CHAPTER 29 Anterior Clinoidal Meningiomas 
INTRODUCTION
Anterior clinoidal meningiomas arise from the meningeal covering of the anterior clinoid process. In the classical neurosurgical literature anterior clinoidal meningiomas have not been separated from medial sphenoid wing or inner sphenoid wing meningiomas. However, accumulating anatomical knowledge and clinical experience has shown that anterior clinoidal meningiomas have unique anatomical and clinical characteristics that puts them apart from meningiomas of the medial sphenoid wing.1–5 Compelling evidence suggests that subtle differences in the patho-anatomy of medial sphenoid wing meningiomas translates into significant differences in surgical outcome. Therefore anterior clinoidal meningiomas should be considered a separate clinical entity. A second level of confusion on the nomenclature stems from the fact that the anterior clinoidal process is situated at the junction of the anterior and middle cranial fossae. Secondary involvement of the anterior clinodial process by anterior or middle fossa meningiomas can create a false impression of primary clinoidal origin. The exact origin of each meningioma can and should be clearly identified. With the help of modern neuroradiology it became increasingly possible to differentiate anterior clinoidal meningiomas. Reported incidence of clinoidal meningiomas ranges from 34%6 to 43.9%7 among sphenoid wing meningiomas.
HISTORY
In 1938, Cushing and Eisenhardt8 broadly classified this group as “deep inner or clinoidal third sphenoid ridge meningiomas,” and this marked the first use of “clinoidal” to distinguish these tumors. In the French literature, Vincent9 referred to them as “sphenocavernous meningiomas” in 1935. The classification of Bonnal and colleagues10 in 1980 identified a group of “sphenocavernous and clinoidal meningiomas” and described them as “extended upward in to the cranial cavity from the dura of cavernous sinus, of the anterior clinoid process and of the internal part of the sphenoid wing.” This group is also similar to the first category of Ojemann’s11 sphenoid ridge meningiomas. Al-Mefty’s1 elegant and more detailed system that was introduced in 1990 classified clinoidal meningiomas as a separate disease entity. Likewise, other reports have attributed unique clinical characteristics and outcomes to sphenocavernous meningiomas (which arise from the lateral wall of the cavernous sinus), cavernous meningiomas (which arise from cavernous sinus proper), planum sphenoidale meningiomas, optic canal meningiomas, and hyperostosing meningiomas of the sphenoid wing.12–16
PRESENTATION
