CHAPTER 35 Anesthesia for Office-Based Pediatric Anesthesia
“A pediatrician’s dream of the ideal world would be to have individuals knowledgeable about the special needs of infants and children assembled wherever children are to be treated” (Avery, 1975). Little did Dr. Avery know back in 1975, as she wrote these words in the journal Anesthesiology as a guest editor introducing a symposium on pediatric anesthesia, to what extent and in what places children were to be anesthetized and by whom. The prophetic nature of her question, “Where will pediatric surgery be done in the future?” and her statement, “Surely the anesthetist should contribute to the definition of what can be done in what setting,” is astounding when considering how rapidly the surgical and anesthetic care of children has progressed from the hospital to the ambulatory surgery center to the office-based setting since Dr. Avery’s remarks (1975).
The concept of office-based anesthesia and surgery is not a novel one, because dentists, oral surgeons, and plastic surgeons have been using office-based procedures for decades. In fact, dentists and oral surgeons have been at the forefront of office anesthesia and surgery dating back to one of the first office-based anesthetics, involving Colton and Wells in 1844, for an extraction of a wisdom tooth (Jacobsohn, 1995; Yagiela, 1999).
Surgical practice has now come full circle, because before the early 1900s many surgical procedures were performed in offices. Subsequently, because surgical procedures became more complex, the hospital became the major site for surgery (Yagiela, 1999). Despite this shift, the development of ambulatory anesthesia continued throughout the twentieth century with the development of the first free-standing surgical center in 1916, by Waters, and ultimately with the first ambulatory surgery center established by Reed and Ford in the late 1960s (White, 1997).
Over the past several years, the concept of office-based anesthesia and surgery has become well publicized. In addition to the increasing number of clinical reports, professional society publications, review articles and texts looking at the efficacy or regarding the subject of office-based anesthesia, there are unfortunately numerous reports of significant morbidity and mortality, as well as near misses both in the medical and dental literature and in the lay press (LaMendola, 1998; Laurito, 1998; Schulte and Bergal, 1998; de Jong, 1999; Neergaard, 1999; Rao et al., 1999; Tang et al., 1999; Arens, 2000; Morrell, 2000; Stoelting, 2000; Vogt, 2000; Hilton, 2001; Joshi, 2003; Gooden, 2006; Kaushal et al., 2006; Hussain, 2006; Hausman and Frost, 2007; Shapiro, 2007; ASA, 2008a, 2008b, 2008c; Coldiron et al., 2008; Heard et al., 2009; Sherry, 2009). The initial reports of mortality in the office-based setting have been highlighted by the unexpected death of the mother of music celebrity Kanye West and consequent legislation in California (Carter, 2007; McGreevy, 2009).
Resident training in office-based anesthesia has also recently been advocated by some within the field of anesthesia. This information, along with the fact that there is an ongoing closed-claim analysis of office-based anesthesia and surgery within the American Society of Anesthesiologists (ASA) (lending itself to the knowledge that this entity does exist and that improvements to existing procedures have been started), must be compelling evidence for the anesthesiologist to realize that office-based surgery and anesthesia are here to stay (Domino, 2001; Hausman et al., 2006; Hausman, 2008; Posner, personal communication, January 2009).
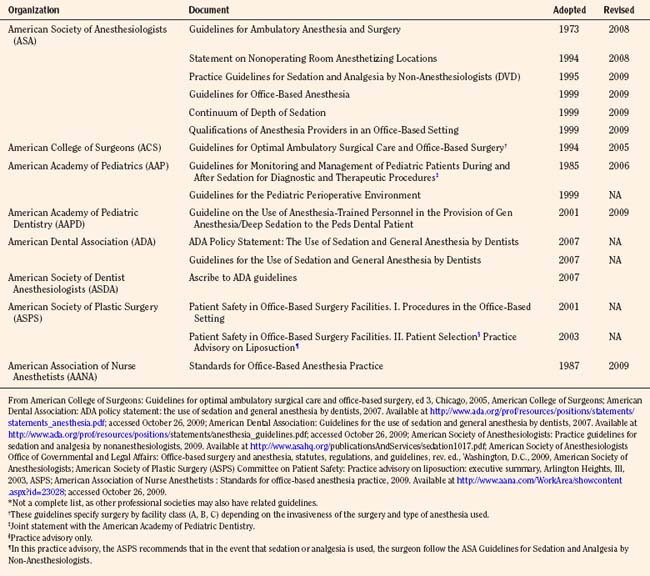
Interest in office-based anesthesia is also manifesting in the increasing number of societies—specifically, the Society for Ambulatory Anesthesia (SAMBA), and the Anesthesia Patient Safety Foundation (APSF)—that promulgate educational and safety-related literature in this relatively new arena for anesthesiologists. Also, the ASA, as well as other groups including, but not limited to, The American College of Surgeons and various surgical subspecialty societies, the American Dental Association, the American Academy of Pediatric Dentistry, and the American Association of Nurse Anesthetists, have published guidelines specifically addressing office-based anesthesia or surgery or both (Table 35-1). Furthermore, the ASA Committee on Ambulatory Surgical Care and SAMBA have published a “how-to” manual for setting up a safe office environment (ASA, 2008d).
TABLE 35-1 Specialty-Specific Guidelines for Sedation and Analgesia and Office-Based Anesthesia and Surgery*
In an effort to establish consistency in the safe administration of sedation and anesthesia to children, some societies have established joint guidelines or updated guidelines outlining the use of anesthesia personnel to its membership (American Academy of Pediatrics, 2006; American Academy of Pediatric Dentistry, 2009). All of these societies have developed their guidelines or policies in response to the overwhelming interest and growing participation by their memberships in this area, as well as an overall common concern for patient safety.
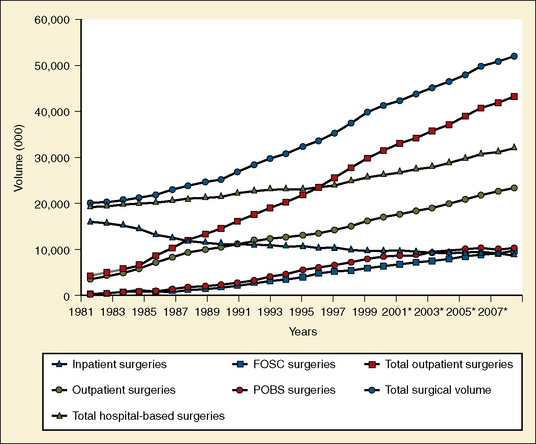
Estimates of the actual number of surgical cases completed, pediatric or otherwise, in an office setting, are difficult to ascertain. It is estimated that there were 10 million office-based surgical procedures performed in 2005, consistent with one marketing study from several years ago, that estimated that by 2006 the total number of outpatient procedures performed would be approximately 40 million, with 11 million of these being office-based procedures (Fig. 35-1) (SMG Marketing-Verispan, L.L.C., 2002; Twersky, 2008). In 2009, these numbers are holding steady; however, the dynamic nature of the reimbursement environment and the development of minimally invasive surgical technologies may make the number of office-based procedures skyrocket over the next several years.
According to a 2009 report from the National Center for Health Statistics, in 2006 there were approximately 35 million ambulatory surgery visits in the United States, with most of these being in hospital-based facilities vs. free-standing facilities (Cullen et al., 2009). Just over 7% of these reported ambulatory surgery visits were by children younger than 15 years of age. Although the total number of ambulatory surgery visits for this age group went up in 2006, the percentage decreased from the 8.5% reported for 1996 (Hall and Lawrence, 1998). As is suggested, without mandatory reporting of all ambulatory surgery procedures requiring anesthesia to a central agency, office-based anesthesia data will remain obscure (Perrott, 2008).
Although many different types of surgical procedures, including otolaryngologic, urologic, cosmetic, ophthalmologic, and radiologic (including single-photon emission computed tomography [SPECT]) scanning are performed on children in the office-based setting, the majority of procedures requiring anesthesia or sedation in the office setting for children are dental procedures (Cartwright et al., 1996; Goldblum et al., 1996; Grevelink et al., 1997; Yagiela, 1999; Siegel et al., 2000; Smith and Smith, 2000; Garin et al., 2001; Friedman et al., 2002; Ross and Eck, 2002; Barinholtz, 2009; Gravningsbraten et al., 2009).
The most common reason for children to require dental procedures is related to early childhood caries (ECC), with an incidence in primary teeth of 41% in children between ages 2 and 11 years (Beltrán-Aguilar et al., 2005). Furthermore, a majority of ECC in the United States occurs in children from low-income households. (Vargas et al., 1998; Nunn et al., 2009). Yagiela (1999) reports that a survey by the American Academy of Pediatric Dentistry estimated in 1995 that the number of children who received anesthesia for dental procedures was close to 200,000. Presently, the actual number of dental procedures performed with patients under general anesthesia is difficult to determine because of underreporting, and because estimates are generated from Medicaid-provided information of hospital-based care (Casamassimo et al., 2009). The actual number of children requiring anesthesia for these procedures may be significantly greater than this, with the discrepancy being because of a paucity of access to the appropriate care secondary to a lack of primary-care access and as important, a lack of insurance coverage. Significantly, this access to care can be positively impacted by state legislation. White and others (2008) report that non–Medicaid and Medicaid dental visits increased by approximately 60% and 30% (p < .05), respectively after the State of North Carolina introduced general-anesthesia legislation for preschool-aged children.
In a 2006 survey of Illinois dentists licensed to give some form of sedation or anesthesia, close to 116,000 cases were performed with some form of sedation and general anesthesia (Flick et al., 2007). Most of these sedation and anesthesia cases (90%) were performed by oral and maxillofacial surgeons (63% of survey respondents); however, general dentist and pediatric dentist respondents (29%) accounted for most of the remaining sedation and anesthesia. The number of pediatric patients cared for, however, was not specified.
Why office-based surgery and anesthesia?
As one can see from the information presented in Figure 35-1, there is a compelling need to be able to provide quality surgical and anesthesia services in the office-based setting. The actual reasons as to why this boom has occurred and continues to evolve and why anesthesiologists are more aggressively entering this arena are severalfold.
Ostensibly, the most persuasive argument for a paradigm shift to the office-based setting is financial. Potential and realized limitations and reductions in reimbursement because of health care reform and economic constraints are causing physicians to become more creative in facilitating cost-effective approaches to surgery. Costs can be reduced by reducing or eliminating facility fees that ordinarily accompany the cost-sharing charges at hospital-based and ambulatory surgery-based centers. Much of office-based surgery, especially cosmetic surgery, is performed on a cash-payment, fee-for-service basis, and therefore cost containment by limiting facility-fee overhead becomes crucial to patient affordability. This affordability is critical in the case of pediatric dental procedures, where in many instances, neither dental nor medical insurance covers out-of-office dental procedures and anesthesia costs (Ross and Eck, 2002).
One surgical study from the United States as early as 1994 compares the cost of performing an inguinal herniorrhaphy in the hospital with that of the cost in an office setting. This study finds the latter a more cost-effective alternative with a reduction in cost of a laparoscopic approach or an open approach of approximately 70% and 60%, respectively when compared with the hospital outpatient setting (Schultz, 1994). In one pediatric study, Lalwani and others (2007) show a per-patient savings of close to $5000 when patients with special health care needs who require anesthesia or sedation undergo dental rehabilitation procedures in an office-based setting when compared with those receiving care in a hospital operating room.
Safety and outcome
With the government, medical community, third-party payers, and the consumers (patients) pushing for more expeditious and cost-effective (and hence potentially more profitable) ways in which to provide surgical and anesthesia services, the anesthesia services provided are moving further away from the hospital safety net. Dr. Ervin Moss (1998), the impetus behind tighter regulation of office-based anesthesia and surgery in the state of New Jersey and perhaps beyond, stated, “Some say the major difference between the office-based and hospital-based anesthesiologist is that the former must be more courageous or foolhardy.”
Whether or not Dr. Moss’s characterization of the office-based anesthesiologist holds true, the fact remains that anesthesia care must be provided safely, if not more vigilantly, than hospital-based or surgery center-based anesthesia. Although some have questioned whether the convenience of office-based surgery and anesthesia is worth the risk, consensus opinion, as this specialty area continues to develop, is that it must be done correctly and consistently (Arens, 2000; Bridenbaugh, 2005).
Although anesthesia-related mortality risk continues to be difficult to assess, perhaps because of methodologic issues, it has nevertheless decreased over the past several decades (Lagasse, 2009). Anesthesia-related mortality risk in the United States has recently been quoted to range from 0.1: 10,000 to approximately 0.8:10,000 procedures performed (Biboulet et al., 2001; Lagasse, 2002, 2009; Newland et al., 2002; Li et al., 2009). These numbers appear slightly lower than the rate published outside the United States, at 1.12:10,000 cases (Braz et al., 2006).
In children, the incidence of cardiac arrest from anesthesia is reported to range from 1.4:10,000 to 4.6:10,000 with the incidence of anesthesia-related mortality being reported by some as between 1.2:10,000 and 7.7:10,000 cases in ASA Physical Status (PS) I-II and ASA PS III-IV, respectively (Morray et al., 2000). This disparity seen between patients of lower and higher ASA PS is consistent with morbidity statistics presented by Murat and others (2004) in a report of over 24,000 anesthesia cases over 2½ years and by Kakavouli and others (2009) in a study comparing adverse events for patients receiving anesthesia in the operating room (2.5%) with those outside the operating room (3.5%). Respiratory events were the leading cause in both studies, 1.7% and 1.9%, respectively (Zuercher and Ummenhofer, 2008; Bharti et al., 2009).
Interestingly, the number of pediatric arrests related to medications decreased almost 20% from 1994 to 1997 (37%) and from 1998 to 2004 (18%) as reported in an update on the pediatric perioperative cardiac arrest (POCA) registry (Bhananker et al., 2007). The authors suggest that this may be because of the decreased use of halothane in pediatric anesthesia. This is important in that the relatively newer, faster onset and offset (desirable characteristics in the office-based setting) volatile agents, sevoflurane and to a lesser extent desflurane, may confer a larger therapeutic index in the office-based setting. Furthermore, these authors report that airway causes for pediatric arrest still occur with a high incidence (27%), reemphasizing the importance of having superior airway management skills in pediatric anesthesia regardless of location.
