CHAPTER 16 Angiographic Evaluation of Meningiomas
INTRODUCTION
For many decades, conventional cerebral angiography has been the major diagnostic modality to depict, diagnose, and investigate the specific features of all intracranial space-occupying lesions including meningiomas. Developed in the 1930s, angiographic technology has evolved considerably. The use of advanced technologies such as digital subtraction and three- dimensional image rendering, and the development of less toxic and better tolerated contrast materials, have greatly increased the diagnostic yield as well as the safety of angiography. Another major evolution in angiography technique has been the development of endovascular therapeutic techniques, which offer unique therapeutic possibilities. Advanced angiographic techniques and equipment have made this examination also more comfortable for the patient. Despite all of these advances, angiography is still an invasive technique that requires special skills and expertise, and one that is associated with a small but significant complication rate and radiation hazard risks. In the last four decades we have evidenced the development and widespread application of newer diagnostic technologies including computed tomography (CT) and magnetic resonance imaging (MRI). The diagnostic success of CT and MRI and their less invasive nature have greatly devaluated the importance of more invasive diagnostic modalities such as angiography, but not completely eliminated it. Newer technologies and modalities are still evolving to provide the information that can be gained from angiography. With the current technology, angiography still yields diagnostic information that CT or MRI cannot provide. Specific tumoral vessels are demonstrated in much better resolution than with alternative, less invasive techniques.1–3 However, angiography provides not merely a high-resolution map of the intracranial vasculature but also a detailed spatiotemporal analysis of the contrast material traveling through the vessels that yields important functional information on the tumor vasculature in addition to an anatomic description. Therefore, we believe that a good appreciation of angiographic technique and the information provided from it will lead to a better understanding of meningiomas. This chapter aims to summarize all available information on angiographic examination of meningiomas.
