CHAPTER 47 Annular Repair
The anulus fibrosus is an important part of the intervertebral disc. Similar to the other disc components, the anulus undergoes changes with aging and with degeneration.1,2 The degenerative process leads to a weakening of the anulus, and delamination, fissures, and cracks occur. Occasionally, the nucleus pulposus can herniate into the cracks and through the entire annular wall. Also, as part of surgical procedures to address contained disc herniations, iatrogenic holes in various shapes and locations are made into the anulus.
In recent years, there has been increasing interest in strengthening the annular structure, which is important to regain the normal function of the disc.3 There is also interest in repairing degenerative and iatrogenic cracks, tears, and holes in the anulus to prevent recurrence of disc herniations and to slow the degenerative process after discectomy. This chapter reviews some of these strategies and puts them in the context of successful treatment of different types of painful spinal conditions. The focus is on the lumbar discs.
Anulus Fibrosus
The anulus fibrosus is a laminate structure surrounding the central nucleus pulposus and inserting into the endplates and the vertebral body. It consists primarily of water, collagen, proteoglycans, and noncollagenous proteins. The laminates are organized in layers mainly composed of type I collagen fibers, which alternate in angles with respect to the transverse plane. Between the layers are so-called interlaminar spaces containing proteoglycans (aggrecan, versican) and other linking elements.4 In the center, the fibers insert into the cartilaginous endplates, and in the periphery they bypass the endplates and insert into the bone, called Sharpey fibers. The inner and outer parts of the anulus differ in that in the inner part the layers are less well organized and more widely spaced. The proportion of type I collagen increases from the inner anulus to the outer anulus, whereas type II collagen is more common in the inner anulus than outer anulus. Small proteoglycans (decorin and biglycan) are found primarily in the outer anulus, whereas elastin is present throughout. Elastin constitutes 1.7% to 2% of the dry weight of the anulus. In the outer anulus, elastin is present within the lamella running parallel to the collagen and in the same direction.5,24 In the inner anulus, elastin is also organized within the lamellae. Fiber networks bind adjacent lamella together preventing them from separating.6,7 Table 47–1 compares the outer anulus and inner anulus.
| Outer Anulus | Inner Anulus | |
|---|---|---|
| Collagen | 40%-60% dw | 25%-40% dw |
| Type of collagen | Type I mainly | Type II mainly |
| Proteoglycan | 5%-8% dw | 11%-20% dw |
| Cells | Fusiform | Chondrocytelike |
dw, dry weight.
The cell density in the anulus pulposus is about twice the cell density of the nucleus pulposus.8 In the outer anulus, the cells are fusiform-shaped and align with the collagen fibers alternating with each lamella. These cells produce mainly type I collagen. In the inner anulus, the cells are more similar to the cells of the nucleus pulposus. They are chrondrocyte-like and produce mainly type II collagen.
Effect of Aging on Anulus Fibrosus
Various chemical and structural changes occur with aging. These changes seem to occur first in the inner anulus, which loses a large part of its proteoglycan and water and gradually assumes a more nucleuslike structure.2 The overall proteoglycan and collagen concentrations decrease with aging, probably reflecting a decrease in cellular biochemical activity. Among smaller nonaggregating proteoglycans, decorin levels decrease with aging in the outer anulus, and biglycan and fibromodulin levels increase. In the inner anulus, decorin levels have been found to increase with aging.
Healing of Anulus
Early studies in dogs showed comparatively poor healing when larger defects were created in the annular wall.9 Smith and Walmsley10 reported that after an incision the outer anulus healed by fibrous tissue ingrowth from the sides and that there was also a gradual healing of the inner anulus over a 1-year period. Long-term collagen fibers gradually invaded the nuclear tissue, of which some remained in the annular incisions. Fazzalari and colleagues,11 using an ovine model, introduced needle punctures and concentric tears and tested the specimen mechanically up to 18 months. Significant changes occurred in disc biomechanics in both cases and remained significant over time. The annular lamellae thickened, and the adjacent vertebral body bone volume fraction increased. Although this model is not a herniation model, it shows the poor healing of concentric tears and their effect on the disc biomechanics. There is limited information on annular repair in humans after discectomy. Current information suggests a limited healing potential after annulotomy. This increases the risk of reherniation. Reoperation rates for recurrent herniations ranging from 3% to 27% have been reported.12–15 The frequency of reoperations seems to be related to the size of the annular defect.
