CHAPTER 26 Anesthesia for Orthopedic Surgery
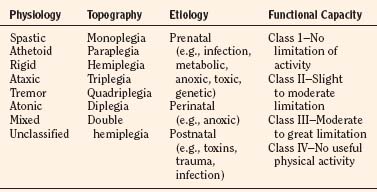
The topic of anesthesia for pediatric orthopedic surgery encompasses the entire age and medical spectrum of pediatrics and includes the newborn and adolescent, the otherwise normal, the chronically ill, the patient with multiple complex congenital anomalies, the patient with emergent trauma, the elective inpatient, and the outpatient. Orthopedic surgeons operate on virtually every area of the body from the cervical spine to the pelvis to the toes. In many instances, the perioperative anesthesia plan for pediatric orthopedic patients depends more on their ages, on the site and emergent nature of surgery, and on the need for perioperative analgesia and sedation, than it does on the underlying disease or the specifics of the surgical procedure. In other cases, the underlying medical condition, associated anomalies, pathophysiology, and surgical procedure dictate the anesthesia plan. Positioning the patient on the operating room table may be difficult because of deformities or contractures, and often patients require special operating tables or frames to achieve the best posture for surgery. The anesthesiologist must be aware of unusual associated syndromes that have clear orthopedic implications, and syndromes with underlying clinical significance unrelated to the orthopedic condition. Conditions that are commonly encountered in pediatric orthopedic surgery and their anesthesia implications are listed in Table 26-1, but the list is by no means comprehensive (Campbell, 2009). The Online Mendelian Inheritance in Man website (www.ncbi.nlm.nih.gov/omim) is a good source for this information.
TABLE 26-1 Anesthetic Implications of Commonly Encountered Orthopedic Disorders
| Disease | Surgical Interventions | Anesthetic Implications |
| Congenital Malformations | ||
| Amniotic band constriction | Soft tissue release | May have facial clefts |
| Clubfoot | Tendon lengthening, release | Dictated by associated malformations |
| Klippel-Feil syndrome | Release, scoliosis | Hemifused or fused vertebra; limited cervical spine mobility; possible difficult intubation; heart defects |
| Radial dysgenesis | Tendon lengthening, pollicization release | Episodic thrombocytopenia; congenital heart disease |
| Sprengel’s deformity | — | Associated only with Klippel-Feil syndrome |
| Trisomy 21 (Down syndrome) | Cervical spine fusion | Large tongue; usually easy intubation; in-line stabilization during intubation; congenital heart disease; opioid sensitivity |
| Acquired Conditions | ||
| Charcot-Marie-Tooth disease | Tendon transfer | Possible sensitivity with nondepolarizing muscle relaxants; succinylcholine may result in hyperkalemia |
| Legg-Calvé-Perthes disease | Osteotomies, pinning | None known |
| Osteomyelitis | Culture, aspiration | Systemic bacterial infection |
| Septic arthritis | Culture, irrigation | Systemic bacterial infection |
| Slipped femoral capital epiphysis | Pinning | Obesity |
| Tumors, benign | Excision, curettage | Possible significant blood loss; pathologic fracture |
| Tumors, malignant | Radical excision, amputation | Blood loss; metastasis: CNS, lung; chemotherapy; cardiotoxicity |
| Syndromes, Inherited Conditions | ||
| Apert syndrome and Crouzon’s disease | Syndactyly repair; craniosynostosis; hypertelorism | Airway usually normal, but occasional mandibular hypoplasia; cardiac defect |
| Ellis-van Creveld syndrome | Polydactyly | Cardiac defects; bronchial collapse |
| Holt-Oram syndrome | Tendon lengthening, pollicization release | Cardiac defects (ASD, VSD) |
| Jeune syndrome (asphyxiating thoracic dystrophy) | Chest reconstruction, scoliosis | Respiratory failure, prolonged mechanical ventilation; renal failure |
| Marfan syndrome | Scoliosis | Cardiac (AI, MR), aortic aneurysm |
| Moebius sequence | Syndactyly | Micrognathia; cleft palate; cranial nerve palsy |
| Osteogenesis imperfecta | Pathologic fractures, scoliosis | Fractures on positioning or intubation; hypermetabolic fever, platelet dysfunction; blood pressure cuff may cause fractures |
| VATER (vertebral, anal, tracheal esophageal fistula, renal, cardiac) | Tendon lengthening, pollicization release | Cardiac defects; tracheoesophageal fistula |
| Short-Stature Syndrome | ||
| Achondroplasia | Spinal fusion, cervical decompression, Ilizarov | Poor cervical mobility, difficult arterial catheterization |
| Morquio-Ullrich disease | Cervical spine fusion | Poor cervical mobility, difficult airway |
| Mucopolysaccharidoses (Hurler’s, Hunter’s, Morquio’s syndromes) | Kyphoscoliosis, bony abnormalities | Very difficult intubations; unstable necks; respiratory failure perioperatively |
| Systemic Disease | ||
| Juvenile rheumatoid arthritis | Varies | TMJ ankylosis; cervical spine immobility or instability; carditis; occasional pulmonary involvement; difficult airway |
| Neurofibromatosis | Scoliosis | CNS tumors; occasional pheochromocytoma |
| Sickle cell anemia | Osteomyelitis, Legg-Calvé-Perthes disease, pathologic fracture | Anemia; vasoocclusive crisis; acute chest syndrome; stroke; hypothermia; hypoxia; hypovolemia; immunocompromised host; avoid tourniquet when possible |
| CNS Diseases | ||
| Arthrogryposis multiplex | Tendon releases (multiple congenital contractures), scoliosis | Difficult intubation (TMJ ankylosis, cervical spine immobility); GE reflux; postoperative upper airway obstruction; congenital heart disease |
| Cerebral palsy | Tendon releases | GE reflux; postoperative upper airway obstruction |
| Myelomeningocele | Lower extremity tendon releases, scoliosis, kyphosis | Hydrocephalus |
| Werdnig-Hoffmann disease | Scoliosis | Respiratory insufficiency; bulbar involvement (poor secretion handling); succinylcholine-induced hyperkalemia |
| Myopathies | ||
| Duchenne’s muscular dystrophy | Tendon releases, scoliosis | Respiratory insufficiency; cardiomyopathy; succinylcholine-induced hyperkalemia; anesthesia induced rhabdomyolysis |
| Myotonia dystrophica | Tendon releases | Succinylcholine-induced myotonic spasm; cardiac conduction system involvement; avoid direct muscle stimulation |
AI, Aortic incompetence; ASD, atrial septal defect; CNS, central nervous system; GE, gastroesophageal; MR, mitral regurgitation; TMJ, temporomandibular joint; VSD, ventricular septal defect.
Scoliosis
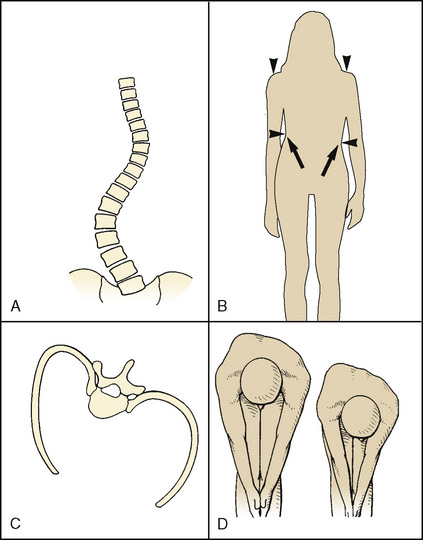
Scoliosis, derived from the Greek root meaning “crooked,” is a lateral and rotational deformity of the thoracolumbar spine. With progression of the lateral spinal curvature, the spinous processes rotate toward the concave side of the curve. The ribs on the convex side are pushed posteriorly by the rotating spine, forming the characteristic gibbous deformity. The ribs on the concave side become prominent anteriorly and are crowded together. Occasionally, scoliosis is associated with kyphosis (“humpback”) or lordosis (“bent backward”) (Fig. 26-1).
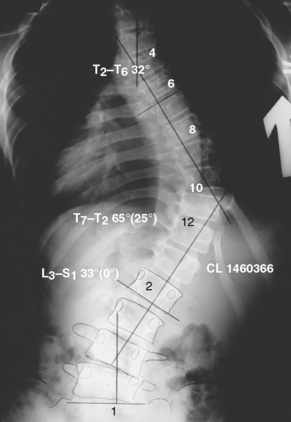
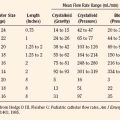
The progression of scoliosis and the severity of its systemic manifestations correlate with the angle of curvature measured by the Cobb method (Table 26-2)—that is, the angle between the upper surface of the top vertebra and the lower surface of the bottom vertebra. The end vertebrae are those that are maximally tilted. Perpendicular lines are extended from these end vertebrae to the center of the curve. The angle formed by the intersecting perpendiculars determines the angle of curvature (Fig. 26-2). The curve is defined as facing to the right or to the left, depending on the convexity of the curve. A lateral curve of greater than 10 degrees is abnormal. Respiratory impairment rarely occurs with a curvature of less than 60 degrees.
TABLE 26-2 Correlation of Angle of Curve and Symptoms in Patients with Scoliosis
| Angle of Curvature | Significance |
| <10 | Normal |
| >25 | Echocardiographic evidence of increased pulmonary artery pressures |
| >40 | Surgical intervention |
| >65 | Restrictive lung disease |
| >100 | Symptomatic lung disease, dyspnea on exertion |
| >120 | Alveolar hypoventilation |
Epidemiology and Etiology
The overall prevalence of spinal deformities in the North American population is between 1% and 2% (Weinstein et al., 2003). Curves can be described on the basis of their anatomic configurations, age of onset, and associated pathology. In the past, polio and tuberculosis infection were the most common causes. Today, most cases of scoliosis are classified as idiopathic because the basic pathophysiology is unknown. Pedigree analysis suggests that scoliosis is a sex-linked trait with variable expression and incomplete penetrance (Xiong and Sevastik, 1998; Lowe et al., 2000). The most common types of scoliosis are listed in Box 26-1.
Box 26-1 Classification of Scoliosis
Congenital scoliosis is a curvature of the spine that is the result of a rib or vertebral anomaly. Idiopathic scoliosis is the most common of the spinal deformities and has three periods of onset, all coincident with periods of rapid growth spurts: infantile (<3 years old), juvenile (3 to 10 years old), and adolescent (>10 years old). Progression of the deformity depends on the age of onset. Infantile idiopathic scoliosis is associated with an increased incidence of mental retardation, inguinal hernias, congenital dislocation of the hip, and congenital heart disease. Juvenile idiopathic scoliosis can usually be managed conservatively (Lowe et al., 2000). Adolescent idiopathic scoliosis is the most common form of scoliosis and occurs most commonly in girls (Weinstein et al., 2003). The curve may resolve, remain stable, or progress in severity. The most significant prognosticators of curve progression in girls are age at onset, premenarchal status, and bone age (Table 26-3) (Lowe et al., 2000; Ahn et al., 2002). Postulated mechanisms for the progression of adolescent idiopathic scoliosis include abnormal vertebral ossification, leptin-induced increased sympathetic nervous system activity, and increases in platelet-derived calmodulin (Lowe et al., 2004; Burwell et al., 2008; Burwell et al., 2009; Gu et al., 2009). Exotic scoliosis is a term introduced by Campbell and Smith (2007) to describe the spinal deformity found in rare pediatric conditions that pose special challenges beyond simply correcting the spinal curve. These very complicated patients with early-onset spinal deformity often tax the multisubspecialty resources of even the most sophisticated pediatric centers. Examples include patients with Jeune and Marfan syndromes.
TABLE 26-3 Incidence of Scoliotic Curve Progression at Time of Diagnosis of 10-Degree Curve
| Age | Menarchal Status | Bone Maturity |
| <11 yr (88%) | Premenarche (53%) | Immature (68%) |
| >15 yr (29%) | Postmenarchal (11%) | Mature (18%) |
Natural History
The natural history of scoliosis varies according to the cause and the pattern of vertebral involvement. Uncorrected, scoliosis results in curve progression, cosmetic deformity, back pain, and physiologic compromise (Weinstein et al., 2003). In most cases of idiopathic scoliosis, the spinal curvature remains small, and conservative nonoperative management is appropriate (Richards et al., 1976). In 0.2% to 0.5% of cases, the curve increases, necessitating surgical intervention (Ahn et al., 2002). In patients with idiopathic scoliosis, only those with thoracic apices and curves of greater than 100 degrees are at increased risk for death from cor pulmonale and right ventricular failure (Asher and Burton, 2006). The grim prognosis of early death and respiratory failure is untrue in most patients with idiopathic scoliosis (Weinstein et al., 1981). The timing of surgery for this condition is controversial. The worse the curve and the more compromised the cardiorespiratory function, the greater the risk for perioperative morbidity and mortality. The pulmonary hypertension of progressive uncorrected idiopathic scoliosis often results in life-compromising respiratory failure in the fourth or fifth decade (Taylor and Gropper, 2006).
Respiratory Sequelae
Even asymptomatic scoliotic patients have demonstrable abnormalities in pulmonary function. As the degree of curvature progresses, vertebral rotation leads to thoracic cage asymmetry and deformation; lung volumes and pulmonary compliance are often but not always inversely related to the degree of this curve (Newton et al., 2005). When the scoliotic curve is greater than 65 degrees, pulmonary function tests demonstrate the characteristic pattern of restrictive lung disease. The first manifestation of this restrictive lung disease is a reduction in vital capacity; in many cases, the vital capacity (normal, 60 mL/kg) is severely reduced, often to less than 60% of predicted. Of the subdivisions of vital capacity, inspiratory capacity is affected to a greater extent than expiratory reserve volume. Functional residual capacity and residual volume are not as severely affected. These alterations in lung volumes are referable to the scoliotic changes in chest wall compliance and the resting position of the thoracic cage, rather than to parenchymal changes. The thoracic deformity of early-onset scoliosis damages pulmonary vascular development and inhibits physiologic alveolar growth, also producing decreases in pulmonary volumes (Charles et al., 1976; Fernandes and Weinstein, 2007).
The pulmonary impairment that results from the scoliosis of neuromuscular disease is exacerbated by coexisting abnormalities in central respiratory drive, coordination of swallowing, and innervation of the upper airway and respiratory musculature. Pulmonary dysfunction in these patients is exacerbated by an increased frequency of respiratory infections, a predilection to aspiration, and an impaired ability to clear pulmonary secretions. Patients who have abnormal results on their pulmonary function test, particularly a forced vital capacity (FVC) of less than 30%, or those who have hypercapnia preoperatively will probably require postoperative (or chronic) ventilation (Almenrader and Patel, 2006). Maximum inspiratory and expiratory mouth pressures (Pimax, Pemax) that the patient can generate against airway occlusion are the important indices for his or her ability to reexpand the lungs (sighs, Pimax ≤ 40 cm H2O) and to expel secretions (coughs, Pemax > 40 cm H2O). Unless the patient can generate more than these threshold pressures preoperatively, postoperative admission to the intensive care unit for ventilatory support should be anticipated.
Cardiovascular Sequelae
Mitral valve prolapse is found in 25% of patients with scoliosis but in less than 10% of age-matched controls. Echocardiographic evidence for increased pulmonary artery pressures has been demonstrated in individuals with only modest degrees of scoliosis in the absence of abnormal pulmonary function. Patients with angles of curvature greater than 70 degrees develop pulmonary hypertension on exercise; those with curves greater than 110 degrees have mean pulmonary artery hypertension at rest. Kafer (1980) proposed that this increase in pulmonary vascular resistance is not just the result of lung compression from thoracic cage abnormalities but is also the result of an increased incidence of hypoxic pulmonary vasoconstriction (Schur et al., 1984). In addition, development of the pulmonary vascular bed may be impaired, resulting in a fundamental reduction in the number of functional vascular units per lung (Kafer, 1980; Schur et al., 1984). Chronic hypoxia induces pulmonary vascular remodeling, which contributes significantly to the pulmonary hypertension in these patients (Morrell et al., 2009).
Any child with a myopathy or borderline respiratory status should have an electrocardiogram and an echocardiogram performed to assess the presence of cor pulmonale, ventricular wall motion, ejection fraction, and ventricular wall thickness. Many myopathies, particularly Duchenne’s muscular dystrophy, involve cardiac muscle and skeletal muscle (Manzur and Muntoni, 2009; Strehle, 2009). Duchenne’s muscular dystrophy is the most common muscular dystrophy in children who present for surgery. An X-linked recessive disorder, this progressive, debilitating disease affects skeletal, cardiac, and smooth muscle. Typically, afflicted boys become wheelchair dependent by the age of 10 years, and in the past, death from respiratory or cardiac failure occurred in this population before the age of 20. With the introduction of contemporary cardiopulmonary interventions, including noninvasive home ventilation, these men are living to previously unprecedented ages (Birnkrant et al., 2007; Birnkrant, 2009). Scoliosis is common, and surgery is often performed to improve the quality of life (see Chapter 3, Respiratory Physiology in Infants and Children).
Numerous anesthesia challenges are presented by patients with Duchenne’s muscular dystrophy. Clinically significant cardiomyopathies and rhythm disturbances manifest by 10 years of age (see Chapter 36, Systemic Disorders). Many of these children are obese because of muscle weakness, fatty degeneration of muscle fibers, and lack of exercise. Succinylcholine can cause a fatal hyperkalemia in these patients, who may present for surgery before the diagnosis has been definitively made; therefore, the routine use of this muscle relaxant is no longer recommended in all children (Birnkrant et al., 2007; Birnkrant, 2009; Gurnaney et al., 2009).
Preoperative Evaluation
The most important aspects of the preoperative evaluation include determination of the location and degree of spinal curvature, the cause of the scoliosis, the patient’s history of exercise tolerance, respiratory symptoms, and the presence of coexisting diseases. A directed physical examination of the cardiorespiratory system should evaluate the presence of tachypnea, crackles, wheezing, and signs of right heart failure, such as hepatomegaly, jugular venous distention, and peripheral edema. Any preoperative neurologic deficits should be recorded. Based on the severity of the curve and the degree of respiratory impairment, the preoperative laboratory studies listed in Box 26-2 should be requested.
Children with myelodysplastic syndromes are likely to develop an allergy to latex products (Kelly et al., 1994; Maxwell, 2004; Dewachter et al., 2009). All children with any of these syndromes should be considered allergic to latex, and nonlatex products (e.g., tourniquets, sterile and nonsterile gloves) should be substituted for the latex equivalents. Corticosteroids and antihistamines are not administered prophylactically.
Surgical Techniques
The treatment of spinal curvature is dictated by the type of scoliosis and by the surgeon’s expertise and preferences. Very few cases of congenital scoliosis can be managed conservatively. The mainstay of therapy is posterior spinal fusion without instrumentation, followed by prolonged immobilization. Instrumentation in these patients has been associated with a prohibitively high rate of paraplegia, which is presumed to be the result of coexisting cord and vertebral anomalies. Although conservative therapy is the most frequently used treatment for idiopathic scoliosis, when rapid curve progression is anticipated, surgical intervention is used for severe truncal deformities and for pain unrelieved by medical therapy (Weinstein et al., 2003).
Posterior Spinal Fusion
Several instrumentation techniques are available for treatment of the scoliotic spine. The Harrington rod, the original instrumentation system, consists of a stainless steel rod that is connected to the inferior facets and pedicles of the spine by multiple ratchet hooks placed at the terminal aspects of the curve. Distraction is adjusted using the ratchet principle (Harrington and Dickson, 1976; Harrington, 1988). The incidence of neurologic complications after this technique is 0.23%. The disadvantages of the Harrington rod include two-dimensional correction, curvature distraction by the end hooks, and the need for prolonged postoperative immobilization. Because of these problems, this technique is rarely used.