Many of the cases brought to the attention of the medical, dental, and lay community involve children undergoing dental procedures (Morell, 2000; Hussain, 2006; Sherry, 2009). This information is not only tied intimately to the present topic but also is the point of origin for much of the controversy over the safety and efficacy of office-based anesthesia for children.
In two reports from the United States, Coté and others (2000a, 2000b) retrospectively analyzed information obtained from the Food and Drug Administration, the United States Pharmacopoeia, and a survey of pediatric specialists to report on contributing factors to critical incidents and medications used in sedation as they relate to adverse sedation events in children undergoing diagnostic and therapeutic procedures.
In the first report, Coté and others (2000a) reported on 95 incidents. Over half of these incidents resulted in death (n = 51) or permanent neurologic injury (n = 9). The remaining patients either had prolonged hospitalization without injury (n = 21) or sustained no harm as a result of the adverse event (n = 14). Of note was that those children who were cared for in non–hospital-based facilities (older and healthier) had a much higher rate of death and permanent neurologic injury (93%) than did those cared for in hospital-based facilities (37%). The authors also reported that inadequate resuscitation was a major factor in the non–hospital-based facilities (57%) compared with the hospital-based setting (2.5%) and that patients cared for in the nonhospital setting were more likely to sustain a cardiac arrest as the second or third event compared with those in the hospital setting (p < .001).
In the second report, Coté and others (2000b) used the same 95 incidents to determine whether there was a relationship between medications and adverse events. Although the authors noted no relationship between the class of drug used, the route of administration, and the incidence of death and permanent neurologic injury, they did note that adverse outcomes were associated with the administration of drug overdoses and administration of three or more sedating medications. Furthermore, 11 of 12 patients (all younger than 6 years of age) sustained adverse outcomes either at home or in a car. Two of the 12 patients, who had received sedative medication at home before their procedure, sustained an adverse event at home before the scheduled procedure.
Of note was that over one third of the reported events occurred during sedation for dental procedures (n = 32). Twenty-nine of these resulted in death or permanent neurologic injury. Most of the involved practitioners were either oral surgeons or dentists, three were pedodontists, and one was a nurse anesthetist under the supervision of a dentist. None of the practitioners was an anesthesiologist (Coté et al., 2000b).
Although these conclusions may seem intuitive to those who regularly care for children, they are reinforced by recent reports suggesting that severe adverse outcomes in pediatric sedation or analgesia outside the operating room may be improved by consistency in guidelines, training, and adverse-event reporting across institutions and specialties that are implemented through well-designed sedation services (Cravero et al., 2006, 2009; Bhatt et al., 2009). In the latter study, Cravero and others (2009) show that whereas the incidence of pulmonary events (e.g., stridor, laryngospasm, excessive secretions, and central and obstructive sleep apnea) is high, they report a low incidence for cardiopulmonary resuscitation (0.4:10,000) and no mortalities in approximately 50,000 sedation procedures performed by various nonanesthesiologist pediatric specialists. Lightdale and others (2006) advocate the use of microstream capnography to enhance the safety of pediatric patients undergoing procedures under sedation or analgesia via early detection of hypoxemia caused by alveolar hypoventilation.
Although there are anecdotal reports and studies of adverse outcomes, complications, and side effects related to sedation and anesthesia in the dental literature, others have reported on a safety record that is as good as, if not better than, that of the anesthesia community (Denman et al., 1968; Yee et al., 1985; Blayney et al., 1999; Girdler and Smith, 1999; Laskin, 1999; Saxen et al., 1999; Whitmire, 1999; Johnson et al., 2001; Manoharan et al., 2001; Yagiela, 2001; Perrott et al., 2003). The incidence of mortality rates in dental anesthesia is reported to be anywhere from 1:250,000 in the United Kingdom to 1:300,000 to less than 1:1 million in the United States (Cartwright, 1999; Brandom and Herlich, 1999; D’Eramo, 1999; Laskin, 1999).
As if there is not enough controversy within the anesthesia community, within the dental community there is also debate over who can best serve the patient requiring anesthesia for a dental procedure. Yagiela (2001) points out that members of the American Society of Dental Anesthesiologists, those who provide “approximately 25,000 pediatric general anesthetics per year,” subscribe closely to the American Academy of Pediatrics and American Society of Anesthesiologists guidelines for sedation and anesthesia. He also states that “since the organization’s inception 2 decades ago, there have been no known incidents of mortality or significant morbidity in children managed in the dental office by a dentist anesthesiologist” and intimates that safety may be improved in dental offices with a greater adherence to these guidelines by the dental community as a whole (Yagiela, 2001).
In contrast to the paucity of information about the safety and outcomes related to office-based anesthesia, the efficacy of anesthesia in pediatric ambulatory surgery in general is well known. As reported by Willetts and others (1997), outpatient surgery may date back to James H. Nicoll, a Glasgow surgeon who in the early 1900s successfully performed several thousand ambulatory procedures on young children at the West Graham Street Dispensary for Children. Contemporary literature continues to confirm over the past 50 years that for a growing variety of surgical procedures, the ambulatory setting for surgery and anesthesia is safe, cost effective, and therefore preferable to inpatient surgery (Postuma et al., 1987; Patel and Hannallah, 1988; Hannallah, 1991; Ghosh and Sallam, 1994; Blacoe et al., 2008; Shah et al., 2008; Mattila et al., 2009).
In one prospective study examining the efficacy of pediatric day surgery at the Children’s Hospital of Eastern Ontario, the overall complication rate was very low. The investigators, over 5 years, prospectively studied children undergoing various outpatient procedures, including myringotomy, tonsillectomy and adenoidectomy, dental procedures, and inguinal hernia repairs. Most of the children were between 2 and 7 years old, and the total number of cases approached 25,000. The reported complication rate was 1.6% per year. The most common complication was postoperative bleeding, primarily secondary to tonsillectomy and adenoidectomy. None of the complications resulted in permanent disability (Letts et al., 2001). In a retrospective study from Children’s National Medical Center focusing on pediatric otolaryngology procedures, Shah and others (2008) report that in close to 5000 cases (55% of total surgical cases) over 7 years, the rate of unanticipated outcomes was 0.2%. Similar to the previous study, the majority of adverse outcomes were related to posttonsillectomy and adenoidectomy bleeding.
Most of the information relative to the outcomes for office-based anesthesia and surgery is related to adult patients. In a retrospective report specifically looking at outcomes for plastic surgical procedures, Hoefflin and others (2001) reported no significant morbidity and no mortality over 18 years in more than 23,000 procedures in which general anesthesia was used. The anesthesia was provided by a board-certified anesthesiologist, and the single facility was accredited by the American Association for Accreditation of Ambulatory Surgical Facilities (AAAASF) (Hoefflin et al., 2001). In this report, most of the patients underwent cosmetic surgery, with some patients having multiple procedures. The only incidents reported as significant by the authors were the rare occurrence of electrocardiograph and oxygen-monitor failure. None of these events resulted in any patient complications, although three patients were hospitalized for custodial care. Minor anesthetic complications included nausea and vomiting (fewer than 5%), postextubation sore throat (fewer than 5%), shivering, one case of dental damage, one case of delayed (10 days) deep vein thrombosis, and one case of carpal tunnel syndrome after intravenous catheter infiltration.
In a retrospective study comparing outcomes in physician offices and ambulatory surgery centers in Florida, Vila and others (2003) found that despite new regulations imposed by the Florida Board of Medicine in the year 2000, between 2000 and 2002 there was a significantly increased risk of adverse incidents and deaths in offices (65.8:100,000 and 5.3:100,000, respectively) compared with ambulatory surgery centers (9.2:100,000 and 0.8:100,000, respectively). Furthermore, the authors estimated that a significant number of injuries and at least six deaths per year could have been prevented had all surgical cases been performed in an ambulatory surgery center.
In another report and as a follow-up study to previous reports, Coldiron and others (2008) reviewed critical surgical incidents that occurred in physicians’ offices in the state of Florida from 2000 to 2007 subsequent to mandatory reporting that began in February 2000 (Coldiron 2001, 2002). This mandatory reporting initially resulted from several newspaper accounts of poor patient outcomes and questions of whether office anesthesia and surgery were safe. Those incidents requiring reporting are listed in Box 35-1.
Box 35-1 Incidents in Surgical Offices Requiring Reporting by the State of Florida
From Coldiron B: Office surgical incidents: 19 months of Florida data, Dermatol Surg 28:710, 2002.
In the data obtained from Florida’s Agency for Health Care Administration, there were 31 deaths and 143 procedure-related complications and hospital transfers. Most of the bad outcomes occurred during “nonmedically-indicated” cosmetic surgery such as liposuction or liposuction with abdominoplasty. Plastic surgeons were responsible for 48% of all deaths and 52% of hospital transfers. Interestingly, most of the deaths (78%) occurred in ASA PS I patients. These authors note that the reported deaths and complications were unrelated to whether the physician was board certified or whether the physician had similar surgical privileges in a hospital (the latter being a mandatory requirement in some states). However, only 40% of facilities reporting adverse events were accredited by an independent agency. Despite the latter data, there are those who suggest that rigid state regulations and licensing laws along with accreditation of office-based surgical and anesthesia practices will help ensure patient safety (Kaushal et al., 2006).
Bitar and others (2003) retrospectively reviewed close to 5000 office-based plastic surgery procedures that were performed under monitored anesthesia care with sedation. The procedures in this study were performed by board-certified plastic surgeons, and anesthesia was provided by a CRNA. Nearly all patients were reported to be ASA PS I or II (99.9%), and the majority of patients were adult females (92%). The most common complications reported in this study were dyspnea (0.05%, n = 2), protracted nausea and vomiting (0.2%, n = 6), and unplanned hospital admission (0.05%, n = 2). One patient required intubation without prolonged sequelae. The author reported no cardiac arrests, deaths, or any incidence of deep vein thrombosis or pulmonary embolus. The author concluded that office anesthesia and surgery were safe when using appropriate protocols and patient selection.
Koch and others (2003) reported on several office-based anesthesia practices from various regions of the United States that were performed between 1981 and 2002. In this report, the authors noted no intraoperative deaths in over 64,000 anesthesia cases. Furthermore, in a subset of pediatric patients that comprised this report, one Chicago-based office anesthesia practice reported no intraoperative deaths, significant perioperative morbidity, or emergent hospital transfers in over 600 pediatric anesthesia cases. This number is now approximately 1200 cases and includes only one pediatric emergent hospital transfer (a possible malignant hyperthermia case). All complications continue to be minor, with a reported incidence of immediate and delayed PONV of less than 0.07% and 1%, respectively. Children in this subset ranged in age from 18 months to 17 years. Most of the children were younger than 6 years old and were classified as ASA PS I or II patients (Barinholtz, personal communication, 2009). These safety results are consistent with those reported by others caring for special-needs dental patients in the office-based setting (Caputo, 2009).
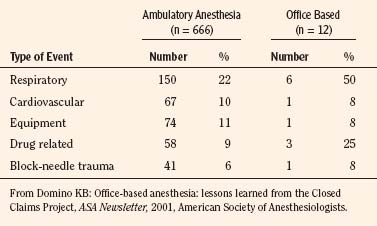
In a review of the ASA’s Closed Claims Project database, Domino (2001) compared claims (all age populations) made against anesthesiologists in the office setting (n = 14) with those made in other ambulatory surgery settings (n = 753). Claims for dental damage and for nonoperative pain management were excluded from this analysis. Although patient demographics were similar in both groups (Table 35-2), most of the claims in the office-based setting were related to plastic surgery or dental procedures, whereas in the ambulatory setting, most claims were related to procedures other than plastic or dental. The severity of injury appeared greater in the office-based setting compared with other ambulatory sites. In this initial report, most of the claims made in other ambulatory sites (62%) were for “temporary or nondisabling injury,” whereas most of the claims from the office-based setting were for death (64%). The author pointed out, however, that without a denominator, a true risk assessment for each site cannot be ascertained. Subsequent data from the ASA Closed Claims Analysis, with more claims in both the ambulatory (total, n = 877) and office-based settings (total, n = 29), continue to show a preponderance of death and brain damage in the office-based setting, although at a reduced (40%) incidence (Posner, personal communication, 2009). Likewise, Jimenez and others (2007), in a closed claims analysis of 532 pediatric cases from 1973 to 2000 (not exclusively ambulatory or office-based, but from 1990 to 2000 the highest percentage of the claims involved dental, otolaryngologic, and maxillofacial surgeries), found that death and brain damage were also the dominant injuries in malpractice claims, although the proportion of these claims decreased in the 10 years before 2001.
TABLE 35-2 Patient Characteristics in Analysis of Claims Made in Office-Based Anesthesia Incidents
| Ambulatory (n = 753) | Office Based (n = 14) | |
| Age (mean yr) | 41 | 45 |
| Female (%) | 58 | 64 |
| ASA PS class I/II (%) | 82 | 89 |
| Elective surgery (%) | 97 | 100 |
| Anesthesia type | ||
| General (%) | 66 | 71 |
| MAC (%) | 10 | 14 |
| Surgical procedure | ||
| Dental (%) | 3 | 21 |
| Plastic surgery (%) | 32* | 64* |
| Other (%) | 64† | 14† |
MAC, Monitored anesthesia care.
* p < 0.05, Ambulatory vs. office based.
† p < 0.01, Ambulatory vs. office based.
From Domino KB: Office-based anesthesia: lessons learned from the Closed Claims Project, ASA Newsletter, 2001, American Society of Anesthesiologists.
In both office-based settings and ambulatory settings, Domino (2001) noted that respiratory events were most common. A summary of airway-related complications and drug-related complications in office-based claims is shown in Table 35-3. Although the timing for injury was similar for both sites, there tended to be fewer claims for events occurring after discharge in the other ambulatory claims (7%) than for the office-based claims (21%).
The Closed Claims Project analysis also discloses that a greater number of office-based claims continue to be potentially preventable with better monitoring (Posner, personal communication, 2009). In the earlier report, a greater percentage of office-based claims involved substandard care compared with other ambulatory settings (not statistically significant), and payment was made in a higher percentage of claims (92% vs. 59%) and for a higher median payment ($200,000 vs. $85,000) for office-based claims than for other ambulatory settings (Domino, 2001).
