15 Anesthesia for Children Undergoing Heart Surgery
Perioperative Challenges in Pediatric Cardiac Anesthesia
Anesthesia Management for Surgery Requiring Cardiopulmonary Bypass
Control of Systemic and Pulmonary Vascular Resistance during Anesthesia
Anesthetic Drugs Used in Pediatric Cardiac Anesthesia
Stress Response to Cardiac Surgery
Preoperative Evaluation
In the United States, 40,000 children are born each year with congenital heart disease (CHD),1 representing an incidence of 6 to 8 cases per 1000 live births.2 Children with chromosomal abnormalities such as trisomy 21 (i.e., Down syndrome) have a greater incidence of CHD. Having a sibling with CHD increases the risk of CHD, as does the presence of other congenital abnormalities.3 With improving diagnostic techniques, many children with CHD are diagnosed in the antenatal or early postnatal period. Associated with this improvement in diagnostic ability is a trend in most centers to undertake definitive repair earlier, with many patients undergoing corrective surgery in the neonatal period. Overall, about one half of all children with CHD undergo cardiac surgery in the first year of life, and about 25% undergo surgery in the first month of life.4,5
For managing children with complex cardiac defects, there is increasing reliance on echocardiography and magnetic resonance imaging (MRI) to acquire diagnostic data. Although fewer children are being subjected to diagnostic angiography, more interventional cardiac catheterization procedures are being performed. Many conditions that would previously have been treated surgically are now treated in the angiography suite by interventional cardiologists, such as atrial septal defects (ASDs), patent ductus arteriosus (PDA), and ventricular septal defects (VSDs). Other interventions include dilating arteries with balloon catheters with and without stents and coiling of aberrant or excessive collateral vessels. The pulmonary artery is commonly balloon dilated and stented, and coarctation of the aorta is treated similarly by balloon dilation. Stenotic valves are also commonly dilated. These procedures have led to the risk being transferred from the angiography suite to the operating room.6 For the individual child, there has been a dramatic decrease in morbidity as increasing numbers of conditions are treated in the angiography suite, but the risks of complications that occur in the angiography suite have increased as more complex procedures are performed (see Chapter 20).
The Preoperative Visit and Evaluation
The preoperative visit is an important part of the overall management of anesthesia for children with CHD.7 The preoperative visit has several aims:
Medical Assessment
In addition to gathering this specific diagnostic information, a directed history and physical examination should be performed to assess the overall condition of the child. Attention should be directed toward assessing the degree of cardiac failure, cyanosis, or risks of pulmonary hypertension and the prior surgical procedures and how this information may alter access to the central circulation and placement of invasive monitors. The general nutritional state of the child should be assessed; poor growth and development may be a sign of severe CHD. Other information should be sought that may have a bearing on the anesthetic plan. For example, is this repeat surgery, and does it require a repeat sternotomy? This has a bearing on line placement because a femoral bypass may be required, and it should be avoided for line placement. The previous use of aprotinin is important because the risk of anaphylaxis is increased in a second exposure and particularly if it has been given in the previous 6 months.8
Prescribing Premedication
The use of sedative premedication can be useful in cardiac anesthesia, but this practice varies widely. Numerous medications may be used, and numerous recommendations exist. Premedication for infants younger than 6 months of age is usually unnecessary. Premedication for older, healthy children who show little anxiety and with whom good preoperative rapport can be established is often unnecessary. However, older children, particularly those who have undergone previous surgery, have fears about anesthesia and surgery. It is important to address the fears of these children. Sedative premedication may play a pivotal role in achieving adequate anxiolysis for separation from the parents and induction of anesthesia. I prefer to avoid premedication in children with severe congestive heart failure. Cyanotic children such as those with tetralogy of Fallot (TOF) often benefit from sedative premedication because crying and struggling during induction of anesthesia may worsen their cyanosis. However, it is important that these cyanotic children are well supervised after premedication because they have a blunted response to hypoxia.9 In the United States, supplemental premedication is sometimes administered under the direct supervision of the anesthesiologist in the preoperative facility, providing for a calm child and gentle separation from the parents. In the United Kingdom, where induction of anesthesia takes place in a dedicated anesthesia room, parents are present until after the induction, often making additional premedication unnecessary.
The most common premedication is oral midazolam (0.5 to 1.0 mg/kg).10 However, the effect of midazolam may be unpredictable and may cause dysphoria. Numerous other medications have been recommended for this purpose, including ketamine, clonidine, temazepam, and chloral hydrate. The use of these drugs is often dictated by local preferences and is not always evidence based.
Giving Information
Providing information to the parents and to the child if they are capable of understanding is a key element of the preoperative visit. This information includes the use of sedative premedication, the type of induction, fasting times, the type and likely position of invasive lines, the need for a stay in an intensive care unit (ICU) postoperatively, and the expected length of that stay. The use of other monitors such as transesophageal echocardiography (TEE) should be outlined and any contraindications sought, along with the probability of needing a blood transfusion. Questions about the risk of anesthesia and surgery should be addressed to the satisfaction of the parents (see Chapter 4).
Upper Respiratory Tract Infection and Cardiac Surgery
Otherwise healthy children undergoing elective noncardiac surgery in the presence of an upper respiratory infection (URI) are more likely to suffer respiratory complications (Table 15-1). These complications typically are minor, are easily managed, and usually result in minimal morbidity.11–13 The decision about whether to proceed with noncardiac surgery in a child with a URI is made on an individual basis.14
TABLE 15-1 Diagnosis of Upper Respiratory Tract Infection
Data from Schreiner MS, O’Hara I, Markakis DA, Politis GD. Do children who experience laryngospasm have an increased risk of upper respiratory tract infection? Anesthesiology 1996;85:475-80.
The decision to proceed with cardiac surgery in children is difficult. Although children with cardiac failure are prone to multiple URIs, they may also have signs that can mimic URIs. Surgery may be relatively urgent, and postponing surgery exposes the child to an increased risk. Cardiac surgery in children with URIs results in a prolonged stay in the ICU and prolonged ventilation times, although overall hospital stay is not prolonged. There is an increased incidence of pulmonary atelectasis and an increase in postoperative bacterial infections. There appears not to be any statistically significant increase in mortality rates (4.2% with URIs versus 1.6% without URIs) or long-term sequelae in children with URIs who undergo cardiac surgery. The URI group was significantly younger and smaller, which may account in part for the greater but statistically insignificant increased mortality rate.15 Although this increase in mortality was not statistically significant, it does raise concerns about the risks posed by URIs before cardiac surgery. Children who are scheduled for a Glenn shunt or completion of the Fontan circulation may be at particular risk because an increase in pulmonary vascular resistance (PVR) can adversely affect outcome. It is prudent to postpone surgery in a child with a URI who is scheduled for elective cardiac surgery. If the surgery is urgent, discussion with the surgical team is required to correctly assess the risks and benefits to the child.
Perioperative Challenges in Pediatric Cardiac Anesthesia
Cyanosis
Children with cyanotic cardiac defects compensate for chronic hypoxia with increased erythropoiesis, increased circulating blood volume, vasodilation, and metabolic adjustments of factors, such as circulating 2,3-diphosphoglycerate (2,3-DPG). These changes allow greater tissue delivery of oxygen. The increase in blood viscosity with polycythemia leads to increased vascular resistance and sludging, which may result in renal, pulmonary, and cerebral thromboses, especially in dehydrated children.16 Long periods without oral intake preoperatively and postoperatively should be avoided in children with polycythemia, unless adequate intravenous hydration is provided.
PVR increases more than systemic vascular resistance (SVR) with an increasing hematocrit, further decreasing pulmonary blood flow in children who already have a compromised pulmonary circulation. Coagulopathies are common in children with cyanotic CHD and may adversely influence surgical hemostasis.17,18 When the hematocrit exceeds 65%, excessive viscosity impairs microvascular perfusion and outweighs the advantages of increased oxygen-carrying capacity. Reduction of red blood cell volume can correct the coagulopathy and improve hemodynamics when increases in hematocrit are extreme.19
Impaired Hemostasis
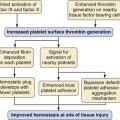
Hemostasis is impaired after bypass in infants and children. This results from a combination of immature coagulation factor synthesis, hemodilution after bypass, and a complex interaction involving consumption of clotting factors and platelets. At birth, the levels of vitamin K–dependent coagulation factors in healthy, full-term neonates are only 40% to 66% of adult values. During the first month of life, these levels increase to 53% to 90% of adult values.20 However, in children with CHD, especially those with cyanosis or systemic hypoperfusion, coagulation factors often continue to be depressed due to impaired hepatic protein synthesis. Although antithrombin III levels are also low, true heparin resistance is rare in infants because of parallel decreases in coagulation factors.
At the onset of cardiopulmonary bypass (CPB), the introduction of the prime volume, which is two to three times greater than the child’s blood volume, dilutes the factor concentrations, particularly fibrinogen, to 50% of values before bypass and the platelet count to 30% of values before bypass. This degree of dilution occurs even when the pump circuit is primed with whole blood. Greater dilution may occur when packed red cells are used in the priming volume. At the conclusion of neonatal bypass, the activity of clotting factors is often extremely low, the fibrinogen concentration is frequently less than 100 mg/dL, and the platelet count has been reduced to 50,000 to 80,000/mm3.21,22 In addition to these quantitative changes, functional changes in the platelets occur during bypass. Extracorporeal circulation causes a loss of platelet adhesion receptors, activation of platelets, and formation of leukocyte-platelet conjugates. Platelet adhesion receptors are more depressed in children with cyanotic compared with acyanotic cardiac defects. Heparin also impairs platelet function independent of CPB.23
Cardiac surgery is associated with significant activation of the fibrinolytic system.24 Inadequate heparin levels during CPB may also contribute to postoperative bleeding because inadequate anticoagulation may allow activation of the hemostatic pathways. Activation causes the consumption of platelets and clotting factors. The standard measurement of anticoagulation, the activated clotting time (ACT), shows a poor correlation with heparin levels in children undergoing CPB.25 In one study, the use of heparin monitoring and heparin titration was associated with larger doses of heparin but smaller doses of protamine for antagonism.26 Activation of clotting cascades is also reduced, decreasing bleeding in the postoperative period.26 As a result of this multifactorial coagulopathy, blood loss is a greater problem in children than in adults and is a particular problem in neonates and small infants (see Chapter 17).27
Strategies to Reduce Bleeding after Bypass
In an effort to normalize factors and platelets to effective concentrations, some medical centers use fresh whole blood in the cardiopulmonary circuit prime. In adult patients and an in vitro aggregation study, transfusion of fresh whole blood provided equal or greater hemostatic and functional benefit when compared with transfusion of platelet concentrates. In children, transfusion of fresh whole blood less than 48 hours from harvest is associated with less blood loss compared with transfusion of reconstituted whole blood (e.g., packed erythrocytes, fresh frozen plasma [FFP], and platelets).28 However, fresh whole blood is often difficult to obtain. The units must be refrigerated for 24 to 48 hours while donor screening is performed, and storage causes significant platelet injury. Insistence on fresh whole blood places tremendous pressures on the transfusion service and donor center to coordinate the matching of donor types with recipient needs.
In practice, individual component therapy is used. In neonates and small infants with dilutional coagulopathy, platelets should be given in combination with cryoprecipitate to correct the defect in clotting. An initial dose of 10 mL of platelets/kg of body weight may need to be repeated. Platelets may be administered if bleeding persists and the platelet count is less than 100,000/mm3.29 Cryoprecipitate contains high concentrations of fibrinogen, factor VIII, von Willebrand factor, and factor XIII. Fibrinogen and von Willebrand factor are required for platelet adhesion and aggregation to occur. Platelet adhesion and aggregation are the fundamental first steps in primary hemostasis (see Chapter 10). The subsequent step of platelet degranulation switches on the entire coagulation cascade and cannot take place without adhesion and aggregation.30 Administration of FFP, for which there is no evidence of effectiveness in treating this type of coagulopathy, to the infant may excessively dilute the red cell mass and platelets.31
Transfusion guidelines have been described for adults and have been shown to reduce postoperative bleeding and transfusion requirements.32,33 However, similar guidelines have not been forthcoming for children in whom the practice appears to be more empirical. This is a less than ideal situation, and more work is urgently needed to produce well-validated guidelines. The thromboelastogram and the platelet count may be used to identify which children are likely to bleed after cardiac surgery.
Antifibrinolytics
The antifibrinolytics used in pediatric cardiac surgery include ε-aminocaproic acid (EACA) and tranexamic acid (TA). In many countries, aprotinin is no longer available, and in other countries, it has only very limited availability after its marketing license was withdrawn due to safety concerns. EACA and TA are lycine analogues that reduce bleeding after cardiac surgery in adults and children.34,35 They do not exert any antiinflammatory activity. Doses for pediatric cardiac surgery have not been clearly established.
Aprotinin is a serine protease inhibitor that has been studied thoroughly in adults. Early evidence demonstrated that it reduces bleeding, reduces the time taken to extubation, shortens ICU stay, and reduces overall mortality rates.36 However, subsequent studies have contradicted these earlier findings.37 The same volume of evidence has not been published for children, although several studies suggest that it is effective in reducing bleeding and that it reduces the time spent on the ventilator and in the ICU.38–41 An increased risk of renal failure or stroke in adults undergoing revascularization surgery has been reported.42 The same investigators reported an increase in the 5-year mortality rate for adults after the use of aprotinin in revascularization surgery.43 Aprotinin has increased the 30-day mortality rate by as much as one third compared with TA or EACA.37 Comparable data for children have not been forthcoming (see Chapter 18). Aprotinin is available for use in New Zealand, Australia, and Canada. It appears that the early data regarding increased death rates have not been supported by subsequent studies and that the benefits may outweigh risks in specific populations.44
Topical Agents
The use of topical agents to promote clot formation and reduce bleeding in children after cardiac surgery is common. The most commonly used topical agents are fibrin sealants. Fibrin sealants mimic the stages of the blood coagulation process. Unlike the synthetic adhesives, they are biocompatible.45 Fibrin sealants are usually sourced from plasma components, and most contain virally inactivated human fibrinogen and thrombin with different quantities of factor XIII, antifibrinolytic agents, and calcium.45 When the fibrinogen and thrombin are mixed during the application process, the fibrinogen is converted to fibrin monomers. This results in the formation of a semirigid fibrin clot. By mimicking the later stages of the coagulation process, these sealants stop bleeding and assist in wound healing.45 They have significantly reduced bleeding in children.46
Ultrafiltration
Ultrafiltration is a process that removes ultrafiltrate from a child during and after CPB. It provides many benefits, including increasing the hematocrit, concentrating the clotting factors and platelets, increasing blood pressure, reducing PVR, and removing inflammatory mediators in the ultrafiltrate. It has significantly reduced bleeding after cardiac surgery in children.47,48
Desmopressin
Desmopressin acts by increasing plasma concentrations of factor VIII and von Willebrand factor. It has been effective in reducing bleeding after CPB in adult cardiac surgery.49 Unfortunately, studies in children failed to demonstrate a similar effectiveness in reducing bleeding or transfusion requirements.50
Anesthesia Management for Surgery Requiring Cardiopulmonary Bypass
Monitoring
Noninvasive monitoring during pediatric cardiac surgery includes pulse oximetry, five-lead electrocardiography, an automated blood pressure cuff, a precordial or esophageal stethoscope, continuous airway manometry, inspired and expired capnography, anesthetic gas and oxygen analysis, multiple-site temperature measurement, and volumetric urine collection. The pulse oximeter is especially important when managing children with congenital cardiac disease. At least two probes should be placed on different limbs in the event that one fails during the procedure. In children with cyanotic heart disease, conventional pulse oximetry overestimates arterial oxygen saturation as saturation decreases51; this error tends to be exacerbated in the presence of severe hypoxemia.52 When monitoring children with a shunt across the ductus arteriosus, a probe should be placed on a right hand digit to measure preductal oxygenation, and a second probe should be placed on a toe to measure postductal oxygenation (children with a right-sided aortic arch may require the probe to be placed on a left-hand digit). Children undergoing repair of coarctation of the aorta should be monitored with a pulse oximeter on the right upper limb, because it may be the only reliable monitor during the repair, and blood pressure cuffs should be placed before and after the coarctation. These two cuffs may be cycled and the differential documented before and after surgical correction.
Monitoring end-tidal carbon dioxide tension (Petco2) is of value in most children. However, in children with cyanotic-shunting cardiac lesions, the Petco2 measurement may be less reflective of Paco2 because of ventilation-perfusion mismatching.53 Arterial blood gases are the most accurate measure of the adequacy of ventilation and oxygenation. To provide rapid decision making, it is helpful to have the blood gas analysis machine located in or near the cardiac operating room.54
Monitoring ionized calcium concentrations is essential during surgical procedures in which significant quantities of citrated blood are infused rapidly or when entire blood volumes are replaced. Neonates are particularly prone to disturbances in their ionized calcium concentration when citrated whole blood, FFP, or platelets are infused. Those with limited cardiac reserve tolerate ionized hypocalcemia poorly because of their greater sensitivity to the myocardial effects of citrate infusion (see Chapter 10).55 In isolation, the total serum calcium concentration is misleading.
Temperature monitoring during CPB is a critical guide to adequate brain cooling and to appropriate rewarming before separation from bypass. Because it is not practical to measure brain temperature directly, surrogate measuring sites are used. The tympanic membrane, nasopharyngeal, and rectum have been used; the nasopharyngeal site most closely matches true brain temperature and is the site at which temperature is most often monitored. The tympanic and rectal sites tend to overestimate the brain temperature.56,57 Measurement of skin temperature gives an indication about peripheral perfusion and provides information about adequate peripheral rewarming.
