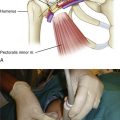
21 Anesthesia for Noncardiac Surgery in Children with Congenital Heart Disease
A wide spectrum of extracardiac anomalies has been described in children with CHD.1–5 A great incidence of chromosomal syndromes and genetic disorders is associated with CHD, and the reported prevalence of associated malformations ranges between 10% and 33%.6–8 The organ systems most often affected include musculoskeletal, central nervous, renal-urinary, gastrointestinal, and respiratory. Although many extracardiac malformations are relatively minor and have limited or no clinical implications, many children with CHD have significant noncardiac comorbidities.9 These pathologic and disease processes may necessitate surgical intervention. Other routine ailments and conditions may affect these children and require diagnostic procedures and surgical care.
The challenges of caring for children with CHD are magnified by the diversity of structural malformations, each with specific physiologic perturbations, hemodynamic consequences, and severity. This is further complicated by the variety of medical and surgical management strategies available. Most children require an individualized approach to anesthetic care.10–12
Clinical outcomes for CHD depend on the nature of the anatomic abnormalities and the possibility of successful palliation or correction.13 The primary goal of palliative surgery is to favorably influence the natural history of the defect and decrease the likelihood of the severe consequences of the disease. However, children continue to have abnormal cardiovascular anatomy and physiology, and their abnormal circulation is associated with an increased risk of perioperative adverse events.14–17
Despite these considerations, for children with good hemodynamic results, the risks associated with noncardiac surgery may not be significantly different from those of others without CHD. These children are considered to be doing well clinically, have a good functional status, require few or no medications, have no exercise restrictions, and undergo routine surveillance. They require minimal or no adjustment in perioperative care compared with that provided to children without CHD. In others, however, residual abnormalities exist. In some who are less fortunate, a pathologic process may remain or develop after cardiac surgery that is related to the primary disease or therapy. This may lead to severe cardiovascular or pulmonary impairment. These residua and sequelae may necessitate further medical or surgical interventions and may increase perioperative morbidity during noncardiac surgery.18 Management of these children is influenced by several factors but to a significant extent by the residual problems of the disease and treatment and associated hemodynamic perturbations.19,20
Many publications have examined the implications of anesthesia for children and adults with CHD undergoing noncardiac surgery.10,19–33 However, only a limited number of studies have provided data on perioperative outcomes.34–38 In contrast to the extensive literature regarding perioperative cardiac assessment and risk stratification during noncardiac surgery in adults with heart disease and the development of guidelines aimed at improving clinical outcomes, the lack of rigorous scientific data on this subject for the pediatric age group has made an equivalent effort challenging.39
Preoperative Assessment
A detailed preoperative evaluation is indispensable for identifying and anticipating factors that may place a child with CHD at increased risk during anesthesia (Table 21-1).40,41 An important goal of this assessment is to gather information regarding the nature of the cardiovascular disease and prior therapeutic interventions. A determination of functional status is based on clinical data. The history and physical examination, in addition to the laboratory data and ancillary tests, provide complementary information about anatomic or hemodynamic status, enabling an overall risk assessment. Based on this clinical assessment and consideration of the major pathophysiologic consequences of a particular condition, a systematic, detailed, organized plan should be formulated for anesthesia and perioperative management. In some cases, the preoperative evaluation may establish the need to delay or defer elective noncardiac surgery, other interventions, or diagnostic procedures.
TABLE 21-1 Factors That Place Children with Congenital Heart Disease at Increased Risk during Anesthesia for Noncardiac Surgery
History of implanted device (pacemaker or defibrillator)
Older age at the time of cardiac intervention
Older type of cardiac surgical procedure
Pulmonary hypertension/pulmonary vascular disease
Significant outflow tract obstruction
Significant sequelae or residua
History and Physical Examination
As for all children undergoing anesthesia, the history and physical examination results are essential components of a thorough preoperative evaluation. In addition to the specifics regarding the present illness and planned procedure, the history should focus on the status of the cardiovascular system. Relevant information includes the type of cardiovascular disease and comorbid conditions, medications, allergies, prior hospitalizations, surgical procedures, anesthesia experiences, and complications. Symptoms, including tachypnea, dyspnea, tachycardia, rhythm problems, and fatigue, should be sought. Feeding difficulties and diaphoresis may represent significant symptoms in infants, whereas decreased activity level or exercise intolerance may be a concern for older children. Palpitations, chest pain, and syncope should be characterized. The history should include an assessment of growth and development because these may be affected in children with CHD. Failure to thrive suggests ongoing cardiorespiratory compromise. Those with decompensated disease, complex pathologies, associated genetic defects, or other syndromes may be particularly vulnerable. Recent illnesses such as intercurrent respiratory infections or pulmonary disease may increase the potential for perioperative complications and require careful appraisal of the risk/benefit ratio in elective cases.42,43
Fasting Guidelines
Although the optimal period of fasting for children before surgery has been the subject of some debate, most centers follow established guidelines to reduce the risk of aspiration.44–47 The same guidelines are applicable to children with CHD with a few additional considerations. Intake of clear fluids or the intravenous administration of maintenance fluids may be required in some children to ensure adequate hydration if the fasting period is prolonged. This is particularly important in small infants and in patients with obstructive lesions, cyanotic disease, or single-ventricle physiology. Maintenance of adequate hydration and ventricular preload may limit potential detrimental hemodynamic changes associated with anesthesia and surgery.
Intraoperative Management
General Considerations
Anesthesia Care Provider
Anesthesia care should be provided by an experienced individual who is familiar with children with CHD, the planned operative procedure, and the surgeon’s usual approach. The most important factor that an anesthesiologist can offer a child with CHD is a comprehensive understanding of the anatomic abnormalities, pathophysiology of the cardiac malformation, and how this may be affected by the anesthetic and surgical procedure. Familiarity with the most likely residua and sequelae is essential.18 Adequate communication among all physicians involved enhances the likelihood of the best possible outcome.
Premedication
The use of premedication to provide sedation and anxiolysis is routine before most surgical procedures because some degree of fear or anxiety is expected. This facilitates parental separation, entry into the operating room, placement of monitors, and induction of anesthesia. The cardiorespiratory effects of premedication in children may be influenced by the underlying systemic disease.48–52
Commonly used premedications include oral or intravenous benzodiazepines, opioids, and small amounts of hypnotic agents. Drugs such as barbiturates and ketamine are occasionally used. Alternative routes for premedication include intramuscular, intranasal, and rectal methods. Children with hemodynamic decompensation may require little or no premedication. Caution should also be exercised for those with a history of cardiovascular pathology associated with significant increases in pulmonary artery pressure or pulmonary arteriolar resistance because hypoventilation and hypoxemia may be detrimental. Conversely, children susceptible to hypercyanotic episodes or those with catecholamine-induced arrhythmias may benefit from heavy premedication. In selected children (e.g., infants and children with cyanotic heart disease), oxygen saturation monitoring after premedication and the administration of supplemental oxygen is recommended.50
Intravenous Access
Secure intravenous access is mandatory for administration of fluids and medications during anesthesia care. In most children with CHD, intravenous access is established after an inhalational induction. In those considered at great risk, such as children with severe outflow tract obstruction, moderate to severe cardiac dysfunction, pulmonary hypertension, or potential for hemodynamic compromise, consideration should be given for placement of intravenous access before induction of anesthesia or very early in the induction. The size of the intravenous catheter should be determined by the anticipated fluid requirements. If peripheral access is poor, central venous access may be necessary, particularly if there is potential for large intravascular volume shifts and to allow monitoring of central venous pressure. Placement of a central venous catheter may be assisted by audio Doppler or two-dimensional ultrasound guidance (see Chapter 48). In the small infant with single-ventricle physiology, central venous cannulation with catheter placement in the superior vena cava may be undesirable in view of concerns about potential vascular complications that may affect pulmonary blood flow or subsequent surgical palliation. In these children, a small catheter or alternative approach (e.g., femoral venous access) should be considered. In children with an existent or potential right-to-left shunt, all air must be removed from intravenous infusion tubing. Air filters may be difficult to use in the operating room because they may restrict the rate at which intravenous fluids or blood may be administered in emergency situations. They may be more useful in the preoperative and postoperative periods.
Monitoring
Arterial Blood Pressure Assessment
Basic blood pressure monitoring begins with pulse palpation. An automated blood pressure cuff is used in most children. The selection of monitoring site may be influenced by vascular anomalies (e.g., aortic arch pathology, aberrant origin and course of aortic arch vessels) or prior surgical interventions (e.g., Blalock-Taussig shunt, arterial cutdown). Direct systemic blood pressure monitoring by an indwelling arterial catheter may be necessary for beat-to-beat assessment and for blood gas analysis. In children, this is usually accomplished after induction of anesthesia. Arterial cannulation can be achieved percutaneously in most circumstances with a reduced risk of complications (see Chapter 48). Use of the radial arteries is preferable, particularly in the neonate, to minimize catheter-related vascular problems. Ultrasound guidance with Doppler or two-dimensional imaging may facilitate cannulation. The decision regarding the need for invasive monitoring is largely based on the child’s clinical condition and nature of the surgical procedure.
Electrocardiography
An ECG is used to monitor heart rate, cardiac rhythm, and ST-segment analysis. One or multiple leads typically are displayed. Most systems use two leads: standard lead II for arrhythmia monitoring plus inferior ischemia detection and precordial lead V5 for lateral ischemia detection. Arrhythmias may occur as a result of hypoxia, electrolyte imbalances, acid-base abnormalities, intravascular or intracardiac catheters, and surgical manipulations near or around the thorax. Ischemia may be evident on direct examination of the ECG or ST-segment analysis.53 Although in the adult population this is associated with worsened outcome, the implication for children is unknown.54,55
Pulse Oximetry
Placement of an oximeter probe is well tolerated, even by uncooperative children, and it is typically one of the earliest monitors applied during induction of anesthesia. Monitoring arterial oxygen saturation is particularly useful in infants, cyanotic children, and those with complex anatomy or significant hemodynamic compromise. In addition to providing continuous assessment of oxygen-hemoglobin saturation and heart rate, the pulse oximeter waveform may indicate the adequacy of peripheral perfusion and cardiac output.56,57 Other parameters that may be reflected by the Spo2 include intracardiac or great artery–level shunting and pulmonary blood flow.
Capnography
Capnography can confirm proper endotracheal tube placement, help to assess the adequacy of ventilation, and aid recognition of pathologic conditions such as bronchospasm, airway obstruction, and malignant hyperthermia. In spontaneously breathing, sedated children receiving supplemental oxygen through a nasal cannula, capnography monitors the end-tidal or exhaled carbon dioxide (Petco2) concentration. A prospective, observational study in children undergoing cardiac catheterization with sedation administered by nonanesthesiologists found that monitored Petco2 values provided a reasonable estimate of arterial blood CO2 values.58 Although the absolute value for Petco2 may not be as reliable as in the presence of an endotracheal tube, the capnograph waveform confirms the presence or absence of respirations and air exchange. End-tidal CO2 monitoring also provides a gross index of pulmonary blood flow. In children with cyanotic heart disease, Petco2 values may underestimate arterial carbon dioxide tension (Paco2) measurements due to altered pulmonary blood flow and ventilation/perfusion mismatch.59,60
Transesophageal Echocardiography
Numerous publications have documented the utility of transesophageal echocardiography as a monitoring device in high-risk adults undergoing noncardiac procedures.61–72 Sporadic reports have demonstrated the utility of this imaging approach in children undergoing noncardiac surgery.73–78 However, the contribution or application of this modality in the pediatric age group in this particular setting has not been well defined and requires further investigation.
Selection of Techniques and Agents
Anesthesia Technique
Regional anesthesia has been safe and effective in children with CHD (see Chapters 41 and 42).76–79 Advantages of regional anesthesia, such as epidural and spinal techniques, include an effect largely limited to the surgical site, decreased number of systemic medications, a potentially brief recovery period, and usually a more pleasant experience for the child. Use of these techniques, however, may not always be effective. Regional anesthesia retains the potential for hemodynamic compromise, particularly in hypovolemic children or those with a fixed cardiac output. It is also contraindicated in those with coagulation defects. The administration of agents such as local anesthetics, opioids, or other adjuvants (e.g., clonidine) into the caudal space may attenuate the sympathetic outflow associated with surgical manipulation and noxious stimuli and facilitate postoperative pain management.
The choice of technique affects termination of the anesthesia and emergence. Anesthesia performed with fewer agents is inherently simpler and usually easier and more predictable to terminate. The availability of ultra-short-acting opioids (e.g., remifentanil) and other agents (e.g., dexmedetomidine) has avoided the need for postoperative ventilation solely related to residual effects of depressant drugs. Ventricular function and the presence of intracardiac shunts can significantly affect uptake and distribution of inhalational anesthetics and the kinetics of intravenous medications (see Chapter 6).
Inhalational Agents
The use of inhalational anesthetics has been at the forefront of pediatric anesthesia practice for many years.80,81 Sevoflurane was introduced in the mid-1990s, replacing halothane for induction of anesthesia in many centers. A study on the safety and efficacy of inhaled agents in infants and children with CHD during cardiac surgery demonstrated twice as many episodes of hypotension, moderate bradycardia, and emergent drug use in those who received halothane compared with those who received sevoflurane.82 These data and those from other studies that demonstrated the potential benefits of sevoflurane on hemodynamic stability and minimal impact on myocardial performance led to sevoflurane becoming the preferred anesthetic agent for children, particularly those with heart disease.83–88 Nonetheless, in some jurisdictions and under some conditions, halothane may remain the primary anesthetic for children.
Intravenous Agents
Propofol is one of the most frequently used medications for intravenous sedation and general anesthesia. It has been used in children with CHD in numerous settings.89–92 The hemodynamic effects of propofol have been investigated in children with normal hearts and in those with cardiovascular disease. An echocardiographic study in infants with normal hearts undergoing elective surgery demonstrated that propofol did not alter heart rate, shortening fraction, rate-corrected velocity of circumferential fiber shortening, or cardiac index after intravenous induction.93 However, this medication decreased arterial blood pressure to a greater extent than thiopental, an effect attributed to a reduction in afterload. A comparison of propofol and ketamine during cardiac catheterization found that propofol caused a transient decrease in mean arterial pressure and mild arterial oxygen desaturation in some children.94 In view of the significantly faster recovery, it was concluded that propofol was a practical alternative to ketamine for elective cardiac catheterization in children.
Another investigation in 30 children with CHD undergoing cardiac catheterization demonstrated significant decreases in mean arterial blood pressure and systemic vascular resistance during propofol administration.89 No changes in heart rate, mean pulmonary artery pressure, or pulmonary vascular resistance were observed. In children with intracardiac shunts, the net result of propofol was a significant increase in the right-to-left shunt, a decrease in the left-to-right shunt, and decreased pulmonary-to-systemic blood flow ratio, resulting in a statistically significant decrease in the Pao2 and arterial oxygen saturation (Sao2), as well as reversal of the shunt direction from left-to-right to right-to-left in two patients. It was also shown that propofol could lead to further hemoglobin desaturation in children with cyanotic heart disease.
The effects of propofol have been examined in children undergoing electrophysiologic testing and radiofrequency catheter ablation for tachyarrhythmias. The drug has no significant effect on sinoatrial or atrioventricular node function or accessory pathway conduction in Wolff-Parkinson-White syndrome.95,96 However, a study documented that ectopic atrial tachycardia may be suppressed during propofol administration in children.97
Thiopental, a rapid-acting barbiturate, was used for many years for induction of anesthesia. Several investigations have documented the cardiovascular responses to this agent in the pediatric age group. In children with normal hearts, the cardiac index remains unchanged, although the shortening fraction decreases and alterations in load-independent parameters of contractility occur.93 The myocardial depressant properties of barbiturates are well established, as are its effects on venodilation and blood pooling in the periphery. These data suggest that a subset of children who receive thiopental may be at risk for hemodynamic instability. It has been suggested that thiopental should be used with caution, particularly in those with limited reserve or increased sympathetic tone. Thiopental is not available for use in the United States.
Etomidate, a carboxylated imidazole derivative, has anesthetic and amnestic properties but no analgesic effects. This agent demonstrates favorable qualities over other intravenous drugs due to its lack of effect on hemodynamics.98,99 This, combined with laboratory and clinical data that support minimal effects on myocardial contractility, makes this drug a particularly desirable agent in critically ill children and in those with limited cardiovascular reserve.100 Despite these benefits, several undesirable adverse effects are associated with etomidate, including pain on intravenous administration, myoclonic movements that may mimic seizure activity, and inhibition of adrenal steroid synthesis perioperatively.101,102 Although used primarily as an induction agent, etomidate has been administered for sedation of children during cardiac catheterization and in other settings.103–105 A concentrated form of this medication is available in Europe but not in the United States.
Several investigations have addressed the concern of potential detrimental changes in pulmonary vascular tone resulting from ketamine although no significant effects have been reported on pulmonary arterial pressures and pulmonary vascular resistance at the usual clinical doses.106–109 Regarding its effect on myocardial performance, in-vitro investigations have shown a direct myocardial depressant effect in animal species and the failing adult human heart. This is considered to be the result of inhibition of L-type voltage-dependent calcium channels in the sarcolemmal membrane and may be a consideration in critically ill infants with severely impaired cardiac reserves. Additional undesirable effects of ketamine include emergence reactions, excessive salivation, vomiting, and increased intracranial pressure.