Legislation and regulations
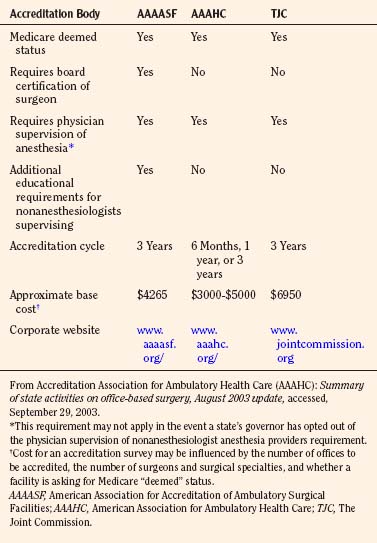
There are many entities presently imparting their influences on the practice of office-based anesthesia and surgery. These include, but are not limited to, state regulatory bodies; federal regulatory agencies such as the Centers for Medicare and Medicaid Services (CMS) and the Office of the Inspector General (OIG); national medical professional societies; national medical and safety organizations such as the Federation of State Medical Boards (FSMB, 2002), the National Committee for Quality Assurance (NCQA), and the National Patient Safety Foundation (NPSF); accrediting organizations such as the American Association for Accreditation of Ambulatory Surgery Facilities (AAAASF), the Accreditation Association for Ambulatory Health Care (AAAHC), and The Joint Commission; and the insurance industry. Also, in 2003, the AMA published the Core Principles for Office-Based Surgery that are currently endorsed by government agencies, the accrediting bodies, and specialty societies.
The main problem with regulation and legislation concerning office-based surgery and anesthesia is the wide regulatory variability that exists among states. Some states are highly regulated, whereas other states have no regulations (Fig. 35-2). In the states that do have regulations, these regulations are often ambiguous. State regulations regarding facility structure, personnel, equipment, and credentialing of individual practitioners differ widely. A more complete compendium of state regulations is available through the ASA Office of Governmental and Legal Affairs (ASA, 2009). In addition to state agencies and legislation, professional societies can regulate practice by establishing practice guidelines, practice standards, and advisories. The disadvantage of professional societies is that they run the risk of being self-interest groups. Consistent regulatory control could also be achieved by recognizing established accrediting bodies as oversight organizations. The organizations most commonly involved in the accreditation of ambulatory surgery centers are AAAASF, AAAHC, and The Joint Commission.

FIGURE 35-2 2009 Office-based surgery and anesthesia requirements.
(From ASA Office-based anesthesia: considerations for anesthesiologists in setting up and maintaining a safe office anesthesia environment.American Society of Anesthesiologists, 2008d.)
Historically, AAAASF, known until the early 1990s as the American Association for Accreditation of Ambulatory Plastic Surgery Facilities (AAAAPSF), was created to accredit only outpatient plastic surgery facilities. However, as other medical specialties moved procedures to free-standing outpatient settings, this organization became active in accrediting these facilities as well (AAAASF, 2003).
In contrast to AAAHC and The Joint Commission, AAAASF accredits facilities over a much narrower spectrum of medical specialties. The surgical specialties include colon and rectal surgery, obstetrics and gynecology, ophthalmology, orthopedic surgery, otolaryngology, plastic surgery, general surgery, and urology. AAAASF is particularly restrictive in its practitioner credentialing. This organization requires all surgeons to be certified by the American Board of Medical Specialties (ABMS) and requires all of its surgeons practicing at an ambulatory or office-based facility to have hospital privileges for the same procedures being performed in the office facility (AAAASF, 2003). AAAASF will only accredit a practice where the surgeons practice in their area of board certification. Furthermore, the AAAASF will not accredit an organization if propofol is not administered by an anesthesiologist or a properly supervised CRNA or anesthesia assistant.
In addition to defining requirements for surgical personnel, all three organizations offer accreditation processes that outline expectations for the facilities as they relate to meeting standards for the facility physical plant (e.g., by ensuring that state, local, Occupational Safety and Health Administration [OSHA], and National Fire Protection Association regulations are followed), anesthesia administration, monitoring, equipment and personnel, ancillary staff, patient transfer policies, patient safety and emergency resuscitation issues, quality improvement, and patient satisfaction issues. A summary of some of the similarities and differences between the three most visible accrediting organizations is given in Table 35-4.
Whether accreditation affects outcomes in office-based surgery and anesthesia is uncertain. In a survey study, the AAAASF sent a questionnaire to its accredited facilities that addressed patient safety in plastic surgery office facilities. Two hundred forty-one of 418 facilities responded to the questionnaire. Over 5 years 400,000 surgical procedures were studied, and the authors reported the risk of significant complications to be 1:213 and the risk of death to be 1:57,000 cases. They concluded that overall risk in an accredited office is comparable with that of other ambulatory surgery sites (free-standing or hospital based) (Morello et al., 1997). In a retrospective review of an accredited office-based plastic surgery facility, Byrd and others (2003) reported no deaths in over 5000 cases. However, these authors noted that several patients required hospital admission for various medical problems.
Clinical aspects
Patient and Procedure Selection
Because most office-based procedures for pediatric patients involve dental procedures, a majority of the children undergoing these procedures are older than 1 year of age. However, as the realm of office surgery for children expands (including tonsillectomies), and because there are no prospective studies as yet in the office-based anesthesia literature looking at outcomes and patient age for children undergoing office-based surgery, it seems that the present guidelines for other ambulatory surgery venues should be used (Gravningsbraten et al., 2009). When taking this latter point into consideration, the minimum age requirement (other parameters such as type of procedure not withstanding) for children undergoing office-based procedures can be determined by whether a child requires prolonged postoperative monitoring based on postconceptual age or the administration of opiate analgesics and other respiratory depressant agents (Welborn and Greenspun, 1994; Galinkin and Kurth, 1998). Although postanesthesia care unit (PACU) complications resulting from respiratory complications may be more common in neonates and infants, any other minimum age requirements are often arbitrary and may reflect the comfort level of the surgeon, office staff, and anesthesia personnel caring for the child (Westman, 1999; Ross and Eck, 2002; Murat et al., 2004). Adherence to policies and guidelines set forth for minimum age requirements for outpatient anesthesia in ambulatory surgery centers and hospital-based ambulatory surgery departments is critical.
Most children cared for in the office-based setting are ASA PS I and II patients. In some situations, it may be acceptable to provide anesthesia care for those children with stable comorbidities who are ASA PS III patients despite the fact that these patients in general may be more susceptible to adverse outcomes from anesthesia (Morray, 2002; Murat et al., 2004; Jimenez et al., 2007). Not surprisingly, getting consensus from pediatric anesthesiologists as to which comorbidities are appropriate for hospital-based or free-standing ambulatory surgery is difficult, but some of the pediatric comorbidities considered high risk for the office-based surgical suite are listed in Box 35-2 (Abu-Shahwan, 2007).
In an early prospective study looking at prolonged recovery stay and unplanned hospital admission after ambulatory surgery in pediatric patients, the authors reported annual rates of approximately 4% and 2%, respectively. PONV and respiratory complications were the most common factors leading to prolonged recovery stay, whereas respiratory complications (32%) and surgical reasons (30%) were the factors most commonly responsible for unplanned hospital admission. These authors also found that higher ASA physical status had a direct relationship to unplanned hospital admission and adverse respiratory events (D’Errico et al., 1998).
Subsequently, Dornhoffer and Manning (2000), in a retrospective chart review, evaluated unplanned hospital admission after four types of otologic procedures (not including myringotomy tube placement) in adult and pediatric outpatients. The unplanned hospital admission rates for children and adults were 5.7% and 2.3%, respectively. The most common reason for unplanned hospital admission was PONV. These findings are relatively consistent over time, and in a more recent study, Blacoe and others (2008) in Scotland found an overall unplanned admission rate of 1.8% in over 13,000 day-surgery cases. The combined percentage of admissions for two surgical complications (postoperative bleeding, 13.9%, and more extensive surgery than planned, 11.8%) exceeded that for the most common anesthesia-related cause for admission, PONV (23.5%).
Dornhoffer and Manning (2000) also noted that tympanomastoidectomy with ossicular reconstruction, procedures lasting longer than 2 hours, and asthma were risk factors predicting the need for postoperative admission. In contrast to this study, Mingus and others (1997) reported that surgical cases lasting as little as 1 hour may be associated with a higher rate of unplanned hospital admission.
In a study by Fortier and others (1998) involving over 15,000 patients, unplanned hospital admission after ambulatory surgery was related to longer duration of anesthesia and surgery, higher ASA PS (classes II and III), postoperative bleeding, excessive pain, and nausea and vomiting. Of note, and similar to the more recent study of Blacoe and others (2008), is that surgical reasons are identified to be more commonly responsible for hospital admission than are anesthesia-related issues (38% and 25%, respectively). The fact that the office-based setting is best suited for those children who are healthy without significant comorbidity and are undergoing minimally invasive procedures may inherently result in few postoperative complications.
Preoperative Preparation
Preanesthetic Interview
As is the case with ambulatory surgery in general, rarely is the preanesthetic interview for office-based surgery for pediatric patients conducted in person. For the anesthesiologist who works in a limited number of offices, preanesthetic interviews may be possible during the patient’s presurgical office visit if the anesthesiologist should be attending that particular office on the same day. This is the exception rather than the rule when anesthesiologists provide itinerant care for a multitude of offices. Although some authors have advocated presurgical evaluation in certain instances on the same day of surgery (Overdyk et al., 1999; Mangia et al., 2009), citing economic and logistic advantages for practitioners and patients, most preanesthetic interviews will be initiated by telephone before the day of surgery.
The preanesthetic interview for office-based procedures does not differ much from that described for other ambulatory settings (Ferrari, 2004; Polaner, 2006). However, there are caveats relative to the preanesthetic interview for an office-based procedure.
Preoperative Laboratory Testing
Because most children receiving office-based anesthesia services are healthy and are undergoing minimally invasive procedures, the necessity for preoperative laboratory testing is rare. As is advocated by other authors, any laboratory testing should be determined on an individual patient basis by clinical need after the preanesthetic interview (Meneghini et al., 1998; Friedberg, 2003; Maxwell, 2004; Polaner, 2006). Despite agreement on the lack of clinical utility and increased cost of routine preoperative laboratory screening among anesthesia professionals, an anesthesiologist providing office-based anesthesia services must be aware of any state and local government mandates for such testing. Additionally, each practitioner must establish a policy for determining the cost-to-benefit yield (especially in the office setting where cost savings are a key advantage) on more controversial laboratory testing such as pregnancy testing, especially in adolescents and teenagers (Wheeler and Coté, 1999; Hennrikus et al., 2001; Kahn et al., 2008; Bodin et al., 2010).
Preoperative Sedation
Despite the ostensibly less intimidating environment of the office, the anxiety that pediatric patients (and their parents) undergoing office-based procedures experience is probably no less than that in other surgical environments. The need for preoperative sedation or some other useful mechanism by which the anesthesiologist can reduce preoperative anxiety is critical for a couple of reasons. First, parents are becoming more educated about the perioperative process via the Internet and the lay press, and second, the space-limited environment of many office practices and the close proximity to office waiting rooms and other patients may make preoperative sedation of children most at risk for preoperative anxiety even more important (Baldauf, 2009).
The group of children most likely to undergo a mask induction of anesthesia is also the age group of children most likely to experience preoperative anxiety (Kain et al., 1996, 2002; McCann et al., 2001; Watson and Visram, 2003). Most children receiving preoperative sedation receive midazolam (McCann et al., 2001; Rosenbaum et al., 2009). Although various routes of administration are advocated, including intranasal and rectal, oral administration of midazolam is the most common (Levine et al., 1993; Davis et al., 1995; Griffith et al., 1998; McGraw and Kendrick, 1998; Marhofer et al., 1999). Ease of administration as determined by acceptance by the child, in most instances, dictates the route of administration. Most often in practice, a dose of 0.5 to 1 mg/kg (maximum dose, 20 mg) orally is effective.
Although some have found that certain routes of administration of midazolam have no impact on discharge times (Davis et al., 1995; Kain et al., 2000), others find that there are significant delays in recovery or discharge times with the use of orally administered midazolam in combination with various anesthetic techniques (Viitanen et al., 1999).
Despite a report on the decreased efficacy in the levels of preoperative sedation using oral melatonin compared with oral midazolam, Kain and others (2009) did show a significant (p < 0.05) dose-related lower incidence of emergence agitation (another office undesirable) in those patients receiving melatonin. Furthermore, the use of combination preoperative sedation, such as intramuscular ketamine and midazolam, in uncooperative children may be inappropriate for the office-based setting because of prolonged discharge times (Verghese et al., 2003).
The benefit of parental presence in reducing preoperative anxiety remains questionable (to the child and parent), especially compared with other nonpharmacologic and pharmacologic approaches to preoperative anxiety reduction (McCann et al., 2001; Kain et al., 2003; Chundamala et al., 2009; Yip et al. 2009). Whatever a practitioner’s personal preference, the office-based environment may provide an excellent opportunity for parental presence during induction of anesthesia, especially in cases where sterility concerns are minimal, such as for dental restoration or radiologic procedures.
When choosing parental presence as the sole method of anxiety reduction, it is important to consider the information available that may enhance the success of this approach. Some authors report that specific patient behavior profiles, specific parent-child anxiety level combinations (e.g., calm parent and anxious child), and the presence of a child’s mother as opposed to the father may have the most impact on reducing the anxiety level of the child (Messeri et al., 2004; Kain et al., 2006a; Chorney and Kain, 2009).
Intraoperative Care
The intraoperative care of the pediatric patient in the office-based setting relative to induction and maintenance of anesthesia does not differ much from that in other ambulatory settings. Despite the minimal standards set forth by most states relative to office-based anesthesia, from the anesthesiologist’s perspective, monitoring and equipment standards and guidelines must not be different for the office than for other surgical and anesthesia sites. The anesthesiologist should adhere to the Standards for Basic Anesthetic Monitoring and Guidelines for Office-Based Anesthesia (ASA, 2005, 2009b) (see Chapters 10 and 11, Equipment and Monitoring). With this in mind, anesthetic technique is limited only by the equipment, supplies, and resources of the physical plant that are available.
Despite liberalizing the fasting period (clear liquids 2 to 3 hours before anesthesia), administration of intravenous fluids both during and after the surgical procedure is important. There is evidence in both adult and pediatric populations that aggressive intraoperative fluid management may in fact decrease the incidence of PONV (Holte et al., 2007; Goodarzi et al., 2009).
