CHAPTER 33 Anesthesia and Sedation for Pediatric Procedures Outside the Operating Room
Advances in imaging and endoscopic technology requests from medical colleagues for support during prolonged or high-risk procedures and concern about liability exposure have led to increasing demands for anesthesiologists’ professional services outside the operating room. In the 14 years since the first publication of this chapter, anesthesia services outside the operating room have increased more than sixfold at Children’s Hospital Boston to over 7000 anesthetic procedures annually. As the demand for sedation services has increased nationally, so also have nonanesthesiologists expressed an interest in providing these services and are often using anesthesia billing codes. Indeed, pediatric radiologists, oncologists, dentists, gastroenterologists, pulmonologists, pediatricians, hospitalists, and intensivists are all able to supervise and deliver sedation. Monitoring techniques, sedative choices, and sedation guidelines vary between specialty organizations. Although the American Academy of Pediatrics (AAP) recently updated its guidelines, the recommendations of the American College of Gastroenterology and the American College of Emergency Physicians differ from those of the AAP (AAP et al., 2008).
Requirements for extramural locations
Organization and Administration
A collaborative relationship between extramural and anesthesiology departments is critical to providing safe patient care. At a minimum, safe patient care requires appropriate anesthesia equipment and monitors, adequate space, and experienced ancillary providers who are knowledgeable in anesthesia and facile in providing assistance if needed. Each off-site area has its own needs, goals, and guidelines. It is ideal to designate a team of anesthesiologists committed to providing extramural anesthesia care and troubleshooting the logistical challenges in the various locations. Each member should rotate regularly through the different extramural sites in order to maintain familiarity with the procedures, to foster a relationship with the physicians and ancillary personnel, to understand the anesthesia demands unique to each site, and sharing information about ongoing advances. Technological advances are particularly expanding in the field of radiology, and complicated imaging studies challenge the anesthesiologist to have an understanding of the unique conditions that each study requires. Understanding these requirements will guide the anesthesiologist in developing a management plan (Lee et al, 2008).
In the past, extramural locations were not designed with the anesthesiologist in mind. The need for anesthesia had not been anticipated when off-site locations were planned. It is only within the past decade that the demand for anesthesia services in these sites has burgeoned. Thus, most off-site locations have not been configured to support the capabilities for anesthesia. Ideally, anesthesiologists should be involved in the early stages of site design to ensure that minimum standards for anesthesia delivery are met and to troubleshoot engineering issues and advocate for adequate space for anesthetic induction and emergence (Committee on Drugs, 1992; American Academy of Pediatrics Section on Anesthesiology, 1999). Physical plant considerations for MRI-site planning have been previously described (Koskinen, 1985). When anesthesia services are requested, these sites may not meet minimum standards and may require reengineering to meet minimum requirements of the American Society of Anesthesiologists (ASA) (House et al., 1994). The anesthesia machine should be equipped with back-up supplies of E cylinders filled with oxygen and nitrous oxide. If pipeline oxygen is not available, then oxygen should be supplied from H cylinders (6600 L) rather than the smaller E tanks (659 L).
Scavenging systems should be carefully evaluated in the extramural location. Unlike the operating room, passive scavenging systems may not always be possible. A safe means of active scavenging may be provided by the vacuum at the wall or wall suction canisters. A scavenging system should be dedicated solely to waste gases. Many MRI scanners do not have wall suction because MRI-compatible wall suction is not widely available. If the suction is located outside the MRI suite, then a mouse-sized hole may be created in the suite’s wall to allow suction tubing to be passed inside (Koskinen, 1985).
Personnel, Support, and Logistics
Leadership is crucial; a director of anesthesia services at an extramural location can orchestrate, facilitate, and coordinate anesthesia services. This director can also serve as a consultant for the other medical and nursing staff. By being available to answer questions, do on-site consultations, examine patients, and provide back-up support or emergency-airway expertise, the anesthesiologist can also support a nurse-administered sedation program. Nurses who provide sedation under the supervision of the ordering extramural physician (e.g., gastrointestinal, radiologic, or dental) should have Pediatric Advanced Life Support (PALS) and Basic Life Support (BLS) certifications. The Joint Commission on Accreditation of Healthcare Organizations (JCAHO) (2009) requires that individuals who administer sedation are able to rescue patients from whatever level of sedation or anesthesia is achieved, whether intentional or unintentional. All children scheduled for nursing sedation during a scan should receive a prescreening telephone call from a radiology nurse the day before the scheduled scan. Often, these telephone calls are made after business hours to ensure that a parent is home. The nurse reviews the medical history, relays fasting instructions, and reminds the parent to administer the child’s routine medications with a sip of clear fluid. The supervising physician must give final approval for sedation after reviewing the child’s medical history and current medical status and before ordering the medications. To minimize the chance of drug-delivery error or miscalculation, it is helpful to have preprinted order sheets or a computerized order-entry template.
Periprocedural patient care: standards of practice and quality assurance
The practice standards adopted by the American Society of Anesthesiologists (ASA) in 1986 for basic intraoperative monitoring apply to extramural locations as well. Practice standards and guidelines promulgated by the AAP are exceeded by established practice standards in anesthesiology (Committee on Drugs, 1992; Anesthesiology and Pediatrics, 1999). Significant variances may exist when practitioners who are not anesthesiologists administer sedation (Keeter et al., 1990). Practice Standards for Non-Anesthetizing Locations were adopted by the ASA in 1994 (ASA House of Delegates, 1994).
Scheduling and Preparation of Patients
Appropriate planning for an anesthetic begins with a familiarity with the procedure. The requesting service orders the procedure and then leaves the logistics of scheduling to the extramural service. Radiologists recognize that involvement with anesthesia lengthens their total time commitment to a patient and potentially limits the number of procedures accomplished in a day (Winter, 1978; Cremin, 1990). A well-coordinated system to screen patients on the day of the procedure is important. Experienced personnel, ideally a certified pediatric nurse practitioner (CPNP), should be designated to take initial vital signs, review recent medical history, begin IV lines if necessary, and familiarize the family with the upcoming procedure, including anesthesia.
It is not always possible for an anesthesiologist to provide sedation and anesthesia for all children when there is a large volume of cases. A structured nursing sedation program can provide safe and effective sedation. In many hospitals, the extramural department (gastroenterology, radiology, cardiology) outsources responsibility for sedation to another department, which could include pediatrics, hospital medicine, anesthesiology, intensive care, or emergency medicine. After screening the patient, an appropriate referral for either general anesthesia or procedural sedation is the usual result. Because MRI is a unique environment, it is more efficient to have the MRI nurses screen patients before and on the day of the procedure. To ensure consistent decision making, the anesthesiology and radiology departments should develop a set of guidelines and easily identifiable “red flags ”to help in this triaging process (Table 33-1). If any questions arise, additional medical history needs to be clarified, or additional studies need to be performed, the nurse and anesthesiologist confer before making the final decision regarding general anesthesia or procedural sedation. In addition, chronically ill children often have electrolyte disturbances, coagulation and hematologic abnormalities, and hemodynamic instability. A consent for the administration of general anesthesia or procedural sedation must be obtained in parity with policies established for anesthesia in the operating room.
| Red Flag | Indications |
| Apnea | Documented by sleep study, strong clinical history, or on an apnea monitor |
| Unstable cardiac disease | Cyanotic, depressed myocardial function, or significant stenotic or regurgitation lesions |
| Respiratory compromise | Recent (<8 weeks) pneumonia, bronchitis, asthma, or respiratory infection |
| Craniofacial defect | Potential for difficult airway |
| History of a difficult airway | |
| Active gastroesophageal reflux or vomiting | In poor control, with or without medical or surgical treatment |
| Hypotonia and lack of head control | Patient may not be able to maintain own airway without assistance |
| Allergies to barbiturates | Usually the mainstay of a sedation protocol; also allergies to other sedatives to be administered |
| Prior failed sedation | Unable to be sedated or unsuccessful imaging study because of excessive movement |
| Tremors | Unlikely to be ablated with sedation |
Selection of Agents and Techniques
The selection of an anesthetic technique in an extramural location depends on the patient’s underlying medical condition, age, drug tolerance, and anticipated procedure. The airway management may be influenced by the procedure itself, anticipated postprocedure course (e.g., intensive care unit [ICU] or postprocedure intubation) and past anesthetic course (e.g., difficult intubation). The assistance of an anesthesiologist is often sought when sedation administered by the radiologist has failed in the past; it is important to be aware that parents and radiologists may have the expectation that anesthesia will provide ideal conditions and guarantee successful completion of the procedure (Hubbard et al., 1992).