Although the visual problems are the major cause of presenting symptoms in anterior clinoidal meningiomas (45.3%, 53.3%, 58% in some series),2,3,17 this rate is not as high as in other meningiomas located in the neighboring region such as tuberculum sellae meningiomas (75.9%–100%).18–21 The growth potential of the latter tumors may result in early impingement on the optic apparatus. However, for the anterior clinoidal meningiomas, this is true only for Al-Mefty group III tumors that encroach on the optic nerve at the optic foramen.
PREOPERATIVE EVALUATION
Clues to Differentiate Anterior Clinoidal Meningiomas from Other Medial Sphenoidal Ring Meningiomas
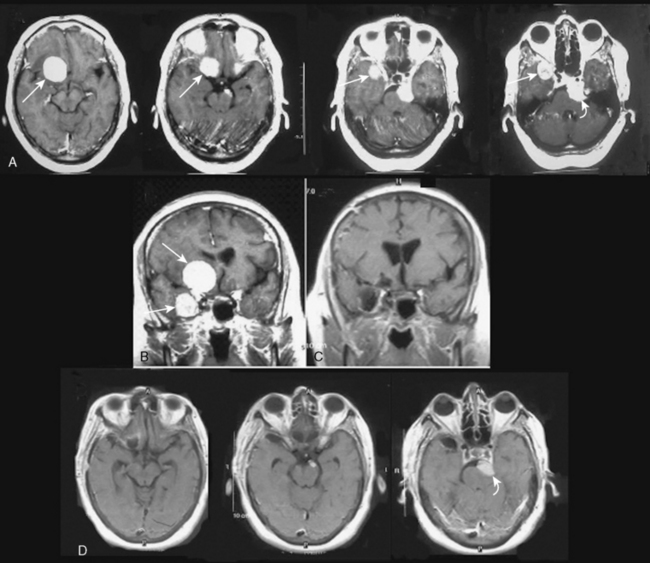
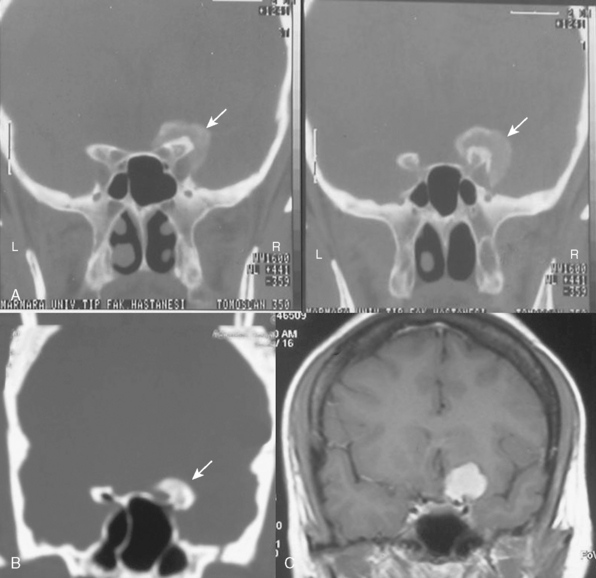
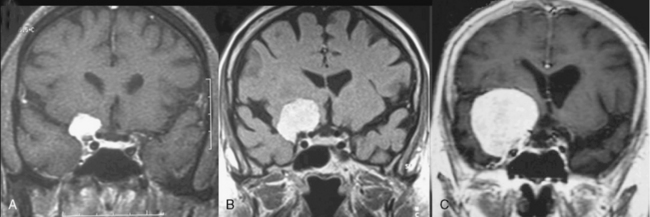
It is difficult to differentiate among meningiomas of this anatomic region before surgery, especially if the tumor is large. However, clues on preoperative imaging can help with diagnosis. With anterior clinoidal meningiomas, the epicenter of the tumor base is on the anterior clinoid process and the tumor typically grows upward, forming a small pedicle toward the suprasellar area and the sylvian fissure. In contrast, meningiomas of the medial third of the sphenoid wing grow in the direction of the anterior aspect of the medial temporal lobe (Fig. 29-1). Another characteristic feature of anterior clinoidal meningiomas is the presence of hyperostosis of the anterior clinoidal process on coronal CT. Accordingly, we recommend coronal CT for any patient with a suspected meningioma at this location (Fig. 29-2).
Cavernous Sinus Invasion
Because anterior clinoidal meningiomas tend to grow upward, true invasion of the cavernous sinus is very rare. The current literature on anterior clinoidal meningiomas documents a very wide range of cavernous sinus invasion rates, ranging from 0% to 44.1%.1–3,22 We believe that this variability is, in part, a reflection of the lack of objective and universal nomenclature among authors. This problem has led to some medial sphenoid wing meningiomas being analyzed as part of the clinoidal group.
Tumor Grading
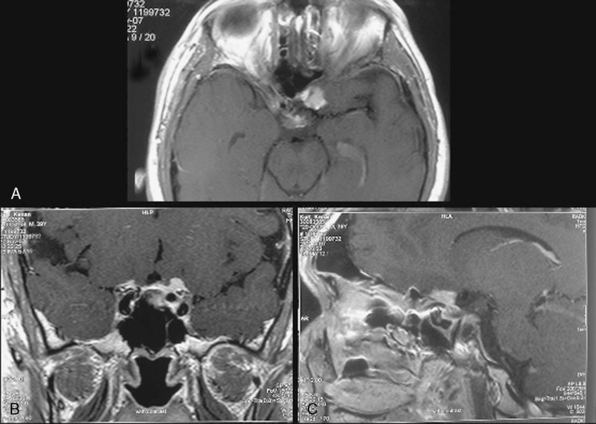
Several classification schemes have been proposed as methods for predicting surgical outcome.1,3,10,20,22 Al-Mefty’s1 elegant classification of clinoidal meningiomas is based on advances in microsurgical anatomy and has been widely accepted. This scheme considers the origin of the tumor and its invasion pattern in the region of the clinoid process as indicators of resectability. It differentiates among lower clinoidal meningiomas (no arachnoidal dissection plane between the internal carotid artery [ICA] and tumor; group I), distal or lateral clinoidal meningiomas (which do have an arachnoidal plane between ICA and tumor; group II), and meningiomas that originate at the optic foramen (group III). In group III tumors, the arachnoidal membrane is present between the ICA and the tumor but may be absent between the optic nerve and the tumor (Fig. 29-3).
