Chapter 54 Anterior and Subtemporal Approaches to the Infratemporal Fossa
The presence of neurovascular structures within the ITF (e.g., ICA) or adjacent to it (e.g., CN VII) is the limiting step for designing a surgical approach to the ITF. Surgical approaches often center on the preservation and identification of these neurovascular entities. The first report in the English literature of a surgical approach to the ITF is attributed to Barbosa,1 who in 1961 described his approach for advanced tumors of the maxillary sinus. Transtemporal approaches described by Fisch and preauricular approaches by Schramm and Sekhar are the basis for other modifications.2–8 Subsequent approaches follow the surgical and anatomic principles shown by these authors.
PREOPERATIVE EVALUATION
Diagnostic and Staging Work-up
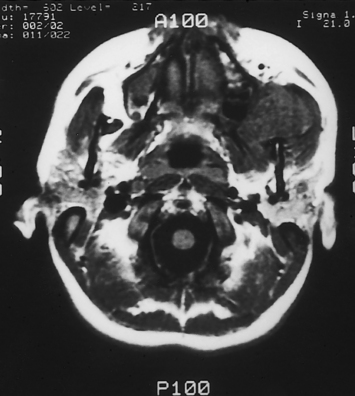
Owing to the inaccessibility of the ITF to physical examination, radiographic imaging is a vital component of the evaluation. Computed tomography (CT) and magnetic resonance imaging (MRI) provide valuable information and are obtained using standard skull base protocols. CT is superior to MRI, showing the remodeling or erosion of neurovascular foramina or other bones of the skull base. MRI better delineates the soft tissue planes, the tumor–soft tissue interface, and the presence of tumor along neural and vascular structures (Fig. 54-1). CT and MRI are often complementary.
If the risk for injury or sacrifice of the ICA is high, the collateral cerebral blood flow may be evaluated using angiography-balloon occlusion with xenon CT (ABOX-CT). A nondetachable balloon is inserted in the ICA via the femoral artery. The balloon is inflated for 15 minutes while the awake patient is monitored for any neurologic deficit. If the patient does not develop any deficit, the balloon is deflated, and the patient is transferred to a CT suite. A mixture of 32% xenon/68% oxygen is administered via facial mask for 4 minutes. CT shows the distribution of xenon, which reflects the blood flow within the cerebral tissue, providing a quantitative assessment of milliliters of blood flow per minute per 100 g of brain tissue. The process is repeated after reinflation of the arterial balloon. A computer calculates the differential of the xenon diffusion in the brain before and after the balloon inflation, identifying patients at risk for an ischemic stroke secondary to reduced blood flow after occlusion of the ipsilateral ICA (Table 54-1).9
| Cerebral Blood Flow (mL/min/100 g Tissue) | Risk | Implication |
|---|---|---|
| >35 | Low | Carotid may be sacrificed |
| 21-35 | Moderate | Patient would tolerate occlusion under controlled circumstances; reconstruction is recommended |
| ≤20 | High | Patient would not tolerate occlusion of internal carotid artery |
Rehabilitation Considerations
Laryngeal framework surgery (thyroplasty) performed during the extirpative surgery or the early postoperative period improves the glottic closure and decreases the risk for aspiration, often obviating the need for a tracheotomy for the sole purpose of tracheopulmonary toilet.10–12 Laryngeal framework surgery allows the patient to compensate for the deficits using the remaining function (contralateral side) more effectively. Laryngeal framework surgery does not restore the motor or the sensory function. These patients remain at a higher risk for aspiration and nutritional deficiencies. Collaboration with an experienced speech-language pathologist, who can assist with the monitoring of the patient and the diet modifications and provide intensive swallowing therapy, is crucial to prevent the pulmonary and nutritional complications of aspiration. In patients with severe deficits or in patients with cognitive problems, strong consideration should also be given to placement of a gastrostomy tube to facilitate postoperative feeding and decrease the risk of prandial aspiration.