Premedication has many purposes: the relief of anxiety, easy separation from parents, sedation, analgesia, amnesia, reduction of salivary and gastric secretions, elevation of gastric pH, and decreased cardiac vagal activity. Medications should be adjusted to the psychological and physiologic condition of the patient and family. Parent-present inductions may be offered when the presence of a parent has a calming effect on the child. In the event of potentially detrimental parent anxiety, premedication may be preferable to a parent-present induction (Kain et al., 2001).
Barbiturates may be useful as the sole method of providing sedation. Pentobarbital, for example, has the advantage of providing sedation, causes minimal respiratory and circulatory depression, and is rarely associated with adverse events (Karian et al., 2002). Barbiturates have no analgesic properties. They can produce paradoxical reactions, especially in children. No antagonist to barbiturates is available, thus dosing should be carefully titrated. IV pentobarbital by titration has been used successfully by radiologists while monitoring oral and nasal air flow, oxygen saturation with a pulse oximeter (SpO2), end-tidal carbon dioxide, and cardiac rate and rhythm, with transient decreases in SpO2 in up to 7.5% of patients; interventions have included stimulation and head repositioning (Strain et al., 1988; Rooks et al., 2003).
Other studies have described the use of pentobarbital both in the oral and IV forms (Chung et al., 2000; Mason et al., 2001a). For infants younger than 1 year of age, oral pentobarbital is more successful and carries a lower rate of adverse events compared with chloral hydrate (Rooks et al., 2003). The long half-life of pentobarbital (approximately 24 hours) requires careful and conservative recovery and discharge guidelines. The dosage of oral pentobarbital is 2 to 6 mg/kg and up to 9 mg/kg in patients who are receiving barbiturate therapy.
Sodium thiopental in a mean induction dose of 6 mg/kg and a mean total dose of 8.5 ± 3 mg/kg has been used successfully as the sole anesthetic for CT and MRI in 200 children from 1 month to 12 years of age (Spear et al., 1993). Methohexital has a shorter recovery time than thiopental and is more effective than oral chloral hydrate (Manuli and Davies, 1993). Methohexital-induced seizures in patients with temporal lobe epilepsy have been reported. Thiopental or pentobarbital are alternatives for these patients (Rockoff and Goudsouzian, 1981). For patients taking barbiturate-containing anticonvulsant medications, a higher dose limit is generally more successful. Methohexital has also been used intramuscularly (IM) for radiotherapy in doses of 8 to 10 mg/kg. The onset time via this route is often twofold to threefold longer than rectally administered methohexital (Jeffries, 1988).
Although propofol does not have a labeled indication for children younger than 3 years of age, propofol has been used extensively in this age group as a means of providing sedation or anesthesia. Propofol sedation by bolus or continuous infusion for MRI scans of the brain can provide successful imaging conditions but with the risk of need for airway intervention and respiratory compromise (Vangerven et al., 1992; Cravero et al., 2009). Fatal metabolic acidosis and myocardial failure associated with lipemic serum have been reported in five children who were admitted to the ICU for respiratory support for upper respiratory tract infections while being sedated with continuous infusion propofol (Parke et al., 1992). Bloomfield and others (1993) have suggested a continuous infusion beginning at a rate of 99 mL/hr until the patients fall asleep (usually over 1 to 3 minutes) and then a decreasing rate during treatment, a strategy that works very well. Patients were typically awake, alert, and taking clear liquids 20 minutes later (Bloomfield et al., 1993).
Opiates reduce anesthetic, preprocedure, and postprocedure analgesic requirements. They are reversible with naloxone. Whereas narcotics may be unnecessary for diagnostic procedures that are not painful, they may be very useful for therapeutic interventions, especially for patients with postprocedural pain. They are also useful after anthracycline chemotherapy, with documented impaired myocardial function (Burrows et al., 1985). Because narcotics depress the ventilatory response to carbon dioxide (CO2), this respiratory depression may be of particular concern for children with increased intracranial pressure. Narcotics may also worsen preexisting nausea and vomiting.
Benzodiazepines have the advantage of anxiolysis with minimal vomiting and cardiorespiratory depression. Diazepam is painful during IV injection and may lead to thrombophlebitis; midazolam is water soluble and therefore may be more suitable intravenously or intramuscularly. The elimination half-life of midazolam averages 2.5 hours, compared with 20 to 70 hours for diazepam (Greenblatt et al., 1981; Reves et al., 1985). Young patients or patients with significant liver disease may have prolonged duration and exaggerated effect of the benzodiazepines.
Preparation of the stomach and aspiration prophylaxis are of particular concern for urgently scheduled cases (outside of fasting guidelines) or when the medical history suggests aspiration risk. If using H2-receptor antagonists, bronchospasm may occur in patients with asthma because of the relative increased availability of H1-receptors. H2-blockers may also inhibit metabolism of other concurrently administered medications. Metoclopramide accelerates gastric emptying and increases tone in the lower esophageal sphincter, but it is associated with a significant incidence of extrapyramidal side effects in children (Sledge et al., 1992). Ondansetron works synergistically with other agents through its vagal blocking actions in the gastrointestinal tract, as well as through its inhibition of the chemoreceptor trigger zone via serotonin receptor antagonism, particularly for patients undergoing radiation therapy with pulses of chemotherapy (Burnette and Perkins, 1992; Figg et al., 1993).
Ketamine has enjoyed great popularity during the past 30 years for sedation, analgesia, or anesthesia outside the operating room because of its support of the cardiovascular and respiratory systems. Ketamine-induced nightmares, hallucinations, delusions, and agitation are rare in children (Sussman, 1974; Hostetler and Davis, 2002). Karian and others (2002) have reported on a ketamine-sedation program for use in interventional radiology. In this program, IV or IM ketamine was administered in the interventional radiology suite by credentialed nurses and radiologists to patients undergoing select procedures. This protocol has allowed painful procedures to be tolerated by patients who previously would have required general anesthesia (Karian et al., 2002).
Dexmedetomidine, although not approved by the Food and Drug Administration (FDA) for pediatric use, obtained approval in October 2008 for adult procedural sedation in areas outside of the ICU. Particularly for pediatric sedation for radiologic imaging studies, dexmedetomidine alone in high dosages can achieve immobility for MRI and CT examinations (Mason et al., 2006, 2008a, 2008b). Its use for procedural sedation has still to be explored further, because its clinical application has been examined only in small studies and often in combination with ketamine (Barton et al., 2008).
Some patients require general anesthesia because of previous sedation failures, the need for a secure airway, or procedural logistics. Newer, less-soluble anesthetic agents such as sevoflurane and desflurane have pharmacokinetic profiles that compare favorably with propofol in adults; there seems little reason to think that this would not be the case with children, although pediatric anesthesiologists often avoid using desflurane because of its pungency and associated airway irritability (Van Hemelrijck et al., 1991). Since its introduction to clinical practice in the mid-1990s, sevoflurane has become the volatile anesthetic of choice in children. Its lack of airway irritability as a side effect and its ability to provide children with stable hemodynamic function, together with its rapid onset and offset make sevoflurane a useful agent for children (Furst et al., 1996).
Regional anesthesia, rarely administered outside the pediatric operating room, nevertheless remains a valid choice in some circumstances. Intercostal nerve blocks may be useful for lung or rib biopsies, chest tubes, biliary or subphrenic drainage procedures, and insertion of biliary stents. Nerve block of the brachial plexus by the axillary, interscalene, or supraclavicular route has been reported for the brachial approach to catheterization and neuraxial block of the lower extremities for femoral catheterizations and percutaneous approaches to the kidneys (Eggers et al., 1967; Ross, 1970; Lind and Mushlin, 1987). Spinal anesthesia in conjunction with regional hyperthermia and limb exsanguination has been successfully used for repeated painful radiotherapy on lower extremities (Spencer and Barnes, 1980).
Resuscitation
The physicians, nurses, anesthesiologists, technologists, and support personnel must know the location of a readily accessible code cart. In addition, a hard board to be placed under the patient during resuscitation should be available. Mock codes should be performed regularly to ensure adequate flow, teamwork, and delineation of responsibilities in the event of an emergency. The MRI scanner poses a special problem. Codes should never be conducted in the scanner because as support personnel rush inside to assist, ferrous materials that are not removed will become projectile and create an even more hazardous situation. Quenching a magnet should not be an alternative, because it requires a minimum of 3 minutes to eliminate the magnetic field. In addition, inadequate exhaust during a quench has been known to produce hypoxic conditions in the scanner and has resulted in patient death. A “black quench” could melt the MRI coils and require replacement of the scanner, a costly and time-consuming undertaking. Defibrillators are not compatible with MRI and may not function properly when they are exposed to the magnetic field (Snowdon, 1989). In an emergency, a patient should be removed from the scanner to an area outside of the magnetic field. This designated area is a safe place for resuscitation and should have not only a wall oxygen source for a self-inflating bag but also access to appropriate monitors.
