling subside and joint motion and muscle function are restored. This delay markedly reduces the incidence of postoperative complications of knee motion limitations and muscle weakness. All patients with acute ruptures are profiled with regard to their desired future activity level to determine whether ACL reconstruction is warranted.139 Those who wish to return to high-risk activities, including strenuous athletics or occupations involving pivoting, cutting, twisting, and turning, are considered for reconstruction. In patients with acute ACL ruptures and a concomitant displaced bucket-handle meniscus tear, surgery within 2 weeks is required to reduce the meniscus to a normal location and repair the tear. The ACL reconstruction may be performed at the same setting; however, knees with excessive swelling and pain undergo meniscus repair first. After an appropriate period of rehabilitation, ACL reconstruction is performed.
Patients involved in low-risk activities or who are willing to avoid strenuous athletic and occupational activities that place the knee at increased risk for giving-way episodes may not require ACL reconstruction. The patient is placed into a conservative treatment program that includes rehabilitation to regain muscle strength and neuromuscular function and counseling on the risk of future giving-way reinjuries and potential damage to the joint. Even with surgical reconstruction, patients are informed that an ACL rupture is a serious injury and it is unlikely that they will ever have a truly normal knee joint. The injury may also involve a bone bruise and chondral damage, with sequelae for future joint symptoms. The goal of conservative management is to prevent recurrent giving-way reinjuries, which are deleterious to the joint because they frequently result in meniscus tears and subsequent meniscectomy. It is important to categorize the knee joint as to the injury that has occurred. Repairable meniscus tears almost always indicate a concurrent ACL reconstruction. Otherwise, the success of the meniscus repair may be compromised.40,108,157 DeHaven and coworkers40 documented higher failure rates of meniscus repairs in knees with ACL deficiency than in knees with normal ACL function (46% and 5%, respectively) in a 10-year follow-up study. A grade III pivot shift and grossly positive Lachman test (increased ≥ 10 mm anterior tibial translation) indicate involvement of the secondary ligamentous restraints and, in the authors’ experience, an increased risk of giving-way reinjuries with recreational activities. Patients with physiologic laxity of other ligaments or partial tears (second-degree) of medial or lateral ligaments frequently have a grossly positive pivot shift test, as discussed in Chapter 3, The Scientific Basis for Examination and Classification of Knee Ligament Injuries.
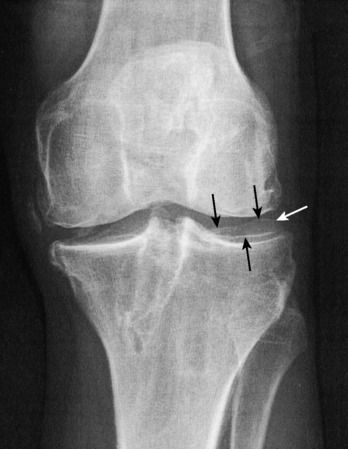
The rationale for the indications for ACL reconstruction is based on a study of 103 patients with chronic ACL ruptures.141 These knees had no other ligament injuries and had not undergone reconstruction. The patients were evaluated a mean of 5.5 years after the initial knee injury; a subgroup of 39 patients presented an average of 11 years postinjury. This study was not a natural history study, because all patients sought treatment owing to symptoms. After the initial injury, 82% of the patients returned to sports activities, which gave an initial false impression of the seriousness of the ACL disruption. After 5 years, only 35% were continuing athletics, often with recurrent symptoms of pain or swelling. A significant reinjury occurred in 36 patients within 6 months and in 53 patients within the 1st year after the initial injury. Moderate to severe symptoms of pain, swelling, and/or giving-way were reported in 32 patients during walking, in 45 patients during activities of daily living, and in 73 patients during turning or twisting athletic activities. Giving-way occurred in 22 patients during walking, in 34 patients during recreational sports, and in 66 patients during strenuous sports. Knees that had undergone meniscectomy had a two- to fourfold increase in pain and swelling symptoms. Radiographic findings lagged behind clinical findings, because 44% of patients in the subgroup 11 years from injury had moderate to severe arthritic changes. The “ring” sign was noted, which represents an osteophyte (Fig. 7-1) that forms around the circumference of the lateral tibial plateau. This is associated with an absence of a pivot shift phenomena, but abnormal anterior tibial translation, because the lateral femoral condyle rotation is restrained by the osteophyte and increased concavity of the lateral tibial plateau.
In a follow-up study, 84 of the initial 103 patients underwent a repeat evaluation 3 years after initiating a conservative and activity modification treatment program.139 The frequently quoted “rule of thirds” represents the outcome of this study regarding the effects of an ACL injury on subsequent functional activities. Approximately one third of the patients showed improvement in symptoms and functional limitations with daily or recreational activities, one third did not improve, and one third became worse with continuing symptoms and limitations. Thirty percent of the patients were noncompliant with the activity modification recommendations and stated they would continue athletics despite symptoms and the increased risk for future arthritis (labeled “knee abusers”). These patients had the poorest overall prognosis with conservative management. This general rule of thirds is not specific to individual patients with chronic ACL insufficiency because it does not take into account the presence of meniscus and arthritis grading, compliance with activity modification, and maintenance of a muscle strengthening program. The frequency of giving-way episodes correlated with chronic pain, swelling, and functional limitations. The conclusion was reached that any giving-way episode with athletics, even if followed by periods of no giving-way or other symptoms, was to be absolutely avoided because meniscus tears and joint deterioration result. The results of this program show the importance of counseling the patient on the expected levels of athletic activity possible and following closely those who do not initially choose to have surgery to lessen the chance of significant arthritis in future years.
Partial ACL tears occur more frequently than suspected and present in a manner similar to complete ACL ruptures. These injuries frequently occur from a noncontact giving-way episode during jumping or pivoting and are often accompanied with a popping sensation and acute hemarthrosis. There is a limitation of full extension with pain on even gentle attempts to passively achieve 0°, mimicking a torn displaced meniscus, which requires magnetic resonance imaging (MRI) to exclude a mechanical block to knee extension. In a study reported by the senior author and associates140 of 32 knees with a partial ACL tear verified arthroscopically, a tear to one or both menisci occurred in 53%. An average of 67 months (range, 24–110 mo) postinjury, 38% had progressed to complete ACL deficiency. The arthroscopic evaluation of the extent of ACL disruption was related to the prognosis and progression to complete ACL deficiency. Even though the arthroscopic evaluation does not define the true extent of ligament injury on a microscopic or functional basis, 86% of knees in which 75% of the ACL was torn progressed to ACL deficiency.136 One half of the knees with an estimated injury involving 50% of the ligament progressed to ACL deficiency, even though major portions of the ACL were still intact, preventing a pivot shift phenomena. Only 12% of knees that had an estimated one fourth or less of the ligament torn progressed to complete ACL deficiency; therefore, these knees had a reasonably good prognosis for return to athletics.
Messner and Maletius104 followed 22 consecutive patients with partial ACL ruptures (no more than 50% of the ACL fibers torn) a mean of 12 years postinjury; all but 1 were also reexamined a mean of 20 years postinjury. No patient required ACL reconstruction. Patients decreased their level of activity from contact sports to recreational activities. Few had an increase in anterior translation or the pivot shift test, and little differences were noted in symptoms between follow-up evaluations.
Revision ACL Reconstruction
Many factors influence the failure rate of ACL surgery.* The definition of a failed ACL reconstruction includes a return of knee instability (increased anterior tibial translation and internal tibial rotation as demonstrated by positive Lachman and pivot shift tests) and, in many cases, the presence of associated pathologies. These include limitation of knee motion with pain and stiffness, muscle atrophy from inadequate rehabilitation, articular cartilage damage, meniscectomy and its effects on joint symptoms and function, undiagnosed lower limb malalignment, and posterolateral (PL) or medial ligament deficiency. When present, each of these pathologies must be addressed and resolved before or during ACL revision. The goal is to restore the best possible function to the knee joint and lower extremity before ACL revision to maximize the success rate and minimize the possibility of failure of the revision procedure. Importantly, the patient and surgeon must address the goals of the revision because associated knee joint damage requires modification or elimination of strenuous, high-impact activities. Often, the patient has had multiple prior surgical procedures that have not achieved the desired outcome.118,126,131 The potential of the revision operation in these cases to accomplish the patient’s goals must be realistically discussed.
CONTRAINDICATIONS
The presence of residual signs and symptoms of a complex regional pain syndrome (CRPS) requires careful examination and questioning regarding the presence of burning pain, abnormal skin hypersensitivity, extremity discoloration, and intolerance to cold and ice. Even after resolution of a prior CRPS, there is an increased frequency for return of the syndrome in these knees that must be promptly recognized in the postoperative period (see Chapter 43, Diagnosis and Treatment of Complex Regional Pain Syndrome).
CLINICAL BIOMECHANICS
Effect of ACL and Lateral Structures on Anterior Tibial Translation and Internal Tibial Rotation Limits
The function of the ACL is discussed in Chapter 3, The Scientific Basis for Examination and Classification of Knee Ligament Injuries. Additional information regarding the restoration of ACL function related to surgical techniques and graft placement issues are discussed in this section. The ACL is the primary restraint to anterior tibial translation, providing 87% of the total restraining force at 30° of knee flexion and 85% at 90° of flexion.29 The iliotibial band (ITB), midmedial capsule, midlateral capsule, medial collateral ligament (MCL), and fibular collateral ligament (FCL) provide a combined secondary restraint to anterior tibial translation. The posteromedial (PM) and PL capsule structures provide added resistance with knee extension. The secondary restraints may become deficient after an ACL injury or repeat giving-way episode, resulting in increased symptoms.
The ACL resists the coupled motions of anterior tibial translation and internal tibial rotation along with the lateral structures. In the authors’ laboratory, Wroble and colleagues196 measured in ACL-deficient knees the simulated effect of lateral soft tissue injuries on internal tibial rotation and anterior tibial translation. The experimental design consisted of a six-degrees-of-freedom instrumented spatial linkage and loading of the knee joint under defined loads of 100 N anteroposterior (AP), 15 Nm varus-valgus, and 5 Nm internal-external tibial rotation torque. The limits of knee motions were determined in the intact knee and then after sectioning the ACL, ITB, lateral capsule, popliteus tendon and popliteofibular ligament, and PL capsule.
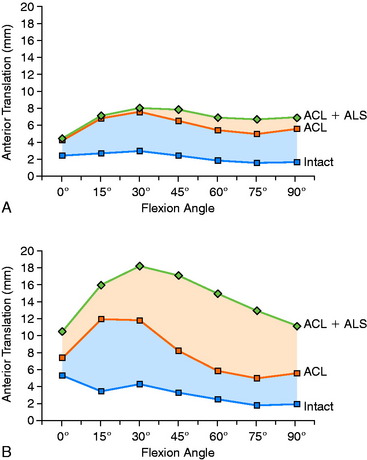
The effect of sectioning the ACL first, followed by the ITB and lateral capsule, is shown in Figure 7-2. Two distinct responses were measured. In the first set of knees (see Fig. 7-2A), there were only moderate increases in anterior tibial translation after ACL sectioning and negligible further increases after sectioning the ITB and lateral capsule. However, in the second set of knees (see Fig. 7-2B), much larger increases were noted in anterior tibial translation after sectioning the ACL and even greater increases were found after sectioning the ITB and lateral capsule. These findings are compatible with clinical tests after ACL injury in which, in some knees, there may be only a moderate increase in anterior tibial translation, whereas in others, a much larger increase is present. This concept is discussed in Chapter 3, The Scientific Basis for Examination and Classification of Knee Ligament Injuries, using the analogy of the bumper model to simulate the effect of the secondary restraints on motion limits once the ACL is removed. Clinically, an ACL graft would be expected to be subjected to greater in vivo forces in the second set of knees without the unloading provided by relatively “tight” secondary restraints. The increase in the magnitude of anterior translation in these physiologically loose knees after loss of the anterolateral structures results in a grade III pivot shift phenomenon. These knees require a high-strength, securely fixated ACL graft to resist these major displacements, and autografts are highly recommended in these grossly unstable knees, as is discussed later.
Critical Points CLINICAL BIOMECHANICS
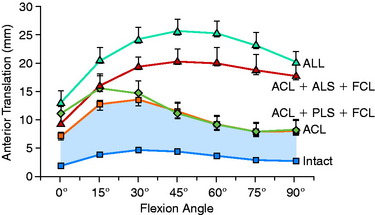
The effect of sectioning the lateral and posterolateral structures (PLS) is shown in Figure 7-3.196 Sectioning of the FCL or PLS (popliteus tendon, popliteofibular ligament, PL capsule) produced statistically significant, but small, increases in anterior tibial translation at low flexion angles. The combined ACL-ALS (anterolateral structures; ITB, anterior and middle portions of the lateral capsule)–FCL-sectioned knee had large increases in anterior tibial translation, with the greatest changes occurring at higher flexion angles.
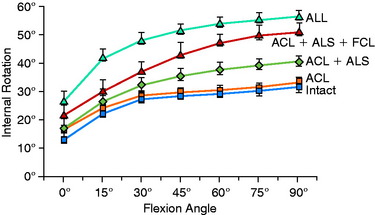
The effect of sectioning the ACL and lateral structures on the limits of internal tibial rotation are shown in Figure 7-4. Sectioning of the ACL produced only a small increase in the final internal rotation limit. Sectioning of the ALS and the FCL produced a sequentially larger increase in internal tibial rotation. In select revision knees that have large increases in internal tibial rotation, the data support the role of a lateral extra-articular procedure to partially unload the ACL graft.