SURGICAL APPROACHES
Preauricular (Subtemporal) Approach
The preauricular approach is suited for tumors that originate in the ITF and intracranial tumors that originate at the anterior aspect of the temporal bone, or greater wing of the sphenoid bone, and that extend into the ITF.5,13,14 It may also be combined with other approaches, such as a subfrontal approach to expose massive tumors that extend to the anterior and middle skull base. The preauricular approach does not provide an adequate exposure for the resection of tumors that invade the tympanic bone, however, and does not provide control of the intratemporal facial nerve or jugular bulb.
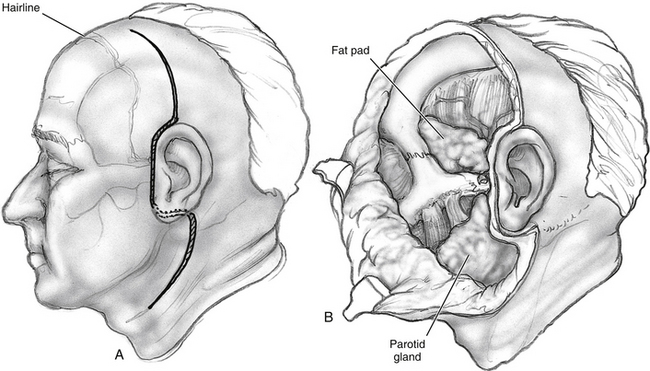
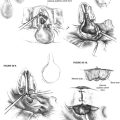
An incision, following a hemicoronal or bicoronal line, is carried through the subcutaneous tissue, galea, and pericranium (Fig. 54-2). Over the temporal area, the incision extends down to the deep layer of the temporal fascia. The anterior branches of the superficial temporal artery are preserved, ensuring adequate blood supply to the scalp flap. Ipsilateral to the tumor, the incision is extended following into the preauricular crease down to the level of the tragus. When proximal control of the ICA is warranted, the incision is extended into the neck using a lazy S pattern, or, alternatively, a separate cervical incision is performed. The scalp is dissected following a subpericranial plane, separating the attachments of the pericranium to the deep layer of the temporal fascia. The scalp flap is elevated from the deep temporal fascia using a broad periosteal elevator.
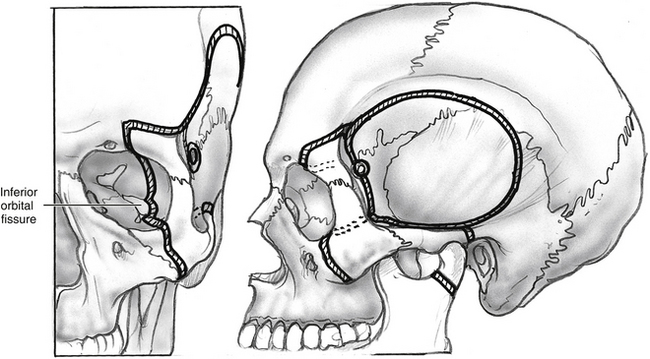
Above the zygoma, the deep temporal fascia splits into superficial and deep layers, which attach to the lateral and medial surfaces of the zygomatic arch. To continue the surgical exposure, the superficial layer of the deep temporal fascia is incised following an imaginary line that joins the superior orbital rim to the zygomatic root. The dissection continues deep to this plane, elevating the superficial layer of the deep temporal fascia off the zygomatic arch (see Fig. 54-2). Fascia and periosteum are reflected anteriorly with the scalp flap. This maneuver protects the frontal branches of the facial nerve that are just lateral to the superficial layer of the deep temporal fascia. Elevation of the periosteum from the lateral surface of the zygomatic arch and malar eminence completes exposure of the orbitozygomatic complex. The periorbita is elevated from the lateral orbit using a Penfield No. 1 dissector, exposing the roof and lateral wall of the orbit down to the inferior orbital fissure.
