CHAPTER 32 Anesthesia for Pediatric Dentistry
In light of the advances in health care, dental disease is still among the most prevalent of diseases, according to the Centers for Disease Control and Prevention (CDC). Although dental caries (tooth decay) is largely preventable, it remains the most common chronic disease of children aged 6 to 11 years, and of adolescents aged 12 to 19 years. Tooth decay is more common than many other common chronic diseases of childhood including asthma. It is four times more common than asthma among adolescents aged 14 to 17 years (CDC Division of Oral Health). Maternal nutritional and behavioral influences are very strong factors that propagate transmission of caries from the mother to her infant (American Academy of Pediatrics, 2003). The impact of caries is pervasive; poor nutrition may cause them or be the result of them. However, fluoridation of community water supplies, use of children’s vitamins containing fluoride, and increased awareness of dental hygiene have produced a significant reduction in dental caries in the general population.
Despite advances in preventive dentistry, some conditions still require more than local anesthesia to facilitate dental treatment. General anesthesia may be required to treat children with severe systemic disease or disabling congenital anomalies, as well as infants and toddlers with milk-bottle caries who require partial or complete oral rehabilitation. General anesthesia may also be required for children and adolescents with severe developmental delay who require a safe and effective environment to render the necessary dental treatment. In addition, the fearful or combative child may require procedural sedation when behavior modification techniques have not succeeded. Proper care for these populations necessitates a care team approach, consisting of properly trained anesthesia and dental providers. A glossary of commonly used dental terms is shown in Table 32-1.
TABLE 32-1 Glossary of Common Dental Terms
| Proper Name | Common Name or Definition |
| Abutment | Tooth or teeth on either side of an edentulous area supporting a bridge |
| Amalgam | Silver-coated restoration |
| Bicuspid | Premolar tooth (older term) |
| Bitewing | Dental radiograph that views several adjacent maxillary and mandibular teeth simultaneously; especially useful in evaluating dental caries |
| Bruxism | Involuntary tooth grinding |
| Burr | Drill bit used to prepare a tooth for caries restoration |
| Caries | Dental cavity or cavities |
| Composite | Tooth-colored restoration |
| Crown | Portion of the tooth seen in the mouth above the gum line; also, term used for the dental restoration of the same anatomic region; popularly known as a cap |
| Cuspid | Canine tooth (older term) |
| Diastemata | Separations between the teeth; commonly seen between the maxillary central incisors |
| Dry socket | Nonhealing extraction site |
| Endodontic therapy | Root canal therapy |
| Exfoliation | Spontaneous loss of a tooth |
| Exodontia | Dental extraction |
| Eye tooth | Canine tooth (familiar term) |
| Gingivitis | Inflammation of superficial aspects of the peridontium |
| Handpiece | Dental drill |
| Ludwig’s angina | Dental infection of the floor of the mouth involving the submandibular, submaxillary, and submental spaces bilaterally |
| Milk tooth | Primary or baby tooth |
| Occlusion | Patient’s “bite” |
| Oral prophylaxis | Dental cleaning |
| Overbite | Degree of vertical overlap of the maxillary teeth over the mandibular teeth |
| Overjet | Degree of horizontal projection of the maxillary teeth beyond the mandibular teeth |
| Periapical | Area surrounding the apex of the root; a periapical dental radiograph also includes the clinical crown of the tooth |
| Periodontium | Soft and hard tissues surrounding and supporting teeth |
| Pulpotomy | Therapeutic removal of the coronal portion of the dental pulp |
| Pyorrhea | Common name for periodontal inflammation, or gum disease; except for gingivitis, periodontal disease is rare in children |
| Rubber dam | Square latex or vinyl sheet used to isolate the teeth from the oral cavity during dental treatments |
Human dentition
Dental Development
Initial calcification of the primary tooth buds may be seen in the fourth month of prenatal life. In general, by the end of the sixth prenatal month, all of the primary teeth have begun to develop. The newborn infant is edentulous, with the rare exception of a mandibular central incisor. This natal or neonatal tooth tends to be quite mobile, and in the past it was thought to require immediate extraction. Recent data suggest that by the end of the neonatal period, this mobile tooth becomes quite stable and capable of normal masticatory function. This is indeed fortunate for the infant, because these neonatal teeth are frequently the only primary teeth that develop in that position (King and Lee, 1989; Cunha et al., 2001).
The sequence of eruption of human teeth may critically affect infant feeding, behavioral, and masticatory skills. Major changes in the appearance of the dentition in the oral cavity probably alter important aspects of neurobehavioral development (Wright, 2000). As an example of eruption sequence alterations, premature infants and neonates requiring prolonged orotracheal intubation have significant defects in both oral and dental structures that may persist past age 5 years, even after removal of the orotracheal tube (Fadavi et al., 1992).
The order of appearance of the teeth in the oral cavity tends to follow generalized patterns (Table 32-2). Usually the teeth erupt in pairs. A mandibular right central incisor erupts at approximately the same time as the mandibular left central incisor, at approximately 6 to 7 months of age. The mandibular teeth usually precede their maxillary counterparts; the maxillary incisors erupt approximately 1 month later than the mandibular incisors. The eruption sequence continues and is usually complete by age 2 to 2½ years. The last tooth to erupt is the deciduous second molar, called the 2-year molar because of its appearance at age 2 years.
| Approximate Age Eruption Begins | Approximate Age Eruption Is Completed | |
| Primary Dentition | ||
| Maxillary | ||
| Central incisor | 7½ mo | 1½ yr |
| Lateral incisor | 9 mo | 2 yr |
| Cuspid | 18 mo | 3¼ yr |
| First molar | 14 mo | 2½ yr |
| Second molar | 24 mo | 3 yr |
| Mandibular | ||
| Central incisor | 6 mo | 1½ yr |
| Lateral incisor | 7 mo | 1½ yr |
| Cuspid | 16 mo | 3¼ yr |
| First molar | 12 mo | 2¼ yr |
| Second molar | 20 mo | 3 yr |
| Permanent Dentition | ||
| Maxillary | ||
| Central incisor | 7–8 yr | 10 yr |
| Lateral incisor | 8–9 yr | 11 yr |
| Cuspid | 11–12 yr | 13–15 yr |
| First bicuspid | 10–11 yr | 12–13 yr |
| Second bicuspid | 10–12 yr | 12–14 yr |
| First molar | 6–7 yr | 9–10 yr |
| Second molar | 12–13 yr | 14–16 yr |
| Mandibular | ||
| Central incisor | 6–7 yr | 9 yr |
| Lateral incisor | 7–8 yr | 10 yr |
| Cuspid | 9–10 yr | 12–14 yr |
| First bicuspid | 10–12 yr | 12–13 yr |
| Second bicuspid | 11–12 yr | 13–14 yr |
| First molar | 6–7 yr | 9–10 yr |
| Second molar | 11–13 yr | 14–15 yr |
From Schour I, Massler M: The development of the human dentition, JADA 28:1153, 1941. Reprinted by permission of ADA Publishing.
When completed, the primary dentition totals 20 teeth (Wright, 2000). As the toddler’s growth continues, the mandible and maxilla enlarge, causing separations, also known as diastemata, between the primary teeth (Zwemer, 1993). The diastemata increase as the primary teeth are beginning to exfoliate and the permanent or succedaneous teeth begin to erupt. The separations also permit sufficient room for the proper alignment of the permanent dentition.
The maintenance of the health and hygiene of the primary teeth is essential to avoid premature tooth loss. When primary teeth are prematurely lost as a result of decay or trauma, the space needed for the permanent tooth eruption is also lost because the natural tendency of the tooth is to tip mesially (toward the midline) in the oral cavity. Subsequently, dental malocclusions tend to occur. Finally, the primary teeth may also function as the permanent teeth if the permanent analogous tooth fails to develop (Wright, 2000).
Development of the secondary, or permanent, dentition begins at birth with calcification of the buds of the permanent first molars. The permanent dentition begins its eruption pattern with the permanent first molar, usually at approximately 6 years of age. Like their primary counterparts, the mandibular teeth usually precede the maxillary teeth, with the permanent mandibular incisors beginning to appear at approximately age 6 to 7 years. Unlike the primary dentition, where there is usually a variability of several months in the timing of eruption, the permanent teeth may vary as much as 1 to 2 years in eruption sequence. After eruption of the permanent first molars, eruption of the remaining permanent teeth then occurs in this sequence: mandibular central incisors, maxillary central incisors, mandibular lateral incisors, maxillary lateral incisors, mandibular cuspids, maxillary and mandibular first premolars, maxillary and mandibular second premolars, maxillary cuspids, and mandibular and maxillary second molars (see Table 32-2). At the completion of the eruption sequence, the permanent dentition consists of 32 teeth (Wright, 2000).
In addition to the frequently absent third molars, two other permanent tooth forms are sometimes congenitally absent. The mandibular premolars and the maxillary lateral incisors may be congenitally absent, either singly or in symmetric pairs (Neville et al., 2002). Occasionally, a tooth that is thought to be congenitally absent is actually impacted in the soft tissues or alveolar bone.
Just as there are congenitally absent teeth, there are supernumerary or accessory teeth. The most common supernumerary tooth is the mesiodens, a conically shaped tooth consistently located in the midline between the maxillary central incisors. Other supernumerary teeth are the third premolars and fourth maxillary molars (Neville et al., 2002).
Dental Identification
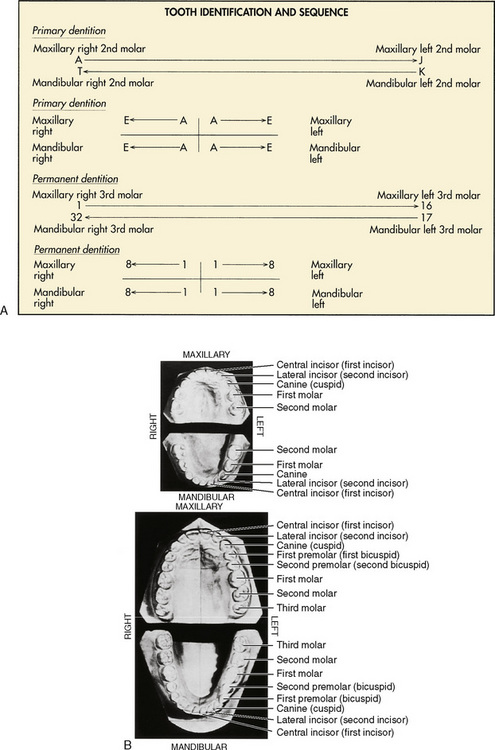
There are two principal universal dental identification systems. In both systems, the primary teeth are designated by letters, and the permanent teeth are designated by numbers. These systems differ in the way that the dental arches (mandible and maxilla) are divided. The first system uses a sequential means for identification, with the primary maxillary right second molar designated as tooth A and followed sequentially around the contralateral side of the maxilla to the left second molar, which is tooth J. The primary mandibular left second molar is tooth K, and the sequence is completed on reaching the mandibular right second molar, tooth T. Similarly, the numbering system for the permanent dentition starts with the maxillary right third molar as tooth 1 and continues to the maxillary left third molar, tooth 16. The sequence continues with the mandibular left third molar, tooth 17, and is completed with the mandibular right third molar, tooth 32 (Herlich, 1990). Both pediatric and general dentists commonly use this system of tooth identification.