Anesthetic Technique
The concepts of “rapid onset, rapid recovery, and minimal side effects,” used often to describe the important characteristics of an appropriate anesthetic technique in an ambulatory setting, is equally important for the office setting. Although all types of anesthesia, including regional anesthesia, can be used in the office setting, general anesthesia and varying levels of sedation are most often used in children (Hausman, 2008).
Even though anesthesiologists debate the clinical (safety and outcome) and cost efficacy of relatively newer anesthetic agents (e.g., sevoflurane, desflurane, remifentanil, and propofol) compared with some of the older agents (e.g., halothane and isoflurane), these newer agents offer a distinct advantage as it relates to their predictability and perhaps their safety (Tang et al., 1999; Moore et al., 2002; Fishkin and Litman, 2003; Bhananker et al., 2007). All levels of the continuum of anesthesia (minimal sedation to general anesthesia) are used in the office setting. The type of anesthesia is determined by the age and cooperation level of the child, the type of procedure, the ease with which local anesthetic can be administered, its efficacy in a particular surgical procedure, the specific routine of the anesthesiologist, and as stated previously, the available equipment.
All of the newer volatile agents are efficacious in pediatric ambulatory surgery (Moore et al., 2002; Polaner 2006). However, if the anesthesiologist is transporting a machine with single-vaporizer capability, then sevoflurane may be the agent of choice because of its efficacy in both induction and maintenance of anesthesia, as well as its rapid onset and offset characteristics. Although some advocate the use of desflurane for maintenance of anesthesia for ambulatory surgery because of its predictability relative to emergence, it is impractical as a complete office-based anesthetic agent in children because of its lack of efficacy for inhalation induction (Zwass et al., 1992; Olssen, 1995; Smiley, 1996; Fishkin and Litman, 2003). Similarly, the intravenous agents propofol and remifentanil have been shown to be useful in the ambulatory setting (Hannallah et al., 1994; Davis et al., 1997, 2000; Pinsker and Caroll, 1999; Cohen et al., 2001).
Although remifentanil can be used successfully in an ambulatory setting when the airway is secured with an endotracheal tube, evidence suggests this narcotic should be used cautiously when the airway is not secured. Litman (1999) evaluated the use of remifentanil for moderate sedation in 17 patients (20 procedures) aged 2 to 12 years who were undergoing short, painful procedures. All patients received intravenous midazolam, 50 mcg/kg, in combination with remifentanil 1 mcg/kg followed by an initial infusion of remifentanil of 0.1 mcg/kg per minute. The remifentanil infusion was then titrated every 5 minutes to provide adequate sedation and analgesia. The average appropriate dose of remifentanil used was 0.4 mcg/kg per minute. Although the author reported successful use of this technique in 17 of 20 procedures, 1 child became unresponsive and required assisted ventilation, and hypoxemia was avoided in 10 of 13 children by continuous stimulation during the procedure.
In adults, the combination of a propofol infusion, titrated to bispectral analysis (BIS) number, and intermittent ketamine boluses has been reported (Friedberg and Sigl, 2000; Friedberg, 2003). In children undergoing dental restoration procedures, Barinholtz (2009) noted that combining propofol (100 to 200 mcg/kg per minute, titrated by BIS) with ketamine (0.5- to 1.0-mg/kg boluses) completely avoided the need for opiate analgesics. In contrast to these latter two reports, successful implementation of sevoflurane for maintenance of anesthesia for dental restorations has been reported by multiple authors (Abu-Shahwan and Chowdary, 2007; König et al., 2009).
Many dental cases are performed with oral sedation and nitrous oxide; however, longer dental restoration procedures may necessitate the use of an endotracheal tube (Saxen et al., 1999; Ross and Eck, 2002; Barinholtz, personal communication, October 2009). This is most prevalent in offices where the anesthesiologist may have limited access to the patient’s airway because of positioning or cramped quarters. Subsequently, ventilation is controlled by hand or mechanical means or the patient is allowed to breathe spontaneously. The anesthesiologist must be judicious in the use of muscle relaxants to facilitate intubation. Prolonged effects of these agents may lead to delayed emergence and consequently delayed discharge and turnover for subsequent cases.
Another concern for the anesthesiologist practicing in an office-based setting is emergence agitation. The incidence of emergence agitation is reported to be approximately 12% to 18%, but Faulk and others (2010) report an incidence of approximately 30% in 400 patients undergoing dental procedures under general anesthesia with sevoflurane. The etiology of emergence agitation appears to be multifactorial and is associated with inadequate postoperative analgesia, high preoperative anxiety levels, rapid emergence, and the newer volatile agents, and it is reported to occur with both intravenous and volatile anesthetic techniques (Kain et al., 2006b; Vlajkovic and Sindjelic, 2007; Kuratani and Oi, 2008; König et al., 2009).
There may be some benefits, although inconsistent, to preoperative sedation with various agents such as midazolam, clonidine, and melatonin in reducing the incidence of emergence agitation (Cox et al., 2006; Almenrader et al., 2007; Kain et al., 2009). The incidence of emergence agitation is also shown to be reduced by the intraoperative use of regional anesthesia, opiates, 5-HT3 receptor antagonists and dexmedetomidine, as well as the administration of ketamine or propofol at emergence from sevoflurane anesthesia (Cohen et al., 2002; Ibacache et al., 2004; Weldon et al., 2004; Lankinen et al., 2006; Abu-Shahwan and Chowdary, 2007; Abu-Shahwan, 2008; Kim et al., 2009).
Depth of Anesthesia Monitoring
The BIS monitor (Aspect Medical; Newton, Massachusetts) was originally described as able to determine the level of sedation, predict the loss of consciousness, and thereby diminish intraoperative awareness when used with various anesthetic agents (Glass et al., 1997). Although in some early reports BIS was shown to minimize awareness, decrease anesthetic use, or hasten recovery in adult patients, a more recent report of a prospective study of 2000 adult patients by Avidan and others (2008) describes no advantage to using accepted target values for BIS (40 to 60) over target values of end-tidal anesthetic concentrations in preventing anesthesia awareness or in reducing the administration of volatile agent (Glass et al., 1997; Song et al., 1997). Consequently, these authors did not recommend routine use of BIS monitoring during anesthesia.
The use of the BIS monitor is also controversial in pediatric anesthesia. A standard BIS number or range of numbers that precludes anesthesia awareness and simultaneously minimizes the amount of anesthesia delivered is difficult to establish, because subgroups of pediatric patients may have different baseline BIS numbers as is reported by Valkenburg and others (2009). In this report, intellectually challenged patients are found to have significantly lower BIS numbers while awake and while under anesthesia compared with patients in a controls group. This inconsistency is relevant, because care for intellectually challenged and special-needs children is commonplace in one of the higher volume areas using pediatric office-based anesthesia, the dental office. (Lalwani et al., 2007).
In a report on an electronic mail survey of members of the British and French pediatric anesthesia societies, over 60% of respondents feel that intraoperative awareness is an important issue in pediatric patients, whereas 10% of respondents report using BIS routinely (Engelhardt et al., 2007). Thus, the vast majority of respondents still continue to use clinical monitoring and end-tidal agent concentrations to assess depth of anesthesia.
There are also pediatric-anesthesia studies reporting advantages to BIS monitoring in patients receiving various types of anesthesia for a range of diagnostic and surgical procedures (Denman et al., 2000; Bannister et al., 2001; McCann et al., 2002; Religa et al., 2002; Messieha et al., 2005; Powers et al., 2005).
Denman and others (2000) found that in children ages 0 to 12 years who were anesthetized with sevoflurane, the BIS value correlated to the depth of anesthesia. Furthermore, this study also found that for a particular level of anesthesia (BIS = 50), children younger than 2 years had a significantly higher end-tidal concentration of sevoflurane than did children ages 2 to 12 years (1.55% vs. 1.25%, respectively). Bannister and others (2001) studied the effect of BIS on anesthetic use and recovery in 240 children. They noted that in patients aged 0 to 6 months the BIS had no effect on anesthetic emergence. However, in older children, BIS was associated with less anesthetic administration and an earlier emergence time.
Religa and others (2002) evaluated the association between BIS and level of consciousness in pediatric patients (aged to 6 years) undergoing dental procedures using a sedation protocol. These authors find that there is a significant association between behavioral responses and levels of sedation. However, the authors note that BIS offered no advantage over routine clinical monitoring and behavioral assessment in this setting.
Messieha and others (2005) report earlier times to extubation (5 ± 2 minutes sooner, p = .04) and PACU discharge (47 ± 17 vs. 63 ± 17 minutes, p = .02) in BIS-monitored patients receiving standardized doses of preoperative sedation with oral midazolam and sevoflurane for induction, with maintenance of anesthesia for dental procedures. Whereas these few “saved” minutes may seem germane to the office-based setting, the cost effectiveness and clinical utility of level-of-consciousness monitoring across pediatric subpopulations still need to be elucidated.
Postanesthesia Care
As in any ambulatory setting, the patient undergoing office-based surgery must meet established criteria before discharge to home, with the goal being to discharge the patient as quickly and as safely as possible (Patel et al., 2001). Actual discharge criteria include an adequate level of consciousness, good pain control, good hydration, minimal to no nausea, and a defined period of time since the last emesis (Ross and Eck, 2002; Fishkin and Litman, 2003). Furthermore, the appropriate personnel (at the minimum that which is outlined by the ASA Guidelines for Office-Based Anesthesia, 2009b) must remain with the child until discharge-ready status is reached.
Postoperative Pain Control
The undertreatment of pain in pediatric patients in traditional surgical and hospital settings continues to be an issue (Stamer et al., 2005; Segerdahl et al., 2008; Fortier et al., 2009). In fact, one study that compared oral ibuprofen (10 mg/kg) with oral acetaminophen with codeine (1 mg/kg per dose of the codeine component) for the emergency room treatment of acute arm fractures found that ibuprofen was more preferable to (because of its side-effect profile) and at least as effective (analgesia) as acetaminophen with codeine; however, the high incidence of treatment failure in both groups, 20.3% and 31.0%, respectively, although not statistically significant between the groups, is clinically relevant to the previous point of potential undertreatment of pain in pediatric patients (Drendal et al., 2009).
Although various opiate analgesics are routinely used in pediatrics and can be administered via conventional (e.g., intravenously, intramuscularly while asleep, or orally) and less conventional (e.g., intranasally) routes in traditional ambulatory surgery settings, their use in the office setting is often minimized or eliminated to help avoid postoperative sedation and PONV that are specific to drugs, dose, and perhaps patients (Weinstein et al., 1994; Anderson et al., 2000; Galinkin et al., 2000; Finkel et al., 2001; Duedahl et al., 2007; Howard et al., 2008a, 2008b; Voronov et al., 2008). Furthermore, because a preponderance of complications leading to unplanned hospital admission after ambulatory surgery in infants is related to the respiratory system, opiate use, which can cause respiratory depression in this age group, could potentially increase these numbers (Westman, 1999).
Because most office-based procedures in children are minimally invasive (at least for now), standard use of nonopioid analgesics such as the nonsteroidal antiinflammatory drugs (NSAIDs) and acetaminophen, when not contraindicated, is advocated. Ketorolac is found to be useful with an excellent safety profile for postoperative analgesia in a variety of pediatric surgical procedures, and it can be administered intravenously in a dose of 0.5 to 0.8 mg/kg (maximum dose, 30 mg) (Lynn et al., 2007).
Relative to pediatric dental procedures, Needleman and others (2008) report that 95% of 90 children undergoing dental rehabilitation under a standardized general anesthesia regimen without local anesthetic infiltration experience postoperative pain. Increased postoperative pain was reported to be more likely in children who had dental extractions, those at least 4 years of age, and those who had experienced a greater number of procedures. It seems intuitive, then, that local anesthetic infiltration should be an excellent analgesic supplement for children undergoing dental rehabilitation. However, Townsend and others (2009) report that in children, 3 to 5½ years old, undergoing oral rehabilitation while under general anesthesia and receiving local anesthetic infiltration in addition to intravenous ketorolac did no better relative to postprocedural pain than those children receiving intravenous ketorolac alone. In fact, these authors report a greater (but not statistically significant) incidence of lip and cheek biting in those children receiving local anesthetic infiltration.
This experience with ketorolac is similar to those of Purday and others (1996) who found ketorolac, 0.75 mg/kg, 1.0 mg/kg, and 1.5 mg/kg to be as efficacious with statistically less PONV as 0.1 mg/kg of morphine sulfate in treating postoperative pain in patients aged 2 to 12 years undergoing dental restoration procedures. Similarly, Maunuksela and others (1992) noted ketorolac to be as efficacious as morphine after pediatric eye surgery. In contrast, Kim and others (2003) report that topical ketorolac is ineffective in treating the pain associated with strabismus surgery. Ibuprofen can also be used to treat postoperative pain, but reports on its effectiveness for some surgical procedures are mixed. Kokki and others (1994) showed that preoperative administration of rectal ibuprofen, 40 mg/kg, divided into four equal doses, was effective in the treatment of postoperative pain in children aged 1 to 4 years in that it reduced the need for supplemental morphine postoperatively. In contrast, Bennie and others (1997), in a double-blind, placebo-controlled study of children older than 6 months who were undergoing bilateral myringotomy and tube placement, found no benefit to the preoperative oral administration of ibuprofen (10 mg/kg) or acetaminophen (15 mg/kg) in the treatment of postoperative pain compared with a placebo group. Joshi and others (2003) reported success with preoperatively administered oral rofecoxib, a cyclooxygenase-2 NSAID, given in a dose of 1 mg/kg, in treating postoperative pain and reducing PONV in children (3 to 11 years old) undergoing tonsillectomy.
Acetaminophen administration is also effective for postoperative analgesia, especially in procedures resulting in mild or moderate pain (Tobias, 2000). Although high-dose acetaminophen (40 mg/kg) administered rectally has been shown to be effective and has opiate-sparing effects, the restrictive quarters of some offices may make this route of administration somewhat prohibitive (Korpela et al., 1999). Similarly, preoperative high-dose acetaminophen (40 mg/kg), administered orally in a one-time dose is shown to be effective in the treatment of postoperative pain after myringotomy and tube placement in children ages 17 months to 6 years, without reaching toxic plasma levels (Bolton et al., 2002). This is contrary to the aforementioned studies, where oral acetaminophen in standard doses (15 mg/kg) was shown to be ineffective (Bennie et al., 1997).