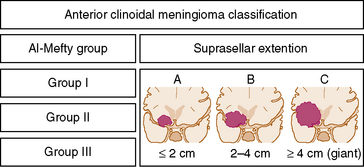
As noted, the current classification scheme considers only tumor origin and invasion pattern around the clinoid process as indicators of resectability.1 However, some reports denote the role of the size in decreasing the success of resection.2,22 Goel and colleagues22 proposed a different method for grading anterior clinoidal meningiomas, one that included tumor size. We wish to emphasize in a very simple and easily remembered manner that pure anterior clinoidal meningiomas grow upward and the size is important. Therefore, we advocate a new classification system that is shown in a simple figure that we think that will be very contributory to the neurosurgical literature. It will also help to reduce the confusion over nomenclature in the current literature. We propose a modification of the classical Al-Mefty system: the addition of subgroups that denote tumor size in coronal plane. This subgrouping can be applied to each of the three groups in the current classification scheme. It should be noted that such absolute cutoff points are irrelevant in biologic systems. This change to the current classification scheme would only provide a more accurate prediction of surgical failure.
According to the new classification the coronal diameter of the tumor is combined with the classical Al-Mefty group designation. Each tumor was graded (typed) with a numerical number denoting the Al-Mefty grouping and a capital letter representing the tumor size on coronal section (Fig. 29-4). A tumor was defined as type A if it measured less than 2 cm, type B if it was 2 cm to 4 cm, and type C (giant) if it was greater than 4 cm (Fig. 29-5). These designations were applied to each group in the current Al-Mefty classification.
Choosing the Optimal Approach
Recent advances in skull-base techniques assisted in resecting these tumors.1–3,22 After operating several cases with pterional and subfrontal approaches, Al-Mefty exclusively used the orbitocranial approach and stated its advantages as: shortest distance to tumor, suitability for surgical attack via multiple routes, and early interception of the tumor’s blood supply through the sphenoid ridge. Many surgeons used various skull-base approaches with or without intra- or extradural removal of anterior clinoid for resecting these challenging tumors. A recent article by Lee and colleagues23 describes a cranial base technique that is a modification of the original “Dolenc approach” and involves extradural clinoidectomy, removal of the roof of the optic canal, and opening of the optic nerve sheath. They stated the advantages of this technique for operating on anterior clinoidal meningiomas and proposed that the conventional pterional approach should be used only for small tumors that cause no visual deficits preoperatively. Mathiesen and Kihlstrom24 also stated that, to achieve or ensure better visual function, it is best to use an extradural approach with drilling of the anterior clinoid and removal of the roof of the optic canal before any intradural steps are performed.
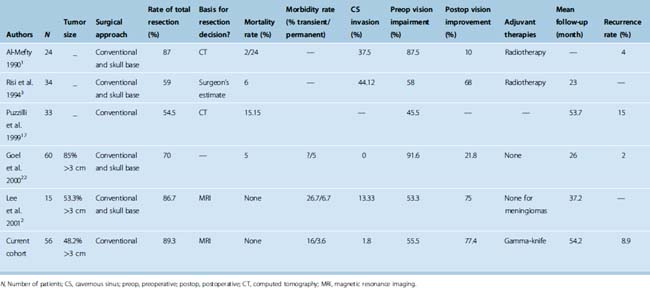
Pure clinoidal meningiomas do not invade the skull base, so the anterior clinoid can be surgically approached with conventional surgical techniques that are fast and efficient and have a relatively low risk of complications. Several reports have documented relatively acceptable results with conventional approaches and, thus, confirm the practical applicability of these techniques.10,20,22,25 Further, two groups of authors who have routinely used skull-base approaches for clinoidal meningiomas stated that conventional techniques would have been sufficient in their cases.3,22 The key features of major series of clinoidal meningiomas published since the introduction of microsurgery are summarized in Table 29-1.