The second designation system divides the dental arch into quadrants. All primary central incisors are tooth A and follow distally or posteriorly, so that all primary second molars are tooth E. To make the designation more specific, the quadrant is also named. For example, the primary maxillary right lateral incisor is designated maxillary right B. Similarly, the permanent dentition is divided into quadrants. All central incisors are tooth 1 and continue posteriorly, so that all third molars are tooth 8. This system is most commonly used by orthodontists (Fig. 32-1).
Dental Anatomy and Physiology
The tooth is composed of a crown, which is usually visible for clinical examination, and a root, which is not seen during routine clinical examination. They are separated by the cementoenamel junction or cervical region of the tooth (Fig. 32-2). The cementoenamel junctions are seen more commonly in adult dentition if gingival (“gum”) recession occurs. The crown is responsible for the slicing, ripping, and grinding of foodstuffs (incisors, canines, and molars, respectively). The root structure imparts stability to the tooth in its surrounding tissues. The anterior teeth, the incisors and the canines, are single rooted with a conical shape. The posterior teeth, the premolars and molars, are multirooted and impart most of their stability by both the number of roots and the subtly divergent directions in which the roots may grow.
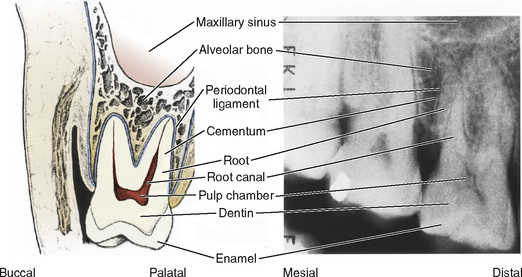
FIGURE 32-2 Schematic (left) and radiographic (right) views of a right maxillary molar.
(Modified with permission from Ash MM Jr, editor: Wheeler’s dental anatomy, physiology, and occlusion, ed 7, Philadelphia, 1993, Saunders, p 6.)
The individual teeth are composed of enamel, dentin, dental pulp, and cementum (Wright, 2000) (see Fig. 32-2). The enamel covers the external surface of the dental crown. It is the hardest substance in the human body and, unlike bone, has no living cells. When intact, enamel functions as a thermal insulator and an impervious barrier to chemicals and microorganisms.
Another difference between the sets of dentition rests in the color of the enamel. The primary teeth are milky white, or opalescent; hence, the name milk teeth. The permanent teeth, on the other hand, are significantly less “milky” because pigment absorption has occurred during their development or has been acquired during the intraoral lifetime of the tooth (Wright, 2000). Two examples are tetracycline staining (developmental) and caffeine staining (acquired).
The pulp chambers of the primary teeth are larger than the permanent teeth because of the relative thinness of the deciduous enamel and dentin (Wright, 2000). Less than meticulous dental restorations or large carious lesions may predispose the primary teeth to pulpal or endodontic therapy earlier than their permanent counterparts.
The root structures of the primary molars are more saber shaped and extend laterally beyond the crown width. This unique root structure allows adequate room for the permanent tooth bud to develop and mature until the exfoliative process is completed for the primary tooth (Wright, 2000).
Dental pathophysiology
Orthodontic Pathology
Malocclusion is the improper alignment of the teeth and jaws or bad bite. A skeletal malocclusion describes a malalignment between the jaws. A dental malocclusion describes the interarch relationship between the upper and lower teeth. A malocclusion may be caused by both hereditary and environmental factors. Crowding, spacing, supernumerary teeth, hypodontia, and asymmetric jaw growth are determined mostly by inheritance. Environmental factors such as thumb sucking, dental caries, premature loss of primary teeth, and trauma also can contribute to malocclusion (Mossey, 1999). An untreated malocclusion can lead to tooth decay, periodontal disease, abnormal wear of teeth, difficulty in chewing and speaking, and poor self-image.
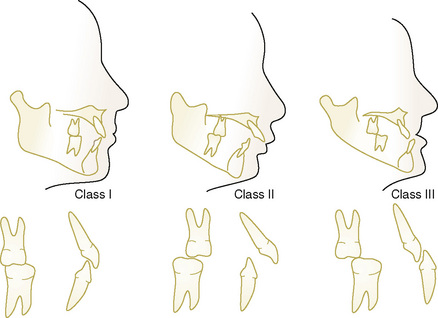
Edward H. Angle, who is regarded as the father of orthodontics, developed a classification system for malocclusion based on the sagittal (anteroposterior) relationship of the teeth and jaws (Angle, 1899). Angle believed the upper first molars were critical to occlusion, and that the upper and lower molars should meet so that the mesiobuccal cusp of the upper molar contacts in the buccal groove of the lower molar. If this molar relationship existed and the teeth were arranged on a smoothly curving line of occlusion, then normal occlusion would result (Fig. 32-3). Angle’s classification system is as follows:
Whether orthodontic treatment should occur in one or two phases of treatment is a source of controversy. Phase I, or early orthodontic treatment, may correct a problem when the patient still has primary teeth or has a combination of primary and permanent teeth. Some practitioners believe it may keep more serious problems from developing, and it may make treatment at a later age shorter or less complicated. Typically, early treatment involves the use of materials and techniques to guide or modify growth as adult teeth are erupting (Kluemper et al., 2000)
Appliances used in early treatment may be removable or fixed (cemented). They may be made of metal, ceramic, or acrylic. Examples of appliances used to address transverse discrepancies include palatal expanders, quad helixes, and transpalatal arches (Fig. 32-4). Headgear therapy may be used to treat anteroposterior discrepancies (class II or class III malocclusions) and are composed of a removable and fixed component. A lower lingual holding arch is a banded appliance used to maintain space for the eruption of permanent teeth in the mandibular arch (Fig. 32-5). Functional appliances change the position of the lower jaw to produce movement of teeth and modification of growth. Examples of functional appliances include the Twin block, Herbst appliance (Fig. 32-6), and Bionator.
Phase II or active treatment involves placement of fixed appliances (braces) or Invisalign in the permanent dentition (Boyd, 2008). Treatment times vary with several factors, including the severity of the problem being corrected, patient response to treatment, and patient compliance. Temporary anchorage devices (Fig. 32-7) are implants used to provide absolute anchorage and help move teeth (Cope, 2005). Patients use retainers to keep teeth in their new positions after treatment.
Combined surgical orthodontic treatment is used to treat patients with severe malocclusions or craniofacial anomalies. Presurgical orthodontic treatment is used to prepare patients for surgery by removing dental compensations and upright teeth over basal bone (Proffit and Miguel, 1995). In a traditional orthognathic procedure, a surgical splint may be fabricated and wired to the patient’s teeth. This splint may help the surgeon determine the final position of the jaws.
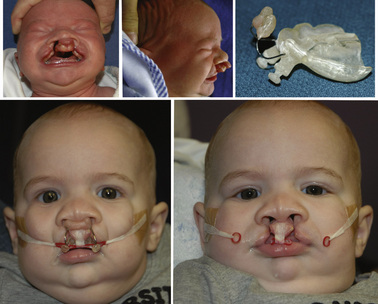
Nasoalveolar molding (NAM) is a presurgical orthopedic technique used to reduce the severity of the cleft deformity in infant patients with complete unilateral or bilateral cleft lip and palate (Grayson et al., 1999). The appliance is composed of a removable acrylic plate fabricated from an intraoral impression, and a nasal stent (Fig. 32-8). The amount of acrylic is sequentially reduced to decrease the separation between the greater and lesser alveolar segments. Once the gap between the alveolar segments is 5 mm or less, the nasal stent is added to mold the nasal cartilage. Lip or nasal repair occurs at 4 to 6 months of age. Patients with NAM can present for cleft lip surgery or ear, nose, and throat procedures such as myringotomy and tubes. The NAM appliance can be left in place during inhalation induction and mask ventilation, or it can be removed before the beginning of induction. The device should be removed before airway instrumentation for a laryngeal mask airway (LMA) or endotracheal tube.
Patient spectrum
The physically handicapped patient most likely to appear for pedodontal treatment has athetoid cerebral palsy, postencephalitis syndrome, profound mental retardation, or autistic behavior (Dougherty et al., 2001; Shenkin et al., 2001; Waldman and Perlman, 2001). For example, patients with cerebral palsy may be wheelchair bound and have significant difficulty in controlling athetoid motion. The use of nitrous oxide, which depresses involuntary movement, may ensure a higher success rate in dental treatments for patients with cerebral palsy (Kaufman et al., 1991). The pedodontist is specially trained to deal with these problems in the kindest and most expedient methods available regardless of the clinical setting (Rosenstein, 1978; Pope and Curzon, 1991).
The pediatric dentist is routinely called on to treat the medically compromised child or adolescent when the general practitioner is reluctant to get involved in treatment. For example, the pediatric dentist primarily treats the child with congenital heart disease, the insulin-dependent diabetic, the patient with craniofacial anomalies, or the child with oncologic diseases in conjunction with the pediatrician or primary care physician. Both fear and lack of training may cause the general dentist to feel quite uncomfortable in treating the compromised child or adolescent. In addition, the general dental practitioner frequently lacks the physical resources, such as specialized equipment, to care for these patients. The pediatric dentist is usually quite comfortable in recommending and prescribing antibiotic prophylaxis for subacute bacterial endocarditis (SBE), for example, and keeps current with appropriate timing in dosage, effectiveness, and relative risks (Wahl, 1994; Hayes and Fasules, 2001; American Academy of Pediatric Dentistry, 2002e).
Dentist’s needs and techniques
The pediatric dental patient requiring anesthesiology services usually needs many dental procedures during a single anesthetic administration. The quality of dental restorations is probably improved under general anesthesia (Tate et al., 2002; Al-Eheideb and Herman, 2003). In addition, the parents of children who have had general anesthesia for pediatric dental care have greater satisfaction than parents of children who did not have general anesthesia (Acs et al., 2001). After induction of general anesthesia and protection of the airway, the anesthesiologist, the anesthesia machine, and the anesthesia equipment cart are positioned at either the patient’s head or side.
