CHAPTER 35 Ankylosing Spondylitis
The association between the major histocompatibility complex antigen HLA-B27 and AS has been well established.1–6 Approximately 90% of AS patients are positive for the HLA-B27 antigen, although less than 10% of patients who are HLA-B27 positive manifest the signs and symptoms of AS. First-degree relatives of AS patients who are HLA-B27 positive who are also positive for the antigen have a 30% risk of having AS, in contrast to the prevalence in the general population, which is 1% to 2%. The exact mechanism of the AS and HLA-B27 connection is unknown, although a bacterial association has been proposed.
Physical Examination and Diagnosis
Sacroiliitis is usually identified on an anteroposterior pelvis film (with or without a Ferguson view). It is widely accepted that the presence of sacroiliitis is crucial for the diagnosis of AS. Sacroiliac joint destruction is the earliest manifestation of AS. The earliest stages of sacroiliitis show some blurring of the cortical margins; this progresses to subcortical erosions (more commonly on the iliac side because it is less robust than the sacral side). In advanced stages, the sacroiliac joints become completely fused, and the cortical erosions disappear. Sacroiliac joint involvement usually is symmetrical and bilateral. Studies have suggested that the use of bony pelvis computed tomography (CT) or magnetic resonance imaging (MRI) in conjunction with plain radiographs may lead to earlier diagnosis of AS.7 It has yet to be determined whether this early diagnosis favorably affects clinical outcomes.
Management of Acute Injury
A patient with AS may present with a progressive neurologic deficit without obvious bony injury or with progression of the deformity and increased pain. Many patients with missed spinal column injuries present at a later time to the clinic or the emergency department with progressive neurologic deficit or worsening of deformity or both. The evaluating clinician must also be aware of possible hyperextension through a fracture at a kyphotic segment, which may result in relatively normal sagittal alignment; attempts to determine the patient’s preexisting deformity from history and prior radiographs should always be made. There have been reports of neurologic injury in patients strapped to spine boards in a position of hyperextension when compared with their preinjury alignment.8–10 Because of the stiff and osteoporotic spine, minor trauma may result in acute angulation or moderately rapid deformity progression. One should refrain from attempting acute correction through such a fracture. The patient should be initially immobilized in a halo vest in the preinjury alignment.
It is generally accepted that AS patients sustain more spinal fractures and dislocations than individuals without AS.11–15 Cooper and colleagues16 retrospectively looked at 158 patients in Rochester, Minnesota, with AS and found a sevenfold increase in the incidence of spinal fractures over that of a cohort of patients without AS. They found no such increase in extremity fractures. The patients with spinal fractures tended to be older and had a greater preinjury involvement of the spine than patients without fractures. Cooper and colleagues16 also noted that this higher incidence was mainly during the first 5 years after diagnosis and suggested that this was due to a greater percentage of bone density loss during this period, resulting in a decreased fracture threshold. In addition, the dampening structures present in a normal spine have lost their load-absorbing qualities in the ankylosed spine. The intervertebral discs are stiff, as are the ligamentous structures, and the facet joints are ankylosed.
Whang and colleagues17compared a cohort of 12 patients with AS who sustained spinal injuries with 18 patients with diffuse idiopathic skeletal hyperostosis (DISH) who sustained spinal injuries. The DISH group represents a group of patients of similar age whose spinal condition results in stiff segments above and below any spinal fracture. Falls from a standing position were the most common mechanism of injury. There was a greater likelihood that the DISH patients did not incur any neurologic deficit (44.4%) compared with AS patients (25% of whom did not have a neurologic deficit). Complication rates were higher in the AS group (42% vs. 33% in the DISH group). There were two deaths in each group related to the injury or its treatment, all of which were considered to be related to the use of the halo vest (aspiration [two deaths], respiratory failure, and multisystem organ failure). Several patients died of unrelated causes during the follow-up period; however, all surviving patients were contacted and were classified as having excellent or good outcomes.
Finkelstein and colleagues18 looked retrospectively at 21 AS patients with a diagnosis of spinal trauma. One third of these patients had a delay in diagnosis; three had complete spinal cord injuries on presentation, and three experienced neurologic deterioration to complete spinal cord injuries after admission. Finkelstein and colleagues18 recommended quick screening cervical and thoracic MRI (one film) and screening lumbosacral spine MRI (one film) for diagnosis, in addition to minimal transfers and immediate stabilization. They did not comment on their definitive protocol for treatment of these patients (operative vs. nonoperative).
Hitchon and colleagues19 retrospectively reviewed 11 patients with AS and thoracic and lumbar fractures. They found 10 of these patients had sustained three-column injuries; 9 patients had extension-type injuries. More than half of these patients had a neurologic deficit (the specifics of which the study authors did not mention); half of these neurologically injured patients had some improvement in function. Hitchon and colleagues19 recommended surgical intervention for stabilization of thoracic and lumbar three-column injuries because of their inherent instability.
