Anesthetic Considerations for Pediatric Surgical Conditions
Risks of Anesthesia
Quantifying the risk of pediatric anesthesia is difficult due to the difficulty in determining whether complications are attributable to the anesthetic, and if so, to what degree. The risk of cardiac arrest for children undergoing anesthesia was estimated in the 1990s to be 1 : 10,000.1,2 However, these studies did not take patient co-morbidity or the surgical condition into consideration. The risk of a healthy child suffering cardiac arrest during myringotomy tube placement is significantly less than the likelihood of a child with complex cardiac disease arresting during a complex cardiac repair.3
A recent review of cardiac arrests in anesthetized children compared 193 events from 1998–2004 to 150 events from 1994–1997.4 A reduction in medication-caused arrests from 37% to 18% was identified, and was attributed to the decline in halothane use (that causes myocardial depression) and the advent of using sevoflurane (that is not associated with myocardial depression). There was also a reduction in unrecognized esophageal intubation as a cause of arrest, due in large part to the advent of end-tidal carbon dioxide (ETCO2) monitoring, pulse oximetry, and an increased awareness of the problem.
Recent large single center reports yield a current estimate of anesthesia-related mortality of 1 : 250,000 in healthy children. To put this into perspective for parents, the risk of a motor vehicle collision on the way to the hospital or surgery center is greater than the risk of death under anesthesia. However, risks of mortality and morbidity are increased in neonates and infants less than one year of age, those who are ASA (American Society of Anesthesiologists) status 3 or greater, and those who require emergency surgery.5
Preoperative Anesthesia Evaluation
All patients presenting for operations under anesthesia benefit greatly from a thorough preanesthetic/preoperative assessment and targeted preparation to optimize any coexisting medical conditions. The ASA physical status (PS) score is a means of communicating the condition of the patient. The PS is not intended to represent operative risk and serves primarily as a common means of communication among care providers (Table 3-1). Any child with an ASA classification of 3 or greater should be seen by an anesthesiologist prior to the day of surgery. This may be modified in cases of hardship due to the distance from the surgical venue or when the patient is well known to the anesthesia service, and the child’s health is unchanged. Finally, outstanding and unresolved medical issues may be significant enough to warrant cancellation of the procedure for optimization of anesthesia and/or further diagnostic workup.
TABLE 3-1
ASA Physical Status Classification
| ASA Classification | Patient Status |
| 1 | A normal healthy patient |
| 2 | A patient with mild systemic disease |
| 3 | A patient with severe systemic disease |
| 4 | A patient with severe systemic disease that is a constant threat to life |
| 5 | A moribund patient who is not expected to survive without the operation |
| 6 | A declared brain-dead patient whose organs are being removed for donor purposes |
| E | An emergency modifier for any ASA classification when failure to immediately correct a medical condition poses risk to life or organ viability |
Criteria for Ambulatory Surgery
Ambulatory surgery comprises 70% or more of the caseload in most pediatric centers. Multiple factors should be considered when evaluating whether a child is suitable for outpatient surgery. Some states regulate the minimum age allowed in an ambulatory surgical center. For example, the minimum age in Pennsylvania is six months. In most cases, the child should be free of severe systemic disease (ASA PS 1 or 2). Other factors that may determine the suitability of a child for outpatient surgery are family and social dynamics. Some institutions utilize a telephone screening evaluation process to determine whether a patient can have their full anesthesia history and physical on the day of surgery rather than being evaluated in a preoperative evaluation clinic prior to surgery.6
Well-controlled systemic illnesses do not necessarily preclude outpatient surgery, but any concerns must be addressed in advance in a cooperative fashion between the surgical and anesthesia services. If a child has a moderate degree of impairment, but the disease is stable and the surgical procedure is of minimal insult, outpatient surgery may be acceptable.
General Principles
In addition to the physical examination, the essential elements of the preoperative assessment in all patients are listed in Box 3-1. Patients and parents may be anxious about recurrence of adverse perianesthetic events such as those listed, and they should be reassured that efforts will be made to prevent these events.
Miscellaneous Conditions
Malignant Hyperthermia Susceptibility
MH is an inherited disorder of skeletal muscle calcium channels, triggered in affected individuals by exposure to inhalational anesthetic agents (e.g., isoflurane, desflurane, sevoflurane), succinylcholine, or both in combination, resulting in an elevation of intracellular calcium. The incidence of an MH crisis is 1 : 15,000 general anesthetics in children. Fifty per cent of patients who have an MH episode have undergone a prior general anesthetic without complication. The resulting MH crisis is characterized by hypermetabolism (fever, hypercarbia, acidosis), electrolyte derangement (hyperkalemia), arrhythmias, and skeletal muscle damage (elevated creatine phosphokinase [CPK]). This constellation of events may lead to death if unrecognized and/or untreated. Dantrolene, which reduces the release of calcium from muscle sarcoplasmic reticulum, when given early in the course of an MH crisis, has significantly improved patient outcomes. With early and appropriate treatment, the mortality is now less than 10%. Current suggested therapy can be remembered using the mnemonic ‘Some Hot Dude Better GIve Iced Fluids Fast” and is summarized in Box 3-2.7 It should be noted that dantrolene must be prepared at the time of use by dissolving in sterile water. It is notoriously difficult to get into solution and the surgeon may be asked to help with this process.
Patients traditionally thought to be MH susceptible are those with a spectrum of muscle diseases listed in Box 3-3. However, many patients who develop MH have a normal history and physical examination. In the past, patients with mitochondrial disorders were thought to be at risk. Anesthetic gases appear safe in this population, but succinylcholine should still be avoided as some patients may have rhabdomyolysis (elevated CPK, hyperkalemia, myoglobinuria) with hyperkalemia without having MH.
Preoperative Fasting Guidelines
Research performed at our institution has demonstrated that intake of clear liquids (i.e., liquids that print can be read through, such as clear apple juice or Pedialyte) up until two hours prior to the induction of anesthesia does not increase the volume or acidity of gastric contents.8 Our policy is to recommend clear liquids until two hours prior to the patient’s scheduled arrival time. Breast milk is allowed up to three hours before arrival for infants up to 12 months of age. Infant formula is allowed until four hours before arrival in infants less than 6 months old, and until six hours before arrival in babies 6–12 months old. All other liquids (including milk), solid food, candy, and gum are not allowed less than eight hours before induction of anesthesia. Although these are the guidelines for our institution, the surgeon should be aware that NPO (nil per os) guidelines are variable and institutionally dependent.
Laboratory Testing
Although serum electrolytes are not routinely screened, electrolytes may be helpful in patients on diuretics. Preoperative glucose should be monitored in insulin-dependent diabetic patients, and also in any patient who has been receiving parenteral nutrition or intravenous (IV) fluids with a dextrose concentration greater than 5% prior to surgery.
Clinical Scenarios and High Risk Populations
Upper Respiratory Tract Infection
One of the most common questions confronting an anesthesiologist is whether to cancel a procedure because of an upper respiratory infection (URI). It is not uncommon for some patients to spend much of their childhood catching, suffering from, or recovering from a URI, with the highest frequency occurring in children under age 6 who attend day care or preschool.9 Patients with a current or recent URI undergoing general anesthesia are theoretically at increased risk for perioperative respiratory complications, including laryngospasm, bronchospasm, and hypoxia, with the youngest patients (<2 years of age) being at greatest risk.10,11 However, anesthetic management may also be tailored to reduce stimulation of a potentially hyper-reactive airway. In addition, cancellation of a procedure imposes an emotional and/or economic burden on patients and families, physicians, and operating rooms. Unless the patient is acutely ill, it is often acceptable to proceed with the anesthetic. Patients with high fever, wheezing, or productive cough may actually have a lower respiratory tract infection and the planned procedure is more likely to be cancelled. Our approach is to discuss the urgency of the scheduled operation with the surgeon, and then to review the risks and benefits of proceeding versus rescheduling with the parents, taking into consideration the possibility that the child may have another URI at the time of the rescheduled procedure. Allowing the parents to participate in the decision-making process (when appropriate) usually leads to mutual satisfaction among all involved parties.