The major indication for cerebral angiography is the demonstration of the multiple feeding vessels of meningiomas, especially of the large ones, for which the possibility of preoperative embolization may also come into consideration.4 Further, meningiomas located in challenging operative areas or adjacent dural sinuses need to be subjected to meticulous angiographic examination.5 In this chapter the digital subtraction angiography (DSA) findings in meningiomas, such as typical angiographic features, feeding and adjacent arteries, dural sinus relationships, and special findings of meningiomas in certain localizations are presented.
ANGIOGRAPHIC TECHNIQUE
The indications for performing angiography in patients with meningiomas are far more limited and therefore much better defined today. Thus each angiographic examination is planned according to these needs and indications, considering the localization of the meningioma. A routine six-vessel examination may not be indicated or may not be sufficient, based on the requirements for the particular patient. A nonselective common carotid injection rarely provides sufficient information. Such a nonselective examination may fail to demonstrate the dural supply.6 A selective angiographic examination of the external carotid artery (ECA), the internal carotid artery (ICA), or the vertebrobasilar circulation is the method of choice. When preoperative embolization is planned, selective injection of all arteries, plus superselective examination of the tumoral vasculature, is required. The angiographic study should display both the tumoral vessels and the vascular anatomy of the related region. Venous phase examinations are important to study the condition of the adjacent veins and dural sinuses.7
ANGIOGRAPHIC FEATURES OF MENINGIOMAS
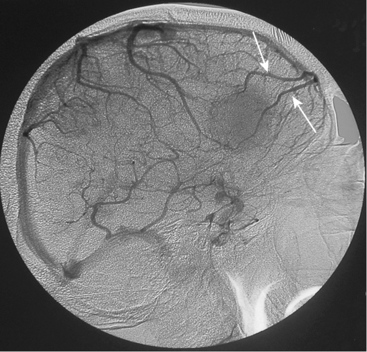
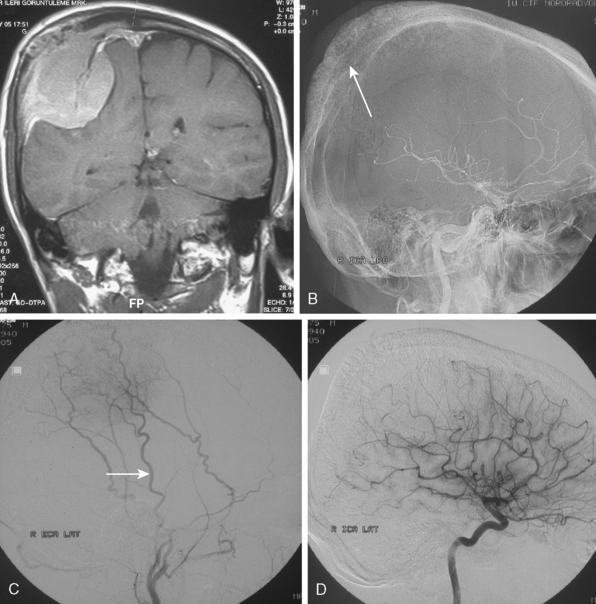
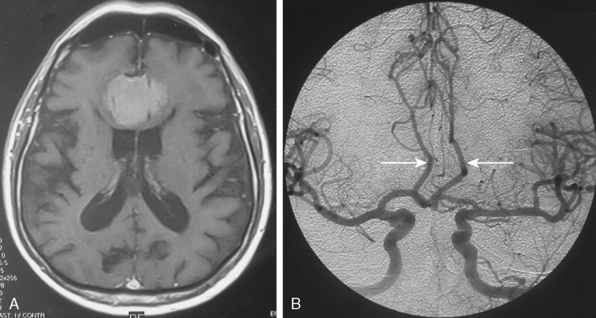
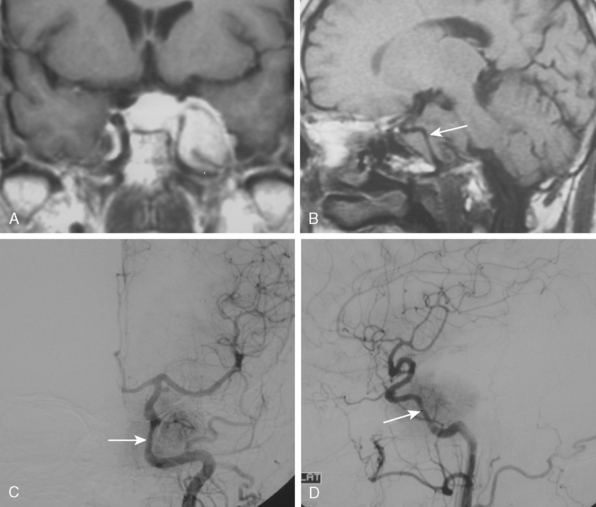
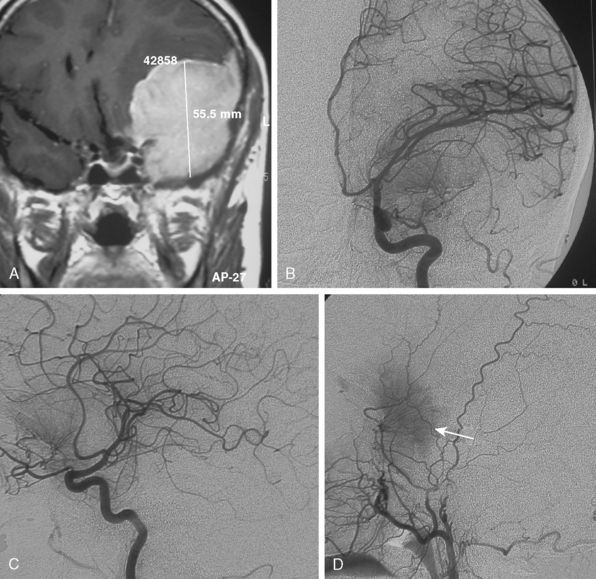
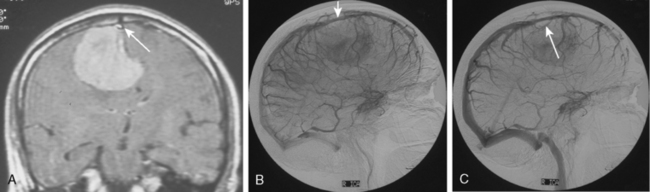
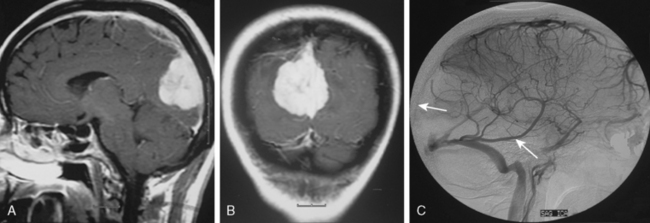
Tumors that have a rich vascular supply can be demonstrated angiographically. Historically, angiography had an important role in the differential diagnosis of intracranial tumors and differentiating benign meningiomas from malignant tumors such as gliomas and metastatic tumors. The most important evidence for such a differentiation was the speed of circulation through the tumor. Tumors with abnormal vascularity are divided into those with an increased speed of circulation through the tumor and those with a normal speed of circulation. An increased speed of circulation is almost invariably associated with a malignant histology. Few exceptions to this are angioblastic meningiomas and hemangioblastomas. Meningiomas may have a pathologically rich vascular tree and in some meningiomas the contrast circulation may be more rapid, and early draining, dilated veins may be observed (Fig. 16-1).8