Graham and van Peteghem20 looked retrospectively at 15 patients over 6 years (1978-1984) comparing types of injuries and treatments. Of patients, 12 had cervical spine injuries; 9 of these had spinal cord injuries. The two patients with thoracic injuries had anterior cord syndromes. There were no compression-type injuries; most injuries resulted from a flexion-extension type of mechanism. The only patient treated with operative intervention was the patient with the lumbar injury, who had hardware failure and had to undergo revision. Two patients died, and three patients had pulmonary complications.
Apple and Anson21 looked at AS patients with spinal fracture and spinal cord injury, comparing operative versus nonoperative treatments. This study was a retrospective, multicenter study of 59 patients. In the operative group, 37 patients were treated with a variety of procedures. Patients in the nonoperative group were placed in halo traction and then halo vests and placed on bed rest. There were no significant differences between the two groups with regard to motor recovery, fusion complications, or mortality rate (22% in both groups). The nonoperative group did have significantly shorter hospital stays. No analysis of the patients according to type of injury or treatment was done, and no discussion of the deaths was presented.
Hunter and Dubo12 reviewed the cases of 19 AS patients who had sustained cervical spine fractures. Five of these patients had a complete spinal cord injury, and all of these patients died after their injury. All of these patients were treated nonoperatively. No patient developed neurologic deterioration, and all of the patients with incomplete cord injury regained some function. Hunter and Dubo12 concluded that nonoperative treatment worked well in these patients, although they suggested that surgery be considered in patients with grossly unstable injuries.
Bohlman retrospectively reviewed 300 patients with cervical spine injuries.21a He found only eight patients who carried a preinjury diagnosis of AS. Five of these patients died of pulmonary or gastrointestinal causes. Clinically significant epidural hematomas were found only in the AS patients. Bohlman recommended decompression for patients with progressive neurologic deficit. There was a delay in diagnosis in four patients, all of whom developed spinal cord injuries.
Complications can arise at the time of injury and from treatment of the injury. Deformity and neurologic injury can occur as a result of the injury; treatment with decompression and internal fixation carries risks of nonunion, hardware failure, failure of the bone-screw interface resulting in loss of fixation, and infection. Even halo management has complications. Skull fractures, pin tract infections, intracerebral hemorrhage, and intracranial air all have been reported with halo immobilization in these patients.9,22 Taggard and Traynelis24 described a posterior cervical fusion (lateral mass plating) they used in seven AS patients who had sustained fractures. The fusions were supplemented with autologous rib grafts. Postoperatively, the patients were immobilized in collars only, with the exception of one, who was placed in a sternal-occipital-mandibular immobilizer. Fusion occurred in all patients; there were two deaths in quadriplegic patients. Taggard and Traynelis24 recommended operative intervention as a means of avoiding postoperative halo immobilization.
Deformity
From a radiographic standpoint, a full spine lateral radiograph with the neck in a neutral position and the hips in a fully extended position is crucial for surgical planning. This radiograph allows measurement of the chin-brow angle, which is formed by a line from the chin-brow to the floor vertical angle. This measurement is helpful when planning any osteotomy. Ideally, the chin-brow angle should be zero. Suk and colleagues23 looked at the significance of the chin-brow measurement in assessing the success of surgical intervention. These investigators evaluated 34 AS patients undergoing lumbar or thoracolumbar osteotomies for correction of sagittal imbalance. Preoperative and postoperative chin-brow angles were measured. Clinical outcome assessment involved the Modified Arthritis Impairment Scales (AIMS). This questionnaire consists of three simple questions plus numerous subscales: function, indoor activity, outdoor activity, psychosocial activity, pain, and overall subjectivity. Suk and colleagues23 found improved postoperative AIMS scores for questions involving looking forward, going up stairs, and going down stairs. There was a negative correlation between chin-brow angles and correction obtained but no correlation between chin-brow angle and clinical outcome. The patients who were overcorrected (to an angle <−10 degrees) had worse scores with regard to looking forward and going down stairs; these results were found to be statistically significant.
Preoperative Assessment
A preoperative nutritional assessment (albumin, prealbumin, total protein) should be performed; perioperative nutritional supplementation (tube feedings or parenteral nutritional assessment) may be indicated. Klein and colleagues25 noted a significant increase in complications such as deep wound infection in patients undergoing lumbar spinal fusion who were malnourished by nutritional parameters preoperatively. Hu and colleagues26 and Lapp and colleagues27 showed that supplementation in the form of parenteral nutrition is beneficial in reducing complication rates after reconstructive spine surgery.