Although NSAIDs alone, or in combination with acetaminophen are opiate-sparing in certain types of surgical procedures, opiate analgesics are not totally precluded from office-based surgery and may in fact at times be necessary (Purday et al. 1996; Hiller et al., 2006; Riad and Moussa, 2007). When either NSAIDs or acetaminophen is inadequate in the treatment of postoperative pain, then traditional combination drugs such as acetaminophen with codeine (0.5 to 1.0 mg/kg of the codeine component) may be appropriate.
Whether interesting alternatives or adjuncts to traditional analgesia regimen, such as innovative nerve blocks and acupuncture, for specific surgical procedures can be time efficient and efficacious in the office setting warrants further study (Voronov et al., 2008; Lin et al., 2009).
Postoperative Nausea and Vomiting
The etiology of PONV is multifactorial, with perhaps a genetic component in some individuals, and remains a major cause of prolonged discharge time and unanticipated hospital admission after ambulatory surgery in children (Awad et al., 2004; Blacoe et al., 2008; Rueffert et al., 2009). Adequate treatment of this side effect may play a significant role in improving patient satisfaction with the perioperative experience (Eberhart et al., 2002). Furthermore, the incidence of postdischarge nausea and vomiting (PDNV) is also significant after ambulatory surgery and has risk factors that do not totally mirror those of PONV. In fact, PDNV occurs in a significant number of individuals who do not experience immediate postoperative vomiting before discharge (Wu et al., 2002; Kolodzie and Apfel, 2009). The optimal treatments of both PONV and PDNV, whether prophylactic or rescue, are essential because of the negative influence these entities can have on an office-based practice (Tang et al., 1999; Kolodzie and Apfel, 2009).
Prophylactic and rescue treatment of PONV and PDNV may not be completely effective, but Engelman and others (2008) show significant risk reduction in postoperative vomiting in a meta-analysis of 11 reports of the use of single- and multiple-drug pharmacologic prophylaxis regimens in children. With this in mind and in addition to the information previously provided on the low incidences of PONV and PDNV anecdotally reported in children and adults by those practitioners intimately involved in the practice of office-based anesthesia, several studies examining the incidence and treatment of PONV and PDNV in this setting are available.
Tang and others (2001) compared the use of propofol-N2O anesthesia with the use of desflurane-N2O anesthesia plus antiemetic prophylaxis on the incidence of PONV in patients undergoing brief, superficial surgical procedures in an office setting. Patients in the propofol group received no PONV prophylaxis, whereas those in the desflurane group received ondansetron (4mg), droperidol (0.625mg), and metoclopramide (10mg) intravenously at the end of surgery. Neither group received opiate analgesics or muscle relaxants, and all patients received local anesthetic at the surgical site and ketorolac for postoperative pain management. The overall incidence of nausea and vomiting was very low in both groups (less than 10%) and did not differ statistically. Patient satisfaction in both groups was excellent.
In another office-based study, Tang and others (2003) compared the addition of 5-HT3 receptor antagonists to a control regimen in patients receiving desflurane-N2O maintenance anesthesia after propofol induction. All patients received droperidol (0.625 mg) and dexamethasone (4 mg) as baseline antiemetic prophylaxis. Subsequently, patients were randomly assigned to receive placebo, dolasetron (12.5 mg), or ondansetron (4 mg) intravenously before emergence from anesthesia. The results of this study show that the incidence of nausea and vomiting was the same in all groups.
It is obviously advantageous to the office-based practitioner to be able to predict which children are more likely to experience PONV and PDNV. A scoring system, included in management guidelines published by the Society for Ambulatory Anesthesia, predicting the risk of postoperative vomiting in children was presented several years ago and prospectively validated in another report (Eberhardt et al., 2004; Gan et al., 2007; Kranke et al., 2007). In this scoring system, the presence of one or more specific risk factors (surgery lasting longer than 30 minutes, strabismus surgery, age older than 3 years, and an immediate family with history of postoperative vomiting or PONV) increases the likelihood of postoperative vomiting.
Although the best regimen for the prophylaxis and rescue treatment of PONV/PDNV in children is not known, the approach to management is multimodal (Kovac, 2007). Treatment includes reducing baseline risk factors (e.g., regional vs. general anesthesia and avoiding opiates, nitrous oxide, volatile agents, and neostigmine) when possible (Gan et al., 2007). Other authors show that appropriate hydration and superhydration decrease the incidence of postoperative vomiting in certain surgeries (Scuderi et al., 2000; Goodarzi et al., 2009). On the other hand, interventions such as routine use of an intraoperative nasogastric tube and its inherent difficulties and routine postoperative fasting may not be effective (Kerger et al., 2009; Radke et al., 2009).
Pharmacologically, a multidrug approach to PONV prophylaxis in children is advocated, and medications from several drug classes are effective in prophylaxis and rescue treatment (Gan et al., 2007; Kovac, 2007). Dexamethasone is shown to be an excellent choice for various procedures in the pediatric and adult populations either by itself or in combination with 5-HT3 receptor antagonists (Splinter, 2001; Subramaniam, 2001; Negri and Ivani, 2002; Sukhani et al., 2002; Liechti et al., 2007). Although the optimal dose for dexamethasone is unknown and reported to be from 0.1 mg/kg when used with ondansetron to 1.5 mg/kg when used alone, Kim and others (2007) report that a dose of 0.0625 mg/kg is as effective as 1 mg/kg (maximum dose, 24 mg) in the treatment of PONV in children undergoing tonsillectomy and adenoidectomy (Splinter and Rhine, 1998; Henzi et al., 2000). Although in a quantitative systematic review, Henzi and others (2000) found the use of dexamethasone to be safe in otherwise healthy patients, a report by Czarnetzki and others (2008) shows that despite a dose-dependent decrease in PONV, there is an increased risk of bleeding in children receiving dexamethasone who undergo tonsillectomy and adenoidectomy.
Rescue treatment should focus on using agents from different classes of medications from those used for prophylaxis in a particular patient, and there is evidence that ondansetron in oral disintegrating tablets may be effective in preventing PONV and PDNV, specifically in those children who do not require rescue therapy before discharge (Wagner et al., 2007; Davis et al., 2008).
Establishing an office-based practice
Equipment and Supplies
Essentially all of the standard anesthesia equipment that is required in hospital ambulatory facilities (operating room and off-site) and surgery centers is required for office-based practice. All monitoring standards must be met in the office-surgical suite. Monitoring equipment must have battery back-up capability, because some offices may not have emergency generator capabilities in the event of power failure. Working in one facility where the equipment is capitalized by the surgeon or capitalized by the anesthesiologist and stored in the surgeon’s office is much easier to deal with than the more common alternative of the anesthesiologist bringing the anesthesia workroom from place to place. Box 35-3 outlines general categories of supplies, standard and emergency, that must be transported to surgical sites by the office-based anesthesiologist. The array of portable equipment that exists these days is more than adequate to meet standards.
Box 35-3 General Categories of Required Supplies Transported by the Office-Based Anesthesiologist
Courtesy D. Barinholtz, Mobile Anesthesiologists, LLC, 2003.
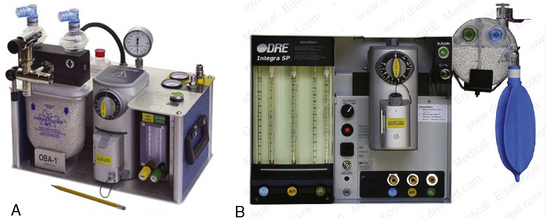
For those practitioners who provide total intravenous anesthesia, an anesthesia machine is not necessary, but the presence of equipment capable of delivering positive-pressure ventilation with oxygen is mandatory. For those anesthesiologists providing care to pediatric patients, a vaporizer-equipped anesthesia machine is invaluable. Most anesthesia machines are moveable, but there are a few machines that are truly portable and available for care of pediatric and adult patients in the office setting. One such machine is the OBA-1 (OBAMED, Cardinal Medical Specialties; Louisville, Kentucky), which weighs approximately 35 pounds, is vaporizer equipped, and is compatible with magnetic resonance imaging (MRI) devices (Fig. 35-3). The OBA-1 allows only spontaneous or manually controlled ventilation, because it is not equipped with an internal ventilator. The Magellan-2200, Model 1/M (Oceanic Medical Products; Atchison, Kansas) and the Narkomed-Mobile (Draeger Medical; Telford, Pennsylvania) are also marketed for office anesthesia, and both contain an internal ventilator. The latter machine, despite its ability to be rolled, is a much heavier unit and may not be practical for the anesthesiologist who is changing office venues on a daily basis.
Whenever a volatile agent or succinylcholine is to be used, one must be prepared to treat malignant hyperthermia. A stock supply of dantrolene must be available, and it is the responsibility of the anesthesiologist or a representative of the practice to proactively educate the surgeon and the surgeon’s office personnel on appropriate protocol in the event of a malignant hyperthermia episode (see Chapter 37, Malignant Hyperthermia).
Reimbursement
Mentioned earlier is the potential for cost savings by moving surgical cases from hospital outpatient or ambulatory surgical facilities to the office. Unfortunately, in some arenas, anesthesia-requiring office procedures are the rule because of the lack of insurance coverage for these procedures. This is especially true in pediatric dentistry. With few exceptions in the United States, dental and medical insurance policies traditionally fail to cover the cost of anesthesia or hospitalization for children requiring dental procedures (Saxen et al., 1999; Yagiela, 1999; White et al., 2008). This has forced dentists to minimize costs for these children and their families by performing procedures in the office with or without the aid of an anesthesia provider.
However, the American Dental Association reports that there are at least 31 states that have passed laws since 1995 (Medically Necessary Care or Special Needs Legislation) requiring that medical insurance plans pay for hospital and connected medical expenses (e.g., general anesthesia services) when the dental treatment occurs in the hospital, ambulatory surgery center, or dental office (Box 35-4) (O’Connor, personal communication, January 2010).
| Arkansas (2005) | Louisiana (1997) | North Carolina (1999) |
| California (1998) | Maine (2001) | North Dakota (1999) |
| Colorado (1998) | Maryland (1998) | Oklahoma (1998) |
| Connecticut (1999) | Michigan (2001) | South Dakota (1999) |
| Florida (1998) | Minnesota (1995) | Tennessee (1997) |
| Georgia (1999) | Mississippi (1999) | Texas (1997) |
| Illinois (2002) | Missouri (1998) | Virginia (2000) |
| Indiana (1999) | Nebraska (2000) | Washington (2001) |
| Iowa (2000) | New Hampshire (1998, 2003) | West Virginia (2009) |
| Kansas (1999) | New Jersey (1999) | Wisconsin (1997) |
| Kentucky (2002) |
Courtesy the American Dental Association, Department of State Government Affairs, January 20, 2010.
Reimbursement and collections for anesthesia and surgery in offices are somewhat complex and often work differently for the surgical provider and the anesthesia provider (Koch et al., 2003). Fee-for-service reimbursement can be lucrative for the anesthesiologist in a high-volume cosmetic surgeon’s office. However, because patients undergoing cosmetic surgery are often charged a set global fee for a specific procedure, some surgeons may look toward a lower-cost anesthesia provider, so the surgeon may recover a greater amount of the preset fee. This may intensify competition among anesthesia providers for these types of procedures.
Reimbursement from third-party payers is at negotiated rates that usually vary by geographic location and payer. Despite the fact that some payers reimburse anesthesia practices for professional fees and supplies or equipment, there are those that invoke the CMS policy that a facility be licensed by the state in order to bill for the latter. Consequently, this may mean a greater cost burden to the patient (Barinholtz, personal communication, 2003). Koch and others (2003) point out that office-based anesthesia practices may become even more ubiquitous and successful by duplicating cost-recovery strategies of surgeons—namely, better collection of facility fees and site-of-service differentials that provide for greater reimbursement for procedures performed in offices.
Abu-Shahwan I. Ambulatory anesthesia and the lack of consensus among Canadian pediatric anesthesiologists: a survey. Paediatr Anaesth. 2007;17:223.
Abu-Shahwan I. Effect of propofol on emergence behavior in children after sevoflurane general anesthesia. Paediatr Anaesth. 2008;18:55.
Abu-Shahwan I., Chowdary K. Ketamine is effective in decreasing the incidence of emergence agitation in children undergoing dental repair under sevoflurane general anesthesia. Paediatr Anaesth. 2007;17:846.
Almenrader N., Passariello M., Coccetti B., et al. Premedication in children: a comparison of oral midazolam and oral clonidine. Paediatr Anaesth. 2007;17:1143.
American Academy of Pediatrics. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: an update. Pediatrics. 2006;118(6):2587.
American Academy of Pediatric Dentistry. Guideline on use of anesthesia personnel in the administration of office-based deep sedation/general anesthesia to the pediatric dental patient. 2009. Available at: http://www.aapd.org/media/Policies_Guidelines/G_AnesthesiaPersonnel.pdf Accessed October 26, 2009
American Association for Accreditation of Ambulatory Surgery Facilities (AAAASF). 2003. Available at http://www.aaaasf.org/history.php
American Society of Anesthesiologists. Standards for basic anesthetic monitoring. 2005. Available at http://www.asahq.org/publicationsAndServices/standards/02.pdf
American Society of Anesthesiologists. Ambulatory surgical care, meeting future challenges today. ASA Newsl. 72(10), 2008. October
American Society of Anesthesiologists. Guidelines for ambulatory anesthesia and surgery. 2008. Available at http://www.asahq.org/publicationsAndServices/standards/04.pdf
American Society of Anesthesiologists. Guidelines for nonoperating room anesthetizing locations. 2008. Available at http://www.asahq.org/publicationsAndServices-Standards/14.pdf
American Society of Anesthesiologists. Office-based anesthesia: considerations for anesthesiologists in setting up and maintaining a safe office anesthesia environment. ed 2. 2008.
American Society of Anesthesiologists. Continuum of depth of sedation: definition of general anesthesia and levels of sedation-analgesia. 2009. Available at http://www.asahq.org/publicationsAndServices/Standards/20.pdf
American Society of Anesthesiologists. Guidelines for office-based anesthesia. 2009. Available at http://www.asahq.org/publicationsAndServices/standards/12.pdf
American Society of Anesthesiologists. Statement on qualifications of anesthesia providers in an office-based setting. 2009. Available at http://www.asahq.org/publicationsAndServices/Standards/29.pdf
Anderson B.J., Ralph C.J., Stewart A.W., et al. The dose-effect relationship for morphine and vomiting after day-stay tonsillectomy in children. Anaesth Intensive Care. 2000;28:155.