Specific extramural sites
Radiology
Computerized Tomography Scan
CT differentiates between high-density (e.g., calcium, iron, bone, and contrast-enhanced vascular and cerebrospinal fluid [CSF] spaces) and low-density (e.g., oxygen, nitrogen, carbon in air, fat, CSF, muscle, white matter, gray matter, and water-containing lesions) structures. Because the scan time is quick, CT may be preferable for patients who are medically unstable and in need of rapid diagnosis—for example, with the child being evaluated for abuse, an intracranial hemorrhage, or abdominal or thoracic mass. Other indications for emergency CT scans may include encephalopathy and a change in neurologic status. In these situations, the issues of a full stomach and increased intracranial pressure usually necessitate a rapid-sequence induction with tracheal intubation. A CT scan of the head is often the preferred study in emergency situations where head trauma is involved (Blankenberg et al., 2000).
The actual scanning sequences are short and can range from 10 to 40 seconds. These short scan times enable many children to complete a CT scan without any sedation, especially with parental presence and distraction techniques. When an anesthesiologist is involved, it is often for airway or failed-sedation issues or for a medically complicated patient. An important aspect of some CT scans is to visualize the sinuses, ears, inner auditory canal, and temporomandibular bones and to evaluate for choanal atresia or craniofacial abnormalities. These scans may require direct coronal imaging with extreme head extension (off the end of the table at an angle between 40 and 70 degrees) or absolute immobility for three-dimensional reconstruction, so it is critical for the treatment team to have a thorough understanding of the specifics of the study. Three-dimensional airway and cardiac studies have evolved and have unique anesthetic requirements. The airway studies require breath holding on inspiration and expiration in order to allow visualization of areas with airway collapse (Lee et al., 2008). The cardiac studies are often done in collaboration with cardiologists and radiologists who are able to make structural and functional assessments of the heart. These scans can also be challenging, because adenosine is often requested in order to briefly pause heart function so that image quality is maximized (Woodard et al., 2006).
Any patient who is at risk for cervical instability should be properly screened before neck extension. Children with Down syndrome are at risk for atlantoaxial instability. The incidence of instability varies from 12% to 32% (Blankenberg et al., 2000). Many children with Down syndrome require cervical spine radiographs before entering grade school or participating in Special Olympics. Usually, the parents are well aware of the radiologic findings. The cervical spine films, however, do not indicate whether or not a child is at risk for dislocation (Davidson, 1988). Rather, those children who exhibit neurologic signs or symptoms such as abnormal gait, increased clumsiness, fatigue with ambulation, or a new preference for sitting games are at risk. In infants, developmental milestones (e.g., crawling, sitting up, and reaching for objects) should be verified. Physical signs of Down syndrome may include clonus, hyperreflexia, quadriparesis, neurogenic bladder, hemiparesis, ataxia, and sensory loss. The asymptomatic Down syndrome child with radiologic evidence of instability may be approved for procedural sedation; however, unnecessary neck movement should be avoided. Any child who displays neurologic signs or symptoms should not be sedated until a neurosurgical or orthopedic consultation is obtained.
Radiologists employ Gastrografin when evaluating abdominal masses. Gastrografin diluted to a concentration of 1.5% is usually considered a clear liquid. The volume that is administered orally is significant; newborns younger than 1 month of age receive 60 to 90 mL, infants between 1 month and 1 year of age may receive up to 240 mL, and children between the ages of 1 and 5 years receive between 240 and 360 mL. Because sedation or anesthesia should usually be accomplished within a window of 1 to 2 hours after ingestion of the contrast, most “elective” fasting guidelines would be violated; however, the scan must be completed while the Gastrografin is still in the gastrointestinal tract. There are no published data to guide optimal induction or sedation techniques as they relate to aspiration risk in these circumstances. Full strength (3%) Gastrografin is hyperosmolar and hypertonic. All Gastrografin should be diluted to an isosmolar and isotonic 1.5% concentration of neutral pH. There is one case report of 1.5% Gastrografin aspiration in a child with no adverse sequelae; therefore, the risk of using a 1.5% concentration of Gastrografin seems low (Friedman et al., 1986; Wells et al., 1991).
Embolization Procedures
Interventional techniques include nonvascular and vascular interventions (Towbin and Ball, 1988). In vascular interventions, embolization and sclerotherapy have become important techniques for treating vascular malformations, aneurysms, fistulas, and hemorrhage; for accomplishing renal ablation; and for presurgical embolization of hypervascular masses. Percutaneous transluminal angioplasty and fibrinolytic therapy are increasing in pediatric institutions; great success is being reported, even in the smallest infants, and the important contribution that adequate sedation and analgesia can make to ultimate outcome has been recognized (Diament et al., 1985).
Vascular malformations are congenital aberrant connections between blood vessels and may be composed of lymphatic, arterial, and venous connections. These lesions, although present at birth, are often discrete and not clearly visible. As the child grows, the vascular malformation may expand rapidly, growing with the child. This rapid proliferative phase may occur in response to hormonal changes (e.g., pregnancy or puberty), trauma, or other stimuli (Jackson et al., 1993). Vascular malformations may be high-flow or low-flow lesions, depending on which vessels are involved. High-flow lesions include arteriovenous fistulas, some large hemangiomas, and arteriovenous malformations. Particularly with large lesions, high-output cardiac failure and congestive heart failure with the potential for pulmonary edema should be anticipated and sought out in the medical history and physical examination. Low-flow lesions consist of venous, intramuscular venous, and lymphatic malformations. Surgical resection of symptomatic vascular malformations may be hazardous as well as unsuccessful—any vascular element not resected may enlarge and cause further problems.
When embolizing vascular malformations, radiologists often aim to cut off not only the feeding vessels but also the central confluence (nidus) where much of the arterial shunting occurs. Embolic agents include stainless-steel minicoils, absorbable gelatin pledgets and powder, detachable silicone balloons, polyvinyl alcohol foam, cyanoacrylate glue, and ethanol. The choice of agent depends on the clinical situation and the size of the blood vessel. When permanent occlusion is the goal, polyvinyl alcohol foam and ethanol are often employed. Both occlude at the level of the arterioles and capillaries. Medium to small-sized arteries may be occluded with coils, the equivalent of surgical ligation. Particularly in trauma situations, when only temporary occlusion (days) is the goal, absorbable gelatin pledgets or powder are used (Coldwell et al., 1994).
Large hemangiomas may be associated with the coagulopathy of Kasabach-Merritt syndrome. In this condition, the hemangioma traps and destroys platelets and other coagulation factors, resulting in thrombocytopenia and an increased risk of bleeding. As the hemangioma involutes, the coagulation status improves (Mulliken and Young, 1988). A condition described as systemic intravascular coagulation (SIC) can occur after the embolization of extensive vascular malformations. This condition is marked by an elevated prothrombin time (PT) with a decrease in coagulation factors and platelets.
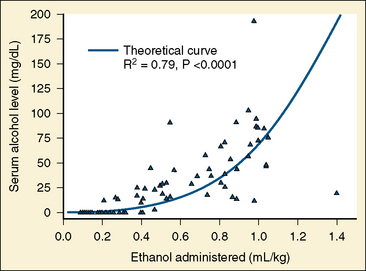
Absolute (99.9%) ethanol is injected in vascular malformations to promote sclerosis. Ethanol may produce a coagulum of blood and cause endothelial necrosis (Becker et al., 1984). Sclerotherapy or embolization with absolute ethanol increases the risk of developing a postprocedure coagulopathy marked by positive D-dimers, elevated PT, and decreased platelets (Mason et al., 2001b). Ethanol causes thrombosis, because it injures the vascular endothelium. Ethanol also denatures blood proteins. Extensive ethanol injections can cause hematuria, and urinary catheters should be inserted to monitor urine output, diuresis, and hematuria. Especially with children scheduled for outpatient surgery, liberal fluid replacement ensures that the hematuria clears before discharge. Ethanol can cause neuropathy and tissue necrosis if it is not injected selectively. Using selective catheterization and direct percutaneous puncture, care is taken not to expose normal blood vessels to the ethanol. In addition to the risk of hematuria, ethanol also can produce significant serum alcohol levels. Mason and others (2000) note that up to 1 mL/kg of ethanol can be administered and that serum ethanol levels have been greater than the intoxication level of .008 mg/dL (Fig. 33-1). Patients with high serum-ethanol levels may be either sedated or extremely agitated, depending on their particular response to intoxication.