As previously mentioned, significant adherence to distal vessels may occur with large tumors. More conservative approach for meningioma cases with significant adherence around distal vessels can be offered to decrease morbidity and mortality. Authors have documented grave results with more aggressive approaches. One group reported that sacrifice of middle cerebral artery (MCA) branches invaded by anterior clinoidal meningioma was associated with 100% mortality.22 Similarly, another study, in which vessel invasion was handled with aggressive surgery, reported an unintended vessel perforation rate of 20.8%, which resulted in 20% mortality.1 Regardless of the surgical approach, significant adherence around distal vessels by anterior clinoidal meningiomas increases the risk of surgical failure and morbidity. The only limitation of conventional approaches is that they do not allow exploration of the cavernous sinus. As noted previously, meningiomas of clinoidal origin rarely invade this sinus. We prefer to address cavernous sinus invasion with adjuvant radiosurgery after maximal surgical debulking of the extracavernous portion. This approach has been proven to be effective for managing primary cavernous sinus meningiomas while minimizing the risk of cranial nerve morbidity.26–35 The literature on sphenocavernous meningiomas also documents a similar shift from the use of aggressive surgery to the use of more conservative multidisciplinary treatment strategies.13,36
SURGICAL TECHNIQUE
Pterional craniotomy and microsurgery with the aim of total tumor removal is performed (Fig. 29-6). The sylvian fissure is split widely and cerebrospinal fluid (CSF) is drained for relaxation of the brain. In cases where the tumor is large, the mass is detached from the orbital floor and then tracked to its origin. Meticulous care is taken when dissecting tumor tissue from cerebral vessels. The MCAs are followed to the carotid bifurcation and then the carotid is traced to its site of passage through the skull base. The tumor is followed along the cerebral vasculature and careful sharp dissection is done along the arachnoidal plane. The dura covering the anterior clinoid is excised. The anterior clinoid is drilled and the optic canal is opened intradurally. Drilling the anterior clinoid and the optic canal opening can be performed extra- or intradurally. Extradural dissection may have the advantage of exposing the ICA segment proximal to the dural ring and this may be of help in following the course of the ICA. In addition, the removal of the anterior clinoid process extradurally may help devascularize the tumor at an earlier stage before removal of the tumor intradurally. However, while performing extradural drilling of the clinoid process or opening the optic canal extradurally, the dura and tumor must be retracted. This puts additional pressure on the optic nerve and thus may cause it to stretch. It may be much safer to use an intradural approach, split the sylvian fissure widely, gradually relax the brain with CSF drainage, resect the tumor, and then drill the anterior clinoid intradurally and remove the roof of the optic canal. However, safety is achieved either way with a good understanding of the anatomy.
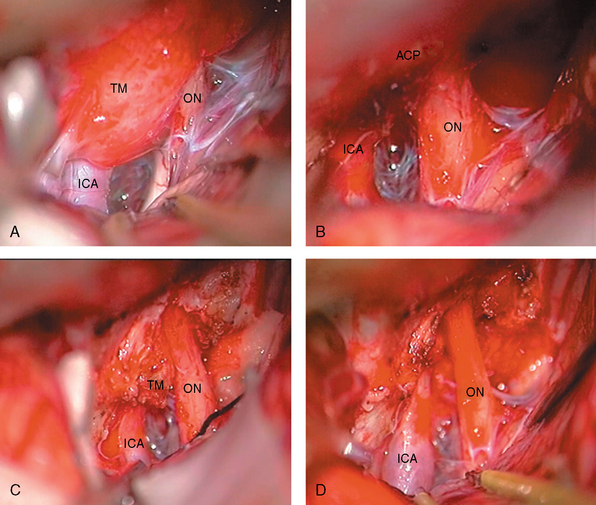
FIGURE 29-6 Operative view of a type III A anterior clinoidal meningioma whose MRI images are shown in Figure 29-3. A, Left-sided pterional approach with the frontal lobe retracted. B, After removal of the tumor. The anterior clinoid process is intact. C, After drilling of the anterior clinoid process. Please note the residual tumor beneath the clinoid within the optic canal. D, End of total tumor removal. ICA, internal carotid artery; ON, optic nerve; TM, tumor.