Arens J.F. Anesthesia for office-based surgery: are we paying too high a price for access and convenience? Mayo Clin Proc. 2000;75:225.
Avery M.E. Editorial views: a pediatrician’s perspective. Anesthesiology. 1975;43:142.
Avidan M.S., Zhang L., Burnside B.A., et al. Anesthesia awareness and the bispectral index. NEJM. 2008;358:1097.
Awad I.T., Moore M., Rusche C., et al. Unplanned hospital admission in children undergoing day-case surgery. Eur J Anaesthesiol. 2004;21:379.
Bannister C.F., Brosius K.K., Sigl J.C., et al. The effect of bispectral index monitoring on anesthetic use and recovery in children anesthetized with sevoflurane in nitrous oxide. Anesth Analg. 2001;92:877.
Baldauf S. Preparing kids and yourself for their hospital visit: 11 tips. Before a test or procedure is needed, getting kids comfortable with medicine makes all the difference. U.S. News and World Report. 2009. July 22 Available at: http://health.usnews.com/articles/health/best-hospitals/2009/07/22/preparing-kidsand-yourselffor-their-hospital-visit-11-tips.html?PageNr=3 Accessed October 25, 2009
Beltrán- Aguilar E.D., Barker L.K., Canto M.T., et al. Surveillance for dental caries, dental sealants, tooth retention, edentulism, and enamel fluorosis: United States, 1988–1994 and 1999–2002. MMWR Surveill Summ. 2005;54(3):1.
Bennie R.E., Boehringer L.A., McMahon S., et al. Postoperative analgesia with preoperative oral ibuprofen or acetaminophen in children undergoing myringotomy. Paediatr Anaesth. 1997;7:399.
Bhananker S.M., Ramamoorthy C., Geiduschek J.M., et al. Anesthesia-related cardiac arrest in children: update from the pediatric perioperative cardiac arrest registry. Anesth Analg. 2007;105:344.
Bharti N., Batra Y.K., Kaur H. Paediatric perioperative cardiac arrest and its mortality: database of a 60- month period from a tertiary paediatric centre. Eur J Anaesthesiol. 2009;26:490.
Bhatt M., Kennedy R.M., Osmond M.H., et al. Consensus-based rcommendations for standardizing terminology and reprting adverse events for emergency department procedural sedation and analgesia in children. Ann Emerg Med. 2009;53:426.
Biboulet P., Aubas P., Dubourdieu J., et al. Fatal and non fatal cardiac arrests related to anesthesia. Can J Anaesth. 2001;48:326.
Bitar G., Mullis W., Jacobs W., et al. Safety and efficacy of office based surgery with monitored anesthesia care-sedation in 4778 consecutive plastic surgery procedures. Plast Reconstr Surg. 2003;111:150.
Blacoe D.A., Cunning E., Bell G. Paediatric day-case surgery: an audit of unplanned hospital admission Royal Hospital for Sick Children, Glasgow. Anaesthesia. 2008;63:610.
Blayney M.R., Malins A.F., Cooper G.M. Cardiac arrhythmias in children during outpatient general anaesthesia for dentistry: a prospective randomised trial. Lancet. 1999;354:1864.
Bodin S.G., Edwards A.F., Roy R.C. False confidences in preoperative pregnancy testing. Anesth Analg. 2010;110:256.
Bolton P., Bridge H.S., Montgomery C.J., et al. The analgesic efficacy of preoperative high dose (40 mg x kg(-1)) oral acetaminophen after bilateral myringotomy and tube insertion in children. Paediatr Anaesth. 2002;12:29.
Brandom B.W., Herlich A. Safety of outpatient dental anaesthesia for children (commentary). Lancet. 1999;354:1836.
Braz L.G., Modolo N.S., do Nascimento P.Jr, et al. Perioperative cardiac arrest: a study of 53,718 anaesthetics over 9yr from a Brazilian teaching hospital. Br J Anaesth. 2006;96:569.
Bridenbaugh P.O. Office-based anesthesia: requirements for patient safety. Anesth Prog. 2005;52:86.
Byrd H.S., Barton F.E., Orenstein H.H., et al. Safety and efficacy in an accredited outpatient plastic surgery facility: a review of 5316 consecutive cases. Plast Reconstr Surg. 2003;112:636.
Caputo A.C. Providing deep sedation and general anesthesia for patients with special care needs in the dental office-based setting. Spec Care Dentist. 2009;29:26.
Carter K.L. Donda West, mom of Chicago rapper Kanye West, former Chicago State professor, department head was inspiration, son’s biggest fan. Chicago Tribune Archive News. 2007. November 12 Available at: http://archives.chicagotribune.com/2007/nov/12/news/chi-hed_west_12_both_nov12 Accessed October 25, 2009
Cartwright D. Death in the dental chair. Anaesthesia. 1999;54:105.
Cartwright P.C., Snow B.W., McNees D.C. Urethral meatotomy in the office using topical EMLA cream for anesthesia. J Urol. 1996;156:857.
Casamassimo P.S., Thikkurissey S., Edelstein B.L., et al. Beyond the dmft: the human and economic cost of early childhood caries. J Am Dent Assoc. 2009;140:650.
Chorney J.M., Kain Z.N. Behavioral analysis of children’s response to induction of anesthesia. Anesth Analg. 2009;109:1434.
Chundamala J., Wright J.G., Kemp S.M. An evidence-based review of parental presence during anesthesia induction and parent/child anxiety. Can J Anaesth. 2009;56:57.
Cohen I.T., Finkel J.C., Hannallah R.S., et al. The effect of fentanyl on the emergence characteristics after desflurane or sevoflurane anesthesia and children. Anesth Analg. 2002;94:1178.
Cohen I.T., Hannallah R.S., Goodale D.B. The clinical and biochemical effects of propofol infusion with and without EDTA for maintenance anesthesia in healthy children undergoing ambulatory surgery. Anesth Analg. 2001;93:106.
Coldiron B. Office surgical incidents: 19 Months of Florida data. Dermatol Surg. 2002;28:710.
Coldiron B. Patient injuries from surgical procedures performed in medical offices. JAMA. 2001;285:2582.
Coldiron B.M., Heely C., Bene N.I. Office surgery incidents: what seven years of Florida data show us. Dermatol Surg. 2008;34:285.
Coté C.J., Notterman D.A., Karl H.W., et al. Adverse sedation events in pediatrics: a critical incident analysis of contributing factors. Pediatrics. 2000;105:805.
Coté C.J., Karl H.W., Notterman D.A., et al. Adverse sedation events in pediatrics: analysis of medications used for sedation. Pediatrics. 2000;106:633.
Cox R.G., Nemish U., Ewen A., et al. Evidence-based clinical update: does premedication with oral midazolam lead to improved behavioural outcomes in children. Can J Anaesth. 2006;53:1213.
Cravero J.P., Blike G.T., Beach M., et al. Incidence and nature of adverse events during pediatric sedation/anesthesia for procedures outside the operating room: report from the pediatric research consortium. Pediatrics. 2006;118:1087.
Cravero J.P., Beach M.L., Blike G.T., et al. The incidence and nature of adverse events during pediatric sedation/anesthesia with propofol for procedures outside the operating room: a report from the pediatric sedation research consortium. Anesth Analg. 2009;108:695.
Cullen K.A., Hall M.J., Golosinskiy A. Ambulatory surgery in the United States, 2006. National Health Statistics Reports. (11):2009. September
Czarnetzki C., Elia N., Lysakowski C., et al. Dexamethasone and risk of nausea and vomiting and postoperative bleeding after tonsillectomy in children: a randomized trial. JAMA. 2008;300:2621.
Davis P.J., Finkel J.C., Orr R.J., et al. A randomized, double blinded study of remifentanil vs. fentanyl for tonsillectomy and adenoidectomy surgery in pediatric ambulatory surgical patients. Anesth Analg. 2000;90:863.
Davis P.J., Lerman J., Suresh S., et al. A randomized multicenter study of remifentanil compared with alfentanil, isoflurane, or propofol in anesthetized pediatric patients undergoing elective strabismus surgery. Anesth Analg. 1997;84:982.
Davis P.J., Tome J.A., McGowan F.X., et al. Preanesthetic medication with intranasal midazolam for brief pediatric surgical procedures: effect on recovery and hospital discharge times. Anesthesiology. 1995;82:2.
Davis P.J., Fertal K.M., Boretsky K.R., et al. The effects of oral ondansetron disintegrating tablets for prevention of at-home emesis in pediatric patients after ear- nose- throat surgery. Anesth Analg. 2008;106:1117.
de Jong R.H. Mega-dose lidocaine dangers seen in “tumescent” liposuction. Anesth Patient Safety Found Newsletter. 1999;14:25.
Denman J.H., Hingson R.A., Tomaro A.J. Cardiac resuscitation in the dental office: report of case. J Oral Surg. 1968;26:470.
Denman W.T., Swanson E.L., Rosow D., et al. Pediatric evaluation of the bispectral index (BIS) monitor and correlation of BIS with end tidal sevoflurane concentration in infants and children. Anesth Analg. 2000;90:872.
D’Eramo E.M. Mortality and morbidity with outpatient anesthesia: the Massachusetts experience. J Maxillofac Surg. 1999;57:531.
D’Errico C., Voepel-Lewis T.D., Siewert M., et al. Prolonged recovery stay and unplanned admission of the pediatric surgical outpatient: an observational study. J Clin Anesth. 1998;10:482.
Domino K.B. Office-based anesthesia: lessons learned from the Closed Claims Project. Am Soc Anesthesiol Newsletter. 2001;65:9.
Dornhoffer J., Manning L. Unplanned hospital admissions following outpatient otologic surgery: the University of Arkansas experience. Ear Nose Throat J. 2000;79:712.
Drendal A.L., Gorelick M.H., Weisman S.J., et al. A randomized clinical trial of ibuprofen vs. acetaminophen with codeine for acute pediatric arm fracture pain. Ann Emerg Med. 2009;54:553.
Duedahl T.H., Hansen E.H. A qualitative systematic review of morphine treatment in children with postoperative pain. Paeditatr Anaesth. 2007;17:756.
Eberhart L.H., Mauch M., Morin A.M., et al. Impact of a multimodal antiemetic prophylaxis on patient satisfaction in high-risk patients for postoperative nausea and vomiting. Anaesthesia. 2002;57:1022.
Eberhardt L.H.J., Geldner G., Kranke P., et al. The development and validation of a risk score to predict probability of postoperative vomiting in pediatric patients. Anesth Analg. 2004;99:1630.
Engelhardt T., Petroz G.C., McCheyne A., et al. Awareness during pediatric anesthesia: what is the position of European pediatric anesthesiologists? Paediatr Anaesth. 2007;17:1066.
Engelman E., Salengros J.C., Barvais L. How much does pharmacologic prophylaxis reduce postoperative vomiting in children? Calculation of prophylaxis effectiveness and expected incidence of vomiting under treatment using Bayesian meta-analysis. Anesthesiology. 2008;109:1023.
Federation of State Medical Boards. Report of the Special Committee on Outpatient (Office-Based) Surgery: executive summary. 2002. Available at http://www.fsmb.org/Policy%20Documents%20and%20White%20Papers/outpatient_surgery_cmt_rpt.htm
Ferrari L.R. Preoperative evaluation of pediatric surgical patients with multisystem considerations. Anesth Analg. 2004;99:1058.
Finkel J.C., Cohen I.T., Hannallah R.S., et al. The effect of intranasal fentanyl on the emergence characteristics after sevoflurane anesthesia in children undergoing surgery for bilateral myringotomy tube placement. Anesth Analg. 2001;92:1164.
Fishkin S., Litman R.S. Current issues in pediatric ambulatory anesthesia. Anesthesiol Clin North Am. 2003;21:305.
Flick W.G., Katsnelson A., Alstrom H. Illinois dental anesthesia and sedation survey for 2006. Anesth Prog. 2007;54:52.
Fortier J., Chung F., Su J. Unanticipated admission after ambulatory surgery: a prospective study. Can J Anaesth. 1998;45:612.
Fortier M.A., MacLaren J.E., Martin S.R., et al. Pediatric pain after ambulatory surgery: where’s the medication? Pediatrics. 2009;124:e588.
Friedberg B.L. Anesthesia for cosmetic facial surgery. Int Anesthesiol Clin. 2003;41:13.
Friedberg B.L., Sigl J.C. Clonidine premedication decreases propofol consumption during bispectral index (BIS)-monitored propofol ketamine technique for office based surgery. Dermatol Surg. 2000;26:848.
Friedman O., Deutsch E.S., Reilly J.S., et al. The feasibility of office based laser assisted tympanic membrane fenestration with tympanostomy tube insertion: the duPont Hospital experience. Int J Pediatr Otorhinolaryngol. 2002;62:31.
Galinkin J.L., Fazi L.M., Cuy R.M., et al. Use of intranasal fentanyl in children undergoing myringotomy and tube placement during halothane and sevoflurane anesthesia. Anesthesiology. 2000;93:1378.
Galinkin J.L., Kurth D. Neonatal and pediatric apnea syndromes. Probl Anesth. 1998;10:444.
Gan T.J., Meyer T.A., Apfel C.C., et al. Society for Ambulatory Anesthesia guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2007;105:1615.
Garin P., Ledeghen S., Van Prooyen-Keyser S., et al. Office-based CO2 laser assisted tympanic membrane fenestration addressing otitis media with effusion. J Clin Laser Med Surg. 2001;19:185.
Ghosh S., Sallam S. Patient satisfaction and postoperative demands on hospital and community services after day surgery. Br J Surg. 1994;81:1635.
Girdler N.M., Smith D.G. Prevalence of emergency events in British dental practice and emergency management skills of British dentists. Resuscitation. 1999;41:159.
Glass P.S., Bloom M., Kearse L., et al. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfantanil in healthy volunteers. Anesthesiology. 1997;86:836-847.
Goldblum T.A., Summers C.G., Egbert J.E., et al. Office probing for congenital nasolacrimal duct obstruction: a study of parental satisfaction. J Pediatr Ophthalmol Strabismus. 1996;33:244.