Cerebral angiography requires motionlessness as well as exquisite control of ventilation. Anesthetic technique, in choice of agent as well as in control of arterial CO2 tension, may affect cerebral blood flow and hence the quality of the scan. Cerebral angiography may be performed in children for the diagnosis or follow-up study of Moyamoya disease, and these children should have anesthetic techniques that minimize the risk of transient ischemic attacks (TIAs) and stroke during the procedure (Soriano et al., 1993). Other considerations include controlled hypercarbia to promote vasodilation and facilitate access and visualization of the vasculature for the radiologist. In the event of vasospasm or difficult access of small, torturous vessels, locally administered (through the catheter) nitroglycerin in small doses (25 to 50 mcg) may facilitate visualization and access. Occlusion of the venous portion of the arteriovenous malformation (AVM) without complete occlusion of the arterial inflow vessels could result in acute swelling and bleeding. Vascularity reduction through occlusion of major feeder vessels is the goal of embolizing large AVMs before planned surgical excision. This may be accomplished as a staged procedure over several days, involving repeated anesthetics or sedation sessions.
Angiographic imaging may be enhanced through the use of glucagon. Glucagon is efficacious for digital subtraction angiography, visceral angiography, and selective arterial injection in the viscera. When needed, glucagon is administered intravenously in divided doses of 0.25 mg to a maximum of 1 mg. Risks include glucagon-induced hyperglycemia, vomiting (particularly when given rapidly), gastric hypotonia, and provocation signs of pheochromocytoma (McLoughlin et al., 1981; Chernish et al., 1990; Jehenson, 1991). Children who receive glucagon should routinely receive prophylactic antiemetics.
The ability to intermittently assess neurologic function and mental status is invaluable during embolization procedures, but it may not be practical in children because of fear, pain, and movement. General anesthesia permits easier control of blood pressure and ventilation and eliminates the concern about patient movement. For children, general anesthesia is often preferred when performing high-risk procedures that require immobility and periods of breath-holding. Preprocedural assessment should include any history of seizures, bleeding, treatment with anticonvulsants or anticoagulants, neurologic symptoms, and evaluation of intracranial-pressure status. It is important to determine whether the patient has had any TIAs or has evidence of cerebrovascular occlusion. Vasodilator agents (calcium channel blockers) or nitrate derivatives may need to be administered after embolization. Because many patients are anticoagulated during the procedure, a preoperative coagulation profile should be obtained. A variety of anticoagulants may have to be on hand as well to prophylaxis for thrombosis (Bidabe et al., 1990).
Morbidity associated with embolization is not negligible. Arteriovenous malformations (AVMs) involving the head and neck often require cannulation of the external carotid artery branches and the thyrocervical trunk. All patients scheduled for embolization should be typed and cross-matched for blood. Those patients who undergo embolizations of AVMs of the head and neck are at risk for stroke, cranial nerve palsies, skin necrosis, blindness, infection, and pulmonary embolism (Riles et al., 1993). It is important to assess and document full return of neurologic status after the patient is extubated.
Magnetic Resonance Imaging
MRI is employed for the evaluation of neoplasms, trauma, skeletal abnormalities, and vascular anatomy (Barnes, 1992). MRIs of the brain are often performed to evaluate developmental delay, behavioral disorders, seizures, failure to thrive, apnea and cyanosis, hypotonia, and mitochondrial or metabolic disorders. Magnetic resonance angiography and venography (MRA and MRV) are especially helpful in evaluating vascular flow and can sometimes replace invasive catheterization studies for follow-up or initial evaluations of vascular malformations, interventional treatment, or radiotherapy (Edelman and Warach, 1993). Functional MRI (fMRI) is an evolving technology that measures the hemodynamic or even metabolic response related to neural activity in the brain or spinal cord. fMRI is often able to localize sites of brain activation and is now dominating brain-mapping techniques because of its low invasiveness and lack of radiation exposure. Some fMRI studies require cognitive facility and are typically interactive with a conscious and responsive patient. fMRI studies on children who are unable to respond appropriately either because of age or cognitive compromise are challenging—how that will evolve in pediatric anesthesiology practice is not yet clear.
Historically, the anesthetic management of children in the MRI suite has been highly dependent and somewhat limited by MRI-compatible monitors and anesthesia gas machines. (Karlik et al., 1988; Menon et al., 1992; Tobin et al., 1992). The American College of Radiology (ACR) established guidelines to minimize the risk of MRI-related mishaps but did not address the needs of the anesthesiologist (Kanal et al., 2007). These guidelines were written in response to fatalities that had occurred when loose, nonferrous oxygen cylinders became projectiles when brought inadvertently into the MRI suite with a patient in the bore of the magnet (Chaljub et al., 2001). In 2008 the ASA assembled a task force composed of anesthesiologists and a radiologist with MRI expertise. This Task Force on Anesthetic Care for Magnetic Resonance Imaging created a document entitled the Practice Advisory on Anesthetic Care for Magnetic Resonance Imaging (ASA Task Force on Anesthetic Care for MRI, 2009). This document establishes important recommendations for safe practice as well as consistency of anesthesia care in the MRI environment. Most important, the Practice Advisory includes all sedation, including monitored anesthesia care, general anesthesia, critical care, and ventilatory support. Conditions of high-risk imaging were defined as imaging in patients with medical or health-related risks or as equipment-related, procedure-related, or surgery-related risks. MRI-guided interventions, cardiac imaging, and airway imaging were all identified as high-risk procedures. The Task Force was designed to promote patient and health care provider safety, prevent MRI mishaps, recognize limitations in physiologic monitoring, optimize patient management, and identify potential equipment and health risks (ASA Task Force on Anesthetic Care for MRI, 2009).
Anesthetic management also depends on the availability of support personnel, equipment and monitors; the personal style and comfort level of the anesthesiologist; and the patient’s particular medical history. Requiring a general anesthetic solely to ensure motionless conditions for a radiologic imaging study is often a frightening concept for parents. Parents often equate pain and surgical interventions with the need for anesthesia and are reluctant to expose their child to a general anesthetic for the sake of an MRI study. Children under the age of 5 years most commonly require moderate or deep sedation or an anesthetic to ensure motionless conditions. Ketamine, narcotics, or benzodiazepines are generally unsuccessful. Pentobarbital, propofol, and dexmedetomidine offer successful alternatives to inhalation anesthesia (Mason et al., 2001a, 2004, 2008a, 2008b; Guenther et al., 2003, Yamamoto, 2008). One technique for general anesthesia is to perform an inhalation induction followed by placement of an LMA. During the scan, the patient maintains spontaneous ventilation. Lidocaine gel (2%) on the LMA cuff is a useful adjunct, because it decreases the incidence of sore throat and retching (Chan and Tham, 1995; Keller et al., 1997). A retrospective study of 200 patients demonstrated the usefulness of this approach (Brimacombe et al., 1995). In children with upper respiratory infections, there was a lower incidence of mild bronchospasm, laryngospasm, breath-holding, and major oxygen desaturation (less than 90%) in the group with LMAs compared with the group that received endotracheal anesthesia (Tait et al., 1998). Temperature monitoring can be accomplished by liquid crystal display (skin temperature). There is still no means of monitoring core temperature, because any temperature probe would risk heat generation and thermal burns to the patient.
Anesthesiologists must be aware of many personal items taken for granted—clipboards, pens, watches, scissors, clamps, credit cards, eyeglasses, and paper clips that should not be in the MRI field (Karlik et al., 1988; Menon et al., 1992; Tobin et al., 1992). Conventional ECG monitoring is not possible, because as the lead wires traverse the magnetic fields, image degradation occurs and, most importantly, the ECG leads heat and cause burns on the patient. Fiberoptic ECG monitoring is necessary to minimize the risk of patient burn. Even with fiberoptic cables, it is important to recognize that the connections between the ECG pads and the telemetry box are still hardwired, and careful attention must be paid to prevent frays, overlap, exposed wires, and knots in the cables (Shellock, 1989). In order to prevent injury to the patient, care must be used to avoid creating a conductive loop between the patient and a conductor (e.g., ECG monitoring and gating leads, plethysmographic gating wire, and fingertip attachments). During the scan, no exposed wires or conductors can touch the patient’s skin, and no imaging coil can be left unconnected to the magnet. Pulse oximeters are also not conventional to the operating room environment; rather they are fiberoptic. Failure to remove the conventional pulse oximeter probes and adhesives has resulted in second- and third-degree burns (Shellock, 1989; Brow et al., 1993).