Central venous lines can be very useful. For cardiac surgical procedures, there are two commonly used methods of obtaining central access. The decision of which to use may be determined in part by institutional bias. In the first method, the cardiac surgeons expose the heart quickly and have it available for inspection and estimation of filling pressures. Central lines can be readily established from the field and handed off to the anesthesia team. These transthoracic central lines are useful but carry a small amount of risk.28 In the second method, percutaneous insertion of central venous lines is particularly indicated for long, complex procedures, especially when access to the infant is limited or the heart is not exposed. Percutaneous cannulation of the central circulation through the internal jugular approach or the subclavian approach has been demonstrated to be safe.29 However, it is important to appreciate that insertion of central venous lines through the internal jugular or subclavian route may fail or may be associated with pneumothorax, hemorrhage, and hematoma formation after puncture of major arteries.29–31 Cannulation of the external jugular vein may avoid some of these serious complications when the catheter can be successfully threaded into the central circulation.32 Increasingly, ultrasound-guided techniques are being employed to establish central venous access (see Chapter 48). In the United Kingdom, the use of ultrasound for the placement of these lines is recommended by the National Institute of Clinical Excellence (NICE), and ultrasound is used routinely for the placement of central lines.
Transesophageal Echocardiography
Use of perioperative echocardiography has become the standard of care in the United States.58,59 In adult practice, anesthesiologists usually perform the TEE, but in children, the TEE is more commonly performed by a pediatric cardiologist. This may reflect the increased complexity of congenital lesions and the difficulty in accurately assessing these lesions and their repairs. TEE has been cost-effective when used routinely during pediatric cardiac surgery.60 The use of TEE can have a significant impact on surgical and medical management. In one large study, a second bypass run was undertaken in 7.3% of cases based on the findings of the TEE. There was a surgical alteration in the management of 12.7% and medical alteration in 18.5% of cases. Pediatric cardiac anesthesiologists usually can perform TEE before and after bypass if they have received adequate training.61
The introduction of small probes with multiplane capability has greatly increased the use of TEE, even in infants and neonates.62,63 In 1999, a survey of centers in the United States indicated that 93% used intraoperative echocardiography and that all but one used TEE.64 The American Society of Echocardiography and the Society of Cardiovascular anesthesiologists have published guidelines for performing a comprehensive intraoperative TEE in adults65 and children.66
Although the use of TEE in children usually is safe, complications do occur and may be more common in small infants.67 Complications include damage to the mouth, oropharynx, esophagus, and stomach. Other complications include hemodynamic disturbance as a result of compression of the left atrium or other structures. Interference with the airway also occurs in a small number of cases. This includes inadvertent extubation, right main-stem intubation, and compression of the tracheal tube. However, the overall incidence is small, approximately 2%.68 Information gathered from the TEE examination takes place before and after bypass and may be divided broadly into two categories: hemodynamic assessment with monitoring and structural diagnostic information. Hemodynamic information includes information about ventricular function and filling.69 Diagnostic information relates to confirmation or otherwise of preoperative findings and assessment of the adequacy of the surgical repair.
Near-Infrared Spectroscopy
Cerebral near-infrared spectroscopy (NIRS) is becoming widely used during cardiopulmonary bypass in children. This noninvasive monitor is used to determine the degree of brain tissue oxygenation.70,71 It is likely to lead to improved neurological outcomes after cardiac surgery although there is no clear evidence in humans (see Chapter 51).
Induction of Anesthesia
The most common inhalational induction agent is sevoflurane. Sevoflurane is very rapid acting and should be used with care in the child with CHD because high concentrations can produce bradycardia, hypotension, and apnea if not titrated carefully. Concentrations should be rapidly reduced after an adequate level of anesthesia is achieved. In children who are cyanotic with a right-to-left shunt and reduced pulmonary blood flow, inhalational inductions are slow. The addition of nitrous oxide can aid an inhalational induction in two ways. First, because it is odorless, it can be started before the introduction of the sevoflurane, allowing the child to be somewhat sedated before the stronger smelling agent is started. Second, it allows a smoother and more rapid induction compared with sevoflurane alone. Concentrations of up to 70% nitrous oxide can be used to smooth induction of anesthesia even in cyanotic children, but the nitrous oxide should be replaced with air and oxygen or 100% oxygen as soon as intravenous access is obtained and a muscle relaxant is given. It is not always necessary to use a mask for an inhalational induction because cupped hands are often more acceptable to the child, particularly one who is frightened of the mask. It is important to tell the child about each event before it happens and to demonstrate the action on yourself, a parent, or toy animal. You should also offer the child the opportunity to hold the mask, or if the child is accompanied by a parent, offer the child the choice of the parent holding the mask. Good premedication often aids this process (see Chapter 4).
For sick children in whom it may be preferable to use an intravenous induction, various options are available. For example, in neonates with coarctation of the aorta or with hypoplastic left heart syndrome who are not ventilated before coming to the operating room, one approach is to administer fentanyl in a dose of 2 to 3 µg/kg, followed by pancuronium and then by a very low dose (i.e., sedative dose) of sevoflurane or isoflurane. Fentanyl obtunds the hypertensive response to intubation, and the pancuronium maintains cardiac output by maintaining the heart rate. The very-low-dose volatile agent provides the sedation or anesthesia. In older children, etomidate is a very good induction agent, providing stable hemodynamics, although it does cause pain on injection. Ketamine is also widely used for intravenous induction in neonates and older children. Ketamine maintains or increases blood pressure, heart rate, and cardiac output. The exact mechanism of these effects of ketamine is unknown; ketamine may stimulate the release of endogenous stores of catecholamines, although it is a negative inotrope in the denervated heart.72 This negative inotropic effect may make ketamine a poor choice in children in whom catecholamine stimulation may already be maximal, such as in severe cardiomyopathy. It may also be a poor choice if tachycardia is undesirable, such as in the case of aortic stenosis.
Monitoring should ideally be applied before induction begins, but applying monitoring can upset the child, which can be detrimental (e.g., the child with TOF who begins to cry and precipitates a “tet spell”). A pulse oximeter probe may be the only monitor that is applied before induction of anesthesia. Sevoflurane or isoflurane may provide another advantage by offering a degree of ischemic preconditioning to the heart and to other organs, particularly the brain and kidney. In a double-blind study of adult patients undergoing coronary bypass grafting, exposure to 4% sevoflurane for 10 minutes before cross-clamping reduced the degree of myocardial dysfunction and renal damage postoperatively.73 It is thought that the same effect is observed in children.74
Control of Systemic and Pulmonary Vascular Resistance during Anesthesia
In some children with hypoplastic left heart syndrome (HLHS) who present for a Norwood procedure, excessive blood flow to the lungs resulting from a relatively low PVR and a relatively high SVR steals blood from the systemic circulation, leading to hypotension, myocardial ischemia, and progressive acidosis. However, when the reverse occurs and the PVR is greater than the SVR, the child develops progressive desaturation. Similar pathophysiology exists with other duct-dependent circulations and to some extent with other shunting lesions. It may prove difficult to manipulate the SVR and PVR predictably because control of PVR is poorly understood, vasoactive drugs usually are distributed on both sides of the circulation, and pharmacologic attempts to modify shunting have produced unpredictable results.75 Despite these problems, several techniques have proved useful in manipulating the relative PVR and SVR. Potent inhalational anesthetics appear to reduce SVR more than PVR. PVR is decreased in children by increasing inspired oxygen to 100% and by hyperventilation to a pH of 7.6 or greater. Positive end-expiratory pressure, acidosis, hypothermia, and the use of 30% or less inspired oxygen can increase PVR. Because vasoconstrictors such as phenylephrine increase SVR more than PVR, they are effective acutely in reducing right-to-left shunting and increasing left-to-right shunting in the operating room.
Anesthetic Drugs Used in Pediatric Cardiac Anesthesia
Inhalational Agents
Sevoflurane
Sevoflurane is the induction agent of choice in pediatric anesthesia.76,77 It is associated with little myocardial depression or dysrhythmias.78–80 It has specific advantages over halothane when used in children with CHD, particularly in children younger than 1 year of age and in cyanotic children.81 In contrast to halothane, sevoflurane causes no reduction in heart rate at 1.0 and 1.5 minimal alveolar concentrations (MACs) in healthy children compared with awake values.82 However, at greater concentrations, it can slow the heart rate and cause respiratory depression. Both features are important in children with CHD because a slow heart rate reduces cardiac output and hypoventilation leads to hypercarbia and hypoxia, which can increase PVR. In the absence of nitrous oxide, sevoflurane causes less depression of myocardial contractility than halothane during induction of anesthesia. Sevoflurane does cause a mild decrease in SVR, but in common with halothane and isoflurane, it does not perturb the shunt between the right and left sides of the heart through an ASD or VSD when it is given in anesthetic concentrations of about 1 MAC in 100% oxygen.83 Sevoflurane has caused conduction abnormalities in susceptible patients.84 It should also be used with great caution in children with severe ventricular outflow tract obstruction (see Chapter 6).85
Isoflurane
At equipotent concentrations, isoflurane causes similar hemodynamic depression in neonates and infants compared with halothane. Isoflurane typically is not used for induction of anesthesia because of the high frequency of laryngospasm (greater than 20%).86 Inadequate ventilation because of laryngospasm or other causes quickly leads to large increases in PVR due to hypoxemia and hypercarbia. This increase in PVR and the resulting pulmonary hypertension is poorly tolerated in small children with heart disease, especially in the presence of right-to-left shunting (see Chapter 6).
Halothane
In the United States and the United Kingdom, the use of halothane has all but ceased, but it is still widely used in other parts of the world. It is included here for completeness. Uptake of halothane in infants younger than 3 months of age is more rapid than it is in adults. This also is the case for the uptake of halothane by the myocardium.87 Although the effects of halothane on the human neonatal myocardium are unknown, young rodents have a reduced cardiovascular tolerance for halothane but require greater amounts for anesthesia.88 Studies have shown a significant incidence of hypotension with bradycardia in infants with normal cardiovascular systems during induction with halothane.89 During induction of anesthesia in normal infants, halothane decreases the cardiac index to 73% of awake values at 1.0 MAC and to 59% at 1.5 MAC.90 The MAC for halothane in infants 1 to 6 months of age is the greatest of any age group.91 This increased anesthetic requirement in infants, combined with the immaturity of their cardiovascular system, explains in part the relative cardiovascular intolerance of halothane by infants. Atropine has been used intramuscularly before induction to partially compensate for the myocardial depression of halothane by reducing bradycardia and hypotension. Although halothane may produce some degree of hypotension, an increase in arterial saturation in children with cyanotic CHD may occur.92
A careful induction with sevoflurane is usually well tolerated in children with mild to moderate heart disease. However, large concentrations of potent inhalational agents may be an unwise choice for induction in young infants with severe cardiac disease. In children of any age with marginal cardiovascular reserve and in those with severe desaturation of systemic arterial blood due to right-to-left shunting, inhalational anesthetic-induced myocardial depression and systemic hypotension are poorly tolerated. A more appropriate use of these anesthetic agents in children with severe heart disease is the addition of low concentrations of the inhalational agent to control hypertensive responses after an intravenous induction (see Chapter 6).
Nitrous Oxide
Nitrous oxide should be avoided for maintenance of anesthesia in children with CHD because of the risk of enlarging intravascular air emboli and the potential to increase the PVR. Nitrous oxide may expand microbubbles and macrobubbles, increasing obstruction to blood flow in arteries and capillaries. In all children with right-to-left shunts, there is a potential for these bubbles to be shunted directly into the systemic circulation and coronaries. Care must be taken to ensure that no air bubbles are accidentally injected into the veins. Adverse outcomes after coronary air embolism are exacerbated by nitrous oxide.93 The hemodynamic effects of venous air embolism are increased by nitrous oxide, even without paradoxical embolization.94 In children with preexisting right-to-left shunts, paradoxical air embolism is clearly a potential problem; but even those with large left-to-right shunts can transiently reverse their shunts. This is particularly true during coughing or a Valsalva maneuver, when the normal transatrial pressure gradient is reversed. Several studies have demonstrated right-to-left shunting of microbubbles of air after injection of saline into the right atrium during these maneuvers.95–97 Because coughing and Valsalva maneuvers may occur during anesthesia induction, even the most rigorous attention to avoiding air bubbles in intravenous lines may not prevent small amounts of air from reaching the systemic circulation. Microbubbles have also been observed after CPB.98
Nitrous oxide can increase PVR in adults.99,100 However, in a 50% inspired concentration, it does not appear to affect PVR or pulmonary artery pressure in infants.101 Nitrous oxide mildly decreases cardiac output at this concentration.102 Avoidance of the use of nitrous oxide has been suggested in children with limited pulmonary blood flow, pulmonary hypertension, or depressed myocardial function. In the well-compensated child who does not require 100% inspired oxygen, nitrous oxide (usually at concentrations of 50%) may be used during induction of anesthesia but discontinued before tracheal intubation. If a reduced inspired oxygen concentration is indicated to maintain an appropriate balance between PVR and SVR after tracheal intubation, air may be added to the inspired gas mixture (see Chapter 6).
Intravenous Induction Agents
Ketamine
Ketamine is a dissociative anesthetic agent that is a good analgesic. It increases blood pressure, heart rate, and cardiac output. Although the mechanism of the stimulation of blood pressure and heart rate has not been established, it is thought to stimulate the release of endogenous stores of catecholamines. Ketamine exerts a negative inotropic effect on the denervated heart.103 I think that this combination of effects makes it a poor choice for children in whom sympathetic stimulation may already be maximal, such as in those with severe cardiomyopathy. It is also a poor choice if tachycardia is undesirable, such as in a child with aortic stenosis. Ketamine is thought to have minimal effect on PVR in children with CHD as long as the airway and ventilation are well preserved,104,105 although it has occasionally increased PVR in children undergoing cardiac catheterization. Ketamine is quite a versatile anesthetic that may be administered intramuscularly and orally when intravenous access is difficult or an inhalational induction is contraindicated. The usual intravenous dose of 2 mg/kg produces a very predictable response, and an intramuscular dose of 8 to 10 mg/kg (combined with 0.1 mg/kg of intramuscular midazolam) is less predictable. The oral dose of ketamine is 5 to 6 mg/kg. The use of ketamine varies greatly from one institution to another, with some units using it extensively and others using it rarely (see Chapter 6).
Etomidate
Etomidate is a very safe drug, with an LD50 to ED50 ratio of 26 in animals models.106 This ratio indicates that the lethal dose (LD) is 26 times greater than the effective dose (ED). It is a short-acting anesthetic with little effect on systemic blood pressure, heart rate, and cardiac output after a single dose in healthy children.107 Etomidate has a favorable hemodynamic profile even when used in shocked children and appears to have a low risk of clinically important myoclonus or status epilepticus, pain on intravenous injection, and nausea and vomiting.108,109 The major concern about etomidate is the increased mortality rates reported when it is administered as a continuous infusion. This grave side effect has been attributed to adrenal suppression.110–112 The inhibition of steroid synthesis occurs after a prolonged infusion and after a single dose of etomidate, and it has created a controversy about its use as an anesthetic agent, particularly in the ICUs in some jurisdictions.113 However, newer analogues of etomidate have addressed these deficiencies and may lead to a surge in its use in the future (see Chapter 6).
Propofol
Propofol is a rapidly acting intravenous hypnotic agent that may be administered as a single dose or by continuous infusion. It has no analgesic properties. Propofol has mild antiemetic properties.114 Its short duration of action is the result of rapid redistribution and metabolism, which also allows the drug to be given by continuous infusion without accumulation. Induction doses decrease SVR, blood pressure, and cardiac output; the effect on heart rate varies. The ED50 for propofol in infants and small children is greater than it is in adults.110–117 If propofol is given very slowly, smaller doses are required to achieve the anesthetic state, although the induction time increases. A slower infusion also results in more stable hemodynamics.118 Pain on injection and involuntary movement after intravenous propofol have been concerns that have been overcome (see Chapter 6). Although propofol can be used safely in children with CHD, it is typically avoided as an induction agent in those with severe CHD because of its effects on SVR and blood pressure. It should be avoided in those with a fixed cardiac output such as severe aortic or mitral stenosis because it may cause severe hypotension. It can be used by infusion during CPB to reduce awareness and may be particularly useful if an early extubation is planned (see Chapter 6).
Opioids
Fentanyl
As in adults with severe cardiac disease, intravenous fentanyl combined with pancuronium and 100% oxygen or air and oxygen provides an excellent induction technique in very sick children with CHD, although it is not an amnestic. Inclusion of intravenous midazolam or another amnestic agent is strongly urged to avoid awareness. In neonates and infants, the use of high-dose opioid anesthesia provides excellent hemodynamic stability, with suppression of the hormonal and metabolic stress response.119,120 When fentanyl or other opioids are combined with nitrous oxide, the negative inotropic effects of nitrous oxide may be evident, particularly in sicker children.121 The high-dose fentanyl technique is effective in preterm neonates undergoing ligation of a PDA.122 In high-risk, full-term neonates and in older infants with severe CHD, the high-dose fentanyl technique in doses of up to 75 µg/kg, combined with pancuronium maintains stable hemodynamics during induction, tracheal intubation, and surgical incision.123 Oxygen saturation is well maintained and often improves during induction, even in cyanotic children.124 The cardiac index, SVR, and PVR in infants given 25 µg/kg of fentanyl do not change significantly.125 Combining pancuronium with fentanyl is desirable because the vagolytic effects of pancuronium offset the potential vagotonic effects of fentanyl. The hemodynamic stability reported in infants with the combination of high-dose fentanyl and pancuronium may not be replicated when other muscle relaxants are used (see Chapter 6).126
Sufentanil
Sufentanil (5 to 20 µg/kg), an alternative to fentanyl, is 5 to 10 times more potent than fentanyl but has a large margin of safety.127 It is highly lipophilic and is rapidly distributed to all tissues. It is infrequently used in infants and children with CHD.
Remifentanil
Remifentanil is an ultra-short-acting opioid that is rapidly metabolized in the plasma and tissue by nonspecific esterases to an inactive metabolite. It has a very brief elimination half-life, with a context-sensitive half-life of only 3 minutes, independent of the duration of infusion. In pediatric cardiac surgery, it is an attractive alternative to fentanyl that provides intense analgesia during the most stimulating parts of surgery but facilitates rapid awakening and weaning from mechanical ventilation without residual opioid effect. Its pharmacodynamics are unaffected by CPB.128 It provides stable hemodynamic conditions in children, although there is a tendency toward bradycardia and systemic hypotension.129–131 It has no negative inotropic effect, even in the failing heart.132
A significant concern is the development of acute tolerance with increasing analgesic requirements after discontinuing remifentanil.133–135 Some studies have suggested that this is not clinically important.136 Strategies to prevent tolerance to remifentanil have included intravenous magnesium infusions as well as nitrous oxide.137,138 Remifentanil is also used for prolonged sedation of children in the ICU. Many units have moved toward early extubation and discharge from the ICU after cardiac surgery (i.e., fast tracking), and remifentanil is a useful drug in this setting (see Chapter 6). Consideration must be given to transitioning to a longer-acting opioid before discontinuation of remifentanil.