Gooden C.K. Office-based anesthesia for the pediatric patient. Seminars in anesthesia, perioperative medicine and pain. 2006;25:19.
Goodarzi M., Matar M.M., Shafa M., et al. A prospective randomized blinded study of the effect of intravenous fluid therapy on postoperative nausea and vomiting in children undergoing strabismus surgery. Paediatr Anaesth. 2009;16:49.
Gravningsbraten R., Nicklasson B., Raeder J. Safety of laryngeal mask airway and short-stay practice in office-based adenotonsillectomy. ACTA Anaesthesiol Scand. 2009;53(2):218.
Grevelink J.M., White V.R., Bonoan R., et al. Pulsed laser treatment in children and the use of anesthesia. J Am Acad Dermatol. 1997;37:75.
Griffith N., Howell S., Mason D.G. Intranasal midazolam for premedication of children undergoing day-case anaesthesia: comparison of two delivery systems with assessment of intraobserver variability. Br J Anaesth. 1998;81:865.
Hall M.J., Lawrence L. Ambulatory surgery in the United States, 1996 advance data. The National Center for Health Statistics. 1998;300:1-16. Available at http://www.cdc.gov/nchs/data/ad/ad300.pdf Accessed March 20, 2005
Hannallah R.S. Selection of patients for paediatric ambulatory surgery. Can J Anaesth. 1991;38:887.
Hannallah R.S., Britton J.T., Schafer P.G., et al. Propofol anesthesia in pediatric ambulatory surgery patients: a comparison with thiopentone and halothane. Can J Anaesth. 1994;41:12.
Hausman L.M. Office-based anesthesia and education. ASA Newsl. 2008;72(10):16.
Hausman L.M., Eisenkraft J.B., Rosenblatt M.A. The safety and efficacy of regional anesthesia in an office-based setting. J Clin Anesth. 2008;20:271.
Hausman L.M., Frost E.A.M. Office-based surgery: expanding role of the anesthesiologist. Middle East J Anesthesiol. 2007;19(2):291.
Hausman L.M., Levine A.I., Rosenblatt M.A. A survey evaluating the training of anesthesiology residents in office-based anesthesia. J Clin Anesth. 2006;18(7):499.
Heard C., Creighton P., Lerman J. Intranasal flumazenil and naloxone to reverse over- sedation in a child undergoing dental restorations. Paediatr Anaesth. 2009;19:795.
Hennrikus W.L., Shaw B.A., Gerardi J.A. Prevalence of positive preoperative pregnancy testing in teenagers scheduled for orthopedic surgery. J Pediatr Orthop. 2001;21:677.
Henzi I., Walder B., Tramèr M.R. Dexamethasone for the prevention of postoperative nausea and vomiting: a quantitative systematic review. Anesth Analg. 2000;90:186.
Hiller A., Meretoja O.A., Korpela R., et al. The analgesia efficacy of acetaminophen, ketoprofen, or their combination for pediatric surgical patients having soft tissue or orthopedic procedures. Anesth Analg. 2006;102:1365.
Hilton L. Office surgery moratorium has doctors debating issue. 2001. January 8, Available at http://bizjournals.com/jacksonville/stories/2001/01/08/story7.html
Hoefflin S.M., Bornstein J.B., Gordon M. General anesthesia in an office-based plastic surgical facility: a report on more than 23,000 consecutive office-based procedures under general anesthesia with no significant anesthetic complications. Plast Reconstr Surg. 2001;107:243.
Holte K., Kristensen B.B., Valentiner L., et al. Liberal vs. restrictive fluid management in knee arthroplasty: a randomized, double-blind study. Anesth Analg. 2007;105:465.
Howard R., Carter B., Curry J., et al. Good practice in postoperative and procedural pain management: postoperative pain. Paediatr Anaesth. 2008;18(s1):36.
Howard R., Carter B., Curry J., et al. Good practice in postoperative and procedural pain management. Analgesia review. Paediatr Anaesth. 2008;18(s1):64.
Hussain R. Anesthesia killed Diamond: examiner. Chicago Sun- Times, 2006. September 29 Available at: http://www.suntimes.com/news/metro/77200,CST-NWS-dentist29.article Accessed: November 4, 2009
Ibacache M.E., Munoz H.R., Brandes V., et al. Single-dose dexmedetomidine reduces agitation after sevoflurane anesthesia in children. Anesth Analg. 2004;98:60.
Jacobsohn P.H. Horace Wells, discoverer of anesthesia. Anesth Prog. 1995;42:73.
Jimenez N., Posner K.L., Cheney F.W., et al. An update on pediatric anesthesia liability: a closed claims analysis. Anesth Analg. 2007;104:147.
Johnson E.L., Roberts M.W., Prasad R. Complication associated with general anesthesia: report of case. ASDC J Dent Child. 2001;68:332.
Joshi G.P. The Society for Ambulatory Anesthesia: 17th Annual meeting report. Anesth Analg. 2003;96:903.
Joshi W., Connelly N.R., Reuben S.S., et al. An evaluation of the safety and efficacy of administering rofecoxib for postoperative pain management. Anesth Analg. 2003;97:35.
Kahn R.L., Stanton M.A., Tong- Ngork S., et al. One- year experience with day-of-surgery pregnancy testing before elective orthopedic procedures. Anesth Analg. 2008;106:1127.
Kain Z.N., Caldwell-Andrews A., Wang S.M. Psychological preparation of the parent and pediatric surgical patient. Anesthesiol Clin North Am. 2002;20:29.
Kain Z.N., Caldwell-Andrews A.A., Maranets I., et al. Predicting which child-parent pair will benefit from parental presence during induction of anesthesia: a decision- making approach. Anesth Analg. 2006;102:81.
Kain Z.N., Mayes L.C., Caldwell-Andrews A.A., et al. Preoperative anxiety, postoperative pain, and behavioral recovery in young children undergoing surgery. Pediatrics. 2006;118:651.
Kain Z.N., Caldwell-Andrews A.A., Mayes L.C., et al. Parental presence during induction of anesthesia: physiological effects on parents. Anesthesiology. 2003;98:58.
Kain Z.N., MacLaren J.E., Herrmann L., et al. Preoperative melatonin and its effects on induction and emergence in children undergoing anesthesia and surgery. Anesthesiology. 2009;111:44.
Kain Z.N., Mayes L., Wang S., et al. Parental presence and a sedative premedicant for children undergoing surgery: a hierarchical study. Anesthesiology. 2000;92:939.
Kain Z.N., Mayes L.C., O’Connor T.Z., et al. Preoperative anxiety in children: predictors and outcomes. Arch Pediatr Adolesc Med. 1996;150:1238.
Kakavouli A., Li G., Carson M., et al. Intraoperative reported adverse events in children. Pediatr Anesth. 2009;19:732.
Kaushal R., Upadhyayula S., Gaba D., et al. The wild west: patient safety in office-based anesthesia. Perspective. May 2006. Agency for Healthcare Research and Quality. Morbidity and Mortality Rounds on the Web Available at: http://www.webmm.ahrq.gov/printviewperspective.aspx?perspectiveID=25&searchStr=office+base+anesthesia Accessed October 25, 2009
Kerger K.H., Mascha E., Steinbrecher B., et al. Routine use of nasogastric tubes does not reduce postoperative nausea and vomiting. Anesth Analg. 2009;109:768.
Kim J., Azavedo L., Bhananker S., et al. Amethociane or ketorolac eyedrops provide inadequate analgesia in pediatric strabismus surgery. Can J Anaesth. 2003;50:819.
Kim J.Y., Chang Y.J., Lee J.Y., et al. Post-induction alfentanil reduces sevoflurane- associated emergence agitation in children undergoing an adenotonsillectomy. Acta Anaesthesiol Scand. 2009;53:678.
Kim M.S., Cote C.J., Cristoloveanu C., et al. There is no dose-escalation response to dexamethasone (0.0625– 1.0 mg/ kg) in pediatric tonsillectomy or adenotonsillectomy patients for preventing vomiting, reducing pain, shortening time to first liquid intake, or the incidence of voice change. Anesth Analg. 2007;104:1052.
Koch M.E., Dayan S., Barinholtz D. Office-based anesthesia: an overview. Anesthesiol Clin North Am. 2003;21:417.
Kokki H., Hendolin H., Maunuksela E.L., et al. Ibuprofen in the treatment of postoperative pain in small children: a randomized double-blind-placebo controlled parallel group study. Acta Anaesthesiol Scand. 1994;38:467.
Kolodzie K., Apfel C.C. Nausea and vomiting after office-based anesthesia. Curr Opin Anaesthesiol. 2009;22:532.
König M.W., Varughese A.M., Brennan K.A., et al. Quality of recovery from two types of general anesthesia for ambulatory dental surgery in children: a double-blind, randomized trial. Paediatr Anaesth. 2009;19:748.
Korpela R., Korvenoja P., Meretoja O.A. Morphine-sparing effect of acetaminophen in pediatric day-case surgery. Anesthesiology. 1999;91:442.
Kovac A.L. Management of postoperative nausea and vomiting in children. Paediatr Drugs. 2007;9:47.
Kranke P., Eberhardt L.H., Toker H., et al. A prospective evaluation of the POVOC score for the prediction of postoperative vomiting in children. Anesth Analg. 2007;105:1592.
Kuratani N., Oi Y. Greater incidence of emergence agitation in children after sevoflurane anesthesia as compared with halothane: a meta-analysis of randomized control trials. Anesthesiology. 2008;109:225.
Lagasse R. Anesthesia safety: model or myth? A review of the published literature and analysis of current original data. Anesthesiology. 2002;97:1609.
Lagasse R.S. Innocent prattle. Anesthesiology. 2009;110:698.
Lalwani K., Kitchin J., Lax P. Office-based dental rehabilitation in children with special healthcare needs using a pediatric sedation model. J Oral Maxillofac Surg. 2007;65(3):427.
LaMendola B. No surgery ever routine, student’s death from cosmetic surgery examined. Fort Lauderdale Sun Sentinel. 1998. Available at http://pqasb.pqarchiver.com/sun_sentinel/ Accessed July 7, 2003
Lankinen U., Avela R., Tarkkila P. The prevention of emergence agitation with tropisetron or clonidine after sevoflurane anesthesia in small children undergoing adenoidectomy. Anesth Analg. 2006;102:1383.
Laskin D.M. The risks of itinerant anesthesia services (editorial). J Oral Maxillofac Surg. 1999;57:363.
Laurito C.E. Report of educational meeting: the Society for Office-Based Anesthesia, Orlando, Florida, March 7, 1998. J Clin Anesth. 1998;10:445.
Liechti M., Feurer R., Gross D., et al. Prevention of postoperative nausea and vomiting in children following adenotonsillectomy, using tropisetron with or without low- dose dexamethasone. J Anesth. 2007;21:311.
Letts M., Davidson D., Splinter W., et al. Analysis of the efficacy of pediatric day surgery. Can J Surg. 2001;44:193.
Levine M., Spahr-Schopfer I., Hartley E., et al. Oral midazolam premedication in children: the minimum time interval for separation from parents. Can J Anaesth. 1993;40:726.
Li G., Warner M., Lang B.H., et al. Epidemiology of anesthesia-related mortality in the United States. Anesthesiology. 2009;110:759.
Lightdale J.R., Goldmann D.A., Feldman H.A., et al. Microstream capnography improves patient monitoring during moderate sedation: a randomized, controlled trial. Pediatrics. 2006;117:e1170.
Lin Y.C., Tassone R.F., Jahng S., et al. Acupuncture management of pain and emergence agitation in children after bilateral myringotomy and tympanostomy tube insertion. Paediatr Anaesth. 2009;19:1096.
Litman R.S. Conscious sedation with remifentanil and midazolam during brief painful procedures in children. Arch Pediatr Adolesc Med. 1999;153:1085.
Lynn A.M., Bradford H., Kantor E.D., et al. Postoperative ketorolac tromethamine use in infants aged 6–18 months: the effect on morphine usage, safety assessment, and stereo- specific pharmacokinetics. Anesth Analg. 2007;104:1040.
Mangia G., Presutti P., Antonucci A., et al. Diagnostic accuracy of anesthesiology evaluation timing: the ‘One-Stop Anesthesia’ in pediatric day-surgery. Paediatr Anaesth. 2009;19:764.
Manoharan G., Moohan V., Adgey J. Ventricular fibrillation in a 12-year-old boy recovering from dental anaesthesia. Int J Cardiol. 2001;81:271.
Marhofer P., Glaser C., Krenn C.G., et al. Incidence of therapy of midazolam induced hiccups in paediatric anaesthesia. Paediatr Anaesth. 1999;9:295.
Mattila K., Hynynen M., et al. Day surgery in Finland: a prospective cohort study of 14 day-surgery units. Acta Anaesthesiol Scand. 2009;53:455.
Maunuksela E.L., Kokki H., Bullingham R.E. Comparison of intravenous ketorolac with morphine for postoperative pain in children. Clin Pharmacol Ther. 1992;52:436.
Maxwell L.G. Age-associated issues in preoperative evaluation, testing, and planning: pediatrics. Anesthsiol Clin North America. 2004;22:27.
McCann M.E., Brustowicz R.M., Bacsik J., et al. The bispectral index and explicit recall during the intraoperative wake-up test for scoliosis surgery. Anesth Analg. 2002;94:1474.
McCann M.E., Kain Z.N. The management of preoperative anxiety in children: an update. Anesth Analg. 2001;93:98.
McGraw T., Kendrick A. Oral midazolam premedication and postoperative behavior in children. Paediatr Anaesth. 1998;8:117.
McGreevy P. Governor’s surprise bill signings: Harvey Milk recognition, paparazzi restrictions and ammo tracking. Los Angeles Times. October 12, 2009. Available at: http://latimesblogs.latimes.com/lanow/2009/10/govs-surprise-bill-signings-harvey-milk-recognition-curbs-on-paparazzi-and-ammo.html
Meneghini L., Zadra N., Zanette G., et al. The usefulness of routine preoperative laboratory tests for one-day surgery in healthy children. Paediatr Anaesth. 1998;8:11.
Messeri A., Caprilli S., Busoni P. Anaesthesia induction in children: a psychological evaluation of the efficiency of parents’ presence. Paediatr Anaesth. 2004;14:551.
Messieha Z.S., Ananda R.C., Hoffman W.E., et al. Bispectral index system (BIS) monitoring reduces time to extubation and discharge in children requiring oral presedation and general anesthesia for outpatient dental rehabilitation. Pediatr Dent. 2005;27:500.