Average noise levels of 95 dB have been measured in a 1.5-T MRI scanner; this level of noise is comparable with the noise level of very heavy traffic (92 dB) or light road work (90 to 110 dB). Exposure to this level of noise has not been considered hazardous if it is limited to less than 2 hours per day (Gangarosa et al., 1987). There are case reports, however, of both temporary and permanent hearing loss after an MRI scan (Brummett et al., 1988; Kanal et al., 1990). Three-Tesla magnets offer the advantage of less image degradation and improved neuroskeletal and musculoskeletal imaging. As the field strength increases, so does the noise (Hattori et al., 2007). In fact, the peak sound-pressure level of a 3-T magnet exceeds 99 dB, the level approved by the International Electrotechnical Commission. Noise reduction did not differ between earplugs and headphones, although the combination of both was more effective at reducing sound (Hattori et al., 2007). Earplugs or MRI-compatible headphones should be offered to all pediatric patients and are required for all patients imaged in the 3-T MRI scanner. fMRI provides additional challenges in the 3-T environment; the noise of the 3 T can interfere with the acoustic stimulation generated for purposes of obtaining the fMRI (Ravicz et al., 2000; Menendez-Colino et al., 2007). Video goggles compatible with the 1.5-T and 3-T environments (Resonance Technology; Los Angeles, California) may be worn by the patient during MR imaging to provide a three-dimensional virtual reality system complete with audio integration. The introduction of this integrated audio-video headset has revolutionized the ability to offer distraction to patients, many of whom are able to tolerate imaging without adjuvant sedation or anesthesia.
Although studies in mice and dogs suggest that exposure to magnetic fields may increase body temperature, it is unlikely that static magnetic fields up to 1.5 T have any effect on core body temperature in adult humans (Sperber et al., 1984; Shellock et al., 1986; Shuman et al., 1988). However, this may be of greater concern in infants and small children, especially in cases like cardiac MRI studies that require a long scan time. The specific absorption rate (SAR) is measured in watts per kilogram and is used to follow the effects of radiofrequency heating. The FDA allows an SAR of 0.4 W/kg averaged over the whole body (Department of Health and Human Services, FDA, 1982). Ex vivo exposure of large metal prostheses to fields over six times that experienced in MRI have not revealed any appreciable heating (Davis et al., 1981). To date, there has been no conclusive evidence that radiofrequency is a significant clinical issue in magnets up to 3 T.
The biological effect of MRI should be considered when offering parent-present induction; however, there are no reports implicating MRI-caused chromosomal aberrations. Studies in amphibians demonstrate that exposure to a 4-T magnetic field does not cause any defects in embryologic development (Prasad et al., 1990). Most hospital’s MRI machines are 1.5 T. Despite these studies, pregnant patients or family members are not usually allowed into the MRI scanner. MRI scans during pregnancy are discouraged by the ACR during the first and second trimesters unless fetal imaging is required or the MRI is necessary for emergent medical care (Woodard et al., 2006).
Additional safety issues for MRI include implanted objects (i.e., cardiac pacemakers), ferromagnetic attraction that causes objects to become projectile, noise, biological effects of the magnetic field, thermal effects, equipment issues, and claustrophobia. Some stainless steel may contain ferritic, austenitic, and martensitic components (Dujovny et al., 1985; Persson et al., 1985). Martensitic alloys contain fractions of a crystal phase known as martensite, which has a body-centered cubic structure, is prone to stress corrosion failure, and is ferromagnetic. Austenite is formed in the hardening process of low carbon and alloyed steels and has ferromagnetic properties. Iron, nickel, and cobalt are also ferromagnetic. For this reason, the components of any implanted device should be carefully researched before entering the magnet. Stainless steel or surgical stainless objects interacting with an external magnetic field may produce translational (attractive) and rotational (torque) forces. Intracranial aneurysm clips, cochlear and stapedial implants, shrapnel, intraorbital metallic bodies, and prosthetic limbs may move and potentially dislodge. Special precaution should be taken with cochlear implants in the 3-T environment, because unremovable magnets may suffer demagnetization in the scanner. These patients should only undergo imaging in the 3-T scanner after special precautions are taken (Majdani et al., 2008). Some eye makeup and tattoos may contain metallic dyes and therefore cause ocular, periorbital, and skin irritation (Scherzinger and Hendee, 1985; Prasad et al., 1990). Some tissue expanders employed in reconstructive surgery have a magnetic port to help identify the location for intermittent injections of saline (Liang et al., 1989). Bivona tracheostomy tubes usually contain ferrous material (although it is not specified in the package insert) and should be replaced with Shiley tracheostomy tubes before patients enter the MRI environment.
Cardiac pacemakers present a special hazard in and around the MRI scanner, especially in patients who are dependent on pacemakers. Most pacemakers have a reed relay switch that can be activated when they are exposed to a magnet of sufficient strength (Pavlicek et al., 1983). This activation could convert the pacemaker to the asynchronous mode. There are at least two known cases of patients with pacemakers who died from cardiac arrest while in an MRI scanner. The autopsy of one patient determined that the death was the result of an interruption of the pacemaker in the magnetic environment (Center for Disease and Radiological Health, 1989). In addition to the risk of pacemaker malfunction, there is also the chance that torque on the pacer or pacing leads may create a disconnect or microshock (Erlebacher et al., 1986). Recent studies demonstrate that with careful preparation, select patients with permanent pacemakers and implantable cardioverter defibrillators may safely undergo imaging in the 1.5-T environment without any inhibition or activation of their device (Nazarian et al., 2006). The ACR formed a Blue Ribbon Panel on MRI Safety in 2001 to review existing MRI safety practices and issue new guidelines. The ACR Guidance Documents for Safe MR Practices was published in 2002 and updated in 2004 and again in 2007 (Kanal et al., 2007). Safety concerns related to pediatric MRI were specifically addressed in the 2007 document, with specific emphasis on patient screening, sedation, and monitoring issues. Implanted cardiac pacemakers or implantable cardioverter defibrillators were considered a relative contraindication to MRI and only scanned in locations staffed with radiologists and cardiologists of appropriate expertise. The recommendation was for radiology and cardiology personnel, along with a fully stocked crash cart, to be readily available, as well as a programmer to adjust the device if necessary. After the MRI, a cardiologist should confirm function of the device and recheck it within 1 to 6 weeks. In general, heart valves are not ferromagnetic and are not a contraindication to MRI. It is critical that everyone entering the vicinity of the MRI scanner fills out a screening form that specifically lists every possible implantable device, alerting the MRI staff to any potential hazards.
The magnetic field may affect the electrocardiogram. The changes in the T wave are not the results of biological effects of the magnetic field but rather to superimposed induced voltages. This effect of the magnetic field on the T wave is not related to cardiac depolarization, because no changes to the P, Q, R, or S waves have ever been observed in patients exposed to fields up to 2 T. There are no reports of MRI affecting heart rate, ECG recordings, cardiac contractility, or blood pressure (Beischer, 1969; Tenforde et al., 1983; McRobbie and Foster, 1985; Gulch and Lutz, 1986). One study, however, found that humans exposed to a 2-T magnet for 10 minutes developed a 17% increase in the cardiac cycle length (CCL). The CCL represented the duration of the RR interval. The CCL reverted to preexposure length within 10 minutes of removing the patient from the magnetic field (Jehenson et al., 1988). The implications of this finding are unclear. This change in CCL in patients with normal hearts may be of no consequence. The implications of this finding for patients with fragile dysrhythmias or sick sinus syndrome, however, have yet to be determined.
In the presence of an external magnetic field, a ferromagnetic object can develop its own magnetic field and become a projectile object. The attractive forces that are created between the intrinsic and extrinsic magnetic fields can propel the ferromagnetic object toward the MRI scanner. Special note should be made of the magnet’s strength. Over the past few years, 1.5-T magnets have been supplanted by 3-T magnets. The field strength and magnetic force generated by a 3-T MRI scanner are unforgiving to the careless or inadvertent introduction of a ferrous object into the environment. Placing a magnet outside the MRI scanner can be a helpful but crude and sometimes inaccurate way to test any objects. If the object is not attracted to the magnet, this is not an absolute indication that there is no ferrous material present. A positive attraction, however, provides critical information to the anesthesiologist. Some unusual objects that have found their way into the MRI suite only to become projectile objects include a metal fan, a pulse oximeter, shrapnel, a wheelchair, a cigarette lighter, a stethoscope, a pager, a hearing aid, a vacuum cleaner, a calculator, a hair pin, an oxygen tank, a prosthetic limb, a pencil, an insulin infusion pump, keys, watches, and steel-tipped or heeled shoes (Kanal, 1992). Small objects can usually be easily removed from the magnet, but large objects may have so much attractive force to the MRI scanner that it is impossible to remove them by manual force. In these circumstances, quenching the magnet may be the only way to release the object once it is attached to the scanner. Quenching the magnet eliminates the magnetic field over a matter of minutes. This process is not without substantial risk; as helium gas is vented, condensation and considerable noise fill the suite. All personnel are required to vacate the suite during a quench, because there is a risk of hypoxic conditions if the helium should inadvertently enter the room.