The dentist’s first step is to obtain necessary intraoral radiographs of the teeth (periapical, bitewing, and occlusal radiographs). The dentist then performs a clinical examination. After placement of a pharyngeal pack (which should be noted on the anesthesia record), dental impressions may be taken if future orthodontic treatment is anticipated. Also, the dentist usually places a rubber dam around the dental arch to be treated. Despite its name, the rubber dam is not usually latex. Nevertheless, if the patient is latex sensitive, care must be taken to ensure that nonlatex products are being used. The rubber dam is held in place by a metal clamp that grasps the dental crown. A substantial length of dental floss or umbilical tape is tied around the clamp before its placement, to prevent inadvertent loss in the aerodigestive tract. Except for extractions and oral prophylaxis, the remainder of the treatment is performed with the rubber dam in place. Caries removal and tooth restoration take place with silver amalgam, tooth-colored composite, or preformed crowns. The rubber dam affords the dentist a dry environment in which the dental materials can cure optimally and achieve their greatest compressive and tensile strength. The rubber dam is also a barrier to protect the patient from iatrogenic dental trauma, including the accidental loss of dental materials or broken instruments and their possible entrance into the aerodigestive tract. The application of topical fluorides takes place after all of the restorative dentistry is completed with the rubber dam still in place (Mathewson and Primosch, 1995).
Pediatric dentistry may also encompass the need for oral and maxillofacial surgery. Oral and maxillofacial surgeons frequently perform these procedures after extensive training. Many oral and maxillofacial surgeons have dual dental and medical training, as well as fellowship training in head and neck surgery or plastic and reconstructive surgery. Because children may have craniofacial anomalies, including orofacial clefts, orthognathic problems, tumors, and blunt or penetrating trauma, such surgical management requires the ability to combine cosmesis, restoration of normal occlusion, and the promotion of normal growth and development of the entire facial skeleton (Kaban, 1993; Vig and Fields, 2000; Ord et al., 2002; Oza et al., 2002; Zeltser et al., 2003).
Clinical Settings for Pediatric Dentists
Most pediatric dental treatment occurs in the dental office without the need for psychological or pharmacologic intervention to address the child’s fear and anxiety. The hallmark of dental pain management is a kind practitioner and staff and the responsible use of adequate local or topical anesthesia, or both. For some patients, simple behavior modification techniques improve the level of cooperation in the dental chair. These techniques include the tell-show-do method and voice control for the fearful, hostile, or disruptive child. The tell-show-do technique involves explaining before the procedure, demonstrating the procedure outside of the child’s mouth, and then actually performing the procedure on the patient. This technique removes the fear of the unknown from the procedure (Lenchner and Wright, 1975). Voice control involves modulation of both the volume and tone of the dentist’s voice to achieve positive behavioral results (Wilson, 1994; American Academy of Pediatric Dentistry, 2008). When behavior modification is deemed necessary for a preschool child, it should be scheduled during the morning, because the child’s longest attention span and optimal level of cooperation are early in the day. Additional techniques include positive reinforcement, distraction, and parental presence (American Academy of Pediatric Dentistry, 2008). Additional behavior modification techniques have been used by the dentist to physically restrain frightened children. This protective stabilization is the restriction of the patients’ freedom of movement, with or without their permission, to decrease the risk of injury and to allow the safe completion of the treatment. Included in these techniques are passive physical restraint with a rigid board (papoose board), and active physical restraint by dental personnel. These techniques are controversial because of their potential psychological trauma and legal implications (Nathan, 1989; Wilson, 1994; Wright, 1994). Continuous monitoring of the patient is essential during protective stabilization.
Most analgesia for pediatric dental procedures is achieved by local anesthetic block. Most blocks are local infiltration in the maxillary region, or mandibular nerve blocks in the mandible. Adverse reactions to local anesthetics seldom occur when they are administered alone. Most commonly, they are related to a relative overdose or lapse in technique. However, even a low dosage, inappropriately injected, can cause palpitations, diaphoresis, or even dizziness (Kaufman et al., 2000). Rarely does vasomotor collapse occur. True allergic reactions, including allergy to metabisulfite or other preservatives of local anesthetics (e.g., paraaminobenzoic acid), are probably the smallest proportion of untoward reactions (Campbell et al., 2001).
Behavior modification may include such novel approaches as hypnosis or music therapy. Highly motivated, intelligent, attentive, or anxious children may have a good emotional and analgesic response to hypnosis when other forms of behavior modification, including pharmacologic forms, are precluded (Kleinhauz and Eli, 1993). Children as young as 3 to 4 years may be successfully hypnotized in the dental office (Lampshire, 1975). Music did not diminish pain, anxiety, or disruptive behavior in a recent study (Aitken et al., 2002), despite anecdotal beliefs of pediatric dentists and parents. Nevertheless, in this study, the patients enjoyed listening to the music and chose to listen to music in subsequent visits.
Electroanesthesia (transcutaneous electronic nerve stimulation [TENS]) has been used successfully for children in the dental office setting. TENS is reported to be effective based on several interrelated theories. These pain control theories include gate control, endorphin release, and serotonin release. For dental procedures, disposable electrode pads are placed bilaterally in the treated dental arch after drying the buccal mucosa. Using a dentally specific TENS device, a pulse rate of 110 Hz, and a pulse width of 225 microseconds in the normal mode, amplitude is slowly increased until the desired response is obtained. Twitching of the lower lip is the amplitude end point in the mandibular arch, and twitching of the orbicularis oculi is the amplitude end point in the maxillary arch. Children are instructed to raise a hand if the amplitude is too uncomfortable, and it is then diminished (te Duits et al., 1993). This technique works best in the area of restorative dentistry in the teeth that have relatively shallow lesions with respect to the dentoenamel junction (Quarnstrom, 1992; te Duits et al., 1993).
The spectrum of use of sedation is illustrated by the fact that medication, including nitrous oxide, is being used less than in previous years and studies. A study by Houpt (2002) suggests that, although more sedation is being used, it is being used by fewer practitioners on more patients in the United States.
A retrospective review of pediatric sedation management suggests that at one U.S. dental school pediatric dental clinic, nonpharmacologic behavioral management is favored more frequently because of its greater success (Eid, 2002). One British study estimated that more than 300,000 general anesthesias are still being administered for dentistry each year in Great Britain, mostly for children. The authors suggest that fewer general anesthetics are available for dentistry now than in the 1970s (Blayney et al., 1999). It is implied that most general anesthesias in Great Britain, however, as opposed to in the United States, are provided in a hospital setting.
Most pediatric dentists and those treating handicapped patients are experienced in the use of nitrous oxide and oral premedication when necessary in the dental office. The reported advantages of nitrous oxide delivered via a Goldman nasal mask include analgesia and sedation (Nathan et al., 1988). The incidence of diffusion hypoxia is minimal after the use of nitrous oxide and oxygen alone, as opposed to nitrous oxide supplementation to parenteral or oral sedatives (Quarnstrom et al., 1991; Dunn-Russell et al., 1993). Hypoxemia may occur when 30% to 50% nitrous oxide is added to chloral hydrate sedation (Litman et al., 1998b). In a recent study, nasal midazolam and nitrous oxide resulted in satisfactory sedation in 96% of the pediatric patients without any clinically relevant oxygen desaturation (Wood, 2010). However, in children with enlarged tonsils, oral midazolam (0.5 mg/kg) and 50% nitrous oxide resulted in significant upper airway obstruction and implied hypoxia (Litman et al., 1998a).
The reports of severe adverse outcomes include hypoxic brain damage and occasional deaths, with the use of nitrous oxide, local anesthesia, and other premedicants. Anesthesiologists should have an active role in the training of pediatric dentists in techniques of anxiolysis and moderate sedation. Adverse outcomes will likely be diminished under these circumstances (Herlich, 2010; Costa et al, 2010). Invariably, these adverse outcomes result from relative or absolute overdosage of one or a combination of nitrous oxide, local anesthetic, and parenteral medication (Goodson and Moore, 1983; Doyle and Goepferd, 1989; Coté et al., 2000a). Because of the widely publicized adverse outcomes of dental office sedation and general anesthesia, the trend in this type of care has been to move away from the dental office unless guidelines for deep sedation and anesthesia by the American Academy of Pediatric Dentistry are followed. These guidelines were promulgated in 1985 and restated in 2006 to promote and ensure that the public is aware and the pediatric patient is protected. The guidelines describe that the anesthesia care provider must be a separate individual with appropriate licensing, credentialing, and training to perform deep sedation and general anesthesia.
In the United Kingdom, a group of investigators performed several retrospective analyses during the 1970s and 1980s. Retrospective data obtained from a national data bank indicated that dental office deaths were infrequent, and the number decreased substantially in the second survey. The decreases in deaths probably result from two factors. First, fewer general anesthesias were being administered in the dental office. Second, the practice of the single individual being both dental practitioner and anesthetist is becoming less frequent because of warnings and suggestions from the General Dental Council of Great Britain. The anesthetist and dental practitioner are more commonly two individuals, each of whose attention is directed toward a single task. Also, the British survey indicated that the person providing anesthesia is more commonly a physician (Coplans and Curson, 1982, 1993). In the United States, closed claims morbidity and mortality involving oral surgeons show that during the period of 1988 to 1999, there were 22 deaths in the office and 136 anesthesia-related claims in total. It was calculated that the death rate was 1 in every 747,732 (0.013 in 10,000) administrations of anesthesia in the dental office (Deegan, 2001). D’Eramo and colleagues (2008) in a survey of oral maxillofacial surgeons in Massachusetts found the adult death rate to be 1 in 1,733,055 from office-based anesthesia. Data from the Pediatric Sedation Research Consortium indicated no mortality in over 49,000 propofol sedations, of which 307 were for dental procedures (Cravero et al., 2009).
Another issue with significant economic ramifications is the cost of multiple treatments in the dental office with procedural sedation as opposed to a single session in the operating room of the hospital under general anesthesia. In studying healthy patients aged 24 to 60 months, Lee and colleagues (2001) found that a patient who required more than three treatment visits with procedural sedation had more cost-effective treatment when all of the treatments were provided in a single visit under general anesthesia.
A reasonable compromise may be a well-equipped hospital or surgicenter dental clinic with standard monitoring devices and resuscitation equipment. Pediatric patients with ASA status I or II may be suitable candidates. With the use of proper equipment and appropriately trained personnel, a large diversity of patients may be safely and satisfactorily treated on an outpatient basis. If the clinic is rationally designed, including recovery areas equipped with oxygen, suction apparatus, and essential monitoring devices, the anesthesiologist can safely administer the anesthetic outside of the traditional operating room setting. It allows more efficient use of time and space as well as reduced cost. From the perspective of the parent and child, outpatient treatment permits a more rapid return to familiar surroundings and activities of daily living (Zuckerberg, 1994). Only the patients with disease or physical conditions that preclude off-site clinical practice need to be treated in the operating room in today’s environment. Some examples of the patients who may need the traditional operating room setting include those with difficult airways, those with coagulopathies, and those with complex anomalies or cardiovascular disease for whom more than standard monitoring is necessary.
