23 Anesthesia for Heart, Lung, and Heart-Lung Transplantation
Heart transplantation
The history of heart transplantation spans almost a century. Canine heterotopic cardiac transplantation was first reported in 1905,1 but such efforts were doomed by ignorance of the workings of the immune system (Box 23-1). Further research in the late 1950s and early 1960s set the stage for the first human cardiac transplant by Barnard in 1966.2 However, there were few long-term survivors in this era because of continued deficiency in understanding and modulating the human immune system, and the procedure fell into general disfavor. Continued research at selected centers (such as Stanford University) and lessons learned from renal transplantation led to greater understanding of the technical issues and immunology required, and by the early 1980s, cardiac transplantation gained widespread acceptance as a realistic option for patients with end-stage cardiomyopathy.
Heart transplantation experienced explosive growth in the mid-to-late 1980s, but the annual number of heart transplants worldwide plateaued by the early 1990s at approximately 3500 per year.3 The factor limiting continued growth has been a shortage of suitable donors. As of January 2010, there were slightly more than 3000 patients on the United Network for Organ Sharing (UNOS) cardiac transplant waiting list (includes all U.S. candidates), whereas only 2028 heart transplants were performed in the United States during the 2009 calendar year. The median waiting time for a cardiac graft varies widely according to blood type (approximately 52 days for type AB recipients in contrast with 242 days for type O recipients listed for the period 2003–2004). In aggregate, approximately 30% to 37% of those patients on the heart transplant list had spent more than a year waiting for a transplant during 2007.4 Adult patients on the heart transplant waiting list are assigned a status of 1A, 1B, or 2. Status 1A patients require mechanical circulatory support, mechanical ventilation, high-dose or multiple inotropes, with continuous monitoring of left ventricular filling pressure. Status 1B patients require mechanical circulatory support beyond 30 days or inotropic support without continuous monitoring of left ventricular filling pressure. All other patients are classified as Status 2.4 The most frequent recipient indications for adult heart transplantation remain either idiopathic or ischemic cardiomyopathy. Other less common diagnoses include viral cardiomyopathy, systemic diseases such as amyloidosis, and complex congenital heart disease (CHD).
The 1-year survival rate after heart transplantation has been reported to be 79%, with a subsequent mortality rate of approximately 4%/year.3 There has been only slight improvement in the survival statistics over the past decade; the Organ Procurement and Transplant Network reports that the 1- and 3-year survival rates after heart transplantation for those transplanted in the United States during the period 1997–2004 was approximately 87% and 78%, respectively, at the time this chapter was written. One-year survival rate after repeat heart transplantation more than 6 months after the original procedure is slightly lower (63%) but substantially worse if performed within 6 months of the original grafting (39%).3 Risk factors for increased mortality have been associated with recipient factors (prior transplantation, poor human leukocyte antigen matching, ventilator dependence, age, and race), medical center factors (volume of heart transplants performed, ischemic time), and donor factors (race, sex, age). Early deaths most frequently are due to graft failure, whereas intermediate-term deaths are caused by acute rejection or infection. Late deaths after heart transplantation most frequently are due to allograft vasculopathy, post-transplant lymphoproliferative disease or other malignancy, and chronic rejection (Box 23-1).
Recipient Selection
Potential candidates for heart transplantation generally undergo a multidisciplinary evaluation including a complete history and physical examination, routine hematology, chemistries (to assess renal and hepatic function), viral serology, electrocardiography, chest radiography, pulmonary function tests, and right- and left-heart catheterization. Ambulatory electrocardiography, echocardiography, and nuclear gated scans are performed if necessary. The goals of this evaluation are to confirm a diagnosis of end-stage heart disease that is not amenable to other therapies and that will likely lead to death within 1 to 2 years, as well as to exclude extracardiac organ dysfunction that could lead to death soon after heart transplantation. Patients typically have New York Heart Association Class IV symptoms and a left ventricular ejection fraction less than 20%. Although most centers eschew a strict age cutoff, the candidate should have a “physiologic” age younger than 60. Detecting pulmonary hypertension and determining whether it is due to fixed elevation of pulmonary vascular resistance (PVR) is crucial; early mortality because of graft failure is threefold greater in patients with increased PVR (transpulmonary gradient > 15 mm Hg or PVR > 5 dynes•sec•cm−5).5 If increased PVR is detected, a larger donor heart, a heterotopic heart transplant, or a heart–lung transplant may be more appropriate. Active infection and recent pulmonary thromboembolism with pulmonary infarction are additional contraindications to heart transplantation. The results of this extensive evaluation should be tabulated and available to the anesthesia team at all times because heart transplantation is an emergency procedure.
Donor Selection and Graft Harvest
Donors can exhibit major hemodynamic and metabolic derangements that can adversely affect organ retrieval.6 Most brain-dead donors will be hemodynamically unstable.7 Reasons for such instability include hypovolemia (secondary to diuretics or diabetes insipidus), myocardial injury (possibly a result of “catecholamine storm” during periods of increased intracranial pressure), and inadequate sympathetic tone because of brainstem infarction. Donors often also have abnormalities of neuroendocrine function such as low T3 and T4 levels. Administration of T3 to brain-dead animals improves ventricular function after transplantation8; T3 administration has enabled decreases in inotropic support in some9,10 but not all human studies.11 Donor volume status should be assiduously monitored, and inotropic and vasopressor therapy should be guided by data from invasive monitors.
Donor cardiectomy is performed through a median sternotomy, usually simultaneously with recovery of other organs such as lungs, kidneys, and liver. Just before cardiac harvesting, the donor is heparinized and an intravenous cannula is placed in the ascending aorta for administration of conventional cardioplegia. The superior vena cava (SVC) is ligated and the inferior vena cava (IVC) transected to decompress the heart, simultaneous with the administration of cold hyperkalemic cardioplegia into the aortic root. The aorta is cross-clamped when the heart ceases to eject. The heart also is topically cooled with ice-cold saline. After arrest has been achieved, the pulmonary veins are severed, the SVC is transected, the ascending aorta is divided just proximal to the innominate artery, and the pulmonary artery (PA) is transected at its bifurcation. The heart is then prepared for transport by placing it in a sterile plastic bag that is placed, in turn, in another bag filled with ice-cold saline, all of which are carried in an ice chest. Of all the regimens tested, conventional cardioplegia has proved most effective in maintaining cardiac performance.12 The upper time limit for ex vivo storage of human hearts appears to be approximately 6 hours.13
Surgical Procedures
Orthotopic Heart Transplantation
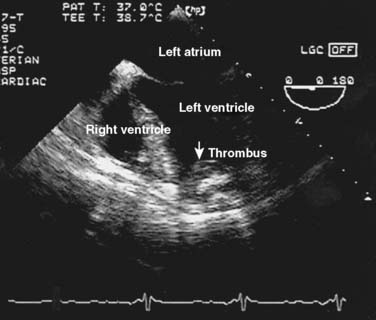
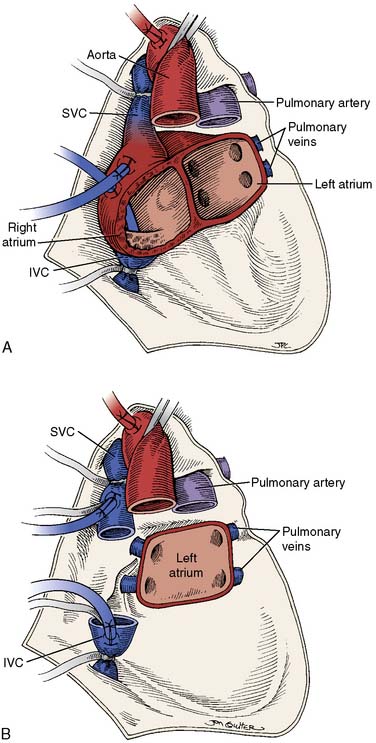
Orthotopic heart transplantation is carried out via a median sternotomy, and the general approach is similar to that used for coronary revascularization or valve replacement. Frequently, patients will have undergone a prior median sternotomy; repeat sternotomy is cautiously performed using an oscillating saw. The groin should be prepped and draped to provide a rapid route for cannulation for cardiopulmonary bypass (CPB) if necessary. After the pericardium is opened, the aorta is cannulated as distally as possible and the IVC and SVC are individually cannulated via the high right atrium. Manipulation of the heart before institution of CPB is limited if thrombus is detected in the heart with transesophageal echocardiography (TEE; Figure 23-1). After initiation of CPB and cross-clamping of the aorta, the heart is arrested and excised (Figure 23-2). The aorta and PA are separated and divided just above the level of their respective valves, and the atria are transected at their grooves. A variant of this classic approach totally excises both atria, mandating bicaval anastomoses. This technique may reduce the incidence of atrial arrhythmias, better preserve atrial function by avoiding tricuspid regurgitation, and enhance cardiac output (CO) after transplantation.14,15
Heterotopic Heart Transplantation
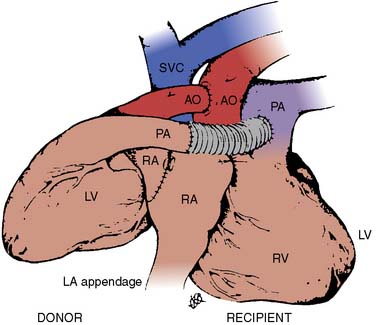
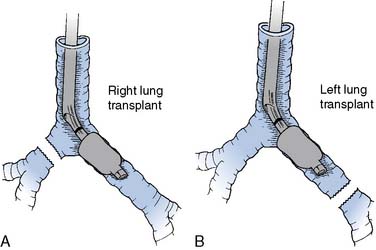
Donor harvesting for heterotopic placement is performed in the previously described manner, except that the azygos vein is ligated and divided to increase the length of the donor SVC; the PA is extensively dissected to provide the longest possible main and right PA; and the donor IVC and right pulmonary veins are oversewn, with the left pulmonary veins incised to create a single large orifice. The operation is performed via a median sternotomy in the recipient, but the right pleura is entered and excised. The recipient SVC is cannulated via the RA appendage, and the IVC via the lower right atrium. After arresting the recipient heart, the LA anastomosis is constructed by incising the recipient left atrium near the right superior pulmonary vein and extending this incision inferiorly, and then anastomosing the respective left atria. The recipient RA-SVC is then incised and anastomosed to the donor RA-SVC, after which the donor aorta is joined to the recipient aorta in an end-to-side manner. Finally, the donor PA is anastomosed to the recipient main PA in an end-to-side manner if it is sufficiently long; otherwise, they are joined via an interposed vascular graft (Figure 23-3).
Special Situations
Mechanical ventricular assist devices (see Chapters 27 and 32) have been used successfully to “bridge” patients who would otherwise die of acute heart failure awaiting transplantation.16 The technique of transplantation is virtually identical in such patients to that for ordinary orthotopic transplantation. However, repeat sternotomy is obligatory. Placement of large-bore intravenous access is prudent because excessive hemorrhage can occur during the transplant procedure.
Rarely, patients will present for cardiac transplantation combined with transplantation of the liver.17 The cardiac allograft usually is implanted first to better enable the patient to survive potential hemodynamic instability associated with reperfusion of the hepatic allograft. Large-bore intravenous access is mandatory. Conventional full heparinization protocols or low-dose heparin with heparin-bonded circuits may be used. A venous cannula can be left in the right atrium at the completion of the heart transplant procedure to serve as a return site for subsequent venovenous bypass during liver transplantation.
Pathophysiology before Transplantation
The pathophysiology of heart transplant candidates is predominantly end-stage cardiomyopathy. Normally, such patients will have both systolic dysfunction (characterized by decreased stroke volume and increased end-diastolic volume) and diastolic dysfunction, characterized by an increased intracardiac diastolic pressure. As compensatory mechanisms to maintain CO fail, the increased LV pressures lead to increases in pulmonary venous pressures and development of pulmonary vascular congestion and edema. A similar process occurs if RV failure also occurs. Autonomic sympathetic tone is increased in patients with heart failure, leading to generalized vasoconstriction, as well as salt and water retention. Vasoconstriction and ventricular dilation combine to substantially increase myocardial wall tension. Over time, the high levels of catecholamines lead to a decrease in the sensitivity of the heart and vasculature to these agents via a decrease in receptor density (i.e., “downregulation”) and a decrease in myocardial norepinephrine stores.18
Therapy of heart failure seeks to reverse or antagonize these processes (see Chapters 10, 32, and 34). Almost all candidates will be maintained on diuretics; hypokalemia and hypomagnesemia secondary to urinary losses are likely, and the anesthesiologist must be alert to the possibility that a patient is hypovolemic from excessive diuresis. Another mainstay of therapy is vasodilators (such as nitrates, hydralazine, and angiotensin-converting enzyme inhibitors), which decrease the impedance to LV emptying and improve cardiac function and survival in patients with end-stage heart failure.19,20 Paradoxically, slow incremental β-blockade with agents such as the β1-antagonist metoprolol also can improve hemodynamics and exercise tolerance in some patients awaiting heart transplantation.21 Patients who are symptomatic despite these measures often will require inotropic therapy. Digoxin is an effective but weak inotrope, and its use is limited by toxic side effects. Phosphodiesterase inhibitors such as amrinone, milrinone, and enoximone are efficacious, but chronic therapy is restricted by concerns about increased mortality in those receiving these agents.22,23 Therefore, inotrope-dependent patients often are treated with intravenous infusions of β-adrenergic agonists such as dopamine or dobutamine. Patients refractory to even these measures may be supported with intra-aortic balloon counterpulsation, but its use is fraught with significant vascular complications and essentially immobilizes the patient. Many patients with low CO are maintained on anticoagulants such as warfarin to prevent pulmonary or systemic embolization, especially if they have atrial fibrillation.
