Anesthesia Techniques, Blood Loss/Fluid Replacement, Airway Management & Convalescence in the Treatment of Dentofacial Deformities
• Use of Deliberate Hypotensive Anesthesia to Limit Blood Loss
• Use of Hemorrhage Depressors to Limit Blood Loss
• Deliberate Hypotensive Anesthesia Used in Orthognathic Surgery: Review of the Literature
• The Need for Blood Replacement in Orthognathic Surgery
• Perioperative Airway Management in the Patient with a Dentofacial Deformity
• In-Hospital Convalescence After Orthognathic Surgery
• At-Home Convalescence and Outpatient Care After Orthognathic Surgery
Use of Deliberate Hypotensive Anesthesia to Limit Blood Loss
Historical Perspective
Deliberate hypotensive anesthesia (DHA) to provide a less bloody field and better operative conditions was first proposed by Harvey Cushing in 1917 for neurosurgical procedures.58 Since then, a spectrum of pharmacologic agents (e.g., nitroglycerine, calcium-channel blocking agents, short-acting beta-adrenergic blockers, purine compounds) and techniques have been used in a variety of combinations in an attempt to accomplish the same objectives.*
In 1950, Enderby and colleagues demonstrated the value of DHA to reduce blood loss in a series of 35 patients.78–81 Eighteen of the 35 (51%) were judged to have an excellent reduction in blood loss during deliberate hypotension, and 8 of the 35 (23%) were judged to have a moderate reduction. The authors attributed this inconsistent effectiveness to individual vascular responses to the hypotensive drugs that were used. They surmised that bleeding at the surgical site could be further minimized if the wound itself was kept uppermost (rather than dependent) through the use of operating room table positioning (e.g., reverse Trendelenburg position for head and neck procedures). Through special positioning, arterial vessels in the operative field would have less pressure, veins would drain more easily, and thus bleeding at the surgical site should be less.
One of the earliest studies to quantify reductions in blood loss with DHA was performed by Eckenhoff and Rich, who completed research in patients who were undergoing a variety of operations, including rhinoplasty, portacaval shunt, and craniotomy for aneurysm.72,73 Half of the patients underwent DHA (n = 115), and the other half (n = 116) went without hypotension. For each of the procedures, blood loss decreased by 50% or more with hypotension techniques. This was the first DHA study to measure blood loss with the use of a control group. By doing so, it greatly added to the power of the research confirming the value of DHA.
Evidence supporting the use of DHA to reduce blood loss has most commonly been found in studies of orthopedic procedures. Thompson and colleagues studied hypotensive anesthesia to reduce blood loss during total hip arthroplasty.255 The mean arterial pressure (MAP) of the patients (n = 30) was reduced to 50 mm Hg. The techniques used to do this included sodium nitroprusside (n = 12) and high inspired concentrations of halothane (n = 9); a control group of patients (n = 9) was normotensive. Blood loss was 1200 mL for the normotensive control subjects but only 400 mL for both hypotensive groups. No anesthesia-specific complications were seen. In 1979, Eerola and colleagues completed a controlled study of 55 patients who were undergoing total hip arthroplasty.76 They found that the 38 patients who were given pentolinium tartrate and halothane for DHA had less blood loss. That same year, Vazeery and colleagues studied patients who were undergoing total hip replacement (n = 25). The patients were given sodium nitroprusside to lower arterial blood pressure.262 They had significantly less blood loss than the control group (n = 25), which was not undergoing deliberate hypotension. The value of DHA to reduce blood loss has also been confirmed for other procedures, including craniotomy, middle-ear surgery, radical cancer operations, and a variety of head and neck surgeries.
Didier and colleagues suggested that the depression of cardiac output correlated better with a dry field than did arterial blood pressure.66 To determine whether a decrease in MAP or cardiac output was the primary cause of the decreased blood loss, Sivarajan and colleagues studied “healthy” (American Society of Anesthesiologist I) subjects (n = 20) who were undergoing bilateral sagittal split ramus osteotomies of the mandible.239 Cardiac output decreased 37% with the use of trimethaphan, but it decreased only 27% with sodium nitroprusside. Interestingly, blood loss was similar for both groups, although cardiac output was two times greater with nitroprusside. The authors concluded that blood pressure rather than cardiac output was the primary determinant of how much blood was lost.
Techniques to Induce Deliberate Hypotensive Anesthesia
Clearly, one can decrease MAP by increasing the amount of inhaled anesthetics such as sevoflurane, isoflurane, and desflurane. Certain drugs that permit moment-to-moment controlled blood pressure are the most popular. The differences in pharmacologic properties among these agents suggest that combinations of these drugs may provide a better pharmacologic profile than could be provided by any agent used alone.234
Effects of Deliberate Hypotensive Anesthesia on Organ Function
Inadequate oxygen supply to the brain and myocardium as a result of inadequate tissue blood flow or perfusion pressure is the principal hazard of DHA, particularly with regard to the effects on cerebral metabolic homeostasis.* Studies to date have shown that the responsible use of DHA does not produce permanent changes in cerebral hemodynamics. For example, the cognitive performance of elderly patients on psychological tests given several days after total hip arthroplasty that involved DHA did not differ from their performance before surgery. The current clinical rationale for studying a lower limit for MAP (e.g., 50 mm Hg to 55 mm Hg) in normotensive patients is based on the belief that this range represents the lowest MAP at which autoregulation of the cerebral blood flow is still intact. However, for chronically hypertensive patients or for patients who are older, the curve is thought to shift to the right. In other words, a higher MAP is required in these individuals to maintain autoregulation. The partial pressure of carbon dioxide in the blood (paCO2) is also an important consideration during DHA. This is because cerebral blood flow changes linearly with paCO2. Whether one drug is better than another for preserving cerebral blood flow is debated.
The preservation of cardiac function is essential, but it is known to be altered by DHA techniques.† Interestingly, cardiac function is better preserved with isoflurane than with equal hypotensive doses of halothane. Studies have shown that isoflurane is suitable for healthy patients who are undergoing DHA. Clinical studies also show that the cardiovascular effects of general anesthetic doses of propofol are similar to those of isoflurane. During deliberate hypotension, the maintenance of an oxygen supply that is sufficient for the metabolic needs of the myocardium is of primary importance. As a general rule, patients with known or suspected ischemic heart disease should not undergo DHA.
Renal blood flow normally equals 20% to 25% of cardiac output. Renal circulation is greatly influenced by autoregulatory mechanisms.61,110,159 Clinical studies suggests that normovolemic patients typically experience the rapid recovery of urine production after the discontinuation of DHA.255 Thompson and colleagues could find no significant changes in serum creatinine, blood urea nitrogen, or serum or urinary electrolytes for patients (n = 30) who had undergone DHA.255
Complications Associated with Deliberate Hypotensive Anesthesia
The precise incidence of complications with the use of DHA is difficult to determine.19,75,41,152,179,248,253 Current data regarding non-fatal complications indicate that hypotension of 50 mm Hg to 65 mm Hg is not a risk factor for young healthy patients (American Society of Anesthesiologists [ASA] 1 and 2). Alternatively, for those individuals with underlying organ dysfunction, the use of DHA may put them at increased risk. Thus, all potential candidates should undergo a complete history and physical examination before surgery. The decision to use DHA should not be made without careful preoperative consideration of patient-specific risk factors.
Special Considerations for the Orthognathic Patient
Before beginning an orthognathic procedure, the logistics of patient positioning, the expected length of the surgery, and the confirmation of patient-specific risk factors for DHA should be discussed by the anesthesiologist and the surgeon.264 Better drugs, more accurate monitoring, a greater level of experience with the techniques, and the consistency of the surgical procedures themselves have permitted a higher percentage of patients to benefit from DHA.* Nevertheless, relative contraindications include a history of cerebrovascular or cardiovascular disease, renal dysfunction, liver dysfunction, peripheral vascular disease, and severe anemia. Fortunately, the orthognathic patient rarely suffers from these dysfunctions. Exceptions include older individuals who are undergoing orthognathic surgery for the treatment of obstructive sleep apnea.228,261 Given current demographics, this is likely to represent an ever-increasing patient population (see Chapter 26).
Use of Hemorrhage Depressors to Limit Blood Loss
Tranexamic Acids
Tranexamic acid is a synthetic amino acid that inhibits fibrinolysis and that has been shown to reduce blood loss and the need for blood transfusion during specific surgical procedures, including total knee arthroplasty, spine surgery, cardiac surgery, and orthognathic surgery.* It has also been used for the treatment of postoperative bleeding in anticoagulated patients after oral surgery (e.g., the extraction of teeth).32,212 Senghore and Harris showed that a single intravenous preoperative dose of tranexamic acid was effective for preventing excessive postoperative bleeding in healthy adult patients who were undergoing third molar extraction.232
Choi and colleagues completed a double-blind, randomized, controlled trial to study the effects of tranexamic acid on blood loss during orthognathic surgery.51 This trial included 73 consecutive patients who were undergoing bimaxillary orthognathic osteotomies. Each study patient received either a bolus of tranexamic acid (20 mg/kg) or a placebo (normal saline) intravenously just before surgery. All patients underwent DHA in accordance with standard hospital protocol. Intraoperative blood loss, operative time, the transfusion of blood products, and perioperative hemoglobin and hematocrit levels were recorded. The total blood loss and the blood loss during the maxillary (Le Fort I) procedure were reduced significantly in the group that received tranexamic acid as compared with the control group. The difference in blood loss during maxillary surgery was 428 mL versus 643 mL. The difference in total blood loss was 878 cc versus 1257 cc. The authors concluded that the use of a preoperative intravenous bolus of tranexamic acid (20 mg/kg) reduces blood loss as compared with a placebo during bimaxillary orthognathic osteotomies.
Desmopressin
Desmopressin acetate is an analog to the naturally occurring hormone vasopressin.4,114,115,138,147,222,225,245 It enhances the vascular endothelial cell release of a number of compounds, including high multimetric von Willebrand factor, factor 7c, factor 7ag, and tissue-type plasminogen activator. Desmopressin by itself has been suggested to be the factor that is responsible for the increased hemostatic activity seen in patients with liver dysfunction and uremia. Additional observations indicate that desmopressin may improve platelet function.
Both of these hemorrhage depressors—desmopressin and tranexamic acid—have been used in combination with DHA during orthognathic surgery at the University of Göteborg Hospital since 1999. Zellin and colleagues retrospectively evaluated and compared the total perioperative blood loss for a group of patients who were assigned to either DHA or a combination of DHA and the use of both of these hemorrhage depressors (i.e., desmopressin and tranexamic acid).276 Thirty orthognathic patients who were consecutively operated on via a standardized Le Fort I osteotomy were assigned to either the control group (n = 15) or the treatment group (n = 15). Both groups underwent DHA. The treatment group received additional hemorrhagic depressors (i.e., both tranexamic acid and desmopressin). The mean blood loss was 740 cc in the control group and 400 cc in the treatment group. This was a statistically significant reduction in blood loss in the treatment group (1 g tranexamic acid intravenously and 0.3 µg per kilogram of body weight of desmopressin subcutaneously).
Deliberate Hypotensive Anesthesia Used in Orthognathic Surgery: Review of the Literature
Schaberg and colleagues were the first to evaluate blood loss and the human physiologic response with the use of DHA in the form of sodium nitroprusside during orthognathic surgery.227 They studied a consecutive series of ASA I patients undergoing Le Fort I maxillary osteotomies. Each was placed in reverse Trendelenburg position (10 degrees), and sodium nitroprusside was used to induce hypotension to not less than 80 mm Hg systolic pressure or 60 mm Hg MAP. The mean estimated blood loss was 256 ± 35 mL. No control group was used for comparison.
Washburn described a retrospective series of 58 individuals who underwent maxillofacial procedures (n = 53 orthognathic patients) in which morphine was used to supplement halothane to induce hypotension.267 The goals were to limit blood loss and to improve the surgical field. There was one death (possibly from halothane-induced liver failure), and only one patient required blood replacement.
Golia and colleagues studied nine patients who underwent either maxillary or combined maxillary and mandibular osteotomies; the DHA techniques that were used included halothane, nitrous oxide, and supplemental doses of muscle relaxants and narcotics.106 Nitroglycerin was added for the purpose of lowering the MAP during periods with a high potential for increased blood loss (e.g., Le Fort I down-fracture). Estimated blood loss averaged 439 mL and the hemoglobin/hematocrit level fell from 13.8 hemoglobin/41 hematocrit to 12 hemoglobin/36 hematocrit (4.9%). The authors concluded that nitroglycerin was a safe and efficacious agent to help control MAP and blood loss in the surgical field during maxillary and mandibular osteotomies. No control group was used.
McNulty and colleagues completed a study of 12 patients who underwent orthognathic procedures (i.e., Le Fort I osteotomy or sagittal split ramus osteotomy of the mandible) under DHA.169 The anesthesia agents that were used included fentanyl, isoflurane, and bolus doses (i.e., 10 mg to 20 mg) of labetalol to control the blood pressure in an attempt to improve the surgical field and limit blood loss. The authors reported clinical satisfaction with the surgical field and no complications. No control group was used.
