Advances in Anesthesia, Vol. 28, No. 1, ** **
ISSN: 0737-6146
doi: 10.1016/j.aan.2010.09.001
The Anesthetic Management of Adult Patients with Organ Transplants Undergoing Nontransplant Surgery
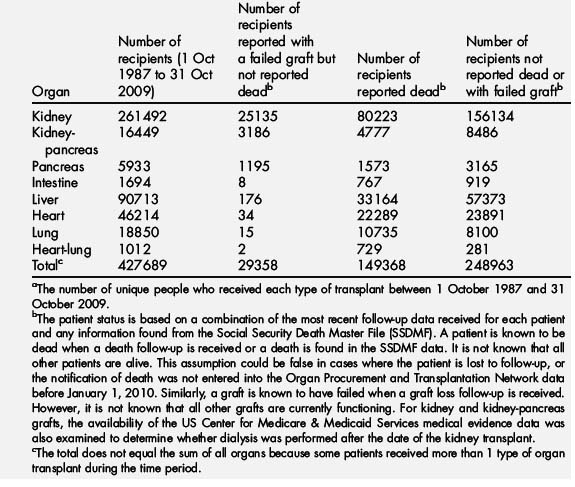
The United Network for Organ Sharing reports that in 2008 and 2009 there were more than 54,000 organs transplanted in the United States. Survival rates have continued to increase in the past several decades as surgical techniques, immunosuppressive therapy, and infection prophylaxis have improved. On October 31, 2009 there were nearly 280,000 surviving organ recipients who underwent transplantation between 1987 and 2009 (Table 1). Several organ types now have 1-year survival rates of 85% or greater, with some approaching 95%, and 3-year survival rates of 80% or better. Living donor kidney transplants, for example, have 1-year survival rates of 95% and 10-year survival rates greater than 75% [1]. As the number of people surviving organ transplant steadily increases, more of these patients are likely to present for nontransplant-related surgery, either elective or emergent, in centers that are not normally involved in transplant procedures. This population may be more likely to present for surgery than those without previous transplant for many reasons. Laparotomy for small bowel obstruction, hip arthroplasty given the increased risk of fracture and avascular necrosis as a result of chronic steroid use, lymph node excision and biopsy because of increased risk of lymphoproliferative disease, ureteral stent placement and removal and native nephrectomy in kidney transplant recipients, bronchoscopy in lung recipients, and biliary tract interventions in liver recipients are just a few of the increased surgical needs in this population. Incisional hernias rates are increased because of the effects of immunosuppressive drugs on wound healing and, abscess drainage because of increased risk of infection are additional problems requiring surgical intervention [2,3].
Table 1 United Network for Organ Sharing: patients transplanted in the United States,a 1 October 1987 to 31 October 2009 by organ received and most recent statusb
Many transplant recipients live relatively normal and productive lives, but often have limited physical reserves. A successful transplant abolishes the symptoms and replaces function of the failed organ, but often there are persistent abnormalities from the underlying or preexisting illness that may have caused the organ failure or chronic physiologic abnormalities resulting from the organ failure itself [4]. Kidney recipients whose renal failure was caused by diabetic nephropathy and pancreas recipients still have persistent complications of diabetes such as gastropathy and neuropathy. Heart recipients whose heart failure resulted from ischemic cardiomyopathy often have extracardiac vascular disease. Cardiovascular disease is also frequently present in patients with chronic kidney disease and those with previous kidney transplant [5,6]. In addition, although immunosuppressive drug use is essential in this population to prevent allograft rejection and protect its function, these drugs have many adverse effects and can lead to renal dysfunction, bone marrow suppression, increased risk of infection, lymphoproliferative disorders, adrenal insufficiency, and pharmacologic interactions. Graft function can deteriorate with time because of chronic rejection and vasculopathy, but at any time graft function can be compromised or suboptimal as a result of acute rejection.
General considerations for preanesthetic evaluation
When a patient with a previous transplant presents for nontransplant surgery, a comprehensive evaluation and survey by the anesthesiologist should include the following key factors: evaluation of the graft function, health and function of other organ systems or the presence of concomitant diseases, presence of infection, and performance or functional status, during the preanesthetic evaluation. Adherence to the fundamental principles of preoperative evaluation along with a high level of vigilance is required [4,7,8]. Information and medical history should be gathered from the medical record, interview with the patient, next of kin or guardian. If medical information is unavailable locally, attempts should be made to contact the transplant center for pertinent history, especially with regard to previous anesthetics. Other useful information from the transplant center includes their most recent evaluations and recent data on graft function and general health of the patient. Close communication with the transplant team may be the single most important step in preparing the patient for surgery and developing a perioperative anesthetic plan [7].
Preoperative laboratory testing in this population should include evaluation of renal function with electrolytes and creatinine, as well as a complete blood count given the adverse effects immune-suppressant medications may have on these organ systems. Serum glucose should also be considered in those patients on corticosteroids or with diabetes mellitus. If neuraxial anesthesia is planned, consideration should be given to obtaining coagulation studies. Focused and functional testing of the transplanted organ is an important part of the preanesthetic evaluation to assess graft function and exclude active rejection. Table 2 suggests tests that may be required to optimize the safe delivery of anesthesia, although preoperative testing need not be limited to these guidelines. Each preoperative evaluation and testing should be considered individually based on the target organ system(s) to be evaluated, the patient’s medical history, and the inherent risks of the upcoming surgical procedure.
| Test target | Essential tests | Consider also |
|---|---|---|
| Blood | Total cell count Hemoglobin |
Hematocrit |
| Kidney | Creatinine Urine analysis |
Urea Creatinine clearance |
| Blood electrolytes | Na+, K+, Mg2+, Ca2+ | |
| Liver | Prothrombin time Partial thromboplastin time Bilirubins Aminotransferases Alkaline phosphatase |
Full coagulation status Ammonia Albumin Prealbumin Cholesterol Lactate dehydrogenase Galactose elimination capacity |
| Pancreas | Amylase | Lipase |
| Lung | Radiography Spirometry for lung and marrow transplant patients |
Spirometry Sputum microbiology Blood gas analysis |
| Heart | Electrocardiography | Echocardiography Coronary angiography |
| Drugs | Cyclosporine or tacrolimus concentration, if applicable | |
| Infections | C-reactive protein Targeted samples |
|
| Other | Blood glucose Blood pressure Pulse Temperature Respiratory rate |
Data from Toivonen HJ. Anaesthesia for patients with a transplanted organ. Acta Anaesthiol Scand 2000;44:819.
Cardiovascular disease is a major cause of mortality and morbidity among organ transplant recipients, especially in those with chronic kidney disease or previous heart transplant, making the risk of a perioperative cardiovascular event a legitimate concern [6,7,9]. After kidney transplantation, a progressive increase in the incidence of ischemic cardiovascular, cerebrovascular, and peripheral vascular events has been documented in epidemiologic studies [5]. Many transplant recipients have undergone complete cardiac testing and in some cases, interventions, before their transplant surgery. Records of the testing and interventions can be easily obtained from the transplant center to be used for comparison and consideration before the upcoming surgery. The question that remains is how long a time period may be allowed to pass before additional cardiac testing should be performed before an upcoming elective surgery. There is, unfortunately, no clear or easy single answer or guideline. Each evaluation should proceed on an individual basis with thorough documentation of the patient’s exercise capacity and functional status along with an electrocardiogram (ECG), taking into consideration any changes, as well as the inherent risk of the surgery itself and the patient’s known cardiovascular history, keeping in mind that many of these patients may have asymptomatic coronary disease as a result of diabetes or the transplant itself. It may be prudent to discuss the upcoming surgery, need for testing, and perioperative optimization, such as initiation of beta-blocker therapy, with the patient’s transplant team or cardiologist, especially if there is documented cardiovascular disease or previous cardiac intervention.