TUMOR VASCULATURE
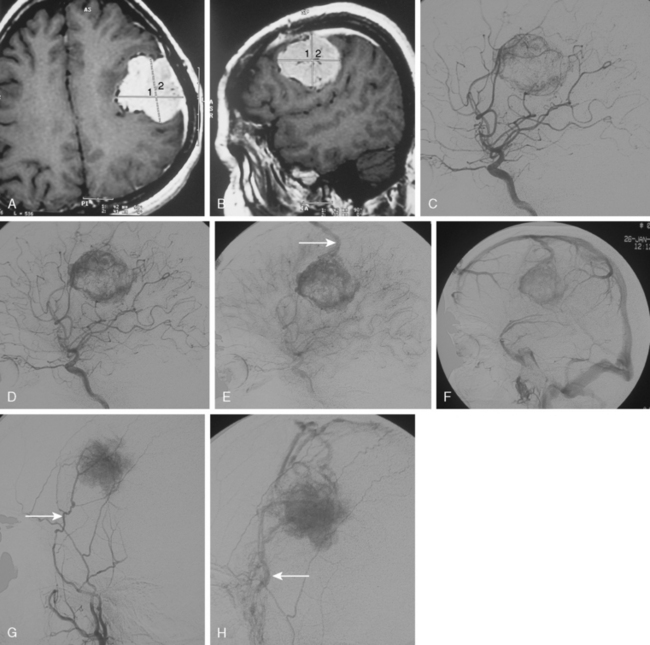
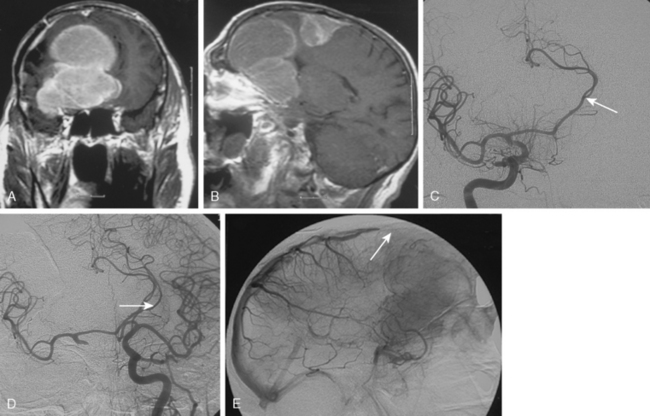
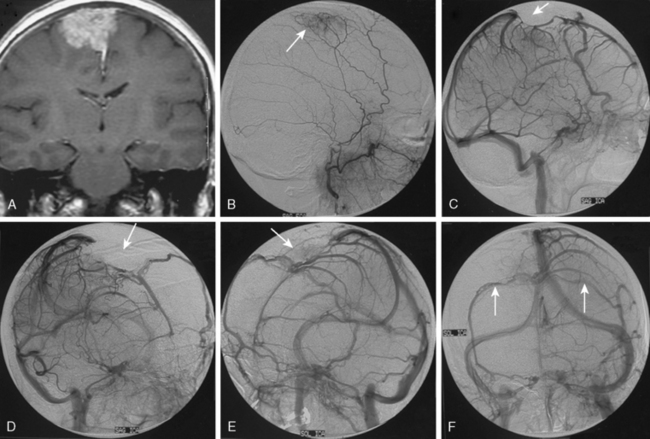
Meningiomas are extra-axial tumors that are based at the dura, and their vascular supply comes from normal arteries that supply the dura at the tumor’s site of attachment. Depending on the epicenter and the extent of meningiomas, these dural arteries may belong to the ECA, ICA, or the vertebrobasilar system.9 With increasing size, most meningiomas acquire blood supply from adjacent pial or leptomeningeal vessels (Fig. 16-2). The majority of meningiomas derive their vascular supply solely form the external carotid circulation. Another large proportion have suppliers from both the ECA and the ICA (or the vertebrobasilary system). Only a very small portion of meningiomas receive no vascular contribution from the ECA. Intraventricular meningiomas constitute fewer than 1% of all meningiomas. These are most commonly located at the atrium and are supplied by the hypertrophic anterior choroideal artery. The drainage is to the subependymal veins.10
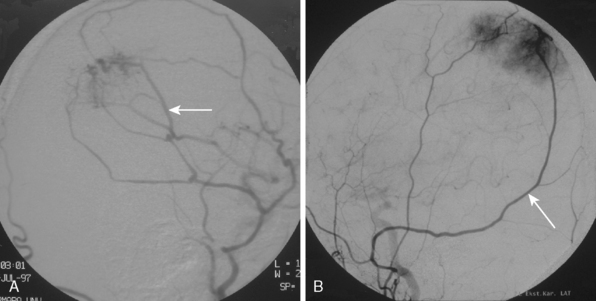
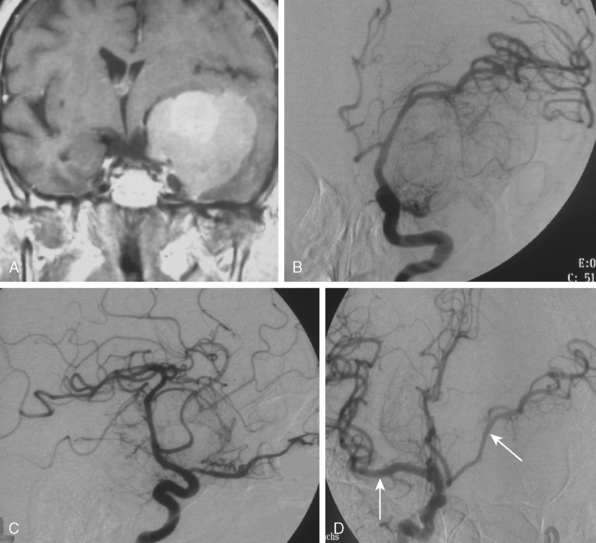
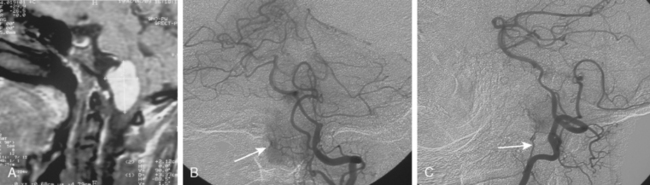
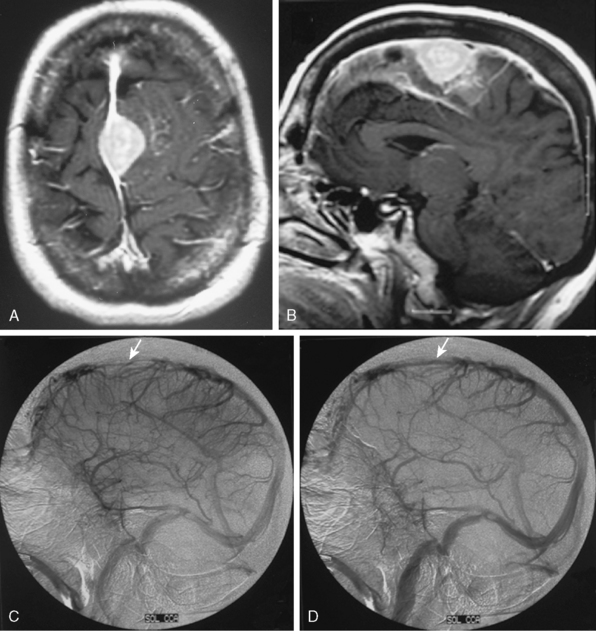
The convexity dura and hence non–skull-base meningiomas are mainly fed by branches of the external carotid system. Such a predominantly external carotid arterial supply is a strong indication that the detected lesion is a meningioma. However; other tumors that invade the dura may also have a prominent ECA supply.11 In these instances, the fast circulation and the bone destruction induced by such malignant metastases or gliomas may aid in the diagnosis. Meningiomas, on the other hand, may cause bone destruction but predominantly with accompanying hyperostosis and thickening of bone. Angiographically occult or avascular meningiomas have also been reported.12 However, exclusion of an angiographic vascular supply requires the injection of all three vascular systems. Even if no specific filling is detected, an area of filling defect may be detected. In our series we have observed an avascular meningioma on a selective ICA catheterization (Fig. 16-3). In general, small, clinoidal, and en plaque meningiomas are the less vascular, whereas convexity and parasagital meningiomas are the more vascular varieties.
