13 Anesthesia for Thoracic Surgery
General Perioperative Considerations
Inhalational anesthetic agents are commonly administered in 100% O2 during maintenance of anesthesia. Isoflurane may be preferred owing to less attenuation of hypoxic pulmonary vasoconstriction compared with other inhalational agents, although this has not been studied in children.1 Nitrous oxide is avoided. IV opioids have a sparing effect on the concentration of inhalational anesthetics required, and therefore may limit the impairment of hypoxic pulmonary vasoconstriction. Alternatively, total IV anesthesia may be used.
A variety of approaches have been described to prevent and treat pain after videoscopic procedures. Bupivacaine infiltration at incision sites before skin incision has been shown to decrease postoperative pain.2,3 Bupivacaine infiltration was found to be superior to IV fentanyl or tenoxicam for reducing postoperative pain.4 The combination of general anesthesia with regional anesthesia that also contributes postoperative analgesia, is particularly desirable for thoracotomy, but may also be beneficial for thoracoscopic procedures. This is especially true when thoracostomy tube drainage, a source of significant postoperative pain, is used after surgery. In addition, regional blockade for postoperative analgesia facilitates deep breathing and coughing, which may limit atelectasis and pneumonia. A variety of regional anesthetic techniques have been described for intraoperative anesthesia and postoperative analgesia, including intercostal and paravertebral blocks, intrapleural infusions, and epidural anesthesia (see Chapters 41, 42, and 43).
Ventilation and Perfusion during Thoracic Surgery
In adults with unilateral lung disease, oxygenation is optimal when the patient is placed in the lateral decubitus position with the healthy lung dependent (“down”) and the diseased lung nondependent (“up”).5 Presumably, this is related to an increase in blood flow to the dependent, healthy lung and a decrease in blood flow to the nondependent, diseased lung because of the hydrostatic pressure (or gravitational) gradient between the two lungs. This phenomenon promotes 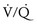 matching in the adult patient undergoing thoracic surgery in the lateral decubitus position.
matching in the adult patient undergoing thoracic surgery in the lateral decubitus position.
In infants with unilateral lung disease, however, oxygenation is improved with the healthy lung “up.”6 Several factors account for this discrepancy between adults and infants. Infants have a soft, easily compressible rib cage that cannot fully support the underlying lung. Therefore functional residual capacity is closer to residual volume, making airway closure likely to occur in the dependent lung even during tidal breathing.7 When the adult is placed in the lateral decubitus position, the dependent diaphragm has a mechanical advantage because it is “loaded” by the abdominal hydrostatic pressure gradient. This pressure gradient is reduced in infants, thereby reducing the functional advantage of the dependent diaphragm. The infant’s small size also reduces the hydrostatic pressure gradient between the nondependent and dependent lungs. Consequently, the favorable increase in perfusion to the dependent, ventilated lung is attenuated in infants.
Finally, the infant’s increased oxygen requirement, coupled with a small functional residual capacity, predisposes to hypoxemia. Infants normally consume 6 to 8 mL of O2/kg/min compared with adult rates of 2 to 3 mL of O2/kg/min.8 For these reasons, infants are at an increased risk of significant hemoglobin desaturation during surgery in the lateral decubitus position.
A modest increase in Paco2 may be beneficial in children during thoracoscopic procedures. In a study of 12 children undergoing video-assisted thoracoscopic surgery for patent ductus arteriosus closure, hypercapnea targeting Paco2 values between 50 and 70 mm Hg increased cardiac output, central venous and arterial oxygen tension.9
Thoracoscopy
With the miniaturization of instruments, progress in video technology, and growing experience among pediatric surgeons, video endoscopic surgery of the chest, or thoracoscopy, is being performed for an increasing number of pediatric surgical indications (Table 13-1). Advantages of thoracoscopy include smaller chest incisions, reduced postoperative pain, and more rapid postoperative recovery compared with thoracotomy (Table 13-2, Fig. 13-1).10,11 Endoscopes that can be passed through a needle and trocar system are now manufactured, and digital video signals can be electronically modified to yield sharp, detailed, color images with a minimum light intensity. Digital cameras are designed to maintain an image in an upright orientation regardless of how the telescope is rotated. They are also equipped with an optical or digital zoom to magnify the image or give the illusion of moving the telescope closer to the object of interest. The smallest of telescopes use fiberoptics and are less than 2 mm in diameter (Fig. 13-2). Two-millimeter disposable ports, mounted on a Veress needle, are used for introduction of these small instruments. Larger instruments and ports are used in larger children and for more complex cases.
TABLE 13-1 Thoracoscopic Procedures in Infants and Children
TABLE 13-2 Advantages of Thoracoscopic versus Open-Chest Surgery
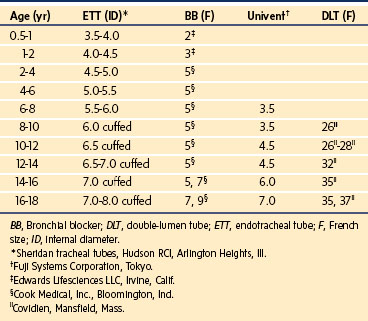
Techniques for Single-Lung Ventilation in Infants and Children
Use of a Single-Lumen Endotracheal Tube
The simplest means of providing SLV is to intentionally intubate the ipsilateral main-stem bronchus with a conventional single-lumen endotracheal tube (ETT).12 When the left bronchus is to be intubated, the bevel of the ETT is rotated 180 degrees and the child’s head is turned to the right.13 The ETT is advanced into the bronchus until breath sounds on the operative side disappear. A fiberoptic bronchoscope (FOB) may be passed through or alongside the ETT to confirm or guide placement. Alternatively, fluoroscopy may be used to guide and position the ETT.14 When a cuffed ETT is used, the distance from the proximal cuff to the tip of the ETT must be less than the length of the main-stem bronchus so that the upper lobe orifice is not occluded (Fig. 13-3).15 This technique is simple and requires no special equipment other than a FOB. This may be the preferred technique of SLV in emergency situations, such as airway hemorrhage or contralateral tension pneumothorax.
Variations of this technique have been described, including intubation of both bronchi independently with small ETTs.16–19 One main-stem bronchus is initially intubated with an ETT, after which another ETT is advanced over a FOB into the opposite bronchus. The disadvantages of these techniques include technical difficulties and trauma to the tracheal and bronchial mucosa. Even after successful bilateral bronchial intubation, the inner diameters of the tubes will be small, limiting gas flow and impeding suctioning of the airways.
Use of Balloon-Tipped Bronchial Blockers
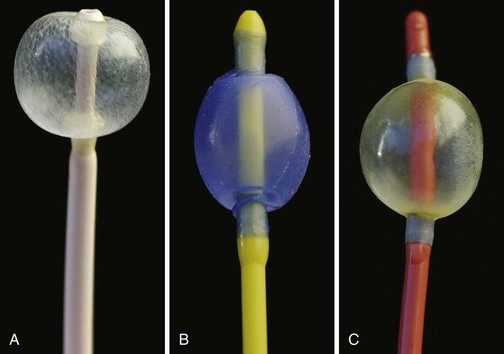
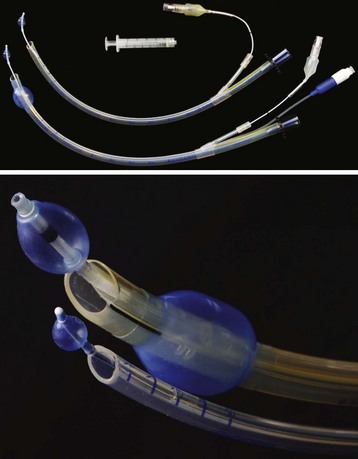
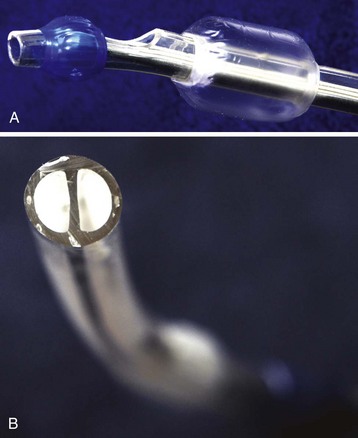
A Fogarty embolectomy catheter or an end-hole, balloon wedge catheter may be used for bronchial blockade to provide SLV (Fig. 13-4).20–23 Placement of a Fogarty catheter is facilitated by bending the tip of its stylet toward the bronchus on the operative side. An FOB may be used to reposition the catheter and confirm appropriate placement. Various techniques for placing an end-hole catheter outside the ETT have been described. Using one such method, the bronchus on the operative side is initially intubated with an ETT.20 A guidewire is then advanced into that bronchus through the ETT. The ETT is removed and the blocker is advanced over the guidewire into the bronchus. An ETT is then reinserted into the trachea alongside the blocker catheter. The catheter balloon is positioned in the proximal main-stem bronchus under fiberoptic visual guidance. Alternatively, if an FOB small enough to pass through the indwelling ETT is not available, fluoroscopy may be used (Fig. 13-5). With an inflated blocker balloon the airway is completely sealed, providing more predictable lung collapse and better operating conditions than with an ETT in the bronchus.
