CHAPTER 34 Anesthesia for Same-Day Surgical Procedures
Historical reports of outpatient surgery date back to the early twentieth century, when Nicholl reported nearly 9000 operations on ambulatory children at Glasgow’s Royal Hospital for Sick Children (Nicholl, 1909). Other early reports from the United States soon followed, but it was not until the 1970s that studies looking at same-day surgery from a systems perspective were published. In addition to examining the patient population, complication rates and surgical procedures, these reports began to look at issues such as cost and delivery of care, as well as the optimization of the nursing and support staff, organization, and physical plant for outpatient surgery. Attention to these details continues to play a central role in the increased use and success of outpatient surgery. In the current economic climate of health care in the United States, there is and will continue to be a major emphasis on cost savings. In addition to the economic advantages of savings on hospital resources, a primary driving force in the popularity of outpatient surgery is satisfaction of the patient’s parents. There are obvious advantages for many parents and children to avoid overnight hospitalization and to have the child back in a familiar home environment on the same day. The decisions that are made in planning the outpatient system will have a major impact on how parents perceive ease of use and quality of care of the entire system, and, as a result, its success. Factors that are not medical at all (e.g., ease of parking, efficiency of check-in procedures, waiting time, parental presence during induction of anesthesia, and early admission to the postanesthetic recovery unit [PACU]) may make impressions on the parent that are equal to the obvious medical issues, such as complication rates, management of postoperative analgesia, postoperative nausea and vomiting (PONV), and rapid return to the preoperative mental state.
Procedures and patients amenable to outpatient surgery and anesthesia
Many operative procedures are well suited to be performed on pediatric outpatients and all share several common characteristics. They all are peripheral procedures that do not involve major violation of a body cavity. They all have limited duration, generally lasting less than 2 hours, and have minimal or moderate amounts of postoperative pain that can easily be managed after discharge from the PACU with oral analgesics, or by either a single-injection regional block placed at the time of surgery or continuous regional analgesia at home. They do not result in major physiologic perturbations or blood loss, nor do they disturb the ability to take oral fluids and nutrition in the immediate postoperative period. They do not require postoperative monitoring beyond the capability of the parents and home. Commonly performed outpatient procedures are listed and categorized in Table 34-1.
TABLE 34-1 Operations Commonly Performed as Outpatient Surgical Procedures by Specialty
| Specialty | Procedures |
| Otolaryngology | Myringotomy and ventilating tubes, adenoidectomy (see text), tonsillectomy (see text), frenulectomy, branchial cleft cysts, endoscopic sinus surgery, examination under anesthesia including some bronchoscopy |
| Ophthalmology | Examination under anesthesia, strabismus repair, nasolacrimal duct probe, intraocular lens implantation, trabeculectomy |
| General pediatric surgery and urology | Herniorrhaphy and hydrocelectomy, orchiopexy, uncomplicated hypospadias, cystoscopy and cystoscopic surgery, circumcision, esophogoscopy, lumps and bumps |
| Gastroenterology | Endoscopy |
| Plastic surgery | Cleft lip and some cleft palate repair, placement of tissue expanders, scar revision, minor reconstructive procedures (otoplasty, septorhinoplasty, etc.) |
| Orthopedics | Hardware removal, casting, percutaneous tenotomy, arthrograms Percutaneous pinnings and simple OFIF |
| Radiology | Imaging studies, radiation therapy |
| Dentistry | Extractions, restorations, examinations Nerve treatments, crowns, sealants, and fillings |
Contraindications for outpatient anesthesia
Expremature Infants and Apnea
The risk of postanesthetic apnea in former premature infants has been well described since the early 1980s, when Liu et al. (1983) published the first prospective study of premature infants anesthetized between 41 and 46 weeks’ postconceptional age (postconceptual age equals the gestational age at birth plus an infant’s current age in weeks). The authors compared a group of premature infants with a control group of term infants of similar ages. The incidence of apnea, defined as pauses in breathing lasting greater than 15 seconds, was 20%. Subsequent studies have approximated this incidence, although some have placed the at-risk period as far out as 60 weeks’ postconceptual age (Welborn et al., 1986; Kurth et al., 1987; Warner et al., 1992). It is now established that infants born before 36 weeks’ conceptional age are at some risk of apnea after general anesthesia. It appears that this risk is because of immaturity in the brainstem’s control of breathing after exposure to general anesthetics, and there may be similar risks after exposure to sedative-hypnotic agents and neuroleptic agents such as ketamine. Numerous studies have tried to define the period of susceptibility in the at-risk population. Several investigators have stratified the risk according to postconceptual age and gestational age at birth, and a meta-analysis of eight studies has reported that the postconceptual age required to reduce the risk to less than 1% with 95% confidence was 54 weeks in infants born at 35 weeks’ gestational age and 56 weeks in those born before 32 weeks’ gestation (Malviya et al., 1993; Coté et al., 1995). In this meta-analysis, anemia was also associated with increased apnea risk, particularly in infants older than 42 weeks’ postconceptual age. The patients in the numerous studies that were included in this meta- analysis may not have all been comparable in terms of underlying state of health, so these data (as in all meta-analyses) must be approached with some caution. Several investigators have suggested that the use of regional anesthesia may eliminate the risk, and a few even advocate discharge of these patients on the day of surgery if no other agents have been administered (Veverka et al., 1991; Webster et al., 1991; Sartorelli et al., 1992; Krane et al., 1995). However, uncontrolled case reports of apnea after spinal anesthesia have been published (Watcha et al., 1989; Tobias et al., 1998). Because these are case reports and there were no control pneumograms, it is unknown if the apnea was related to the anesthetic or not, but these reports have still prompted most clinicians and consultants to continue to recommend admission and monitoring of these patients for 24 hours after any anesthetic. Krane et al. (1995), in a prospective randomized controlled study, compared former premature infants receiving general or spinal anesthesia with preoperative and postoperative impedance respirometry, oxygen saturation, and electrocardiography (ECG). In this small sample of 18 patients, no central apnea differences were detected, but the group receiving spinal anesthesia had fewer desaturations and bradycardic events than those receiving general anesthesia. Caffeine, which has a long history of effective use in apnea of prematurity, has also been suggested to increase central respiratory drive after anesthesia in these patients, although it is still not commonly used (Welborn et al., 1988, 1989).
Obstructive Sleep Apnea
One of the most common indications for tonsillectomy is upper-airway obstruction during sleep. In many centers, obstructive sleep apnea (OSA) accounts for 50% or more of all children who come for tonsillectomy and adenoidectomy (Messner, 2003). These children may have abnormal ventilatory responses to both hypoxia and hypercarbia as a result of the chronic exposure to hypoxic and hypercarbic conditions during sleep (Strauss et al., 1999; Kerbl et al., 2001). These responses can take up to several weeks to revert to normal after resolution of the obstruction. There are concerns, therefore, about the ability to maintain adequate ventilation and oxygenation in the period immediately after the exposure to general anesthetics and to opioids given for postoperative analgesia. A study of 15 otherwise healthy children, ages 1 to 18, with mild OSA used preoperative and postoperative pneumograms to assess respiratory status on the night after adenotonsillectomy. Nine of these children received a halothane-based anesthetic, and six received a fentanyl-based technique. The number of obstructive events decreased, and the nadir of oxygen saturation improved from 78% to 92%. The authors concluded that in cases of mild OSA without other underlying disorders, intensive postoperative monitoring is not necessary (Helfaer et al., 1996). In another group of 134 children selected for outpatient tonsillectomy, 83% of whom carried the diagnosis and indication for surgery for OSA, 11 (8.2%) were admitted for inpatient observation after experiencing respiratory problems in the postanesthetic care unit (Lalakea et al., 1999). These patients as a group were significantly younger than those discharged home (an average age of 4 vs. 6.3 years). Preoperative evaluation and assessment of OSA was not described. Most otolaryngologists consider significant (as opposed to mild or moderate) OSA to be a relative contraindication to outpatient management of adenotonsillectomy, especially in children younger than 3 years of age, although in one small study the postoperative complications in these younger children were not related to obstructive events (Slovik et al., 2003). Those investigators suggested that the severity of OSA, rather than a patient’s age, may be a more predictive factor, but this is in conflict with other reports that recommend that an age younger than 3 years should be considered an independent discriminator (Shott et al., 1987; Biavati et al., 1997). One large retrospective analysis of 2315 patients younger than 6 years of age undergoing adenotonsillectomy for OSA found a higher rate of respiratory complications in those younger than 3 years (9.8% vs. 4.9%) (Statham et al., 2006). The only criterion that appears to be unequivocally accurate in the diagnosis and stratification of severity in OSA is polysomnography; neither the history nor pulse oximetry alone is specific or sensitive enough (American Thoracic Society 1996; Schechter, 2002; Subcommittee on Obstructive Sleep Apnea, American Academy of Pediatrics, 2002). In the absence of objective data, it is worthwhile to elicit a history of significant snoring or sleep-disordered breathing during the preoperative interview with the parent. Even though such historical data may not be accurate in all cases, it serves to alert the anesthesiologist to the potential for problems (Sinha et al., 2008).
Recent data on opioids in the patient with chronic hypoxemia are particularly relevant to the child with OSA, whether the child is to undergo tonsillectomy or another surgical procedure. In an animal model, rats exposed to chronic hypoxic conditions had greater respiratory sensitivity to opioids, exhibiting diminished respiratory drive (Moss et al., 2006). Confirming these animal data, children who experienced significant hypoxia during sleep had lower opioid requirements to achieve analgesia after tonsillectomy (Brown et al., 2006). In this prospective study of 22 children with OSA undergoing tonsillectomy, the opioid dose required to achieve equal analgesic scores on a behavioral pain scale was directly correlated with the nadir of their oxygen saturation on the preoperative polysomnogram. Those with the lowest saturations required the least opioid, demonstrating that chronic recurrent hypoxemia during sleep was associated with increased analgesic sensitivity to morphine. The key implication is that not only are ventilatory responses to hypoxia and hypercarbia with opioids blunted, but actual analgesic requirements are less, and lowering opioid and other sedative medication doses still results in adequate analgesia while potentially reducing the risk of untoward effects.
Preoperative evaluation and planning
The implications of cancellation, particularly on the day of surgery, go far beyond the efficiency of the operating room. A survey by Tait et al. (1997) found that nearly half of parents whose children’s operations were cancelled on the day of scheduled surgery missed a day of work, and about half of these went unpaid as a result. Many drove long distances to get to the hospital, and nearly 25% were frustrated or angry as a result of the cancellation. A small number of dissatisfied or angry parents can have an adverse impact on the success of an outpatient surgery program well out of proportion to their numbers, and great attention must be paid to minimizing these events by using systems that work effectively.
A preoperative visit to the surgeon alone, however, will not optimize the preoperative evaluation process for the anesthesiologist or for the same-day surgery process as a whole. A case-control study of pediatric outpatient cancellations found that 10% of all day surgery patients at a children’s hospital were cancelled on the day of surgery, and half of those were for preventable reasons. Cancelled patients who had inadequate preoperative preparation were more likely to have been seen only in the surgeon’s office and not in the hospital’s preoperative program (Macarthur et al., 1995). It is clear that further screening is optimal to address general medical and anesthetic concerns.
Background
A short telephone interview with the patient’s parent before scheduled surgery, whether conducted by the anesthesiologist, a physician’s assistant, or a nurse practitioner, can be not only a source of clinical information about the patient and about the parent’s concerns, but can also forestall unanticipated problems that can cause delays, cancellations, or complications on the day of surgery. Knowing in advance, for example, that a child with asthma had a mild upper respiratory infection (URI) the week before surgery, can allow the anesthesiologist to prescribe a short preoperative course of steroids with ample time for the drug to take effect. The child who has sickle cell disease with an active URI, on the other hand, might have surgery postponed, thus saving the parents a trip to the hospital and allowing the schedule to be rearranged before the day begins. A well-organized system for conducting these calls should be established, so patients are not missed and communications with the anesthesiologist scheduled to care for a particular patient can be easily accomplished. It is important to organize the system so that calls are most effective. A study of over 5000 patients conducted at National Children’s Hospital in 1992 found that calls made during the evening were far more likely to successfully reach the parents—not an unexpected finding (Patel and Hannallah, 1992). In Great Britain and in some hospitals in the United States, preoperative clinics rather than telephone screening are used, but this may necessitate an additional visit by the family. The inconvenience may outweigh the advantages of a face-to-face visit for some families, and success may be predicated on ease of use of the system. Preoperative screening and evaluation can also improve throughput on the day of surgery, particularly if there is a long list of short cases, such as myringotomy and ventilation-tube placement. The duration of those cases is so short that the time it takes to do a preoperative evaluation may be longer than the operative time. Saving even 10 or 15 minutes per hour might allow the team to perform an additional operative procedure each hour.
In some locations, it is common for a child’s pediatrician to be responsible for “clearing” the child for surgery. This can be a considerable help, because the pediatrician often has the best knowledge of the child’s underlying illnesses and conditions. The pediatrician, however, often has little understanding of the issues that are of greatest concern to the anesthesiologist and may actually miss or ignore problems that can impact anesthetic management, to the detriment of the preoperative evaluation process. The anesthetic implications and preoperative optimization of airway anatomy and function, gastroesophageal reflux, upper respiratory illness, and asthma, as well as of syndromes and chronic conditions such as Trisomy 21 and the former premature infant, all may be underappreciated by pediatricians who do not work in the operating room or administer anesthetics and have usually had little or no training in perioperative medicine (Fisher, 1991). Several recent reviews (written by pediatric anesthesiologists) in the pediatric literature have discussed the preoperative screening process and what the pediatrician needs to know about preparing the child for anesthesia (Fisher, 1991; Fisher et al., 1994; Maxwell et al., 1994; Section on Anesthesiology, 1996). If a hospital or surgery center relies on pediatricians as a major link in the preoperative assessment chain, pediatricians must be familiar with this literature and should have ongoing communication with their anesthesiology colleagues. Collaborative efforts, such as continuing medical education conferences and residency training about these topics, can reap significant rewards.
Urinalysis
A 1990 study of nearly 500 children scheduled for elective surgery found abnormalities in 15% of children; however, more than 80% of those were historically known, clinically insignificant, or false positives. The authors concluded that preoperative urinalysis should not be routinely performed on healthy children for preoperative assessment (O’Conner and Drasner, 1990).
Hematocrit and Complete Blood Count
Unless a surgery is expected to result in significant blood loss (highly unlikely for outpatient surgery), the complete blood count (CBC) screening has little or no value (Roy et al., 1990, 1991; Hackmann et al., 1991). Several studies have demonstrated that the presence of mild to moderate anemia has little to no effect on the conduction of anesthesia or outcome in children undergoing same-day surgical procedures. The presence of anemia in former premature infants of fewer than 54 weeks’ postconceptual age has been found to correlate with an increased risk of postanesthetic apnea, and screening for anemia in this population may detect those at increased risk (Welborn et al., 1991). It is not known, however, if the anemia is the cause of increased apnea or only an associated finding. There are no data to determine whether correction of anemia in these patients reduces the risk of apnea. All former premature infants who are at risk should be admitted for postoperative monitoring in any case, thus screening may not alter clinical practice. Children with sickle cell disease (not trait) need to have their hemoglobin level measured, because both management and outcomes of these patients are dependent on an adequate level of hemoglobin A or F (see the following section).