Neuromuscular Blocking Drugs
Pancuronium has been studied in depth in children with CHD. When administered over a 60- to 90-second interval, pancuronium maintains heart rate and blood pressure.140 An intubating bolus dose of pancuronium may produce tachycardia and increase cardiac output. This bolus dose effect is sometimes desirable to support cardiac output in infants in congestive heart failure because their stroke volume is fixed. Pancuronium may be the muscle relaxant of choice when high-dose opioid techniques are used to offset the vagotonic effects of opioids such as fentanyl. Other muscle relaxants are also widely used, particularly if they are to be extubated in the operating room or early in the ICU.
Long-Term Neurocognitive-Developmental Outcomes Associated with Anesthesia
Concerns have been raised about the possibility that many of the anesthetic agents such as inhalational anesthetics, propofol, ketamine, and midazolam may cause long-term neurocognitive-developmental problems in neonates and young infants.139 This effect is thought to result from the neuronal apoptosis caused by these agents. The opioids have not been implicated in these changes, but this may change in time. There is no evidence to directly link anesthetic exposure in infancy to long-term neurocognitive defects. There is much ongoing research in this area (see Chapter 23).
Regional Anesthesia
The use of regional anesthesia to provide pain relief during and after cardiac surgery in adults also reduces the stress response to surgery and may reduce morbidity and mortality. In adults undergoing cardiac surgery, the benefits of regional anesthesia include earlier extubation, fewer respiratory complications, a reduction in renal failure, fewer strokes, and less myocardial damage after CPB.141–143 In animals, thoracic epidural anesthesia reduces myocardial damage after coronary occlusion.144 The same benefits may be achieved by using intrathecal (spinal) analgesia. High spinal anesthesia using bupivacaine reduces the stress response to CPB and β-adrenergic dysfunction and improves cardiac performance after cardiac surgery in adults.145
Good research into regional anesthesia and analgesia in pediatric cardiac surgery is limited. Caudal morphine has been used to provide postoperative analgesia and has produced good analgesia for about 6 hours while reducing analgesic requirements for up to 24 hours.146 Two retrospective studies in children147,148 included a variety of regional anesthetic techniques. Most children were extubated in the operating room, although ∼4% of them required reintubation within 24 hours. Adverse effects included emesis (39%), pruritus (10%), urinary retention (7%), postoperative transient paresthesia (3%), and respiratory depression (1.8%). The rate of adverse effects was less with a thoracic catheter epidural approach compared with various caudal, lumbar epidural, and spinal approaches. Hospital duration of stay was unaffected by the presence of regional anesthesia complications. Although this study appears to indicate that regional analgesia is safe, the numbers in the study are too small to conclude that regional analgesia is safe for pediatric cardiac surgery.
The use of regional anesthesia in cardiac surgery for children remains controversial.149,150 The main concern is the risk of bleeding and the potential for disastrous neurologic complications. The risks may be greater in children than in adults because of the presence of collateral vessels, increased venous pressure, coagulopathy related to cyanosis, and the use of aspirin. There remain many unanswered questions regarding neuraxial block in children, such as the true incidence of epidural hematoma, the time delay required between placement of the epidural catheter and full anticoagulation, and the correct management of a bloody tap. The estimated risk of epidural hematoma during cardiac surgery in adults is 1 case per 1000 patients and 1 case per 2400 patients for spinal and epidural block, respectively.151 Whether the risks are similar or greater in children cannot be determined because, the numbers of children involved in studies are too small. A large, randomized, prospective study to evaluate a true risk-benefit ratio without bias is needed; until such data are available, various commentators have advised great caution with the use of regional analgesia for cardiac surgery, and some have suggested that it may not be possible to perform the study required because of ethical considerations.152
Fast Tracking
Early extubation of pediatric patients after cardiac surgery offers advantages in terms of cost and reduced morbidity associated with longer ICU stays.153–157 The success of this approach depends on the close teamwork of a multidisciplinary team, with every member of the team working toward the same goal. Successful fast tracking usually requires the development of care pathways to ensure that the quality of patient care is not compromised.158 Early extubation and discharge from the ICU requires preplanning and the adoption of a technique that facilitates this goal. The use of very large doses of fentanyl is not appropriate; alternative techniques have been used, including smaller doses of fentanyl in combination with inhalational agents159,160 or the use of remifentanil in combination with inhalational agents or with propofol. Others have advocated regional anesthesia as a means of speeding extubation, but this approach remains controversial. It is important to choose a neuromuscular blocking drug with a shorter duration of action than pancuronium to ensure that it is easy to reverse the neuromuscular block at the end of surgery. Other important considerations to ensure that early extubation is a success include adequate pain relief in the form of intravenous paracetamol (where available), patient-controlled or nurse-controlled analgesia, and antiemetics because nausea appears to be more of a problem in children who are extubated early.
Some clinicians advocate extubating the trachea in the operating room, whereas others advocate waiting until the child is in the ICU. Delaying the extubation until the child is in the ICU may save operating room time and may reduce the risks of cardiovascular instability, bleeding, and hypothermia.161 Despite these concerns, the tracheas of many children are extubated in the operating room with good outcomes.
Stress Response to Cardiac Surgery
Cardiac surgery and CPB are altered physiologic conditions associated with exaggerated stress responses characterized by the release of numerous metabolic and hormonal substances, including catecholamines, cortisol, growth hormone, prostaglandins, complement, glucose, insulin, and β-endorphins.162,163 The cause of the elaboration of these substances is multifactorial: contact of blood with foreign surfaces, low perfusion pressure, anemia, hypothermia, myocardial ischemia, low levels of anesthesia, and nonpulsatile flow. Other factors that contribute to the increase in stress hormones are delayed renal and hepatic clearance and exclusion of the pulmonary circulation during extracorporeal circulation.164
Neonates of all viable gestational ages, older infants, and children have nociceptive systems that are sufficiently developed and integrated with brainstem cardiovascular control centers to trigger humoral and circulatory responses to pain and stress.165 Substantial humoral, metabolic, and cardiovascular responses to painful and stressful stimulation during surgery have been documented in neonates of all gestational ages and in older infants.166,167 Hormonal stress responses in neonates subjected to cardiac and noncardiac operations are threefold to fivefold greater than those in adults after similar surgeries. Circulatory responses to stressful stimuli in children include systemic and pulmonary hypertension.
Humoral stress responses are particularly extreme during and after cardiac surgery. These responses are characterized by increases in circulating catecholamines, glucagon, cortisol, β-endorphins, growth hormone, and insulin; circulating concentrations of catecholamines may increase by as much as 400% over baseline preoperative concentrations. This is evidence of a massive activation of sympathetic outflow in response to surgical stimulation. Some of these responses may continue for several days postoperatively.168
It has been suggested that such extreme stress responses and neuroendocrine activation may be associated with greater morbidity and mortality. In adults, intraoperative adrenergic activation of 50% above baseline is associated with significant postoperative alterations in β-adrenergic receptor function, including increased β-receptor density and decreased receptor affinity. Mortality among adults with severe congestive failure is associated with increased levels of hormones regulating cardiovascular function, including aldosterone, epinephrine, and norepinephrine.169 In neonates undergoing cardiac surgery, increased concentrations of stress hormones may be associated with increased mortality rates.168
Another factor is the potential effect of stress-induced hyperglycemia on the neurologic outcome. Neonates and young infants are capable of substantial rates of glucose production, mainly from glycogenolysis and gluconeogenesis during surgical stress that can result in hyperglycemia. Such hyperglycemic responses may be associated with poorer neurologic outcomes, particularly after a period of cerebral ischemia.170 The use of high doses of fentanyl (more than 50 µg/kg) has reduced the hormonal stress response and resultant hyperglycemia and may lessen the risk of neurologic injury.171
In sufficient doses, opioids can blunt the stress responses in neonates, infants, and adults.172–174 This blunting results in a more normal, homeostatic humoral and metabolic milieu in the circulation by reducing neuroendocrine activation and levels of regulating hormones. In infants, the use of high-dose opioids for major surgical procedures and postoperative sedation substantially attenuates the neuroendocrine response to surgically induced pain and stress. Catecholamine release that results from intraoperative stress responses may predispose the vulnerable myocardium to dysrhythmias. In neonates with HLHS, sudden ventricular fibrillation occurred in 50% during surgical manipulation under halothane anesthesia. This incidence was dramatically reduced when high doses of fentanyl were introduced as the primary analgesic/sedative.175 With the use of high-dose opioids, intraoperative ventricular fibrillation has virtually disappeared as a problem in this group of neonates.176 In several studies, opioids have been shown to increase the ventricular fibrillation threshold in isolated cardiac Purkinje fibers and to alter action potential duration similar to that with class III antiarrhythmic agents.177,178 Even electrophysiologic events in the neonatal heart, in addition to humoral and hemodynamic responses, may be altered by using high-dose fentanyl anesthesia to attenuate the effects of pain and stress.
Reducing the Stress Response to Surgery and Bypass
Corticosteroids
Corticosteroids are used in many centers in an attempt to reduce the inflammatory response to surgery and bypass.179 However, there is a huge variability in the formulation of the corticosteroids used, the doses, the timing of administration, and the indications for their use. The literature lacks adequate evidence for the use of corticosteroids, although several small studies in humans and animals suggest they confer a benefit.180,181 Many investigators have called for a large multicenter study to determine the benefit of corticosteroids before bypass and the optimal dose and timing.182
Aprotinin
Aprotinin, which was originally used to reduce bleeding after CPB, is now also appreciated to confer significant antiinflammatory effects.183–187 In adults, it reduces mortality and length of ICU stay.188 In children, it improves pulmonary function in the postoperative period and reduces the time to extubation and ICU stay. However, aprotinin is no longer available for routine use in the United States or continental Europe. In the United Kingdom, it is available for use on a named patient basis, but its use has been dramatically reduced as a result.
Allopurinol
Allopurinol is thought to provide protection against oxygen free radicals during reperfusion by inhibiting xanthine oxidase. It reduces oxygen free radical production and may reduce neurologic and cardiac damage after deep hypothermic cardiac arrest.189 This strategy does not appear to have developed widespread use.
Ischemic Preconditioning
The heart is capable of short-term rapid adaptation to brief ischemia such that during a subsequent, more severe ischemic insult, myocardial necrosis is delayed. The infarct-delaying properties of ischemic preconditioning have been observed in all species studied. Five minutes of ischemia is sufficient to initiate preconditioning, and the protective period lasts for 1 to 2 hours. Laboratory experiments have demonstrated that the stimulation of adenosine receptors initiates preconditioning and the intracellular signal transduction mechanisms involve protein kinase C and adenosine triphosphate (ATP)-dependent potassium channels, although there may be some differences between species. An analysis of studies of myocardial infarction in humans has demonstrated that some adults who report having had angina in the days before infarction have a better outcome after their infarction in part due to the ischemic preconditioning. More direct evidence has come from an investigation of adults undergoing percutaneous transluminal angioplasty in whom the ST-segment changes induced by balloon inflation were more marked during the first inflation than the second. In adults undergoing coronary artery bypass grafting, the decline in ATP content during the first 10 minutes of ischemia was reduced in those subjected to a brief preconditioning protocol.190–194
It may be possible to protect organs other than the heart by ischemic preconditioning. It may even be possible to protect organs remotely by producing a period of ischemia in one area such as a limb, which then confers protection to remote organs.195 Cheung and coworkers have demonstrated that the use of a blood pressure cuff to produce short periods of limb ischemia can produce beneficial effects on the heart, lungs, and generalized inflammatory response.196
Glucose-Insulin and Potassium
The use of glucose-insulin and potassium has been advocated for more than 40 years in adult cardiac surgery. It is thought to protect the myocardium from the effects of ischemia caused by aortic cross-clamping.197–200 Its effects have not been studied in children undergoing cardiac surgery.
Anesthesia Considerations for Specific Cardiac Defects
Discussion of anesthesia considerations for repair of every form of CHD is beyond the scope of this chapter. However, a brief discussion of the problems that may be encountered during repair of the more common congenital heart lesions is presented. It is useful to group lesions together because the management principles can be applied more generally within groups (Table 15-2).
TABLE 15-2 Classification of Congenital Heart Disease
Simple Left-to-Right Shunts
Simple left-to-right shunts increase pulmonary blood flow. If the shunt is large, blood flow to the lungs can be as much as threefold to fourfold greater than normal, resulting in volume loading of the right heart. This can lead to right atrial enlargement and right ventricular enlargement that is perhaps associated with tricuspid and pulmonary regurgitation. This combination results in cardiac failure (Table 15-3).
TABLE 15-3 Clinical Features of Cardiac Failure in Children
Medical management of these children is primarily achieved with diuretics. If pulmonary blood flow is large and left untreated, pulmonary vascular disease begins to develop, resulting in pulmonary hypertension. In the early stages, the changes are reversible, but in time, the changes may become irreversible.201–206 Eisenmenger syndrome refers to severe pulmonary hypertension that leads to suprasystemic pulmonary artery pressures that cause the shunt to reverse, leading to cyanosis. The previous left-to-right shunt reverses to become a right-to-left shunt. At this point, the child’s condition becomes inoperable.
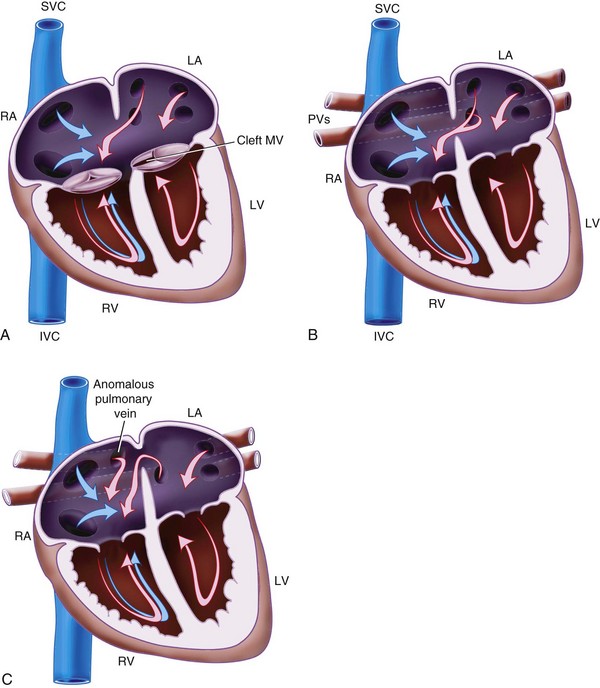
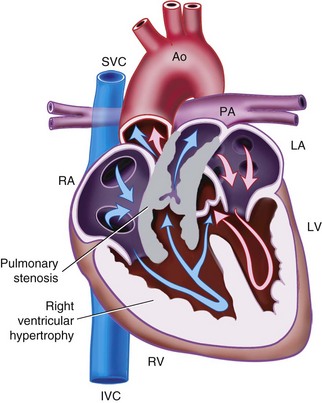
Atrial Septal Defect
ASD is a common heart defect in children, occurring in 1 of 1500 live births and accounting for approximately 10% of all CHD.207 Several types of ASDs exist.
 Patent foramen ovale (PFO) is a normal fetal communication between the two atria that usually closes soon after birth. The PFO remains patent in up to 30% of people. PFO is usually left untreated in children.
Patent foramen ovale (PFO) is a normal fetal communication between the two atria that usually closes soon after birth. The PFO remains patent in up to 30% of people. PFO is usually left untreated in children.
 Primum ASD (Fig. 15-1, A) is located in the inferior part of the atrial septum close to the AV valve and may be associated with a cleft mitral valve. This is a variant of AV septal defect (AVSD).
Primum ASD (Fig. 15-1, A) is located in the inferior part of the atrial septum close to the AV valve and may be associated with a cleft mitral valve. This is a variant of AV septal defect (AVSD).
 Secundum ASD (see Fig. 15-1, B) is found in the region of the fossa ovalis and results from a deficiency in the septum secundum.
Secundum ASD (see Fig. 15-1, B) is found in the region of the fossa ovalis and results from a deficiency in the septum secundum.
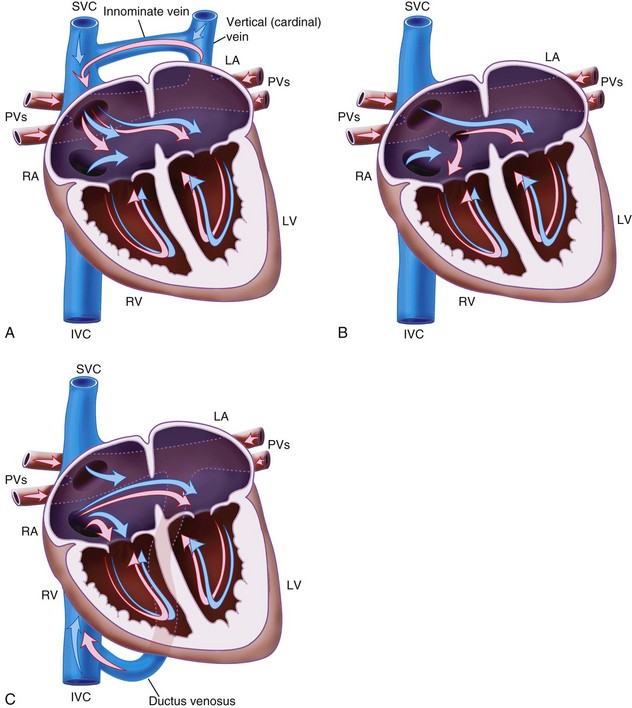
 Sinus venosus ASD (see Fig. 15-1, C) occurs high in the atrial septum, often close to the opening of the SVC. It may be associated with partial anomalous pulmonary venous drainage.
Sinus venosus ASD (see Fig. 15-1, C) occurs high in the atrial septum, often close to the opening of the SVC. It may be associated with partial anomalous pulmonary venous drainage.