One potential problem with this technique is dislodgement of the blocker balloon into the trachea, blocking ventilation to both lungs and/or preventing collapse of the operative lung. The balloons of most catheters currently used for bronchial blockade have low compliance properties (i.e., low volume, high pressure). They require 1 to 3 mL of air or saline to fully inflate. Overdistention of the balloon can damage or even rupture the airway.24 A recent study, however, reported that bronchial blocker cuffs produced lower “cuff-to-tracheal” pressures than double-lumen tubes.25 When closed-tip bronchial blockers are used, the operative lung cannot be suctioned and continuous positive airway pressure (CPAP) cannot be provided to the operative lung if needed.
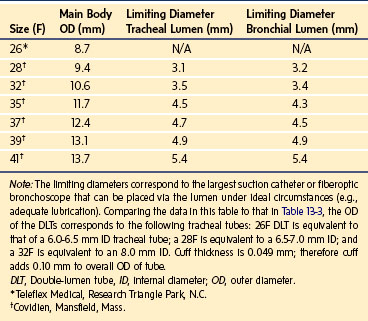
When a bronchial blocker is placed outside the ETT, care must be taken to avoid injury caused by compression and resultant ischemia of the tracheal mucosa. The sum of the catheter diameter and the outer diameter of the ETT should not significantly exceed the tracheal diameter. Outer diameters for pediatric-size ETTs are shown in Table 13-3. These numbers provide an estimate of the predicted tracheal diameter, which should approximate the size of the uncuffed ETT predicted to produce a seal in the trachea.
| ID (mm)* | OD (mm) | Equivalent French Size† |
|---|---|---|
| 3.0 | 4.3 | 13 |
| 3.5 | 4.9 | 15 |
| 4.0 | 5.5 | 17 |
| 4.5 | 6.2 | 19 |
| 5.0 | 6.8 | 21 |
| 5.5 | 7.5 | 23 |
| 6.0 | 8.2 | 25 |
| 6.5 | 8.9 | 27 |
| 7.0 | 9.6 | 29 |
| 7.5 | 10.2 | 31 |
| 8.0 | 10.8 | 32 |
Note: Cuffed tubes have approximately 0.5-mm additional outer diameter. The external diameter may also vary by manufacturer.
ID, Internal diameter; OD, outer diameter.
*Sheridan tracheal tubes (Hudson RCI, Arlington Heights, Ill.).
†French (F) size is 3 x OD (mm).
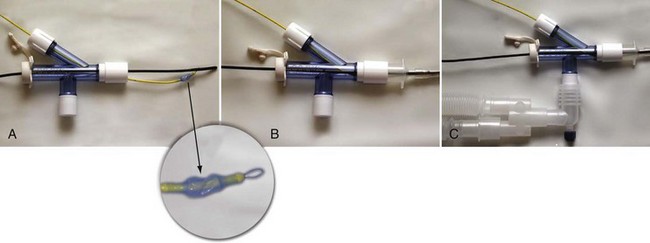
Recently, adapters have been developed that facilitate ventilation during placement of a bronchial blocker through an indwelling ETT.26,27 Use of a 5F endobronchial blocker that is designed for use in children, and has a multiport adapter and FOB, has been described (Cook Medical, Inc., Bloomington, Ind.).28 The balloon is elliptical so that it conforms to the bronchial lumen when inflated. The blocker catheter has a maximum outer diameter of 2.5 mm (including the deflated balloon), a central lumen with a diameter of 0.7 mm, and a distal balloon with a capacity of 3 mL. The balloon has a length of 1.0 cm, corresponding to the length of the right main-stem bronchus in children approximately 2 years of age.29 The blocker is placed coaxially through a dedicated port in the adapter, which also has a port for passage of a FOB and ports for connection to the anesthesia breathing circuit and ETT (Fig. 13-6). The FOB port has a plastic sealing cap, whereas the blocker port has a Tuohy-Borst connector that locks the catheter in place and maintains an airtight seal. Because oxygen can be administered during passage of the blocker and FOB, the risk of hypoxemia during blocker placement is diminished, and repositioning of the blocker may be performed with fiberoptic guidance during surgery.
When a FOB is used to guide the placement of a bronchial blocker, both the blocker catheter and FOB must pass through the indwelling ETT. The smallest ETT through which the catheter and FOB can be passed must be larger than the sum of the outer diameters of the catheter and the FOB. The 5F Cook bronchial blocker and an FOB with a 2.2-mm diameter, for example, may be inserted through an ETT with an internal diameter as small as 5.0 mm; for children with an indwelling ETT smaller than this, a blocker catheter can be positioned under fluoroscopy (see Fig. 13-5).30
Use of a Univent Tube
The Univent tube (Fuji Systems Corporation, Tokyo) is a conventional ETT with a second lumen containing a small blocker catheter that can be advanced into a bronchus (Fig. 13-7).31,32 A balloon located at the distal end of this small tube serves as a blocker. Univent tubes require a FOB for successful placement. Univent tubes are now available in sizes with internal diameters as small as 3.5 and 4.5 mm, for use in children older than 6 years of age.33 Because the blocker tube is firmly attached to the main ETT, displacement of the Univent blocker balloon is less likely than when other blocker techniques are used. The blocker tube has a small lumen that allows egress of gas and can be used to insufflate oxygen or suction the operated lung.
One disadvantage of the Univent tube is the large cross-sectional area occupied by the blocker channel, especially in the smaller size tubes. Therefore Univent tubes have a large outer diameter with respect to their inner (luminal) diameters (Table 13-4). Smaller Univent tubes have a disproportionately high resistance to gas flow.34 The Univent tube’s blocker balloon has low-volume, high-pressure characteristics, so mucosal injury can occur during normal inflation.35,36
| ID (mm) | OD (mm)* |
|---|---|
| 3.5 | 7.5/8.0 |
| 4.5 | 8.5/9.0 |
| 6.0 | 10.0/11.0 |
| 6.5 | 10.5/11.5 |
| 7.0 | 11.0/12.0 |
| 7.5 | 11.5/12.5 |
| 8.0 | 12.0/13.0 |
| 8.5 | 12.5/13.5 |
| 9.0 | 13.0-14.0 |
ID, Internal diameter; OD, outer diameter.
Use of Double-Lumen Tubes
All double-lumen tubes (DLTs) are essentially two tubes of unequal length molded together. The shorter tube ends in the trachea, and the longer tube ends in the bronchus (Fig. 13-8). DLTs for older children and adults have cuffs located on the tracheal and bronchial lumens. The tracheal cuff, when inflated, allows positive-pressure ventilation. The inflated bronchial cuff allows ventilation to be diverted to either or both lungs and protects each lung from contamination from secretions, purulent material, or blood originating from the contralateral side.