Segmental spinal instrumentation was introduced to provide three-dimensional correction and to achieve differential distraction at multiple levels. The Luque instrumentation system consists of sublaminar wires on each side of the spinous process and a long, L-shaped rod that can be contoured three dimensionally. The curve is corrected as the wires are tightened (Luque, 1986; Luque and Rapp, 1988). The internal fixation achieved is more rigid than that obtained with the Harrington system, and it can be extended to the pelvis. The most common deficit after Luque rod instrumentation is a dysesthesia, which is usually observed late (2 to 6 days) in the postoperative period. The proposed mechanism for these findings is expansion of an epidural hematoma in the area of the sublaminar wires (Johnston et al., 1986).
The Cotrel-Dubousset segmental spinal instrumentation system uses multiple laminar and pedicular hooks attached to a double-rod frame (Richards and Johnston, 1987). This system enables three-dimensional correction of complex curves and obviates the need for postoperative immobilization. It is more time consuming than the Harrington system, increases intraoperative blood loss, and has a lower incidence (0.6%) of neurologic complications than Luque rods. Double-curve patterns are more complex and require multiple hooks at multiple fixation sites, necessitating more extensive decortication and contributing to additional blood loss.
Pedicle screws are the most recent advance in posterior spinal fusion (Kim and Noonan, 2009). Initially used for lumbar curves, they are now used for total curve correction. The limitation of posterior spinal fusion with or without instrumentation is that the anterior growth plates, which play a major role in the development of the deformity, are not affected. Late torsional deformities can result.
Anterior Spinal Surgery
The anterior approach to spinal deformities has been advocated for several specific deformities, including severe kyphosis and lordotic paralytic curves in patients with cerebral palsy. Surgery consists of discectomies with or without instrumentation, performed alone or in combination with a posterior spinal fusion. Video-assisted thoracoscopic surgery can be used for this procedure if instrumentation is not being used (Sucato, 2003). The surgical approach used to expose the anterior portion of the spine depends on the exact spinal deformity. Thoracic curves are usually approached through a left thoracotomy, and the procedure is facilitated by insertion of a double-lumen endotracheal tube and one-lung ventilation. Alternatively, single-lung ventilation in young children is performed by advancing a tracheal tube into the main stem bronchus opposite the side of surgery, or by positioning a bronchial blocker into the main stem bronchus on the operative side.
Many techniques for placing a variety of bronchial blockers outside the tracheal tube have been described for use in children (see Chapter 23, Anesthesia for General Abdominal, Thoracic, Urologic, and Bariatric Surgery) (Hammer, 2001, 2004; Hammer et al., 2002). The combined curve of the thoracolumbar spine is exposed transdiaphragmatically by means of a high subcostal incision that necessitates taking the diaphragm down from its bony insertion. Lumbar curves can be approached extraperitoneally or transabdominally. In general, complications of the anterior approach include great vessel disruption, hemothorax, pneumothorax, paralytic interruption of spinal cord perfusion, and excessive angulation or compression of the spinal cord by rapid distraction of the curvature. Spinal cord injury can result from mechanical damage by a screw or disruption of segmental spinal arteries. At the beginning of the 21st century, performance of anterior scoliosis surgery diminished because of the corrective power of pedicle screw constructs and better surgical methods designed to improve flexibility, such as posterior release, posterior osteotomy, and vertebral resection (Kim and Noonan, 2009).
As an alternative to open thoracotomy, a video-assisted thoracoscopic surgery (VATS) approach is being used to perform anterior thoracic spine release in patients who require both anterior and posterior procedures to correct their scoliosis. The appropriate use of pedicle screws has resulted in a 50% to 66% curve correction, with good maintenance of the curve correction for a minimum of 3 years (Lehman et al., 1976).
Anesthesia Management of Scoliosis Surgery
Monitoring and Intraoperative Complications
Scoliosis surgery is very high-risk surgery. Common intraoperative problems are listed in Table 26-4. Complications are related to the surgery (blood loss and its therapy), the prone position, and cardiovascular collapse from myriad causes. Practiced crisis management is essential if patient survival is to be ensured.
TABLE 26-4 Potential Intraoperative Complications During Scoliosis Surgery
| Problem | Monitoring Solution |
| Endotracheal tube malposition | Securely tape tube before turning Benzoin Waterproof tape Bite block (rolled 4×4 gauze pads) After turning prone Hand ventilate and listen to both lung fields DO NOT ALLOW STRETCHER TO LEAVE THE OPERATING ROOM until you are satisfied that the endotracheal tube has not migrated Hourly arterial blood gas determinations Esophageal stethoscope |
| Alteration in pulmonary compliance in the prone position | Proper position on bed frame: ensure chest can expand unimpeded Hourly arterial blood gas determinations |
| Alteration in cardiac function in the prone position | Proper position on bed frame to ensure that venous return is not compromised Indwelling arterial catheter Central venous catheter Bladder catheter |
| Hypotension | Blood loss until proven otherwise Ensure that typed and cross-matched blood is available when surgery starts. Two large-bore peripheral IV catheters and blood warmer Central venous catheter Hourly measurement of hemoglobin/hematocrit Consider Amicar |
| Coagulopathy | Platelet count Prothrombin time, activated partial thromboplastin time, fibrin split products Thromboelastogram |
| Electrolyte abnormalities (usually from blood transfusions) | Frequent measurements of sodium, potassium, and ionized calcium Avoid using “old” packed red blood cells NEVER use hypotonic solutions (including for maintenance fluid requirements) |
| Excessive heat loss | Measure core temperature Heat conservation Active warming (forced air) |
| Neurologic injuries | Proper positioning, with particular attention to eyes and elbows (brachial plexus) Intraoperative assessment of cord function (e.g., sensory and motor evoked potentials) |
Neurologic Monitoring
Postoperative paralysis or sensory loss is the most feared, devastating, and often unpredictable complication of scoliosis surgery (Owen, 1999). Neurologic injury may result from direct injury to the spinal cord or nerves during instrumentation, from excessive traction during distraction, or from compromised perfusion of the spinal cord. Because the ramifications associated with motor deficit are significantly greater than those associated with sensory deficit, surgically induced paraplegia has always been the major concern of scoliosis surgery.
Spinal Cord Blood Flow
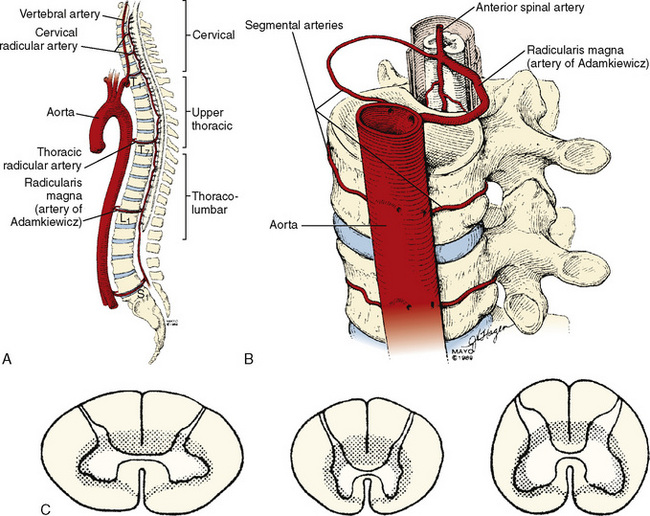
The organization of the spinal cord blood supply is segmental in a cross-sectional and rostral-caudal fashion (Fig. 26-3). The intrinsic spinal cord vasculature consists of the anterior median and the paired posterior spinal arteries. The vasculature supplying these vessels arises from the segmental arteries of the aorta and branches of the subclavian—the vertebral arteries—and the internal iliac arteries. The solitary anterior median spinal artery runs along the entire length of the cord in the anterior sulcus, giving off penetrating branches that supply the ventral two thirds of the spinal cord. Blood flow in the anterior spinal artery is not continuous throughout its span; instead, the anterior spinal artery functions as an anastomotic channel between the terminal branches of successive radicular arteries. Blood that leaves the terminal aspects of these radicular arteries courses upward and downward in the anterior spinal artery. At points between adjacent radicular arteries, blood flows in either direction. The paired posterior spinal arteries, which supply the dorsal third of the cord, also have discontinuous segments and appear more like a plexus of pial vessels than paired arteries.
The regional circulation of the spinal cord is divided into four segments. The cervical and lumbosacral regions each receive double the blood flow received by the thoracic region (see Fig. 26-3). Although each vertebral level has paired segmental arteries, only six to eight important medullary arteries are formed. These medullary arteries join the spinal arteries. The segmental arteries at all other levels are functionally nonsuppliers of blood to the spinal cord itself. The vertebral arteries form the rostral origins of the anterior and posterior spinal arteries and represent the principal supply to the cervical cord. Branches of the thyrocervical and costovertebral arteries supply the lower cervical and upper thoracic cord. A radicular artery arising from T7 provides perfusion for the middle thoracic cord. The most consistent and important of the anterior medullary arteries is the artery of Adamkiewicz—the arteria radicularis magna—which usually joins the anterior spinal artery between T8 and L3. This artery is the predominant source of blood supply to the lower two thirds of the spinal cord. The implications of this design dictate the clinical manifestations of impaired cord perfusion. Watershed areas, which are subject to ischemia during low-flow states, exist between the anterior and posterior circulations and between the four different spinal segments. The segments of T4 to T7 appear to be highly susceptible to injury during periods of hypoperfusion. The dependence of the lower two thirds of the cord on the artery of Adamkiewicz puts this region at particular risk during surgical manipulation of the thoracolumbar aorta and spinal column, and this is referred to as the lumbar artery enlargement syndrome. Although the clinical picture of this syndrome is not constant, it is marked by the development of flaccid paraplegia or quadriplegia (depending on the level of the lesion) and dissociated sensory impairment in which heat and pain sensations are affected but deep sensation is spared.
The same principles that regulate the cerebral blood flow apply to spinal cord blood flow. Thus, cord blood flow depends on the perfusion pressure (i.e., mean arterial pressure [MAP] minus cerebrospinal fluid pressure), integrity of the circulation, microcirculatory autoregulation, and intrinsic regulation. If the perfusion pressure falls below 50 mm Hg, spinal cord blood flow is reduced. Spinal cord blood flow autoregulates within the range of a MAP of 60 to 150 mm Hg. Spinal cord blood flow is also regulated on an intrinsic basis in response to arterial oxygen and carbon dioxide tensions, pH, and cord temperature in a fashion identical to that of the cerebral circulation. Hypercapnia increases flow, whereas a Pao2 below 60 mm Hg results in a vasodilation that overrides the effects of hypocarbia and autoregulation (see Chapter 22, Anesthesia for Neurosurgery).
Minimizing Postoperative Neurologic Complications
The estimated risk for postoperative neurologic injury in patients undergoing spinal instrumentation is 0.72% to 1.6% (Cervellati et al., 1996). In a study of 7885 patients who underwent instrumentation or fusion without instrumentation, 87 patients developed acute neurologic changes, and 36% of these patients recovered without sequelae. Individuals with nonidiopathic scoliosis are at higher risk for neurologic injury. Children with congenital scoliosis suffer neurologic complications disproportionately (Cervellati et al., 1996).
Wake-Up Test
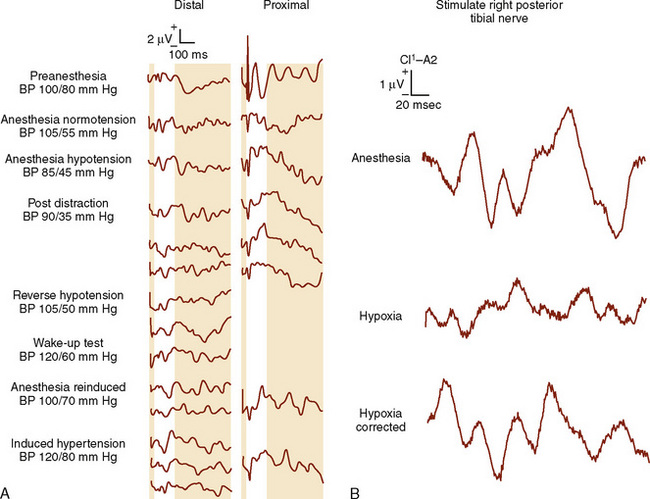
Vauzelle and colleagues (1973) first described the use of the wake-up test to assess the integrity of the spinal cord. In this technique, patients are awakened intraoperatively to assess spinal cord motor function. The wake-up test requires an anesthetic that allows rapid recovery of consciousness and motor function. Ideally, the test is rehearsed preoperatively. During rehearsal, patients are informed that they will be momentarily awakened at the time of rod insertion to test the function of the spinal cord. Patients must be reassured that they will neither remember the event nor experience pain while they are “awake.” Preoperative preparation increases the speed and success of the test. The effectiveness of neurophysiologic monitoring during spinal deformity surgery is now so well established that the wake-up test is rarely used except to confirm monitoring changes (Schwartz et al., 2007).
When a wake-up test is performed, the operating room must be quiet, the surgeon must stop operating, and an observer is positioned (usually under the drapes) to look for foot movement. After discontinuation of the anesthetic, the patient is first asked to move the hands (“squeeze my fingers”) to evaluate the level of consciousness and is then asked to move the feet (“wiggle your toes”). If the patient is unable to move the feet but can move the hands, spinal cord compromise is presumed, and the spinal rod instrumentation is removed immediately. Spinal cord perfusion is maximized by raising the MAP, increasing the hemoglobin concentration, and normalizing arterial carbon dioxide and oxygen tensions. In one series of 166 patients in whom the wake-up test was used, three patients had demonstrable neurologic deficits when awakened (Hall et al., 1978). These deficits disappeared immediately on release of the distracting force (i.e., rods) (Hall et al., 1978).
Neurometric Monitoring
Sensory-Evoked Potentials
Electrophysiologic (neurometric) monitoring provides real-time, continuous assessment of spinal cord function and does not require patient movement, arousal, or cooperation (see Chapter 11, Monitoring) (Gonzalez et al., 2009). The most common technique uses somatosensory evoked potentials (SSEPs), in which the cortical and subcortical responses to peripheral nerve stimulation are monitored (Banoub et al., 2003). Typically, a peripheral mixed nerve (i.e., posterior tibial nerve, peroneal nerve, or median nerve) is stimulated at fixed intervals during a procedure. SSEPs are recorded repeatedly during surgery, and their amplitude (height) and latency (time of occurrence) are compared with baseline values (Gonzalez et al., 2009; Mendiratta and Emerson, 2009). Based on changes in these characteristics, it is possible to determine the functional status of the spinal cord sensory tracts. SSEP monitoring requires specialized technology and expertise. To resolve the very low amplitude evoked potentials from background, random, or spontaneous cortical activity, computer signal averaging of repetitive sensory responses is required. The processed evoked-potential waveform is plotted as voltage against time and is characterized by the poststimulus latency and amplitude. The poststimulus latency reflects the time required for impulse transmission from the site of sensory stimulation. A reduction in amplitude of greater than 50% or an increase in latency of less than 10% relative to baseline values is generally considered significant (Banoub et al., 2003; Gonzalez et al., 2009; Mendiratta and Emerson, 2009).
SSEPs monitor only the dorsal columns of the spinal cord and provide no direct evidence of loss of motor function or anterior spinal cord injury. Motor deficits may occur in the absence of alterations in SSEPs, and numerous case reports have recorded the postoperative finding of paralysis despite unchanged intraoperative SSEPs (i.e., false-negative results). In the setting of spinal cord ischemia, the time to loss of SSEPs was almost three times longer than the time to motor evoked potential (MEP) loss (Banoub et al., 2003; Shine et al., 2008). The most comprehensive information regarding the false-negative rate of SSEPs comes from a survey of spine surgeons by the Scoliosis Research Society and the European Spinal Deformity Society, in which definite neurologic deficits, despite stable SSEPs, occurred during surgery in 0.063% of patients (Nuwer et al., 1995). Children with neuromuscular scoliosis frequently have unreliable SSEP data. Despite these limitations, SSEP monitoring reduces postoperative paraplegia by more than 50% (Nuwer et al., 1995). When SSEP monitoring is equivocal, many recommend an intraoperative wake-up test to assess motor function (Grundy, 1983).
Many pharmacologic and physiologic variables affect the latency and amplitude of SSEPs and have been estimated to account for up to 44% of intraoperative SSEP changes. The most important of these are the anesthesia agents, blood pressure, and body temperature (Table 26-5) (Grundy, 1983; Banoub et al., 2003; Gonzalez et al., 2009). All of the potent inhaled anesthesia agents produce dosage-dependent increases in latency and decreases in amplitude. These effects are less for sevoflurane and desflurane, permitting dosages of 1.5 minimum alveolar concentration (MAC) with minimal SSEP changes. Nitrous oxide compounds the effects of volatile anesthetics on cortical SSEPs (Banoub et al., 2003). Alone, nitrous oxide has no effect on SSEP latency but does decrease its amplitude by 50% (Schwartz et al., 2007). Substantial recovery of latency and amplitude is achievable with discontinuance of nitrous oxide and the inhaled vapors (da Costa et al., 2001). In general, the IV agents affect SSEP less than inhaled agents do; at high dosages, they produce slight to moderate decreases in amplitude and increases in latency. Midazolam has no effect on latency (Laureau et al., 1999; Banoub et al., 2003). Ketamine (Langeron et al., 1997) and etomidate (Thakor et al., 1991) augment SSEP amplitude. Propofol has no effect on amplitude or latency and is highly recommended as a component of total IV anesthesia for scoliosis surgery (Laureau et al., 1999; Boisseau et al., 2002). Opioids given in either analgesic or anesthetic dosages produce minimal SSEP effects (Banoub et al., 2003). In a very small study, dexmedetomidine had no effect on SSEP or MEP (Tobias et al., 2008).
TABLE 26-5 Effects of Anesthesia Agents on Somatosensory Evoked Potentials
| Agent | Amplitude | Latency |
| Desflurane | ↓ | ↑ |
| Isoflurane | ↓ | ↑ |
| Sevoflurane | ↓ | ↑ |
| Nitrous oxide | ↓ | ↔ |
| Barbiturates | ↓ | ↑ |
| Etomidate | ↑ | ↔ |
| Ketamine | ↑ | ↔ |
| Midazolam | ↓ | ↔ |
| Opioids | ↔ | ↔ |
| Propofol | ↔ | ↔ |
| Dexmedetomidine | ↔ | ↔ |
↓, Decreases; ↑, increases; ↔, remains the same.
The amplitude and latency of the waveform are also affected by age, preexisting neurologic deficits, body temperature, Paco2, hypoxia, and blood pressure (Fig. 26-4) (Banoub et al., 2003). The reliability of spinal cord monitoring may be dramatically affected by the variability of the evoked responses. Muscle relaxants have no direct deleterious effects on the SSEP but may produce a more reliable recording by providing “quieter” conditions.
An anesthesia milieu that is compatible with adequate neurometric monitoring and that allows rapid awakening can be created using a variety of approaches. Combining desflurane or sevoflurane with a remifentanil infusion produces ideal SSEPs and still allows for rapid wake-up if a wake-up test is required. Alternatively, the physician can substitute a continuous propofol infusion for the potent inhaled anesthetics in combination with an opioid (Kalkman et al., 1991a; Kalkman et al., 1991b). Because etomidate augments SSEP amplitude, it is particularly useful in patients with abnormal preoperative SSEPs. These individuals are at greatest risk for the development of postoperative neurologic catastrophes (Sloan et al., 1988; Samra and Sorkin, 1991).