Lumbar Osteotomies
The first lumbar osteotomy was described in 1945 by Smith-Petersen.33 This osteotomy is an opening osteotomy, meaning that the apex of the wedge lies posteriorly, opening up the anterior column during correction (osteoclasis through ossified disc space and anterior longitudinal ligament). Smith-Petersen and colleagues performed multilevel osteotomies in six patients. These osteotomies were V-shaped in the coronal plane, with the point of the “V” at the midline in the interlaminar space. The osteotomies are carried out through the articular processes bilaterally at two or three levels. It is imperative that adequate amounts of lamina and flavum are resected before correction so that compression of the neural elements on closure does not occur.
Cauda equina syndrome has been reported by Simmons28 as a result of a decrease in canal dimensions. The posteriorly based closing wedge type of osteotomy results in anterior opening at the level of the disc space. This anterior opening can be better achieved in an AS patient than a patient without AS because of the stiffness of the disc space. Complications of an opening wedge osteotomy include superior mesentery artery syndrome and aortic rupture owing to stretching of the abdominal vasculature.29,30 Vascular complications are rare and tend to occur in older patients with calcific, adherent abdominal vessels.
Lichtblau and Wilson29 described a patient who underwent closed osteoclasis followed by cast placement. This patient died in the immediate postoperative period of an aortic rupture. His history was significant for a large dose of radiation that was used to treat the ankylosed spine. Fazl and colleagues30 described an AS patient who sustained a fracture through the T12-L1 disc space. He was treated with Harrington rod instrumentation and fusion but died 2 days postoperatively from an aortic rupture at the level of the injury. Aortic necrosis was present at autopsy, as were adhesions of the vessels to the spine. More common complications reported include ileus, pneumonia, and root traction injury. Cauda equina syndrome with flaccid paralysis below the level of injury, although rare, was reported in these studies as well.
Patients originally were immobilized in plaster; segmental instrumentation currently is indicated for these patients. Many investigators have reported their results after multilevel Smith-Petersen osteotomies.31–33 Nonunion rates resulting in recurrence and progression of deformity were significant. Soon after the original description of this technique, reports of “plugging up” the open disc spaces with interbody fusions showed increased fusion rates and decreased complications.34 The complications associated with opening wedge osteotomies led to modifications of Smith-Petersen’s techniques. In 1949, Wilson and Turkell34a described a procedure similar to the Smith-Petersen procedure in which less bone is removed but more osteotomies are created. The anterior longitudinal ligament is not ruptured; the anterior column length is not changed. In 1962, McMaster32 described the addition of Harrington compression instrumentation to Smith-Petersen osteotomies in 14 patients. This instrumentation was used to close the wedges produced after osteotomy. Postoperatively, the patients were placed in casts for 9 months. Mean correction was 33 degrees at final follow-up. Subjective improvement was found in horizontal gaze and height and posture. McMaster32 suggested that a slow controlled osteotomy closure was beneficial in terms of overall stability and protection of neural elements. Püschel and Zielke34b also performed multiple wedge-shaped Smith-Petersen type osteotomies and used Zielke instrumentation to close the osteotomies. They also recommended a slow correction with a gradual lordosis.
After reports of nonunions and concerns about stretching of the abdominal vasculature and viscera,35,36,39 Thomasen37 described a closing wedge osteotomy. He reported on 11 patients in whom he preformed a complete laminectomy at L2, transected the transverse processes, and resected the ankylosed facets at L2-3. The pedicles of L2 were removed in their entirety down to the posterior aspect of the vertebral body. The entire vertebral body was decancellated, followed by removal of the posterior cortex and osteotomies of both lateral cortices. After careful mobilization of the dura above and below L2, Thomasen37 closed the wedge by gradual flexion of the table. Internal fixation (plates and wiring) was used in six patients; all patients were placed in casts. One patient had a fracture-dislocation above the level of the osteotomy resulting in a cauda equina syndrome; this patient had almost complete return of neurologic function after revision decompression and internal fixation. Correction ranged from 12 to 50 degrees. All patients had subjective improvement of posture and horizontal gaze.
Heinig’s eggshell procedure was described in 1984 as a monosegmental osteotomy to be used in the same situations in which one would use Thomasen’s procedure.37 Thomasen leaves the anterior vertebral body cortex intact, whereas Heinig actually describes fracturing this cortex, which decreases the length of the anterior column and the posterior column. As long as more bone is removed posteriorly, restoration of lordosis occurs.
Bradford and colleagues38 reported in 1987 on a series of 21 patients with AS who underwent single-level or multilevel lumbar or thoracic osteotomies with or without anterior discectomies. All patients had internal fixation posteriorly with a thoracolumbosacral orthosis. Average corrections ranged from 9 to 36 degrees. Complications were noted more frequently in the closing wedge–type osteotomies (neurapraxias and fracture during hook placement). Wake-up tests were used in all patients. In addition, Bradford and colleagues38 recommended closing-type osteotomies to avoid traction on the spinal cord, wide decompression, and internal fixation to avoid neurologic complications.