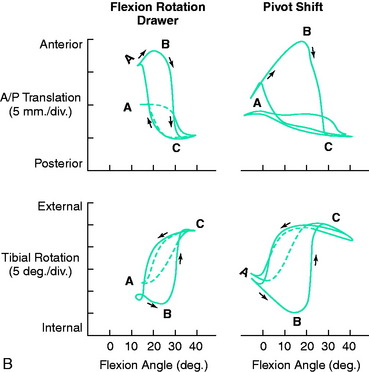
The motions that occur during the pivot shift maneuver are shown in Figure 7-5. At the beginning of the tests, the lower extremity is simply supported against gravity. After ACL disruption, both anterior tibial translation and internal tibial rotation increase as the femur drops back and externally rotates with subluxation of the lateral tibiofemoral compartment. This position is accentuated as the tibia is lifted anteriorly. At approximately 30° knee flexion, the tibia is pushed posteriorly, reducing the tibia into a normal position with the femur. From position C to position A (see Fig. 7-5B), the knee is extended to again produce the subluxated position. In addition to major increases in anterior tibial translation, there is also an increase in internal tibial rotation (midrange limit increase).
The effect of the ACL in providing rotational stability to the motions of anterior translation and internal tibial rotation is shown in Figure 7-6. After ACL sectioning, there is an increase in medial and lateral compartment translation as the center of rotation shifts from inside the knee joint to outside the medial compartment. The medial ligamentous structures influence the new center of rotation. With injury to the medial ligament structures, the center of rotation shifts so far medially that a rotational motion is essentially lost, resulting in a gross anterior subluxation of both compartments. The data show the important surgical concept of restoring injured medial and lateral ligament structures to restore anterior knee stability in conjunction with the ACL.
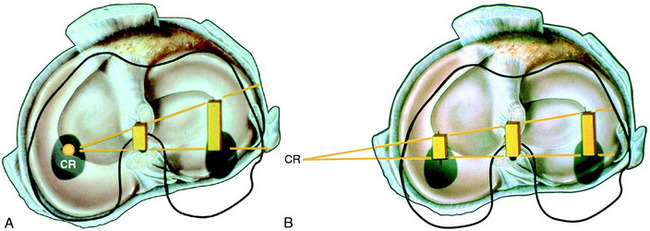
FIGURE 7-6 Intact knee and after ACL sectioning: response to coupled motions of anterior tibial translation and internal tibial rotation. A, Intact knee. The center of rotation may vary between the medial aspect of the PCL and the meniscus border, based on the loads applied and physiologic laxity of the ligaments. B, ACL sectioned; note shift in center of tibial rotation medially. The effect of the increase in tibial translation and internal tibial rotation produces an increase in medial and lateral tibiofemoral compartment translation (anterior subluxation). The millimeters of anterior translation of the tibiofemoral compartment represents the most ideal method to define knee rotational stability (see Chapter 3, The Scientific Basis for Examination and Classification of Knee Ligament Injury). The center of rotation under a pivot shift type of test shifts to the intact medial ligament structures. If these are deficient, the center of rotation shifts outside the knee joint.
Division of the ACL into Anteromedial and Posterolateral Bundles
Controversy exists in published studies on the division of the ACL into two distinct fiber bundles. Some authors provide evidence of both an anatomic and a functional division, whereas others doubt that this division exists and argue that ACL fiber function is too complex to be artificially divided into two bundles. In some studies,4,32 the anteromedial (AM) bundle is identified functionally at its femoral location as the proximal half of the attachment (knee in extension) that tightens with knee flexion. The PL bundle is identified as the distal half of the ACL femoral attachment that tightens with knee extension. The PL bundle is described to relax with knee flexion, as the ACL femoral attachment changes from a vertical to a horizontal structure. The problem is that this classic description of a reciprocal tightening and relaxation of the AM and PL bundles occurs under no anterior loading conditions. With substantial anterior tibial loading, and in particular with a coupled motion of anterior translation and internal tibial rotation, the majority of the ACL fibers are brought into a load-sharing configuration to a differing percentage, as is presented later.
The authors believe the characterization of the ACL into two fiber bundles represents a gross oversimplification not supported by biomechanical length-tension laboratory studies.70,169 Zavras and coworkers205 published a comparative study on previously published isometric points for the ACL and concluded that the ACL isometric zone was high and proximal in the ACL attachment, close to the posterior end of Blumensaat’s line. This data, along with other studies, focused on placing grafts in the proximal aspect of the ACL attachment, in a so-called isometric position. The problem with applying published isometric data to the clinical situation is that the data are valid only for knee flexion-extension and do not indicate the most effective ACL graft position for controlling knee rotational loading as occurs during the pivot shift test. To date, data regarding the most effective graft positions to resist the coupled motions of anterior translation and internal rotation are not available. However, there are data on what graft placement positions are ineffective and should be avoided.
The length-tension behavior of ACL fibers is primarily controlled by the femoral attachment in reference to the center of femoral rotation, the coupled motions applied, the resting length of ACL fibers, and tibial attachment locations. In Chapter 3, The Scientific Basis for Examination and Classification of Knee Ligament Injuries, the concept of an isometric transition zone or contour plot is presented. The zone represents a central transition zone in which fibers undergo 2 mm of length change during knee flexion and extension. This zone is not an isometric zone, because the length change is not zero. All ACL fibers anterior to the zone lengthen with knee flexion, whereas the posterior fibers lengthen with knee extension. Under loading conditions, fibers in both the AM and the PL divisions contribute to resist tibial displacements. ACL fibers in the PL division attach in part posterior to the transitional zone and lengthen with knee extension. Note that the function of the ACL fibers is determined by the anterior-to-posterior direction (knee at extension) as well as the proximal-to-distal femoral attachment. Placement of a graft in an anterior or a posterior position has a large effect in producing deleterious lengthening and graft failure. The obvious example is a graft placed anterior to “residents’ ridge,” which is anterior to the femoral ACL attachment. The proximal-to-distal division used in two-bundle descriptions as the control of fiber length in knee flexion and extension oversimplifies the functional behavior of the ACL fibers, which are even more dependent on the AP femoral attachment.
The division of the ACL into two bundles was historically based on the tibial attachment site and not on a corresponding femoral attachment site. Recent studies project the tibial bundles onto two corresponding femoral sites. In these studies, to be described, the authors usually divide the ACL femoral attachment site at a midpoint into a corresponding AM and PL bundle. In the authors’ opinion, there is not at present convincing anatomic data to support a division of the ACL into two separate bundles, although one study50 reported a bony ridge between the two femoral attachment bundles. Because of the lack of a clear anatomic division of the ACL into two bundles, there is discrepancy among authors on anatomic descriptions of the ACL “bundles” and recommendations for the surgical technique on tibial and femoral tunnels for two bundle graft reconstructions.
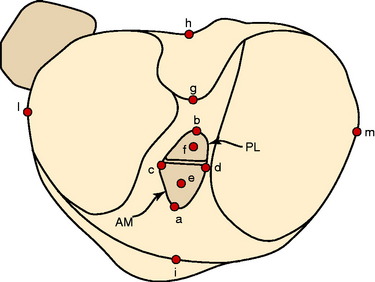
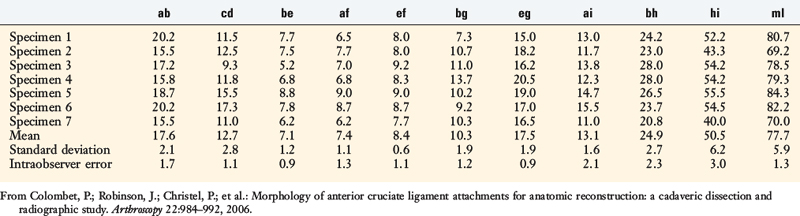
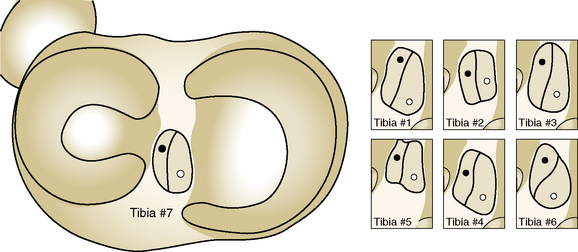
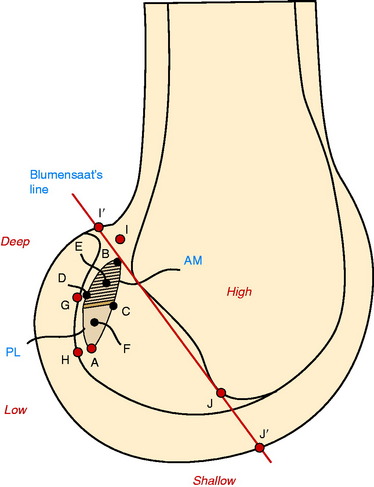
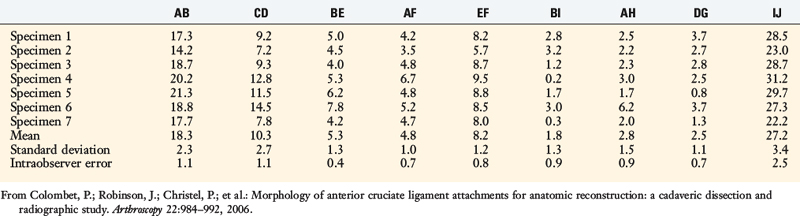
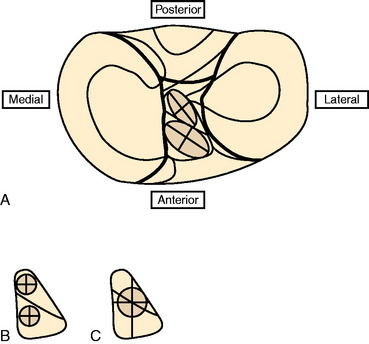
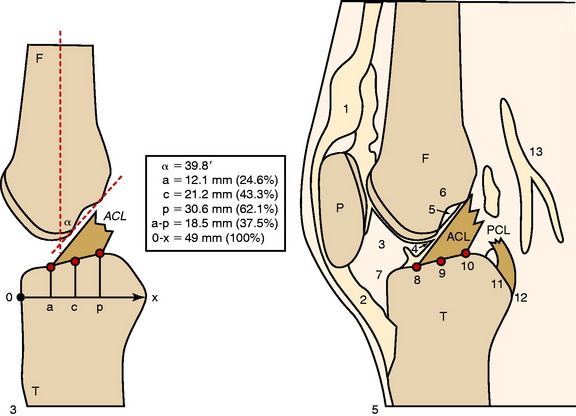
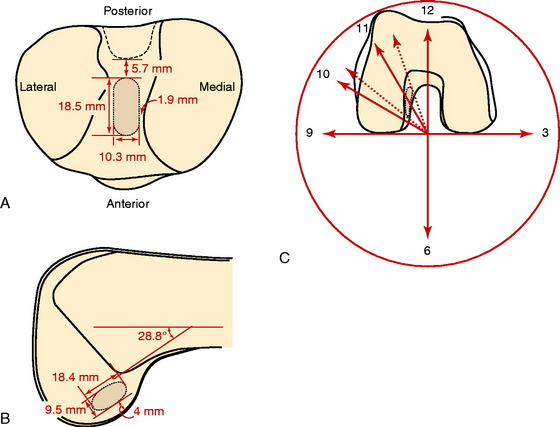
Colombet and associates32 measured the ACL femoral and tibial attachments of the AM and PL bundles in seven unpaired cadaveric knees. The reference position of the retroeminence ridge (RER), representing the posterior interspinous ridge anterior to the posterior cruciate ligament (PCL) attachment, provided an important landmark for measuring from this point to the posterior ACL attachment. The tibial measurement points are shown in Figure 7-7 and the mean values are displayed in Table 7-1. Note the ACL extends posteriorly on the tibia to a distance 10.3 ± 1.9 mm from the RER line (coordinate bg). There is a discrepancy in the published text where this measurement is reported to be 7.1 mm. The mean length of the ACL (coordinate ag) was 17.6 ± 2.1 mm. The distance between the center of the AM and the center of the PL bundle (coordinate ef) was 8.4 ± 0.6 mm. The authors reported that the AM bundle was an average of 17.8 ± 1.7 mm anterior to the RER. The center of the ACL attachment was 19 mm anterior to the RER line. The center of the PL and AM bundles was determined to lie at 52% and 36% of the tibial width (line described by Amis and Jakob5 that passes through the posterior corner of the tibial plateau and parallel to the medial tibial surface). The appearance of the ACL tibial attachments of the AM and PL bundles are shown in Figure 7-8 and represents a medial-to-lateral division, different from other authors who depict an anterior-to-posterior division.
The corresponding femoral attachments of the ACL from this study are shown in Figures 7-9 and 7-10 and the mean values are provided in Table 7-2. The overall length of the ACL was 18.3 ± 2.3 mm, with the center of the AM and PL bundles a mean of 8.2 mm apart and approximately 5 mm from the proximal and distal end of the ACL attachment. The authors recommended that the center of the PL bundle be located 8 mm lower and “shallower” in the notch than the center of the AM bundle. The femoral attachment was 2.5 mm from the articular cartilage. Using the grid system of Bernard and colleagues19 (shown in Fig. 7-10), the center of the AM bundle was 26.4% ± 2.6%, and the center of the PL bundle was 32.3% ± 3.9% the length of Blumensaat’s line.