Posttransplantation diabetes mellitus (PTDM) is a common metabolic consequence of the agents of immunosuppressive therapy. PTDM is defined as sustained hyperglycemia that meets the diagnostic criteria of the American Diabetic Association (ADA) in any posttransplant patient who was not previously diabetic [10,11]. PTDM is generally assigned to the classification of type 2 diabetes because its pathophysiology is a combination of insulin resistance and insulin secretion defects. Risk factors for PTDM include age, nonwhite ethnicity, increased body mass index (BMI, calculated as weight in kilograms divided by the square of height in meters), chronic hepatitis C infection, pretransplant glucose intolerance, and use of glucocorticoids and calcineurin inhibitors. The incidence of PTDM is reported to be as high as 25% and possibly higher in patients with hepatitis C [4,11]. Unfortunately, PTDM has a negative effect on graft survival. Preexisting diabetes has implications for perioperative complications and morbidity, especially increased infection risk and poor wound healing. It is imperative to institute a plan of glycemic control before surgery with close attention to intraoperative and postoperative glucose management. Hyperglycemia suppresses various aspects of immune function, causes endothelial dysfunction and altered vascular reactivity, increases circulating inflammatory mediators, and is associated with a procoagulant state. If hyperglycemia is identified during the preoperative evaluation, the patient’s primary care provider may be contacted to assist in the effort to achieve glycemic control before elective surgery. It is not within the scope of this document to debate the literature and the issues surrounding intensive glycemic control. Most of the information gathered is taken from studies of glycemic control in the intensive care unit and has been extrapolated to the perioperative period. The actual level of hyperglycemia that is predictive of adverse outcome is debated, but perioperative serum glucose levels greater than 200 mg/dL seem to be consistently associated with adverse outcomes including stroke, myocardial infarction, poor wound healing, infection, and death [12,13]. Maintaining perioperative glucose between 120 and 180 mg/dL is a conservative approach that might avoid the perils of intensive glucose control yet control hyperglycemia. If the patient has a serum glucose level of greater than 300 mg/dL documented on 2 or more consecutive checks, consideration should be given to postponing elective surgery until adequate glycemia is achieved.
General considerations of anesthetic management
There is no ideal or generic anesthetic plan that can be used for all transplant recipients undergoing nontransplant surgery. There are no prospective studies comparing anesthesia techniques, but most anesthetics have been used with success including general (inhalational, balanced, and total intravenous), neuraxial, regional, and monitored anesthesia. A successful anesthetic plan requires a clear understanding of the medical and pharmacologic problems that accompany this population, the function and physiology of the allograft, functionality and general health of the patient, the underlying surgical condition, and proposed surgical procedure [4,14].
Monitoring
As a general rule, special equipment is not needed and anesthesia should be performed using standard American Society of Anesthesiology’s monitoring guidelines. The decision to use invasive hemodynamic monitors, placement of central venous access, pulmonary artery catheters or other procedures such as transesophageal echocardiography should be made on a case by case basis. This decision should be guided by consideration of the patient’s comorbidities, hemodynamic stability, the expertise of the anesthesiologist in placing the invasive device and interpreting the data, the inherent risk of the surgery, and the overall risk to benefit ratio of the proposed monitor [15]. It is important to consider that central venous access may be difficult as many of these patients have had long-standing illnesses with the need for central venous catheters for parenteral nutrition, hemodialysis, esophageal variceal hemorrhage, sepsis, and other clinical problems that required central venous access. These may have been complicated by thrombosis, stenosis, and infection making reaccessing these veins difficult or impossible [14]. Meticulous hygiene practice and aseptic technique are of utmost importance to minimize exposure to infectious organisms and bacteremia when attempting any invasive procedures in this population [4,8,16].
Airway Management
Airway management of transplant patients may pose a concern for several reasons. Many patients may have preexisting diabetes mellitus before transplant or acquire diabetes after transplant (PTDM). Diabetic patients can develop limitations in joint mobility caused by glycosylation of the connective tissue within their joints. Several retrospective and prospective evaluations performed at transplant institutions in patients with diabetes mellitus undergoing kidney and/or pancreas transplantation report increased rates of difficult laryngoscopy, including a retrospective analysis from our institution [17,19]. The airway management plan should be formulated after careful airway examination and review of previous anesthetic records. If recent records are not available locally, they may be obtained from the transplant center keeping in mind that if extended time has elapsed since the last anesthetic, the ongoing effects of diabetes on the mandibular and atlanto-occipital joints may result in a difficult intubation not previously reported. Graft-versus-host disease can also create limited joint mobility creating a similar scenario. This population is also at increased risk for lymphoproliferative disorders secondary to immune-suppressant drugs, and lymphoproliferative growth may compromise any part of the airway or mediastinum and cause life-threatening airway obstruction during sedation and anesthesia. Aspiration risk may be increased in transplanted patients as a result of delayed gastric emptying and gastropathy [4,8]. These potential problems should all be taken into consideration when constructing the anesthetic plan for airway management.
Antibiotic Prophylaxis
Antibiotic prophylaxis has not been studied extensively in transplant recipients undergoing nontransplant surgery. Although transplant patients are considered at-risk hosts for infection because of their immune status, there is no evidence to suggest different bacteriology of surgical site infections than for the general population. However, the use of prophylaxis even for clean cases in this higher risk population is advocated and has some supporting evidence [20,21]. It is recommended that the guidelines for surgical antibiotic prophylaxis from the National Surgical Infection Project be followed and should be given before incision.(Table 3). Antimicrobial coverage need not be expanded to include atypical or opportunistic organisms as long as active infection with such an organism is not present or suspected [7,21,22].
Table 3 Antimicrobial prophylaxis for selected surgical procedures
| Operation | Recommended antibiotic prophylaxis | Comments |
|---|---|---|
| Cardiothoracic surgery | Cefazolin, cefuroxime, or cefamandole. If patient has a β-lactam allergy: vancomycin or clindamycin | Most of the guidelines agree that prophylaxis for cardiac surgery should be administered for >24 h after surgery. The ASHP suggests continuation of prophylaxis for cardiothoracic surgery up to 72 h; however, its authors suggest that prophylaxis for <24 h may be appropriate. Cefamandole is not available in the United States |
| Vascular surgery | Cefazolin or cefuroxime. If patient has a β-lactam allergy: vancomycin with or without gentamycin, or clindamycin | |
| Colon surgery | Oral: neomycin plus erythromycin base, or neomycin plus metronidazole. Parenteral: cefoxitin or cefotetan, or cefazolin plus metronidazole | Currently, none of the guidelines address antimicrobial prophylaxis for those patients with β-lactam allergy. Cefmetazole is not available in the United States. Although a recent study indicates that the combination of oral prophylaxis with parenteral antibiotics may result in lower wound infection rates, this is not specified in any of the published guidelines |
| Hip or knee arthroplasty | Cefazolin or cefuroxime. If the patient has a β-lactam allergy: vancomycin or clindamycin | Although not addressed in any of the published guidelines, the workgroup recommends that prophylactic antimicrobial be completely infused before inflation of the tourniquet. Cefuroxime is recommended as a choice for patients undergoing total hip arthroplasty |
| Vaginal or abdominal hysterectomy | Cefazolin, cefotetan, cefoxitin, or cefuroxime | Metronidazole monotherapy is recommended in the ACOG Practice Bulletin as an alternative to cephalosporin prophylaxis for patients undergoing hysterectomy. Trovafloxin, although still available in the United States, is recommended only for serious infections |
Abbreviations: ACOG, American College of Obstetricians and Gynecologists; ASHP, American Society of Health-System Pharmacists.