Sickle Cell Testing
In many states, newborns in at-risk populations are screened for sickle cell disease, thus the sickle cell status of all infants and children in those locations is known. In locations where such newborn screening is not universal, it is prudent to obtain sickle cell testing in any infant or child under the age of 3 years of at-risk ethnicity whose status is unknown. Children older than this are likely to have had symptoms if they are affected. In children who do have sickle cell disease, a preoperative hemoglobin level is mandated to determine the need for preoperative transfusion. A large multicenter trial found that simple transfusion, if the hemoglobin was less than 10g/dL, is as effective as exchange transfusion in these patients (Vichinsky et al., 1995) (see Chapter 36, Systemic Disorders). One study has reported that minor surgical procedures like those usually performed on outpatients could be safely performed in patients with sickle cell disease without preoperative transfusion; complication rates were significantly higher in children undergoing abdominal, thoracic, or airway procedures (Griffin and Buchanan, 1993). However, sickle cell trait, which usually does not cause any symptoms or illness, has rarely been associated with the complications of surgery and anesthesia, and hemoglobin determination in these patients is unnecessary (Konotey-Ahulu, 1969; McGarry and Duncan, 1973; Atlas, 1974; Gibson and Love, 1974).
Heart Murmurs and Cardiology Consultation
At least 25% of healthy children have an audible heart murmur at some time during childhood, and the question of when to refer a child to a pediatric cardiologist for the evaluation of a new murmur is often raised during the preoperative evaluation. The vast majority of these are functional (“innocent”) murmurs, not associated with any structural heart disease. Innocent murmurs are soft (less than grade 3), blowing, and loudest along the left sternal border. They tend to decrease or disappear during inspiration. Most congenital heart lesions appear before the first several months of life, so an asymptomatic murmur in an older child is less likely—but not entirely impossible—to be significant. Exceptions include atrial septal defects, small ventricular septal defects, coarctation of the aorta, some valvular lesions that have no hemodynamic symptoms during normal activity, and in rare instances, other lesions. The ability of a pediatric cardiologist to distinguish between a functional murmur and one caused by a structural heart lesion by examination alone was evaluated and found to be high, so more extensive (and expensive) evaluation, such as echocardiography, is rarely necessary (Newburger et al., 1983). If the child is older than 6 months of age, without any symptoms referable to the cardiac system, most skilled clinicians should be able to evaluate these murmurs and rule out hemodynamically significant congenital heart disease. Cardiomyopathy can also bring on a new murmur, so a previously undetected murmur accompanied by symptoms suggestive of impaired myocardial performance or irritability, such as dysrhythmias, especially if it follows a viral illness, should be evaluated by a cardiologist.
Children with some congenital heart lesions require antibiotic prophylaxis for the prevention of endocarditis when undergoing operations during which they will be at risk. In 2007 there was a major revision in the recommendations for antibiotic prophylaxis of congenital heart disease that significantly reduced the number of conditions that require treatment (Box 34-1). The current American Heart Association recommendations are available online at http://circ.ahajournals.org/cgi/reprint/CIRCULATIONAHA.106.18309.. It should be noted that for optimal treatment, the intravenous (IV) antibiotic should be administered 30 minutes before the procedure’s start, which can pose a considerable problem in day surgery when an IV port is not present before induction. Antibiotic prophylaxis is no longer recommended for routine procedures of the gastrointestinal (GI) or genitourinary (GU) tract, but it is still advisable for dental and oral procedures during which there is manipulation of the gingivae and periapical region of the teeth or perforation of the oral mucosa, procedures of the respiratory tract, and procedures on infected skin or musculoskeletal tissues. For these procedures, oral antibiotics can be given 1 hour before the procedure, thereby eliminating the problem of timing. When IV antibiotics are desired, our current practice is to begin their administration as soon as IV access is obtained.
Adapted from Wilson et al.: Prevention of infective endocarditis: guidelines from the American Heart Association, Circulation 115:1736–1754, 2007.
Underlying illnesses and complicating factors
Upper Respiratory Tract Infections
Probably the most common problem to confront the anesthesiologist caring for children in outpatient surgery is the child with a URI. Viral respiratory tract illness is virtually ubiquitous in children, particularly during the winter months when close indoor contact in schools and daycare facilities with other children with colds is impossible to avoid. The average preschool child contracts between 6 and 8 URIs per year. Both upper and lower respiratory tract viral infections can increase airway inflammation, irritability, and respiratory tract secretions by mechanisms as diverse as increased production and decreased degradation of tachykinins and other neuropeptides, viral induced damage to M2 muscarinic receptors in the airways leading to vagal-mediated hyperreactivity, and increased volume and viscidity of airway secretions causing subsegmental atelectasis (Empey et al., 1976; Dusser et al., 1989; Williams et al., 1992). Increased airway reactivity and hyperresponsiveness occur in the lower airways even in patients with respiratory viral illness clinically limited to the upper airway and even in those with no history of asthma (de Kluijver et al., 2002). After the apparent resolution of the URI, increased airway hyperresponsiveness and irritability may persist for as long as 8 weeks (Empey et al., 1976; Empey, 1983). In children with underlying respiratory disease, such as asthma, bronchopulmonary dysplasia, or other chronic lung diseases, these responses may be further exaggerated. Other risk factors that may be associated with more serious or common complications are age younger than 1 year and sickle cell disease (Cohen and Cameron, 1991). In what is perhaps one of the most comprehensive investigations of URI and anesthesia, 1078 infants and children were prospectively studied (Tait et al., 2001). Independent risk factors for respiratory complications were endotracheal intubation, history of prematurity, reactive airways disease, parental smoking, airway surgery and nasal congestion, and the presence of copious secretions. Of interest is that a history of prematurity was a risk factor even in children who were several years old and no longer had ongoing problems referable to their premature birth.
Numerous studies have documented that children who either have URIs or who have recently recovered from one have more minor airway complications during or after anesthesia compared with healthy children. Mild oxygen desaturation and coughing, as well as more potentially serious complications such as bronchospasm, laryngospasm, and respiratory failure are particularly likely to occur if the airway is stimulated. Tait and Knight (1987) prospectively studied a large cohort of children undergoing myringotomy and ventilating-tube placement for chronic or recurrent otitis media under general mask anesthesia with halothane. The group that had URIs had no difference in the incidence of respiratory problems, no increase in the severity of respiratory illness, and no increase in the duration of URI symptoms. When compared with matched unanesthetized controls, URI symptoms actually decreased in the group receiving halothane anesthesia. These beneficial results may have been influenced by the effects of myringotomy on the course of the infection, but there is also some laboratory evidence that halothane has viricidal properties in tissue-culture preparations. It is not known if the newer volatile anesthetics have similar effects.
Coté et al., in their investigation of the utility of capnometry and pulse oximetry in detecting adverse events during anesthesia and in a subsequent paper further analyzing these data, found that children with URIs commonly had mild oxygen desaturation both during surgery and in recovery (Coté et al., 1991; Rolf and Coté, 1992). Others have noted that postoperative oxygen requirements in these children are commonly transiently increased (Levy et al., 1992). It is possible that the cause is related to subsegmental atelectasis from increased quantity and viscidity of secretions, and that with deep breathing and coughing after emergence, reexpansion of these segments occurs. In the prospective study previously cited, patients with current or recent URIs had a greater incidence of respiratory complications, including breath-holding and desaturation less than 90%, although none of the complications was associated with long-term sequelae. Both the authors and an accompanying editorial concluded that most children with URIs who were not overtly ill and had no other complicating medical issues could, with judicious attention to anesthetic technique, be safely anesthetized with increased risk for only mild transient sequelae (Coté, 2001; Tait et al., 2001).
Although most children with clinically mild URIs can undergo anesthesia safely, the potential for more serious complications in children with URIs should not be overlooked. A prospective study of over 15,000 children found that children who developed laryngospasm were twice as likely to have a URI (Schreiner et al., 1996). The investigators found that the incidence of laryngospasm was most clearly related to the parent’s subjective assessment of a URI, and that younger age and surgeries involving the airway were additive risk factors. A prospective case-controlled study of 1283 children with URI who underwent general anesthesia found a two- to sevenfold increase in respiratory complications during the perioperative course when compared with their counterparts without URIs (Cohen and Cameron, 1991). The incidence was 11-fold higher if the patient was intubated. A very small minority of children with URIs who do not appear to be ill during the preoperative examination may develop acute respiratory failure after the induction of anesthesia or some time during the anesthetic course. Severe hypoxia, bronchospasm, ventilatory insufficiency, and reduced compliance may occur and may even require postoperative ventilation and critical-care management. Some of these children have an unrecognized underlying lower tract disease, such as pneumonia, and others may experience shunt and ventilation-perfusion mismatching from atelectasis and pulmonary collapse as a result of inspissation of secretions and mucus plugging (Campbell, 1990; Williams et al., 1992). In rare instances, cardiomyopathy may follow viral illness. There are several reports of cardiac dysrhythmias or collapse occurring after induction of general anesthesia that were attributed to postviral myocarditis. The onset of abnormal rhythms on the ECG tracing or sudden deterioration of blood pressure or perfusion should alert the anesthesiologist to this possibility (Brampton and Jago, 1990; Terasaki et al., 1990).
Asthma
The prevalence and severity of asthma, especially in children, remains a significant health problem in the United States. As of 1998, 6.4% of the U.S. population carried the diagnosis; two thirds of those were children. A more recent assessment of prevalence rates from 1980 to 2007 found that the prevalence plateaued in 1997, but that 9.1% (or 6.7 million) of American children carried the diagnosis (Akinbami et al., 2009). Nearly one-half million patients are hospitalized yearly with exacerbations of asthma, and almost half of those are children. The prevalence has increased by about 60% in the past 20 years before leveling off in the past 10 years. The death rate, although small, more than doubled from 1975 to 1995, but it appears to have stabilized in the past decade. Children with asthma can be safely and effectively anesthetized for same-day surgery, but careful preoperative preparation and evaluation, as well as intraoperative management, are crucial to avoid exacerbations and complications. Although it was previously common to think of asthma in terms of bronchospasm, current definitions of the disease emphasize the role or airway inflammation in pathogenesis, progression, and management. Recent consensus conferences of the National Institutes of Health (NIH) National Heart, Lung, and Blood Institute have defined asthma as a chronic inflammatory disorder of the airways that comprises many cells beyond those structural elements of the airways themselves, including mast cells, eosinophils, and T lymphocytes. The inflammatory processes that are involved in both the pathogenesis of the disorder, as well as in the progression of disease, are now addressed much more effectively in therapy for all patients with asthma and not only those with severe disease. The mainstay of therapy in the past was the chronic use of bronchodilator therapy, with antiinflammatory drugs reserved for the more severe cases; current thinking is that the first line of treatment should target inflammation with drugs such as inhaled steroids and newer drugs targeting inflammatory mediators (see Chapter 36, Systemic Disorders).
Anesthetizing the child with asthma for outpatient surgery involves the same general principles as for inpatient procedures (Pradal et al., 1995). It is critical for the asthmatic patient to closely adhere to their medication regimen before surgery. Inhaled steroids and agents like leukotriene inhibitors and cromolyn all require regular use for efficacy. The patient must use these medicines regularly and faithfully in the days (and weeks) before undergoing anesthesia. For those who have required systemic steroids in the past, a short course of steroids, beginning 24 hours before induction of anesthesia, may be advisable, particularly if intubation of the trachea will be required. Preoperative treatment with a short acting β-agonist such as albuterol may be helpful as well, even if the patient is not symptomatic, because events may occur during surgery that are likely to provoke airway irritability, especially intubation (Maslow et al., 2000). Much like the child with a URI, avoidance of intubation and airway stimulation, if possible, reduces the potential for exacerbation of airway irritability.
Volatile anesthetics, which have bronchodilatory properties, have obvious advantages in the child with asthma. Propofol, which has been shown to relax tracheal smooth muscle in vitro and to decrease airway resistance in subjects with and without asthma during induction of anesthesia, is an excellent choice when an IV induction is used (Pizov et al., 1995; Eames et al., 1996). This effect on airway smooth muscle has been shown to be even greater than that of ketamine and was also demonstrated during maintenance when an infusion was continued during the anesthetic (Pedersen et al., 1993). A propofol-based anesthetic, combined with either regional anesthesia or a non–histamine-releasing opioid, is a good alternative to volatile anesthesia when a total IV technique is indicated or desired. Some caution must be taken with the sulfite-containing preparation of propofol, as one study in adults has demonstrated a significant increase in airway resistance with this formulation compared with the non–sulfite-containing drug, although adverse clinical events have not been often reported despite widespread use (Rieschke et al., 2003).
Diabetes
Type I diabetes in children is relatively common, occurring in approximately 1 in 500 school-aged children, but data on the optimal intraoperative management of children with this disease are scant. Although there are no prospective investigations published in the English language literature on the subject, there are two recent reviews and consensus guidelines on the perioperative management of insulin-dependent diabetes in children that offer excellent and well-reasoned guidance on their care (Rhodes et al., 2005; Betts et al., 2007). Although many of the problems in anesthetizing adults relate to the late complications of this condition (e.g., damage to many end-organ systems and autonomic dysfunction), these problems are less prevalent in children, and the most common issue is that of glucose control. Children with diabetes can be safely anesthetized as outpatients if great care is taken to maintain good glucose homeostasis. The child should observe the usual fasting guidelines for elective surgery and should be scheduled for surgery early in the day. If the child is receiving a split-dose insulin regimen with short- and intermediate-acting insulins, the child may receive half of the usual intermediate- or long-acting insulin dose on the morning of surgery and omit any short-acting insulin (McAnulty et al., 2000; McAnulty and Hall, 2003). For children receiving a basal insulin regimen, there should be no short-acting insulin administered at all on the morning of surgery, but they should be given the usual dose of insulin glargine, an insulin for basal control with no true peak and a very long duration of action of about 24 hours (Chase et al., 2008; Hirsch, 2005). In all cases, the blood glucose level should be checked upon awakening and again 2 to 3 hours later. Intravenous infusions containing 5% glucose have often been recommended for intraoperative fluid management, but it has generally been found easier to use a non–glucose-containing IV fluid into which a glucose-containing IV is “piggybacked.” In this manner, the patient’s fluid requirements and glucose requirements can be independently regulated. Because the most potentially catastrophic complication of diabetes during surgery is unrecognized hypoglycemia, and symptoms of hypoglycemia may be undetectable during anesthesia, blood glucose levels should be checked every 30 to 60 minutes during the procedure. Hypoglycemia should be treated promptly by reducing or stopping any insulin administration and increasing the IV glucose rate, and hyperglycemia (blood glucose levels over 250 mg/dL) should be treated with a continuous insulin infusion titrated to effect, usually beginning at a rate of 0.05 units/kg per hour. The very short duration of action of IV regular insulin (about 5 minutes) makes glucose control much easier with this method (Barnett et al., 1980). The same management scheme is continued in the PACU until the patient is awake and taking oral fluids without difficulty. At that time, a dose of subcutaneous regular insulin can be administered and an oral diet begun. The patient should check blood glucose levels often during the postoperative day, because the stress of surgery often alters insulin requirements. The dose should be adjusted accordingly. The usual insulin regimen can often be restarted on the day after surgery.
Malignant Hyperthermia
The advent of improved and short-acting IV anesthetics has made the management of patients with malignant hyperthermia (MH) considerably simpler. Current recommendations for the care of patients with MH no longer includes prophylactic therapy with dantrolene, and the use of nontriggering techniques coupled with proper preparation of the anesthesia machine can assure that these patients are not exposed to triggering agents. A 10-year review of 303 patients with the diagnosis of MH who underwent trigger-free anesthesia found that none developed fever in the perioperative period that was attributable to an MH crisis, and none required treatment with dantrolene (Yentis et al., 1992). The authors concluded that patients with MH are suitable candidates for outpatient anesthesia (see Chapter 37, Malignant Hyperthermia).