In 1990, Hehne and colleagues39 reported on 177 patients with AS in whom multisegmental opening wedge lumbar and thoracolumbar osteotomies were performed. Hehne and colleagues39 were the first to report on the use of pedicle screw fixation. Casting and bracing were used postoperatively. Average correction at follow-up (18 to 42 months) was 43 degrees, with horizontal gaze subjectively restored in all cases. Complications included deaths, transient paresis, transient and permanent nerve root injuries, implant failures, and infections. These authors suggested that pedicle screw fixation with multisegmental osteotomies can produce a smoother lordosis than that produced by a monosegmental osteotomy.
In 1992, Jaffray and colleagues39a presented three patients in whom a decancellation closing wedge osteotomy was performed. They did not remove the entire pedicle; rather, the inferior aspect of the pedicle was preserved to ensure protection for the exiting nerve root. Pedicle screw fixation plus a postoperative cast was used. Jaffray and colleagues recommended two-level osteotomies (L2 and L4) for patients who needed more correction. Horizontal gaze was corrected in two patients; one patient required a cervicothoracic osteotomy for complete gaze correction. Complications were not discussed.
More recently, Van Royen and De Gast40 mathematically analyzed the sagittal plane corrections of two patients and determined that the amount of correction needed depends on three parameters: sacral endplate angle, C7 plumb line, and chin-brow angle. The sacral endplate angle reflects the amount of sagittal plane deformity that can be attributed to the hip joints: As the flexion contracture at the hip increases, the pelvis must rotate posteriorly to keep the body center of mass over the pelvis, decreasing the sacral endplate angle. The chin-brow angle has been shown to be a quantifiable parameter that reflects the restoration of horizontal gaze.40 A mathematical formula was found that determines the ideal location and angle for each particular patient for a closing wedge–type osteotomy centered on the anterior longitudinal ligament.
There have been many retrospective reviews of AS patients treated with various osteotomies for sagittal imbalance. Van Royen and De Gast40 performed a meta-analysis of 856 AS patients. They found three different techniques described: multisegment (two to three levels) opening wedge osteotomies with rupture of the anterior longitudinal ligament (i.e., Smith-Petersen), multisegment closing wedge osteotomies (Wilson-Turkell), and closing wedge–type osteotomy with pedicle resection and an anterior hinge (Thomasen). After a thorough and careful review, Van Royen and De Gast40 concluded that although no single technique was clearly superior to the others, the complications associated with closing wedge osteotomies were less serious than the complications associated with the other two groups. In addition, loss of correction was more prevalent in patients treated with opening wedge and polysegmental wedge osteotomies and the closing wedge types.
A handful of studies have attempted to quantify results in terms of patient outcomes using a standardized grading system. In 1995, Halm and colleagues41 used the modified AIMS questionnaire to evaluate 175 patients retrospectively after lumbar osteotomy. Treatment groups were multisegment Smith-Petersen with Harrington compression instrumentation (n = 34), multisegment Smith-Petersen with transpedicular fixation (n = 136), and monosegmental Thomasen with segmental fixation (n = 4). The investigators found statistically significant improvement in 47 of 60 items. Kim and colleagues42 used the AIMS questionnaire prospectively in 45 patients with AS who were treated with Thomasen osteotomies at one or two levels. Osteotomies were mainly performed in the lumbar spine (usually L3). Average increase in lumbar lordosis was 34 degrees, with no significant increase in thoracic kyphosis. All parameters measured were significantly improved. Clinical outcome scores were significantly improved in all five categories; no correlation was found between the amount of radiographic correction obtained and clinical outcome as measured by the questionnaire.
