Fig. 3.1
Pathobiology of circulating Angptl4 in nephrotic syndrome (From [21]). Diagram representing the production of circulating Angptl4 protein and its biological effects. The circulating, sialylated form of Angptl4 is secreted from peripheral organs (mostly the skeletal muscle, heart, and adipose tissue) in minimal-change nephrotic syndrome (MCNS), membranous nephropathy (MN), focal and segmental glomerulosclerosis (FSGS), and non-HIV collapsing glomerulopathy (CG). In addition, podocytes in MCNS secrete a hyposialylated form of the protein that remains restricted to the kidney and induces proteinuria [4] and a normosialylated form that enters the circulation. Circulating Angptl4 binds to glomerular endothelial αvβ5 integrin to reduce proteinuria or inactivates endothelium-bound lipoprotein lipase (LPL) in the skeletal muscle, heart, and adipose tissue to reduce the hydrolysis of plasma triglycerides to free fatty acids (FFA), resulting in hypertriglyceridemia. Some Angptl4 and LPL are lost in the urine
3.1.3.2 aP2-Angptl4 Transgenic Rats
Under normal conditions, major Angptl4-secreting tissues in the body are the adipose tissue, skeletal muscle, heart, and liver. These organs secrete a sialylated form of Angptl4 that enters circulation and affects plasma triglyceride levels.
Rats that specifically overexpress Angptl4 from adipose tissue (aP2-Angptl4 transgenic rats) have high levels of circulating sialylated Angptl4 protein. High circulating sialylated Angptl4 derived from these organs is increased in many forms of human nephrotic syndrome, including MCNS, membranous nephropathy, focal and segmental glomerulosclerosis, and collapsing glomerulopathy [21]. The only difference between the other conditions and MCNS is that the podocyte is an additional source of circulating sialylated Angptl4 in MCNS. As discussed later, podocytes produce a combination of hyposialylated and sialylated Angptl4.
aP2-Angptl4 transgenic rats do not develop proteinuria, since circulating sialylated Angptl4 has an anti-proteinuric effect. These transgenic rats have normal glomerular morphology on light and electron microscopy, and they do not have any modification in the GBM charge. They have significant hypertriglyceridemia, since circulating Angptl4 is a potent inhibitor of lipoprotein lipase (LPL), the endothelium-bound enzyme that hydrolyzes triglycerides to release free fatty acids (FFA).
3.1.4 Study of Angptl4 in the PAN Model of Human MCNS
The PAN model is the most commonly used model of MCNS, induced by a single intravenous injection of puromycin aminonucleoside into rats [24]. These rats present some features of the human MCNS: explosive onset of proteinuria, no visible glomerular lesions by light microscopy, foot process effacement by electron microscopy, hypertriglyceridemia and hypercholesterolemia, and loss of GBM charge. However, proteinuria is not selective and is only partially glucocorticoid sensitive [5].
Confocal microscopy shows co-localization of Angptl4 with nephrin, indicating its expression in podocyte. There is low constitutive Angptl4 protein expression in the normal rat and human podocyte. In both human MCNS and PAN model, Angptl4 secreted from podocytes enters the GBM (co-localizes with proteoglycans) and the blood circulation and is also lost in urine.
In the PAN model, upregulation of Angptl4 gene expression and amount of proteinuria induced are dependent on the dose of puromycin aminonucleoside injected into rats. The proteinuria induced in the model is relatively nonselective, since only 66 % of urinary proteins comprise for albumin, compared to about 86 % in human MCNS relapse patient and 92 % in NPHS-Angptl4 transgenic rats. Regarding selectivity of proteinuria, this is not such a good model to study MCNS.
3.1.5 Molecular Mechanisms of Angptl4 in Proteinuria in MCNS: The Role of Sialylation
Two-dimensional electrophoresis gel studies showed that glomeruli from PAN rats express two forms of the Angptl4 protein: a positively charged form migrating at a high isoelectric point (pI) (8–8.5) and a neutral form migrating at pI between 6 and 7. The lack of adequate sialylation in the high-pI form is an important difference between these two forms of Angptl4. NPHS2-Angptl4 transgenic rats similarly express both forms of Angptl4 in glomeruli, making them suitable to further study the role of Angptl4 in MCNS.
In addition to the transgenic rats, two types of stable cell lines overexpressing Angptl4 were developed in mouse glomerular epithelial cells (GEC) and human embryonic kidney cells (HEK293). Cells secrete high-pI form of Angptl4 in the medium from where it can be harvested. Incubation of both cell lines with the naturally occurring sialic acid precursor N-acetyl-D-mannosamine (ManNAc) allows the conversion of the high-pI hyposialylated Angptl4 into the neutral-pI sialylated form within the cells and secretion of sialylated Angptl4 in the supernatant.