In 2008, the JCAHO recognized the potential and existent hazards of the MRI environment when they published the Sentinel Event Alert (The Joint Commission, 2009). This alert identified 5 MRI-related cases of injury and 4 MRI-related deaths—one from a projectile and three cardiac events. The alert was designed to prevent accidents and injuries in the MRI suite, specifically identifying eight types of possible injuries (Box 33-1).
Box 33-1 The Joint Commission Sentinel Event Alert
Preventing accidents and injuries in the mri suite
From The Joint Commission: Preventing accidents and injuries in the MRI suite, 2008. Available at http://www.jointcommission.org/SentinelEvents/SentinelEventAlert/sea_38.htm.
Special attention has recently been devoted to the topic of the IV gadolinium contrast that is used to enhance MR images. Gadolinium (in the form of gadopentetate dimeglumine) was approved by the FDA in 1998 as a contrast agent for MRI. With an elimination half-life of between 1.3 and 1.6 hours, gadolinium is excreted via the kidneys after forming a complex with chelating agents (Baker et al., 2004). Adults and children have similar elimination half-lives of gadolinium, with 95% excreted within 72 hours (Weinmann et al., 1984; Van Wagoner et al., 1990). Because gadolinium does not contain iodine, it does not produce an osmotic load and is generally considered safer and less allergenic than iodine (Brasch, 1993; Murphy et al., 1996; Dorta et al., 1997). Important warnings from the FDA suggest that patients with advanced kidney failure are at risk of developing nephrogenic systemic fibrosis or nephrogenic fibrosing dermopathy after gadolinium-based MR contrast agents. The FDA first notified health care professionals and the public about this risk in June 2006. There are currently five gadolinium-based contrast agents approved for use in the United States: Magnevist (gadopentetate dimeglumine), Ominiscan (gadodiamide); OptiMARK (gadoversetamide); MultiHance (gadobenate dimeglumine); and Prohance (gadoteridol). To date there are no reports of nephrogenic systemic fibrosis in those with normal kidney function or those with mild-to-moderate kidney insufficiency. The FDA suggests that gadolinium only be used when absolutely necessary in those with advanced kidney failure and that these patients undergo dialysis after vascular studies that require a large amount of gadolinium contrast.
Some patients experience claustrophobia and have difficulty cooperating during the study. Anxiety reactions are estimated to occur in 4% to 30% of patients (Granet and Gelber, 1990; Melendez and McCrank, 1993). As already discussed, new advances in technology now offer patients with anxiety or claustrophobia distraction with MRI-safe video goggles (Resonance Technology; Los Angeles, California). Patients with extreme skeletal abnormalities such as advanced scoliosis or flexion contractures, although motivated, may be unable to lie motionless or supine on the solid, uncushioned MRI table for the extended duration of a spinal MRI. These patients may require general anesthesia for positioning and comfort or may need adjunctive pain medication.
Nuclear Medicine
Nuclear medicine is one of the oldest functional imaging disciplines. Scans obtained through nuclear medicine are useful for identification of epileptic foci in refractory epilepsy, evaluation of cerebrovascular disease (e.g., Moyamoya disease), and the evaluation of cognitive and behavioral disorders (O’Tuama and Treves, 1993). Anesthesiologists become involved when the child’s medical history suggests that procedural sedation would not be appropriate. In order to complete these scans, the child must remain motionless for at least 1 hour.
The two most common nuclear medicine studies that require the administration of an anesthetic are single-photon emission computed tomography (SPECT) scans and positron emission tomography (PET) scans. SPECT scans use single-photon gamma-emitting radioisotopes and rotating gamma cameras to produce three-dimensional brain images. SPECT scans involve the use of radiolabeled technetium-99 (half-life, 6 hours), which has a high rate of first-pass extraction as well as intracellular trapping in proportion to regional cerebral blood flow (Chiron et al., 1989). SPECT scans are ideal when seeking seizure foci, and they often precede surgical resection of an identified focus. The technetium radionuclide is ideal because it remains intracellular and can be visualized on scan hours after a seizure has occurred. Ideally, the child should be scanned within 1 to 6 hours of the seizure. The radionuclides are physiologically harmless and not allergenic. Caregivers should, however, wear gloves to minimize contact with radiation-containing secretions.
PET scans use positron emission tomography and radionuclide tracers of metabolic activity, such as oxygen or glucose metabolism (Chugani, 1993; Griffeth et al., 1993). Unlike SPECT scans, PET scans should be performed during the seizure itself. Because of the short half-life of the glucose tracer (110 minutes), the scan is best completed during the seizure or within 1 hour thereafter.
Radiation Oncology
Fractionated radiation therapy is the strategy of dividing the total radiation therapy course into discrete daily sessions, allowing normal tissue repair between sessions while the tumor burden is lessened or destroyed. Hyperfractionated, or multiple-session daily radiation therapy, is a modality reported primarily in adults for head and neck cancers. The rationale for twice-daily fractionation in children is that fractionation to growing bone in rats reduces the growth deficit by 25% to 30%; the hope is that other normal tissues may be similarly spared during growth (Eifel, 1988; Eifel et al., 1990). Whereas one successful approach has been to give infants an initial formula feeding 6 hours before their first treatment and keep them fasting until recovery from their second anesthetic (6 hours after the first), with the current liberalization of fasting guidelines it is preferable to give children clear liquids during their recovery from the first anesthetic and keep them fasting thereafter for 4 hours before the second anesthetic (Menache et al., 1990).
Stereotactic radiosurgery (with a Gamma Knife) is a major advance in the treatment of select intracranial arteriovenous malformations and tumors in children (Loeffler et al., 1990). Stereotactic radiosurgery differs from external-beam radiotherapy in several important ways. A focused, single, large fraction of radiation is used instead of smaller, daily fractions. Stereotactic radiosurgery uses gamma rays of relatively weak intensity that are produced by 201 cobalt-60 sources that intersect at a single point where all 201 beams converge to destroy tumors, vascular malformations, or abnormal tissue sites within the brain. Normal brain tissue surrounding the abnormality is therefore relatively protected from radiation effects. In pediatrics as well as in adults, most radiosurgery originally concentrated on treatment of small, histologically benign lesions such as vascular malformations, acoustic neuromas, and pituitary adenomas. This has more recently been expanded to include malignant tumors such as solitary metastases, ependymomas, glioblastomas, and several other tissue types. For optimal results, the tumor’s volume should be small (14 cm3) (Coffey et al., 1992).
Calculations for dose and the three-dimensional coordinates for the beam may take several hours to compute after the initial radiologic study and head-frame placement. Some patients do well with sedation and spontaneous breathing; however, younger patients are usually mechanically ventilated. An initial CT scan is followed by computer calculations. Once the calculations are complete, the patient is transferred to the radiosurgery suite. After the irradiation, the patient is allowed to emerge from the anesthetic (Loeffler et al., 1990). The most common perioperative problem is nausea and vomiting, probably because of radiation sensitivity of the chemoreceptor trigger zone.
Total body irradiation (TBI) is generally performed twice a day over a 6-week period. It is usually in preparation for a bone-marrow transplant. For those patients who require anesthesia, the commitment is large—not only for the twice daily anesthetic but also for the coincident fasting status required. As these patients progress with their TBI treatment and become more immunocompromised, there is an increased risk of acquiring an illness during the course of treatment. Vomiting, respiratory illness, poor nutrition, or hypovolemia are all possible. Cancelling a TBI treatment because of an associated illness is discouraged, because it disrupts the course of treatment and could compromise the patient’s overall prognosis. Although anesthesiologists are wary of the risks of aspiration, both sedation and general endotracheal anesthesia have been found to decrease the incidence of vomiting with TBI (Westbrook et al., 1987; Whitwam et al., 1978).