Using the criterion of a decrease in amplitude of greater than 40% amplitude as a significant change, excellent specificity and sensitivity are achievable. In patients with idiopathic scoliosis (i.e., neurologically intact), SSEPs are reliable and can be obtained in more than 98% of patients (Padberg et al., 1998). However, in patients with preexisting diseases such as neuromuscular scoliosis, the reliability of the SSEP is less than 75% but can be improved with the addition of MEP monitoring (Sarwark and Sarwahi, 2007). Precise communication and coordination of efforts among the surgeon, anesthesiologist, and neurometric specialist are imperative when a change in SSEP is observed. Normalization of the SSEPs may occur spontaneously with relaxation of the distraction instrumentation or by improving spinal cord perfusion (e.g., increasing blood pressure and Paco2 levels).
Motor Evoked Potentials
SSEPs are not the modality of choice for monitoring motor tract function or for detecting the presence of a surgically induced motor deficit. The motor pathways can be activated by transcranial stimulation of the motor cortex or by spinal cord stimulation. Transcranial MEPs (TcMEPs) are exquisitely sensitive to compromised spinal cord function and should be used in combination with SSEPs to improve the accuracy of spinal cord monitoring. In a series of 1121 adolescents with idiopathic scoliosis, nine children awoke with a postoperative neurologic deficit, seven had motor or sensorimotor deficit, and two had pure sensory impairment. All seven patients with motor impairments had significant TcMEP changes; only three of the seven had significant SSEP changes. When changes were detected by both modalities, SSEPs lagged behind TcMEPs by approximately 5 minutes (Schwartz et al., 2007). As a result, some centers have abandoned using SSEPs entirely. Finally, the false-positive rate attributed to MEP monitoring during spinal surgery is less than 10% (Langeloo et al., 2003).
Anesthesia agents have profound effects on MEP recordings. Volatile anesthetics, benzodiazepines, etomidate, barbiturates, and even high-dosage propofol affect MEP fidelity (Sloan and Heyer, 2002; Sloan et al., 2008). A maintenance anesthesia that is based on a continuous infusion of propofol and remifentanil is titrated to maintain a MAP of at least 65 mm Hg, and that avoids nitrous oxide and muscle relaxants, provides adequate conditions for uncompromised TcMEP monitoring. Alternatively, high-dosage remifentanil in combination with low-dosage sevoflurane (0.4 to 0.7 MAC), or continuous infusions of propofol, fentanyl, and dexmedetomidine, are also regimens compatible with reliable monitoring (Joseph et al., 1976; Langeloo et al., 2003, 2007; Anschel et al., 2008).
Blood Loss
Exposure of a vast area of decorticated, raw, cancellous bone during spinal surgery results in extensive blood loss that can exceed 25 mL/kg, even in uncomplicated surgery. Children with neuromuscular scoliosis often require more extensive procedures, including pelvic stabilization, than children with idiopathic scoliosis and can easily have blood loss that exceeds a blood volume (Kannan et al., 2002; Meert et al., 2002). However, even if the extent of surgery is controlled, patients with neuromuscular diseases have an almost seven times higher risk for losing greater than 50% of their estimated total blood volume during scoliosis surgery than do normal controls (Edler et al., 2003).
An estimation of circulating blood volume should be made before the induction of anesthesia. The estimated blood volume (EBV) is calculated by multiplying the patient’s weight by the approximate blood volume based on age (Table 26-6). From the EBV, the initial hematocrit, and the minimum acceptable hematocrit, the maximum allowable blood loss can be estimated before packed red blood cell transfusion is indicated.
| Age | Total Blood Volume (mL/kg) |
| Premature infant | 90-105 |
| Term newborn | 80-85 |
| 1 to 12 months | 70-80 |
| Older child | 70-80 |
| Adult | 55-65 |
Multiple strategies have been described to reduce intraoperative blood loss and the need for blood transfusions of homologous blood in the perioperative period (see Chapter 14, Blood Conservation). Techniques that may decrease intraoperative blood loss include induced hypotension, alteration of the operative position, changes in surgical technique, and administration of antifibrinolytic agents such as ε-aminocaproic acid (Amicar) or tranexamic acid (Cyklokapron). Techniques aimed at decreasing the use of homologous blood products include intraoperative blood salvage, preoperative autologous blood donation, and perioperative normovolemic hemodilution and apheresis (Joseph et al., 1976). Each of these techniques has demonstrated efficacy in some clinical situations.
Preoperative autologous donation with and without erythropoietin treatment has been used extensively in children (Helfaer et al., 1998; Vitale et al., 1998). As this has become more widespread for pediatric scoliosis, 70% of children and adolescents have been able to donate sufficient units for their procedures, a figure similar to that for adults (Murray et al., 1997). Using preoperative autologous donation as the sole blood conservation method limited the number of adolescents undergoing scoliosis surgery who required allogeneic transfusions to 11% to 23%. The addition of autologous donation to deliberate hypotension further reduced allogeneic exposure to 10% (Murray et al., 1997).
Alternatively, perioperative hemodilution and apheresis, isovolemic hemodilution, or acute normovolemic hemodilution can substitute for or augment autologous blood donation (Bryson et al., 1998; Copley et al., 1999). Before incision, the patient’s blood is withdrawn through a large-bore peripheral IV cannula or through a central venous catheter and replaced with an isotonic balanced salt solution or colloid in a ratio of 3:1. When using this technique, cardiac index and oxygen extraction increase, and systemic vascular resistance, oxygen delivery, and mixed venous saturation decrease. Lactic acidosis develops when oxygen delivery falls to critical. Moderate acute normovolemic hemodilution, with a minimum hematocrit of 17%, did not result in changes in either oxygen delivery or oxygen consumption in healthy children. The removed blood is stored in anticoagulated bags and is administered as needed to maintain the hematocrit at a predetermined level, usually greater than 20%. Complications of this technique include postoperative pulmonary edema, anasarca, and prolonged postoperative mechanical ventilation.
Perioperative salvage of blood (i.e., use of a cell-saver system) allows blood lost in the operative field to be returned to the patient during the perioperative period (Huet et al., 1999). The efficiency of this technique, its benefits in limiting blood transfusion, and the survivability of red blood cells are unclear (Vitale et al., 2002). When using salvaged blood, it is imperative that the blood be processed and washed adequately to avoid life-threatening bleeding and pulmonary complications caused by transfusion of cellular and tissue debris. Salvaged blood can also cause profound hypotension (e.g., citrate, air embolism) and hemolytic and bleeding complications from centrifugation, cellular debris, or anticoagulant overdose.
Pharmacologic manipulation of the hemostatic mechanism is effective in limiting perioperative blood loss. A series of prospective, randomized, double-blind studies demonstrated that the antifibrinolytic agent ε-aminocaproic acid significantly reduces perioperative blood loss and transfusion requirements in patients with either idiopathic or neuromuscular scoliosis who are undergoing spinal fusion (Florentino-Pineda et al., 1976; Thompson et al., 1976a, 1976b). Similarly, tranexamic acid, 100 mg/kg over 15 minutes, followed by an infusion of 10 mg/kg per hour, significantly reduces blood loss during posterior spinal surgery in children and adolescents with idiopathic and secondary (congenital and neuromuscular) scoliosis but may not affect the actual amount of blood transfused (Sethna et al., 2005). The main safety issues related to antifibrinolytic drugs are hypersensitivity reactions, effects on the kidneys, potential for thrombotic events, and effect on mortality.
Deliberate Hypotension
The most common blood-preservation technique is deliberate hypotension (see Chapter 14, Blood Conservation) (Yaster et al., 1986; Tobias, 2002). Numerous techniques to reduce blood pressure have been used, typically with drugs that are direct venous and arterial vasodilators such as nitroprusside and nitroglycerin. Ganglionic blocking drugs (e.g., trimethaphan), α2-adrenergic agonists (e.g., clonidine), β- and α1-antagonists (e.g., propranolol, esmolol, prazosin, hydralazine), dopamine agonists (e.g., fenoldopam), inhaled anesthetics (e.g., halothane, isoflurane, sevoflurane, desflurane), major conduction blockade (i.e., epidural), and calcium-channel blockers (e.g., nicardipine) are used alone or in combination with the direct-acting venous and arterial vasodilators to lower blood pressure (Yaster et al., 1986; Tobias, 2002; Hammer et al., 2008).
Deliberate hypotension can be used in any patient who is otherwise healthy. It is contraindicated in the presence of evidence of end-organ injury or ischemia. Blood pressure is usually lowered to an MAP of 60 mm Hg. In the past, the most commonly used technique was a continuous infusion of nitroprusside (1 to 8 mcg/kg per minute) combined with β-blockade, using propranolol (1 to 2 mg), labetalol, or an esmolol infusion. β-Blockade is necessary when hypotension is induced with nitroprusside, because nitroprusside produces a reflex tachycardia and increases cardiac index. These effects combine to limit the ability of nitroprusside to lower blood pressure (Yaster et al., 1986). Nitroglycerin, which is often used for deliberate hypotension in adults, fails to reduce MAPs to below 60 mm Hg in children when administered in dosages as high as 40 mcg/kg per minute (Yaster et al., 1986). Potent inhalation agents such as sevoflurane can also be used to induce hypotension in children and adolescents, but they interfere with intraoperative monitoring and delay the ability to rapidly perform a wake-up test. When combined with low-dosage sevoflurane or desflurane, remifentanil is extremely effective. Regardless of the technique used to induce deliberate hypotension, the appropriate use of adjuvants in the anesthesia technique can aid in achieving the desired effect of hypotension (Tobias, 2002). Children can be premedicated with oral clonidine (4 to 8 mcg/kg) 2 hours before surgery to reduce anxiety and to facilitate the induction and maintenance of hypotension during surgery (Hackmann et al., 2003).
Although deliberate hypotension clearly reduces blood loss, provides a relatively bloodless surgical field, and facilitates surgical dissection, its use in scoliosis surgery is controversial. Animal studies have suggested an additive effect of hypotension and surgical pressure on the spinal cord in producing neurologic injury (Brodkey et al., 1972). In dogs, Griffiths and colleagues (1979) showed that cord compression can alter dorsal column conduction at perfusion pressures that did not affect blood flow. Use of deliberate hypotension mandates normocarbia and adequate oxygen-carrying capacity.
Invasive monitoring is essential when deliberate hypotension is used. An arterial catheter for beat-to-beat monitoring of blood pressure and for frequent hematocrit determinations and sampling of arterial blood gas are essential. Because patients must remain normovolemic at all times, a central venous pressure catheter is a useful monitor. Nitroprusside has been shown to decrease arterial oxygen tension in children and to increase the alveolar to pulmonary artery (Pao2-to-Pao2) pressure difference (Yaster et al., 1986). We can assume that the lowered oxygen-carrying capacity of low-hematocrit blood, when coupled with hypoxemia and hypocapnia from overzealous ventilation, can only exacerbate the cord ischemia caused by surgical traction or hypotension. Other end organs that must be protected from hypotension include the heart and kidneys. The heart is easily monitored by following the ST segment of the V5 lead of the electrocardiogram. The kidneys are monitored by bladder catheterization and hourly measurement of urine output. Renal perfusion pressure can be assumed to be inadequate if urine output falls to less than 1 mL/kg per hour.
Positioning
Anesthetized patients are extremely vulnerable during positioning for scoliosis surgery because of their inability to feel the extreme discomfort of certain positions that alter the normal mechanics of the body. Vulnerable parts that must be especially protected to avoid injury by pressure or stretching include the peripheral (ulnar) nerves, the male genitalia, the nipples, and the anterior superior iliac spine (to protect the lateral femoral cutaneous nerve of the thigh). Of the peripheral nerves, the brachial plexus is most vulnerable to stretch in the prone position (Martin and Warner, 1997). Stretching of the lower trunks of the brachial plexus is most likely to occur when the head is turned to the contralateral side, the ipsilateral shoulder is abducted, and the ipsilateral elbow is bent. Although efforts to prevent neuropathies are frequently debated, there is little hard evidence to support specific management recommendations.
The patient’s eyes are vulnerable to corneal abrasion, optic vein engorgement, and retinal ischemia. Postoperative visual loss is a devastating and poorly understood injury. Over the past decade, speculation has grown that the frequency of perioperative blindness has been increasing among patients undergoing major spine surgery. Roth and colleagues found a much higher incidence of blindness (1 in 600) among patients undergoing complex spinal surgery (Roth et al., 1996, 1997; Roth and Barach, 2001). However, a report from the Mayo Clinic did not support this finding (Nuttall et al., 2001). In an effort to help analyze the risk factors involved, the American Society of Anesthesiologists has established an anonymous postoperative visual loss registry (www.asaclosedclaims.org).
Ischemic optic neuropathy, which affects the anterior or posterior portions of the optic nerve, is the most common cause of postoperative visual loss. Visual loss may also be caused by retinal arterial occlusion and cortical blindness. Awaking with visual impairment is one of the most frightening and catastrophic postanesthesia complications that a patient may sustain. It is also an enormous medicolegal liability problem. Commonly cited risk factors in patients who undergo scoliosis surgery in the prone position include hypotension, anemia, and external compression of the eye. Unfortunately, blindness has occurred even when these risk factors were not present (Lee and Lam, 2001; Roth and Barach, 2001).
The prone position increases intraabdominal pressure, which impairs ventilation by decreasing chest compliance and by limiting chest expansion. It also engorges epidural veins, which increases intraoperative bleeding and potentially increases the risk for postoperative epidural hematoma formation. Increased intraabdominal pressure leads to compression of the inferior vena cava, which impedes venous return to the heart, thereby decreasing cardiac output and engorging epidural veins. In an effort to facilitate venous return and avoid increased intraabdominal pressure, specially designed frames have been developed that allow the abdomen to hang free and facilitate respiratory movement (Relton and Hall, 1967).
Anesthesia Techniques
Patients with preexisting neurologic deficits such as cerebral palsy, paralytic scoliosis, and congenital scoliosis have variable SSEP waveforms of weak amplitude. The use of a ketamine or an etomidate infusion in these patients can augment the amplitude of the evoked responses and increase the reliability of SSEP monitoring (Banoub et al., 2003; Lotto et al., 2004).
Postoperative Management
Although pulmonary failure and the need for postoperative ventilation are the primary problems of scoliosis surgery, other common postoperative concerns include ongoing blood loss, disseminated intravascular coagulation, hypovolemia, and development of syndrome of inappropriate secretion of antidiuretic hormone, paralytic ileus, and pain. The most important initial decision at the conclusion of surgery is whether to extubate the trachea. This decision is based in large part on the patient’s preoperative pulmonary function and on the intraoperative course. Scoliosis surgery produces an immediate and transient decrease in pulmonary function of up to 60% of baseline values. The nadir of pulmonary function occurs on the third postoperative day, and it remains at approximately half of baseline values through the first postoperative week. These changes can resolve within 6 months of the procedure (Yuan et al., 1976). Patients with preoperative FVC levels of less than 50% of predicted and patients with nonidiopathic scoliosis should be extubated in the pediatric intensive care unit after cognitive faculties and respiratory muscle strength have returned to baseline. After spinal fusion, 18 children with severe restrictive lung disease (FVC < 45%) required a mean of 9 days of postoperative ventilation (Wazeka et al., 1976). Other common pulmonary complications in the postoperative period include atelectasis, pneumothorax, and pleural effusions.
Syndrome of inappropriate secretion of antidiuretic hormone is common and manifests as hyponatremia, hypoosmolality, decreased urine output, and increased urine osmolality. The increased plasma volume reduces the hemoglobin concentration and can be identified by following the red blood cell’s mean corpuscular volume. As the level of antidiuretic hormone increases, plasma water increases, resulting in free water entry into red blood cells. The mean corpuscular volume increases, and the hemoglobin and mean corpuscular hemoglobin content decrease. The mean corpuscular volume should not change in the face of postoperative blood loss (Mason et al., 1989).
Because the surgery is so extensive, pain is an expected complication in all scoliosis patients. Furthermore, if remifentanil was used intraoperatively without loading the patient with a longer-acting opioid, an exaggerated pain response is to be expected on awaking. Remifentanil produces opioid-induced hyperalgesia, a paradoxical process by which opioid administration, even for short periods of time, increases the sensitivity to pain and worsens pain when the opioid is discontinued (Crawford et al., 2006). Postoperative pain can be treated with IV opioid therapy (i.e., patient-controlled analgesia [PCA]), regional anesthesia techniques, or both (Tobias, 2004). To use PCA, patients are started on a basal infusion of morphine (0.02 mg/kg per hour) or hydromorphone (0.004 mg/kg per hour) combined with a low-dosage naloxone infusion (0.25 mcg/kg per hour) (Maxwell and Yaster, 2003). The patient or nurse can trigger the PCA machine’s bolus button, which provides an additional dose of morphine (0.02 mg/kg) or hydromorphone (0.004 mg/kg) (Monitto et al., 1998). Many centers are using neuraxial techniques for postoperative analgesia (Tobias, 2004). Typically, a single epidural catheter is inserted by the surgeon at the T8 or T9 level before wound closure. Alternatively, two catheters are placed at the top and bottom of the wound by the surgeon. These catheters are intermittently or continuously infused with opioids (i.e., morphine, hydromorphone, or fentanyl), local anesthetics (i.e., 0.625% to 1.25% bupivacaine solutions), or both.
Nonsteroidal antiinflammatory drugs (NSAIDs) such as ketorolac have a morphine-sparing effect and result in fewer opioid-induced side effects such as somnolence, constipation, and pruritus (Reuben et al., 1997, 1998). The use of ketorolac does not increase bleeding, the need for transfusions, or reoperation (Reuben et al., 1997; Vitale et al., 2003). Nevertheless, for reasons that are discussed later, the use of ketorolac in scoliosis patients should be avoided, because NSAIDs significantly inhibited spinal fusion in human and laboratory studies (Glassman et al., 1998; Martin et al., 1999). NSAIDs such as ketorolac are thought to have deleterious effects on bone healing and fracture repair, although the actual clinical effects appear to be controversial at this point. In some studies, chronic NSAID use for more than 3 months was associated with lower fusion and success rates (Deguchi et al., 1998; Harder and An, 2003).
NSAIDs are thought to principally affect the bone morphogenetic proteins (BMPs) (Harder and An, 2003), which are a class of osteoinductive proteins that play an important role in bone growth and are essential for the growth and development of skeletal tissue and for bone regeneration during fracture repair (Kanakaris and Giannoudis, 2008; Dean et al., 2009). Martin and colleagues (1999), using a rabbit model of spinal fusion, investigated the effects of ketorolac on graft healing. Compared with a saline control group, less than half of the rabbits receiving ketorolac had a stable bone graft. However, the coadministration of a recombinant BMP with ketorolac to a third group of rabbits resulted in a 100% successful fusion rate. The investigators concluded that the effects of the NSAID could be completely reversed by the administration of a BMP (Martin et al., 1999). Clinical trials using recombinant BMPs to improve graft healing are in only the preliminary stage (Khan et al., 2002; Sandhu and Khan, 2003). Initial efforts to evaluate the effects of cyclooxygenase-2 (COX-2)–specific inhibitors of bone healing have produced similar results (Gilron et al., 2003). The healing of stabilized tibial fractures in COX-2–deficient mice was significantly delayed, as was intramembranous calvarial bone formation. These bone-healing deficiencies were reversed by the administration of prostaglandin E2 and BMP-2 (Zhang et al., 2002; Clark et al., 2009). In some studies, COX-2 inhibition seemed to have less severe effects on bone healing than nonspecific COX-1 and COX-2 NSAIDs (Gerstenfeld et al., 2003).