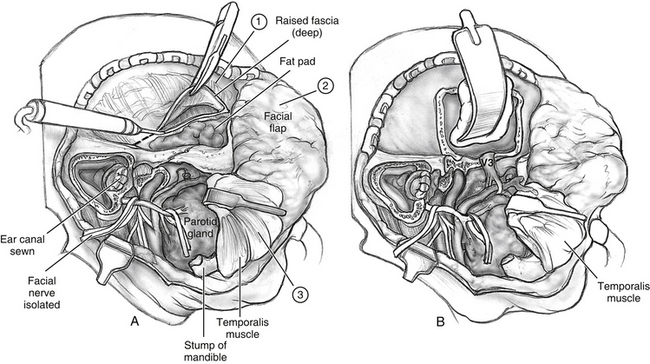
The fascial attachments of the temporalis and masseter muscles to the zygomatic arch are transected using electrocautery. The attachments of the temporalis muscle to the cranium are transected with the electrocautery, and the muscle is elevated off the temporal fossa. If the temporalis muscle is to be returned to its original position at the completion of the surgery, a curved titanium plate (1.5 to 1.7 mm) is screwed at the temporal line, leaving some screw holes empty to facilitate suturing from the plate to the muscle (Fig. 54-3). Then the masseteric fascia is dissected from the masseter muscle, elevating the overlying parotid gland with a broad periosteal elevator (see Fig. 54-2). To increase the arc of rotation of the scalp flap, any soft tissue anterior to the tympanic bone can be transected from superior to inferior, down to the level of the facial nerve. The facial nerve is identified and preserved using a standard technique. It is helpful to preserve a cuff of soft tissue around the main trunk of the facial nerve to prevent a traction injury to the main trunk of the facial nerve.
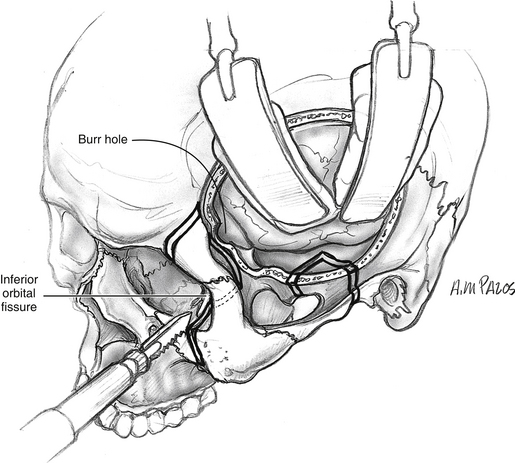
Orbitozygomatic osteotomies are performed at the zygomatic root posteriorly, the zygomaticofrontal suture superiorly, and the zygomaticomaxillary buttress at the level of the zygomaticofacial nerve medially (see Fig. 54-3). Prior periorbital elevation off the lateral and inferior walls is necessary to identify the inferior orbital fissure and to complete the osteotomies of the orbitozygomatic complex. The tip of the reciprocating saw is placed in the most lateral aspect of the inferior orbital fissure; an osteotomy is performed through the malar eminence following a vertical imaginary line medial to the zygomaticofacial foramen. This osteotomy separates the zygoma from the maxilla. Accidental entry into the maxillary sinus may occur, requiring closure of the defect using fascia or pericranium free grafting or both. These free tissue grafts are held in place by compression against the opening when orbitozygomatic bone graft is replaced and plated at the completion of the surgery. All osteotomies are completed with a reciprocating saw transecting the bone in beveled or V-shaped manner in such a way that maximizes the exposure and facilitates replacement of the bone graft at the completion of the surgery. If tumor involvement of the orbit is present, the osteotomies are modified to secure a complete resection.