Dexmedetomidine is a selective α2-adrenergic agonist agent being increasing used in the pediatric age group. Compared with clonidine, the drug exhibits greater specificity for the α2-adrenergic receptor over the α1-adrenergic receptor. Favorable effects of the drug include sedation, anxiolysis, and analgesia. This medication provides hemodynamic stability, although adverse effects have been reported, including bradycardia, hypertension, and hypotension. A study of the hemodynamic effects in children undergoing dexmedetomidine sedation for radiologic imaging demonstrated modest decreases in heart rate and blood pressure. These changes in response to moderate doses were independent of age, required no pharmacologic interventions, and did not result in any adverse events; however, high dose dexmedetomidine can be associated with significant bradycardia.110,110a In addition, treatment of dexmedetomidine-induced bradycardia with glycopyrrolate (5 μg/kg) has been associated with severe persistent hypertension.110b
Dexmedetomidine is used as a premedication agent, during diagnostic studies and procedural sedation, to reduce emergence delirium, in the treatment of symptoms associated with opioid withdrawal, and as an adjuvant agent in the operating room and postoperative settings.111 In children with CHD, its benefits have been reported during monitored anesthesia care, diagnostic and interventional cardiac catheterization, intraoperative sedation, after cardiac and thoracic surgery, as a primary agent during invasive procedures, and in the treatment of perioperative atrial and junctional tachyarrhythmias.112–119 This medication has also been used in children with pulmonary hypertension with good results.120,121
The effects of dexmedetomidine on cardiac electrophysiology have been examined in children; the drug significantly depresses sinus and atrioventricular nodal function.122 Other findings included a reduction in the heart rate and increases in arterial blood pressure. Hammer and colleagues concluded that this medication should be considered undesirable for electrophysiologic studies and that it could be associated with adverse effects in patients at risk for bradycardia or atrioventricular block.122 In contrast, another study concluded that dexmedetomidine was not associated with any significant or any atypical ECG interval abnormalities, except for a trend toward a decrease in heart rate in children with CHD.123 Until additional data are available, it may be prudent to exercise caution when considering the use of dexmedetomidine in children with conduction abnormalities.
Although the experience suggests an overall safety profile in children with CHD, fragile patients may not tolerate the heart rate and blood pressure fluctuations associated with dexmedetomidine administration. Significant adverse effects have been described that include severe bradycardia progressing to asystole.124
Opioids and benzodiazepines are widely used medications in pediatric anesthesia practice. Opioids attenuate the neuroendocrine stress response associated with anesthesia and surgery.125,126 After repair of CHD, these medications have been shown to blunt the stress response in the pulmonary circulation elicited by airway manipulations.127 Morphine administration may be associated with histamine release and hypotension. The synthetic opioids are devoid of these effects and provide excellent hemodynamic stability with minimal changes in heart rate and blood pressure in children with CHD.128 The primary concern about opioid administration is their central respiratory depressant effects because their primary cardiovascular manifestations are minimal. Benzodiazepines provide sedation and amnesia during the perioperative period. Midazolam administration may allow a reduction in the inspired concentration of inhalational anesthetic agents, which is a desirable feature in children with labile hemodynamics or in those considered at great risk for the myocardial depressant properties of inhalational anesthetics. Studies of the effects of benzodiazepines in children with CHD are limited.129
Neuromuscular blocking drugs facilitate endotracheal intubation and prevent reflex movement during surgery if the anesthetics alone are insufficient. All inhalational anesthetics potentiate the effects of nondepolarizing muscle relaxants. These medications have various onsets and durations of action and diverse hemodynamic effects. The cardiovascular and autonomic effects of muscle relaxants have been characterized mainly in adults with acquired cardiovascular disease130–133 (see also Chapter 6). Drug selection is based on the need to facilitate endotracheal intubation and surgical relaxation, hemodynamic side effects, and the anticipated duration of surgery.
Induction of Anesthesia
Induction of anesthesia in children with CHD most commonly can be accomplished using the inhaled or intravenous route. The intramuscular route (i.e., ketamine administration) may be preferable in some cases, particularly in an uncooperative, developmentally delayed, or combative child. Less common induction techniques include subcutaneous, intranasal, and rectal administration of agents. These various approaches may also be used in combination (see Chapter 4).
If intravenous access is not available, an inhalational induction is performed in most cases. A carefully titrated inhalational induction and early placement of an intravenous catheter usually is safe even in children with moderate hemodynamic disturbances, particularly after premedication has been given. This produces loss of consciousness, with acceptable conditions for establishing intravenous access. Inhalational induction may be delayed in cyanotic children and those with right-to-left shunts, particularly for anesthetics with reduced blood solubility, because the decreased pulmonary blood flow limits the rate of increase in the concentration of the anesthetic in the systemic arterial blood. The rapidity of an inhalational induction is increased in the presence of a reduced cardiac output because the anesthetic partial pressure in the alveoli increases more rapidly as less anesthetic is removed by the smaller pulmonary blood flow (see Chapter 6). Left-to-right intracardiac shunts have limited effects on the speed of induction of inhaled anesthetics.
Postoperative Care
Hemoglobin or hematocrit values are monitored as a measure of oxygen-carrying capability in cases in which significant blood loss or the administration of fluids might have occurred. Serum electrolytes are screened if fluid shifts have taken place during the surgical and postoperative periods. Although digoxin is now used less frequently, particular attention should be given to the avoidance of hypokalemia in children receiving this drug. Serum glucose levels should be followed in neonates and small infants and dextrose-containing fluids administered as appropriate. Determination of ionized calcium (iCa2+) levels may be indicated for patients with a history of DiGeorge sequence because of a propensity for hypocalcemia. The required fluid replacement is dictated by the child’s heart defect, type of surgery performed, and volume losses (see Chapters 8 and 10).
Perioperative Problems and Special Considerations
Cyanosis
Cyanosis is a common finding in children with defects characterized by limited pulmonary blood flow or intracardiac mixing. As surgical management strategies evolve to target the youngest of infants, the effects of cyanosis may be limited in these children. However, in those requiring delayed surgery, palliation, or staged correction of their defects, the effects of cyanosis may be long lasting. Chronic hypoxemia affects all major organ systems. Compensatory mechanisms that attempt to provide adequate systemic oxygen delivery in the presence of chronic hypoxemia include polycythemia, increases in blood volume, alterations in oxygen uptake and delivery, and neovascularization. Despite the favorable effects of the adaptive responses, these alterations may be detrimental. Polycythemia, the most significant compensatory response, is associated with increases in blood viscosity and red cell sludging. The common occurrence of iron-deficiency anemia in cyanotic children further enhances hyperviscosity and the unfavorable consequences of this condition. Several hemostatic abnormalities (e.g., thrombocytopenia, altered platelet function, and clotting factor abnormalities) have been documented as a result of hypoxemia and erythrocytosis that may affect the coagulation system and increase perioperative risks.134–139 This is compounded by increased tissue vascularity, with a large number of blood vessels per unit of tissue.
The increased blood viscosity in children with cyanosis is associated with stasis and a risk for thrombotic events.140 If the hematocrit exceeds 65% preoperatively, some clinicians advocate phlebotomy to reduce the hematocrit to 60% to 65%. This limits sludging of red blood cells and increases oxygen delivery to tissues. If blood is removed by preoperative phlebotomy, it may be saved for autologous transfusion in the perioperative period.
Tetralogy Spells
Hypercyanotic episodes may result from further decreases in pulmonary blood flow in children with tetralogy (i.e., tet spells) and significant dynamic right ventricular outflow tract obstruction. Tet spells are rare during noncardiac surgery, probably because general anesthesia attenuates the triggers. Occasionally, however, increased cyanosis may occur without warning in response to obscure stimuli. Whatever the cause, worsening cyanosis implies increases in dynamic obstruction and exacerbation of ventricular-level right-to-left shunting. Factors that decrease systemic blood pressure and systemic vascular resistance, such as hypovolemia and extreme vasodilation, should be avoided. Therapy consists of increasing blood volume and systemic vascular resistance, the latter using either phenylephrine 5 µg/kg IV initially and then 1 to 5 µg/kg/min by continuous infusion or norepinephrine 0.5 µg/kg IV initially and then 0.1 to 0.5 µg/kg/min by continuous infusion. Increasing the inspired oxygen concentration and reducing inspiratory ventilatory pressures may also produce clinical improvement. Additional therapies include increasing the level of sedation or anesthetic depth and β-adrenergic blockade (esmolol [50 μg/kg/min] has largely replaced propranolol in this setting) (see also Chapters 15 and 16). Pulmonary vascular resistance does not play a major role in the physiology of hypercyanotic episodes in tetralogy of Fallot.
Heart Failure
In a retrospective review of 21 children with severe heart failure who underwent 28 general anesthetics, 10% had a cardiac arrest requiring unplanned postoperative admission to the intensive care unit, and 96% required perioperative inotropic support. The investigators concluded that general anesthesia for children with severe heart failure is associated with a significant complication rate.141
Ventricular Dysfunction
In children with cardiomyopathy that was accompanied by severe ventricular dysfunction, general anesthesia for noncardiac procedures was associated with an increased frequency of complications.142 They often required hospital support before and after the procedure that in many cases included intensive care management. Hospital stay was prolonged for children with severe ventricular dysfunction compared with those with a lesser degree of impairment. Based on these findings, early consideration of perioperative intensive care support was recommended for monitoring and optimization of cardiovascular therapy.142
Pulmonary Hypertension
A less common entity is increased pulmonary vascular resistance, which may be reactive or fixed. The diagnosis is formally determined at cardiac catheterization and involves pulmonary vascular reactivity testing. Pulmonary hypertension and increased pulmonary vascular resistance represent risks for major perioperative complications in children, regardless of cause.143,144
Several factors can increase pulmonary vascular tone (Table 21-2). Therapy should be aggressive in the acute setting, aimed at reducing pulmonary artery pressures with interventions such as additional sedation, hyperventilation, hyperoxygenation, and treatment of acidosis. The use of selective pulmonary vasodilators (e.g., inhaled nitric oxide, other agents) and inotropic support of the right ventricle may be indicated. Manipulation of pulmonary hemodynamics is challenged by the difficulty of directly measuring these parameters in children. Management of critical situations requires a thorough understanding of the pathophysiologic process and experienced clinical judgment. Because of the significant morbidity and potential periprocedural mortality for children with a history of severe pulmonary hypertension, an in-depth evaluation of the risk/benefit ratio of the planned procedure and its impact on the overall quality of life is essential.
TABLE 21-2 Factors Associated with Increased Pulmonary Vascular Tone
Endocarditis Prophylaxis
The latest guidelines of the American Heart Association for the prevention of infective endocarditis indicate that routine antibiotic prophylaxis is no longer needed for most children with CHD (see Table 14-4).145 In contrast to earlier guidelines, the administration of antibiotics solely to prevent endocarditis is not recommended for children undergoing genitourinary or gastrointestinal tract procedures, although neither of these subspecialty professional bodies have fully adopted the new guidelines. It is important to discuss the need for prophylaxis with the responsible physician.
The guidelines target individuals at increased risk for a poor outcome if they develop endocarditis (see Chapter 14). Preventive antibiotics for dental procedures are recommended for children with the following conditions:
 History of infective endocarditis
History of infective endocarditis
 Unrepaired cyanotic CHD, including palliative shunts and conduits
Unrepaired cyanotic CHD, including palliative shunts and conduits
 Completely repaired congenital heart defect with prosthetic material or a prosthetic device, whether placed by surgery or by catheter intervention, during the first 6 months after the procedure
Completely repaired congenital heart defect with prosthetic material or a prosthetic device, whether placed by surgery or by catheter intervention, during the first 6 months after the procedure
 Repaired CHD with residual defects at the site or adjacent to the site of a prosthetic patch or prosthetic device (which inhibit endothelialization)
Repaired CHD with residual defects at the site or adjacent to the site of a prosthetic patch or prosthetic device (which inhibit endothelialization)
 Cardiac transplant recipients who develop cardiac valvulopathy
Cardiac transplant recipients who develop cardiac valvulopathy
Anticoagulation
Anticoagulants, antiplatelet drugs, and thrombolytic agents are increasingly being used in children, particularly in those with CHD.146–150 Decisions regarding management are primarily influenced by the nature of the procedure, urgency of the intervention, specific drug therapy, and expected effects or laboratory data. The major concern is the potential for bleeding. Recommendations for the management of children taking warfarin (Coumadin) vary widely.151–153 The proposed strategies are quite heterogeneous, and the lack of consensus reflects the paucity of randomized trials addressing this issue. The problem is further complicated by the lack of guidelines specific to pediatric practice.154
If the indications for anticoagulation are for native valve disease or atrial arrhythmias, the risk of a major thromboembolic event is considered to be relatively small, and warfarin may be discontinued 1 to 2 weeks before the day of surgery. In those with mechanical prosthetic valves, the risk of thromboembolic events is greater. Many recommend discontinuing the oral anticoagulant a few days before surgery and allowing the prothrombin time to return to within 20% of normal.151 Administration of parenteral vitamin K or clotting factors, including fresh frozen plasma (FFP), may be required to restore the prothrombin time within an acceptable range, especially in those with liver disease and in emergency cases. Some experts advise preoperative hospitalization, particularly in high-risk individuals such as those with mitral or combined valve prostheses, to discontinue warfarin therapy and to initiate a heparin infusion, which is continued up until a few hours before surgery. Others suggest that low-molecular-weight heparin may be the better option instead of unfractionated heparin because the perioperative conversion from warfarin therapy to heparin can be accomplished without the need for hospitalization.152
The risk of bleeding from the surgical intervention versus the risk of a thromboembolism from a reduced anticoagulant dose determines to what extent and for what duration the anticoagulant therapy should be reduced. In some cases, the cardiologist and surgeon may decide to temporarily use aspirin therapy before and after surgery. There is disagreement regarding whether antiplatelet therapy is preferable to anticoagulation in children with prosthetic aortic valves or after certain surgical interventions.155–158
Conduction Disturbances and Arrhythmias
Acute rhythm disturbances may occur with the use of any anesthetic agent or technique and may be related to several factors. The administration of agents with vagolytic or sympathomimetic properties requires consideration in patients with prior history of or pathology associated with arrhythmias. Bradycardia may occur during induction of anesthesia, laryngoscopy, and endotracheal intubation, particularly in infants and in children with Down syndrome.159–161 In most cases, bradycardia is self-limited and requires no therapy.
Pacemakers and Implantable Cardioverter-Defibrillators
An in-depth discussion of perioperative considerations related to implanted pacemakers or defibrillators can be found in Chapter 14. Consultation with a cardiologist or electrophysiologist is essential when caring for children with implanted devices. Unit interrogation and programming are required in most cases. The main goal is to avoid problems with hardware malfunction related to electromagnetic interference (i.e., electrocautery). Chronotropic agents and backup pacing modalities (e.g., transvenous, epicardial, transcutaneous) should be readily available and carefully considered in the event of pacemaker malfunction associated with an inadequate underlying heart rate. A magnet should be accessible to enable asynchronous pacing if required. Perioperative ECG monitoring is essential, as well as the use of modalities that can confirm pulse generation during pacing. Implanted devices should be interrogated and reprogrammed after the procedure.
Eisenmenger Syndrome
Eisenmenger syndrome is characterized by irreversible pulmonary vascular disease and cyanosis related to reversal in the direction of an intracardiac or arterial level shunt.162,163 This is unlikely to occur in the current surgical era, but it may occasionally occur in older children, adolescents, or adults with CHD. Morbidity is linked to problems associated with chronic cyanosis and erythrocytosis. Other problems include hemoptysis, gout, cholelithiasis, hypertrophic osteoarthropathy, and decreased renal function. Variables associated with poor outcome include syncope, increased right ventricular end-diastolic pressure, and significant hypoxemia (i.e., systemic arterial oxygen saturation less than 85%). Life expectancy is significantly reduced.164 Most succumb suddenly, probably from ventricular tachyarrhythmias. Surgical modalities that have been advocated in selected patients include combined heart and lung transplantation165 and lung transplantation alone.166
Despite the overall poor prognosis and the extremely high risk of a bad outcome, several reports have documented successes with a variety of anesthetic techniques and agents.167–171 Nevertheless, it is vital that the family and patient, if of appropriate age, understand these risks before undertaking any procedure requiring anesthesia or deep sedation.
Cardiac Transplantation
Cardiac transplantation may be considered the best option in end-stage cardiac pathology as a result of congenital or acquired disease.172,173 A major consideration in the care of these children is the lack of external nerve supply of the transplanted heart. The physiology of the denervated heart implies that the usual autonomic regulatory mechanisms are not operational, increasing the vulnerability of transplanted children to hemodynamic alterations.174 Compensatory responses may be delayed, further increasing the potential for compromise.
Outcomes of Noncardiac Surgery
Limited data regarding the risks of noncardiac surgery and anesthesia in children with CHD raises concerns. A review of 110 children with CHD who underwent 135 anesthesias over a 1-year period found a 47% incidence of adverse events and more than one adverse event in a significant number of children.34 Continuous monitoring of perioperative rhythm abnormalities in 70 children with CHD and a history of ventricular arrhythmias documented a 35% and 87% incidence of intraoperative and postoperative ventricular arrhythmias, respectively.175 Other studies have reported cyanosis, treatment for congestive heart failure, poor health, and young age as risk factors.35 A retrospective review of a large number of children documented that these risks involved major and minor interventions.37
Data from the Pediatric Perioperative Cardiac Arrest (POCA) registry shed further insight into this subject. Anesthesia-related cardiac arrests in children with congenital and acquired heart disease were examined. Causes of cardiac arrest were primarily cardiovascular in nature, occurring more frequently in those with heart disease than those without. Events occurred more frequently in the general operating room, usually during the maintenance period. Among children who suffered a cardiac arrest, the most common type of CHD was a single ventricle (i.e., Fontan physiology), particularly those managed early with palliation. The overall mortality rate for children with heart disease was greater than for those without heart disease, and children with aortic stenosis (i.e., Williams syndrome) and cardiomyopathy have the greatest mortality rate.176
Specific Congenital Heart Defects
The anesthetic care of children with CHD undergoing noncardiac surgery is strongly influenced by the particular nature of the cardiovascular malformations, pathophysiology of the lesions, operative state (e.g., unoperated, prior palliative, definitive procedure), complications associated with the primary pathology or treatment, and other comorbidities.12
This section addresses relevant anatomic features, hemodynamic consequences of selected cardiac defects, and treatment strategies. Potential residua, sequelae (Table 21-3), and long-term outcomes of the lesions are discussed, focusing on their implications for perioperative care.