SURGICAL OUTCOME
In the older series, surgical mortality was unacceptably high with low total resection rate. In 1980, Bonnal and colleagues10 reported a mortality rate of 42% with 0% total resection rate. Similarly, the mortality rate was 32% in Uihlein and Weyand’s series.37 Considering this, a significant number of surgeons accepted and recommended subtotal removal with more conservative strategies. However, with the advent of skull-base exposure, anesthesia, microsurgical techniques, and imaging, surgical outcomes improved. In addition, when speaking of better surgical outcome, one should also consider the point that small differences in the exact origin of the tumor (anterior clinoidal vs. medial or lateral sphenoidal wing) and the cavernous sinus invasion pattern translate to significant differences in surgical outcome. In this regard, significant progress has been made toward understanding the patho-anatomy of anterior clinoidal meningiomas; thus this is another reason for the difference of better surgical outcomes with time. Total resection rates improved ranging from 54.5% to 89.3% with a permanent morbidity rate of 5% (in average) in series published during the microsurgery era.
Research has shown that tumor size is also important with regard to resectability.22 With large tumors, invasion of vascular and neural structures beyond the immediate periclinoidal area becomes a concern.1,22 To predict the surgical outcome of clinoidal meningiomas that are already of considerable size, it is necessary to introduce another classification parameter: distal adherence. When a clinoidal meningioma of any origin breaks through its arachnoidal covering, there is potential for significant adherence of distal branches of the ICA, MCA, anterior cerebral artery (ACA), and their perforators. As it is the case in ICA, distal vessel invasion precludes total surgical resection with acceptable morbidity. Goel and colleagues22 indicated a high risk of surgical failure for tumors larger than 3 cm (35.29% vs. none in smaller tumors). Such a difference was also evident in the cohort reported by Lee and colleagues2 (22.22% vs. none in smaller tumors).
Despite pessimistic reports in the earlier series,1 most recent series report good visual outcome in these tumors ranging from 68% to 76.9%.2–4 The optic nerve that was under ischemia by compression of the tumor may remain in ischemic penumbra and may have a potential for recovery after decompression. Likewise, a partially demyelinated optic nerve may remyelinate after surgery. This is also supported with the findings of other series that preoperative duration of visual loss adversely affects the visual outcome.2,19 We believe that direct compression is the most important factor that causes visual problems in the anterior clinoidal meningioma patients, and these are reversible if the decompression is performed within the period of ischemic penumbra.
MANAGEMENT OF RESIDUAL OR RECURRENT TUMORS AND THE ROLE OF STEREOTACTIC RADIOSURGERY
There is not a single study that deals only with the natural history of anterior clinoidal meningiomas. However, it is possible to extract knowledge from the studies of skull-base or other meningiomas series. Nakamura and colleagues studied the natural history of 41 incidental meningiomas.38 They found that in a mean follow-up of 43 months, mean absolute growth rate is 1.34 cm3/year (0.19–2.62 cm) and mean tumor doubling time is 13.6 years (1.73–49.6 years). In the largest series in the literature, which presents the results of the 40 skull-base meningioma patients, most of them involving the cavernous sinus, anterior clinoid, and petroclival region, the authors found that 7 patients (17%) experienced tumor growth and 11 patients (27.5%) experienced neurologic progression.39 In this study, mean radiologic follow-up was 76 months and mean clinical follow-up was 83 months. The results showed that these tumors may be very indolent tumors, and impairment may be much less than the imaging studies would suggest. However, the results also showed that these tumors grow in time.
A second operation carries a significantly higher mortality and failure rate.1,26,40 In patients who had undergone a previous surgery, the arachnoid membrane may have been violated; subsequently the dissection plane is lost and the tumor will be direct contact with the adventitia.1 In this case the difficulty in Al-Mefty group II cases become similar to that in group I cases. Thus, more conservative strategies are chosen for the second-stage treatment. Gamma-knife radiosurgery is a treatment option and effective for the residual or recurrent anterior clinoidal meningiomas (cavernous or extracavernous, wherever the location).29,41 Fractionated stereotactic radiosurgery is an additional treatment option of high efficacy with only few side effects. In the case of tumors that are not appropriate for gamma-knife radiosurgery, such as with adjacent optic nerve (< 2 mm), fractionated stereotactic radiosurgery is recommended.41–46
[1] Al-Mefty O. Clinoidal meningiomas. J Neurosurg. 1990;73(6):840-849.
[2] Lee J.H., Jeun S.S., Evans J., Kosmorsky G. Surgical management of clinoidal meningiomas. Neurosurgery. 2001;48(5):1012-1019. discussion 1019–21
[3] Risi P., Uske A., de Tribolet N. Meningiomas involving the anterior clinoid process. Br J Neurosurg. 1994;8(3):295-305.