Pathophysiology after Transplantation
The physiology of patients after heart transplantation is of interest not only to anesthesiologists in cardiac transplant centers but to the anesthesiology community at large because a substantial portion of these patients return for subsequent surgical procedures.24,25
Cardiac denervation is an unavoidable consequence of heart transplantation. Many long-term studies indicate that reinnervation is absent,26,27 or at best partial or incomplete,28 in humans. Denervation does not significantly change baseline cardiac function,29,30 but it does substantially alter the cardiac response to demands for increased CO. Normally, increases in heart rate can rapidly increase CO, but this mechanism is not available to the transplanted heart. Heart rate increases only gradually with exercise, and this effect is mediated by circulating catecholamines.26 Increases in CO in response to exercise are instead mostly mediated via an increase in stroke volume.31 Therefore, maintenance of adequate preload in cardiac transplant recipients is crucial. Lack of parasympathetic innervation probably is responsible for the gradual decrease in heart rate after exercise seen in transplant recipients, rather than the usual sharp decline.
Denervation has important implications in the choice of pharmacologic agents used after cardiac transplantation. Drugs that act indirectly on the heart via either the sympathetic (ephedrine) or parasympathetic (atropine, pancuronium, edrophonium) nervous systems generally will be ineffective. Drugs with a mixture of direct and indirect effects will exhibit only their direct effects (leading to the absence of the normal increase in refractory period of the atrioventricular node with digoxin,32 tachycardia with norepinephrine infusion, and bradycardia with neostigmine).33 Thus, agents with direct cardiac effects (such as epinephrine or isoproterenol) are the drugs of choice for altering cardiac physiology after transplantation. However, the chronically high catecholamine levels found in cardiac transplant recipients may blunt the effect of α-adrenergic agents, as opposed to normal responses to β-adrenergic agents.34
Allograft coronary vasculopathy remains the greatest threat to long-term survival after heart transplantation. Allografts are prone to the accelerated development of an unusual form of coronary atherosclerosis that is characterized by circumferential, diffuse involvement of entire coronary arterial segments, as opposed to the conventional form of coronary atherosclerosis with focal plaques often found in eccentric positions in proximal coronary arteries.35 The pathophysiologic basis of this process remains elusive, but it is likely due to an immune cell–mediated activation of vascular endothelial cells to upregulate the production of smooth muscle cell growth factors.36 More than half of all heart transplant recipients have evidence of concentric atherosclerosis 3 years after transplant, and more than 80% at 5 years.37 Because afferent cardiac reinnervation is rare, a substantial portion of recipients with accelerated vasculopathy will have silent ischemia.38 Noninvasive methods of detecting coronary atherosclerosis are insensitive for detecting allograft vasculopathy.39 Furthermore, coronary angiography often underestimates the severity of allograft atherosclerosis40; other diagnostic regimens such as intravascular ultrasound and dobutamine stress echocardiography may detect morphologic abnormalities or functional ischemia, respectively, in the absence of angiographically significant lesions.35,40,41 Therefore, the anesthesiologist should assume that there is a substantial risk for coronary vasculopathy in any heart transplant recipient beyond the first 2 years, regardless of symptoms, the results of noninvasive testing, and even angiography.
Anesthetic Management
Induction
Most patients presenting for heart transplantation will not be in a fasting state and should be considered to have a “full stomach.” Therefore, the induction technique should aim to rapidly achieve control of the airway to prevent aspiration while avoiding myocardial depression. A regimen combining a short-acting hypnotic with minimal myocardial depression (etomidate, 0.3 mg/kg), a moderate dose of narcotic to blunt the tachycardic response to laryngoscopy and intubation (fentanyl, 10 μg/kg), and succinylcholine (1.5 mg/kg) is popular42; high-dose narcotic techniques with or without benzodiazepines also have been advocated.43,44 Vasodilation should be countered with an α-agonist. Anesthesia can be maintained with additional narcotic and sedatives (benzodiazepines or scopolamine).44,45
Intraoperative Management
After induction, the stomach can be decompressed with an orogastric tube and a TEE probe introduced while the bladder is catheterized. A complete TEE examination often will reveal useful information not immediately available from other sources, such as the presence of cardiac thrombi (see Figure 23-1), ventricular volume and contractility, and atherosclerosis of the ascending aorta and aortic arch. Cross-matched blood should be immediately available once surgery commences, especially if the patient has had a previous sternotomy; patients not previously exposed to cytomegalovirus should receive blood from donors who are likewise cytomegalovirus negative. Sternotomy and cannulation for CPB are performed as indicated earlier. The period before CPB often is uneventful, apart from arrhythmias and slow recovery of coronary perfusion because of manipulation of the heart during dissection and cannulation. The PA catheter should be withdrawn from the right heart before completion of bicaval cannulation.
Weaning from bypass begins after ventilation is resumed and the cannula in the SVC is removed. The donor heart should be paced if bradycardia is present despite the inotropic infusion. Once the patient is separated from CPB, the PA catheter can be advanced into position. Patients with increased PVR are at risk for acute RV failure and may benefit from a pulmonary vasodilator such as prostaglandin E1 (0.05 to 0.15 μg/kg/min).46 Rarely, such patients will require support with a RV assist device.47 TEE often will provide additional useful information about right- and left-heart function and volume, and document normal flow dynamics through the anastomoses. Unless a bicaval anastomosis was created, a ridge of redundant tissue will be evident in the left atrium and should not cause alarm (see Videos 1A and 1B, available online).
Protamine then is given to reverse heparin’s effect after satisfactory weaning from CPB. Continued coagulopathy despite adequate protamine is common after heart transplantation, especially if there has been a prior sternotomy. Treatment is similar to that used for other postbypass coagulopathies: meticulous attention to surgical hemostasis, empiric administration of platelets, and subsequent addition of fresh-frozen plasma and cryoprecipitate guided by subsequent coagulation studies (see Chapters 17, 30 and 31). After adequate hemostasis is achieved, the wound is closed in standard fashion and the patient transported to the intensive care unit (ICU).
Postoperative Management and Complications
Management in the ICU after the conclusion of the procedure essentially is a continuation of the anesthetic management after CPB.48 The electrocardiogram; arterial, central venous, and/or PA pressures; and arterial oxygen saturation are monitored continuously. Cardiac recipients will continue to require β-adrenergic infusions for chronotropy and inotropy for up to 3 to 4 days. Vasodilators may be necessary to control arterial hypertension and decrease impedance to LV ejection. Patients can be weaned from ventilatory support and extubated when the hemodynamics are stable and hemorrhage has ceased. The immunosuppressive regimen of choice (typically consisting of cyclosporine, azathioprine, and prednisone, or tacrolimus and prednisone) should be started after arrival in the ICU. Invasive monitoring can be withdrawn as the inotropic support is weaned, and mediastinal tubes removed after drainage subsides (usually after 24 hours). Patients usually can be discharged from the ICU after 2 or 3 days (see Chapters 33–35).
Early complications after heart transplantation include acute and hyperacute rejection, cardiac failure, systemic and pulmonary hypertension, cardiac arrhythmias, renal failure, and infection. Hyperacute rejection is an extremely rare but devastating syndrome mediated by preformed recipient cytotoxic antibodies against donor heart antigens. The donor heart immediately becomes cyanotic from microvascular thrombosis and ultimately ceases to contract.49 This syndrome is lethal unless the patient can be supported mechanically until a suitable heart is found. Acute rejection is a constant threat in the early postoperative period and may present in many forms (e.g., low CO, arrhythmias). Acute rejection occurs most frequently during the initial 6 months after transplantation, so its presence is monitored by serial endomyocardial biopsies, with additional biopsies to evaluate any acute changes in clinical status. Detection of rejection mandates an aggressive increase in the level of immunosuppression, usually including pulses of glucocorticoid or a change from cyclosporine to tacrolimus. Low CO after transplantation may reflect a number of causative factors: hypovolemia, inadequate adrenergic stimulation, myocardial injury during harvesting, acute rejection, tamponade, or sepsis. Therapy should be guided by invasive monitoring, TEE, and endomyocardial biopsy. Systemic hypertension may be caused by pain, so adequate analgesia should be obtained before treating blood pressure with a vasodilator. Because fixed pulmonary hypertension will have been excluded during the recipient evaluation, pulmonary hypertension after heart transplantation usually will be transient and responsive to vasodilators such as prostaglandin E1, nitrates, or hydralazine after either orthotopic or heterotopic placement.50,51 Atrial and ventricular tachyarrhythmias are common after heart transplantation52; once rejection has been ruled out as a cause, antiarrhythmics are used for conversion or control (except those acting via indirect mechanisms such as digoxin, or those with negative inotropic properties such as β-blockers and calcium channel blockers). Almost all recipients will require either β-adrenergic agonists or pacing to increase heart rate in the immediate perioperative period, but 10% to 25% of recipients also will require permanent pacing.53,54 Renal function often improves immediately after transplantation, but immunosuppressives such as cyclosporine and tacrolimus may impair renal function.55,56 Finally, infection is a constant threat to immunosuppressed recipients. Bacterial pneumonia is frequent early in the postoperative period, with opportunistic viral and fungal infections becoming more common after the first several weeks (see Chapter 37).
Pediatric Considerations
In the pediatric population, dilated cardiomyopathy and complex congenital heart defects are the primary indications for heart transplantation. Although the number of donors and recipients has remained stable in recent years, the overall survival has improved in children undergoing heart transplantation. Factors that contribute to this trend are enhanced preservation of the donor heart, improved selection of recipients and donors, and refinements in surgical techniques and immunosuppressive therapy.57
Cardiac transplantation is recommended when the child’s expected survival is less than 1 year. In some centers, this therapy is offered as the primary intervention to the infant born with hypoplastic left-heart syndrome. The perioperative and intraoperative management of these infants undergoing heart transplant have been extensively reviewed.58
The preoperative assessment for heart transplantation in the patient with complex CHD might be more extensive depending on the heart defect and previous corrective or palliative procedures. Similar to the child with dilated cardiomyopathy, assessment of the indexed pulmonary vascular resistance (PVRI) is essential.57 In adults, a PVRI greater than 5 units and a transpulmonary gradient greater than 15 mm Hg are contraindications for transplantation. In children, the acceptable PVRI is less than 10 units, but it is not unusual for a pediatric heart transplant candidate to have a PVRI greater than 10 units. In one pediatric cardiac center, 20% of the transplanted patients had PVRIs greater than 6 units. However, in the 6 to 10 unit range, the child is at risk for acute RV failure because the donor’s right ventricle is thin walled and the myocardium has been ischemic. If the PVRI decreases significantly in the catheterization laboratory, with a trial of vasodilator testing, hyperventilation, 100% O2, and nitric oxide, the candidate is acceptable for transplant. If the PVRI remains borderline, the candidate is admitted to the hospital for a 1- or 2-week trial with milrinone and dobutamine. If the PVRI then falls, transplantation is offered. These patients might benefit from pulmonary vasodilation therapy during weaning from CPB and in the ICU.
Besides determining the blood type (ABO), it is important to assess for the presence of antibodies against human histocompatability leukocyte antigen.59 Antibodies against human histocompatability leukocyte antigen may have developed in the recipient who was exposed to blood products during palliation for complex CHD. Hyperacute rejection may lead to graft loss in the operating room in this setting. The risk for primary graft failure is greater if more than mild systolic dysfunction was present in the donor heart before transplantation. In pediatrics, the donor-recipient heart size matching in weight ranges between 80% and 300%. At surgery, the bicaval technique is preferred.