Sickle Cell Anemia
Children with sickle cell disease have increased risks in the perioperative period; however, they can usually undergo anesthesia and surgery as outpatients. Preoperative testing and management of transfusion was discussed above. The major risk factors for inducing a crisis in the perioperative period are dehydration, hypoxia, diminished perfusion, and acidosis. If close attention is paid to avoiding these risk factors, most patients with sickle cell disease can be managed as outpatients for suitable operations. In particular, good hydration and analgesia are important for stable recovery. Caregivers must be more strict than usual in ensuring that the child can take oral fluids without difficulty before discharge to home. The use of surgical tourniquets for orthopedic surgery in patients with sickle cell disease is controversial, but they should probably be avoided in outpatient surgery where postoperative acid-base status, perfusion, and the development of late-onset complications cannot be closely monitored (Adu-Gyamfi et al., 1993). Tonsillectomy and adenoidectomy in patients with sickle cell disease and OSA appear to entail increased risks and probably should not be performed on an outpatient basis (Sidman and Fry, 1988; Derkay et al., 1991; Halvorson et al., 1997) (see Chapter 36, Systemic Disorders).
Preoperative preparation of the child and family
Family-Centered Care
There has been increasing emphasis in pediatric medicine on the care of the child within the context of the family. This is in part behind the current vogue for including the parents of the patient in the experience of induction of anesthesia and early admission to the PACU. When one considers outpatient surgery, however, this concept is extended even further, because the family is more intimately involved in the postoperative care of the child than ever before. The parent or primary caregiver becomes the surrogate nurse once the child is discharged home and therefore must be involved to a greater degree in the postoperative experience even before discharge from the day-surgery unit. It has become the norm in most pediatric institutions and general hospitals that have sizable pediatric surgical programs for parental involvement to include preoperative tours of the operating room and PACU, parental presence during induction of anesthesia, and admission of the parents to the PACU very soon after the child’s arrival and emergence from anesthesia. In most cases, experience with these programs have found them to ease, not complicate, the care of the child, and disruptive parents are the rare exception (Schofield and White, 1989).
Preoperative Teaching and Parental Presence
Outpatient surgery is an intense experience for both parents and children. Many things happen within a short time span, and the emphasis on efficiency and throughput can limit the time that staff can spend in preparing each parent and child for all that will happen. Preoperative teaching programs have become common methods of education to help families understand what to expect on the day of surgery. These programs include preoperative tours of the outpatient surgery center, preoperative telephone calls, written brochures, and videotapes (Karl et al., 1990; Kleinfeldt, 1990; O’Byrne et al., 1997; Cassady et al., 1999; Bellew et al., 2002; Koinig, 2002). Whereas the explicit goals of these programs are education and the efficient transmission of information, an implicit goal is reduction of anxiety and undesirable behavioral consequences of the stress of the perioperative experience (Margolis et al., 1998). The first objective can be met by many, if not all of these programs, but the more far-reaching ones may be more difficult to attain. A study of 143 2- to 6-year-old children who were randomized to receive either an interactive teaching book or no intervention found more, not less, preoperative anxiety in the children who had received the book, but less aggression during induction and fewer behavioral changes 2 weeks after surgery (Margolis et al., 1998). A well-controlled and designed study found that preoperative teaching programs of various modalities had an effect of anxiolysis only in the holding area on the day of surgery; that effect did not extend effectively to the induction period itself (Kain et al., 1998b). Although parental satisfaction was clearly increased by parental presence during induction, and highly anxious children benefited from presence of a parent during induction, children’s anxiety and behavior were more effectively modulated by the use of premedication (Kain et al., 1996; Kain et al. 1998a). Although these data might suggest that the expense and effort of elaborate teaching programs, when examined in a critical and rigorous manner, may not be as cost-effective as more modest programs combined with premedication, one must recognize that limited benefits have value as well. For the parent and child who are waiting for an hour in the preoperative area, a reduction in stress for that period alone is meaningful. Furthermore, the norm in many communities is that such programs are welcomed and expected by many parents; they can also serve as opportunities to educate and improve compliance with preoperative procedures and thereby reduce the incidence of case cancellation.
Part of the art of pediatric anesthesia, of course, is the ability to rapidly establish effective and reassuring communication with the parent and child. The rapport and trust that the anesthesiologist creates during the preoperative interview is also an important and effective method of reassurance and anxiolysis that can enhance the transition to the operating room. In the outpatient setting, where time is more constrained, the value of a quick game, magic trick, kind word, or even brief induction of hypnotic suggestion should not be underestimated. Bringing a security item, such as a blanket or favorite toy into the operating room, can provide additional comfort to the child. Having this item immediately available at the time of emergence may also be helpful (see Chapter 8, Psychological Aspects of Pediatric Anesthesia).
Premedication
As was noted above, the use of sedative premedication has been shown to be the most effective means of reducing preoperative anxiety, postoperative recall, and maladaptive behavior in children undergoing outpatient surgery. Oral midazolam has become the most commonly used premedicant in the United States since 2000 (Kain et al., 2004). Significant reduction in postoperative recall and establishment of anterograde amnesia has been demonstrated with 0.5 mg/kg of midazolam administered orally as little as 10 minutes before induction (Kain et al., 2000). Oral doses as low as 0.25 mg/kg have been demonstrated to be as effective as larger doses with only a slightly slower time of onset (Coté et al., 2002). Of particular concern in the outpatient setting, however, is the problem of delayed emergence. Several studies using 0.5 mg/kg of oral midazolam have found that the drug delayed recovery, but the actual time of discharge from the hospital was not prolonged (Bevan et al., 1997; Viitanen et al., 1999a, 1999b). In institutions where the PACU is divided into phase I (initial recovery from the operating room until the child has reached an awake state with stable vital signs and is ready to take oral fluids) and phase II (less intensive observation and readying for discharge to home) areas, this translates to a longer stay in phase I recovery only. Such delays have the potential to affect throughput and cause bottlenecks for patients arriving from the operating room, but they do not effect total hospital time. The use of lower doses may reduce this problem, but data are not yet available. Young children who return to the operating room for repeated procedures (e.g., those with recurrent laryngeal papillomas) may especially benefit from premedication, even with relatively low doses. In this population the benefits largely outweigh any disadvantage of delayed emergence.
The benefits of oral administration, fairly rapid onset, and reliability of effect give midazolam considerable advantage over other agents. It does have several disadvantages, however, that must be considered. Midazolam has an extremely bitter, unpleasant taste. Although both the commercially available oral product and products compounded by the hospital mask the flavor to some degree, acceptance by some children remains poor. Alternative nonparenteral routes of administration have been studied, including nasal (0.2 to 0.3 mg/kg), transmucosal (0.2 mg/kg), and rectal (0.3 mg/kg), but each of these also has disadvantages, so that the oral administration remains the most commonly used and best-tolerated method for the majority of children (Saint-Maurice et al., 1986; Karl et al., 1993; Pandit et al., 2001).
Other agents and routes of administration, although less commonly used, have a place in the armamentarium. Other medications have been used in conjunction with midazolam, notably ketamine (Funk et al., 2000). Its advantage over a single-drug regimen appears to be in the child who is exceptionally uncooperative and requires a deeper level of sedation resulting from a dissociative state. Oral and transmucosal fentanyl has been used with success as well. Both the oral administration of the IV preparation and the commercially available oral transmucosal fentanyl “lollipop” have been shown to be effective; however, postoperative nausea may be increased compared with other agents, limiting its usefulness in the outpatient setting (Howell et al., 2002; Tamura et al., 2003). Similar results have been seen with nasally administered sufentanil, which also may cause nasal burning and chest wall rigidity. This agent appears to be less useful than others for these reasons and has largely fallen out of favor with most pediatric anesthesiologists. Intramuscular administration of premedication is uncommonly used in children for obvious reasons—children have an intense dislike and fear of needles. In some cases, however, when a child is exceedingly uncooperative and unmanageable, there may be no better alternative, and it is safer and more humane to administer a quick intramuscular injection with a small needle than to force the anesthesia mask on the face of an awake struggling child for what will surely appear to be a very long 60 seconds. Ketamine, often in combination with midazolam and glycopyrrolate, is the most commonly used agent. The usual doses range from 3 to 5 mg/kg; the lower doses are administered in combination with 0.1 mg/kg of midazolam. A high concentration (100 mg/mL) of ketamine should be used to minimize the injected volume. The anterior thigh is the most common site of administration (see Chapter 9, Preoperative Preparation).
Anesthetic techniques
Induction
The vast majority of children in the United States have an inhalation induction of anesthesia for outpatient procedures. The advantages are obvious—relatively rapid induction without any painful stimulus. Most children are needle-phobic, and are quite relieved when informed that they need not have anything painful done to them while they are conscious. The technique of inhalation induction is described in detail in Chapter 13, Induction, Maintenance, and Recovery. If the child uses a pacifier, it may be kept in the mouth until consciousness is lost, and the face mask may be placed over it. Scented lip gloss, such as bubble gum or fruit flavors, may be added to the facemask to disguise the odor of the volatile agent. The room should be otherwise quiet and free from conversation or other disruptions; the anesthesiologist should maintain continuous verbal contact with the child, telling a story in a soft, soothing modulated voice until the child falls asleep. Distracting modalities, such as movies or cartoons projected on the operating room’s video monitors or on hand-held video devices can be used to good effect as well.
Sevoflurane has replaced halothane, which is no longer available in the United States, as the agent of choice for inhalation induction. Induction is rapid, and because sevoflurane is nonpungent, it causes very little airway irritation and coughing. It is possible to quickly increase the inspired concentration or even begin with the vaporizer set at 8% and still avoid coughing. Sevoflurane does have a mildly unpleasant odor, but it is still relatively easy to breathe. Sevoflurane, however, causes some interference with ventilatory drive and respiratory muscle function, reducing the effectiveness of spontaneous ventilation (Brown et al., 1998). In many cases, even if a total IV anesthetic is planned, an inhalation induction with sevoflurane is performed until IV access can be established.
Airway Management
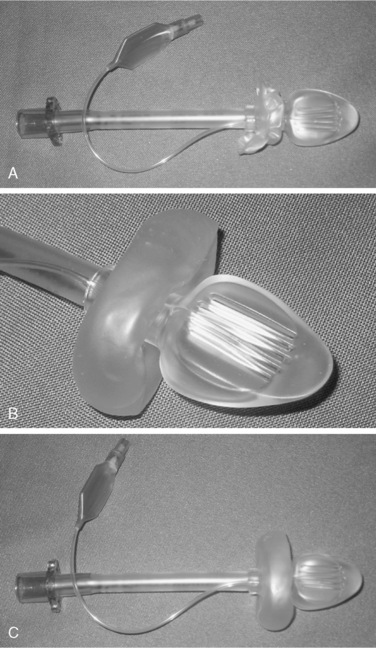
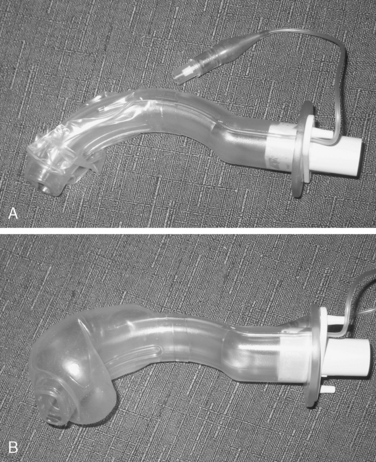
Supraglottic airway devices may cause less laryngeal irritation than endotracheal tubes and can be placed without visualization of the airway (Brimacombe, 1995). The LMA, developed by Dr. Brain, is the first of these devices and is available in multiple pediatric sizes. Several competitors, including the COBRA Perilaryngeal Airway and the COPA Oropharyngeal Airway, are now marketed (Figs. 34-1 and 34-2). All of these devices offer a less stimulating means of maintaining the airway while freeing the hands of the anesthesiologist for other tasks. As was mentioned above, a number of studies have demonstrated that the ability to maintain a stable airway without stimulating the larynx and trachea can decrease the incidence of adverse respiratory events in children with active or recent URIs (Tait et al., 1998). The same might be true for patients with asthma, where the airway is also hyperirritable, although there are no data in children at this time. Airway pressure and resistance in anesthetized adults without lung disease have been shown to be lower with the LMA compared with an endotracheal tube, and it has been shown to induce less bronchoconstriction (Berry et al., 1999). Although the LMA may diminish lower respiratory tract stimulation, it does not appear to decrease the incidence of postoperative sore throat (Splinter et al., 1994). The findings by Tait et al. that endotracheal tubes are more stimulating than LMAs, which are in turn more stimulating than a face mask, may serve to guide the decision of how to manage the airway if all other factors are equal.
Infants appear to have a greater incidence of problems with supraglottic airway devices than older children. There is a high incidence of infolding of the epiglottis and malposition in children under 10 kg and a significantly higher incidence of airway complications such as laryngospasm, breath-holding, obstruction, and coughing when compared with a conventional mask with oral airway (Harnett et al., 2000; Bagshaw, 2002; Polaner et al., 2006).
Anesthetic Maintenance
Halothane is no longer available in the United States and has been replaced by sevoflurane as the primary volatile anesthetic for inhalation induction. It has been argued that sevoflurane’s lower blood-gas solubility and blood:gas partition coefficient give it significant advantage over older, more soluble agents such as isoflurane for emergence, where a more rapid return to consciousness is desired in the outpatient setting. Whereas return to wakefulness may indeed be quicker with sevoflurane (and is even more rapid with desflurane), for short cases the speed to awakening with isoflurane can approach that of the less soluble agents if the isoflurane is turned off sooner. Perhaps more importantly, time to awakening has no relationship to time to discharge from the hospital (Lerman et al., 1996; Sury et al., 1996; Welborn et al., 1996). The latter is the metric that reflects both day-surgery unit efficiency and cost savings, and anesthesia with sevoflurane has not been shown to produce shorter discharge times, which is generally related not to speed of emergence but more to other factors, including premedication, complications of recovery such as postoperative nausea and vomiting, emergence agitation, and analgesic needs (Bacher et al., 1997).
Sevoflurane has one additional characteristic that limits its effectiveness as a maintenance agent in outpatient anesthesia—the problem of emergence agitation. Several investigators have studied this problem, which can be exceedingly disruptive and disturbing for PACU caregivers and parents. With the increased use of sevoflurane, it became apparent that there was an increase in emergence agitation or delirium in the PACU, and the majority of studies, including a recent meta-analysis (see Chapter 13, Induction, Maintenance, and Recovery, Figure 13-4), support this clinical impression (Mayer et al., 2006; Rieger et al., 1996; Beskow and Westrin, 1999; Cravero et al., 2000; Kuratani and Oi, 2008; Bortone et al., 2006). However, there are a few studies that have not found an increased incidence compared with isoflurane (Meyer et al., 2007; Voepel et al., 2003). The child with emergence agitation appears wild and incoherent; he is inconsolable and does not appear to recognize familiar people. This phenomenon has clearly been distinguished from inadequate analgesia. Cravero et al. (2000) compared emergence characteristics of sevoflurane to halothane anesthesia in children undergoing magnetic resonance imaging. This prospective randomized study design effectively eliminated pain or dysphoria of neural blockade as potential confounding variables, leaving only the choice of volatile agent as a factor (Cravero et al., 2000). Using either low- or high-threshold criteria to define agitation and delirium, the investigators found much higher rates (33% vs. 0% with high-threshold criteria and 88% vs. 12% applying low-threshold criteria) with sevoflurane as compared with halothane. Any time advantage gained by more rapid emergence was eliminated by the difficulty in caring for the agitated child in the PACU, and hospital discharge times were not different. Another study of sevoflurane in 100 children undergoing myringotomy and tube placement found that even with very short times under anesthesia, the incidence of emergence agitation was unacceptably high (Lapin et al., 1999). Although discharge times in this study were faster in the sevoflurane group, 67% demonstrated emergence agitation, leading them to conclude that sevoflurane was unsuitable for use as a sole agent for this procedure. They found that the addition of midazolam reduced this problem while lengthening recovery but not discharge times. Other investigators have found that midazolam does not prevent emergence agitation, and have found better responses to opioids, dexmedetomidine, low-dose ketamine, and propofol and ketorolac (Guler et al., 2005; Hung et al., 2005; Aouad et al., 2007; Breschan et al., 2007; Tsai et al., 2008; Davis et al., 1999). It is possible that the cause is related to the different effects of these agents on brain function that has been noted on electroencephalogram; speed of emergence does not seem to be a cause (Constant et al., 1999; Cohen et al., 2003; Oh et al., 2005).