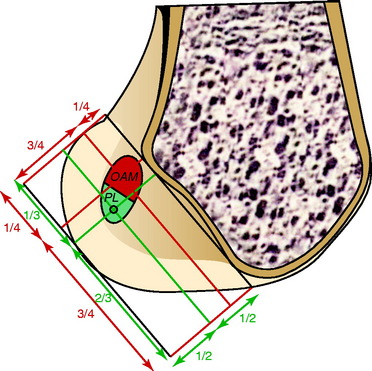
FIGURE 7-10 Position of the centers of the AM and PL bundles on the grid described by Bernard et al.19
(From Colombet, P.; Robinson, J.; Christel, P.; et al.: Morphology of anterior cruciate ligament attachments for anatomic reconstruction: a cadaveric dissection and radiographic study. Arthroscopy 22:984–992, 2006.)
In the most extensive study to date, Edwards and coworkers45 defined the ACL tibial attachment in 55 cadaveric specimens. In Figure 7-11, the tibial bony landmarks are depicted. The “over-the-back” ridge (RER) again corresponds to the interspinous ridge just anterior and proximal to the PCL attachment. In Figure 7-12, the authors present their recommendations for best-fit central and two tunnel positions. The center of the ACL attachment was 15 ± 2 mm (range, 11–18 mm) from the RER at 36% of the AP depth of the tibia. The center of the PL bundle anterior from the RER was 10 ± 1 mm (range, 8–13 mm) and the AM bundle was 17 ± 2 mm (range, 13–19 mm), corresponding to 29% and 46% of the tibial depth, respectively. The authors noted, in disagreement with other publications, that they were not able to define two separate anatomic bundles. Instead, they defined two functional bundles by observing the tightening and slackening behavior of the ACL fibers during flexion and extension.
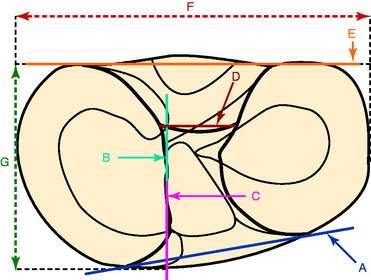
Stabuli and Rauschning174a reported the center of the ACL tibial attachment at 43% of the AP depth, which extended from 25% to 62% of the tibial width. These important landmarks provide a reference to measure lateral radiographs before and after ACL graft placement to avoid a posterior and vertical graft placement (Fig. 7-13). Siebold and associates171 performed a cadaveric study in 50 knees and documented the ACL tibial insertion using a digital image analysis system and divided the ACL into AM and PL bundles. The average AP length of the ACL tibial attachment was 14 mm (range, 9–18 mm). The average male ACL tibial insertion area was 130 mm2 versus the average female knee of 106 mm2. The average AP length of the ACL footprint in females was 14 mm (range, 9–18 mm), and in males, 15 mm (range, 12–18 mm). The average width of the ACL in all knees was 10 mm. The study concluded that two tibial bone tunnels cannot be placed at the anatomic centers of the AM and PL bundles because the space is too narrow and the tunnels would overlap. This highlights the difficulty of the ACL double-bundle technique in re-creating the native ACL fiber attachment.
Zantop and colleagues204 performed a cadaveric study in 20 knees that outlined the AM and PL bundle locations at the tibial and femoral sites. The authors arrived at different conclusions from those of Siebold and associates.171 The tibia ACL division into the AM bundle was oriented from medial to lateral and occupied the anterior one half of the ACL footprint and was aligned with the anterior horn of the lateral meniscus. The center of the PL bundle was located 11 mm posterior and 4 mm medial to the anterior insertion of the lateral meniscus. The center of the AM bundle was located at 30% and the PL bundle was located at 40% on the lateral transtibial line.
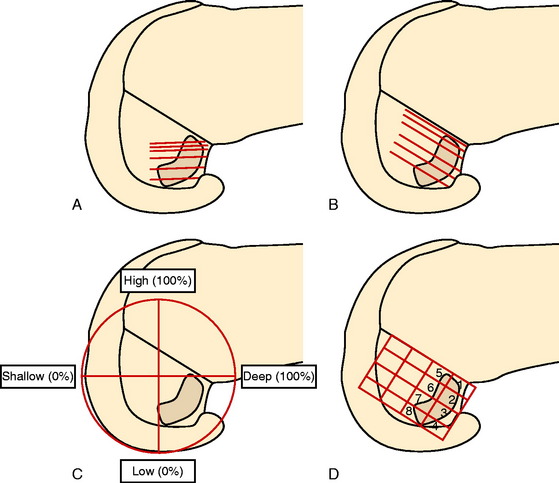
Edwards and coworkers46 described the anatomic locations of the ACL femoral attachments of the AM and PL bundles in a companion study in 22 cadaveric knees using a measurement grid system shown in Figure 7-14. The authors reported a wide variation in the size and shape of the ACL attachment and bundle arrangement that was selected (Fig. 7-15). Note that the AM and PL bundle divisions differ from the prior study. The femoral attachment was oriented at 37° to the long axis of the femur. The authors reported that the AM bundle extended to the posterior proximal limit of the femoral notch between the 10:30 and the 11:30 positions. The PL bundle was located between the 9:00 and the 10:30 positions. The authors reported that a 6-mm graft tunnel had the best fit if the AM bundle was located at 11 o’clock, 6 mm from the posterior outlet, and the PL bundle was located at 10 o’clock, 9 mm from the posterior outlet. The authors noted that other studies had different recommendations for the placement of two femoral tunnels in the double-bundle technique.
Rue and associates159 in a cadaveric study used a transtibial-drilled femoral tunnel, placed at the 10:30 position, and determined the location of a single-graft femoral tunnel in relation to the AM and PL bundles. The authors placed the guide pin for the tibial tunnel approximately 7 mm anterior to the PCL, which would represent a posterior one third tibial attachment. The authors reported the transtibial drilling of the femoral tunnel resulted in the footprint of the AM occupying an average of 32% (range, 3%–49%) of the area of the tunnel, and the footprint of the PL bundle occupying an average of 26% (range, 7%–41%). In addition, the remainder of the area of the 10-mm tunnel did not overlap the ACL footprint. The wide ranges reported in this study for the location of the femoral tunnel in terms of the native ACL femoral attachment highlight the problems of using the tibial tunnel to drill the femoral tunnel.
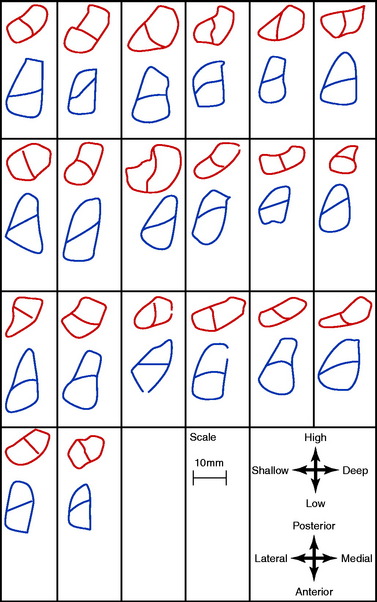
Heming and colleagues71 reported the ACL tibial and femoral footprints in 12 cadaveric knees (Fig. 7-16). Note in Figure 7-16C, the orientation of the clock face places the 9 to 3 o’clock horizontal line at the base of the femoral condyles and not within the center of the notch, which is typically used by other authors. A guide pin placed between the ACL anatomic femoral and tibial attachment centers (as in a transtibial drilling technique) was only possible if the tibial tunnel started in a medial position close to the joint line that would be too short to be functional for ACL grafts. The study concluded that transtibial drilling techniques resulted in a more vertical graft orientation.
ACL Function Defined by the Role of AM and PL Divisions
Sakane and coworkers,160 in one of the first cadaveric robotic studies, reported the PL bundle provided the primary restraint to anterior tibial translation at low flexion angles. Increasing forces were reported in the AM bundle with knee flexion; these remained lower than the calculated in situ forces of the PL bundle.
The role of the ACL bundles in restraining rotational motions was examined by Zantop and associates203 in a robotic study. A 134-N anterior tibial load and a combined 10-Nm valgus and 4-Nm internal tibial torque were applied to cadaveric knees. Sectioning the PL bundle at 30° of flexion resulted in an approximately 7-mm increase in anterior tibial translation, whereas sectioning the AM bundle produced at 60° an approximately 9-mm increase in anterior translation. Similar data were reported at low flexion angles for the combined rotational loads. These data imply a near-absence of function of the remaining “intact” bundle. In contrast, Markolf and colleagues,97 in a cadaveric study, reported that cutting the PL bundle resulted in an increase of only 1.1 mm at 10° flexion and 0.5 mm at 30° flexion. These authors questioned the need to reconstruct the PL bundle for restoration of normal ACL laxity.
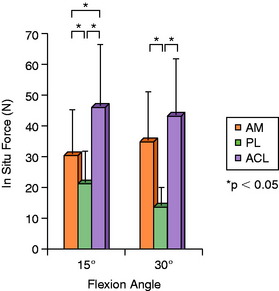
Gabriel and coworkers,55 in a robotic cadaveric experiment, removed all of the soft tissues to produce a femur-ACL-tibia specimen. For anterior tibial loading (Fig. 7-17), the AM bundle was reported to be nearly equal to the PL bundle at 15° of flexion, with increasing AM forces with knee flexion. Under the rotation loads of valgus and internal tibial rotation, the forces in the AM bundle exceeded those of the PL bundle (Fig. 7-18).
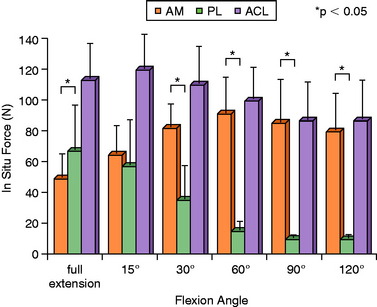
FIGURE 7-17 In situ force in the intact ACL and its AM and PL bundles in response to 134 N anterior tibial load.
(From Gabriel, M. T.; Wong, E. K.; Woo, S. L.; et al.: Distribution of in situ forces in the anterior cruciate ligament in response to rotatory loads. J Orthop Res 22:85–89, 2004; reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.)
Li and associates90 used MRI and fluroscopic images of subjects performing a lunge to create three-dimensional models in which the AM and PL ACL attachments were outlined. The study reported that the AM and PL bundles reached their maximum elongation between full extension and 30° of flexion and did not demonstrate a reciprocal behavior with knee flexion-extension.
Giron and colleagues,61 in a cadaveric experiment, determined that a “deep” (proximal) femoral tunnel position could be achieved with either one of three techniques (double incision, transtibial, or anteromedial portal). However, Arnold and coworkers9 showed in cadaveric experiments that the ACL is entirely attached to the lateral femoral wall and that this attachment position was not able to be accessed through a transtibial tunnel (40% AP tibial measurement). The transtibial tunnel method resulted in the guide pin and drill hole placed at the junction of the proximal ACL attachment and femoral roof (Fig. 7-19).
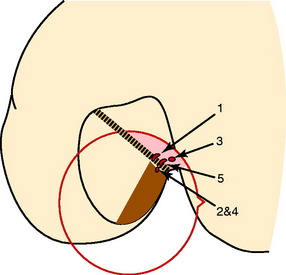
FIGURE 7-19 Notch view, summary guiding pinholes; knees no. 1–5, schematic drawing.
(From Arnold, M. P.; Kooloos, J.; van Kampen, A.: Single-incision technique misses the anatomical femoral anterior cruciate ligament insertion: a cadaver study. Knee Surg Sports Traumatol Arthrosc 9:194–199, 2001; with kind permission of Springer Science+Business Media.)
Mae and associates,96 in a cadaveric knee study, used a robotic simulator to study the effect of single- versus two-femoral tunnel ACL graft reconstruction. A single tibial tunnel was placed in the center of the ACL tibial attachment. The locations of the single and two tunnels were described at the 1:00 and 2:30 positions. The data for AP laxity and AP in situ graft forces at 44 N graft pretension showed little difference between the single- and the double-bundle grafts, both of which produced a mild to moderate overconstraint that limited anterior tibial translation. The study reported on the load-sharing between the normal ACL bundles (Fig. 7-20), which demonstrated that the PL bundle functioned more at low flexion angles, equal to the AM bundle at 10° of flexion, with a further increase in AM bundle function with increasing knee flexion. A study of load-sharing between the two bundles of a simulated pivot shift type of loading was not performed.
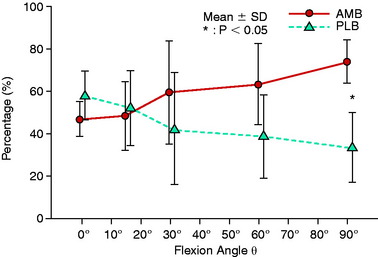
FIGURE 7-20 Graph shows force sharing between the normal ACL bundles under 100 N of anterior load.
(From Mae, T.; Shino, K.; Miyama, T.; et al.: Single- versus two-femoral socket anterior cruciate ligament reconstruction technique: biomechanical analysis using a robotic simulator. Arthroscopy 17:708–716, 2001.)
Yagi and colleagues199 compared single- and double-bundle ACL hamstring reconstructions. A double-looped single-bundle hamstring graft was placed at approximately the 11 o’clock position and tensioned to 44 N, whereas each bundle of the double-bundle graft was tensioned to 44 N. The double-bundle graft had a total of 88 N of tension versus 44 N for the single-graft reconstruction. In response to a 134-N anterior load at 30° of flexion, the data showed an unexplained residual anterior tibial translation (intact, 6.4 mm ± 2.4 mm: single-bundle, 10.2 ± 2.5 mm), which would not be anticipated from a single graft in terms of resisting translation. The finding of a lax single graft most likely explains the reported decreased ability of the single graft to resist the combined rotation-translation motions.