Sedation and anesthesia for dental procedures
Procedural Sedation
With these goals in mind, procedural sedation of the pediatric dental patient may be considered. The foundations of pharmacologic sedation for the pediatric dental patient are monitoring standards to which all practitioners should adhere (Wilson, 2000). Minimum or moderate procedural sedation intended in the sensitive patient may become deep sedation or general anesthesia if vigilance is not applied. These standards have been promulgated by several organizations, including the ASA (2002), The American Academy of Pediatric Dentistry and American Academy of Pediatrics (McGuire, 2007), and the American Academy of Pediatric Dentistry (2002d) (Consensus Conference, National Institutes of Health, 1985; Rosenberg and Campbell, 1991; Council on Scientific Affairs, AMA, 1993). The American Academy of Pediatric Dentistry guidelines suggest that children who are status ASA III or IV should have treatment that necessitates sedation performed in a hospital environment.
Before any sedation is administered, including nitrous oxide with oxygen, appropriate fasting guidelines must be given to the parents or guardians of the patient. Data suggest that prolonged fasts meant to reduce the likelihood of vomiting and aspiration are somewhat deleterious to patient outcome. Guidelines for fasting from solid foods, milk, and milk products remain at a minimum of 8 hours. However, clear liquids, including pulpless juices, plain gelatin, and ice popsicles, are encouraged and acceptable until 2 hours before the anticipated arrival in the care facility. The pediatric patient is more cooperative and the parents are more satisfied as a result of these suggested guidelines (Schreiner, 1994). All of these guidelines are predicated on normal gastrointestinal function. If the patient has abnormal gastrointestinal function, more conservative fasting orders must be considered.
Oral, intranasal or transoral, parenteral, and rectal routes for administration of sedative medications are used during procedural sedation. Pediatric dentists have traditionally and preferentially used the oral route to administer premedication (Primosch and Bender, 2001). The old practice of having the parents administer a prescribed oral medication at home has been fraught with danger to the toddler and young child. Airway obstruction and emesis with aspiration were real complications of that practice. For reasons of safety, the practice has changed. Children are now brought to the treatment facility or dental office 1 hour ahead of the scheduled procedure time, and the oral premedication is administered under the guidance of the pediatric dentist. Two of the most popular agents have been hydroxyzine (1 to 2 mg/kg) and chloral hydrate (50 to 75 mg/kg; maximal dosage, 2 g). These agents have had a good success rate and a reasonable margin of safety. In addition, nitrous oxide may be given in conjunction with the usual administration of local anesthetic blocks (Moore et al., 1984; Shapira et al., 1992). More potent oral agents, such as ketamine, diazepam, and midazolam, are also given in the treatment facility or dental office. The practitioner must allow a reasonable time for onset of action before dental treatment (Sullivan et al., 2001). Oral midazolam has gained widespread popularity because of its reasonable margin of safety in addition to its rapid onset for either premedication before general anesthesia or as the main agent for procedural sedation (Kupietzky and Houpt, 1993; Levine et al., 1993). These agents may be given alone or with another agent (Dallman et al., 2001; Bui et al., 2002; Nathan and Vargas, 2002).
Mild oxygen desaturation has been noted and is easily treated with supplemental oxygen and repositioning of the patient’s airway. In some cases, when nitrous oxide was added to the oral premedication, the degree of hypoxemia increased (Litman et al., 1998a). The use of traditional monitors such as clinical observation, blood pressure, and pulse was clearly insufficient to assess the degree of hypoxemia. Pulse oximetry, capnography, and precordial stethoscopes have become necessary to adequately assess and prevent poor outcomes despite limitations in the pediatric dental environment (Anderson and Vann, 1988; Poiset et al., 1990; Wilson, 1990; Dunn-Russell et al., 1993). The use of supplemental oxygen also helps to reduce hypoxemia. Novel means of improving oxygenation include the delivery of supplemental oxygen via the saliva injector and the use of external nasal dilators (Milnes, 2002; Moses and Lieberman, 2003).
Intranasal or transoral administration of water-soluble agents such as ketamine, midazolam, or sufentanil produces effective sedation and premedication for procedural sedation. However, sufentanil produces a significantly high incidence of respiratory depression, even in relatively small dosages (1.0 mcg/kg) and is not recommended (Abrams et al., 1993). Midazolam (0.5 mg/kg orally, or 0.2 to 0.3 mg/kg intranasally) is ideal for creating a milieu in which the child is easily separated from the parent. It also transforms a disruptive child into a quiescent child in the dental chair with minimal desaturation (Abrams et al., 1993; Levine et al., 1993). However, once the handpiece (dental drill) was activated, the noise distracted the child sufficiently that the pediatric dentist could not efficiently treat the child (Theroux et al., 1994). Despite the popularity of oral sedation, one group of investigators found that there was no relationship between oral sedation and behavior of the children in the dental office on subsequent visits (McComb et al., 2002).
The rectal route of administration for procedural sedation and premedication has enjoyed popularity with only a small number of practitioners. Midazolam (1.0 mg/kg or even higher dosages) has been used for procedural sedation and for premedication. Onset of action usually occurs within 15 to 30 minutes (Roelofse and Van der Bijl, 1991). The main drawback to rectal administration of these agents is the risk of expulsion of the sedative and the unreliable uptake from the distal colonic mucosa.
Many drugs have been used as parenteral agents for procedural sedation in pediatric dentistry. Opioids, benzodiazepines, antihistamines, ultra-short-acting barbiturates, and dissociatives have been used successfully. All have had some negative features as well. Short-acting agents with acceptable margins of safety in dental sedation include methohexital, meperidine, ketamine, diazepam, and midazolam. Respiratory depression and concomitant hypoxia have been the recurrent theme in parenteral sedation by pediatric dentists (Allen, 1992; Coté et al., 2000a, 2000b). As previously mentioned, clinical observation was insufficient and the airway was subsequently lost. Because of the fine line between moderate procedural sedation, deep sedation, and general anesthesia, the dentist and assistant who administer procedural sedation must be experienced in recognizing and handling cardiorespiratory depression. Electrocardiography, pulse oximetry, blood pressure, capnography, and precordial stethoscope are essential monitors (American Academy of Pediatrics and American Academy of Pediatric Dentistry, 2006).
Other drawbacks to intravenous sedation in pediatric dentistry have been the potential for inflicted pain to achieve intravenous access and the lack of familiarity with drug combinations on the part of the practitioner. The child’s fear of pain from a needle puncture has been successfully addressed by inhalation of nitrous oxide in oxygen or the transmucosal administration of midazolam. Use of EMLA (eutectic mixture of local anesthetics, 2.5% lidocaine, and 2.5% prilocaine) cream or topical (ELA-Max) lidocaine cream before venipuncture has been successful in reducing or eliminating needle puncture pain (Nilsson et al., 1994; Koh et al., 2004). Also, EMLA- and lidocaine-impregnated patches have been used intraorally with varying success before local anesthetic blocks and local procedures before the insertion of rubber dam clamps (Stecker et al., 2002). The main disadvantage of the use of the transdermal local anesthetics in EMLA cream is that it requires application at least 45 minutes to 1 hour before a painful procedure. ELA-Max is faster and requires approximately 30 minutes to take effect (Koh et al., 2004).
General Anesthesia
The American Academy of Pediatric Dentistry (2002d) has issued guidelines for the indications for general anesthesia for children having dental procedures (Box 32-1). Nunn et al. (1995) also described the indications for general anesthesia in 265 pediatric patients. These indications include extensive treatment needs, behavior management issues, medically compromised children, extreme anxiety, and physical disabilities. The most common indication, accounting for 30.6% of the anesthesias, was intellectual impairment and autism. Dental phobia accounted for 21.4% of the patients.
From the American Academy of Pediatric Dentistry: Guidelines for the elective use of conscious sedation, deep sedation and general anesthesia in pediatric dental patients, Pediatr Dent 24:74, 2002.
Fear of dental procedures affects all ages. For some it may be so strong that dental visits are avoided altogether. In children, anxiety prior to a surgical or dental procedure centers on the fear of pain, loss of control, and separation from the parent or guardian. Preoperative anxiety and its risk factors are discussed in Chapter 8, Psychological Aspects of Pediatric Anesthesia, and Chapter 9, Preoperative Preparation. Children most at risk for heightened anxiety are between 1 and 5 years old and have divorced parents, previous negative medical experiences, and heightened emotionality (Kain et al, 2007).
Autism is an abnormality in neurodevelopment that results in abnormal social interactions and recurrent repetitive behavior. The diagnostic criteria include age of onset of less than 3 years old, severe abnormality of social reciprocity, severe abnormality of communication development, and restrictive, repetitive patterns of behavior and imagination (Klein and Nowak, 1998). The incidence of autism is increasing and is now estimated to be 1 to 2 per 1000 (Newschaffer et al., 2007). Autism spectrum disorders are a heterogeneous group of disorders that include autism, Asperger’s syndrome, Rett syndrome, and Pervasive Developmental Disorder-Not Otherwise Specified (PDD-NOS). Children with autism and autism spectrum disorders can be uncooperative and often require behavioral interventions, sedation, or general anesthesia to complete a dental examination or procedure.
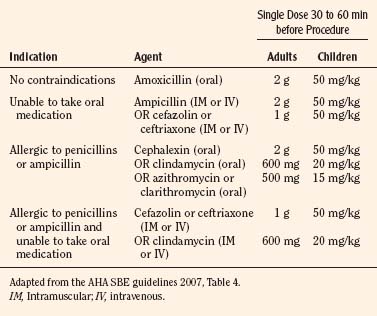
The child with significant medical disorders may require the assistance of an anesthesiologist during dental care. Some of these medical disorders may include the child with a known difficult airway, congenital cardiac disease, or multiple coexisting disorders. Children with cyanotic congenital heart disease may require SBE prophylaxis (Box 32-2). The appropriate antibiotic selection is summarized in Table 32-3. Patients with one of the cardiac conditions in Box 32-2 having dental procedures that manipulate the gingival tissue or periapical region of the teeth or perforate the oral mucosa require SBE prophylaxis. Procedures that do not require prophylaxis include routine anesthetic injections through noninfected tissue, taking dental radiographs, placement of removable prosthodontic or orthodontic appliances, adjustment of orthodontic appliances, placement of orthodontic brackets, shedding of deciduous teeth, and bleeding from trauma to the lips (Wilson et al., 2007).
Box 32-2 Indications for the Use of Subacute Bacterial Endocarditis Prophylaxis
Adapted from the AHA SBE guidelines, 2007.