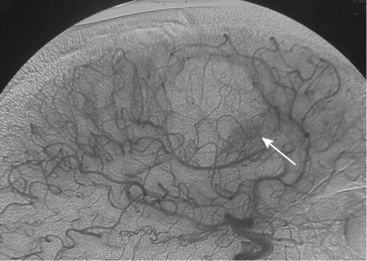
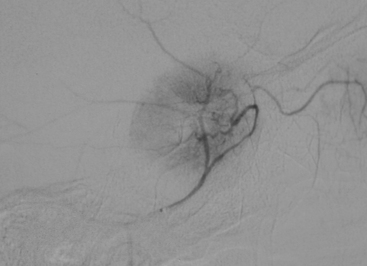
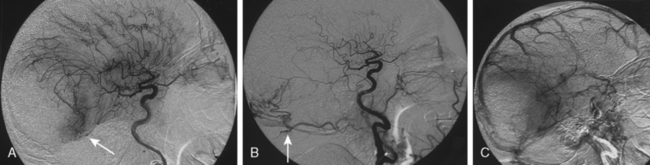
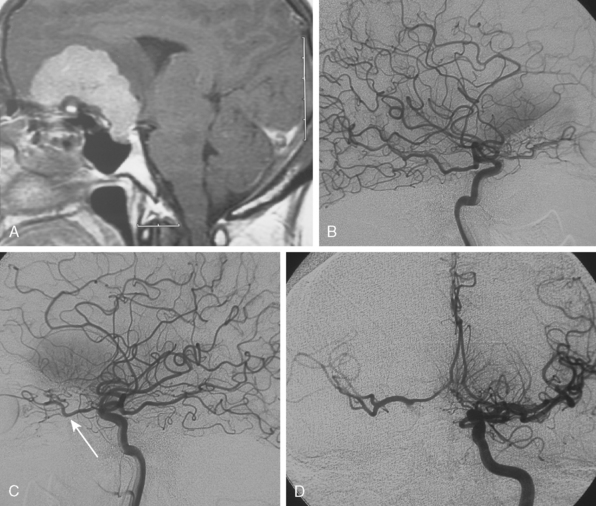
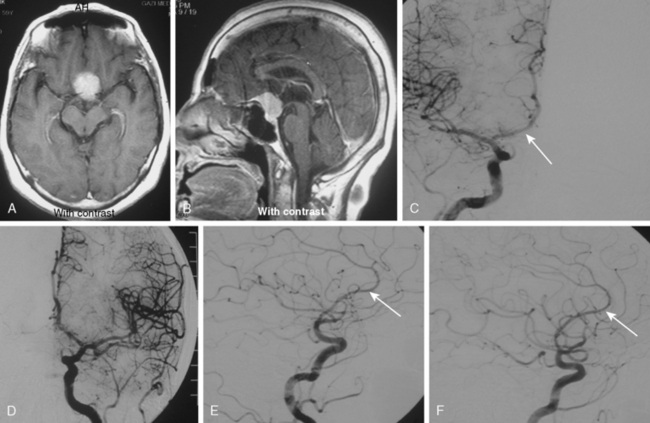
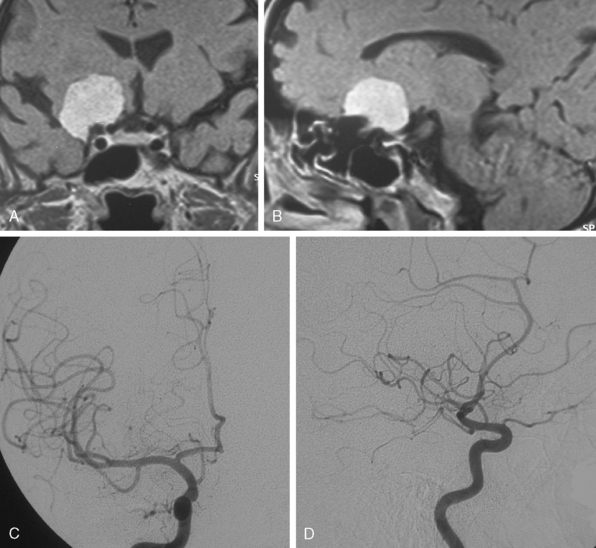
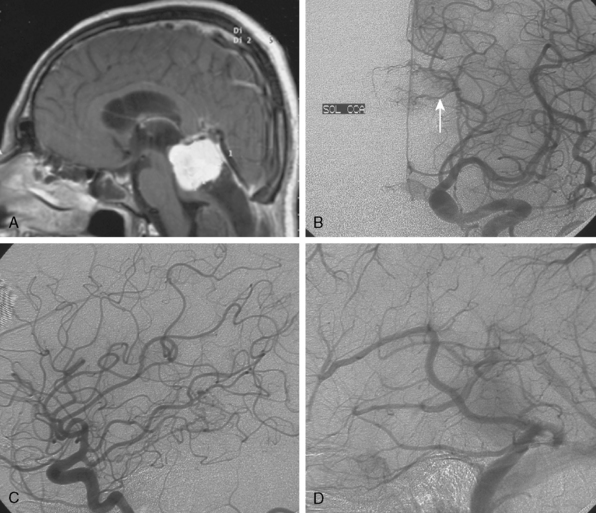
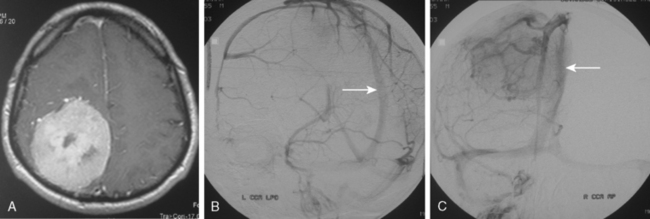
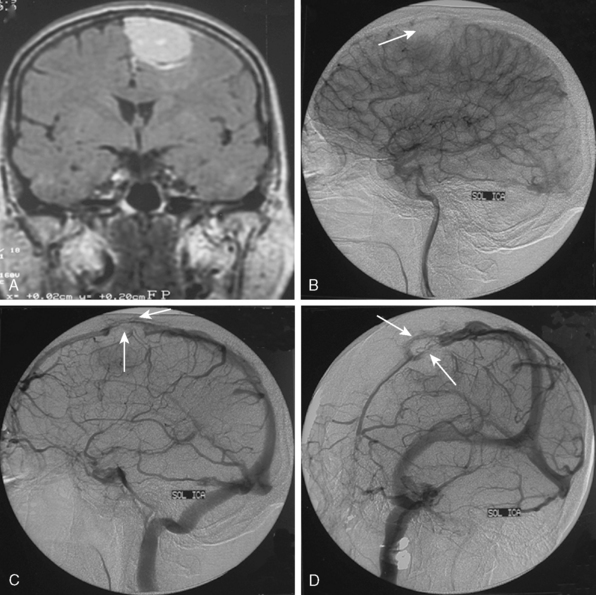
Parasagittal and convexity meningiomas can be the prototype to discuss the angiographic features of meningiomas. The convexity dura is supplied mainly by the middle meningeal artery (MMA), and this is the most common artery to be enlarged due to the increased vascular supply to a meningioma (Fig. 16-4).13 Convexity meningiomas derive their blood supply mostly from the MMA and accessory meningeal artery.14,15 At the midline location the vascular supply from the MMA may be bilateral. These tumors may frequently show invasion of the adjacent bone. Meningiomas that involve the adjacent bone may demonstrate perforating feeder branches from scalp vessels such as from the superficial temporal and external occipital arteries (Fig. 16-5). The blood flow in the ICA is comparatively faster than in the ECA. But the meningioma will result in earlier filling of the ECA branches, which is best appreciated on common carotid injections. The increased flow commonly results in a tortuous initial portion of the artery, before it bifurcates. Taveras and Wood have reported that such tortuosity may also be a normal variation, but that the persistence of this tortuosity in a segment longer than 1 to 2 cm is pathologic.16 Enlargement and earlier filling is also observed in branches of the middle meningeal trunk if they predominantly feed the tumor. Near its termination, the principal vessel feeding the meningioma will give off multiple radial vascular branches. This radial branching point is called the hilus and marks the epicenter of the tumor. In meningiomas that receive their vascular supply from both the ECA and ICA, the hilus is almost invariably fed by the ECA.16 In the early arterial phase, fine radially distributed tumor vessels become conspicuous, which is then followed by a “capillary blush.” The main feeder characteristically displays extensive branching at the hilus but the fine tumoral neovasculature displays linear centrifugal course without further branching (Fig. 16-6). This group of radially branching fine vessels is called the “sunburst pattern.”17 Frequently, the center of the sunburst pattern may correspond to a point of hyperostosis or osteolysis on the adjacent bone.18 This is characteristic of meningiomas but can also be seen in hemangiopericytomas. A fairly even distribution of the contrast material within the tumor in the capillary phase creates the characteristic capillary blush. Some meningiomas exhibit a more homogeneous blush in which vessels cannot be identified at all, probably due to their small size.19 In contrast to more invasive tumors such as gliomas, the capillary blush in meningiomas is sharply demarcated. In meningiomas that have a vascular supply from independent vessels originating from the ECA and ICA, the capillary blush may develop in a piecemeal fashion. The periphery of the tumor, especially of the large meningiomas, may be supplied by pial vessels that contribute to the gradual dense opacification of the meningioma (Figs. 16-7 and 16-8).13 The tumor blush continues to persist into the late venous phase, which is also a characteristic finding of meningiomas (see Fig. 16-1). Despite an abundant arterial supply and a well developed capillary tree, large and prominent venous structures are usually absent in meningiomas. In some meningiomas, venous drainage into the deep Galenic venous system may be observed.
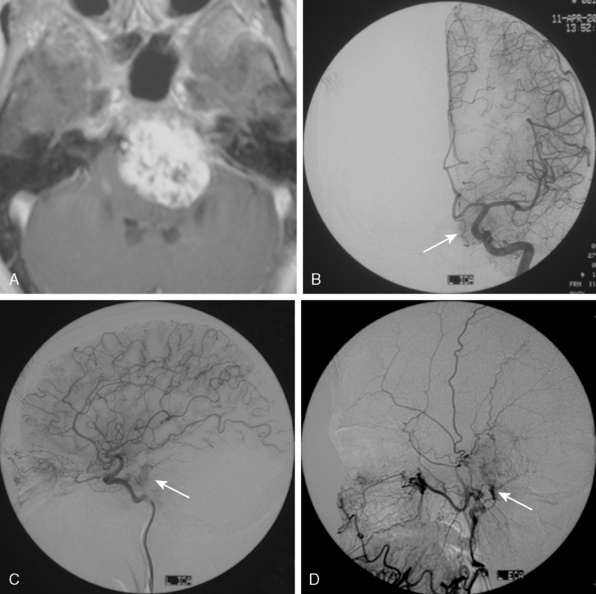