Marraro described a bilumen tube for infants.37 This tube consists of two separate uncuffed tracheal tubes of different lengths attached longitudinally. This tube is not available in the United States. The smallest cuffed DLT commercially available in the United States is a 26F size (Teleflex Medical, Research Triangle Park, N.C.). This DLT may be used in children as young as 8 years old. DLTs are also available in sizes 28 and 32F (Nellcor brand [Covidien, Mansfield, Mass.]); these are suitable for children 10 years of age and older. Numerous manufacturers produce clear, disposable, PVC Robertshaw-design DLTs, which are available in sizes 35 to 41F (Table 13-5). Essentially, they consist of similar features with small modifications in cuff shape and location. A colored bronchial cuff, commonly blue, permits easy identification by fiberoptic bronchoscopy. In general, the cuffs used in DLTs are high-compliance cuffs that are designed to exert less pressure on the tracheal and bronchial mucosa compared with low-compliance cuffs. For right-sided DLTs, the endobronchial cuff is donut shaped and allows the right upper lobe ventilation opening to be positioned at the right upper lobe orifice. Despite this design, right upper lobe occlusion may occur because of the shorter length of the right main-stem bronchus.1,38 Therefore right-sided DLTs are used infrequently.
DLTs are inserted in children using the same technique as in adults, and left DLTs are almost exclusively used.39 The tip of the tube is inserted just past the vocal cords, and the stylet is withdrawn. The DLT is rotated 90 degrees to the appropriate side and then advanced into the bronchus. After intubation, the tracheal cuff is inflated first and equal breath sounds should be confirmed. To prevent mucosal damage from excessive pressure applied by the bronchial cuff, the cuff is inflated with incremental volumes to seal air leaks around the bronchial cuff into the trachea. Inflation of the bronchial cuff seldom requires more than 2 mL of air. After inflation of the bronchial cuff, bilateral breath sounds should be rechecked to confirm that the bronchial cuff is not herniating across the carina to impede contralateral lung ventilation. Fiberoptic bronchoscopy can be used to directly visualize the proximal edge of the bronchial cuff in the left bronchus, just distal to the carina. One simple way to verify that the tip of the bronchial lumen is located in the designated bronchus is to clamp the tracheal lumen at the level of the connector and then observe and auscultate left and right lungs. Usually, inspection will reveal unilateral movement of the ventilated hemithorax. With the tracheal lumen clamped, auscultation of the chest will demonstrate air entry in the left lung and no ventilation in the right lung. After auscultation and release of the tracheal clamp, the bronchial lumen is clamped and the tracheal lumen is ventilated to confirm movement and breath sounds of the right lung. Whenever a right-sided DLT is used, ventilation of the right upper lobe must be verified. This can be accomplished by careful auscultation over the right upper lung field or, more accurately, by fiberoptic bronchoscopy. When a left-sided DLT is used, the risk of occluding the left upper lobe bronchus by advancement of the bronchial tip into the distal left main bronchus should be considered.
DLTs may be malpositioned in up to 48% of cases despite careful inspection and auscultation.40 The simplest way to evaluate proper positioning of a left-sided DLT is to perform fiberoptic bronchoscopy through the tracheal lumen. The carina is then visualized, and only the proximal edge of the bronchial cuff should be identified just distal to the carina. Herniation of the bronchial cuff over the carina to partially occlude the contralateral main-stem bronchus should be excluded. Fiberoptic bronchoscopy should then be performed via the bronchial lumen to identify the patent left upper lobe orifice. When a right-sided DLT is used, the right upper lobe bronchial orifice must be identified while the bronchoscope is passed through the right upper lobe ventilating slot.
The use of FOB to facilitate positioning of DLTs in children is dependent on the availability of small instruments. FOBs with an external diameter of 3.6 mm are commonly available and will pass through a 35F DLT. Smaller FOBs are needed when 26F, 28F, and 32F DLTs are used (see Table 13-5 for “limiting diameters”).
In the adult population, the depth of insertion of the DLT is directly related to the height of the patient.41 No equivalent measurements are available as yet in children. Fortunately, there are very few reports in children of airway damage from DLTs.
A disadvantage of DLTs is the need to change the DLT to a single-lumen ETT if mechanical ventilation is required after surgery. This is a particular problem for children in whom tracheal intubation was difficult initially because of anatomic or functional limitations. Even when an airway was not classified as difficult preoperatively, it may become difficult secondary to facial and supraglottic edema, the presence of secretions and/or blood in the airway, and laryngeal trauma from the initial intubation. The use of an ETT exchange catheter may facilitate the exchange of a DLT for a single lumen ETT.42 These devices are commercially available in a variety of sizes (Cook Medical, Inc., Bloomington, Ind.) and allow oxygen insufflation and jet ventilation (see E-Fig. 12-3, A–H).
Several important caveats should be considered before using an ETT exchange catheter. First, it must be small enough to pass through the tracheal lumen of the DLT. This should be tested in vitro before the procedure is performed in vivo. Second, it should never be advanced against resistance, and the clinician must always be cognizant of the depth of insertion; perforations of the tracheobronchial tree have been reported.43 Third, a jet ventilator should be immediately available in case the new ETT does not follow the exchange catheter into the trachea and oxygenation via the catheter is needed. The jet ventilator should be preset to a peak inspiratory pressure of 25 psi (172 kPa) by an inline regulator. When passing an ETT over an ETT exchange catheter, a laryngoscope should be used to facilitate passage of the ETT into the trachea. It should be noted that the tip of the ETT may hang up on the laryngeal inlet and may require 90 degrees rotation clockwise or counter-clockwise to successfully pass, should this occur.
General Considerations in the Management of Single-Lung Ventilation
After the child has been placed in the lateral decubitus position, proper ETT, bronchial blocker, or DLT position should be reconfirmed, because malpositioning may occur when turning the patient. Two-lung ventilation should be maintained for as long as possible before switching to SLV. When SLV is required, an Fio2 of 1.0 is generally used. Assuming an intact hypoxic pulmonary vasoconstriction response, Pao2 during SLV should be between 150 and 210 mm Hg.44 The lungs should be ventilated with a tidal volume of 8 to 10 mL/kg at a ventilatory rate that maintains the Paco2 between 45 and 60 mm Hg, unless this degree of hypercapnia cannot be tolerated because of other physiologic factors (e.g., concomitant metabolic acidosis). Inadequate tidal volumes may lead to atelectasis in the ventilated lung (reduced functional residual capacity), and increased intrapulmonary shunting, resulting in hypoxemia. Large tidal volumes may force blood to the nondependent lung (similar to the application of positive end-expiratory pressure), thereby increasing the intrapulmonary shunt.45,46
After the institution of SLV, Pao2 may continue to decrease for up to 45 minutes. Should hypoxemia develop, proper positioning of the indwelling blocker or tube should be reconfirmed by fiberoptic bronchoscopy, if possible. Several techniques can be employed to improve oxygenation. The most effective maneuver for improving Pao2 is the application of CPAP to the nondependent lung.47 Insufflation of oxygen to achieve a CPAP of 10 cm H2O, for example, produces alveolar inflation and decreases intrapulmonary shunt fraction. This can usually be accomplished without significant expansion of the lung and interference with surgical conditions. If the Pao2 continues to decrease despite the application of CPAP to the deflated lung, a malpositioned bronchial blocker or tube should be considered. This may be signaled by a sudden increase in the inflation pressure, a decrease in tidal volume, and/or a change in the capnogram. When a DLT is in place, the surgeon may aid repositioning. The surgeon can palpate the bronchi and manually occlude the main bronchial lumens, thereby guiding the tip of the DLT into the correct position. When the cause of the hypoxemia and/or hypercarbia cannot be readily identified, the balloon or cuff should be deflated and both lungs should be ventilated after informing the surgeon of the problem.
Guidelines for selecting appropriate tubes (or catheters) for SLV in children are shown in Table 13-6. There is significant variability in overall size and airway dimensions in children, particularly in teenagers. The recommendations shown in Table 13-6 are based on average values for airway dimensions. Larger DLTs may be safely used in large adolescents.
Surgical Lesions of the Chest
Neonates and Infants
Tracheal and/or esophageal compression may occur as a result of a variety of lesions in the chest, including vascular rings and slings (see also Chapters 14 and 31).
A FOB is used to evaluate the severity of the stenosis and exclude other causes of stridor (e.g., vocal cord paralysis or laryngomalacia). When general anesthesia is required, inhalational anesthesia may be administered via a face mask, with the FOB inserted through an adapter in the mask and into the nasopharynx. This is usually performed while the infant breathes spontaneously.48 Noninvasive imaging studies are being increasingly utilized for the diagnosis of a variety of congenital airway lesions, including vascular rings.48 Bronchography and “virtual” computed tomography (CT) scanning may also be useful.49,50
A cricoid split procedure may be performed for infants with acquired subglottic stenosis. After diagnostic bronchoscopy, the infant is either intubated with an ETT or a rigid bronchoscope is left in place during the operation. Anesthesia may be maintained with inhalational agents or an IV anesthetic technique, such as with propofol and remifentanil.51 Typically, an ETT 0.5 mm larger than the original ETT is placed after the repair.