Lung transplantation
History and Epidemiology
Although the first human lung transplant was performed in 1963, surgical technical problems and inadequate preservation and immunosuppression regimens prevented widespread acceptance of this procedure until the mid-1980s (Box 23-2). Advances in these areas have since made lung transplantation a viable option for many patients with end-stage lung disease. For the period January 1, 1985, to June 30, 2008, a total of 29,732 lung transplants were reported to the Registry of the International Society for Heart and Lung Transplantation.3 The frequency of both single- and double-lung transplants increased exponentially during the period up until 1993, with the sharpest growth in unilateral transplants. According to data collected by UNOS between 2000 and 2002, the annual frequency of lung transplantation has remained stagnant, with the total number still averaging in the vicinity of 1000. This is unchanged from the time between 1993 and 1995, when the numbers first leveled off. Further growth in lung transplantation is constrained by a shortage of donor organs, with demand for organs still vastly exceeding supply. This may potentially be exacerbated by data that were published in 2009, revealing that double-lung transplant afforded fewer hospitalizations and potentially better long-term survival.60
It is estimated that in excess of a million individuals with end-stage lung disease are potential recipients of lung transplants.61 Some had hoped that non–heart-beating donors would provide an alternative source of organs, but this has not been the case. The Organ Procurement and Transplantation Network currently registers approximately 4000 patients for lung transplantation. This number does not accurately reflect the number of organs required because some patients will require bilateral lung transplantation. Average time to transplant increased to as much as 451 days in 1999; however, recently, that time has again decreased significantly. Currently, about one fourth of patients are transplanted within 251 days. Most of this improvement has been seen with recipients who are 50 years and older. One explanation for this may be increasing leniency in organ-selection criteria. This seems not to have been associated with increasing mortality rates. Mortality for patients on the waiting list also has continued to decline, from a 1993 high of close to 250 per 1000 patient-years to approximately 140 in 2002. Although some of this improvement may be ascribed to better medical management of patients on the waiting list, it is also likely due to broadened criteria for acceptance for transplantation and subsequent inclusion of patients with less severe illness.
Increased experience with lung transplantation has been accompanied by a decrease in both operative and long-term mortality. For example, 30-day mortality rate for double-lung transplantation decreased from 44% in 1988 to 13.6% in 1991, whereas that for single-lung transplantation decreased from 22.7% to 12.6%.62 As of the end of 2008, 3-year actuarial survival for recipients of both single- and double-lung transplants performed in the era 1992 to 1995 was approximately between 56% and 67% depending on recipient age percentage, which is a trend of continuing improvement of the periods preceding 2005.3 Even better survival data have been reported from centers with extensive experience with these procedures (1-year survival rates of 82% for double-lung recipients and 90% for single-lung recipients).63 Infection is the most frequent cause of death in the first year after transplant, but this is superseded in later years by bronchiolitis obliterans.3 Notable is that 21% of all lung transplants were performed at 21 centers around the world averaging 50 procedures per year.3
Some of the most challenging patients are those with cystic fibrosis. The 1-year survival rate of 79% and 5-year survival rate of 57% after lung transplantation has shown that despite the high incidence of poor nutrition and the almost ubiquitous colonization by multidrug-resistant organisms, these patients can still successfully undergo lung transplantation with acceptable outcomes data.64
It is a sign of the maturity of lung transplantation procedures that survival data for “redo” lung transplantation also are becoming available. A late-1991 survey of centers reported that actuarial survival after redo transplantation was significantly worse than that of first-time recipients (e.g., 35% vs. > 75% at 1 year),65 and subsequent data have confirmed this observation.3 Infection and multiorgan failure before repeat transplant are associated with an almost uniformly fatal outcome. Subsequent data from UNOS, however, have shown an improvement, with the 1-year survival rate at 66.3% in the retransplant patients as compared with 83.8% in the primary transplant population. This is, however, significantly worse at 3 years, with repeat survival rate at 38.8% compared with 63.2%.
Recipient Selection
Potential recipients undergo a multidisciplinary assessment of their suitability, including pulmonary spirometry, radiography (plain film and chest CT scan), and echocardiography or multigated image acquisition scan. Patients older than 40 years and those with pulmonary hypertension usually undergo left-heart catheterization to exclude significant coronary atherosclerosis or an intracardiac shunt. TEE may yield data (e.g., unanticipated atrial septal defect) that will alter subsequent surgical approach in approximately one quarter of patients with severe pulmonary hypertension.66 Candidates who are accepted often are placed on a physical conditioning regimen to reverse muscle atrophy and debilitation and kept within 20% of their ideal body weight. Because lung transplantation is an emergency procedure (limited by a lung preservation time of 6 to 8 hours),67 results of this comprehensive evaluation should be readily available to the anesthesia team at all times. Weiss68 published data in 2009 that supported the cautious transplantation of patients older than 60 years but recommended against transplantation of patients older than 70. Data from the same authors suggested that race-matching also provided a survival benefit that manifested itself in the first 2 years after transplant.69
Donor Selection and Graft Harvest
The ongoing shortage of suitable donor organs has led to a liberalization of selection criteria. Prospective lung donors who were cigarette smokers are no longer rejected simply based on a pack-year history. Computed tomography has been used to assess the structural integrity of the lung, particularly in donors who have suffered traumatic chest injury. Lungs that have contusion limited to less than 30% of a single lobe can be considered adequate.70 Greater use also has been made of organs from older but otherwise healthy donors (55 to 60 years old), especially when the ischemic period will be short.71 A clear chest radiograph, normal blood gas results, unremarkable findings on bronchoscopy, sputum stain, and direct intraoperative evaluation confirm satisfactory lung function. The lungs are matched to the recipient for ABO blood type and size (oversized lungs can result in severe atelectasis and compromise of venous return in the recipient, especially after double-lung transplantation). Donor serology and tracheal cultures will guide subsequent antibacterial and antiviral therapy in the recipient.
Surgical Procedures
Because of the relative shortage of lung donors, and the finding that recipients can gain significant exercise tolerance even with only one transplanted lung,72 single-lung transplantation is the procedure of choice for all lung transplant candidates, except when leaving one of the recipient’s lungs in place would predispose to complications. For example, the presence of lung disease associated with chronic infection (cystic fibrosis and severe bronchiectasis) mandates double-lung transplantation to prevent the recipient lung from acting as a reservoir of infection and subsequently cross-contaminating the allograft. Patients with severe air trapping may require double-lung transplantation if uncontrollable ventilation/perfusion mismatching will be likely after transplantation. Lobar transplantation into children and young adults from living-related donors is discussed separately later in this chapter.
Single-Lung Transplant
The choice of which lung to transplant is usually based on multiple factors, including avoidance of a prior operative site, preference for removing the native lung with the worst ventilation/perfusion ratio, and donor lung availability. The recipient is positioned for a posterolateral thoracotomy, with the ipsilateral groin prepped and exposed in case CPB becomes necessary. With the lung deflated, a pneumonectomy is performed, with special care to preserve as long a PA segment as possible. After removal of the diseased native lung, the allograft is positioned in the chest with precautions to maintain its cold tissue temperature. The bronchial anastomosis is performed first. A “telescoping” anastomosis is used if there is significant discrepancy in size between the donor and the recipient. The object of the technique is to minimize the chance of dehiscence. Although it was once common to wrap bronchial anastomoses with omentum, wrapping produces no added benefit when a telescoping anastomosis is performed. The PA is anastomosed next, and finally the pericardium is opened and the allograft LA cuff containing the pulmonary venous orifices is anastomosed to the native left atrium. The pulmonary circuit is then flushed with blood and de-aired. The initial flush solution is usually cold (4° C) but is followed by a warm (37° C) flush. The warm flush usually is performed during final completion of the vascular anastomoses. The goal of the flushing is to achieve a controlled reperfusion.73 The contents of this solution are listed in Box 23-3.
Double-Lung Transplant
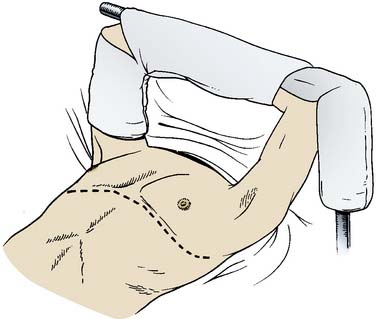
Early attempts at double-lung transplantation using an en bloc technique via a median sternotomy were plagued by frequent postoperative airway dehiscence because of poor vascular supply of the tracheal anastomosis, by hemorrhage caused by extensive mediastinal dissection (which also resulted in cardiac denervation), by the requirement for complete CPB and cardioplegic arrest (to facilitate pulmonary arterial and venous anastomoses), and by poor access to the posterior mediastinum. The subsequent development of the bilateral sequential lung transplant technique via a “clamshell” thoracosternotomy (essentially two single-lung transplants performed in sequence) has avoided many of the problems inherent in the en bloc technique.74,75 An alternative to using a clamshell incision in slender patients is an approach through two individual anterolateral thoracotomies. This results in a particularly pleasing cosmetic result in female patients because the scar falls in the breast crease. Use of CPB is optional, exposure of the posterior mediastinum is enhanced (improving hemostasis), and cardiac denervation usually can be avoided. Pleural scarring usually is extensive in patients with cystic fibrosis, and postoperative hemorrhage and coagulopathy are the rule if CPB is required. Transplantation of both lungs is performed in the supine position. The groin is prepped and exposed in case CPB is required. If a clamshell incision is utilized, the arms are padded and suspended over the head on an ether screen (Figure 23-4). In the slender patient whose anteroposterior chest dimensions are normal, the arms may be tucked at the patient’s sides. Recipient pneumonectomy and implantation of the donor lung are performed sequentially on both lungs in essentially the same manner as described earlier for a single-lung transplant. The native lung with the worst function should be transplanted first. In patients whose indication for transplantation is suppurative disease, the pleural cavity is pulse-lavaged with antibiotic-containing solution that has been tailored to that patient’s antimicrobial sensitivity profile. In addition to this, the anesthesiologist irrigates the trachea and bronchi with diluted iodophor solution before the donor lung is brought onto the surgical field.
Pathophysiology before Transplantation
Patients with highly compliant lungs and obstruction of expiratory airflow cannot completely exhale the delivered tidal volume, resulting in positive intrapleural pressure throughout the respiratory cycle (“auto-PEEP” [positive end-expiratory pressure] or “intrinsic PEEP”), which decreases venous return and causes hypotension.76 The presence of auto-PEEP is highly negatively correlated with forced expiratory volume in 1 second (FEV1; percentage predicted) and highly positively correlated with pulmonary flow resistance and resting hypercarbia.77 Hyperinflation is a frequent complication of single-lung ventilation during lung transplantation in patients with obstructive lung disease. Hyperinflation-induced hemodynamic instability can be diagnosed by turning off the ventilator for 30 seconds and opening the breathing circuit to the atmosphere. If the blood pressure returns to its baseline value, hyperinflation is the underlying cause. Hyperinflation can be ameliorated with deliberate hypoventilation (decreasing both the tidal volume and rate).78 Although this may result in profound hypercarbia, high carbon dioxide tensions are well tolerated in the absence of hypoxemia. PEEP also may decrease air trapping because it decreases expiratory resistance during controlled mechanical ventilation.79 However, the application of PEEP requires close monitoring because if the level of extrinsic PEEP applied exceeds the level of auto-PEEP, further air trapping may result.
RV failure frequently is encountered in lung-transplant recipients with pulmonary hypertension because of chronically increased RV afterload. The response of the right ventricle to a chronic increase in afterload is to hypertrophy, but eventually this adaptive response is insufficient. As a result, RV stroke volume decreases and chamber dilation results. The following should be kept in mind when caring for patients with severe dysfunction (Box 23-4). First, increases in intrathoracic pressure may markedly increase PVR,80 leading to frank RV failure in patients with chronic RV dysfunction. Changes in RV function may occur immediately after adding PEEP, increasing tidal volume, or decreasing expiratory time, and can have devastating consequences. In addition, although intravascular volume expansion in the presence of normal PVR increases CO, overzealous infusion in patients with increased PVR will increase RV end-diastolic pressure and RV wall stress, decreasing CO.81 Inotropes with vasodilating properties (such as dobutamine or milrinone) often are a better choice than volume for augmenting CO in the setting of increased PVR. Furthermore, the right ventricle has a greater metabolic demand yet a lower coronary perfusion pressure than normal. RV performance can be augmented by improving RV coronary perfusion pressure with α-adrenergic agents, provided these vasoconstrictors do not disproportionately increase PVR. This can sometimes be a better choice than augmenting the perfusion pressure with β-adrenergic agents because the oxygen supply is increased without a large increase in oxygen demand. Finally, vasodilators such as nitroprusside or prostaglandin E1 may be effective in decreasing PVR and improving RV dysfunction early in the disease process, when only mild-to-moderate pulmonary hypertension is present. However, they are of notably limited value in the presence of severe, end-stage pulmonary hypertension. Systemic vasodilation and exacerbation of shunting often limit their use. Inhaled nitric oxide has shown promise as a means of acutely decreasing PVR without altering systemic hemodynamics both during the explantation phase and after lung transplantation.82,83 Nitric oxide decreases both PA pressure and intrapulmonary shunting. Further, the combination of inhaled nitric oxide and aerosolized prostacyclin had a synergistic effect, without causing deleterious effects on the systemic perfusion pressure. The use of nitric oxide with or without inhaled prostacyclin may be helpful in avoiding CPB in patients having lung transplantation (see Chapters 10, 24, and 34).