The dural vascular supply is more complicated at the skull base, and most skull-base meningiomas have multiple suppliers. Tumors of the anterior fossa such as falx and olfactory groove meningiomas may derive their blood supply from the anterior meningeal artery, which is a branch of the anterior ethmoidal and respectively of the ophthalmic artery.20 Angiographic examination of midline anterior fossa tumors requires injection of both internal maxillary arteries. The dural branches of the ICA can supply the meningiomas extending more posteriorly. For midline tumors bilateral carotid examination is imperative to show a possible contralateral supply (Fig. 16-9). Middle fossa meningiomas such as those of the greater and lesser wings of the sphenoid bone are supplied by both the ECA and the ICA. Anastomotic connections between dural and pial vessels should also be considered.9 Examination of the arteries of the middle and anterior fossa meningiomas should systematically explore the origin of the ophthalmic artery, which is very variable. Middle fossa meningiomas can be supplied by the recurrent meningeal artery, which is a branch of the ophthalmic artery and/or small branches of the cavernous carotid artery. Meningiomas of the sellar and parasellar regions may be supplied by branches of the ECA such as the artery of foramen rotundum, the vidian artery, the accessory and MMAs, and the ascending pharyngeal artery. The tentorium is supplied mainly by the ICA.21 For meningiomas of the posterior fossa and the foramen magnum, catheterization of the ECA and its branch the ascending pharyngeal artery that supply the posterior fossa with the posterior meningeal artery is appropriate.22,23 However, the posterior meningeal artery can arise also as a branch of the middle meningeal or vertebral arteries and supplies the dura up to the falx cerebelli. Meningeal branches of the occipital artery supply the lateral regions of the posterior fossa. The anterior and inferior aspects of the clivus as well as the foramen magnum may derive their blood supply through the anterior meningeal branch of the vertebral artery. For clival meningiomas, bilateral injection of the ICA may be necessary because of contributions from both cavernous ICA segments. Cerebellopontine angle meningiomas may be supplied by the dural branches of the MMAs but may also receive a contribution from the branches of the ascending pharyngeal and occipital arteries. Examination of this area may be improved by superselective catheterization of these arteries.
RELATION OF THE TUMOR TO NONTUMORAL VESSELS
Mass Effect
Special patterns of arterial displacement are characteristic of certain meningiomas. Olfactory groove meningiomas displace both A1 segments of the ACA upwards and A2 and A3 segments posteriorly (Fig. 16-10).20 Conversely, tuberculum sella meningiomas displace only the supracavernous segment of the ICA posteriorly (Fig. 16-11). Petroclival meningiomas do not usually cause any change in the contour or in the course of the ICA (Fig. 16-12), while cavernous sinus meningiomas displace the petrous and cavernous segments medially (Fig. 16-13).24 Sphenoid wing meningiomas elevate the M1 segment of the middle cerebral artery (MCA) and displace the supracavernous segment of the ICA medially (Fig. 16-14). In contrast, anterior clinoidal meningiomas generally have no effect on the course of the ICA (Fig. 16-15). Pineal region meningiomas can cause both upward and downward displacement of the internal cerebral veins and vein of Galen according to the tumor location in relation to the great vein of Galen (Fig. 16-16).25
Local Arterial Involvement
It is very well demonstrated that meningiomas can invade the adventitia of major vessels traveling through the tumor. Clinically, this information is extremely important as attempts at total resection of such meningiomas can result in intraoperative rupture or may require alternative strategies for the resection of meningioma at the involved segment.24,26 In most cases of arterial encasement, no changes are evident on angiographic examination. However; in a small subset of patients, narrowing or obliteration, pseudoaneurysm formation, a rough surface, or a beaded appearance may be observed in large arteries or their smaller branches. Such arterial involvement may be suspected from MRI and CT studies and the angiographic workup can be modified accordingly. In these cases, if a radical surgical procedure is planned, angiographic findings indicating involvement of the major arteries are sought, but also cross compression or balloon occlusion studies are performed to guide the surgical strategy. These functional studies are discussed in the text that follows.