Petersen and coworkers146 concluded that the location of the ACL double-bundle grafts in clinical studies varied markedly at both the femoral and the tibial sites. In a cadaveric study, Petersen and coworkers146 found that a two-tunnel tibial technique provided an advantage in resisting combined rotatory loads, simulating the pivot shift (anterior tibial translation, 7.5 mm vs. 9.5 mm, 30° flexion, 5 Nm internal rotation, 10 Nm valgus torque). Of note, the single tibial tunnel and graft was placed 7 to 8 mm anterior to the PCL; the authors stated that this was a position recommended by some surgeons.74,107 As previously discussed, the posterior tibial tunnel position results in a more vertical ACL graft, with portions of the graft possibly even posterior to the native ACL tibial attachment. The addition of a second graft placed in a more anterior tibial position within the ACL footprint would be expected to provide better resistance to the loading conditions. Although the data do not provide evidence for a double-bundle ACL procedure, it does provide evidence to avoid placement of a single ACL graft in the posterior one third of the ACL tibial attachment.
Yamamoto and associates200 conducted a cadaveric robotic study that compared a single ACL graft placed in the 10:00 position on the lateral wall with an anatomic double-bundle reconstruction. This is one of the few published studies in which a single-graft lateral wall placement was used, avoiding a more proximal graft placement. Similar loading conditions were used as in the other robotic studies (anterior tibial load 134 N, combined rotatory load 10 Nm valgus and 5 Nm internal tibial torque). There was no statistical difference in the anterior tibial translation or in situ force at 30° knee flexion between the intact ACL, the single bundle, or the anatomic double-bundle reconstructions. Under the combined rotatory loading conditions, there were no differences in anterior tibial translation, internal tibial rotation, and ACL in situ graft forces, which is in direct contrast to other robotic experiments.
Markolf and colleagues98 measured in cadaver knees a simulated pivot shift in the intact knee and then after single-bundle and double-bundle ACL reconstruction. The single-bundle reconstruction (placed at the anatomic AM bundle site) restored mean tibial rotations and lateral plateau displacements to levels similar to those of the intact knee, whereas the double-bundle reconstructions reduced coupled rotations and displacements to levels less than those in the intact knee. The authors concluded that the overconstraint induced by the double-bundle reconstructions has unknown clinical consequences and that the need for the added complexity of this procedure is questionable.
Cuomo and coworkers38 reported on the effects of tensioning single- and double-bundle ACL reconstructions in cadaveric knees instrumented with a six-degrees-of-freedom system under defined loading conditions (anterior translation, 90 N; 5 Nm internal rotation torque). Different flexion positions were used for tensioning each of the grafts in the double-bundle construct. The single graft was tensioned at 20° knee flexion. All grafts had sufficient tension applied to restore the intact knee laxity (translation). Tensioning each of the grafts individually (AM bundle at 90°, PL bundle at 20°, varying which was tensioned first) resulted in overconstraining AP translation. In contrast, tensioning both the AM and the PL grafts simultaneously at 20° provided the best match for the intact knee AP translation throughout knee flexion. The data show the difficulty in tensioning two ACL grafts at the time of surgery in terms of matching the intact ACL load-sharing between fibers. With anterior loading, both the AM and the PL fibers participate in load-sharing, but at different percentages, as already discussed. For single-bundle ACL grafts placed within the center of the femoral and tibial attachments, higher overall graft tension occurred under anterior loading compared with the two graft bundles tensioned at 20° flexion. Importantly, the data show that tensioning of the AM and PL grafts under low tensions (20 N) provided the best results. The single graft required 38 ± 27 N to restore the native knee laxity.
Scopp and associates,164 in cadaveric experiments, reported that a more oblique femoral tunnel placement (60° from vertical) was more effective in resisting internal rotational torques (difference 4.4°, 6.5 Nm) than a “standard” tunnel placement (30° from vertical). The femoral tunnel was drilled using a transtibial technique and, most likely, a posterior tibial tunnel placement. Anterior tibial translation values were not restored to normal (increased 2.5 and 2.2 mm, standard and oblique femoral tunnels).
Simmons and colleagues172 in cadaver experiments showed the deleterious effects of a more vertical tibial tunnel (70°, 80°) with impingement against the PCL and higher graft tension. A more oblique tibial tunnel and femoral tunnel in the 60° coronal plane did not impinge against the PCL. The study recommended a posterior tibial tunnel placement and more proximal ACL femoral attachment with use of a transtibial drilling technique, again showing the predominance of studies in the literature in which a more vertical graft orientation results.
Arnold and coworkers10 showed in cadavers that the passive ACL tension flexion-extension tension curve was not reproduced by femoral tunnels placed at the 10 and 11 o’clock position, but was reproduced by grafts in the 9 o’clock–positioned tunnels. A posterior tibial tunnel was used in this experiment.
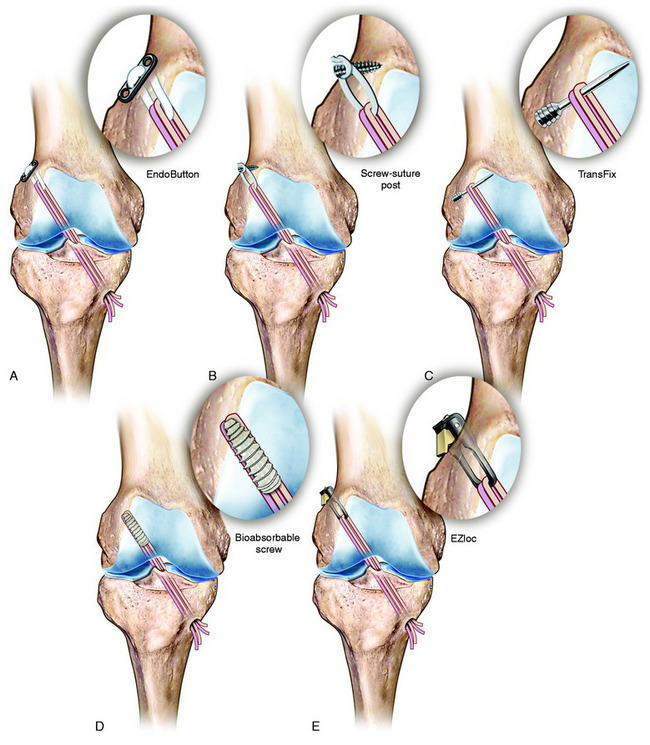
There are incomplete in vitro data on the comparison of a anatomic single-graft construct (within central tibial and femoral tunnels) with a double-bundle construct.94 There are also incomplete experimental data on the function of individual regions of ACL fibers in resisting coupled motions as occurs in the pivot shift phenomena, which is an important area for future study (Fig. 7-21). Other biomechanical studies reviewed show that a single ACL graft placed within the anatomic femoral and tibial attachments (avoiding a high femoral and posterior tibial position) restores rotatory stability and question the need for a double-bundle procedure.98
One theoretical advantage of a double-graft construct tensioned appropriately is that there is initially less graft tension in each of the graft strands compared with the overall tension in a single graft. Stated differently, a single graft will always exhibit much higher graft tension to resist anterior loading than two graft arms in which load-sharing occurs. There is also the theoretical advantage of tensioning the two graft strands at different knee flexion positions to exhibit a different percentage of sharing of the overall tension than a single graft. From an experimental standpoint, either a single-graft or a double-graft construct can be tensioned to restore normal motion limits; however, this may occur at the expense of high graft tensions, particularly in a single-graft construct. Thus, the advantage of a double-graft construct (either ACL or PCL) is to restore normal knee motion limits under the lowest graft tensile loads, which is advantageous during the graft healing and remodeling. A graft under lower loads has the theoretical advantage of less risk in stretching out with return of the abnormal motion limits. In addition, an ACL or a PCL graft under higher tension under cyclical loading conditions is at high risk of graft stretching and failure.167 Again, it should be noted that either one or two ACL grafts do not restore native ACL fiber length-tension properties.
Clinical Measurement of ACL Graft Function during and after ACL Surgery
The assessment of ACL graft function must take into account the restoration of the normal coupled motion limits of anterior tibial translation and internal tibial rotation. During KT-2000 testing, only anterior tibial translation is assessed. If the knee joint has a 3-mm or lower increase in anterior tibial translation over the opposite knee, it can be assumed that there is not a positive pivot shift because this amount of constraint to anterior tibial translation also limits internal rotation. Conversely, if greater than 5 mm of increased anterior tibial translation exists, there is usually an abnormal increase in internal tibial rotation that results in a positive pivot shift test and patient complaints of giving-way. The problem is in knees that demonstrate 3 to 5 mm of increased anterior tibial translation, which may represent 20% to 30% of patients in clinical investigations,2,7,143,165,166 especially when allografts are used.131 This mild to moderate increase in anterior tibial translation results in a mildly positive Lachman test with a hard endpoint. However, along with an increase in internal tibial rotation, these knees may demonstrate a positive pivot shift and giving-way symptoms. Because the pivot shift test is highly subjective and variable between examiners (see Chapter 3, The Scientific Basis for Examination and Classification of Knee Ligament Injuries), an author may report a successful result based on the KT-2000 (anterior tibial translation) or a pivot shift test, whereas another examiner may grade the knee as a failure based on the method by which the pivot shift test is performed. There is a pressing need for clinical examination tools that incorporate tibial translations and rotations and the resultant subluxations in millimeters of the medial and lateral tibiofemoral compartments under defined loading conditions.
Bull and associates25 were among the first authors to report intraoperative measurement of tibial translations and rotations using a three-dimensional motion analysis system. Robinson and colleagues156 performed an ACL double-bundle reconstruction using computerized navigation techniques in 22 patients. Both the AM and the PL bundles contributed to resisting anterior tibial translation during the pivot shift tests. However, the PL bundle was more important than the AM bundle in controlling abnormal tibial rotation. It is probable that the AM bundle was placed in a more vertical proximal position adjacent to the intercondylar roof. Computerized navigation techniques allow for a highly accurate measurement of knee translations and rotations that may lead to the most objective means to measure knee kinematics during surgery. However, the exact location of ACL grafts and the loads applied by the examiner are variables still to be determined.
In a frequently quoted study, Tashman and coworkers179 devised a unique methodology of dynamic in vivo measurements of patients after ACL reconstruction on a treadmill while running downhill, employing a biplanar radiographic system to measure tibiofemoral displacements. The reconstructed knees showed an increase in mean values of external tibial rotation (3.8° ± 2.3°) and adduction (2.8° ± 1.6°). The effect of these small differences from a clinical standpoint is unknown and future studies are required that involve more dynamic rotational movements in comparison with straight-ahead running to measure the rotational kinematics of the knee joint. The specific ACL graft femoral and tibial tunnel placement was not identified in this study.
Ristanis and associates155 assessed 11 patients after a bone–patellar tendon–bone (B-PT-B) reconstruction using kinematic data from a six-camera optoelectronic system obtained during a jump landing and pivoting maneuver. The ACL reconstruction did not restore tibial rotation to normal values of the opposite uninvolved extremity or controls. The femoral tunnel was placed through an AM approach with the knee joint flexed to 120° to achieve a 10 to 11 o’clock lateral wall position. The tibial tunnel was placed into the center of the ACL footprint. The authors noted the limitation of the study in the movement of skin markers accurately representing true tibiofemoral joint rotations.
Monaco and colleagues106 in patients undergoing ACL reconstruction evaluated the effect of a single-bundle reconstruction with an extra-articular procedure to a double-bundle ACL reconstruction. Computerized navigation instrumentation was used in the operating room before and after the ACL procedures. The addition of the PL bundle after the AM bundle did not have an effect in reducing AP translation or the limit of internal tibial rotation, and there were no significant differences between the single- and the double-bundle reconstructions. The extra-articular reconstruction significantly reduced the internal tibial rotation limit, as would be expected. The tibial placement of the graft was performed with the guide pin 7 mm anterior to the PCL insertion into the posterior ACL attachment. The authors did not determine the effect of either graft configuration on the coupled motions of the pivot shift test but only on the limit of internal tibial rotation alone.
The authors’ conclusions of single-graft versus double-bundle graft ACL studies are summarized in Table 7-3. The hypothesis presented is that an ideally placed single-graft construct (avoiding a vertical graft orientation) provides control of the abnormal pivot shift motions, avoiding the necessity and added complexity of a double-bundle graft reconstruction.
TABLE 7-3 Authors’ Conclusions of Single- versus Double-Bundle Anterior Cruciate Ligament Reconstructions
Recommended Tibial and Femoral ACL Graft Locations
The important landmarks for the ACL tibial attachments are the medial tibial spine, posterior interspinous ridge (RER) of the proximal PCL fossa, and the attachment of the lateral meniscus. The PCL is a poor soft tissue landmark for the posterior extent of the native ACL attachment. It should be noted that some authors75 have advocated a guide pin and tibial tunnel placement 6 to 8 mm from the PCL, which would place the tibial tunnel in the posterior portion of the ACL. In some knees, the tibial tunnel would be posterior to the native ACL attachment and just a few millimeters from the RER or interspinous ridge. This posterior tibial tunnel produces a near-vertical ACL graft that would not be expected to resist rotational loads in obliterating the pivot shift phenomena, as already discussed.