Blau and colleagues compared esmolol in 15 patients with sodium nitroprusside in 15 other patients as the primary drugs for DHA to limit blood loss during Le Fort I osteotomy.31 All study patients were physically rated as ASA I or II. The mean MAP averaged 58 mm Hg to 60 mm Hg, with a target range set at 55 mm Hg to 65 mm Hg. The mean heart rate was maintained at approximately 70 beats/min with esmolol and 100 beats/min with nitroprusside. The surgeons generally rated bleeding as mild to moderate and the surgical field as “drier” with esmolol. The total measured blood loss was less with esmolol (i.e., 436 mL with esmolol versus 895 mL with nitroprusside). The authors found the advantages of esmolol as compared with nitroprusside to include greater control of the blood pressure, the reduction of blood loss, and a drier surgical field.
Precious and colleagues studied 50 orthognathic surgery patients in a prospective, randomized, blocked, stratified, and single-blind fashion.206 All patients underwent either sagittal split ramus osteotomies of the mandible, Le Fort I osteotomy, or genioplasty. One group of patients (n = 25) received DHA, whereas the other group (n = 25) received anesthesia with no specific attempt to reduce blood pressure during the operation. The surgeon, who was unaware of which group the patient had been assigned to, rated the surgical field every 15 minutes. At the completion of surgery, three different methods were used to estimate or calculate blood loss. The duration of the procedure was recorded from the time of the first incision to the time of the last stitch placement. The estimated blood loss was significantly less when DHA was used. The surgical field was also rated better, but there was no significant difference in the duration of the procedure with or without the use of DHA. The authors concluded that DHA results in both reduced blood loss and improved visibility in the surgical field.
Enlund and colleagues studied 36 patients who were undergoing orthognathic surgery.84 Each patient was randomly assigned to be either hypotensive (n = 18) or normotensive (n = 18) during surgery. Hypotension was achieved with the use of isoflurane to a MAP of 50 mm Hg to 64 mm Hg. The hypotensive group had significantly less blood loss per minute. Only two of the patients in each group underwent bimaxillary osteotomies. A majority of the patients in each group underwent only mandibular ramus osteotomies. None of the patients in either group required blood replacement.
Dolman and colleagues completed a prospective, randomized, blinded-to-the-surgeon clinical trial of DHA in a consecutive series of orthognathic patients.69 The purpose of the study was to compare the quality of the surgical field, the blood loss, and the operative time with either hypotensive or normotensive anesthesia during Le Fort I osteotomy. The study patients (n = 23) were randomized into normotensive or hypotensive anesthesia treatment groups. No local anesthesia with a vasoconstrictor was used by the surgeon. In the experimental group, hypotensive anesthesia was maintained with propofol (3 mg/kg/hr), sufentanyl (0.5 mg/kg/hr), and isoflurane (0.5% in 100% oxygen). The quality of the surgical field in both groups was assessed intraoperatively by direct observation and again postoperatively with video imaging. The surgical time was measured on the videotape, and blood loss was measured by volumetric and gravimetric techniques. There was a statistically significant correlation between the surgeon’s perception of the quality of the surgical field and the blood pressure. There was a statistically significant reduction in blood loss when using hypotensive anesthesia. However, there was no significant reduction in operative time when using hypotensive anesthesia. The authors concluded that hypotensive anesthesia is a valuable technique for reducing blood loss and improving the quality of the surgical field during Le Fort I osteotomy.
Praveen and colleagues completed a prospective randomized clinical study to assess DHA and blood loss during orthognathic surgery.204 A total of 53 patients who were undergoing orthognathic surgery were included in the study. The patient ages ranged from 15 to 33 years. They were randomly allocated to have either normotensive anesthesia or to be given DHA. Median blood loss with DHA was 200 mL; under normotensive anesthesia, it was 350 mL. The authors concluded that there was a pronounced reduction in blood loss during orthognathic operations with the patient under DHA as compared with normotensive anesthesia.
Manola and colleagues completed a prospective randomized comparison of three types of pharmacologic agents to induce hypotension during functional endoscopic sinus surgery.165 The patients were divided into three equal groups in accordance with the agents used: 1) sufentanyl/sevoflurane; 2) remifentanil/propofol; and 3) fentanyl/isoflurane. Evaluation included surgical field rating and measured blood loss. The authors found that the quantity of blood loss and the visibility of the surgical field were improved with the use of either remifentanil and sufentanyl as compared with fentanyl.
Choi and colleagues completed a comparative study to report about the use of three different pharmacologic drugs to induce DHA in patients who were undergoing orthognathic surgery (i.e., Le Fort I and sagittal split ramus osteotomies of the mandible).49 The 50 adult patients were randomly allocated to receive either nitroglycerin (n = 25) or remifentanil (n = 25). The MAP and the heart rate were measured at intervals. The heart rates and blood pressures were significantly better controlled with the use of remifentanil as compared with nitroglycerin. Interestingly, there were no significant differences in the surgical field ratings or the measured blood loss between the two groups.
Farah and colleagues completed a comparative study to report on the use of two different pharmacologic protocols to induce DHA in patients submitted to orthognathic surgery.90 Twenty ASA 1 patients between the ages of 17 and 44 years were randomly assigned into two groups: group 1 (clonidine and remifentanil) and group 2 (dexmedetomidine and isoflurane). Other drugs that were used as part of the anesthesia regimen were common to both groups. Specific patient parameters were assessed, including arterial blood pressure; heart rate; temperature during the intraoperative and postoperative periods; incidence of nausea and vomiting; postoperative pain; awakening time; extubation time; and postanesthesia recovery time. The results of the study showed that there were no significant differences between the two groups with respect to physiologic responses or surgical time. Both study protocols proved to be effective and safe. Each was suggested as a satisfactory option to achieve DHA during orthognathic procedures when there is an expectation of significant blood loss.
The Need for Blood Replacement in Orthognathic Surgery
Background
For individuals who are undergoing complex orthognathic surgery (i.e., Le Fort I and sagittal split ramus osteotomies of the mandible)—often in combination with other simultaneous procedures (e.g., genioplasty, liposuction, septoplasty, inferior turbinate reduction, removal of wisdom teeth)—a preoperative doctor–patient discussion of the need for intraoperative blood replacement is not just an academic issue.92,103,107,149,163,201 Published studies indicate that individuals who are undergoing bimaxillary surgery require blood replacement in the range of 2.5% to 75%.15,64,138,142,148,156,200,218,223,226,235,259,274 Rummasak and colleagues retrospectively studied patients who underwent bimaxillary osteotomies (n = 208) during a 4-year timeframe from 2005 to 2009 at a single institution. The authors reviewed possible factors for intraoperative blood loss and looked for statistical significance. They documented that, with consistent anesthesia techniques, the two factors of greatest importance for blood loss were 1) the experience of the surgeon and 2) the overall operative time.223
Madsen and colleagues conducted a prospective study to evaluate the predictive value of the viscoelastic properties of whole blood samples collected preoperatively in relation to intraoperative blood loss in patients who were undergoing orthognathic surgery (n = 41). They concluded that, with other variables controlled for, the etiology of intraoperative bleeding can be predicted by means of preoperative thromboelastography. This is a global method that addresses the complex interplay among coagulation factors, blood platelets, and components of the fibrinolytic system. Blood platelet count, activated partial thromboplastin time, prothrombin time, plasma fibrinogen concentration, and D-dimer concentration were determined by routine methods. The authors conclude that thromboelastography results in individual patients can be used for the evaluation of bleeding risk. Further studies will be required to confirm the validity of their findings.162
Methods to limit the need for blood replacement continue to evolve and include refinements in DHA techniques as well as in surgical management (i.e., patient selection, collaborative clinical efforts, and the efficiency of the operation and the postoperative care).102 Despite stringent donor screening criteria and the rigorous testing of every unit of blood, there remains a risk for the transmission of several pathogens.70,93,104,108,134,177 For viruses such as the human immunodeficiency virus (1 in 2,135,000), hepatitis B virus (1 in 205,000), and hepatitis C virus (1 in 2,000,000), this is due mainly to “window-period” donations.34,247 The risks of newly emerging pathogens that can affect blood safety (e.g., Creutzfeldt-Jakob disease) remain unclear. There are other pathogens (e.g., cytomegalovirus, parvovirus B19) that are common in the general donor population and that may pose a risk in the immunosuppressed patient.213 In addition, allogenic transfusion has been shown quantitatively to be an important contributor to postoperative infection.94,260
The options of autogenous and donor-directed blood banking and their popularity for elective surgery are attributed to their perceived safety, to refinements in blood bank administration, and to public concern about the hazards of allogenic blood replacement.16,86,91,105,118,167,184,210,240 In at least one state (California) and one nation (Germany), the preoperative discussion of blood replacement options is mandated by law and must be documented in the medical record. Other alternatives to allogenic transfusion include acute normovolemic hemodilution, intraoperative blood salvage, and perioperative therapy with recombinant human erythropoietin (Procrit).35,101,136,170,173,180,230,231
The absolute need for and actual use of blood transfusion in the practice of orthognathic surgery remains dependent on considerations that relate to total whole body oxygen delivery and its ability to meet the body’s metabolic needs.56,121,175,189 Many individual hospitals have instituted strict criteria for the transfusion of blood products.215 Although mandated criteria may decrease the use of blood transfusions, it remains unclear if complications or delays in recovery have occurred as a result.250
Review of Study
Posnick and colleagues completed a retrospective study to review blood replacement practices in a consecutive series of a single surgeon’s experience with patients who all underwent, at a minimum, simultaneous Le Fort I maxillary osteotomy, bilateral sagittal split ramus osteotomies of the mandible, septoplasty, and inferior turbinate reduction procedures.202 Many patients also underwent additional adjunctive procedures.
A chart review of a consecutive series of the surgeon’s patients (n = 34) who underwent elective bimaxillary orthognathic surgery (Le Fort I osteotomy and sagittal split osteotomies of the mandible) in combination with intranasal (septoplasty and inferior turbinate reduction) and often other simultaneous procedures during a 5-month time frame at a single institution was carried out (see Table 11-1). The chart review occurred at least 3 months after surgery for all patients.
TABLE 11-1
Blood Replacement for Complex Orthognathic Surgery
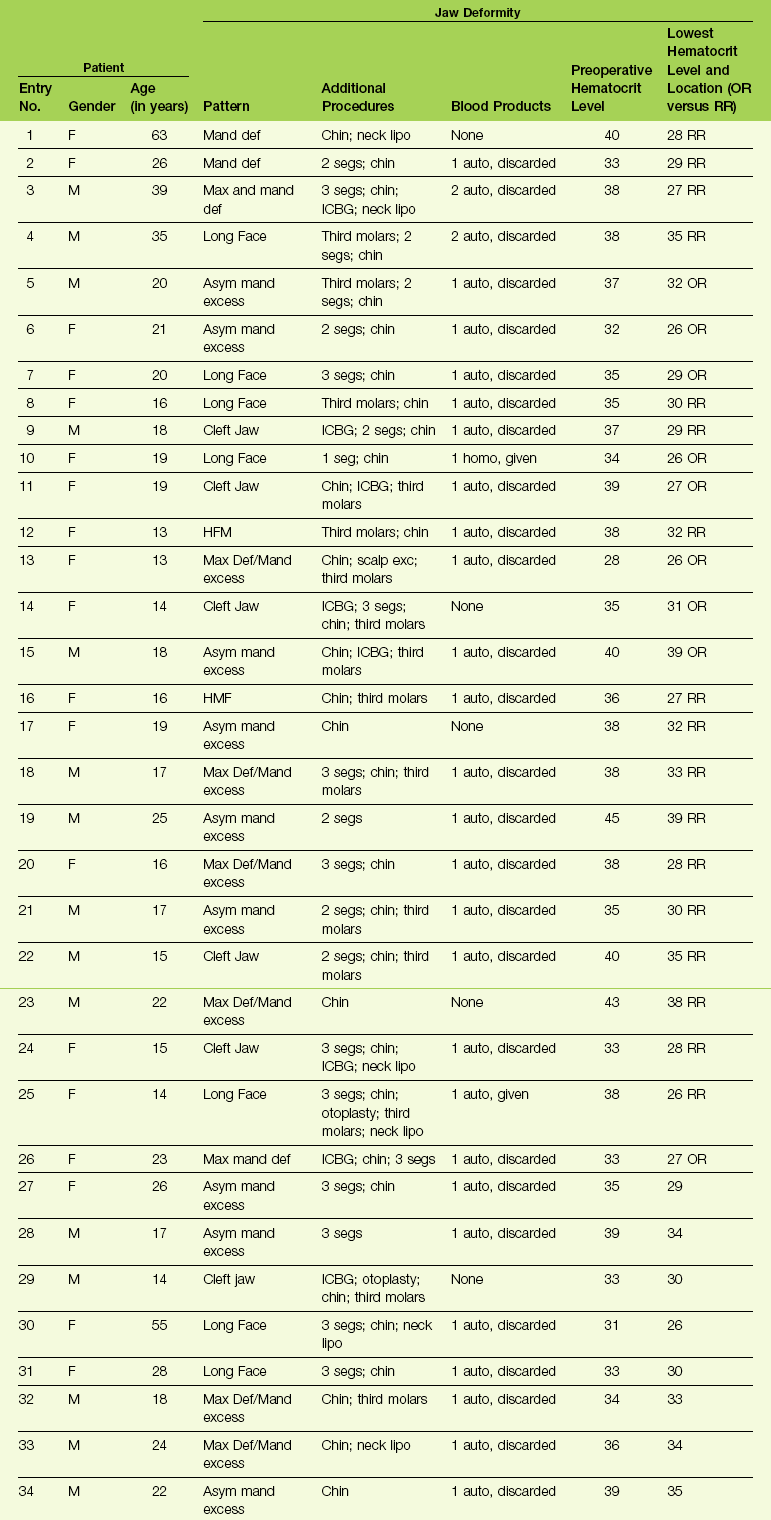
From Posnick JC, Rabinovich A, Richardson DT: Blood replacement for complex orthognathic surgery, J Oral Maxillofac Surg 68:54–59, 2010.