Mingus M.L., Bodian C.A., Bradford C.N., et al. Prolonged surgery increases the likelihood of admission of scheduled ambulatory surgery patients. J Clin Anesth. 1997;9:446.
Moore E.W., Pollard B.J., Elliott R.E. Anaesthetic agents in pediatric day case surgery: do they affect outcome? Eur J Anaesthsiol. 2002;19:9.
Morell R.C. OBA questions, problems just now recognized, being defined. Anesth Patient Safety Newsletter. 2000;15:1.
Morello D.C., Colon G.A., Fredericks S., et al. Patient safety in accredited office surgical facilities. Plast Reconstr Surg. 1997;99:1496.
Morray J.P. Anesthesia-related cardiac arrest in children: an update. Anesthesiol Clin North Am. 2002;20:1.
Morray J.P., Geiduschek J.M., Ramamoorthy M.B., et al. Anesthesia-related cardiac arrest in children: initial findings of the Pediatric Perioperative Cardiac Arrest (POCA) registry. Anesthesiology. 2000;93:6.
Moss E. Revelations: New Jersey office regulations adopted. Am Soc Anesthesiol Newsletter. 1998;62:17.
Murat I., Constant I., Maud’huy H. Perioperative anesthetic morbidity in children: a database of 24,165 anaesthetics over a 30-month period. Paediatr Anaesth. 2004;14:158.
Needleman H.L., Harpavat S., Wu S., et al. Postoperative pain and other sequelae of dental rehabilitations performed on children under general anesthesia. Pediatr Dent. 2008;30:111.
Neergaard L. Sedation safety alert: anesthesia in doctor’s office riskier. Chicago Sun-Times, 1999. Friday, March 19
Negri P.D., Ivani G. Management of postoperative nausea and vomiting in children. Paediatr Drugs. 2002;4:717.
Newland M.C., Ellis S.J., Lydiatt C.A., et al. Anesthesia-related cardiac arrest and its mortality: a report covering 72,959 anesthetics over 10 years from a U.S. teaching hospital. Anesthesiology. 2002;97:108.
Nunn M.E., Dietrich T., Singh H.K., et al. Prevalenve of early childhood caries among very young urban Boston children compared with U.S. children. J Public Health Dent. 2009;69(3):156.
Olsson G.L. Inhalational anaesthesia at the extremes of age: paediatric anaesthesia. Anaesthesia. 1995;50(Suppl):34.
Overdyk F.J., Napoleon B., Tagge E.P., et al. “One-stop” surgery: implications for anesthesiologists of an expedited pediatric surgical process. South Med J. 1999;92:308.
Patel R.I., Hannallah R.S. Anesthetic complications following pediatric ambulatory surgery: A 3-yr study. Anesthesiology. 1988;69:1009.
Patel R.I., Verghese S.T., Hannallah R.S., et al. Fast-tracking children after ambulatory surgery. Anesth Analg. 2001;92:918.
Perrott D.H. Anesthesia outside the operating room in the office-based setting. Curr Opin Anaesthesiol. 2008;21:480.
Perrott D.H., Yuen J.P., Andersen R.V., et al. Office-based ambulatory anesthesia: outcomes of clinical practice of oral and maxillofacial surgeons. J Oral Maxillofac Surg. 2003;61:983.
Pinsker M.C., Caroll N.V. Quality of emergence from anesthesia and incidence of vomiting with remifentanil in a pediatric population. Anesth Analg. 1999;89:71.
Polaner D. Anesthesia for pediatric same-day procedures. In: Motoyama E.K., Davis P.J., editors. Smith’s anesthesia for infants and children. Philadelphia: Mosby-Elsevier; 2006:874-894.
Postuma R., Ferguson C.C., Stanwick R.S., et al. Pediatric day-care surgery: a 30 year hospital experience. J Pediatr Surg. 1987;22:304.
Powers K.S., Nazarian E.B., Tapyrik S.A., et al. Bispectral index as a guide for titration of propofol during procedural sedation among children. Pediatrics. 2005;115:1666.
Purday J.P., Reichert C.C., Merrick P.M. Comparative effects of three doses of intravenous ketorolac or morphine on emesis and analgesia for restorative dental surgery in children. Can J Anaesth. 1996;43:221.
Radke O.C., Biedler A., Kolodzie K., et al. The effect of postoperative fasting on vomiting in children and their assessment of pain. Paediatr Anaesth. 2009;19:494.
Rao R.B., Ely S.F., Hoffman R.S. Deaths related to liposuction. N Engl J Med. 1999;340:1471.
Religa Z.C., Wilson S., Ganzberg S.I., et al. Association between bispectral analysis and level of conscious sedation of pediatric dental patients. Pediatr Dent. 2002;24:221.
Riad W., Moussa A. Pre-operative analgesia with rectal diclofenac and/or paracetamol in children undergoing inguinal hernia repair. Anaesthesia. 2007;62:1241.
Rosenbaum A., Kain Z.N., Larsson P., et al. The place of premedication in pediatric practice. Paediatr Anaesth. 2009;19:817.
Ross A.K., Eck J.B. Office-based anesthesia for children. Anesthesiol Clin North Am. 2002;20:195.
Rueffert H., Thieme V., Wallenborn J., et al. Do variations in the 5-HT3A and 5-HT3B serotonin receptor genes (HTR3A and HTR3B) influence the occurrence of postoperative vomiting. Anesth Analg. 2009;109:1442.
Saxen M.A., Wilson S., Paravecchio R. Anesthesia for pediatric dentistry. Dent Clin North Am. 1999;43:231.
Schulte F., Bergal J. For these patients, the ultimate price. Cosmetic surgery: the hidden dangers. 1998. Available at http://pqasb.pqarchiver.com/sun_sentinel/ Accessed July 7, 2003
Schultz L.S. Cost analysis of office surgery clinic with comparison to hospital outpatient facilities for laparoscopic procedures. Int Surg. 1994;79:273.
Scuderi P.E., James R.L., Harris L., et al. Multimodal antiemetic management prevents early postoperative vomiting after outpatient laparoscopy. Anesth Analg. 2000;91:1408.
Segerdahl M., Warren-Stomberg M., Rawal N., et al. Children in day surgery: clinical practice and routines. The results from a nation-wide surgery. Acta Anesthesiol Scand. 2008;52:821.
Shah R.K., Welborn L., Ashktorab S., et al. Safety and outcomes of pediatric otolaryngology procedures at an ambulatory surgery center. Laryngoscope. 2008;118:1937.
Shapiro F.E. Manual of office-based anesthesia procedures. Philadelphia: Lippincott Williams & Wilkins, 2007.
Sherry J. Child deaths from anesthesia. Dental Office Magazine. February, 2009. Available at http://www.dentalofficemag.com/articles/print.html?id=354853&bPool=DE.pennnet.com%2farticle_tool_bar Accessed October 24, 2009
Siegel G., Brodsky L., Waner M., et al. Office-based laser assisted tympanic membrane fenestration in adults and children: pilot data to support an alternative to traditional approaches to otitis media. Int J Pediatr Otorhinolaryngol. 2000;53:111.
SMG Marketing-Verispan, L.L.C. Freestanding outpatient surgery center market report, 2002 edition. Yardley, PA: Verispan, 2002.
Smiley R.M. An overview of induction and emergence of characteristics of desflurane in pediatric, adult, and geriatric patients. Anesth Analg. 1996;75:S38.
Smith C., Smith D.P. Office pediatric urologic procedures from a parental perspective. Urology. 2000;55:272.
Song D., Joshi G.P., White P.F. Titration of volatile anesthetics using bispectral index facilitates recovery after ambulatory anesthesia. Anesthesiology. 1997;87:842-848.
Splinter W.M. Prevention of vomiting after strabismus surgery in children: dexamethasone alone vs. dexamethasone plus low-dose ondansetron. Paediatr Anaesth. 2001;11:591.
Splinter W.M., Rhine E.J. Low-dose ondansetron with dexamethasone more effectively decreases vomiting after strabismus surgery in children than does high-dose ondansetron. Anesthesiology. 1998;88:72.
Stamer U.M., Mpasios N., Maier C., et al. Postoperative analgesia in children-current practice in Germany. Eur J Pain. 2005;9:555.
Stoelting R.K. Special Issue: OBA safety: office-based anesthesia provokes safety fears. Anesth Patient Safety Found Newsletter. 2000;15:1.
Subramaniam B., Madan R., Sadhasivam S., et al. Dexamethasone is a cost-effective alternative to ondansetron in preventing PONV after paediatric strabismus repair. Br J Anaesth. 2001;86:84.
Sukhani R., Pappas A.L., Lurie J., et al. Ondansetron and dolasetron provide equivalent postoperative vomiting control after ambulatory tonsillectomy in dexamethasone-pretreated children. Anesth Analg. 2002;95:1230.
Tang J., Chen L., White P.F., et al. Recovery profiles, costs, and patient satisfaction with propofol and sevoflurane for fast-track office-based anesthesia. Anesthesiology. 1999;91:253.
Tang J., Chen X., White P.F., et al. Antiemetic prophylaxis for office-based surgery: are the 5-HT3 receptor antagonists beneficial? Anesthesiology. 2003;98:293.
Tang J., White P.F., Wender R.H., et al. Fast-track office-based anesthesia: a comparison of propofol vs. desflurane with antiemetic prophylaxis in spontaneously breathing patients. Anesth Analg. 2001;92:95.
Tobias J.D. Weak analgesics and non-steroidal anti-inflammatory agents in the management of children with acute pain. Pediatr Clin North Am. 2000;47:527.
Townsend J.A., Ganzberg S., Thikkurissy S. The effect of local anesthetic on quality of recovery characteristics following dental rehabilitation under general anesthesia. Anesth Prog. 2009;56:115.
Twersky R.S. OBA: Guidelines for a growing subspecialty. ASA Newsl. 2008;72(10):8.
Vargas C.M., Crall J.J., Schneider D.A. Sociodemographic distribution of pediatric dental caries: NHANES III, 1988–1994. J Am Dent Assoc. 1998;129(9):1229.
Valkenburg A.J., de Leeuw T.G., Tibboel D., et al. Lower bispectral index values in children who are intellectually disabled. Anesth Analg. 2009;109:1428.
Verghese S.T., Hannallah R.S., Patel R., et al. Ketamine and midazolam is an inappropriate preinduction combination in uncooperative children undergoing brief ambulatory procedures. Paediatr Anaesth. 2003;13:228.
Viitanen H., Annila P., Viitanen M., et al. Midazolam premedication delays recovery from propofol-induced sevoflurane anesthesia in children 1–3 yr. Can J Anaesth. 1999;46:766.
Vila H., Soto R., Cantor A.B., et al. Comparative outcomes analysis of procedures performed in physicians offices and ambulatory surgery centers. Arch Surg. 2003;138:991.
Vlajkovic G.P., Sindjelic R.P. Emergence delirium in children: many questions, few answers. Anesth Analg. 2007;104:84.
Vogt A. $4.5 Million dollars in boy’s death. 2000. Available at http://pqasb.pqarchiver.com/chicagotribune/
Voronov P., Tobin M.J., Billings K., et al. Postoperative pain relief in infants undergoing myringotomy and tube placement; comparison of a novel regional anesthetic block to intranasal fentanyl: a pilot study. Paediatr Anaesth. 2008;18:1196.
Wagner D.S., Gauger V., Chiravuri D., et al. Ondansetron oral disintegrating tablets for the prevention of postoperative vomiting in children undergoing strabismus surgery. Ther clin Risk Manag. 2007;3:691.
Watson A.T., Visram A. Children’s preoperative anxiety and postoperative behaviour. Pediatr Anaesth. 2003;13:188.
Weinstein M.S., Nicolson S.C., Schreiner M.S. A single dose of morphine sulfate increases the incidence of vomiting after outpatient inguinal herniorrhaphy. Anesthesiology. 1994;81:572.
Welborn L.G., Greenspun J.C. Anesthesia and apnea: perioperative considerations in the former premature infant. Pediatr Clin North Am. 1994;41:188.
Weldon B.C., Bell M., Craddock T. The effect of caudal anesthesia on emergence agitation in children after sevoflurane vs. halothane anesthesia. Anesth Analg. 2004;98:321.
Westman H.R. Postoperative complications and unanticipated hospital admissions. Semin Pediatr Surg. 1999;8:23.
Wheeler M., Coté C.J. Preoperative pregnancy testing in a tertiary care children’s hospital: a medico-legal conundrum. J Clin Anesth. 1999;11:56.
White H.R., Lee J.Y., Rozier R.G. The effects of general anesthesia legislation on operating room visits by preschool children undergoing dental treatment. Pediatr Dent. 2008;30:70.
White P.F. Ambulatory anesthesia and surgery: past, present and future. In: White P.F., editor. Ambulatory anesthesia and surgery. London: WB Saunders; 1997:3-34.
Whitmire H.C.Jr. Medicolegal considerations for office-based anesthesia in dentistry. Dent Clin North Am. 1999;43:361.
Willetts I.E., James H., Nicoll, et al. Pioneer pediatric surgeon. Ann R Coll Surg Engl. 1997;79(Suppl):164.
Wu C.L., Berenholtz S.M., Pronovost P.J., et al. Systematic review and analysis of postdischarge symptoms after outpatient surgery. Anesthesiology. 2002;96:994.
Yagiela J.A. Adverse sedation events in pediatrics (letter). Pediatrics. 2001;107:1494.
Yagiela J.A. Office-based anesthesia in dentistry. Dent Clin North Am. 1999;43:201.
Yee K.F., Holland R.B., Carrick A., et al. Morbidity following day-stay dental anaesthesia. Aust Dent J. 1985;30:333.
Yip P., Middleton P., Cyna A.M., et al. Non- pharmacological interventions for assisting the induction of anaesthesia in children. Cochrane Database System Rev. 8, 2009. CD006447, Available at: http://www.mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD006447/frame.html Accessed December 24, 2009
Zuercher M., Ummenhofer W. Cardiac arrest during anesthesia. Curr Opin Crit Care. 2008;14:269.
Zwass M.S., Fisher D.M., Welborn L.G., et al. Induction and maintenance characteristics of anesthesia with desflurane and nitrous oxide in infants and children. Anesthesiology. 1992;76:373.