Premedication
Patients presenting for general anesthesia often require a premedication to reduce anxiety and enhance cooperation. Benzodiazepines are a very effective anxiolytic. Adults or adolescents can be given diazepam. In one study, patients with autism were sedated more effectively with midazolam (0.5 mg/kg) than with diazepam (0.3 mg/kg) (Pisalchaiyong et al., 2005). In younger children, midazolam can be administered orally in a commercially flavored liquid or the concentrated intravenous solution can be given intranasally. The recommended dosage for midazolam is 0.5 mg/kg and 0.2 mg/kg for oral and nasal administration, respectively (Davis et al, 1995; Kain et al, 2000). Buccal midazolam (0.2 mg/kg) was described for dental procedures but found not to be effective (Hosey et al., 2009). Ketamine can also be administered alone or in combination with midazolam. This combination is effective and provides both anxiolysis and analgesia. The dosage of oral ketamine is 3 to 10 mg/kg. When combined with oral midazolam, the dosage is typically 3 to 6 mg/kg. This combination has been described to be very effective in patients with developmental delay. In this case report, the authors suggest using a flavored soda such as Dr. Pepper to mask the bitter flavor of the medication (Shah et al., 2009).
Clonidine has also been described as a premedicant for anxiolysis for children prior to surgical procedures. There are some significant advantages. It has no bitter taste, it may provide some analgesia, and it may decrease the incidence of emergence agitation, and it may decrease postoperative nausea and vomiting. A significant disadvantage is its slow onset time (30 to 45 minutes) (Almenrader et al., 2007; Dahmani et al., 2010). α2-Agonists have been used in patients with autism, autism spectrum disorders, and attention deficit hyperactivity disorder (ADHD) and have been shown to have some benefit (Ming et al., 2008; Scahill, 2009). Clonidine may be an effective premedication for these patients when they present for dental procedures. The dosage of oral clonidine for sedation is 4 mcg/kg.
Induction and Maintenance
There is no standard anesthetic for children having dental procedures. Patients that require a general anesthetic require medications that will allow them to remain motionless during repeated painful stimuli without feeling pain or having recall. This can be achieved with intravenous or inhalational anesthetics. The principles that guide the selection of anesthetics depend on the underlying medical disorders and the procedure being performed. In the United States, the traditional induction technique for general anesthesia in the pediatric age group has been inhalational anesthesia. The anesthesia mask is coated with a pleasant scent such as a fruit-scented lip balm. Sevoflurane, nitrous oxide, and oxygen is administered using high flows and concentrations while the anesthesiologist tells a story or employs another distraction technique. Intravenous induction techniques may include the use of a transdermal local anesthetic (ELA-Max), followed by the insertion of an intravenous cannula and subsequent administration of an appropriate intravenous agent, most commonly propofol or thiopental (Zuckerberg, 1994). Propofol can be mixed with lidocaine (1 mL of 1% lidocaine added to 9 mL of 1% propofol) to minimize local irritation and pain (see Chapter 13, Induction, Maintenance, and Recovery). Other, less commonly used techniques for the induction of general anesthesia include rectal administration of methohexital, thiopental, ketamine, or midazolam (Martone et al., 1991; Roelofse and Van der Bijl, 1991; Zuckerberg, 1994). The nasal or oral transmucosal administration of water-soluble agents such as ketamine or midazolam has also been used (Levine et al., 1993).
Once general anesthesia is induced, intravenous access and the airway are secured. All monitors may be placed before or after induction of anesthesia, depending on the cooperation of the patient. When possible, the first monitors that are placed, regardless of the timing of their placement, should be pulse oximetry and a precordial stethoscope. Careful positioning, padding, and application of thermal conservation devices must be accomplished before beginning dental treatment (see Chapter 13, Induction, Maintenance, and Recovery).
Maintenance of anesthesia for the pediatric patient having dental surgery can be accomplished with either an inhalation technique or an intravenous technique. Sevoflurane is used very commonly as an inhaled anesthetic for outpatient anesthesia. Its use has been described in children having dental procedures. Desflurane is also commonly used for outpatient procedures because of its relatively low blood gas solubility. There does not appear to be a significant advantage of either of these agents over the other for ambulatory anesthesia in terms of clinically significant discharge times. A potential concern for desflurane is its effect on airway reactivity. Patients recovering from desflurane may experience more coughing than those receiving sevoflurane (White et al., 2009). This occurs because, unlike sevoflurane, desflurane increases airway resistance in children having anesthesia. Patients with bronchial hyperreactivity (asthma, upper respiratory tract infections, and bronchopulmonary dysplasia) may be particularly prone to the bronchospastic effects of desflurane and should probably avoid this agent (von Ungern-Sternberg et al., 2008). If an intravenous agent is chosen for maintenance, an ideal agent may be propofol. It has the advantage of a short duration of action along with its antiemetic benefits (Coté, 1994). Remifentanil is also an appropriate choice.
Studies have not demonstrated benefit of one anesthetic technique over another for dental procedures. Konig et al, (2009), in a double-blind study, compared a sevoflurane-based anesthetic with a propofol-based anesthetic and found no difference in emergence delirium or postoperative pain. However, there was significantly less postoperative nausea and vomiting in the propofol group. Children with autism may have increased propofol requirements when compared with other children with intellectual impairments. In a retrospective study, Asahi and others (2009) found that an increased amount of propofol was required to achieve adequate sedation in children with autism.
Postoperative pain management for children having dental surgery is best accomplished with a multimodal technique. Intraoperatively, the dentist can place dental nerve blocks prior to dental extractions. Nonsteroidal antiinflammatory agents such as ketorolac are very effective for dental pain. The pharmacology of ketorolac has been established in infants and children. A dosage of 0.5 mg/kg provides adequate analgesic plasma levels, and it is cleared at a faster rate in infants, even in those less than 6 months old, than in adults (Lynn et al., 2007; Zuppa et al., 2009). Analgesia may also be supplemented with morphine (0.05 to 0.10 mg/kg), or fentanyl (1 to 4 mcg/kg).
Airway Management
Preparation of the nasal cavity is essential to minimize the risk of bleeding and to optimize the laryngoscopic or fiberoptic view. Once the child has been anesthetized, a mucosal vasoconstrictor such as oxymetazoline (Afrin) is applied to the nasal mucosa in both nares. The dosage for the child between 2 to 5 years of age is 2 to 3 drops of a 0.025% solution in each nostril (Mcgee et al., 2009). Oxymetazoline can be administered as drops or it can be applied via nasal cottonoids. There is controversy regarding the use of topical vasoconstrictors. Some pediatric anesthesiologists choose not to use topical vasoconstrictors (phenylephrine or oxymetazoline) because of concern about cardiac complications (e.g., pulmonary edema and cardiac arrest) (Thrush, 1995; Groudine et al., 2000). Systemic absorption of the vasoconstrictor can result in profound systemic vasoconstriction and hypertension. Pulmonary edema occurs secondary to β-blockade treatment of the hypertension.
Before introduction of the nasal endotracheal tube, each naris should be assessed to determine which is the most patent. A lubricated nasal pharyngeal airway can be passed into each naris to accomplish this. The most effective way to determine the best anatomic route for nasal intubation is to fiberoptically inspect each nares. Previously unidentified anatomic abnormalities are most likely to be found with this technique (Smith and Reid, 1999).
Other techniques to minimize bleeding during nasotracheal intubation in children include reducing the size of the endotracheal tube (ETT) by a half to a full size, warming it in warmed saline, and telescoping its end into a rubber catheter (Elwood et al., 2002; Watt et al., 2007). The incidence of bleeding appears to be reduced when the ETT is warmed in saline. The reduction in bleeding is even more pronounced when its tip is covered with a rubber catheter prior to introduction into the naris. In Watt’s study, 56% of children who had a room temperature ETT placed had clinically significant bleeding. This was reduced to 39% if the ETT was exposed to warm saline, and it was reduced even further (to 5%) when the tube was telescoped with a rubber catheter. A final technique is to use an obturator, which can be an esophageal stethoscope (Seo et al., 2007), a suction catheter (Herlich et al., 1996), or a fiberoptic scope. Beside bleeding, complications of nasotracheal intubation include bacteremia, dislodgment of adenoidal tissue, and laceration of aerodigestive mucosa with subsequent false passage. Turbinate ulceration may also occur. Fiberoptic guidance of nasotracheal intubation may reduce some of the attendant comorbidities that the anesthesiologist may face (Herlich, 1991). Once the tip of the ETT is positioned in the posterior pharynx, it can be guided into the larynx with direct laryngoscopy or with a flexible fiberoptic scope.
Nasotracheal tubes need to be secured in a way that avoids alar pressure (Fig. 32-9). Prolonged alar pressure can result in tissue ischemia and loss. In addition, the eyes must be protected and the forehead padded.
Fiberoptic intubation may become the rule rather than the exception in patients with facial trauma (Kaban, 1993), mandibulofacial dysostosis (Treacher Collins syndrome), and other congenital craniofacial anomalies (Pierre Robin sequence, Goldenhar’s syndrome). These patients frequently have palatal clefts and severe dental problems that require dental therapy and possibly orthognathic surgery (Gendelman and Herlich, 1993).
The armored version of the LMA, or flexible LMA, may be indicated for some pediatric dental patients who need dental care under general anesthesia. The advantages of the LMA are its ease of placement and tolerance in the spontaneously ventilating patient. Like an orotracheal tube, it has disadvantages, including its presence in the oral cavity, the interference with rubber dam placement, and its larger size in comparison with a standard orotracheal tube. With a skilled pediatric dentist performing restorative dentistry or surgical procedures, minimal hemorrhage may be seen on the LMA at the end of the procedure (Alexander, 1990; Webster et al., 1993).
Postoperative problems
Most postoperative problems related to pediatric dentistry are common to many other surgical procedures. Postoperative pain, prolonged emergence difficulties with voiding and ambulation, and nausea and vomiting are all seen in pediatric dental patients. However, even after brief general anesthetics for dental procedures, significant hypoxemia may be encountered that is not relieved by administering supplemental oxygen alone. A British study demonstrated that experienced postanesthesia nursing after dental procedures was the most effective means of preventing and treating hypoxemia (Lanigan, 1992).
Postoperative pain may be obviated by the early intraoperative administration of analgesics such as morphine (0.05 to 0.1 mg/kg) or fentanyl (1 to 2 mcg/kg). In addition, an acetaminophen suppository (30 to 40 mg/kg), given shortly before the end of the procedure, confers additional analgesia with minimal side effects. Oral acetaminophen (10 to 15 mg/kg) may be even more effective if given preoperatively (Yaster et al., 1994).
Postoperative nausea and vomiting have numerous causes in the pediatric dental population. A common cause is swallowed blood. Once intraoral bleeding has ceased, the nausea and vomiting from this cause usually abate. Opioid use and abdominal distention caused by bag-mask ventilation with upper airway obstruction or with excessive pressure may also produce postoperative nausea and vomiting. Nitrous oxide is a controversial cause of postoperative nausea and vomiting. A cause of emesis or nausea unique to dentistry is the inadvertent ingestion of intraoperatively administered topical fluorides to reduce dental caries (Mathewson and Primosch, 1995).