Each patient was also evaluated for complications specific to the procedures that were carried out. Potential complications specific to the orthognathic procedures included infection that required the extended use of antibiotics or drainage procedures; dental injury; fibrous union; aseptic necrosis; bleeding that required secondary treatment; and oroantral, oronasal, and orocutaneous fistulas. Potential complications specific to the intranasal procedures included postoperative nasal bleeding that required packing or cauterization; the need for postoperative blood transfusion specific to nasal bleeding; the presence of septal perforation; and postoperative nasal obstruction that required additional procedures within 3 months after orthognathic surgery. Potential perioperative airway compromise was also reviewed, including the need for delayed extubation, reintubation, or tracheotomy. Potential complications specific to transfusions, including infections and autoimmune reactions, were also sought. The patient records that were reviewed included the office chart; the hospital records; and the data stored at the Red Cross (hospital) blood bank. Specific study data collected for each patient (n = 34) can be found in Table 11-1.
Controversies in Blood Replacement for Orthognathic Surgery
The current recommendation of the National Heart, Lung and Blood Institute’s conference report is that autogenous blood donations are indicated for patients who are having surgical procedures for which blood is usually cross-matched.185 The less likely the transfusion for a specific procedure, the more likely that donated blood will not be used. The recommendations are that patients should not be encouraged to donate blood for autogenous use during surgery unless there is a greater than 10% likelihood that they will need it. The historic literature indicates a wide range (i.e., 2.5% to 75%) with regard to the use of transfusion during bimaxillary orthognathic surgery. The review study performed by Posnick and colleagues indicated only a 6% need.202 In current practice, the need for blood replacement during bimaxillary orthognathic surgery may be closer to 2%. The high-risk individual (e.g., an adult with obstructive sleep apnea and other comorbidities) can usually be recognized from the outset, and preparations can be made in advance (i.e., blood typing and cross-matching).
Another common question is, “Should the indications for the use of autogenous blood transfusion differ from those for the use of allogenic blood transfusion?” If precise indications for a blood transfusion could be defined, autogenous blood would not be given more frequently than allogenic. Although the benefits of allogenic and autogenous blood transfusions are the same, the risks are not. Therefore, the risk-to-benefit ratio clearly supports the more liberal use of autogenous blood. It has been documented that patients who preoperatively donate blood are more likely to be transfused earlier and more frequently than patients who do not auto-donate. Studies have challenged the use of autologous blood donations for elective surgery, because there is the possibility of blood overcollection, the wasting of autologous units, and the costs of preparation and storage. Cohen developed an interesting mathematical model to analyze the use of preoperative autogenous blood donation.53 The model clarifies how clinicians can customize autogenous blood collection for individual patients to minimize waste (i.e., the need to discard non-transfused blood).
Perioperative Airway Management in the Patient with a Dentofacial Deformity
A basic airway decision for the surgeon and the anesthesiologist to make for the individual who is to undergo orthognathic surgery and who therefore requires general anesthesia is whether to use induced anesthesia and then directly intubate with or without the use of a Glide Scope (Bell Medical Inc., St. Louis, MO); to use awake fiber-optic intubation; or to use a percutaneous technique (e.g., cricothyroidotomy and tracheostomy). The patient with a dentofacial deformity who presents for general anesthesia should be considered to have a difficult airway until it is proven otherwise (Fig. 11-1).45,48,123,127,141,146,172,203,207,217
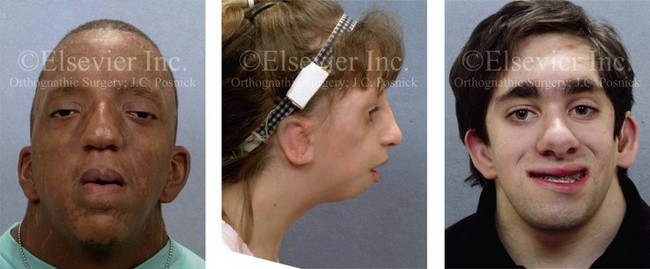
Figure 11-1 Perioperative risk to the airway and its management should be considered during all phases of orthognathic surgery, including before nasotracheal intubation, during surgery, at the time of extubation, and soon after extubation. Individuals who are scheduled for orthognathic surgery who have been recognized to be at high risk for airway compromise should be discussed in advance with the anesthesia team to arrange for special needs. Three patient examples in which these issues came into play are shown: an adult with Noonan syndrome; a teenage girl with Treacher Collins syndrome; and a teenager with the Klippel–Feil anomaly. Each of these patients also has the potential for cervical spine anomalies with limited neck range of motion. The Risk of spinal cord injury should also be evaluated before orthognathic surgery.
Head and Neck Examination
The head and neck examination of a patient who is scheduled to undergo endotracheal intubation should include his or her mouth-opening ability; the ability to protrude the lower jaw; any limitations of neck extension; the measurement of the thyromental distance; and the Mallampati test.140,164,165 These parameters cover the standard reference points to determine whether or not to expect a difficult airway. These parameters represent components of the basic head and neck airway evaluation recommended in the guidelines of the American Society of Anesthesiologists.6
1. Mouth-opening ability. This is measured as the interincisor distance. A value of less than 4 cm has been proposed as an indicator of possible difficult intubation. Fibrous or bony temporomandibular joint ankylosis will absolutely diminish mouth opening. Muscle spasms or pain may also limit mouth opening, but these factors can be overcome with muscle relaxants and analgesics.
2. The inability of the individual to protrude the lower jaw forward. When the mandibular incisors cannot protrude in front of the maxillary incisors, this is considered to be an indicator of a difficult intubation. It is often an indirect test for mandibular retrognathia. In addition, it can be a deceiving indicator of normal jaw morphology in the individual with a normal mandible but with maxillary deficiency.
3. Limited neck extension. An angle of less than 20 degrees as measured between the occlusal surface of the maxillary teeth and the neck when it is in full extension is felt to be suggestive of a difficult direct laryngoscopy. A percentage of dentofacial deformity patients will have cervical spine anomalies that prevent adequate and safe neck extension.
4. The Mallampati test. The visibility of the pharyngeal structures when the mouth is wide open is considered to be of limited use as an individual assessment. However, when this is considered in combination with other physical findings, it is of some value. The dentofacial deformity patient with baseline obstructive sleep apnea is likely to have a Class IV Mallampati classification. The Mallampati classification is determined by having the awake patient in the sitting position open his or her mouth widely and then protrude the tongue completely forward. The clinician then documents which structures can be visualized.
Class I: The soft palate, the fauces, the entire uvula, and the pillars are visualized.
Class II: The soft palate, the fauces, and a portion of the uvula are visualized.
Class III: The soft palate and the base of the uvula are visualized.
5. The thyromental distance. This is often used as an indirect gauge of mandibular morphology. The ratio of a patient’s overall height to his or her thyromental distance may be used as an indirect assessment of mandibular deficiency.
• The status of the teeth (e.g., mobility, missing teeth, removable and fixed appliances, general oral heath);
• The neck circumference (this is an indirect indicator of obstructive sleep apnea)
• Any presenting dentofacial deformity (e.g., maxillo-mandibular deficiency)
• Any anatomic cause of nasal airway obstruction (e.g., septal deviation, posterior choanal atresia, enlarged turbinates)
Prevention of Complications during Tracheal Intubation
1. The maintenance of oxygenation is essential. Preoxygenation is performed before the induction of anesthesia. Mask ventilation is also used in between attempts at tracheal intubation.
2. Trauma to the upper airway must be prevented. The first attempt at tracheal intubation should be performed under optimal conditions (i.e., patient positioning, preoxygenation, and equipment preparation).
3. The anesthesiologist should have a backup plan in place before initiation of the primary technique. He or she must have the skills and equipment needed to execute the backup plan for the patient with a difficult airway. The recent introduction of video-assisted laryngoscopy instruments have lessened the need for more invasive methods to secure the airway, such as the use of a fiber-optic bronchoscope and percutaneous techniques. The backup plan should include the eventuality that, if noninvasive techniques do not restore oxygenation, then cricothyroidotomy (which is generally the percutaneous airway method of choice) is ready to go. In an actual “cannot intubate—cannot ventilate” situation, immediate percutaneous access to the cricothyroid membrane is essential.
4. The anesthesiologist should seek the best help available as soon as difficulty with tracheal intubation is experienced. He or she should be aware of available backup personnel or colleagues who can be quickly called in to assist during an emergency.
The practical guidelines for the management of the difficult airway have been reported by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway.6 The guidelines recommend the following: 1) a detailed evaluation of the airway; 2) a general physical examination; 3) additional patient-specific evaluations, as needed; 4) basic preparation for difficult airway management; 5) a strategy for the intubation of the difficult airway; 6) a strategy for the extubation of the difficult airway; and 7) arranging for follow-up care.
1. An assessment of the likelihood and anticipated clinical impact of four basic problems that may occur alone or in combination:
2. A consideration of the relative clinical merits and feasibility of the three basic airway management choices:
• Awake fiber-optic intubation versus intubation after the induction of general anesthesia
• The use of noninvasive techniques for the initial approach to intubation (e.g., direct conventional laryngoscopy, laryngeal mask airway–assisted airway attainment, video-assisted laryngoscopy) versus the use of invasive techniques (e.g., percutaneous cricothyroidotomy, tracheostomy)
• The preservation of spontaneous ventilation during intubation attempts versus the ablation of spontaneous ventilation during intubation attempts
3. The anesthesiologist must select a primary approach to the difficult airway:
• Awake fiber-optic intubation in the patient who cannot be adequately ventilated and who will be difficult to intubate
• The patient who can be adequately ventilated but who will be difficult to intubate
• The life-threatening situation in which the patient cannot be ventilated or intubated and who will require immediate cricothyroidotomy or tracheostomy
4. The anesthesiologist should formulate in advance an alternative plan in the event that the primary approach is ineffective.
Special Airway Considerations in the Patient with a Dentofacial Deformity
The patient with a dentofacial deformity who is scheduled to undergo orthognathic surgery requires nasotracheal intubation as a first choice. The consideration of patient-specific intubation difficulties should be discussed between the surgeon and the anesthesiologist before arrival in the operating room (see Figure 11-1).
Difficulty with passing the endotracheal tube through the external nasal valve and the nasal cavity:
• The dentofacial deformity patient will often have an obstructed nasal airway as a result of a combination of scarred external nasal valves; a deviated septum; hypertrophic turbinates; a constricted bony nasal cavity (e.g., floor of the nose, lateral nasal walls); or posterior choanal atresia.
• In the patient with a cleft palate, a pharyngoplasty may be in place. This will result in limited lateral ports if a superiorly based pharyngeal flap is in place. There may be a limited central port if an orticochea (sphincteroplasty) flap is in place. Either of these forms of pharyngoplasty in the patient with a cleft palate may interfere with the passage of a nasotracheal tube.
• If the endotracheal tube cannot be passed through the nose and into the oropharynx, then the insertion of the endotracheal tube through a submental (skin) incision maybe carried out. This will accomplish intubation objectives but avoid interference with the occlusion during surgery. To accomplish the submental approach, the endotracheal tube is first passed through the oral cavity and into the pharynx. With the use of a laryngoscope, it is then passed through the vocal cords. The patient will be adequately ventilated while the submental approach is accomplished. A sharp submental incision is made, and this is followed by blunt dissection just behind the mandible and then into the mouth. The end of the endotracheal tube is pulled through the floor of the mouth and out of the submental incision for the administration of anesthesia.