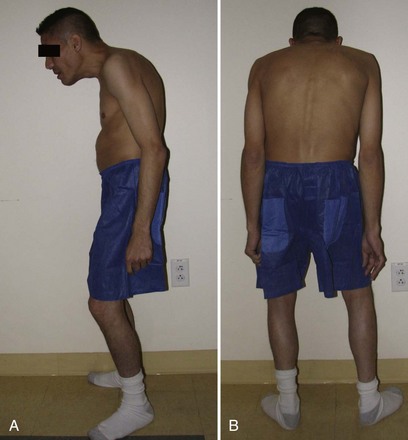
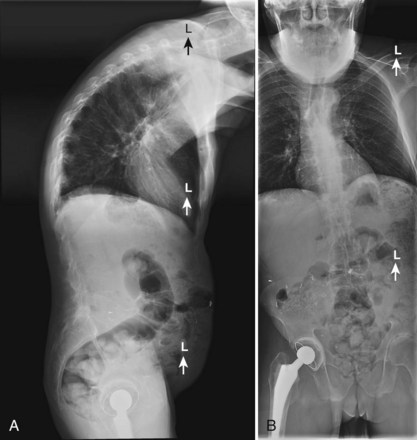
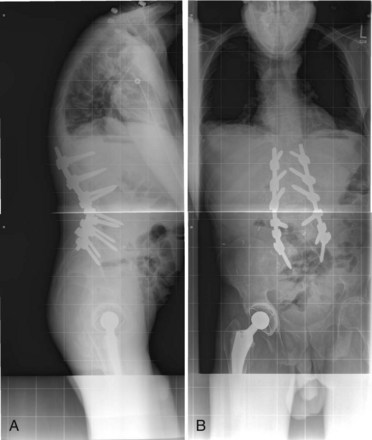
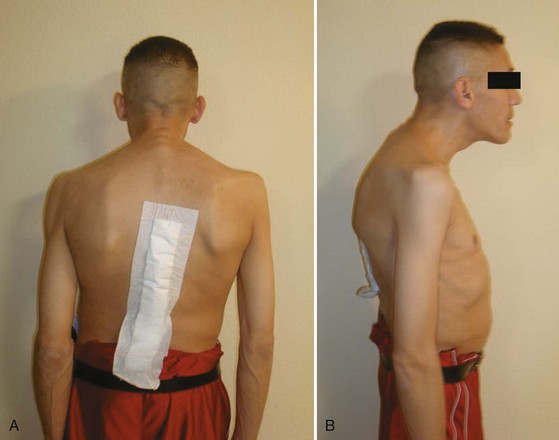
Berven and colleagues43 looked at 13 patients undergoing transpedicular wedge resection. Three of these patients had AS and were having spine surgery for the first time. These investigators also used outcome measures (modified Scoliosis Research Society questionnaire) in a retrospective manner (Figs. 35-1 to 35-4). After 2 years, most of these patients was satisfied and would have the surgery again. The changes in C7 plumb line and lumbar lordosis were statistically significant. Complications included dural tear, transient nerve root injury, pulmonary embolus, and loss of sagittal balance. None of the AS patients showed a loss of sagittal balance at follow-up.
Bridwell and colleagues44 looked at 27 patients undergoing pedicle subtraction osteotomy, also in a retrospective fashion. Two of these patients had AS. Outcome data (Oswestry and SRS-24) were also obtained retrospectively. Bridwell and colleagues44 found a significant improvement in sagittal balance and lumbar lordosis and a high level of patient satisfaction. Complications included deep vein thrombosis, myocardial infarction, compartment syndrome, visual field loss, pseudarthrosis, loss of correction, urinary retention, and neurologic deficits (root lesions). The patients with the latter two complications all responded to a central canal decompression.
Thoracic Osteotomies
Kawahara and colleagues45 described a closing-opening wedge osteotomy in the thoracic and thoracolumbar spine. They used this procedure on seven patients with sagittal imbalance. The osteotomy consisted of a partial vertebrectomy with a large posterior wedge that is performed in a manner similar to a costotransversectomy. After bony resection, pedicle screw instrumentation plus temporary correction rods are used to facilitate a closing wedge correction of about 30 degrees. An opening wedge–type maneuver is facilitated, again through the instrumentation, and a spacer or allograft is inserted. Kawahara and colleagues45 noted good improvement in kyphosis, lordosis, and plumb line. They had no neurologic complications, no nonunions, and no loss of correction (follow-up of 2.2 to 7.5 years).
Cervicothoracic Osteotomy
If the primary deformity has been determined to be in the cervical spine, an osteotomy at the cervicothoracic junction can be done. Patients with these deformities, in addition to the problems with horizontal gaze, also can experience dysphagia and problems related to poor oral intake. The chin-brow angle is of paramount importance when planning a corrective osteotomy in the cervicothoracic region. A key point is not to overcorrect the horizontal gaze because this can lead to inability of patients to see the floor ahead of them. These patients may function better when corrected to a chin-brow angle of about 10 degrees. In 1958, Urist46 described an osteotomy in the cervicothoracic region, noting that the canal at this level is quite large and that the C8 nerve root is quite mobile compared with the upper cervical roots. In addition, potential loss of the lower cervical roots is less morbid than loss of the upper cervical roots. Lastly, the vertebral arteries are typically extraosseous at these levels, making resection of the lateral masses less risky.