[4] Tobias S., Kim C.H., Kosmorsky G., Lee J.H. Management of surgical clinoidal meningiomas. Neurosurg Focus. 2003;14(6):e5.
[5] Pamir M.N., Belirgen M., Ozduman K., Kiliç T., Ozek M. Anterior clinoidal meningiomas: analysis of 43 consecutive surgically treated cases. Acta Neurochir (Wien). 2008;150:625-635.
[6] Al-Mefty O., Ayoubi S. Clinoidal meningiomas. Acta Neurochir Suppl (Wien). 1991;53:92-97.
[7] Cophignon J., Lucena J., Clay C., Marchac D. Limits to radical treatment of spheno-orbital meningiomas. Acta Neurochir Suppl (Wien). 1979;28(2):375-380.
[8] Cushing H., Eisenhardt L. Meningiomas: Their Classification, Regional Behavior, Life History and Surgical End Results. Springfield, IL: Charles C Thomas, 1938.
[9] David M.. Les Meningiomes De La Petit aile du sphenoide (considerations anatomo-cliniques et therapeutiques). 1935;111-130.
[10] Bonnal J., Thibaut A., Brotchi J., Born J. Invading meningiomas of the sphenoid ridge. J Neurosurg. 1980;53(5):587-599.
[11] Ojemann R.G. Meningiomas of the basal parapituitary region: technical considerations. Clin Neurosurg. 1980;27:233-262.
[12] Wilson W.B., Gordon M., Lehman R.A. Meningiomas confined to the optic canal and foramina. Surg Neurol. 1979;12(1):21-28.
[13] Abdel-Aziz K.M., Froelich S.C., Dagnew E., et al. Large sphenoid wing meningiomas involving the cavernous sinus: conservative surgical strategies for better functional outcomes. Neurosurgery. 2004;54(6):1375-1383. discussion 1383–4
[14] Shrivastava R.K., Sen C., Costantino P.D., Della Rocca R. Sphenoorbital meningiomas: surgical limitations and lessons learned in their long-term management. J Neurosurg. 2005;103(3):491-497.
[15] Fahlbusch R., Schott W. Pterional surgery of meningiomas of the tuberculum sellae and planum sphenoidale: surgical results with special consideration of ophthalmological and endocrinological outcomes. J Neurosurg. 2002;96(2):235-243.
[16] Sekhar L.N., Janecka I.P. Surgery of cranial base tumors. Raven Press, New York, 1993..
[17] Puzzilli F., Ruggeri A., Mastronardi L., et al. Anterior clinoidal meningiomas: report of a series of 33 patients operated on through the pterional approach. Neuro-oncol. 1999;1(3):188-195.
[18] Grisoli F., Diaz-Vasquez P., Riss M., et al. Microsurgical management of tuberculum sellae meningiomas. Results in 28 consecutive cases. Surg Neurol. 1986;26(1):37-44.
[19] Pamir M.N., Ozduman K., Belirgen M., et al. Outcome determinants of pterional surgery for tuberculum sellae meningiomas. Acta Neurochir (Wien). 2005;147(11):1121-1130. discussion 1130
[20] Yasargil M. Microneurosurgery. Stuttgart-New York: Thieme Verlag, 1996.
[21] Zevgaridis D., Medele R.J., Mueller A., et al. Meningiomas of the sellar region presenting with visual impairment: impact of various prognostic factors on surgical outcome in 62 patients. Acta Neurochir Suppl. 2001;143:471-476.
[22] Goel A., Gupta S., Desai K. New grading system to predict resectability of anterior clinoid meningiomas. Neurol Med Chir (Tokyo). 2000;40(12):610-616. discussion 616–7
[23] Lee J.H., Sade B., Park B.J. A surgical technique for the removal of clinoidal meningiomas. Neurosurgery. 2006;59(Suppl. 1):ONS108-ONS114. discussion ONS108–14
[24] Mathiesen T., Kihlstrom L. Visual outcome of tuberculum sellae meningiomas after extradural optic nerve decompression. Neurosurgery. 2006;59(3):570-576. discussion 570–6
[25] Samii M., Ammirati M. Medial sphenoid wing meningiomas. In: Surgery of Skull Base Meningiomas. Berlin: Springer-Verlag; 1993:35-41.
[26] Mirimanoff R.O., Dosoretz D.E., Linggood R.M., et al. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985;62(1):18-24.