Clinically, because of limited data, the effects of NSAIDs on bone healing remain controversial (Vuolteenaho et al., 2008). In a retrospective analysis of more than 300 patients undergoing spinal fusion who received a COX-2 inhibitor (ketorolac) or no NSAIDs for the first 5 postoperative days, patients who received ketorolac had a threefold increase in nonunion rate compared with COX-2 recipients or controls (Maxy and Glassman, 2001; Gajraj, 2003). On the other hand, in a retrospective study of 405 patients undergoing spinal fusion, no difference was noted in fusion rates between those who received ketorolac for the first 48 hours postoperatively and those who did not (Pradhan et al., 1976).
Joint disorders
Arthrogryposis Multiplex Congenita
Arthrogryposis multiplex congenita (AMC) consists of a heterogeneous group of disorders characterized by nonprogressive congenital joint contractures. Fetal akinesia results in the birth of a baby with multiple curved, rigid joints. The incidence is 1 in 3000 live births. Because of their extensive contractures, tense skin, and minimal muscle mass and subcutaneous tissue, these children have been described as looking like thin wooden dolls. Arthrogryposis is a clinical finding that is characteristic of a vast number of disorders (Bamshad et al., 2009). In the past, an in utero, self-limited anterior horn cell disease was proposed (Bonilla-Felix, 2004). AMC is now recognized as resulting from fetal neurogenic, myogenic, or connective tissue abnormalities, as well as maternal disorders such as maternal myasthenia gravis. The child’s neurologic examination is fundamental to determining the etiology of AMC. The AMC of children with normal neurologic examinations results from amyoplasia, a distal arthrogryposis, a generalized connective tissue disorder, or fetal crowding. Children with amyoplasia frequently have midfacial hemangiomata, and 10% have gastroschisis or intestinal atresia. The distal arthrogryposes are a group of 10 autosomal dominant disorders with mutations in the genes that encode proteins of the contractile apparatus of fast-twitch myofibers (Sung et al., 1993). Children with these disorders have characteristic congenital contractures of two or more different body areas. The AMC of children with abnormal neurologic examinations is the result of developmental central nervous system abnormalities, spinal muscular atrophies, peripheral neuropathies, and myopathies (Takano et al., 2001). Mutations in the genes that encode for the neuromuscular junction’s acetylcholine receptor proteins appear as AMC (Burke et al., 2003). AMC has also been associated with spinal cord dysplasia, lung hypoplasia, renal tubular dysfunction, and micrognathia (Narkis et al., 2007a, 2007b).
The anesthesia management of children with AMC is complicated by associated congenital abnormalities, an abnormal upper airway, and positioning difficulties (Martin and Tobias, 2006). Arthrogryposis is associated with congenital heart disease, pulmonary hypertension, cor pulmonale, and urogenital anomalies. The distal arthrogryposis disorders can be associated with Freeman-Sheldon syndrome, as well as the presence of trismus, cleft palate, or pulmonary hypertension (Laishley and Roy, 1986; Stevenson et al., 2006; Toydemir et al., 2006; Toydemir and Bamshad, 2009). Decreased pulmonary reserve that results from pulmonary hypoplasia and scoliotic restrictive lung disease may potentiate hypoxemia and may necessitate postoperative ventilatory support.
Patients with arthrogryposis have micrognathia, a high arched palate, and a short and rigid neck, making tracheal intubation difficult and at times impossible (Szmuk et al., 2001). Direct laryngoscopy and intubation become more difficult as the patient ages, because craniofacial involvement often progresses with growth. Alternative strategies to direct laryngoscopy and tracheal intubation, such as the use of the laryngeal mask airway with or without the use of a tube exchanger, or fiberoptics, have been employed successfully in this disorder (Szmuk et al., 2001). The extensive contractures, tense skin, and minimal muscle mass and subcutaneous tissue pose challenges for intraoperative positioning and IV access.
Children with Freeman-Sheldon syndrome, in particular, are prone to hyperthermia and muscular rigidity responses to inhaled anesthetics and a neuroleptic malignant syndromic response to metoclopramide (Madi-Jebara et al., 2007). Although the response to nondepolarizing relaxants has been reported to be extremely variable, the use of short-acting nondepolarizing agents in association with careful monitoring of neuromuscular function has been successful in these patients.
The association of intraoperative hyperthermic crises with AMC has been sporadically reported. Hopkins and colleagues, in reporting three cases of hyperpyrexia, reviewed the literature of nine additional cases (Hopkins et al., 1991). In nine cases, hyperthermia, tachycardia, and hypercarbia were observed intraoperatively. Six episodes were assumed to be malignant hyperthermia, and dantrolene therapy was immediately instituted. Two cases responded rapidly to aggressive cooling. One patient received a nontriggering anesthetic. The halothane contracture test was not performed in any of these reported cases. In two large series of children with AMC who were anesthetized with triggering agents, no confirmed cases of malignant hyperthermia were reported (Baines et al., 1986). In light of the absence of laboratory confirmation, the occurrence of a hyperthermic crisis without exposure to triggering agents, and a large number of affected patients without hyperthermic response, Hopkins and colleagues (1991) concluded that these hypermetabolic responses are distinct from malignant hyperthermia. Patients with AMC are not considered to be at an increased risk for malignant hyperthermia (Laishley and Roy, 1986; Benca and Hogan, 2009).
Juvenile Idiopathic Arthritis (Formerly Juvenile Rheumatoid Arthritis)
Juvenile idiopathic arthritis (JIA) is the most common autoimmune disease of childhood, affecting 20 to 150 per 100,000 children (Helmick et al., 2008). It is defined as the presence of joint pain, stiffness, and swelling that persists for longer than 6 weeks, first occurring when the patient is younger than 16 years. The estimated incidence is 14 cases per 100,000 children per year. The International League of Associations for Rheumatology reclassified this disease as juvenile idiopathic arthritis to distinguish it from adult-onset rheumatoid arthritis (Weiss and Ilowite, 2005; Hayward and Wallace, 2009).
Therapy
Physical and occupational therapies are important adjuncts to medication in the management of JIA, because they help to maintain and improve range of motion, muscle strength, and skills for activities of daily living. Splints may be used to prevent contractures and to help in the work of improving range of motion. Arthroplasty may be needed for patients with severe deformities. Medications used to achieve these goals include NSAIDs, glucocorticoids, and disease-modifying antirheumatic drugs (methotrexate, sulfasalazine, leflunomide, cyclophosphamide, gold). Finally, biological agents, including tumor necrosis factor inhibitors (etanercept, infliximab, adalimumab, the interleukin-1 inhibitor anakinra, and the B-cell depleter rituximab), have all been added to the treatment armamentarium with great success (Hashkes and Laxer, 2005, 2006; Weiss and Ilowite, 2005).
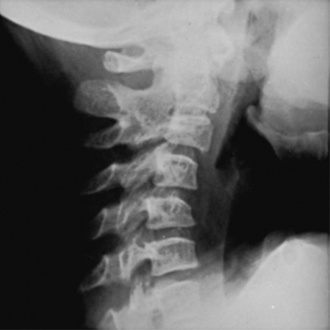
Anesthesia considerations in the child with JIA are principally focused on airway management. JIA involvement of the mandibular head and the temporomandibular joint (TMJ), which limits mouth opening, occurs in over 60% of children (Pedersen et al., 2001; Sidiropoulou-Chatzigianni et al., 2008). As a result of TMJ disease, the mandible’s growth is stunted, producing micrognathia in as many as 30% of children with JIA (Arabshahi and Cron, 2006). Magnetic resonance imaging (MRI) and ultrasound are useful imaging modalities to detect TMJ disease. Cervical spine disease is commonly seen in the systemic and the polyarticular forms of JIA, sometimes resulting in spinal fusion, which reduces cervical mobility (Fig. 26-5). Cervical stiffness is reported in 46% to 60% of patients. Radiographic changes are usually seen in the late stages of the disease and only in children with severe involvement. As a component of atlantoaxial rotatory subluxation or as a solitary manifestation, torticollis can develop, further increasing the degree of difficulty in airway management in these patients (Subach et al., 1998). Cricoarytenoiditis, an unusual manifestation of systemic JIA, can result in airway obstruction and severe distortion of the glottic anatomy (Jacobs and Hui, 1977; Vetter, 1994). Children with JIA should undergo a complete preoperative evaluation of the TMJ and cervical spine to assess for evidence of limited range of motion. The assessment should include dynamic radiographs.
Marfan Syndrome
Marfan syndrome (MFS) is an autosomal dominant connective tissue disorder caused by mutations of the gene FBN1 on chromosome 15q21, which is responsible for the production of fibrillin-1, a complex glycoprotein that is a major constituent of various connective tissue types (Dietz et al., 2005; Judge and Dietz, 2005). Mouse models of MFS have revealed that fibrillin-1 mutations perturb the signaling of local transforming growth factor-β (TGFβ), in addition to impairing tissue integrity. This discovery has led to the identification of a new disorder (Loeys-Dietz syndrome), which has significantly worse cardiovascular consequences than MFS, and which has led to new avenues of therapeutic intervention (Dietz et al., 2005; Judge and Dietz, 2005; Ramirez and Dietz, 2007). Cardiovascular (mitral valve prolapse), ocular, craniofacial, and musculoskeletal systems are frequently affected in these patients. The cardiovascular complications of MFS and Loeys-Dietz syndrome, specifically aortic root disease (dilation, aneurysm), aneurysms and dissections throughout the arterial tree, and generalized arterial tortuosity, are the major causes of morbidity and premature death of these patients (Dietz et al., 2005; Judge and Dietz, 2005; Ramirez and Dietz, 2007). The clinical features of the musculoskeletal abnormalities of MFS include scoliosis, joint hypermobility, and craniofacial abnormalities. The prevalence of MFS is 2 to 3 cases per 10,000 individuals; about 25% of patients have no family history.
Aortic size appears to correlate with central pulse pressure, and historically, children with MFS were maintained on β-blockade to slow aortic root growth. Recent literature is in conflict as to the efficacy of this approach. Ladouceur and colleagues (2007) found that children treated with β-blockade had decreased aortic root growth; however, Selamet Tierney and colleagues (2007) found that the rate of aortic root dilation was no different between treated and untreated children. A promising new approach to slow the progression of aortic root dilation uses the angiotensin II–receptor blocker losartan, an antihypertensive medication known to inhibit TGFβ signaling (Brooke et al., 2008). The long-term survival of patients with MFS and ascending aortas greater than 50 mm is increased with long-term medical therapy in combination with prophylactic cardiac surgery.
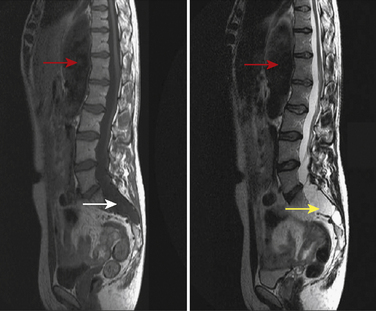
MFS affects the spine in several ways. The prevalence of scoliosis in MFS is greater than 50%; only 10% to 20% of these patients require any treatment. The scoliosis of MFS progresses at a faster rate than in the general population. Sagittal plane spinal deformities are also common in MFS; 40% of patients with MFS have a kyphosis greater than 50 degrees (Demetracopoulos and Sponseller, 2007). These patients are at risk for atlantoaxial translocation with neck flexion and extension (Hobbs et al., 1997). Patients undergoing seemingly routine, uneventful direct laryngoscopy and endotracheal intubation can develop atlantoaxial rotatory subluxation, manifested as unresolved torticollis and neck pain in the postoperative period. These events are thought to result from abnormal bone morphology, the abnormal shape of the atlantoaxial facet, or the laxity of ligaments (Herzka et al., 2000). In a series of 100 patients with MFS, greater than 50% had evidence of increased atlantoaxial translation. The preadolescent population had a greater range of motion than did the adolescent or adult groups (Hobbs et al., 1997). Atlantoaxial subluxation has been reported as a cause of sudden death in patients with MFS (MacKenzie and Rankin, 2003). MFS is also associated with dural ectasia, a widening of the dural sac and nerve roots in the caudal portion of the spine. The MRIs of children with MFS suggest dural ectasia in 40% of cases. Dural ectasia can manifest as headache, proximal leg pain, leg weakness and numbness, and abdominal, genital, and rectal pain. Bony erosion can lead to myelomeningocele formation (MacKenzie and Rankin, 2003). As demonstrated in Figure 26-6, the presence of a dural ectatic segment (dilation of the dural sac) could have dramatic implications for neuraxial regional anesthetics, and MRI of the lumbosacral spine should be obtained preoperatively when this technique is contemplated in patients with MFS.
Syndromes of disproportionate short stature: dwarfism
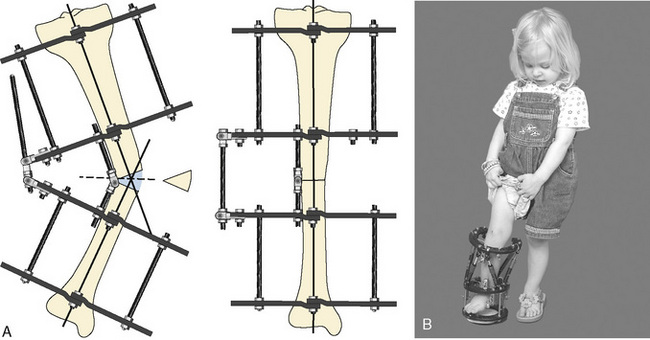
Children with dwarfism are unified solely by their phenotype of disproportionate short stature and associated limb deformities. More than 35,000 dwarfs are estimated to live in the United States, and the individual osteochondrodysplasias and mucopolysaccharidoses that produce this phenotype number well over 350 (Berkowitz et al., 1990). Achondroplasia is the most common form of dwarfism, with an incidence of 1 in 30,000 live births. It affects more than 250,000 individuals worldwide (Shirley and Ain, 2009). More than 95% of patients have the same point mutation in the FGFR3 gene, on chromosome 4p16. FGFR3 codes for fibroblast growth factor receptors, which normally function as an inhibitor to linear bone growth; achondroplastic mutations exaggerate this normal physiologic function (Horton et al., 2007). The mucopolysaccharidosis (MPS) syndromes (i.e., Hurler’s syndrome [MPS I-H], Hunter’s syndrome [MPS II], Morquio’s syndrome [MPS IV], and Scheie’s syndrome [MPS V]) are few in number but pose significant anesthesia challenges. Limb-lengthening techniques, cervical decompression, joint replacement, limb realignment, and bone marrow transplantation for patients with mucopolysaccharidoses are but a few of the procedures that are increasingly being performed on these patients. The number of diseases that constitute the dwarfing syndrome is enormous, and the limited anecdotal anesthesia experience for many of these precludes an encyclopedic review of all of the syndromes. Nevertheless, the common pathologic conditions to a large number of the dwarfing syndromes listed in Box 26-3 should be evaluated in the preoperative preparation of an affected patient.
Box 26-3 Systemic Manifestations of the Dwarfing Syndromes
Airway Abnormalities
The anesthesia management of dwarfs is frequently complicated by anatomic abnormalities of the upper airway and by difficulty in visualization of the larynx during direct laryngoscopy. Inability to intubate is the major cause of morbidity and mortality when anesthetizing dwarfs (Berkowitz et al., 1990). Upper airway obstruction is frequently a result of thickened pharyngeal and laryngeal structures, narrowed nasal passages, micrognathia, copious secretions, pharyngeal hypoplasia, and tracheal narrowing. It is seen most frequently in patients with mucopolysaccharidoses, diastrophic dysplasia, camptomelic dysplasia, severe diastrophic dysplasia, and Russell-Silver syndrome. Some patients demonstrate upper airway obstruction even in the awake state. Airway patency can be severely affected by positional changes alone. Some patients with achondroplasia, Morquio’s syndrome, and metatropic dysplasia maintain a patent airway with the neck extended but completely obstruct when the neck is flexed. Sedation and general anesthesia often result in complete upper airway obstruction. Direct laryngoscopy and tracheal intubation are extremely difficult to perform in many dwarfs. Children with mucopolysaccharidoses have been described as presenting the worst airway problem in pediatric anesthesia; 54% of patients with MPS I are difficult intubations, and 23% are intubation “failures” (Martins et al., 2009). Endotracheal intubation is often hampered by inadequate laryngeal exposure from a shortened neck and protruding tongue, infiltration of the glottic and epiglottic structures with abnormal mucopolysaccharide, enlarged tonsils and adenoids, and subglottic, tracheal, and bronchial narrowing. In contrast, the patient with achondroplasia, who may also have narrowed nasal passages and pharyngeal hypoplasia because of cranial base angulation and midface hypoplasia, rarely develops periinduction airway obstruction and can be easily managed with a face mask (Monedero et al., 1997).
Anesthesia Management
Preoperative sedative drugs should be avoided in patients prone to upper airway obstruction. Before induction, IV access is obtained, and an antisialagogue is administered. If the potential for severe upper airway obstruction and difficult intubation is anticipated, the equipment and personnel required to establish an emergency airway should be present before the induction of anesthesia (see Chapter 13, Induction, Maintenance, and Recovery, and Chapter 12, Airway Management). Spontaneous ventilation is mandatory. Often, the only way to identify the glottis is by observation of the air bubbles during spontaneous ventilation. An inhalational induction with high concentrations of oxygen and sevoflurane or a continuous IV infusion of propofol or ketamine are equally effective approaches in this situation. After an adequate anesthesia plane is achieved, endotracheal intubation can be accomplished by direct laryngoscopy or by fiberoptic-guided bronchoscopy. Alternatives include insertion of the lightwand and fiberoptic intubation by means of a laryngeal mask airway. Neuromuscular relaxants are avoided until the airway is secured. Rarely, a tracheostomy performed while the patient is awake may be the safest approach. The examiner must avoid neck manipulation, particularly neck flexion during laryngoscopy, in patients with atlantoaxial instability or foramen magnum stenosis.
Pulmonary Dysfunction
The pulmonary dysfunction common to children with a dwarfing syndrome is multifactorial in origin. Restrictive lung disease is a consequence of thoracic cage dystrophy (e.g., Jeune’s syndrome) or scoliosis, and it results in reduced lung volumes, ventilation/perfusion mismatching, progressive hypoxemia, and hypercarbia. Obstructive or central sleep apnea causes pulmonary hypertension, behavioral disorders, substantial morbidity, and sudden death (Sisk et al., 1999). Structural abnormalities, particularly in patients with MPS, may cause intrathoracic obstruction. Children with MPS frequently suffer from chronic sinus infections that result from mucosal alterations and an increased viscosity of their secretions, a combination that produces a milieu of chronic airway inflammation (Martins et al., 2009). The preoperative assessment of these patients must include an evaluation of pulmonary function and a sleep study. The finding of central apnea necessitates a neuroradiologic evaluation of the cervical spine and foramen magnum. The presence and severity of pulmonary hypertension can be determined by an electrocardiography and echocardiography.
Cardiac Dysfunction
Children with dwarfing syndromes have a variety of causes for cardiac dysfunction. A high incidence of coexisting structural heart disease, such as atrial septal defects, occurs in a number of the dysplasias. Acquired valvular heart disease is a common complicating feature of children with MPS and is occasionally found in osteogenesis imperfecta. Ischemic heart disease may result from infiltrative mucopolysaccharides or from the consequences of cor pulmonale and longstanding pulmonary hypertension. Cardiomyopathy is a severe problem for children with MPS (Mohan et al., 2002; Rigante and Segni, 2002). Physical examination, chest radiography, electrocardiography, and especially echocardiography are useful in diagnosing the extent of cardiovascular involvement. The most reliable indicators of pulmonary hypertension are the presence of tricuspid regurgitation and prolongation of the right systolic time interval. The electrocardiogram should be reviewed for evidence of myocardial ischemia, particularly in patients with Hurler’s syndrome or Hunter’s syndrome.