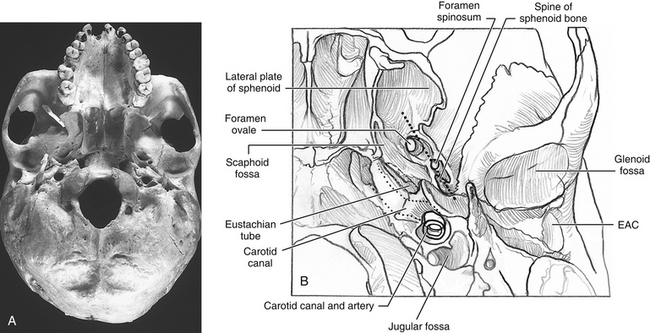
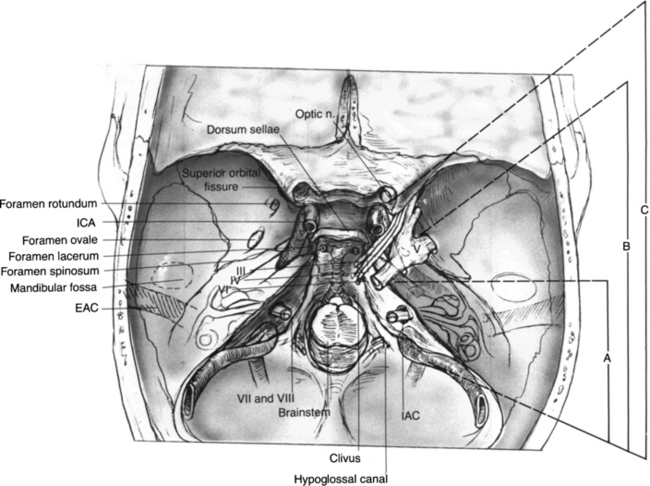
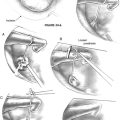
A subtemporal craniectomy may aid in the identification of neurovascular structures piercing the infratemporal skull base and to augment the exposure. The lateralmost bone is removed using rongeurs. The origin of the lateral pterygoid plate at the skull base is identified anteriorly. Anatomic relationships that are useful for the identification of infratemporal skull base structures are shown in Figure 54-4, including the posterior curve of the attachment of the lateral pterygoid plate that is in alignment with the foramen ovale, foramen spinosum, and the spine of the sphenoid bone. These structures lie in a straight “line of sight” that is lateral to the canal of the ICA. The inferolateral aspect of the sphenoid sinus may be accessed by removing the bone (i.e., pterygoid plates) between the second and third divisions of the trigeminal nerve. The extirpation of the tumor can now proceed, including the involved soft tissue and bone. To continue the subtemporal exposure, the middle meningeal artery is clipped or cauterized using bipolar electrocautery and transected. Bleeding from the venous plexus that accompanies V3 through the foramen ovale may be controlled with absorbable knitted fabric (Surgicel) packing.
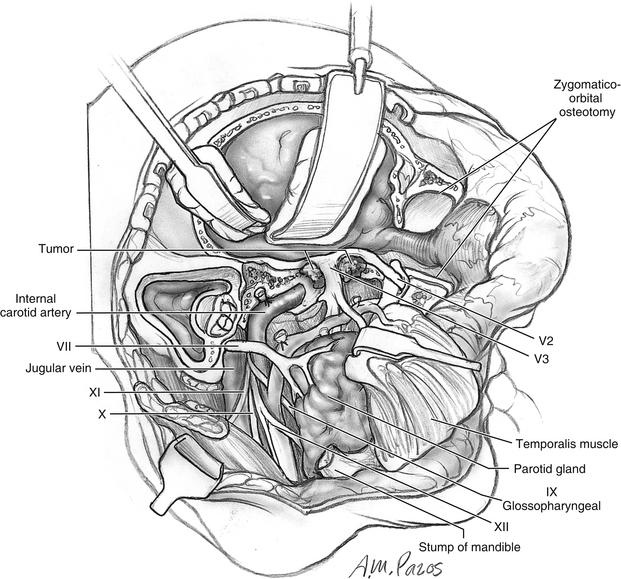
Lesions that do not involve the temporal bone or petrous portion of the ICA are adequately exposed with this stepwise approach. Dissection of the petrous ICA is necessary, however; the glenoid fossa is removed as part of the orbitozygomatic bone graft. It is first necessary to perform a temporal craniotomy for exposure of the superior aspect of the glenoid fossa (Fig. 54-5). The capsule of the TMJ is dissected free from the fossa and displaced inferiorly. If possible, the capsule and meniscus are preserved. With use of a reciprocating saw, osteotomies are made through the glenoid fossa, incorporating the lateral two thirds of the fossa (see Fig. 54-5). This maneuver avoids potential injury to the ICA that is located medial to the fossa. In addition, this modification provides stability for the mandibular condyle after reconstruction, although it can be prone to anterior dislocation. Injury to the cochlea is possible if the osteotomies are made too posteriorly. If additional exposure is necessary (i.e., carotid canal and extratemporal ICA), the condylar neck and contents of the condylar fossa can be transected at the level of the sigmoid notch and removed (Fig. 54-6).