With prolonged postoperative nausea and vomiting, increased hydration and antiemetics may be administered with their attendant caveats, including bladder distention, extrapyramidal effects, and prolonged sedation (Herlich et al., 1996). Ondansetron (100 to 150 mcg/kg) is effective at lessening the severity of postoperative nausea and vomiting when administered prophylactically. Dexamethasone (0.1 to 0.2 mg/kg, up to a maximum of 10 mg) is another highly effective medication for the prevention and treatment of postoperative nausea and vomiting, especially in conjunction with ondansetron (see Chapter 13, Induction, Maintenance, and Recovery). Moderate dose metoclopramide (0.5 mg/kg) has been described prophylactically in children having tonsillectomies, but it is less effective than ondansetron (0.1 mg/kg) (Bolton et al., 2007). If the emesis is severe enough to warrant admission to the hospital for control and rehydration, the pediatric dentist requires the services of a primary care physician (presumably a pediatrician) to assume the overall management of the pediatric dental patient. Fluid and electrolyte management, as well as general patient welfare issues, may be beyond the scope and comfort level of the pediatric dentist. Patients with refractory vomiting requiring admission should have their serum sodium measured to look for hyponatremia.
Some postoperative problems appear more frequently among patients who have had dental rehabilitation, surgical removal of impacted teeth, or other surgical lesions. Postoperative hyperpyrexia seems to occur with greater frequency in patients in whom intraoral dental procedures have been performed. One group investigated preschool-age children to ascertain the etiology of such febrile states. In a randomized fashion, some children were given oral antibiotics 1 hour before their procedure. All children received general anesthesia with nasotracheal intubation and subsequent packing of the oropharynx to reduce the incidence of aspiration of gastric contents and blood. Ventilation was controlled to reduce the likelihood of atelectasis as a cause of postoperative temperature elevation. Intravenous fluid therapy was administered to both groups to reduce the contribution of dehydration as a cause of postoperative temperature elevation. The two groups were found to have equal rates of significant postoperative temperature elevation. The authors suggested that other perioperative etiologies should be investigated, including anesthetic effects on temperature regulation during dental procedures (Holan et al., 1993). There may be certain dentally induced pyrogens, or an antibiotic with a broader spectrum may be needed to cover the organism causing the hyperpyrexic bacteremia.
Nasotracheal intubation, as previously described, is the preferred method of airway protection. However, transient postoperative epistaxis is not uncommon in patients with boggy turbinates, traumatic intubation or extubation, or relatively stenotic nares (Herlich et al., 1996). Usually, direct pressure adequately treats the problem. Rarely, vasoconstrictors and intranasal packing are needed to treat the epistaxis.
Postobstruction or negative pressure pulmonary edema may be seen in children with large muscle mass or obesity. The patients are usually adolescents, but this can occur in children as young as 5 years (Van Kooy and Gargiulo, 2000; Ciavarro and Kelly, 2002).
Dental Complications of Anesthesia
The dental complications of anesthetic care are varied, usually minor, but a frequent source of malpractice claims. Most minor injuries are settled without going through malpractice litigation. The incidence of periperative dental injury is based on retrospective data and varies from 0.02% to 0.05% (Lockhart et al., 1986; Warner et al., 1999; Newland et al., 2007). In one study of a large tertiary medical center, the frequency of perianesthetic dental trauma requiring repair, stabilization, or removal of the tooth was approximately 1 in 4500 cases (Warner et al., 1999). Nevertheless, the anesthesiologist must be aware of the potential pitfalls and take appropriate safeguards. If a dental complication of anesthesia does occur, a dental consultation should be obtained as soon as possible. Also, the chief of the anesthesia department, the hospital’s risk manager, and the patient’s family should be notified. Patients old enough to understand what has transpired should also be informed.
The neonate is not immune from the dental complications of anesthesia. Laryngoscopy of the neonatal oral cavity may result in excoriation or laceration of the gum pads. Unilateral right- or left-sided hypoplastic enamel defects may be seen in the primary maxillary incisors as a result of laryngoscopy during the neonatal period (Angelos et al., 1989). Oropharyngeal airways, as well as suction devices, may also cause lacerations or excoriation of the intraoral soft tissues. Prophylactic use of water-soluble lubricants or saline solution applied to any of these devices before their placement reduces the likelihood of intraoral trauma.
Many children and their parents are aware of loose primary teeth during the preoperative visit. However, a careful examination, including a mobility check of each primary tooth before induction of general anesthesia, is appropriate (Maxwell et al., 1994).
If an excessively loose primary tooth is noted during the exfoliative phase, the parents should be informed, and it may be safely removed by the anesthesiologist once general anesthesia has been induced. In this increasingly litigious society, a separate, written consent may be necessary for removal of the loose primary tooth by the anesthesiologist. A gauze barrier is placed lingually to prevent inadvertent introduction of the tooth into more distal locations in the respiratory or gastrointestinal tract. Subsequently, a second gauze is wrapped around the loose tooth to be extracted. With a twisting and snapping action, the tooth is easily removed. The tooth is usually missing most or all of its root structure. The reason for the root structure loss is the natural resorptive processes that occur from the underlying permanent tooth that is beginning to erupt. If some of the root structure remains in the extraction site, no attempt should be made to retrieve it. The retrieval process may cause damage to the erupting permanent tooth bud. Also, the remaining root fragment naturally and harmlessly sequesters into the oral cavity (Herlich et al., 1996).
Conditions that may predispose the pediatric patient to dental avulsions under general anesthetic conditions include the scissors-like action of the anesthesiologist’s fingers in the mouth opening before laryngoscopy. If this maneuver is accomplished using the incisors, the likelihood of inadvertent avulsion is increased. The mouth-opening maneuvers should be accomplished by using the molars whenever possible to take advantage of their inherent dental stability as well as to effect the largest opening possible. The use of oropharyngeal airways in the pediatric or adult patient as a bite block should be avoided for similar reasons. The anterior teeth are single rooted. If the patient closes the mouth with excessive force, the force transmitted by the tooth is essentially perpendicular to the airway and leads to increased risk of avulsion or fracture. The ideal technique uses gauze bite blocks with a long retrieval tag placed along the molar teeth. The forces are now directed toward softer material and the multirooted molar teeth, which are more likely to sustain and evenly disperse the vertical, shear forces (Herlich, 1990; Herlich et al., 1996).
The inadvertent avulsion of loose primary teeth may nevertheless be unavoidable during airway manipulations. If a primary tooth is avulsed, it is imperative that it be retrieved. The tooth is usually found elsewhere in the mouth or outside of the oral cavity. It may also be found on the patient’s gown or bed sheets or on the floor. If it cannot be located in these likely places, anteroposterior and lateral thoracoabdominal radiographs are necessary to locate the tooth. If the tooth is found in the digestive tract, it should pass without incident within several days. If the tooth is found in the tracheobronchial tree, however, it must be retrieved by whatever means necessary, including thoracotomy. The sequelae of leaving a foreign body in the tracheobronchial tree are extremely dangerous (Herlich, 1990; Herlich et al., 1996).
Reimplantation also includes splinting of the tooth to one or two adjacent teeth on each side of the reimplanted tooth to confer stability. Despite early reimplantation, failures exist and may necessitate root canal therapy or extraction at an unspecified later date. The time course of reimplantation failure is unpredictable (Herlich, 1990), but if reimplantation occurs within 30 minutes, success may be as high as 90% (Kainuma et al., 1996).
Interceptive orthodontic appliances, such as mandibular lingual arch wires or maxillary segmental orthodontic wires, or both, are bonded by brackets to the teeth. These appliances may become loosened or avulsed during airway maneuvers. Like other prosthetic devices, they may also be recemented or bonded postoperatively with little harm to the patient or dentition (Herlich, 1990).
Head and neck radiation result in significant xerostomia (dry mouth) because of the destruction of the salivary glands. Because normal salivary flow has been eliminated, these children are at a very high risk for cervical (gumline) caries and possible dental complications during anesthesia. Regardless of the severity or nature of the injury, any head, neck, and oral trauma may predispose a patient to dental complications of anesthesia. With proper planning and care, the patient will have fewer and less severe dental complications (Herlich et al., 1996).
Summary
For questions and answers on topics in this chapter, go to “Chapter Questions” at www.expertconsult.com.
Abrams R., Morrison J.E., Villasenor A., et al. Safety and effectiveness of intranasal administration of sedative medications (ketamine, midazolam, or sufentanil) for urgent brief pediatric dental procedures. Anesth Prog. 1993;40:63.
Acs G., Pretzer S., Foley M., et al. Perceived outcomes and parental satisfaction following rehabilitation under general anesthesia. Pediatr Dent. 2001;23:419.
Aitken J.C., Wilson S., Coury D., et al. The effect of music distraction on pain, anxiety, and behavior in pediatric dental patients. Pediatr Dent. 2002;24:114.
Al-Eheideb A.A., Herman N.G. Outcomes of dental procedures performed on children under general anesthesia. J Clin Dent. 2003;27:181.
Alexander C.A. A modified Intavent laryngeal mask for ENT and dental anaesthesia [letter]. Anaesthesia. 1990;45:892.
Allen G.D. Diagnosis and treatment of respiratory problems in sedation and anesthesia in dentistry. Anesth Prog. 1992;39:150.
Almenrader N., Passariello M., Coccetti B., et al. Premedication in children: a comparison of oral midazolam and oral clonidine. Pediatric Anesthesia. 2007;17:1143-1149.
American Academy of Pediatric Dentistry. Guidelines for the elective use of conscious sedation, deep sedation, and general anesthesia in pediatric patients. ASDC J Dent Child. 1985;53:21-22.
American Academy of Pediatric Dentistry. Guideline on antibiotic prophylaxis for patients at risk. Revised. Pediatr Dent. 2002;24:107.
American Academy of Pediatric Dentistry. Guideline on behavior management. Pediatr Dent. 2002;24:68.
American Academy of Pediatric Dentistry. Guideline on prevention of bacterial endocarditis. Pediatr Dent. 2002;24:109.
American Academy of Pediatric Dentistry. Guidelines for the elective use of conscious sedation, deep sedation and general anesthesia in pediatric dental patients. Pediatr Dent. 2002;24:74.
American Academy on Pediatric Dentistry Clinical Affairs Committee, Behavior Management Subcommittee. Guideline on behavior guidance for the pediatric dental patient. Pediatr Dent. 2008;30(7):125-133.
American Academy of Pediatrics. Oral health risk assessment timing and establishment of the dental home. Pediatrics. 2003;111:1113.
American Academy of Pediatrics, American Academy of Pediatric Dentistry. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures. Pediatrics. 2006;118(6):2587-2602.
American Society of Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists: an updated report by the American Society of Anesthesiologists task force on sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004.
Anderson J.A., Vann W.F.Jr. Respiratory monitoring during pediatric sedation: pulse oximetry and capnography. Pediatr Dent. 1988;10:94.
Angelos G., Smith D.R., Jorgenson R., et al. Oral complications associated with oral tracheal intubation. Pediatr Dent. 1989;11:133.