 Coronary sinus ASD (i.e., unroofed coronary sinus) is a defect in the atrial wall allows blood to flow from the left atrium to right atrium through the coronary sinus.
Coronary sinus ASD (i.e., unroofed coronary sinus) is a defect in the atrial wall allows blood to flow from the left atrium to right atrium through the coronary sinus.
 Common atrium has a complete absence of the atrial septum. The AV valves may be abnormal or unaffected.
Common atrium has a complete absence of the atrial septum. The AV valves may be abnormal or unaffected.
Anesthesia Considerations
 These children can frequently be extubated on the operating table or early in the ICU, and smaller doses of opioids can be used. Alternatively, short-acting drugs (e.g., remifentanil) administered by infusion and possibly in combination with propofol are useful if early extubation is planned.
These children can frequently be extubated on the operating table or early in the ICU, and smaller doses of opioids can be used. Alternatively, short-acting drugs (e.g., remifentanil) administered by infusion and possibly in combination with propofol are useful if early extubation is planned.
 The problems of postoperative pulmonary hypertension are seldom encountered.
The problems of postoperative pulmonary hypertension are seldom encountered.
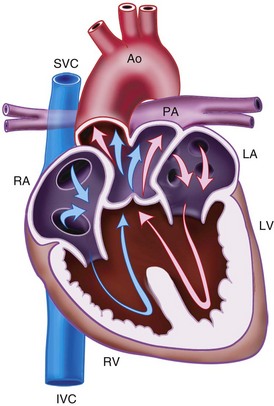
Ventricular Septal Defect
VSD is the most common congenital defect in children, occurring in 1.5 to 3.5 of 1000 live births and accounting for 20% of CHD (Fig. 15-2, A).208 Four types are described: subarterial (5%), perimembranous (80%), inlet (5%), and muscular (10%). If the flow through the VSD is small, it is referred to as restrictive, but if the flow is large, it is called unrestrictive. It is possible to close a small percent of VSDs using a percutaneous, transcatheter device.
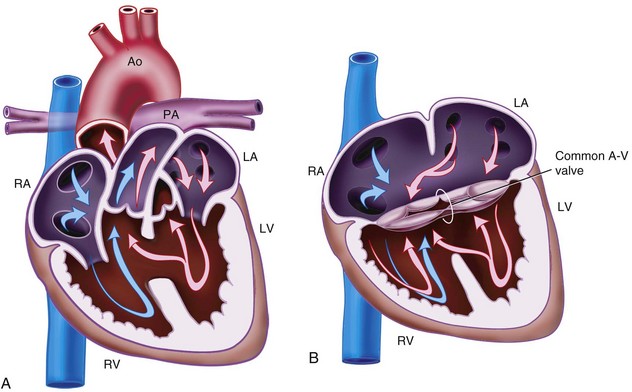
Atrioventricular Septal Defect
AVSDs are also known as AV canal defects or endocardial cushion defects, and they result from a defect in the AV septum. The incidence is about 0.2 cases per 1000 live births, and they account for about 3% of CHD. They are commonly associated with trisomy 21, TOF, and DiGeorge syndrome (Table 15-4). Two common types of AVSD exist:
 Partial AVSD usually consists of a primum ASD with a cleft in the anterior mitral valve leaflet (see Fig. 15-1, A).
Partial AVSD usually consists of a primum ASD with a cleft in the anterior mitral valve leaflet (see Fig. 15-1, A).
 Complete AVSD consists of a large septal defect with atrial and ventricular components and a common AV valve (see Fig. 15-2, B).
Complete AVSD consists of a large septal defect with atrial and ventricular components and a common AV valve (see Fig. 15-2, B).
TABLE 15-4 Clinical Features and Concerns of DiGeorge Syndrome
Aortopulmonary Window
Aortopulmonary window is a rare CHD defect in which there is a communication between the main pulmonary artery and the ascending aorta, and it accounts for 0.1% of CHD (Fig. 15-3). Four types are classified according to the size and exact position of the defect.209 A left-to-right shunt is usually present. These children present with heart failure and are at risk for pulmonary vascular disease if not treated early. It is frequently associated with other cardiac and noncardiac anomalies:
 VACTERL: vertebral anomalies, anal atresia, cardiac defect, tracheoesophageal atresia, renal anomalies, and limb abnormalities
VACTERL: vertebral anomalies, anal atresia, cardiac defect, tracheoesophageal atresia, renal anomalies, and limb abnormalities
 CHARGE: coloboma of the eye and central nervous system anomalies, heart defects, atresia of the choanae, retardation of growth and development, genital or urinary defects, and ear anomalies
CHARGE: coloboma of the eye and central nervous system anomalies, heart defects, atresia of the choanae, retardation of growth and development, genital or urinary defects, and ear anomalies
 CATCH-22 association (i.e., mnemonic for DiGeorge syndrome): cardiac defect, abnormal facies, thymic hypoplasia, cleft palate, hypocalcemia (velocardiofacial syndrome), with 22q11 chromosome microdeletion (see Table 15-4)
CATCH-22 association (i.e., mnemonic for DiGeorge syndrome): cardiac defect, abnormal facies, thymic hypoplasia, cleft palate, hypocalcemia (velocardiofacial syndrome), with 22q11 chromosome microdeletion (see Table 15-4)
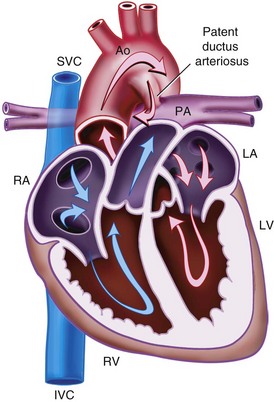
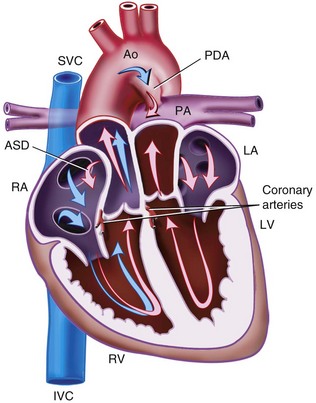
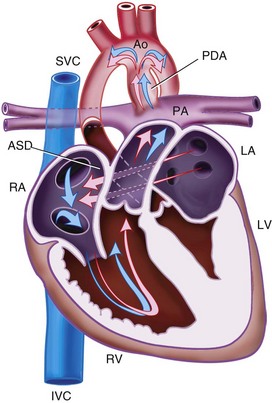
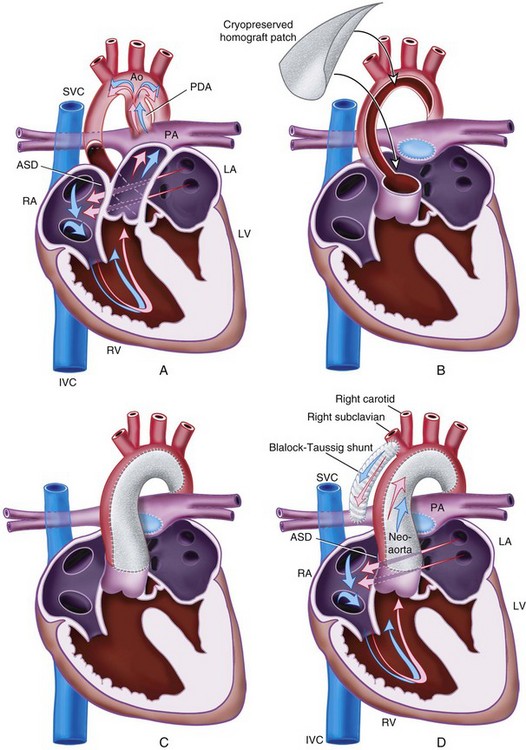
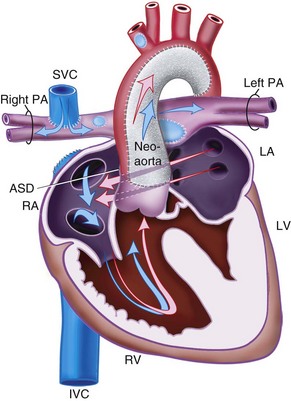
Patent Ductus Arteriosus
The ductus arteriosus, a remnant from the fetal circulation, extends from the descending aorta to the main pulmonary artery and usually closes soon after birth. However, it remains patent in approximately 1 of 2500 live births and accounts for about 10% of all CHD (Fig. 15-4). In the fetus, blood from the right ventricle is directed into the pulmonary artery, but because of the high PVR, it flows into the descending aorta. After birth, the PVR decreases, and blood flows from the aorta to the lungs. PDA is common in preterm infants, and its presence may explain an ongoing requirement for mechanical ventilation. In these infants, a left thoracotomy is required to ligate or divide the PDA. It may also occur in older children; but at this age, percutaneous closure by an interventional cardiologist is the preferred approach. The anesthesia implications are similar to those of other lesions described with left-to-right shunts preoperatively.
Preoperative requirements include the following:
Particular perioperative risks include the following:
 Difficulty ventilating or hemoglobin desaturation because of lung retraction
Difficulty ventilating or hemoglobin desaturation because of lung retraction
 Tearing the PDA with massive hemorrhage
Tearing the PDA with massive hemorrhage
Anesthesia Considerations
 A dedicated intravenous line for fluids and drugs with a long (100 to 150 cm), low-caliber extension to allow access from a distance (space around the cots in NICU is limited)
A dedicated intravenous line for fluids and drugs with a long (100 to 150 cm), low-caliber extension to allow access from a distance (space around the cots in NICU is limited)
 The tracheal tube should have only a small air leak. A large leak may prevent adequate ventilation during lung retraction (recheck security and correct position of the tip of the tube after repositioning in the decubitus position, before starting surgery).
The tracheal tube should have only a small air leak. A large leak may prevent adequate ventilation during lung retraction (recheck security and correct position of the tip of the tube after repositioning in the decubitus position, before starting surgery).
 Intercostal nerve block by surgeon at the completion of surgery
Intercostal nerve block by surgeon at the completion of surgery
Simple Right-to-Left Shunts
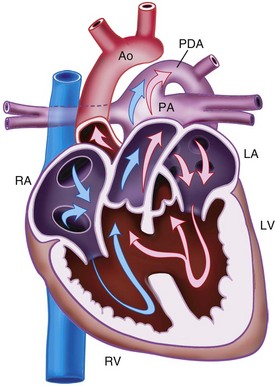
Tetralogy of Fallot
TOF is the most common cyanotic CHD defect and accounts for 6% to 11% of CHD. It has four features (Fig. 15-5):
The right-to-left shunt and cyanosis observed in children with TOF results from a combination of the RVOTO and VSD. The degree of hypoxemia depends on the relationship between the RVOTO and the SVR that determines the degree of right-to-left shunting. TOF may be associated with a large number of other cardiac and extracardiac anomalies. Extracardiac anomalies include DiGeorge syndrome (see Table 15-4) and trisomy 21.
Hypercyanotic Episodes
 Sedation or analgesia (e.g., fentanyl, morphine)
Sedation or analgesia (e.g., fentanyl, morphine)
 Phenylephrine is given as a 0.5-µg/kg bolus and doubled at 1-minute intervals until a satisfactory response is achieved; this is followed by an infusion at 1 to 5 µg/kg/min (larger bolus doses may be required in small preterm infants).
Phenylephrine is given as a 0.5-µg/kg bolus and doubled at 1-minute intervals until a satisfactory response is achieved; this is followed by an infusion at 1 to 5 µg/kg/min (larger bolus doses may be required in small preterm infants). β-Blockers are administered to relax infundibular spasm and reduce the heart rate.
β-Blockers are administered to relax infundibular spasm and reduce the heart rate.
Surgical Management
The optimal surgical management of children with TOF remains controversial. The choice is between initial palliation with a systemic-to-pulmonary shunt followed by a complete repair when the infant is older and complete repair during the neonatal or early infant period. The current trend is toward early complete repair.210,211 Complete repair involves closure of the VSD and relief of the RVOTO. Relief of the RVOTO most commonly requires a transannular patch that involves a right ventriculotomy. Right ventricular dysfunction is a particular problem after repair, and a degree of pulmonary regurgitation is usually present if a transannular patch has been done. Junctional ectopic tachycardia (JET) is a particular risk after complete correction.
Anesthesia Considerations
Systemic-to-Pulmonary Shunt.
 The patient is usually a neonate or small infant.
The patient is usually a neonate or small infant.
 Sedative premedication is useful to prevent crying during induction, which may provoke a hypercyanotic spell.
Sedative premedication is useful to prevent crying during induction, which may provoke a hypercyanotic spell.
 There is a risk of a hypercyanotic spell during induction and surgery.
There is a risk of a hypercyanotic spell during induction and surgery.
 Inhalational or intravenous induction is appropriate.
Inhalational or intravenous induction is appropriate.
 Surgery is usually performed through a thoracotomy (left or right) but may be through a sternotomy.
Surgery is usually performed through a thoracotomy (left or right) but may be through a sternotomy.
 Arterial and central venous access is required.
Arterial and central venous access is required.
 Tracheal tube should be snug, with no or minimal air leak because lung retraction during surgery makes ventilation very difficult.
Tracheal tube should be snug, with no or minimal air leak because lung retraction during surgery makes ventilation very difficult.
 The arterial line should not be in the arm on the side that the shunt will be placed because the subclavian artery will be clamped, and the arterial pressure will be lost.
The arterial line should not be in the arm on the side that the shunt will be placed because the subclavian artery will be clamped, and the arterial pressure will be lost.
 Hemodynamic and respiratory disturbance can be problematic during surgery.
Hemodynamic and respiratory disturbance can be problematic during surgery.
 The surgeon may request a small dose of heparin.
The surgeon may request a small dose of heparin.
 Bleeding may occur after clamps are released; be prepared for a blood transfusion.
Bleeding may occur after clamps are released; be prepared for a blood transfusion.
 Postoperatively, the pulmonary blood supply predominantly depends on the size of the shunt. If the shunt is too small, the infant may have a low saturation level; if the shunt is too large, the infant may develop heart failure or pulmonary edema and hypotension.
Postoperatively, the pulmonary blood supply predominantly depends on the size of the shunt. If the shunt is too small, the infant may have a low saturation level; if the shunt is too large, the infant may develop heart failure or pulmonary edema and hypotension.
 Pulmonary blood flow also depends on systemic blood pressure; the greater the blood pressure, the more blood flows to the lungs and the higher the saturation.
Pulmonary blood flow also depends on systemic blood pressure; the greater the blood pressure, the more blood flows to the lungs and the higher the saturation.
 Milrinone is often combined with norepinephrine because the norepinephrine increases the low diastolic pressure created by the shunt but does not produce the unwanted tachycardia seen with epinephrine.
Milrinone is often combined with norepinephrine because the norepinephrine increases the low diastolic pressure created by the shunt but does not produce the unwanted tachycardia seen with epinephrine.
Complete Repair.
 Both intravenous and inhalational induction agents are appropriate.
Both intravenous and inhalational induction agents are appropriate.
 Right ventricular dysfunction and pulmonary regurgitation may be postoperative problems.
Right ventricular dysfunction and pulmonary regurgitation may be postoperative problems.
 Too much of an inotrope may worsen RVOTO postoperatively by dynamic narrowing of the RVOT.
Too much of an inotrope may worsen RVOTO postoperatively by dynamic narrowing of the RVOT.
 Milrinone may be particularly useful because it promotes diastolic relaxation of the stiff right ventricle.
Milrinone may be particularly useful because it promotes diastolic relaxation of the stiff right ventricle.
 Pyrexia and excessive β-adrenergic stimulation may help precipitate junctional ectopic tachycardia postoperatively.
Pyrexia and excessive β-adrenergic stimulation may help precipitate junctional ectopic tachycardia postoperatively.
 Surgeons frequently measure right ventricular pressure to assess the quality of the repair.
Surgeons frequently measure right ventricular pressure to assess the quality of the repair.
 Perioperative echocardiography is useful in assessing repair and right ventricular function.
Perioperative echocardiography is useful in assessing repair and right ventricular function.
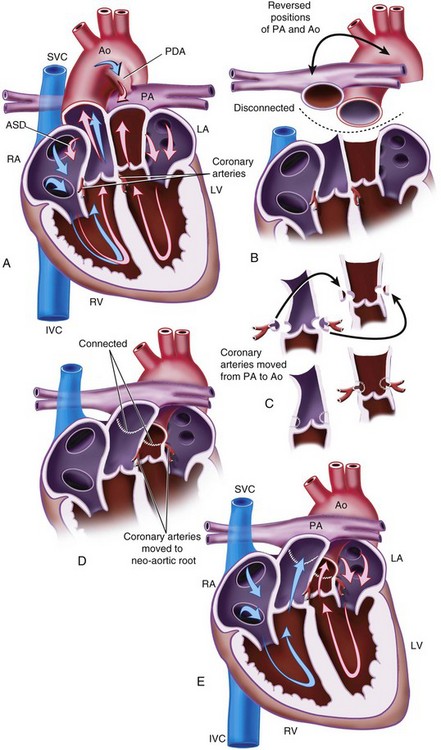
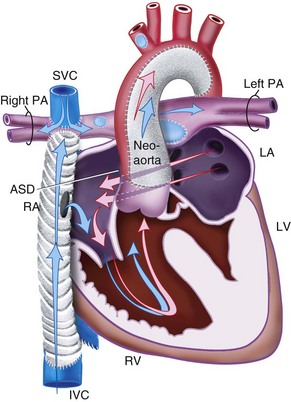
Transposition of the Great Arteries
TGA refers to the situation in which the aorta arises from the morphologic right ventricle and the pulmonary artery arises from the morphologic left ventricle (Fig. 15-6). In this ventriculoarterial (VA) discordance, the atria are related to the ventricles in the normal way (i.e., AV concordance). This results in two circulations that run in parallel rather than in series, which is the normal anatomic arrangement. Without some mixing of the two circulations, the systemic circulation would remain completely deoxygenated. However, some mixing does occur through the PDA or through a VSD that is present in approximately 25% of cases. If there is no VSD and mixing is inadequate, ductal patency is maintained after birth with an intravenous prostaglandin E1 infusion, and a balloon atrial septostomy is performed urgently in the neonatal period.