To dissect the petrous segment of the ICA, it is necessary to transect the mandibular division of the trigeminal nerve at the foramen ovale (see Fig. 54-6). When the ICA is mobilized from its horizontal canal, it can be transposed or retracted to facilitate the resection of tumor, or to gain access to the petrous apex.
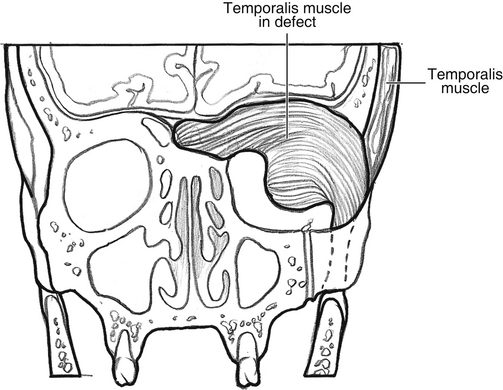
After extirpation of the tumor, it is necessary to close any communication with the upper aerodigestive tract. If viable, a temporalis muscle flap is used to obliterate the dead space and protect the ICA (Fig. 54-7). Because of the branching pattern of the blood supply to the temporalis muscle, the muscle can be divided vertically, and the anterior half of the muscle may be transposed with an intact blood supply. The remaining posterior half of the muscle is transposed anteriorly to fill the temporal fossa defect.
Postauricular (Transtemporal) Approach
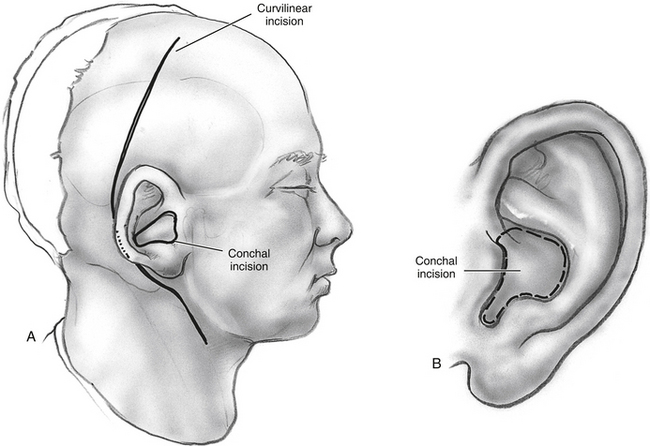
The postauricular approach is designed to expose and resect lesions involving the temporal bone and extending into the ITF.3,14 A question mark–shaped or C-shaped incision is started in the temporal area and extended, postauricularly, into the mastoid region, curving down to follow one of the midneck horizontal skin creases (Fig. 54-8).
If the middle ear is to be sacrificed as part of the approach or the tumor resection, and there is a risk of a postoperative CSF leak (intradural work), the external auditory canal (EAC) is closed permanently to prevent CSF otorrhea. The EAC is divided at the bony-cartilaginous junction and then closed using everting stitches. This closure is reinforced with a myoperiosteal U-shaped flap based on the posterior margin of the EAC. Alternatively, if the middle ear is to be spared, the canal may be preserved by placing the incisions in the conchal area (Fig. 54-8B). The incision follows the margin of the conchal bowl and tragus so that the scar would be hidden.
A mastoidectomy and dissection of the vertical portion of the facial nerve allows the transposition of the facial nerve, providing a wider access to the infratemporal fossa (see Fig. 54-9). In patients who require a radical parotidectomy, a mastoidectomy provides the means to obtain proximal control of the neural margins and to graft the nerve. It also provides access to the jugular bulb and adjacent lower cranial nerves.