Angle E.H. Classification of malocclusion. Dental Cosmos. 1899;41:248-264.
Asahi Y., Kubota K., Omichi S., et al. Dose requirements for propofol anaesthesia for dental treatment for autistic patients compared with intellectually impaired patients. Anaesth Intensive Care. 2009;37(1):70-73.
Blayney M.R., Malins A.F., Cooper G.M. Cardiac arrhythmias in children during outpatient general anaesthesia for dentistry: a prospective randomized trial. Lancet. 1999;354:1864.
Bolton C.M., Myles P.S., Carlin J.B., et al. Randomized, double-blind study comparing the efficacy of moderate-dose metoclopramide and ondansetron for the prophylactic control of postoperative vomiting in children after tonsillectomy. Br J Anaesth. 2007;99(5):699-703.
Boyd R.L. Esthetic Orthodontic treatment using the Invisalign appliance for moderate to complex malocclusions. J Dent Educ. 2008;72(8):948-967.
Bui T., Redden R.J., Murphy S., et al. A comparison study between ketamine and ketamine-promethazine in pediatric dental patients. Anesth Prog. 2002;49:14.
Campbell J.R., Maestrello C.L., Campbell R.L., et al. Allergic response to metabisulfite in lidocaine anesthetic solution. Anesth Prog. 2001;48:21.
Ciavarro C., Kelly J.PW. Postobstructive pulmonary edema in an obese child after an oral surgery procedure under general anesthesia: a case report. J Oral Maxillofac Surg. 2002;60:1503.
Consensus Conference, National Institutes of Health. Anesthesia and sedation in the dental office. JAMA. 1985;254:1073.
Cope J. Temporary anchorage devices in orthodontics: a paradigm shift. Semin Orthod. 2005;11(1):3-9.
Coplan M.P., Curson I. Deaths associated with dentistry and dental disease 1980–1989. Anaesthesia. 1993;48:435.
Coplans M.P., Curson I. Deaths associated with dentistry. Br Dent J. 1982;153:357.
Costa P.S., Valadao-Junior W.J., Costa L.R. Dental sedation by dentists: a view from anesthesiologists working in Central Western Brazil. Anesth Analg. 2010;110:110-114.
Coté C.J., Karl H.W., Notterman D.A., et al. Adverse sedation events in pediatrics: analysis of medications used for sedation. Pediatrics. 2000;106:633.
Coté C.J., Notterman D.A., Karl H.W., et al. Adverse sedation events in pediatrics: a critical incident analysis of contributing factors. Pediatrics. 2000;105:805.
Coté C.J. Sedation for the pediatric patient. Pediatr Clin North Am. 1994;41:31.
Council on Scientific Affairs, American Medical Association. The use of pulse oximetry during conscious sedation. JAMA. 1993;270:1463.
Cravero J., Beach M.L., Blike G.T., et al. The incidence and nature of adverse events during pediatric sedation/anesthesia with propofol for procedures outside the operating room: a report from the pediatric sedation research consortium. Anesth Analg. 2009;108:795.
Cunha R.F., Carrilho-Boer F.A., Dias-Torriani D., et al. Natal and neonatal teeth: review of the literature. Pediatr Dent. 2001;23:158.
D’Eramo E., Bontempi W.J., Howard J.B., et al. Experience among Massachusetts oral and maxillofacial surgeons. J Oral Maxillofac Surg. 2008;66:2421-2433.
Dahmani S., Brasher C., Stany I., et al. Premedication with clonidine is superior to benzodiazepines: a meta analysis of published studies. Acta Anaesthesiol Scand. 2010;54:397-402.
Dallman J.A., Ingelzi M.A.Jr, Briskie D.M., et al. Comparing the safety, efficacy and recovery of intranasal midazolam vs. oral chloral hydrate and promethazine. Pediatr Dent. 2001;23:424.
Davis P.J., Tome J.A., McGowan F.X., et al. Preanesthetic medication with intranasal midazolam for brief pediatric surgical procedures: effect on recovery and hospital discharge times. Anesthesiology. 1995;82(1):2-5.
Deegan A.E. Anesthesia morbidity and mortality, 1988–1999: Claims statistics from AAOMS National Insurance Company. Anesth Prog. 2001;48:89.
Dougherty N., Romer M., Lee R.S., et al. Trends in special care training in pediatric dental residencies. J Dent Child. 2001;68:384.
Doyle K.A., Goepferd S.J. An allergy to local anesthetics? The consequences of misdiagnosis. J Dent Child. 1989;56:103.
Dunn-Russell T., Adair S.M., Sams D., et al. Oxygen saturation and diffusion hypoxia in children following nitrous oxide sedation. Pediatr Dent. 1993;15:88.
Eid H. Conscious sedation in the 21st century. J Clin Pediatr Dent. 2002;26:179.
Elwood T., Stillions D.M., Woo D.W., et al. Nasotracheal intubation: a randomized trial of two methods. Anesthesiology. 2002;96:51-53.
Fadavi S., Adeni S., Dziedzic K., et al. The oral effects of orotracheal intubation in prematurely born preschoolers. J Dent Child. 1992;59:420.
Gendelman M.S., Herlich A. Anesthesia for oral and maxillofacial surgery. Prog Anesthesiol. 1993;7:18.
Goodson J.M., Moore P.A. Life-threatening reactions after pedodontic sedation: an assessment of narcotic, local anesthetic, and antiemetic drug interaction. J Am Dent Assoc. 1983;107:239-245.
Grayson B.H., Santiago P.E., Brecht L.E., et al. Presurgical nasoalveolar molding in infants with cleft lip and palate. Cleft Palate Craniofac J. 1999;36(6):486-498.
Groudine S.B., Hollinger I., Jones J., The Phenylephrine Advisory Committee. New York State Guidelines on the Topical Use of Phenylephrine in the Operating Room. Anesthesiology. 2000;92:859.
Hayes P.A., Fasules J. Dental screening of pediatric cardiac surgical patients. J Dent Child. 2001;68:255.
Herlich A., Garber J.G., Orkin F.K., et al. Dental and salivary complications. In: Gravenstein N., Kirby R.R., editors. Complications in anesthesiology. ed 2,. Philadelphia: Lippincott-Raven; 1996:163.
Herlich A. How do we bridge the gap? Anesth Analg. 2010;110:11-12.
Herlich A. Fiberoptic for head and neck patients. Anesthesiol Clin North Am. 1991;9:111.
Herlich A. Dental complications of anesthesia. Prog Anesthesiol. 1990;11:250.
Holan G., Kadari A., Engelhard D., et al. Temperature elevation in children following dental treatment under general anesthesia with or without prophylactic antibiotics. Pediatr Dent. 1993;15:99.
Hosey M.T., Asbury A.J., Bowman A.J., et al. The effect of transmucosal 0.2 mg/kg midazolam premedication on dental anxiety, anaesthetic induction and psychological morbidity in children undergoing general anaesthesia for tooth extraction. 2009;207(1):1-6.
Houpt M. Project USAP 2000–Use of sedative agents by pediatric dentists: a 15-year follow-up survey. Pediatr Dent. 2002;24:4.
Kaban L.B. Diagnosis and treatment of fractures of the facial bones in children 1943–1993. J Oral Maxillofac Surg. 1993;51:722.
Kain Z., MacLaren J., McClain B., et al. Effects of age and emotionality on the effectiveness of midazolam administered preoperatively to children. Anesthesiology. 2007;107(4):545-552.
Kain Z.N., Hostadter M.B., Mayes L.C., et al. Midazolam: effects on amnesia and anxiety in children. Anesthesiology. 2000;92(4):939-946.
Kainuma M., Yamada M., Miyake T., et al. Early application of the cross-suture splint to teeth avulsed at tracheal intubation [Letter]. Anesthesiology. 1996;84:1516.
Kaufman E., Goharian S., Katz Y., et al. Adverse reactions triggered by dental local anesthetics: a clinical survey. Anesth Prog. 2000;47:134.
Kaufman E., Meyer S., Wolnerman J.S., et al. Transient suppression of involuntary movements in cerebral palsy patients during dental treatment. Anesth Prog. 1991;38:200.
Klein J., Nowak A.J. Autistic disorder: a review for the pediatric dentist. Pediatr Dent. 1998;20:312-317.
Kleinhauz M., Eli I. When pharmacologic anesthesia is precluded: the value of hypnosis as a sole anesthetic agent in dentistry. Spec Care Dent. 1993;13:15.
Kluemper G.T., Beeman C.S., Hicks E.P., et al. Early orthodontic treatment: what are the imperatives? J Am Dental Assoc. 2000;131:613-620.
Koh J.L., Harrison D., Myers R., et al. A ranodomized double-blind comparison study of EMLA and ELA-Max for topical anesthesia in children undergoing intravenous insertion. Paediatr Anaesth. 2004;14(12):977-982.
Konig M.W., Varughese A.M., Brennen K.A., et al. Quality of recovery from two types of general anesthesia for ambulatory dental surgery in children: a double-blind, randomized trial. Pediatr Anesthesia. 2009;19:748-755.
Kupietzky A., Houpt M.I. Midazolam: a review of its use for conscious sedation of children. Pediatr Dent. 1993;15:237.
Lampshire E.L. Hypnosis in dentistry for children. In: Wright G.Z., editor. Behavior management in dentistry for children. Philadelphia: WB Saunders; 1975:115.
Lanigan C.J. Oxygen desaturation after dental anesthesia. Br J Anaesth. 1992;68:142.
Lee J.Y., Vann W.F.Jr, Roberts M.W., et al. A cost analysis of treating pediatric dental patients using general anesthesia versus conscious sedation. Anesth Prog. 2001;48:82.
Lenchner V., Wright G.Z. Nonpharmaco-therapeutic approaches to behavior management. In: Wright G.Z., editor. Behavior management in dentistry for children. Philadelphia: WB Saunders; 1975:91.
Levine M.F., Spahr-Schopfer I.A., Hartley E., et al. Oral midazolam premedication in children: the minimum time for separation from parents. Can J Anæsth. 1993;40:726.
Litman R.S., Kottra J.A., Berkowitz R.J., et al. Upper airway obstruction during midazolam/nitrous oxide sedation in children with enlarged tonsils. Pediatr Dent. 1998;20:318.
Litman R.S., Kottra J.A., Verga K.A., et al. Chloral hydrate sedation: the additive sedative and respiratory depressant effects of nitrous oxide. Anesth Analg. 1998;86:724.
Lockhart P.B., Feldbau E.V., Gabel R.A., et al. Dental complications during and after tracheal intubation. J Am Dent Assoc. 1986;112:480-483.
Lynn A.M., Bradford H., Kantor E.D., et al. Postoperative ketorolac tromethamine use in infants aged 6–18 months: the effect on morphine usage, safety assessment, and stereo-specific pharmacokinetics. Anesth Analg. 2007;104:1040-1051.