Data from Bratzler DW, Houck PM. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis 2004;38:1706–15.
Intravenous Anesthetics
The selection and administration of intravenous anesthetics should be guided by the patient’s hemodynamic status, the drug’s cardiovascular effects and pharmacokinetic properties, especially the biotransformation and elimination profiles of the drug. Drugs that undergo primarily hepatic metabolism, such as barbiturates, should be dose adjusted if administered to avoid prolonged effects in patients with hepatic insufficiency. Care should be taken when using barbiturates as cardiac output and mean arterial pressures can decrease precipitously as a result of venous pooling and loss of cardiac preload. Propofol is extensively metabolized by the liver to inactive glucuronic acid metabolites that are excreted by the kidneys. Despite the known mechanisms of biotransformation, there seems to be no need for dose adjustments in patients with hepatic or renal failure indicating an extrahepatic route of elimination as well [23]. Care should be used in patients with cardiovascular compromise as propofol can worsen cardiac contractility, compromise cardiac preload, cause bradycardia, and lower systemic vascular resistance culminating in diminished cardiac output and mean arterial pressure. Etomidate does not have the cardiac depressant effect of barbiturates and propofol. Etomidate is metabolized rapidly by hydrolysis within the liver and by plasma esterases, and also does not require dosage adjustment in renal or hepatic disease. One unique characteristic of etomidate is its ability to inhibit the 11-β-hydroxylase, an enzyme necessary for the synthesis of cortisol, for 5 to 8 hours after administration. The clinical significance in patients who already have adrenal suppression as a result of exogenous steroid use is unclear, but heightened attention should be paid to the need for perioperative stress-dose corticosteroids [23]. Ketamine is metabolized via the hepatic cytochrome P-450 system to norketamine, which has approximately one-third of the clinical activity of its parent and therefore the clinical effects of ketamine are prolonged in the presence of hepatic insufficiency. The metabolites of norketamine are excreted by the kidneys. The usual cardiac stimulating effects caused by central stimulation of the sympathetic system are not present in the denervated heart, but ketamine can still increase systemic vascular tone. Ketamine has neuroexcitatory effects and is known to cause myoclonic activity, although its ability to actually cause seizures is debated [23]. However, there are case reports of seizures after its administration in patients who have previously had liver transplant and in the presence of cyclosporine, an immunosuppressant drug with the potential for neurotoxicity [24]. Table 4 is an abbreviated list of drugs commonly used in the perioperative period that have active metabolites that require renal excretion and accumulate in patients with renal insufficiency.
Table 4 Drugs with renally excreted active metabolites and their actions
| Morphine | Morphine 6-glucuronide | Antianalgesic |
| Morphine 3-glucuronide | Potent sedative-analgesic | |
| Normorphine | Neuroexcitatory and seizures | |
| Meperidine | Normeperidine | Neuroexcitatory and seizures |
| Diazepam | Oxazepam | Sedative |
| Midazolam | 1-Hydroxymidazolam | Sedative |
| Vecuronium | Desacetyl-vecuronium | Neuromuscular blockade |
| Ketamine | Norketamine | Neuroexcitatory and psychomimmetic actions |
Data from Littlewood K. The immunocompromised adult and surgery. Best Pract Res Clin Anaesth 2008;22(3):585–609.
Inhalational Anesthetics
All inhaled anesthetics have been used in transplanted patients with success. Among the inhaled anesthetics currently used, only halothane requires a truly cautionary note given its potential for hepatotoxicity and direct cardiac depressant effects. Of the most commonly used volatile anesthetics in the United States today, isoflurane, desflurane, and sevoflurane, there does not seem to be a significant clinical advantage or disadvantage of one over the others. The choice of inhaled anesthetic can be dictated by the anesthesiologist’s preference, experiences, and comfort with the anesthetic [25]. The decision whether or not to use a volatile anesthetic need not be imposed by the presence of a transplanted organ, but should be guided by other patient factors, such as hemodynamic stability and history or risk of malignant hyperthermia. The theoretic risk of nephrotoxicity caused by the liberation of free fluoride and Compound A following sevoflurane metabolism does not seem to be a true clinical concern outside of laboratory animals [4,25]. The decision to use nitrous oxide (N2O) as part of the anesthetic should be considered with caution. Whether or not to use N2O as part of the anesthetic should be based on clinical findings of the patient and type of surgical procedure, such as the potential for postoperative nausea and for expanding air-filled cavities such as pneumothorax, intestinal obstruction, and tympanic membrane grafts. It is probably prudent to avoid prolonged use of N2O because of the potential risk of bone marrow suppression and the potential for altered immunologic response (impaired chemotaxis and motility of polymorphonuclear leukocytes) [25]. Although these effects were seen in laboratory animals receiving N2O for more than 24 hours, they may be potentiated in immune-compromised patients. N2O can also worsen preexisting pulmonary hypertension, which may be present in patients who have undergone lung, heart, or liver transplant, by increasing pulmonary vascular resistance and potentiating hypoxemia. N2O is best avoided in patients who have known or suspected pulmonary hypertension. Although there is little data on the use of N2O in the transplanted population, the brief use of N2O during induction or emergence is probably acceptable and without clinical consequence but may be best avoided if there are alternatives [25,26].
Neuromuscular Blockade
The decision to use neuromuscular blockade should be based on the type of surgery and actual need for muscle relaxation during the procedure or the need to optimize intubating conditions. The choice of specific neuromuscular blocking agent to be used should be dictated by length of surgery, underlying medical illnesses such as myasthenia or other neuromuscular disorders, history of malignant hyperthermia, and the functional state of the patient’s kidney and liver. Of the nondepolarizing agents, the metabolism and clearance of mivacurium, a short-acting agent, and cisatracurium and atracurium, intermediate-acting agents, are independent of kidney and liver function (Table 5). In the presence of a normally functioning kidney or liver graft, other nondepolarizing agents, such as vecuronium, rocuronium, and pancuronium, exhibit normal clinical activity, but can have prolonged effects in the face of hepatic or renal insufficiency and require dose adjustments and close neuromuscular monitoring and evidence of full reversal before extubation [27]. Some immune-suppressant drugs can have effects on neuromuscular blockade. Azathioprine can increase the dose required to achieve and maintain muscle relaxation and cyclosporine can prolong the action of the neuromuscular blocking agents [28]. Succinylcholine, the only depolarizing agent available in the United States currently, is used frequently in this population given the need for rapid sequence intubation and rapid airway control. There are no contraindications in patients who have undergone organ transplant with the possible exception of previous cardiac transplant, in which its actions can be complex. Otherwise, succinylcholine should be avoided only if there are other clinical reasons, such as hyperkalemia, muscular dystrophy, or history of malignant hyperthermia [27,28].
Table 5 Drugs not solely dependent on renal and/or hepatic elimination
| Drug | Metabolism |
|---|---|
| Succinylcholine | Pseudocholinesterase |
| Propofol | Extrahepatic and hepatic metabolism |
| Esmolol | Red cell esterase |
| Remifentanil | Nonspecific esterases |
| Cisatracurium | Hofmann elimination |
| Mivacurium | Pseudocholinesterase |
| Atracurium | Non specific plasma esterase |
| Etomidate | Plasma esterases and hepatic metabolism |
Regional and Neuraxial Anesthesia
There are few contraindications to performing a regional or neuraxial anesthetic in previously transplanted patients. The consideration of spinal or epidural anesthesia is appropriate in this population as long as there is no increased risk for bleeding complications. There may be several advantages to choosing a neuraxial or regional technique in this population. Superior analgesia over systemic opioids, especially in patients who may have narcotics tolerance as a result of long-term opioid use, reduced pulmonary complications, decreased incidence of graft occlusion, and improved joint mobility are just a few of the benefits of regional and neuraxial anesthesia [29]. Clinically relevant doses of bupivicaine and ropiviciane, which are commonly used local anesthetics for neuraxial anesthesia, do not seem to result in toxic levels or increased risk of toxic effects in renal and liver transplant recipients [8,14]. However, it is important to be prepared for the risk of hypotension because of preexisting autonomic neuropathy and cardiac denervation in this population when performing a neuraxial anesthetic [9,29]. Concurrent hemodynamic monitoring is imperative during the procedure. Direct and indirect-acting adrenergic agonists should be readily available along with emergency airway supplies. Cautious correction of hypovolemia before epidural or spinal anesthesia may help to attenuate the hypotension.