For infants with severe congenital tracheal stenosis, a laryngotracheoplasty may be performed. This procedure involves the placement of a costal, auricular, or laryngeal cartilage graft into the anterior and/or posterior trachea.52 In some cases, a stent may be positioned within the trachea. These infants may require a tracheal tube and mechanical ventilation for a variable period of time postoperatively. In these cases, sedation, analgesia, and at times neuromuscular blockade are maintained after surgery.
Congenital cystic lesions in the thorax may be classified into three categories.53 Bronchogenic cysts result from abnormal budding or branching of the tracheobronchial tree. They may cause respiratory distress, recurrent pneumonia, and/or atelectasis because of lung compression. Dermoid cysts are clinically similar to bronchogenic cysts but differ histologically because they are lined with keratinized, squamous epithelium rather than respiratory (ciliated columnar) epithelium. They usually manifest later in childhood or adulthood. Cystic adenomatoid malformations are structurally similar to bronchioles but lack associated alveoli, bronchial glands, and cartilage.54 Because these lesions communicate with the airways, they may become overdistended as a result of gas trapping, leading to respiratory distress in the first few days of life. When they are multiple and air filled, cystic adenomatoid malformations may resemble congenital diaphragmatic hernias radiographically. Treatment is surgical resection of the affected lobe. As with congenital diaphragmatic hernias, prognosis depends on the amount of remaining lung tissue, which may be hypoplastic because of compression in utero.55
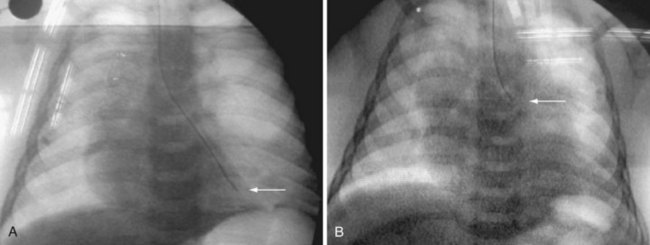
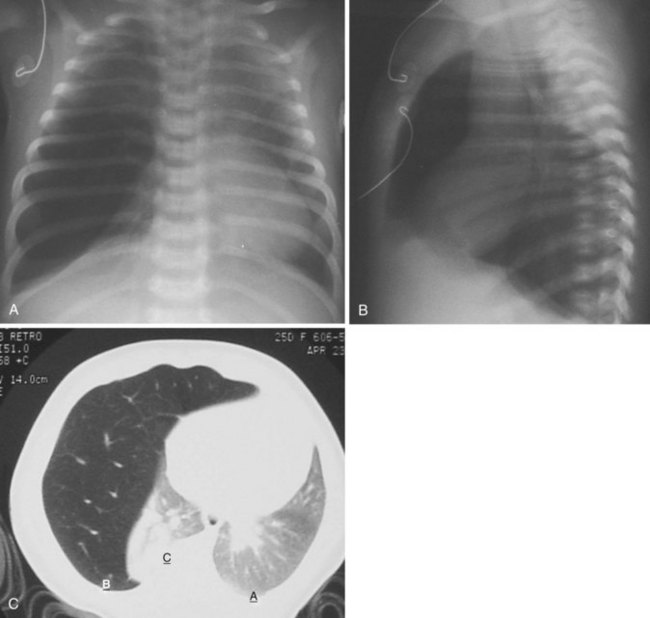
Congenital lobar emphysema often manifests with respiratory distress shortly after birth.56 This lesion may be caused by “ball-valve” bronchial obstruction in utero, causing progressive distal overdistention with fetal lung fluid. The resultant emphysematous lobe may compress lung tissue bilaterally, resulting in a variable degree of hypoplasia. Congenital cardiac deformities are present in about 15% of children with congenital lobar emphysema.57 Radiographic signs of hyperinflation may be misinterpreted as tension pneumothorax or atelectasis on the contralateral side (Fig. 13-9). Positive-pressure ventilation may exacerbate lung hyperinflation. Nitrous oxide is contraindicated, and isolation of the lungs during anesthesia is desirable (see also chapter 36).
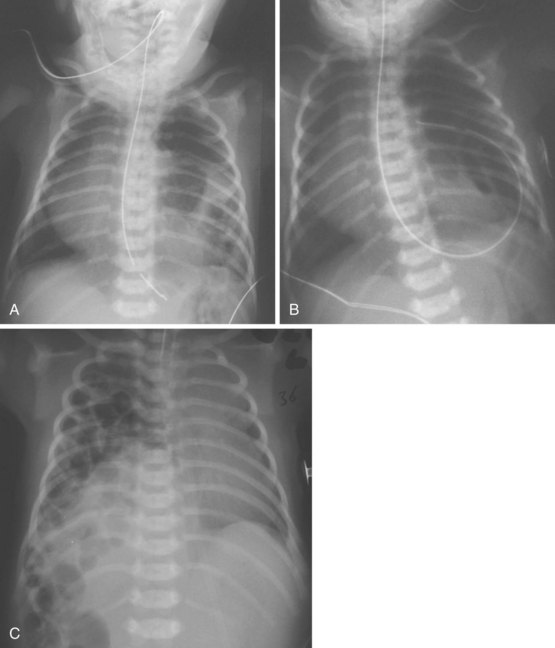
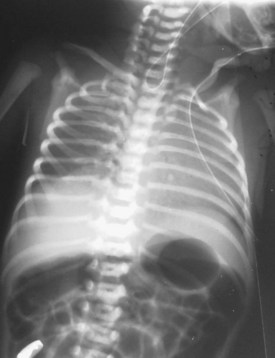
Congenital diaphragmatic hernia is a life-threatening condition that occurs in approximately 1 in 2000 live births. Failure of a portion of the fetal diaphragm to develop allows abdominal contents to enter the thorax, interfering with normal lung growth. In 70% to 80% of diaphragmatic defects, a portion of the left posterior diaphragm fails to close, forming a triangular defect known as the foramen of Bochdalek. Hernias through the foramen of Bochdalek that occur early in fetal life usually cause respiratory failure immediately after birth owing to pulmonary hypoplasia. Distention of the gut postnatally with bag-and-mask ventilation exacerbates the ventilatory compromise by further compressing the lungs. The diagnosis is often made prenatally, and fetal surgical repair has been described.58 Neonates present with tachypnea, a scaphoid abdomen, and absent breath sounds over the affected side. Chest radiography typically shows bowel in the left hemithorax, with deviation of the heart and mediastinum to the right and compression of the right lung (Fig. 13-10). Right-sided hernias (see Fig. 13-10, C) may occur late and manifest with milder signs. In the presence of significant respiratory distress, bag-and-mask ventilation should be avoided and immediate tracheal intubation should be performed (see also Chapter 36).
Because pulmonary hypertension with right-to-left shunting contributes to severe hypoxemia in neonates with congenital diaphragmatic hernia, a variety of pulmonary vasodilators have been used. These include tolazoline, prostacyclin, dipyridamole, and nitric oxide.59–63 High-frequency oscillatory ventilation has been used in conjunction with pulmonary vasodilator therapy to improve oxygenation before surgery.64 Occasionally, prostaglandin E1 is used to maintain a patent ductus arteriosus and reduce right ventricular afterload. In cases of severe lung hypoplasia and pulmonary hypertension refractory to these therapies (e.g., Pao2 less than 50 mm Hg with Fio2 of 1.0), extracorporeal membrane oxygenation (ECMO) should be initiated early to avoid progressive lung injury. Improved outcomes have been associated with early use of ECMO followed by delayed surgical repair.65
A particularly poor prognosis is predicted if congenital diaphragmatic hernia is associated with cardiac deformities, preoperative alveolar-to-arterial oxygen gradient greater than 500 mm Hg, or severe hypercarbia despite vigorous ventilation.66,67 Prognosis has also been correlated with pulmonary compliance and radiographic findings.68,69
Surgical correction via a subcostal incision with ipsilateral chest tube placement may be performed before, during, or immediately after ECMO.70,71 In neonates undergoing surgical repair without ECMO, pulmonary hypertension is the major cause of morbidity and mortality. Hyperventilation to induce a respiratory alkalosis and 100% oxygen may be administered to decrease pulmonary vascular resistance. The anesthetic should be designed to minimize sympathetic discharge, which may exacerbate pulmonary hypertension (e.g., a high-dose opioid technique). The lungs of these infants should be ventilated with small tidal volumes and low inflating pressures, to avoid pneumothorax on the contralateral (usually right) side. Both nitric oxide and high-frequency oscillatory ventilation have been used during surgical repair.72,73 A high index of suspicion of right-sided pneumothorax should be maintained, and a thoracostomy tube should be placed in the event of acute deterioration of respiratory or circulatory function. It is also imperative that normal body temperature, intravascular volume, and acid-base status be maintained. Mechanical ventilation is continued postoperatively in nearly all cases, because lung compliance is markedly reduced after surgery.