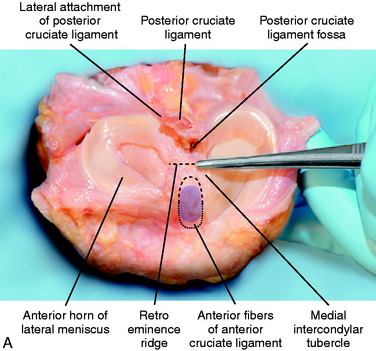
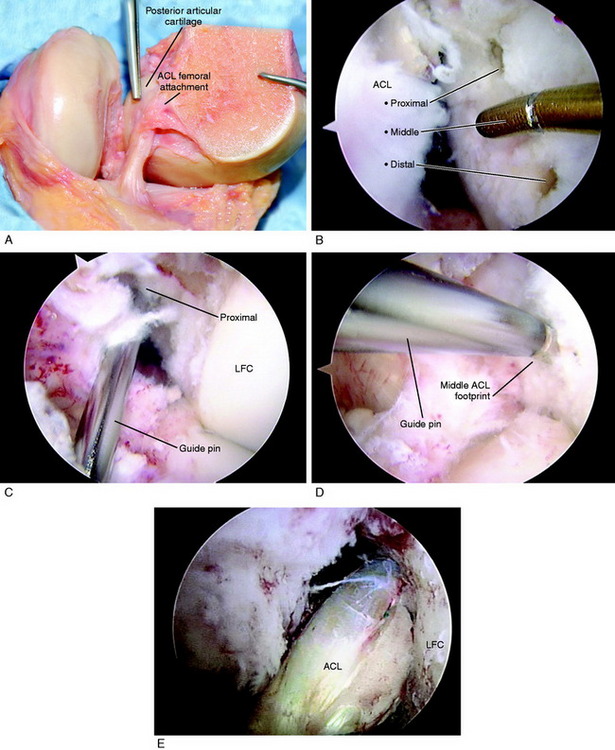
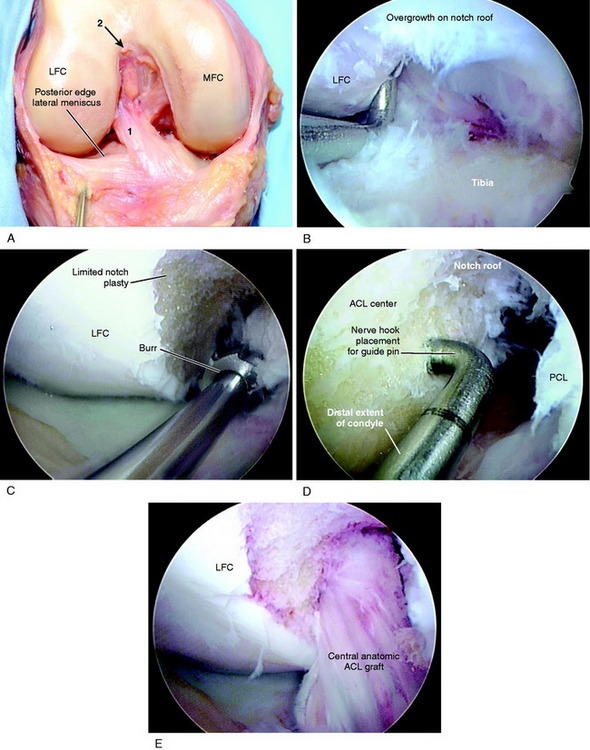
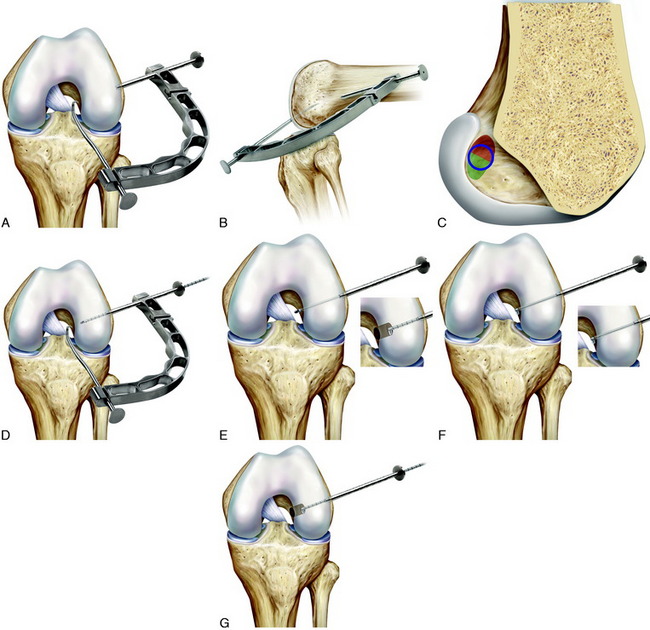
The ACL tibial attachment location recommended by the authors for a single graft is directly adjacent to the lateral meniscus anterior horn attachment as shown in Figure 7-22. The ACL attachment can be easily mapped at surgery based on the anatomic references provided. In some knees, the anterior extent of the ACL attachment may be obscured by soft tissues, and in these cases, the RER or posterior interspinous ridge of the PCL fossa is an important landmark. The center of the ACL will be 16 to 20 mm anterior to the RER or posterior interspinous ridge. The guide pin is most ideally placed eccentric and 2 to 3 mm anterior and medial to the true ACL center, because the ACL graft displaces to the posterior and lateral aspect of the tibial tunnel.31 The eccentric tunnel places the majority of the graft within the central tibial attachment and avoids the posterior attachment location. It is important that an impingement of the graft with the anterior intercondylar notch does not occur because the circular graft may occupy a portion of the native flattened ACL tibial attachment. A limited anterior notchplasty is often required. In many ACL revision knees, the bony ridge posterior to the ACL attachment (“no-man’s land”; Fig. 7-23) has been disrupted by a prior graft tunnel that extends 1 to 2 mm from the RER and requires a bone graft prior to the revision procedure. It is important that during the ACL tibial tunnel preparation (primary or revision surgery), the tibial drill not inadvertently penetrate into or beyond the posterior one third ACL attachment and adjacent posterior interspinous ridge to avoid a vertical graft orientation.
For single grafts, the authors recommend a central anatomic ACL placement with the femoral guide pin 2 to 3 mm above the midpoint of the proximal-to-distal length of the ACL attachment and 8 mm from the posterior articular cartilage edge (see Fig. 7-23). A key is to define the ACL attachment at 20° to 30° of flexion with the arthroscope in the AM portal. After the joint is marked, the knee can be placed in 120° of flexion if an endoscopic technique is selected. A 9- to 10-mm-diameter tunnel occupies the central ACL attachment, leaving only the most proximal and distal few millimeters of the attachment not occupied by a graft. It is important that the ACL tunnel not be too distal because this shortens the intra-articular graft tibiofemoral length. The location for a single-tunnel ACL reconstruction is more distal than recommendations for a “one o’clock” tunnel that replaces only the proximal portion of the ACL femoral attachment. However, it has not been determined in clinical studies whether a difference exists between the one o’clock and the two o’clock femoral graft placement sites.
In Figure 7-24A, the hybrid graft position of a proximal femoral and posterior tibial position is shown, which the authors do not recommend. The preferred central anatomic tibial is presented in Figure 7-24B and femoral position is presented in Figure 7-24C. The placement of a double-bundle ACL reconstruction is shown in Figure 7-24D.
CLINICAL EVALUATION
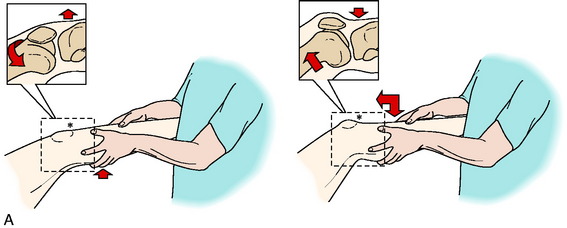
A thorough history is performed, including a detailed account of all knee injuries and the operative procedures that have been performed. In revision knees, prior operative records are obtained to verify the surgical findings, condition of the articular cartilage and menisci, and graft placement. A comprehensive physical examination is performed, including assessment of knee flexion and extension, patellofemoral indices, tibiofemoral crepitus, tibiofemoral joint line pain, muscle strength, and gait abnormalities. The surgeon must determine all of the abnormal translations and rotations in the knee joint. The medial posterior tibiofemoral step-off on the posterior drawer test is done at 90° of flexion. Quantification of posterior tibial translation may be performed using stress radiography in knees with PCL ruptures to determine whether the rupture is partial or complete (see Chapter 21, Posterior Cruciate Ligament: Diagnosis, Operative Techniques, and Clinical Outcomes).73 The appropriate tests to determine the integrity of the ACL are performed, including KT-2000 arthrometer testing at 20° of flexion (134 N force) to quantify total AP displacement. The pivot shift test is recorded on a scale of 0 to III, with a grade of 0 indicating no pivot shift; grade I, a slip or glide; grade II, a jerk with gross subluxation or clunk; and grade III, gross subluxation with impingement of the posterior aspect of the lateral side of the tibial plateau against the femoral condyle.
Critical Points CLINICAL EVALUATION
Insufficiency of the PLS and medial ligament structures are determined by varus and valgus stress testing at 0° and 30° of knee flexion. The surgeon estimates the amount of joint opening (in millimeters) between the initial closed contact position of each tibiofemoral compartment, performed in a constrained manner avoiding internal or external tibial rotation, and the maximally opened position. The result is recorded according to the increase in the tibiofemoral compartment of the affected knee compared with that of the opposite normal knee. Abnormal medial or lateral joint opening may be measured with stress radiographs. The tibiofemoral rotation dial test at 30° and 90° detects increases in external tibial rotation with posterior subluxation of the lateral tibial plateau or anterior subluxation of the medial tibial plateau due to medial ligament injury. A word of caution is necessary, because it is possible to confuse increased external tibial rotation for a PL injury and miss the medial side ligament injury that requires correction.135 The presence of a varus recurvatum in both the supine and the standing positions is carefully assessed. Gait analysis is performed to detect a varus or valgus thrust or hyperextension thrust. Patients can often demonstrate their knee instability on standing, with a few degrees of knee flexion, by producing an external femoral rotation that reproduces the pivot shift phenomena. Abnormal varus or valgus tibiofemoral joint opening may also be demonstrated by the patient.
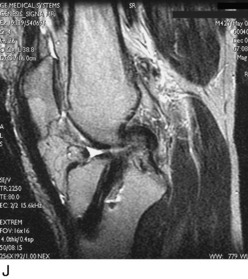
Radiographs taken during the initial examination include standing AP at 0°, lateral at 30° of knee flexion, weight-bearing PA at 45° of knee flexion, and patellofemoral axial views. Double-stance standing radiographs of both lower extremities, from the femoral heads to the ankle joints, are obtained in knees in which varus or valgus lower extremity alignment is detected on clinical examination. The mechanical axis and weight-bearing line are measured as discussed in Chapter 31, Primary, Double, and Triple Varus Knee Syndromes: Diagnosis, Osteotomy Techniques, and Clinical Outcomes. MRI is performed to provide further details of tunnel placement and the condition of the articular cartilage and menisci. Fast-spin-echo techniques are available to obtain superior-quality articular cartilage images.148,149 The tibial tunnel placement is visualized on sagittal views and measurements are made to determine whether the tunnel is too posterior, which requires a staged bone-graft procedure.
At the authors’ center, patients complete questionnaires and are interviewed for the assessment of symptoms, functional limitations, sports and occupational activity levels, and patient perception of the overall knee condition according to the Cincinnati Knee Rating System (CKRS; see Chapter 44, The Cincinnati Knee Rating System).17 The forms are available online for patients to obtain prior to their consultation.
PREOPERATIVE PLANNING
Considerable counseling and patient education are required on the expected results and outcomes from the primary and revision procedures, as previously discussed. This is especially important in knees with preexisting arthritis or loss of meniscal function or those that require additional major operative procedures. In nearly one half of revision knees, a major concomitant procedure will be required.126 Patients need to understand the technical difficulties inherent in the operative procedures and that the results are typically not as favorable as primary ACL reconstruction. A surgeon-rehabilitation team is required to provide instruction on rehabilitation to ensure that the postoperative exercise program will be successfully followed by the patient.
A lateral extra-articular ITB procedure is recommended with allografts in knees that demonstrate a grade III pivot shift test or greater than 10 mm of increased AP translation on KT-2000 testing to reduce the high failure rate noted after revision ACL allograft reconstruction81,131,177 and the delay in allograft healing and maturation.8,78,79,207 Knees with grossly positive clinical laxity tests have involvement of the secondary ligament restraints, primarily the lateral structures, as shown in the “Clinical Biomechanics” section. Associated medial or lateral ligament laxity is an indication for medial or lateral ligament reconstruction. In the Authors’ ACL Primary Reconstruction Clinical Studies section that follows, the results of autograft and allografts are discussed in further detail.
INTRAOPERATIVE EVALUTION
The lateral and medial gap tests are done during the arthroscopic examination (see Chapter 22, Posterolateral Ligament Injuries: Diagnosis, Operative Techniques, and Clinical Outcomes).130 The knee is flexed to 25° to 30° and a varus load of approximately 89 N applied. A calibrated nerve hook is used to measure the amount of tibiofemoral compartment opening. Knees that have 12 mm or more of joint opening at the periphery or 10 mm at the midpoint of the tibiofemoral compartment require a PL or medial ligament reconstructive procedure.
OPERATIVE TECHNIQUES
Graft Selection
The principles of ACL surgery are summarized in Table 7-4. Currently, there is no standard graft choice for ACL reconstruction. Autograft tissue sources include B-PT-B, quadriceps tendon–patellar bone (QT-PB), and semitendinosus-gracilis (STG) tendons. The authors prefer B-PT-B autogenous grafts in athletes, a recommendation supported by several long-term studies42,69,72,89,197 and in recent studies showing a higher rate of ACL primary reconstruction failure after allografts compared with allografts, especially in younger patients.83,95 A B-PT-B autograft is not recommended if there is associated patellofemoral arthritis (Grade 2B classification; see Chapter 47, Articular Cartilage Rating Systems), anterior knee pain, or a history of patellar subluxation or dislocation. A B-PT-B autograft is not performed when patient issues suggest a decreased ability to follow the more involved rehabilitation program and initial pain related to this graft. In recreational athletes and more sedentary patients, a four-strand STG autograft is recommended. Newer fixation methods have increased the success rate, and the rehabilitation postoperatively is easier on the patient.