Although the risk of epidural abscess complicating epidural anesthesia is low (about 1 in 2000 or less), there seems to be a very slight increase of the incidence in immunocompromised patients in many epidemiologic series. Staphylococcus aureus is isolated in approximately two-thirds of the epidural abscesses indicating infection from normal skin flora. Meningitis after spinal anesthesia seemingly does not have a greater disposition for immunocompromised hosts compared with healthy patients. When meningitis occurs after spinal anesthesia, the organism cultured is usually an oropharyngeal bacteria implying iatrogenic infection from the proceduralist [29,30]. Therefore, if a neuraxial block is included in the anesthetic plan, it is imperative that strict aseptic technique is practiced and a mask should be worn. Although the risk of infectious complications is very low, it is important to be highly vigilant when monitoring these patients after a neuraxial anesthetic as the attenuated inflammatory response may diminish the typical signs and symptoms of epidural abscess or meningitis [29].
There is limited information available on the incidence of infectious complications after peripheral nerve block procedures in immunocompromised patients, other than a few case reports and series. Overall, the incidence of infectious complications seems to be extremely low, even in immune-suppressed patients. When they do occur, the organisms cultured are similar to those found in infections after neuraxial anesthesia, Staphylococcus aureus and mouth organisms, indicating skin or mouth flora from the anesthesiologist or patient as the source [31]. Again, aseptic technique and a mask should be considered essential when performing these procedures.
There may be other, albeit less well understood benefits of using a neuraxial or regional anesthetic technique in this population. There is consistent evidence that surgical stress suppresses both cellular and humoral immune function for several days after surgery. This effect may be exaggerated or prolonged in patients with preexisting immune dysfunction. General anesthetics alone do not diminish the surgical stress response [20,29]. Neuraxial anesthesia and postoperative analgesia are associated with some preservation of cell-mediated immunity and attenuated inflammatory response. Local anesthetics themselves lessen the inflammatory response to stress and injury [20,32]. The risk to benefit ratio of neuraxial anesthesia or regional block may, therefore, be altered in previously transplanted patients. The decision to perform a regional or neuraxial anesthetic technique in a previously transplanted patient must be made on an individual basis. Anesthetic alternatives, the risks of the technique that may be increased, balanced with the potential for greater benefit should all be carefully considered when constructing the anesthetic plan in this population.
Postoperative Pain Management
In addition to the use of neuraxial anesthesia and peripheral nerve blocks, opiates are also reasonable options for intraoperative and postoperative analgesia, keeping in mind the patient’s renal and hepatic function. Delayed clearance of opioids can result in prolonged sedation in patients with underlying hepatic or renal insufficiency. Several of the narcotics have active metabolites that are renally excreted and can accumulate, such as morphine 3-glucuronide, which is 40 times as potent as morphine, and morphine 6-glucuoronide. Normeperidine, a metabolite of meperidine, is neuroexcitatory and has the potential to cause seizures as a result of delayed clearance in patients with renal insufficiency [9,33]. Remifentanil, which is cleared by plasma ester hydrolysis, is not affected by hepatic or renal function and may be an acceptable choice of opioid in this population (see Table 5) [33]. In the presence of a normally functioning kidney or liver graft, these drugs are metabolized and eliminated in a normal fashion and in usual clinical doses there should be no prolonged effects. Nonsteroidal antiinflammatory drugs are best avoided or used cautiously in this population of patients given their potential for hepatotoxicity and nephrotoxicity [4,8].
Perioperative management of immunosuppressive regimen
Almost all survivors of organ transplant are immunocompromised, whether by the antirejection drugs or underlying illness such as an autoimmune disease or diabetes mellitus that caused their organ failure or both. Their immune competence can be further altered by a period of acute illness, the stress of surgery, and disruption of their immunosuppressive regimen by inexperienced providers. This instability can readily result in acute rejection, acute infection, or both [16]. The first step in perioperative planning requires a call to the transplant center to discuss the perioperative management of the patient’s immune-suppressant drug regimen. In all instances, a transplant coordinator or other member of the transplant team will assist in devising the perioperative management plan for the immune-suppressant regimen.
It is helpful to have a basic understanding of the drugs most commonly used and their side-effect profile and drug interactions. Standard maintenance immunosuppression regimens usually consist of a combination of 2 or 3 drugs from the following classes: corticosteroids, calcineurin inhibitors, antiproliferatives, and inhibitors of the protein kinase mTOR. The number of drugs used and variations of the regimen are based on the recipient’s risk of rejection, immunogenicity of the allograft (liver being the least allogeneic), and adverse reactions or toxicities that develop while on the drugs [20,28]. Corticosteroids are a key component of most maintenance regimens and are associated with numerous well-known adverse effects including hypertension, central obesity, hyperlipidemia, diabetes mellitus, and osteoporosis. Adrenal suppression occurs as the result of chronic use of exogenous corticosteroids and can manifest as overt adrenal insufficiency during times of stress, illness, surgery, or abrupt withdrawal. The decision to give stress-dose steroid prophylaxis is controversial. Despite the lack of data, most investigators recommend a preoperative augmentation of corticosteroids and in transplant recipients, a postoperative increase in the corticosteroid dose, with a rapid return to baseline dose based on the extent or stress of the surgery, time to recovery, and ability to take oral medications. In the transplant recipient, the prevention of postoperative allograft rejection is just as crucial as prevention of intraoperative adrenal crisis [7,21,28]. At our institution, dexamethasone 10 mg intravenously (IV) or hydrocortisone 50 to 100 mg IV is given at the time of surgery, followed by dexamethasone 3 mg IV every 12 hours for 2 or 3 days, or until the patient can resume their normal oral immunosuppressive regimen.
Calcineurin inhibitors have been an integral part of antirejection treatment since the 1980s. Tacrolimus is available in oral and intravenous preparations. Tacrolimus has replaced the use of cyclosporine in many instances. If the prospective surgical patient’s regimen includes tacrolimus and the recommendation from the transplant center includes a course of IV tacrolimus, it is wise to communicate with your local pharmacy to determine if this drug is readily available. Monitoring of serum levels of tacrolimus is also required because of the narrow therapeutic index. It is also suggested to determine the turnaround time for this information as this drug level may not be immediately available at your institution, which may also pose a challenge in the care of these patients. The most common adverse effect of calcineurin inhibitors is nephrotoxicity. Other adverse effects include neurotoxicity and diabetes mellitus [28].
Azathioprine was the first immunosuppressive agent to be used with clinical success and is an inhibitor of purine nucleotide synthesis or an antiproliferative. It has been widely replaced by mycophenylate mofetil (MMF). The major side effects of MMF are gastrointestinal upset, leukopenia, anemia, and increased incidence of invasive cytomegalovirus infections, especially the gastrointestinal tract. MMF does not require therapeutic drug monitoring [20,28].