Desflurane is another new volatile agent with rapid onset and offset characteristics caused by an exceptionally low blood: gas partition coefficient and solubility. It, too, appears to have a higher incidence of emergence agitation than older agents, although less than has been reported with sevoflurane (Mayer et al., 2006; Davis et al., 1994; Welborn et al., 1996; Valley et al., 2003). Emergence is significantly faster than with sevoflurane, however. This is because of its low solubility in tissues such as muscle and brain as compared with sevoflurane. Because sevoflurane is similar to halothane in its solubility in vessel-rich tissue groups, after discontinuance of the agent, significant blood concentrations are maintained as the agent returns from these depot storage sites to the bloodstream along its concentration gradient. This does not occur to a significant degree with desflurane because of its low tissue solubility, thereby speeding emergence time. Desflurane has also been found to decrease the ability to maintain spontaneous ventilation at concentrations greater than 1 MAC (Behforouz et al., 1998). Although desflurane is a potent airway irritant and is contraindicated for inhalation induction because of a very high incidence of severe laryngospasm, it does not appear to cause problems with deep extubation, although the incidence of coughing and other symptoms of airway irritability appear to be somewhat higher with desflurane (Zwass et al., 1992; Sneyd et al., 1998; Valley et al., 2003).
Nitrous oxide continues to be used as an adjunctive agent for outpatient anesthesia in combination with both volatile and IV agents. It is useful as a sedative when placing IV cannulae in situations where a pure IV technique is used, and it can ease the introduction of the pungent volatile agents when performing inhalation induction. Its utility as an agent for maintenance, however, is limited by its capacity to increase the incidence of postoperative nausea and vomiting. For this reason, its use in the outpatient setting should be constrained (Divatia et al., 1996).
The development of propofol heralded a new era in the maintenance of anesthesia in outpatients. It is not hyperbole to suggest that it thrust total IV anesthesia (TIVA) into the mainstream of anesthetic techniques, allowing rapid titration of anesthetic depth and prompt emergence without the use of volatile agents. Propofol is a potent antiemetic and can reduce the incidence of nausea and vomiting when used in combination with both other anesthetic agents and other antiemetics (Sneyd et al., 1998; Barst et al., 1999). Despite its rapid emergence characteristics when used for anesthetics of short or moderate duration, the incidence of delirium and agitation is very low when compared with sevoflurane or desflurane (Nakayama et al., 2007). Propofol’s major limitation is its lack of analgesic properties, and it must be used with an opioid or regional technique to provide adequate depth of anesthesia for most stimulating procedures. It is an excellent (and perhaps the ideal) agent for use in imaging, radiation treatment, or interventional radiologic procedures where there is a minimum or absence of stimulation (Aldridge and Gordon, 1992; Martin et al., 1992; Vangerven et al., 1992; Frankville et al., 1993). It is particularly attractive for anesthesia for radiation therapy, where children require daily repeated general anesthetics for up to 6 consecutive weeks. In this situation, children are able to be ready for discharge to home within 20 minutes of the end of the treatment session, and in contrast to other agents such as barbiturates, children receiving propofol have no evidence of either drug accumulation or development of tolerance and dose escalation (Glauber and Audenaert, 1987; Mills and Lord, 1992; Fassoulaki et al., 1994).
Remifentanil is a unique IV opioid with rapid onset and elimination. In contrast to other opioids, its degradation is independent of organ metabolism, instead relying on hydrolysis by plasma esterases. The drug permits the anesthesiologist to provide intense intraoperative levels of opioid analgesia with no residual respiratory depression after emergence (Roulleau et al., 2003). Other postoperative side effects usually associated with opioids, such as nausea and vomiting, excessive sedation, and respiratory depression are absent or extremely rare (Pinsker and Carroll, 1999; Eltzschig et al., 2002). Intraoperative conditions are notable for hemodynamic stability, although both bradycardia and hypotension can occur at higher infusion rates. The most significant caveat to the use of remifentanil is that its rapid degradation provides no postoperative analgesia whatsoever (Davis et al., 2000). It is essential, therefore, to use another agent or technique for this purpose, such as a long-acting opioid, a regional block, or a nonsteroidal such as ketorolac, and to administer it with adequate time for action before the patient’s emergence and the dissipation of remifentanil’s effect.
Perhaps the most effective manner in which to use remifentanil is to combine it with propofol. The combination of the two agents provides a balanced anesthetic that is easily titratable and results in significant reductions in the dose of both (Grundmann et al., 1998; Keidan et al., 2001). The two drugs can be mixed in the same syringe and administered via syringe pump. Because remifentanil degrades in propofol over time, aliquots small enough to be infused within 1 hour should be used (Stewart et al., 2000). For procedures of mild to moderate stimulation, 10 mcg of remifentanil per 1 mL (10 mg) of propofol is used, with infusion rates beginning at 100 mcg/kg per minute of propofol (0.1 mcg/kg per minute of remifentanil). This concentration permits the maintenance of spontaneous ventilation in most patients (Peacock et al., 1998; Reyle-Hahn et al., 2000). In particular, spontaneous ventilation is well maintained in children younger than 3 years old, even at higher infusion rates and even though remifentanil requirements to block the somatic response to skin incision are close to twofold higher in children than in adults (0.15 vs. 0.08 mcg/kg) (Barker et al., 2007; Munoz et al., 2007). For more stimulating procedures, the remifentanil concentration is doubled to 20 mcg/mL of propofol, and the infusion begins at the same rate of 100 mcg/kg per minute of propofol (0.2 mcg/kg per minute of remifentanil). Many patients breathe spontaneously with this concentration as well, although slow respiratory rates are common, and clinicians must be vigilant to avoid hypoventilation. This technique provides a stable intraoperative course, combined with an exceptionally smooth emergence and rapid return to baseline function, especially for patients undergoing procedures such as bone marrow aspirations, lumbar punctures, interventional radiology, upper and lower GI endoscopies, and incision and drainage of abscesses (Glaisyer and Sury, 2005).
Regional anesthesia (usually in combination with a general anesthetic) can be used to great advantage in outpatients. Although the prime advantage and reason for its use is the provision of postoperative analgesia (as is later discussed in more detail), the modest reduction in the depth of general anesthesia can speed recovery and reduce the incidence of opioid-related untoward effects. Motor blockade is, in most cases, not a contraindication to discharge to home and can be reduced or eliminated by the use of low concentration local anesthetics. Most children have their motor block resolved before discharge from the PACU, although this may not be the case for longer-acting peripheral nerve blocks (Burns et al., 1990). For those who are discharged with residual blockade, clear and explicit instructions to the parents as to how to guard the limb and good follow-up inquiry via telephone are critical. Other reported advantages of regional blockade include decreased intraoperative blood loss and improved operating conditions during hypospadias repair (Gunter et al., 1990). For operations shorter than 1 hour in duration, preoperative blockade did not affect the duration of postoperative analgesia compared with blockade administered at the end of the case (Rice et al., 1990).
Nearly all regional anesthetics in children in the United States are administered after the induction of general anesthesia, although Marhofer and others (2004) reports the extensive use of regional anesthesia with sedation in Austria. See Chapter 16, Regional Anesthesia, for details on performing the various regional blocks. In most cases, regional blockade for ambulatory surgery is performed with local anesthetics only, omitting opioids and adjunctive agents, although there is growing experience with caudal clonidine (2 mcg/kg or less) in outpatient anesthesia in children over 6 months of age. The risks of respiratory depression that can occur with those additives must always be considered, an important safety factor in the patient who will not be monitored after discharge from the PACU.
Neuromuscular Blockade
In most outpatient procedures in infants and children, neuromuscular blockade is not necessary. The majority of surgical procedures that are done in this setting can be performed without it, and intubation can most commonly be achieved with inhalation anesthesia, sometimes combined with a single dose of propofol in older children. Alternatively, a single dose of remifentanil can be administered in combination with propofol for intubation and has been shown to facilitate rapid sequence induction without muscle relaxants (Batra et al., 2004). When muscle relaxants are employed, it is best to avoid any of the longer-acting nondepolarizers and rely on intermediate acting drugs such as atracurium or cisatracurium, rocuronium or vecuronium. These agents are discussed in detail in Chapter 7, Pharmacology of Pediatric Anesthesia. When a muscle relaxant is used, adequacy of reversal must be assured. A recent investigation in adults found that incomplete reversal and mild degrees of residual neuromuscular blockade were common (Debaene et al., 2003). At least with some agents, children are less prone to inadequate spontaneous reversal. In children receiving mivacurium (no longer available in the United States), residual weakness was not observed, whereas the finding was present in the adults (Bevan et al., 1996). Stringent criteria for adequacy of spontaneous reversal must be sought, and reversal agents administered if necessary (Baurain et al., 1998; Ali, 2003).
Fluids
As fasting times become shorter, the consequences of preoperative fasting are less problematic, but there remain occasional patients who come to surgery with varying degrees of dehydration (Coté, 1990; Cook-Sather et al., 2003). Additionally, the patient who is to be discharged home may not be interested in drinking large amounts of fluids in the hours immediately after surgery. It is useful, therefore, to provide adequate hydration not only to correct the fluid deficit but also to provide a cushion for the postoperative period. This is particularly the case for operations that may disrupt the ability to drink easily, such as tonsillectomy. Isotonic fluids should be administered, and the IV catheter may be kept in place until just before discharge. It is rarely necessary to provide glucose supplementation in the IV fluids in children outside the neonatal period (Sandstrom et al., 1993). The deficit plus current maintenance requirements should be repleted within 2 to 3 hours. In some cases, children are discharged home before that, but those are generally the ones at least risk for inadequate intake.
Postoperative analgesia
If there is any issue that has the potential to completely sabotage the success of an outpatient surgery it is inadequate postoperative analgesia. No child can be discharged to home if the parents cannot adequately manage their pain using simple interventions. Inadequate analgesia has been identified in several studies as one of the most common causes for unanticipated admission to the hospital after surgery (Grenier et al., 1998). In another study, postoperative pain was identified by parents as the major problem they encountered after discharge to home (Kokinsky et al., 1999). Only 28% of the patients in that study had received regional blocks, however.
Regional and Local Anesthesia
Regional and local anesthesia has become one of the key modalities for postoperative analgesia for amenable procedures. Among the commonly performed operations in pediatric outpatient surgery that are associated with significant postoperative pain, only adenotonsillectomy and tympanostomy tube placement are not suitable for local or regional anesthesia. A prospective randomized study of glossopharyngeal nerve block, previously reported as an effective technique in adults, was stopped before its completion because of a 50% incidence of upper airway obstruction in the treatment group. The authors terminated the study and concluded that glossopharyngeal block is dangerous in children after tonsillectomy because of the common occurrence of inadvertent blockade of the vagus and recurrent laryngeal nerves (Bean-Lijewski, 1997).
Strict attention to the limits of local anesthetic dose must be observed with any regional block, and aspiration should precede any injection. When large volumes of local anesthetic are injected for any regional block, both a test dose and incremental injection technique should be employed to minimize the risk of intravascular injection. A maximum of 2.5 mg/kg of bupivacaine can be administered to children over 6 months of age; younger infants should have the dose reduced by 30% (1.8 mg/kg) because of decreased levels of plasma binding proteins (Lerman et al., 1989; Luz et al., 1998). In addition, there appears to be an increased toxicity risk during general anesthesia with volatile agents (Badgwell et al., 1990). In these younger infants an additional margin of safety may be gained by the use of ropivacaine or levobupivacaine (no longer available in the United States), which appear to have less toxic potential (Bardsley et al., 1998; Kohane et al., 1998; Gunter et al., 1999; Morrison et al., 2000).
Although usually performed by the surgeon rather than the anesthesiologist, the value of wound infiltration with local anesthetic should not be underestimated. There are numerous limited procedures for which a regional nerve block would be more intervention than necessary, such as simple hardware removals in orthopedics or excisional biopsies of small lesions, where infiltration of the wound can be used to great advantage. Local anesthetic blood levels with this technique have been found to be low when dose limits are adhered to (Mobley et al., 1991). When a peripheral nerve or regional block can be performed, however, those techniques may offer superior analgesia. A study of caudal analgesia compared with wound infiltration for analgesia after inguinal herniorrhaphy found better analgesia and quicker emergence times, fewer pain-related behaviors, and earlier hospital discharge times with the caudal block. Less supplementation with systemic analgesics was required (Conroy et al., 1993).
Caudal anesthesia is probably the most commonly performed block in pediatric anesthesia practice. It is usually easy to administer, has an acceptably low incidence of complications, and is highly effective for surgical procedures below the level of the umbilicus (Dalens and Hasnaoui, 1989). The duration of effective analgesia is considerably longer than one would expect based on the usual length of action of the local anesthetic alone. A study that compared 0.25% bupivacaine with and without epinephrine found that the addition of epinephrine markedly prolonged the analgesia, and that prolonged duration of analgesia was correlated with both younger age and lower surgical site (penoscrotal vs. inguinal) (Warner et al., 1987). Duration of analgesia ranged from as short as 5 hours (inguinal surgery in patients older than 11 years of age) to as long as 23 hours (penoscrotal operation in patients 1 to 5 years of age) as judged by the time to first requirement for supplemental analgesia. In a study of caudal blockade for analgesia after club foot repair, analgesia lasted at least 8 hours (Foulk et al., 1995). The addition of clonidine to the local anesthetic has been found by some, but not all, investigators to prolong the duration of caudal analgesia (Constant et al., 1998; Ansermino et al., 2003; Tripi et al., 2005; Wheeler et al., 2005). Controversy remains, however, regarding the safety of this drug for outpatient use, particularly in smaller infants and at higher doses because of the risk of oversedation (Hansen and Henneberg, 2004). It appears prudent to limit the dose to less than 2 mcg/kg if caudal clonidine is used in a patient who will be discharged home and to avoid it altogether in infants younger than 6 months of age.
Side effects of caudal blockade in children are unusual. Multiple studies have confirmed that urinary retention does not occur after caudal block using local anesthetic without central neuraxis opioids (Warner et al., 1987; Fisher et al., 1993). No differences in side effects were seen between caudal blockade and ilioinguinal-iliohypogastric nerve block; PACU stays were longer by less than 5 minutes and hospital stays by less than 10 minutes with the caudal block (Splinter et al., 1995). Motor function is not significantly impaired at the time of discharge and does not preclude or delay discharge (Burns et al., 1990).