Pathophysiology after Lung Transplantation
The implantation of the donor lung(s) causes marked alterations in recipient respiratory physiology. In single-lung recipients, the pattern of ventilation/perfusion matching depends on the original disease process. For example, with pulmonary fibrosis, blood flow and ventilation gradually divert to the transplanted lung, whereas in patients transplanted for diseases associated with pulmonary hypertension, blood flow is almost exclusively diverted to the transplanted lung, which still receives only half of the total ventilation.84 In such patients the native lung represents mostly dead-pace ventilation. Transplantation results in obligatory sympathetic and parasympathetic denervation of the donor lung and, therefore, alters the physiologic responses of airway smooth muscle. Exaggerated bronchoconstrictive responses to the muscarinic agonist methacholine have been noted in some (but not all) studies of denervated lung recipients.85,86 The mechanism of hyper-responsiveness may involve cholinergic synapses, inasmuch as they are the main mediators of bronchoconstriction. For example, electrical stimulation of transplanted bronchi (which activates cholinergic nerves) produces a hypercontractile response.87 This suggests either enhanced release of acetylcholine from cholinergic nerve endings because of an increased responsiveness of parasympathetic nerves or else loss of inhibitory innervation. Such effects are unlikely to be postsynaptic in origin because the number and affinity of muscarinic cholinergic receptors on transplanted human bronchi are similar to controls.88 Reinnervation during subsequent weeks to months has been demonstrated in several animal models,89,90 but there was no definitive evidence concerning reinnervation of transplanted human lungs until a small study was published in 2008 that showed return of cough reflex to noxious stimuli (distal to the anastomosis) within 12 months. The presence of nerve cells in the anastomoses of deceased patients also was noted.91 Mucociliary function is transiently severely impaired after lung transplantation and remains depressed for up to a year after the procedure.92 Thus, transplant recipients require particularly aggressive endotracheal suctioning to remove airway secretions.
Lung transplantation also profoundly alters the vascular system. The ischemia and reperfusion that are an obligatory part of the transplantation process damage endothelia. Cold ischemia alone decreases β-adrenergic cyclic adenosine monophosphate–mediated vascular relaxation by approximately 40%, and subsequent reperfusion produces even greater decreases in both cyclic guanosine monophosphate–mediated and β-adrenergic cyclic adenosine monophosphate–mediated pulmonary vascular smooth muscle relaxation.93 Endothelial damage in the pulmonary allograft also results in “leaky” alveolar capillaries and the development of pulmonary edema. Pulmonary endothelial permeability is approximately three times greater in donor lungs than in healthy volunteers.94 Regulation of pulmonary vasomotor tone solely by circulating humoral factors is another side effect of denervation. Changes in either the levels of circulating mediators or in the responsiveness of the pulmonary vasculature to such mediators may result in dramatic effects on the pulmonary vasculature. An example of the former is the finding that the potent vasoconstrictor endothelin is present at markedly increased levels (two to three times normal) immediately after transplantation and remains increased for up to a week thereafter.95 Alterations in the response of denervated pulmonary vasculature to α1-adrenergic agents96 and prostaglandin E1,97 as well as a reduction in nitric oxide activity, also have been demonstrated in acutely denervated lung.96 Dysfunctional responses to mediators may be exaggerated if CPB is required.96 Pulmonary vacular resistance can be substantially decreased with the administration of inhaled nitric oxide after reperfusion. It remains unclear whether nitric oxide also ameliorates reperfusion injury. Several studies suggest that nitric oxide prevents or modulates reperfusion injury as measured by decreased lung water, lipid peroxidase activity, neutrophil aggregation in the graft, and decreased IL-6, IL-8, and IL-10.98–101 However, a number of studies suggest that although nitric oxide has an effect on pulmonary hemodynamics, it does not ameliorate reperfusion injury.102–104
Aerosolized inhaled prostacyclin also decreases PVR after reperfusion and improves oxygenation without the added theoretic risk for worsening reperfusion injury.105 Inhaled prostacyclin has approximately the same effectiveness as nitric oxide in treating lungs damaged by reperfusion injury and offers the added benefit of being cheaper.106
A number of other agents have shown promise in decreasing postreperfusion injury in animal studies. Tetrahydrobiopterin, an essential cofactor in the nitric oxide synthase pathway, decreased the intracellular water, myeloperoxidase activity, and lipid peroxidation, and increased cyclic guanosine monophosphate levels when given during reperfusion.107 The administration of surfactant into the donor lung before harvest also appeared to ameliorate ischemia/reperfusion injury in pigs. There was a decrease in the PVR, less inflammatory cellular infiltrate, and an increase in nitric oxide levels in the group that received surfactant.108
Given these pathophysiologic derangements, it is not surprising that PVR increases in the transplanted lung.109,110 However, what the clinician observes in the lung-transplant patient will depend on the severity of pulmonary vascular dysfunction present before surgery. PA pressures decrease dramatically during lung transplantation in patients who had pulmonary hypertension before transplantation111 and remain so for weeks to months thereafter.111–117 Concomitant with the decrease in PA pressure, there is an immediate decrease in RV size after lung transplantation in those patients with preexisting pulmonary hypertension, as well as a return to a more normal geometry of the interventricular septum.111 Both of these effects are sustained over several weeks to months.111–117 Although echocardiographic indices of RV function (RV fractional area change) have not shown a consistent improvement in the immediate post-transplant period,111 several other studies have documented improvement in RV function during the first several months after lung transplantation.111,112,114–117 One striking finding was that persistent depression of RV function (defined as baseline RV fractional area change of less than 30% with failure to increase after transplant by either at least 5% or by 20% of baseline) was statistically associated with death in the immediate perioperative period.111
Anesthetic Management
Preoperative Evaluation and Preparation
Equipment necessary for this procedure is analogous to that used in any procedure in which CPB and cardiac arrest are a real possibility. Special mandatory pieces of equipment include some method to isolate the ventilation to each lung; although bronchial blockers have their advocates, double-lumen endobronchial tubes offer the advantages of easy switching of the ventilated lung, suctioning of the nonventilated lung, and facile independent lung ventilation after surgery. A left-sided double-lumen endobronchial tube is suitable for virtually all lung transplant cases (even left-lung transplants; Figure 23-5). Regardless of whether a bronchial blocker or double-lumen tube is used, a fiberoptic bronchoscope is absolutely required to rapidly and unambiguously verify correct tube positioning, evaluate bronchial anastomoses, and clear airway secretions. An adult-sized bronchoscope offers better field of vision and superior suctioning capability but can be used only with 41 or 39 French double-lumen tubes. A ventilator with low internal compliance is necessary to adequately ventilate the noncompliant lungs of recipients with restrictive lung disease or donor lungs suffering from reperfusion injury. The added capability of the ventilator to deliver pressure-controlled ventilation also is important, especially for the patients who have pulmonary fibrotic disease or reperfusion injury. Single-lung recipients with highly compliant lungs may require independent lung ventilation with a second ventilator after transplantation (discussed in detail later). A PA catheter capable of estimating right ventricular ejection fraction (RVEF) can be useful in diagnosing RV failure and its response to inotropes and vasodilators, as well as the response of the right ventricle to clamping of the PA. However, RVEF catheters are not accurate in the presence of significant tricuspid regurgitation or when malpositioned. Continuous mixed venous oximetry is beneficial in evaluating tissue oxygen delivery in patients subject to sudden, severe cardiac decompensation in the course of the operation, as well as the responses to therapy. A rapid-infusion system can be lifesaving in cases in which major hemorrhage occurs because of anastomotic leaks, inadequate surgical ligation of mediastinal collateral vessels, chest wall adhesions, or coagulopathy after CPB.
Induction of Anesthesia
Patients presenting for lung transplantation frequently arrive in the operating room area without premedication. Indeed, many will be admitted directly to the operating room from home. Because of the nature of the procedure planned, and many months on the transplant waiting list, these patients are often extremely anxious. Considering the risk for respiratory depression from sedatives in patients who are chronically hypoxic or hypercapnic, or both, only the most judicious use of intravenous benzodiazepines or narcotics is warranted. Assiduous administration of adequate local anesthesia during placement of invasive monitoring will also considerably improve conditions for both the patient and anesthesiologist. The standard noninvasive monitoring typical of cardiovascular procedures (two electrocardiogram leads including a precordial lead, blood pressure cuff, pulse oximetry, capnography, and temperature measurement) is used. Intravenous access sufficient to rapidly administer large volumes of fluid is required. Generally, two large-bore (16- or, preferably, 14-gauge catheters, or a 9 French introducer sheath) intravenous catheters are placed. Patients for bilateral sequential lung transplantation who will receive a “clamshell” thoracosternotomy (see Figure 23-4) should have intravenous catheters placed in the internal or external jugular veins, because peripherally placed intravenous catheters often are unreliable when the arms are bent at the elbow and suspended from the ether screen. An intra-arterial catheter is an absolute requirement for blood pressure monitoring and for obtaining specimens for arterial blood gases. Continuous monitoring via a fiberoptic electrode placed in the arterial catheter occasionally may be useful if this technology is available. The femoral artery should be avoided, if possible, because the groin may be needed as a site for cannulation for CPB. Although the radial or brachial artery may be used in single-lung transplantation patients, these sites are not optimal in those who will require CPB (e.g., en bloc double-lung transplants or patients with severe pulmonary hypertension) because the transduced pressure may inaccurately reflect central aortic pressure during and after CPB, as well as in patients undergoing a clamshell thoracosternotomy, because of the positioning of the arms. In the authors’ institution, the majority of patients now have bilateral limited thoracotomies instead of the thoracosternotomy. An axillary arterial catheter may be useful in the latter situations because it provides a more accurate measure of central aortic pressure and allows sampling blood closer to that perfusing the brain. This may be important if partial CPB with a femoral arterial cannula is used because differential perfusion of the upper and lower half of the body may result. A PA catheter is inserted via the internal or external jugular veins. A TEE probe is placed after the airway is secured. PA pressure monitoring is most useful in patients who have preexisting pulmonary hypertension, especially during induction and during initial one-lung ventilation (OLV) and PA clamping. Position of the PA catheter can be verified by TEE to ensure that it is residing in the main PA.
Three main principles should guide the formulation of a plan for induction: (1) protection of the airway; (2) avoidance of myocardial depression and increases in RV afterload in patients with RV dysfunction; and (3) avoidance and recognition of lung hyperinflation in patients with increased lung compliance and expiratory airflow obstruction (Box 23-5). All lung transplants are done on an emergency basis, and the majority of patients will have recently had oral intake and must be considered to have “full stomachs.” Because aspiration during induction would be catastrophic, every measure must be taken to protect the airway. Patients with known or suspected abnormalities of airway anatomy should be intubated awake after topical anesthesia is applied to the airway. Although a conventional rapid-sequence intravenous induction with a short-acting hypnotic (such as etomidate, 0.2 to 0.3 mg/kg), a small amount of narcotic (e.g., up to 10 μg/kg fentanyl), and succinylcholine usually will be tolerated, patients with severe RV dysfunction may exhibit profound hemodynamic instability in response to this induction regimen. For such patients, a more gradual induction is recommended, with greater reliance on high doses of narcotics and ventilation with continuous application of cricoid pressure. Patients with bullous disease or fibrotic lungs requiring high inflation pressures may develop a pneumothorax during initiation of positive-pressure ventilation. Acute reductions in Sao2 accompanied by difficulty in ventilating the lungs and refractory hypotension should generate strong suspicions that a tension pneumothorax has developed. RV function can be impaired during induction by drug-induced myocardial depression, increases in afterload, or by ischemia secondary to acute RV dilation. Agents that act as myocardial depressants (such as thiopental) should be avoided in such patients. Increases in RV afterload can result from inadequate anesthesia, exacerbation of chronic hypoxemia and hypercarbia and metabolic acidosis, as well as increases in intrathoracic pressure because of positive-pressure ventilation. Systemic hypotension is poorly tolerated because increased RV end-diastolic pressure will diminish net RV coronary perfusion pressure. In addition, chronic increase of RV afterload increases the metabolic requirements of RV myocardium. Once the trachea is intubated and positive-pressure ventilation initiated, the avoidance of hyperinflation in patients with increased pulmonary compliance or bullous disease is crucial. Small tidal volumes, low respiratory rates, and inspiratory/expiratory (I:E) ratios should be used (“permissive hypercapnia”). If hemodynamic instability does occur with positive-pressure ventilation, the ventilator should be disconnected from the patient. If hyperinflation is the cause of hypotension, blood pressure will increase within 10 to 30 seconds of the onset of apnea. Ventilation then can be resumed at a tidal volume and/or rate compatible with hemodynamic stability.