TABLE 21-3 Potential Issues after Interventions for Selected Congenital Heart Defects
Persistent right ventricular dilation and abnormal motion of interventricular septum
Atrial arrhythmias, ventricular dysfunction if late repair
Pulmonary venous obstruction (sinus venosus defect associated with anomalous pulmonary venous return)
Mitral valve problems, left ventricular outflow tract obstruction (ostium primum defect with cleft mitral valve)
Residual pathology (e.g., intracardiac shunts, outflow tract obstruction)
After atrial baffle procedure: baffle leak, obstruction of systemic or pulmonary venous pathways, progressive right ventricular dilation or failure, tricuspid regurgitation, sinus node dysfunction, or atrial arrhythmias
After arterial switch operation: aortic root dilation, aortic regurgitation, supravalvar stenosis (pulmonary or aortic), or coronary insufficiency
After aortopulmonary shunt: shunt stenosis with associated hypoxemia, ventricular volume overload, systemic ventricular dilation, distortion of pulmonary artery anatomy, or pulmonary hypertension
After bidirectional Glenn connection or hemi-Fontan procedure: progressive cyanosis due to venous collaterals or other vascular communications, allowing venous pathways to bypass the pulmonary circuit or due to development of pulmonary arteriovenous malformations (more likely with classic Glenn anastomosis)
After Fontan procedure: increased systemic venous pressures, right atrial hypertension (with atriopulmonary connection), sinus node dysfunction, atrial rhythm disturbances, atrioventricular valve regurgitation, hepatic dysfunction, thrombotic complications, coagulation defects, protein losing enteropathy, or progressive systemic ventricular dilation or dysfunction
Residual or recurrent pathology (e.g., intracardiac shunts, right ventricular outflow tract obstruction, distal pulmonary artery bed abnormalities)
Progressive pulmonary regurgitation with need for repeat intervention (e.g., right-sided heart dilation, dysfunction)
Arrhythmias associated with poor hemodynamics
Atrial Septal Defects
Anatomy and Pathophysiology
Defects in the interatrial septum, or atrial septal defects (ASDs) (see Fig. 15-1), are among the most common congenital cardiac anomalies (30% to 40% of CHD) in childhood. ASDs occur in 1 of 1500 live births. Based on their location, several types of defects have been identified:
1. Ostium secundum or fossa ovalis defect, the most common type (75%), results from a deficiency in the region of the fossa ovalis (see Fig. 15-1, B). These defects may be associated with mitral valve prolapse or mitral regurgitation.177–179
2. Ostium primum defect, a form of atrioventricular septal (canal or endocardial cushion) defect (see Fig. 15-1, A),180 is characterized by a deficiency in the inferior portion of the interatrial septum. They account for 15% to 20% of ASDs. They frequently are associated with a commissure or cleft in the anterior leaflet of the mitral valve and various degrees of regurgitation.
3. Sinus venosus defect, which occurs in 5% to 10% of ASDs, typically is located at the superior aspect of the interatrial septum, below the region where the superior vena cava joins the right atrium (i.e., sinus venosus defect of the superior vena cava-type) (see Fig. 15-1, C).181 It is frequently associated with anomalous pulmonary venous drainage.182
4. Coronary sinus defect is uncommon and consists of a communication between the left atrium and mouth of the coronary sinus. It is commonly associated with an unroofed coronary sinus and persistent left superior vena cava that drains directly into the left atrium, resulting in a right-to-left shunt.183
5. Other entities that may allow interatrial shunting include a patent foramen ovale (PFO) at one end of the spectrum and a common atrium at the other. A PFO has been identified in as many of 25% of individuals. This communication may have implications for perioperative care because it has the potential for right-to-left shunting and paradoxical emboli.184,185 In a common atrium, there is complete or near-total absence of the interatrial septum. This may be found in complex CHD.
Treatment Options, Residua, Sequelae, and Long-Term Outcomes
Surgical closure of secundum ASDs in childhood provides excellent results, almost normal long-term survival, and negligible mortality.186–189 Normal ventricular function should be anticipated after repair of these defects. Rarely, children may demonstrate persistent right ventricular dilation and abnormal ventricular septal motion, but this may not be associated with a functional deficit.190 Atrial arrhythmias and ventricular dysfunction may occur after late repairs as a result of chronic volume overload. Delayed defect closure may be a risk factor for the rare development of pulmonary hypertension or pulmonary vascular disease later in life due to the abnormally increased pulmonary blood flow.191
Transcatheter approaches are an alternative to surgery for closure of secundum ASDs in selected children, with excellent success rates for relatively small communications (see Chapter 20).192–194 Complications are rare and may be associated with the intervention or occur at a later time.195
After surgical closure of ostium primum defects, morbidity manifests primarily as mitral valve dysfunction (e.g., mitral regurgitation) and left ventricular outflow tract obstruction.196–200 In most children, outcomes are favorable after repair at an early age. After closure of sinus venosus defects, potential problems include pulmonary venous obstruction and loss of sinus node function.201–203 Repair of coronary sinus defects consists of patch closure of the atrial communication at the mouth of the coronary sinus. This leaves a small right-to-left shunt as deoxygenated blood from the coronary sinus continues to drain directly into the left atrium.204 If a connection between the left superior vena cava and left atrial connection persists, a variety of surgical approaches may allow redirection of the abnormal systemic venous return. For most children with atrial communications, significant postoperative sequelae are unlikely to occur, and outcomes are generally good. In most cases, no major repercussion from the repaired defect should be expected for future anesthesia care.
Ventricular Septal Defects
Anatomy and Pathophysiology
Ventricular septal defects (VSDs) are the most common of all congenital cardiac anomalies, comprising 30% to 60% of CHD (excluding a bicuspid aortic valve) and occurring in 2 to 6 cases per 1000 live births (see Fig. 15-2, A).205 VSDs can be found in isolation or in the context of other structural malformations. Large defects require early attention for symptoms related to congestive heart failure or pulmonary hypertension. VSDs have a greater rate of spontaneous closure in childhood—about 75% closure by 6 months to 1 year.206,207
Various classification schemes have been proposed for VSDs based on their anatomic location, size, restrictive or nonrestrictive nature, and hemodynamic significance.208,209 The following scheme categorizes defects as four major morphologic types based on their anatomic location. In some cases, the boundaries of a defect may extend beyond the margin of a particular region of the ventricular septum into another.
1. Perimembranous defects (most common type) are located in the membranous region, under the septal leaflet of the tricuspid valve and just below the level of the aortic valve. They are frequently associated with redundant septal tricuspid valve tissue (e.g., aneurysm) that may limit shunting or eventually result in complete defect closure.
2. Muscular defects are located anywhere within the trabecular or muscle-bound component of the ventricular septum. Multiple defects give the appearance of a “Swiss cheese” septum, which complicates surgical closure.
3. Doubly committed, subarterial or supracristal defects are found within the region of the subpulmonary infundibulum. They may be associated with aortic valve herniation or prolapse into the defect and aortic regurgitation.210
4. Inlet defects are located in the posterior aspect of the ventricular septum close to the atrioventricular valves. Associated anomalies of the atrioventricular valves frequently coexist.
Small defect: The pulmonary-to-systemic systolic pressure ratio is less than 0.3, and the 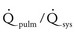 is less than 1.4. The defect causes negligible to minimal hemodynamic changes. Normal right ventricular systolic pressure, pulmonary vascular resistance, and left ventricular size are typically found.
is less than 1.4. The defect causes negligible to minimal hemodynamic changes. Normal right ventricular systolic pressure, pulmonary vascular resistance, and left ventricular size are typically found.
Moderate defect: The systolic pressure ratio is greater than 0.3, and the 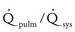 is 1.4 to 2.2. The defect may be associated with volume overload and congestive symptoms. Some degree of left atrial and left ventricular dilation exists, as well as increased pulmonary artery pressures.
is 1.4 to 2.2. The defect may be associated with volume overload and congestive symptoms. Some degree of left atrial and left ventricular dilation exists, as well as increased pulmonary artery pressures.
Large defect: The systolic pressure ratio is greater than 0.3, and the 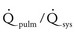 is greater than 2.2. The defect is associated with significant symptoms (e.g., failure to thrive, congestive heart failure). Cardiomegaly and increased pulmonary vascularity are frequent findings.
is greater than 2.2. The defect is associated with significant symptoms (e.g., failure to thrive, congestive heart failure). Cardiomegaly and increased pulmonary vascularity are frequent findings.
Treatment Options, Residua, Sequelae, and Long-Term Outcomes
Surgical closure of VSDs early in childhood results in excellent outcomes, usually without sequelae.211–213 Surgical intervention in older children may lead to reduced left ventricular function and increased left ventricular mass.214 Small communications, although regarded as hemodynamically insignificant, may not be benign. This has led to ongoing controversy regarding the need for definitive intervention. Several young children with moderate defects remain relatively asymptomatic until later life, when gradual decompensation may ensue related to increased end-diastolic volume and ventricular dilation. In these children, defect closure is indicated if the magnitude of the increase in pulmonary vascular resistance is not prohibitive, which is rarely the case. Severely increased pulmonary vascular resistance (more than 7 Wood units per m2) augments the risks associated with the surgical procedure, and it may not return to normal levels after the intervention.215 If postoperative pulmonary hypertension persists, the prognosis is unfavorable, with the potential for eventual right ventricular failure.216 Development of Eisenmenger syndrome (i.e., pulmonary vascular obstructive disease and reversal in the direction of the ventricular level shunt) has become rare due to early recognition and management of children with these defects.
Postoperative sequelae after VSD closure include residual or, less commonly, recurrent defects, arrhythmias or other conduction system disturbances, subaortic obstruction, and valvar regurgitation.217 Although surgical closure is considered the gold standard, transcatheter closure by device placement is feasible for selected muscular and postoperative residual defects defects.218,219 Early data demonstrate excellent closure rates with reduced rates of complications220 (see also Chapter 20). Limited experience and follow-up are available with catheter-based interventions for closure of membranous communications.221–224
Atrioventricular Septal Defects
Anatomy and Pathophysiology
Atrioventricular septal defects (AVSDs), also known as atrioventricular canal defects or endocardial cushion defects, result in deficiency of the atrioventricular septum and altered formation of the atrioventricular valves (see Fig. 15-2, B).225 These rare defects comprise only 4% of CHD cases, although they have a prevalence among patients with Down syndrome of 25%. AVSDs can be classified as follows:
1. The complete form (i.e., common atrioventricular canal defect) consists of an ostium primum defect, an interventricular communication at the superior aspect of the inlet or posterior muscular septum, and a common atrioventricular valve. They are frequently associated with various degrees of atrioventricular valve regurgitation.
2. The partial form (i.e., incomplete form) is characterized by an ostium primum ASD accompanied by a cleft or commissure in the left-sided atrioventricular valve. Two functionally distinct atrioventricular valvar orifices are usually identified (see “Atrial Septal Defects”).
3. The transitional or intermediate form is a combination of a partial AVSD with a small, interventricular communication and two distinct atrioventricular valve components.
Treatment Options, Residua, Sequelae, and Long-Term Outcomes
The surgical approach for complete defects has evolved from a two-stage intervention (i.e., initial pulmonary artery banding to limit pulmonary blood flow and subsequent complete repair) to a single strategy of primary repair in infancy. For a complete defect, this consists of partition of the common atrioventricular valve, patch closure of the intracardiac communications, and closure of the left-sided valvar cleft. The long-term outlook after repair is generally good, with a small likelihood of residual dysfunction.226–228
Postoperative problems include left atrioventricular valve regurgitation or stenosis, residual intracardiac shunting, atrioventricular block, and subaortic obstruction.199,200 Occasionally, pulmonary hypertension persists or develops postoperatively; it is more likely in children with Down syndrome. In the remote past, uncorrected defects resulted in Eisenmenger physiology, accounting for significant late morbidity and early death.229 Considerations for noncardiac surgery in children after surgical repair include the residual effects of the prior ventricular volume load, the status of the atrioventricular valve, and patency of the left ventricular outflow tract.
Right Ventricular Outflow Tract Obstructions
Anatomy and Pathophysiology
Pulmonary valve stenosis is the most common pathology among children with right ventricular outflow tract obstruction.230 Other lesions that result in obstruction to pulmonary blood flow include infundibular stenosis, muscle bundles within the body of the right ventricle, and anatomic alterations in the pulmonary arterial bed. These pathologies may be found in isolation or occur as part of more complex malformations. Such is the case in tetralogy of Fallot (discussed later), in which multiple anatomic levels of right ventricular outflow tract obstruction are typically encountered (see Fig. 15-5).
Treatment Options, Residua, Sequelae, and Long-Term Outcomes
Percutaneous balloon valvuloplasty is very effective and currently considered the treatment of choice for valvar pulmonary stenosis, replacing surgical valvotomy in most cases. Outcomes are excellent, and long-term issues are rare.231–233 Dysplastic valves have a less favorable response to catheter-based interventions, and affected children are more likely to require surgery. Indications for repeat intervention include residual right ventricular outflow tract obstruction and progressive pulmonary regurgitation.234
Left Ventricular Outflow Tract Obstructions
Anatomy and Pathophysiology
Left ventricular outflow tract obstruction may occur at the level of the aortic valve, supravalvar region, or subvalvar region. It may take place in isolation or as part of complex cardiovascular disease. A bicuspid aortic valve is the most common of all congenital cardiac anomalies, occurring in approximately 2% of the general population.235 Although it may not necessarily imply valvar stenosis, this abnormality can be associated with progressive obstruction or regurgitation. A bicuspid valve may be found in asymptomatic individuals or within the context of severe left heart obstruction. The prevalence of coexistent defects is relatively large and frequently includes patent ductus arteriosus, VSD, aortic coarctation, and other abnormalities of the aorta and its branches.
In supravalvar aortic stenosis, the narrowing typically occurs at the sinotubular junction. The coronary arteries arise proximal to the area of obstruction and are subjected to increased systolic pressures equal to that of the left ventricle. The arteriopathy found in many of these children may involve the origin of the coronary arteries or other systemic and pulmonary vessels.236 This malformation may occur as part of Williams syndrome, which is characterized by elfin facies, mental retardation, idiopathic hypercalcemia, and other features. Several reports have described unexpected complications in these children, including death during anesthesia care.237–240 Affected children should be considered at increased risk for any procedure.
Hypoplastic left heart syndrome (HLHS) represents an extreme form of left ventricular outflow tract obstruction (see Fig. 15-10). It encompasses a constellation of malformations, affecting left-sided cardiac structures (e.g., mitral and aortic valves, aorta, arch) (see “Single Ventricle”).
Common features of the anomalies that result in obstruction to left ventricular output include a pressure gradient across the involved region, increased left ventricular systolic pressure, increased myocardial force, and left ventricular wall stress. With chronic obstruction, the hypertrophied myocardium is at risk for subendocardial ischemia as a consequence of an imbalance in the ratio of myocardial oxygen supply and demand. Factors such as increases in left ventricular afterload, inadequate hypertrophic remodeling, and decreases in myocardial systolic or diastolic performance may compromise stroke volume and contribute to cardiac dysfunction and heart failure in this setting.241 These issues are relevant for children with more than mild obstruction, and they influence anesthesia care during noncardiac surgery or other interventions.
Treatment Options, Residua, Sequelae, and Long-Term Outcomes
Individuals with a bicuspid aortic valve may remain asymptomatic for many years but are at risk for aortic stenosis or regurgitation and concomitant hemodynamic alterations. Some of those requiring surgical intervention during childhood undergo reoperation for recurrent stenosis or progressive regurgitation in the next 25 years.242 Percutaneous balloon valvuloplasty may be a treatment option for critical or severe aortic valve disease.243 Surgical alternatives include valvotomy, mechanical or bioprosthetic valve placement, and root replacement with homograft or autograft material. In the Ross operation, the native, diseased root is replaced by a pulmonary autograft, and an extracardiac conduit establishes continuity between the right ventricle and main pulmonary artery.244 Repeat intervention for eventual failure of the right ventricular conduit is anticipated in these children.245 In addition to surveillance of the right ventricular outflow tract, monitoring for aortic root dilation and concomitant regurgitation is an important component of follow-up.246,247
Management of discrete subaortic stenosis remains a challenge, and the timing of surgery is controversial.248 Postoperative complications include residual or recurrent obstruction and progressive aortic regurgitation. For severe supravalvar obstruction, surgical intervention is recommended, and it results in adequate relief of the obstruction in most cases.249
Myocardial fibrosis and ventricular dysfunction may be a feature of severe aortic outflow obstruction in infancy. Although adequate relief of the obstruction results in significant clinical improvement, abolition of congestive heart failure, and myocardial remodeling in most children, ventricular hypertrophy or dilation persists along with various degrees of systolic or diastolic impairment in many. Other problems include myocardial ischemia, ventricular failure, and risk of sudden death.250,251 Important concerns related to anesthesia and surgery are the potentially limited left ventricular functional reserve and alterations of the fine balance between myocardial oxygen supply and demand. Maintenance of coronary perfusion and ventricular contractile function is key in the care of these children. Pharmacologic agents with vasoactive and inotropic properties should be readily available during anesthesia care.
Patent Ductus Arteriosus
Anatomy and Pathophysiology
The ductus arteriosus is a vascular structure connecting the pulmonary trunk and thoracic aorta (see Fig. 15-4). It enables right ventricular output into the descending aorta during fetal life, within the context of typically increased pulmonary vascular resistance. Persistent patent ductus arteriosus (PDA) may be an isolated finding or associated with other forms of heart disease. Prematurity is an important risk factor.
Treatment Options, Residua, Sequelae, and Long-Term Outcomes
Children with a tiny or small PDA have a normal life expectancy.252 Those with hemodynamically significant communications eventually develop symptoms related to left ventricular volume overload. In some cases, this predisposes them to moderate or severe pulmonary hypertension. Although unlikely in the current era, in the past, the long-standing, high-pressure, and high-flow states associated with a moderate or large communication resulted in Eisenmenger syndrome in some children.
Ductal closure can be performed by surgical ligation or division. This is the favored approach in preterm infants and those with large communications.253 Percutaneous catheter occlusion can also be accomplished with a good success rate254 (see also Chapter 20). Video-assisted thoracoscopic surgery has been used for ductal ligation.255,256 Regardless of the approach, interruption of this vascular structure is rarely associated with long-term issues. Children can expect a normal cardiovascular reserve and should be managed accordingly during future anesthesia care.
Coarctation of the Aorta
Anatomy and Pathophysiology
Coarctation of the aorta is characterized by narrowing of the aortic lumen in the thoracic region. The constriction may be discrete or diffuse. In infants, a long, narrowed aortic segment often is associated with hypoplasia of the transverse arch and aortic isthmus, in which case other structural cardiac malformations may also exist.257 Associated defects include a bicuspid aortic valve, VSD, mitral valve abnormalities, and other types of left-sided obstructive lesions.