For central skull-base meningiomas, such as in the sellar and parasellar regions, careful anteroposterior and lateral angiograms of the ICA should be obtained to examine the possibility of ICA encasement. Sphenoid wing meningiomas may invade or encase the MCA. Further, the displacement of the MCA cranially is a diagnostic feature for these tumors (Fig. 16-17). Both anterior cerebral arteries (ACAs) and their relationships to the tumor must be carefully evaluated for meningiomas of the anterior cranial fossa (Fig. 16-18). The evaluation of the patency of the anterior communicating artery via the compression technique is imperative also to avoid an inadvertent sacrifice of one of the A1 segments of ACAs during surgery. Encasement of the vertebral arteries is not uncommon in foramen magnum meningiomas (Fig. 16-19). In cases of focal narrowing of the artery that indicates adventitial invasion, the contralateral vertebral artery and the origin of the posterior ICA in relation to the tumor should be carefully evaluated. Checking the function of the posterior communicating arteries with carotid compression can be beneficial.
Dural Sinus Involvement by Meningiomas
Major indications for DSA are the demonstration of the dural sinuses and major veins next to a meningioma and to determine the patency or occlusion of these structures.27 Approximately one third of supratentorial meningiomas are located near the superior sagittal sinus (SSS); half of parasagittal meningiomas are located on both sides of the falx.28 Knowledge of this common spatial relationship deserves a meticulous angiographic study such as multiple oblique exposures of the SSS and the demonstration of the compensatory outflow pathways in case of sinus occlusion. The radiologic findings can demonstrate the progressive changes in dural sinus involvement such as: (1) a unilateral meningioma is attached to the sinus wall (Fig. 16-20); (2) the sinus wall is invaded and the contrast flow has become thinner (Fig. 16-21); (3) the meningioma has partially occupied the venous sinus, the contrast column of the sinus is irregular, and the blood flow is diminished (Figs. 16-22 and 16-23); (4) the meningioma totally occludes the sinus (Fig. 16-24), or (5) the sinus is embedded in the meningioma29,30 (Fig. 16-25). Venous sinus occlusions by meningiomas may result in development of new collateral venous drainage that should be demonstrated on preoperative studies to safeguard a surgical ligation (see Fig. 16-25).
The major angiographic features typical for meningiomas presented in this chapter are summarized in Table 16-1.
| Arterial phase characteristics |
CROSS COMPRESSION AND BALLOON OCCLUSION STUDIES
In meningiomas that present with encasement of major arteries such as the internal carotid or the vertebral arteries, the surgical procedure caries the risks of intraoperative arterial rupture or unintended intraoperative arterial ligation, or the surgical procedure may include a planned vascular bypass. In such cases, DSA can provide important information on the contralateral arterial circulation and the vascular reserve. It is crucial to demonstrate contralateral ICA and the vertebral artery with ipsilateral carotid artery compression in order to evaluate the collateral circulation of the circle of Willis (see Fig. 16-9). Balloon occlusion studies are more reliable than simple manual compression to evaluate cross-flow. To increase the diagnostic yield the balloon occlusion studies can be combined with perfusion studies such as single photon emission computed tomography (SPECT).31,32
Embolization
The optimal treatment for meningiomas is complete resection, when possible. Because meningiomas are usually quite vascular, which in turn can complicate tumor extirpation, preoperative embolization can contribute to complete tumor resection by diminishing operative time and intraoperative blood loss.4,33,34 In the literature, data on the results of embolization for the meningioma surgery is limited. Two retrospective studies showed a beneficial effect in terms of estimated blood loss at surgery.35,36 In another prospective study, embolized and nonembolized meningiomas treated with surgery were compared and the only difference found was the reduced intraoperative blood loss in completely devascularized meningiomas, whereas the other parameters did not differ significantly.37
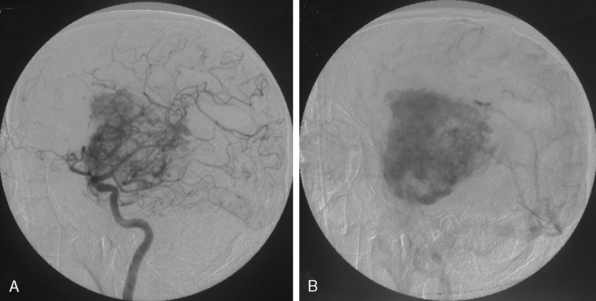
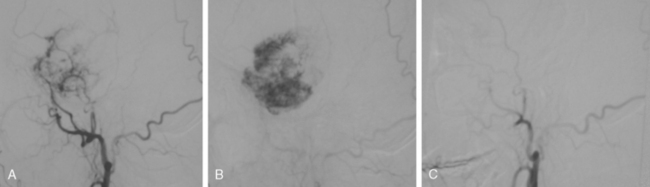
Embolization involves the devascularization of a tumor’s blood supply through the placement of an embolic agent via a microcatheter into the feeding arteries (Fig. 16-26).
To date a variety of embolic materials have been used, with polyvinyl alcohol (PVA) particles the most widely used one. Trisacryl gelatin microspheres (Embospheres, Guerbet Biomedical, Louvres, France) are new, commercially available, hydrophilic, nonabsorbable collagen-coated embolic agents.34 These particles are precisely calibrated, deformable, and tend not to aggregate, properties that enable this agent to easily penetrate distally within the vessel. Embolization with other materials, such as gelatin sponges, lyophilized dura, n-butyl cyanoacrylate, silastic spheres, fibrin glue preparation, and liquid material, has also been described.38–40 After the microcatheter is removed, postembolization angiography is performed to evaluate the angiographic completeness of embolization.