In revision knees, if the ipsilateral patellar tendon was previously harvested, the contralateral patellar tendon is a valid graft source. Ipsilateral patellar tendon graft reharvest is not recommended owing to persistent changes on MRI and graft morphology reported in these knees 7 to 10 years postoperatively.20,85,88,91,93 A second option is a QT-PB graft taken from the same or the opposite knee, with grafting of any residual patellar bone defect. The quadriceps tendon has the greatest cross-sectional area (100 mm2) and is ideal in revision knees if the prior tunnels are in the desired anatomic ACL site but are slightly expanded. If autograft tissues are not available or the patient refuses a contralateral knee harvest, then a B-PT-B or Achilles tendon–bone allograft is recommended. A posterior or anterior tibialis allograft is not recommended.173 It is desirable to have one portion of the graft with a bone plug for increased rate of graft healing in one of the tunnels. The authors use allografts that have not undergone irradiation sterilization.
Patient Setup and Positioning
Critical Points OPERATIVE TECHNIQUES: GRAFT SELECTION, PATIENT SETUP AND POSITIONING, GRAFT HARVEST
Graft Selection
Graft Harvest, B-PT-B Autograft
A single femoral nerve block is administered preoperatively or in the recovery room, which markedly decreases the need for analgesic medication. The patient is instructed to use crutches with decreased weight-bearing for 24 hours owing to decreased quadriceps function. Bupivacaine (Marcaine) or lidocaine installation is not recommended because high local doses may alter chondrocyte function and viability.68,84,184 A fluid inflow–pressure-regulated pump is recommended over gravity infusion because hemostasis can be controlled and tourniquet use avoided in most cases. An electrocautery device is always available to control bleeding points.
With the patient in a supine position on the operative table and all extremities well padded, a tourniquet is placed on the middle to proximal thigh (Fig. 7-25A). A leg holder is used for the initial arthroscopic examination to provide for maximum opening of the medial and lateral tibiofemoral compartments during the gap tests and to provide necessary distraction for a meniscus repair or partial resection. A leg holder provides better visualization of the posterior meniscus regions over a lateral post. A low-profile leg holder is used, which is pressed into the operative bed mattress to decrease posterior thigh pressure; this is removed after the initial diagnostic arthroscopic procedure. The knee portion of the bed is flexed 60° to 90° and the bed midportion is retroflexed 15° to allow flexion of the hips to relieve undue tension on the femoral neurovascular structures. The uninvolved extremity is placed in a well-cushioned padded holder. An alternative approach described in the literature is to place the uninvolved extremity in an abducted and flexed position with a thigh and leg holder.
After the arthroscopic procedures and removal of the leg holder, two positions may be used for the lower limb. The first is to adjust the operative bed to allow 90° of knee flexion. The knee can be flexed to 120° or more during the procedure, if necessary, by having the operative assistant flex the hip joint and knee joint. The second option is to position the operative bed flat and use an Alverado foot and leg holder or sandbag taped to the table to allow the knee joint to be flexed to the desired position (see Fig. 7-25B). The senior author prefers the second position when any associated medial, lateral, or PCL procedures are performed. The second position allows the surgical assistants to easily view and assist the associated ligament reconstructive procedures. With concurrent medial or lateral reconstructions, the surgical procedure is performed at 20° to 30° of flexion with a roll placed beneath the thigh. The knee joint can also be brought to full extension to determine that posterior capsular reconstructions do not constrain full knee extension.
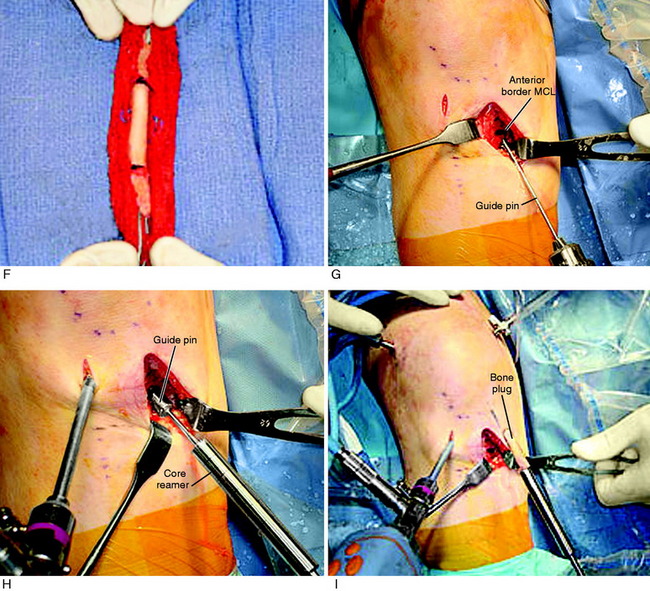
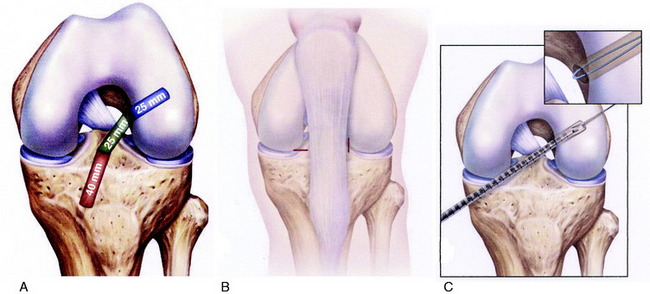
Graft Harvest: B-PT-B Autograft
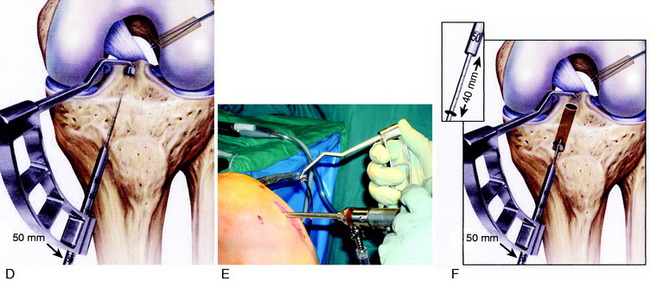
A tourniquet is inflated to 275 mm of pressure. This is usually the only time the tourniquet is inflated in the reconstructive procedure. A 3- to 4-cm vertical medial incision is made just adjacent to the medial border of the patella tendon, avoiding the tibial tubercle (Fig. 7-26). The incision is located just medial to the inferior pole of the patella. A proximal skin incision over the patella is not required because the patella is easily displaced distally during removal of the patella bone plug.
Graft Harvest: QT-PB Autograft
A 5- to 6-cm longitudinal incision is made from the superior pole of the patella, extending to the midline proximally. The prepatellar retinaculum is reflected and protected for later closure over the grafted patellar defect. The quadriceps tendon and its junction with the vastus medialis obliquus and vastus lateralis obliquus (VLO) are identified. The proximal portion of the quadriceps tendon is identified and the graft harvest is carried 15 mm distal to the rectus femoris muscle-tendon attachment in order not to weaken this site. A 10-mm-wide tendon graft, through all three layers, is removed to a length of 60 to 70 cm (Fig. 7-27). The tendon attachment to the anterior superior pole of the patella is carefully identified and the synovial attachment protected. A power saw with the cutting blade marked with paper tape to a depth of 10 mm is used to cut the anterior cortex to produce a patellar bone graft 22 to 24 mm long by 9 to 10 mm wide. The bone graft is sized to 9 to 10 mm in diameter. The quadriceps tendon defect is closed with interrupted 0-Ethibond suture (Ethicon, Somerville, NJ). Two sutures of 0-nonabsorbable material are placed just proximal to the patellar bone defect to create a pocket for the bone graft obtained from the coring reamer. The core bone graft completely obliterates the patellar defect, and a meticulous closure of anterior tissues over the graft is performed, as already described.
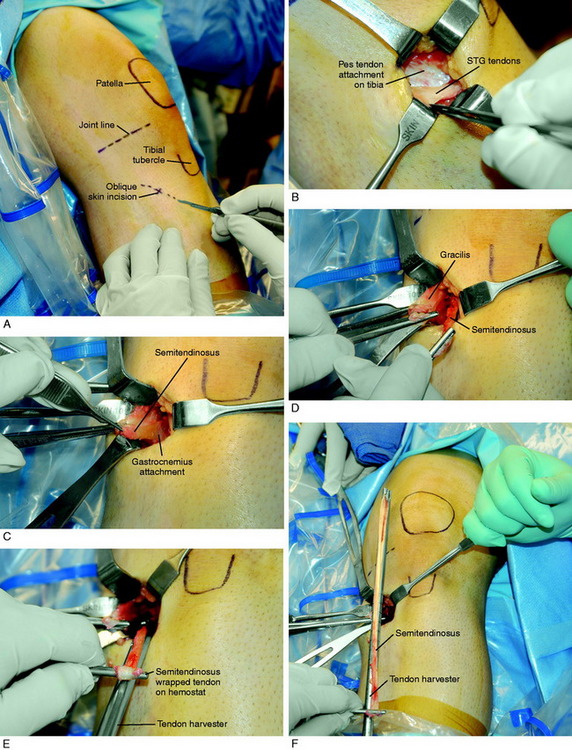
Graft Harvest: STG Autograft
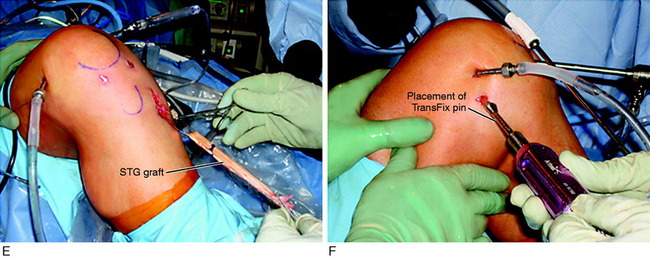
The STG graft harvest procedure is shown in Figure 7-28. A 3- to 4-cm oblique incision is made over the palpated pes tendons. The incision is cosmetic and the plane beneath the subcutaneous tissues carefully dissected. The surgeon is seated, the bed is flexed 60°, and a headlight is routinely used. The sartorius fascia is incised directly proximal to the semitendinosus and gracilis palpated tendons over the distal superficial medial collateral ligament (SMCL) attachment. This provides a window and opening to protect the SMCL. The overlying fascia is incised proximally in the same oblique plane of the STG tendons.
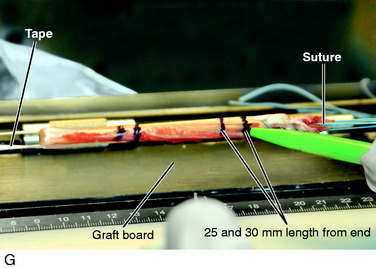
The proximal extent of each tendon is palpated and superficial tissues removed, protecting the overlying sartorius fascia. The dissection plane is never carried close to the inferior sartorius or superior gracilis muscles to avoid injury to the saphenous nerve. The headlight provides excellent illumination in the proximal portion of the incision to protect any neurovascular structures. The proximal fascia about each tendon is bluntly dissected and the semitendinosus tendon attachment to the medial gastrocnemius fascia is incised. With tension on each tendon and a repetitive pulling motion, each tendon will freely displace 10 cm. It is important to determine that each tendon is completely free of tissues to allow the tendon harvester to pass freely. The closed-end graft harvester is passed along the trajectory of each tendon and each tendon is transected at 20 to 22 cm. There is a commercially available tendon harvester with a blunt tip that protects against inadvertent tendon transection and has a tendon cutter in its distal tip activated by the mechanism at the handle. The STG tendons are prepared (see Fig. 7-28). Each tendon is looped about a 3-mm tape and the tendon end sutured to itself with a No. 2 nonabsorbable suture. A third suture is added between both sutured tendon ends. A running 0-nonabsorbable suture is used to produce a tubed structure running from proximal to distal and then back to the proximal starting point. A metal graft-tensioning board (25–30 N tension) is used rather than a graft preparation board with plastic holders because the latter is difficult to sterilize to avoid a graft contamination problem. The graft is marked 25 mm from each end, wrapped in a blood-soaked sponge, and placed in a secure place on the back table.
ITB Extra-articular Tenodesis
Two clinical studies (Noyes and Barber112 and Ferretti and coworkers49) have shown statistically significant improvements in knee stability in knees with ACL revision or with gross instability and loss of secondary restraints that had an extra-articular tenodesis procedure for additional restraint of tibial internal rotation. The procedure is described here and the rationale for its use at the time of ACL reconstruction is discussed later in this chapter.