• The presenting dentofacial malformation may result in limited mouth opening (e.g., Treacher Collins syndrome, hemifacial microsomia)
• Temporomandibular joint pathology (bony or fibrous ankylosis) will limit the extent of mouth opening (i.e., the interincisal distance).
• Masticatory muscle spasm will limit mouth opening and may be confused with bony or fibrous temporomandibular joint ankylosis.
• The presenting mandibular malformation or deformity (e.g., moderate to severe hypoplasia) will result in the retro and superior positioning of all of the soft tissues of the floor of the mouth and the anterior neck. This will result in limited visualization of the vocal cords. This may be true even for a non-obese patient in the neck-extended position during a full jaw-thrust maneuver.
• A percentage of patients with dentofacial deformities will have congenital limitations in the range of motion of the neck (e.g., hemifacial microsomia [Goldenhar variant], Klippel–Feil anomaly). This should be assessed before the patient arrives in the operating room.
• For the patient with baseline cervical spine anomalies, evaluation by a spine surgeon and specialized radiograph studies may be indicated in the outpatient setting before arrival in the operating room.
• Possible advantages of intraoperative spinal monitoring should be discussed before arrival in the operating room.
It is likely that the orthognathic surgeon will be most familiar with the nasal, oral, pharyngeal, temporomandibular joint, and cervical spine patient-specific anatomy. Potential locations of obstruction and limitations in neck and jaw range of motion should be discussed before arrival in the operating room. The surgeon should be ready to assist with the passage of the nasotracheal tube through the external nasal valve and the nasal cavity and into the oropharynx. The anesthesiologist will then complete direct laryngoscopy, video-assisted laryngoscopy (i.e., GlideScope), or fiber-optic intubation to pass the endotracheal tube through the vocal cords.144,146 In anticipation of the possible need for percutaneous airway management, the team should have the skill and readiness of equipment to complete either a cricothyroidotomy or a formal tracheostomy, as indicated.
Immediate Preintubation Preparation of the Airway
• Pretreatment with the judicious use of a topical vasoconstrictor (e.g., oxymetazoline, Neo-Synephrine) to shrink the nasal mucosa
• Pretreatment with a topical anesthetic, including topical lidocaine jelly
• Sequential dilation with lubricated nasopharyngeal airways (i.e., the nasal trumpets) to ease the passage of the nasotracheal tube
Strategy for Extubation after Orthognathic Surgery
After orthognathic surgery, all patients should be considered to have a difficult airway at the time of extubation. An extubation strategy is a logical extension of the planned intubation strategy for the patient with a difficult airway. The extubation strategy should be specific to the patient, and it should take into account preoperative anatomic factors; preexisting medical conditions; intraoperative findings; patient-specific swelling and intra-oral bleeding; and the patient’s response to the anesthesia techniques that were used.37,43,62,124,257,278
Factors to consider include the following:
• Baseline preoperative obstructive sleep apnea
• Baseline limited neck range of motion
• Excessive sedation with the inadequate return of reflexes
• Continued intranasal or intraoral hemorrhage with pooling in the pharynx, which may go unrecognized and cannot be easily suctioned
• Excessive swelling within the intranasal, intraoral, and pharyngeal components of the upper airway
• The potential for immediate postextubation vomiting
• The patient-specific presence of in-place intermaxillary wires, elastics, or intra-oral splints
The formulated extubation strategy should include the following:
• Strong consideration of the advantages of awake extubation versus extubation before the return of consciousness
• The formulation of an airway management plan to be implemented if the patient is not able to maintain adequate ventilation after extubation
• An evaluation of the general and patient specific clinical factors that may result in difficulty with mask ventilation after extubation
• Consideration for the short-term maintenance of a device that can serve as a guide for expedited reintubation. A semi-rigid airway device called a tube exchanger can be inserted through the lumen of the nasotracheal tube and into the trachea before the nasotracheal tube is removed. This device can then be used to facilitate the reinsertion of the nasotracheal tube should reintubation be required. It also allows for the limited ability to both oxygenate and ventilate the patient during the performance of reintubation maneuvers.
• A plan for percutaneous intubation (i.e., cricothyroidotomy or tracheostomy) with available equipment at the location of patient extubation (i.e., operating room, recovery room, or intensive care unit) with the skilled personnel required to complete the procedure, if required
• Discussion between surgeon and the anesthesiologist regarding patient-specific limitations in mouth opening (e.g., intermaxillary wires, elastics, masticatory muscle spasms, intra-oral splints)
Extubation Criteria for the Orthognathic Patient
• Stage I is present when there is analgesia and amnesia but no loss of consciousness.
• Stage II is the period after a loss of consciousness at which point the patient’s reflexes are still present, the breathing pattern is irregular, and there may be excitability. The pupils will be dilated, and there will be a disconjugate gaze.
• Stage III is considered surgical anesthesia, when there will be lack of response to surgical stimulation. Loss of the eyelash reflex is a reliable indicator of this stage. Within Stage III, there are additional planes or levels of consciousness that correspond with the amount of anesthetic delivered. The plane of anesthesia that is desired depends on the surgery being performed.
• Stage IV describes cardiovascular and respiratory failure as a result of the overadministration of anesthetic, which results in brain stem and medullary depression. This is potentially fatal.
Negative pressure pulmonary edema is an uncommon complication that occurs during the extubation of the trachea in approximately 0.1% of cases.29 It is believed to be a result of laryngospasm. Upper airway obstruction from glottis closure leads to marked inspiratory efforts, which generates negative intrathoracic pressure. This may cause negative-pressure pulmonary edema and, rarely, hemoptysis. Immediately after extubation, the patient will be noted to have marked respiratory distress followed by edema fluid and decreased arterial oxygen saturation, with rising partial pressure of CO2 levels. A chest radiograph will likely show bilateral pulmonary infiltrates. The most effective form of management is reintubation followed by positive end-expiratory pressure for 12 to 24 hours.
Before the Extubation of the Orthognathic Patient
• Any deliberate hypotensive techniques should be ceased with a return to preoperative hemodynamic stability.
• Any hypothermia should be addressed with rewarming techniques, including temperature-controlled intravenous fluids and warming blankets.
• The patient’s breathing pattern should be regular, with adequate tidal volume (i.e., 6 to 10 mL/kg) and rate of respiration (i.e., 10 to 20 breaths/min).
• If bispectral index monitoring is used to assess the depth of anesthesia, a return to preoperative levels should be documented.
• Adequate analgesia is maintained with appropriate opioids, but titration with maintenance to a physiologic respiratory rate and tidal volume is required.
• The patient should be sufficiently awake and should have spontaneous eye opening with a conjugate gaze.
• The patient should be able to follow commands (e.g., sustained head lift and hand grip).
• A nasopharyngeal airway (i.e., a nasal trumpet) may be placed to assist with ventilation after extubation.
Checklist of Benchmark Airway Parameters Before Extubation
2. Paralysis reversed adequately (i.e., sustained tetany without fade for >5 sec)
4. No longer in Stage II anesthesia
5. Negative inspiratory force of <−20 mm Hg (i.e., you can take off the bag, cover the hole with your hand, and have the patient suck in while you are watching the pressure gauge)
6. “Leak test” for the evaluation of pharyngeal and laryngeal edema
In-Hospital Convalescence After Orthognathic Surgery
After extubation, initial management takes place in a recovery room setting. Transfer to a monitored bed either in an intensive care unit or a step-down or telemetry unit is generally preferred. Continuous awareness of the potential for impending respiratory compromise and the need for monitoring to confirm hemodynamic stability is essential early after extubation.263
Airway Needs
Respiratory issues are of immediate postoperative concern in the orthognathic surgery patient.* The individual must be able to maintain his or her airway as documented by the ability to adequately oxygenate and ventilate. Multiple factors may compromise the airway, including limitations in nasal breathing as a result of the following: 1) the Le Fort I down-fracture with tears in the nasal mucosa; 2) specific intranasal procedures that were carried out (i.e., septoplasty, inferior turbinate reduction); and 3) edema after nasotracheal tube removal. The oral airway will be partially obstructed by the following: 1) ongoing bleeding, secretions, and swelling; 2) the use of guiding elastics; and 3) the presence of an acrylic intra-oral occlusal splint secured to the maxillary dentition. A nasopharyngeal trumpet may be helpful to improve the nasal airway early after extubation. The guiding elastics should be removed, if needed, to improve the oral airway. Active and frequent bedside suctioning is carried out. Supplemental oxygen given via a face tent provides an additional reserve through the nose and mouth; it is titrated to maintain pulse oximetry (i.e., oxygen saturation) of more than 95%, as described later in this chapter.
Monitoring Equipment
Sequential compression devices placed on the lower extremities are used intraoperatively and maintained during the postoperative period to limit the formation of deep venous thrombosis. These devices increase the velocity of venous blood return to the heart, thereby decreasing venous stasis. In addition, the mechanical compression results in increased fibrinolytic activity within the vascular system. Stockings should be maintained while the patient is immobilized. On postoperative day 1, the patient should be ambulated with assistance, at which point the compression stockings may be discontinued. Multiple studies have documented that early ambulation improves recovery and results in a decreased risk of deep venous thrombosis, the return of bowel function, and earlier hospital discharge.6
Diet
Surgical trauma to the lips and cheeks initially results in the diminished ability to achieve full lip seal. Without sufficient lip seal, the ease with which one can swallow liquids or drink from a cup is limited (see Chapter 8). A feeding syringe with a soft wide bore tip is initially used. It is placed in the vestibule between the cheek and the posterior teeth to facilitate the direction and depth of entry of liquids. The fluid bolus is injected, and the patient then seals the lips and swallows. The clear liquid diet is rapidly advanced to full liquids to achieve the needed caloric and nutritional intake. The patient transitions from syringe feeding to the use of a “sippy cup” or a squeezable water bottle and then to drinking from a soft glass as soon as he or she is able.
Nausea and Vomiting Management
Postoperative nausea and vomiting are common anesthetic concerns for the patient. If these conditions are not managed, they may in rare cases result in aspiration.236,256 Steps taken to decrease their likelihood include gastric suctioning at the end of the procedure before extubation and the use of prophylactic medications. All orthognathic patients are given prophylactic antiemetic therapy. A medication regimen may include the following: glucocorticoids such as dexamethasone at the onset of surgery; ondansetron (Zofran) or granisetron (Kytril) 15 minutes before the conclusion of surgery; metoclopramide (Reglan); and the judicious intake of liquids immediately after extubation. Multimodal antiemetic therapy in the recovery room should be employed for refractory cases of nausea and vomiting; this may include promethazine, compazine, and repeat doses of ondansetron-like agents. Appropriate postoperative patient monitoring and bedside access to a mouth-suctioning device are essential. As long as the patient can maintain protective airway reflexes, episodes of vomiting (with only liquids on the stomach) should have minimal serious sequelae.
Antibiotics
Multiple studies have confirmed the benefit of prophylactic doses of antibiotics for clean-contaminated (Class II) surgery and specifically for orthognathic surgery to decrease wound infection rates. This is typically initiated as a prophylactic dose of the first-generation cephalosporin cefazolin (Ancef); 15 to 20 mg/kg and up to 1 g is given before surgical incision and then every 6 hours. The postoperative course of intravenous antibiotics continues while the patient is still in the hospital. Antibiotics are then given orally upon discharge for a total of no more than a 5-day course. Opinions vary with regard to the extent of use of antibiotics for orthognathic surgery. Bentley and colleagues performed a randomized controlled trial that compared a 1-day regimen with a 5-day regimen of antibiotics for patients who were undergoing orthognathic surgery.24 A group of 30 patients who were undergoing combined maxillary and mandibular osteotomies was divided equally. Nine out of 15 patients in the 1-day antibiotic group developed postoperative infection, whereas only 1 out of 15 patients in the 5-day antibiotic group did. The authors concluded that a 5-day course of antibiotics is useful to decrease the risk of postoperative infection (see Chapter 16).
Criteria for Discharge from Hospital
• The ability to ingest sufficient liquids orally (i.e., hydration and nutrition)
• The absence of significant fever
• Stable vital signs, including the maintenance of pulse-oximetry documented oxygenation while sleeping
• The absence of continued nausea and vomiting
• Sufficient ambulation to carry out necessary activities of daily living in conjunction with a designated family member, friend, or caregiver
• Adequate pain control with oral analgesia while in the hospital
• The demonstrated ability to achieve required body and oral hygiene
References
1. Ablad, B. A study of the mechanism of the hemodynamic effects of hydralazine in man. Acta Pharmacol Toxicol. 1963; 20:1.
2. Acampora, G, Shah, N, Del Valle, O, et al. Myocardial depression by esmolol during nitroprusside hypotension [abstract]. Anesthesiology. 1989; 71:A19.
3. Adams, AP. Techniques of vascular control for deliberate hypotension during anaesthesia. Br J Anaesth. 1975; 47:777–792.
4. Agnelli, G, Berrettini, M, de Cunto, M, et al. Desmopressin-induced improvement of abnormal coagulation in chronic liver disease [letter]. Lancet. 1983; 1:645.