Preoperative embolization of the pial arteries is not usually practiced because of the risk of stroke.41 Palliative embolization has been conducted as an alternative to definitive resection in patients who are not candidates for surgery.42
The risks of meningioma embolization are small in carefully selected cases. Data on the procedure-related complications are limited and controversial. In a small series of patients, neurologic deficit was absent,43 or seen at rates of 12% to 16%.35–37,44 In another large series of unselected cases of meningiomas, the reported rate of overall neurologic adverse events was 6.5%, the rate of permanent deficits was 2.2%, and the mortality rate was 0.5%.45 Neurologic deficits have been reported after embolization as a result of improperly identified vascular territories, reflux of embolic agents, or tumor swelling and hemorrhage after embolization. There are some dangerous anastomotic pathways between the branches of the ECA and ICA.12 The distal internal maxillary artery, which has potential communication with the ICA; the neuromeningeal trunk of the ascending pharyngeal artery, which supplies the 9th, 10th, and 11th cranial nerves; the odontoid branch of the ascending pharyngeal artery, which has potential vertebral artery anastomosis; and the meningolacrimal branch of the MMA, which has a potential retinal supply, should be identified clearly to avoid potentially disastrous ischemic complications caused by inadvertent passage of embolizing particles through these dangerous anastomosis. Therefore, some authors generally do not recommend the use of ultrasmall particles that are smaller than 100 μm.45
In some cases, provocative testing may be practiced by injection of small-dose lidocaine through the microcatheter.46 The development of a temporary cranial nerve deficit increases the risk of embolization and may warrant catheter repositioning before embolization.
Other complications including tumoral47 and subarachnoid48 hemorrhage, scalp necrosis,49 retinal embolus,50 and iatrogenic carotid-cavernous fistula51 have been described in case reports. A cystic or necrotic component within a large meningioma should be considered a risk factor for postembolization hemorrhage.47 The relatively increased risk of hemorrhage is probably due to a higher incidence of abnormal fragile vessels occurring within these tumors.
Another important issue is the optimal time interval between the embolization process and the surgery, although general agreement is lacking, and the review of the literature shows a wide range from 1 to 2 days to 10 days.52,53 With the old absorbable embolic materials such as Gelfoam, the interval cannot be prolonged due to rapid recanalization.54 With the advent of new particles like PVA or Embospheres that cause permanent embolization, the interval can be as long as the effect of devascularization. The main final effect of embolization is the softening of the tumor that is caused by the process of coagulative necrosis and the degree of necrotic change tends to increase with time after ischemia. Kai and colleagues reported that the necrotic process does not progress further after 7 to 9 days, and prolonging the interval had no further beneficial effects.53
[1] Abe T., Matsomoto K., Hanakawa K., et al. Role of 3D-TOF magnetic resonance angiography for intracranial meningioma. J Clin Neurosci. 1998;5:476-497.
[2] Goldmann A., Kunz U., Bader C., Leibing U., Friedrich J.M., Oldenkott P. MR imaging and MR angiography in preoperative evaluation of intracranial meningiomas. Eur Radiol. 1994;4:538-544.
[3] Tsuchiya K., Hachiya J., Mizutani J., Yoshino A. Three-dimensional helical CT angiography of skull base meningiomas. Am J Neuroradiol. 1996;17:933-936.
[4] Gruber A., Killer M., Mazal P., et al. Preoperative embolization of intracranial meningiomas: a 17-year single center experience. Minim Invasive Neurosurg. 2000;43:18-29.
[5] Oka K., Go Y., Kimura H., Tomonaga M. Obstruction of the superior sagittal sinus caused by parasagittal meningiomas: the role of collateral venous pathways. J Neurosurg. 1994;81:520-524.
[6] Maxwell R.E., Chou S.N. Preoperative evaluation and management of meningiomas. In: Schmidek H.H., editor. Meningiomas and Their Surgical Management. Philadelphia: WB Saunders; 1991:109-117.
[7] Zimmerman R.D. MRI of intracranial meningiomas. In: Al-Mefty O., editor. Meningiomas. New York: Raven Press; 1991:209-223.
[8] Tanaka M., Imhof H.G., Schucknecht B., Kollias S., Yonekawa Y., Valavanis A. Correlation between the efferent venous drainage of the tumor and peritumoral edema in intracranial meningiomas: superselective angiographic analysis of 25 cases. J Neurosurg. 2006;104:382-388.
[9] Wilson G., Weidner W., Hanafee W. The demonstration and diagnosis of meningiomas by selective carotid angiography. AJR Am J Radiol. 1965;95:868-873.
[10] Bhatoe H.S., Singh P., Dutta V. Intraventricular meningiomas: a clinicopathological study and review of the literature. Neurosurg Focus. 2006;20:1-6.
[11] Taveras J.M. Neuroradiology, 3rd ed. Baltimore: Williams & Wilkins, 1996.
[12] Osborn A.G. Introduction to Cerebral Angiography. Philadelphia: Harper & Row, 1980.
[13] Osborne A.G. Meningiomas and other nonglial neoplasms. In: Osborn A.G., editor. Diagnostic Neuroradiology. St. Louis: Mosby Year Book; 1994:579-625.
[14] Logue V. Parasagittal meningiomas. Adv Techn Stand Neurosurg. 1975;2:171-198.
[15] Wilkins R.H. Parasagittal meningiomas. In: Al-Mefty O., editor. Meningiomas. New York: Raven Press; 1991:329-344.
[16] Taveras J.M., Wood E.H. Diagnostic Neuroradiology. Vol. II, 2nd ed. Baltimore: Williams & Wilkins, 1976.
[17] Hattori K., Miyachi S., Kobayashi N., et al. Contralateral meningeal artery supply of paramedian meningiomas. Surg Neurol. 2005;64:242-248.
[18] Krayenbühl H.A., Yaşargil M.G. Cerebral Angiography, 2nd ed. London: Butterworths, 1968.
[19] Jacobs J.M., Harnesberger H.R. Diagnostic angiography and meningiomas. In: Al-Mefty O., editor. Meningiomas. New York: Raven Press; 1991:225-241.
[20] Tsikoudas A., Martin-Hirsch D.P. Olfactory groove meningiomas. Clin Otolaryngol. 1999;24:507-509.