Treatment Options, Residua, Sequelae, and Long-Term Outcomes
Symptoms associated with severe aortic arch obstruction or concomitant cardiovascular pathology lead to early intervention in some children.258 Alterations in ventricular systolic function associated with a neonatal presentation usually resolve after relief of the obstruction. Systemic hypertension and a residual gradient that exceeds 25 to 30 mm Hg are regarded as indications for repeat intervention. Various catheter-based and surgical approaches have been applied to the management of this lesion; each has advantages and disadvantages259 (see also Chapter 20).
Repair at an early age is advocated in view of reduced surgical risks for the younger age group, and early repair minimizes late morbidity.260 Long-term issues include systemic hypertension (independent of the hemodynamic result) and residual or recurrent aortic arch obstruction.261 Left ventricular hypertrophy may persist in some children after repair, particularly in those undergoing interventions later in childhood. Abnormalities in diastolic ventricular function have been reported after successful repair.262 Catheter techniques (i.e., balloon angioplasty with and without stent implantation) have been effective in relieving the obstruction and normalizing blood pressure.263 This approach may be used as primary therapy or to address residual or recurrent disease.264 Aortic aneurysms can occur around the area of coarctation or elsewhere in the aorta after surgical intervention or balloon angioplasty. Additional long-term problems result from coexistent defects, such as bicuspid aortic valve and premature development of coronary artery disease. Aortic coarctation has been associated with cerebral aneurysms.
Tetralogy of Fallot
Anatomy and Pathophysiology
Tetralogy of Fallot (TOF) is the most common cyanotic cardiac lesion (see Fig. 15-5).265 This malformation is characterized by right ventricular outflow tract obstruction, an interventricular communication, right ventricular hypertrophy, and aortic override. There is considerable variation in the severity of the disease, accounting for the spectrum of clinical manifestations. The subpulmonary obstruction arises from anterior deviation of the infundibular septum and may have dynamic and fixed components.266 Pulmonary valve stenosis almost invariably exists, and the main pulmonary artery and distal branches often demonstrate various degrees of hypoplasia. The limitation of pulmonary blood flow and magnitude of ventricular level right-to-left shunting account for the degree of cyanosis.
Treatment Options, Residua, Sequelae, and Long-Term Outcomes
The surgical management of TOF has evolved from a strategy of a staged approach with initial palliation using a systemic-to-pulmonary shunt to a single-stage, definitive repair in infants. Ongoing controversy exists about the favored approach in the neonate or very young infant in need of surgical therapy.267,268 In selected cases, percutaneous balloon pulmonary valvuloplasty may be performed as a palliative, temporizing measure.269 The definitive repair of TOF, although a successful operation enabling most children to be free of symptoms, may be associated with significant postoperative residua.270,271 Volume loads may arise from pulmonary regurgitation, residual shunts, and the presence of aortopulmonary collaterals. Ventricular pressure loads, however, may result from residual or recurrent right ventricular outflow tract or obstruction to the pulmonary artery bed. This situation is associated with right ventricular hypertension, myocardial hypertrophy, and reduced ventricular compliance.
Conditions that may require repeat intervention include pulmonary regurgitation of significant severity, residual or recurrent pulmonary outflow tract obstruction, and residual, hemodynamically significant intracardiac shunts. Catheter-based procedures may be effective in the management of obstruction of the pulmonary vasculature, and they have been applied to rehabilitate the vascular tree in cases of significant underdevelopment. Children who have undergone right ventricle–to–pulmonary artery reconstruction by means of placement of an extracardiac conduit eventually develop conduit failure (i.e., stenosis or regurgitation) requiring reoperation.272 Aortic root dilation can lead to increasing degrees of regurgitation and the need for surgical intervention.
On late follow-up, pulmonary regurgitation has been identified as a major cause of morbidity, and it may result in progressive right ventricular dysfunction due to significant volume overload, ventricular arrhythmias with their associated disabilities, and even death. In recognition of the long-term morbidity linked to severe pulmonary regurgitation, the surgical strategy for this defect has undergone reappraisal and modification over the years.273 A current method uses a transatrial approach for closure of the VSD, minimizing the size of an infundibular incision (if one is required) and avoiding or limiting the size of the transannular patch.274,275
Postoperatively, a subset of children develops a pattern that is characterized by right ventricular diastolic noncompliance, which is known as restrictive right ventricular physiology. It is associated with a reduced likelihood of progressive pulmonary regurgitation and right ventricular dilation. In these children, the right ventricle operates at a greater end-diastolic pressure, and the children demonstrate superior exercise performance in addition to a reduced likelihood of developing ventricular rhythm abnormalities.276
D-Transposition of the Great Arteries
Anatomy and Pathophysiology
In d-transposition of the great arteries (d-TGA), the aorta arises from the anatomic right ventricle, and the pulmonary artery arises from the left ventricle (see Fig. 15-6). This anomaly accounts for the most common cause of cyanotic heart disease in the neonatal period. Associated defects include VSDs, left ventricular outflow tract obstruction, and coronary artery anomalies.
Treatment Options, Residua, Sequelae, and Long-Term Outcomes
Several decades ago, the approach to d-TGA consisted of an atrial baffle (i.e., atrial switch) or redirection procedure (i.e., Mustard or Senning operations). Physiologic correction was accomplished by allowing systemic venous blood to drain into the left ventricle and pulmonary artery while pulmonary venous blood was rerouted through the tricuspid valve into the right ventricle and aorta. The right ventricle remained as the chamber ejecting against systemic afterload. These procedures provided relief of cyanosis and reasonably good survival.277 In the long term, however, this approach led to complications such as sinus node dysfunction and atrial rhythm disturbances.278 Progressive right ventricular dilation, tricuspid (i.e., systemic atrioventricular valve) annular dilation, associated regurgitation, and eventual right ventricular dysfunction or failure were causes of major morbidity.279–281 In addition to the rhythm abnormalities and conduction defects, this problem was thought to account for sudden death in some individuals later in life. Other problems included progressive obstruction of venous pathways and intracardiac shunting through atrial baffle leaks. Abnormal right and left ventricular responses to exercise have also been documented in these patients.282
The arterial switch operation (i.e., Jatene procedure) is the standard surgical approach in neonates with d-TGA. The repair establishes a normal, concordant relationship between the ventricles and their respective great arteries, achieving anatomic correction. The procedure involves transection of the arterial trunks above the level of the semilunar valves, anastomotic connections to their appropriate outflows, translocation of the coronary arteries to the neoaortic root, and closure of any intracardiac communications (see Fig. 15-7, A-E). Normal physiology is restored, enabling the left ventricle to function as the systemic pump. This procedure can be performed with good results, and long-term outcomes usually are very favorable.283–286 Potential postoperative problems include supravalvar pulmonary or aortic obstruction. Neoaortic root dilation and aortic regurgitation may be identified on follow-up. Ventricular function is normal in most cases.287
Anesthetic management of most children after the arterial switch operation should be the same as in those without structural or functional abnormalities. However, there is some concern about coronary complications in these children that may not be evident clinically or identified by routine surveillance methods. Investigations have demonstrated postoperative regional left ventricular wall motion abnormalities, evidence of myocardial perfusion defects, and pathologic changes in the coronary vasculature, suggesting a risk for coronary insufficiency.288–292
Congenitally Corrected Transposition of the Great Arteries
Anatomy and Pathophysiology
Cyanosis is absent because the circulations are physiologically corrected. The anatomic right ventricle functions as the systemic pump. Associated defects are frequently present and include pulmonary outflow tract obstruction, a ventricular communication, and tricuspid (left-sided) abnormalities. In some individuals, this lesion may remain undetected until the onset of arrhythmias or syncope due to complete atrioventricular block or the effects of concomitant pathology.293
Treatment Options, Residua, Sequelae, and Long-Term Outcomes
Without associated defects, children with corrected transposition may do well for many years. Development of complete atrioventricular block is common with increasing age.294 Selected children, particularly those at a young age or with coexistent defects that maintain left ventricular pressure at systemic levels, may be suitable candidates for a surgical intervention that restores the left ventricle as the systemic chamber.295 This complex repair, known as the double-switch operation or a variation thereof, combines redirection of the systemic and pulmonary venous flows in an atrial baffle procedure with the arterial switch operation. This strategy, however, may not affect mortality compared with conservative management.296
Issues that require long-term surveillance in children with congenitally corrected transposition include right ventricular performance and tricuspid valve competency.297 The overall long-term survival of individuals with this condition is substantially reduced compared with age-matched controls.298,299
Truncus Arteriosus
Anatomy and Pathophysiology
Truncus arteriosus is characterized by a single arterial trunk that gives rise to the aorta, pulmonary root, and coronary arteries (see Fig. 15-8). A ventricular communication usually exists underneath the single arterial root or truncal valve. Various anatomic types are identified according to the origin of the pulmonary arteries from the arterial trunk.300,301 Associated pathology includes a right aortic arch, aortic arch interruption, abnormalities of the truncal valve (e.g., abnormal number of cusps, stenosis, regurgitation), and coronary artery anomalies. Approximately one third of children with truncus arteriosus have DiGeorge syndrome (see Chapter 14).
Clinical features of the neonate with this defect largely depend on the status of the pulmonary vasculature. If the resistance is increased, the infant is well compensated. The normal decrease in pulmonary vascular resistance leads to symptoms related to pulmonary overcirculation and congestive heart failure, accounting for the need for surgical intervention early in life. Truncus arteriosus is one of the structural malformations associated with a significant risk for adverse events before correction, because balancing the pulmonary and vascular resistances may be quite challenging.302 The physiology that characterizes a reduced pulmonary vascular resistance and a significant runoff setting is that of an increased arterial oxygen saturation, reduced diastolic arterial pressures (potentially leading to myocardial ischemia), systemic hypotension, impaired cardiac output, and hypoperfusion of distal beds.
Treatment Options, Residua, Sequelae, and Long-Term Outcomes
Surgery for truncus arteriosus consists of detaching the main pulmonary artery segment from the truncal root, repairing the ensuing aortic wall defect, closing the VSD to allow left ventricular output through the arterial root, and placing an extracardiac right ventricle–to–pulmonary artery conduit. Alternative approaches to establishing this continuity have been performed without the use of conduits.303
Neonates undergoing truncus arteriosus repair have excellent survival rates.304–307 Late complications include conduit failure, residual or recurrent pulmonary artery obstruction, and truncal valve problems. Truncal valve dysfunction may require repair or replacement. The main issues of concern are the status of the right ventricle–to–pulmonary artery conduit and truncal root, consequences related to semilunar valve problems, and biventricular function.
Ebstein Anomaly
Anatomy and Pathophysiology
The classic findings in Ebstein anomaly for the tricuspid valve include a large sail-like anterior leaflet and apically displaced septal and posterior leaflets.308,309 This configuration commonly results in an atrialized portion of the right ventricle and tricuspid regurgitation. Some degree of right ventricular dysplasia is common. An interatrial communication is a frequent finding, and it may produce right-to-left shunting and clinical cyanosis. The spectrum of disease ranges from minimal or no symptoms to intractable congestive heart failure.310 A neonatal presentation implies a major clinical problem and usually portends a poor prognosis. Symptoms in older children include cyanosis, palpitations, dyspnea, and exercise intolerance. Initial symptoms may be related to supraventricular tachycardia.
Treatment Options, Residua, Sequelae, and Long-Term Outcomes
Children with Ebstein anomaly may require only conservative management and follow-up. In most cases, however, surgery is indicated for tricuspid regurgitation, closure of interatrial communications, or other associated problems. Procedures to ablate arrhythmias may be indicated. In contrast to adults, children are less likely to require valve replacement.311 A cavopulmonary or Glenn connection may be performed in some cases as part of a so-called one and a half ventricle approach to limit the right-sided volume load associated with severe tricuspid valve regurgitation.312,313 One report indicated good functional outcomes and long-term survival after surgery for Ebstein anomaly.314 Atrial arrhythmias (including Wolf-Parkinson-White syndrome) are common before and after surgery.
Interrupted Aortic Arch
Anatomy and Pathophysiology
Interrupted aortic arch is an uncommon malformation characterized by discontinuity between the ascending and descending thoracic aorta (see Fig. 15-14). Ductal patency is essential for systemic perfusion beyond the area of interruption. This anomaly is classified in terms of the site of interruption. It is type A if it occurs distal to the left subclavian artery, type B if between the left carotid and left subclavian arteries, and type C if between the carotid arteries. Type B interruption is the most common variant, followed in frequency by types A and C.
Treatment Options, Residua, Sequelae, and Long-Term Outcomes
Surgical intervention is necessary for interrupted aortic arch during the first few days of life. The goal is to establish aortic arch continuity and to address coexistent defects. The current approach favors a one-stage repair.315–317 Survival in uncomplicated cases is excellent.318 Problems after repair mainly involve the left ventricular outflow tract.319 Reoperation may be required and in some cases may consist of left ventricular outflow tract enlargement (i.e., Konno procedure). Eventual aortic root or valve replacement or a Ross-Konno procedure may be necessary.
Congenital Anomalies of the Coronary Arteries
Anatomy and Pathophysiology
Congenital anomalies of the coronary arteries include an abnormal origin of one of the main branches, aberrant vascular course, or pathologic communications that involve the coronary circulation.320,321 The most common anomalies detected during childhood include anomalous origin of the left main coronary artery from the pulmonary artery (ALCAPA), coronary artery–to–pulmonary artery fistulas, and coronary cameral fistulas (i.e., connection between a coronary artery and cardiac chamber). Although rare, anomalous origin of a coronary artery from the incorrect (contralateral) sinus of Valsalva may occur in asymptomatic children and adolescents.322 In some instances, a major coronary artery courses between the great arteries. This situation may be associated with compromised coronary blood flow and myocardial ischemia during exercise, presumably related to dilation of the arterial roots to accommodate the increased stroke volume.
Single Ventricle
Treatment Options, Residua, Sequelae, and Long-Term Outcomes
Norwood Procedure
In this setting, maneuvers that increase pulmonary vascular resistance are indicated to improve hemodynamics. Measures employed include limiting inspired oxygen concentrations, the administration of subambient gas mixtures, and increasing the partial pressure of carbon dioxide (Pco2) by hypoventilation or the administration of inspired carbon dioxide. A comparison of hypoxia versus hypercarbia in infants with HLHS under conditions of anesthesia and paralysis demonstrated that although 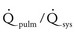 decreases in both conditions, inspired CO2 was more effective than hypoxic gas mixtures at increasing parameters associated with improved systemic output.323 The administration of inspired CO2 may be favored over hypoventilation as a means of increasing pulmonary vascular resistance and improving the overall clinical condition.
decreases in both conditions, inspired CO2 was more effective than hypoxic gas mixtures at increasing parameters associated with improved systemic output.323 The administration of inspired CO2 may be favored over hypoventilation as a means of increasing pulmonary vascular resistance and improving the overall clinical condition.
The Norwood procedure is considered the first step among the three stages in the palliative pathway for infants with HLHS or similar cardiac malformations.324 The intervention, also referred to as stage I single-ventricle palliation or reconstruction, is typically performed within the first few days of life. Surgery consists of aortic reconstruction or creation of a neoaorta, establishing continuity between the native main pulmonary artery and aortic arch to provide for unobstructed systemic outflow from the right ventricle; the creation of an unrestricted atrial communication by means of an atrial septectomy; and establishing a source of pulmonary blood flow (see Fig. 15-11). For many years, pulmonary blood flow was established by fashioning a modified Blalock-Taussig shunt. Currently, a right ventricle–to–pulmonary artery conduit (i.e., Sano modification and similar variations) is used as an alternative to provide pulmonary blood flow. Although the potential benefits of one approach over the other have been elucidated, additional studies, some with long-term follow-up, are required to provide further information.325–331
Another approach, a hybrid stage I strategy, has been applied to selected neonates. In this procedure, a median sternotomy is performed, and both branched pulmonary arteries are banded. A stent is then delivered across the ductus arteriosus under fluoroscopic guidance,332,333 and the interatrial communication is then enlarged.
Outcomes after the Norwood procedure vary; good results imply operative survival for 85% to 90% of infants.334 Immediate postoperative problems include systemic hypoxemia, decreased myocardial performance, and excessive pulmonary blood flow. Monitoring of mixed venous oxygen saturation and cerebral near-infrared spectroscopy are helpful in balancing the pulmonary and systemic circulations in this setting. Occasionally, aortic arch obstruction occurs, and less commonly, the atrial septum becomes restrictive. Interstage mortality accounts for attrition among Norwood survivors.335 Among infants who have undergone placement of a right ventricle–to–pulmonary artery conduit, stenosis of the conduit associated with progressive cyanosis may account for significant interstage morbidity and often requires intervention or early second-stage palliation.336,337
The anticipated arterial oxygen saturation after stage I surgery is expected to be in the range of 75% to 85%. During perioperative care, blood pressure monitoring should consider the potential presence of a Blalock-Taussig shunt that may compromise ipsilateral subclavian artery flow. In these infants, the right ventricle ejects into the pulmonary and systemic circulations. Although this is a more stable arrangement compared with that before the Norwood procedure, it remains a relatively fragile parallel circulation. These infants display little tolerance to even the most common childhood conditions, and ailments such as dehydration, febrile illnesses, or other stresses that may have catastrophic consequences. Despite these challenges, successful outcomes have been reported during noncardiac surgery for a variety of procedures, including those that may be associated with significant hemodynamic perturbations, such as laparoscopic surgery.338
Glenn Anastomosis or Hemi-Fontan Procedure
A cavopulmonary connection or Glenn procedure (i.e., stage II palliation) consists of the creation of a direct anastomosis between the superior vena cava and one of the pulmonary artery branches (see Fig. 15-12). This is considered an intermediary step in the sequential diversion of the systemic venous blood into the pulmonary vasculature in children with single-ventricle physiology. The original or classic operation consisted of an end-to-end anastomosis of the transected superior vena cava onto a disconnected right pulmonary artery, and it was complicated by increasing desaturation that was attributed in many cases to the development of pulmonary arteriovenous fistulae.339 The current approach is to attach the superior vena cava to the right pulmonary artery in end-to-side fashion, preserving pulmonary artery continuity (i.e., bidirectional cavopulmonary anastomosis [BCPA] or bidirectional Glenn connection). Depending on the specific anatomic abnormalities, right, left, or bilateral BCPAs may be indicated.
The second-stage intervention requires a reduced pulmonary vascular resistance because of the passive nature of the pulmonary blood flow. This approach provides adequate palliation to a significant number of infants at an early age while conferring favorable hemodynamic benefits.340 Diverting a portion of the systemic venous return directly into the pulmonary bed reduces the output requirements of the single ventricle while decreasing the ventricular volume load and myocardial work.