Intraoperative Management
Institution of OLV occurs before hilar dissection and may compromise hemodynamics or gas exchange, or both (Box 23-6). Patients with diminished lung compliance often can tolerate OLV with normal tidal volumes and little change in hemodynamics. In contrast, patients with increased lung compliance and airway obstruction often will exhibit marked hemodynamic instability, unless the tidal volume is decreased and the expiratory time is increased. The magnitude of hypoxemia generally peaks about 20 minutes after beginning OLV. Hypoxemia during OLV may be treated with continuous positive airway pressure applied to the nonventilated lung,118 PEEP to the ventilated lung, or both. Continuous positive airway pressure attempts to oxygenate the shunt fraction but may interfere with surgical exposure. PEEP attempts to minimize atelectasis in the ventilated lung, but may concomitantly increase shunt through the nonventilated lung. Definitive treatment of shunt in the nonventilated lung is provided by rapid isolation and clamping of the PA of the nonventilated lung. Pneumothorax on the nonoperative side may result during OLV if a large tidal volume is used.
BOX 23-6 Management Principles for One-Lung Ventilation During Lung Transplantation
CPAP, continuous positive airway pressure; PEEP, positive end-expiratory pressure.
PA clamping usually is well tolerated, except in the face of pulmonary hypertension with diminished RV reserve. If the degree of RV compromise is uncertain, a 5- to 10-minute trial of PA clamping is attempted; then the RV is evaluated by serial COs and RVEF measurements and inspection by TEE. A significant decrease in CO may predict patients who will require extracorporeal support.119 Other indications for CPB in lung transplantation are listed in Box 23-7.120
BOX 23-7 Indications for Cardiopulmonary Bypass During Lung Transplantation
| Cardiac index | < 2 L/min/m2 |
| SvO2 | < 60% |
| Mean arterial pressure | < 50 to 60 mm Hg |
| SaO2 | < 85% to 90% |
| pH | < 7.00 |
Patients with severe pulmonary hypertension (greater than two thirds of systemic pressure) generally will be placed on CPB before PA clamping. The intraoperative use of nitric oxide (20 to 40 parts per million [ppm]) may allow some procedures to proceed without the use of CPB.121
Lung transplantation usually can be performed without the aid of CPB; even during bilateral sequential lung transplantation, experienced teams utilize CPB for only about one quarter of patients.122,123 Although CPB may provide stable hemodynamics, it is associated with an increased transfusion requirement.78 In addition, graft function (as reflected by alveolar-arterial oxygen gradient) may be compromised,124 endothelium-dependent cyclic guanosine monophosphate–mediated and β-adrenergic cyclic adenosine monophosphate–mediated pulmonary vascular relaxation may be impaired to a greater degree,125 and a longer period of mechanical ventilation may be necessary.122 Several exceptional circumstances require CPB: the presence of severe pulmonary hypertension because clamping of the PA will likely result in acute RV failure and “flooding” of the nonclamped lung, the repair of associated cardiac anomalies (e.g., patent foramen ovale, atrial or ventricular septal defects), treatment of severe hemodynamic or gas exchange instabilities, and living-related lobar transplantation. Hypercarbia generally is well tolerated and should not be considered a requirement for CPB per se.78 Thus, the frequency of CPB will depend on recipient population factors such as prevalence of end-stage pulmonary vascular disease and associated cardiac anomalies.126 The use of femoral venous and arterial cannulae for CPB during lung transplantation may lead to poor venous drainage and/or “differential perfusion” of the lower and upper body. Moreover, native pulmonary blood flow continues and may act as an intrapulmonary shunt during CPB. In this case, the cerebral vessels receive this desaturated blood, whereas the lower body is perfused with fully oxygenated blood from the CPB circuit. This effect is detectable by blood gas analysis of samples drawn from suitable arteries or by appropriately located pulse oximeter probes. Treatment includes conventional measures to increase venous return and augment bypass flow, or placing a venous cannula in the right atrium if this is feasible. The anesthesiologist also should maximize the inspired oxygen concentration and add PEEP to decrease intrapulmonary shunt. If all other measures fail, ventricular fibrillation can be induced using alternating current.127
ECMO also has been suggested as an alternative method of CPB during lung transplantation. It has been suggested that the use of ECMO with heparin-bonded circuits might improve the outcome of both single- and double-lung transplants by lessening the amount of pulmonary edema, especially in those patients who need CPB because of hemodynamic instability or who have primary pulmonary hypertension. An added benefit of this technique is that it clears the operative field of bypass cannulae, making left-sided transplant as unimpeded as right-sided transplant. There is no apparent increase in transfusion requirement.128 Another added benefit of using ECMO in situ is that reperfusion of the lungs can be more easily controlled because the CO transiting the newly transplanted lung can be precisely controlled. This is especially the case for patients with advanced pulmonary hypertension.129
If CPB is used, weaning from circulatory support occurs when the graft anastomoses are complete. Ventilation is resumed with a lung protection strategy similar to that used in the ARDSnet (Acute Respiratory Distress Syndrome Network) trial.71 This demonstrated that patients with decreased compliance related to acute respiratory distress syndrome had a 22% decrease in mortality rate when applying tidal volumes of 6 mL/kg and a plateau pressure less than 30 cm H2O.71 Minimizing the inspired fraction of O2 may help prevent generation of oxygen free radicals and modulate reperfusion injury. Fio2 can be decreased to the minimum necessary to maintain the Spo2 greater than 90%. Special attention should be directed to assessing and supporting RV function during this period, inasmuch as RV failure is the most frequent reason for failure to wean. Although the right ventricle often can be seen in the surgical field, TEE is more valuable for visualizing this structure’s functional properties at this juncture. TEE also allows the evaluation of PA (see Video 2, available online) and pulmonary vein anastomoses. The PA diameter should be greater than 1 cm. Interrogating the pulmonary veins should demonstrate a two-dimensional diameter that is at least 0.5 cm with the presence of flow as measured by color-flow Doppler. In addition, pulse wave Doppler interrogation should yield flow rates less than 100 cm/sec to indicate adequacy of anastomosis. The operator must ensure that they align the Doppler beam angle with the pulmonary vein flow because misalignment may lead to underestimation of the true peak venous flow (see Video 3, available online). Care should be taken to measure these flow rates with both lungs being perfused because the measurements could be erroneous if measured with one PA clamped (Figure 23-6).130,131 Inotropic support with dobutamine or epinephrine, as well as pulmonary vasodilation with nitroglycerin, nitroprusside, milrinone, or nitric oxide, may be necessary if RV dysfunction is evident. Milrinone has the advantage of providing both inotropic and vasodilatory effects; however, its administration can be complicated by significant systemic hypotension, necessitating the concomitant use of epinephrine or norepinephrine (see Chapters 10, 24, 27, 32, and 34).
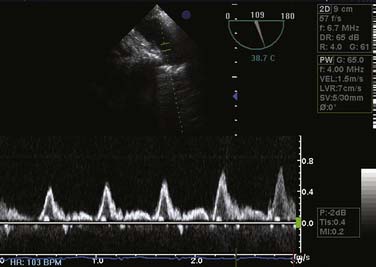
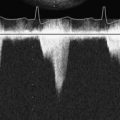
Figure 23-6 Pulsed-wave Doppler interrogation of the left superior pulmonary vein after lung transplantation.
Coagulopathy after weaning from CPB is common. The severity of coagulopathy may be worse after double- than single-lung transplantation, probably because of the more extensive dissection, presence of collaterals and scarring, and the longer duration of CPB. Factors under the anesthesiologist’s control include incomplete reversal of heparin’s effects, which should be assayed by the activated coagulation time. Similarly, preexisting deliberate anticoagulation (e.g., caused by warfarin) should be aggressively corrected with fresh-frozen plasma. Because platelet dysfunction is common after CPB, empiric administration is justified if coagulopathy persists. The thrombotic and fibrinolytic systems are activated during lung transplantation, especially if CPB is used, and although aprotinin can reduce this activation and perhaps reduce perioperative hemorrhage,132–134 it has been withdrawn from production. The utility of ε-aminocaproic acid, tranexamic acid, and desmopressin (DDAVP) in replacing aprotinin in this setting remains unknown (see Chapter 31), although some preliminary data suggest that tranexamic acid may be similar in efficacy to aprotinin.
Although some degree of pulmonary edema commonly is detected by chest radiograph after surgery, it is uncommon to encounter severe pulmonary edema in the operating room immediately after reperfusion of the graft. However, when it does occur, postreperfusion pulmonary edema can be dramatic and life-threatening. Copious pink frothy secretions may require almost constant suctioning to maintain a patent airway and may be accompanied by severe gas exchange and compliance abnormalities. Treatment includes high levels of PEEP using selective lung ventilation, diuresis, and volume restriction. Occasionally, patients may require support with ECMO for several days until reperfusion injury resolves; a high percentage of patients so treated ultimately survive.135,136 Adequate analgesia is crucial for these patients to facilitate the earliest possible extubation, ambulation, and participation in spirometric exercises to enhance or preserve pulmonary function. Lumbar or thoracic epidural narcotic analgesia provides excellent analgesia while minimizing sedation. Epidural catheters can be placed before the procedure if time permits or after conclusion of the procedure. Placement of epidural catheter in cases in which a high expectation exists for the necessity of CPB remains a controversial topic. If CPB has been used or coagulopathy has developed, placement should be deferred until coagulation tests have normalized (see Chapter 38).
Fluid therapy also can impact the outcomes of lung transplantation as was demonstrated by McIlroy et al, who showed that the greater the amount of colloid (gelatin) that was used, the greater the A-a gradient, and the greater likelihood of delayed extubation. It was unclear, though, whether this effect extended to other colloids.137
Postoperative Management and Complications
Surgical technical complications are uncommon immediately after lung transplantation but may be associated with high morbidity.138 Pulmonary venous obstruction usually presents as acute, persistent pulmonary edema of the transplanted lung.139 Color-flow and Doppler TEE will show narrowed pulmonary venous orifices with turbulent, high-velocity flow and loss of the normal phasic waveform. PA anastomotic obstruction should be suspected if PA pressures fail to decrease after reperfusion of the lung graft. If the right PA is obstructed, this usually is evident on a TEE examination in the same way as for pulmonary venous obstruction; it is usually much more difficult to adequately inspect the left PA anastomosis with TEE, although some centers have reported a high success rate.140 The diagnosis can be definitively made by measuring the pressure gradient across the anastomosis either by inserting needles on both sides of the anastomosis to transduce the respective pressures or by advancing the PA catheter across it. However, care should be taken not to measure this gradient while the contralateral PA is clamped, because the shunting of the entire CO through one lung will exaggerate the gradient present.141 Angiography and perfusion scanning also are useful for making this diagnosis but are not immediately available in the operating room. Bronchial dehiscence or obstruction is extremely rare in the immediate perioperative period and can be evaluated by fiberoptic bronchoscopy.
Tension pneumopericardium and postoperative hemothorax with complete ventilation/perfusion mismatch are other rare complications that have been reported after lung transplantation.142,143 Patients with pulmonary hypertension and RV hypertrophy occasionally may develop dynamic RV outflow obstruction when transplantation acutely decreases RV afterload; the diagnosis can be confirmed using TEE.144 Hyperacute rejection of a kind similar to that seen with heart transplantation has not been noted with lung transplantation.
The most common cause of death in the immediate perioperative period is graft dysfunction from reperfusion injury, which usually presents with hypoxemia, pulmonary infiltrates, poor lung compliance, pulmonary hypertension, and RV failure. If there are no technical reasons to account for pulmonary hypertension and RV failure, then graft dysfunction must be suspected. Unfortunately, few treatments will specifically ameliorate graft dysfunction and therapy is largely supportive. Vasodilator therapy to directly decrease PVR and, therefore, RV afterload may improve hemodynamics and, in some cases, may improve gas exchange. Both prostaglandin E1 and nitrates can reverse severe hypoxemia and pulmonary hypertension after lung transplantation, and the latter attenuate the increase in transcription of vasoconstrictor genes (such as for endothelin and platelet-derived growth factor) induced by hypoxia.145 Indeed, a “prophylactic” low-dose infusion of prostaglandin E1 has been reported to preserve arterial oxygen tension without altering pulmonary hemodynamics in dogs after single-lung transplantation.146 Improvement in pulmonary hemodynamics and gas exchange in patients with graft dysfunction also have been reported with the administration of nitric oxide.82,147,148 Compared with historic control patients who developed graft dysfunction before the advent of nitric oxide, inhalation of nitric oxide decreased the duration of mechanical ventilation, frequency of airway complications, and mortality.148 Improved hemodynamics and gas exchange may reflect the ability of nitric oxide to compensate for the decrease in endothelium-derived relaxant factor activity after transplantation. If nitric oxide has been used to control pulmonary hypertension after surgery, it should be weaned gradually to avoid any rebound pulmonary vasoconstriction.149 Finally, ECMO may be used to support the patient until there is adequate recovery of pulmonary function.135,136
Infection is a constant threat in these immunosuppressed patients. Prophylactic antibiotic coverage is aimed at agents commonly causing nosocomial and aspiration pneumonias because these are common in donors. Coverage can be modified once culture results from the donor trachea are available. Patients with cystic fibrosis should receive antibiotics targeted at bacteria found in the native lungs before transplantation. Infection should be suspected as the cause of any infiltrate found on chest radiograph, especially if fever or leukocytosis develops, but distinguishing infection from reperfusion injury and rejection may be difficult. Diagnostic bronchoscopy and bronchoalveolar lavage are useful in defining therapy and differentiating infection from rejection,150,151 but open-lung biopsy occasionally is necessary for definitive diagnosis. Patients who are seronegative to viral agents to which the donor was seropositive (e.g., cytomegalovirus) will require prophylactic antiviral therapy. Vadnerkar et al’s152 study showed that 43% of patients who had undetected mold infections at the time of transplant were at risk for very poor outcomes, with a mortality rate of 29%.