[21] Asari S., Maeshiro T., Tomita S., et al. Meningiomas arising from the falcotentorial junction. Clinical features, neuroimaging studies, and surgical treatment. J Neurosurg. 1995;82:726-738.
[22] Rhoton A.L.Jr. Meningiomas of the cerebellopontine angle and foramen magnum. Neurosurg Clin N Am. 1994;5:349-377.
[23] McDermott M.W., Wilson C.B. Meningiomas. In: Youmans J.R., editor. Neurological Surgery. 4th ed. Philadelphia: WB Saunders; 1996:2782-2825.
[24] Shaffrey M., Dolenc V., Lanzino G., et al. Invasion of the internal carotid artery by cavernous sinus meningiomas. Surg Neurol. 1999;52:167-171.
[25] Lozier A.P., Bruce J.N. Meningiomas of the velum interpositum: surgical considerations. Neurosurg Focus. 2003;15:1-9.
[26] Ishikawa M., Nishi S., Aoki T., et al. Predictability of internal carotid artery (ICA) dissectability in cases showing ICA involvement in parasellar meningioma. J Clin Neurosci. 2001;8(Suppl. 1):22-25.
[27] Sindou M.P., Alvernia J.E. Results of attempted radical tumor removal and venous repair in 100 consecutive meningiomas involving the major dural sinuses. J Neurosurg. 2006;105:514-525.
[28] Schmidek H.H. Meningiomas and Their Surgical Management. Philadelphia: WB Saunders Company, 1991.
[29] Bonnal J., Brotchi J. Surgery of the superior sagittal sinus in parasagittal meningiomas. J Neurosurg. 1978;48:935-945.
[30] Sindou M., Auque J. The intracranial venous system as a neurosurgeons perspective. Adv Tech Stand Neurosurg. 2000;26:131-216.
[31] Yamamoto Y., Nishiyama Y., Toyama Y., Satoh K., Irie K., Ohkawa M. Preliminary results of Tc-99m ECD SPECT to evaluate cerebral collateral circulation during balloon test occlusion. Clin Nucl Med. 2002;27(9):633-637.
[32] Witt J.P., Yonas H., Jungreis C. Cerebral blood flow response pattern during balloon test occlusion of the internal carotid artery. Am J Neuroradiol. 1994;15:847-856.
[33] Hieshima G.B., Everhart F.R., Mehringer C.M., et al. Preoperative embolization of meningiomas. Surg Neurol. 1980;14:119-127.
[34] Bendszus M., Klein R., Burger R., et al. Efficacy of trisacryl gelatin microspheres versus polyvinyl alcohol particles in the preoperative embolization of meningiomas. Am J Neuroradiol. 2000;21:255-261.
[35] Dean B., Flom R.A., Wallace R.C., et al. Efficacy of endovascular treatment of meningiomas: evaluation with matched samples. Am J Neuroradiol. 1993;15:1675-1680.
[36] Macpherson P. The value of pre-operative embolisation of meningioma estimated subjectively and objectively. Neuroradiology. 1991;33:334-337.
[37] Bendszus M., Rao G., Burger R., et al. Is there a benefit of preoperative meningioma embolization? Neurosurgery. 2000;47:1306-1312.
[38] Manelfe C., Lasjaunias P., Ruscalleda J. Preoperative embolization of intracranial meningiomas. Am J Neuroradiol. 1986;7:963-972.
[39] Teasdale E., Patterson J., McLellan D., Macpherson P. Subselective preoperative embolization for meningiomas. J Neurosurg. 1984;60:506-511.
[40] Richter H.-P., Schachenmayr W. Preoperative embolization of intracranial meningiomas. Neurosurgery. 1983;13:261-268.
[41] Dowd C.F., Halbach Van V., Higashida R.T. Meningiomas: the role of preoperative angiography and embolization. Neurosurg Focus. 2003;1:1-4.
[42] Koike T., Sasaki O., Tanaka R., et al. Long-term results in a case of meningioma treated by embolization alone—case report. Neurol Med Chir. 1990;30:173-177.
[43] Hieshima G.B., Everhart F.R., Mehringer C.M., et al. Preoperative embolization of meningiomas. Surg Neurol. 1980;14:119-127.
[44] Richter H.P., Schachenmayr W. Preoperative embolization of intracranial meningiomas. Neurosurgery. 1983;13:261-268.
[45] Bendszus M., Monoranu C.M., Schutz A., Nolte I., Vince G.H., Solymosi L. Neurologic complications after particle embolization of intracranial meningiomas. Am J Neuroradiol. 2005:1413-1419.
[46] Halbach V.V., Hieshima G.B., Higashida R.T., et al. Endovascular therapy of head and neck tumors. In: Viñuela F., Halbach V.V., Dion J.E., editors. Interventional Neuroradiology: Endovascular Therapy of the Central Nervous System. New York: Raven Press; 1992:17-28.
[47] Yu S.C.H., Boet R., Wong G.K.C., Lam W.W.M., Poon W.S. Postembolization hemorrhage of a large and necrotic meningioma. Am J Neuroradiol. 2004;25:506-508.
[48] Hayashi T., Shojima K., Utsunomiya H., et al. Subarachnoid hemorrhage after preoperative embolization of a cystic meningioma. Surg Neurol. 1987;27:295-300.
[49] Adler J.R., Upton J., Wallman J., et al. Management and prevention of necrosis of the scalp after embolization and surgery for meningioma. Surg Neurol. 1986;25:357-360.
[50] Turner T., Trobe J.D., Deveikis J.P. Sequential branch retinal artery occlusions following embolization of an intracranial meningioma. Arch Ophthalmol. 2002;120:857-860.
[51] Barr J.D., Mathis J.M., Horton J.A. Iatrogenic carotid-cavernous fistula occurring after embolization of a cavernous sinus meningioma. Am J Neuroradiol. 1995;16:483-485.
[52] Brismar J., Conqvist S. Therapeutic embolization in the external carotid artery region. Acta Radiol Diagn. 1978;19:715-731.
[53] Kai Y., Hamada J., Morioka M., Yano S., Todaka T., Ushio Y. Appropriate interval between embolization and surgery in patients with meningioma. Am J Neuroradiol. 2002;23:139-142.
[54] Djindjian R., Merland J.J., Rey A., Thurel J., Houdart R. Superselective arteriography of the external carotid artery. Importance of this new technic in neurological diagnosis and in embolization. Neuro Chirurg. 1973:165-171.