Neurologic Dysfunction
Cervicomedullary compression and hydrocephalus are the main neurologic concerns in patients with dwarfism. The causes of cervical cord compression include atlantooccipital instability, odontoid process hypoplasia, foramen magnum stenosis, abnormal meningeal glycosaminoglycan deposition, and, infrequently, cervical scoliosis (Kachur and Del, 2000). Foramen magnum stenosis is a frequent complication of achondroplasia. Physical findings or a history consistent with upper motor neuron weakness (e.g., progressive weakness, hyperreflexia, abnormal plantar response), sleep apnea, cyanosis, or respiratory distress is suggestive of cervicomedullary compression. Flexion and extension neck films should be obtained to determine the degree of cervical spine instability in affected patients. Achondroplasia and MPS are associated with increased intracranial pressure; hydrocephalus arises from the impaired absorption of cerebrospinal fluid by the arachnoid granulations (Sheridan and Johnston, 1994).
Osteogenesis imperfecta
Osteogenesis imperfecta (OI) is another of the dwarfing syndromes that involves unique problems. It is a heritable disorder of collagen production with varying degrees of severity. Its cardinal manifestation is bone fragility, and it frequently appears in childhood with multiple fractures after little or no trauma. Ninety percent of individuals with OI have mutations of the pro-α1 or pro-α2 chains of type I collagen (COL1A1 or COL1A2) genes. A number of those without collagen mutations have mutations involving the enzyme complex responsible for posttranslational hydroxylation of the position 3 proline residue of COL1A1. Two of the genes encoding proteins involved in that enzyme complex, LEPRE1 and cartilage-associated protein, when mutated, have been shown to cause autosomal-recessive osteogenesis imperfecta, which has a moderate to severe clinical phenotype, often indistinguishable from OI types II or III (Basel and Steiner, 2009; Shapiro and Sponsellor, 2009). The pathogenetic molecular mechanism of these deletions dictates the severity of the clinical features. Chain exclusion, in which the abnormal chain is not incorporated into the collagen triple helix, produces a milder phenotype. Chain inclusion, in which the abnormal chain is incorporated into the collagen triple helix, results in a defective helix and a more severe phenotype (Basel and Steiner, 2009). The effects of OI manifest as abnormalities of bone, teeth, sclera, and ligaments. The four clinical subtypes are listed in Table 26-7 (Cohen, 2002).
| Type | Features |
| I | Mildest form Mild bone fragility; bimodal fracture curve (first peak: between 1 year and puberty; second, smaller peak: postmenopausally in women and after 70 years in men); normal stature; blue sclerae; deafness in some cases, most commonly occurring in second decade; dominant inheritance |
| II | Most severe form: perinatal lethal form Half do not survive day 1, and 90% are dead by 1 week; extreme short stature; short, bowed long bones, particularly lower limbs; ribs have beaded appearance from recurrent fractures; respiratory insufficiency; absence of calvarial mineralization; dominant mutation |
| III | Progressively deforming type Many fractures; thin ribs with discrete fractures; curvature of spine that may be severe enough to reduce pulmonary reserve; severe short stature; deafness; dentinogenesis imperfecta; dominant mutation; rare recessives unlinked to type I collagen genes |
| IV | Mild-to-moderate bone fragility; short stature; deafness in some cases; dentinogenesis imperfecta; dominant inheritance |
Adapted from Cohen MM Jr: Some chondrodysplasias with short limbs: molecular perspectives, Am J Med Genet 112:304, 2002.
The hallmarks of this disease are bony fragility and multiple fractures after even innocuous trauma. Scoliosis and kyphosis are common, producing significant restrictive lung disease and pulmonary hypertension, especially in OI type III. A majority of patients with OI develop valvular regurgitant lesions. Medical management includes the use of bisphosphonates, which are anti-bone-resorptive drugs (Phillipi et al., 2008; Castillo and Samson-Fang, 2009). Some data suggest their efficacy in reducing fracture frequency, increasing bone density, promoting remodeling of previously crush-fractured vertebrae, reducing chronic pain, and improving mobility in children and infants with osteogenesis imperfecta (Cheung and Glorieux, 2008; Phillipi et al., 2008; Castillo and Samson-Fang, 2009).
Anesthesia Management
The mainstay of OI management is orthopedic surgery and rehabilitative physiotherapy (Cheung and Glorieux, 2008; Basel and Steiner, 2009). The hallmark of anesthesia management is to handle these patients very gently. Indeed, fractures may occur from simple procedures such as applying a tourniquet or measuring blood pressure or while positioning the patient on the operating room table. Airway management may cause fractures, and the physician must pay particular attention to the teeth, mandible, and cervical spine. Occasionally, visualization of the airway is difficult, and the use of a laryngeal mask airway, as in other patients with difficult airways, may be very helpful (Kostopanagiotou et al., 2000; Asai and Shingu, 2001). Patients with OI have a hypermetabolic state and become hyperthermic during anesthesia (Benca and Hogan, 2009). This condition is not malignant hyperthermia, even though a few case reports of true malignant hyperthermia have been reported in these patients (Rampton et al., 1984; Porsborg et al., 1996). Many have recommended the use of a total IV anesthesia technique (Karabiyik et al., 2002). The routine pediatric anesthesia practice of preventing intraoperative hypothermia, such as using warming blankets and heated, humidified gasses, should be tempered, and antimuscarinics such as atropine and glycopyrrolate should be used judiciously. Some patients with OI bruise easily as a result of a presumed platelet abnormality. Bleeding and hemorrhage are rare, but approximately 30% of these patients have abnormal bleeding times, capillary fragility, and reduced levels of factor VIII.
Osteopetrosis
Osteopetrosis (i.e., marble bone disease) is an inherited disease with diminished bone resorption resulting from osteoclastic abnormalities, whose clinical hallmark is hard and brittle bones that fracture very easily (Balemans et al., 2005; Del et al., 2008). The metabolic disorders in this group are genetically heterogeneous. Autosomal-dominant osteopetrosis is the result of either abnormal osteoclast resorption or abnormal low-density lipoprotein receptor–related proteins (Balemans et al., 2005; Del et al., 2008). Clinically, osteopetrosis appears in three forms: infantile autosomal recessive (“malignant”), intermediate autosomal recessive, and autosomal dominant. Children with the severe form of osteopetrosis have insufficient bone marrow to support normal hematopoiesis; thrombocytopenia, anemia, and infectious complications are life threatening in the first decade of life. Patients with all forms of osteopetrosis are at risk for pathologic fractures (Tolar et al., 2004). Stem-cell transplantation is the only curative treatment for malignant osteopetrosis (Driessen et al., 2003). Patients with osteopetrosis frequently require anesthetics for bone marrow examinations and for treatment of their pathologic fractures.
Airway and cervical spine issues dominate the perioperative concerns of those caring for a child with osteopetrosis. Burt and coworkers (1999), in a series of 65 anesthesias for children with osteopetrosis, reported that the rate of airway management difficulties was much higher for this group of children than for the other children anesthetized at their institution. Mandibular abnormalities and TMJ immobility contributed to the difficulty of orotracheal intubation, and abnormalities of the nasal turbinates made nasotracheal intubation difficult. Cervicomedullary stenosis limited optimal head positioning, and concurrent thrombocytopenia exacerbated airway instrumentation and limited the options of a regional anesthetic. Afflicted children are also at risk for spontaneous cervical fractures. Children with the malignant infantile osteopetrosis variant have obstructive sleep apnea and nocturnal hypoxemia (Kasow et al., 2008). No particular anesthesia technique was deemed superior, but there was an emphasis on meticulous preoperative airway preparation with the ready availability of the resources for emergency airway management. Placement of intraosseous needles for emergency vascular access is difficult and nearly impossible in patients with this disease.
Cerebral palsy
Cerebral palsy (CP) is a static encephalopathy defined as a nonprogressive disorder of posture and movement that manifests as poor muscle control, weakness, and increased muscle tone (Rosenbaum, 2007). Epilepsy and abnormalities of speech, vision, and intellect that result from a defect or lesion of the developing brain are often associated. It is the most common childhood motor disability, occurring in 2 of 1000 live births (Paneth et al., 2006). The CP phenotype results from multiple etiologies. In premature infants, periventricular leukomalacia is commonly associated with the development of CP. In term infants, early antenatal insults are attributed to be the cause of CNS injury and can manifest as events at the time of delivery. The most common etiologic factors are prematurity and birth weight greater or less than ideal weight for gestational age (Pharoah et al., 1996; Paneth et al., 2006; Rosenbaum, 2007).
CP can be classified by a description of the motor handicap in terms of physiology (major motor abnormality), topography (extremity involved), etiology, and functional capacity (Table 26-8). Although the CNS lesion is static, the degree of impairment can change with time. CP is commonly associated with a spectrum of developmental disabilities, including mental retardation, epilepsy, and visual, hearing, speech, cognitive, and behavioral abnormalities. The motor handicap may be the least of the child’s problems. The necessity to treat each patient’s problems uniquely and to avoid generalization cannot be overemphasized. Some children with CP may be of normal intelligence but limited in their ability to communicate. Others with marked developmental delay may be difficult to separate from their parents because of natural fear and an inability to reason in a way expected of children with normal intelligence (see Chapter 9, Preoperative Preparation).
Several drugs are commonly used in treating spasticity, athetosis, dystonia, and seizures in patients with CP, and many of these drugs have significant anesthesia implications. Drugs used to treat spasticity include dantrolene, benzodiazepines, and baclofen. Incapacitating athetosis is treated with levodopa; dystonia, with carbamazepine and trihexyphenidyl. Seizures are commonly treated with phenobarbital, phenytoin, clonazepam, carbamazepine, and sodium valproate. The muscle spasticity of CP is thought to be caused by inadequate release of the inhibitor γ-aminobenzoic acid (GABA) in the dorsal horn of the spinal cord, resulting in a relative excess of excitatory glutamate on the alpha motor neurons, which produces simultaneous contraction of agonist and antagonist muscle groups. The symptoms of spastic diplegia can be treated surgically with rhizotomy, a procedure in which the roots of the spinal nerves are divided (Albright, 1992). Spasticity can also be treated medically by administering a continuous intrathecal infusion of baclofen or by local intramuscular injection of botulinum toxin (Albright, 1996a). Baclofen is a GABA agonist that binds to the GABA(B) receptor located in the dorsal horn of the spinal cord, where the primary sensory fibers end, producing presynaptic inhibition of monosynaptic and polysynaptic reflexes. Continuous intrathecal baclofen infusions are used to manage intractable and generalized spasticity while minimizing side effects (Albright, 1996b; Verrotti et al., 2006). Most patients will have improved range of motion, decreased painful muscle spasms, and improvements in independent function.
Intramuscular botulinum toxin A (BTX-A) is also used to relieve dynamic deformities that result from muscle spasticity. After injection, BTX-A is taken up in presynaptic terminals, inhibiting acetylcholine release and functionally denervating muscle fibers within 2 to 3 cm of the injection site. Focal controlled muscle weakness is produced, which reduces spasticity for up to 6 months (Bjornson et al., 2007). Tight heel cords may be treated conservatively with serial casting, with botulinum injection, or surgically with an Achilles tenotomy. The child’s particular spasticity and resulting joint contractures require vigilant positioning and padding in the operating room to avoid neurovascular compromise and skin ulcerations.
Preoperative concerns for children with CP focus on their pulmonary, gastrointestinal, and neurologic problems. This population is prone to aspiration pneumonia from gastroesophageal reflux and nasopharyngeal aspiration, as demonstrated by the finding that 75% of children with gastroesophageal reflux and delayed gastric emptying are neurologically impaired (Gisel et al., 2003; Ceriati et al., 2006). Many have chronic lung disease with a reactive component, and they suffer from frequent respiratory infections. Obstructive sleep apnea, seen in 20% to 50% of this patient population, has many causes, including bulbar dysfunction, neurogenic laryngomalacia, and decreased pharyngeal tone (Schwengel et al., 2009). Children with developmental disabilities are more than three times more likely to develop sedation-related hypoxia (Kannikeswaran et al., 2009).
Airway management can be complicated by restricted TMJ range of motion and by poor and malpositioned dentition. Patients with CP commonly have gastroesophageal reflux disease, dysfunctional swallowing, and severe food refusal, all contributing to suboptimal nutritional status that portends increased perioperative complications. Children with severe CP have a significant narrowing of their palates, placing them at increased risk for airway obstruction. Children with CP have a higher incidence of latex allergy than the general population. Thirty percent of children with CP require antiepileptic medications (Singhi et al., 2003).
In most orthopedic surgical procedures performed in patients with CP, almost any anesthesia technique and combination of drugs can be used. Potent inhaled anesthetics, muscle relaxants (including succinylcholine), hypnotics, sedatives, opioids, and local anesthetics have been used safely. Children with CP appear to have a lower MAC than unaffected children (Frei et al., 1997). Succinylcholine does not cause hyperkalemia, and it can be safely administered to patients with CP. They have a slightly increased sensitivity to succinylcholine compared with normal children (Dierdorf et al., 1985; Theroux et al., 1994). Resistance to nondepolarizing muscle relaxants and rapid recovery from neuromuscular blockade have been reported in this patient population, which may be explained by the increase in extrajunctional acetylcholine receptors (Theroux et al., 2002). Propofol requirements are significantly lower in noncommunicative CP children than in their unaffected peers (Saricaoglu et al., 2005).
Postoperative pain management is important in the care of patients with CP. The surgical procedures, particularly those for relieving spasticity, are extremely painful. The child severely affected with CP may be unable to communicate his or her pain, and health care providers are often unable to accurately assess the severity of postoperative pain. Parents and other routine caregivers are invaluable in assessing the pain of these patients. The Non-Communicating Children’s Pain Checklist–Postoperative Version has been validated for children with intellectual disabilities (Breau et al., 2002, 2003). Postoperative pain is treated with continuous epidural (caudal or lumbar) infusions. Lidocaine (1.5 to 2.0 mg/kg per hour) plus fentanyl (0.5 mcg/kg per hour), chloroprocaine (3 mg/kg per hour) plus fentanyl (0.5 mcg/kg per hour), and bupivacaine (0.625 to 1 mg/mL, 0.2 to 0.4 mg/kg per hour) with or without fentanyl (0.5 mcg/kg per hour) or hydromorphone (2 to 4 mcg/kg per hour) have all been used. Muscle spasms are virtually universal and are treated prophylactically with IV diazepam. The addition of clonidine to the postoperative epidural infusion at a dosage of 0.08 to 0.12 mcg/kg per hour is also effective at relieving muscle spasm. The management of posterior rhizotomy requires special attention. Often used in severe spasticity, this surgical procedure requires stimulation of the dorsal roots intraoperatively and observation of muscle response (see Chapter 22, Anesthesia for Neurosurgery) (Farmer and Sabbagh, 2007).
Ilizarov method
Professor G. A. Ilizarov introduced the concept of distraction osteogenesis in the 1950s (Herbert et al., 1995). Working as a general practitioner in Siberia, he found himself treating many patients with chronic osteomyelitis associated with bone loss and many veterans of World War II who had developed fracture nonunions. Using materials from the metal factories at which many of his patients were employed, he fashioned external fixators and transosseous wires to induce the formation of new bone between freshly cut osseous surfaces that are gradually pulled apart. Using this technique, he was able to salvage limbs that otherwise would have been amputated. For many years, he worked in isolation, with his techniques remaining unknown in most of the world (Fig. 26-7).
Over the past 20 years, this method has undergone significant refinements, and it is now employed in the treatment of congenital limb and other skeletal deformities, acquired short limbs, and angular deformities, and in the reconstruction of large bony defects that result from trauma, tumor excision, infection, and fracture nonunions (Herbert et al., 1995). Distraction osteogenesis has also been used to treat the numerous syndromes associated with micrognathia and retrognathia, such as the Pierre Robin syndrome, Treacher Collins syndrome, Nager’s syndrome, velocardiofacial syndrome, and Pfeiffer’s syndrome, all of which can result in airway obstruction (McCarthy et al., 2001; Sidman et al., 2001).
The success of the Ilizarov technique of distraction osteogenesis depends on adherence to the principles of tension-stress phenomena. These include a low-energy osteotomy to preserve periosteal blood supply; a slow, incremental distraction rate to preserve soft tissue blood supply; and maintenance of full function of the extremity. Bone healing is promoted by the biological stress of walking on or flexing a broken limb, causing a trampoline-like effect of pulling and contracting that stimulates bone growth and healing. Using a corticotomy that preserves blood flow to the periosteum and the medullary canal, a gap between healthy, vascular-sufficient bone is created. Wires are inserted into the bone above and below the osteotomy and are attached under tension to an external fixator at 90-degree angles to the plane of the deformity. After an initial latency period, the osteotomy is gradually distracted at a rate of 1 mm/day in four incremental steps. The external fixator serves as the distracting device and provides optimal mechanical stability so that weight bearing and range of motion on the operative limb are possible on the second hospital day. The early functional use of the affected limb stimulates callus formation and osteoblastic activity (Birch and Samchukov, 2004).
Anesthesia Considerations
Because the Ilizarov procedure is applied to children with a wide spectrum of diseases, including those with complex congenital musculoskeletal anomalies, the anesthesia implications of their coexisting diseases often take precedence over those of the operative procedure. These operative procedures are often long and complex but are associated with few hemodynamic perturbations and only modest blood loss. The perioperative complications of the Ilizarov procedure that are affected by anesthesia management include nerve injury and the need for intact motor function and optimal analgesia in the postoperative period. Nerve injury can occur during pin placement and during the distraction process. To recognize inadvertent surgical trauma to nerves in the operative field, neuromuscular relaxation is avoided so that muscle contractions can be recognized. Postoperative surgical pain can be intense in the first 48 hours, and these patients are encouraged to begin physical therapy on the first postoperative day, with an emphasis on passive range of motion and weight bearing (Paley, 1990).
Children undergoing an Ilizarov procedure are often anesthetized with a general anesthetic supplemented with an epidural or peripheral nerve catheter. In most cases, endotracheal intubation is accomplished with short-acting neuromuscular blockade or with deep inhalational or IV anesthesia. Children with concurrent airway or cervical spine anomalies are intubated with a fiberoptic bronchoscope or with a laryngeal mask airway–guided approach during spontaneous breathing. After the induction of general anesthesia, an epidural or peripheral nerve catheter is placed. If an epidural is used, a continuous epidural infusion of 0.8 to 1 mg/mL of bupivacaine with 1 mcg/mL of fentanyl can be started in the operating room at an infusion rate of 1 mL/kg per hour. Anesthesia is maintained with small amounts of opioids and a low concentration of inhaled anesthesia agents. The epidural infusion of bupivacaine and fentanyl is continued for the first 24 to 36 hours postoperatively and augmented with acetaminophen (see Chapter 16, Regional Anesthesia). In patients who do not have a neuraxial or peripheral nerve catheter in place, IV PCA can be used.