[27] Jaaskelainen J. Seemingly complete removal of histologically benign intracranial meningioma: late recurrence rate and factors predicting recurrence in 657 patients. A multivariate analysis. Surg Neurol. 1986;26(5):461-469.
[28] Pendl G., Eustacchio S., Unger F. Radiosurgery as alternative treatment for skull base meningiomas. J Clin Neurosci. 2001;1(Suppl. 8):12-14.
[29] Kondziolka D., Lunsford L.D., Coffey R.J., Flickinger J.C. Stereotactic radiosurgery of meningiomas. J Neurosurg. 1991;74(4):552-559.
[30] Kondziolka D., Lunsford L.D., Coffey R.J., Flickinger J.C. Gamma knife radiosurgery of meningiomas. Stereotact Funct Neurosurg. 1991;57(1–2):11-21.
[31] Kobayashi T., Kida Y., Mori Y. Long-term results of stereotactic gamma radiosurgery of meningiomas. Surg Neurol. 2001;55(6):325-331.
[32] Hodes J.E., Sanders M., Patel P., Patchell R.A. Radiosurgical management of meningiomas. Stereotact Funct Neurosurg. 1996;66(1–3):15-18.
[33] Eustacchio S., Trummer M., Fuchs I., et al. Preservation of cranial nerve function following gamma knife radiosurgery for benign skull base meningiomas: experience in 121 patients with follow-up of 5 to 9.8 years. Acta Neurochir Suppl. 2002;84:71-76.
[34] Chang J.H., Chang J.W., Choi J.Y., et al. Complications after gamma knife radiosurgery for benign meningiomas. J Neurol Neurosurg Psychiatry. 2003;74(2):226-230.
[35] Mathiesen T., Lindquist C., Kihlstrom L., Karlsson B. Recurrence of cranial base meningiomas. Neurosurgery. 1996;39(1):2-7. discussion 8–9
[36] Pamir M.N., Kilic T., Bayrakli F., Peker S. Changing treatment strategy of cavernous sinus meningiomas: experience of a single institution. Surg Neurol. 2005;2(Suppl. 64):S58-S66.
[37] Uihlain A., Weyand W.R. Meningiomas of anterior clinoid process as a cause of unilateral loss of vison. Surgical considerations. Arch Ophthalmol. 1953;49:261-270.
[38] Nakamura M., Roser F., Jacobs C., et al. Medial sphenoid wing meningiomas: clinical outcome and recurrence rate. Neurosurgery. 2006;58(4):626-639. discussion 626–39
[39] Bindal R., Goodman J.M., Kawasaki A., et al. The natural history of untreated skull base meningiomas. Surg Neurol. 2003;59(2):87-92.
[40] MacCarty C.S., Taylor W.F. Intracranial meningiomas: experiences at the Mayo Clinic. Neurol Med Chir (Tokyo). 1979;19(7):569-574.
[41] Torres R.C., Frighetto L., De Salles A.A., et al. Radiosurgery and stereotactic radiotherapy for intracranial meningiomas. Neurosurg Focus. 2003;14(5):e5.
[42] Behbehani R.S., McElveen T., Sergott R.C., et al. Fractionated stereotactic radiotherapy for parasellar meningiomas: a preliminary report of visual outcomes. Br J Ophthalmol. 2005;89(2):130-133.
[43] Jalali R., Loughrey C., Baumert B., et al. High precision focused irradiation in the form of fractionated stereotactic conformal radiotherapy (SCRT) for benign meningiomas predominantly in the skull base location. Clin Oncol (R Coll Radiol). 2002;14(2):103-109.
[44] Lo S.S., Cho K.H., Hall W.A., et al. Single dose versus fractionated stereotactic radiotherapy for meningiomas. Can J Neurol Sci. 2002;29(3):240-248.
[45] Shrieve D.C., Hazard L., Boucher K., Jensen R.L. Dose fractionation in stereotactic radiotherapy for parasellar meningiomas: radiobiological considerations of efficacy and optic nerve tolerance. J Neurosurg. 2004;3(Suppl. 101):390-395.
[46] Milker-Zabel S., Zabel-du Bois A., Huber P., et al. Fractionated stereotactic radiation therapy in the management of benign cavernous sinus meningiomas: long-term experience and review of the literature. Strahlenther Onkol. 2006;182(11):635-640.