Sirolimus was introduced in 2000 and complexes with the protein kinase mTOR causing an arrest in a certain phase of the cell cycle. Sirolimus is effective when used in combination with a calcineurin inhibitor or antiproliferative drug. When used in conjunction with a calcineurin inhibitor it allows lower doses of the calcineurin inhibitor to be used, decreasing the risk of nephrotoxicity. Its use is limited by the development of hepatic artery thrombosis immediately after liver transplant, and thrombotic microangiopathy when used with cyclosporine concomitantly in kidney and kidney-pancreas recipients. The most common side effects of sirolimus include thrombocytopenia, anemia, leukopenia, diarrhea, and mouth ulcers. It is also associated with wound complications. The most serious and potentially fatal complication is interstitial alveolar pneumonitis [20,28].
Organ-specific considerations for anesthetic management of the transplanted patient
Thoracic Organs: Heart and Lung
Anesthesia for patients after heart transplant
According to the International Society for Heart and Lung Transplantation (ISHLT), more than 80,000 heart transplants have been performed since the first in 1967. In the ISHLT’s 25th Official Adult Heart Transplant Report, the total number of heart transplants performed annually is estimated at 5000 worldwide. This report highlights several interesting trends in heart transplantation. First, the primary indication for transplantation has shifted from a balance between both ischemic and nonischemic cardiomyopathy to most patients being transplanted for nonischemic cardiomyopathy (50%). Ischemic cardiomyopathy now accounts for only 34% of transplantations. Other indications include adult congenital heart disease (3%), retransplantation (2%), and valvular heart disease (2%). Second, the average age of transplant recipients is increasing. Currently, 25% of all heart transplant recipients are age 60 years or more. Third, survival of transplant recipients continues to improve. The projected half-life for the most recent cohort of recipients is approximately 11 years; recipients transplanted between 1982 and 1991 had a half-life of 8.8 years. Fourth, morbidity data show the incidence of hyperlipidemia, renal insufficiency, and diabetes in this patient population continues to increase. Fifth, mortality data highlight the difficulty in striking a balance between inadequate and excessive immunosuppression. Cardiac allograft vasculopathy (CAV), believed to be an immune-mediated process, and late graft failure accounted for 33% of deaths in patients surviving beyond 5 years. Malignancy (23%) and infection (11%), both unfortunate consequences of immunosuppression, continue to have a significant effect on morbidity in long-term survivors [34].
As the number of heart transplant recipients increases, so does their average age, average life expectancy, and number of significant comorbidities. Anesthesiologists can expect to see them with increasing frequency in the operating room. A single-center study from the University of Toronto followed 86 heart transplant patients for 4 years and found that 18 of them returned to the operating room for a total of 32 noncardiac operations requiring anesthesia. Surgical cases included 9 cataract surgeries; 3 pacemaker insertions and laparotomies; 2 central line insertions, dental extractions, cholecystectomies, and inguinal herniorrhaphies; and 1 scleral buckle, brain tumor biopsy, thrombectomy, osteotomy, ileostomy, bronchoscopy, lung resection, and transurethral resection of prostate [35].
Surgical technique
This technique is associated with significant morphologic changes of the right atrium and subsequent changes in tricuspid annular morphology. This results in significant tricuspid regurgitation (TR) in a high percentage of posttransplant patients. Unfortunately, tricuspid regurgitation is associated with decreased long-term survival in this patient population. As a result, several modifications to the classic technique have been proposed including the addition of a tricuspid annular ring or replacement of the tricuspid valve at the time of surgery. More recently, interest has shifted to a technique requiring bicaval anastomoses rather than the anastomosis of a right atrial cuff to the donor heart. Investigations of this technique show a significant decrease in the incidence of TR (31% vs 70%), no stenosis at the level of the venous anastomoses, and fewer atrial arrhythmias (15% vs 55%). This does not lead to significant increase in CPB time or donor organ ischemic time [36].
Immunosuppression
The ISHLT’s 25th Official Adult Heart Transplant Report follows trends in posttransplant immunosuppression. These patients receive a course of induction immunosuppression followed by a separate regimen of maintenance immunosuppression. Maintenance therapy typically involves 2 immunosuppressive drugs with or without the addition of a corticosteroid (prednisone). The ISHLT reports there is no standardized regimen for immunosuppressive therapy for patients after heart transplantation. The most commonly used therapy is the combination of tacrolimus and mycophenolate mofetil (MMF) with or without corticosteroids (46%); 30% of patients were on the combination of cyclosporine and MMF with or without corticosteroids. Other common immunosuppressive drugs used in maintenance therapy include rapamycin and azathioprine [34].
Cyclosporine is a highly nephrotoxic drug. It can also cause hypertension, hepatotoxicity, tremors, and seizures. The gingival hyperplasia and gastric atony, as well as the hyperkalemia and hypomagnesemia caused by cyclosporine can have obvious anesthetic implications. Cyclosporine has been reported to dramatically increase the duration of action of vecuronium and pancuronium [8]. It is available as an intravenous drug for patients unable to take the parenteral form. Cyclosporine blood levels should be closely monitored in the perioperative period.
Azathioprine’s most significant side effects are myelosuppression and hepatotoxicity. Laboratory investigations suggest a slight antagonistic effect with nondepolarizing neuromuscular blockers, but this has been deemed clinically insignificant [37]. Tacrolimus is both nephrotoxic and neurotoxic. It has also been shown to cause hypertension, hyperlipidemia and hyperglycemia. MMF causes leukopenia and a variety of gastrointestinal symptoms. Rapamycin is known to cause myelosuppression and hyperlipidemia.
Posttransplant physiology
There are several, notable physiologic differences between a native heart and a transplanted heart. The most remarkable difference, and arguably that with the most anesthetic implications, is denervation. Efferent denervation ablates the resting parasympathetic tone responsible for maintaining baseline heart rate. Because of this, transplanted patients generally have an increased baseline heart rate of 90 to 100 beats per minute [38]. The loss of direct sympathetic innervation means that the cardiac response to physiologic stressors (exercise, hypovolemia, vasodilatation, pain, light anesthesia) is mediated by circulating catecholamines and, as a result, tends to occur much less quickly. Predominantly parasympathetic responses (visceral traction, abdominal insufflation, oculocardiac reflex, vasovagal bradycardia, hypertension-induced bradycardia, cardiac response to carotid massage, Valsalva maneuvers) are absent. Denervation also has pharmacologic ramifications. The denervated heart no longer responds normally to indirect-acting medications (medications that mediate effects via the autonomic nervous system). Administration of drugs such as neostigmine, physostigmine, pyridostigmine, edrophonium, glycopyrrolate, atropine, digoxin, and nifedipine no longer produce their anticipated heart rate effects. Indirect-acting drugs such as ephedrine have a decreased effect. Direct-acting drugs such as glucagon, norepinephrine, epinephrine, isoproterenol, dopamine, and beta-blockers are effective choices for managing hemodynamics in these patients. Reflex responses such as the bradycardia expected after administration of phenylephrine may not be present. Also, there is no evidence for alpha and beta receptor upregulation or denervation hypersensitivity after heart transplant [35]. There are several case reports suggesting reinnervation months to years after heart transplantation [39]. One dramatic report describes asystole after administration of neostigmine [40]. It is recommended that a muscarinic antagonist be administered in conjunction with an anticholinesterase in all heart transplant patients. Hemodynamically significant bradycardia will still respond to isoproterenol and transcutaneous pacing.
Afferent denervation in heart transplant patients is most notable for eliminating anginal symptoms. These high-risk patients will not reliably experience or report anginal symptoms with ischemia; complicating preoperative evaluation and decision making. As previously mentioned, there is evidence of reinnervation in patients months to years after transplant. There are several case reports of transplanted patients suffering anginal symptoms years after surgery [39]. Afferent denervation has also been shown to blunt renin-angiotensin-aldosterone regulation and vascular responses to changes in filling pressures.