5. Ahlering, TE, Henderson, JB, Skinner, DG. Controlled hypotensive anesthesia to reduce blood loss in radical cystectomy for bladder cancer. J Urol. 1983; 129:953–954.
6. American Society of Anesthesiologists. Practice guidelines for management of the difficult airway. A report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 1993; 78:597–602.
7. Anderson, JA. Deliberate hypotensive anesthesia for orthognathic surgery: controlled pharmacologic manipulation of cardiovascular physiology. Int J Adult Orthodon Orthognath Surg. 1986; 1:133–159.
8. Angaran, DM, Schultz, NJ, Tschida, VH. Esmolol hydrochloride: an ultrashort-acting, B-adrenergic blocking agent. Clin Pharm. 1986; 5:288–303.
9. Artru, AA. Cerebral vascular responses to hypocapnia during nitroglycerin-induced hypotension. Neurosurgery. 1985; 16:468–472.
10. Artru, AA. Cerebral metabolism and EEG during combination of hypocapnia and isoflurane-induced hypotension in dogs. Anesthesiology. 1986; 65:602–608.
11. Artru, AA. Cerebral metabolism and the electroencephalogram during hypocapnia plus hypotension induced by sodium nitroprusside or trimethaphan in dogs. Neurosurgery. 1986; 18:36–44.
12. Artru, AA. Partial preservation of cerebral vascular responsiveness to hypocapnia during isoflurane-induced hypotension in dogs. Anesth Analg. 1986; 65:660–666.
13. Artru, AA, Colley, PS. Cerebral blood flow response to hypocapnia during hypotension. Stroke. 1984; 15:878–883.
14. Artru, AA, Wright, K, Colley, PS. Cerebral effects of hypocapnia plus nitroglycerin-induced hypotension in dogs. J Neurosurg. 1986; 64:924–931.
15. Ash, DC, Mercuri, LG. The relationship between blood ordered and blood administered in orthognathic surgery: a retrospective study. J Oral Maxillofac Surg. 1985; 43:944.
16. Avantika, N, Pogrel, MA. Preoperative autologous blood donation for oral and maxillofacial surgery: an analysis of 913 patients. J Oral Maxillofac Surg. 2005; 63:347.
17. Aziz, SR, Agnihotri, N, Ziccardi, VB. Lobar collapse immediately after orthognathic surgery. J Oral Maxillofac Surg. 2010; 68:2335.
18. Bahlman, SH, Eger El, II., Halsey, MJ, et al. The cardiovascular effects of halothane in man during spontaneous ventilation. Anesthesiology. 1972; 36:494–502.
19. Beadnell, SW, Saunderson, JR, Sorenson, DC. Compartment syndrome following oral and maxillofacial surgery. J Oral Maxillofac Surg. 1988; 46:232.
20. Bemard, JM, Pinaud, M, Francois, T, et al. Deliberate hypotension with nicardipine or nitroprusside during total hip arthroplasty. Anesth Analg. 1991; 73:341–345.
21. Benoni, G, Fredin, H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomized, double-blind study of 86 patients. J Bone Joint Surg Br. 1996; 78:434.
22. Benoni, G, Lethagen, S, Fredin, H. The effect of tranexamic acid on local and plasma fibrinolysis during total knee arthroplasty. Thromb Res. 1997; 85:195.
23. Benoni, G, Fredin, H, Knebel, R, et al. Blood conservation with tranexamic acid in total hip arthroplasty: a randomized, double-blind study in 40 primary operations. Acta Orthop Scand. 2001; 72:442.
24. Bentley, KC, Head, TW, Aiello, GA. Antibiotic prophylaxis in orthognathic surgery: A 1-day versus 5-day regimen. J Oral Maxillofac Surg. 1999; 57:226.
25. Bergman, S, Hoffman, WE, Gans, BJ, et al. Blood flow to oral tissues: an experimental study with enflurane, sodium nitroprusside, and nitroglycerin. J Oral Maxillofac Surg. 1982; 40:13–17.
26. Bernard, JM, Pinaud, M, Maquin-Mavier, I, et al. Hypotensive anesthesia with isoflurane and enflurane during total hip replacement: a comparative study of catecholamine and renin angiotensin responses. Anesth Analg. 1989; 69:467–472.
27. Bernet, F, Carrel, T, Marbet, G, et al. Reduction of blood loss and transfusion requirements after coronary artery bypass grafting: similar efficacy of tranexamic acid and aprotinin in aspirin-treated patients. J Card Surg. 1999; 14:92.
28. Berntorp, E, Follrud, C, Lethagen, S. No increased risk of venous thrombosis in women taking tranexamic acid. Thromb Haemost. 2001; 86:714.
29. Bhavani-Shankar, K, Hart, NS, Mushlin, PS. Negative pressure induced airway and pulmonary injury. Can J Anaesth. 1997; 44:78–81.
30. Bigoli, SJ, Dumont, L, Mattys, M, et al. A serious anesthetic complication of a Le Fort I osteotomy. Eur J Anaesthesiol. 1999; 16:201.
31. Blau, WS, Kafer, ER, Anderson, JA. Esmolol is more effective than sodium nitroprusside in reducing blood loss during orthognathic surgery. Anesth Analg. 1992; 75:172–178.
32. Blinder, D, Manor, Y, Martinowitz, U, et al. Dental extractions in patients maintained on continued oral anticoagulant: comparison of local hemostatic modalities. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999; 88:137.
33. Bourreli, B, Pinaud, M, Passuti, N, et al. Additive effects of dihydralazine during enflurane or isoflurane hypotensive anaesthesia for spinal fusion. Can J Anaesth. 1988; 35:242–248.
34. Brand, A. Immunological aspects of blood transfusion. Blood Rev. 2000; 14:130–144.
35. Bridgens, JP, Evans, CR, Dobson, PM, et al. Intraoperative red blood-cell salvage in revision hip surgery. A case-matched study. J Bone Joint Surg Am. 2007; 89:270.
36. Brittain, RT, Levy, GP. A review of the animal pharmacology of labetalol, a combined alpha- and beta-adrenoceptor-blocking drug. Br J Clin Pharmacol. 1976; 3(4 Suppl 3):681–699.
37. Broccard, AF, Liaudet, L, Aubert, JD, et al. Negative pressure post-tracheal extubation alveolar hemorrhage. Anesth Analg. 2001; 92:273–275.
38. Bulloch, SE, Fridrich, KL. Capillary blood flow in the maxilla during hypotensive anesthesia for maxillary surgery. J Oral Maxillofac Surg. 1993; 51(Suppl 3):142–143.
39. Camarasa, MA, Olle, G, Serra-Prat, M, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth. 2006; 96:576.
40. Carswell, DJ, Varkey, GP, Drake, CG. Labetalol for controlled hypotension in surgery for intracranial aneurysm. Can Anaesth Soc J. 1981; 28:505.
41. Casati, V. About dosage schemes and safety of tranexamic acid in cardiac surgery. Anesthesiology. 2003; 99:236.
42. Casati, V, Guzzon, D, Oppizzi, M, et al. Hemostatic effects of aprotinin, tranexamic acid and epsilon-aminocaproic acid in primary cardiac surgery. Ann Thorac Surg. 1999; 68:2252.
43. Cascade, PN, Alexander, GD, Mackie, DS. Negative-pressure pulmonary edema after endotracheal intubation. Radiology. 1993; 186:671–675.
44. Casthely, PA, Lear, S, Cottrell, JE, Lear, E. Intrapulmonary shunting during induced hypotension. Anesth Analg. 1982; 61:231–235.
45. Cavusoglu, T, Yazici, I, Demirtas, Y, et al. A rare complication of nasotracheal intubation: accidental middle turbinectomy. J Craniofac Surg. 2009; 20:566.
46. Chan, W, Smith, DE, Ware, WH. Effects of hypotensive anesthesia in anterior maxillary surgery. J Oral Surg. 1980; 38:504.
47. Chebel, NA, Ziade, D, Achkouty, R. Bilateral pneumothorax and pneumomediastinum after treatment with continuous positive airway pressure after orthognathic surgery. Br J Oral Maxillofac Surg. 2010; 48:e14.
48. Chemello, PD, Nelson, SR, Wolford, LM. Finger injury resulting from pulse oximeter probe during orthognathic surgery. Oral Surg Oral Med Oral Pathol. 1990; 69:161.
49. Choi, SH, Lee, WK, Lee, KY, et al. Efficacy of remifentanil-induced controlled hypotension for orthognathic two jaw surgery. Korean J Anesthesiol. 2007; 52(1):62–66.
50. Choi, WS, Samman, N. Risks and benefits of deliberate hypotension in anaesthesia: a systematic review. Int J Oral Maxillofac Surg. 2008; 37:687–703.
51. Choi, WS, Irwin, MG, Samman, N. The effect of tranexamic acid on blood loss during orthognathic surgery: a randomized controlled trial. J Oral Maxillofac Surg. 2009; 67:125–133.
52. Chua, W, Chidambaram, A, Doyle, PT, et al. Voicing concern: an unusual sequelae of orthognathic surgery. Prog Orthod. 2006; 7:220.
53. Cohen, JA, Brecher, ME. Preoperative autologous blood donation: benefit or detriment? A mathematical analysis. Transfusion. 1995; 35:640.
54. Cole, PV. The safe use of sodium nitroprusside. Anaesthesia. 1978; 33:473–477.
55. Cole, PV. Sodium nitroprusside. In: Hewer CL, Atkinson RS, eds. Recent advances in anaesthesia and analgesia. London: Churchill Livingstone; 1979:139–149.
56. Consensus conference. Perioperative red blood cell transfusion. JAMA. 1988; 260:2700.
57. Cottrell, JE, Casthely, PA, Brodie, JD, et al. Prevention of nitroprusside-induced cyanide toxicity with hydroxocobalamin. N Engl J Med. 1978; 298:809–811.
58. Cushing, H. Tumors of the nervus acusticus. Philadelphia: W. B. Saunders Company; 1917.
59. Dalmau, A, Sabate, A, Acosta, F, et al. Tranexamic acid reduces red cell transfusion better than epsilon-aminocaproic acid or placebo in liver transplantation. Anesth Analg. 2000; 91:29.
60. Davies, DW, Kadar, D, Steward, DJ, Munro, IR. A sudden death associated with the use of sodium nitroprusside for induction of hypotension during anaesthesia. Can Anaesth Soc J. 1975; 22:547–552.
61. Dedrick, DF, Mans, AM, Campbell, PA, et al. Does ATP-induced hypotension cause potentially serious metabolic complications? Anesthesiology. 1982; 57:A66.
62. Deepika, K, Kenaan, CA, Barrocas, AM, et al. Negative pressure pulmonary edema after acute upper airway obstruction. J Clin Anesth. 1997; 9:403–408.
63. Del Valle, O, Edmondson, R, Reinsel, R, et al. Esmolol vs nitroprusside for hypotension: dose response during isoflurane anesthesia [abstract]. Anesthesiology. 1990; 72:A143.
64. Dhariwal, DK, Gibbons, AJ, Kittur, MA, et al. Blood transfusion requirements in bimaxillary osteotomies. Br J Oral Maxillofac Surg. 2004; 42:231.
65. Diaz, JH, Lockhart, CH. Hypotensive anaesthesia for craniectomy in infancy. Br J Anaesth. 1979; 51:233–235.
66. Didier, EP, Clagett, OT, Theye, RA. Cardiac performance during controlled hypotension. Anesth Analg. 1965; 44:379.
67. Dinmore, P. Combined use of trimethaphan and sodium nitroprusside. Br J Anaesth. 1977; 49:1070.
68. Dodson, TB, Neuenschwander, MC, Bays, RA. Intraoperative assessment of maxillary perfusion during Le Fort I osteotomy. J Oral Maxillofac Surg. 1994; 52:827–831.
69. Dolman, RM, Bentley, KC, Head, TW, et al. The effects of hypotensive anesthesia on blood loss and operative time during Le Fort I osteotomies. J Oral Maxillofac Surg. 2000; 58:834.
70. Domen, RE. Adverse reactions associated with autologous blood transfusion: evaluation and incidence at a large academic hospital. Transfusion. 1998; 38:296.
71. Dunn, CJ, Goa, KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999; 57:1005.
72. Eckenhoff, JE, Enderby, GEH, Larson, A, et al. Pulmonary gas exchange during deliberate hypotension. Br J Anaesth. 1963; 35:750–759.
73. Eckenhoff, JE, Rich, JC. Clinical experiences with deliberate hypotension. Anesth Analg. 1966; 45:21.
74. Edmondson, R, Del Valle, O, Shah, N, et al. Esmolol for potentiation of nitroprusside-induced hypotension: impact on the cardiovascular, adrenergic, and random-angiotensin systems in man. Anesth Analg. 1989; 69:202–206.
75. Edwards, DB, Scheffler, RB, Jackler, I. Postoperative pneumomediastinum and pneumothorax following orthognathic surgery. J Oral Maxillofac Surg. 1986; 44:137.