The choice of using ManNAc for sialylation-based therapeutics was determined by several factors [25]. First, ManNAc has a neutral charge allowing it to cross cell membranes easily. Once in the cell, it is converted into sialic acid and then incorporated into glycoproteins as Angptl4 [4]. Furthermore, ManNAc enters the sialic acid biosynthesis pathway after the rate-limiting enzymatic step catalyzed by GNE (UDP-N-acetylglucosamine-2-epimerase/N-acetylmannosamine kinase) [26] (Fig. 3.2). This surmounts the intrinsic limitations of the pathway during high-demand states. Second, podocytes are nondividing cells, and therefore, cellular ManNAc content is not divided by cell division. Lastly, orally administrated ManNAc is rapidly lost in the urine (90 % within 4 h). During this process, the podocyte, being the outermost layer of the glomerular filter, gets normal exposure during transit across this filter.
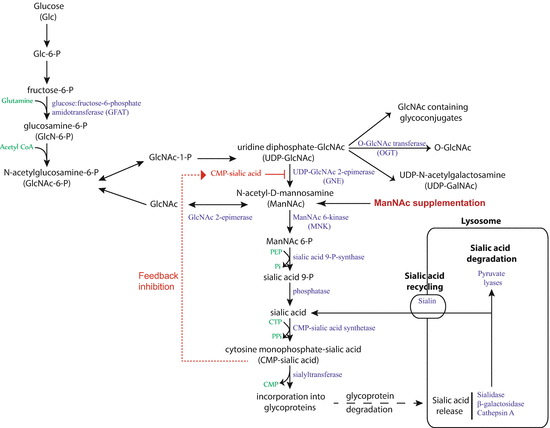

Fig. 3.2
Sialic acid biosynthesis and recycling pathway (From [27]). In humans, sialic acid is synthesized from glucose. The rate-limiting step, catalyzed by UDP-N-acetylglucosamine-2-epimerase/N-acetylmannosamine kinase (GNE), is subject to feedback inhibition. ManNAc is the product of this rate-limiting step, so exogenous ManNAc supplementation enters the pathway after this step. A substantial amount of sialic acid is recycled via the anion transporter sialin following lysosomal degradation of glycoproteins and glycolipids
To answer the question of the biological significance of Angptl4 sialylation, in vivo studies were conducted to verify its effects on albuminuria. Treatment of NPHS2-Angptl4 transgenic rats with daily oral ManNAc supplementation results in an over 40 % decrease in albuminuria from baseline over 12 days. Concomitantly, conversion of significant amounts of high-pI to neutral-pI Angptl4 occurs in the glomeruli of these ManNAc-treated rats compared to untreated rats. The neutral fraction reacts with sialic acid-binding lectin from Sambucus nigra (SNA I), confirming the increase in sialylation of Angptl4 in ManNAc-treated rats. This form of therapy has a significant memory effect, since it took 24 days for the proteinuria to rise back to the level of control-treated NPHS2-Angptl4 rats. To verify the biological effects of ManNAc on sialylation in the glomeruli, the two-dimensional electrophoresis gel patterns of another important protein in the podocyte were studied. Podocalyxin is a structural sialoglycoprotein expressed in podocytes. The non-modification of the overall charge of podocalyxin suggests that podocalyxin was not affected by ManNAc therapy [4].
The lack of sialylation of Angptl4 in MCNS is still under investigation and could have several origins. A simple possible explanation is based on the low constitutive expression of Angptl4 in normal podocytes. Therefore, only small amounts of sialic acid are required by this pathway at baseline. Due to severe and rapid upregulation of Angptl4 expression in experimental MCNS disease (70-fold increase in messenger RNA expression), demand exceeds supply, resulting in the secretion of hyposialylated high-pI protein. Another possible explanation for the lack of sialylation of Angptl4 is that the activity of a class of enzymes called sialyltransferases, which add sialic acid residues to proteins, is decreased in podocytes in MCNS.