Failure of the central and lateral portions of the diaphragm to fuse results in a retrosternal defect, the foramen of Morgagni. This usually manifests as signs of bowel obstruction rather than respiratory distress. Repair is usually performed via an abdominal incision (see also Chapter 36).
Tracheoesophageal fistula and/or esophageal atresia occurs in approximately 1 in 4000 live births. In 80% to 85% of afflicted infants, this lesion includes esophageal atresia with a distal esophageal pouch and a proximal tracheoesophageal fistula.74,75 The fistula is usually located one to two tracheal rings above the carina. Afflicted neonates present with spillover of pooled oral secretions from the pouch and may develop progressive gastric distention and tracheal aspiration of acidic gastric contents via the fistula. A common association is the VACTERL complex, consisting of vertebral, anorectal, cardiac, tracheal, esophageal, renal, and/or limb defects.76 Esophageal atresia is confirmed when an orogastric tube passed through the mouth cannot be advanced more than about 7 cm (Fig. 13-11). The proximal pouch tube should be secured and continuous suction applied, after which a chest radiograph is diagnostic (see also Chapter 36).
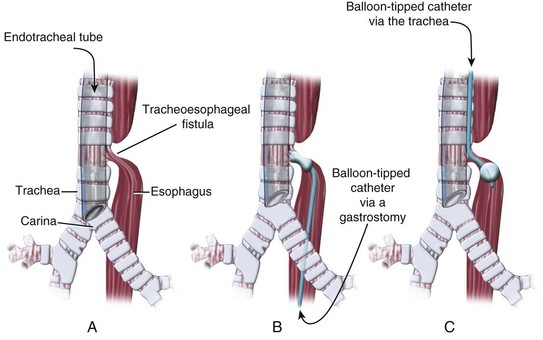
Mask ventilation and tracheal intubation are avoided before surgery if possible, because they may exacerbate gastric distention and further compromise respirations. Once the trachea is intubated, it is occasionally necessary to occlude the tracheal orifice of the fistula with the tracheal tube. The tip of the tracheal tube is positioned just above the carina by auscultation of diminished breath sounds over the left axilla as the tube is advanced into the right main-stem bronchus, after which the tube is retracted until breath sounds are increased over the left chest (Fig. 13-12, A). A small FOB may be passed through the tracheal tube to confirm appropriate placement. Rarely, an emergency gastrostomy is performed because of massive gastric distention. Placement of a balloon-tipped catheter in the fistula via the gastrostomy may be performed under guidance with an FOB, to prevent further gastric distention and/or enable effective positive-pressure ventilation in cases of significant lung disease (see Fig. 13-12, B).77 “Antegrade” occlusion of a tracheoesophageal fistula has also been reported with a balloon-tipped catheter advanced through the trachea into the fistula (see Fig. 13-12, C).78 Preoperative evaluation should be performed to diagnose associated anomalies, particularly cardiac, musculoskeletal, and gastrointestinal defects, which occur in 30% to 50% of afflicted infants.79 A poor prognosis for infants with tracheoesophageal fistula and esophageal atresia has been associated with prematurity and underlying lung disease, as well as the coexistence of other congenital anomalies.80
Surgical repair usually involves a right thoracotomy and extrapleural dissection of the posterior mediastinum. In most cases, the fistula is ligated and primary esophageal anastomosis is performed (“short gap atresia”). In cases in which the esophageal “gap” is long, the proximal segment is preserved for subsequent staged anastomosis, with or without intestinal interposition.75 The trachea may be intubated with the infant breathing spontaneously, or during gentle positive-pressure ventilation with small tidal volumes to avoid gastric distention. If a gastrostomy tube is in place, occlusion of the fistula may be confirmed by cessation of bubbling via underwater tubing connected to the gastrostomy or the appearance of CO2 in the end-tidal gas.55 Alternatively, the tracheal tube may be positioned in the main-stem bronchus, opposite the side of the thoracotomy incision, until the fistula is ligated.
Esophageal atresia without connection to the trachea occurs much less commonly. These lesions are generally diagnosed by radiography after inability to pass an orogastric tube, at which time an absence of gas in the abdomen may be noted (see Fig. 36-4). So-called H-type tracheoesophageal fistula without esophageal atresia is relatively rare. Infants with H-type lesions may present later in childhood or adulthood with recurrent pneumonias or gastric distention during positive-pressure ventilation (see Chapter 36).81,82
Childhood
Anterior mediastinal masses include neoplasms of the lung, mediastinum, and pleura. These tumors may be primary or metastatic. Perhaps the most common primary tumors are lymphoblastic lymphoma, a form of non-Hodgkin lymphoma, and Hodgkin disease. Less commonly, teratomas (germ cell tumors), thymomas, as well as thyroid, parathyroid, and mesenchymal tumors may manifest as anterior mediastinal masses.83 Signs and symptoms that result from vascular and/or airway compression may include dyspnea, orthopnea, pain, coughing, pleural effusion, and/or superior vena cava syndrome (swelling of the upper arms, face, and neck).84,85
Preoperative evaluation should include CT, echocardiography, and flow-volume studies whenever feasible. Tracheal, bronchial, and/or vascular (superior vena cava or pulmonary outflow tract) compression, as detected by CT, is associated with a high incidence of serious complications during induction of anesthesia.85 However, CT scans are static pictures that may not identify dynamic compression of an airway or vascular outflow tract. These tumors may occur as extrathoracic or intrathoracic, variable obstruction or fixed obstruction. Echocardiography identifies compression of the superior vena cava or pulmonary outflow tract. Flow-volume loops may be effective in detecting dynamic compression of the airways, although their utility in adult patients has been questioned86 (see Chapter 11 and Fig. 11-6).
Establishing the correct diagnosis often requires a tissue biopsy. More often than not, there is an urgency to secure the tissue for diagnosis, because T-cell type non-Hodgkin lymphoblastic lymphomas, which constitute 30% to 40% of non-Hodgkin lymphoma, have a 12-hour doubling time.87 A rapid diagnosis and chemotherapy prescription may prevent widespread dissemination of the tumor. Indeed, today, the 5-year survival of Hodgkin and non-Hodgkin lymphoblastic lymphoma exceeds 80%. Every effort should be made to secure a tissue diagnosis by lymph node or bone marrow biopsy under local anesthesia or sedation, thereby precluding the need for general anesthesia and facilitating early treatment.88 If peripheral tissue diagnosis cannot be obtained and signs of severe airway and/or circulatory compromise are present, careful consideration should be given to administering a 12- to 24-hour burst of corticosteroids, initiating chemotherapy, and/or treating with limited radiation to decrease the size of the tumor and reduce the risk of life-threatening compression of the airway or major vessels under anesthesia. The risk is that any or all of these interventions may cause involution of the tumor and compromise the tissue diagnosis; thus some oncologists prefer that none of these interventions is used prebiopsy.89,90 Corticosteroids effect a reduction in tissue mass (i.e., tumor lysis) of lymphomas by inducing apoptosis in the tumor via a number of mechanisms.91 One study found that four features were predictive of perianesthesia complications in these children: orthopnea, upper body edema, great vessel compression, and main-stem bronchial compression (odds ratio of 5.1 to 8).92 A second study found that the extent of vascular and airway compression, according to radiologic investigations, was predictive of perianesthesia complications.88 It must be emphasized that adolescents and adults have different risk factors than small children under the age of 8 years. In adults, for example, intraoperative complications have been associated with pericardial effusions diagnosed by CT scans, whereas postoperative respiratory complications have been associated with greater than 50% tracheal compression on preoperative CT.92a Great care must be taken to properly prepare these children and the families for general anesthesia, together with the attendant risks.