Rejection episodes are common and may occur as early as several days after transplantation. Rejection often presents as new infiltrates on chest radiograph in the setting of deteriorating gas exchange. Bronchoscopy with transbronchial biopsy helps to rule out other causes of deterioration and document acute changes consistent with rejection. Therapy for acute lung rejection consists of large pulses of steroids such as methylprednisolone or changing the immunosuppressive agents (cyclosporine to tacrolimus or vice versa). Expired nitric oxide has been shown to be an indicator of chronic rejection in post–lung-transplant patients. Measurements of expired nitric oxide have been shown to decrease with the switch of cyclosporine to tacrolimus, reflecting a decrease in the inflammation in the pulmonary mucosa.153 Expired nitric oxide may be a useful tool to observe patients for the presence or change in chronic graft rejection.154
One of the most serious complications of lung transplantation occurs late. Bronchiolitis obliterans is a syndrome characterized by alloimmune injury leading to obstruction of small airways with fibrous scar.155 Patients with bronchiolitis obliterans present with cough, progressive dyspnea, obstruction on flow spirometry, and interstitial infiltrates on chest radiograph. Therapy for this syndrome includes augmentation of immunosuppression,156 cytolytic agents (which have been used with varying degrees of success),157,158 or retransplantation in refractory cases.
Living-Related Lung Transplantation
The scarcity of suitable donor lungs has resulted in waiting times on transplant lists in excess of 2 years, during which time up to 30% of candidates succumb to their illness.159 Living-related lung transplantation programs have developed to address the needs of lung transplant candidates with acute deterioration expected to preclude survival. Successful grafting of a single lobe for children with bronchopulmonary dysplasia or Eisenmenger syndrome, or two lobes for children and young adults with cystic fibrosis, has encouraged several centers to consider such procedures. 73,160 The anesthetic management issues related to such undertakings have been reviewed.64 Donor candidates will have undergone a rigorous evaluation to ensure that there are no contraindications to lobe donation and that the donation is not being coerced. Donor lobectomy is performed via a standard posterolateral thoracotomy.83 Of special note to the anesthesiologist during such procedures is the requirement for OLV to optimize surgical exposure, the continuous infusion of prostaglandin E1 to promote pulmonary vasodilation, and the administration of heparin and steroids just before lobe harvest. Anesthetic management of the recipient is identical to that for a standard lung transplant, except that the use of CPB is mandatory for bilateral lobar transplant.
Pediatric Considerations
The number of pediatric lung transplants has decreased since the late 1990s. Cystic fibrosis, CHD, and primary pulmonary hypertension are the main indications for lung transplantation in children.161 In infants, CHD is the most common indication for lung transplantation, whereas cystic fibrosis is the most common indication in adolescents. Because of the underlying pulmonary vascular disease or infectious process in these children, bilateral single-lung transplantation is the operation of choice. Single-lung transplantation is less commonly performed in this age group. Transplantation is recommended when the life expectancy of the child is 2 years or less.
Bilateral single-lung transplantation surgery is performed via a bilateral anterior thoracotomy with transverse division of the sternum (“clamshell” incision) with the patient lying supine.162 Size disparities between the donor and recipient are not uncommon because of the limited donor pool. Accommodation of a large donor lung is achieved by parenchymal reduction. Placement of a small donor lung is facilitated by intraoperative and postoperative pulmonary arterial vasodilation with nitric oxide.
The average experience in pediatric lung transplantation is two to three patients per center per year.163 The majority of these patients are scheduled as emergencies the day of transplantation. The family and patient usually are very anxious. Judicious premedication is given to the recipient before general anesthesia. Morbidity during induction of anesthesia is increased by hypoxemia, hypercapnia, and systemic hypotension in these critically ill patients.
After placement of routine monitors, anesthesia is induced with a hypnotic agent (e.g., etomidate), an opioid, and titration of an inhalation agent. A muscle relaxant is added to facilitate tracheal intubation. Cardiac arrest and circulatory collapse may occur after induction of anesthesia in the recipient with primary pulmonary hypertension and CHD with pulmonary hypertension.164 An inhalation agent, additional opioid, benzodiazepines, and muscle relaxants are used throughout the procedure. A thickened hypertrophic right ventricle requires additional volume administration. Frequent endotracheal tube suctioning usually is required intraoperatively before transplantation in the recipient with cystic fibrosis.
Heart-lung transplantation
History and Epidemiology
The diminished frequency of heart-lung transplantation since 1990 reflects that it is being supplanted by lung transplantation. The number of heart-lung transplants worldwide peaked at 241 in 1989, and there has been a continual decline in subsequent years to approximately half that number.3 Approximately only 173 heart-lung transplant candidates were registered with UNOS as of early March 2005, less than 5% of the number on the lung transplant list. The most common recipient indications remain primary pulmonary hypertension, CHD (including Eisenmenger syndrome), and cystic fibrosis.
One-year survival rate after heart-lung transplantation is 60%, significantly less than that for isolated heart or lung transplantation.3 Mortality in subsequent years is approximately 4% per year, similar to that for heart transplantation. Risk factors for increased mortality after heart-lung transplant are recipient ventilator dependence, male recipient sex, and a donor age older than 40 years.3 Early deaths are most often due to graft failure or hemorrhage, whereas midterm and late deaths primarily are due to infection and bronchiolitis obliterans, respectively. Repeat heart-lung transplant is a rare procedure and likely to remain so because the 1-year survival rate after repeat heart-lung transplant is dismal (28%).3
Postoperative Management and Complications
Infection is a more frequent and serious complication in heart-lung recipients than in isolated heart recipients. Bacterial and fungal infections are especially common in the first month after transplantation, with viral and other pathogens (Pneumocystis carinii and Nocardia) occurring in subsequent months.165
Similar to isolated heart or lung transplants, rejection episodes are common early after heart-lung transplantation. Rejection may occur independently in either the heart or lung.106 Therapy is similar to that for rejection of isolated heart or lung grafts.
Pediatric Considerations
Although the number of heart-lung transplants performed worldwide has been decreasing since 1990, of the 49 cases performed in the United States in 1992, more than half were in children.166 The overall decline has been attributed to earlier referral for isolated lung transplantation, before cor pulmonale becomes irreversible. However, there are still a number of uncorrectable types of CHD that inevitably lead to Eisenmenger syndrome in children; thus, the need for pediatric heart-lung transplantation will continue for the foreseeable future.107,108
The number of pediatric heart and lung transplantations continue to decrease.167 Only 10 pediatric heart and lung transplants were performed in 2001 worldwide. Since 1990, the majority of recipients were adolescents. In this group of patients, cystic fibrosis and CHD were the main indications. Only 20% of the recipients are alive 10 years after the transplantation. Early mortality is caused by graft failure; after 1 year after transplantation, the cause is bronchiolitis obliterans. The lack of survival improvement in these patients calls for reassessment of heart and lung transplantation in children.
1 Carrel A., Guthrie C.C. The transplantation of veins and organs. Am J Med. 1905;1:1101.
2 Barnard C.N. The operation. A human cardiac transplant: An interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. S Afr Med J. 1967;41:1271-1274.
3 Hosenpud J.D., Novick R.J., Bennett L.E., et al. The Registry of the International Society for Heart and Lung Transplantation: Thirteenth Official Report. J Heart Lung Transplant. 1996;15:655.
4 Organ Procurement and Transplantation Network, OPTN 2008 Annual Report, Available at: http://optn.transplant.hrsa.gov/data/annualreport.asp
5 Murali S., Kormos R.L., Uretsky B.F., et al. Preoperative pulmonary hemodynamics and early mortality after orthotopic cardiac transplantation: The Pittsburgh experience. Am Heart J. 1993;126:896.
6 Darby J.M., Stein K., Grenvik A., et al. Approach to management of the heartbeating brain dead organ donor. JAMA. 1989;261:2222.
7 Nygaard C.E., Townsend R.N., Diamond D.L. Organ donor management and organ outcome a 6 year review from a level 1 trauma center. J Trauma. 1990;30:728.
8 Votapka T.V., Canvasser D.A., Pennington D.G., et al. Effect of triodothyronine on graft function in a model of heart transplantation. Ann Thoracic Surg. 1996;62:78.
9 Novitzky D. Transplantation, euthyroid sick syndrome, and triiodothyronine replacement. J Heart Lung Transplant. 1992;11:S196.
10 Novitzky D., Cooper D.K.C., Reichart B. Hemodynamic and metabolic responses to hormonal therapy in brian-dead potential organ donors. Transplantation. 1987;43:852.
11 Randell T.T., Hockerstedt K.A. Triiodothyronine treatment in brain-dead multiogran donors—a controlled study. Transplantation. 1992;54:736.
12 Hardesty R.L., Griffith B.P., Deep G.M., et al. Improved cardiac function using cardioplegia during procurement and transplantation. Transplant Proc. 1983;15:1253.
13 Watson D.C., Reitz B.A., Baumgartner W.A. Distant heart procurement for transplantation. Surgery. 1979;86:56.
14 Grant S.C.D., Khan M.A., Yonan N., et al. Technique of anastomosis and incidence of atrial tachyarrythmias following heart transplantation. Br Heart J. 1994;71:40.
15 El-Gamel A., Deiraniya A.K., Rahman A.N., et al. Orthotopic heart transplantation hemodynamics: Does atrial preservation improve cardiac output after transplantation? J Heart Lung Transplant. 1996;15:564.
16 Mehta S.M., Aufiero T.X., Pae W.E. Combined registry for the clinical use of mechanical ventricular assist pumps and the total artificial heart in conjunction with heart transplantation: Sixth official report. J Heart Lung Transplant. 1994;14:585.
17 Shaw B.W.Jr, Bahnson H.T., Hardesty R.L., et al. Combined transplantation of the heart and liver. Ann Surg. 1985;202:667.
18 Bristow M.R., Ginsburg R., Minobe W., et al. Decreased catecholamine sensitivity and beta adrenergic receptor density in failing human hearts. N Engl J Med. 1982;307:205.
19 Cohn J.N., Archibald D.G., Ziesche S., et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. N Engl J Med. 1986;314:1547.
20 Anonymous. Effects of enalapril on mortality in severe congestive heart failure. Results of Cooperative North Scandinavian Enalapril Survival Study (Consensus). N Engl J Med. 1987;316:1429.
21 Kalman J., Buchholz C., Steinmetz M., et al. Safety and efficacy of beta-blockade in patients with chronic congestive heart failure awaiting transplantation. J Heart Lung Transplant. 1995;14:1212.
22 Remme W.J. Inodilator therapy for heart failure. Early, late or not at all? Circulation. 1993;87:IV97.
23 Packer M., Carver J.R., Rodeheffer R.J., et al. Effect of oral milrinone on mortality in severe chronic heart failure. N Engl J Med. 1991;325:1468.
24 Steed D.L., Brown B., Reilly J.J., et al. General surgical complications in heart and heart-lung transplantation. Surgery. 1985;98:739.
25 Isono S.S., Woolson S.T., Schurman D.J. Total joint arthroplasty for steroid-induced osteonecrosis in cardiac transplant patients. Clin Orthop. 1987;217:201.
26 Stinson E.B., Griepp R.B., Schroeder J.S. Hemodynamic observations one and two years after cardiac transplantation in man. Circulation. 1972;45:1183.
27 Rowan R.A., Billingham M.E. Myocardial innervation in long-term heart transplant survivors: A quantitative ultrastructural survey. J Heart Transplant. 1988;7:448.
28 Wilson R.F., Christensen B.V., Olivari M.T., et al. Evidence for structural sympathetic reinnervation after orthotopic cardiac transplantation in humans. Circulation. 1991;83:1210.
29 Stinson E.B., Griepp R.B., Clark D.A., et al. Cardiac transplantation in man. VIII. Survival and function. J Heart Transplant. 1970;7:145.
30 Verani M.S., George S.E., Leon C.A., et al. Systolic and diastolic ventricular performance at rest and during exercise in heart transplantation recipients. J Heart Transplant. 1988;7:145.
31 Kent K.M., Cooper T. The denervated heart: A model for studying autonomic control of the heart. N Engl J Med. 1974;291:1017.