The Former Preterm Infant
Postanesthetic Apnea
The risk of apnea is increased in ex-premature infants because of immaturity of the central and peripheral chemoreceptors with blunted responses to hypoxia and hypercapnia, even without the additional burden of anesthetic/opioid-induced respiratory depression. In addition, anesthetic agents decrease muscle tone in the upper airway, chest wall, and diaphragm, thereby further depressing the ventilatory response to hypoxia and hypercapnia. In the immediate neonatal period, immaturity of the diaphragmatic musculature causes early fatigability, which may also contribute to apnea.12 Although postanesthetic apnea may be brief and resolve either spontaneously or with minor stimulation, in ex-premature infants even brief apnea may result in significant hypoxia. Although most apneic episodes occur within the first two hours after anesthesia, apnea can be seen up to 18 hours postoperatively.
This increased risk of apnea affects the postanesthetic care of infants born prematurely, mandating that those at risk be admitted for cardiorespiratory monitoring. Despite numerous studies on this issue, the postnatal age at which this increased risk of apnea disappears is still being debated. The results of a meta-analysis of pertinent studies indicated that a significant reduction occurred in the incidence of apnea at 52 to 54 weeks’ postconceptual age.13 A hematocrit less than 30% was identified as an independent risk factor, and it was recommended that ex-premature infants with this degree of anemia be hospitalized postoperatively for observation regardless of the postconceptual age. However, conclusions drawn from this meta-analysis have been challenged. Moreover, the sample size of this study may not have been large enough to draw valid conclusions.14
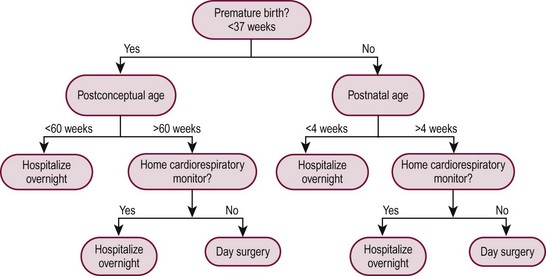
Although the risk of apnea can be decreased with regional anesthesia and/or caffeine, our practice is to admit all at-risk patients (those with a postconceptual age of younger than 60 weeks), regardless of the anesthetic technique used, to monitored, high-surveillance inpatient units for 23 hours after anesthesia and operation. Similarly, infants born at term must be at least 1 month of age to be candidates for outpatient surgery because postanesthetic apnea has been reported in full-term infants up to 44 weeks postconceptual age.13 Figure 3-1 shows an algorithm useful for decision making regarding eligibility for day surgery in young infants.
Anterior Mediastinal Mass
It has long been recognized that the anesthetic management of the child with an anterior mediastinal mass is very challenging and fraught with the risk of sudden airway and cardiovascular collapse. Signs and symptoms of positional airway compression and cardiovascular dysfunction may, or may not, be present. However, the absence of signs and symptoms does not preclude the possibility of life-threatening collapse of the airway or cardiovascular obstruction upon induction of anesthesia. Patients presenting with anterior mediastinal masses (e.g., lymphoma) are at particularly high risk of airway compromise and cardiovascular collapse with the induction of general anesthesia due to compression of the trachea or great vessels when intrinsic muscle tone is lost and spontaneous respiration ceases.15–17 When this occurs, there may not be airway compromise, but rather obstruction of vascular inflow to the right atrium and/or outflow tract obstruction from the right or left ventricle.
Positioning the child is an important part of the anesthetic plan for these patients. The sitting position favors gravitational pull of the tumor toward the abdomen rather than allowing the tumor to fall posteriorly onto the airway and major vessels as occurs in the supine position. However, the sitting position makes intubation challenging. Thus, positioning the symptomatic child in the lateral decubitus position is recommended. Turning the child lateral or prone, or lifting the sternum, have been shown to alleviate acute deterioration in ventilation or cardiovascular collapse secondary to tumor compression.18,19 In any patient with an increased potential for such obstruction, provision should be made for the availability of a rigid bronchoscope, the ability to move the operating room table to effect position changes, and the ability to institute cardiopulmonary bypass or extracorporeal membrane oxygenation (ECMO). Compression of greater than 50% of the cross-sectional area of the trachea on CT imaging has been suggested to identify a population at risk of airway collapse during induction of general anesthesia.20
When possible, percutaneous biopsy of the mass using local anesthesia with or without judicious doses of sedative medication is often ideal and poses the least risk to the patient. In patients who have additional tissue sites from which a biopsy can be obtained (e.g., cervical, axillary, or inguinal lymph nodes), it may be safer to proceed with the patient in a semi-sitting position using local anesthesia and carefully titrating sedation so that spontaneous ventilation is preserved. Recently, ketamine and dexmedetomidine have been shown to provide good sedation with preservation of airway patency and spontaneous respiration in this setting.21 If progression to general anesthesia is required and airway and/or vascular compression exists, standby ECMO capability is strongly recommended.
The inherent conflict between the need to obtain an accurate and timely tissue diagnosis and the very real concern regarding the safe conduct of the anesthetic requires an open dialogue between the anesthesiologist, surgeon, and oncologist to reach an agreement on strategies to achieve these goals. Many experts recommend the development and utilization of an algorithm for anesthetic management of the child with an anterior mediastinal mass (Fig. 3-2). The algorithm addresses assessment of signs and symptoms, evaluation of cardiopulmonary compromise, and treatment options.18,22,23
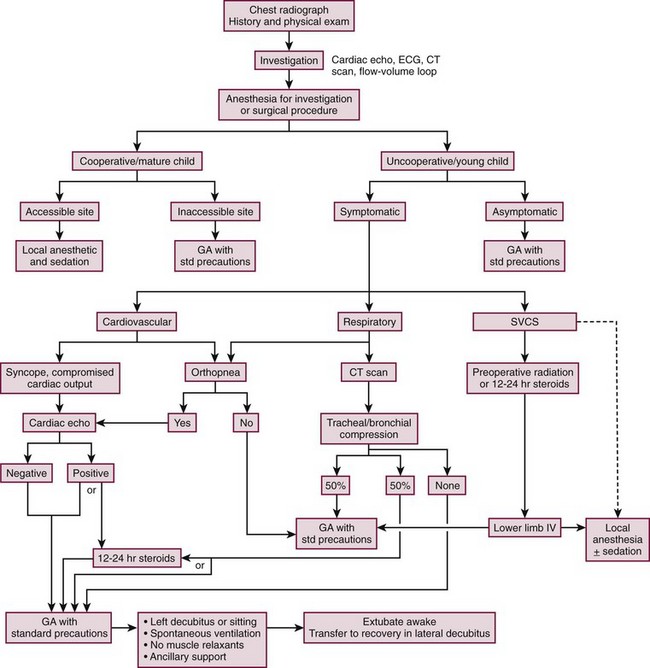
FIGURE 3-2 This algorithm describes management of the patient with a large anterior mediastinal mass. GA, general anesthesia. SVCS, superior vena cava syndrome. (Adapted from Cheung S, Lerman J. Mediastinal masses and anesthesia in children. In: Riazi J, editor. The Difficult Pediatric Airway. Anesthesiol Clin North Am 1998;16:893–910.)
Patients with Congenital Heart Disease
Each year in the U.S., nearly 32,000 children are born with CHD. Extracardiac anomalies are seen in up to 30% of infants with CHD,24,25 and may necessitate operative intervention in the neonatal period prior to repair or palliation of the cardiac lesion. Although physiologically well-compensated patients may undergo noncardiac surgery with minimal risk, certain patient groups have been identified as high risk: children less than 1 year of age, especially premature infants; patients with severe cyanosis, poorly compensated congestive failure or pulmonary hypertension; patients requiring emergency surgery and patients with multiple coexisting diseases.26
Endocarditis Prophylaxis
The most recent American Heart Association (AHA) guidelines for perioperative antibiotic prophylaxis emphasize evidence-based practice. Current opinion reflects the view that endocarditis is more likely to result from frequent exposure to bacteremias occurring as a consequence of activities of daily living than those due to dental, gastrointestinal, or genitourinary tract procedures.27–30 Except for the conditions listed in Box 3-4, the AHA no longer recommends routine antibiotic prophylaxis for any other form of CHD. For a more comprehensive discussion, the reader is referred to the original publications.31,32
Special Issues in Patients with CHD
Pulmonary Hypertension
Prolonged exposure of the pulmonary vascular bed to high flows secondary to left-to-right shunting, pulmonary venous obstruction, or high left atrial pressures can lead to elevated pulmonary artery (PA) pressures and the development of pulmonary veno-occlusive disease.