If untreated, most infants with TGA die in the first year of life of hypoxia and heart failure. Pulmonary vascular disease develops early and contributes to this high mortality rate.212,213 The mechanism for the early development of pulmonary vascular disease is complex and not simply related to high pulmonary blood flow. However, the presence of a VSD further accelerates this process. These infants are at risk for pulmonary hypertensive crises in the postoperative period.214
Surgical Options
Arterial Switch Operation.
An ASO is the operation of choice if the intracardiac anatomy is appropriate. The ASO involves transecting the two main arterial trunks distal to their respective valves and switching them to produce VA concordance (Fig. 15-7). It also involves disconnecting the coronary arteries from the “old” aorta and reconnecting them to the neoaorta. This restores anatomic and physiologic normality. The coronary anatomy varies widely in TGA but must be well assessed preoperatively because moving the coronary arteries to the neoaorta is difficult but crucial to a successful outcome. In some cases, the coronary arteries run in the wall of the aorta (intramural), and this poses particular difficulties for the surgeon. Ventricular function after surgery depends largely on unrestricted flow in the coronary arteries.
Mustard and Senning Procedures.
The Mustard and Senning procedures are ASOs. They involve the use of intraatrial baffles to redirect deoxygenated blood from the venae cavae to the left atrium, left ventricle, and pulmonary artery and oxygenated pulmonary venous blood to the right atrium, right ventricle, and aorta. They create AV discordance, restore physiologic but not anatomic normality, and leave the physiologic right ventricle as the systemic ventricle. These procedures were performed as definitive procedures before the arterial switch became successful but are rarely used today as definitive repairs. They are still used as palliation in children with TGA, VSD, and pulmonary vascular disease.215 In these cases, the VSD is left open.
Anesthesia Considerations for the Arterial Switch Procedure
 The patient is a neonate in the first few weeks of life.
The patient is a neonate in the first few weeks of life.
 Inhalational or intravenous induction is possible.
Inhalational or intravenous induction is possible.
 Invasive arterial and central venous lines are required. If VSD closure is required, bicaval cannulation may be required for CPB, and because an internal jugular central venous pressure line may interfere with this, a femoral central venous pressure line may be preferable. If the patient does not have a VSD, a single venous cannula in the atrium can be used for CPB, and the internal jugular vein can be used.
Invasive arterial and central venous lines are required. If VSD closure is required, bicaval cannulation may be required for CPB, and because an internal jugular central venous pressure line may interfere with this, a femoral central venous pressure line may be preferable. If the patient does not have a VSD, a single venous cannula in the atrium can be used for CPB, and the internal jugular vein can be used.
 Myocardial ischemia occurring after the cross-clamp is removed may be related to coronary air emboli or poor coronary anastomoses. A generous perfusion pressure after removal of cross-clamp encourages flushing air from coronary arteries. If ischemia results from an anatomic problem with the coronary arteries, they may need to be redone with a second bypass run.
Myocardial ischemia occurring after the cross-clamp is removed may be related to coronary air emboli or poor coronary anastomoses. A generous perfusion pressure after removal of cross-clamp encourages flushing air from coronary arteries. If ischemia results from an anatomic problem with the coronary arteries, they may need to be redone with a second bypass run.
 TEE or epicardial echo is useful in assessing adequate de-airing, myocardial function, and adequacy of coronary anastomoses.
TEE or epicardial echo is useful in assessing adequate de-airing, myocardial function, and adequacy of coronary anastomoses.
 Post-CPB myocardial dysfunction may result from one or more of the following:
Post-CPB myocardial dysfunction may result from one or more of the following:
 The anesthesiologist should anticipate pulmonary hypertension.
The anesthesiologist should anticipate pulmonary hypertension.
 Inotropes are almost always required. Dopamine or epinephrine can be used, and milrinone is a particularly useful agent in these cases because it is an inodilator.
Inotropes are almost always required. Dopamine or epinephrine can be used, and milrinone is a particularly useful agent in these cases because it is an inodilator.
 After the repair, the pulmonary artery is anterior to the aorta. Any dilation of the pulmonary artery as a result of pulmonary hypertension can lead to coronary compression and myocardial ischemia.
After the repair, the pulmonary artery is anterior to the aorta. Any dilation of the pulmonary artery as a result of pulmonary hypertension can lead to coronary compression and myocardial ischemia.
 The left ventricle is frequently noncompliant, and volume should be increased with care and in small amounts. Left atrial pressure can rise quickly if fluid is given injudiciously.
The left ventricle is frequently noncompliant, and volume should be increased with care and in small amounts. Left atrial pressure can rise quickly if fluid is given injudiciously.
Truncus Arteriosus
Truncus arteriosus is a rare congenital heart defect that occurs in about 0.7 of 1000 live births and accounts for about 1% of all CHD. The basic lesion is that of a common arterial outlet for the aorta and pulmonary artery associated with a single valve and a VSD (Fig. 15-8). The three types depend on how the pulmonary arteries arise from the aorta and on the size of the aorta. Blood mixes at the arterial level with a resultant high pulmonary blood flow. This leads to heart failure and early development of pulmonary hypertension. Surgery must be performed early in life to prevent pulmonary hypertension from becoming irreversible. Truncus arteriosus is associated with DiGeorge syndrome (see Table 15-4). Irradiated blood products should be used and calcium concentrations carefully monitored.
Anesthesia Considerations
 The patient is a small neonate in the first month of life.
The patient is a small neonate in the first month of life.
 Repair is a high-risk procedure.
Repair is a high-risk procedure.
 Risks include a postoperative pulmonary hypertensive crisis.
Risks include a postoperative pulmonary hypertensive crisis.
 Patients may already be intubated and ventilated and may be on inotropes.
Patients may already be intubated and ventilated and may be on inotropes.
 If the child is not ventilated, premedication is probably best avoided.
If the child is not ventilated, premedication is probably best avoided.
 Circulatory arrest may be required.
Circulatory arrest may be required.
Anomalous Pulmonary Venous Connection
 In supracardiac TAPVC, the pulmonary veins connect to the SVC through an ascending vertical vein (Fig. 15-9, A).
In supracardiac TAPVC, the pulmonary veins connect to the SVC through an ascending vertical vein (Fig. 15-9, A).
 In cardiac TAPVC, the pulmonary veins connect to the right atrium through the coronary sinus (see Fig. 15-9, B).
In cardiac TAPVC, the pulmonary veins connect to the right atrium through the coronary sinus (see Fig. 15-9, B).
 In infracardiac TAPVC, the pulmonary veins connect to the inferior vena cava (IVC) through a common vein, which traverses the diaphragm (see Fig. 15-9, C).
In infracardiac TAPVC, the pulmonary veins connect to the inferior vena cava (IVC) through a common vein, which traverses the diaphragm (see Fig. 15-9, C).
Hypoplastic Left Heart Syndrome
The anatomic features of HLHS (Fig. 15-10) include the following:
The prognosis for infants who are born with HLHS has improved dramatically. Previously, virtually all of these infants died of this condition, but in some centers, most children now survive at least into childhood.216 The longer-term outlook has not been fully determined, and many hurdles remain.
Surgical Palliation
Norwood Stage I.
The Norwood stage I operation is performed in the neonatal period. It involves reconstructing the aortic arch so that it arises from the pulmonary trunk. The pulmonary valve becomes the neoaortic valve. The branch pulmonary arteries are disconnected from the pulmonary trunk, and a new pulmonary blood supply is provided by a shunt from the subclavian artery (i.e., Blalock-Taussig shunt) or from the right ventricle (i.e., Sano modification) (Fig. 15-11).217 If the ASD is restrictive, it is enlarged.
Norwood Stage II.
The Norwood stage II (hemi-Fontan) operation takes place at about 6 months of age. It involves taking down the shunt that was created at the first operation and creating a new connection from the SVC to the pulmonary arteries (i.e., a bidirectional or Glenn shunt). The result is a pulmonary blood supply that is provided by systemic venous blood from the SVC. Flow is passive and depends on pulmonary artery pressures remaining low. The infants remain cyanotic with arterial saturations in the mid-80s because desaturated blood from the IVC continues to flow into the heart and the systemic circulation (Fig. 15-12).
Norwood Stage III.
The Norwood stage III (Fontan) operation converts the anatomy into a Fontan circulation. The surgery involves connecting the IVC through an extracardiac or intracardiac conduit to the pulmonary artery. This creates a single-ventricle or Fontan circulation (Fig. 15-13). The single right ventricle pumps blood to the systemic circulation, and the pulmonary blood supply is provided by passive flow by systemic venous blood from the SVC and IVC. The PVR must remain low because any increase will dramatically reduce pulmonary blood flow. It is common for a small hole (i.e., fenestration) to be created between the extracardiac conduit and the right atrium so that if the PVR rises, blood will be directed to the right atrium and allow cardiac output to be maintained. In this situation, the child becomes cyanotic, but cardiac output is maintained, a much safer situation than a state of low cardiac output. Postoperatively, increased systemic venous pressure may cause pleural effusions, an enlarged liver, or protein-losing enteropathy. Later, if PVR remains consistently low, the fenestration can be closed with a transvenous device.
The long-term problem for these children is that the morphologic right ventricle that becomes the systemic ventricle fails over time. The only recourse is heart transplantation.218,219
Anesthesia Considerations
Norwood Stage I.
 The anesthesiologist must understand the anatomy and physiology of HLHS.
The anesthesiologist must understand the anatomy and physiology of HLHS.
 Balance between systemic and pulmonary circulations is maintained by balancing PVR and SVR. If the PVR decreases, blood flow will be directed away from the systemic circulation and the lungs will be flooded. This results in hypotension and hypoperfusion with increasing acidosis. If PVR increases, cyanosis will increase. Before anesthesia, these infants are best managed spontaneously breathing in room air with a prostaglandin E1 infusion to maintain ductal patency. However, if mechanical ventilation is required, it is important to maintain normal to high Paco2 and very low Fio2, usually with air.
Balance between systemic and pulmonary circulations is maintained by balancing PVR and SVR. If the PVR decreases, blood flow will be directed away from the systemic circulation and the lungs will be flooded. This results in hypotension and hypoperfusion with increasing acidosis. If PVR increases, cyanosis will increase. Before anesthesia, these infants are best managed spontaneously breathing in room air with a prostaglandin E1 infusion to maintain ductal patency. However, if mechanical ventilation is required, it is important to maintain normal to high Paco2 and very low Fio2, usually with air.
 Air should be available for transfer to the operating room, or a self-inflating bag should be used.
Air should be available for transfer to the operating room, or a self-inflating bag should be used.
 High-dose opioid technique is preferred.
High-dose opioid technique is preferred.
 Venous access is gained through the femoral or umbilical veins. The internal jugular vein is avoided because narrowing of the SVC would jeopardize the Glenn shunt.
Venous access is gained through the femoral or umbilical veins. The internal jugular vein is avoided because narrowing of the SVC would jeopardize the Glenn shunt.
 Profound hypothermia may be required.
Profound hypothermia may be required.
 Postoperative myocardial dysfunction is common, and inotropes are required.
Postoperative myocardial dysfunction is common, and inotropes are required.
 Balancing systemic and pulmonary blood flow remains an issue after bypass. Some centers use the long-acting α-adrenergic blocker phenoxybenzamine after bypass to reduce SVR variability, allowing greater concentrations of oxygen to be used and an overall increase in oxygen delivery.220 The alternative approach is to combine a vasodilator such as milrinone with dopamine or epinephrine to achieve a similar effect. I prefer to deliver a bolus dose of milrinone (25 µg/kg over 20 minutes during rewarming), followed by an infusion of 0.3 µg/kg/min in combination with epinephrine, 0.05 to 0.1 µg/kg/min.
Balancing systemic and pulmonary blood flow remains an issue after bypass. Some centers use the long-acting α-adrenergic blocker phenoxybenzamine after bypass to reduce SVR variability, allowing greater concentrations of oxygen to be used and an overall increase in oxygen delivery.220 The alternative approach is to combine a vasodilator such as milrinone with dopamine or epinephrine to achieve a similar effect. I prefer to deliver a bolus dose of milrinone (25 µg/kg over 20 minutes during rewarming), followed by an infusion of 0.3 µg/kg/min in combination with epinephrine, 0.05 to 0.1 µg/kg/min.
 Coagulopathy may occur after bypass.
Coagulopathy may occur after bypass.
 Antifibrinolytics are often used
Antifibrinolytics are often used
 The sternum is frequently left open, and closure may be delayed for several days.
The sternum is frequently left open, and closure may be delayed for several days.
Norwood Stage II.
 The procedure is carried out with CPB.
The procedure is carried out with CPB.
 Cardioplegia is not used. The heart remains beating, and inotropes are seldom required.
Cardioplegia is not used. The heart remains beating, and inotropes are seldom required.
 Venous access is achieved through the femoral veins. However, only a single side should be used because femoral bypass is occasionally required. A short temporary cannula is useful in the internal jugular vein. It reflects the pulmonary artery pressure after anastomosis of the SVC to the pulmonary artery. It is removed early in the postoperative period to avoid any possibility of thrombosis in the SVC.
Venous access is achieved through the femoral veins. However, only a single side should be used because femoral bypass is occasionally required. A short temporary cannula is useful in the internal jugular vein. It reflects the pulmonary artery pressure after anastomosis of the SVC to the pulmonary artery. It is removed early in the postoperative period to avoid any possibility of thrombosis in the SVC.
 This is repeat surgery, and external defibrillator pads should be attached.
This is repeat surgery, and external defibrillator pads should be attached.
 The aim is early extubation. Positive intrathoracic pressure reduces flow in the Glenn shunt.
The aim is early extubation. Positive intrathoracic pressure reduces flow in the Glenn shunt.
 Infants should be nursed with the head up at 30 degrees after surgery.
Infants should be nursed with the head up at 30 degrees after surgery.
Norwood Stage III.
 Surgery is carried out with CPB but usually without cross-clamping the aorta.
Surgery is carried out with CPB but usually without cross-clamping the aorta.
 PVR must remain low postoperatively, careful management of the lungs is important to minimize atelectasis, and nitric oxide is occasionally required.221
PVR must remain low postoperatively, careful management of the lungs is important to minimize atelectasis, and nitric oxide is occasionally required.221
 If an inotrope is required, milrinone is a good choice because of its beneficial effects on PVR.
If an inotrope is required, milrinone is a good choice because of its beneficial effects on PVR.
 Early extubation is beneficial in terms of hemodynamics.
Early extubation is beneficial in terms of hemodynamics.
 Large amounts of fluid may be required in the early postoperative period.
Large amounts of fluid may be required in the early postoperative period.
Aortic Stenosis
Obstruction to the LVOT can occur at the valvular, subvalvular, or supravalvular area or in various combinations, and it is common, accounting for up to 10% of CHD.222,223 Congenital valvar aortic stenosis is frequently associated with a bicuspid valve. Severe critical aortic stenosis in neonates occurs in approximately 10% of cases and requires urgent treatment. Supravalvular aortic stenosis may be associated with Williams syndrome.224
Despite the many anatomic varieties of aortic stenosis, the resulting pathophysiology remains essentially the same. There is an increasing imbalance between oxygen supply and demand (i.e., impaired coronary blood flow due to low coronary perfusion pressure plus increased workload on the left ventricle leading to subendocardial ischemia), left ventricular hypertrophy, and a risk of left ventricular failure. There is always the risk of sudden death, especially with Williams syndrome. The age at which the child presents is a risk factor; younger children are most at risk. Two thirds of those presenting in the first 3 months of life will require inotropic or ventilatory support before treatment.225
Treatment Options
Treatment options depend on the patient’s age and the severity and type of lesion. In neonates with critical aortic stenosis, an urgent valvuloplasty is required. It can be performed surgically using CPB, but it typically is performed using transluminal balloon angioplasty in the cardiac catheterization laboratory.226 Complications in this age group include ventricular fibrillation, aortic incompetence, or residual aortic stenosis.
In the older child, several surgical approaches may be used, depending on the anatomy. Transluminal balloon valvuloplasty commonly is performed in older patients. The most common complications of valvuloplasty are aortic incompetence and residual aortic stenosis. Valve replacement with a mechanical valve or bioprosthetic valve is delayed as long as possible because of the long-term problems associated with the anticoagulation needed with a mechanical valve and because of the inevitable calcification of the bioprosthetic valve. An alternative surgical option is the Ross procedure, which involves moving the pulmonary valve into the aortic valve position and using a homograft in the pulmonary position. The need for reoperation with the Ross procedure is reduced because the systemic valve (i.e., neoaortic valve) grows with the child and calcification of the homograft in the pulmonary position is slow. There is no need for anticoagulation.227–229
Anesthesia Considerations
 A crucial aim of anesthesia is to maintain the balance of oxygen supply and demand. This involves maintaining a normal heart rate (no tachycardia or bradycardia), maintaining SVR to preserve coronary perfusion, avoiding hypertension, and avoiding myocardial depression.
A crucial aim of anesthesia is to maintain the balance of oxygen supply and demand. This involves maintaining a normal heart rate (no tachycardia or bradycardia), maintaining SVR to preserve coronary perfusion, avoiding hypertension, and avoiding myocardial depression.
 Anesthesia for neonates having surgery with CPB is similar to other neonatal cardiac surgery.
Anesthesia for neonates having surgery with CPB is similar to other neonatal cardiac surgery.
 For transluminal balloon valvuloplasty
For transluminal balloon valvuloplasty
 The catheter crossing the aortic valve and inflation of the balloon can lead to dramatic cardiovascular changes. Cardiac output decreases, myocardial ischemia occurs, and bradycardia is common. In neonates, ventricular fibrillation may occur after passing the wire across the valve. The anesthesiologist must be prepared to resuscitate the neonate quickly, and drugs, particularly epinephrine, should be immediately available.
The catheter crossing the aortic valve and inflation of the balloon can lead to dramatic cardiovascular changes. Cardiac output decreases, myocardial ischemia occurs, and bradycardia is common. In neonates, ventricular fibrillation may occur after passing the wire across the valve. The anesthesiologist must be prepared to resuscitate the neonate quickly, and drugs, particularly epinephrine, should be immediately available. It is possible the child will remain ventilated after the procedure because ventricular function can remain poor for some time.