76. Eerola, R, Eerola, M, Kaukinen, L, et al. Controlled hypotension and moderate haemodilution in major hip surgery. Ann Chir Gynaecol. 1979; 69:109.
77. Ekback, G, Axelsson, K, Ryttberg, L, et al. Tranexamic acid reduces blood loss in total hip replacement surgery. Anesth Analg. 2000; 91:1124.
78. Enderby, GEH. Controlled circulation with hypotensive drugs and posture to reduce bleeding in surgery: preliminary results with pentamethonium iodide. Lancet. 1950; 1:1145–1147.
79. Enderby, GEH. Pentolinium tartrate in controlled hypotension. Lancet. 1954; 2:1097–1098.
80. Enderby, GEH. A report of mortality and morbidity following 9107 hypotensive anaesthetics. Br J Anaesth. 1961; 33:109–113.
81. Enderby, GEH. Some observations on the practice of deliberate hypotension. Br J Anaesth. 1975; 47:743–744.
82. Endrich, M, Franke, N, Peter, K, Messmer, K. Induced hypotension: action of sodium nitroprusside and nitroglycerin on the microcirculation. Anesthesiology. 1987; 66:605–613.
83. Enlund, M, Mentell, O, Engström, et al. Occurrence of adenylate kinase in cerebrospinal fluid after isoflurane anaesthesia and orthognathic surgery. Ups J Med Sci. 1996; 101:97.
84. Enlund, MG, Ahlstedt, BL, Andersson, LG, et al. Induced hypotension may influence blood loss in orthognathic surgery, but it is not crucial. Scand J Plast Reconstr Surg Hand Surg. 1997; 31:311.
85. Eriksson, O, Kjellman, H, Pilbrant, A, et al. Pharmacokinetics of tranexamic acid after intravenous administration to normal volunteers. Eur J Clin Pharmacol. 1974; 7:375.
86. Etchason, J, Petz, L, Keeler, E, et al. The cost effectiveness of preoperative autologous blood donations. N Engl J Med. 1995; 332:719.
87. Fahmy, NM. Indications and contraindications for deliberate hypotension with a review of its cardiovascular effects. Int Anesthesiol Clin. 1979; 17:175–187.
88. Fahmy, NR. Nitroglycerin as a hypotensive drug during general anesthesia. Anesthesiology. 1978; 49:17–20.
89. Fahmy, NR. Nitroprusside versus nitroprusside-trimethaphan mixture for induced hypotension: hemodynamic effects and cyanide release. Clin Pharmacol Ther. 1985; 37:264–270.
90. Farah, GJ, de Moraes, M, Filho, LI, et al. Induced hypotension in orthognathic surgery: a comparative study of 2 pharmacological protocols. J Oral Maxillofac Surg. 2008; 66:2261–2269.
91. Feagan, B. The efficacy, safety and acceptance of autologous blood donation as a blood conservation strategy. Vox Sang. 2002; 83(Suppl 1):11–12.
92. Flood, TR, Ilankovan, V, Moos, KF, El-Attar, A. Cross-match requirements in orthognathic surgery: an audit. Br J Oral Maxillofac Surg. 1990; 28:292.
93. Fong, J, Gurewitsch, ED, Kang, HJ, et al. An analysis of transfusion practice and the role of intraoperative red blood cell salvage during cesarean delivery. Anesth Analg. 2007; 104:666.
94. Fordyce, AM, Telfer, MR, Stassen, LF. Cross-matched blood for major head and neck surgery: an analysis of requirements. Br J Oral Maxillofac Surg. 1998; 36:103–106.
95. Fridrich, KL. Anesthetic techniques to reduce blood loss and transfusion therapy. Oral Maxillofac Surg Clin North Am. 1992; 4:863–864.
96. Fridrich, KL. Induced hypotensive anesthesia of adolescent orthognathic surgery patients [discussion]. J Oral Maxillofac Surg. 1996; 54:683.
97. Fromme, GA, MacKenzie, RA, Gould, AB, et al. Controlled hypotension for orthognathic surgery. Anesth Analg. 1986; 65:683–686.
98. Fukunaga, AF, Facke, WE, Bloor, BC. Hypotensive effects of adenosine and adenosine triphosphate compared to sodium nitroprusside. Anesth Analg. 1982; 61:273–278.
99. Fukunaga, AF, Ikeda, K, Matsuda, L. ATP-induced hypotensive anesthesia during surgery. Anesthesiology. 1982; 57:A65.
100. Gallagher, DM, Milliken, RA. Induced hypotension for orthognathic surgery. J Oral Surg. 1979; 37:47–51.
101. Gallandat Huet, RCG, Siemons, AW, Baus, D, et al. A novel hydroxyethyl starch for effective perioperative plasma volume substitution in cardiac surgery. Can J Anesth. 2000; 47:1207.
102. Gardner, WJ. The control of bleeding during operation by induced hypotension. JAMA. 1946; 132:572–574.
103. Gibbons, AJ, Dhariwal, DK, Benton, A, et al. Blood usage in maxillofacial surgery. Br J Oral Maxillofac Surg. 2002; 40:350.
104. Gillon, G, Greenburg, AG. Transfusions: infectious complications. Complications in Surgery. 1992; 11:19.
105. Goldman, M, Savard, R, Long, A, et al. Declining value of preoperative autologous donation. Transfusion. 2002; 41:819.
106. Golia, JK, Woo, R, Farole, A, Seltzer, J. Nitroglycerin-controlled circulation in orthognathic surgery. J Oral Maxillofac Surg. 1985; 43:342–345.
107. Gong, SG, Krishnan, V, Waack, D. Blood transfusions in bimaxillary orthognathic surgery: are they necessary? Int J Adult Orthodon Orthognath Surg. 2002; 17:314.
108. Goodnaugh, LT. Risks of blood transfusion. Crit Care Med. 2003; 31(Suppl):678.
109. Goodson, ML, Manemi, R, Paterson, AW. Pneumothorax after orthognathic surgery. Br J Oral Maxillofac Surg. 2010; 48:180.
110. Goto, F, Otani, E, Kato, S, Fujita, T. Prostaglandin El as a hypotensive drug during general anaesthesia. Anaesthesia. 1982; 37:530–535.
111. Grace, AH. Prostatectomy under hypotensive anaesthesia. Proc R Soc Med. 1961; 54:1130–1132.
112. Grime, PD, Tyler, C. An obstructed airway: cuff herniation during nasotracheal anesthesia for a bimaxillary osteotomy. Br J Oral Maxillofac Surg. 1991; 29:14.
113. Gruvstad, M, Kebbon, L, Lof, BA. Changes in mental function after induced hypotension. Acta Psych Scand. 1962; 37(Suppl 163):1–112.
114. Guay, J, Reinberg, C, Poitras, B, et al. A trial of desmopressin to reduce blood loss in patients undergoing spinal fusion for the idiopathic scoliosis. Anesth Analg. 1992; 75:405.
115. Guyuron, B, Vaughan, C, Schlecter, B. The role of DDAVP (desmopressin) in orthognathic surgery. Ann Plast Surg. 1996; 37:516.
116. Hackmann, T, Friesen, M, Allen, S, et al. Clonidine facilitates controlled hypotension in adolescent children. Anesth Analg. 2003; 96:976.
117. Harp, JR, Wollman, H. Cerebral metabolic effects of hyperventilation and deliberate hypotension. Br J Anaesth. 1973; 45:256–262.
118. Hegtverdt, AK, Collins, ML, White, RP, Turvey, TA. Minimizing the risk of transfusion in orthognathic surgery: use of predeposited autologous blood. Int J Adult Orthodon Orthognath Surg. 1987; 2:185.
119. Hewitt, PB, Lord, PW, Thornton, HL. Propranolol in hypotensive anaesthesia. Anaesthesia. 1967; 22:82–90.
120. Hiippala, ST, Strid, LJ, Wennerstrand, MI, et al. Tranexamic acid radically decreases blood loss and transfusions associated with total knee arthroplasty. Anesth Analg. 1997; 84:839.
121. Hines, R, Barash, PG. Infusion of sodium nitroprusside induces platelet dysfunction in vitro. Anesthesiology. 1989; 70:611–615.
122. Ho, KM, Ismail, H. Use of intravenous tranexamic acid to reduce allogeneic blood transfusion in total hip and knee arthroplasty: a meta-analysis. Anaesth Intensive Care. 2003; 31:529.
123. Holdgaard, HO, Pedersen, J, Schurizek, BA, et al. Complications and late sequelae following nasotracheal intubation. Acta Anaesthesiol Scand. 1993; 37:475.
124. Holmes, JR, Hensinger, RN, Wojtys, EW. Postoperative pulmonary edema in young, athletic adults. Am J Sports Med. 1991; 19:365–371.
125. Horrow, JC, van Riper, DF, Strong, MD, et al. Hemostatic effects of tranexamic acid and desmopressin during cardiac surgery. Circulation. 1991; 84:2063.
126. Horrow, JC, Van Riper, DF, Strong, MD, et al. The dose-response relationship of tranexamic acid. Anesthesiology. 1995; 82:383.
127. Hughes, C, Johnson, C, Irvine, G. A technique to reduce airway risk in patients undergoing maxillofacial surgery. Anaesthesia. 2001; 56:1127.
128. Hunter, AR. Neurosurgical anaesthesia. Oxford: Blackwell Scientific Publications; 1964.
129. Husted, H, Blond, L, Sonne-Holm, S, et al. Tranexamic acid reduces blood loss and blood transfusions in primary total hip arthroplasty: a prospective randomized double-blind study in 40 patients. Acta Orthop Scand. 2003; 74:665.
130. Hynes, M, Calder, P, Scott, G. The use of tranexamic acid to reduce blood loss during total knee arthroplasty. Knee. 2003; 10:375.
131. Ickx, BE, van der Linden, PJ, Melot, C, et al. Comparison of the effects of aprotinin and tranexamic acid on blood loss and red blood cell transfusion requirements during the late stages of liver transplantation. Transfusion. 2006; 46:595.
132. Ido, K, Neo, M, Asada, Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000; 120:518.
133. Ishikawa, T, Funatsu, N, Okamoto, K, et al. Blood-brain barrier function following drug-induced hypotension in the dog. Anesthesiology. 1983; 59:526–531.
134. Joint statement on acquired immune deficiency syndrome (AIDS) related to transfusion. Transfusion. 1983; 23:87.
135. Jordan, WS, Graves, CL, Boyd, WA, et al. Cardiovascular effects of three techniques for inducing hypotension during anesthesia. Anesth Analg. 1971; 50:1059–1068.
136. Jovic, MD, Calija, BM, Radomir, BJ, et al. The use of acute normovolemic hemodilution in patients undergoing cardiac surgery. Cardiovasc Surg. 2003; 11:201.
137. Kademani, D, Voiner, JL, Quinn, PD. Acute hypertensive crisis resulting in pulmonary edema and myocardial ischemia during orthognathic surgery. J Oral Maxillofac Surg. 2004; 62:240.
138. Karnezis, TA, Stulberg, SD, Vixon, RL, et al. The hemostatic effect of desmopressin on patients who had total joint arthroplasty: a double-blind randomized trial. J Bone Joint Surg. 1994; 76:1545.
139. Kassell, NF, Boarini, DJ, Olin, JJ, Sprowell, JA. Cerebral and systemic circulatory effects of arterial hypotension induced by adenosine. J Neurosurg. 1983; 58:69–76.
140. Keats, AS. The ASA classification of physical status: a recapitulation. Anesthesiology. 1978; 49:233.
141. Keiser, GJ, Bozentka, NE, Gold, BD. Laryngeal granuloma: a complication of prolonged endotracheal intubation. Anesth Prog. 1991; 38:232.
142. Kessler, P, Hegewald, J, Adler, W, et al. Is there a need for autogenous blood donation in orthognathic surgery? Plast Reconstr Surg. 2006; 117:571.
143. Kien, ND, White, DA, Reitan, JA, Eisele, JH, Jr. Cardiovascular function during controlled hypotension induced by adenosine triphosphate or sodium nitroprusside in the anesthetized dog. Anesth Analg. 1987; 66:103–110.
144. Kim, HJ, Kim, JT, Kim, HS, et al. A comparison of Glide Scope videolaryngoscopy and direct laryngoscopy for nasotracheal intubation in children. Paediatr Anaesth. 2011; 21:417.
145. Kitaguchi, K, Nakajima, T, Takaki, O, et al. The change in cerebral blood flow during hypotensive anesthesia induced by prostaglandin El. Masui. 1992; 41:766–771.
146. Cha, KJ, Lee, SC. Thermosoftening treatment of the nasotracheal tube before intubation can reduce epistaxis and nasal damage. Anesth Analg. 2000; 91:698.
147. Kohler, M, Hellestern, P, Miyashita, C, et al. Comparative study of intranasal, subcutaneous and intravenous administration of desamino-arginine vasoprassine (DDAVP). Thromb Haemost. 1986; 55:108.