The pathophysiology and anesthetic implications of pulmonary hypertension have been well reviewed,33–35 and there is no ideal sedative/anesthetic agent for these patients. A frank discussion of the high risk of anesthesia in these patients should be held with the patient’s family when the anesthetic consent is obtained.
Anesthetic management strategies are guided by three considerations: (1) appropriate manipulation of factors affecting pulmonary vascular resistance (PVR); (2) the effect of anesthetic agents on PVR; and (3) maintenance of cardiac output (CO) and coronary perfusion pressures. Increases in PVR can potentially culminate in right ventricular (RV) failure if excessive.36–38 Ventilatory strategies can profoundly alter cardiovascular pathophysiology via complex interactions influencing cardiac function and output due to alterations in RV preload and afterload. Given the propensity for desaturation and increases in PCO2 with spontaneous ventilation, controlled ventilation is recommended intraoperatively with maintenance of lung volumes at or around functional residual capacity (FRC) with minimal positive end expiratory pressure (PEEP) and avoidance of high inspiratory pressures, hypercarbia, or hypoxemia. Normal preload should be maintained and hypotension avoided in these patients in order to optimize CO, coronary artery flow, and oxygen supply to the RV. Dopamine, epinephrine, and milrinone should be available to further improve cardiac function if necessary.
Cyanosis and Polycythemia
Increased bleeding tendencies, and a variety of associated laboratory abnormalities, have long been noted in cyanotic patients. When compared to acyanotic children a disproportionate number of cyanotic children are thrombocytopenic, with the degree of thrombocytopenia directly related to the severity of polycythemia. Abnormalities in prothrombin time, partial thromboplastin time, and individual factor deficiencies have also been described and defy simple classification.39 Although these deficiencies may cause no symptoms other than bruising, severely cyanotic patients should have clotting studies prior to operation.
Pacemakers/Implantable Cardioverter-Defibrillators
Increasing numbers of infants and children have pacemakers or implantable cardioverter-defibrillators (ICDs). In recent years increasing numbers of children have had ICDs placed for prevention of sudden cardiac death due to congenital or acquired long QT syndrome.40 Necessary preoperative information for these patients includes the indication for device placement, the date of last device check, and the current underlying cardiac rate and rhythm without device support. Indications for permanent pacemakers in patients with CHD include congenital or postsurgical complete heart block, and sinus node or AV node dysfunction.
The American College of Cardiology/American Heart Association (ACC/AHA) guidelines advocate pre- and postoperative interrogation of permanent pacemakers.41 All patients with an ICD should undergo preoperative device interrogation with disabling of defibrillation capability intraoperatively and resumption in the postoperative period. Bipolar electrocautery should be utilized whenever possible in the patient with a pacemaker or ICD. If monopolar electrocautery is used, the electrocautery return pad should be placed as far away from the pacing generator as possible, and the pacemaker generator/leads axis should not be located between the operative site and the grounding pad. If the pacemaker cannot be placed in an asynchronous mode and electrocautery adversely affects it, cautery current should be applied for not more than 1 second at a time, with 10 seconds between burses of current, to allow for maintenance of CO.42,43
The Difficult Pediatric Airway
The patient with a ‘difficult airway’ may require advanced airway management techniques in order to secure his/her airway including the lighted stylet, the fiberoptic intubating stylet, the flexible fiberoptic bronchoscope, direct laryngoscopy with intubating stylet, fiberoptic rigid laryngoscopy, an anterior commissure scope, the laryngeal mask airway, cricothyrotomy, and tracheostomy. Anesthesiologists and facilities do not need availability of all of the listed techniques. When a difficult airway is anticipated, it is important to have all necessary airway equipment present in the operating room (OR) before induction of anesthesia, as well as communication of the difficult airway potential to all members of the OR team. Indirect intubation methods should be utilized rather than repeated attempts at direct laryngoscopy because airway edema and bleeding increase with each attempt, decreasing the likelihood of success with subsequent indirect methods.44
Patients that require additional approaches to obtain an airway require additional OR time and, in certain cases, continuation of intubation postoperatively may be necessary, mandating ICU admission. Most difficult airways in the pediatric age group can be anticipated. Unlike in adults, it is rare to encounter an unanticipated difficult airway in a normal-appearing child. Some congenital syndromes associated with difficult airway management are listed in Table 3-2.
TABLE 3-2
Syndromes and Craniofacial Abnormalities Associated with Difficult Intubation
| Syndrome | Associated Features |
| Arthrogryposis | Limited mouth opening and cervical mobility |
| Beckwith–Wiedemann | Macroglossia |
| Freeman–Sheldon (whistling face) | Microstomia |
| Goldenhar syndrome (hemifacial microsomia) | Hemifacial microsomia, mandibular hypoplasia (uni- or bilateral) |
| Klippel–Feil | Limited cervical mobility |
| Mucopolysaccharidoses (e.g., Hurler) | Macroglossia, limited cervical mobility, Infiltration of tongue, supraglottis |
| Pierre–Robin | Micrognathia, glossoptosis, cleft palate |
| Treacher–Collins | Maxillary/mandibular hypoplasia |
| Trisomy 21 (Down) | Macroglossia, subglottic stenosis, atlanto-axial instability |
The ASA has developed practice guidelines and an algorithm for management of the difficult airway. This guideline and algorithm are continually updated and well known to anesthesiologists.44 Although the guidelines and algorithm are intended for use in adult patients, their emphasis on the importance of having a clear primary plan with multiple back-up contingency plans is equally applicable to infants and children.
Intraoperative Management
Monitoring and Vascular Access
Standard monitoring in pediatric anesthesia follows the ASA ‘Standards for Basic Anesthetic Monitoring’45 and includes pulse oximetry, noninvasive automated blood pressure measurement, electrocardiography, capnography, and temperature monitoring. Temperature monitoring is indicated in most pediatric anesthetics because of the increased prevalence of both MH and, more commonly, hypothermia in infants and children exposed to ambient OR temperatures.
Anesthetic Considerations for Specific Surgical Approaches
Two features of laparoscopic intervention create concern in the anesthetic management of infants and children: (1) the creation of a pneumoperitoneum with the concomitant increase in intra-abdominal pressure and resultant changes in ventilatory parameters; and (2) the extremes of patient positioning that may be required for optimal exposure of intra-abdominal structures.46 An appreciation of the physiologic, hemodynamic, and ventilatory consequences during and after a laparoscopic operation is an important part of careful patient selection.
Carbon dioxide is the gas of choice for insufflation for several reasons. Carbon dioxide is noncombustible and is cleared more rapidly from the circulation than the other options. The cardiovascular consequences of intravascular gas embolism present less risk with CO2 than with an insoluble gas such as helium or air. However, cardiovascular collapse has been reported in several infants following insufflation, with end-tidal gas monitoring implying that these events were due to gas embolism.47,48 Neonates and very young infants may be uniquely at risk for such events because of possible patency and large caliber of the ductus venosus. Carbon dioxide uptake may be significantly greater in children, owing to the greater absorptive area of the peritoneum in relation to body weight, and the smaller distance between capillaries and peritoneum. Regardless, hypercarbia has been demonstrated in pediatric studies during CO2 insufflation.49 Increases in minute ventilation by as much as 60% may be required to maintain baseline ETCO2, but the goal for an appropriate CO2 level need not be the baseline value. Instead, ETCO2 can safely rise into the 50s.