Induction of anesthesia in children with anterior mediastinal masses may be associated with severe airway obstruction and circulatory collapse.88 This may occur even in children without signs or symptoms of respiratory or cardiovascular compromise.93,94 Therefore a preoperative assessment of what position provides the most reliable and consistent good gas exchange should be sought from the child or the parents (nocturnal sleep position). Recommended anesthetic techniques for children with anterior mediastinal masses include inhalation induction or a slow IV induction (with ketamine or propofol), with maintenance of spontaneous respiration. The latter offsets the effect of gravity, which pulls the tumor onto the pulmonary artery, superior vena cava, and tracheobronchial tree, causing life-threatening cardiopulmonary consequences.87,90,95 The use of continuous positive airway pressure while maintaining spontaneous respiration, maintains functional residual capacity that is otherwise reduced under anesthesia.96 In adolescents and adults, neuromuscular blockade is commonly used to facilitate tracheal intubation and prevent coughing associated with DLT placement. The morbidity associated with neuromuscular blockade appears to be minimal as long as appropriate precautions are taken. The actual risks in young children, however, have not been studied.92a Keeping the head of the bed elevated may decrease the deleterious effects of supine positioning, including cephalad displacement of the diaphragm and secondary reduction of thoracic volume.97 Placing the child in a partial or even full left lateral decubitus position may help to maintain airway patency and reduce cardiovascular and/or tracheal compression.87 Performing tracheal intubation while deeply anesthetized, without the use of muscle relaxants and positive-pressure ventilation, preserves the normal transpulmonary pressure gradient and improves flow through conducting airways.98–100 The loss of negative intrathoracic pressure associated with neuromuscular blockade increases the risk of severe airway compression and reduction in pulmonary blood flow (i.e., cardiac output).101 As an alternative to tracheal intubation, use of a laryngeal mask airway has been described.102 The use of a helium-oxygen (70%/30%) mixture has been recommended to decrease the resistance to breathing and to increase hemoglobin saturation when an anterior mediastinal tumor compresses the trachea and/or bronchi (where turbulent gas flow exists).102 In the event of tracheal or bronchial collapse under anesthesia, lateral or prone positioning and/or rigid bronchoscopy may be lifesaving.87 Performing a median sternotomy and cardiopulmonary bypass in this situation has been recommended but is quite impractical unless access for partial bypass has been established before induction of anesthesia.90
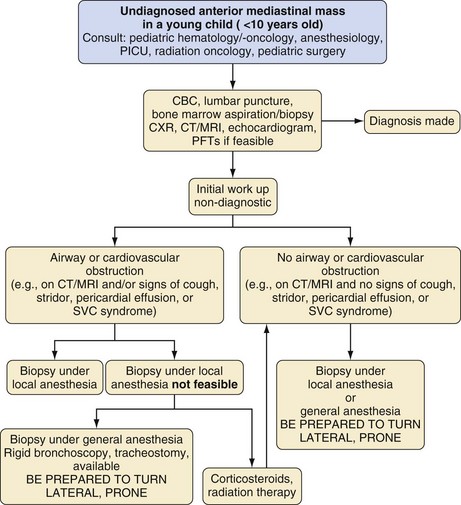
Institutions should have an algorithm in place for the evaluation of children with anterior mediastinal masses that includes a multidisciplinary approach (Fig. 13-13).
Capan LM, Turndorf H, Patel C, et al. Optimization of arterial oxygenation during one-lung anesthesia. Anesth Analg. 1980;59:847–851.
Rees DI, Wansbrough SR. One-lung anesthesia and arterial oxygen tension during continuous insufflation of oxygen to the nonventilated lung. Anesth Analg. 1982;61:507–512.
Hammer GB, Harrison TK, Vricella LA, et al. Single lung ventilation in children using a new paediatric bronchial blocker. Pediatr Anesth. 2002;12:69–72.
Heaf DP, Helms P, Gordon MB, Turner HM. Postural effects on gas exchange in infants. N Engl J Med. 1983;28:1505–1508.
Keon TP. Death on induction of anesthesia for cervical node biopsy. Anesthesiology. 1981;55:471–472.
1 Benumof JF, Augustine SD, Gibbons JA. Halothane and isoflurane only slightly impair arterial oxygenation during one-lung ventilation in patients undergoing thoracotomy. Anesthesiology. 1987;67:910–914.
2 Kato J, Ogawa S, Katz J. Effects of presurgical local infiltration of bupivacaine in the surgical field on postsurgical wound pain in laparoscopic gynecologic examinations: a possible preemptive analgesic effect. Clin J Pain. 2000;16:12–17.
3 Moiniche S, Jorgensen H, Wetterslev J, Dahl JB. Local anesthetic infiltration for postoperative pain relief after laparoscopy: a qualitative and quantitative systematic review of intraperitoneal, port-site infiltration and mesosalpinx block. Anesth Analg. 2000;90:899–912.
4 Salman MA, Yücebas ME, Coskun F, Aypar Ü. Day-case laparoscopy: a comparison of prophylactic opioid, NSAID or local anesthesia for postoperative analgesia. Acta Anaesthesiol Scand. 2000;44:536–542.
5 Remolina C, Khan AU, Santiago TV, Edelman NH. Positional hypoxemia in unilateral lung disease. N Engl J Med. 1981;304:523–525.
6 Heaf DP, Helms P, Gordon MB, Turner HM. Postural effects on gas exchange in infants. N Engl J Med. 1983;28:1505–1508.
7 Mansell A, Bryan C, Levison H. Airway closure in children. J Appl Physiol. 1972;33:711–714.
8 Dawes GS. Fetal and neonatal physiology. Chicago: Year Book Medical; 1973.
9 Mukhtar AM, Obaya GM, Elmasry A, Dessouky N. The therapeutic potential of intraoperative hypercapnea during video-assisted thoracoscopy in pediatric patients. Anesth Analg. 2008;106:84–88.
10 Mouroux J, Clary-Meinesz C, Padovani B. Efficacy and safety of videothoracoscopic lung biopsy in the diagnosis of interstitial lung disease. Eur J Cardiothorac Surg. 1997;11:22–26.
11 Mackinlay TA, Lyons GA, Chimondeguy DJ, et al. VATS débridement versus thoracotomy in the treatment of loculated postpneumonia empyema. Ann Thorac Surg. 1996;61:1626–1630.
12 Rowe R, Andropoulos D, Heard M, et al. Anesthetic management of pediatric patients undergoing thoracoscopy. J Cardiothorac Vasc Anesth. 1994;8:563–566.
13 Kubota H, Kubota Y, Toyoda Y, et al. Selective blind endobronchial intubation in children and adults. Anesthesiology. 1987;67:587–589.
14 Cohen DE, McCloskey JJ, Motas D, Archer J, Flake AW. Fluoroscopic-assisted endobronchial intubation for single lung ventilation in infants. Pediatr Anesth. 2011;21:681–684.
15 Lammers CR, Hammer GB, Brodsky JB, Cannon WB. Failure to isolate the lungs with an endotracheal tube positioned in the bronchus. Anesth Analg. 1997;85:946–947.
16 Cullum AR, English CW, Branthwaite MA. Endobronchial intubation in infancy. Anaesthesia. 1973;28:66–70.
17 McLellan I. Endobronchial intubation in children. Anaesthesia. 1974;29:757–758.
18 Yeh TF, Pildes RS, Salem MR. Treatment of persistent tension pneumothorax in a neonate by selective bronchial intubation. Anesthesiology. 1978;49:37–38.
19 Watson CB, Bowe EA, Burk W. One-lung anesthesia for pediatric thoracic surgery: a new use for the fiberoptic bronchoscope. Anesthesiology. 1982;56:314–315.
20 Hammer GB, Manos SJ, Smith BM, et al. Single lung ventilation in pediatric patients. Anesthesiology. 1996;84:1503–1506.