Biologic Repair
At least four types of annular repair are discussed in the literature: collagen modification, cell therapy, gene therapy, and tissue engineered scaffolds. Gene and cell therapies are unlikely to repair existing cracks, tears, and incisions unless combined with scaffolds. Numerous studies have been published in which scaffolds have been populated with cells, and various success rates have been reported. Most of these studies have been performed in vitro, but a few animal studies have been reported. Gene and growth factor therapies can repair annular needle punctures in the early stages. Many growth factors seem to have a stronger effect on proteoglycan than collagen. Zhang and colleagues16 reported that collagen synthesis was enhanced by BMP-13 and Sox9.
Bron and colleagues3 listed requirements for anulus fibrosus scaffolds. The scaffolds should:
To date, no single approach has met all these requirements, but several scaffolds are promising.17–20
Surgical Repair
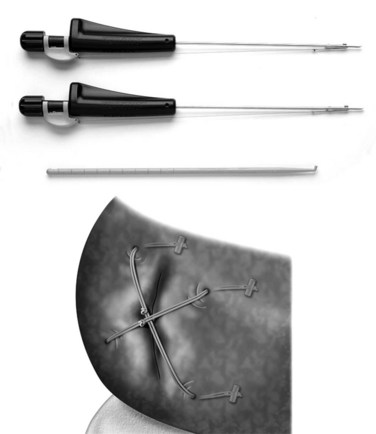
A few alternatives have been developed to address the closure of an annular defect caused by a discectomy. Wang and colleagues21 put absorbable gelatin sponge (Gelfoam), platinum core, bone cement, and tissue glue into an 18-gauge needle defect. Gelfoam seemed to have the best result. The size of the defect in this model is so small, however, that it is difficult to extrapolate this model to a discectomy. Sutures have been used in a sheep model.22 The healing effect was not statistically significant. More recently, sutures with anchors (Xclose and Inclose; Anulex Technologies, Inc., Minnetonka, MN) have been introduced commercially and are currently undergoing a U.S. Food and Drug Administration (FDA) trial (Fig. 47–1). A retrospective case series comparing 133 microdiscectomy cases without anulus repair with 59 cases with anulus repair was reported at a Society meeting.23 There were 16 reoperations within 12 months in the nonrepair group (12.9%) compared with 4 in the annular repair group (6.8%).23 To what degree sutures can be used when larger defects exist is unknown. It is also unclear how well they reverse the biomechanical changes caused by the defect.
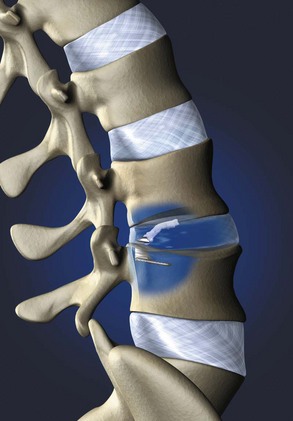
Barricaid (Intrinsic Therapeutics, Inc., Woburn, MA) is another commercially available implant that anchors into the vertebral body and supports a woven mesh barrier inserted into the defect (Fig. 47–2). Clinical data are not yet available, but an investigational device exemption (IDE) trial is planned for 2010. Other implants are in various stages of development and likely to be presented at meetings in the near future.
Summary
Key Points
1 Osti OL, Vernon-Roberts B, Moore R, et al. Annular tears and disc degeneration in the lumbar spine: A post-mortem study of 135 discs. J Bone Joint Surg Br. 1992;74:678-682.
This is a classic study of anatomic findings of the anulus fibrosus of humans.
2 Bron JL, Heider MN, Meisel H-J, et al. Repair, regenerative and supportive therapies of the annulus fibrosus: Achievements and challenges. Eur Spine J. 2009;18:301-313.
This is a review article on repair of the anulus fibrosus.
3 Carragee EJ, Han MY, Suen PW, et al. Clinical outcomes after lumbar discectomy for sciatica: The effects of fragment type and anular competence. J Bone Joint Surg Am. 2007;85:102-108.
4 Hartman L, Griffith SL, Melone B, et al. Surgical outcome of lumbar microdiscectomy with emphasis on annular repair techniques. Annual Meeting of Congress of Neurological Surgeons (CNS). New Orleans. 2009.
This study of a mechanical repair technique shows promising results.
1 Osti OL, Vernon-Roberts B, Moore R, et al. Annular tears and disc degeneration in the lumbar spine: A post-mortem study of 135 discs. J Bone Joint Surg Br. 1992;74:678-682.
2 Singh K, Masuda K, Thonar EJ-M, et al. Age-related changes in the extracellular matrix of mucleus pulposus and annulus fibrosus of human intervertebral disc. Spine (Phila Pa 1976). 2008;34:10-16.