148. Kretschmer, W, Koster, U, Dietz, K, et al. Factors for intraoperative blood loss in bimaxillary osteotomies. J Oral Maxillofac Surg. 2008; 66:1399.
149. Kurian, A, Ward-Booth, P. Blood transfusion and orthognathic surgery—a thing of the past? Br J Oral Maxillofac Surg. 2004; 42:369.
150. Lagerkranser, M, Andreen, M, Irestedt, L, Sollevi, A. Controlled hypotension with the combination of dipyridamole and adenosine. Anaesthesia. 1982; 37(Suppl):303.
151. Lam, AM. Induced hypotension. Can Anaesth Soc J. 1984; 31:S56.
152. Laureano-Filho, JR, Godoy, F, O’Ryan, F. Orthodontic bracket lost in the airway during orthognathic surgery. Am J Orthod Dentofacial Orthop. 2008; 134:288.
153. Laureano-Filho, JR, de Oliveiro Neto, PJ, Duarte, N, et al. Successful management of malignant hyperthermia during orthognathic surgery: a case report. J Oral Maxillofac Surg. 2008; 66:1485.
154. Lessard, MR, Trepanier, CA, Baribault, JP, et al. Isoflurane-induced hypotension in orthognathic surgery. Anesth Analg. 1989; 69:379–383.
155. Litfle, DM. Induced hypotension during anesthesia and surgery. Anesthesiology. 1955; 16:320–332.
156. Lupori, JP, Van Sickels, JE, Holmgreen, WC. Outpatient orthognathic surgery: review of 205 cases. J Oral Maxillofac Surg. 1997; 155:558.
157. Mackie, D. Hypotensive anesthesia and orthognathic surgery. Anesth Analg. 1993; 76:667.
158. Macnab, MSP, Manninen, PH, Lam, AM, Gelb, AW. The stress response to induced hypotension for cerebral aneurysm surgery: a comparison of two hypotensive techniques. Can J Anaesth. 1988; 35:111–115.
159. Macrae, WR, Owen, M. Severe metabolic acidosis following hypotension induced with sodium nitroprusside. Br J Anaesth. 1974; 46:795–797.
160. Macrae, WR, Wildsmith, JAW, Dale, BAB. Induced hypotension with a mixture of sodium nitroprusside and trimethaphan camsylate. Anaesthesia. 1981; 36:312–315.
161. Madsen, JB, Cold, GE, Hansen, ES, et al. Cerebral blood flow and metabolism during isoflurane-induced hypotension in patients subjected to surgery for cerebral aneurysms. Br J Anaesth. 1987; 59:1204–1207.
162. Madsen, DE, Ingerslev, J, Sidelmann, JJ, et al. Intraoperative blood loss during orthognathic surgery is predicted by thromboelastography. J Oral Maxillofac Surg. 2012; 70:e547–e552.
163. Magness, A, Yashon, D, Locke, G, et al. Cerebral function during trimethaphan-induced hypotension. Neurology. 1973; 23:506–509.
164. Mallampati, SR, Gatt, SP, Gugino, LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985; 32(4):429–434.
165. Manola, M, De Luca, E, Moscillo, L, Mastella, A. Using remifentanil and sufentanil in functional endoscopic sinus surgery to improve surgical conditions. ORL J Otorhinolaryngol Relat Spec. 2005; 67:83–86.
166. Marchal, JM, Gomez-Luque, A, Martos-Crespo, F, et al. Clonidine decreases intraoperative bleeding in middle ear microsurgery. Anesthesiol Scand. 2001; 45:627.
167. Marciani, RD, Dickson, LG. Autologous transfusion in orthognathic surgery. J Oral Maxillofac Surg. 1985; 43:201.
168. McNeill, TW, DeWald, RL, Kuo, KN, et al. Controlled hypotensive anesthesia in scoliosis surgery. J Bone Joint Surg. 1974; 56:1167–1172.
169. McNulty, S, Sharifi-Azad, S, Farole, A. Induced hypotension with labetalol for orthognathic surgery. J Oral Maxillofac Surg. 1987; 45:309–311.
170. Mead, JH, Anthony, CD, Sattler, M. Hemotherapy in elective surgery: an incidence report, review of the literature, and alternatives for guideline appraisal. Am J Clin Pathol. 1980; 74:223.
171. Melissari, E, Scully, MF, Paes, T, et al. The influence of DDAVP infusion on the coagulation and fibrinolytic response to surgery. Thromb Haemost. 1986; 55:54.
172. Meoli, AL, Rosen, CL, Kristo, D, et al. Report of the AASM clinical practice review committee. Upper airway management of the adult patient with obstructive sleep apnea in the perioperative period—avoiding complications. Sleep. 2003; 26(8):1060–1065.
173. Messmer, KF. Acceptable haematocrit levels in surgical patients. World J Surg. 1987; 11:41–46.
174. Michenfelder, JD, Theye, RA. Canine systemic and cerebral effects of hypotension induced by hemorrhage, trimethaphan, halothane, or nitroprusside. Anesthesiology. 1977; 46:188–195.
175. Moenning, JE, Bussard, DA, Lapp, TH, et al. Average blood loss and the risk of requiring perioperative blood transfusion in 506 orthognathic surgical procedures. J Oral Maxillofac Surg. 1995; 53:880.
176. Monaghan, A, Hindle, I. Malignant hyperpyrexia in oral surgery: case report and literature review. Br J Oral Maxillofac Surg. 1994; 32:190.
177. Moor, AC, Dubbleman, TM, VanSteveninck, J, Brand, A. Transfusion-transmitted diseases: risks, prevention and perspectives. Eur J Haematol. 1999; 62:1–18.
178. Moraca, P, Bitte, EM, Hale, DE, et al. Clinical evaluation of sodium nitroprusside as a hypotensive agent. Anesthesiology. 1962; 23:193–199.
179. Murphy, MA. Bilateral posterior ischemic optic neuropathy after lumbar spine surgery. Ophthalmology. 2003; 110:1454–1457.
180. Murray, D. Acute normovolemic hemodilution. Eur Spine J. 2004; 13(Suppl):S72.
181. Murtagh, GP. Controlled hypotension with halothane. Anaesthesia. 1960; 15:235–244.
182. Nakazawa, K, Taneyama, C, Benson, KT, et al. Mixtures of sodium nitroprusside and trimethaphan for induction of hypotension. Anesth Analg. 1991; 73:59–63.
183. Nanni, V, Sachs, S. Mediastinal emphysema following Le Fort I osteotomy: report of a case. Oral Surg Oral Med Oral Pathol. 1986; 62:508.
184. Nath, A, Pogrel, MA. Preoperative autologous blood donation for oral and maxillofacial surgery: an analysis of 913 patients. J Oral Maxillofac Surg. 2005; 63:347.
185. National Heart, Lung, and Blood Institute Expert Panel on the use of Autologous Blood. Transfusion alert: use of autologous blood. Transfusion. 1995; 3:703–711.
186. Neilipovitz, DT. Tranexamic acid for major spinal surgery. Eur Spine J. 2004; 13(Suppl):S62.
187. Neilipovitz, DT, Murto, K, Hall, L, et al. A randomised trial of tranexamic acid to reduce blood transfusion for scoliosis surgery. Anesth Analg. 2001; 93:82.
188. Newman, B, Gelb, AW, Lam, AM. The effect of isoflurane-induced hypotension on cerebral blood flow and cerebral metabolic rate for oxygen in humans. Anesthesiology. 1986; 64:307–310.
189. Nkenke, E, Kessler, P, Wiltfang, J, et al. Hemoglobin value reduction and necessity of transfusion in bimaxillary orthognathic surgery. J Oral Maxillofac Surg. 2005; 63:623.
190. Omstein, E, Matteo, RS, Weinstein, JA, Schwartz, AE. A randomized controlled trial of esmolol for deliberate hypotension. Anesthesiology. 1987; 67:A423.
191. Ornsiein, E, Matteo, RS, Weinstein, JA, Schwartz, AE. A controlled trial of esmolol for the induction of deliberate hypotension. J Clin Anesth. 1988; 1:31–35.
192. O’Ryan, F, Ebker, BN. Prolonged apnea after orthognathic surgery due to atypical cholinesterase. Int J Oral Surg. 1981; 10:338.
193. Owall, A, Lagerkranser, M, Sollevi, A. Effects of adenosine-induced hypotension on myocardial hemodynamics and metabolism during cerebral aneurysm surgery. Anesth Analg. 1988; 67:228–232.
194. Pagar, DM, Kupperman, AW, Stern, M. Cutting of nasoendotracheal tube: an unusual complication of maxillary osteotomies. J Oral Surg. 1978; 36:314.
195. Pasch, T, Huk, W. Cerebral complications following induced hypotension. Eur J Anaesthesiol. 1986; 3:299.
196. Patel, NJ, Patel, BS, Paskin, S, Laufer, S. Induced moderate hypotensive anesthesia for spinal fusion and Harrington rod instrumentation. J Bone Joint Surg. 1985; 67:1384–1387.
197. Petrozza, PH. Induced hypotension. Int Anesthesiol Clin. 1990; 28:223–229.
198. Pfleiderer, T. Na-nitroprussid. A very potent platelet disaggregating substance. Acta Univ Carol Med Monogr. 1972; 53:247.
199. Piecuch, JF, West, RA. Spontaneous pneumomediastinum associated with orthognathic surgery: a case report. Oral Surg Oral Med Oral Pathol. 1979; 48:506.
200. Pineiro-Aguilar, A, Somoza-Martin, M, et al. Blood loss in orthognathic surgery: a systematic review. J Oral Maxillofac Surg. 2011; 69:885–892.
201. Posnick, JC, Fantuzzo, JJ, Troost, T. Simultaneous intranasal procedures to improve chronic obstructive nasal breathing in patients undergoing maxillary (Le Fort I) osteotomy. J Oral Maxillofac Surg. 2007; 65:2273.
202. Posnick, JC, Rabinovich, A, Richardson, DT. Blood replacement practices for complex orthognathic surgery: a single surgeon’s experience. J Oral Maxillofac Surg. 2010; 68:54–59.
203. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2003; 98:1269–1277.
204. Praveen, K, Narayanan, V, Muthusekhar, MR, Baig, MF. Hypotensive anaesthesia and blood loss in orthognathic surgery: a clinical study. Br J Oral Maxillofac Surg. 2001; 39:138.
205. Precious, DS, Bosco, DA, Splinter, W, Muir, J. Induced hypotensive anesthesia for adolescent orthognathic surgery patients. J Oral Maxillofac Surg. 1993; 51(Suppl 3):94–95.
206. Precious, DS, Splinter, W, Bosco, D. Induced hypotensive anaesthesia for adolescent orthognathic surgery patients. J Oral Maxillofac Surg. 1996; 54:680–683.
207. Prior, S, Heaton, J, Jatana, KR, Rashid, RG. Parker flex-tip and standard-tip endotracheal tubes: a comparison during nasotracheal intubation. Anesth Prog. 2010; 57:18.
208. Prys-Roberts, C, Lloyd, JW, Fisher, A, et al. Deliberate profound hypotension induced with halothane: studies of haemodynamics and pulmonary gas exchange. Br J Anaesth. 1974; 46:105–116.
209. Prys-Roberts, C, Millard, RK. Self-tuning adaptive control of induced hypotension in humans: a comparison of isoflurane and sodium nitroprusside. J Clin Monit. 1990; 6:236–240.
210. Puelacher, W, Hinteregger, G, Nussbaumer, W, et al. Preoperative autologous blood donation in orthognathic surgery: a follow-up study of 179 patients. J Craniomaxillofac Surg. 1998; 26:121.
211. Purdham, RS. Reduced blood loss with hemodynamic stability during controlled hypotensive anesthesia for Le Fort I maxillary osteotomy using high-dose fentanyl: a retrospective study. CRNA. 1996; 7:33.
212. Ramstrom, G, Sindet-Pedersen, S, Hall, G, et al. Prevention of postsurgical bleeding in oral surgery using tranexamic acid without dose modification of oral anticoagulants. J Oral Maxillofac Surg. 1993; 51:1211.
213. Regan, F, Taylor, C. Recent developments blood transfusion medicine. BMJ. 2002; 318:1435.
214. Reid, RW, Zimmerman, AA, Laussen, PC, et al. The efficacy of tranexamic acid versus placebo in decreasing blood loss in pediatric patients undergoing repeat cardiac surgery. Anesth Analg. 1997; 84:990.
215. Renner, S, Howantiz, PL. Preoperative autologous blood donation in 612 hospitals. Arch Pathol Lab Med. 1992; 116:613.
216. Rodrigo, C. Induced hypotension during anesthesia with special reference to orthognathic surgery. Anesth Prog. 1995; 42:41–58.
217. Rodrigo, C. Anesthetic considerations for orthognathic surgery with evaluation of difficult intubation and technique for hypotensive anesthesia. Anesth Prog. 2000; 47:151.