Although one study found that placing the block at the end of the case resulted in better analgesia, other well-controlled studies have shown that there is no decrement in the duration of analgesia with blocks administered at the beginning or end of surgery for procedures lasting less than 1 hour (Rice et al., 1990; Holthusen et al., 1994). A study of 0.5 mL/kg vs. 1 mL/kg of 0.125% bupivacaine with epinephrine for penile and scrotal surgery found no difference in the duration of analgesia up to 8 hours postoperatively (Malviya et al., 1992).
Peripheral nerve blocks can be used with considerable efficacy for outpatient surgery in children and can be administered either by the anesthesiologist or by the surgeon on the operative field. Ilioinguinal-iliohypogastric nerve block combined with wound infiltration showed similar efficacy to a caudal block for analgesia after inguinal herniorrhaphy in two randomized studies (Schindler et al., 1991; Splinter et al., 1995). Paraumbilical block was shown to provide excellent analgesia for umbilical herniorrhaphy, although the duration of analgesia was less than 8 hours in three of 11 subjects (Courreges et al., 1997). The transverse abdominis plane block, which can be reliably placed under ultrasound guidance, has been shown to be effective for prolonged analgesia after abdominal operations, including herniorrhaphy (Suresh and Chan, 2009). Fascia iliaca block is an effective and easy to place block for surgery of the leg above the level of the knee (Dalens et al., 1989). Ophthalmic surgery is often associated with considerable postoperative pain. Placement of a peribulbar block or a subconjunctival injection has been shown to provide long-lasting analgesia with a minimum of side effects in pediatric patients (Ates et al., 1998; Coppens et al., 2002; Subramaniam et al., 2003). Penile nerve blocks are often used in older patients undergoing distal hypospadias repairs or for circumcision. The dorsal nerve block has been shown to result in better postoperative analgesia than a penile ring block (Holder et al., 1997). Greater auricular nerve block has been shown to be efficacious after tympanomastiod surgery (Suresh et al., 2002). Many peripheral blocks of the extremities are very useful for analgesia after ambulatory surgery because of their long duration of action (Marhofer et al., 2004). If children have residual motor blockade at the time of discharge, follow-up care is necessary until the block has completely resolved.
An emerging practice in regional anesthesia is the use of continuous ambulatory peripheral nerve blocks and field blocks for postoperative analgesia (Ganesh et al., 2007). This has been facilitated by two technological innovations: the increased use of ultrasound to accurately place peripheral nerve or plexus cannulae, and the development of elastomeric infusion devices that permit delivery of local anesthetic at a controlled rate without the use of high-cost pumps. These devices contain a reservoir of local anesthetic large enough to last several days. The reservoir is surrounded by a balloon-like bulb that when filled with air compresses the reservoir and infuses the drug. A flow limiter in the tubing controls the infusion rate (Fig. 34-3). Children can be sent home, and with daily telephone follow-up conversations, can receive either a long-acting nerve block or the infusion of the local anesthetic into their wound for several days. The parent can easily remove the catheter when the infusion is complete.
The development of parenterally administered nonsteroidal antiinflammatory drugs, principally ketorolac, has allowed the anesthesiologist to administer long-acting nonopioid analgesics so that they have taken effect by the time the patient awakens. There is a sizable literature on the use of ketorolac in pediatric patients over the age of 1 year, but no randomized studies to date of COX-2 type drugs in pediatrics. Many of these studies have specifically examined the role of ketorolac in outpatient surgery. Ketorolac has been shown to have several advantages over opioid analgesics for postoperative analgesia in pediatric outpatients. There is a significant reduction in nausea and vomiting in patients receiving ketorolac as compared with morphine, fentanyl, or other opioids (Mendel et al., 1995; Purday et al., 1996). The duration of a single dose of ketorolac is longer (6 hours) than most of the commonly used opioid analgesics (Dsida et al., 2002). Lack of respiratory depression, of course, is one ketorolac’s major advantages over opioids. Ketorolac’s major problem for use in outpatient analgesia is that it causes reversible dysfunction of platelet adhesion, and bleeding problems have been noted to be more common in children undergoing adenotonsillectomy (Gallagher et al., 1995; Judkins et al., 1996; Splinter et al., 1996; Splinter and Roberts, 1996). Bleeding problems have not been reported in other types of surgery, but caution should be used when administering ketorolac after other oral surgical procedures. Ketorolac appears to offer significant benefits to the pediatric outpatient after select operations.
Opioids remain the gold standard for postoperative analgesia for moderate to severe pain, and they are still commonly prescribed for pediatric outpatients, both parenterally during anesthesia and by oral, transmucosal, and other routes for postoperative analgesia. Their principal disadvantages are the side effects of nausea and respiratory depression; at the doses prescribed for outpatients, ileus is rarely a problem. Even a single dose of morphine administered for postoperative analgesia after inguinal hernia surgery has been reported to increase the incidence of PONV (Weinstein et al., 1994). Adjunctive nonopioid analgesics such as acetaminophen have an opioid-sparing effect and can reduce the amount of opioid necessary to achieve analgesia, thereby reducing the risk of side effects.
Intranasal fentanyl (2 mcg/kg, administered intraoperatively) has been used primarily for analgesia after myringotomy and tube surgery. Because these procedures are commonly performed without obtaining IV access, this provides an easy route for the administration of a potent analgesic. In a study comparing intranasal fentanyl to placebo, postoperative conditions were superior with fentanyl (Henderson et al., 1988). Children who received intranasal fentanyl were not only more comfortable and less agitated, but they did not have an increase in PONV, a problem that has been noted with intranasal sufentanil (Henderson et al., 1988; Galinkin et al., 2000).
Numerous oral opioid analgesics are used for postoperative analgesia in children. The surgeon and not the anesthesiologist often prescribes these drugs, but the anesthesiologist’s expertise in the use of analgesics should be sought or offered when protocols are devised. Commonly used agents include oxycodone, hydrocodone, and tramadol (Table 34-2). All of these agents can be given in combination with acetaminophen and nonsteroidal antiinflammatory drugs for added efficacy. These agents are generally administered for 1 to 3 days; operations that result in more severe pain are less commonly performed on outpatients and require analgesic inpatient management. Codeine is still commonly prescribed for pain, but its use should be discouraged. Codeine is actually a prodrug—methylmorphine—that must be demethylated to its active form. Bioavailability varies widely depending on genetic variants of demethylation ability. Because of these pharmacogenetic differences, some patients achieve no analgesia at all as a result of an inability to demethylate the drug, whereas some suffer from increased side effects or even achieve potentially toxic drug levels because of more extensive demethylation (Quiding et al., 1992; Fagerlund and Braaten, 2001; El-Tahtawy et al., 2006) (see Chapter 15, Pain Management).
| Oxycodone | 0.1-0.2 mg/kg | PO q3-4 hours |
| Hydrocodone | 0.5 mg/kg | PO q3-4 hours |
| Tramadol | 1-2 mg/kg | PO q4-6 hours |
Pacu, recovery, and discharge issues
Nausea and Vomiting
PONV is perhaps the most common complication after anesthesia, although the reported incidence varies widely from approximately 8% to 50% (Heyland et al., 1997; Villeret et al., 2002). The variance is most likely because of study methodology and definitions. It is usually not an intractable problem, but it certainly is the cause of considerable distress, and if not well controlled may be a cause of unanticipated admission (Patel et al., 1997; Rose and Watcha, 1999). Numerous factors have been identified as increasing the risk of postoperative vomiting, some related to the surgical procedure, some to the anesthetic agents and some to the patient (Eberhart et al., 2004; Gan et al., 2007; Kranke et al., 2007). The most common of these are listed in Table 34-3. Eberhart et al. (2004) noted the following risk factors for pediatric PONV: surgery lasting longer than 30 minutes, age older than 3 years, family or patient history of PONV, and strabismus surgery. The incidence of postoperative vomiting was 9%, 10%, 30%, 55%, and 70% if zero, one, two, three, or four risk factors were present. Because discharge to home is so dependent on the child returning to a baseline condition that allows the intake of fluids and nutrients, it is essential that nausea and vomiting be kept to a minimum for outpatient surgeries.
TABLE 34-3 Common Causes of Postoperative Nausea and Vomiting in Outpatients
| Surgical Factors | Anesthetic Factors | Patient Factors |
| Adenotonsillectomy | Use of volatile agents | Prior history of PONV |
| Middle ear surgery | Use of nitrous oxide | History of motion sickness |
| Testicular surgery | Use of opioids | Age over 2 years |
| Laparoscopic surgery | Insufflation of the stomach (difficult mask ventilation) | Girls > boys |
| Insufflation of the bowel (endoscopy) | Reversal of neuromuscular blockade (cholinergics) | |
| Unrelieved pain |
PONV, Postoperative nausea and vomiting.
Anesthetic technique has been correlated with the incidence of PONV, with propofol-based techniques having the lowest incidence (Sneyd et al., 1998; Barst et al., 1999; Gurkan et al., 1999). The use of volatile agents, opioids, nitrous oxide, and cholinergic drugs for the reversal of neuromuscular blockade all increase the risk, although one study found that desflurane had less PONV than reported with other volatile anesthetics (Mendel et al., 1995; Divatia et al., 1996; Kuhn et al., 1999). Remifentanil, in contrast with other opioids, does not appear to increase PONV (Pinsker and Carroll, 1999).
Dose-response studies of ondansetron suggest that for maximal efficacy, prophylactic doses of 0.1 to 0.15 mg/kg up to 4 mg should be administered (Rose et al., 1996; Patel et al., 1997; Sadhasivam et al., 2000). Lower doses were either not as effective, or were no more effective than placebo. Timing the dose before or after manipulation of the extraocular muscles in strabismus surgery (one of the most emetogenic operations) did not appear to make a difference, although the best effect has been reported when the drug is given at the end of the procedure (Madan et al., 2000). Another HT3-antagonist, granisetron, was effective when 0.2 mg/kg was administered orally before induction of anesthesia (Munro et al., 1999). When a single dose was not adequate, controversy exists as to whether a second dose is efficacious (Rose and Martin, 1996; Kovac et al., 1999). Dexamethasone is effective at preventing PONV, and has the additional advantage of costing substantially less than the 5–HT3-antagonists (Splinter and Roberts, 1996; Subramaniam et al., 2001). Dosing recommendations vary widely, but two-dose ranging studies found no additional benefits to using more than 0.25 mg/kg for decreasing postoperative nausea and vomiting; higher doses may be justified after tonsillectomy for the additional benefit of improving the ability to take oral fluids (Madan et al., 2005; Kim et al., 2007; Karaman et al., 2009). Despite a recent controversial study that found an increased incidence of bleeding after a single dose of dexamethasone, a Cochrane Database meta-analysis found benefit with no adverse effects of this therapy (Steward et al., 2003; Czarnetzki et al., 2008).
Metaclopramide is thought to act by increasing gastric emptying. Although it appears to be effective, several investigators have found it to be less so than ondansetron; in a recent meta-analysis, metaclopramide was found to be less effective than either the 5-HT blocking agents or dexamethasone (Broadman et al., 1990; Furst and Rodarte, 1994; Bolton et al., 2006). It has a higher incidence of side effects, primarily sleepiness and occasional extrapyramidal effects, than either of the other agents; therefore, it should be viewed as a second-line therapy. Cisapride, which also is a prokinetic agent, was found to be ineffective at treating PONV (Cook-Sather et al., 2002).
Several investigators recommend the use of multiple agents in combination for those at greatest risk of PONV. It appears that combining multiple drugs with different mechanisms of action yield the best results (Rose and Watcha, 1999; Gan et al., 2007). A factorial analysis comparing the use ondansetron, metaclopramide, and dexamethasone alone and in combination found that although each of the drugs decreased the odds of vomiting, and the addition of dexamethasone to ondansetron further decreased the odds of postoperative emesis, the combination of metaclopramide and ondansetron actually had a negative effect, and the authors therefore recommend against that particular combination (Gunter et al., 2006). Similarly, the use of a low-risk anesthetic, especially propofol, which appears to have antiemetic properties of its own, in combination with prophylaxis, may result in the lowest incidence of all (Barst et al., 1999). Low infusion rates of propofol as an anesthetic adjunct have been shown to have similar effects (Erdem et al., 2008).
An additional factor that has been found to promote PONV is the insistence on oral intake in the PACU before discharge. Although it appears sensible to want to demonstrate that a child is able to take and retain oral intake before discharge to home, investigators found that insistence on oral intake before discharge increased the incidence of PONV and lengthened hospital stay (Schreiner et al., 1992; Schreiner and Nicolson, 1995). This common practice probably deserves a critical reexamination.
Adu-Gyamfi Y., Sankarankutty M., Marwa S. Use of a tourniquet in patients with sickle-cell disease. Can J Anaesth. 1993;40(1):24-27.
Akinbami L.J., Moorman J.E., Garbe P.L., Sondik E.J. Status of childhood asthma in the United States, 1980-2007. Pediatrics. 2009;123(Suppl 3):S131-S145.
Aldridge L.M., Gordon N.H. Propofol infusions for radiotherapy. Paediatr Anaesth. 1992;2:133-137.
Ali H.H. Criteria of adequate clinical recovery from neuromuscular block. Anesthesiology. 2003;98(5):1278-1280.
American Thoracic Society. Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med. 1996;153:866-878.
Ansermino M., Basu R., Vandebeek C., Montgomery C. Nonopioid additives to local anaesthetics for caudal blockade in children: a systematic review. Paediatr Anaesth. 2003;13(7):561-573.
Aouad M.T., Yazbeck-Karam V.G., Nasr V.G., et al. A single dose of propofol at the end of surgery for the prevention of emergence agitation in children undergoing strabismus surgery during sevoflurane anesthesia. Anesthesiology. 2007;107(5):733-738.
Ates Y., Unal N., Cuhruk H., Erkan N. Postoperative analgesia in children using preemptive retrobulbar block and local anesthetic infiltration in strabismus surgery. Reg Anesth Pain Med. 1998;23(6):569-574.
Atlas S.A. The sickle cell trait and surgical complications: a matched-pair patient analysis. JAMA. 1974;229(8):1078-1080.
Bacher A., Burton A.W., Uchida T., Zornow M.H. Sevoflurane or halothane anesthesia: can we tell the difference? Anesth Analg. 1997;85(6):1203sP1206.
Badgwell J., Heavner J., Kytta J. Bupivacaine toxicity in young guinea pigs is age-dependent and is affected by volatile anesthetics. Anesthesiology. 1990;73:297-303.
Bagshaw O. The size 1.5 laryngeal mask airway (LMA) in paediatric anaesthetic practice. Paediatr Anaesth. 2002;12(5):420-423.
Bardsley H., Gristwood R., Baker H., et al. A comparison of the cardiovascular effects of levobupivacaine and rac-bupivacaine following IV administration to healthy volunteers. Br J Clin Pharmacol. 1998;46(3):245-249.
Barker N., Lim J., Amari E., et al. Relationship between age and spontaneous ventilation during IV anesthesia in children. Paediatr Anaesth. 2007;17(10):948-955.
Barnett A.H., Robinson M.H., Harrison J.H., Watkins P.J. Mini-pump: method of diabetic control during minor surgery under general anaesthesia. Br Med J. 1980;280(6207):78-79.
Barst S.M., Leiderman J.U., Markowitz A., et al. Ondansetron with propofol reduces the incidence of emesis in children following tonsillectomy. Can J Anaesth. 1999;46(4):359-362.
Batra Y.K., Al Qattan A.R., Ali S.S., et al. Assessment of tracheal intubating conditions in children using remifentanil and propofol without muscle relaxant. Paediatr Anaesth. 2004;14(6):452-456.