32 Goodman D.J., Rossen R.M., Cannom D.S., et al. Effect of digoxin on atrioventricular conduction. Studies in patients with and without cardiac autonomic innervation. Circulation. 1975;51:251.
33 Backman S.B., Ralley F.E., Fox G.S. Neostigmine produces bradycardia in a heart transplant recipient. Anesthesiology. 1993;78:777.
34 Borow K.M., Neumann A., Arensman F.W., et al. Cardiac and peripheral vascular responses to adrenoceptor stimulation and blockade after cardiac transplantation. J Am Coll Cardiol. 1989;14:1229.
35 Tuzcu E.M., DeFranco A.C., Hobbs R., et al. Prevalence and distribution of transplant coronary artery disease. J Heart Lung Transplant. 1995;14:S202.
36 Hosenpud J.D., Morris T.E., Shipley G.D., et al. Cardiac allograft vasculopathy transplantation. Transplantation. 1996;61:939.
37 Gao S.Z., Hunt S.A., Schroeder J.S., et al. Early development of accelerated graft coronary artery disease: Risk factors and course. J Am Coll Cardiol. 1996;28:673.
38 Stark R.P., McGinn A.L., Wilson R.F. Chest pain in cardiac-transplant recipients. N Engl J Med. 1991;324:1791.
39 Smart F.W., Ballantyne C.M., Cocanougher B., et al. Insensitivity of noninvasive test to detect coronary artery vasculopathy after heart transplant. Am J Cardiol. 1991;67:243.
40 St Goar F.G., Pinto F.J., Alderman E.L., et al. Intracoronary ultrasound in cardiac transplant recipients: In vivo evidence of angiographically silent intimal thickening. Circulation. 1992;85:979.
41 Akosah K.O., Mohanty P.K., Funai J.T., et al. Noninvasive detection of transplant coronary artery disease by dobutamine stress echocardiography. J Heart Lung Transplant. 1994;13:1024.
42 Waterman P.M., Bjerke R. Rapid-sequence induction technique in patients with severe ventricular dysfunction. J Cardiothorac Anesth. 1988;2:602.
43 Murkin J.M., Moldenhauer C.C., Hug C.C.Jr. High-dose fentanyl for rapid induction of anaesthesia in patients with coronary artery disease. Can Anaesth Soc J. 1985;32:320.
44 Hensley F.A., Martin D.E., Larach D.R., et al. Anesthetic management for cardiac transplantation in North America. J Cardiothorac Anesth. 1987;1:429.
45 Berberich J.J., Fabian J.A. A retrospective analysis of fentanyl and sufentanil for cardiac transplantation. J Cardiothorac Anesth. 1987;1:200.
46 Armitage J.M., Hardesty R.L., Griffith B.P. An effective treatment of right heart failure after orthotopic heart transplantation. J Heart Transplant. 1987;6:348.
47 Fonger J.D., Borkon A.M., Baumgartner W.A. Acute right ventricular failure following heart transplantation: Improvement with PGE1 and right ventricular assist. J Heart Transplant. 1986;5:317.
48 Stein KL, Armitage J.M., Martich G.D. Intensive care of the cardiac transplant recipient. In: Ayres S.M., Grenvik A., Holbrook P.R., Shoemaker W.C., editors. Textbook of Critical Care. ed 3. WB Saunders; 1995:1649.
49 Weil R.III, Clarke D.R., Iwaki Y., et al. Hyperacute rejection of a transplanted human heart. Transplantation. 1981;32:71.
50 Bhatia S.J.S., Kirshenbaum J.M., Shemin R.J., et al. Time course of resolution of pulmonary hypertension and right ventricular remodeling after orthotopic cardiac transplantation. Circulation. 1987;76:819.
51 Villanueva F.S., Murali S., Uretsky B.F., et al. Resolution of severe pulmonary hypertension after heterotopic cardiac transplantation. J Am Coll Cardiol. 1989;14:1239.
52 Jacquet L., Ziady G., Stein K., et al. Cardiac rhythm disturbances early after orthotopic heart transplantation: Prevalence and clinical importance of the observed abnormalities. J Am Coll Cardiol. 1990;16:832.
53 Scott C.D., Omar I., McComb J.M., et al. Long-term pacing in heart transplant recipients is usually unnecessary. Pacing Clin Electrophysiol. 1991;14:1792.
54 Romhilt D.W., Doyle M., Sagar K.B., et al. Prevalence and significance of arrhythmias in long-term survivors of cardiac transplantation. Circulation. 1982;66:I219.
55 Chomette G., Auriol M., Beaufil H., et al. Morphology of cyclosporine nephrotoxicity in human heart transplant recipients. J Heart Transplant. 1986;5:273.
56 Platz K.P., Mueller A.R., Blumhardt G., et al. Nephrotoxicity following orthotopic liver transplantation. A comparison between cyclosporine and FK506. Transplantation. 1994;58:170.
57 Burch M., Aurora P. Current status of paediatric heart, lung, and heart-lung transplantation. Arch Dis Child. 2004;89:386-389.
58 Lillehei C.W., Mayer J.E.Jr, Shamberger R.C., et al. Pediatric lung transplantion and “Lessons from Green Surgery”. Ann Thoracic Surg. 1999;68:S25-S27.
59 Spray T.L., Bridges N.D. Lung transplantation for pediatric pulmonary hypertension. Prog Pediatr Cardiol. 2001;12:319-325.
60 Weiss E.S., Allen J.G., Merlo C.A., et al. Factors indicative of long-term survival after lung transplantation: A review of 836 10 year survivors. J Heart Lung Transplant. 2010;29:240-246.
61 Olson C.M. Diagnostic and therapeutic technology assessment: Lung transplantation. JAMA. 1993;269:931.
62 Kaye M.P. The Registry of the International Society for Heart and Lung Transplantation: Ninth official report. J Heart Lung Transplant. 1992;11:599.
63 Trulock E.P., Cooper J.D., Kaiser L.R., et al. The Washington University–Barnes Hospital experience with lung transplantation. JAMA. 1991;266:1943.
64 Quinlan J.J., Gasior T.A., Firestone S., et al. Anesthesia for living-related (lobar) lung transplantation. J Cardiothorac Vasc Anesth. 1996;10:391.
65 Novick R.J., Kaye M.P., Patterson G.A., et al. Redo lung transplantation: A North American-European experience. J Heart Lung Transplant. 1993;12:5.
66 Gorcsan J.III, Edward T.D., Ziady G.M., et al. Transesophageal echocardiography to evaluate patients with severe pulmonary hypertension for lung transplantation. Ann Thoracic Surg. 1995;59:717.
67 Hardesty R.L., Aeba R., Armitage J.M., et al. A clinical trial of University of Wisconsin solution for pulmonary preservation. J Thorac Cardiovasc Surg. 1993;105:660.
68 Weiss E.S., Merlo C.A., Shah A.S. Impact of advanced age in lung transplantation: An analysis of United Network for Organ Sharing data. J Am Coll Surg. 2009;208:400-409.
69 Allen J.G., Weiss E.S., Merlo C.A., et al. Impact of donor-recipient race matching on survival after Lung Transplantation: Analysis of over 11000 patients. J Heart Lung Transplant. 2009;28:1063-1071.
70 Gilbert S., Dauber J., Hattler B., et al. Lung and heart-lung transplantation at the University of Pittsburgh 1982–2002. Clin Transpl. 2002:253-261.
71 Brower R.G., Matthay M.A., Morris A., et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301-1308.
72 Low D.E., Trulock E.P., Kaiser L.R., et al. Morbidity, mortality, and early results of single versus bilateral lung transplantation for emphysema. J Thorac Cardiovasc Surg. 1992;103:1119.
73 Starnes V.A., Barr M.L., Cohen R.G. Lobar transplantation: Indications, technique and outcome. J Thorac Cardiovasc Surg. 1994;108:403.
74 Kaiser L.R., Pasque M.K., Trulock E.P., et al. Bilateral sequential lung transplantation: The procedure of choice for double-lung replacement. Ann Thorac Surg. 1991;52:438.
75 Bisson A., Bonnette P. A new technique for double-lung transplantation. J Thorac Cardiovasc Surg. 1992;103:40.
76 Pepe P.E., Marini J.J. Occult positive end-expiratory pressure in mechanically ventilated patients with airflow obstruction. The Auto-PEEP effect. Am Rev Respir Dis. 1982;126:166.
77 Haluszka J., Chartrand D.A., Grassino A.E., et al. Intrinsic PEEP and arterial PCO2 in stable patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1990;141:1194.
78 Quinlan J.J., Buffington C.W. Deliberate hypoventilation in a patient with air trapping during lung transplantation. Anesthesiology. 1993;78:1177.
79 Smith T.C., Marini J.J. Impact of PEEP on lung mechanics and work of breathing in severe airflow obstruction. J Appl Physiol. 1988;65:1488.
80 Biondi J.W., Hines R.L., Matthay R.A. Comparative right ventricular function during assist control, intermittent mandatory and spontaneous ventilation. Anesth Analg. 1986;65:S18.
81 Prewitt R., Ghignone M. Treatment of right ventricular dysfunction in acute respiratory failure. Crit Care Med. 1983;5:346.
82 Adatia I., Lillehei C., Arnold J.H., et al. Inhaled nitric oxide in the treatment of postoperative graft dysfunction after lung transplantation. Ann Thoracic Surg. 1994;57:1311.
83 Cohen R.G., Barr M.L., Schenkel F.A., et al. Living-related donor lobectomy for bilateral lobar transplantation in patients with cystic fibrosis. Ann Thorac Surg. 1994;57:1423.
84 Kramer M.R., Marshall S.E., McDougall I.R., et al. The distribution of ventilation and perfusion after single-lung transplantation in patients with pulmonary fibrosis and pulmonary hypertension. Transplant Proc. 1991;23:1215.
85 Maurer J.R., McLean P.A., Cooper J.D., et al. Airway hyperactivity in patients undergoing lung and heart/lung transplantation. Am Rev Respir Dis. 1989;139:1038.
86 Herve P., Picard N., Le Roy Ladurie M., et al. Lack of bronchial hyperresponsiveness to methacholine and to isocapnic dry air hyperventilation in heart/lung and double-lung transplant recipients with normal lung histology. Am Rev Respir Dis. 1992;145:1503.
87 Tavakoli R., Buvry A., Le Gall G., et al. In vitro bronchial hyperresponsiveness after lung transplantation in the rat. Am J Physiol. 1992;262:L322.
88 Stretton C.D., Mak J.C.W., Belvisi M.G., et al. Cholinergic control of human airways in vitro following extrinsic denervation of the human respiratory tract by heart-lung transplantation. Am Rev Respir Dis. 1990;142:1030.
89 Takichi T., Maeda M., Shirakusa T., et al. Sympathetic reinnervation of unilaterally denervated rat lung. Acta Physiol Scand. 1995;154:43.
90 Clifford P.S., Coon R.L., Von Colditz J.H., et al. Pulmonary denervation in the dog. J Appl Physiol. 1983;54:1451.
91 Duarte A.G., Terminella L., Smith J.T., et al. Restoration of cough reflex in lung transplant recipients. Chest. 2008;134:310-316.
92 Dolovich M., Rossman C., Chambers C. Muco-ciliary function in patients following single lung or lung/heart transplantation. Am Rev Respir Dis. 1987;135:A363.
93 Fullerton D.A., Mitchell M.B., McIntyre R.C., et al. Cold ischemia and reperfusion each produce pulmonary vasomotor dysfunction in the transplanted lung. J Thorac Cardiovasc Surg. 1993;106:1213.
94 Hunter D.N., Morgan C.J., Yacoub M., et al. Pulmonary endothelial permeability following lung transplantation. Chest. 1992;102:417.
95 Shennib H., Serrick C., Saleh D., et al. Plasma endothelin-1 levels in human lung transplant recipients. J Cardiovasc Pharmacol. 1995;26(Suppl 3):S516.
96 Flavahan N.A., Aleskowitch T.D., Murray P.A. Endothelial and vascular smooth muscle responses are altered after left lung autotransplantation. Am J Physiol. 1994;266:H2026.
97 Kukkonen S., Heikkila L., Verkkala K., et al. Pulmonary vasodilatory properties of prostaglandin e1 are blunted after experimental single-lung transplantation. J Heart Lung Transplant. 1995;14:280.
98 Ueda K., Date H., Fujita T., et al. Effects of inhaled nitric oxide in a canine living-donor lobar lung transplant model. Jpn J Thorac Cardiovasc Surg. 2000;48:693-699.
99 Lang J.D.Jr, Leill W. Pro: Inhaled nitric oxide should be used routinely in patients undergoing lung transplantation. J Cardiothorac Vasc Anesth. 2001;15:785-789.
100 Karamasetty M., Klinger J. NO: More than just a vasodilator in lung transplantation. Am J Respir Cell Mol Biol. 2002;26:1-5.