218. Rohling, RG, Haers, PE, Zimmermann, AP, et al. Multimodal strategy for reduction of homologous transfusions in cranio-maxillofacial surgery. Int J Oral Maxillofac Surg. 1999; 28:137–142.
219. Rollason, WN, Hough, JM. A study of hypotensive anaesthesia in the elderly. Br J Anaesth. 1960; 32:276–285.
220. Rollason, WN, Hough, JM. A re-examination of some electrocardiographic studies during hypotensive anaesthesia. Br J Anaesth. 1969; 41:985–986.
221. Rollason, WN, Robertson, GS, Cordiner, CM, Hall, DJ. A comparison of mental function in relation to hypotensive and normotensive anaesthesia in the elderly. Br J Anaesth. 1971; 43:561–566.
222. Ruggeri, ZM, Mannucci, PM, Lombardi, R. Multimeric composition of factor VIII/von Willebrand factor following administration of DDAVP: implication for pathophysiology and therapy of von Willebrand’s disease subtypes. Blood. 1982; 59:1272.
223. Rummasak, D, Apipan, B, Kaewpradup, P. Factors that determine intraoperative blood loss in bimaxillary osteotomies and the need for preoperative blood preparation. J Oral Maxillofac Surg. 2011; 69:e456–e460.
224. Saamivaara, L, Brander, P. Comparison of three hypotensive anaesthetic methods for middle ear microsurgery. Acta Anaesthesiol Scand. 1984; 28:435–442.
225. Salzman, EW, Weinstein, MJ, Weintraub, RM, et al. Treatment with desmopressin acetate to reduce blood loss after cardiac surgery: a double-blind randomised trial. N Engl J Med. 1986; 314:1402.
226. Samman, N, Cheung, LK, Tong, ACK, et al. Blood loss and transfusion requirements in orthognathic surgery. J Oral Maxillofac Surg. 1996; 54:21.
227. Schaberg, SJ, Kelly, JF, Terry, B, et al. Blood loss and hypotensive anesthesia in oral-facial corrective surgery. J Oral Surg. 1976; 34:147–156.
228. Schendel, S, Powell, N, Jacobson, R. Maxillary, mandibular and chin advancement: Treatment planning based on airway anatomy in obstructive sleep apnea. J Oral Maxillofac Surg. 2011; 69:663–676.
229. Schindler, I, Andel, H, Leber, J, Kimla, T. Moderate induced hypotension provides satisfactory operating conditions in maxillofacial surgery. Acta Anaesthesiol Scand. 1994; 38:384–387.
230. Sculco, PT, Galliana, J. Blood management experience: relationship between autologous blood donation and transfusion in orthopedic surgery. Orthopedics. 1999; 22(Suppl):s129.
231. Segal, JB, Blasco-Colmenares, E, Norris, EJ, et al. Preoperative acute normovolemic hemodilution: a meta-analysis. Transfusion. 2004; 44:632.
232. Senghore, N, Harris, M. The effect of tranexamic acid (cyclokapron) on blood loss after third molar extraction under a day case general anaesthetic. Br Dent J. 1999; 186:634.
233. Sethna, NF, Zurakowski, D, Brustowicz, RM, et al. Tranexamic acid reduces intraoperative blood loss in pediatric patients undergoing scoliosis surgery. Anesthesiology. 2005; 102:727.
234. Shah, N, DelValle, O, et al. Esmolol or sodium nitroprusside for induced hypotension during Isoflurane anesthesia. Vasc Endovascular Surg. 1993; 27:681–687.
235. Shay Bess, R, Lenke, L, Bridwell, K, et al. Wasting of preoperatively donated autologous blood in the surgical treatment of adolescent idiopathic scoliosis. Spine. 2006; 31:2375.
236. Silva, AC, O’Ryan, F, Poor, DB. Postoperative nausea and vomiting (PONV) after orthognathic surgery: a retrospective study and literature review. J Oral Maxillofac Surg. 2006; 64:1385.
237. Simpson, DA, Ireland, J. Hypotensive and normotensive anaesthesia in total hip replacement: a comparative study. Br J Clin Pract. 1983; 37:16–18.
238. Sindet-Pedersen, S, Ramstrom, G, Bernvil, S, et al. Hemostatic effect of tranexamic acid mouthwash in anticoagulant-treated patients undergoing oral surgery. N Engl J Med. 1989; 320:840.
239. Sivarajan, M, Amory, DW, Everett, GB, Buffington, C. Blood pressure, not cardiac output, determines blood loss during induced hypotension. Anesth Analg. 1980; 59:203–206.
240. Slater, JL. Autologous transfusion: its role in transfusion medicine in Canada. Ann RCPSC. 1988; 21:209.
241. Sollevi, A. Hypotensive anesthesia and blood loss. Acta Anaesthesiol Scand. 1988; 32(Suppl 89):39–43.
242. Sollevi, A, Lagerkranser, M, Irestedt, L, et al. Controlled hypotension with adenosine in cerebral aneurysm surgery. Anesthesiology. 1984; 61:400–405.
243. Sood, S, Jayalaxmi, TS, Vijayaraghavan, S, Nundy, S. Use of sodium nitroprusside induced hypotensive anaesthesia for reducing blood loss in patients undergoing lienorenal shunts for portal hypertension. Br J Surg. 1987; 74:1036–1038.
244. Stead, SW, Bloor, BC. The effects of captopril on the renin-angiotensin system and the sympathetic nervous system during sodium nitroprusside-induced hypotension in the halothane-anesthetized rabbit. J Cardiovasc Pharmacol. 1990; 15:465–471.
245. Stewart, A, Newman, L, Sneddon, K, et al. Aprotinin reduces blood loss and the need for transfusion in orthognathic surgery. Br J Oral Maxillofac Surg. 2001; 39:365.
246. St-Hilaire, H, Montazem, AH, Diamond, J. Pneumomediastinum after orthognathic surgery. J Oral Maxillofac Surg. 2004; 62:892.
247. Stramer, SL. Current risks of transfusion-transmitted agents. Arch Pathol Lab Med. 2007; 131:702.
248. Strickland, SM, Westrich, GH. Spontaneous compartment syndrome occurring postoperatively in 2 oral surgery patients. J Oral Maxillofac Surg. 2000; 58:814.
249. Strunn, L. Organ perfusion during controlled hypotension. Br J Anaesth. 1975; 47:793–798.
250. Sudhindran, S. Perioperative blood transfusion: a plea for guidelines. Ann R Coll Surg Engl. 1997; 79:299–302.
251. Suttner, SW, Piper, SN, Lang, K, et al. Cerebral effects and blood sparing efficiency of sodium nitroprusside-induced hypotension alone and in combination with acute normovolemic hemodilution. Br J Anaesth. 2001; 87:699.
252. Tanaka, N, Sakahashi, H, Sato, E, et al. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br. 2001; 83:702.
253. Teeples, TJ, Rallis, DJ, Rieck, KL, et al. Lower extremity compartment syndrome associated with hypotensive anesthesia for orthognathic surgery: a case report and review of the disease. J Oral Maxillofac Surg. 2010; 68:1166.
254. Thiagarajamurthy, S, Levine, A, Dunning, J. Does prophylactic tranexamic acid safely reduce bleeding without increasing thrombotic complications in patients undergoing cardiac surgery? Interact Cardiovasc Thorac Surg. 2004; 3:489.
255. Thompson, GE, Miller, RD, Stevens, WC, Murray, WR. Hypotensive anesthesia for total hip arthroplasty: a study of blood loss and organ function (brain, heart, liver, and kidney). Anesthesiology. 1978; 48:91–96.
256. Thompson, HJ. The management of post-operative nausea and vomiting. J Adv Nurs. 1999; 29:1130.
257. Tobias, JD. Nicardipine for controlled hypotension during orthognathic surgery. Plast Reconstr Surg. 1997; 99:1539–1543.
258. Tough, ICK. Induced hypotension and post operative bleeding. Anaesthesia. 1960; 15:154–157.
259. Ueki, K, Marukawa, K, Shimada, M, et al. The assessment of blood loss in orthognathic surgery for prognathia. J Oral Maxillofac Surg. 2005; 63:350.
260. Vamvakas, EC. Meta-analysis of randomized controlled trials comparing the risk of postoperative infection between recipients of allogenic and autologous blood transfusion. Vox Sang. 2002; 83:339.
261. Van de Perre, JPA, Stoelinga, PJW, Blijdorp, PA, et al. Perioperative morbidity in maxillofacial orthopaedic surgery: a retrospective study. J Craniomaxillofac Surg. 1996; 24:263.
262. Vazeery, AK, Lunde, O. Controlled hypotension in hip joint surgery. An assessment of surgical hemorrhage during sodium nitroprusside infusion. Acta Orthop Scand. 1979; 50(4):433–441.
263. Venugoplan, SR, Nanda, V, Turkistani, K, et al. Discharge patterns of orthognathic surgeries in the United States. J Oral Maxillofac Surg. 2012; 70:e77–e86.
264. Wagner, JD, Moore, DL. Preoperative laboratory testing for the oral and maxillofacial surgery patient. J Oral Maxillofac Surg. 1991; 49:177.
265. Wang, HH, Liu, LMP, Katz, RL. A comparison of the cardiovascular effects of sodium nitroprusside and trimethaphan. Anesthesiology. 1977; 46:40–48.
266. Warner, WA, Shumrick, DA, Caffrey, JA. Clinical investigation of prolonged induced hypotension in head and neck surgery. Br J Anaesth. 1970; 42:39–44.
267. Washburn, MC, Hyer, RL. Deliberate hypotension for elective major maxillofacial surgery: a balanced halothane and morphine technique. J Maxillofac Surg. 1982; 10:50–55.
268. Weerasinghe, JU, Gunawardana, RH. Delayed tracheal extubation in oral and maxillofacial surgery. Br J Oral Maxillofac Surg. 1999; 37:152.
269. Wildsmith, JAW, Drummond, GB, MacRae, WR. Blood-gas changes during induced hypotension with sodium nitroprusside. Br J Anaesth. 1975; 47:1205–1210.
270. Wildsmith, JAW, Sinclair, CJ, Thom, J, et al. Hemodynamic effects of induced hypotension with a nitroprusside-trimethaphan mixture. Br J Anaesth. 1983; 55:381–389.
271. Wilson, ME, Ellis, FR. Predicting malignant hyperpyrexia. Br J Anaesth. 1979; 51:66.
272. Wood, M, Hyman, S, Wood, AJJ. A clinical study of sensitivity to sodium nitroprusside during controlled hypotensive anesthesia in young and elderly patients. Anesth Analg. 1987; 66:132.
273. Yamada, S, Brauer, F, Knierim, D, et al. Safety limits of controlled hypotension in humans. Acta Neurochir Suppl. 1988; 42:14–17.
274. Yu, CN, Chow, TK, Kwan, AS, et al. Intra-operative blood loss and operating time in orthognathic surgery using induced hypotensive general anesthesia: prospective study. Hong Kong Med J. 2000; 6:307.
275. Yun, KI, Lee, JA, Park, JU. Intubation granuloma: report of a case. J Oral Maxillofac Surg. 2008; 66:1263.
276. Zellin, G, Rasmusson, L, Palsson, J, et al. Evaluation of hemorrhage depressors on blood loss during orthognathic surgery: a retrospective study. J Oral Maxillofac Surg. 2004; 62:662.
277. Zonis, Z, Seear, M, Reichert, C, et al. The effect of preoperative tranexamic acid on blood loss after cardiac operations in children. J Thorac Cardiovasc Surg. 1996; 111:982.
278. Zulian, MA, Chisum, JW, Mosby, EL, et al. Extubation criteria for oral and maxillofacial surgery patients. J Oral Maxillofac Surg. 1989; 47:616.
*References 5, 8, 20, 26, 33, 36, 40, 54, 55, 63, 65, 67, 82, 88, 89, 98, 99, 111, 116, 119, 128, 150, 151, 155, 160, 166, 168, 178, 181, 182, 190, 191, 196–198, 209, 219, 221, 224, 237, 241–244, 258, 265, 270, 272.
*References 13, 14, 10, 11, 9, 12, 83, 113, 117, 133, 135, 139, 145, 158, 161, 163, 174, 178, 188, 195, 221, 249, 251, 269, 273.
†References 1–3, 18, 44, 57, 60, 74, 87, 143, 193, 208, 220.
*References 7, 25, 38, 46, 49, 50, 68, 95–97, 100, 154, 157, 205, 211, 216, 229, 257, 266, 274.
*References 21–23, 27, 28, 39, 41, 42, 59, 71, 77, 85, 120, 122, 125, 126, 129–132, 171, 186, 187, 214, 233, 238, 252, 254, 277.
*References 17, 30, 47, 52, 72, 109, 112, 137, 141, 148, 153, 176, 183, 192, 194, 199, 246, 268, 271, 275.