Baurain M.J., Hennart D.A., Godschalx A., et al. Visual evaluation of residual curarization in anesthetized patients using one hundred-hertz, five-second tetanic stimulation at the adductor pollicis muscle. Anesth Analg. 1998;87(1):185-189.
Bean-Lijewski J.D. Glossopharyngeal nerve block for pain relief after pediatric tonsillectomy: retrospective analysis and two cases of life-threatening upper airway obstruction from an interrupted trial. Anesth Analg. 1997;84(6):1232-1238.
Behforouz N., Dubousset A.M., Jamali S., Ecoffey C. Respiratory effects of desflurane anesthesia on spontaneous ventilation in infants and children. Anesth Analg. 1998;87(5):1052-1055.
Bellew M., Atkinson K.R., Dixon G., Yates A. The introduction of a paediatric anaesthesia information leaflet: an audit of its impact on parental anxiety and satisfaction. Paediatr Anaesth. 2002;12(2):124-130.
Berry A., Brimacombe J., Keller C., Verghese C. Pulmonary airway resistance with the endotracheal tube vs. laryngeal mask airway in paralyzed anesthetized adult patients. Anesthesiology. 1999;90(2):395-397.
Beskow A., Westrin P. Sevoflurane causes more postoperative agitation in children than does halothane. Acta Anaesthesiol Scand. 1999;43(5):536-541.
Betts P., Brink S.J., Swift P.G., et al. Management of children with diabetes requiring surgery. Pediatr Diabetes. 2007;8(4):242-247.
Bevan D.R., Kahwaji R., Ansermino J.M., et al. Residual block after mivacurium with or without edrophonium reversal in adults and children. Anesthesiology. 1996;84(2):362-367.
Bevan J.C., Veall G.R., Macnab A.J., et al. Midazolam premedication delays recovery after propofol without modifying involuntary movements. Anesth Analg. 1997;85(1):50-54.
Biavati M.J., Manning S.C., Phillips D.L. Predictive factors for respiratory complications after tonsillectomy and adenoidectomy in children. Arch Otolaryngol Head Neck Surg. 1997;123(5):517-521.
Bolton C.M., Myles P.S., Nolan T., Sterne J.A. Prophylaxis of postoperative vomiting in children undergoing tonsillectomy: a systematic review and meta-analysis. Br J Anaesth. 2006;97(5):593-604.
Bortone L., Ingelmo P., Grossi S., et al. Emergence agitation in preschool children: double-blind, randomized, controlled trial comparing sevoflurane and isoflurane anesthesia. Paediatr Anaesth. 2006;16(11):1138-1143.
Brampton W.J., Jago R.H. Acute viral myocarditis: a death associated with anaesthesia. Anaesthesia. 1990;45(3):215-217.
Breschan C., Platzer M., Jost R., et al. Midazolam does not reduce emergence delirium after sevoflurane anesthesia in children. Paediatr Anaesth. 2007;17(4):347-352.
Brimacombe J. The advantages of the LMA over the tracheal tube or facemask: a meta-analysis. Can J Anaesth. 1995;42(11):1017-1023.
Broadman L.M., Ceruzzi W., Patane P.S., et al. Metoclopramide reduces the incidence of vomiting following strabismus surgery in children. Anesthesiology. 1990;72(2):245-248.
Brown K., Aun C., Stocks J., et al. A comparison of the respiratory effects of sevoflurane and halothane in infants and young children. Anesthesiology. 1998;89(1):86-92.
Brown K.A., Laferriere A., Lakheeram I., Moss I.R. Recurrent hypoxemia in children is associated with increased analgesic sensitivity to opiates. Anesthesiology. 2006;105(4):665-669.
Burns A.M., Shelly M.P., Dewar A.K. Caudal analgesia for pediatric day case surgery: assessment of motor function prior to discharge. J Clin Anesth. 1990;2(1):27-30.
Campbell N.N. Respiratory tract infection and anaesthesia: haemophilus influenzae pneumonia that developed under anaesthesia. Anaesthesia. 1990;45(7):561-562.
Cassady J.F.Jr, Wysocki T.T., Miller K.M., et al. Use of a preanesthetic video for facilitation of parental education and anxiolysis before pediatric ambulatory surgery. Anesth Analg. 1999;88(2):246-250.
Chase H.P., Arslanian S., White N.H., Tamborlane W.V. Insulin glargine vs. intermediate-acting insulin as the basal component of multiple daily injection regimens for adolescents with type 1 diabetes mellitus. J Pediatr. 2008;153(4):547-553.
Cohen I.T., Finkel J.C., Hannallah R.S., et al. Rapid emergence does not explain agitation following sevoflurane anaesthesia in infants and children: a comparison with propofol. Paediatr Anaesth. 2003;13(1):63-67.
Cohen M.M., Cameron C.B. Should you cancel the operation when a child has an upper respiratory tract infection? [see comments]. Anesth Analg. 1991;72(3):282-288.
Conroy J.M., Othersen H.B.Jr, Dorman B.H., et al. A comparison of wound instillation and caudal block for analgesia following pediatric inguinal herniorrhaphy. J Pediatr Surg. 1993;28(4):565-567.
Constant I., Dubois M.C., Piat V., et al. Changes in electroencephalogram and autonomic cardiovascular activity during induction of anesthesia with sevoflurane compared with halothane in children. Anesthesiology. 1999;91(6):1604-1615.
Constant I., Gall O., Gouyet L., et al. Addition of clonidine or fentanyl to local anaesthetics prolongs the duration of surgical analgesia after single shot caudal block in children. Br J Anaesth. 1998;80(3):294-298.
Cook-Sather S.D., Harris K.A., Chiavacci R., et al. A liberalized fasting guideline for formula-fed infants does not increase average gastric fluid volume before elective surgery. Anesth Analg. 2003;96(4):965-969. table of contents
Cook-Sather S.D., Harris K.A., Schreiner M.S. Cisapride does not prevent postoperative vomiting in children. Anesth Analg. 2002;94(1):50-54. table of contents
Coppens M., Versichelen L., Mortier E.. Treatment of postoperative pain after ophthalmic surgery. Bull Soc Belge Ophtalmol. 2002(285):27-32.
Coté C. The upper respiratory tract infection (URI) dilemma: fear of a complication or litigation? Anesthesiology. 2001;95(2):283-285.
Coté C.J. Fasting after midnight for children: a reappraisal [see comments]. Anesthesiology. 1990;72(4):589-592.
Coté C.J., Cohen I.T., Suresh S., et al. A comparison of three doses of a commercially prepared oral midazolam syrup in children. Anesth Analg. 2002;94(1):37-43. table of contents
Coté C.J., Rolf N., Liu L.M., et al. A single-blind study of combined pulse oximetry and capnography in children. Anesthesiology. 1991;74(6):980-987.
Coté C.J., Zaslavsky A., Downes J.J., et al. Postoperative apnea in former preterm infants after inguinal herniorrhaphy: a combined analysis [see comments]. Anesthesiology. 1995;82(4):809-822.
Courreges P., Poddevin F., Lecoutre D. Para-umbilical block: a new concept for regional anaesthesia in children. Paediatr Anaesth. 1997;7(3):211-214.
Cravero J., Surgenor S., Whalen K. Emergence agitation in paediatric patients after sevoflurane anaesthesia and no surgery: a comparison with halothane. Paediatr Anaesth. 2000;10(4):419-424.
Czarnetzki C., Elia N., Lysakowski C., et al. Dexamethasone and risk of nausea and vomiting and postoperative bleeding after tonsillectomy in children: a randomized trial. JAMA. 2008;300(22):2621-2630.
Dalens B., Hasnaoui A. Caudal anesthesia in pediatric surgery: success rate and adverse effects in 750 consecutive patients. Anesth Analg. 1989;68(2):83-89.
Dalens B., Vanneuville G., Tanguy A. Comparison of the fascia iliaca compartment block with the 3-in-1 block in children. Anesth Analg. 1990;70(4):474. published erratum appears in [see comments], Anesth Analg 69(6):705–713, 1989
Davis P.J., Cohen I.T., McGowan F.X.Jr, Latta K. Recovery characteristics of desflurane vs. halothane for maintenance of anesthesia in pediatric ambulatory patients. Anesthesiology. 1994;80(2):298-302.
Davis P.J., Finkel J.C., Orr R.J., et al. A randomized, double-blinded study of remifentanil vs. fentanyl for tonsillectomy and adenoidectomy surgery in pediatric ambulatory surgical patients. Anesth Analg. 2000;90(4):863-871.
Davis P.J., Greenberg J.A., Gendelman M., Fertal K. Recovery characteristics of sevoflurane and halothane in preschool aged children undergoing bilateral myringotomy and pressure equalization tube insertion. Anesth Analg. 1999;88:34-38.
de Kluijver J., Grunberg K., Sont J.K., et al. Rhinovirus infection in nonasthmatic subjects: effects on intrapulmonary airways. Eur Respir J. 2002;20(2):274-279.
Debaene B., Plaud B., Dilly M.P., Donati F. Residual paralysis in the PACU after a single intubating dose of nondepolarizing muscle relaxant with an intermediate duration of action. Anesthesiology. 2003;98(5):1042-1048.
Derkay C.S., Bray G., Milmoe G.J., Grundfast K.M. Adenotonsillectomy in children with sickle cell disease. South Med J. 1991;84(2):205-208.
Divatia J.V., Vaidya J.S., Badwe R.A., Hawaldar R.W. Omission of nitrous oxide during anesthesia reduces the incidence of postoperative nausea and vomiting: a meta-analysis. Anesthesiology. 1996;85(5):1055-1062.
Dsida R.M., Wheeler M., Birmingham P.K., et al. Age-stratified pharmacokinetics of ketorolac tromethamine in pediatric surgical patients. Anesth Analg. 2002;94(2):266-270. table of contents
Dusser D., Jacoby D., Djokic T., et al. Virus induces airway hyperresponsiveness to tachykinins: role of neutral endopeptidase. J Appl Physiol. 1989;67:1504-1511.
Eames W.O., Rooke G.A., Wu R.S., Bishop M.J. Comparison of the effects of etomidate, propofol, and thiopental on respiratory resistance after tracheal intubation. Anesthesiology. 1996;84(6):1307-1311.
Eberhart L.H., Geldner G., Kranke P., et al. The development and validation of a risk score to predict the probability of postoperative vomiting in pediatric patients. Anesth Analg. 2004;99:1630-1637.
El-Tahtawy A., Kokki H., Reidenberg B.E. Population pharmacokinetics of oxycodone in children 6 months to 7 years old. J Clin Pharmacol. 2006;46(4):433-442.
Eltzschig H.K., Schroeder T.H., Eissler B.J., et al. The effect of remifentanil or fentanyl on postoperative vomiting and pain in children undergoing strabismus surgery. Anesth Analg. 2002;94(5):1173-1177. table of contents
Empey D.W. Effect of airway infections on bronchial reactivity. Eur J Respir Dis Suppl. 1983;128(Pt 1):366-368.
Empey D.W., Laitinen L.A., Jacobs L. Mechanisms of bronchial hyperreactivity in normal subjects after upper respiratory infections. Am Rev Respir Dis. 1976;113:131-139.
Erdem A.F., Yoruk O., Alici H.A., et al. Subhypnotic propofol infusion plus dexamethasone is more effective than dexamethasone alone for the prevention of vomiting in children after tonsillectomy. Paediatr Anaesth. 2008;18(9):878-883.
Fagerlund T.H., Braaten O. No pain relief from codeine…? An introduction to pharmacogenomics. Acta Anaesthesiol Scand. 2001;45(2):140-149.
Fassoulaki A., Farinotti R., Mantz J., Desmonts J.M. Does tolerance develop to the anaesthetic effects of propofol in rats? Br J Anaesth. 1994;72(1):127-128.
Fisher Q.A. Clear for surgery: current attitudes and practices of pediatricians. Clin Pediatr (Phila). 1991;30(5):326. published erratum appears in Clin Pediatr (Phila) 30(1):35–41, 1991
Fisher Q.A., Feldman M.A., Wilson M.D. Pediatric responsibilities for preoperative evaluation. J Pediatr. 1994;125(5 Pt 1):675-685.
Fisher Q.A., McComiskey C.M., Hill J.L., et al. Postoperative voiding interval and duration of analgesia following peripheral or caudal nerve blocks in children. Anesth Analg. 1993;76(1):173-177.
Foulk D.A., Boakes J., Rab G.T., Schulman S. The use of caudal epidural anesthesia in clubfoot surgery. J Pediatr Orthop. 1995;15(5):604-607.
Frankville D.D., Spear R.M., Dyck J.B. The dose of propofol required to prevent children from moving during magnetic resonance imaging [see comments]. Anesthesiology. 1993;79(5):953-958.
Funk W., Jakob W., Riedl T., Taeger K. Oral preanaesthetic medication for children: double-blind randomized study of a combination of midazolam and ketamine vs midazolam or ketamine alone. Br J Anaesth. 2000;84(3):335-340.
Furst S.R., Rodarte A. Prophylactic antiemetic treatment with ondansetron in children undergoing tonsillectomy. Anesthesiology. 1994;81(4):799-803.
Galinkin J.L., Fazi L.M., Cuy R.M., et al. Use of intranasal fentanyl in children undergoing myringotomy and tube placement during halothane and sevoflurane anesthesia. Anesthesiology. 2000;93(6):1378-1383.
Gallagher J.E., Blauth J., Fornadley J.A. Perioperative ketorolac tromethamine and postoperative hemorrhage in cases of tonsillectomy and adenoidectomy. Laryngoscope. 1995;105(6):606-609.
Gan T.J., Meyer T.A., Apfel C.C., et al. Society for ambulatory anesthesia guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2007;105:1615-1628.
Ganesh A., Rose J.B., Wells L., et al. Continuous peripheral nerve blockade for inpatient and outpatient postoperative analgesia in children. Anesth Analg. 2007;105(5):1234-1242. table of contents
Gibson I.H., Love S.H. Sickle cell trait: a rare cause of post-operative death in Northern Ireland. Ulster Med J. 1974;43(2):143-147.
Glaisyer H.R., Sury M.R. Recovery after anesthesia for short pediatric oncology procedures: propofol and remifentanil compared with propofol, nitrous oxide, and sevoflurane. Anesth Analg. 2005;100(4):959-963.
Glauber D.T., Audenaert S.M. Anesthesia for children undergoing craniospinal radiotherapy. Anesthesiology. 1987;67(5):801-803.
Grenier B., Dubreuil M., Siao D., Meymat Y. Paediatric day case anaesthesia: estimate of its quality at home. Paediatr Anaesth. 1998;8(6):485-489.
Griffin T.C., Buchanan G.R. Elective surgery in children with sickle cell disease without preoperative blood transfusion. J Pediatr Surg. 1993;28(5):681-685.
Grundmann U., Uth M., Eichner A., et al. Total IV anaesthesia with propofol and remifentanil in paediatric patients: a comparison with a desflurane-nitrous oxide inhalation anaesthesia. Acta Anaesthesiol Scand. 1998;42(7):845-850.
Guler G., Akin A., Tosun Z., et al. Single-dose dexmedetomidine reduces agitation and provides smooth extubation after pediatric adenotonsillectomy. Paediatr Anaesth. 2005;15(9):762-766.
Gunter J.B., Forestner J.E., Manley C.B. Caudal epidural anesthesia reduces blood loss during hypospadias repair. J Urol. 1990;144(2 Pt 2):517-519. discussion 530
Gunter J.B., Gregg T., Varughese A.M., et al. Levobupivacaine for ilioinguinal/iliohypogastric nerve block in children. Anesth Analg. 1999;89(3):647-649.