Careful preoperative planning with radiographic studies is of paramount importance when performing a cervicothoracic osteotomy. Full-length standing lateral spine radiographs are needed to measure the chin-brow angle. Lateral tomography or fine-cut CT with sagittal and coronal reconstructions is performed to delineate the anatomy.28,46 Axial CT scans can be very helpful in characterizing the distorted anatomy often seen in patients with AS, especially with regard to placement of instrumentation. MRI can help to rule out occult fractures, which should be suspected if there is recent onset of pain or rapid progression of deformity; MRI should be obtained if there is any neurologic deficit. In addition, flexion and extension lateral cervical spine radiographs should be obtained to look for any instability occurring at the occipitocervical and atlantoaxial levels. A subset of AS patients develop instability in these areas as a result of excessive stiffness of the entire spinal column because the stress placed on these upper cervical areas can be quite high. Although the number of AS patients who have occipitocervical or atlantoaxial instability is not as high as the number of AS patients with rheumatoid arthritis, missing this instability can be catastrophic.
The surgeon must carefully examine the preoperative radiographs and CT scans to determine the amount of correction needed. As described by Simmons,28 the measured chin-brow angle should be transposed onto a neutral cervicothoracic film, with the apex of the angle centered on the posterior longitudinal ligament at the C7-T1 level. By extrapolation, the extent of posterior elements to be resected can be determined.
As originally described by Urist46 and popularized by Simmons, the procedure was performed under local anesthesia only, with the patient awake in a seated position, in seated halo traction. The seated position carries with it the risk of air embolus in a patient with a patent foramen ovale so that continuous cardiac monitoring is indicated during the procedure.28,46 With the widespread use of neurophysiologic monitoring, the awake seated position is almost never used for these procedures at the present time except in the case where the severe spinal deformity precludes prone positioning. A baseline set of somatosensory evoked potentials and motor evoked potentials is obtained, and the patient’s head is placed in a rigid head holder (three-pin Mayfield). After the flip, a repeat run of neurophysiologic monitoring is obtained.
The literature concerning cervicothoracic osteotomies in AS patients is sparse. Simmons46a in his original article in 1972 reported on 42 patients who underwent cervicothoracic osteotomy as described by Urist. The operations were performed in the seated position under local anesthesia, and postoperative immobilization consisted of a halo vest. Simmons reported two nonunions successfully treated with anterior fusion, one pulmonary embolus, two myocardial infarctions (one fatal), and one root injury treated with repeat decompression. The patients who did not experience complications all were quite satisfied with their outcomes and had their horizontal gaze restored.
McMaster32 reported retrospectively on 15 patients with abnormal horizontal gaze who were treated with an extension osteotomy at the cervicothoracic junction. These surgeries were performed in the prone position with a halo jacket in place before the osteotomy. Only three patients had internal fixation. All patients had their horizontal gaze restored. Complications included one patient with delayed postoperative quadriparesis, two nonunions, a C8 nerve root lesion, and subluxation at the osteotomy site. McMaster32 also treated their nonunions with anterior fusion with subsequent good results.
The remainder of the literature dealing with cervicothoracic osteotomies in AS patients is in the form of case reports. Sengupta and colleagues46b addressed the complication of overcorrection resulting in the inability of the patient to look down. They performed a same-day four-stage procedure in the lateral decubitus position with transparent drapes and reported restoration of horizontal gaze in one patient. They recommended this procedure only in extreme cases.
Summary
Pearls
Pitfalls
Key Points
1 Graham B, Van Peteghem PK. Fractures of the spine in ankylosing spondylitis. Spine (Phila Pa 1976). 1989;14:803-807.
2 Halm H, Metz-Stavenhagen P, Zielke K. Results of surgical correction of kyphotic deformities of the spine in ankylosing spondylitis on the basis of the modified Arthritis Impact Measurement Scales. Spine (Phila Pa 1976). 1995;20:1612-1619.
3 Hehne H, Zielke K, Bohm H. Polysegmental lumbar osteotomies and transpedicled fixation for correction of long-curved kyphotic deformities in ankylosing spondylitis. Clin Orthop Relat Res. 1990;258:49-55.
4 Simmons EH. Kyphotic deformity of the spine in ankylosing spondylitis. Clin Orthop Relat Res. 1977;128:65-77.
5 Thomasen E. Vertebral osteotomy for correction of kyphosis in ankylosing spondylitis. Clin Orthop Relat Res. 1985;194:142-153.
1 Brewerton DA, Hart FD, Nicholls A, et al. Ankylosing spondylitis and HL-A27. Lancet. 1973;1:904-907.
2 Calin A. Ankylosing spondylitis. Clin Rheum Dis. 1985;11:41-60.
3 Carbone LD, Cooper C, Michet CJ, et al. Ankylosing spondylitis in Rochester, Minnesota, 1935-1989. Is the epidemiology changing? Arthritis Rheum. 1992;35:1476-1482.