It is possible the child will remain ventilated after the procedure because ventricular function can remain poor for some time.Coarctation of the Aorta
Anesthesia Considerations
Neonatal Repair.
 These infants are sick, with poor left ventricular function. They should be treated very carefully, and the anesthesiologist should not be misled by an infant who looks reasonably well.
These infants are sick, with poor left ventricular function. They should be treated very carefully, and the anesthesiologist should not be misled by an infant who looks reasonably well.
 Some infants are already intubated and ventilated, and some may be receiving an inotrope such as dopamine.
Some infants are already intubated and ventilated, and some may be receiving an inotrope such as dopamine.
 Intravenous access often is established to give prostaglandin E1; this intravenous line can also be used to administer induction agents. I prefer to give incremental doses of fentanyl (up to 5 µg/kg) and then a muscle relaxant and to supplement this with a very low dose of isoflurane (0.3% to 0.5%). This can be omitted if hypotension ensues.
Intravenous access often is established to give prostaglandin E1; this intravenous line can also be used to administer induction agents. I prefer to give incremental doses of fentanyl (up to 5 µg/kg) and then a muscle relaxant and to supplement this with a very low dose of isoflurane (0.3% to 0.5%). This can be omitted if hypotension ensues.
 Inotropes may be required before surgery, and they should be available.
Inotropes may be required before surgery, and they should be available.
 Ideally, the arterial line should be placed in the right arm to allow blood pressure measurement during arterial cross-clamping. The left subclavian may be partially obstructed during the repair. Some have advocated an arterial line below the coarctation to measure perfusion pressure during cross-clamping, but this may be very difficult in practice because femoral pulses are usually absent.
Ideally, the arterial line should be placed in the right arm to allow blood pressure measurement during arterial cross-clamping. The left subclavian may be partially obstructed during the repair. Some have advocated an arterial line below the coarctation to measure perfusion pressure during cross-clamping, but this may be very difficult in practice because femoral pulses are usually absent.
 A central venous line should be placed.
A central venous line should be placed.
 Surgery usually takes place through a left thoracotomy without the use of CPB. The lung is retracted, and ventilation may be problematic. The endotracheal tube must fit snugly and have a minimal leak because a tracheal tube with a large leak may make ventilation very difficult.
Surgery usually takes place through a left thoracotomy without the use of CPB. The lung is retracted, and ventilation may be problematic. The endotracheal tube must fit snugly and have a minimal leak because a tracheal tube with a large leak may make ventilation very difficult.
 Paraplegia may occur in about 1% of cases and is thought to result from hypoperfusion during aortic cross-clamping.230,231 To reduce the chance of spinal cord damage, infants should be cooled to 34° C or 35° C before the cross-clamp is applied, although this approach is not evidence based. Normocarbia and upper limb blood pressure should be maintained. Low-dose anticoagulation may be applied. A short cross-clamp time is thought to be important.
Paraplegia may occur in about 1% of cases and is thought to result from hypoperfusion during aortic cross-clamping.230,231 To reduce the chance of spinal cord damage, infants should be cooled to 34° C or 35° C before the cross-clamp is applied, although this approach is not evidence based. Normocarbia and upper limb blood pressure should be maintained. Low-dose anticoagulation may be applied. A short cross-clamp time is thought to be important.
 Epidural anesthesia is occasionally used, but I have not adopted this approach.
Epidural anesthesia is occasionally used, but I have not adopted this approach.
 Postoperative hypertension may be a problem, and a vasodilator such as sodium nitroprusside may be required.
Postoperative hypertension may be a problem, and a vasodilator such as sodium nitroprusside may be required.
Anesthesia for the Older Child.
 These children usually are not as sick as the neonates.
These children usually are not as sick as the neonates.
 Issues about vascular lines are similar to those in neonatal repair.
Issues about vascular lines are similar to those in neonatal repair.
 Careful intravenous induction with a combination of fentanyl and an induction agent of choice is standard. Etomidate is a good anesthetic because of its cardiovascular stability.
Careful intravenous induction with a combination of fentanyl and an induction agent of choice is standard. Etomidate is a good anesthetic because of its cardiovascular stability.
 Although a collateral blood supply is present, the spinal cord remains at risk during the cross-clamping, and the same precautions taken with neonates should be taken with these children.
Although a collateral blood supply is present, the spinal cord remains at risk during the cross-clamping, and the same precautions taken with neonates should be taken with these children.
 An oral cuffed tracheal tube is useful because early extubation is the norm.
An oral cuffed tracheal tube is useful because early extubation is the norm.
 Postoperative hypertension is a common problem, and good analgesia combined with sodium nitroprusside and β-blockers is usually required. Up to 30% of children eventually develop long-term hypertension that will require therapy.
Postoperative hypertension is a common problem, and good analgesia combined with sodium nitroprusside and β-blockers is usually required. Up to 30% of children eventually develop long-term hypertension that will require therapy.
Interrupted Aortic Arch
Interrupted aortic arch is a rare anomaly, accounting for less than 1% of CHD. In this condition, disruption occurs between the ascending aorta and descending aorta (Fig. 15-14). The three types depend on where the disruption takes place. A PDA is present and is required to supply the descending aorta. A VSD is also common. Interrupted aortic arch is frequently associated with a 22q11 deletion and results in the DiGeorge syndrome (see Table 15-4).232
These children are often small for gestational age and are started on a prostaglandin infusion to maintain ductal patency. They are often sick with progressive acidosis and poor cardiac output. There is increasing pulmonary blood flow as the duct closes. Surgical repair depends on the presence of associated lesions, particularly a VSD. In the single-stage repair, the arch is reconstructed, and the VSD is closed. The two-stage repair involves repair of the aortic arch and banding of the pulmonary artery to limit blood flow to the lungs. The VSD is closed in a later stage of the procedure. Either way, deep hypothermic circulatory arrest is likely to be employed. Some centers use selective regional perfusion to try and limit neurologic injury.233 The early and late mortality rates are high. Higher mortality rates correlate with small size, acidosis preoperatively, and associated cardiac lesions.234
Anesthesia Considerations
 The patient is a small, sick neonate.
The patient is a small, sick neonate.
 DiGeorge syndrome is identified (particularly hypocalcemia and the need for irradiated blood products).
DiGeorge syndrome is identified (particularly hypocalcemia and the need for irradiated blood products).
 High-dose opioid technique is standard.
High-dose opioid technique is standard.
 Ideally, blood pressure should be monitored above and below the interruption, but this is often difficult in practice.
Ideally, blood pressure should be monitored above and below the interruption, but this is often difficult in practice.
 Deep hypothermic circulatory arrest may be used.
Deep hypothermic circulatory arrest may be used.
 An antifibrinolytic is frequently used.
An antifibrinolytic is frequently used.
 Coagulopathy may occur after bypass.
Coagulopathy may occur after bypass.
 Anticipate poor renal function postoperatively.
Anticipate poor renal function postoperatively.
 There is a risk of postoperative pulmonary hypertensive crises.
There is a risk of postoperative pulmonary hypertensive crises.
Transport and Transfer to a Pediatric Intensive Care Unit
After surgery is completed, cardiac surgical patients need a period of intensive care. The first phase of this care is transport of the children from the operating room to the PICU. This is a potentially hazardous time and requires good organization, teamwork, and appropriate equipment. Guidelines exist for the safe transport of these children.235
Transport to the PICU can be defined as a preparatory phase, transport phase, and stabilization phase.236 During the preparatory phase, the estimated time of arrival in the PICU is communicated with the PICU. The bed space is prepared, ventilator and monitors are configured in an appropriate way, and any additional interventions that may be required are made ready. In my institution, a form is sent to the PICU that indicates the child’s age and weight, the ventilator settings that will be required, the number of transducers that will be required, and the infusions that are running. After arrival in the PICU, two basic tasks need to be undertaken: transfer of technology and transfer of information. It is better to allow the technology transfer to occur before the information handover. This includes ensuring that all the monitors are connected and working appropriately, that the ventilator is connected and delivering adequate ventilation, that all infusions are working, and that drains and urinary catheter are all in place with baseline readings documented. After this has been accomplished, a single handover of information should be done with all of the appropriate personnel present, and it should include information given by the anesthesiologist and surgeon. In my institution, a checklist is followed to ensure that no important information is omitted. It is important to avoid a large number of information handovers between individuals rather than a single, comprehensive handover with all the relevant personnel present.
Arnold DM, Fergusson DA, Chan AK, et al. Avoiding transfusions in children undergoing cardiac surgery: a meta-analysis of randomized trials of aprotinin. Anesth Analg. 2006;102:731–737.
Andropoulos DB, Stayer SA, Diaz LK, Ramamoorthy C. Neurological monitoring for congenital heart surgery. Anesth Analg. 2004;99:1365–1375.
Bettex DA, Pretre R, Jenni R, Schmid ER. Cost-effectiveness of routine intraoperative transesophageal echocardiography in pediatric cardiac surgery: a 10-year experience. Anesth Analg. 2005;100:1271–1275.
Hoffman TM, Wernovsky G, Atz AM, et al. Prophylactic intravenous use of milrinone after cardiac operation in pediatrics (PRIMACORP) study. Prophylactic Intravenous Use of Milrinone After Cardiac Operation in Pediatrics. Am Heart J. 2002;143:15–21.
Kern FH, Morana NJ, Sears JJ, Hickey PR. Coagulation defects in neonates during cardiopulmonary bypass. Ann Thorac Surg. 1992;54:541–546.
Malviya S, Voepel-Lewis T, Siewert M, et al. Risk factors for adverse postoperative outcomes in children presenting for cardiac surgery with upper respiratory tract infections. Anesthesiology. 2003;98:628–632.
Mangano DT, Tudor IC, Dietzel C. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353–365.
Naik SK, Knight A, Elliott M. A prospective randomized study of a modified technique of ultrafiltration during pediatric open-heart surgery. Circulation. 1991;84(Suppl):III422–III431.
1 Benson DWJ. Changing profile of congenital heart disease. Pediatrics. 1989;83:790–791.
2 Fixler DE. Epidemiology of congenital heart disease. In: Oski FA, ed. Principles and practice of pediatrics. Philadelphia: JB Lippincott; 1990:349–352.
3 Bernstein D. Evaluation of the cardiovascular system. In: Behrman RE, ed. Nelson textbook of pediatrics. 17th ed. Philadelphia: WB Saunders; 2004:1481–1488.
4 Di Donato RM, Jonas RA, Lang P, et al. Neonatal repair of tetralogy of Fallot with and without pulmonary atresia. J Thorac Cardiovasc Surg. 1991;10:126–137.
5 Benson DW. Changing profile of congenital heart disease. Pediatrics. 1975;55:485–491.
6 Bennett D, Marcus R, Stokes M. Incidents and complications during pediatric cardiac catheterization. Paediatr Anaesth. 2005;15:1083–1088.
7 Practice advisory for preanesthesia evaluation: a report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2002;96:485–496.
8 Jaquiss RD, Ghanayem NS, Zacharisen MC, et al. Safety of aprotinin use and reuse in pediatric cardiothoracic surgery. Circulation. 2002;106(12 Suppl 1):I90–I94.
9 Edelman NH, Lahiri S, Braudo L, et al. The blunted ventilatory response to hypoxia in cyanotic congenital heart disease. N Engl J Med. 1970;282:405–411.
10 Bergendahl H, Lonnqvist PA, Eksborg S. Clonidine in paediatric anaesthesia: review of the literature and comparison with benzodiazepines for premedication. Acta Anaesthesiol Scand. 2006;50:135–143.
11 Tait AR, Malviya S, Voepel-Lewis T, et al. Risk factors for perioperative adverse respiratory events in children with upper respiratory tract infections. Anesthesiology. 2001;95:299–306.
12 Coté CJ. The upper respiratory tract infection (URI) dilemma: fear of a complication or litigation? Anesthesiology. 2001;95:283–285.
13 Rolf N, Coté CJ. Frequency and severity of desaturation during general anaesthesia in children with and without upper respiratory tract infection. J Clin Anaesth. 1992;4:200–203.
14 von Ungern-Sternberg BS, Boda K, Chambers NA, et al. Risk assessment for respiratory complications in paediatric anaesthesia: a prospective cohort study. Lancet. 2010;376:773–783.
15 Malviya S, Voepel-Lewis T, Siewert M, et al. Risk factors for adverse postoperative outcomes in children presenting for cardiac surgery with upper respiratory tract infections. Anesthesiology. 2003;98:628–632.
16 Phornphutkul C, Rosenthal A, Nadas AS, Berenberg W. Cerebrovascular accidents in infants and children with cyanotic congenital heart disease. Am J Cardiol. 1973;32:329–334.
17 Williams GD, Bratton SL, Ramamoorthy C. Factors associated with blood loss and blood product transfusions: a multivariate analysis in children after open-heart surgery. Anesth Analg. 1999;89:57–64.
18 Miller BE, Mochizuki T, Levy JH, et al. Predicting and treating coagulopathies after cardiopulmonary bypass in children. Anesth Analg. 1997;85:1196–1202.
19 Maurer HM, McCue CM, Robertson LW, Haggins JC. Correction of platelet dysfunction and bleeding in cyanotic congenital heart disease by simple red cell volume reduction. Am J Cardiol. 1975;35:831–835.
20 Andrew M, Paes B, Milner R, et al. Development of the human coagulation system in the full-term infant. Blood. 1987;70:165–172.
21 Kern FH, Morana NJ, Sears JJ, Hickey PR. Coagulation defects in neonates during cardiopulmonary bypass [see comment]. Ann Thorac Surg. 1992;54:541–546.
22 Moganasundram S, Hunt BJ, Sykes K, et al. The relationship among thromboelastography, hemostatic variables, and bleeding after cardiopulmonary bypass surgery in children. Anesth Analg. 2010;110(4):995–1002.
23 Khuri SF, Valeri CR, Loscalzo J, et al. Heparin causes platelet dysfunction and induces fibrinolysis before cardiopulmonary bypass. Ann Thorac Surg. 1995;60:1008–1014.
24 Tabuchi N, de Haan J, Boonstra PW, van Oeveren W. Activation of fibrinolysis in the pericardial cavity during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1993;106:828–833.
25 Chan AK, Leaker M, Burrows FA, et al. Coagulation and fibrinolytic profile of paediatric patients undergoing cardiopulmonary bypass [erratum appears in Thromb Haemost 1997 May;77:1047]. Thromb Haemost. 1997;77:270–277.
26 Codispoti M, Ludlam CA, Simpson D, Mankad PS. Individualized heparin and protamine management in infants and children undergoing cardiac operations. Ann Thorac Surg. 2001;71:922–927. discussion 927-8
27 Williams GD, Bratton SL, Riley EC, Ramamoorthy C. Association between age and blood loss in children undergoing open heart operations. Ann Thorac Surg. 1998;66:870–875. discussion 875-6
28 Manno CS, Hedberg KW, Kim HC, et al. Comparison of the hemostatic effects of fresh whole blood, stored whole blood, and components after open heart surgery in children. Blood. 1991;77:930–936.
29 Williams GD, Bratton SL, Riley EC, Ramamoorthy C. Coagulation tests during cardiopulmonary bypass correlate with blood loss in children undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 1999;13:398–404.
30 Lind SE, Marks PW, Ewenstein BM, et al. Hemostatic system. In: Handin RI, Lux SE, Stossel TP, eds. Blood: principles and practice of hematology. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2003:959–981.
31 Casbard AC, Williamson LM, Murphy MF, et al. The role of prophylactic fresh frozen plasma in decreasing blood loss and correcting coagulopathy in cardiac surgery: a systematic review. Anaesthesia. 2004;59:550–558.
32 Nuttall GA, Oliver WC, Santrach PJ, et al. Efficacy of a simple intraoperative transfusion algorithm for nonerythrocyte component utilization after cardiopulmonary bypass. Anesthesiology. 2001;94:773–781.
33 Shore-Lesserson L, Manspeizer HE, DePerio M, et al. Thrombo-elastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg. 1999;88:312–319.
34 Chauhan S, Kumar BA, Rao BH, et al. Efficacy of aprotinin, epsilon aminocaproic acid, or combination in cyanotic heart disease. Ann Thorac Surg. 2000;70:1308–1312.
35 Reid RW, Zimmerman AA, Laussen PC, et al. The efficacy of tranexamic acid versus placebo in decreasing blood loss in pediatric patients undergoing repeat cardiac surgery. Anesth Analg. 1997;84:990–996.
36 Levi M, Cromheecke ME, de Jonge E, et al. Pharmacological strategies to decrease excessive blood loss in cardiac surgery: a meta-analysis of clinically relevant endpoints. Lancet. 1999;354:1940–1947.
37 Fergusson DA, Hebert PC, Mazer CD, et al. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008;29:2319–2331.
38 Boldt J, Knothe C, Zickmann B, et al. Comparison of two aprotinin dosage regimens in pediatric patients having cardiac operations: influence on platelet function and blood loss. J Thorac Cardiovasc Surg. 1993;105:705–711.
39 D’Errico CC, Shayevitz JR, Martindale SJ, et al. The efficacy and cost of aprotinin in children undergoing reoperative open heart surgery. Anesth Analg. 1996;83:1193–1199.
40 Dietrich W, Mossinger H, Spannagl M, et al. Hemostatic activation during cardiopulmonary bypass with different aprotinin dosages in pediatric patients having cardiac operations [see comment]. J Thorac Cardiovasc Surg. 1993;105:712–720.
41 Jaquiss RD, Ghanayem NS, Zacharisen MC, et al. Safety of aprotinin use and reuse in pediatric cardiothoracic surgery. Circulation. 2002;106(12 Suppl 1):I90–I94.
42 Mangano DT, Tudor IC, Dietzel C. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353–365.
43 Mangano DT, Miao Y, Vuylsteke A, et al. Mortality associated with aprotinin during 5 years following coronary artery bypass graft surgery. JAMA. 2007;297:471–479.
44 Update 2011-124 [press release]. Health Canada decision on Trasylol (aprotinin), Ottawa, Health Canada Information Update, September 21, 2011.