Hydrocephalic patients warrant special mention in regard to CO2 insufflation. Although patients with VP shunts have been shown to have intracranial pressure increases associated with a modest decrease in cerebral perfusion pressure at an intra-abdominal pressure of 10 mmHg or less,50 a recent review of laparoscopic compared to open abdominal surgery in children with shunts showed no pneumocephalus or increase in the incidence of shunt infection in the laparoscopic group.51 This is due to the fact that most VP shunts now have a one-way valve that will not allow gas entry. Interestingly, one group recently reported a case of pneumocephalus that occurred in a patient with such a shunt and valve that was inserted 20 years earlier.52
The increase in intra-abdominal pressure seen with laparoscopy is associated with well-documented cardiorespiratory changes. Changes in ventilatory dynamics occur due to cephalad displacement of the diaphragm. This results in a reduction in lung volume, ventilation-perfusion mismatch, and altered gas exchange. Bozkurt and coworkers demonstrated statistically significant decreases in pH and PaO2 and increased PaCO2 after 30 minutes of pneumoperitoneum.53 These changes are additive to the 20% reduction in FRC that occurs with induction of general anesthesia. The magnitude of the pulmonary effects correlates directly with intraperitoneal pressures, and may be further exacerbated by steep Trendelenburg positioning.54
Significant cardiovascular changes have been demonstrated in response to increased intra-abdominal pressure and patient position. In the supine or Trendelenburg position, the venous return is less impaired when the intra-abdominal pressure is kept below 15 mmHg. The position preferred for upper abdominal procedures is reverse-Trendelenburg or supine. The head-up position reduces venous return and CO.55 Several pediatric studies have utilized echocardiography (supine),56 impedance cardiography (15° head-down),57 and continuous esophageal aortic blood flow echo-Doppler (supine)58 to assess hemodynamic changes during laparoscopic surgery. These studies demonstrated significant reductions in stroke volume and cardiac index (CI), along with a significant increase in SVR. Pneumoperitoneum was found to be associated with significant increases in left ventricular end-diastolic volume, left ventricular end-systolic volume, and left ventricular end-systolic wall stress.56 All three studies demonstrated a decrease in cardiac performance and an increase in vascular resistance in healthy patients undergoing laparoscopy for lower abdominal procedures. The cardiovascular changes seen with pneumoperitoneum (Box 3-5) occur immediately with creation of the pneumoperitoneum and resolve on desufflation.
Thoracoscopy
Thoracoscopy has advantages over open thoracotomy, including reduced postoperative pain, decreased duration of hospitalization, improved cosmetic results, and decreased incidence of chest wall deformity.59,60 An optimal anesthetic plan considers potential respiratory derangements including ventilation-perfusion mismatch which may result from positioning, CO2 insufflation into the pleural cavity, and single-lung ventilation. In addition, much like insufflation during laparoscopy, hemodynamic changes during chest insufflation can compromise preload, stroke volume, CI, and blood pressure.60
In a study of 50 pediatric patients undergoing thoracoscopy for a variety of operations, systolic and diastolic blood pressures were significantly lower, and ETCO2 was significantly higher during thoracoscopy.60 After intrapleural CO2 insufflation, there was a statistically significant increase in ETCO2 during one-lung ventilation (OLV) compared with two-lung ventilation. On the other hand, two-lung ventilation with CO2 insufflation was associated with a lower systolic and diastolic pressure than OLV. The increase in ETCO2 correlated with the duration of the insufflation. These factors should be considered along with any pre-existing preoperative respiratory or cardiovascular compromise in planning the operation and anesthetic management. The magnitude of the physiologic changes induced by either one-lung or two-lung ventilation with insufflation is impacted by the patient’s age, underlying co-morbid conditions, and anesthetic agents utilized.
Many thoracic procedures require lung deflation and minimal lung excursion on the operative side while ventilating the contralateral lung. OLV is useful if the surgeon requires additional exposure. In the pediatric patient, there are several options for attaining unilateral lung isolation (Fig. 3-3).61
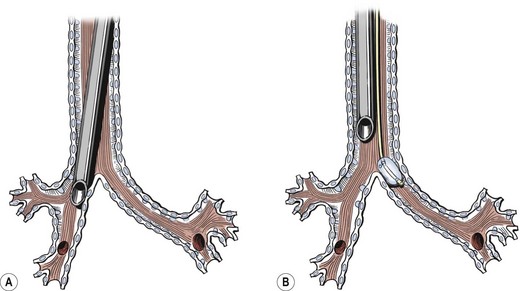
FIGURE 3-3 There are several methods available for single-lung ventilation in infants and children. (A) The most common method is to use a conventional single-lumen endotracheal tube to intubate a main-stem bronchus. (B) Another technique is to position the endotracheal tube in the trachea followed by insertion of a balloon-tipped bronchial blocker that is passed along the endotracheal tube and occludes the ipsilateral main-stem bronchus. The position of the bronchial blocker is usually confirmed using fiberoptic bronchoscopy.
Complications related to anesthetic management are usually related to mechanical factors such as airway injury and malposition of the ETT. Additional problems related to physiologic alterations include hypoxemia and hypercapnia. An unusual complication was reported during attempted thoracoscopic resection of a congenital cystic adenomatoid malformation in a 3.5 kg infant.62 During CO2 insufflation, there was a sharp rise in ETCO2 accompanied by severe hypoxemia and bradycardia. This was due to occlusion of the ETT by blood. After immediate conversion, it was discovered that there had been direct insufflation into the cyst and that the cyst communicated directly with the tracheobronchial tree.
It is important to try to maintain a reasonable range of elevated CO2 in neonates undergoing thoracoscopic procedures. Mukhtar and colleagues reported that permissive hypercapnia with ETCO2 50–70 mmHg was associated with improved cardiac output and arterial oxygen tension in neonates undergoing thoracoscopic ligation of patent ductus arteriosus.63 A case series in which high-frequency oscillating ventilation (HFOV) was used in neonates undergoing thoracoscopic procedures has been reported.64 HFOV enables better CO2 elimination while optimizing the visualization for the surgeons.
Postanesthesia Care
The recovery period for infants and children may be more crucial than for adult patients with 3–4% of infants and children developing major complications in the recovery period, compared to only 0.5% of adults. Most of these complications occur in the youngest children (<2 years of age) and are most commonly respiratory in nature.65
Common Postanesthesia Problems
Postoperative Nausea and Vomiting
PONV is the most common cause of delayed discharge from the postanesthesia care unit (PACU) and the most common reason for unanticipated hospitalization following outpatient surgery.66,67 Certain procedures, such as strabismus surgery, middle ear surgery, orchiopexy, and umbilical hernia repair are associated with a greater than 50% incidence of postoperative vomiting. Similarly, the perioperative use of any opioid is associated with a very high incidence of PONV, even when general anesthetic drugs associated with a lower incidence of nausea, such as propofol, are used.68 Common approaches to treat or prevent PONV include alteration of the anesthetic technique, perioperative administration of antiemetics (either prophylactically or as treatment), and limitation of postoperative oral intake.69,70
Respiratory Complications
Postintubation croup, or postextubation subglottic edema, has been a well-recognized entity since airways were first secured with endotracheal tubes. Children are more prone to develop croup following intubation than adults because of the differences in their airway anatomy. Children have narrower laryngeal and tracheal lumens that are more easily compromised by mucosal edema. Children with trisomy 21 may be at increased risk for this complication due to the increased incidence of occult subglottic narrowing. Other contributing factors to the development of croup include traumatic or repeated intubation attempts, coughing (‘bucking’) on the ETT, changes in patient position after intubation, and general anesthesia in children with a current or recent upper respiratory tract infection.71–73
The incidence of post-intubation croup has decreased from 6% to 1% of all endotracheally intubated children.74 This reduction has occurred because of the development and use of sterile, implant-tested ETTs, the routine intraoperative use of humidified gases, and by using an appropriately sized (air leak pressure of less than 25 cm water) ETT in children younger than 5 years of age.
Laryngospasm, while possibly life threatening, is almost always transient and treatable by early application of continuous positive airway pressure (CPAP) by mask combined, if necessary, with a small dose of propofol (1–2 mg/kg). Rescue with succinylcholine is indicated if oxygen desaturation persists despite CPAP and propofol. Laryngospasm may also occur in the OR during anesthetic induction or emergence from anesthesia. Effective maneuvers for management of laryngospasm have recently been outlined in a helpful algorithm accompanying a case scenario publication.75 Bronchospasm is more common in children with poorly controlled asthma and those exposed to second hand smoke. It is most often managed with administration of nebulized β-agonists such as albuterol.73
Intraoperative Awareness
Intraoperative awareness is a rare but disturbing condition in which patients undergoing an operation and anesthesia can recall surroundings, sounds, events, and sometimes even pain. The definition of intraoperative awareness is becoming conscious during a procedure performed under general anesthesia, with subsequent explicit memory of specific events that took place during that time. A Sentinel Event Alert was issued by the Joint Commission (JC) regarding the prevention and management of intraoperative awareness in October 2004. The ASA published a Practice Advisory for Intraoperative Awareness and Brain Functioning Monitoring in April 2006.76
The incidence of intraoperative awareness in adults has been reported to be 0.1–0.9% in older studies, and 0.0068% or 1 per 14,560 patients in a 2007 report of 87,361 patients.77 Most experts estimate the true incidence in adults to be 0.1–0.2%. There is a dearth of literature about intraoperative awareness in infants and children, but there is a 2005 study of 864 children in which the incidence was reported as 0.8%.78 Some of these data may be confounded by the memory of entering the OR after administration of preoperative sedation or a memory of events and sensations during emergence. Certainly, the likelihood of a clear memory of a painful event during surgery is a much rarer event than the other events more commonly reported. However, there are multiple adverse consequences of intraoperative awareness, including post-traumatic stress disorder and medical-legal implications.