101 Moreno I., Vicente R., Mir A., et al. Effects of inhaled nitric oxide on primary graft dysfunction in lung transplantation. Transplant Proc. 2009;41:2210-2212.
102 Meade M.O., Granton J., Matte-Martyn A., et al. A randomized trial of inhaled nitric oxide to prevent ischemia reperfusion injury after lung transplantation. Am J Respir Crit Care Med. 2003;167:1483-1489.
103 Ardehali A., Laks H., Levine M., et al. A prospective trial of inhaled nitric oxide in clinical lung transplantation. Transplantation. 2001;72:112-115.
104 Botha P., Jeykanthan M., Rao J.N., et al. Inhaled nitric oxide for modulation of ischemia-reperfusion injury in lung transplantation. J Heart Lung Transplant. 2007;26:1199-1205.
105 Royston D. High-dose aprotinin therapy: A review of the first five years’ experience. J Cardiothorac Vasc Anesth. 1992;6:76.
106 Griffith B.P., Hardesty R.L., Trento A., et al. Asynchronous rejection of heart and lungs following cardiopulmonary transplantation. Ann Thoracic Surg. 1985;40:488.
107 Smyth R.L., Scott J.P., Whitehead B. Heart-lung transplantation in children. Transplant Proc. 1990;22:1470.
108 Starnes V.A., Marshall S.E., Lewiston N.J., et al. Heart-lung transplantation in infants, children and adolescents. J Pediatr Surg. 1991;26:434.
109 Corris P.A., Odom N.J., Jackson G., et al. Reimplantation injury after lung transplantation in a rat model. J Heart Transplant. 1987;6:234.
110 Jones M.T., Hsieh C., Yoshikawa K., et al. A new model for assessment of lung preservation. J Thorac Cardiovasc Surg. 1988;96:608.
111 Katz W.E., Gasior T.A., Quinlan J.J., et al. Immediate effects of lung transplantation on right ventricular morphology and function in patients with variable degrees of pulmonary hypertension. J Am Coll Cardiol. 1996;27:384.
112 Yeoh T.K., Kramer M.R., Marshall S., et al. Changes in cardiac morphology and function following single-lung transplantation. Transplant Proc. 1991;23:1226.
113 Frist W.H., Lorenz C.H., Walker E.S., et al. MRI complements standard assessment of right ventricular function after lung transplantation. Ann Thoracic Surg. 1995;60:268.
114 Carere R., Patterson G.A., Liu P.P., et al. Right and left ventricular performance after single and double-lung transplantation. J Thorac Cardiovasc Surg. 1991;102:115.
115 Ritchie M., Waggoner A.D., Davila-Roman V.G., et al. Echocardiographic characterization of the improvement in right ventricular function in patients with severe pulmonary hypertension after single-line transplantation. J Am Coll Card. 1993;22:1170.
116 Scuderi L.J., Bailey S.R., Calhoon J.H., et al. Echocardiographic assessment of right and left ventricular function after single-line transplantation. Am Heart J. 1994;127:636.
117 Kramer M.R., Valatine H.A., Marshall S.E., et al. Recovery of the right ventricle after single-lung transplantation in pulmonary hypertension. Am J Cardiol. 1994;73:494.
118 Benumof J.L. Anesthesia for Thoracic Surgery. Philadelphia: WB Saunders, 1987.
119 Hirt S.W., Haverich A., Wahlers T., et al. Predictive criteria for the need of extracorporeal circulation in single-lung transplantation. Ann Thorac Surg. 1992;54:676.
120 Chetham P. Anesthesia for heart or single or double lung transplantation. J Card Surg. 2000;15:167-174.
121 Myles P.S., Venama H. Avoidance of cardiopulmonary bypass during bilateral sequential lung transplantation using inhaled nitric oxide. J Cardiothorac Vasc Anesth. 1995;9:571.
122 Myles P.S., Weeks A.M., Buckland M.R., et al. Anesthesia for bilateral sequential lung transplantation: Experience of 64 cases. J Cardiothorac Vasc Anesth. 1997;11:177.
123 Triantafillou A.N., Pasque M.K., Huddleston C.B., et al. Predictors, frequency, and indications for cardiopulmonary bypass during lung transplantation in adults. Ann Thoracic Surg. 1994;57:1248.
124 Aeba R., Griffith B.P., Kormos R.L., et al. Effect of cardiopulmonary bypass on early graft dysfunction in clinical lung transplantation. Ann Thoracic Surg. 1994;57:715.
125 Fullerton D.A., McIntyre R.C.J., Mitchell M.B., et al. Lung transplantation with cardiopulmonary bypass exaggerates pulmonary vasomotor dysfunction in the transplanted lung. J Thorac Cardiovasc Surg. 1995;109:212.
126 Firestone L., Carrera J., Firestone S., et al. Single-lung transplants: Who needs bypass? Anesthesiology. 1993;79:A46.
127 Sekela M.E., Noon G.P., Holland V.A., et al. Differential perfusion: Potential complication of femoral-femoral bypass during single lung transplantation. J Heart Lung Transplant. 1991;10:322.
128 Ko W.J., Chen Y.S., Lee Y.C. Replacing cardiopulmonary bypass with extracorporeal membrane oxygenation in lung transplantation operations. Artif Organs. 2001;25:607-612.
129 Pereszlenyi A., Lang G., Steltzer H., et al. Bilateral lung transplantation with intra and postoperatively prolonged ECMO support in patients with pulmonary hypertension. Eur J Cardiothorac Surg. 2002;21:858-863.
130 Michel-Cherqui M., Brusset A., Liu N., et al. Intraoperative transesophageal echocardiographic assessment of vascular anastomoses in lung transplantation. A report of 18 cases. Chest. 1997;111:1229-1235.
131 Hausmann D., Daniel W.G., Mugge A. Imaging of pulmonary artery and vein anastomoses by transesophageal echocardiography after lung transplantation. Circulation. 1992;86(5 Suppl):II251-II258.
132 Royston D. Aprotinin therapy in heart and heart-lung transplantation. J Heart Lung Transplant. 1993;12:S19.
133 Gu Y.J., de Haan J., Brenken U.P., et al. Clotting and fibrinolytic disturbance during lung transplantation: Effect of low-dose aprotinin. J Thorac Cardiovasc Surg. 1996;112:599.
134 Jaquiss R.D., Huddleston C.B., Spray T.L. Use of aprotinin in pediatric lung transplantation. J Heart Lung Transplant. 1995;14:302.
135 Zenati M., Pham S.M., Keenan R.J., et al. Extracorporeal membrane oxygenation for lung transplant recipients with primary severe donor lung dysfunction. Transpl Int. 1996;9:227.
136 Glassman L.R., Keenan R.J., Fabrizio M.C., et al. Extracorporeal membrane oxygenation as an adjunct treatment for primary graft failure in adult lung transplant recipients. J Thorac Cardiovasc Surg. 1995;110:723.
137 McIlroy D.R., Pilcher D.V., Snell G.I. Does anaesthetic management affect early outcomes after lung transplant? An exploratory analysis. Br J Anesth. 2009;102:506-514.
138 Leibowitz D.W., Smith C.R., Michler R.E., et al. Incidence of pulmonary vein complications after lung transplantation: A prospective transesophageal echocardiographic study. J Am Coll Cardiol. 1994;24:671.
139 Malden E.S., Kaiser L.R., Gutierrez F.R. Pulmonary vein obstruction following single-lung transplantation. Chest. 1992;102:671.
140 Hausmann D., Daniel W.G., Mugge A., et al. Imaging of pulmonary artery and vein anastomoses by transesophageal echocardiography after lung transplantation. Circulation. 1992;86:II-251.
141 Despotis G.J., Karanikolas M., Triantafillou A.N., et al. Pressure gradient across the pulmonary artery anastomosis during lung transplantation. Ann Thorac Surg. 1995;60:630.
142 Cohrane L.J., Mitchell M.E., Raju S., et al. Tension pneumopericardium as a complication of single-lung transplantation. Ann Thorac Surg. 1990;50:808.
143 Jellinek H., Klepetko W., Hiesmayr M. Komplettes Ventilations/Perfusions Mibverhaltnis Nachy Einseitiger Lungentransplantation und Postoperativem Hamatothorax. Anaesthetist. 1992;41:134.
144 Gorcsan J.III, Reddy S.C., Armitage J.M., et al. Acquired right ventricular outflow tract obstruction after lung transplantation: Diagnosis by transesophageal echocardiography. Am Soc Echocardiogr. 1993;6:324.
145 Mentzer S.J., Reilly J.J., DeCamp M., et al. Potential mechanism of vasomotor dysregulation after lung transplantation for primary pulmonary hypertension. J Heart Lung Transplant. 1995;14:387.
146 Aoe M., Trachiotis G.D., Okabayashi K., et al. Administration of prostaglandin E1 after lung transplantation improves early graft function. Ann Thorac Surg. 1994;58:655.
147 MacDonald P., Mundy J., Rogers P., et al. Successful treatment of life-threatening acute reperfusion injury after lung transplantation with inhaled nitric oxide. J Thorac Cardiovasc Surg. 1995;110:861.
148 Date H., Triantafillou A.N., Trulock E.P., et al. Inhaled nitric oxide reduced human lung allograft dysfunction. J Thorac Cardiovasc Surg. 1996;111:913.
149 Lindberg L., Sjoberg T., Ingemansson R., et al. Inhalation of nitric oxide after lung transplantation. Ann Thorac Surg. 1996;61:956-962.
150 Scott J.P., Fradet G., Smyth R.L., et al. Prospective study of transbronchial biopsies in the management of heart-lung and single lung transplant patients. J Heart Lung Transplant. 1991;10:626.
151 Higgenbottam T., Stewart S., Penketh A., et al. Transbronchial lung biopsy for the diagnosis of rejection in heart-lung transplant patients. Transplantation. 1988;46:532.
152 Vadnerkar A., Clancy C.J., Celik U., et al. Impact of mold infection in explanted lungs on outcomes of lung transplantation. Transplantation. 2010;89:253-260.
153 Verleden G., Dupont L., Raemdonck D.V., et al. Effect of switching from cyclosporine to tacrolimus on exhaled nitric oxide and pulmonary function in patients with chronic rejection after lung transplantation. J Heart Lung Transplant. 2002;22:908-913.
154 Gabbay E., Walters E.H., Orsida B., et al. Post-lung transplant bronchiolitis obliterans syndrome (BOS) is characterized by increased exhaled nitric oxide levels and epithelial inducible nitric oxide synthetase. Am J Respir Crit Care Med. 2000;162:2182-2187.
155 Reichenspurner H., Girgis R.E., Robbins R.C., et al. Obliterative bronchiolitis after lung and heart-lung transplantation. Ann Thorac Surg. 1995;60:1845.
156 Allen M.D., Burke C.M., McGregor C.G.A., et al. Steroid-responsive bronchiolitis after human heart-lung transplantation. J Thorac Cardiovasc Surg. 1986;92:449.
157 Snell G.I., Esmore D.S., Williams T.J. Cytolytic therapy for the bronchiolitis obliterans syndrome complicating lung transplantation. Chest. 1996;109:874.
158 Kesten S., Rajagopalan N., Maurer J. Cytolytic therapy for the treatment of bronchiolitis obliterans syndrome following lung transplantation. Transplantation. 1996;61:427.
159 Paradis I., Manzetti J., Foust D. When to refer for lung transplantation? Characteristics of candidates who die vs. those who survive to receive a transplant. Am Rev Respir Dis. 1993;147:A597.
160 Starnes V.A., Lewiston N.J., Luikart H., et al. Current trends in lung transplantation: Lobar transplantation and expanded use of single lungs. J Thorac Cardiovasc Surg. 1992;104:1060.
161 Webber S.A. The current state of, and future prospects for, cardiac transplantation in children. Cardiol Young. 2003;13:64-83.
162. Quinlan J.J., Firestone S., Firestone L.L. Anesthesia for heart, lung, and heart-lung transplantation. In: Kaplan J.A., Reich D.L., Konstadt S.N., editors. Cardiac Anesthesia. Philadelphia: WB Saunders, 1998.
163 Brawn W.J., Barron D.J. Management and outcome in hypoplastic left heart syndrome. Curr Paediatr. 2004;14:26-32.
164 Reddy S.C., Webber S.A. Pediatric heart and lung transplantation. Indian J Pediatr. 2003;70:19-25.
165 Kramer M.R., Marshall S.E., Starnes V.A., et al. Infectious complications in heart-lung transplantation. Arch Intern Med. 1993;153:2010.
166 Bailey L.L. Heart transplantation techniques in complex congenital heart disease. J Heart Lung Transplant. 1993;12:S168.
167 Boucek M.M., Edwards L.B., Keck B.M., et al. The registry of the International Society for Heart and Lung Transplantation: Sixth official pediatric report 2003. J Heart Lung Transplant. 2003;22:636-652.