3.1.6 Link Between Proteinuria and Hypertriglyceridemia in Nephrotic Syndrome
There are large gaps of knowledge in our understanding of the molecular relationship between proteinuria, the primary driver in nephrotic syndrome, and most of the other components. Only the link between proteinuria and hypertriglyceridemia has been clearly elucidated [21, 27], revealing the intrinsic role of circulating Angptl4 in nephrotic syndrome. This relationship is strongly influenced by the link between FFA and albumin. Albumin is the most abundant plasma protein that circulates as a 69 kDa monomer and serves as a vehicle to transport cations, hormones, and FFA. FFA are a critical energy source for the body and also serve as an important molecular mediator in nephrotic syndrome. FFA can be used as a source of energy by organs like the skeletal muscle and heart [28] or can be recycled by adipose tissue, since it both releases and takes up FFA for storage as triglycerides. These organs also have high expression for LPL, Angptl4, and peroxisome proliferator-activated receptor (PPAR) family members, which regulate Angptl4 expression in response to FFA uptake.
Normal sources of fatty acids include diet, mobilization from adipose tissue, and conversion of excess carbohydrates into fat by the liver. Some fatty acids are coupled with glycerol to form triglycerides (or triacylglycerols) for transport or storage and can be converted back into FFA by lipases. FFA that are not part of triglycerides circulate in the blood, mostly coupled non-covalently with albumin. Each albumin molecule has six high-affinity FFA-binding sites and many low-affinity-binding sites, and up to ten FFA molecules can be bound to an albumin molecule at any given time [29]. Adipose tissue releases FFA into circulation after serial conversion of triglycerides to diglycerides by adipose triglyceride lipase and diglycerides to monoglycerides by hormone-sensitive lipase. After digestion of dietary fat, medium-chain fatty acids (8–12 carbon chain) are transported coupled with albumin, whereas long-chain fatty acids (14 or more carbon chains) are converted back to triglycerides, incorporated into chylomicrons, and transferred to the circulation via the thoracic duct.
There are two sources of FFA for uptake by organs: albumin-bound FFA and conversion of circulating triglycerides into FFA by the endothelium-anchored enzyme LPL (Fig. 3.3). The balance between these two sources of FFA uptake is significantly altered in nephrotic syndrome. For reasons that are unknown and need to be explored in the future, albumin with a low FFA content is preferentially lost in urine by proteinuric kidneys, but albumin with high FFA content is not, so the result is a progressive retention of albumin with high FFA content [29, 21]. As proteinuria reaches nephrotic range, hypoalbuminemia develops, and a combination of high FFA containing albumin and hypoalbuminemia raises the plasma ratio of FFA to albumin [21]. This elevated plasma FFA-to-albumin ratio induces increased FFA uptake in the skeletal muscle, heart, and adipose tissue, which in turn increases Angptl4 expression and secretion from these tissues. Since Angptl4 is a known PPAR target gene [30–32], and PPAR expression is increased during the nephrotic phase in these tissues [21], at least part of this Angptl4 upregulation is likely to be PPAR mediated. Angptl4 secreted from these organs into the circulation has two effects presented in a local and a systemic feedback loops (Fig. 3.4). First, it binds to the αvβ5 integrin in glomerular endothelium and reduces proteinuria (Fig. 3.4, systemic feedback loop). The precise mechanism by which the Angptl4–αvβ5 integrin interaction reduces proteinuria is not known, but it is possible that additional feedback loops within the glomerulus are involved. Second, Angptl4 inactivates LPL activity in these organs, thereby reducing the conversion of triglycerides into FFA, which reduces FFA uptake by this pathway (Fig. 3.4, local feedback loop), and also results in hypertriglyceridemia. Overall, it looks like the local feedback loop, in which Angptl4 decreases LPL activity and then reduces the availability of FFA generated from triglycerides, reduces the effectiveness of the systemic feedback loop by limiting the extent of Angptl4 upregulation [21]. This attempt to reduce proteinuria by Angptl4 represents a systemic response against rising proteinuria.