Tourniquets
Pneumatic tourniquets are commonly used to provide a dry operative field and limit intraoperative blood loss during extremity surgery (Kam et al., 2001). Modern pneumatic tourniquets consist of three basic components: a cuff that is similar to a blood pressure cuff and is wrapped around a patient’s limb and then inflated, a compressed gas source, and a mechanism with a pressure gauge that is designed to maintain pressure in the cuff at a set value. After elevation and application of an Esmarch’s bandage to exsanguinate the limb, the tourniquet is applied over smooth padding and inflated. Older methods inflate the tourniquet to a pressure based on the patient’s systolic pressure. Recent practice is to determine the limb occlusion pressure and add a margin of 75 mm Hg for lower extremity surgery and 50 mm Hg for upper extremity procedures. Limb occlusion pressure, first suggested by Cushing, is the minimum pressure necessary to occlude arterial blood flow, as determined automatically or via palpation or pulse oximeter. To prevent accidental injury, the cuff should have a width that is greater than one half of the limb’s diameter and an accurate pressure gauge, and the cuff should be inflated to the lowest possible inflation pressure recommended. The duration of inflation should also be carefully monitored (Tuncali et al., 2006; Reilly et al., 2009).
The length of time that the tourniquet can remain safely inflated is controversial. The most common recommendation, 2 hours, is based on the finding that cellular ischemic changes such as mitochondrial swelling, myelin degeneration, glycogen storage depletion, and Z-line lysis are reversible if the tourniquet is inflated for no more than 1 to 2 hours (Patterson and Klenerman, 1979). The deleterious effects of tourniquet inflation include pain while the tourniquet is inflated (“tourniquet pain”), metabolic and hemodynamic changes that occur during tourniquet inflation and deflation, and damage to blood vessels and muscle if the tourniquet is inflated for excessive periods. The use of pneumatic tourniquets has been associated with perioperative neuropathy (Welch et al., 2009).
The hemodynamic consequences of tourniquet application include increases in blood and central venous pressures. Kaufman and Walts (1982) reported an overall 30% increase in blood pressure during tourniquet inflation. The blood pressure response is more exaggerated in patients under general anesthesia than in those undergoing regional blockade (Valli et al., 1987). Tourniquet-induced hypertension can be prevented by the preoperative administration of 0.25 mg/kg of ketamine (Satsumae et al., 2001). Limb exsanguination and tourniquet inflation can redistribute 15% of the total blood volume to the general circulation rapidly. Central venous pressure increases of up to 14 mm Hg have been reported in adults with the application of bilateral tourniquets. The clinical significance of central venous pressure reduction that accompanies tourniquet deflation primarily depends on the presence of preexisting cardiac dysfunction.
Tourniquet-induced hyperthermia, usually 1° to 2° C, occurs within 90 minutes of tourniquet inflation and appears to be the result of decreased cutaneous heat loss from skin distal to the tourniquet. The effect is more profound if bilateral tourniquets are used (Estebe et al., 1996). The combination of metabolic acidosis and hypercarbia that occurs after tourniquet release is the result of reperfusion and the washing out and reentry into the central circulation of lactic acid, potassium, and other toxic substances that accumulated in tissues during tourniquet-induced limb ischemia. Accompanying hemodynamic effects include hypertension and hypotension, tachycardia, bradycardia, and, rarely, ventricular dysrhythmias. These effects are self-limited and usually resolve over a few minutes. Other than increasing minute ventilation in patients who are being mechanically ventilated, most pediatric patients rarely or never require specific therapy for tourniquet deflation.
Clubfoot
Clubfoot (talipes equinovarus) is a relatively common congenital deformity that occurs in 1 of 1000 live births (Alvarez et al., 2008; Dobbs and Gurnett, 2009). Most clubfoot deformities are bilateral and can occur in otherwise normal children who have no syndrome, cytogenetic abnormality, or extrinsic cause for the deformity (Drvaric et al., 1989; Cummings et al., 2002). Clubfoot is also commonly seen in patients with neuropathies and myopathies such as myelodysplasia, CP, arthrogryposis, spinal muscular atrophy, and muscular dystrophy (Drvaric et al., 1989; Cummings et al., 2002). Clubfoot has degrees of severity, and the treatment is individualized in each patient. In some patients, manipulation and casting can restore the bony architecture. In others, surgery is required. When to perform surgery is controversial. Some surgeons prefer to operate on neonates; others operate when the affected child is 3 months, 6 months, or older than 1 year.
The patient is positioned prone (for Cincinnati and two-incision techniques) or supine (for Turco incision), and the procedure is performed with a tourniquet. The surgery involves soft tissue release, including posterior, medial, plantar, and lateral releases; tendon transfer and lengthening; and pin fixation (Drvaric et al., 1989; Cummings et al., 2002). At the completion of surgery, the foot and the calf to the middle thigh are well padded and casted. Postoperatively, patients experience intense pain. Virtually any general anesthesia technique can and has been used for this surgery. Because postoperative pain is such an important aspect of the care of these patients, a combined regional (epidural or sciatic nerve block) and general anesthesia technique is commonly used. The epidural catheter is used intraoperatively and postoperatively.
The percutaneous Ponseti approach to the clubfoot involves weekly stretching of the deformity, followed by application of a long leg cast. By 4 to 5 weeks, all components of the deformity are corrected, with the exception of the equinus. The equinus is addressed with a percutaneous Achilles tenotomy, followed by a final long leg cast (Herzenberg et al., 2002; Janicki et al., 2009).
Developmental dysplasia of the HIP
Developmental dysplasia of the hip (i.e., congenital hip dislocation) is a spectrum of abnormalities of the developing hip joint that ranges from shallowness of the acetabulum to capsular laxity and instability to frank dislocation (Eastwood, 2003; Scherl, 2004). Developmental dysplasia of the hip is relatively common, occurring in 1.5 to 20 of 1000 live births (Swaroop and Mubarak, 2009). Previously known as congenital hip dislocation, it is now understood to be a condition that is not purely congenital but one that develops over time. It is common in children born by breech delivery. Screening in the newborn period consists of looking for asymmetries in skin folds, range of abduction, and height of the knees, as well as using provocative testing. The latter, known as the Ortolani test, elicits a click or clunk as the femoral head is moved in and out of the acetabulum. In the absence of other developmental disabilities, developmental dysplasia of the hip does not cause significant functional disability even if the diagnosis is missed or delayed; however, if untreated, it can lead to degenerative hip arthritis. Ultrasound and MRI are useful for detecting hip dysplasia within the first weeks of life and valuable in following the course of treatment (Harding et al., 1997; Dwek, 2009).
Treatment is designed to relocate and stabilize the femoral head in the acetabulum. Bracing with the Pavlik harness (which prevents extension and adduction of the hip joint while allowing movement in the safe zone) and body casting are used for the first 6 months to 1 year of life. Virtually any general anesthesia technique, including caudal epidural blockade, can and has been used for casting and surgery (Castillo-Zamora et al., 2005).
Slipped capital femoral epiphysis
Slipped capital femoral epiphysis (SCFE) is a displacement of the femoral head in relation to the femoral neck through the growth plate during a period of rapid growth in adolescence (Aronsson et al., 2006). SCFE is common in obese teenagers and manifests with pain localized to the groin, the knee, or the distal thigh (Kocher et al., 2004). On physical examination, these children limp, and diagnosis is made by obtaining anteroposterior and frog-leg lateral radiographs of the pelvis. In 20% of patients, SCFE is bilateral on presentation, although only one side may be symptomatic.
Surgical management consists of placing one or two screws across the growth plate of the affected hip to prevent further slippage (Kocher et al., 2004; Aronsson et al., 2006). The pinning is done in situ, meaning that no attempt is made to reduce the epiphysis back to its original position; such maneuvers damage the blood supply to the femoral head and lead to avascular necrosis (Boero et al., 2003). Virtually any general anesthesia technique can and has been used for this surgery. Many of these patients have full stomachs when they present emergently; therefore, patients are at risk for pulmonary aspiration of gastric contents, necessitating a rapid-sequence induction of general anesthesia.
Fractures
The method by which to anesthetize a patient with a fracture depends on the urgency of the procedure, the risk for vomiting and aspiration, the child’s maturity, and the wishes of the parents and surgeon. Regional or general anesthesia is possible. Regional anesthesia may make it impossible to evaluate motor function even if dilute concentrations of local anesthetics are used. With general anesthesia, full-stomach precautions should be taken to minimize the risks of vomiting and pulmonary aspiration of gastric contents; thus, rapid-sequence induction and airway protection with an endotracheal tube are in order. A complete discussion on how to perform blocks of the upper and lower extremity, and how to manage peripheral nerve catheters, can be found in Chapter 16, Regional Anesthesia.
Fat embolism syndrome
Fat embolism develops in nearly all patients with bone fractures, or during orthopedic procedures, but is usually asymptomatic (Akhtar, 2009). Fat embolism syndrome (FES) is a collection of respiratory, hematologic, neurologic, and cutaneous symptoms and signs that are associated with trauma and other disparate surgical and medical conditions such as sickle cell acute chest syndrome and acute pancreatitis (Georgopoulos and Bouros, 2003). The incidence of the clinical syndrome is low (<1% in retrospective reviews), and embolization of marrow fat appears to be an almost inevitable consequence of closed long-bone fractures (Mellor and Soni, 2001; Parisi et al., 2002). Multiple fractures increase the incidence of FES to 30%. FES is characterized by the triad of hypoxemia, neurologic abnormalities, and a petechial rash (Box 26-4). The challenge to the pediatric anesthesiologist is to recognize the intraoperative manifestations of FES in a multiple-trauma patient or in a patient with an isolated long-bone fracture.
Pathophysiology
The pathophysiologic mechanisms that produce the FES remain controversial. In the most accepted mechanical hypothesis, bone injury disrupts the medullary canal, the adipose tissue, and the bone’s vasculature; a hematoma forms at the site of this injury. When the intramedullary pressure exceeds venous pressure, fat globules are forced into the circulation (Arai et al., 2007). Chylomicrons are destabilized by the effects of fat intravasation and form very large, circulating fat globules. Fat can be detected in pulmonary arterial samples in up to 70% of patients with long-bone or pelvic fractures, especially if the pulmonary artery catheter is wedged (Byrick et al., 1989). The embolic particles can then obstruct right ventricular outflow or, in the setting of a patent foramen ovale, the systemic circulation. The biochemical hypothesis attributes the manifestations of the FES to the toxic effects of free fatty acids on the pulmonary microcirculation. In the pulmonary vascular bed, these fat particles are hydrolyzed to free fatty acids, which produce pulmonary vasculitis and hemorrhagic pneumonitis—a combination physiologically indistinguishable from acute respiratory distress syndrome. Surfactant activity is compromised, functional residual capacity is decreased, and endothelial integrity is violated, producing a large Pao2–Pao2 gradient and increased pulmonary vascular resistance. Fat particles and free fatty acids can enter the systemic circulation through pulmonary arteriovenous shunts to generate the central, renal, and cutaneous manifestations of this syndrome. As compared with air, the volume of fat associated with cardiopulmonary failure is 20-fold less (Husebye et al., 2006).
FES can be demonstrated in 90% of patients with long-bone fractures, but symptomatic FES occurs in only 10% to 22% of patients with long-bone or pelvic fractures. Classically, it develops 24 to 72 hours after an injury and is characterized by acute respiratory insufficiency with diffuse pulmonary infiltrates, global neurologic dysfunction, and petechiae. The pulmonary compromise is usually followed by the neurologic changes. If a petechial rash develops, it occurs 48 to 72 hours after the onset of FES. This complete presentation is seen in less than 10% of cases. Respiratory insufficiency may be the only manifestation of this syndrome, and it may occur in only one third of patients. This unexplained hypoxia is how FES manifests during general anesthesia (van Besouw and Hinds, 1989). Few patients with FES have a fulminant course, in which severe pulmonary hypertension and progressive right heart failure develop within hours of the injury. Vasopressor infusions and cardiopulmonary bypass support may be necessary to treat these sequelae.
The diagnosis of FES is a clinical one, and it may be difficult to establish (Georgopoulos and Bouros, 2003). Supportive laboratory tests include an inexplicable drop in Pao2, hematocrit, platelet counts, and fibrinogen levels. The characteristic radiograph of bilateral fluffy pulmonary infiltrates may not be apparent for 24 to 48 hours after the onset of FES. Fat is seen in the urine in 50% of patients within 3 days. The usefulness of serum and urinary measurements of fat and lipase activity is limited by their poor sensitivity and specificity and by a lack of availability. Identification of fat droplet cells in bronchoalveolar lavage is the only rapid and specific method of identifying the development of this syndrome (Mimoz et al., 1995). The retinal changes of bilateral cotton-wool spots and intraretinal hemorrhages are seen in 60% of patients with FES.
End-tidal CO2 monitoring does not seem to be as sensitive to fat emboli as it is in other embolic states. Although end-tidal CO2 does change with a massive fat embolism, monitoring it has not been as effective as echocardiography in detecting smaller emboli. Transesophageal echocardiography can detect fat emboli during surgical manipulation of the operative bone, and it can demonstrate the regional wall motion abnormalities and right ventricular dilation that are harbingers of the FES physiologic perturbations (Capan and Miller, 2001).
After FES develops, treatment is nonspecific and supportive. It consists of early resuscitation and stabilization, administration of 100% oxygen, application of positive end-expiratory pressure, and the use of inverse-ratio ventilation. Bronchoscopy and bronchoalveolar lavage are useful in establishing a diagnosis and in removing the intraluminal debris and hemorrhagic exudate that accompany a fulminant presentation. An adequate intravascular volume must be maintained, and inotropic infusions and red blood cell transfusions are often required. Historically, advocated therapies have included IV alcohol, heparin, low-molecular-weight dextrans, and steroids. Limited data support the efficacy of any of these therapies once FES has begun. Early administration of methylprednisolone may decrease the incidence of FES (Lindeque et al., 1987). A 10% mortality rate has been reported for all patients; among children, the mortality rate is 33%.
For questions and answers on topics in this chapter, go to “Chapter Questions” at www.expertconsult.com.
Ahn U.M., Ahn N.U., Nallamshetty L., et al. The etiology of adolescent idiopathic scoliosis. Am J Orthop. 2002;31:387-395.
Akhtar S. Fat embolism. Anesthesiol Clin. 2009;27:533-550.
Albright A.L. Baclofen in the treatment of cerebral palsy. J Child Neurol. 1996;11:77-83.
Albright A.L. Neurosurgical treatment of spasticity: selective posterior rhizotomy and intrathecal baclofen. Stereotact Funct Neurosurg. 1992;58:3-13.
Albright A.L. Spasticity and movement disorders in cerebral palsy. J Child Neurol. 1996;11(Suppl 1):S1-S4.
Almenrader N., Patel D. Spinal fusion surgery in children with non-idiopathic scoliosis: is there a need for routine postoperative ventilation? Br J Anaesth. 2006;97:851-857.
Alvarez C., De V.M., Varghese R. Review of current methods used in the treatment of clubfoot at initial presentation and at recurrence. J Surg Orthop Adv. 2008;17:107-114.
Anschel D.J., Aherne A., Soto R.G., et al. Successful intraoperative spinal cord monitoring during scoliosis surgery using a total intravenous anesthetic regimen including dexmedetomidine. J Clin Neurophysiol. 2008;25:56-61.
Arabshahi B., Cron R.Q. Temporomandibular joint arthritis in juvenile idiopathic arthritis: the forgotten joint. Curr Opin Rheumatol. 2006;18:490-495.
Arai F., Kita T., Nakai T., et al. Histopathologic features of fat embolism in fulminant fat embolism syndrome. Anesthesiology. 2007;107:509-511.
Aronsson D.D., Loder R.T., Breur G.J., et al. Slipped capital femoral epiphysis: current concepts. J Am Acad Orthop Surg. 2006;14:666-679.
Asai T., Shingu K. Tracheal intubation through the intubating laryngeal mask in patients with unstable necks. Acta Anaesthesiol Scand. 2001;45:818-822.
Asher M.A., Burton D.C. Adolescent idiopathic scoliosis: natural history and long term treatment effects. Scoliosis. 2006;1:2.
Baines D.B., Douglas I.D., Overton J.H. Anaesthesia for patients with arthrogryposis multiplex congenita: what is the risk of malignant hyperthermia? Anaesth Intensive Care. 1986;14:370-372.
Balemans W., Van W.L., Van H.W. A clinical and molecular overview of the human osteopetroses. Calcif Tissue Int. 2005;77:263-274.
Bamshad M., Van Heest A.E., Pleasure D. Arthrogryposis: a review and update. J Bone Joint Surg Am. 2009;91(Suppl 4):40-46.
Banoub M., Tetzlaff J.E., Schubert A. Pharmacologic and physiologic influences affecting sensory evoked potentials: implications for perioperative monitoring. Anesthesiology. 2003;99:716-737.
Basel D., Steiner R.D. Osteogenesis imperfecta: recent findings shed new light on this once well-understood condition. Genet Med. 2009;11:375-385.
Benca J., Hogan K. Malignant hyperthermia, coexisting disorders, and enzymopathies: risks and management options. Anesth Analg. 2009;109:1049-1053.
Berkowitz I.D., Raja S.N., Bender K.S., et al. Dwarfs: pathophysiology and anesthetic implications. Anesthesiology. 1990;73:739-759.
Birch J.G., Samchukov M.L. Use of the Ilizarov method to correct lower limb deformities in children and adolescents. J Am Acad Orthop Surg. 2004;12:144-154.
Birnkrant D.J., Panitch H.B., Benditt J.O., et al. American College of Chest Physicians consensus statement on the respiratory and related management of patients with Duchenne muscular dystrophy undergoing anesthesia or sedation. Chest. 2007;132:1977-1986.
Birnkrant D.J. The American College of Chest Physicians consensus statement on the respiratory and related management of patients with Duchenne muscular dystrophy undergoing anesthesia or sedation. Pediatrics. 2009;123(Suppl 4):S242-S244.
Bjornson K., Hays R., Graubert C., et al. Botulinum toxin for spasticity in children with cerebral palsy: a comprehensive evaluation. Pediatrics. 2007;120:49-58.
Boero S., Brunenghi G.M., Carbone M., et al. Pinning in slipped capital femoral epiphysis: long-term follow-up study. J Pediatr Orthop B. 2003;12:372-379.
Boisseau N., Madany M., Staccini P., et al. Comparison of the effects of sevoflurane and propofol on cortical somatosensory evoked potentials. Br J Anaesth. 2002;88:785-789.
Bonilla-Felix M. Development of water transport in the collecting duct. Am J Physiol Renal Physiol. 2004;287:F1093-F1101.
Breau L.M., Camfield C.S., McGrath P.J., et al. The incidence of pain in children with severe cognitive impairments. Arch Pediatr Adolesc Med. 2003;157:1219-1226.
Breau L.M., Finley G.A., McGrath P.J., et al. Validation of the non-communicating children’s pain checklist-postoperative version. Anesthesiology. 2002;96:528-535.
Brodkey J.S., Richards D.E., Blasingame J.P., et al. Reversible spinal cord trauma in cats. Additive effects of direct pressure and ischemia. J Neurosurg. 1972;37:591-593.
Brooke B.S., Habashi J.P., Judge D.P., et al. Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome. N Engl J Med. 2008;358:2787-2795.
Bryson G.L., Laupacis A., Wells G.A. Does acute normovolemic hemodilution reduce perioperative allogeneic transfusion? A meta-analysis. The International Study of Perioperative Transfusion. Anesth Analg. 1998;86:9-15.
Burke G., Cossins J., Maxwell S., et al. Rapsyn mutations in hereditary myasthenia: distinct early- and late-onset phenotypes. Neurology. 2003;61:826-828.
Burt N., Haynes G.R., Bailey M.K. Patients with malignant osteopetrosis are at high risk of anesthetic morbidity and mortality. Anesth Analg. 1999;88:1292-1297.