Martone C.H., Nagelhout J., Wolf S.M. Methohexital: a practical review for outpatient dental anesthesia. Anesth Prog. 1991;38:195.
Mathewson R.J., Primosch R.E. Fundamentals of pediatric dentistry, ed 3. Chicago: Quintessence, 1995.
Maxwell L.G., Deshpande J.K., Wetzel R.C., et al. Preoperative evaluation of children. Pediatr Clin North Am. 1994;41:93.
McComb M., Koenigsberg S.R., Broder H.L., et al. The effects of oral conscious sedation on future behavior and anxiety in pediatric dental patients. Pediatr Dent. 2002;24:3.
McGee W., Howrie D., Schmitt C., et al. Pediatric drug therapy handbook and formulary, ed 5. Department of Pharmacy, Children’s Hospital of Pittsburgh, 2009.
McGuire E.J. American Academy of Pediatric Dentistry and American Academy of Pediatrics Issue: Co-endorsed sedation guidelines. Pa Dent J. 2007;74(2):24-27.
Milnes A.R. Delivering supplemental oxygen during sedation via a saliva injector. Pediatr Dent. 2002;24:340.
Ming X., Gordon E., Kang N., et al. Use of clonidine in children with autism spectrum disorders. Brain and Development. 2008;30(7):454-460.
Moore P.A., Mickey E.A., Hargreaves J.A., et al. Sedation in pediatric dentistry: a practical assessment procedure. J Am Dent Assoc. 1984;109:564.
Moses A.J., Lieberman M. The effect of external nasal dilators on blood oxygen levels in dental patients. J Am Dent Assoc. 2003;134:97.
Mossey P.A. The hereditability of malocclusion: part 2. The influence of genetics in malocclusion. Br J Orthod. 1999;26(3):195-203.
Nathan J.E. Management of the difficult child: A survey of pediatric dentists’ use of restraints, sedation, and general anesthesia. J Dent Child. 1989;56:293.
Nathan J.E., Vargas K.G. Oral midazolam with and without meperidine for management of the difficult young pediatric dental patient: a retrospective study. Pediatr Dent. 2002;24:129.
Nathan J.E., Venham L.L., West M.S., et al. The effects of nitrous oxide on anxious young pediatric patients across sequential visits: a double-blind study. J Dent Child. 1988;55:220.
Neville B.W., Damm D.D., Allen C.M., et al. Oral and maxillofacial pathology, ed 2. Philadelphia: WB Saunders, 2002.
Newland M.C., Ellis S.J., Peers K.R., et al. Dental injury associated with anesthesia report of 161,687 anesthetics given over 14 years. J Clin Anesth. 2007;19:339-345.
Newschaffer C.J., Croen L.A., Daniels J., et al. The epidemiology of autism spectrum disorders. Annu Rev Public Health. 2007;28:235-258.
Nilsson A., Boman I., Wallin B., et al. The EMLA patch: A new type of local anaesthetic application for dermal analgesia for children. Anaesthesia. 1994;49:70.
Nunn J.H., Davidson G., Gordon P.H., et al. A retrospective review of a service to provide comprehensive dental care under general anesthesia. Spec Care Dentist. 1995;15:97-101.
Ord R.A., Blanchaert R.H.Jr, Nikitakis N.G., et al. Ameloblastoma in children. J Oral Maxillofac Surg. 2002;60:762.
Oza N., Agarwal K., Panda K.N., et al. An unusual mode of injury: implantation of a broken toothbrush medial to ramus: report of a case. J Dent Child. 2002;68:193.
Pisalchaiyong T., Trairatvorakul C., Jirakijja J., et al. Comparison of the effectiveness of oral diazepam and midazolamfor the sedation of autistic patients during dental treatment. Pediatr Dent. 2005;27(3):198-206.
Poiset M., Johnson R., Nakamura R., et al. Pulse rate and oxygen saturation in children during routine dental procedures. J Dent Child. 1990;57:279.
Pope J.EC., Curzon M.E. The dental status of cerebral palsied children. Pediatr Dent. 1991;13:156.
Primosch R.E., Bender F. Factors associated with administration route when using midazolam for pediatric conscious sedation. J Dent Child. 2001;68:233.
Proffit W.R., Miguel J.A. The duration and sequencing of surgical-orthodontic treatment. Int J Adult Orthodon Orthognath Surg. 1995;10(1):35-42.
Quarnstrom F. Electronic dental anesthesia. Anesth Prog. 1992;39:162.
Quarnstrom F.C., Milgrom P., Bishop M.J., et al. Clinical study of diffusion hypoxia after nitrous oxide analgesia. Anesth Prog. 1991;38:21.
Roelofse J.A., Van der Bijl P. Adverse reactions to midazolam and ketamine premedication in children [letter]. Anesth Prog. 1991;38:73.
Rosenberg M.B., Campbell R.L. Guidelines for intraoperative monitoring of dental patients undergoing conscious sedation, deep sedation, and general anesthesia. Oral Surg Oral Med Oral Pathol. 1991;71:2.
Rosenstein S. Dentistry in cerebral palsy and related handicapping conditions. Springfield, IL: Charles C Thomas, 1978.
Scahill L. Alpha-2 adrenergic agonists in children with inattention, hyperactivity and impulsiveness. CNS Drugs. 2009;23(Suppl 1):43-49.
Schreiner M.S. Preoperative and postoperative fasting in children. Pediatr Clin North Am. 1994;41:111.
Seo K.S., Kim J., Yang S.M., et al. A new technique to reduce epistaxis and enhance navigability during nasotracheal intubation. Anesth Analg. 2007;105:1420.
Shah S., Shah S., Apuya J., et al. Combination of oral ketamine and midazolam as a premedication for a severely autistic and combative patient. J Anesth. 2009;23:126.
Shapira J., Holan G., Guelmann M., et al. Evaluation of the effect of nitrous oxide and hydroxyzine in controlling the behavior of the pediatric dental patient. Pediatr Dent. 1992;14:167.
Shenkin J.D., Davis M.J., Corbin S.B., et al. The oral health of special needs children: dentistry’s challenge to provide care. J Dent Child. 2001;68:201.
Smith J.E., Reid A.P. Asymptomatic intranasal abnormalities influencing the choice of nostril for nasotracheal intubation. Br J Anaesth. 1999;83(6):882-886.
Stecker S.S., Swift J.Q., Hodges J.S., et al. Should a mucoadhesive patch (Dentipatch) be used for gingival anesthesia in children? Anesth Prog. 2002;49:3.
Sullivan D.C., Wilson C.FG., Webb M.D., et al. A comparison of two oral ketamine-diazepam regimens for the sedation of anxious pediatric dental patients. Pediatr Dent. 2001;23:223.
Tate A.R., Ng M.W., Needleman H.L., et al. Failure rates of restorative procedures following dental rehabilitation under general anesthesia. Pediatr Dent. 2002;24:69.
te Duits E., Goepfor S., Donly K., et al. The effectiveness of electronic dental anesthesia in children. Pediatr Dent. 1993;15:191.
Theroux M.C., West D.W., Corddry D.H., et al. Efficacy of intranasal midazolam in facilitating dental procedures in preschool children. SAMBA Mtg Abstract. 1994;9:2.
Thrush D.N. Cardiac arrest after oxymetazoline nasal spray. J Clin Anesth. 1995;7:512-514.
Van Kooy M.A., Gargiulo R.F. Postobstructive pulmonary edema. Am Fam Phys. 2000;62:401-404.
Vig K.WL., Fields H.W. Facial growth and management of orthodontic problems. Pediatr Clin North Am. 2000;47:1085.
von Ungern-Sternberg S., Saudan, Petak F., et al. Desflurane but not sevoflurane impairs airway and respiratory tissue mechanics in children with susceptible airways. Anesthesiology. 2008;108:216-224.
Wahl M.J. Myths of dental-induced endocarditis. Arch Intern Med. 1994;154:137.
Waldman H.B., Perlman S.P. Children with both mental retardation and mental illnesses live in our communities and need dental care. J Dent Child. 2001;68:360.
Warner M.E., Benefeld S.M., Warner M.A., et al. Perianesthetic dental injuries: frequency, outcomes, and risk factors. Anesthesiology. 1999;90:1302.
Watt S., Pickhardt D., Lerman J., et al. Telescoping tracheal tubes into catheters minimize epistaxis during nasotracheal intubation in children. Anesthesiology. 2007;106:238.
Webster A.C., Morley-Forster P.K., Dain S., et al. Anaesthesia for adenotonsillectomy: a comparison between tracheal intubation and the armoured laryngeal mask airway. Can J Anaesth. 1993;40:1171.
White P.F., Tang J., Wender R.H., et al. Desflurane versus sevoflurane for maintenance of outpatient anesthesia: the effect on early versus late recovery and perioperative coughing. Anesth Analg. 2009;109(2):387-393.
Wilson S. Conscious sedation and pulse oximetry: false alarms? Pediatr Dent. 1990;12:228.
Wilson S. Nonpharmacologic issues in pain perception and control. In: Pinkham J.R., editor. Pediatric dentistry: Infancy through adolescence. ed 2,. Philadelphia: WB Saunders; 1994:88.
Wilson S. Pharmacologic behavior management for pediatric dental treatment. Pediatr Clin North Am. 2000;47:1159.
Wilson W., Taubert K.A., Gewitz M., et al. Prevention of infective endocarditis: guidelines from American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, American Heart Association Council on Cardiovascular Disease in the Young; American Heart Association Council on Clinical Cardiology, American Heart Association Council on Cardiovascular Surgery and Anesthesia, Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116(15):e376-e377.
Wood M. The safety and efficacy of intranasal midazolam sedation combined with inhalation sedation with nitrous oxide and oxygen in paediatric dental patients as an alternative to general anesthesia. SAAD Dig. 2010;26:12-22.
Wright G.Z. Psychologic management of children’s behaviors. In: McDonald R.E., Avery D.R., editors. Dentistry for the child and adolescent. ed 6,. St Louis: Mosby–Year Book; 1994:32-52.
Wright J.T. Normal formation and development of defects of the human dentition. Pediatr Clin North Am. 2000;47:975.
Yaster M., Sola J.E., Pegrolli W.Jr, et al. The night after surgery: postoperative management of the pediatric outpatient-surgical and anesthetic aspects. Pediatr Clin North Am. 1994;41:199.
Zeltser R., Kalter A., Casap N., et al. Oropharyngeal impalement injuries in children: report of 2 cases. J Oral Maxillofac Surg. 2003;61:510.
Zuckerberg A.L. Perioperative approach to children. Pediatr Clin North Am. 1994;41:15.
Zuppa A.F., Mondick J.T., Davis L., et al. Population pharmacokinetics of ketorolac in neonates and infants. Am J Therapeutics. 2009;16:143-146.
Zwemer T.J. Boucher’s clinical dental terminology, ed 4. St Louis: Mosby–Year Book, 1993.