4 Kahn MF, Chamot AM. SAPHO syndrome. Rheum Dis Clin North Am. 1992;18:225-246.
5 Reveille JD. HLA-B27 and the seronegative spondyloarthropathies. Am J Med Sci. 1998;316:239-249.
6 Schlosstein L, Terasaki PI, Bluestone R, et al. High association of an HL-A antigen, W27, with ankylosing spondylitis. N Engl J Med. 1973;288:704-706.
7 Devogelaer JP, Maldague B, Malghem J, et al. Appendicular and vertebral bone mass in ankylosing spondylitis: A comparison of plain radiographs with single- and dual-photon absorptiometry and with quantitative computed tomography. Arthritis Rheum. 1992;35:1062-1067.
8 Broom MJ, Raycroft JF. Complications of fractures of the cervical spine in ankylosing spondylitis. Spine (Phila Pa 1976). 1988;13:763-766.
9 Moreau W, Wilcox N, Brown MF. Immobilization of spinal fractures in patients with ankylosing spondylitis. Injury. 2003;34:372-373.
10 Podolsky SM, Hoffman JR, Pietrafesa CA. Neurologic complications following immobilization of cervical spine fractures in patients with ankylosing spondylitis. Ann Emerg Med. 1983;12:578-580.
11 Hanson JA, Mirza S. Predisposition for spinal fracture in ankylosing spondylitis. AJR Am J Roentgenol. 2000;174:150.
12 Hunter T, Dubo HI. Spinal fractures complicating ankylosing spondylitis: A long-term followup study. Arthritis Rheum. 1983;26:751-759.
13 Surin VV. Fractures of the cervical spine in patients with ankylosing spondylitis. Acta Orthop Scand. 1980;51:79-84.
14 Verlaan JJ, Diekerhof CH, Buskens E, et al. Surgical treatment of traumatic fractures of the thoracic and lumbar spine: A systematic review of the literature on techniques, complications, and outcome. Spine (Phila Pa 1976). 2004;29:803-814.
15 Wade W, Saltzstein R, Maiman D. Spinal fractures complicating ankylosing spondylitis. Arch Phys Med Rehabil. 1989;70:398-401.
16 Cooper C, Carbone L, Michet CJ, et al. Fracture risk in patients with ankylosing spondylitis: A population based study. J Rheumatol. 1994;21:1877-1882.
17 Whang PG, Golberg G, Lawrence JP, et al. The management of spinal injuries in patients with ankylosing spondylitis or diffuse idiopathic skeletal hyperostitis: A comparison of treatment methods and clinical outcomes. J Spine Disord Tech. 2009;22:77-85.
18 Finkelstein JA, Chapman JR, Mirza S. Occult vertebral fractures in ankylosing spondylitis. Spinal Cord. 1999;37:444-447.
19 Hitchon PW, From AM, Brenton MD, et al. Fractures of the thoracolumbar spine complicating ankylosing spondylitis. J Neurosurg Spine. 2002;97:218-222.
20 Graham B, van Peteghem PK. Fractures of the spine in ankylosing spondylitis: Diagnosis, treatment, and complications. Spine (Phila Pa 1976). 1989;14:803-807.
21 Apple DFJr, Anson C. Spinal cord injury occurring in patients with ankylosing spondylitis: A multicenter study. Orthopedics. 1995;18:1005-1011.
21a Bohlman HH. Acute fractures and dislocations of the cervical spine. An analysis of three hundred hospitalized patients and review of the literature. J Bone Joint Surg Am. 1979;61:1119-1142.
22 Schroder L, Liljenqvist U, Greiner C, et al. Complications of halo fixation for cervical spine injuries in patients with ankylosing spondylitis—report of three cases. Arch Orthop Trauma Surg. 2003;123:112-114.
23 Suk S, Kim KT, Lee SH, et al. Significance of chin-brow vertical angle in correction of kyphotic deformity of ankylosing spondylitis patients. Spine (Phila Pa 1976). 2003;28:2001-2005.
24 Taggard DA, Traynelis VC. Management of cervical spinal fractures in ankylosing spondylitis with posterior fixation. Spine (Phila Pa 1976). 2000;25:2035-2039.
25 Klein JD, Hey LA, Yu CS, et al. Perioperative nutrition and postoperative complications in patients undergoing spinal surgery. Spine (Phila Pa 1976). 1996;21:2676-2682.
26 Hu SS, Fontaine F, Kelly B, et al. Nutritional depletion in staged spinal reconstructive surgery: The effect of total parenteral nutrition. Spine (Phila Pa 1976). 1998;23:1401-1405.