Burwell R.G., Aujla R.K., Grevitt M.P., et al. Pathogenesis of adolescent idiopathic scoliosis in girls―a double neuro-osseous theory involving disharmony between two nervous systems, somatic and autonomic expressed in the spine and trunk: possible dependency on sympathetic nervous system and hormones with implications for medical therapy. Scoliosis. 2009;4:24.
Burwell R.G., Dangerfield P.H., Moulton A., et al. Etiologic theories of idiopathic scoliosis: autonomic nervous system and the leptin-sympathetic nervous system concept for the pathogenesis of adolescent idiopathic scoliosis. Stud Health Technol Inform. 2008;140:197-207.
Byrick R.J., Kay J.C., Mullen J.B. Capnography is not as sensitive as pulmonary artery pressure monitoring in detecting marrow microembolism: studies in a canine model. Anesth Analg. 1989;68:94-100.
Campbell R.M.Jr. Spine deformities in rare congenital syndromes: clinical issues. Spine (Phila Pa). 2009;34:1815-1827.
Campbell R.M.Jr, Smith M.D. Thoracic insufficiency syndrome and exotic scoliosis. J Bone Joint Surg Am. 2007;89(Suppl 1):108-122.
Capan L.M., Miller S.M. Monitoring for suspected pulmonary embolism. Anesthesiol Clin North America. 2001;19:673-703.
Castillo H., Samson-Fang L. Effects of bisphosphonates in children with osteogenesis imperfecta: an AACPDM systematic review. Dev Med Child Neurol. 2009;51:17-29.
Castillo-Zamora C., Castillo-Peralta L.A., Nava-Ocampo A.A. Dose minimization study of single-dose epidural morphine in patients undergoing hip surgery under regional anesthesia with bupivacaine. Paediatr Anaesth. 2005;15:29-36.
Ceriati E., De P.F., Ciprandi G., et al. Surgery in disabled children: general gastroenterological aspects. Acta Paediatr Suppl. 2006;95:34-37.
Cervellati S., Bettini N., Bianco T., et al. Neurological complications in segmental spinal instrumentation: analysis of 750 patients. Eur Spine J. 1996;5:161-166.
Charles Y.P., Dimeglio A., Marcoul M., et al. Influence of idiopathic scoliosis on three-dimensional thoracic growth. Spine (Phila Pa). 1976;33:1209-1218.
Cheung M.S., Glorieux F.H. Osteogenesis Imperfecta: update on presentation and management. Rev Endocr Metab Disord. 2008;9:153-160.
Clark C.A., Li T.F., Kim K.O., et al. Prostaglandin E2 inhibits BMP signaling and delays chondrocyte maturation. J Orthop Res. 2009;27:785-792.
Cohen M.M.Jr. Some chondrodysplasias with short limbs: molecular perspectives. Am J Med Genet. 2002;112:304-313.
Copley L.A., Richards B.S., Safavi F.Z., et al. Hemodilution as a method to reduce transfusion requirements in adolescent spine fusion surgery. Spine. 1999;24:219-222.
Crawford M.W., Hickey C., Zaarour C., et al. Development of acute opioid tolerance during infusion of remifentanil for pediatric scoliosis surgery. Anesth Analg. 2006;102:1662-1667.
Cummings R.J., Davidson R.S., Armstrong P.F., et al. Congenital clubfoot. Instr Course Lect. 2002;51:385-400.
da Costa V.V., Saraiva R.A., de Almeida A.C., et al. The effect of nitrous oxide on the inhibition of somatosensory evoked potentials by sevoflurane in children. Anaesthesia. 2001;56:202-207.
Dean D.B., Watson J.T., Moed B.R., et al. Role of bone morphogenetic proteins and their antagonists in healing of bone fracture. Front Biosci. 2009;14:2878-2888.
Deguchi M., Rapoff A.J., Zdeblick T.A. Posterolateral fusion for isthmic spondylolisthesis in adults: analysis of fusion rate and clinical results. J Spinal Disord. 1998;11:459-464.
Del F.A., Cappariello A., Teti A. Genetics, pathogenesis and complications of osteopetrosis. Bone. 2008;42:19-29.
Demetracopoulos C.A., Sponseller P.D. Spinal deformities in Marfan syndrome. Orthop Clin North Am. 2007;38:563-572. vii
Dewachter P., Mouton-Faivre C., Emala C.W. Anaphylaxis and anesthesia: controversies and new insights. Anesthesiology. 2009;111:1141-1150.
Dierdorf S.F., McNiece W.L., Rao C.C., et al. Effect of succinylcholine on plasma potassium in children with cerebral palsy. Anesthesiology. 1985;62:88-90.
Dietz H.C., Loeys B., Carta L., et al. Recent progress towards a molecular understanding of Marfan syndrome. Am J Med Genet C Semin Med Genet. 2005;139C:4-9.
Dobbs M.B., Gurnett C.A. Update on clubfoot: etiology and treatment. Clin Orthop Relat Res. 2009;467:1146-1153.
Driessen G.J., Gerritsen E.J., Fischer A., et al. Long-term outcome of haematopoietic stem cell transplantation in autosomal recessive osteopetrosis: an EBMT report. Bone Marrow Transplant. 2003;32:657-663.
Drvaric D.M., Kuivila T.E., Roberts J.M. Congenital clubfoot: etiology, pathoanatomy, pathogenesis, and the changing spectrum of early management. Orthop Clin North Am. 1989;20:641-647.
Dwek J.R. The hip: MR imaging of uniquely pediatric disorders. Radiol Clin North Am. 2009;47:997-1008.
Eastwood D.M. Neonatal hip screening. Lancet. 2003;361:595-597.
Edler A., Murray D.J., Forbes R.B. Blood loss during posterior spinal fusion surgery in patients with neuromuscular disease: is there an increased risk? Paediatr Anaesth. 2003;13:818-822.
Estebe J.P., Le N.A., Malledant Y., et al. Use of a pneumatic tourniquet induces changes in central temperature. Br J Anaesth. 1996;77:786-788.
Farmer J.P., Sabbagh A.J. Selective dorsal rhizotomies in the treatment of spasticity related to cerebral palsy. Childs Nerv Syst. 2007;23:991-1002.
Fernandes P., Weinstein S.L. Natural history of early onset scoliosis. J Bone Joint Surg Am. 2007;89(Suppl 1):21-33.
Florentino-Pineda I., Thompson G.H., Poe-Kochert C., et al. The effect of amicar on perioperative blood loss in idiopathic scoliosis: the results of a prospective, randomized double-blind study. Spine (Phila Pa). 1976;29:233-238.
Frei F.J., Haemmerle M.H., Brunner R., et al. Minimum alveolar concentration for halothane in children with cerebral palsy and severe mental retardation. Anaesthesia. 1997;52:1056-1060.
Gajraj N.M. The effect of cyclooxygenase-2 inhibitors on bone healing. Reg Anesth Pain Med. 2003;28:456-465.
Georgopoulos D., Bouros D. Fat embolism syndrome: clinical examination is still the preferable diagnostic method. Chest. 2003;123:982-983.
Gerstenfeld L.C., Thiede M., Seibert K., et al. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2003;21:670-675.
Gilron I., Milne B., Hong M. Cyclooxygenase-2 inhibitors in postoperative pain management: current evidence and future directions. Anesthesiology. 2003;99:1198-1208.
Gisel E.G., Tessier M.J., Lapierre G., et al. Feeding management of children with severe cerebral palsy and eating impairment: an exploratory study. Phys Occup Ther Pediatr. 2003;23:19-44.
Glassman S.D., Rose S.M., Dimar J.R., et al. The effect of postoperative nonsteroidal anti-inflammatory drug administration on spinal fusion. Spine. 1998;23:834-838.
Gonzalez A.A., Jeyanandarajan D., Hansen C., et al. Intraoperative neurophysiological monitoring during spine surgery: a review. Neurosurg Focus. 2009;27:E6.
Griffiths I.R., Trench J.G., Crawford R.A. Spinal cord blood flow and conduction during experimental cord compression in normotensive and hypotensive dogs. J Neurosurg. 1979;50:353-360.
Grundy B.L. Intraoperative monitoring of sensory-evoked potentials. Anesthesiology. 1983;58:72-87.
Gu S.X., Wang C.F., Zhao Y.C., et al. Abnormal ossification as a cause the progression of adolescent idiopathic scoliosis. Med Hypotheses. 2009;72:416-417.
Gurnaney H., Brown A., Litman R.S. Malignant hyperthermia and muscular dystrophies. Anesth Analg. 2009;109:1043-1048.
Hackmann T., Friesen M., Allen S., et al. Clonidine facilitates controlled hypotension in adolescent children. Anesth Analg. 2003;96:976-981.
Hall J.E., Levine C.R., Sudhir K.G. Intraoperative awakening to monitor spinal cord function during Harrington instrumentation and spine fusion: description of procedure and report of three cases. J Bone Joint Surg Am. 1978;60:533-536.
Hammer G.B. Selective bronchial blockade in small infants. Anesth Analg. 2001;93:1624-1625.
Hammer G.B. Single-lung ventilation in infants and children. Paediatr Anaesth. 2004;14:98-102.
Hammer G.B., Harrison T.K., Vricella L.A., et al. Single lung ventilation in children using a new paediatric bronchial blocker. Paediatr Anaesth. 2002;12:69-72.
Hammer G.B., Verghese S.T., Drover D.R., et al. Pharmacokinetics and pharmacodynamics of fenoldopam mesylate for blood pressure control in pediatric patients. BMC Anesthesiol. 2008;8(6):6.
Harder A.T., An Y.H. The mechanisms of the inhibitory effects of nonsteroidal anti-inflammatory drugs on bone healing: a concise review. J Clin Pharmacol. 2003;43:807-815.
Harding M.G., Harcke H.T., Bowen J.R., et al. Management of dislocated hips with Pavlik harness treatment and ultrasound monitoring. J Pediatr Orthop. 1997;17:189-198.
Harrington P.R. The history and development of Harrington instrumentation, by Paul R. Harrington, 1973. Clin Orthop. 1988;227:3-5.
Harrington P.R., Dickson J.H. Spinal instrumentation in the treatment of severe progressive spondylolisthesis. Clin Orthop. 1976:157-163.
Hashkes P.J., Laxer R.M. Medical treatment of juvenile idiopathic arthritis. JAMA. 2005;294:1671-1684.
Hashkes P.J., Laxer R.M. Update on the medical treatment of juvenile idiopathic arthritis. Curr Rheumatol Rep. 2006;8:450-458.
Hayward K., Wallace C.A. Recent developments in anti-rheumatic drugs in pediatrics: treatment of juvenile idiopathic arthritis. Arthritis Res Ther. 2009;11:216.
Helfaer M.A., Carson B.S., James C.S., et al. Increased hematocrit and decreased transfusion requirements in children given erythropoietin before undergoing craniofacial surgery. J Neurosurg. 1998;88:704-708.
Helmick C.G., Felson D.T., Lawrence R.C., et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15-25.
Herbert A.J., Herzenberg J.E., Paley D. A review for pediatricians on limb lengthening and the Ilizarov method. Curr Opin Pediatr. 1995;7:98-105.
Herzenberg J.E., Radler C., Bor N. Ponseti versus traditional methods of casting for idiopathic clubfoot. J Pediatr Orthop. 2002;22:517-521.
Herzka A., Sponseller P.D., Pyeritz R.E. Atlantoaxial rotatory subluxation in patients with Marfan syndrome. A report of three cases. Spine. 2000;25:524-526.
Hobbs W.R., Sponseller P.D., Weiss A.P., et al. The cervical spine in Marfan syndrome. Spine. 1997;22:983-989.
Hopkins P.M., Ellis F.R., Halsall P.J. Hypermetabolism in arthrogryposis multiplex congenita. Anaesthesia. 1991;46:374-375.
Horton W.A., Hall J.G., Hecht J.T. Achondroplasia. Lancet. 2007;370:162-172.
Huet C., Salmi L.R., Fergusson D., et al. A meta-analysis of the effectiveness of cell salvage to minimize perioperative allogeneic blood transfusion in cardiac and orthopedic surgery. International Study of Perioperative Transfusion (ISPOT) Investigators. Anesth Analg. 1999;89:861-869.
Husebye E.E., Lyberg T., Roise O. Bone marrow fat in the circulation: clinical entities and pathophysiological mechanisms. Injury. 2006;37(Suppl 4):S8-S18.
Jacobs J.C., Hui R.M. Cricoarytenoid arthritis and airway obstruction in juvenile rheumatoid arthritis. Pediatrics. 1977;59:292-294.
Janicki J.A., Narayanan U.G., Harvey B., et al. Treatment of neuromuscular and syndrome-associated (nonidiopathic) clubfeet using the Ponseti method. J Pediatr Orthop. 2009;29:393-397.
Johnston C.E., Happel L.T., Norris R.Jr, et al. Delayed paraplegia complicating sublaminar segmental spinal instrumentation. J Bone Joint Surg Am. 1986;68:556-563.
Joseph S.A.Jr, Berekashvili K., Mariller M.M., et al. Blood conservation techniques in spinal deformity surgery: a retrospective review of patients refusing blood transfusion. Spine (Phila Pa). 1976;33:2310-2315.
Judge D.P., Dietz H.C. Marfan’s syndrome. Lancet. 2005;366:1965-1976.
Kachur E., Del M.R. Mucopolysaccharidoses and spinal cord compression: case report and review of the literature with implications of bone marrow transplantation. Neurosurgery. 2000;47:223-228.
Kafer E.R. Respiratory and cardiovascular functions in scoliosis and the principles of anesthetic management. Anesthesiology. 1980;52:339-351.
Kalkman C.J., ten Brink S.A., Been H.D., et al. Variability of somatosensory cortical evoked potentials during spinal surgery: effects of anesthetic technique and high-pass digital filtering. Spine. 1991;16:924-929.
Kalkman C.J., Traast H., Zuurmond W.W., et al. Differential effects of propofol and nitrous oxide on posterior tibial nerve somatosensory cortical evoked potentials during alfentanil Anaesthesia. Br J Anaesth. 1991;66:483-489.
Kam P.C., Kavanagh R., Yoong F.F., et al. The arterial tourniquet: pathophysiological consequences and anaesthetic implications. Anaesthesia. 2001;56:534-545.
Kanakaris N.K., Giannoudis P.V. Clinical applications of bone morphogenetic proteins: current evidence. J Surg Orthop Adv. 2008;17:133-146.
Kannan S., Meert K.L., Mooney J.F., et al. Bleeding and coagulation changes during spinal fusion surgery: a comparison of neuromuscular and idiopathic scoliosis patients. Pediatr Crit Care Med. 2002;3:364-369.
Kannikeswaran N., Mahajan P.V., Sethuraman U., et al. Sedation medication received and adverse events related to sedation for brain MRI in children with and without developmental disabilities. Paediatr Anaesth. 2009;19:250-256.
Karabiyik L., Parpucu M., Kurtipek O. Total intravenous anaesthesia and the use of an intubating laryngeal mask in a patient with osteogenesis imperfecta. Acta Anaesthesiol Scand. 2002;46:618-619.
Kasow K.A., Stocks R.M., Kaste S.C., et al. Airway evaluation and management in 7 children with malignant infantile osteopetrosis before hematopoietic stem cell transplantation. J Pediatr Hematol Oncol. 2008;30:225-229.
Kaufman R.D., Walts L.F. Tourniquet-induced hypertension. Br J Anaesth. 1982;54:333-336.
Kelly K.J., Pearson M.L., Kurup V.P., et al. A cluster of anaphylactic reactions in children with spina bifida during general anesthesia: epidemiologic features, risk factors, and latex hypersensitivity. J Allergy Clin Immunol. 1994;94:53-61.
Khan S.N., Sandhu H.S., Lane J.M., et al. Bone morphogenetic proteins: relevance in spine surgery. Orthop Clin North Am. 2002;33:447-463. ix
Kim Y.J., Noonan K.J. What’s new in pediatric orthopaedics. J Bone Joint Surg Am. 2009;91:743-751.
Kocher M.S., Bishop J.A., Weed B., et al. Delay in diagnosis of slipped capital femoral epiphysis. Pediatrics. 2004;113:e322-e325.
Kostopanagiotou G., Coussi T., Tsaroucha N., et al. Anaesthesia using a laryngeal mask airway in a patient with osteogenesis imperfecta. Anaesthesia. 2000;55:506.
Ladouceur M., Fermanian C., Lupoglazoff J.M., et al. Effect of beta-blockade on ascending aortic dilatation in children with the Marfan syndrome. Am J Cardiol. 2007;99:406-409.
Laishley R.S., Roy W.L. Freeman-Sheldon syndrome: report of three cases and the anaesthetic implications. Can Anaesth Soc J. 1986;33:388-393.
Langeloo D.D., Journee H.L., de K.M., et al. Criteria for transcranial electrical motor evoked potential monitoring during spinal deformity surgery: a review and discussion of the literature. Neurophysiol Clin. 2007;37:431-439.
Langeloo D.D., Lelivelt A., Louis J.H., et al. Transcranial electrical motor-evoked potential monitoring during surgery for spinal deformity: a study of 145 patients. Spine (Phila Pa). 2003;28:1043-1050.
Langeron O., Lille F., Zerhouni O., et al. Comparison of the effects of ketamine-midazolam with those of fentanyl-midazolam on cortical somatosensory evoked potentials during major spine surgery. Br J Anaesth. 1997;78:701-706.
Laureau E., Marciniak B., Hebrard A., et al. Comparative study of propofol and midazolam effects on somatosensory evoked potentials during surgical treatment of scoliosis. Neurosurgery. 1999;45:69-74.
Lee L.A., Lam A.M. Unilateral blindness after prone lumbar spine surgery. Anesthesiology. 2001;95:793-795.
Lehman R.A.Jr, Lenke L.G., Keeler K.A., et al. Operative treatment of adolescent idiopathic scoliosis with posterior pedicle screw-only constructs: minimum three-year follow-up of one hundred fourteen cases. Spine (Phila Pa). 1976;33:1598-1604.
Lindeque B.G., Schoeman H.S., Dommisse G.F., et al. Fat embolism and the fat embolism syndrome: a double-blind therapeutic study. J Bone Joint Surg Br. 1987;69:128-131.
Lotto M.L., Banoub M., Schubert A. Effects of anesthetic agents and physiologic changes on intraoperative motor evoked potentials. J Neurosurg Anesthesiol. 2004;16:32-42.
Lowe T.G., Burwell R.G., Dangerfield P.H. Platelet calmodulin levels in adolescent idiopathic scoliosis (AIS): can they predict curve progression and severity? Summary of an electronic focus group debate of the IBSE. Eur Spine J. 2004;13:257-265.
Lowe T.G., Edgar M., Margulies J.Y., et al. Etiology of idiopathic scoliosis: current trends in research. J Bone Joint Surg Am. 2000;82-A:1157-1168.
Luque E.R. Segmental spinal instrumentation of the lumbar spine. Clin Orthop. 1986:126-134.
Luque E.R., Rapp G.F. A new semirigid method for interpedicular fixation of the spine. Orthopedics. 1988;11:1445-1450.
MacKenzie J.M., Rankin R. Sudden death due to atlantoaxial subluxation in marfan syndrome. Am J Forensic Med Pathol. 2003;24:369-370.
Madi-Jebara S., El-Hajj C., Jawish D., et al. Anesthetic management of a patient with Freeman-Sheldon syndrome: case report. J Clin Anesth. 2007;19:460-462.
Manzur A.Y., Muntoni F. Diagnosis and new treatments in muscular dystrophies. J Neurol Neurosurg Psychiatry. 2009;80:706-714.