Gunter J.B., McAuliffe J.J., Beckman E.C., et al. A factorial study of ondansetron, metoclopramide, and dexamethasone for emesis prophylaxis after adenotonsillectomy in children. Paediatr Anaesth. 2006;16(11):1153-1165.
Gurkan Y., Kilickan L., Toker K. Propofol-nitrous oxide vs. sevoflurane-nitrous oxide for strabismus surgery in children. Paediatr Anaesth. 1999;9(6):495-499.
Hackmann T., Steward D.J., Sheps S. Anemia in pediatric day-surgery patients: prevalence and detection. Anesthesiology. 75(1), 1991.
Halvorson D.J., McKie V., McKie K., et al. Sickle cell disease and tonsillectomy. Preoperative management and postoperative complications. Arch Otolaryngol Head Neck Surg. 1997;123(7):689-692.
Hansen T.G., Henneberg S.W. Caudal clonidine in neonates and small infants and respiratory depression. Paediatr Anaesth. 2004;14(6):529-530.
Harnett M., Kinirons B., Heffernan A., et al. Airway complications in infants: comparison of laryngeal mask airway and the facemask-oral airway. Can J Anaesth. 2000;47(4):315-318.
Helfaer M.A., McColley S.A., Pyzik P.L., et al. Polysomnography after adenotonsillectomy in mild pediatric obstructive sleep apnea. Crit Care Med. 1996;24(8):1323-1327.
Henderson J.M., Brodsky D.A., Fisher D.M., et al. Pre-induction of anesthesia in pediatric patients with nasally administered sufentanil. Anesthesiology. 1988;68(5):671-675.
Heyland K., Dangel P., Gerber A.C. Postoperative nausea and vomiting (PONV) in children. Eur J Pediatr Surg. 1997;7(4):230-233.
Hirsch I.B. Insulin analogues. N Engl J Med. 2005;352(2):174-183.
Holder K.J., Peutrell J.M., Weir P.M. Regional anaesthesia for circumcision: subcutaneous ring block of the penis and subpubic penile block compared. Eur J Anaesthesiol. 1997;14(5):495-498.
Holthusen H., Eichwede F., Stevens M., et al. Pre-emptive analgesia: comparison of preoperative with postoperative caudal block on postoperative pain in children. Br J Anaesth. 1994;73(4):440-442.
Howell T.K., Smith S., Rushman S.C., et al. A comparison of oral transmucosal fentanyl and oral midazolam for premedication in children. Anaesthesia. 2002;57(8):798-805.
Hung W.T., Chen C.C., Liou C.M., Tsai W.Y. The effects of low-dose fentanyl on emergence agitation and quality of life in patients with moderate developmental disabilities. J Clin Anesth. 2005;17(7):494-498.
Judkins J.H., Dray T.G., Hubbell R.N. Intraoperative ketorolac and posttonsillectomy bleeding. Arch Otolaryngol Head Neck Surg. 1996;122(9):937-940.
Kain Z.N., Mayes L.C., Wang S.M., et al. Parental presence during induction of anesthesia vs. sedative premedication: which intervention is more effective? Anesthesiology. 1998;89(5):1147-1156. discussion 1149A-1110A
Kain Z.N., Caramico L.A., Mayes L.C., et al. Preoperative preparation programs in children: a comparative examination. Anesth Analg. 1998;87(6):1249-1255.
Kain Z.N., Hofstadter M.B., Mayes L.C., et al. Midazolam: effects on amnesia and anxiety in children. Anesthesiology. 2000;93(3):676-684.
Kain Z.N., Mayes L.C., Caramico L.A., et al. Parental presence during induction of anesthesia. A randomized controlled trial. Anesthesiology. 1996;84(5):1060-1067.
Kain Z.N., Caldwell-Andrews A.A., Krivutza D.M., et al. Trends in the practice of parental presence during induction of anesthesia and the use of preoperative sedative premedication in the United States, 1995-2002: results of a follow-up national survey. Anesth Analg. 2004;98(5):1252-1259.
Karaman M., Ilhan A.E., Dereci G., Tek A. Determination of optimum dosage of intraoperative single dose dexamethasone in pediatric tonsillectomy and adenotonsillectomy. Int J Pediatr Otorhinolaryngol. 2009;73(11):1513-1515.
Karl H.W., Pauza K.J., Heyneman N., Tinker D.E. Preanesthetic preparation of pediatric outpatients: the role of a videotape for parents. J Clin Anesth. 1990;2(3):172-177.
Karl H.W., Rosenberger J.L., Larach M.G., Ruffle J.M. Transmucosal administration of midazolam for premedication of pediatric patients: comparison of the nasal and sublingual routes. Anesthesiology. 1993;78(5):885-891.
Keidan I., Berkenstadt H., Sidi A., Perel A. Propofol/remifentanil vs. propofol alone for bone marrow aspiration in paediatric haemato-oncological patients. Paediatr Anaesth. 2001;11(3):297-301.
Kerbl R., Zotter H., Schenkeli R., et al. Persistent hypercapnia in children after treatment of obstructive sleep apnea syndrome by adenotonsillectomy. Wien Klin Wochenschr. 2001;113(7–8):229-234.
Kim M.S., Coté C.J., Cristoloveanu C., et al. There is no dose-escalation response to dexamethasone (0.0625-1.0 mg/kg) in pediatric tonsillectomy or adenotonsillectomy patients for preventing vomiting, reducing pain, shortening time to first liquid intake, or the incidence of voice change. Anesth Analg. 2007;104(5):1052-1058. tables of contents
Kleinfeldt A.S. Preoperative phone calls: reducing cancellations in pediatric day surgery. Aorn J. 1990;51(6):1559-1564.
Kohane D.S., Sankar W.N., Shubina M., et al. Sciatic nerve blockade in infant, adolescent, and adult rats: a comparison of ropivacaine with bupivacaine. Anesthesiology. 1998;89(5):1199-1208. discussion 1110A
Koinig H. Preparing parents for their child’s surgery: preoperative parental information and education. Paediatr Anaesth. 2002;12(2):107-109.
Kokinsky E., Thornberg E., Ostlund A.L., Larsson L.E. Postoperative comfort in paediatric outpatient surgery. Paediatr Anaesth. 1999;9(3):243-251.
Konotey-Ahulu F.I. Anaesthetic deaths and the sickle-cell trait. Lancet. 1969;1(7588):267-268.
Kovac A.L., O’Connor T.A., Pearman M.H., et al. Efficacy of repeat IV dosing of ondansetron in controlling postoperative nausea and vomiting: a randomized, double-blind, placebo-controlled multicenter trial. J Clin Anesth. 1999;11(6):453-459.
Krane E.J., Haberkern C.M., Jacobson L.E. Postoperative apnea, bradycardia, and oxygen desaturation in formerly premature infants: prospective comparison of spinal and general anesthesia. Anesth Analg. 1995;80(1):7-13.
Kranke P., Eberhart L.H., Toker H., et al. A prospective evaluation of the POVOC score for the prediction of postoperative vomiting in children. Anesth Analg. 2007;105:1592-1597.
Kuhn I., Scheifler G., Wissing H. Incidence of nausea and vomiting in children after strabismus surgery following desflurane anaesthesia. Paediatr Anaesth. 1999;9(6):521-526.
Kuratani N., Oi Y. Greater incidence of emergence agitation in children after sevoflurane anesthesia as compared with halothane: a meta-analysis of randomized controlled trials. Anesthesiology. 2008;109(2):225-232.
Kurth C.D., Spitzer A.R., Broennle A.M., Downes J.J. Postoperative apnea in preterm infants. Anesthesiology. 1987;66(4):483-488.
Lalakea M.L., Marquez-Biggs I., Messner A.H. Safety of pediatric short-stay tonsillectomy. Arch Otolaryngol Head Neck Surg. 1999;125(7):749-752.
Lapin S.L., Auden S.M., Goldsmith L.J., Reynolds A.M. Effects of sevoflurane anaesthesia on recovery in children: a comparison with halothane. Paediatr Anaesth. 1999;9(4):299-304.
Lerman J., Davis P.J., Welborn L.G., et al. Induction, recovery, and safety characteristics of sevoflurane in children undergoing ambulatory surgery: a comparison with halothane. Anesthesiology. 1996;84(6):1332-1340.
Lerman J., Strong H.A., LeDez K.M., et al. Effects of age on the serum concentration of alpha 1-acid glycoprotein and the binding of lidocaine in pediatric patients. Clin Pharmacol Ther. 1989;46(2):219-225.
Levy L., Pandit U.A., Randel G.I., et al. Upper respiratory tract infections and general anaesthesia in children: peri-operative complications and oxygen saturation. Anaesthesia. 1992;47(8):678-682.
Liu L.M.P., Coté C.J., Goudsouzian N.G., et al. Life-threatening apnea in infants recovering from anesthesia. Anesthesiology. 1983;59:506-510.
Luz G., Wieser C., Innerhofer P., et al. Free and total bupivacaine plasma concentrations after continuous epidural anaesthesia in infants and children. Paediatr Anaesth. 1998;8(6):473-478.
Macarthur A.J., Macarthur C., Bevan J.C. Determinants of pediatric day surgery cancellation. J Clin Epidemiol. 1995;48(4):485-489.
Madan R., Bhatia A., Chakithandy S., et al. Prophylactic dexamethasone for postoperative nausea and vomiting in pediatric strabismus surgery: a dose ranging and safety evaluation study. Anesth Analg. 2005;100(6):1622-1626.
Madan R., Perumal T., Subramaniam K., et al. Effect of timing of ondansetron administration on incidence of postoperative vomiting in paediatric strabismus surgery. Anaesth Intensive Care. 2000;28(1):27-30.
Malviya S., Fear D.W., Roy W.L., Lerman J. Adequacy of caudal analgesia in children after penoscrotal and inguinal surgery using 0.5 or 1.0 ml.kg-1 bupivacaine 0.125%. Can J Anaesth. 1992;39(5 Pt 1):449-453.
Malviya S., Swartz J., Lerman. Are all preterm infants younger than 60 weeks postconceptual age at risk for postanesthetic apnea? Anesthesiology. 1993;78(6):1076-1081.
Margolis J.O., Ginsberg B., Dear G.L., et al. Paediatric preoperative teaching: effects at induction and postoperatively. Paediatr Anaesth. 1998;8(1):17-23.
Marhofer P., Sitzwohl C., Greher M., Kapral S. Ultrasound guidance for infraclavicular brachial plexus anaesthesia in children. Anaesthesia. 2004;59(7):642-646.
Martin L.D., Pasternak L.R., Pudimat M.A. Total intraveous anesthesia with propofol in pediatric patients outside the operating room. Anes Analg. 1992;74:609-612.
Maslow A.D., Regan M.M., Israel E., et al. Inhaled albuterol, but not IV lidocaine, protects against intubation-induced bronchoconstriction in asthma. Anesthesiology. 2000;93(5):1198-1204.
Maxwell L.G., Deshpande J.K., Wetzel R.C. Preoperative evaluation of children. Pediatr Clin North Am. 1994;41(1):93-110.
Mayer J., Boldt J., Rohm K.D., et al. Desflurane anesthesia after sevoflurane inhaled induction reduces severity of emergence agitation in children undergoing minor ear-nose-throat surgery compared with sevoflurane induction and maintenance. Anesth Analg. 2006;102(2):400-404.
McAnulty G.R., Hall G.M. Anaesthesia for the diabetic patient. Br J Anaesth. 2003;90(4):428-429.
McAnulty G.R., Robertshaw H.J., Hall G.M. Anaesthetic management of patients with diabetes mellitus. Br J Anaesth. 2000;85(1):80-90.
McGarry P., Duncan C. Anesthetic risks in sickle cell trait. Pediatrics. 1973;51(3):507-512.
Mendel H.G., Guarnieri K.M., Sundt L.M., Torjman M.C. The effects of ketorolac and fentanyl on postoperative vomiting and analgesic requirements in children undergoing strabismus surgery. Anesth Analg. 1995;80(6):1129-1133.
Messner A.H. Treating pediatric patients with obstructive sleep disorders: an update. Otolaryngol Clin North Am. 2003;36(3):519-530.
Meyer R.R., Munster P., Werner C., et al. Isoflurane is associated with a similar incidence of emergence agitation/delirium as sevoflurane in young children: a randomized controlled study. Paediatr Anaesth. 2007;17(1):56-60.
Mills D.C., Lord W.D. Propofol for repeated burns dressings in a child: a case report. Burns. 1992;18(1):58-59.
Mobley K.A., Wandless J.G., Fell D. Serum bupivacaine concentrations following wound infiltration in children undergoing inguinal herniotomy. Anaesthesia. 1991;46(6):500-501.
Morrison S.G., Dominguez J.J., Frascarolo P., Reiz S. A comparison of the electrocardiographic cardiotoxic effects of racemic bupivacaine, levobupivacaine, and ropivacaine in anesthetized swine. Anesth Analg. 2000;90(6):1308-1314.
Moss I.R., Brown K.A., Laferriere A. Recurrent hypoxia in rats during development increases subsequent respiratory sensitivity to fentanyl. Anesthesiology. 2006;105(4):715-718.
Munoz H.R., Cortinez L.I., Ibacache M.E., Altermatt F.R. Remifentanil requirements during propofol administration to block the somatic response to skin incision in children and adults. Anesth Analg. 2007;104(1):77-80.
Munro H.M., D’Errico C.C., Lauder G.R., et al. Oral granisetron for strabismus surgery in children. Can J Anaesth. 1999;46(1):45-48.
Nakayama S., Furukawa H., Yanai H. Propofol reduces the incidence of emergence agitation in preschool-aged children as well as in school-aged children: a comparison with sevoflurane. J Anesth. 2007;21(1):19-23.
Newburger J.W., Rosenthal A., Williams R.G., et al. Noninvasive tests in the initial evaluation of heart murmurs in children. N Engl J Med. 1983;308(2):61-64.
Nicholl J. The surgery of infancy. Br Med J. 1909;2:753.
O’Byrne K.K., Peterson L., Saldana L. Survey of pediatric hospitals’ preparation programs: evidence of the impact of health psychology research. Health Psychol. 1997;16(2):147-154.
O’Conner M.E., Drasner K. Preoperative laboratory testing of children undergoing elective surgery. Anesth Analg. 1990;70:176-180.
Oh A.Y., Seo K.S., Kim S.D., et al. Delayed emergence process does not result in a lower incidence of emergence agitation after sevoflurane anesthesia in children. Acta Anaesthesiol Scand. 2005;49(3):297-299.
Pandit U.A., Collier P.J., Malviya S., et al. Oral transmucosal midazolam premedication for preschool children. Can J Anaesth. 2001;48(2):191-195.
Patel R.I., Davis P.J., Orr R.J., et al. Single-dose ondansetron prevents postoperative vomiting in pediatric outpatients. Anesth Analg. 1997;85(3):538-545.
Patel R.I., Hannallah R.S. Preoperative screening for pediatric ambulatory surgery: evaluation of a telephone questionnaire method. Anesth Analg. 1992;75(2):258-261.
Peacock J.E., Luntley J.B., O’Connor B., et al. Remifentanil in combination with propofol for spontaneous ventilation anaesthesia. Br J Anaesth. 1998;80(4):509-511.
Pedersen C.M., Thirstrup S., Nielsen-Kudsk J.E. Smooth muscle relaxant effects of propofol and ketamine in isolated guinea-pig trachea. Eur J Pharmacol. 1993;238(1):75-80.