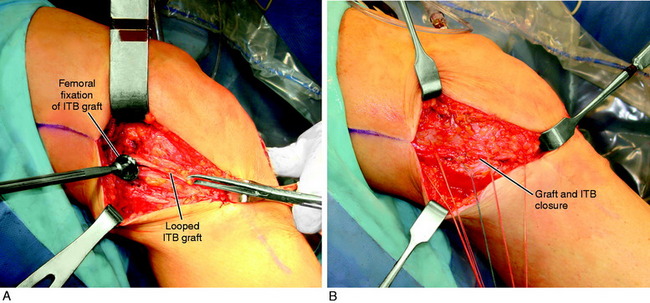
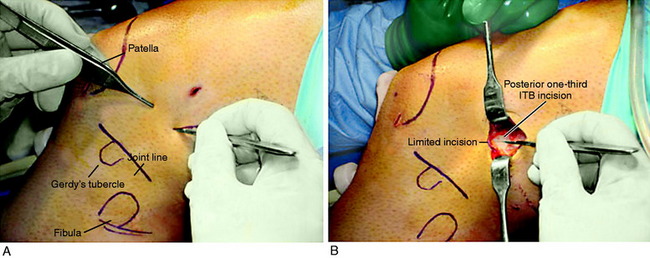
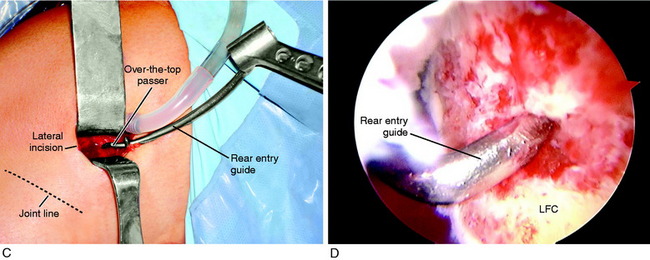
A lateral incision 8 to 10 cm in length is made at the midlateral aspect of the knee extending from Gerdy’s tubercle to the tibia proximally. The skin incision is undermined by subcutaneous dissection to increase the skin mobility and shorten the incision for cosmetic purposes. A strip of ITB is incised proximal to distal from the posterior one third of the band, which is 12 mm wide and 18 to 20 cm long with the tibial attachment intact (Fig. 7-29).
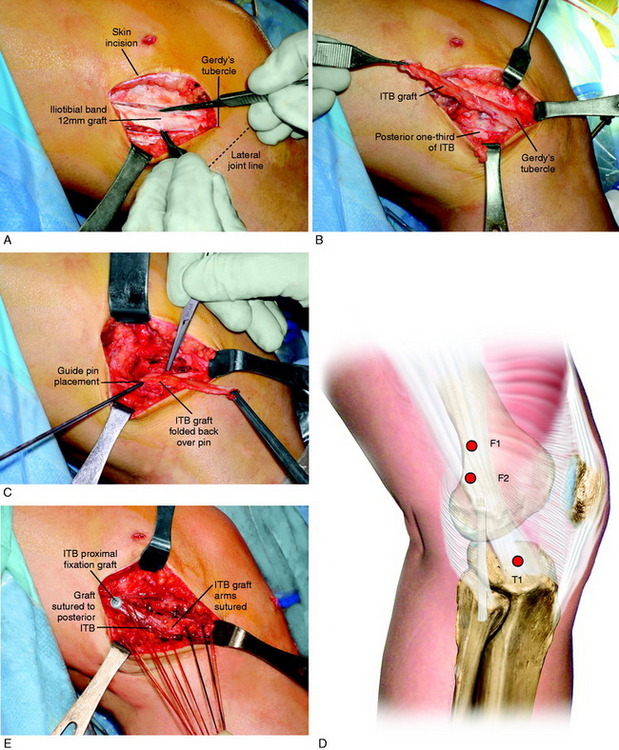
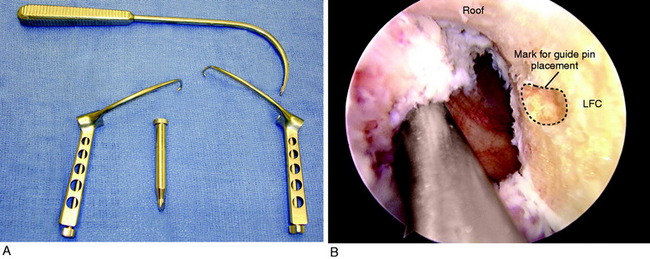
FIGURE 7-29 A, Cosmetic lateral incision is made just proximal to Gerdy’s tubercle. A 10-mm strip along the posterior one half of the iliotibial band (ITB) is harvested. The proximal skin flap is undermined to obtain a 20-mm-long graft. B, The ITB graft (10 mm wide) is dissected distally to Gerdy’s tubercle, where the tibial attachment is left intact. C, The ITB graft is looped around a guide pin placed just distal to the lateral intermuscular and proximal to the FCL attachment. The knee is flexed from 0 to 135°, neutral tibial rotation. D, The “isometric points” graft attachment sites for an extra-articular reconstruction measured by Kurosawa et al.87a The points T1–F1 provided the least change in length with knee flexion (maximum % of strain, 11.6 ± 3.0%). The points T1–F2 offer a second choice, except that there is an increase in the percentage of strain with knee flexion compared with that at T1–F1. E, The graft is looped around a soft tissue screw and washer and both graft ends are sutured to each other and to the remaining posterior ITB. This forms a strong femoral-tibial structure that both incorporates the graft and reproduces the normal posterior ITB femoral tibial attachments. The knee is in neutral tibial rotation and graft overtensioning is avoided.
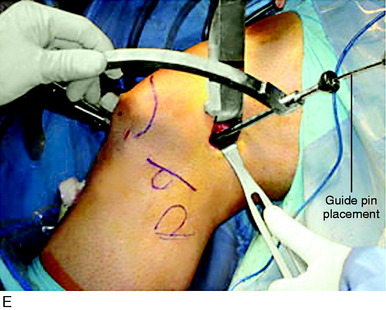
The most isometric point on the proximal and posterior aspect of the lateral femoral condyle is located (see Fig. 7-29D) and tested by using a guide pin placed at the proximal and posterior aspect of the lateral femoral condyle. The point for the femoral attachment of the ITB is usually at the anatomic ITB deep fiber insertion to the femur. This region is usually just distal to the lateral intramuscular septum and posterior and proximal to the lateral epicondyle and anterior to the gastrocnemius tubercle lateral attachment. The error is to place the ITB graft too anterior, which blocks knee flexion. The posterior placement allows the ITB graft to undergo increasing tension with knee extension. This area is curetted to remove soft tissues to allow for the ITB strip to be in contact with the overlying bone.
It is important in tensioning the ITB graft not to overconstrain the joint and block normal internal tibial rotation. After the ACL procedure is completed, the extra-articular tensioning and fixation is performed. The knee is placed at 30° of flexion and neutral rotation. The ITB strip is fixated around a soft tissue washer (Fig. 7-30) and cancellous screw and brought back upon itself to Gerdy’s tubercle. The screw is angulated anteriorly away from the ACL tunnel. The ITB strip is then sutured to itself with only mild tension applied to the ITB graft. The sutures are placed through the posterior one third of the remaining intact ITB just posterior to where the graft was harvested. This produces a strong restraint to abnormal internal tibial translation and anterior tibial translation. The knee is taken through a normal internal-external tibial rotation to ensure that there is no abnormal constraint to internal tibial rotation. The knee is flexed to 135°; usually, increasing knee flexion will provide for increased tension in the graft. There is no true isometric point for the femoral attachment of the graft for flexion-extension and tibial rotation motions. It is probable that even with precautions taken to not overconstrain knee motion, regaining full knee flexion may be more difficult when the extra-articular procedure is added.
Critical Points OPERATIVE TECHNIQUES: ILIOTIBIAL BAND EXTRA-ARTICULAR TENODESIS
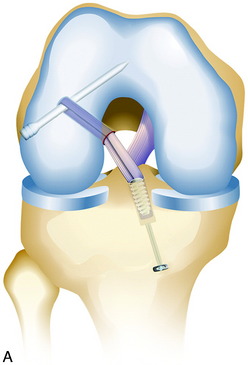
ACL B-PT-B Graft Anatomic Tibial and Femoral Technique
A single-graft procedure is described in which the graft is located into the central anatomic tibial and femoral attachment locations, as already discussed. The graft placement for primary and revision knees specifically avoids the proximal ACL femoral attachment site and the posterior ACL tibial attachment site to avoid a vertical graft and allow the graft to resist rotational coupled motions in addition to anterior tibial translation (Fig. 7-31). This section describes using a single B-PT-B autograft or allograft procedure. The authors’ clinical studies support the concept of a single-graft technique for primary and revision procedures. One exception in revision knees is when a double-bundle procedure may be used in a knee in which a prior vertical ACL graft placement was performed that limits anterior tibial translation and a second coronal graft is added to provide control of tibial rotation.
Placement of Tibial Tunnel
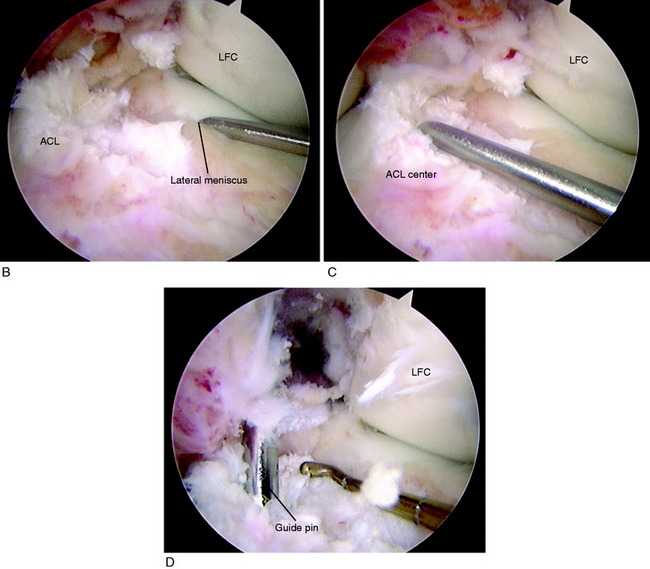
As previously described, the ideal central ACL tibial attachment location is directly adjacent and anterior to the posterior edge of the lateral meniscus anterior horn attachment (see Fig. 7-24). The ACL attachment can be easily mapped at surgery based on the anatomic reference maps provided, with the ACL center location based on the anterior-to-posterior and medial-to-lateral attachments. The anterior extent of the ACL attachment may be obscured by soft tissues, and the RER or posterior interspinous ridge of the PCL fossa is an important landmark. The center of the ACL will be 16 to 20 mm anterior to the RER or interspinous ridge. The guide pin is placed eccentric and 2 to 3 mm anterior and medial to the true ACL center as the ACL graft displaces to the posterior and medial aspects of the tibial tunnel.31 This eccentric tunnel places the majority of the graft within the ideal central tibial attachment.
Placement of Tibial Tunnel
Placement of Femoral Tunnel
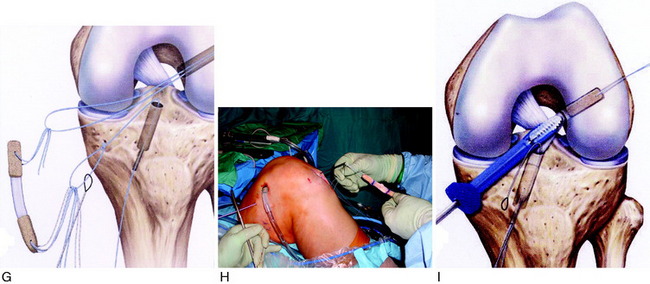
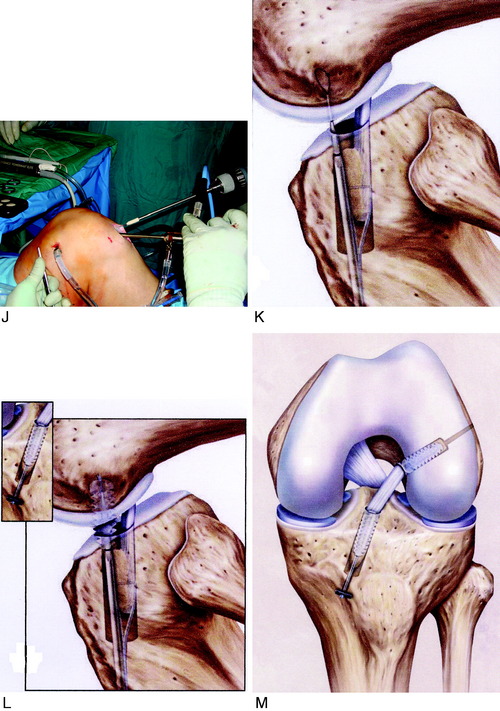
It is important to determine the graft length of the autograft or allograft to ensure that a mismatch does not occur in terms of tunnel and intra-articular length and graft length. The most common problem is with a patella alta and a B-PT-B length greater than 100 to 110 mm based on the patient’s body habitus. The length of the patellar tendon is determined on preoperative lateral radiographs. The normal patellar length based on the Linclau technique92 (patellar cartilage length to anterior vertical tibial prominence; see Chapter 41, Prevention and Treatment of Knee Arthrofibrosis) is a 1:1 ratio with the patellar tendon in the 35- to 45-mm range. The intra-articular ACL length is measured on the lateral MRI, and this length is matched with an autograft or allograft. It is possible to accommodate a shorter length patellar tendon by adjusting the proximity of the tibial tunnel. A patella alta with a tendon length greater than 50 mm is usable with the bone portion of the graft adjusted in the tibia and femoral tunnels. A two-incision technique allows adjustment of graft length by proximal advancement in the femoral tunnel and is ideal when there is graft mismatch due to an excessively long patellar tendon. In rare instances, with a long B-PT-B graft, the tibial bone plug is rotated 180° onto the tendon, sutured to the bone plug, and the tibial tunnel resized. The bone plug sutures are tied to a tibial post. With a graft length mismatch, the authors do not recommend removal of the bone plug to shorten overall graft length or multiple twisting of the graft.
Placement of Femoral Tunnel
As previously described, either a two-incision technique or an AM portal femoral tunnel placement with knee hyperflexion technique is used in primary and revision knees. Baer and associates13 recently reported in a cadaver study that at least 110° of knee flexion is required for femoral tunnel drilling through the AM portal to avoid potential injury to the peroneal nerve, the lateral condylar articular surface, the FCL, and the popliteus tendon. The use of the AM portal to place the femoral tunnel has the problems in some knees of difficulty with visualization at 100° to 120° of flexion, the potential for the drill to damage the medial femoral condyle, and a shorter femoral socket which may be a problem with a B-PT-B autograft. As with any of the ACL techniques, operator experience is necessary to achieve a successful result.