Clinic and Office Procedures
Endoscopic Procedures
Gastrointestinal endoscopy has become a routine part of patient care, and as such it constitutes the bulk of procedures performed by a pediatric gastroenterologist (Fox, 1998). Depending on the patient and the type of procedure contemplated (therapeutic vs. diagnostic), children may require no sedation, minimal to moderately deep sedation, or general anesthesia. Minimal sedation may impair cognitive function and coordination while ventilatory and cardiovascular functions are relatively unaffected. However, pediatric patients often are uncooperative and do not tolerate endoscopic procedures with minimal or moderate sedation, necessitating deeper sedation or general anesthesia to successfully accomplish the procedure (American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists, 2002). Over the past 15 years, the volume of endoscopic procedures has increased by twofold to fourfold in the adult community, and has most likely increased at a similar rate in children (Cohen et al., 2006).
The inability to independently maintain ventilatory function and to respond purposefully increases the risk involved with deep sedation. Although some recommend that deep sedation be limited to anesthesiologist delivery only, gastroenterologists have demonstrated that they too are able to safely administer sedation (Saint-Maurice and Hamza, 1992; Wolfe and Rao, 1992; Hassall, 1993, 1994; Dillon et al., 1998; Bouchut et al., 2001; Koh et al., 2001; Paspatis et al., 2006). Propofol, considered by the ASA to be within the scope of anesthesia practice only, is considered by the American Society for Gastrointestinal Endoscopy (ASGE) to be an acceptable sedative for gastroenterologist administration (ASGE, 2002; Walker et al., 2003; Rex et al., 2005; Perera et al., 2006; Rex, 2006; Abu-Shahwan and Mack, 2007). Propofol is an important sedative for gastroenterologists, and it is estimated that one quarter of all adult endoscopies are performed with propofol sedation (Cohen et al., 2006). Children with more complex medical problems, anticipated airway difficulties, morbid obesity, or behavioral problems can undergo their procedures in the operating room. Regardless of the site of the procedure, all patients scheduled for endoscopy should be evaluated in advance to confirm that they are appropriate candidates. In addition, the anesthetic technique depends on the procedure, the patient, and the skill of the endoscopist, as well as the limitations and capabilities of the endoscopy suite. There is a wide range of sedation agents for children as well as adults, and there is no optimal sedation regimen for either age group (Lightdale et al., 2007).
Procedural sedation is readily achieved with an IV anesthetic combining a sedative (e.g., midazolam), opioid (e.g., fentanyl, alfentanil, remifentanil, or dexmedetomidine), and a hypnotic (e.g., propofol). Spontaneous ventilation without the patient’s airway being intubated has been shown to be a safe and effective technique (Bouchut et al., 2001; Koh et al., 2001; Koroglu et al., 2006). The majority of complications are respiratory and usually occur during an esophagogastroduodenoscopy (EGD). These complications include apnea, laryngospasm, bronchospasm, and airway obstruction. Bradycardia has been observed with dexmedetomidine use in children. Most problems resolve after withdrawal of the endoscope and positive pressure ventilation with a tightly-fitting mask; however, some patients may require endotracheal intubation to secure an airway and safely complete the procedure.
Esophagogastroduodenoscopy
A majority of the respiratory complications noted previously occur during EGD when compared with colonoscopy, especially in infants and younger children. It has been suggested that this results from a combination of factors, including the large size of the endoscope and partial airway obstruction resulting in hypoxemia. Abdominal distention as a result of air introduced into the stomach may impair diaphragmatic excursion, leading to hypoventilation. This has led several groups to select 6 months of age, as a result of the higher respiratory complication rate in this age group, as the time before which general anesthesia with endotracheal intubation is required for the procedure (Wolfe and Rao, 1992; Koh et al., 2001).
Endoscopic Retrograde Cannulation of the Pancreas
Although many institutions report success of procedural sedation in pediatric patients undergoing endoscopic retrograde cannulation of the pancreas, general anesthesia with endotracheal intubation may make the procedure easier to perform, especially if the procedure is of long duration, the patient has significant comorbid diseases, or the procedure is performed with the patient in the prone position (Teng et al., 2000; Prasil et al., 2001).
Psychiatric Interviews
IV sodium amobarbital has a long history as an adjunct to psychotherapy, having found its peak use during World War II and immediately thereafter in helping soldiers deal with the stresses and trauma of combat (Zonana, 1979). Although this technique has enjoyed a resurgence for diagnostic and therapeutic interventions in adults, pediatric reports are rare. Weller et al. (1985) described the successful use of sodium Amytal in psychiatric interviews in prepubescent children. The induction of a tranquil state and sedation (e.g., slurred speech, a sense of fatigue, difficulty counting backwards, and basal vital signs) is similar to the anxiolysis achieved during monitored anesthesia care. A bispectral index (BIS) monitor may prove to be a useful adjunct as well (Palmer et al., 2001). The psychiatric interview process under these conditions is fascinating to participate in, if only as an observer. Memory retrieval, for instance the uncovering of relationships between current psychopathology and earlier traumatic life events, or symptom removal via therapeutic suggestions, are examples of interventions facilitated by the pharmacologically induced relaxed state made possible by the anesthesiologist during the interview.
Safety issues for patients and their anesthesiologists
Use of Intravascular Contrast Media
In a comprehensive review, Goldberg (1984) noted that approximately 5% of radiologic examinations with radiocontrast media (RCM) are complicated by adverse reactions, with one third of these being severe and requiring immediate treatment. Reactions occur most commonly in patients between 20 and 50 years of age and are relatively rare in children. The male/female ratio is about 2.5:1, not dissimilar to the gender distribution of other allergies such as latex, aspirin, and neuromuscular blocking agents. With a history of atopy or allergy, the risk of a reaction is increased from 1.5- to 10-fold. Reactions vary from mild, subjective sensations of restlessness, nausea, and vomiting to a rapidly evolving, angioedema-like picture accompanied by respiratory distress, bronchospasm, arrhythmias, and cardiac arrest. Because of the high osmolar concentrations of these agents (often higher than 1000 mOsm, and sometimes more than 2000 mOsm), caution should be exercised with patients who have a limited cardiovascular reserve (such as patients in congestive heart failure or those with cardiomyopathy). In addition, volume-depleted young children who have been kept fasting for prolonged intervals or who have had bowel preparations should be prehydrated before RCM administration. Those patients who are dependent on a full intravascular volume status (e.g., patients with sickle cell disease or restricted pulmonary circuit volume with cyanotic congenital heart disease and patients with arteriovenous shunts) should be monitored carefully for an initial rise in filling pressures and intravascular volume and subsequent diuresis after an osmolar load. Patients with impaired excretory mechanisms, such as those in renal failure, must be monitored closely after high osmolar loads. Low-osmolar RCM administrations are relatively safe with regard to life-threatening reactions, but moderate non–life-threatening reactions requiring some treatment occur 0.2% to 0.4% of the time, and a severe life-threatening reaction can occur in 0.04% of patients (Thomsen and Bush, 1998).
RCM contains iodine, because iodine’s high density and low toxicity make it an ideal agent for visualization and differentiation. The iodine is filtered through the glomeruli and is not reabsorbed by the glomeruli or the tubules. Because the contrast agent is hypertonic relative to plasma, an initial hypertensive response is usually followed by a hyperosmotic diuresis with the potential for hypotension. Equilibration with the extracellular fluid compartment occurs within 10 minutes, heralded by the onset of diuresis. Special attention should be paid when administering RCM with iodine to any child with a history of congestive heart failure. Patients with hepatic or renal dysfunction should be observed closely for signs of impaired excretion of the RCM. In sickle cell disease, the increase in blood osmolarity may precipitate shrinkage, clumping, and ultimately sickling of erythrocytes and vascular occlusion. Sickled cells are known to align with external magnetic fields to which they are exposed; it is unknown how this theoretic concern compares, for example, with the normal forces of deformation imposed on red cells of patients with sickle cell disease in their normal course through the vascular tree (Kanal et al., 1990).
Gadolinium diethylenetriaminepentaacetic acid (DTPA) is a low osmolar ionic contrast medium used for MRI, with a slower clearance in neonates and young infants than adults, yielding longer windows for imaging (Elster, 1990). Free gadolinium has a biological half-life of several weeks, with uptake and excretion taking place in the kidneys and liver. Unfortunately, free gadolinium is quite toxic and is therefore chelated to another structure that restricts the ion and decreases its toxicity. The most common adverse reactions are nausea, vomiting, hives, and headache. Local injection site symptoms include irritation, focal burning, or a cool sensation. Transient elevations in serum bilirubin (3% to 4% of patients) have been reported, and a transient elevation in iron for Magnevist and Omniscan (15% to 30% of patients) occurs, which tends to reverse spontaneously within 24 to 48 hours (Van Wagoner and Worah, 1993). Anaphylactoid reactions occur on the order of 1:100,000 to 1:500,000 and are more rare (< 1:100,000 doses) in children.