Martin G.J.Jr, Boden S.D., Titus L. Recombinant human bone morphogenetic protein-2 overcomes the inhibitory effect of ketorolac, a nonsteroidal anti-inflammatory drug (NSAID), on posterolateral lumbar intertransverse process spine fusion. Spine. 1999;24:2188-2193.
Martin J.T., Warner M.A. The ventral decubitus (prone) position. In: Martin J.T., Warner M.A., editors. Positioning in anesthesia and surgery. Philadelphia: WB Saunders; 1997:155.
Martin S., Tobias J.D. Perioperative care of the child with arthrogryposis. Paediatr Anaesth. 2006;16:31-37.
Martins A.M., Dualibi A.P., Norato D., et al. Guidelines for the management of mucopolysaccharidosis type I. J Pediatr. 2009;155:S32-S46.
Mason R.J., Betz R.R., Orlowski J.P., et al. The syndrome of inappropriate antidiuretic hormone secretion and its effect on blood indices following spinal fusion. Spine. 1989;14:722-726.
Maxwell L.G. Age-associated issues in preoperative evaluation, testing, and planning: pediatrics. Anesthesiol Clin North America. 2004;22:27-43.
Maxwell L.G., Yaster M. Effects of a low-dose naloxone infusion on opioid-induced side effects and analgesia in children and adolescents treated with intravenous patient-controlled analgesia: a prospective, randomized, controlled, double-blind study. Anesthesiology. 2003;99:A1453.
Maxy R.J., Glassman S.D. The effect of nonsteroidal anti-inflammatory drugs on osteogenesis and spinal fusion. Reg Anesth Pain Med. 2001;26:156-158.
McCarthy J.G., Stelnicki E.J., Mehrara B.J., et al. Distraction osteogenesis of the craniofacial skeleton. Plast Reconstr Surg. 2001;107:1812-1827.
Meert K.L., Kannan S., Mooney J.F. Predictors of red cell transfusion in children and adolescents undergoing spinal fusion surgery. Spine. 2002;27:2137-2142.
Mellor A., Soni N. Fat embolism. Anaesthesia. 2001;56:145-154.
Mendiratta A., Emerson R.G. Neurophysiologic intraoperative monitoring of scoliosis surgery. J Clin Neurophysiol. 2009;26:62-69.
Mimoz O., Edouard A., Beydon L., et al. Contribution of bronchoalveolar lavage to the diagnosis of posttraumatic pulmonary fat embolism. Intensive Care Med. 1995;21:973-980.
Mohan U.R., Hay A.A., Cleary M.A., et al. Cardiovascular changes in children with mucopolysaccharide disorders. Acta Paediatr. 2002;91:799-804.
Monedero P., Garcia-Pedrajas F., Coca I., et al. Is management of anesthesia in achondroplastic dwarfs really a challenge? J Clin Anesth. 1997;9:208-212.
Monitto C.L., Greenberg R.S., Kost-Byerly S., et al. Safety and efficacy of parent/nurse controlled analgesia in patients less than 6 years of age. Anesthesiology. 1998;89:1324.
Morrell N.W., Adnot S., Archer S.L., et al. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S20-S31.
Murray D.J., Forbes R.B., Titone M.B., et al. Transfusion management in pediatric and adolescent scoliosis surgery: efficacy of autologous blood. Spine. 1997;22:2735-2740.
Narkis G., Landau D., Manor E., et al. Genetics of arthrogryposis: linkage analysis approach. Clin Orthop Relat Res. 2007;456:30-35.
Narkis G., Ofir R., Landau D., et al. Lethal contractural syndrome type 3 (LCCS3) is caused by a mutation in PIP5K1C, which encodes PIPKI gamma of the phophatidylinsitol pathway. Am J Hum Genet. 2007;81:530-539.
Newton P.O., Faro F.D., Gollogly S., et al. Results of preoperative pulmonary function testing of adolescents with idiopathic scoliosis: a study of six hundred and thirty-one patients. J Bone Joint Surg Am. 2005;87:1937-1946.
Nuttall G.A., Garrity J.A., Dearani J.A., et al. Risk factors for ischemic optic neuropathy after cardiopulmonary bypass: a matched case/control study. Anesth Analg. 2001;93:1410-1416.
Nuwer M.R., Dawson E.G., Carlson L.G., et al. Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: results of a large multicenter survey. Electroencephalogr Clin Neurophysiol. 1995;96:6-11.
Owen J.H. The application of intraoperative monitoring during surgery for spinal deformity. Spine. 1999;24:2649-2662.
Padberg A.M., Wilson-Holden T.J., Lenke L.G., et al. Somatosensory- and motor-evoked potential monitoring without a wake-up test during idiopathic scoliosis surgery: an accepted standard of care. Spine. 1998;23:1392-1400.
Paley D. Problems, obstacles, and complications of limb lengthening by the Ilizarov technique. Clin Orthop. 1990:81-104.
Paneth N., Hong T., Korzeniewski S. The descriptive epidemiology of cerebral palsy. Clin Perinatol. 2006;33:251-267.
Parisi D.M., Koval K., Egol K. Fat embolism syndrome. Am J Orthop. 2002;31:507-512.
Patterson S., Klenerman L. The effect of pneumatic tourniquets on the ultrastructure of skeletal muscle. J Bone Joint Surg Br. 1979;61-B:178-183.
Pedersen T.K., Jensen J.J., Melsen B., et al. Resorption of the temporomandibular condylar bone according to subtypes of juvenile chronic arthritis. J Rheumatol. 2001;28:2109-2115.
Pharoah P.O., Platt M.J., Cooke T. The changing epidemiology of cerebral palsy. Arch Dis Child Fetal Neonatal Ed. 1996;75:F169-F173.
Phillipi C.A., Remmington T., Steiner R.D. Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst Rev,. 2008. CD005088
Porsborg P., Astrup G., Bendixen D., et al. Osteogenesis imperfecta and malignant hyperthermia. Is there a relationship? Anaesthesia. 1996;51:863-865.
Pradhan B.B., Tatsumi R.L., Gallina J., et al. Ketorolac and spinal fusion: does the perioperative use of ketorolac really inhibit spinal fusion? Spine (Phila Pa). 1976;33:2079-2082.
Ramirez F., Dietz H.C. Marfan syndrome: from molecular pathogenesis to clinical treatment. Curr Opin Genet Dev. 2007;17:252-258.
Rampton A.J., Kelly D.A., Shanahan E.C., et al. Occurrence of malignant hyperpyrexia in a patient with osteogenesis imperfecta. Br J Anaesth. 1984;56:1443-1446.
Reilly C.W., McEwen J.A., Leveille L., et al. Minimizing tourniquet pressure in pediatric anterior cruciate ligament reconstructive surgery: a blinded, prospective randomized controlled trial. J Pediatr Orthop. 2009;29:275-280.
Relton J.E., Hall J.E. An operation frame for spinal fusion: a new apparatus designed to reduce haemorrhage during operation. J Bone Joint Surg Br. 1967;49:327-332.
Reuben S.S., Connelly N.R., Lurie S., et al. Dose-response of ketorolac as an adjunct to patient-controlled analgesia morphine in patients after spinal fusion surgery. Anesth Analg. 1998;87:98-102.
Reuben S.S., Connelly N.R., Steinberg R. Ketorolac as an adjunct to patient-controlled morphine in postoperative spine surgery patients. Reg Anesth. 1997;22:343-346.
Richards B.S., Bernstein R.M., D’Amato C.R., et al. Standardization of criteria for adolescent idiopathic scoliosis brace studies: SRS Committee on Bracing and Nonoperative Management. Spine (Phila Pa). 1976;30:2068-2075.
Richards B.S., Johnston C.E. Cotrel-Dubousset instrumentation for adolescent idiopathic scoliosis. Orthopedics. 1987;10:649-654.
Rigante D., Segni G. Cardiac structural involvement in mucopolysaccharidoses. Cardiology. 2002;98:18-20.
Rosenbaum P. The natural history of gross motor development in children with cerebral palsy aged 1 to 15 years. Dev Med Child Neurol. 2007;49:724.
Roth S., Barach P. Postoperative visual loss: still no answers—yet. Anesthesiology. 2001;95:575-577.
Roth S., Nunez R., Schreider B.D. Unexplained visual loss after lumbar spinal fusion. J Neurosurg Anesthesiol. 1997;9:346-348.
Roth S., Thisted R.A., Erickson J.P., et al. Eye injuries after nonocular surgery: a study of 60,965 anesthetics from 1988 to 1992. Anesthesiology. 1996;85:1020-1027.
Samra S.K., Sorkin L.S. Enhancement of somatosensory evoked potentials by etomidate in cats: an investigation of its site of action. Anesthesiology. 1991;74:499-503.
Sandhu H.S., Khan S.N. Recombinant human bone morphogenetic protein-2: use in spinal fusion applications. J Bone Joint Surg Am. 2003;85-A(Suppl 3):89-95.
Saricaoglu F., Celebi N., Celik M., et al. The evaluation of propofol dosage for anesthesia induction in children with cerebral palsy with bispectral index (BIS) monitoring. Paediatr Anaesth. 2005;15:1048-1052.
Sarwark J., Sarwahi V. New strategies and decision making in the management of neuromuscular scoliosis. Orthop Clin North Am. 2007;38:485-496. v
Satsumae T., Yamaguchi H., Sakaguchi M., et al. Preoperative small-dose ketamine prevented tourniquet-induced arterial pressure increase in orthopedic patients under general anesthesia. Anesth Analg. 2001;92:1286-1289.
Scherl S.A. Common lower extremity problems in children. Pediatr Rev. 2004;25:52-62.
Schur M.S., Brown J.T., Kafer E.R., et al. Postoperative pulmonary function in children: comparison of scoliosis with peripheral surgery. Am Rev Respir Dis. 1984;130:46-51.
Schwartz D.M., Auerbach J.D., Dormans J.P., et al. Neurophysiological detection of impending spinal cord injury during scoliosis surgery. J Bone Joint Surg Am. 2007;89:2440-2449.
Schwengel D.A., Sterni L.M., Tunkel D.E., et al. Perioperative management of children with obstructive sleep apnea. Anesth Analg. 2009;109:60-75.
Selamet Tierney E.S., Feingold B., Printz B.F., et al. Beta-blocker therapy does not alter the rate of aortic root dilation in pediatric patients with Marfan syndrome. J Pediatr. 2007;150:77-82.
Sethna N.F., Zurakowski D., Brustowicz R.M., et al. Tranexamic acid reduces intraoperative blood loss in pediatric patients undergoing scoliosis surgery. Anesthesiology. 2005;102:727-732.
Shapiro J.R., Sponsellor P.D. Osteogenesis imperfecta: questions and answers. Curr Opin Pediatr. 2009;21:709-716.
Sheridan M., Johnston I. Hydrocephalus and pseudotumour cerebri in the mucopolysaccharidoses. Childs Nerv Syst. 1994;10:148-150.
Shine T.S., Harrison B.A., De Ruyter M.L., et al. Motor and somatosensory evoked potentials: their role in predicting spinal cord ischemia in patients undergoing thoracoabdominal aortic aneurysm repair with regional lumbar epidural cooling. Anesthesiology. 2008;108:580-587.
Shirley E.D., Ain M.C. Achondroplasia: manifestations and treatment. J Am Acad Orthop Surg. 2009;17:231-241.
Sidiropoulou-Chatzigianni S., Papadopoulos M.A., Kolokithas G. Mandibular condyle lesions in children with juvenile idiopathic arthritis. Cleft Palate Craniofac J. 2008;45:57-62.
Sidman J.D., Sampson D., Templeton B. Distraction osteogenesis of the mandible for airway obstruction in children. Laryngoscope. 2001;111:1137-1146.
Singhi P., Jagirdar S., Khandelwal N., et al. Epilepsy in children with cerebral palsy. J Child Neurol. 2003;18:174-179.
Sisk E.A., Heatley D.G., Borowski B.J., et al. Obstructive sleep apnea in children with achondroplasia: surgical and anesthetic considerations. Otolaryngol Head Neck Surg. 1999;120:248-254.
Sloan T.B., Heyer E.J. Anesthesia for intraoperative neurophysiologic monitoring of the spinal cord. J Clin Neurophysiol. 2002;19:430-443.
Sloan T.B., Janik D., Jameson L. Multimodality monitoring of the central nervous system using motor-evoked potentials. Curr Opin Anaesthesiol. 2008;21:560-564.
Sloan T.B., Ronai A.K., Toleikis J.R., et al. Improvement of intraoperative somatosensory evoked potentials by etomidate. Anesth Analg. 1988;67:582-585.
Stevenson D.A., Carey J.C., Palumbos J., et al. Clinical characteristics and natural history of Freeman-Sheldon syndrome. Pediatrics. 2006;117:754-762.
Strehle E.M. Long-term management of children with neuromuscular disorders. J Pediatr (Rio J). 2009;85:379-384.
Subach B.R., McLaughlin M.R., Albright A.L., et al. Current management of pediatric atlantoaxial rotatory subluxation. Spine. 1998;23:2174-2179.
Sucato D.J. Thoracoscopic anterior instrumentation and fusion for idiopathic scoliosis. J Am Acad Orthop Surg. 2003;11:221-227.
Sung Y.F., Wetchler B.V., Duncalf D., et al. A double-blind, placebo-controlled pilot study examining the effectiveness of intravenous ondansetron in the prevention of postoperative nausea and emesis. J Clin Anesth. 1993;5:22-29.
Swaroop V.T., Mubarak S.J. Difficult-to-treat Ortolani-positive hip: improved success with new treatment protocol. J Pediatr Orthop. 2009;29:224-230.
Szmuk P., Ezri T., Warters D.R., et al. Anesthetic management of a patient with arthrogryposis multiplex congenita and limited mouth opening. J Clin Anesth. 2001;13:59-60.
Takano T., Aotani H., Takeuchi Y. Asymmetric arthrogryposis multiplex congenita with focal pachygyria. Pediatr Neurol. 2001;25:247-249.
Taylor J.M., Gropper M.A. Critical care challenges in orthopedic surgery patients. Crit Care Med. 2006;34:S191-S199.
Thakor N.V., Vaz C.A., McPherson R.W., et al. Adaptive Fourier series modeling of time-varying evoked potentials: study of human somatosensory evoked response to etomidate anesthetic. Electroencephalogr Clin Neurophysiol. 1991;80:108-118.
Theroux M.C., Akins R.E., Barone C., et al. Neuromuscular junctions in cerebral palsy: presence of extrajunctional acetylcholine receptors. Anesthesiology. 2002;96:330-335.
Theroux M.C., Brandom B.W., Zagnoev M., et al. Dose response of succinylcholine at the adductor pollicis of children with cerebral palsy during propofol and nitrous oxide anesthesia. Anesth Analg. 1994;79:761-765.
Thompson G.H., Florentino-Pineda I., Poe-Kochert C., et al. Role of amicar in surgery for neuromuscular scoliosis. Spine (Phila Pa). 1976;33:2623-2629.
Thompson G.H., Florentino-Pineda I., Poe-Kochert C. The role of amicar in decreasing perioperative blood loss in idiopathic scoliosis. Spine (Phila Pa). 1976;30:S94-S99.
Tobias J.D., Goble T.J., Bates G., et al. Effects of dexmedetomidine on intraoperative motor and somatosensory evoked potential monitoring during spinal surgery in adolescents. Paediatr Anaest. 2008;18:1082-1088.
Tobias J.D. A review of intrathecal and epidural analgesia after spinal surgery in children. Anesth Analg. 2004;98:956-965.
Tobias J.D. Controlled hypotension in children: a critical review of available agents. Paediatr Drugs. 2002;4:439-453.
Tolar J., Teitelbaum S.L., Orchard P.J. Osteopetrosis. N Engl J Med. 2004;351:2839-2849.
Toydemir R.M., Bamshad M.J. Sheldon-Hall syndrome. Orphanet J Rare Dis. 2009;4(11):11.
Toydemir R.M., Rutherford A., Whitby F.G., et al. Mutations in embryonic myosin heavy chain (MYH3) cause Freeman-Sheldon syndrome and Sheldon-Hall syndrome. Nat Genet. 2006;38:561-565.
Tuncali B., Karci A., Tuncali B.E., et al. A new method for estimating arterial occlusion pressure in optimizing pneumatic tourniquet inflation pressure. Anesth Analg. 2006;102:1752-1757.
Valli H., Rosenberg P.H., Kytta J., et al. Arterial hypertension associated with the use of a tourniquet with either general or regional Anaesthesia. Acta Anaesthesiol Scand. 1987;31:279-283.
van Besouw J.P., Hinds C.J. Fat embolism syndrome. Br J Hosp Med. 1989;42:304. 1
Vauzelle C., Stagnara P., Jouvinroux P. Functional monitoring of spinal cord activity during spinal surgery. Clin Orthop. 1973;93:173-178.
Verrotti A., Greco R., Spalice A., et al. Pharmacotherapy of spasticity in children with cerebral palsy. Pediatr Neurol. 2006;34:1-6.
Vetter T.R. Acute airway obstruction due to arytenoiditis in a child with juvenile rheumatoid arthritis. Anesth Analg. 1994;79:1198-1200.
Vitale M.G., Choe J.C., Hwang M.W., et al. Use of ketorolac tromethamine in children undergoing scoliosis surgery. an analysis of complications. Spine J. 2003;3:55-62.
Vitale M.G., Levy D.E., Park M.C., et al. Quantifying risk of transfusion in children undergoing spine surgery. Spine J. 2002;2:166-172.
Vitale M.G., Stazzone E.J., Gelijns A.C., et al. The effectiveness of preoperative erythropoietin in averting allogenic blood transfusion among children undergoing scoliosis surgery. J Pediatr Orthop B. 1998;7:203-209.
Vuolteenaho K., Moilanen T., Moilanen E. Non-steroidal anti-inflammatory drugs, cyclooxygenase-2 and the bone healing process. Basic Clin Pharmacol Toxicol. 2008;102:10-14.
Wazeka A.N., DiMaio M.F., Boachie-Adjei O. Outcome of pediatric patients with severe restrictive lung disease following reconstructive spine surgery. Spine (Phila Pa). 1976;29:528-534.
Weinstein S.L., Dolan L.A., Spratt K.F., et al. Health and function of patients with untreated idiopathic scoliosis: a 50-year natural history study. JAMA. 2003;289:559-567.
Weinstein S.L., Zavala D.C., Ponseti I.V. Idiopathic scoliosis: long-term follow-up and prognosis in untreated patients. J Bone Joint Surg Am. 1981;63:702-712.
Weiss J.E., Ilowite N.T. Juvenile idiopathic arthritis. Pediatr Clin North Am. 2005;52:413-442.
Welch M.B., Brummett C.M., Welch T.D., et al. Perioperative peripheral nerve injuries: a retrospective study of 380,680 cases during a 10-year period at a single institution. Anesthesiology. 2009;111:490-497.
Xiong B., Sevastik J.A. A physiological approach to surgical treatment of progressive early idiopathic scoliosis. Eur Spine J. 1998;7:505-508.
Yaster M., Simmons R.S., Tolo V.T., et al. A comparison of nitroglycerin and nitroprusside for inducing hypotension in children: a double-blind study. Anesthesiology. 1986;65:175-179.
Yuan N., Fraire J.A., Margetis M.M., et al. The effect of scoliosis surgery on lung function in the immediate postoperative period. Spine (Phila Pa). 1976;30:2182-2185.
Zhang X., Schwarz E.M., Young D.A., et al. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest. 2002;109:1405-1415.