Other physiologic changes in the transplanted heart include a mild decrease in ventricular function (by 3 months most recipients have returned to New York Heart Association (NYHA) class I functional capacity [35]), mild to moderate diastolic dysfunction, a dependence on preload for maintenance of cardiac output, and an increase in resting coronary blood flow as a result of loss of adrenergic tone. Surprisingly, several physiologic factors remain intact or unchanged; myocardial metabolism is normal, contractile reserve is normal, autoregulation of coronary blood flow remains intact, and the Frank-Starling mechanism is normal [37].
Early posttransplant patients can have significant ECG abnormalities. If a portion of the patient’s native right atrium was retained during surgery, the ECG may demonstrate 2 P waves: 1 from the native atria (not conducted beyond the suture line) and another from the donor SA node (conducted normally via the AV node) [38]. Ventricular ectopy is common in the first several weeks after transplant, but usually diminishes. Supraventricular dysrhythmias (atrial premature beats, atrial fibrillation, and atrial flutter) are common after transplantation, but are also associated with episodes of acute rejection. New-onset supraventricular dysrhythmias, therefore, should heighten suspicion for rejection. First-degree atrioventricular block is common, as is either incomplete or complete right bundle branch block. Several studies quote a significant incidence of bradyarrythmias requiring pacemaker insertion (3.5%–20%) [41]. Antiarrythmic drugs such as verapamil, procainamide, and quinidine are useful for the treatment of supraventricular tachyarrythmias from atrial flutter and atrial fibrillation. Because of its negative inotropic effects, lidocaine should be used with caution in these patients.
Posttransplant morbidities
Heart transplant patients suffer significant posttransplant morbidities. The most ominous of these morbidities is rejection. There are 3 types of cardiac allograft rejection: hyperacute, acute vascular, and acute cellular rejection [37]. Most rejection episodes occur within the first 6 months after transplant. Most commonly, the severity of rejection episodes decreases with time. There are no specific clinical signs or symptoms associated with acute rejection, however, suspicion is increased with any of the following: fatigue, lethargy, nausea, fever, anorexia, hypotension, peripheral edema, dyspnea, S3 gallop, increased jugular venous pressure, decreased systolic function, worsening diastolic dysfunction, pericardial effusion, and new-onset ventricular or supraventricular dysrhythmias. There is evidence to show that acute allograft rejection significantly increases intraoperative morbidity [35]. All transplant recipients are rigorously screened for evidence of rejection. Endomyocardial biopsies, the gold standard for evaluating rejection, are performed weekly for the first 4 weeks after transplant, every other week for the second month after transplant, monthly from 2 to 6 months postoperatively, and then every 3 months through the first postoperative year. Screening is continued at least annually from then on.
CAV is the major manifestation of chronic rejection in heart transplant patients and is one of the leading late causes of death after heart transplant. The diffuse, concentric intimal thickening of the coronary arteries in CAV is believed to be caused by a combination of both immune-mediated and nonimmunologic risk factors [42]. At 1 year after transplant, angiographically significant coronary disease is present in 10% to 20% of patients, and at 5 years more than 50% of patients have angiographically significant disease [34]. The lack of afferent innervation can eliminate anginal symptoms causing silent ischemia in these patients.
Infection, another significant posttransplant morbidity, is a constant threat to heart transplant patients on immunosuppressive therapy. The highest incidence of infection occurs in the first several months postoperatively. This coincides with aggressive induction immunosuppression. Within the first year after transplant, non-CMV infection accounted for 33% of deaths in heart transplant recipients. After 5 years, infection accounted for 11% of deaths [34]. Infection can be difficult to diagnose in this population. Immunosuppressive therapy can mask the normal signs and symptoms (leukocytosis, fever, peritonitis) so one must have a high index of suspicion.
Malignancy is another unfortunate consequence of immunosuppression. It accounts for 23% of deaths in heart transplant patients at 5 years. At 10 years after transplant, 33% of patients had some form of malignancy, mostly skin cancers [34].
The ISHLT has reported that the incidence of renal insufficiency, hyperlipidemia, and diabetes has been increasing in this patient population. By 10 years after transplantation, 99% of recipients have hypertension, 14% have severe renal insufficiency (creatinine >2.5 in 8%, chronic dialysis in 5%, and renal transplant in 1%), 93% have hyperlipidemia, and 37% have diabetes. In their report, the ISHLT caution that because their analysis focused only on surviving recipients, the incidence of these morbidities is likely underestimated [34].
Preoperative evaluation
Dysrhythmias are common in this patient population. A preoperative ECG is necessary. Ventricular dysrhythmias are prevalent in the early postoperative period and decrease with time. Supraventricular dysrhythmias are also common postoperatively. The new onset of any dysrhythmia, especially supraventricular in origin should raise suspicion for an episode of acute rejection. Right bundle branch blocks are common and are of no real significance [41]. Several patients require pacemaker placement for bradyarrhythmias after transplant. The presence of a pacemaker warrants its interrogation, an understanding of its current settings, and possible adjustment of those settings for surgery. Biphasic or 2 separate P waves are a common finding on ECG and are of no anesthetic significance.
Intraoperative care
No single anesthetic technique has been proven to be superior in caring for heart transplant patients having nontransplant surgery [43]. Spinal anesthesia, epidural anesthesia, regional anesthesia, general anesthesia, and sedation have all been safely performed in these patients. Because of their strict dependence on preload for cardiac output, the literature suggests that general anesthesia is preferred over neuraxial techniques. When a neuraxial technique is used, epidural anesthesia is preferred over spinal anesthesia. Anesthesiologists should consider careful preoperative volume loading when planning a neuraxial technique.
Another goal in caring for these patients is to minimize invasive techniques that increase the risk for infection. Oral intubation is preferred over nasal intubation. One should carefully consider the need for invasive monitoring (arterial lines, central lines). If needed, these lines should be placed with strict adherence to aseptic technique and should be removed as soon as possible. Groin sites should be avoided whenever possible because of the high risk of infection. Preoperative antibiotics should be given as per guidelines and before invasive line placement if possible. If large fluid shifts are expected, consider the use of a pulmonary artery catheter and/or transesophageal echocardiography. All other standard monitors are indicated. Intraoperative ECG is especially helpful for detecting ischemic events and dysrhythmias [44].
Anesthesia for patients after lung transplant
According to the ISHLT, nearly 26,000 lung transplants have been performed since the first in 1983. In the ISHLT’s 25th Official Adult Lung and Heart/Lung Transplant Report, the total number of lung transplants performed annually is estimated at nearly 2200 worldwide. This report highlights several interesting trends in lung transplantation. First, chronic obstructive pulmonary disease (COPD) continues to be the primary indication for single lung transplantation (SLT) (50%). Other indications for SLT include idiopathic pulmonary fibrosis (IPF) (28%) and alpha-1 antitrypsin disease (A1AT) (7.1%). The primary indication for double lung transplantation (DLT) is cystic fibrosis (CF) (28%). Other indications for DLT include COPD (24%) and IPF (14%). Second, the average age of the lung transplant recipient is increasing. Currently, 24.1% of lung transplant recipients are aged 60 years or older and 3.7% are age 65 years or older. Third, survival of transplant recipients continues to improve. The half-life of an SLT recipient is now 4.5 years and the conditional half-life (half-life of those recipients alive 1 year after transplant) is 6.4 years. The half-life of a DLT recipient is 6.2 years and the conditional half-life of a DLT recipient is 8.8 years. Fourth, 5-year morbidity analysis shows continued prevalence of hypertension (85.3%), hyperlipidemia (53%), renal dysfunction (37%), diabetes (35%), and bronchiolitis obliterans (BO) (33.7%). Malignancy is another common morbidity among transplant recipients. At 5 years after transplant, 17% of recipients reported a malignancy and at 10 years after transplant 34% of recipients had reported a malignancy. Fifth, mortality analysis continues to show that BO, believed to be a manifestation of acute and chronic rejection, accounts for 28.5% of deaths in patients surviving 3 to 5 years. Other causes of death include noncytomegalovirus infection (19.2%), graft failure (19%), and nonlymphoma malignancy (7.9%) [45].