45 Berrevoet FdeHB. Use of topical hemostatic agents during liver resection. Dig Surg. 2007;24:288–293.
46 Codispoti M, Mankad PS. Significant merits of a fibrin sealant in the presence of coagulopathy following paediatric cardiac surgery: randomised controlled trial. Eur J Cardiothorac Surg. 2002;22:200–205.
47 Naik SK, Knight A, Elliott MJ. A successful modification of ultrafiltration for cardiopulmonary bypass in children. Perfusion. 1991;6:41–50.
48 Naik SK, Knight A, Elliott M. A prospective randomized study of a modified technique of ultrafiltration during pediatric open-heart surgery. Circulation. 1991;84(5 Suppl):III422–III431.
49 Andersson TL, Solem JO, Tengborn L, Vinge E. Effects of desmopressin acetate on platelet aggregation, von Willebrand factor, and blood loss after cardiac surgery with extracorporeal circulation. Circulation. 1990;81:872–878.
50 Oliver WC, Jr., Santrach PJ, Danielson GK, et al. Desmopressin does not reduce bleeding and transfusion requirements in congenital heart operations. Ann Thorac Surg. 2000;70:1923–1930.
51 Praud JP, Carofilis A, Bridey F, et al. Accuracy of two wavelength pulse oximetry in neonates and infants. Pediatr Pulmonol. 1989;6:180–182.
52 Fanconi S. Pulse oximetry for hypoxemia: a warning to users and manufacturers. Intensive Care Med. 1989;15:540–542.
53 Lazzell VA, Burrows FA. Stability of the intraoperative arterial to end-tidal carbon dioxide partial pressure difference in children with congenital heart disease. Can J Anaesth. 1991;38:859–865.
54 Stewart FC, Morana NJ, Sears JJ, Hickey PR. Anesthesia laboratory for the pediatric operating room. Int Anesthesiol Clin. 1992;30:177–188.
55 Rebeyka IM, Yeh T, Jr., Hanan SA, et al. Altered contractile response in neonatal myocardium to citrate-phosphate-dextrose infusion. Circulation. 1990;82:IV367–IV370.
56 Stone JG, Young WL, Smith CR, et al. Do standard monitoring sites reflect true brain temperature when profound hypothermia is rapidly induced and reversed? Anesthesiology. 1995;82:344–351.
57 Adar R, Rubinstein N, Blieden L. Immediate complications and late sequelae of arterial catheterization in children with congenital heart disease. Pediatr Cardiol. 1983;4:25–28.
58 Cheitlin MD, Alpert JS, Armstrong WF, et al. ACC/AHA guidelines for the clinical application of echocardiography: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Clinical Application of Echocardiography). Developed in collaboration with the American Society of Echocardiography. Circulation. 95, 1997. 1686–1644
59 Fyfe DA. Transesophageal echocardiography for congenital heart disease. J Invasive Cardiol. 1992;4:459–467.
60 Bettex DA, Pretre R, Jenni R, Schmid ER. Cost-effectiveness of routine intraoperative transesophageal echocardiography in pediatric cardiac surgery: a 10-year experience. Anesth Analg. 2005;100:1271–1275.
61 Bettex DA, Schmidlin D, Bernath MA, et al. Intraoperative transesophageal echocardiography in pediatric congenital cardiac surgery: a two-center observational study. Anesth Analg. 2003;97:1275–1282.
62 Rice MJ, McDonald RW, Li X, et al. New technology and methodologies for intraoperative, perioperative, and intraprocedural monitoring of surgical and catheter interventions for congenital heart disease. Echocardiography. 2002;19:725–734.
63 Shiota T, Lewandowski R, Piel JE, et al. Micromultiplane transesophageal echocardiographic probe for intraoperative study of congenital heart disease repair in neonates, infants, children, and adults. Am J Cardiol. 1999;83:292–295.
64 Stevenson JG. Utilization of intraoperative transesophageal echocardiography during repair of congenital cardiac defects: a survey of North American centers. Clin Cardiol. 2003;26:132–134.
65 Shanewise JS, Cheung AT, Aronson S, et al. ASE/SCA guidelines for performing a comprehensive intraoperative multiplane transesophageal echocardiography examination: recommendations of the American Society of Echocardiography Council for Intraoperative Echocardiography and the Society of Cardiovascular Anesthesiologists Task Force for Certification in Perioperative Transesophageal Echocardiography. Anesth Analg. 1999;89:870–884.
66 Fyfe DA, Ritter SB, Snider AR, et al. Guidelines for transesophageal echocardiography in children. J Am Soc Echocardiogr. 1992;5:640–644.
67 Randolph GR, Hagler DJ, Connolly HM, et al. Intraoperative transesophageal echocardiography during surgery for congenital heart defects. J Thorac Cardiovasc Surg. 2002;124:1176–1182.
68 Stevenson JG. Incidence of complications in pediatric transesophageal echocardiography: experience in 1650 cases. J Am Soc Echocardiogr. 1999;12:527–532.
69 Schiller NB. Hemodynamics derived from transesophageal echocardiography (TEE). Cardiol Clin. 2000;18:699–709.
70 Andropoulos DB, Stayer SA, Diaz LK, Ramamoorthy C. Neurological monitoring for congenital heart surgery. Anesth Analg. 2004;99:1365–1375.
71 Booth EA, Dukatz C, Ausman J, Wider M. Cerebral and somatic venous oximetry in adults and infants. Surg Neurol Int. 2010;1:75.
72 Morray JP, Lynn AM, Stamm SJ, et al. Hemodynamic effects of ketamine in children with congenital heart disease. Anesth Analg. 1984;63:895–899.
73 Julier K, da Silva R, Garcia C, et al. Preconditioning by sevoflurane decreases biochemical markers for myocardial and renal dysfunction in coronary artery bypass graft surgery: a double-blinded, placebo-controlled, multicenter study. Anesthesiology. 2003;98:1315–1327.
74 Malagon I, Hogenbirk K, van Pelt J, et al. Effect of three different anaesthetic agents on the postoperative production of cardiac troponin T in paediatric cardiac surgery. Br J Anaesth. 2005;94:805–809.
75 Morray JP, Lynn AM, Mansfield PB. Effect of pH and Pco2 on pulmonary and systemic hemodynamics after surgery in children with congenital heart disease and pulmonary hypertension. J Pediatr. 1988;113:474–479.
76 Sarner JB, Levine M, Davis PJ, et al. Clinical characteristics of sevoflurane in children: a comparison with halothane. Anesthesiology. 1995;82:38–46.
77 Lerman J, Davis PJ, Welborn LG, et al. Induction, recovery, and safety characteristics of sevoflurane in children undergoing ambulatory surgery: a comparison with halothane. Anesthesiology. 1996;84:1332–1340.
78 Epstein RH, Stein AL, Marr AT, Lessin JB. High concentration versus incremental induction of anesthesia with sevoflurane in children: a comparison of induction times, vital signs, and complications. J Clin Anesth. 1998;10:41–45.
79 Sigston PE, Jenkins AM, Jackson EA, et al. Rapid inhalation induction in children: 8% sevoflurane compared with 5% halothane. Br J Anaesth. 1997;78:362–365.
80 Holzman RS, van der Velde ME, Kaus SJ, et al. Sevoflurane depresses myocardial contractility less than halothane during induction of anesthesia in children. Anesthesiology. 1996;85:1260–1267.
81 Russell IA, Miller Hance WC, Gregory G, et al. The safety and efficacy of sevoflurane anesthesia in infants and children with congenital heart disease. Anesth Analg. 2001;92:1152–1158.
82 Lerman J, Sikich N, Kleinman S, Yentis S. The pharmacology of sevoflurane in infants and children. Anesthesiology. 1994;80:814–824.
83 Laird TH, Stayer SA, Rivenes SM, et al. Pulmonary-to-systemic blood flow ratio effects of sevoflurane, isoflurane, halothane, and fentanyl/midazolam with 100% oxygen in children with congenital heart disease. Anesth Analg. 2002;95:1200–1206.
84 Maruyama K, Agata H, Ono K, et al. Slow induction with sevoflurane was associated with complete atrioventricular block in a child with hypertension, renal dysfunction, and impaired cardiac conduction. Paediatr Anaesth. 1998;8:73–78.
85 Driessen JJ, van Oort AM, Booij LH. Severe myocardial ischaemia during mask induction of anaesthesia in an infant with unknown critical supravalvular aortic stenosis. Anaesthesia. 2003;58:568–570.
86 Friesen RH, Lichtor JL. Cardiovascular effects of inhalation induction with isoflurane in infants. Anesth Analg. 1983;62:411–414.
87 Brandom BW, Brandom RB, Cook DR. Uptake and distribution of halothane in infants: in vivo measurements and computer simulations. Anesth Analg. 1983;62:404–410.
88 Cook DR, Brandom BW, Shiu G, Wolfson B. The inspired median effective dose, brain concentration at anesthesia, and cardiovascular index for halothane in young rats. Anesth Analg. 1981;60:182–185.
89 Friesen RH, Lichtor JL. Cardiovascular depression during halothane anesthesia in infants: study of three induction techniques. Anesth Analg. 1982;61:42–45.
90 Murray DJ, Forbes RB, Mahoney LT. Comparative hemodynamic depression of halothane versus isoflurane in neonates and infants: an echocardiographic study. Anesth Analg. 1992;74:329–337.
91 Lerman J, Robinson S, Willis MM, Gregory GA. Anesthetic requirements for halothane in young children 0-1 month and 1-6 months of age. Anesthesiology. 1983;59:421–424.
92 Greeley WJ, Bushman GA, Davis DP, Reves JG. Comparative effects of halothane and ketamine on systemic arterial oxygen saturation in children with cyanotic heart disease. Anesthesiology. 1986;65:666–668.
93 Tuman KJ, McCarthy RJ, Spiess BD, et al. Effects of nitrous oxide on coronary perfusion after coronary air embolism. Anesthesiology. 1987;67:952–959.
94 Mehta M, Sokoll MD, Gergis SD. Effects of venous air embolism on the cardiovascular system and acid-base balance in the presence and absence of nitrous oxide. Acta Anaesthesiol Scand. 1984;28:226–231.
95 Banas JS, Jr., Meister SG, Gazzaniga AB, et al. A simple technique for detecting small defects of the atrial septum. Am J Cardiol. 1971;28:467–471.
96 Kronik G, Mosslacher H. Positive contrast echocardiography in patients with patent foramen ovale and normal right heart hemodynamics. Am J Cardiol. 1982;49:1806–1809.
97 Lynch JJ, Schuchard GH, Gross CM, Wann LS. Prevalence of right-to-left atrial shunting in a healthy population: detection by Valsalva maneuver contrast echocardiography. Am J Cardiol. 1984;53:1478–1480.
98 Topol EJ, Humphrey LS, Borkon AM, et al. Value of intraoperative left ventricular microbubbles detected by transesophageal two-dimensional echocardiography in predicting neurologic outcome after cardiac operations. Am J Cardiol. 1985;56:773–775.
99 Schulte-Sasse U, Hess W, Tarnow J. Pulmonary vascular responses to nitrous oxide in patients with normal and high pulmonary vascular resistance. Anesthesiology. 1982;57:9–13.
100 Lappas DG, Buckley MJ, Laver MB, et al. Left ventricular performance and pulmonary circulation following addition of nitrous oxide to morphine during coronary-artery surgery. Anesthesiology. 1975;43:61–69.
101 Hickey PR, Hansen DD, Strafford M, et al. Pulmonary and systemic hemodynamic effects of nitrous oxide in infants with normal and elevated pulmonary vascular resistance. Anesthesiology. 1986;65:374–378.
102 Murray DJ, Forbes RB, Dull DL, Mahoney LT. Hemodynamic responses to nitrous oxide during inhalation anesthesia in pediatric patients. J Clin Anesth. 1991;3:14–19.
103 Morray JP, Lynn AM, Stamm SJ, et al. Hemodynamic effects of ketamine in children with congenital heart disease. Anesth Analg. 1984;63:895–899.
104 Gassner S, Cohen M, Aygen M, et al. The effect of ketamine on pulmonary artery pressure: an experimental and clinical study. Anaesthesia. 1974;29:141–146.
105 Hickey PR, Hansen DD, Cramolini GM, et al. Pulmonary and systemic hemodynamic responses to ketamine in infants with normal and elevated pulmonary vascular resistance. Anesthesiology. 1985;62:287–293.
106 Doenicke A, Lorenz W, Beigl R, et al. Histamine release after intravenous application of short-acting hypnotics: a comparison of etomidate, Althesin (CT1341) and propanidid. Br J Anaesth. 1973;45:1097–1104.
107 Sarkar M, Laussen PC, Zurakowski D, et al. Hemodynamic responses to etomidate on induction of anesthesia in pediatric patients. Anesth Analg. 2005;101:645–650.
108 Zuckerbraun NS, Pitetti RD, Herr SM, et al. Use of etomidate as an induction agent for rapid sequence intubation in a pediatric emergency department. Acad Emerg Med. 2006;13:602–609.
109 Guldner G, Schultz J, Sexton P, et al. Etomidate for rapid-sequence intubation in young children: hemodynamic effects and adverse events. Acad Emerg Med. 2003;10:134–139.
110 Wagner RL, White PF. Etomidate inhibits adrenocortical function in surgical patients. Anesthesiology. 1984;61:647–651.
111 Wagner RL, White PF, Kan PB, et al. Inhibition of adrenal steroidogenesis by the anesthetic etomidate. N Engl J Med. 1984;310:1415–1421.
112 Watt I, Ledingham IM. Mortality amongst multiple trauma patients admitted to an intensive therapy unit. Anaesthesia. 1984;39:973–981.
113 Longnecker DE. Stress free: to be or not to be? Anesthesiology. 1984;61:643–644.
114 Reimer EJ, Montgomery CJ, Bevan JC, et al. Propofol anaesthesia reduces early postoperative emesis after paediatric strabismus surgery. Can J Anaesth. 1993;40:927–933.
115 Manschot HJ, Meursing AE, Axt P, et al. Propofol requirements for induction of anesthesia in children of different age groups. Anesth Analg. 1992;75:876–879.
116 Hannallah RS, Baker SB, Casey W, et al. Propofol: effective dose and induction characteristics in unpremedicated children. Anesthesiology. 1991;74:217–219.
117 Westrin P. The induction dose of propofol in infants 1-6 months of age and in children 10-16 years of age. Anesthesiology. 1991;74:455–458.
118 Stokes DN, Hutton P. Rate-dependent induction phenomena with propofol: implications for the relative potency of intravenous anesthetics. Anesth Analg. 1991;72:578–583.
119 Anand KJ, Hansen DD, Hickey PR. Hormonal-metabolic stress responses in neonates undergoing cardiac surgery. Anesthesiology. 1990;73:661–670.
120 Anand KJ. Neonatal stress responses to anesthesia and surgery. Clin Perinatol. 1990;17:207–214.
121 Motomura S, Kissin I, Aultman DF, Reves JG. Effects of fentanyl and nitrous oxide on contractility of blood-perfused papillary muscle of the dog. Anesth Analg. 1984;63:47–50.
122 Robinson S, Gregory GA. Fentanyl-air-oxygen anesthesia for ligation of patent ductus arteriosus in preterm infants. Anesth Analg. 1981;60:331–334.
123 Hickey PR, Hansen DD. Fentanyl- and sufentanil-oxygen-pancuronium anesthesia for cardiac surgery in infants. Anesth Analg. 1984;63:117–124.
124 Laishley RS, Burrows FA, Lerman J, Roy WL. Effect of anesthetic induction regimens on oxygen saturation in cyanotic congenital heart disease. Anesthesiology. 1986;65:673–677.
125 Hickey PR, Hansen DD, Wessel DL, et al. Pulmonary and systemic hemodynamic responses to fentanyl in infants. Anesth Analg. 1985;64:483–486.
126 Salmenpera M, Peltola K, Takkunen O, Heinonen J. Cardiovascular effects of pancuronium and vecuronium during high-dose fentanyl anesthesia. Anesth Analg. 1983;62:1059–1064.
127 Leysen JE, Gommeren W, Niemegeers CJ. [3H]Sufentanil, a superior ligand for mu-opiate receptors: binding properties and regional distribution in rat brain and spinal cord. Eur J Pharmacol. 1983;87:209–225.
128 Davis PJ, Wilson AS, Siewers RD, et al. The effects of cardiopulmonary bypass on remifentanil kinetics in children undergoing atrial septal defect repair. Anesth Analg. 1999;89:904–908.
129 Akpek EA, Erkaya C, Donmez A, et al. Remifentanil use in children undergoing congenital heart surgery for left-to-right shunt lesions. J Cardiothorac Vasc Anesth. 2005;19:60–66.
130 Bell G, Dickson U, Arana A, et al. Remifentanil vs fentanyl/morphine for pain and stress control during pediatric cardiac surgery. Paediatr Anaesth. 2004;14:856–860.
131 Donmez A, Kizilkan A, Berksun H, et al. One center’s experience with remifentanil infusions for pediatric cardiac catheterization. J Cardiothorac Vasc Anesth. 2001;15:736–739.
132 Ogletree ML, Sprung J, Moravec CS. Effects of remifentanil on the contractility of failing human heart muscle. J Cardiothorac Vasc Anesth. 2005;19:763–767.
133 Crawford MW, Hickey C, Zaarour C, et al. Development of acute opioid tolerance during infusion of remifentanil for pediatric scoliosis surgery. Anesth Analg. 2006;102:1662–1667.
134 Guignard B, Bossard AE, Coste C, et al. Acute opioid tolerance: intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology. 2000;93:409–417.
135 Vinik HR, Kissin I. Rapid development of tolerance to analgesia during remifentanil infusion in humans. Anesth Analg. 1998;86:1307–1311.
136 Gustorff B, Nahlik G, Hoerauf KH, Kress HG. The absence of acute tolerance during remifentanil infusion in volunteers. Anesth Analg. 2002;94:1223–1228.
137 Song JW, Lee YW, Yoon KB, Park SJ, Shim YH. Magnesium sulfate prevents remifentanil-induced postoperative hyperalgesia in patients undergoing thyroidectomy. Anesth Analg. 2011;113:390–397.
138 Echevarria G, Elgueta F, Fierro C, et al. Nitrous oxide (N2O) reduces postoperative opioid-induced hyperalgesia after remifentanil-propofol anaesthesia in humans. Br J Anaesth. 2011;107:959–965.