In the two-incision technique, there are two approaches based on the necessity to add additional suture after fixation at the femoral site. The two choices are to drill the tunnel in a retrograde or antegrade procedure. In the retrograde-drilling procedure (Fig. 7-32), a lateral incision of 2 to 3 cm in length is made at the posterior one third of the ITB. The posterior one third of the ITB is incised for 4 to 6 cm to allow exposure. The interval posterior to the vastus lateralis is entered and the muscle protected. An S retractor is placed beneath the VLO to gently lift the muscle anteriorly, avoiding entering the proximal joint capsule. The proximal edge of the lateral femoral condyle is bluntly palpated with an instrument (over-the-top location), and the goal is to locate the tunnel entrance just anterior and not distal to this point. A 15-mm periosteal incision is made and an elevator used to remove soft tissues from the site for the tunnel proximal entrance.
In most chronic ACL-deficient knees and revision knees, there is an overgrowth of cartilage and spur formation in the femoral notch, requiring a notchplasty to prevent ACL graft impingement. The notchplasty rules taught by the senior author since the mid 1980s and used in all the clinical studies reported later in this chapter are described in Table 7-5. In primary ACL knees, an anterior notchplasty of a few millimeters is almost always required owing to the central placement of the tibial tunnel and ACL graft within the central tibial attachment. A lateral notchplasty is performed when required when there is insufficient width (9–10 mm) between the PCL and the lateral femoral notch wall to accommodate the ACL graft.
The ACL femoral attachment is mapped based on the bony landmarks already described. The location of the guide pin for an ACL central femoral tunnel is shown in Figures 7-23, 7-24, 7-31, and 7-33. The guidewire is placed within the central ACL attachment, which is midway between the lateral notch roof and the distal articular cartilage edge (2:00 to 2:30 position), 8 mm from the posterior articular cartilage edge. Clock locations are actually an inaccurate description of the tunnel location.45,47 With the central femoral tunnel, the posterior back wall is 3 to 4 mm thick and the graft occupies approximately two thirds to three fourths of the ACL footprint. Always preserve 4 to 5 mm of the posterior back wall of the tunnel so that the graft is not placed too far posteriorly. Grafts placed too far posteriorly will have increased tension with knee extension and may potentially block full extension. A guide pin placed 8 mm from the posterior articular cartilage at the central ACL attachment will have a 4 mm posterior back wall for a 8-mm graft. A guide pin placed 10 mm from the posterior articular cartilage at the central ACL attachment would have a 5 mm posterior back wall for a 10-mm graft. One key to define the ACL attachment is to place the knee in 20° to 30° of flexion viewed through the AM portal and map out the oval attachment and center point, measuring the distance to the posterior articular cartilage. Once the ideal center point is selected, the knee is flexed to the position desired. It is easier to mark out the ACL femoral attachment when it is viewed in a vertical plane rather than the horizontal attachment plane with knee flexion. The tunnel is drilled to the appropriate diameter for a snug graft fit in the tunnel. The edges of the tunnel are chamfered to prevent graft abrasion. The technique with a rear-entry guide drill is shown in Figure 7-33.
Graft Tunnel Passage, Conditioning, and Fixation
Li and associates90 reported the ACL three-dimensional morphologic changes in humans during weight-bearing flexion using a fluoroscopic and MRI-based system. The ACL tibial insertion twisted internally relative to the femoral insertion. The ACL internal twist amounted to approximately 10° at full extension to 44° at 90° flexion. The data were based on insertion site measurements and not actual ACL fiber microgeometry. Even though some authors5 have recommended an ACL graft orientation to reproduce an internal twist of the ACL fibers, this effect in terms of clinical outcome is unknown. The B-PT-B orientation is usually placed in the sagittal plane on the femur and tibia with the cancellous surface lateral in the femoral tunnel to lessen abrasion of the tendon against the femoral condyle.
Techniques Using Other Grafts
A variety of techniques for femoral fixation (Fig. 7-34) and tibial fixation (Fig. 7-35) are available, based on the preference of the surgeon. The two-incision technique is preferred over the EndoButton technique. The tibial fixation is usually the weakest, and especially in revision surgery, a suture post is added. Interference screw fixation alone of an STG or soft tissue allograft is also not recommended. A suture post is commonly required to achieve adequate fixation.
Alternative Procedures
Outside-In “Flip-Drill” for Femoral Tunnel
An alternative approach is to use a two-incision technique with the proximal skin incision only of sufficient length to accommodate the guide pin “flip-drill” to locate and drill the tunnel from inside-out (Fig. 7-36). This procedure provides a long femoral tunnel for graft healing and incorporation. It also allows control of the guide pin for the femoral tunnel to be oriented into the most ideal position to achieve good-quality bone for interference screw fixation and, in revision knees, to avoid prior tunnels. This technique avoids the necessity for tunnel drilling in a hyperflexed knee position, which is less ideal in revision knees when the femoral tunnel has to be controlled in an exact manner to avoid a prior misplaced tunnel.
The steps for this technique are shown in Figure 7-36. The steps already described for locating the central femoral ACL guide pin placement are performed. A 10-mm incision is made at the posterior one third of the thigh and at the proximal margin of the lateral femoral condyle. The drill guide is placed and the blunt proximal guide sleeve bluntly advanced to the femur. The guide pin flip-drill is advanced and the position and entrance determined with the arthroscope in the AM portal. The drill socket is created. The tunnel joint entrance is chamfered to prevent graft abrasion. The graft is passed in a retrograde manner and fixed either endoscopically or retrograde with the tunnel extending proximally and using the second incision. When the bone quality on the femur is not ideal and a femoral suture post is desired, it is easy to enlarge the incision for visualization, extend the femoral tunnel to exit proximally, and provide a graft suture post.
Endoscopic ACL Reconstruction
Multiple endoscopic ACL reconstructive techniques have been described in the literature for B-PT-B and soft tissue grafts. The approach taken in this chapter is to describe some of these techniques without specifically endorsing one over another. Regardless of the technique the surgeon selects, the anatomic placement of the tibial and femoral tunnels is exactly what has previously been described and illustrated. In Figure 7-37, the all-inside B-PT-B ACL “RetroConstruction” procedure (Arthrex, Naples, FL) is shown as an example of an advanced endoscopic technique that the senior author has used. A complete description of this technique is available from the manufacturer. There is a learning curve for the tibial interference screw fixation. The femoral tunnel technique is the same as already described.
Critical Points OPERATIVE TECHNIQUES: ALTERNATIVE PROCEDURES
Endoscopic Transfix ACL Reconstruction
Numerous procedures are described in which a cross-femoral pin is used to provide proximal graft fixation. The author has found this represents an alternative technique to the two-incision procedure with the use of a four-strand STG graft. An example of this procedure is shown in Figure 7-38. A complete description of this technique and videotape demonstration are available from the manufacturer. It is recommended that this technique be performed in a bioskills setting prior to patient application, because there are specific steps to master. Placement of the cross-pin requires caution to ensure that the tunnel is on the lateral femoral wall and central ACL attachment location, similar to the outside-in two-incision technique previously described. This means that the cross-pin will be entering in a relatively distal position on the femoral condyle and placed posteriorly to avoid damaging the FCL attachment. The common mistake is to locate the femoral tunnel high in the attachment, adjacent to the roof.
Management of Graft Malposition
Critical Points OPERATIVE TECHNIQUES: MANAGEMENT OF GRAFT MALPOSITION AND ENLARGEMENT
B-PT-B, bone–patellar tendon–bone; STG, semitendinosus-gracilis tendons.
Identification of Tibial Tunnel Problems in Revision Knees and Need for Staged Bone-Grafting
It is usually not difficult to identify the prior tibial tunnel entrance into the joint after removal of the soft tissues and graft material at the ACL attachment location. The adjacent lateral meniscus attachment may be encased in scar tissue and is preserved. The PCL, posterior ridge, medial tibial spine, and medial articular cartilage are all identified. The farthest extent of the posterior ACL tibial attachment is approximately 8 mm anterior to the posterior ridge (RER), as already discussed. This is a critical measurement because many primary ACL operative procedures place the ACL graft into the posterior one third of the ACL attachment. It is often the case that during the drilling procedure for the posterior tibial tunnel, or in using this tunnel for the femoral tunnel placement, the tunnel is enlarged posteriorly out of the native ACL footprint and extends to the RER. This situation results in a vertical ACL graft. Often, the preoperative MRI will allow measurements of the tunnel location relative to the AP width, as already described, and the surgeon is aware preoperatively of this problem (Fig. 7-39).
It may initially appear that the tibial tunnel is acceptable; however, after removing all of the soft tissues or when the tibial tunnel drilling is performed, the surgeon may discover that a posterior tibial tunnel exists that extends all the way to or through the RER, which is not acceptable for the revision graft. These cases require staged bone-grafting (Fig. 7-40). On occasion, the posterior tibial tunnel is located in the posterior one third of the native ACL attachment, with only a 5-mm bridge between the tunnel and the RER. If the tibial tunnel is not dilated requiring grafting, it is possible to drill and relocate the tibial tunnel approximately 8 mm anteriorly into a more advantageous central ACL attachment position. A cortical allograft dowel is positioned to occupy and fill the posterior one third of the tunnel, maintaining the graft in the ideal central position. Another technique is to fashion the bone plug of a revision allograft, maintaining an enlarged bone block to fill the posterior portion of the tibial tunnel and prevent the collagen portion of the graft displacing posteriorly. A third technique is to perform a double-bundle reconstruction with the posterior tibial tunnel used for the PL bundle (assuming the graft is placed into the native ACL tibial footprint and not located in an abnormal posterior position). A second anterior tibial tunnel is used for the AM bundle. Use of double-bundle grafts in a revision procedure requires that the femoral tunnel location also be ideal, which is often a problem. A misplaced femoral tunnel may prevent two additional tunnels to be adequately placed or the two additional tunnels could possibly weaken the femoral condyle.
Identification of Prior Femoral Tunnel in Revision Knees and Need for Staged Bone-Grafting
In a majority of revision cases, the index graft is placed in a vertical nonanatomic position on the roof of the intercondylar notch (Fig. 7-41), and it is possible to place the revision tunnel in the ideal central ACL anatomic attachment. The author uses the two-incision approach to reorient the tibial tunnel entirely away from the index tunnel. It is sometimes possible to perform an endoscopic approach using an AM portal to drill the femoral tunnel with a hyperflexed knee; however, the two-incision technique is preferable in most revision procedures. In posterior wall femoral blow-out situations, a reoriented tunnel wall can often be used and the bone portion of the graft fashioned so that the bone is placed in the posterior portion of the tunnel to maintain the collagen portion of the graft within the native ACL femoral footprint.
Tibial Tunnel
Femoral Tunnel
Bone-Grafting Procedure for Tibial and Femoral Enlarged Tunnels
Principles are available to follow in performing bone-grafting of enlarged tunnels. The first is to adequately prepare the graft site by removing all soft tissues, curet, and place small drill holes into the cortical tunnel walls to provide the most ideal surface for bone-graft healing. Cultures of the removed soft tissues are obtained. Rarely, a gram stain and frozen section are necessary to exclude an occult infectious process within a tibial or femoral tunnel, particularly when an allograft was used. Second, it is ideal to use autogenous bone that is supplemented by allograft bone in larger defects. Third, the posterior tibial RER, when deficient, is replaced with good-integrity cortical-cancellous bone (such as a bicortical iliac crest autograft) and not cancellous bone, which is a weaker construct. The goal is to prevent the revision graft in the newly placed tunnel from enlarging the tunnel posteriorly by a windshield-wiper effect against poor-quality bone. The same situation applies to an enlarged dilated femoral tunnel in which the anterior shallow portion of the tunnel is grafted with a combined cortical-cancellous autograft to obtain a good-quality bone construct. The senior author prefers a “minimally invasive” iliac crest autograft procedure, removing only the superior and outer portions and not disturbing the inner table and muscle attachments. This procedure is described in detail in Chapter 31, Primary, Double, and Triple Varus Knee Syndromes: Diagnosis, Osteotomy Techniques, and Clinical Outcomes. The outer iliac crest can be fashioned into a rectangular or circular dowel that is wedged into the enlarged tibial or femoral tunnel, providing a near-complete filling of the expanded tunnel and likelihood of the revision procedure being performed in 3 to 5 months. Strips of cortical cancellous bone are packed into any remaining defects and, if necessary, supplemented with allograft iliac crest bone. In major defects, cancellous bone in the range of 5 to 8 mm fragments are packed between the cortical-cancellous strips. The bone-grafting procedure is easily performed in an arthroscopically assisted manner, enlarging the portals as necessary to deliver the bone graft into the femoral tunnel. The tibial tunnel is packed in a retrograde manner, observing the final position of the graft from an appropriate arthroscopic portal.
Thomas and colleagues181 performed staged ACL revision reconstruction in a series of 49 knees, all of which required bone-grafting of previous tibial tunnels to avoid overlap into correctly placed revision tunnels. The authors stressed the importance of ensuring good-quality bone stock to achieve anatomic ACL graft placement and adequate fixation. At follow-up a mean of 6 years postoperatively, only 2 of the revision knees had failed.
The postoperative rehabilitation program is described in Chapter 13, Rehabilitation of Primary and Revision Anterior Cruciate Ligament Reconstructions. The authors do not administer preoperative or intraoperative intra-articular anesthetic injections or use pain pump catheters postoperatively owing to the possible complication of adverse effects on articular cartilage.68,84,154