One study demonstrated a 12% rate of interstage attrition between BCPA and the Fontan procedure in children with HLHS palliation.341 Risk factors included tricuspid valve regurgitation and low weight at the time of the BCPA. These factors may affect the anesthesia-related risks for noncardiac procedures required between these two stages of palliation.
Considerations include the passive nature of the pulmonary blood flow, the importance of maintaining adequate intravascular volume (i.e., minimal fasting) to enhance pulmonary blood flow, and limiting significant increases in pulmonary vascular tone. Pulmonary blood flow and systemic arterial oxygenation are significantly influenced by the interplay between pulmonary artery pressure (i.e., equal to the pressure in the superior vena cava), pulmonary venous pressure, and pulmonary vascular resistance. The expected systemic arterial oxygen saturation ranges between 75% and 85%. Although factors that increase pulmonary vascular resistance may negatively influence pulmonary blood flow, the observation has been made that early after BCPA, moderate hypercapnia with respiratory acidosis improves arterial oxygenation and reduces oxygen consumption, enhancing overall oxygen transport in these children.342 Hyperventilation can decrease cerebral oxygenation and should be avoided.343 Postoperative issues include hypoxemia related to the development of collateral vessels that bypass the pulmonary circulation, atrioventricular valve regurgitation, and impaired ventricular function.
Fontan Procedure
The Fontan procedure is the final step (i.e., stage III reconstruction) in the separation of the pulmonary and systemic circulations in children with a functional single ventricle. This intervention allows passive blood flow from the inferior vena cava into the pulmonary vascular bed while bypassing the heart and achieves a circulation in series (see Fig. 15-13). A fenestration, or communication, between the systemic venous pathway and physiologic common atrium may be created in some cases. It allows right-to-left shunting, which provides cardiac output that is not solely dependent on pulmonary blood flow. It also alleviates potential problems associated with chronically increased systemic venous pressures. Common features of the numerous Fontan modifications are separation of the pulmonary and systemic circulations and relief of hypoxemia.344 Pulmonary blood flow occurs without an intervening ventricular chamber. It depends critically on the transpulmonary pressure gradient (or driving pressure across the pulmonary bed) and is influenced by pulmonary vascular resistance. This blood flow determines cardiac output, emphasizing the importance of adequate hydration and maintenance of central venous pressure.
Several anatomic and hemodynamic variables influence Fontan physiology. Critical factors include unobstructed systemic venous return, status of the pulmonary vasculature, reduced intrathoracic pressures, systemic atrioventricular valve competency, systemic ventricular function, unobstructed systemic outflow, and atrial contribution to ventricular filling.345 Long-term problems are related to sinus node dysfunction, loss of atrioventricular synchrony, atrial arrhythmias, atrioventricular valve regurgitation, ventricular dysfunction, venous pathway obstruction or thrombotic complications, and symptoms resulting from a chronic reduced cardiac output state.346 Long-standing increases in systemic venous pressures in children after the Fontan procedure can produce hepatic dysfunction, coagulation defects, protein-losing enteropathy, and rhythm disturbances. The quality of life after the Fontan operation may be compromised by a late decline in functional status, reoperations, arrhythmias, and thromboembolic events.347–352 Decreased exercise tolerance in most children represents limited cardiopulmonary reserve, which manifests as an inability to increase cardiac output to meet the metabolic demands associated with increased work. In some cases, surgical revision to a more hemodynamically favorable Fontan modification is indicated.353,354
Several considerations are important in the perioperative care of children with Fontan circulation.355–357 Even mild alterations in factors that influence cardiac output, such as ventricular preload, atrioventricular synchrony, contractile function, afterload, and stress response, may adversely impact hemodynamics. Ensuring the adequacy of hydration, preserving sinus rhythm, and limiting the stress response are key goals. Maintenance of adequate ventricular function may require the administration of inotropic or vasoactive agents perioperatively. Because systemic venous pressures are typically increased, the potential for bleeding and its effects on ventricular filling should be considered. The likelihood of blood loss with ensuing hemodynamic instability is exacerbated by the coagulation defects in these children.358 The potential for end-organ dysfunction related to chronically decreased organ perfusion, particularly in the renal and hepatic systems, should be considered, and problems may require interventions to minimize perioperative morbidity. Drugs or devices appropriate for cardiac rhythm or arrhythmia management should be accessible.
Baum VC, Barton DM, Gutgesell HP. Influence of congenital heart disease on mortality after noncardiac surgery in hospitalized children. Pediatrics. 2000;105:332–335.
Carmosino MJ, Friesen RH, Doran A, Ivy DD. Perioperative complications in children with pulmonary hypertension undergoing noncardiac surgery or cardiac catheterization. Anesth Analg. 2007;104:521–527.
Coté CJ, Wax DF, Jennings MA, et al. End-tidal carbon dioxide monitoring in children with congenital heart disease during sedation for cardiac catheterization by nonanesthesiologists. Pediatr Anesth. 2007;17:661–666.
Galantowicz M, Cheatham JP, Phillips A, et al. Hybrid approach for hypoplastic left heart syndrome: intermediate results after the learning curve. Ann Thorac Surg. 2008;85:2063–2070. discussion 2070-71
Ohye RG, Sleeper LA, Mahony L, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–1992.
Ramamoorthy C, Haberkern CM, Bhananker SM, et al. Anesthesia-related cardiac arrest in children with heart disease: data from the Pediatric Perioperative Cardiac Arrest (POCA) registry. Anesth Analg. 2010;110:1376–1382.
Williams GD, Maan H, Ramamoorthy C, et al. Perioperative complications in children with pulmonary hypertension undergoing general anesthesia with ketamine. Paediatr Anaesth. 2010;20:28–37.
1 Wallgren EI, Landtman B, Rapola J. Extracardiac malformations associated with congenital heart disease. Eur J Cardiol. 1978;7:15–24.
2 Ferencz C, Rubin JD, McCarter RJ, et al. Cardiac and noncardiac malformations: observations in a population-based study. Teratology. 1987;35:367–378.
3 Pradat P. Noncardiac malformations at major congenital heart defects. Pediatr Cardiol. 1997;18:11–18.
4 Tennstedt C, Chaoui R, Korner H, Dietel M. Spectrum of congenital heart defects and extracardiac malformations associated with chromosomal abnormalities: results of a seven year necropsy study. Heart. 1999;82:34–39.
5 Gucer S, Ince T, Kale G, et al. Noncardiac malformations in congenital heart disease: a retrospective analysis of 305 pediatric autopsies. Turk J Pediatr. 2005;47:159–166.
6 Greenwood RD, Rosenthal A, Parisi L, et al. Extracardiac abnormalities in infants with congenital heart disease. Pediatrics. 1975;55:485–492.
7 Hoffman JI, Christianson R. Congenital heart disease in a cohort of 19,502 births with long-term follow-up. Am J Cardiol. 1978;42:641–647.
8 Pearson GD, Neill CA, Beittel TM, Kidd L. Determinants of outcome in hospitalized infants with congenital heart disease. Am J Cardiol. 1991;68:1055–1059.
9 Massin MM, Astadicko I, Dessy LH. Noncardiac comorbidities of congenital heart disease in children. Acta Paediatr. 2007;96:753–755.
10 Scallan M. Congenital heart disease: Anesthetic considerations in noncardiac surgery. Middle East J Anesthesiol. 1993;12:205–209.
11 Litman RS. Anesthetic considerations for children with congenital heart disease undergoing noncardiac surgery. Anesth Clin North Am. 1997;15:93–103.
12 White MC. Approach to managing children with heart disease for noncardiac surgery. Paediatr Anaesth. 2011;21:522–529.
13 Benson DWJ. Changing profile of congenital heart disease. Pediatrics. 1989;83:790–791.
14 Karl HW, Hensley FAJ, Cyran SE, Franked CA, Myers JL. Hypoplastic left heart syndrome: anesthesia for elective noncardiac surgery. Anesthesiology. 1990;72:753–757.
15 Nicolson SC, Steven JM, Kurth CD, Kruclak CP, Jobes DR. Anesthesia for noncardiac surgery in infants with hypoplastic left heart syndrome following hemi-Fontan operation. J Cardiothorac Vasc Anesth. 1994;8:334–336.
16 Torres AJ, DiLiberti J, Pearl RH, et al. Noncardiac surgery in children with hypoplastic left heart syndrome. J Pediatr Surg. 2002;37:1399–1403.
17 Walker SG, Stuth EA. Single-ventricle physiology: perioperative implications. Semin Pediatr Surg. 2004;13:188–202.
18 Somerville J. The physician’s responsibilities: residua and sequelae. J Am Coll Cardiol. 1991;18:325–327.
19 Laussen PC. Anesthetic considerations following surgical repair of congenital heart defects. Prog Anesthesiol. 1994;8:131–148.
20 McGowan FXJ. Perioperative issues in patients with congenital heart disease. IARS Rev Course Lect. 2006:68–75.
21 al Khudhairi D. Anesthetic management of patients with congenital heart disease coming for noncardiac surgery. Middle East J Anesthesiol. 1990;10:585–593.
22 Burrows FA. Anaesthetic management of the child with congenital heart disease for noncardiac surgery. Can J Anaesth. 1992;39:R60–R70.
23 Haselby KA, Moorthy SS. Noncardiac surgery in the patient with congenital heart disease. Semin Pediatr Surg. 1992;1:65–73.
24 Baum VC, Perloff JK. Anesthetic implications of adults with congenital heart disease. Anesth Analg. 1993;76:1342–1358.
25 Baum VC. The adult patient with congenital heart disease. J Cardiothorac Vasc Anesth. 1996;10:261–282.
26 Jonmarker C. Patients with congenital heart malformations for noncardiac surgery. Acta Anaesthesiol Scand Suppl. 1997;110:104–105.
27 Galli KK, Myers LB, Nicolson SC. Anesthesia for adult patients with congenital heart disease undergoing noncardiac surgery. Int Anesthesiol Clin. 2001;39:43–71.
28 Teske DW, Caniano DA. Noncardiac surgery in patients with heart disease. In: Allen HD, Gutgesell HP, Clark EB, Driscoll DJ, eds. Moss and Adams’ heart disease in infants, children, and adolescents including the fetus and young adult. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2001:408–413.
29 Mohindra R, Beebe DS, Belani KG. Anaesthetic management of patients with congenital heart disease presenting for noncardiac surgery. Ann Cardiac Anaesth. 2002;5:15–24.
30 Lovell AGT. Anaesthetic implications of grown-up congenital heart disease. Br J Anaesth. 2004;93:129–139.
31 Chassot PG, Bettex DA. Anesthesia and adult congenital heart disease. J Cardiothorac Vasc Anesth. 2006;20:414–437.
32 Heggie J, Karski J. The anesthesiologist’s role in adults with congenital heart disease. Cardiol Clin. 2006;24:571–585.
33 Sumpelmann R, Osthaus WA. The pediatric cardiac patient presenting for noncardiac surgery. Curr Opin Anaesthesiol. 2007;20:216–220.
34 Strafford MA, Henderson KLH. Anesthetic morbidity in congenital heart disease patients undergoing noncardiac surgery. Anesthesiology. 1991;75:A1056.
35 Warner MA, Lunn RJ, O’Leary PW, Schroeder DR. Outcomes of noncardiac surgical procedures in children and adults with congenital heart disease. Mayo Perioperative Outcomes Group. Mayo Clin Proc. 1998;73:728–734.
36 Coran DL, Rodgers WB, Keane JF, Hall JE, Emans JB. Spinal fusion in patients with congenital heart disease: predictors of outcome. Clin Orthop Relat Res. 1999;364:99–107.
37 Baum VC, Barton DM, Gutgesell HP. Influence of congenital heart disease on mortality after noncardiac surgery in hospitalized children. Pediatrics. 2000;105:332–335.
38 Carmosino MJ, Friesen RH, Doran A, Ivy DD. Perioperative complications in children with pulmonary hypertension undergoing noncardiac surgery or cardiac catheterization. Anesth Analg. 2007;104:521–527.
39 Weiss BM, Hess O. Perioperative cardiovascular evaluation for noncardiac surgery: congenital heart diseases and heart diseases in pregnancy deserve better guidelines. Circulation. 1997;95:530–531.
40 Werner JC, Fripp RR, Whitman V. Evaluation of the pediatric surgical patient with congenital heart disease. Surg Clin North Am. 1983;63:1003–1015.
41 Mossad EB, Joglar J. Preoperative evaluation and preparation. In: Andropoulos DB, Stayer SA, Russell IA, Mosaad EB, eds. Anesthesia for congenital heart disease. 2nd ed. Oxford, UK: Wiley-Blackwell Publishing; 2010:223–243.
42 Tait AR. Anesthetic management of the child with an upper respiratory tract infection. Curr Opin Anaesthesiol. 2005;18:603–607.
43 Tait AR, Malviya S. Anesthesia for the child with an upper respiratory tract infection: still a dilemma? Anesth Analg. 2005;100:59–65.
44 Emerson BM, Wrigley SR, Newton M. Preoperative fasting for paediatric anesthesia: a survey of current practice. Anaesthesia. 1998;53:326–330.
45 Ferrari LR, Rooney FM, Rockoff MA. Preoperative fasting practices in pediatrics. Anesthesiology. 1999;90:978–980.
46 Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: a report by the American Society of Anesthesiologist Task Force on Preoperative Fasting. Anesthesiology. 1999;90:896–905.
47 Cook-Sather SD, Litman RS. Modern fasting guidelines in children. Best Pract Res Clin Anaesthesiol. 2006;20:471–481.
48 Audenaert SM, Wagner Y, Montgomery CL, et al. Cardiorespiratory effects of premedication for children. Anesth Analg. 1995;80:506–510.
49 Stow PJ, Burrows FA, Lerman J, Roy WL. Arterial oxygen saturation following premedication in children with cyanotic congenital heart disease. Can J Anaesth. 1988;35:63–66.
50 DeBock TL, Davis PJ, Tome J, et al. Effect of premedication on arterial oxygen saturation in children with congenital heart disease. J Cardiothorac Anesth. 1990;4:425–429.
51 Levine MF, Hartley EJ, Macpherson BA, et al. Oral midazolam premedication for children with congenital cyanotic heart disease undergoing cardiac surgery: a comparative study. Can J Anaesth. 1993;40:934–938.
52 Masue T, Shimonaka H, Fukao I, et al. Oral high-dose midazolam premedication for infants and children undergoing cardiovascular surgery. Paediatr Anaesth. 2003;13:662–667.
53 Bell C, Rimar S, Barash P. Intraoperative ST-segment changes consistent with myocardial ischemia in the neonate: a report of three cases. Anesthesiology. 1989;71:601–604.
54 Slogoff S, Keats AS. Does perioperative myocardial ischemia lead to postoperative myocardial infarction? Anesthesiology. 1985;62:107–114.
55 Pasternack PF, Grossi EA, Baumann FG, et al. The value of silent myocardial ischemia monitoring in the prediction of perioperative myocardial infarction in patients undergoing peripheral vascular surgery. J Vasc Surg. 1989;10:617–625.
56 Barker SJ, Tremper KK. Pulse oximetry: applications and limitations. Int Anesthesiol Clin. 1987;25:155–175.
57 Tremper KK, Barker SJ. Pulse oximetry. Anesthesiology. 1989;70:98–108.
58 Coté CJ, Wax DF, Jennings MA, Gorski CL, Klippstein K. Endtidal carbon dioxide monitoring in children with congenital heart disease during sedation for cardiac catheterization by nonanesthesiologists. Pediatr Anesth. 2007;17:661–666.
59 Burrows FA. Physiologic dead space, venous admixture, and the arterial to end-tidal carbon dioxide difference in infants and children undergoing cardiac surgery. Anesthesiology. 1989;70:219–225.
60 Lazzell VA, Burrows FA. Stability of the intraoperative arterial to end-tidal carbon dioxide partial pressure difference in children with congenital heart disease. Can J Anaesth. 1991;38:859–865.
61 Eisenberg MJ, London MJ, Leung JM, et al. Monitoring for myocardial ischemia during noncardiac surgery: a technology assessment of transesophageal echocardiography and 12-lead electrocardiography. The Study of Perioperative Ischemia Research Group. JAMA. 1992;268:210–216.
62 Ellis JE, Shah MN, Briller JE, et al. A comparison of methods for the detection of myocardial ischemia during noncardiac surgery: automated ST-segment analysis systems, electrocardiography, and transesophageal echocardiography. Anesth Analg. 1992;75:764–772.
63 Kolev N, Brase R, Swanevelder J, et al. The influence of transoesophageal echocardiography on Intraoperative decision making: a European multicentre study. European Perioperative TOE Research Group. Anaesthesia. 1998;53:767–773.
64 Suriani RJ, Neustein S, Shore-Lesserson L, Konstadt S. Intraoperative transesophageal echocardiography during noncardiac surgery. J Cardiothorac Vasc Anesth. 1998;12:274–280.
65 Skiles JA, Griffin BP. Transesophageal echocardiographic (TEE) evaluation of ventricular function. Cardiol Clin. 2000;18:681–697.
66 Bert AA, Maslow A. Utility of transesophageal echocardiography in noncardiac surgery. Med Health R I. 2001;84:332–335.
67 Denault AY, Couture P, McKenty S, et al. Perioperative use of transesophageal echocardiography by anesthesiologists: impact in noncardiac surgery and in the intensive care unit. Can J Anaesth. 2002;49:287–293.
68 Maslow A, Bert A, Schwartz C, Mackinnon S. Transesophageal echocardiography in the noncardiac surgical patient. Int Anesthesiol Clin. 2002;40:73–132.
69 Hofer CK, Zollinger A, Rak M, et al. Therapeutic impact of intraoperative transoesophageal echocardiography during noncardiac surgery. Anaesthesia. 2004;59:3–9.
70 Patteril M, Swaminathan M. Pro: intraoperative transesophageal echocardiography is of utility in patients at high risk of adverse cardiac events undergoing noncardiac surgery. J Cardiothorac Vasc Anesth. 2004;18:107–109.
71 Memtsoudis SG, Rosenberger P, Loffler M, et al. The usefulness of transesophageal echocardiography during intraoperative cardiac arrest in noncardiac surgery. Anesth Analg. 2006;102:1653–1657.