Immunosuppression
Most lung transplant recipients receive a course of induction (high-dose) immunosuppression followed by a maintenance phase of lower-dose therapy. The ISHLT reports that maintenance therapy for most recipients consists of a 3-drug regimen. A corticosteroid (prednisone) is usually combined with either tacrolimus or cyclosporine and either MMF or azathioprine. The most common 3-drug combination for maintenance is prednisone, tacrolimus, and MMF [45]. All these drugs and their anesthetic implications are discussed in the section on anesthesia for noncardiac surgery after heart transplantation.
Posttransplant physiology
At the time of transplantation, the donor lung is denervated. This denervation is arguably the most significant physiologic difference between a transplanted and a native lung. It results in the loss of the cough reflex distal to the site of the bronchial anastomosis. This, of course, places the recipient at risk for both aspiration and infection. In the normal lung, autonomic innervation is responsible for regulating airway tone, however, there seems to be no clinically significant change in bronchomotor tone after transplantation nor is there an increase in the incidence of airway hyperreactivity [46]. Bronchodilators continue to be effective although some investigations suggest their effect is slightly decreased. Studies have also shown that denervation has little, if any, effect on respiratory rate or tidal volume. Evidence suggests that hypoxic pulmonary vasoconstriction (HPV) is unaffected by denervation [47].
In DLT recipients, pulmonary blood flow is normal. In SLT recipients, the transplanted lung receives 60% to 70% of pulmonary perfusion. In patients transplanted for primary pulmonary hypertension (PPH) blood flow to the transplanted lung can increase to as much as 99% of cardiac output [48].
In DLT patients, pulmonary function tests (PFTs), arterial blood gases (ABGs) and alveolar-arterial oxygen gradient return to normal within 9 months of transplant. Only a slight decrease in carbon dioxide diffusing capacity (DLco2) remains. In SLT patients, PFTs continuously improve in the first 9 months after transplant. SLT recipients are generally left with a slight restrictive defect and a mildly decreased DLco2 [48].
Mucociliary clearance is significantly decreased in lung transplant recipients. This has been shown in both short-term and long-term survivors. Together with the loss of the cough reflex, this places the lung transplant recipient at high risk for both mucus plugging and infection [49].
Posttransplant morbidities
Episodes of acute rejection are common among lung transplant recipients. During the first few months after transplant, most patients experience at least 1 episode of acute rejection. Nearly 60% of all rejection episodes occur within the first 3 months after transplant. Rejection is often hard to distinguish from infection. It often presents with cough, fever, malaise, dyspnea, hypoxia, wheezing, worsening PFTs, and infiltrates on chest radiograph. The clinical criteria for diagnosing rejection include increase in temperature 0.5°C above baseline, Pao2 decrease of greater than 10 mmHg below baseline, new or changing infiltrates on chest radiograph, and a decrease in FEV1 (forced expiratory volume in the first second of expiration) of more than 10% below stable baseline [48]. Infection has to be excluded as a cause. Differentiating between rejection and infection is usually quite difficult and often only possible with bronchoscopy, bronchial-alveolar lavage, and cultures. Occasionally lung biopsy is required to make a definitive diagnosis. Treatment involves a prolonged burst of high-dose corticosteroids.
Infection is a significant cause of both morbidity and mortality in lung transplant recipients. It is the leading cause of death in the first year after transplant (39.5%) [45]. The high rate of infection in this period coincides with the administration of aggressive induction chemotherapy. In the 3- to 5-year posttransplant group, infection accounted for 19.5% of deaths [45]. As previously mentioned, it can be difficult to differentiate infection from rejection. In addition, immunosuppression often masks the clinical hallmarks of infection including fever and leukocytosis. Physicians caring for these patients must have a high index of suspicion for infection.
BO is a syndrome characterized by immune injury to the transplanted lung resulting in the obstruction of small airways with fibrous scar tissue. Patients with BO typically present with progressive cough, evidence of obstruction on PFTs, and interstitial infiltrates on chest radiograph [50]. Although exceedingly rare in the first year, BO is one of the leading causes of death in transplant recipients surviving beyond 1 year (21.5% of deaths in those surviving 1–3 years after transplant). At 5 years after transplant, 33.7% of recipients have a diagnosis of BO [45]. First-line treatment is generally corticosteroid therapy, but the only definitive treatment is retransplantation [51].
As with any group of immunosuppressed patients, malignancy rates in lung transplant recipients increase as duration of therapy increases. 3.6% of 1-year survivors have at least 1 form of malignancy. In those who survive 5 years, the incidence increases to 12.3%. In early survivors, lymphoma accounts for the greatest number of malignancies, whereas skin cancer leads in those surviving 5 years or more [45].
Other significant comorbidities reported by the ISHLT in 5-year survivors include hypertension (85.3%), hyperlipidemia (53.6%), renal dysfunction (37%), and diabetes (35.5%). The ISHLT’s report looks only at survivors, and therefore may significantly underestimate the prevalence of these comorbidities [45].
Intraoperative care
Positive-pressure ventilation can cause disruption of anastomotic suture lines, emphysematous bleb rupture or hyperinflation of the native, diseased lung. Increasing ventilatory pressures, therefore, raise concern for pneumothorax or hyperinflation of the native lung with subsequent compression of the transplanted lung and even cardiac compression resulting in hypotension [52]. An effort should be made to keep peak inspiratory pressures low. Because of differences in compliance between the native and transplanted lung, it is not unusual to see a biphasic capnography tracing in SLT recipients. The administration of humidified gases may help decrease mucus plugging in the perioperative period. Frequent, careful endotracheal tube suctioning and bronchodilator administration is also recommended.
Abdominal Organs: Kidney, Liver, Pancreas, and Intestine
Kidney
As kidney transplant is the most commonly performed organ transplant, it is feasible that the nontransplant anesthesiologist will be required to perform an anesthetic on one of these patients. Many have preexisting multisystem diseases that persist after transplant. Diabetes mellitus, hypertension, hyperlipidemia, and cardiovascular disease are extremely common comorbidities in this patient subset [3,16].
When planning an anesthetic for this population, it is essential to assess kidney function preoperatively. After kidney transplant many patients experience a 20% reduction in the function of the transplanted organ as a result of chronic rejection, the toxic effects of immunosuppressive drugs, or both [3]. Although serum creatinine level is generally normal or near normal in an adequately functioning renal allograft, glomerular filtration rate may be diminished and drugs that require renal excretion, especially drugs with active metabolites, should be used judiciously with avoidance of repeated doses [3,9]. Table 4 is a list of potential perioperative medications with active metabolites that are cleared via the kidneys.
Renal allograft function is at risk from potentially nephrotoxic drugs, such as nonsteroidal antiinflammatory drugs and intravenous contrast agents. These medications are best avoided or used with extreme caution. A recent exposure to intravenous contrast should prompt a reevaluation of kidney function before proceeding to elective surgery [3,4,8]. The transplanted kidney is also vulnerable to other insults, such as ischemia, so it is important to maintain normovolemia and avoid intraoperative hypotension. Diuretics should not be given without careful consideration of the patient’s intravascular volume status [3,9].