21 Ginsberg RJM. New technique for one-lung anesthesia using a bronchial blocker. J Thorac Cardiovasc Surg. 1981;82:542–546.
22 Lin YC, Hackel A. Paediatric selective bronchial blocker. Pediatr Anesth. 1994;4:391–392.
23 Turner MWH, Buchanon CCR, Brown SW. Paediatric one-lung ventilation in the prone position. Pediatr Anesth. 1997;7:427–429.
24 Borchardt RA, LaQuaglia MP, McDowall RH, Wilson RS. Bronchial injury during lung isolation in a pediatric patient. Anesth Analg. 1998;87:324–325.
25 Guyton DC, Besselievre TR, Devidas M, et al. A comparison of two different bronchial cuff designs and four different bronchial cuff inflation methods. J Cardiothorac Vasc Anesth. 1997;11:599–603.
26 Takahashi M, Horinouchi T, Kato M. Double-access-port endotracheal tube for selective lung ventilation in pediatric patients. Anesthesiology. 2000;93:308–309.
27 Arndt GA, Delessio ST, Kranner PW, et al. One-lung ventilation when intubation is difficult—presentation of a new endobronchial blocker. Acta Anaesthesiol Scand. 1999;43:356–358.
28 Hammer GB, Harrison TK, Vricella LA, et al. Single-lung ventilation in children using a new paediatric bronchial blocker. Pediatr Anesth. 2002;12:69–72.
29 Scammon RE. Dimensions of the respiratory tract at various ages in man. In: Abt IA, ed. Pediatrics. Philadelphia: WB Saunders; 1923:257.
30 Marciniak B, Fayoux P, Hebrard A, et al. Fluoroscopic guidance of Arndt endobronchial blocker placement for single-lung ventilation in small children. Acta Anaesth Scand. 2008;52:1003–1005.
31 Kamaya H, Krishna PR. New endotracheal tube (Univent tube) for selective blockade of one lung. Anesthesiology. 1985;63:342–343.
32 Gayes JM. Pro: one-lung ventilation is best accomplished with the Univent endotracheal tube. J Cardiothorac Vasc Anesth. 1993;7:103–107.
33 Hammer GB, Brodsky JB, Redpath J, Cannon WB. The Univent tube for single lung ventilation in children. Pediatr Anesth. 1998;8:55–57.
34 Slinger PD, Lesiuk L. Flow resistances of disposable double-lumen, single-lumen, and Univent tubes. J Cardiothorac Vasc Anesth. 1998;12:142–144.
35 Kelley JG, Gaba DM, Brodsky JB. Bronchial cuff pressures of two tubes used in thoracic surgery. J Cardiothorac Vasc Anesth. 1992;6:190–194.
36 Benumof JL, Gaughan SD, Ozaki GT. The relationship among bronchial blocker cuff inflation volume, proximal airway pressure, and seal of the bronchial blocker cuff. J Cardiothorac Vasc Anesth. 1992;6:404–408.
37 Marraro G. Selective bronchial intubation in paediatrics: the Marraro paediatric bilumen tube. Pediatr Anesth. 1994;4:255–258.
38 Cohen E. Con: right-sided double lumen tubes should not be used routinely in thoracic surgery. J Cardiothorac Vasc Anesth. 2002;16:249–252.
39 Brodsky JB, Mark JBD. A simple technique for accurate placement of double-lumen endobronchial tubes. Anesth Rev. 1983;10:26–30.
40 Smith GB, Hirsch NP, Ehrenwerth J. Placement of double-lumen endobronchial tubes: Correlation between clinical impressions and bronchoscopic findings. Br J Anaesth. 1986;58:1317–1320.
41 Brodsky JB, Macario A, Mark JBD. Tracheal diameter predicts double-lumen tube size: a method for selecting left double-lumen tubes. Anesth Analg. 1996;82:861–864.
42 Ho AC, Chung HS, Lu PP, et al. Facilitation of alternative one-lung and two-lung ventilation by use of an endotracheal tube exchanger for pediatric empyema during video-assisted thoracoscopy. Surg Endosc. 2004;18:1752–1756.
43 Fetterman D, Dubovoy A, Reay M. unforeseen esophageal misplacement of airway exchange catheter leading to gastric perforation. Anesthesiology. 2006;104:1111–1112.
44 Cohen E. Oxygenation and hemodynamic changes during one-lung ventilation. J Cardiothorac Anesth. 1988;2:134–140.
45 Flacke JW. Influence of tidal volume and pulmonary artery occlusion on arterial oxygenation during anesthesia. South Med J. 1976;69:619–626.
46 Capan LM, Turndorf H, Patel C, et al. Optimization of arterial oxygenation during one-lung anesthesia. Anesth Analg. 1980;59:847–851.
47 Rees DI, Wansbrough SR. One-lung anesthesia and arterial oxygen tension during continuous insufflation of oxygen to the nonventilated lung. Anesth Analg. 1982;61:507–512.
48 Gaafar AH, El-Noueam KI. Bronchoscopy versus multi-detector computed tomography in the diagnosis of congenital vascular ring. J Laryngol Otol. 2011;125:301–308.
49 Mok Q, Negus S, McLaren CA, et al. Computed tomography versus bronchography in the diagnosis and management of tracheobronchomalacia in ventilator dependent infants. Arch Dis Child Fetal Neonatal Ed. 2005 Jul;90(4):F290–F293.
50 Heyer CM, Nuesslein TG, Jung D, et al. Tracheobronchial anomalies and stenoses: detection with low-dose multidetector CT with virtual tracheobronchoscopy—comparison with flexible tracheobronchoscopy. Radiology. 2007;242(2):542–549.
51 Hammer GB,. Pediatric general surgery—anesthetic considerations. In: Jaffe RA, Samuels SI, eds. The anesthesiologist’s manual of surgical procedures. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 1999:872–875.
52 Vinograd I, Klim B, Efrati Y. Airway obstruction in neonates and children: surgical treatment. J Cardiovasc Surg. 1994;35:7–12.
53 Kravitz RM. Congenital malformations of the lung. Pediatr Clin North Am. 1994;41:453–472.
54 Ryckman FC, Rosenkrantz JG. Thoracic surgical problems in infancy and childhood. Surg Clin North Am. 1985;65:1423–1454.
55 Schwartz MZ, Ramachandran P. Congenital malformations of the lung and mediastinum—a quarter century of experience from a single institution. J Pediatr Surg. 1997;32:44–47.
56 Raynor AC, Capp MP, Sealy WC. Lobar emphysema of infancy: diagnosis, treatment, and etiologic aspects. Ann Thorac Surg. 1967;4:374–385.
57 Lincoln JC, Stark J, Subramanian S. Congenital lobar emphysema. Ann Surg. 1971;173:55–62.
58 Mychaliska GB, Bullard KM, Harrison MR. In utero management of congenital diaphragmatic hernia. Clin Perinatol. 1996;23:823–841.
59 Sumner E, Frank JD. Tolazoline in the treatment of congenital diaphragmatic hernias. Arch Dis Child. 1981;56:350–353.
60 Kaapa P, Koivisto M, Ylikorlaka O, Kouvalainen K. Prostacyclin in the treatment of neonatal pulmonary hypertension. J Pediatr. 1985;107:951–953.
61 Roberts JD, Polaner DM, Lang P, Zapol WM. Inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Lancet. 1992;340:818–819.
62 Ivy DD, Ziegler JW, Kinsella JP. Dipyridamole attenuates rebound pulmonary hypertension after inhaled nitric oxide withdrawal in postoperative congenital heart disease. J Thorac Cardiovasc Surg. 1998;115:875–882.
63 Mariani G, Barefield ES, Carlo WA. The role of nitric oxide in the treatment of neonatal pulmonary hypertension. Curr Opin Pediatr. 1996;8:118–125.
64 Miguet D, Claris O, Lapillonne A. Preoperative stabilization using high-frequency oscillatory ventilation in the management of congenital diaphragmatic hernia. Crit Care Med. 1994;22:S77–S82.
65 Frenckner B, Ehren H, Granholm T. Improved results in patients who have congenital diaphragmatic hernia using preoperative stabilization, extracorporeal membrane oxygenation, and delayed surgery. J Pediatr Surg. 1997;32:1185–1189.