72 Schulmeyer MC, Santelices E, Vega R, Schmied S. Impact of intraoperative transesophageal echocardiography during noncardiac surgery. J Cardiothorac Vasc Anesth. 2006;20:768–771.
73 Alexianu D, Skolnick ET, Pinto AC, et al. Severe hypotension in the prone position in a child with neurofibromatosis, scoliosis and pectus excavatum presenting for posterior spinal fusion. Anesth Analg. 2004;98:334–335.
74 Galas JM, van der Velde ME, Chiravuri SD, et al. Echocardiographic diagnosis of right ventricular inflow compression associated with pectus excavatum during spinal fusion in prone position. Congenit Heart Dis. 2009;4:193–195.
75 Neira VM, Gardin L, Ryan G, et al. A transesophageal echocardiography examination clarifies the cause of cardiovascular collapse during scoliosis surgery in a child. Can J Anaesth. 2011;58:451–455.
76 Ecoffey C. Pediatric regional anesthesia-update. Curr Opin Anaesthesiol. 2007;20:232–235.
77 Holzman RS, Nargozian CD, Marnach R, McMillan CO. Epidural anesthesia in patients with palliated cyanotic congenital heart disease. J Cardiothorac Vasc Anesth. 1992;6:340–343.
78 Sacrista S, Kern D, Fourcade O, et al. Spinal anaesthesia in a child with hypoplastic left heart syndrome. Paediatr Anaesth. 2003;13:253–256.
79 Katznelson R, Mishaly D, Hegesh T, Perel A, Keidan I. Spinal anesthesia for diagnostic cardiac catheterization in high-risk infants. Paediatr Anaesth. 2005;15:50–53.
80 Lerman J. Inhalational anesthetics. Paediatr Anaesth. 2004;14:380–383.
81 Lerman J. Inhalation agents in pediatric anesthesia—an update. Curr Opin Anaesthesiol. 2007;20:221–226.
82 Russell IA, Miller-Hance WC, Gregory G, et al. The safety and efficacy of sevoflurane anesthesia in infants and children with congenital heart disease. Anesth Analg. 2001;92:1152–1158.
83 Holzman RS, van der Velde ME, Kaus SJ, et al. Sevoflurane depresses myocardial contractility less than halothane during induction of anesthesia in children. Anesthesiology. 1996;85:1260–1267.
84 Wodey E, Pladys P, Copin C, et al. Comparative hemodynamic depression of sevoflurane versus halothane in infants: an echocardiographic study. Anesthesiology. 1997;87:795–800.
85 Chiu CL, Wang CY. Sevoflurane for dental extraction in children with tetralogy of Fallot. Paediatr Anaesth. 1999;9:268–270.
86 Rivenes SM, Lewin MB, Stayer SA, et al. Cardiovascular effects of sevoflurane, isoflurane, halothane, and fentanyl-midazolam in children with congenital heart disease: an echocardiographic study of myocardial contractility and hemodynamics. Anesthesiology. 2001;94:223–229.
87 Laird TH, Stayer SA, Rivenes SM, et al. Pulmonary-to-systemic blood flow ratio effects of sevoflurane, isoflurane, halothane, and fentanyl/midazolam with 100% oxygen in children with congenital heart disease. Anesth Analg. 2002;95:1200–1206.
88 Ikemba CM, Su JT, Stayer SA, et al. Myocardial performance index with sevoflurane-pancuronium versus fentanyl-midazolam-pancuronium in infants with a functional single ventricle. Anesthesiology. 2004;101:1298–1305.
89 Williams GD, Jones TK, Hanson KA, Morray JP. The hemodynamic effects of propofol in children with congenital heart disease. Anesth Analg. 1999;89:1411–1416.
90 Poortmans G. Anaesthesia for children with congenital heart disease undergoing diagnostic and interventional procedures. Curr Opin Anaesthesiol. 2004;17:335–338.
91 Akin A, Esmaoglu A, Guler G, et al. Propofol and propofol-ketamine in pediatric patients undergoing cardiac catheterization. Pediatr Cardiol. 2005;26:553–557.
92 Mart CR, Parrish M, Rosen KL, et al. Safety and efficacy of sedation with propofol for transoesophageal echocardiography in children in an outpatient setting. Cardiol Young. 2006;16:152–156.
93 Wodey E, Chonow L, Beneux X, et al. Haemodynamic effects of propofol vs thiopental in infants: an echocardiographic study. Br J Anaesth. 1999;82:516–520.
94 Lebovic S, Reich DL, Steinberg LG, et al. Comparison of propofol versus ketamine for anesthesia in pediatric patients undergoing cardiac catheterization. Anesth Analg. 1992;74:490–494.
95 Lavoie J, Walsh EP, Burrows FA, et al. Effects of propofol or isoflurane anesthesia on cardiac conduction in children undergoing radiofrequency catheter ablation for tachydysrhythmias. Anesthesiology. 1995;82:884–887.
96 Sharpe MD, Dobkowski WB, Murkin JM, et al. Propofol has no direct effect on sinoatrial node function or on normal atrioventricular and accessory pathway conduction in Wolff-Parkinson-White syndrome during alfentanil/midazolam anesthesia. Anesthesiology. 1995;82:888–895.
97 Lai LP, Lin JL, Wu MH, et al. Usefulness of intravenous propofol anesthesia for radiofrequency catheter ablation in patients with tachyarrhythmias: infeasibility for pediatric patients with ectopic atrial tachycardia. Pacing Clin Electrophysiol. 1999;22:1358–1364.
98 Guldner G, Schultz J, Sexton P, et al. Etomidate for rapid-sequence intubation in young children: hemodynamic effects and adverse events. Acad Emerg Med. 2003;10:134–139.
99 Sarkar M, Laussen PC, Zurakowski D, et al. Hemodynamic responses to etomidate on induction of anesthesia in pediatric patients. Anesth Analg. 2005;101:645–650.
100 Schechter WS, Kim C, Martinez M, et al. Anaesthetic induction in a child with endstage cardiomyopathy. Can J Anaesth. 1995;42:404–408.
101 Gancher S, Laxer KD, Krieger W. Activation of epileptogenic activity by etomidate. Anesthesiology. 1984;61:616–618.
102 Donmez A, Kaya H, Haberal A, et al. The effect of etomidate induction on plasma cortisol levels in children undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 1998;12:182–185.
103 Rupprecht T, Kuth R, Bowing B, et al. Sedation and monitoring of paediatric patients undergoing open low-field MRI. Acta Paediatr. 2000;89:1077–1081.
104 Rothermel LK. Newer pharmacologic agents for procedural sedation of children in the emergency department-etomidate and propofol. Curr Opin Pediatr. 2003;15:200–203.
105 Di Liddo L, D’Angelo A, Nguyen B, et al. Etomidate versus midazolam for procedural sedation in pediatric outpatients: a randomized controlled trial. Ann Emerg Med. 2006;48:433–440.
106 Morray JP, Lynn AM, Stamm SJ, et al. Hemodynamic effects of ketamine in children with congenital heart disease. Anesth Analg. 1984;63:895–899.
107 Hickey PR, Hansen DD, Cramolini GM, et al. Pulmonary and systemic hemodynamic responses to ketamine in infants with normal and elevated pulmonary vascular resistance. Anesthesiology. 1985;62:287–293.
108 Williams GD, Philip BM, Chu LF, et al. Ketamine does not increase pulmonary vascular resistance in children with pulmonary hypertension undergoing sevoflurane anesthesia and spontaneous ventilation. Anesth Analg. 2007;105:1578–1584.
109 Williams GD, Maan H, Ramamoorthy C, et al. Perioperative complications in children with pulmonary hypertension undergoing general anesthesia with ketamine. Paediatr Anaesth. 2010;20:28–37.
110 Mason KP, Zgleszewski SE, Prescilla R, et al. Hemodynamic effects of dexmedetomidine sedation for CT imaging studies. Paediatr Anaesth. 2008;18:393–402.
110a Mason KP, Zurakowski D, Zgleszewski SE, et al. High dose dexmedetomidine as the sole sedative for pediatric MRI. Paediatr Anaesth. 2008;18:403–411.
110b Mason KP, Zgleszewski S, Forman RE, Stark C, Dinardo JA. An exaggerated hypertensive response to glycopyrrolate therapy for bradycardia associated with high-dose dexmedetomidine. Anesth Analg. 2009;108:906–908.
111 Yuen VM. Dexmedetomidine: perioperative applications in children. Paediatr Anaesth. 2010;20:256–264.
112 Chrysostomou C, Di Filippo S, Manrique AM, et al. Use of dexmedetomidine in children after cardiac and thoracic surgery. Pediatr Crit Care Med. 2006;7:126–131.
113 Munro HM, Tirotta CF, Felix DE, et al. Initial experience with dexmedetomidine for diagnostic and interventional cardiac catheterization in children. Paediatr Anaesth. 2007;17:109–112.
114 Barton KP, Munoz R, Morell VO, Chrysostomou C. Dexmedetomidine as the primary sedative during invasive procedures in infants and toddlers with congenital heart disease. Pediatr Crit Care Med. 2008;9:612–615.
115 Chrysostomou C, Beerman L, Shiderly D, et al. Dexmedetomidine: a novel drug for the treatment of atrial and junctional tachyarrhythmias during the perioperative period for congenital cardiac surgery: a preliminary study. Anesth Analg. 2008;107:1514–1522.
116 Klamt JG, de Andrade Vicente WV, Garcia LV, Ferreira CA. Effects of dexmedetomidine-fentanyl infusion on blood pressure and heart rate during cardiac surgery in children. Anesthesiol Res Pract. 2010 Aug 19. [Epub ahead of print]
117 Hosokawa K, Shime N, Kato Y, et al. Dexmedetomidine sedation in children after cardiac surgery. Pediatr Crit Care Med. 2010;11:39–43.
118 Potts AL, Anderson BJ, Holford NH, et al. Dexmedetomidine hemodynamics in children after cardiac surgery. Paediatr Anaesth. 2010;20:425–433.
119 Su F, Nicolson SC, Gastonguay MR, et al. Population pharmacokinetics of dexmedetomidine in infants after open heart surgery. Anesth Analg. 2010;110:1383–1392.
120 Nathan AT, Marino BS, Hanna B, Nicolson SC. Novel use of dexmedetomidine in a patient with pulmonary hypertension. Paediatr Anaesth. 2008;18:782–784.
121 Shinohara H, Hirota K, Sato M, et al. Monitored anesthesia care with dexmedetomidine of a patient with severe pulmonary arterial hypertension for inguinal hernioplasty. J Anesth. 2010;24:611–613.
122 Hammer GB, Drover DR, Cao H, et al. The effects of dexmedetomidine on cardiac electrophysiology in children. Anesth Analg. 2008;106:79–83.
123 Chrysostomou C, Komarlu R, Lichtenstein S, et al. Electrocardiographic effects of dexmedetomidine in patients with congenital heart disease. Intensive Care Med. 2010;36:836–842.
124 Zhang X, Schmidt U, Wain JC, Bigatello L. Bradycardia leading to asystole during dexmedetomidine infusion in an 18-year-old double-lung transplant recipient. J Clin Anesth. 2010;22:45–49.
125 Anand KJ, Hickey PR. Halothane-morphine compared with highdose sufentanil for anesthesia and postoperative analgesia in neonatal cardiac surgery. N Engl J Med. 1992;326:1–9.
126 Wolf AR, Doyle E, Thomas E. Modifying infant stress responses to major surgery: spinal vs extradural vs opioid analgesia. Paediatr Anaesth. 1998;8:305–311.
127 Hickey PR, Hansen DD, Wessel DL, et al. Blunting of stress responses in the pulmonary circulation of infants by fentanyl. Anesth Analg. 1985;64:1137–1142.
128 Hickey PR, Hansen DD. Fentanyl- and sufentanil-oxygen-pancuronium anesthesia for cardiac surgery in infants. Anesth Analg. 1984;63:117–124.
129 Gruber EM, Laussen PC, Casta A, et al. Stress response in infants undergoing cardiac surgery: a randomized study of fentanyl bolus, fentanyl infusion, and fentanyl-midazolam infusion. Anesth Analg. 2001;92:882–890.
130 Morris RB, Cahalan MK, Miller RD, et al. The cardiovascular effects of vecuronium (ORG NC45) and pancuronium in patients undergoing coronary artery bypass grafting. Anesthesiology. 1983;58:438–440.
131 Salmenpera M, Peltola K, Takkunen O, Heinonen J. Cardiovascular effects of pancuronium and vecuronium during high-dose fentanyl anesthesia. Anesth Analg. 1983;62:1059–1064.
132 Scott RP, Basta SJ. Cardiovascular and autonomic effects of muscle relaxants. Compr Ther. 1985;11:56–67.
133 Sethna DH, Starr NJ, Estafanous FG. Cardiovascular effects of nondepolarizing neuromuscular blockers in patients with coronary artery disease. Can Anaesth Soc J. 1986;33:280–286.
134 Horigome H, Hiramatsu Y, Shigeta O, et al. Overproduction of platelet microparticles in cyanotic congenital heart disease with polycythemia. J Am Coll Cardiol. 2002;39:1072–1077.
135 Tempe DK, Virmani S. Coagulation abnormalities in patients with cyanotic congenital heart disease. J Cardiothorac Vasc Anesth. 2002;16:752–765.
136 Territo MC, Rosove MLH. Cyanotic congenital heart disease: hematologic management. J Am Coll Cardiol. 1991;18:320–322.
137 Goel M, Shome DK, Singh ZN, et al. Haemostatic changes in children with cyanotic and acyanotic congenital heart disease. Indian Heart J. 2000;52:559–563.
138 Suarez CR, Menendez CE, Griffin AJ, et al. Cyanotic congenital heart disease in children: hemostatic disorders and relevance of molecular markers of hemostasis. Semin Thromb Hemost. 1984;10:285–289.
139 Lill MC, Perloff JK, Child JS. Pathogenesis of thrombocytopenia in cyanotic congenital heart disease. Am J Cardiol. 2006;98:254–258.
140 Olgun N, Uysal KM, Irken G, et al. Platelet activation in congenital heart diseases. Acta Paediatr Jpn. 1997;39:566–569.
141 Murphy TW, Smith JH, Ranger MR, Haynes SR. General anesthesia for children with severe heart failure. Pediatr Cardiol. 2011;32:139–144.
142 Kipps AK, Ramamoorthy C, Rosenthal DN, Williams GD. Children with cardiomyopathy: Complications after noncardiac procedures with general anesthesia. Paediatr Anaesth. 2007;17:775–781.
143 Friesen RH, Williams GD. Anesthetic management of children with pulmonary arterial hypertension. Paediatr Anaesth. 2008;18:208–216.
144 Williams GD, Maan H, Ramamoorthy C, et al. Perioperative complications in children with pulmonary hypertension undergoing general anesthesia with ketamine. Paediatr Anaesth. 2010;20:28–37.
145 Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association. a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. J Am Dent Assoc. 2007;138:739–745. 747-60
146 Streif W, Andrew M, Marzinotto V, et al. Analysis of warfarin therapy in pediatric patients: a prospective cohort study of 319 patients. Blood. 1999;94:3007–3014.
147 Albisetti M, Andrew M. Low molecular weight heparin in children. Eur J Pediatr. 2002;161:71–77.
148 Monagle P, Chan A, Massicotte P, et al. Antithrombotic therapy in children: The seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:645S–687S.
149 Palareti G. Warfarin anticoagulation in children made easier. Thromb Res. 2006;118:667–669.
150 Bontadelli J, Moeller A, Schmugge M, et al. Enoxaparin therapy for arterial thrombosis in infants with congenital heart disease. Intensive Care Med. 2007;33:1978–1984.
151 Tinker JH, Tarhan S. Discontinuing anticoagulant therapy in surgical patients with cardiac valve prostheses: observations in 180 operations. JAMA. 1978;239:738–739.
152 Jafri SM. Periprocedural thromboprophylaxis in patients receiving chronic anticoagulation therapy. Am Heart J. 2004;147:3–15.
153 Jaffer AK. Anticoagulation management strategies for patients on warfarin who need surgery. Cleve Clin J Med. 2006;73(Suppl 1):S100–S105.
154 Tait RC, Ladusans EJ, El-Metaal M, et al. Oral anticoagulation in paediatric patients: dose requirements and complications. Arch Dis Child. 1996;74:228–231.
155 McGrath LB, Gonzalez-Lavin L, Eldredge WJ, et al. Thromboembolic and other events following valve replacement in a pediatric population treated with antiplatelet agents. Ann Thorac Surg. 1987;43:285–287.
156 Reller MD. Congenital heart disease: current indications for antithrombotic therapy in pediatric patients. Curr Cardiol Rep. 2001;3:90–95.
157 Jacobs ML, Pourmoghadam KK, Geary EM, et al. Fontan’s operation: is aspirin enough? Is coumadin too much? Ann Thorac Surg. 2002;73:64–68.
158 Seipelt RG, Franke A, Vazquez-Jimenez JF, et al. Thromboembolic complications after Fontan procedures: comparison of different therapeutic approaches. Ann Thorac Surg. 2002;74:556–562.
159 Borland LM, Colligan J, Brandom BW. Frequency of anesthesia-related complications in children with Down syndrome under general anesthesia for noncardiac procedures. Paediatr Anaesth. 2004;14:733–738.
160 Bai W, Voepel-Lewis T, Malviya S. Hemodynamic changes in children with Down syndrome during and following inhalation induction of anesthesia with sevoflurane. J Clin Anesth. 2010;22:592–597.
161 Kraemer FW, Stricker PA, Gurnaney HG, et al. Bradycardia during induction of anesthesia with sevoflurane in children with Down syndrome. Anesth Analg. 2010;111:1259–1263.
162 Wood P. The Eisenmenger syndrome or pulmonary hypertension with reversed central shunt. BMJ. 1958;2:755–762.
163 Previte J, Haran P. Eisenmenger syndrome. Semin Cardiothorac Vasc Anesth. 2001;5:67–78.
164 Saha A, Balakrishnan KG, Jaiswal PK, et al. Prognosis for patients with Eisenmenger syndrome of various aetiology. Int J Cardiol. 1994;45:199–207.
165 Griffith BP, Hardesty RL, Trento A, et al. Heart-lung transplantation: lessons learned and future hopes. Ann Thorac Surg. 1987;43:6–16.