Pain Management
The incidence of postoperative pain in the pediatric population, although difficult to evaluate objectively, is probably similar to that in the adult population. It is reasonable, therefore, to assume that about 75% of children will report significant pain on the first postoperative day.79 Many studies looking at pain in hospitalized children report under-treatment in both medical and surgical patients.80 This under-treatment may be related to: (1) inadequate analgesia provided intraoperatively; (2) underestimation of an infant’s ability to experience pain (primarily in neonates who are erroneously believed to be incapable of experiencing or remembering painful experiences); (3) fear of analgesic (primarily opioid) side effects; (4) fear of addiction by both caregivers and parents; (5) inadequate knowledge or utilization of pain assessment scales in children who are either pre-verbal or unable to use numerical rating scales; (6) failure to appreciate the benefit of nonopioid analgesics in provision of effect pain relief while reducing total opioid dose and attendant adverse effects; and (7) failure to utilize basic regional analgesic techniques that are easily applied even in the ambulatory setting.
The management of pain in infants and children is hampered by the difficulty that exists in assessing pain. Many children may respond to pain by emotionally withdrawing from their surroundings, and this may be misinterpreted by the medical and nursing staff as evidence that they have no pain. In addition, when questioned as to their degree of pain, children may not volunteer useful information for fear of painful interventions (e.g., ‘shots’). To circumvent these difficulties, pain assessment scales have been developed for use in infants and children that are more objective and depend on caregiver assessment of body positions, facial expression, and physiologic variables. Although there are many scales available, an institution should adopt one scale for each stage of development, and ensure that caregivers are trained so that they are used reproducibly in settings where pain is treated. Examples of these pain scales include CRIES for neonates (until 1 month of age), FLACC from 1 month to age 4 years, FACES for age 5 to 9 years and in children who are developmentally appropriate, and a numerical scale for those older than 10 years of age.81,82
Opioids
The mainstay in pain control remains the use of opioids, although increasingly regional analgesic techniques (epidural or peripheral nerve block) are being used in infants and children. There are many opioids available for both IV and oral administration, but they all have common adverse effects. These include dose-dependent respiratory depression as mentioned above, which may be more prominent in neonates and young infants and in patients with OSA.12,83,84 Other side effects that vary in prevalence among drugs and patients are dysphoria, somnolence, nausea and vomiting, pruritus, constipation, and urinary retention.
Morphine remains the standard by which the potency of other opioids is measured. Equipotent analgesic doses of commonly used IV opioids are listed in Table 3-3. As the plasma concentration of morphine correlates poorly with its desired analgesic effect—a fourfold variation has been measured in the plasma concentration of morphine at which patients express the need for additional pain medication—many clinicians believe that morphine is best administered in a patient-controlled device (patient-controlled analgesia [PCA]) to allow self-titration of medication according to the level of pain experienced.
A discussion of patient selection and dosing for PCA is beyond the scope of this chapter, but can be found in many textbooks of pediatric anesthesiology and pain management.81 Patients receiving PCA should be continuously monitored for cardiorespiratory depression by monitoring the echocardiogram, respiratory rate, and pulse oximetry.85
Fentanyl is a synthetic opioid that usually has a relatively short duration of action as a result of its rapid distribution into fat and muscle due to its high lipid solubility. With repeated dosing, the duration of action appears to increase.86 When compared with morphine, fentanyl is about 100 times more potent. (Fentanyl dosages are calculated in micrograms rather than milligrams.) In controlled comparisons with equipotent dosages, morphine is generally found to provide better, more long-lasting analgesia than fentanyl, but with more side effects such as pruritus, nausea, and vomiting.87–89 Opioids with short half-lives like fentanyl may also demonstrate the development of much more rapid tolerance to its analgesic effects than morphine or hydromorphone.
Hydromorphone is a well-tolerated alternative to morphine and fentanyl, and is felt to cause less pruritus and sedation than morphine, with the few adult studies that exist suggesting equivalence rather than superiority.90 It is five to seven times more potent than morphine, and its duration of action is similar to morphine, and longer than fentanyl.
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
As more and more pediatric operations are being performed on an outpatient basis, and with the goal of minimizing opioid dosing to reduce adverse effects, significant interest has developed in the role of nonopioid analgesics for management of postoperative pain. Acetaminophen is an effective analgesic for mild to moderate pain, and can be administered rectally in the perioperative period, especially to infants. Rectal absorption is variable and bioavailability is lower, mandating a higher initial dose (30–40 mg/kg) than that administered orally (10–15 mg/kg).91,92 A rectal dose of 30 mg/kg of acetaminophen has proved to have analgesic properties similar to 1 mg/kg of ketorolac.93 In 2011, intravenous acetaminophen was approved for use in adults and children older than 2 years of age in the U.S.
Ketorolac is an oral and parenteral NSAID shown to have excellent pain control characteristics unassociated with PONV, or respiratory depression.94–96 Dosage recommendations are 0.5 mg/kg intravenously (maximum dose 30 mg) every 6 to 8 hours for 48 hours. Due to its effects on renal blood flow and tubular function, ketorolac is contraindicated in patients with pre-existing impairment of renal function. Likewise, it should not be administered to patients at risk for coagulopathy or a history of gastric ulcers. As NSAIDs such as ketorolac and ibuprofen affect platelet aggregation and adhesiveness, their use is limited in many patients that are at risk for postoperative bleeding, particularly children who have undergone tonsillectomy.93,97 In addition, many orthopedic surgeons forbid the use of NSAIDS during and after operations in which new bone formation is important (fractures, spine fusions) because NSAIDS have been shown to impair osteoblastic activity.98 The extent to which this effect is clinically important is unclear.99,100
Regional and Local Anesthetic Techniques
As general anesthesia is nearly universal in children, pure regional anesthesia is less common than in adults. However, pediatric patients, including outpatients, are excellent candidates for a host of regional blocks.101–103 Some blocks require specialized equipment like a nerve stimulator or ultrasound, but others such as an ilioinguinal block can be performed by landmarks alone. Local infiltration by the surgeon is encouraged when a neuraxial or peripheral block is not performed.
Regional anesthetic techniques used concomitantly with general anesthesia have had resurgence in both adult and pediatric patients. These techniques include peripheral nerve blocks, and caudal, epidural, or spinal blocks. These blocks include the rectus sheath block for umbilical procedures, ilioinguinal block for inguinal procedures, and the transversus abdominis plane block for lower abdominal procedures.104–106
Clonidine has gained favor as an adjunct in regional anesthesia. A centrally acting alpha-2 agonist with anti-emetic and mild sedative effects, clonidine confers an analgesic benefit as well. It has been shown to increase the analgesic duration of caudal blocks to as long as 18 hours.107 Clonidine has also been used effectively in epidural infusions. Moreover, rather than causing nausea or pruritus, clonidine actually decreases the incidence of postoperative nausea. In higher doses (≥2 µg/kg) given epidurally, clonidine may cause sedation, with some authors recommending that children receiving this dose be admitted for observation. Clonidine is not recommended for use in infants under 6 months of age.
In selected cases, peripheral nerve blocks appear to be a superior pain control modality. They offer the benefit of no systemic side effects (nausea, pruritus, sedation, urinary retention) and often allow for faster recovery. It is increasingly common for these blocks to be performed under ultrasound guidance, which confers increased accuracy of placement, which in turn allows the use of reduced local anesthetic volume, greater efficacy, and improved efficiency. For orthopedic extremity surgery, some children are being discharged home with peripheral nerve catheters which are removed at home by the parents two days postoperatively.108
Prescribing Discharge Analgesics
The surgeon or surgeon’s designee must take seriously the responsibility of prescribing pain medications to be administered by the parents at home after discharge. This is important for all patients, but especially for ambulatory surgery patients because of the rapid transition from PACU to home. It is imperative to clearly communicate with the parent/guardian regarding the nature of the medications prescribed, assessment of pain, and realistic expectations for the course of pain in the days after surgery. It is important to emphasize the same issues that are of concern when giving analgesics in the hospital: right drug, right dose, right time.