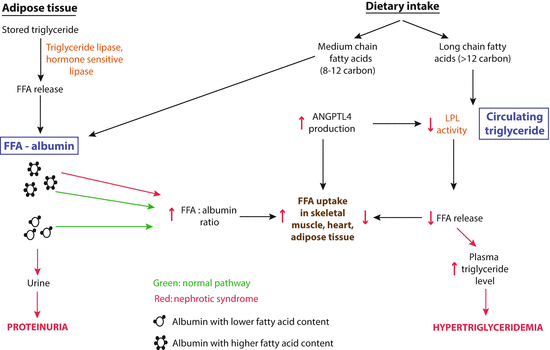
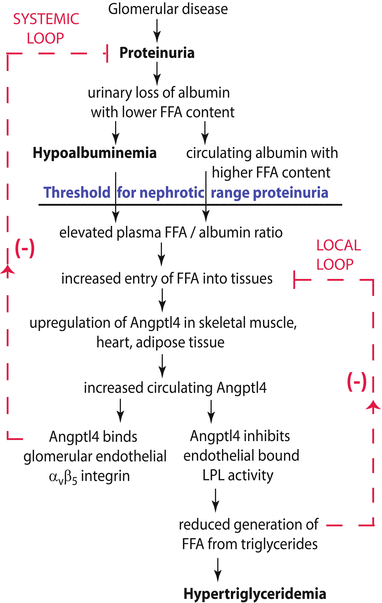

Fig. 3.3
Schematic illustration of the two sources of free fatty acids (FFA) available for uptake by the skeletal muscle, heart, and adipose tissue in the normal and nephrotic state (From [41]). Green shows normal conditions, and red illustrates changes in nephrotic syndrome. The balance shifts significantly to albumin-bound free fatty acids (FFA) because of retention of albumin with high FFA content in nephrotic syndrome. Angptl4 secreted from these organs reduces the conversion of triglycerides to FFA by inactivating lipoprotein lipase (LPL), thereby reducing use of triglycerides and resulting in hypertriglyceridemia

Fig. 3.4
Circulating Angptl4: link between proteinuria and hypertriglyceridemia in nephrotic syndrome (From [21]). Schematic illustration of negative feedback loops in the link between proteinuria, hypoalbuminemia, and hypertriglyceridemia that are mediated by Angptl4 and free fatty acids (FFA) (unesterified fatty acids with a free carboxylate group). Plasma FFAs are non-covalently bound to albumin, and because of the preferential loss of albumin with low FFA content during proteinuria, albumin with higher FFA content is retained in circulation. As glomerular disease progresses and proteinuria increases, hypoalbuminemia develops, and the combination of high albumin-FFA content and lower plasma albumin levels increases the plasma ratio of FFAs to albumin. This increased available FFA enters the skeletal muscle, heart, and adipose tissue to induce upregulation of Angptl4, mediated at least in part by peroxisome proliferator-activated receptor (PPAR) transcription factors. Angptl4 secreted from these organs participates in two feedback loops. In the systemic loop, it binds to glomerular endothelial αvβ5 integrin and reduces proteinuria. In a local loop, it inhibits lipoprotein lipase (LPL) activity in the same organs from which it is secreted to reduce the uptake of FFAs, thereby curtailing the stimulus for its own upregulation
3.1.7 Importance of the Nephrotic-Range Proteinuria Threshold
In patients, manifestation of the other components of nephrotic syndrome starts only after proteinuria crosses the nephrotic-range proteinuria threshold, which is usually defined as about 3.5 g/d in adults, but it is likely to be quite variable between different individuals and within the same individual among different components. Until recently, the molecular basis for this threshold was not known. It is, at present, only possible to explain the nephrotic threshold in the context of hypertriglyceridemia [21]. Hypertriglyceridemia is dependent on the retention of albumin with high FFA content, resulting in a plasma FFA-to-albumin ratio that is high enough to induce upregulation of Angptl4 expression in the skeletal muscle, heart, and adipose tissue. Studies in experimental animals reveal that, during mild proteinuria, plasma FFA-to-albumin ratio, plasma Angptl4 levels, peripheral organ Angptl4, and PPAR mRNA expression are similar to control non-proteinuric animals. During severe proteinuria, all these parameters are significantly elevated, suggesting that the threshold for nephrotic-range proteinuria, in the context of hypertriglyceridemia, correlates with the downstream effects of an increased plasma FFA-to-albumin ratio.
3.1.8 Current and Future Therapies for MCNS
3.1.8.1 Current Therapies
Current therapies for kidney disease related to proteinuric disorders rely on agents used in other fields than nephrology. In one category, glucocorticoids, cyclophosphamide, azathioprine, chlorambucil, mycophenolate mofetil, cyclosporine, tacrolimus, and the anti-CD20 antibody have been used to treat glomerular disease because of their immunosuppressive properties. Over the past decade, it became clear that several of these drugs have direct effects on glomerular cells [33, 4]. In another category, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, spironolactone, and renin inhibitors such as aliskiren block the renin–angiotensin system and are used for supportive therapy.
Among these drugs, glucocorticoids are usually the first line of therapy for MCNS. Indeed, up to 95 % of patients with MCNS are sensitive to glucocorticoid therapy [1], which suggests that the key mediators of this disease are either encoded by glucocorticoid-sensitive genes or controlled by glucocorticoid-sensitive pathways. Glomerular ANGPTL4 upregulation noted in the PAN model is glucocorticoid sensitive, since administration of glucocorticoids in PAN reduces glomerular ANGPTL4 expression and proteinuria [4]. There is limited value in treating the NPHS2-ANGPTL4 transgenic rat with glucocorticoids to study glucocorticoid sensitivity in the context of MCNS because transgenic expression is not driven by the native ANGPTL4 promoter.