Pancreas
Pancreas transplantation has evolved slowly in the past 45 years to treat type 1 diabetes mellitus. In general, pancreas transplant takes place in 1 of 3 scenarios: simultaneous kidney transplantation (SPK), pancreas transplant in a patient who has previously received a kidney allograft or pancreas transplantation alone in a patient with preserved renal function. Emergence of superior surgical techniques and immune suppression in pancreas transplantation has improved outcomes since the first dismal attempts. Pancreatic graft survival rates at 5 years are best in the SPK group at about 69% [1]. Pancreas transplantation also continues to have the highest complication rates of solid organ transplants other than intestinal transplant [16].
Patients who have undergone pancreas transplant are often those with the most severe diabetic complications, which persist despite transplant and may negatively affect anesthetic management. The anesthetic plan should reflect that diabetic stiff joint syndrome, gastropathy and gastroparesis, and autonomic neuropathy persist after transplant [16,18]. Pancreas recipients are at high risk for coronary artery disease, which is frequently silent or asymptomatic. The results of previous cardiac testing and interventions, as well as a thorough evaluation of the patient’s current ECG and functional status are imperative. Preoperative assessment should also include evaluation of metabolic status, serum glucose, renal function, including electrolytes and acid-base status, and volume status. Individuals with a functioning pancreatic graft should have effective glucose metabolism and intraoperative supplementation is likely not required [7,16]. In patients with failed or poorly functioning grafts, a plan for perioperative glucose control should be established. Intraoperative monitoring of glucose to detect hyperglycemia and hypoglycemia and treatment with insulin to avoid diabetic ketoacidosis is essential in patients with failed grafts. Previous abdominal surgery in this population increases the risk of intraoperative bleeding and adequate intravenous access, and up-to-date typing and screening should be considered an important part of the anesthetic management plan. One caveat of special concern in patients with bladder drainage of the exocrine pancreas is the potential for severe metabolic acidosis caused by bicarbonate losses. These patients may require intraoperative monitoring of acid-base status and possible bicarbonate replacement.
Liver
The first successful human liver transplantation was performed in 1967 [1]. In 2008 and 2009 there were nearly 13,000 liver transplants performed in more than 140 institutions in the United States with almost 57,500 recipients surviving at October 31, 2009 (see Table 1). After transplantation, tests of synthetic liver function and aminotransferases should normalize. The bilirubin level generally normalizes within 3 months and transaminases in 2 weeks after transplant if the allograft is functioning normally. Drug metabolism returns to normal soon after reperfusion of the allograft. Synthesis and clearance of coagulation factors, such as factors I, II, V, VII, VIII, IX, X, XI, XII, and XIII, and regulatory proteins, such as antithrombin III, protein C, protein S, and plasminogen, also return to normal soon after transplantation in a well-functioning allograft [53]. Platelet function normalizes after transplant, but platelet count may be persistently less than normal after transplant. However, the absolute platelet count is typically adequate for normal hemostasis. Some patients may undergo splenectomy after liver transplantation if severe thrombocytopenia and splenomegaly persist. Preoperative testing in this patient population should include evaluation of aspartate aminotransferase, alanine aminotransferase, γ-glutamyl transferase, internationalized normalized ratio, bilirubin, alkaline phosphatase, albumin, and platelet count. Evidence of abnormalities or inadequate hepatic function seen on basic testing may be indicators of rejection, infection, or biliary stasis, and communication with the transplant center is important along with postponement of elective surgery [4,16].
Intravenous anesthetics and other drugs that are hepatically metabolized or cleared can be safely administered as long as all evidence points to a well-functioning allograft without biliary stasis [3,54]. Both general and regional anesthesia techniques may be used with success and without deterioration in allograft function as long as there are no contraindications to the technique chosen and adequate hepatic perfusion is maintained throughout the procedure [54,55].
Abdominal operations in liver transplant recipients have the potential for major bleeding so it is important to have prepared adequately with at least large bore intravenous catheters and possibly central venous access, and an up-to-date type and cross. It is important to maintain adequate systemic blood pressure and volume as hypoperfusion and ischemic insult are poorly tolerated by the liver allograft because the normal physiologic mechanisms that maintain and control blood flow in the liver are blunted [16]. It may be necessary to communicate with the institutional blood bank to determine the availability of blood products before surgery. Many facilities may have limited available resources. Platelets are often not stored on site and must be transported from a regional center when needed. If intraoperative bleeding is considered a viable risk, then it is prudent to ensure availability of blood products before the day of surgery. Some transplanted patients may have had multiple transfusions previously and are at risk for acquiring antibodies that may further delay the ability to obtain blood products in an emergency. Therefore, adequate preparation should be given to the potential need for transfusion and blood products before the patient presents to the operating suite.
Liver recipients with pretransplant hepatopulmonary syndrome and portopulmonary hypertension usually have resolution of hypoxemia and pulmonary hypertension some time after transplant. However, some may have residual hypoxemia or persistent pulmonary hypertension. If a patient is on a long-term pulmonary vasodilator, such as sildenafil or epoprostenol, it should be continued in the perioperative period. Despite continuing the medications, pulmonary hypertension can be worsened perioperatively by hypoxemia, hypercapnia, or acidosis, and additional measures such as nitric oxide should be available to treat pulmonary hypertension. It may be prudent to contact the transplant center or the patient’s pulmonologist for assistance in constructing a perioperative plan for management of the patient’s pulmonary hypertension and to discuss possible supplemental measures during the operative procedure [16]. Intensive care admission following the surgical procedure should be strongly considered in this population for close respiratory and hemodynamic monitoring.
Intestinal
Since 1987 there have been approximately 1700 intestinal transplants with slightly more than 900 reported surviving with a functional graft (see Table 1). More than half of these are children. The transplanted bowel elicits a significant alloimmune response and rejection is an all too common problem with this type of transplant. There is also a significant incidence of graft-versus-host disease in this population [12,16]. This subset of transplanted patients requires a high level of multidisciplinary support. Other than absolute emergent situations, it is unlikely that these patients will present for surgery outside of a transplant center. Should an anesthesiologist be confronted with this particular transplanted patient, there are several important factors to consider when constructing the anesthetic plan. Gastric and intestinal motility are often significantly delayed, and this should be reflected in the plan for airway management [16]. Chronic liver dysfunction is a common finding. Chronic renal dysfunction is also frequently present as a result of multiple insults of hypovolemia, sepsis, need for immunosuppressants, and frequent therapy with antibiotics [12,16]. If abdominal surgery is necessary, the anesthesiologist should be prepared for a difficult surgical dissection and the potential for hemorrhagic complications. These patients have invariably been subjected to multiple central venous catheters for chronic parenteral nutrition and administration of medication. Recurrent episodes of infection and venous thromboses cause venous damage and the reality of difficult venous access should be anticipated [16]. Denervation and impaired lymphatic drainage of the transplanted intestine affect intestinal permeability and absorption [8]. These patients frequently have derangements in their acid-base status and electrolytes and suffer malnutrition. Hypoproteinemia is common and is associated with pleural effusions, ascites, and edema. Infection and sepsis are an unfortunate but common morbidity in this population because of bacteremia from bowel translocation, and the need for vasopressor support should also be anticipated [12,16].
Preoperative testing should include evaluation of electrolytes, acid-base status, glucose, liver and kidney function, complete blood count, and coagulation studies. As these patients are often weak and debilitated at baseline, postoperative mechanical ventilatory support may be necessary. Many of these patients required tracheostomy during the peritransplant period because of the need for prolonged mechanical ventilation, which may effect airway management. Although early extubation is desirable, extubation should not be attempted unless electrolyte, fluid, and acid-base status are optimal, oxygenation is adequate, there is hemodynamic stability, and pain management is optimal [12,16]. Communication with the intensive care staff before surgery to alert them to the possibility of admission to the unit will likely facilitate the patient’s care. Open lines of communication with the transplant center during all phases of care should be established.