Numerous studies looking at parental home analgesic administration after surgery have shown that parents commonly do not understand that some children may become withdrawn and immobile in response to pain instead of crying.109 In addition, many parents fail to administer prescribed pain medication even when they recognize their child is having pain, in part because of lack of specific instructions or because of fear of adverse effects, including misperceptions about the potential for ‘addiction’.110,111 Care must be taken to avoid advising time-contingent (especially around the clock) dosing of opioids because of the increased risk of nausea, vomiting, constipation, but most importantly somnolence and respiratory depression.112
With regard to choice of opioid, prescribers should be knowledgeable about recommended dosage and formulations available for various oral opioids. The most commonly prescribed opioid in children has been codeine (more specifically acetaminophen with codeine). A recent publication has noted concerns about a number adverse effects of codeine administration.113 These include lack of analgesic efficacy in approximately 5–10% of the population in whom low CYP2D6 activity leads to low or no conversion of codeine to morphine in the body, which is required for analgesia.114 More worrisome is the fact that up to a third of individuals (depending on their ethnic origin) are ultrarapid metabolizers because of increased CYP2D6 activity. Codeine administration in these individuals results in high plasma levels of morphine which can cause respiratory depression, which is especially worrisome in children and especially in children with OSA. The risk of codeine administration to children who may be unidentified ultrarapid metabolizers led the U.S. Food and Drug Administration to issue a safety alert in August, 2012 regarding the risk of adverse events or death in children given codeine after tonsillectomy and/or adenoidectomy.115,116
Discharge Criteria
In general, children should be comfortable, awake, and stable, on room air or back to baseline oxygen supplementation, have age-appropriate vital signs, and be well hydrated before discharge from outpatient surgery. These variables have been quantified with the modified Aldrete score (Table 3-4), which lists the important factors taken into consideration for discharge. Most institutions require a modified Aldrete score of 9 or greater for discharge to floor, but criteria for discharge home should be stricter, comprising the elements listed in Box 3-6.
References
1. Chopra, V, Bovill, JG, Spierdijk, J. Accidents, near accidents and complications during anesthesia: A retrospective analysis of a 10-year period in a teaching hospital. Anaesthesia. 1990; 45:3–6.
2. Aubas, S, Biboulet, P, Daures, JP, et al. Incidence and etiology of cardiac arrest occurring during the peroperative period and in the recovery room: Apropos of 102,468 anesthesia cases. Ann Fr Anesth Reanim. 1991; 10:436–442.
3. Odegard, KC, DiNardo, JA, Kussman, BD, et al. The frequency of anesthesia-related cardiac arrests in patients with congenital heart disease undergoing cardiac surgery. Anesth Analg. 2007; 105:335–343.
4. Bhananker, SM, Ramamoorthy, C, Geiduschek, JM, et al. Anesthesia-related cardiac arrest in children: Update from the Pediatric Perioperative Cardiac Arrest Registry. Anesth Analg. 2007; 105:344–350.
5. Flick, RP, Sprung, J, Harrison, TE, et al. Perioperative cardiac arrests in children between 1988 and 2005 at a tertiary referral center. Anesthesiology. 2007; 106:226–237.
6. Patel, RI, Hannallah, RS. Preoperative screening for pediatric ambulatory surgery: Evaluation of a telephone questionnaire method. Anesth Analg. 1992; 75:258–261.
7. Zuckerberg, AL. A hot mnemonic for the treatment of malignant hyperthermia. Anesth Analg. 1993; 77:1077.
8. Cook-Sather, SD, Harris, KA, Chiavacci, R, et al. A liberalizaed fasting guideline for formula-fed infants does not increase average gastric fluid volume before elective surgery. Anesth Analg. 2003; 96:965–969.
9. Tait, AR, Reynolds, PI, Gutstein, HB. Factors that influence an anesthesiologist’s decision to cancel elective surgery for the child with an upper respiratory tract infection. J Clin Anesth. 1995; 7:491–499.
10. Parnis, SJ, Barker, DS, Van Der Walt, JH. Clinical predictors of anaesthetic complications in children with respiratory tract infections. Paediatr Anaesth. 2001; 11:29–40.
11. Tait, AR, Malviya, S, Voepel-Lewis, T, et al. Risk factors for perioperative adverse respiratory events in children with upper respiratory tract infections. Anesthesiology. 2001; 95:299–306.
12. Rigatto, H, Brady, JP. Periodic breathing and apnea in preterm infants: Evidence for hypoventilation possibly due to central respiratory depression. Pediatrics. 1972; 50:202–218.
13. Coté, CJ, Zaslavsky, A, Downes, JJ, et al. Postoperative apnea in former preterm infants after inguinal herniorrhaphy: A combined analysis. Anesthesiology. 1995; 82:809–822.
14. Fisher, D. When is the ex-premature infant no longer at risk for apnea? Anesthesiology. 1995; 82:807–808.
15. Hammer, GB. Anaesthetic management for the child with a mediastinal mass. Paediatr Anaesth. 2004; 14:95–97.
16. Yamashita, M, Chin, I, Horigome, H. Sudden fatal cardiac arrest in a child with an unrecognized anterior mediastinal mass. Resuscitation. 1990; 19:175–177.
17. Viswanathan, S, Campbell, CE, Crok, RC. Asymptomatic undetected mediastinal mass: A death during ambulatory anesthesia. J Clin Anesth. 1995; 7:151–155.
18. Lerman, J. Anterior mediastinal masses in children. Semin Anesth. 2007; 26:133–140.
19. Cho, Y, Suzuki, S, Yokoi, M, et al. Lateral position prevents respiratory occlusion during surgical procedure under general anesthesia in the patient of huge anterior mediastinal lymphoblastic lymphoma. J Thorac Cardiovasc Surg. 2004; 52:476–479.
20. Shamberger, RC, Holzman, RS, Griscom, NT, et al. CT quantitation of tracheal cross-sectional area as a guide to the surgical and anesthetic management of children with anterior mediastinal masses. J Pediatr Surg. 1991; 26:138–142.
21. Mahmoud, M, Tyler, T, Sadhasivam, S. Dexmedetomidine and ketamine for large anterior mediastinal mass biopsy. Paediatr Anaesth. 2008; 18:1011–1013.
22. Cheung, S, Lerman, J. Mediastinal masses and anesthesia in children. Anesthesiol Clin North Am. 1998; 16:893–910.
23. Ricketts, RR. Clinical management of anterior mediastinal tumors in children. Semin Pediatr Surg. 2001; 10:161–168.
24. Greenwood, RD, Rosenthal, A, Parisi, L, et al. Extracardiac abnormalities in infants with congenital heart disease. Pediatrics. 1975; 55:485–492.
25. Hoffman, JI, Christianson, R. Congenital heart disease in a cohort of 19,502 births with long-term follow-up. Am J Cardiol. 1978; 42:641–647.
26. Hennein, HA, Mendeloff, EN, Cilley, RE, et al. Predictors of postoperative outcome after general surgical procedures in patients with congenital heart disease. J Pediatr Surg. 1994; 29:866–870.
27. Strom, BL, Abrutyn, E, Berlin, JA, et al. Dental and cardiac risk factors for infective endocarditis. A population based, case-control study. Ann Intern Med. 1998; 129:761–769.
28. Roberts, GJ. Dentists are innocent! Everyday bacteremia is the real culprit: A review and assessment of the evidence that dental surgical procedures are the principal cause of endocarditis in children. Paediatr Cardiol. 1999; 20:317–325.
29. Seymour, RA, Lowry, R, Whitworth, JM, et al. Infective endocarditis, dentistry and antibiotic prophylaxis; Time for a rethink? Br Dent J. 2000; 189:610–615.
30. Durack, D. Antibiotics for prevention of endocarditis during dentistry: Time to scale back? Ann Intern Med. 1998; 129:829–831.
31. Horskotte, D, Follath, F, Gutschik, E, et al. Guidelines on the prevention, diagnosis and treatment of infective endocarditis: Executive summary. The task force on infective endocarditis of the European Society of Cardiology. European Heart Journal. 2004; 25:267–276.