3 Bron JL, Heider MN, Meisel H-J, et al. Repair, regenerative and supportive therapies of the annulus fibrosus: Achievements and challenges. Eur Spine J. 2009;18:301-313.
4 Pezowicz CA, Robertson PA, Broom ND. The structural basis of interlamellar cohesion in the intervertebral disc well. J Anat. 2006;208:317-330.
5 Smith LJ, Byers S, Costi JJ, et al. Elastic fibers enhance the mechanical integrity of the human lumbar annulus fibrosus in the radial direction. Ann Biomed Eng. 2008;36:214-223.
6 Yu J, Tirlapur U, Fairbank J, et al. Microfibrils, elastin fibres and collagen fibres in the human intervertebral disc and bovine tail disc. J Anat. 2007;210:460-471.
7 Melrose J, Smith SM, Appleyard RC, et al. Aggrecan, verSICAN and type VI collagen are components of annular translamellar cross bridges in the intervertebral disc. Eur Spine J. 2008;17:314-324.
8 Roughley PJ. Biology of intervertebral disc aging and degeneration: Involvement of the extracellular matrix. Spine (Phila Pa 1976). 2004;29:2691-2699.
9 Key JA, Ford LT. Experimental intervertebral-disc lesions. J Bone Joint Surg Am. 1948;30:621-630.
10 Smith JW, Walmsley R. Experimental incision of the intervertebral disc. J Bone Joint Surg Br. 1951;33:612-625.
11 Fazzalari NL, Costi JJ, Hearn TC, et al. Mechanical and pathologic consequences of induced concentric anular tears in an ovine model. Spine (Phila Pa 1976). 2001;26:2572-2581.
12 Carragee EJ, Han MY, Suen PW, et al. Clinical outcomes after lumbar discectomy for sciatica: The effects of fragment type and anular competence. J Bone Joint Surg Am. 2007;85:102-108.
13 Ebeling U, Kalbarcyk H, Reulen HJ. Microsurgical re-operation following lumbar disc surgery: Timing, surgical findings, and outcome in 92 patients. J Neurosurg. 2007;70:397-404.
14 Ambrossi GLG, McGirt MJ, Sciobba DA, et al. Recurrent lumbar disc herniation after single level discectomy: Incidence and health care cost analysis. Neurosurgery. 2009;65:574-578.
15 Watters WC, McGirt MJ. An evidence-based review of the literature on the consequences of conservative versus aggressive discectomy for the treatment of primary disc herniation with radiculopathy. Spine J. 2009;9:240-257.
16 Zhang Y, Anderson DB, Phillips FM, et al. Comparative effects of bone morphogenetic proteins and Sox9 overexpression on matrix accumulation by bovine annulus fibrosus cells: Implications for annular repair. Spine. 2007;32:2515-2520.
17 Nerurkar NL, Elliott DM, Mauck RL. Mechanics of oriented electrospun nanofibrous scaffolds for annulus fibrosus tissue engineering. J Orthop Res. 2007;25:1018-1028.
18 Sato M, Asazuma T, Ishihara M, et al. An atelocollagen honeycomb-shaped scaffold with a membrane seal (ACHMS-scaffold) for the culture of annulus fibrosus cells from an intervertebral disc. J Biomed Mater Res A. 2003;64:248-256.
19 Sato M, Asazuma T, Ishihara M, et al. An experimental study of the regeneration of the intervertebral disc with an allograft of cultured annulus fibrosus cells using a tissue-engineering method. Spine (Phila Pa 1976). 2003;28:548-553.
20 Sato M, Kikuchi M, Ishihara M, et al. Tissue engineering of the intervertebral disc with cultured annulus fibrosus cells using atelocollagen honeycomb-shaped scaffold with a membrane seal (ACHMS scaffold). Med Bio Eng Comput. 2003;41:365-371.
21 Wang YH, Kuo TF, Wang JL. The implantation of non-cell-based materials to prevent the recurrent disc herniation: An in vivo porcine model using quantitative discomanometry examination. Eur Spine J. 2007;16:1021-1027.
22 Ahlgren BD, Lui W, Herkowitz HN, et al. Effect of annular repair on the healing strength of the intervertebral disc: A sheep model. Spine (Phila Pa 1976). 2000;25:2165-2170.
23 Hartman L, Griffith SL, Melone B, et al. Surgical outcome of lumbar microdiscectomy with emphasis on annular repair techniques. Annual Meeting of Congress of Neurological Surgeons (CNS). New Orleans. 2009.
24 Smith LJ, Fazzalari NL. The elastin fibre network of the human lumbar annulus fibrosus: Architecture, mechanical function and potential role in the progression of intervertebral disc degeneration. Eur Spine J. 2009;18:439-448.