However, prolonged and repeated exposure to glucocorticoids has numerous serious side effects. Since MCNS patients relapse frequently and often become glucocorticoid dependent, it is more appropriate to explore additional ways of treating this disease.
3.1.8.2 ManNAc: A New Therapeutic Agent for MCNS
The upregulation of podocyte-secreted Angptl4 is present in human and experimental MCNS and induces heavy proteinuria. Podocyte-secreted hyposialylated Angptl4 induces proteinuria, and conversion of hyposialylated Angptl4 to its sialylated form reduces proteinuria in NPHS2-Angptl4 transgenic rats. Therefore, sialylation of Angptl4 using ManNAc is a reasonable approach toward proteinuria.
Sialic acid is commonly present as terminal carbohydrate on glycoproteins. ManNAc is the precursor of all physiological sialic acids. In biological systems it is the precursor compound for the biosynthesis of the sialic acids especially N-acetylneuraminic acid (Neu5Ac/NANA). Sialic acid is essential for a variety of cellular functions. In humans, mutations of the GNE gene result in a severe neuromuscular disorder called hereditary inclusion body myopathy (HIBM) [34]. These patients never develop kidney disease. Mice with mutations at the same site in the mouse GNE gene develop kidney disease, which suggests that mice are very different from humans in this aspect, and data from such mutant mice bears no relevance to human disease. This is further strengthened by the observation of the GNE knockout mice, with a knock-in of the human gene with the common HIBM mutation, that develop muscle disease only and have no sign of kidney disease [34].
Sialylation of proteins is a natural event in human cells. A substantial amount of sialic acid in cells is recycled, which reduces tremendously the requirement for de novo sialic acid synthesis [35]. Sialic acid is most commonly incorporated at O- and N-glycosylation sites of glycoproteins and in glycosphingolipids (gangliosides). The predominant form of sialic acid present in humans is NANA/Neu5Ac [36]. Humans synthesize sialic acid from glucose since there is no major nutritional source of NANA/Neu5Ac. Animals convert NANA into N-glycolylneuraminic acid (NGNA/Neu5Gc) using the enzyme NANA hydroxylase. This enzyme is nonfunctional in humans due to an exon deletion/frameshift mutation [37]. Therefore, under most circumstances, NGNA present in food is not incorporated into human proteins, excluding diet as a source of sialic acid in humans. During normal condition and during times of high demand (e.g., fasting, nephrotic syndrome), sialylated Angptl4 is secreted into the circulation from organs having very active sialic acid biosynthesis pathway like the liver and adipose tissue. Podocytes likely have a less active sialic acid biosynthesis pathway, and in condition of severe upregulation of Angptl4, like in MCNS, this pathway is unable to sialylate newly produced Angptl4, despite a slight increase in the expression of the rate-limiting enzyme GNE. This results in the secretion of a combination of hyposialylated and sialylated Angptl4 from podocytes. The fact that exogenously administered ManNAc enters the sialic acid biosynthesis pathway after the rate-limiting step catalyzed by UDP–GNE allows the cells to synthetize sialic acid when it is needed.
ManNAc could be used as a therapy in diseases involving the podocyte for two reasons. First, unlike most other cells in the body, podocyte division is very rare. This cellular specificity would allow the podocyte to accumulate ManNAc, and consequently, ManNAc therapy would require lower oral dose compared to therapy-targeting dividing cells. Finally, 90 % of the ManNAc oral dose is secreted into urine within 4 h [34]. This rapid urinary excretion is an advantage, since podocytes are exposed to most of the oral dose of ManNAc while it is filtered through the glomerulus. Overall chances of toxicity from ManNAc are dramatically decreased by the combination of low-dose requirement for podocyte disease and rapid urinary excretion.