32. Danchin, N, Duval, X, Leport, C. Prophylaxis of infective endocarditis: French recommendations 2002. Heart. 2005; 91:715–718.
33. Blaise, G, Langleben, D, Hubert, B. Pulmonary arterial hypertension. Anesthesiology. 2003; 99:1415–1432.
34. Fischer, LG, Van Aken, H, Burkle, H. Management of pulmonary hypertension: Physiological and pharmacological considerations for anesthesiologists. Anesth Analg. 2003; 96:1603–1616.
35. Friesen, RH, Williams, GD. Anesthetic management of children with pulmonary arterial hypertension. Pediatric Anesthesia. 2008; 18:208–216.
36. Hakim, TS, Michel, RP, Chang, HK. Effect of lung inflation on pulmonary vascular resistance by arterial and venous occlusion. J Appl Physiol. 1982; 53:1110–1115.
37. Luce, JM. The cardiovascular effects of mechanical ventilation and positive end expiratory pressure. JAMA. 1984; 252:807–811.
38. Jardin, F, Vieillard-Baron, A. Right ventricular function and positive pressure ventilation in clinical practice: From hemodynamic subsets to respiratory settings. Intensive Care Med. 2003; 29:1426–1434.
39. Henriksson, P, Varendh, G, Lundstron, MR. Haemostatic defects in cyanotic congenital heart disease. Br Heart J. 1979; 41:23–27.
40. Silka, MJ, Bar-Cohen, Y. Pacemakers and implantable cardioverter-defibrillators in pediatric patients. Heart Rhythm. 2006; 3:1360–1366.
41. Epstein, AE, DiMarco, JP, Ellenbogen, KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): Developed in Collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008; 117:e350–e408.
42. Madigan, JD, Choudhri, AF, Chen, J, et al. Surgical management of the patient with an implanted cardiac device: Implications of electromagnetic interference. Ann Surg. 1999; 230:639–647.
43. Practice Advisory for Perioperative Management of Patients with Cardiac Rhythm Management Devices. Pacemakers and Implantable Cardioverter-Defibrillators. Anesthesiology. 2005; 103:186–198.
44. Litman, RS. The difficult pediatric airway. In: Litman RS, ed. Pediatric Anesthesia—The Requisites in Anesthesiology. Philadelphia: Elsevier Mosby; 2004:135–146.
45. Standards for basic anesthetic monitoring (Approved by the ASA House of Delegates on October 21, 1986 and last amended on October 20, 2010.
46. Pennant, JH. Anesthesia for laparoscopy in the pediatric patient. Anesth Clin North Am. 2001; 19:69–74.
47. Kudsi, OY, Jones, SA, Brenn, BR. Carbon dioxide embolism in a 3-week-old neonate during laparoscopic pyloromyotomy: A case report. J Pediatr Surg. 2009; 44:842–845.
48. Taylor, SP, Hoffman, GM. Gas embolus and cardiac arrest during laparoscopic pyloromyotomy in an infant. Can J Anaesth. 2010; 57:774–778.
49. McHoney, MC, Corizia, L, Eaton, S, et al. Carbon dioxide elimination during laparoscopy in children is age dependent. J Pediatr Surg. 2003; 38:105–110.
50. Uzzo, RG, Bilsky, M, Mininberg, DT, et al. Laparoscopic surgery in children with ventriculoperitoneal shunts: Effect of pneumoperitoneum on intracranial pressure— preliminary experience. Pediatr Urol. 1997; 49:753–757.
51. Fraser, JD, Aguayo, P, Sharp, SW, et al. The safety of laparoscopy in pediatric patients with ventriculoperitoneal shunts. J Laparoendosc Adv Surg Tech. 2009; 19:675–678.
52. Raskin, J, Guillaume, DJ, Ragel, BT. Laparoscopic-induced pneumocephalus in a patient with a ventriculoperitoneal shunt. Pediatr Neurosurg. 2010; 46:390–391.
53. Bozkurt, P, Kaya, G, Altintas, F, et al. Systemic stress response during operations for acute abdominal pain performed via laparoscopy or laparotomy in children. Anaesthesia. 2000; 55:5–9.
54. Bannister, CF, Brosius, KK, Wulkan, M. The effect of insufflation pressure on pulmonary mechanics in infants during laparoscopic surgical procedures. Paediatr Anaesth. 2003; 13:785–789.
55. Joris, JL, Noirot, DP, Legrand, MJ, et al. Hemodynamic changes during laparoscopic cholecystectomy. Anesth Analg. 1993; 76:1067–1071.
56. Gentili, A, Iannettone, CM, Pigna, A, et al. Cardiocirculatory changes during videolaparoscopy in children: An echocardiographic study. Paediatr Anaesth. 2000; 10:399–406.
57. Kardos, A, Vereczkey, G, Pirot, L, et al. Use of impedance cardiography to monitor haemodynamic changes during laparoscopy in children. Paediatr Anaesth. 2001; 11:175–179.
58. Gueugniaud, P, Abisseror, M, Moussa, M, et al. The hemodynamic effects of pneumoperitoneum during laparoscopic surgery in healthy infants: Assessment by continuous esophageal aortic blood flow echo-Doppler. Anesth Analg. 1998; 86:290–293.
59. Haynes, SR, Bonner, S. Anaesthesia for thoracic surgery in children. Paediatr Anaesth. 2000; 10:237–251.
60. Gentili, A, Lima, M, De Rose, R, et al. Thoracoscopy in children: Anaesthesiological implications and case reports. Minerva Anesthesiol. 2007; 73:161–171.
61. Hammer, GB. Single-lung ventilation in infants and children. Paediatr Anaesth. 2004; 14:98–102.
62. Mukhtar, AM, Dessouky, NM. Unusual complication during pediatric thoracoscopy. Pediatr Anesth. 2006; 16:986–988.
63. Mukhtar, AM, Obayah, GM, Elmasry, A, et al. The therapeutic potential of intraoperative hypercapnia during video-assisted thoracoscopy in pediatric patients. Anesth Analg. 2008; 106:84–88.
64. Mortellaro, VE, Fike, FB, Adibe, OO, et al. The use of high-frequency oscillating ventilation to facilitate stability during neonatal thoracoscopic operations. J Laparoendosc Adv Surg Tech. 2011; 21:877–879.
65. Murat, I, Constant, I, Maud’huy, H. Perioperative anaesthetic morbidity in children: A database of 24,165 anaesthetics over a 30-month period. Paediatr Anaesth. 2004; 14:158–166.
66. Patel, RI, Hannallah, RS. Anesthetic complications following pediatric ambulatory surgery: A 3-year study. Anesthesiology. 1988; 69:1009–1012.
67. Watcha, MF, White, PF. Postoperative nausea and vomiting: Its etiology, treatment, and prevention. Anesthesiology. 1992; 77:162–184.
68. Weir, PM, Munro, HM, Reynolds, PI, et al. Propofol infusion and the incidence of emesis in pediatric outpatient strabismus surgery. Anesth Analg. 1993; 76:760–764.
69. Schreiner, MS. Preoperative and postoperative fasting in children. Pediatr Clin North Am. 1994; 41:111–120.
70. Schreiner, MS, Nicolson, SC, Martin, T, et al. Should children drink before discharge from day surgery? Anesthesiology. 1992; 76:528–533.
71. Koka, BV, Jeon, IS, Andre, JM, et al. Postintubation croup in children. Anesth Analg. 1977; 56:501–505.
72. Schreiner, MS, O’Hara, I, Markakis, DA, et al. Do children who experience laryngospasm have an increased risk of upper respiratory tract infection? Anesthesiology. 1996; 85:475–480.
73. von Ungern-Sternberg, BS, Boda, K, Chambers, NA, et al. Risk assessment for respiratory complications in paediatric anaesthesia: A prospective cohort study. Lancet. 2010; 376:773–783.
74. Khine, HH, Corddry, DH, Kettrick, RG, et al. Comparison of cuffed and uncuffed endotracheal tubes in young children during general anesthesia. Anesthesiology. 1997; 86:627–631.
75. Orliaquet, GA, Gall, O, Savoldelli, GL, et al. Case scenario: Perianesthetic management of laryngospasm in children. Anesthesiology. 2012; 116:458–471.
76. Practice advisory for intraoperative awareness and brain function monitoring: A report by the American Society of Anesthesiologists Task Force on Intraoperative Awareness. Anesthesiology. 2006; 104:847–864.