166 Ueno T, Smith JA, Snell GI, et al. Bilateral sequential single lung transplantation for pulmonary hypertension and Eisenmenger’s syndrome. Ann Thorac Surg. 2000;69:381–387.
167 Lyons B, Motherway C, Casey W, Doherty P. The anaesthetic management of the child with Eisenmenger’s syndrome. Can J Anaesth. 1995;42:904–909.
168 Raines DE, Liberthson RR, Murray JR. Anesthetic management and outcome following noncardiac surgery in nonparturients with Eisenmenger’s physiology. J Clin Anesth. 1996;8:341–347.
169 Ammash NM, Connolly HM, Abel MD, Warnes CA. Noncardiac surgery in Eisenmenger syndrome. J Am Coll Cardiol. 1999;33:222–227.
170 Martin JT, Tautz TJ, Antognini JF. Safety of regional anesthesia in Eisenmenger’s syndrome. Reg Anesth Pain Med. 2002;27:509–513.
171 Subramaniam K, Yared JP. Management of pulmonary hypertension in the operating room. Semin Cardiothorac Vasc Anesth. 2007;11:119–136.
172 Alkhaldi A, Chin C, Bernstein D. Pediatric cardiac transplantation. Semin Pediatr Surg. 2006;15:188–198.
173 Schure AY, Kussman BD. Pediatric heart transplantation: demographics, outcomes, and anesthetic implications. Pediatr Anesth. 2011;21:594–603.
174 DePaolis-Lutzo M, Neill AL. Anesthetic considerations for the postcardiac transplant patient. AANA J. 1991;59:47–52.
175 Mahla E, Rotman B, Rehak P, et al. Perioperative ventricular dysrhythmias in patients with structural heart disease undergoing noncardiac surgery. Anesth Analg. 1998;86:16–21.
176 Ramamoorthy C, Haberkern CM, Bhananker SM, et al. Anesthesia-related cardiac arrest in children with heart disease: data from the Pediatric Perioperative Cardiac Arrest (POCA) registry. Anesth Analg. 2010;110:1376–1382.
177 Leachman RD, Cokkinos DV, Cooley DA. Association of ostium secundum atrial septal defects with mitral valve prolapse. Am J Cardiol. 1976;38:167–169.
178 Boucher CA, Liberthson RR, Buckley MJ. Secundum atrial septal defect and significant mitral regurgitation: incidence, management and morphologic basis. Chest. 1979;75:697–702.
179 Suchon E, Podolec P, Plazak W, et al. Mitral valve prolapse associated with ostium secundum atrial septal defect—a functional disorder. Acta Cardiol. 2004;59:237–238.
180 Pillai R, Ho SY, Anderson RH, Lincoln C. Ostium primum atrioventricular septal defect: an anatomical and surgical review. Ann Thorac Surg. 1986;41:458–461.
181 Li J, Al Zaghal AM, Anderson RLH. The nature of the superior sinus venosus defect. Clin Anat. 1998;11:349–352.
182 Pascoe RD, Oh JK, Warnes CA, et al. Diagnosis of sinus venosus atrial septal defect with transesophageal echocardiography. Circulation. 1996;94:1049–1055.
183 Ootaki Y, Yamaguchi M, Yoshimura N, et al. Unroofed coronary sinus syndrome: diagnosis, classification, and surgical treatment. J Thorac Cardiovasc Surg. 2003;126:1655–1656.
184 Kerut EK, Norfleet WT, Plotnick GD, Giles TD. Patent foramen ovale: a review of associated conditions and the impact of physiological size. J Am Coll Cardiol. 2001;38:613–623.
185 Sukernik MR, Mets B, Bennett-Guerrero E. Patent foramen ovale and its significance in the perioperative period. Anesth Analg. 2001;93:1137–1146.
186 Murphy JG, Gersh BJ, McGoon MD, et al. Long-term outcome after surgical repair of isolated atrial septal defect: follow-up at 27 to 32 years. N Engl J Med. 1990;323:1645–1650.
187 Meijboom F, Hess J, Szatmari A, et al. Long-term follow-up (9 to 20 years) after surgical closure of atrial septal defect at a young age. Am J Cardiol. 1993;72:1431–1434.
188 Guntheroth WG. Atrial septal defects: surgical closure and outcomes. Am J Cardiol. 1999;84:1142–1143.
189 Bolz D, Lacina T, Buser P, et al. Long-term outcome after surgical closure of atrial septal defect in childhood with extensive assessment including MRI measurement of the ventricles. Pediatr Cardiol. 2005;26:614–621.
190 Pearlman AS, Borer JS, Clark CE, et al. Abnormal right ventricular size and ventricular septal motion after atrial septal defect closure: etiology and functional significance. Am J Cardiol. 1978;41:295–301.
191 Motiwala A, Fatimi SH, Akhtar N, et al. Patients with congenital atrial septal defects: effect of age at repair and defect size on pulmonary artery pressures prior to repair. Thorac Cardiovasc Surg. 59, 2011. 289–286
192 Masura J, Gavora P, Podnar GT. Long-term outcome of transcatheter secundum-type atrial septal defect closure using Amplatzer septal occluders. J Am Coll Cardiol. 2005;45:505–507.
193 Pawelec-Wojtalik M, Wojtalik M, Mrowczynski W, et al. Comparison of cardiac function in children after surgical and Amplatzer occluder closure of secundum atrial septal defects. Eur J Cardiothorac Surg. 2006;29:89–92.
194 Butera G, Lucente M, Rosti L, et al. A comparison between the early and midterm results of surgical as opposed to percutaneous closure of defects in the oval fossa in children aged less than 6 years. Cardiol Young. 2007;17:35–41.
195 Du ZD, Hijazi ZM, Kleinman CS, et al. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J Am Coll Cardiol. 2002;39:1836–1844.
196 Portman MA, Beder SD, Ankeney JL, et al. A 20-year review of ostium primum defect repair in children. Am Heart J. 1985;110:1054–1058.
197 Ceithaml EL, Midgley FM, Perry LW. Long-term results after surgical repair of incomplete endocardial cushion defects. Ann Thorac Surg. 1989;48:413–416.
198 Agny M, Cobanoglu A. Repair of partial atrioventricular septal defect in children less than five years of age: late results. Ann Thorac Surg. 1999;67:1412–1414.
199 Murashita T, Kubota T, Oba J, et al. Left atrioventricular valve regurgitation after repair of incomplete atrioventricular septal defect. Ann Thorac Surg. 2004;77:2157–2162.
200 Welke KF, Morris CD, King E, et al. Population-based perspective of long-term outcomes after surgical repair of partial atrioventricular septal defect. Ann Thorac Surg. 2007;84:624–628.
201 Russell JL, LeBlanc JG, Deagle ML, Potts JE. Outcome following repair of sinus venosus atrial septal defects in children. Asian Cardiovasc Thorac Ann. 2002;10:231–234.
202 Attenhofer Jost CH, Connolly HM, Danielson GK, et al. Sinus venosus atrial septal defect: long-term postoperative outcome for 115 patients. Circulation. 2005;112:1953–1958.
203 Gaynor JW. Management of sinus venosus defects. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2006;9:35–39.
204 Quaegebeur J, Kirklin JW, Pacifico AD, Bargeron LMJ. Surgical experience with unroofed coronary sinus. Ann Thorac Surg. 1979;27:418–425.
205 Hoffman JI. Incidence of congenital heart disease. I. Postnatal incidence. Pediatr Cardiol. 1995;16:103–113.
206 Turner SW, Hunter S, Wyllie JP. The natural history of ventricular septal defects. Arch Dis Child. 1999;81:413–416.
207 Freedom RM, Yoo SJ, Coles JG, Konstantinov I. Ventricular septal defect. In: Freedom RM, Yoo SJ, Mikailian H, Williams WG, eds. The natural and modified history of congenital heart disease. New York: Blackwell Publishing; 2004:16–30.
208 Becker AE, Anderson RLH. Classification of ventricular septal defects—a matter of precision. Heart Vessels. 1985;1:120–121.
209 Soto B, Ceballos R, Kirklin JW. Ventricular septal defects: a surgical viewpoint. J Am Coll Cardiol. 1989;14:1291–1297.
210 Rhodes LA, Keane JF, Keane JP, et al. Long follow-up (to 43 years) of ventricular septal defect with audible aortic regurgitation. Am J Cardiol. 1990;66:340–345.
211 Kuribayashi R, Sekine S, Aida H, et al. Long-term results of primary closure for ventricular septal defects in the first year of life. Surg Today. 1994;24:389–392.
212 Scully BB, Morales DL, Zafar F, et al. Current expectations for surgical repair of isolated ventricular septal defects. Ann Thorac Surg. 2010;89:544–549. discussion 550-1
213 Meijboom F, Szatmari A, Utens E, et al. Long-term follow-up after surgical closure of ventricular septal defect in infancy and childhood. J Am Coll Cardiol. 1994;24:1358–1364.
214 Jarmakani JM, Graham TPJ, Canent RVJ, Capp MP. The effect of corrective surgery on left heart volume and mass in children with ventricular septal defect. Am J Cardiol. 1971;27:254–258.
215 Neutze JM, Ishikawa T, Clarkson PM, et al. Assessment and follow-up of patients with ventricular septal defect and elevated pulmonary vascular resistance. Am J Cardiol. 1989;63:327–331.
216 Friedli B, Kidd BS, Mustard WT, Keith JD. Ventricular septal defect with increased pulmonary vascular resistance: late results of surgical closure. Am J Cardiol. 1974;33:403–409.
217 Chiu SN, Wang JK, Lin MT, et al. Progression of aortic regurgitation after surgical repair of outlet-type ventricular septal defects. Am Heart J. 2007;153:336–342.
218 Holzer R, Balzer D, Cao QL, et al. Device closure of muscular ventricular septal defects using the Amplatzer muscular ventricular septal defect occluder: immediate and mid-term results of a U.S. registry. J Am Coll Cardiol. 2004;43:1257–1263.
219 Aleem IS, Karamlou T, Benson LN, McCrindle BW. Transcatheter device versus surgical closure of ventricular septal defects: a clinical decision analysis. Catheter Cardiovasc Interv. 2006;67:630–636.
220 Butera G, Chessa M, Carminati M. Percutaneous closure of ventricular septal defects. Cardiol Young. 2007;17:243–253.
221 Fu YC, Bass J, Amin Z, et al. Transcatheter closure of perimembranous ventricular septal defects using the new Amplatzer membranous VSD occluder: results of the U.S. phase I trial. J Am Coll Cardiol. 2006;47:319–325.
222 Holzer R, de Giovanni J, Walsh KP, et al. Transcatheter closure of perimembranous ventricular septal defects using the Amplatzer membranous VSD occluder: immediate and midterm results of an international registry. Catheter Cardiovasc Interv. 2006;68:620–628.
223 Thanopoulos BV, Rigby ML, Karanasios E, et al. Transcatheter closure of perimembranous ventricular septal defects in infants and children using the Amplatzer perimembranous ventricular septal defect occluder. Am J Cardiol. 2007;99:984–989.
224 Walsh MA, Bialkowski J, Szkutnik M, et al. Atrioventricular block after transcatheter closure of perimembranous ventricular septal defects. Heart. 2006;92:1295–1297.
225 Craig B. Atrioventricular septal defect: from fetus to adult. Heart. 2006;92:1879–1885.
226 Goldfaden DM, Jones M, Morrow AG. Long-term results of repair of incomplete persistent atrioventricular canal. J Thorac Cardiovasc Surg. 1981;82:669–673.
227 Hanley FL, Fenton KN, Jonas RA, et al. Surgical repair of complete atrioventricular canal defects in infancy: twenty-year trends. J Thorac Cardiovasc Surg. 1993;106:387–394.
228 El-Najdawi EK, Driscoll DJ, Puga FJ, et al. Operation for partial atrioventricular septal defect: a forty-year review. J Thorac Cardiovasc Surg. 2000;119:880–889.
229 Young D, Mark LH. Fate of the patient with the Eisenmenger syndrome. Am J Cardiol. 1971;28:658–669.
230 Mack G, Silberbach M. Aortic and pulmonary stenosis. Pediatr Rev. 2000;21:79–85.
231 Hayes CJ, Gersony WM, Driscoll DJ, et al. Second natural history study of congenital heart defects: results of treatment of patients with pulmonary valvar stenosis. Circulation. 1993;87(Suppl):I28–I37.
232 Anand R, Mehta AV. Natural history of asymptomatic valvar pulmonary stenosis diagnosed in infancy. Clin Cardiol. 1997;20:377–380.
233 Harrild DM, Powell AJ, Tran TX, et al. Long-term pulmonary regurgitation following balloon valvuloplasty for pulmonary stenosis risk factors and relationship to exercise capacity and ventricular volume and function. J Am Coll Cardiol. 2010;55:1041–1047.
234 Roos-Hesselink JW, Meijboom FJ, Spitaels SE, et al. Long-term outcome after surgery for pulmonary stenosis (a longitudinal study of 22-33 years). Eur Heart J. 2006;27:482–488.
235 Tutar E, Ekici F, Atalay S, Nacar N. The prevalence of bicuspid aortic valve in newborns by echocardiographic screening. Am Heart J. 2005;150:513–515.
236 Horowitz PE, Akhtar S, Wulff JA, et al. Coronary artery disease and anesthesia-related death in children with Williams syndrome. J Cardiothorac Vasc Anesth. 2002;16:739–741.
237 Bragg K, Fedel GM, DiProsperis A. Cardiac arrest under anesthesia in a pediatric patient with Williams syndrome: a case report. AANA J. 2005;73:287–293.
238 Medley J, Russo P, Tobias JD. Perioperative care of the patient with Williams syndrome. Paediatr Anaesth. 2005;15:243–247.
239 Monfared A, Messner A. Death following tonsillectomy in a child with Williams syndrome. Int J Pediatr Otorhinolaryngol. 2006;70:1133–1135.
240 Burch TM, McGowan FXJ, Kussman BD, et al. Congenital supravalvular aortic stenosis and sudden death associated with anesthesia: what’s the mystery? Anesth Analg. 2008;107:1848–1854.
241 Carabello BA. Aortic stenosis: from pressure overload to heart failure. Heart Fail Clin. 2006;2:435–442.
242 Keane JF, Driscoll DJ, Gersony WM, et al. Second natural history study of congenital heart defects: results of treatment of patients with aortic valvar stenosis. Circulation. 1993;87:I16–I27.
243 McLean KM, Lorts A, Pearl JM. Current treatments for congenital aortic stenosis. Curr Opin Cardiol. 2006;21:200–204.
244 Kadner A, Raisky O, Degandt A, et al. The Ross procedure in infants and young children. Ann Thorac Surg. 2008;85:803–808.
245 Raja SG, Pollock JC. Current outcomes of Ross operation for pediatric and adolescent patients. J Heart Valve Dis. 2007;16:27–36.
246 Laudito A, Brook MM, Suleman S, et al. The Ross procedure in children and young adults: a word of caution. J Thorac Cardiovasc Surg. 2001;122:147–153.
247 Pasquali SK, Shera D, Wernovsky G, et al. Midterm outcomes and predictors of reintervention after the Ross procedure in infants, children, and young adults. J Thorac Cardiovasc Surg. 2007;133:893–899.
248 Brauner R, Laks H, Drinkwater DCJ, et al. Benefits of early surgical repair in fixed subaortic stenosis. J Am Coll Cardiol. 1997;30:1835–1842.
249 Scott DJ, Campbell DN, Clarke DR, et al. Twenty-year surgical experience with congenital supravalvar aortic stenosis. Ann Thorac Surg. 2009;87:1501–1507. discussion 1507-8
250 Brown JW, Stevens LS, Holly S, et al. Surgical spectrum of aortic stenosis in children: a thirty-year experience with 257 children. Ann Thorac Surg. 1988;45:393–403.
251 Wheller JJ, Hosier DM, Teske DW, et al. Results of operation for aortic valve stenosis in infants, children, and adolescents. J Thorac Cardiovasc Surg. 1988;96:474–477.
252 Campbell M. Natural history of persistent ductus arteriosus. Br Heart J. 1968;30:4–13.
253 Ewert P. Challenges encountered during closure of patent ductus arteriosus. Pediatr Cardiol. 2005;26:224–229.
254 Masura J, Tittel P, Gavora P, Podnar GT. Long-term outcome of transcatheter patent ductus arteriosus closure using Amplatzer duct occluders. Am Heart J. 2006;151:755. e7-10
255 Burke RP, Jacobs JP, Cheng W, et al. Video-assisted thoracoscopic surgery for patent ductus arteriosus in low birth weight neonates and infants. Pediatrics. 1999;104:227–230.
256 Jacobs JP, Giroud JM, Quintessenza JA, et al. The modern approach to patent ductus arteriosus treatment: complementary roles of video-assisted thoracoscopic surgery and interventional cardiology coil occlusion. Ann Thorac Surg. 2003;76:1421–1427.
257 Abbruzzese PA, Aidala E. Aortic coarctation: an overview. J Cardiovasc Med (Hagerstown). 2007;8:123–128.
258 Campbell M. Natural history of coarctation of the aorta. Br Heart J. 1970;32:633–640.
259 Gibbs JL. Treatment options for coarctation of the aorta. Heart. 2000;84:11–13.
260 Presbitero P, Demarie D, Villani M, et al. Long term results (15-30 years) of surgical repair of aortic coarctation. Br Heart J. 1987;57:462–467.
261 Hauser M, Kuehn A, Wilson N. Abnormal responses for blood pressure in children and adults with surgically corrected aortic coarctation. Cardiol Young. 2000;10:353–357.
262 Florianczyk T, Werner B. Assessment of left ventricular diastolic function in children after successful repair of aortic coarctation. Clin Res Cardiol. 2011;100:493–499.
263 Golden AB, Hellenbrand WE. Coarctation of the aorta: stenting in children and adults. Catheter Cardiovasc Interv. 2007;69:289–299.
264 Rao PS. Stents in the management of aortic coarctation in young children. JACC Cardiovasc Interv. 2009;2:884–886.
265 Shinebourne EA, Babu-Narayan SV, Carvalho JS. Tetralogy of Fallot: from fetus to adult. Heart. 2006;92:1353–1359.
266 Anderson RH, Jacobs ML. The anatomy of tetralogy of Fallot with pulmonary stenosis. Cardiol Young. 2008;18(Suppl 3):12–21.