In MCNS, ManNAc could be used as a complementary therapy to glucocorticoids. Indeed, glucocorticoids reduce podocyte Angptl4 gene expression, but ManNAc improves sialylation of the protein itself. ManNAc therapy would be very useful in patients who have frequent relapses, those developing resistance to glucocorticoids, or patients in whom complete remission is not achieved or who require very prolonged glucocorticoid therapy. ManNAc therapy could be considered for maintenance therapy, either as a daily very low-dose regimen or intermittent low-dose therapy. Knowing that long-term glucocorticoid therapy is associated with multi-organ complications, the first episode of MCNS would still be treated with glucocorticoids, especially in children, at least in part to prove that the disease is indeed glucocorticoid sensitive. Another mechanism by which glucocorticoids are effective in MCNS is by increasing Angptl4 secretion from the skeletal muscle, heart, and adipose tissue [38], which has anti-proteinuric effects. Knowing that 80–90 % of children relapse after the first remission [39, 40], ManNAc is perfectly suited as a maintenance drug to reduce the frequency and intensity of relapse.
3.1.9 Can Angptl4 Be Used as a Novel Biomarker to Diagnose the Type of INS?
The development of novel biomarkers for MCNS is important. Hyposialylated Angptl4 is lost in the urine in MCNS patients. Detailed studies would need to be conducted to see if high-pI hyposialylated Angptl4 in the urine can be used as a biomarker for MCNS.
3.2 Conclusion
Even if the podocyte Angptl4 upregulation explains most of the cardinal manifestations of MCNS, it clearly does not explain the acute onset of symptoms. For this phenomenon, additional mechanisms need to be explored.
Angptl4 has been conclusively implicated in the pathogenesis of proteinuria in human and experimental MCNS. When it is overexpressed in the podocyte, it is secreted within two forms, a hyposialylated and a sialylated form, and the hyposialylated induces proteinuria. This lack of sialylation can be corrected by supplementation of animal drinking water with the sialic acid precursor ManNAc. Furthermore, it is implicated in other hallmarks of MCNS like hyperlipidemia, effacement of foot process, and loss of GBM charge.
In the near future, Angptl4 could be used as a biomarker of MCNS depending on the ability to measure hyposialylated Angptl4 in urine from patients. Sialylation-based therapeutic strategy holds significant promise in the treatment of common forms of proteinuric chronic kidney disease. This is a novel area, innovative and mechanism based, and has been extensively studied in vivo in appropriate human disease models. Manipulating Angptl4-related pathways in the context of therapeutics seems to have a high chance of success. This new therapy could be used to treat relapse patients and could prevent prolonged and repeated exposure to corticosteroids and their drug-related adverse events.
References
1.
Nachman PH, Jennett JC, Falk RJ. Primary glomerular disease. In: Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu ASL, Brenner BM, editors. The kidney. 9th ed. Philadelphia, PA: Elsevier; 2012. p. 1100–91.
2.
Miner JH. Glomerular filtration: the charge debate charges ahead. Kidney Int. 2008;74:259–61. doi:10.1038/ki.2008.260.CrossRefPubMed
3.
Shalhoub RJ. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet. 1974;2:556–60. doi:10.1016/S0140-6736(74)91880-7.CrossRefPubMed
4.
Clement LC, Avila-Casado C, Macé C, Soria E, Bakker WW, Kersten S, Chugh SS. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med. 2011;17:117–22. doi:10.1038/nm.2261.PubMedCentralCrossRefPubMed
5.
Clement LC, Liu G, Perez-Torres I, Kanwar YS, Avila-Casado C, Chugh SS. Early changes in gene expression that influence the course of primary glomerular disease. Kidney Int. 2007;72:337–47. doi:10.1038/sj.ki.5002302.CrossRefPubMed
6.
Abraham P, Isaac B. Ultrastructural changes in the rat kidney after single dose of cyclophosphamide – possible roles for peroxisome proliferation and lysosomal dysfunction in cyclophosphamide-induced renal damage. Hum Exp Toxicol. 2011;30:1924–30. doi:10.1177/0960327111402240.CrossRefPubMed
7.
Chugh S, Yuan H, Topham PS, Haydar SA, Mittal V, Taylor GA, Kalluri R, Salant DJ. Aminopeptidase A: a nephritogenic target antigen of nephrotoxic serum. Kidney Int. 2001;59:601–13. doi:10.1046/j.1523-1755.2001.059002601.x.CrossRefPubMed
8.
Avila-Casado MC, Perez-Torres I, Auron A, Soto V, Fortoul TI, Herrera-Acosta J. Proteinuria in rats induced by serum from patients with collapsing glomerulopathy. Kidney Int. 2004;66:133–43. doi:10.1111/j.1523-1755.2004.00715.x.CrossRef
9.
Yoshida K, Shimizugawa T, Ono M, Furukawa H. Angiopoietin-like protein 4 is a potent hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein lipase. J Lipid Res. 2002;43:1770–2. doi:10.1194/jlr.C200010-JLR200.CrossRefPubMed
10.