27 Lapp MA, Bridwell KH, Lenke LG, et al. Prospective randomization of parenteral hyperalimentation for long fusions with spinal deformity: Its effect on complications and recovery from postoperative malnutrition. Spine (Phila Pa 1976). 2001;26:809-817. discussion 817
28 Simmons EH. Kyphotic deformity of the spine in ankylosing spondylitis. Clin Orthop Relat Res. 1977;128:65-77.
29 Lichtblau PO, Wilson PD. Possible mechanism of aortic rupture in orthopaedic correction of rheumatoid spondylitis. J Bone Joint Surg Am. 1956;38:123-127.
30 Fazl M, Bilbao JM, Hudson AR. Laceration of the aorta complicating spinal fracture in ankylosing spondylitis. Neurosurgery. 1981;8:732-734.
31 Law WA. Osteotomy of the spine. Clin Orthop Relat Res. 1969;66:70-76.
32 McMaster MJ. A technique for lumbar spinal osteotomy in ankylosing spondylitis. J Bone Joint Surg Br. 1985;67:204-210.
33 Smith-Petersen MN, Larson CB, Aufranc OE. Osteotomy of the spine for correction of flexion deformity in rheumatoid arthritis. Clin Orthop Relat Res. 1969;66:6-9.
34 Herbert JJ. Technique and results of vertebral osteotomy in 26 cases. Acta Orthop Scand. 1952;22:36-58.
34a Wilson MJ, Turkel JH. Multiple spinal wedge osteotomy; its use in a case of Marie-Strumpell spondylitis. Am J Surg. 1949;77:777-782.
34b Püschel J, Zielke K. [Corrective surgery for kyphosis in bekhterev’s disease—indication, technique, results (author’s transl)] [Article in German]. Z Orthop Ihre Grenzgeb, 120. 1982:338-342.
35 Adams JC. Technique, dangers and safeguards in osteotomy of the spine. J Bone Joint Surg Br. 1952;34:226-232.
36 Camargo FP, Cordeiro EN, Napoli MM. Corrective osteotomy of the spine in ankylosing spondylitis: Experience with 66 cases. Clin Orthop Relat Res. 1986;208:157-167.
37 Thomasen E. Vertebral osteotomy for correction of kyphosis in ankylosing spondylitis. Clin Orthop Relat Res. 1985;194:142-152.
38 Bradford DS, Schumacher WL, Lonstein JE, et al. Ankylosing spondylitis: Experience in surgical management of 21 patients. Spine (Phila Pa 1976). 1987;12:238-243.
39 Hehne HJ, Zielke K, Bohm H. Polysegmental lumbar osteotomies and transpedicled fixation for correction of long-curved kyphotic deformities in ankylosing spondylitis: Report on 177 cases. Clin Orthop Relat Res. 1990;258:49-55.
39a Jaffray D, Becker V, Eisenstein S. Closing wedge osteotomy with transpedicular fixation in ankylosing spondylitis. Clin Orthop Relat Res. 1992;279:122-126.
40 Van Royen BJ, De Gast A. Lumbar osteotomy for correction of thoracolumbar kyphotic deformity in ankylosing spondylitis: A structured review of three methods of treatment. Ann Rheum Dis. 1999;58:399-406.
41 Halm H, Metz-Stavenhagen P, Zielke K. Results of surgical correction of kyphotic deformities of the spine in ankylosing spondylitis on the basis of the modified Arthritis Impact Measurement Scales. Spine (Phila Pa 1976). 1995;20:1612-1619.
42 Kim KT, Suk KS, Cho YJ, et al. Clinical outcome results of pedicle subtraction osteotomy in ankylosing spondylitis with kyphotic deformity. Spine (Phila Pa 1976). 2002;27:612-618.
43 Berven SH, Deviren V, Smith JA, et al. Management of fixed sagittal plane deformity: Results of the transpedicular wedge resection osteotomy. Spine (Phila Pa 1976). 2001;26:2036-2043.
44 Bridwell KH, Lewis SJ, Lenke LG, et al. Pedicle subtraction osteotomy for the treatment of fixed sagittal imbalance. J Bone Joint Surg Am. 2003;85:454-463.
45 Kawahara N, Tomita K, Baba H, et al. Closing-opening wedge osteotomy to correct angular kyphotic deformity by a single posterior approach. Spine (Phila Pa 1976). 2001;26:391-402.
46 Urist MR. Osteotomy of the cervical spine; report of a case of ankylosing rheumatoid spondylitis. J Bone Joint Surg Am. 1958;40:833-843.
46a Simmons EH. The surgical correction of flexion deformity of the cervical spine in ankylosing spondylitis. Clin Orthop Relat Res. 1972;86:132-143.
46b Sengupta DK, Khazim R, Grevitt MP, Webb JK. Flexion osteotomy of the cervical spine: a new technique for correction of iatrogenic extension deformity in ankylosing spondylitis. Spine (Phila Pa 1976). 2001;26:1068-1072.