Lateral Fisch Infratemporal Fossa Approaches
Fisch has described an array of lateral infratemporal fossa approaches that are the prototypic otologic approaches to the ITF (Fig. 54-8,9,10)3. The hallmark of these approaches is temporal bone management emphasizing facial nerve rerouting and subtemporal dural exposure for wide access to the lateral skull base. Figure 54-10 illustrates the anatomic regions appropriate for the Fisch type A, B, and C approaches. The Fisch type A approach has been described in detail in Chapter 46 regarding its application in glomus jugulare surgery. The Fisch type B and C approaches are designed to approach more anterior pathology involving the petrous apex and clivus. The type C approach is an extension of the type B approach, and is used for lesions of the anterior ITF, sella, and nasopharynx. The Fisch type D is a preauricular ITF approach using an orbitozygotomy and resection of the floor of the middle fossa for medial dural exposure without a lateral temporal craniotomy.
Fisch Type B
The principal exposure maneuver in the Fisch type B ITF approach is reflection of the zygomatic arch and temporalis muscle inferiorly and removal of the bone of the skull base floor to provide access to the ITF. The incision is wide and C-shaped beginning at the angle of the mandible and extending retroauricularly and anterior laterally to the eyebrow. The ear canal is transected and closed in the same manner as described in Chapter 47 in a two-layer technique.
Anterior Transfacial Approach (Facial Translocation)
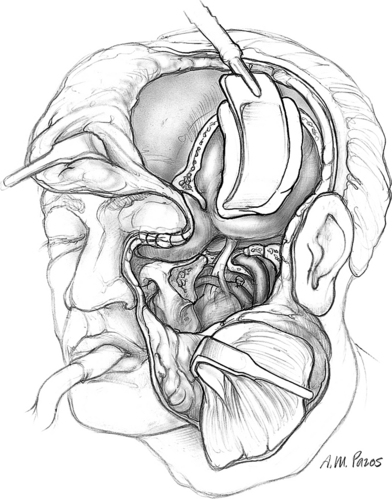
The anterior transfacial technique is best used to approach sinonasal tumors invading the ITF, the masticator space, or the pterygomaxillary fossa, and for tumors of the nasopharynx extending into the ITF (Fig. 54-11).7,13,14 A bicoronal incision with an ipsilateral preauricular extension is performed and extended through the subcutaneous tissue (see the section on preauricular approach). A Weber-Fergusson incision is completed and extended down to the periosteum of the maxilla, nasal bones, and orbital rim. During a “traditional” translocation approach, a horizontal incision is carried over the superior edge of the zygomatic bone, extending into the lateral canthus, to meet the Weber-Fergusson incision (see Fig. 54-11). The frontal branches of the facial nerve are identified and dissected as they cross over the zygomatic arch. They are then entubulated with silicone tubing and transected. These nerve branches are reanastomosed at the end of the case, using an entubulation technique.
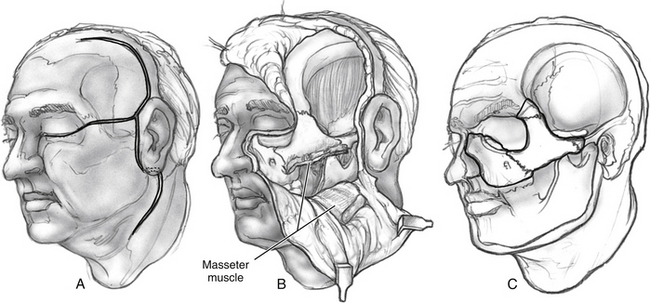
FIGURE 54-11 Incisions and osteotomies for facial translocation approach as described by Janecka.13,14
Subperiosteal dissection of the anterior maxilla exposes the infraorbital nerve, which is then transected and tagged to facilitate its identification and reanastomosis at the end of the case. Then, an inferiorly based flap including the upper third of the upper lip, entire cheek, lower eyelid, parotid gland, and facial nerve is reflected inferiorly. The frontotemporal scalp flap is elevated in a subpericranial plane. This flap is reflected anteriorly, exposing the superior orbital rims (see Fig. 54-11). Alternatively, the exposure can be achieved without the temporal incision by combining the preauricular approach with the anterior exposure provided by the Weber-Fergusson incision.