Goh YY, Pal M, Chong HC, Zhu P, Tan MJ, Punugu L, Tan CK, Huang RL, Sze SK, Tang MB, Ding JL, Kersten S, Tan NS. Angiopoietin-like 4 interacts with matrix proteins to modulate wound healing. J Biol Chem. 2010;285:32999–3009. doi:10.1074/jbc.M110.108175.PubMedCentralCrossRefPubMed
11.
Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, Massagué J. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi:10.1016/j.cell.2008.01.046.PubMedCentralCrossRefPubMed
12.
Yin W, Romeo S, Chang S, Grishin NV, Hobbs HH, Cohen JC. Genetic variation in ANGPTL4 provides insights into protein processing and function. J Biol Chem. 2009;284:13213–22. doi:10.1074/jbc.M900553200.PubMedCentralCrossRefPubMed
13.
Ge H, Yang G, Huang L, Motola DL, Pourbahrami T, Li C. Oligomerization and regulated proteolytic processing of angiopoietin-like protein 4. J Biol Chem. 2004;279:2038–45. doi:10.1074/jbc.M307583200.CrossRefPubMed
14.
Yau MH, Wang Y, Lam KS, Zhang J, Wu D, Xu A. A highly conserved motif within the NH2-terminal coiled-coil domain of angiopoietin-like protein 4 confers its inhibitory effects on lipoprotein lipase by disrupting the enzyme dimerization. J Biol Chem. 2009;284:11942–52. doi:10.1074/jbc.M809802200.PubMedCentralCrossRefPubMed
15.
Kersten S, Mandard S, Tan NS, Escher P, Metzger D, Chambon P, Gonzalez FJ, Desvergne B, Wahli W. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J Biol Chem. 2000;275:28488–93. doi:10.1074/jbc.M004029200.CrossRefPubMed
16.
Yoon JC, Chickering TW, Rosen ED, Dussault B, Qin Y, Soukas A, Friedman JM, Holmes WE, Spiegelman BM. Peroxisome proliferator-activated receptor gamma target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol Cell Biol. 2000;20:5343–9. doi:10.1128/MCB.20.14.5343-5349.2000.PubMedCentralCrossRefPubMed
17.
Kim I, Kim HG, Kim H, Kim HH, Park SK, Uhm CS, Lee ZH, Koh GY. Hepatic expression, synthesis and secretion of a novel fibrinogen/angiopoietin-related protein that prevents endothelial-cell apoptosis. Biochem J. 2000;346:603–10.PubMedCentralCrossRefPubMed
18.
Romeo S, Pennacchio LA, Fu Y, Boerwinkle E, Tybjaerg-Hansen A, Hobbs HH, Cohen JC. Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat Genet. 2007;39:513–6. doi:10.1038/ng1984.PubMedCentralCrossRefPubMed
19.
Romeo S, Yin W, Kozlitina J, Pennacchio LA, Boerwinkle E, Hobbs HH, Cohen JC. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J Clin Invest. 2009;119:70–9. doi:10.1172/JCI37118.PubMedCentralPubMed
20.
Mandard S, Zandbergen F, van Straten E, Wahli W, Kuipers F, Müller M, et al. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J Biol Chem. 2006;281:934–44. Epub 4 Nov 2005. Erratum in: J Biol Chem. 2006;281:21575. doi:10.1074/jbc.M506519200.
21.
Clement LC, Macé C, Avila-Casado C, Joles JA, Kersten S, Chugh SS. Circulating angiopoietin-like 4 links proteinuria with hypertriglyceridemia in nephrotic syndrome. Nat Med. 2014;20:37–46. doi:10.1038/nm.3396.PubMedCentralCrossRefPubMed
22.
Michael AF, Blau E, Vernier RL. Glomerular polyanion. Alteration in aminonucleoside nephrosis. Lab Invest. 1970;23:649–57.PubMed
23.
Cazes A, Galaup A, Chomel C, Bignon M, Bréchot N, Le Jan S, Weber H, Corvol P, Muller L, Germain S, Monnot C. Extracellular matrix-bound angiopoietin-like 4 inhibits endothelial cell adhesion, migration, and sprouting and alters actin cytoskeleton. Circ Res. 2006;99:1207–15. doi:10.1161/01.RES.0000250758.63358.91.PubMedCentralCrossRefPubMed
24.
Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, Krofft RD, Logar CM, Marshall CB, Ohse T, Shankland SJ. Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol. 2009;296:F213–29. doi:10.1152/ajprenal.90421.2008.CrossRefPubMed
25.