Pinsker M.C., Carroll N.V. Quality of emergence from anesthesia and incidence of vomiting with remifentanil in a pediatric population. Anesth Analg. 1999;89(1):71-74.
Pizov R., Brown R.H., Weiss Y.S., et al. Wheezing during induction of general anesthesia in patients with and without asthma: a randomized, blinded trial. Anesthesiology. 1995;82(5):1111-1116.
Polaner D.M., Ahuja D., Zuk J., Pan Z. Video assessment of supraglottic airway orientation through the perilaryngeal airway in pediatric patients. Anesth Analg. 2006;102(6):1685-1688.
Pradal M., Vialet R., Soula F., et al. The risk of anesthesia in the asthmatic child. Pediatr Pulmonol Suppl. 1995;11:51-52.
Purday J.P., Reichert C.C., Merrick P.M. Comparative effects of three doses of IV ketorolac or morphine on emesis and analgesia for restorative dental surgery in children. Can J Anaesth. 1996;43(3):221-225.
Quiding H., Olsson G.L., Boreus L.O., Bondesson U. Infants and young children metabolise codeine to morphine: a study after single and repeated rectal administration. Br J Clin Pharmacol. 1992;33(1):45-49.
Rachel Homer J., Elwood T., Peterson D., aRampersad S. Risk factors for adverse events in children with colds emerging from anesthesia: a logistic regression. Paediatr Anaesth. 2007;17(2):154-161.
Reyle-Hahn M., Niggemann B., Max M., et al. Remifentanil and propofol for sedation in children and young adolescents undergoing diagnostic flexible bronchoscopy. Paediatr Anaesth. 2000;10(1):59-63.
Rhodes E.T., Ferrari L.R., Wolfsdorf J.I. Perioperative management of pediatric surgical patients with diabetes mellitus. Anesth Analg. 2005;101(4):986-999. table of contents
Rice L.J., Pudimat M.A., Hannallah R.S. Timing of caudal block placement in relation to surgery does not affect duration of postoperative analgesia in paediatric ambulatory patients. Can J Anaesth. 1990;37(4 Pt 1):429-431.
Rieger A., Schroter G., Philippi W., et al. A comparison of sevoflurane with halothane in outpatient adenotomy in children with mild upper respiratory tract infections. J Clin Anesth. 1996;8(3):188-197.
Rieschke P., LaFleur B.J., Janicki P.K. Effects of EDTA- and sulfite-containing formulations of propofol on respiratory system resistance after tracheal intubation in smokers. Anesthesiology. 2003;98(2):323-328.
Rolf N., Coté C.J. Frequency and severity of desaturation events during general anesthesia in children with and without upper respiratory infections. J Clin Anesth. 1992;4(3):200-203.
Rose J.B., Brenn B.R., Corddry D.H., Thomas P.C. Preoperative oral ondansetron for pediatric tonsillectomy. Anesth Analg. 1996;82(3):558-562.
Rose J.B., Martin T.M. Posttonsillectomy vomiting: ondansetron or metoclopramide during paediatric tonsillectomy: are two doses better than one? Paediatr Anaesth. 1996;6(1):39-44.
Rose J.B., Watcha M.F. Postoperative nausea and vomiting in paediatric patients. Br J Anaesth. 1999;83(1):104-117.
Roulleau P., Gall O., Desjeux L., et al. Remifentanil infusion for cleft palate surgery in young infants. Paediatr Anaesth. 2003;13(8):701-707.
Roy W.L., Lerman J., McIntyre B.G. Preoperative hemoglobin values in minor paediatric surgery. Can J Anaesth. 1990;37(4 Pt 2):S7.
Roy W.L., Lerman J., McIntyre B.G. Is preoperative haemoglobin testing justified in children undergoing minor elective surgery? [see comments]. Can J Anaesth. 1991;38(6):700-703.
Sadhasivam S., Shende D., Madan R. Prophylactic ondansetron in prevention of postoperative nausea and vomiting following pediatric strabismus surgery: a dose-response study. Anesthesiology. 2000;92(4):1035-1042.
Saint-Maurice C., Meistelman C., Rey E., et al. The pharmacokinetics of rectal midazolam for premedication in children. Anesthesiology. 1986;65(5):536-538.
Sandstrom K., Nilsson K., Andreasson S., et al. Metabolic consequences of different perioperative fluid therapies in the neonatal period. Acta Anaesthesiol Scand. 1993;37(2):170-175.
Sartorelli K.H., Abajian J.C., Kreutz J.M., Vane D.W. Improved outcome utilizing spinal anesthesia in high-risk infants. J Pediatr Surg. 1992;27(8):1022-1025.
Schechter M.S. Technical report: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109(4):e69.
Schindler M., Swann M., Crawford M. A comparison of postoperative analgesia provided by wound infiltration or caudal analgesia. Anaesth Intensive Care. 1991;19(1):46-49.
Schofield N.M., White J.B. Interrelations among children, parents, premedication, and anaesthetists in paediatric day stay surgery. BMJ. 1989;299(6712):1371-1375.
Schreiner M.S., Nicolson S.C. Pediatric ambulatory anesthesia: fasting—before or after surgery? J Clin Anesth. 1995;7(7):589-596.
Schreiner M.S., Nicolson S.C., Martin T., Whitney L. Should children drink before discharge from day surgery? Anesthesiology. 1992;76(4):528-533.
Schreiner M.S., O’Hara I., Markakis D.A., Politis G.D. Do children who experience laryngospasm have an increased risk of upper respiratory tract infection? Anesthesiology. 1996;85(3):475-480.
Section on Anesthesiology, A. A. o. P. Evaluation and preparation of pediatric patients undergoing anesthesia. Pediatrics. 1996;98(3):502-508.
Shott S.R., Myer C.M.3rd, Cotton R.T. Efficacy of tonsillectomy and adenoidectomy as an outpatient procedure: a preliminary report. Int J Pediatr Otorhinolaryngol. 1987;13(2):157-163.
Sidman J.D., Fry T.L. Exacerbation of sickle cell disease by obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 1988;114(8):916-917.
Sinha R., Bose S., Thangaswamy C.R. Unrecognized obstructive sleep apnea in children: caught on the wrong foot and lessons learnt!!!. Paediatr Anaesth. 2008;18(10):984-986.
Slovik Y., Tal A., Shapira Y., et al. Complications of adenotonsillectomy in children with OSAS younger than 2 years of age. Int J Pediatr Otorhinolaryngol. 2003;67(8):847-851.
Sneyd J.R., Carr A., Byrom W.D., Bilski A.J. A meta-analysis of nausea and vomiting following maintenance of anaesthesia with propofol or inhalational agents. Eur J Anaesthesiol. 1998;15(4):433-445.
Splinter W.M., Bass J., Komocar L. Regional anaesthesia for hernia repair in children: local vs caudal anaesthesia. Can J Anaesth. 1995;42(3):197-200.
Splinter W.M., Rhine E.J., Roberts D.W., et al. Preoperative ketorolac increases bleeding after tonsillectomy in children. Can J Anaesth. 1996;43(6):560-563.
Splinter W.M., Roberts D.J. Dexamethasone decreases vomiting by children after tonsillectomy. Anesth Analg. 1996;83(5):913-916.
Splinter W.M., Smallman B., Rhine E.J., Komocar L. Postoperative sore throat in children and the laryngeal mask airway. Can J Anaesth. 1994;41(11):1081-1083.
Statham M.M., Elluru R.G., Buncher R., Kalra M. Adenotonsillectomy for obstructive sleep apnea syndrome in young children: prevalence of pulmonary complications. Arch Otolaryngol Head Neck Surg. 2006;132(5):476-480.
Steward D.L., Welge J.A., Myer C.M. Steroids for improving recovery following tonsillectomy in children. Cochrane Database Syst Rev. (1):2003. CD003997
Stewart J.T., Warren F.W., Maddox F., et al. The stability of remifentanil hydrochloride and propofol mixtures in polypropylene syringes and polyvinylchloride bags at 22o-24oC. Anesthesia & Analgesia. 2000;90(6):1450sP1451.
Strauss S.G., Lynn A.M., Bratton S.L., Nespeca M.K. Ventilatory response to CO2 in children with obstructive sleep apnea from adenotonsillar hypertrophy. Anesth Analg. 1999;89(2):328-332.
Subcommittee on Obstructive Sleep Apnea American Academy of Pediatrics. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109(4):704-712.
Subramaniam B., Madan R., Sadhasivam S., et al. Dexamethasone is a cost-effective alternative to ondansetron in preventing PONV after paediatric strabismus repair. Br J Anaesth. 2001;86(1):84-89.
Subramaniam R., Subbarayudu S., Rewari V., et al. Usefulness of pre-emptive peribulbar block in pediatric vitreoretinal surgery: a prospective study. Reg Anesth Pain Med. 2003;28(1):43-47.
Suresh S., Barcelona S.L., Young N.M., et al. Postoperative pain relief in children undergoing tympanomastoid surgery: is a regional block better than opioids? Anesth Analg. 2002;94(4):859-862. table of contents
Suresh S., Chan V.W. Ultrasound guided trans vs. abdominis plane block in infants, children and adolescents: a simple procedural guidance for their performance. Paediatr Anaesth. 2009;19(4):296-299.
Sury M.R., Black A., Hemington L., et al. A comparison of the recovery characteristics of sevoflurane and halothane in children. Anaesthesia. 1996;51(6):543-546.
Tait A.R., Knight P.R. The effects of general anesthesia on upper respiratory tract infections in children. Anesthesiology. 1987;67(6):930-935.
Tait A.R., Malviya S., Voepel-Lewis T., et al. Risk factors for perioperative adverse respiratory events in children with upper respiratory tract infections. Anesthesiology. 2001;95(2):299-306.
Tait A.R., Pandit U.A., Voepel-Lewis T., et al. Use of the laryngeal mask airway in children with upper respiratory tract infections: a comparison with endotracheal intubation. Anesth Analg. 1998;86(4):706-711.
Tait A.R., Voepel-Lewis T., Munro H.M., et al. Cancellation of pediatric outpatient surgery: economic and emotional implications for patients and their families. J Clin Anesth. 1997;9(3):213-219.
Tamura M., Nakamura K., Kitamura R., et al. Oral premedication with fentanyl may be a safe and effective alternative to oral midazolam. Eur J Anaesthesiol. 2003;20(6):482-486.
Terasaki F., Kitaura Y., Hayashi T., et al. Arrhythmias in Coxsackie B3 virus myocarditis. Continuous electrocardiography in conscious mice and histopathology of the heart with special reference to the conduction system. Heart Vessels Suppl. 1990;5:45-50.
Tobias J.D., Burd R.S., Helikson M.A. Apnea following spinal anaesthesia in two former pre-term infants. Can J Anaesth. 1998;45(10):985-989.
Tripi P.A., Palmer J.S., Thomas S., Elder J.S. Clonidine increases duration of bupivacaine caudal analgesia for ureteroneocystostomy: a double-blind prospective trial. J Urol. 2005;174(3):1081-1083.
Tsai P.S., Hsu Y.W., Lin C.S., et al. Ketamine but not propofol provides additional effects on attenuating sevoflurane-induced emergence agitation in midazolam premedicated pediatric patients. Paediatr Anaesth. 2008;18(11):1114-1115.
Valley R.D., Freid E.B., Bailey A.G., et al. Tracheal extubation of deeply anesthetized pediatric patients: a comparison of desflurane and sevoflurane. Anesth Analg. 2003;96(5):1320-1324. table of contents
Vangerven G., Van Hemelrijch J., Wouters P., et al. Light anesthesia with propofol for paediatric MRI. Anaesthesia. 1992;47:706-707.
Veverka T.J., Henry D.N., Milroy M.J., et al. Spinal anesthesia reduces the hazard of apnea in high-risk infants. Am Surg. 1991;57(8):531-534. discussion 534-535
Vichinsky E.P., Haberkern C.M., Neumayr L., et al. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. The Preoperative Transfusion in Sickle Cell Disease Study Group [see comments]. N Engl J Med. 1995;333(4):206-213.
Viitanen H., Annila P., Viitanen M., Yli-Hankala A. Midazolam premedication delays recovery from propofol-induced sevoflurane anesthesia in children 1-3 yr. Can J Anaesth. 1999;46(8):766-771.
Viitanen H., Annila P., Viitanen M., Tarkkila P. Premedication with midazolam delays recovery after ambulatory sevoflurane anesthesia in children. Anesth Analg. 1999;89(1):75-79.
Villeret I., Laffon M., Duchalais A., et al. Incidence of postoperative nausea and vomiting in paediatric ambulatory surgery. Paediatr Anaesth. 2002;12(8):712-717.
Voepel-Lewis T.S., Tait M., Tate A.R. A prospective cohort study of emergence agitation in the pediatric postanesthesia care unit. Anesth Analg. 2003;96(6):1625-1630.
Warner L.O., Teitelbaum D.H., Caniano D.A., et al. Inguinal herniorrhaphy in young infants: perianesthetic complications and associated preanesthetic risk factors. J Clin Anesth. 1992;4(6):455-461.
Warner M., Kunkel S., Offord K., et al. The effects of age, epinephrine, and operative site on duration of caudal analgesia in pediatric patients. Anesth Analg. 1987;66:995-998.
Watcha M.F., Thach B.T., Gunter J.B. Postoperative apnea after caudal anesthesia in an ex-premature infant. Anesthesiology. 1989;71(4):613-615.
Webster A.C., McKishnie J.D., Kenyon C.F., Marshall D.G. Spinal anaesthesia for inguinal hernia repair in high-risk neonates [see comments]. Can J Anaesth. 1991;38(3):281-286.
Weinstein M.S., Nicolson S.C., Schreiner M.S. A single dose of morphine sulfate increases the incidence of vomiting after outpatient inguinal surgery in children. Anesthesiology. 1994;81(3):572-577.
Welborn L.G., de Soto H., Hannallah R.S., et al. The use of caffeine in the control of post-anesthetic apnea in former premature infants. Anesthesiology. 1988;68(5):796-798.
Welborn L.G., Hannallah R.S., Fink R., et al. High-dose caffeine suppresses postoperative apnea in former preterm infants. Anesthesiology. 1989;71(3):347-349.
Welborn L.G., Hannallah R.S., Luban N.L. Anemia and postoperative apnea in former preterm infants. Anesthesiology. 1991;74(6):1003-1006.
Welborn L.G., Hannallah R.S., Norden J.M., et al. Comparison of emergence and recovery characteristics of sevoflurane, desflurane, and halothane in pediatric ambulatory patients. Anesth Analg. 1996;83(5):917-920.
Welborn L.G., Ramirez N., Oh T.H., et al. Postanesthetic apnea and periodic breathing in infants. Anesthesiology. 1986;65(6):658-661.
Wheeler M., Patel A., Suresh S., et al. The addition of clonidine 2 microg.kg-1 does not enhance the postoperative analgesia of a caudal block using 0.125% bupivacaine and epinephrine 1:200,000 in children: a prospective, double-blind, randomized study. Paediatr Anaesth. 2005;15(6):476-483.
Williams O.A., Hills R., Goddard J.M. Pulmonary collapse during anaesthesia in children with respiratory tract symptoms. Anaesthesia. 1992;47(5):411-413.
Yentis S.M., Levine M.F., Hartley E.J. Should all children with suspected or confirmed malignant hyperthermia susceptibility be admitted after surgery? A 10-year review. Anesth Analg. 1992;75(3):345-350.
Zwass M.S., Fisher D.M., Welborn L.G., et al. Induction and maintenance characteristics of anesthesia with desflurane and nitrous oxide in infants and children. Anesthesiology. 1992;76(3):373-378.