139 Davidson AJ. Anesthesia and neurotoxicity to the developing brain: the clinical relevance. Paediatr Anaesth. 2011;21:716–721.
140 Maunuksela EL, Gattiker RI. Use of pancuronium in children with congenital heart disease. Anesth Analg. 1981;60:798–801.
141 Scott NB, Turfrey DJ, Ray DA, et al. A prospective randomized study of the potential benefits of thoracic epidural anesthesia and analgesia in patients undergoing coronary artery bypass grafting. Anesth Analg. 2001;93:528–535.
142 Staats PS, Panchal SJ. Pro: the anesthesiologist should provide epidural anesthesia in the coronary care unit for patients with severe angina. J Cardiothorac Vasc Anesth. 1997;11:105–108.
143 Loick HM, Schmidt C, Van AH, et al. High thoracic epidural anesthesia, but not clonidine, attenuates the perioperative stress response via sympatholysis and reduces the release of troponin T in patients undrgoing coronary artery bypass grafting. Anesth Analg. 1999;88:701–709.
144 Davis RF, DeBoer LW, Maroko PR. Thoracic epidural anesthesia reduces myocardial infarct size after coronary artery occlusion in dogs. Anesth Analg. 1986;65:711–717.
145 Lee TW, Grocott HP, Schwinn D, Jacobsohn E. High spinal anesthesia for cardiac surgery: effects on beta-adrenergic receptor function, stress response, and hemodynamics. Anesthesiology. 2003;98:499–510.
146 Rosen KR, Rosen DA. Caudal epidural morphine for control of pain following open heart surgery in children. Anesthesiology. 1989;70:418–421.
147 Hammer GB, Ngo K, Macario A. A retrospective examination of regional plus general anesthesia in children undergoing open heart surgery. Anesth Analg. 2000;90:1020–1024.
148 Peterson KL, DeCampli WM, Pike NA, et al. A report of two hundred twenty cases of regional anesthesia in pediatric cardiac surgery. Anesth Analg. 2000;90:1014–1019.
149 Steven JM, McGowan FX, Jr. Neuraxial blockade for pediatric cardiac surgery: lessons yet to be learned. Anesth Analg. 2000;90:1011–1013.
150 Bosenberg A. Neuraxial blockade and cardiac surgery in children. Paediatr Anaesth. 2003;13:559–560.
151 Ho AM, Chung DC, Joynt GM. Neuraxial blockade and hematoma in cardiac surgery: estimating the risk of a rare adverse event that has not (yet) occurred. Chest. 2000;117:551–555.
152 Steven JM, McGowan FX, Jr. Neuraxial blockade for pediatric cardiac surgery: lessons yet to be learned. Anesth Analg. 2000;90:1011–1013.
153 Vricella LA, Dearani JA, Gundry SR, et al. Ultra fast track in elective congenital cardiac surgery. Ann Thorac Surg. 2000;69:865–871.
154 Marianeschi SM, Seddio F, McElhinney DB, et al. Fast-track congenital heart operations: a less invasive technique and early extubation. Ann Thorac Surg. 2000;69:872–876.
155 Kloth RL, Baum VC. Very early extubation in children after cardiac surgery. Crit Care Med. 2002;30:787–791.
156 Heinle JS, Fox LS. Early extubation of neonates and young infants after cardiac surgery. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 1998;1:103–108.
157 Shekerdemian LS, Penny DJ, Novick W. Early extubation after surgical repair of tetralogy of Fallot. Cardiol Young. 2000;10:636–637.
158 Laussen PC, Reid RW, Stene RA, et al. Tracheal extubation of children in the operating room after atrial septal defect repair as part of a clinical practice guideline. Anesth Analg. 1996;82:988–993.
159 Burrows FA, Taylor RH, Hillier SC. Early extubation of the trachea after repair of secundum-type atrial septal defects in children. Can J Anaesth. 1992;39:1041–1044.
160 Schuller JL, Bovill JG, Nijveld A, et al. Early extubation of the trachea after open heart surgery for congenital heart disease: a review of 3 years’ experience. Br J Anaesth. 1984;56:1101–1108.
161 Lake CL. Fast tracking the paediatric cardiac surgical patient. Paediatr Anaesth. 2000;10:231–236.
162 Anand KJ, Hansen DD, Hickey PR. Hormonal-metabolic stress responses in neonates undergoing cardiac surgery. Anesthesiology. 1990;73:661–670.
163 Hindmarsh KW, Sankaran K, Watson VG. Plasma beta-endorphin concentrations in neonates associated with acute stress. Dev Pharmacol Ther. 1984;7:198–204.
164 Anand KJ, Sippell WG, Aynsley-Green A. Pain, anaesthesia, and babies. Lancet. 1987;2:1210.
165 Anand KJ, Hickey PR. Pain and its effects in the human neonate and fetus. N Engl J Med. 1987;317:1321–1329.
166 Anand KJ, Sippell WG, Schofield NM, Aynsley-Green A. Does halothane anaesthesia decrease the metabolic and endocrine stress responses of newborn infants undergoing operation? BMJ (Clin Res Ed). 1988;296:668–672.
167 Anand KJ, Hickey PR. Halothane-morphine compared with highdose sufentanil for anesthesia and postoperative analgesia in neonatal cardiac surgery. N Engl J Med. 1992;326:1–9.
168 Anand KJ, Sippell WG, Aynsley-Green A. Randomised trial of fentanyl anaesthesia in preterm babies undergoing surgery: effects on the stress response. Lancet. 1987;1:62–66.
169 Swedberg K, Eneroth P, Kjekshus J, Wilhelmsen L. Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. CONSENSUS Trial Study Group. Circulation. 1990;82:1730–1736.
170 Steward DJ, Da Silva CA, Flegel T. Elevated blood glucose levels may increase the danger of neurological deficit following profoundly hypothermic cardiac arrest. Anesthesiology. 1988;68:653.
171 Ellis DJ, Steward DJ. Fentanyl dosage is associated with reduced blood glucose in pediatric patients after hypothermic cardiopulmonary bypass. Anesthesiology. 1990;72:812–815.
172 Hickey PR, Hansen DD, Wessel DL, et al. Blunting of stress responses in the pulmonary circulation of infants by fentanyl. Anesth Analg. 1985;64:1137–1142.
173 Giesecke K, Hamberger B, Jarnberg PO, et al. High- and low-dose fentanyl anaesthesia: hormonal and metabolic responses during cholecystectomy. Br J Anaesth. 1988;61:575–582.
174 Walsh ES, Paterson JL, O’Riordan JB, Hall GM. Effect of high-dose fentanyl anaesthesia on the metabolic and endocrine response to cardiac surgery. Br J Anaesth. 1981;53:1155–1165.
175 Hickey PR, Hansen DD. High-dose fentanyl reduces intraoperative ventricular fibrillation in neonates with hypoplastic left heart syndrome. J Clin Anesth. 1991;3:295–300.
176 Hansen DD, Hickey PR. Anesthesia for hypoplastic left heart syndrome: use of high-dose fentanyl in 30 neonates. Anesth Analg. 1986;65:127–132.
177 Blair JR, Pruett JK, Introna RP, et al. Cardiac electrophysiologic effects of fentanyl and sufentanil in canine cardiac Purkinje fibers. Anesthesiology. 1989;71:565–570.
178 Saini V, Carr DB, Hagestad EL, et al. Antifibrillatory action of the narcotic agonist fentanyl. Am Heart J. 1988;115:598–605.
179 Checchia PA, Bronicki RA, Costello JM, Nelson DP. Steroid use before pediatric cardiac operations using cardiopulmonary bypass: an international survey of 36 centers. Pediatr Crit Care Med. 2005;6:441–444.
180 Lodge AJ, Chai PJ, Daggett CW, et al. Methylprednisolone reduces the inflammatory response to cardiopulmonry bypass in neonatal piglets: timing is important. J Thorac Cardiovasc Surg. 1999;117:515–522.
181 Schroeder VA, Pearl JM, Schwartz SM, et al. Combined steroid treatment for congenital heart surgery improves oxygen delivery and reduces postbypass inflammatory mediator expression. Circulation. 2003;107:2823–2828.
182 Pearl JM, Schwartz SM, Nelson DP, et al. Preoperative glucocorticoids decrease pulmonary hypertension in piglets after cardiopulmonary bypass and circulatory arrest. Ann Thorac Surg. 2004;77:994–1000.
183 Levy JH, Sypniewski E. Aprotinin: a pharmacologic overview. Orthopedics. 2004;27(6 Suppl):s653–s658.
184 Arnold DM, Fergusson DA, Chan AK, et al. Avoiding transfusions in children undergoing cardiac surgery: a meta-analysis of randomized trials of aprotinin. Anesth Analg. 2006;102:731–737.
185 McDonough J, Gruenwald C. The use of aprotinin in pediatric patients: a review. J Extra Corpor Technol. 2003;35:346–349.
186 Mojcik CF, Levy JH. Aprotinin and the systemic inflammatory response after cardiopulmonary bypass. Ann Thorac Surg. 2001;71:745–754.
187 Yun TJ, Rho JR. Aprotinin attenuates the elevation of pulmonary vascular resistance after cardiopulmonary bypass. J Korean Med Sci. 2006;21:25–29.
188 Levy JH. Efficacy and safety of aprotinin in cardiac surgery. Orthopedics. 2004;27:s659–s662.
189 Clancy RR, McGaurn SA, Goin JE, et al. Allopurinol neurocardiac protection trial in infants undergoing heart surgery using deep hypothermic circulatory arrest. Pediatrics. 2001;108:61–70.
190 Alkhulaifi AM, Jenkins DP, Pugsley WB, Treasure T. Ischaemic preconditioning and cardiac surgery. Eur J Cardiothorac Surg. 1996;10:792–798.
191 Hawaleshka A, Jacobsohn E. Ischaemic preconditioning: mechanisms and potential clinical applications. Can J Anaesth. 1998;45:670–682.
192 Wu ZK, Pehkonen E, Laurikka J, et al. Protective effect of unstable angina in coronary artery bypass surgery. Scand Cardiovasc J. 2000;34:486–492.
193 Wu ZK, Tarkka MR, Pehkonen E, et al. Ischaemic preconditioning has a beneficial effect on left ventricular haemodynamic function after a coronary artery bypass grafting operation. Scand Cardiovasc J. 2000;34:247–253.
194 Wu ZK, Tarkka MR, Eloranta J, et al. Effect of ischaemic preconditioning, cardiopulmonary bypass and myocardial ischaemic/reperfusion on free radical generation in CABG patients. Cardiovasc Surg. 2001;9:362–368.
195 Kharbanda RK, Mortensen UM, White PA, et al. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106:2881–2883.
196 Cheung MM, Kharbanda RK, Konstantinov IE, et al. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol. 2006;47:2277–2282.
197 Schipke JD, Friebe R, Gams E. Forty years of glucose-insulin-potassium (GIK) in cardiac surgery: a review of randomized, controlled trials. Eur J Cardiothorac Surg. 2006;29:479–485.
198 Szabo Z, Hakanson E, Maros T, Svedjeholm R. High-dose glucose-insulin-potassium after cardiac surgery: a retrospective analysis of clinical safety issues. Acta Anaesthesiol Scand. 2003;47:383–390.
199 Bothe W, Olschewski M, Beyersdorf F, Doenst T. Glucose-insulin-potassium in cardiac surgery: a meta-analysis. Ann Thorac Surg. 2004;78:1650–1657.
200 Quinn DW, Pagano D, Bonser RS. Glucose and insulin influences on heart and brain in cardiac surgery. Semin Cardiothorac Vasc Anesth. 2005;9:173–178.
201 Friedman WF, Heiferman MF. Clinical problems of postoperative pulmonary vascular disease. Am J Cardiol. 1982;50:631–636.
202 Wagenvoort CA. Hypertensive pulmonary vascular disease complicating congenital heart disease: a review. Cardiovasc Clin. 1973;5:43–60.
203 Wilson SK, Hutchins GM, Neill CA. Hypertensive pulmonary vascular disease in Down syndrome. J Pediatr. 1979;95:722–726.
204 Celermajer DS, Cullen S, Deanfield JE. Impairment of endothelium-dependent pulmonary artery relaxation in children with congenital heart disease and abnormal pulmonary hemodynamics. Circulation. 1993;87:440–446.
205 Yamaki S, Abe A, Tabayashi K, et al. Inoperable pulmonary vascular disease in infants with congenital heart disease. Ann Thorac Surg. 1998;66:1565–1570.
206 Dexter L. Pulmonary vascular disease in acquired and congenital heart disease. Arch Intern Med. 1979;139:922–928.
207 Samanek M, Slavik Z, Zborilova B, et al. Prevalence, treatment, and outcome of heart disease in liveborn children: a prospective analysis of 91,823 liveborn children. Pediatr Cardiol. 1989;10:205–211.
208 Mavroudis C, Backer CL, Jacobs JP, et al. Ventricular septal defect. In: Mavroudis C, Backer CL, eds. Pediatric cardiac surgery. 3rd ed. Philadelphia: Mosby; 2003:298–338.
209 Jacobs JP, Quintessenza JA, Gaynor JW, et al. Congenital Heart Surgery Nomenclature and Database Project: aortopulmonary window. Ann Thorac Surg. 2000;69(4 Suppl):S44–S49.
210 Reddy VM, Hanley FL. Cardiac surgery in infants with very low birth weight. Semin Pediatr Surg. 2000;9:91–95.
211 Reddy VM, McElhinney DB, Sagrado T, et al. Results of 102 cases of complete repair of congenital heart defects in patients weighing 700 to 2500 grams. J Thorac Cardiovasc Surg. 1999;117:324–331.
212 Kumar A, Taylor GP, Sandor GG, Patterson MW. Pulmonary vascular disease in neonates with transposition of the great arteries and intact ventricular septum. Br Heart J. 1993;69:442–445.
213 Haworth SG. Pulmonary vascular disease in different types of congenital heart disease: implications for interpretation of lung biopsy findings in early childhood. Br Heart J. 1984;52:557–571.
214 Clarkson PM, Neutze JM, Wardill JC, Barratt-Boyes BG. The pulmonary vascular bed in patients with complete transposition of the great arteries. Circulation. 1976;53:539–543.
215 Dhasmana JP, Stark J, de Laval M, et al. Long-term results of the “palliative” Mustard operation. J Am Coll Cardiol. 1985;6:1138–1141.
216 Pearl JM, Nelson DP, Schwartz SM, Manning PB. First-stage palliation for hypoplastic left heart syndrome in the twenty-first century. Ann Thorac Surg. 2002;73:331–339. discussion 339-40
217 Mahle WT, Cuadrado AR, Tam VK. Early experience with a modified Norwood procedure using right ventricle to pulmonary artery conduit. Ann Thorac Surg. 2003;76:1084–1088.
218 Tanoue Y, Kado H, Shiokawa Y, et al. Midterm ventricular performance after Norwood procedure with right ventricular-pulmonary artery conduit. Ann Thorac Surg. 2004;78:1965–1971.
219 Fraser CD, Jr., Mee RB. Modified Norwood procedure for hypoplastic left heart syndrome. Ann Thorac Surg. 1995;60:S546–S549.
220 Tweddell JS, Hoffman GM, Fedderly RT, et al. Phenoxybenzamine improves systemic oxygen delivery after the Norwood procedure. Ann Thorac Surg. 1999;67:161–167. discussion 167-8
221 Gamillscheg A, Zobel G, Urlesberger B, et al. Inhaled nitric oxide in patients with critical pulmonary perfusion after Fontan-type procedures and bidirectional Glenn anastomosis. J Thorac Cardiovasc Surg. 1997;113:435–442.
222 Botto LD, Correa A, Erickson JD. Racial and temporal variations in the prevalence of heart defects. Pediatrics. 2001;107:E32.
223 Maizza AF, Ho SY, Anderson RH. Obstruction of the left ventricular outflow tract: anatomical observations and surgical implications [see comment]. J Heart Valve Dis. 1993;2:66–79.
224 Medley J, Russo P, Tobias JD. Perioperative care of the patient with Williams syndrome. Paediatr Anaesth. 2005;15:243–247.
225 Bauer EP, Schmidli J, Vogt PR, et al. Valvotomy for isolated congenital aortic stenosis in children: prognostic factors for outcome. Thorac Cardiovasc Surg. 1992;40:334–339.
226 Rao PS, Thapar MK, Galal O, Wilson AD. Follow-up results of balloon angioplasty of native coarctation in neonates and infants. Am Heart J. 1990;120(6 Pt 1):1310–1314.
227 Elkins RC, Knott-Craig CJ, Ward KE, et al. Pulmonary autograft in children: realized growth potential. Ann Thorac Surg. 1994;57:1387–1393. discussion 1393-4
228 Elkins RC, Lane MM, McCue C. Ross operation in children: late results. J Heart Valve Dis. 2001;10:736–741.
229 Gerosa G, McKay R, Ross DN. Replacement of the aortic valve or root with a pulmonary autograft in children. Ann Thorac Surg. 1991;51:424–429.
230 Watterson KG, Dhasmana JP, O’Higgins JW, Wisheart JD. Distal aortic pressure during coarctation operation. Ann Thorac Surg. 1990;49:987–990.
231 Park SC, Neches WH. The neurologic complications of congenital heart disease. Neurol Clin. 1993;11:441–462.
232 Momma K, Matsuoka R, Takao A. Aortic arch anomalies associated with chromosome 22q11 deletion (CATCH 22). Pediatr Cardiol. 1999;20:97–102.
233 Lim C, Kim WH, Kim SC, et al. Aortic arch reconstruction using regional perfusion without circulatory arrest. Eur J Cardiothorac Surg. 2003;23:149–155.
234 Tlaskal T, Hucin B, Hruda J, et al. Results of primary and two-stage repair of interrupted aortic arch [see comment]. Eur J Cardiothorac Surg. 1998;14:235–242.
235 Warren J, Fromm RE, Jr., Orr RA, et al. Guidelines for the inter- and intrahospital transport of critically ill patients. Crit Care Med. 2004;32:256–262.
236 Venkataraman ST. Intrahospital transport of critically ill children: should we pay attention? Crit Care Med. 1999;27:694–695.