77. Pollard, RJ, Coyle, JP, Gilbert, RL, et al. Intraoperative awareness in a regional medical system: A review of 3 years’ data. Anesthesiology. 2007; 106:269–274.
78. Davidson, AJ, Huang, GH, Czarnecki, C, et al. Awareness during anesthesia in children: A prospective cohort study. Anesth Analg. 2005; 100:653–661.
79. Mather, L, Mackie, J. The incidence of postoperative pain in children. Pain. 1983; 15:271–282.
80. Groenewald, CB, Rabbitts, JA, Schroeder, DR, et al. Prevalence of moderate-severe pain in hospitalized children. Paediatr Anaesth. 2012; 22:661–668.
81. Malviya, S, Polaner, DM, Berde, C. Acute Pain. In: Cote CJ, Lerman J, Todres ID, eds. A Practice of Anesthesia for Infants and Children. 4th ed. Philadelphia: Saunders Elsevier; 2009:939–978.
82. Merkel, SI, Voepel-Lewis, T, Shayevitz, JR, et al. The FLACC: A behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997; 23:293–297.
83. Pasternak, GW, Zhang, A, Tecott, L. Developmental differences between high and low affinity opiate binding sites: Their relationship to analgesia and respiratory depression. Life Sci. 1980; 27:1185–1190.
84. Brown, KA, Laferriere, A, Lakheeram, I, et al. Recurrent hypoxemia in children is associated with increased analgesic sensitivity to opiates. Anesthesiology. 2006; 105:665–669.
85. Nelson, KL, Yaster, M, Kost-Byerly, S, et al. A national survey of American Pediatric Anesthesiologists: Patient-controlled analgesia and other intravenous opioid therapies in pediatric acute pain management. Anesth Analg. 2010; 110:754–760.
86. Kay, B, Rolly, G. Duration of action of analgesia supplement of anesthesia: A double-blind comparison between morphine, fentanyl, and sufentanil. Acta Anaesthesiol Belg. 1977; 28:25–32.
87. Claxton, AR, McGuire, G, Chung, F, et al. Evaluation of morphine versus fentanyl for postoperative analgesia after ambulatory surgical procedures. Anesth Analg. 1997; 84:509–514.
88. Sanford, Jr.TJ, Smith, NT, Dec-Silver, H, et al. A comparison of morphine, fentanyl, and sufentanil anesthesia for cardiac surgery: Induction, emergence, and extubation. Anesth Analg. 1986; 65:259–266.
89. Lejus, C, Roussiere, G, Testa, S, et al. Postoperative extradural analgesia in children: Comparison of morphine with fentanyl. Br J Anesth. 1994; 72:156–159.
90. Rapp, SE, Egan, KJ, Ross, BK, et al. A multidimensional comparison of morphine and hydromorphone patient-controlled analgesia. Anesth Analg. 1996; 82:1043–1048.
91. Birmingham, PK, Tobin, MJ, Henthom, TK, et al. Twenty-four hour pharmacokinetics of rectal acetaminophen in children. Anesthesiology. 1997; 87:244–252.
92. Montgomery, CJ, McCormack, JP, Reichert, CC, et al. Plasma concentrations after high-dose (45 mg•kg-1) rectal acetaminophen in children. Can J Anaesth. 1995; 42:982–986.
93. Rusy, LM, Houck, CS, Sullivan, LJ, et al. A double-blind evaluation of ketorolac tromethamine versus acetaminophen in pediatric tonsillectomy: Analgesia and bleeding. Anesth Analg. 1995; 80:226–229.
94. Forrest, JB, Heitlinger, EL, Revell, S. Ketorolac for postoperative pain management in children. Drug Saf. 1997; 16:309–329.
95. Gillis, JC, Brogden, RN. Ketorolac: A reappraisal of its pharmacodynamic and pharmacokinetic properties and therapeutic use in pain management. Drugs. 1997; 53:139–188.
96. Yaster, M. Non-steroidal anti-inflammatory drugs. In: Yaster M, Krane EJ, Kaplan RF, Cote CJ, Lappe DG, eds. Pediatric pain management and sedation handbook. St Louis: Mosby Year Book; 1997:19–27.
97. Gunter, JB, Varughese, AM, Harrington, JF, et al. Recovery and complications after tonsillectomy in children: A comparison of ketorolac and morphine. Anesth Analg. 1995; 81:1136–1141.
98. Chang, JK, Wang, GJ, Tsai, ST, et al. Nonsteroidal anti-inflammatory drug effects on osteoblastic cell cycle, cytotoxicity, and cell death. Connect Tissue Res. 2005; 46:200–210.
99. Sucato, DJ, Lovejoy, JF, Agrawal, S, et al. Postoperative ketorolac does not predispose to pseudoarthrosis following posterior spinal fusion and instrumentation for adolescent idiopathic scoliosis. Spine. 2008; 33:1119–1124.
100. Li, Q, Zhang, Z, Cai, Z. High-dose ketorolac affects adult spinal fusion: A meta-analysis of the effect of perioperative nonsteroidal anti-inflammatory drugs on spinal fusion. Spine. 2011; 36:E461–E468.
101. Marhofer, P, Ivani, G, Suresh, S, et al. Everyday regional anesthesia in children. Paediatr Anaesth. 2012; 22:995–1001.
102. Lonnqvist, PA. Blocks for pain management in children undergoing ambulatory surgery. Curr Opin Anaesthesiol. 2011; 24:627–632.
103. Willschke, H, Marhofer, P, Machata, AM, et al. Current trends in paediatric regional anaesthesia. Anaesthesia. 2010; 65(Suppl 1):97–104.
104. Willschke, H, Kettner, S. Pediatric regional anesthesia: Abdominal wall blocks. Paediatr Anaesth. 2012; 22:88–92.
105. Gurnaney, HG, Maxwell, LG, Kraemer, FW, et al. Prospective randomized observer-blinded study comparing the analgesic efficacy of ultrasound-guided rectus sheath block and local anaesthetic infiltration for umbilical hernia repair. Br J Anaesth. 2011; 107:790–795.
106. Mai, CL, Young, MJ, Quraishi, SA. Clinical implications of the transversus abdominis plane block in pediatric anesthesia. Paediatr Anaesth. 2012; 22:831–840.
107. Tripi, PA, Palmer, JS, Thomas, S, et al. Clonidine increases duration of bupivacaine caudal analgesia for ureteroneocystostomy: A double-blind prospective trial. J Urol. 2005; 174:1081–1083.
108. Gurnaney, H, Kraemer, FW, Maxwell, L, et al. Ambulatory continuous peripheral nerve blocks in children and adolescents-A longitudinal eight year single center study. Anesth Analg. 2013.
109. Zisk, RY, Grey, M, Medoff-Cooper, B, et al. The squeaky wheel gets the grease: Parental pain management of children treated for bone fractures. Pediatr Emerg Care. 2008; 24:89–96.
110. Kankkunen, P, Vehvilainen-Julkunen, K, Pietila, AM, et al. Is the sufficiency of discharge instructions related to children’s postoperative pain at home after day surgery? Scand J Caring Sci. 2003; 17:365–372.
111. Fortier, MA, MacLaren, JE, Martin, SR, et al. Pediatric pain after ambulatory surgery: Where’s the medication? Pediatrics. 2009; 124:e588–e595.
112. Sutters, KA, Holdridge-Zeuner, D, Waite, S, et al. A descriptive feasibility study to evaluate scheduled oral analgesic dosing at home for the management of postoperative pain in preschool children following tonsillectomy. Pain Med. 2012; 13:472–483.
113. Baugh, RF, Archer, SM, Mitchell, RB, et al. Clinical practice guideline: Tonsillectomy in children. Otolaryngol Head Neck Surg. 2011; 144:S1–30.
114. Williams, DG, Patel, A, Howard, RF. Pharmacogenetics of codeine metabolism in an urban population of children and its implications for analgesic reliability. Br J Anaesth. 2002; 89:839–845.
115. Ciszkowski, C, Madadi, P, Phillips, MS, et al. Codeine, ultrarapid-metabolism genotype, and postoperative death. NEJM. 2009; 361:827–828.
116. Kelly, LE, Rieder, M, van den Anker, J, et al. More codeine fatalities after tonsillectomy in North American children. Pediatrics. 2012; 129:e1343–e1347.