Chugh SS, Clement LC, Macé C. New insights into human minimal change disease: lessons from animal models. Am J Kidney Dis. 2012;59:284–92. doi:10.1053/j.ajkd.2011.07.024.PubMedCentralCrossRefPubMed
26.
Luchansky SJ, Yarema KJ, Takahashi S, Bertozzi CR. GlcNAc 2-epimerase can serve a catabolic role in sialic acid metabolism. J Biol Chem. 2003;278:8035–42. doi:10.1074/jbc.M212127200.CrossRefPubMed
27.
Chugh SS, Macé C, Clement LC, Del Nogal Avila M, Marshall CB. Angiopoietin-like 4 based therapeutics for proteinuria and kidney disease. Front Pharmacol. 2014;5:23. doi:10.3389/fphar.2014.PubMedCentralCrossRefPubMed
28.
Mattijssen F, Kersten S. Regulation of triglyceride metabolism by angiopoietin-like proteins. Biochim Biophys Acta. 1821;2012:782–9. doi:10.1016/j.bbalip.2011.10.010.
29.
30.
Kersten S, Lichtenstein L, Steenbergen E, Mudde K, Hendriks HF, Hesselink MK, Schrauwen P, Müller M. Caloric restriction and exercise increase plasma ANGPTL4 levels in humans via elevated free fatty acids. Arterioscler Thromb Vasc Biol. 2009;29:969–74. doi:10.1161/ATVBAHA.108.182147.CrossRefPubMed
31.
Staiger H, Haas C, Machann J, Werner R, Weisser M, Schick F, Machicao F, Stefan N, Fritsche A, Häring HU. Muscle-derived angiopoietin-like protein 4 is induced by fatty acids via peroxisome proliferator-activated receptor (PPAR)-delta and is of metabolic relevance in humans. Diabetes. 2009;58:579–89. doi:10.2337/db07-1438.PubMedCentralCrossRefPubMed
32.
Georgiadi A, Lichtenstein L, Degenhardt T, Boekschoten MV, van Bilsen M, Desvergne B, Müller M, Kersten S. Induction of cardiac Angptl4 by dietary fatty acids is mediated by peroxisome proliferator-activated receptor beta/delta and protects against fatty acid-induced oxidative stress. Circ Res. 2010;106:1712–21. doi:10.1161/CIRCRESAHA.110.217380.CrossRefPubMed
33.
Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14:931–8. doi:10.1038/nm.1857.PubMedCentralCrossRefPubMed
34.
Malicdan MC, Noguchi S, Hayashi YK, Nonaka I, Nishino I. Prophylactic treatment with sialic acid metabolites precludes the development of the myopathic phenotype in the DMRV-hIBM mouse model. Nat Med. 2009;15:690–5. doi:10.1038/nm.1956.CrossRefPubMed
35.
Bertozzi CR, Freeze HH, Varki A, Esko JD. Glycans in biotechnology and the pharmaceutical industry. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of glycobiology. 2nd ed. New York: Cold Spring Harbor Press; 2009. p. 719–32.
36.
Varki A, Schauer R. Sialic acids. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of glycobiology. 2nd ed. New York, NY: Cold Spring Harbor Press; 2009. p. 199–217.
37.
Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, Muchmore EA, Nelson DL, Warren ST, Varki A. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci U S A. 1998;95:11751–6. doi:10.1073/pnas.95.20.11751.PubMedCentralCrossRefPubMed
38.
Koliwad SK, Kuo T, Shipp LE, Gray NE, Backhed F, So AY, Farese Jr RV, Wang JC. Angiopoietin-like 4 (ANGPTL4, fasting-induced adipose factor) is a direct glucocorticoid receptor target and participates in glucocorticoid-regulated triglyceride metabolism. J Biol Chem. 2009;284:25593–601. doi:10.1074/jbc.M109.025452.PubMedCentralCrossRefPubMed
39.
Koskimies O, Vilska J, Rapola J, Hallman N. Long-term outcome of primary nephrotic syndrome. Arch Dis Child. 1982;57:544–8. doi:10.1136/adc.57.7.544.PubMedCentralCrossRefPubMed
40.
Tarshish P, Tobin JN, Bernstein J, Edelmann Jr CM. Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. J Am Soc Nephrol. 1997;8:769–76.PubMed
41.
Macé C, Chugh SS. Nephrotic syndrome: components, connections, and angiopoietin-like 4-related therapeutics. J Am Soc Nephrol. 2014;25:2393–8. doi:10.1681/ASN.2014030267.CrossRefPubMed