267 Sousa Uva M, Chardigny C, Galetti L, et al. Surgery for tetralogy of Fallot at less than six months of age: is palliation “old-fashioned”? Eur J Cardiothorac Surg. 1995;9:453–459.
268 Kantorova A, Zbieranek K, Sauer H, et al. Primary early correction of tetralogy of Fallot irrespective of age. Cardiol Young. 2008;18:153–157.
269 Wu ET, Wang JK, Lee WL, et al. Balloon valvuloplasty as an initial palliation in the treatment of newborns and young infants with severely symptomatic tetralogy of Fallot. Cardiology. 2006;105:52–56.
270 Karamlou T, McCrindle BW, Williams WG. Surgery insight: late complications following repair of tetralogy of Fallot and related surgical strategies for management. Nat Clin Pract Cardiovasc Med. 2006;3:611–622.
271 Kadner A, Tulevski II, Bauersfeld U, et al. Chronic pulmonary valve insufficiency after repaired tetralogy of Fallot: diagnostics, reoperations and reconstruction possibilities. Expert Rev Cardiovasc Ther. 2007;5:221–230.
272 Sano S, Karl TR, Mee RB. Extracardiac valved conduits in the pulmonary circuit. Ann Thorac Surg. 1991;52:285–290.
273 Aboulhosn J, Child JS. Management after childhood repair of tetralogy of fallot. Curr Treat Options Cardiovasc Med. 2006;8:474–483.
274 Morales DL, Zafar F, Heinle JS, et al. Right ventricular infundibulum sparing (RVIS) tetralogy of fallot repair: a review of over 300 patients. Ann Surg. 2009;250:611–617.
275 Bove T, Francois K, Van De Kerckhove K, et al. Assessment of a right-ventricular infundibulum-sparing approach in transatrial-transpulmonary repair of tetralogy of Fallot. Eur J Cardiothorac Surg. 2011;41:126–133.
276 Gatzoulis MA, Clark AL, Cullen S, et al. Right ventricular diastolic function 15 to 35 years after repair of tetralogy of Fallot: restrictive physiology predicts superior exercise performance. Circulation. 1995;91:1775–1781.
277 Wilson NJ, Clarkson PM, Barratt-Boyes BG, et al. Long-term outcome after the mustard repair for simple transposition of the great arteries. 28-year follow-up. J Am Coll Cardiol. 1998;32:758–765.
278 Bender HWJ, Stewart JR, Merrill WH, et al. Ten years’ experience with the Senning operation for transposition of the great arteries: physiological results and late follow-up. Ann Thorac Surg. 1989;47:218–223.
279 Gelatt M, Hamilton RM, McCrindle BW, et al. Arrhythmia and mortality after the Mustard procedure: a 30-year single-center experience. J Am Coll Cardiol. 1997;29:194–201.
280 Murphy DJJ. Transposition of the great arteries: long-term outcome and current management. Curr Cardiol Rep. 2005;7:299–304.
281 Warnes CA. Transposition of the great arteries. Circulation. 2006;114:2699–2709.
282 Ensing GJ, Heise CT, Driscoll DJ. Cardiovascular response to exercise after the Mustard operation for simple and complex transposition of the great vessels. Am J Cardiol. 1988;62:617–622.
283 Hutter PA, Kreb DL, Mantel SF, et al. Twenty-five years’ experience with the arterial switch operation. J Thorac Cardiovasc Surg. 2002;124:790–797.
284 Hovels-Gurich HH, Seghaye MC, Ma Q, et al. Long-term results of cardiac and general health status in children after neonatal arterial switch operation. Ann Thorac Surg. 2003;75:935–943.
285 Hraska V, Mattes A, Haun C, et al. Functional outcome of anatomic correction of corrected transposition of the great arteries. Eur J Cardiothorac Surg. 2011;40:1227–1234.
286 Williams WG, McCrindle BW, Ashburn DA, et al. Outcomes of 829 neonates with complete transposition of the great arteries 12-17 years after repair. Eur J Cardiothorac Surg. 2003;24:1–9.
287 Colan SD, Trowitzsch E, Wernovsky G, et al. Myocardial performance after arterial switch operation for transposition of the great arteries with intact ventricular septum. Circulation. 1988;78:132–141.
288 Vogel M, Smallhorn JF, Trusler GA, Freedom RM. Echocardiographic analysis of regional left ventricular wall motion in children after the arterial switch operation for complete transposition of the great arteries. J Am Coll Cardiol. 1990;15:1417–1423.
289 Bonhoeffer P, Bonnet D, Piechaud JF, et al. Coronary artery obstruction after the arterial switch operation for transposition of the great arteries in newborns. J Am Coll Cardiol. 1997;29:202–206.
290 Weindling SN, Wernovsky G, Colan SD, et al. Myocardial perfusion, function and exercise tolerance after the arterial switch operation. J Am Coll Cardiol. 1994;23:424–433.
291 Bartoloni G, Bianca S, Patane L, Mignosa C. Pathology of coronary narrowing after arterial switch operation: autopsy findings in two patients who died within 3 months of surgical treatment and review of the literature. Cardiovasc Pathol. 2006;15:49–54.
292 Raisky O, Bergoend E, Agnoletti G, et al. Late coronary artery lesions after neonatal arterial switch operation: results of surgical coronary revascularization. Eur J Cardiothorac Surg. 2007;31:894–898.
293 Kafali G, Elsharshari H, Ozer S, et al. Incidence of dysrhythmias in congenitally corrected transposition of the great arteries. Turk J Pediatr. 2002;44:219–223.
294 Huhta JC, Maloney JD, Ritter DG, et al. Complete atrioventricular block in patients with atrioventricular discordance. Circulation. 1983;67:1374–1377.
295 Rutledge JM, Nihill MR, Fraser CD, et al. Outcome of 121 patients with congenitally corrected transposition of the great arteries. Pediatr Cardiol. 2002;23:137–145.
296 Yeh TJ, Connelly MS, Coles JG, et al. Atrioventricular discordance: results of repair in 127 patients. J Thorac Cardiovasc Surg. 1999;117:1190–1203.
297 Graham TPJ, Bernard YD, Mellen BG, et al. Long-term outcome in congenitally corrected transposition of the great arteries: a multi-institutional study. J Am Coll Cardiol. 2000;36:255–261.
298 Huhta JC, Danielson GK, Ritter DG, Ilstrup DM. Survival in atrioventricular discordance. Pediatr Cardiol. 1985;6:57–60.
299 Hraska V, Duncan BW, Mayer JEJ, et al. Long-term outcome of surgically treated patients with corrected transposition of the great arteries. J Thorac Cardiovasc Surg. 2005;129:182–191.
300 Colett RW, Edwards JE. Persistent truncus arteriosus: a classification according to anatomic types. Surg Clin North Am. 1949;29:559–568.
301 Calder L, Van Praagh R, Van Praagh S, et al. Truncus arteriosus communis: Clinical, angiocardiographic, and pathologic findings in 100 patients. Am Heart J. 1976;92:23–38.
302 Odegard KC, DiNardo JA, Kussman BD, et al. The frequency of anesthesia-related cardiac arrests in patients with congenital heart disease undergoing cardiac surgery. Anesth Analg. 2007;105:335–343.
303 Chen JM, Glickstein JS, Davies RR, et al. The effect of repair technique on postoperative right-sided obstruction in patients with truncus arteriosus. J Thorac Cardiovasc Surg. 2005;129:559–568.
304 Reddy VM, Hanley F. Late results of repair of truncus arteriosus. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 1998;1:139–146.
305 Thompson LD, McElhinney DB, Reddy M, et al. Neonatal repair of truncus arteriosus: continuing improvement in outcomes. Ann Thorac Surg. 2001;72:391–395.
306 Ullmann MV, Gorenflo M, Sebening C, et al. Long-term results after repair of truncus arteriosus communis in neonates and infants. Thorac Cardiovasc Surg. 2003;51:175–179.
307 Rajasinghe HA, McElhinney DB, Reddy VM, et al. Long-term follow-up of truncus arteriosus repaired in infancy: a twenty-year experience. J Thorac Cardiovasc Surg. 1997;113:869–878.
308 Seward JB. Ebstein’s anomaly: ultrasound imaging and hemodynamic evaluation. Echocardiography. 1993;10:641–664.
309 Driscoll DJ, Fuster V, Danielson GK. Ebstein’s anomaly of the tricuspid valve. In: Giuliani ER, Gersh BJ, McGoon MD, Hayes DL, Schaff HV, eds. Mayo Clinic practice of cardiology. St Louis: CV Mosby; 1996:1598–1605.
310 Shiina A, Seward JB, Edwards WD, et al. Two-dimensional echocardiographic spectrum of Ebstein’s anomaly: detailed anatomic assessment. J Am Coll Cardiol. 1984;3:356–370.
311 Chen JM, Mosca RS, Altmann K, et al. Early and medium-term results for repair of Ebstein anomaly. J Thorac Cardiovasc Surg. 2004;127:990–998.
312 Marianeschi SM, McElhinney DB, Reddy VM, et al. Alternative approach to the repair of Ebstein’s malformation: intracardiac repair with ventricular unloading. Ann Thorac Surg. 1998;66:1546–1550.
313 Kreutzer C, Mayorquim RC, Kreutzer GO, et al. Experience with one and a half ventricle repair. J Thorac Cardiovasc Surg. 1999;117:662–668.
314 Brown ML, Dearani JA, Danielson GK, et al. Functional status after operation for Ebstein anomaly: the Mayo Clinic experience. J Am Coll Cardiol. 2008;52:460–466.
315 Sandhu SK, Beekman RH, Mosca RS, Bove EL. Single-stage repair of aortic arch obstruction and associated intracardiac defects in the neonate. Am J Cardiol. 1995;75:370–373.
316 Flint JD, Gentles TL, MacCormick J, et al. Outcomes using predominantly single-stage approach to interrupted aortic arch and associated defects. Ann Thorac Surg. 2010;89:564–569.
317 Hirooka K, Fraser CDJ. One-stage neonatal repair of complex aortic arch obstruction or interruption. Recent experience at Texas Children’s Hospital. Tex Heart Inst J. 1997;24:317–321.
318 Oosterhof T, Azakie A, Freedom RM, et al. Associated factors and trends in outcomes of interrupted aortic arch. Ann Thorac Surg. 2004;78:1696–1702.
319 Hirata Y, Quaegebeur JM, Mosca RS, et al. Impact of aortic annular size on rate of reoperation for left ventricular outflow tract obstruction after repair of interrupted aortic arch and ventricular septal defect. Ann Thorac Surg. 2010;90:588–592.
320 Greenberg MA, Fish BG, Spindola-Franco LH. Congenital anomalies of the coronary arteries: classification and significance. Radiol Clin North Am. 1989;27:1127–1146.
321 Frommelt PC, Frommelt MA. Congenital coronary artery anomalies. Pediatr Clin North Am. 2004;51:1273–1288.
322 Davis JA, Cecchin F, Jones TK, Portman MA. Major coronary artery anomalies in a pediatric population: incidence and clinical importance. J Am Coll Cardiol. 2001;37:593–597.
323 Tabbutt S, Ramamoorthy C, Montenegro LM, et al. Impact of inspired gas mixtures on preoperative infants with hypoplastic left heart syndrome during controlled ventilation. Circulation. 2001;104:I159–I164.
324 Bove EL. Current status of staged reconstruction for hypoplastic left heart syndrome. Pediatr Cardiol. 1998;19:308–315.
325 Ballweg JA, Dominguez TE, Ravishankar C, et al. A contemporary comparison of the effect of shunt type in hypoplastic left heart syndrome on the hemodynamics and outcome at stage 2 reconstruction. J Thorac Cardiovasc Surg. 2007;134:297–303.
326 Ohye RG, Devaney EJ, Hirsch JC, Bove EL. The modified Blalock-Taussig shunt versus the right ventricle-to-pulmonary artery conduit for the Norwood procedure. Pediatr Cardiol. 2007;28:122–125.
327 Wernovsky G, Ghanayem N, Ohye RG, et al. Hypoplastic left heart syndrome: consensus and controversies in 2007. Cardiol Young. 2007;17(Suppl 2):75–86.
328 Ruffer A, Danch A, Gottschalk U, et al. The Norwood procedure—does the type of shunt determine outcome? Thorac Cardiovasc Surg. 2009;57:270–275.
329 Ohye RG, Sleeper LA, Mahony L, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–1992.
330 Menon SC, Minich LL, Casper TC, et al. Regional myocardial dysfunction following norwood with right ventricle to pulmonary artery conduit in patients with hypoplastic left heart syndrome. J Am Soc Echocardiogr. 2011;24:826–833.
331 Mroczek T, Malota Z, Wojcik E, et al. Norwood with right ventricle-to-pulmonary artery conduit is more effective than Norwood with Blalock-Taussig shunt for hypoplastic left heart syndrome: mathematic modeling of hemodynamics. Eur J Cardiothorac Surg. 2011;30:1412–1417.
332 Bacha EA, Daves S, Hardin J, et al. Single-ventricle palliation for high-risk neonates: the emergence of an alternative hybrid stage I strategy. J Thorac Cardiovasc Surg. 2006;131:163–171.
333 Galantowicz M, Cheatham JP, Phillips A, et al. Hybrid approach for hypoplastic left heart syndrome: Intermediate results after the learning curve. Ann Thorac Surg. 2008;85:2063–2070. discussion 2070-1
334 Galantowicz M, Cheatham JP. Lessons learned from the development of a new hybrid strategy for the management of hypoplastic left heart syndrome. Pediatr Cardiol. 2005;26:190–199.
335 Simsic JM, Bradley SM, Stroud MR, Atz AM. Risk factors for interstage death after the Norwood procedure. Pediatr Cardiol. 2005;26:400–403.
336 Desai T, Stumper O, Miller P, et al. Acute interventions for stenosed right ventricle-pulmonary artery conduit following the right-sided modification of Norwood-Sano procedure. Congenit Heart Dis. 2009;4:433–439.
337 O’Connor MJ, Ravishankar C, Ballweg JA, et al. Early systemic-to-pulmonary artery shunt intervention in neonates with congenital heart disease. J Thorac Cardiovasc Surg. 2011;142:106–112.
338 Mariano ER, Boltz MG, Albanese CT, et al. Anesthetic management of infants with palliated hypoplastic left heart syndrome undergoing laparoscopic nissen fundoplication. Anesth Analg. 2005;100:1631–1633.
339 Trusler GA, Williams WG, Cohen AJ, et al. William Glenn lecture. The cavopulmonary shunt: evolution of a concept. Circulation. 1990;82:IV131–IV138.
340 Scheurer MA, Hill EG, Vasuki N, et al. Survival after bidirectional cavopulmonary anastomosis: analysis of preoperative risk factors. J Thorac Cardiovasc Surg. 2007;134:82–89.
341 Carlo WF, Carberry KE, Heinle JS, et al. Interstage attrition between bidirectional Glenn and Fontan palliation in children with hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2011;142:511–516.
342 Li J, Hoskote A, Hickey C, et al. Effect of carbon dioxide on systemic oxygenation, oxygen consumption, and blood lactate levels after bidirectional superior cavopulmonary anastomosis. Crit Care Med. 2005;33:984–989.
343 Mott AR, Alomrani A, Tortoriello TA, et al. Changes in cerebral saturation profile in response to mechanical ventilation alterations in infants with bidirectional superior cavopulmonary connection. Pediatr Crit Care Med. 2006;7:346–350.
344 Giroud JM, Jacobs JP. Fontan’s operation: evolution from a procedure to a process. Cardiol Young. 2006;16(Suppl 1):67–71.
345 Hosein RB, Clarke AJ, McGuirk SP, et al. Factors influencing early and late outcome following the Fontan procedure in the current era. The “two commandments”? Eur J Cardiothorac Surg. 2007;31:344–352.
346 Ono M, Boethig D, Goerler H, et al. Clinical outcome of patients 20 years after Fontan operation—effect of fenestration on late morbidity. Eur J Cardiothorac Surg. 2006;30:923–929.
347 Alphonso N, Baghai M, Sundar P, et al. Intermediate-term outcome following the Fontan operation: a survival, functional and risk-factor analysis. Eur J Cardiothorac Surg. 2005;28:529–535.
348 Barker PC, Nowak C, King K, et al. Risk factors for cerebrovascular events following Fontan palliation in patients with a functional single ventricle. Am J Cardiol. 2005;96:587–591.
349 Cheung YF, Chay GW, Chiu CS, Cheng LC. Long-term anticoagulation therapy and thromboembolic complications after the Fontan procedure. Int J Cardiol. 2005;102:509–513.
350 Mahnke CB, Boyle GJ, Janosky JE, et al. Anticoagulation and incidence of late cerebrovascular accidents following the Fontan procedure. Pediatr Cardiol. 2005;26:56–61.
351 Giannico S, Hammad F, Amodeo A, et al. Clinical outcome of 193 extracardiac Fontan patients: the first 15 years. J Am Coll Cardiol. 2006;47:2065–2073.
352 Setty SP, Herrington CS. Fontan procedure: old lessons and new frontiers. Expert Rev Cardiovasc Ther. 2006;4:515–521.
353 Backer CL, Deal BJ, Mavroudis C, et al. Conversion of the failed Fontan circulation. Cardiol Young. 2006;16(Suppl 1):85–91.
354 Mavroudis C, Backer CL, Deal BJ, et al. Evolving anatomic and electrophysiologic considerations associated with Fontan conversion. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2007:136–145.
355 Taylor KL, Holtby H, Macpherson B. Laparoscopic surgery in the pediatric patient post Fontan procedure. Paediatr Anaesth. 2006;16:591–595.
356 Bailey PDJ, Jobes DR. The Fontan patient. Anesthesiol Clin. 2009;27:285–300.
357 Yuki K, Casta A, Uezono S. Anesthetic management of noncardiac surgery for patients with single ventricle physiology. J Anesth. 2011;25:247–256.
358 Odegard KC, McGowan FXJ, Zurakowski D, et al. Coagulation factor abnormalities in patients with single-ventricle physiology immediately prior to the Fontan procedure. Ann Thorac Surg. 2002;73:1770–1777.


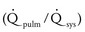 that exceeds 2 to 1 and the potential detrimental effects of chronic right ventricular volume overload are indications for intervention.
that exceeds 2 to 1 and the potential detrimental effects of chronic right ventricular volume overload are indications for intervention.