Orbitozygomatic osteotomies are performed and joined with the maxillary osteotomies to free the anterior face of the ipsilateral maxilla en bloc with the orbitozygomatic complex. Alternatively, the maxillary bone graft can be elevated as a vascularized graft attached to the cheek flap, as described by Catalano and Biller.8 The temporalis and masseter muscles are dissected from the zygomatic bone with electrocautery. Osteotomies are completed, and the bone graft is removed. The temporalis muscle is reflected inferiorly. Removal of the coronoid process increases the caudal arc of rotation of the temporalis muscle. After completion of these steps, the anterior, medial, and lateral boundaries of the ITF are well exposed.
In selected cases, the pterygoid plates can be excised to provide further access to the medial ITF or nasopharynx. A temporosubtemporal craniotomy provides additional exposure superiorly and allows dissection of intracranial structures (Fig. 54-12). After the tumor resection, the temporalis muscle may be used to obliterate the surgical defect and provide separation of the cranial cavity from the upper aerodigestive tract, as previously described.
1. Barbosa J.F. Surgery of extensive cancer of paranasal sinuses: Presentation of a new technique. Arch Otolaryngol. 1961;73:129-138.
2. Terz J.J., Young H.F., Lawrence W.Jr. Combined craniofacial resection for locally advanced carcinoma of the head and neck, II: Carcinoma of the paranasal sinuses. Am J Surg. 1980;140:618-624.
3. Fisch U. The infratemporal fossa approach for the lateral skull base. Otolaryngol Clin North Am. 1984;17:513-552.
4. Biller H.F., Shugar J.M.A., Krespi Y.P. A new technique for wide-field exposure of the base of the skull. Arch Otolaryngol. 1981;107:698-707.
5. Sekhar L.N., Schramm V.L., Jones N.F. Subtemporal-preauricular infratemporal fossa approach to large lateral and posterior cranial base neoplasms. J Neurosurg. 1987;67:499.
6. Cocke E.W.Jr., Robertson J.H., Robertson J.T., Crooke J.P.Jr. The extended maxillotomy and subtotal maxillectomy for excision of skull base tumors. Arch Otolaryngol Head Neck Surg. 1990;116:92-104.
7. Janecka I.P., Sen C.N., Sekhar L.N., Arriaga M. Facial translocation: A new approach to the cranial base. Arch Otolaryngol Head Neck Surg. 1990;103:413-419.
8. Catalano P.J., Biller H.F. Extended osteoplastic maxillotomy: A versatile new procedure for wide access to the central skull base and infratemporal fossa. Arch Otolaryngol Head Neck Surg. 1993;119:394-400.
9. Snyderman C.H., Carrau R.L., de Vries E.J.. Carotid artery resection: Update on preoperative evaluation. Johnson J.T., Derkay C.S., Mandell-Brown M.K., Newman R.K., editors. AAO-HNS Instructional Courses, 6. 1993, 341-346.
10. Netterville J.L., Jackson G., Civantos F. Thyroplasty in the functional rehabilitation of neurotologic skull base surgery patients. Am J Otol. 1993;14:460-464.
11. Carrau R.L., Pou A., Eibling D.E., et al. Laryngeal framework surgery for the management of aspiration. Head Neck. 1999;21:139-145.
12. Pou A., Carrau R.L., Eibling D.E., Murry T. Laryngeal framework surgery for the management of aspiration in high vagal lesions. Am J Otolaryngol. 1998;19:1-8.
13. Nuss D.W., Janecka I.P., Sekhar L.N., Sen C.N. Craniofacial disassembly in the management of skull-base tumors. Otolaryngol Clin North Am. 1991;24:1465-1497.
14. Sekhar L.N., Sen C., Snyderman C.H., Janecka I.P. Anterior, anterolateral, and lateral approaches to extradural petroclival tumors. In: Sekhar L.N., Janecka I.P., editors. Surgery of Cranial Base Tumors. New York: Raven Press; 1993:157-223.