66 Harrison MR, Bjordal RI, Langmark F. Congenital diaphragmatic hernia: the hidden mortality. J Pediatr Surg. 1978;13:227–230.
67 Adelman S, Benson CD. Bochdalek hernias in infants: factors determining mortality. J Pediatr Surg. 1976;11:569–573.
68 Geary MP, Chitty LS, Morrison JJ. Perinatal outcome and prognostic factors in prenatally diagnosed congenital diaphragmatic hernia. Ultrasound Obstet Gynecol. 1998;12:107–111.
69 Donnelly LF, Sakurai M, Klosterman LA. Correlation between findings on chest radiography and survival in neonates with congenital diaphragmatic hernia. AJR Am J Roentgenol. 1999;173:1589–1593.
70 Truog RD, Schena JA, Hershenson MB, et al. Repair of congenital diaphragmatic hernia during extracorporeal membrane oxygenation. Anesthesiology. 1990;72:750–753.
71 Clark RH, Hardin WD, Jr., Hirschl RB. Current surgical management of congenital diaphragmatic hernia: a report from the Congenital Diaphragmatic Hernia Study Group. J Pediatr Surg. 1998;33:1004–1009.
72 Leveque C, Hamza J, Berg AE, et al. Successful repair of a severe left congenital diaphragmatic hernia during continuous inhalation of nitric oxide. Anesthesiology. 1994;80:1171–1175.
73 Bouchut JC, Dubois R, Moussa M, et al. High frequency oscillatory ventilation during repair of neonatal congenital diaphragmatic hernia. Pediatr Anesth. 2000;10:377–379.
74 Cumming WA. Esophageal atresia and tracheoesophageal fistula. Radiol Clin North Am. 1975;13:277–295.
75 Holder TM, Ashcraft KW, Sharp RJ. Care of infants with esophageal atresia, tracheoesophageal fistula, and associated anomalies. J Thorac Cardiovasc Surg. 1987;94:828–835.
76 Barry JE, Auldist AW. The VATER association: one end of a spectrum of anomalies. Am J Dis Child. 1974;128:769–771.
77 Bloch EC, Filston HC. A thin fiberoptic bronchoscope as an aid to occlusion of the fistula in infants with tracheoesophageal fistula. Anesth Analg. 1988;67:791–793.
78 Filston HC, Chitwood WR, Jr., Schkolne B. The Fogarty balloon catheter as an aid to management of the infant with esophageal atresia and tracheoesophageal fistula complicated by severe RDS or pneumonia. J Pediatr Surg. 1982;17:149–151.
79 Humphreys GH, Hogg BM, Ferrer J. Congenital atresia of the esophagus. J Thorac Surg. 1956;32:332–348.
80 Franken EA, Jr., Smith JA, Smith WL. Tumors of the chest wall in infants and children. Pediatr Radiol. 1977;6:13–18.
81 Grant DM, Thompson GE. Diagnosis of congenital tracheoesophageal fistula in the adolescent and adult. Anesthesiology. 1978;49:139–140.
82 Broemling N, Campbell F. Anesthetic management of congenital tracheoesophageal fistula. Pediatr Anesth. 2011;21:1092–1099.
83 Furst SR, Burrows PE, Holzman RS. General anesthesia in a child with a dynamic, vascular anterior mediastinal mass. Anesthesiology. 1996;4:976–979.
84 Azarow KS, Pearl RH, Zurcher R. Primary mediastinal masses: a comparison of adult and pediatric populations. J Thorac Cardiovasc Surg. 1993;106:67–72.
85 Azizkhan RG, Dudgeon DL, Colombani PM. Life-threatening airway obstruction as a complication to the management of mediastinal masses in children. J Pediatr Surg. 1985;20:816–822.
86 Slinger P, Karsli G. Management of the patient with a large anterior mediastinal mass: recurring myths. Curr Opin Anaesthesiol. 2007;20:1–3.
87 Lerman J. Anterior mediastinal masses in children. Sem Anesth, Periop Med Pain. 2007;26:133–140.
88 Ng A, Bennett J, Bromley P, Davies P, Morland B. Anaesthetic outcome and predictive risk factors in children with mediastinal tumours. Pediatr Blood Cancer. 2007;48:160–164.
89 Borenstein SH, Gerstle T, Malkin D, Thorner P, Filler RM. The effects of prebiopsy corticosteroid treatment on the diagnosis of mediastinal lymphoma. J Pediatr Surg. 2000;35:973–976.
90 Hack HA, Wright NB, Wynn RF. The anaesthetic management of children with anterior mediastinal masses. Anaesthesia. 2008;63:837–846.
91 Greenstein S, Ghias K, Krett NL, Rosen ST. Mechanisms of glucocorticoid-mediated apoptosis in hematological malignancies. Clin Cancer Res. 2002;8:1681–1694.
92 Anghelsescu DL, Burgoyne LL, Liu T, et al. Clinical and diagnostic imaging findings predict anesthetic complications in children presenting with malignant mediastinal masses. Pediatr Anesth. 2007;17:1090–1098.
92a Bechard P, Letourneau L, Lacasse Y, Cote D, Bussieres JS. Perioperative cardiorespiratory complications in adults with mediastinal mass. Anesthesiology. 2004;100:826–834.
93 Yamashita M, Chin I, Horigome H. Sudden fatal cardiac arrest in a child with an unrecognized anterior mediastinal mass. Resuscitation. 1990;19:175–177.
94 Viswanathan S, Campbell CE, Cork RC. Asymptomatic undetected mediastinal mass: a death during ambulatory anesthesia. J Clin Anesth. 1995;7:151–155.
95 Ferrari LR, Bedford RF. Anterior mediastinal mass in a pregnant patient: anesthetic management and considerations. J Clin Anesth. 1989;1:460–463.
96 Covino BG, Fozzard HA, Rehder K, eds. Effects of anesthesia, Bethesda, Md., American Physiological Society, 1985:91–106.
97 Pulleritz J, Holzman RS. Anesthesia for patients with mediastinal masses. Can Anaesth Soc J. 1989;36:681–688.
98 Sibert KS, Biondi JW, Hirsch NP. Spontaneous respiration during thoracotomy in a patient with a mediastinal mass. Anesth Analg. 1987;66:904–907.
99 Piro AH, Weiss DR, Hellman S. Mediastinal Hodgkin’s disease: a possible danger for intubation anesthesia. Int J Radiat Oncol Biol Phys. 1976;1:415–419.
100 Cheung SLW, Lerman J. Mediastinal masses and anesthesia in children. Anesthesiol Clin North America. 1998;16:893–909.
101 Neuman GG, Weingarten AE, Abramowitz RM. The anesthetic management of the patient with a mediastinal mass. Anesthesiology. 1984;60:144–147.
102 Polaner DM. The use of heliox and the laryngeal mask airway in a child with an anterior mediastinal mass. Anesth Analg. 1996;82:208–210.

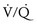 ) mismatch. During thoracic surgery, several factors act to further increase
) mismatch. During thoracic surgery, several factors act to further increase 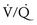 mismatch. General anesthesia, neuromuscular blockade, and mechanical ventilation may cause a decrease in functional residual capacity of both lungs. Compression of the dependent lung in the lateral decubitus position may cause atelectasis. Surgical retraction and/or SLV collapse the operative lung. Hypoxic pulmonary vasoconstriction, which acts to divert blood flow away from underventilated lung, thereby minimizing
mismatch. General anesthesia, neuromuscular blockade, and mechanical ventilation may cause a decrease in functional residual capacity of both lungs. Compression of the dependent lung in the lateral decubitus position may cause atelectasis. Surgical retraction and/or SLV collapse the operative lung. Hypoxic pulmonary vasoconstriction, which acts to divert blood flow away from underventilated lung, thereby minimizing 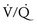 mismatch, may be diminished by inhalational anesthetic agents and other vasodilating drugs. These factors apply equally to infants, children, and adults. The overall effect of the lateral decubitus position on
mismatch, may be diminished by inhalational anesthetic agents and other vasodilating drugs. These factors apply equally to infants, children, and adults. The overall effect of the lateral decubitus position on 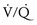 mismatch, however, is different in infants compared with older children and adults.
mismatch, however, is different in infants compared with older children and adults.