The treatment of severe allergic reactions, whether anaphylactoid or anaphylactic, is no different than for any other allergic reaction. Epinephrine, aminophylline, atropine, diphenhydramine, and steroids have all been employed in order to control varying degrees of adverse reactions. A patient who requires RCM administration and who has had a previous reaction to RCM has an increased (35% to 60%) risk for a reaction on reexposure. Pretreatment of these high-risk patients with prednisone and diphenhydramine 1 hour before RCM administration reduces the risk of reactions to 9%; the addition of ephedrine 1 hour before RCM administration further reduces the rate to 3.1% (Kelly et al., 1978; Greenberger, 1984).
In 2007, the ACR updated their practice guidelines for the use of intravascular contrast media that they published in the Manual on Contrast Media (ACR, 2008). Iodinated low-osmolality contrast media have a lower incidence of adverse effects as compared with ionic high-osmolality contrast media. The management of acute reactions in children is clearly outlined by the ACR (Box 33-2).
Box 33-2 Management of Acute Reactions in Children
Facial edema
Laryngeal edema or bronchospasm
Pulmonary edema
Hypotension with tachycardia
Hypotension with bradycardia (vagal reaction)
IM, Intramuscular; IO, intraosseous; IV, intravenous; SC, subcutaneous; PO, orally.
From American College of Radiology 2008 “Manual on Contrast Media.” Version 6. Reston, Va, p 75-76.
Ionizing Radiation
Radiation exposure is directly proportional to the duration of the procedure and inversely proportional to the square of the distance from the source. Henderson et al. (1991) monitored the radiation exposure of 16 pediatric anesthesia fellows during a – month period. Fellows assigned to the cardiac catheterization laboratory had a fluoroscopy exposure time of 14 to 85 minutes per case, typically for two to three cases per day. For these anesthesiologists, badge readings ranged from 20 to 180 mrem/month. All noncardiac anesthesia fellows had undetectable (<10 mrem/month) levels. All fellows wore lead aprons, 50% wore a thyroid shield, and one stepped at least 10 feet away from the source during every exposure; this latter fellow had a reading of 30 mrem, despite having spent 26 hours in the catheterization laboratory. The annual maximum permissible dose (MPD) for nonradiation workers (including anesthesiologists) is 100 mrem or 1 mSievert (mSv, Systeme Internationale Units). For comparison, the MPD for radiation workers is 50 mSv annually and 10 mSv times age cumulatively. MPD during pregnancy for radiation workers (per gestation) is 5 mSv. Limits of exposure and comparative sources of exposure are listed in Table 33-2.
| Limits for Exposures | Exposure | Range |
| Occupational dose limit (U.S. NRC) | 5000 mrem/year | |
| Occupational exposure limits for minors | 500 mrem/year | |
| Occupational exposure limits for fetus | 500 mrem | |
| Source of exposure | ||
| Average dose to U.S. public from all sources | 630 mrem/year | |
| Average dose to U.S. public from natural sources | 330 mrem/year | |
| Average dose to U.S. public from medial sources | 53 mrem/year | |
| Chest x-ray | 8 mrem | 5-20 mrem |
| Extremities x-rays | 1 mrem | |
| Dental x-ray | 10 mrem | |
| Head/neck x-ray | 20 mrem | |
| Cervical spine x-ray | 22 mrem | |
| Lumbar spinal x-rays | 130 mrem | |
| Pelvis x-ray | 44 mrem | |
| Hip x-ray | 83 mrem | |
| Upper GI series | 245 mrem | |
| Lower GI series | 405 mrem | |
| CT (head and body) | 1100 mrem | |
| Expected 50% death without medical attention | 400,000 mrad | 300,000-500,000 mrem |
| Doubling dose for genetic effects | 100,000 mrad | |
| Doubling dose for cancer | 500,000 mrad | (8% per mSv, natural level at 20%) |
| Dose for increase cancer risk 1:1,000 | 1250 mrem | (8% per mSv) |
| Consideration of therapeutic abortion threshold (dose in utero) | 10,000 mrem |
1 mrem = 10 mSv; 1 rad is the amount of radiation to deposit 0.01 joules of energy per kilogram.
NRC, Nuclear Regulatory Commission; U.S., United States.
High-Intensity Magnetic Fields
MRI exposes the patient (and the health care workers surrounding the patient) to a static magnetic field, a rapidly switched spatial-gradient magnetic field, and radiofrequency magnetic fields. The static magnetic field, which causes alignment of unpaired tissue protons, may cause movement of ferromagnetic devices such as vascular clips, ventricular shunt connectors, casings for pacemakers, and control devices for pacemakers. Metallic devices in other areas, particularly when invested with fibrous tissue, are less problematic (Shellock and Crues, 1988; Shellock, 1989). As mentioned previously, tissue expanders may have magnetic ports to facilitate identification of the injection site. Despite their low mass, such ports have a potential for torque and movement in the presence of a strong magnetic field; therefore, the specific type of tissue expander should be identified before patient evaluation in an MRI (Liang et al., 1989). Assessment of risk in patients with implants or other possibly ferromagnetic devices or objects consists of a careful history including penetrating wounds, physical examination to look for scars, and possibly a plain radiograph of the region in question (Pohost et al., 1992). Other concerns have been increased blood pressure, cardiac arrhythmias, and impaired mental function. Although described or theorized on an experimental basis, little clinical documentation is available.
The magnetic field generates an electrical current two to three orders of magnitude less than a defibrillator (10 mA/M2, compared with, 000 to 10,000 mA/M2). This current strength may nevertheless reprogram a programmable pacemaker and interfere with its function (Erlebacher et al., 1986). Exposure to a strong external magnetic or electromagnetic field can lead to conversion of a demand pulse generator from synchronous to asynchronous mode, damage to the reed switch (that activates the fixed- rate pulse generator), reprogramming of pacemaker parameters, induction of currents in the electrode wires, or displacement of the generator itself. Indeed, it is the sensitivity of some reed switches that has determined the safety boundary of MRI devices as being 5 Gauss (5 × 10-4 T). Patients with implantable defibrillators or cardioverters, implantable infusion pumps (e.g., for insulin), cochlear implants, and neurostimulators are all at risk for having the implant device reprogrammed on exposure to the magnetic field. Defibrillator failure has been reported in the MRI environment (Snowdon, 1989).
Radiofrequency pulses cause heat production in metallic implants and coiled wires such as ECG cables or pulse oximeter cables if they are looped and laying on the patient’s skin. Patients with compromised thermoregulatory abilities, such as those with cardiac problems, fever, or taking certain drugs, may be at particular risk. Included in this group are infants, whose SAR is greater than that of adults because of the greater ratio of body surface area to body mass, and whose thermoregulatory abilities may be interfered with during a general anesthetic (Kussman et al., 2004). SAR refers to the energy absorption (e.g., increasing body temperature) with an increase in the total amount of radiofrequent energy absorbed (Fitzsimmons, 1992).
Increased reports of vertigo, nausea, and a metallic taste have been found in a study on human exposure to the 4-T magnetic field of a whole-body scanner (Schenck et al., 1992). Fertilized frog embryos exposed to a 4-T magnetic field did not demonstrate any adverse effects on early development (Prasad et al., 1990). An increase in CCL of 17% was found in healthy volunteers in a 2-T environment after 10 minutes of exposure, causing speculation about the effect of the 2-T environment on the sinus node (Jehenson et al., 1988). This may be of particular concern in patients with a preexisting arrhythmia history. Of more significant concern in pediatric patients is the potential for hypothermia, because of the air flow directed through the scanner cavity and the inability to control room temperature or use radiant warmers. The use of warm IV fluid bags, thermal packs, and blankets can decrease heat loss. Excellent reviews of monitoring considerations and equipment choices in the MRI environment, as well as patient safety principles are available, and the ACR (with a representative from the ASA) has published a recent white paper on MRI safety (Kanal et al., 1990, 2007; International Non-Ionizing Radiation Committee of the International Radiation Protection Association, 1991; Fitzsimmons, 1992; Menon et al., 1992; Patteson and Chesney, 1992; Pohost et al., 1992).
MRI and spectroscopy do not employ ionizing radiation. However, secondary harmful effects, such as magnetic objects becoming projectiles within the magnetic field as they approach the bore of the magnet and potentially causing injury, are a consideration (Chu and Sangster, 1986; Chaljub et al., 2001). Patients (and anesthesiologists) with metallic implants such as vascular clamps, hemostatic clips, dental devices, heart valve prostheses, intravascular coils, filters and stents, ocular implants, orthopedic implants, otologic implants, shrapnel, penile implants, and vascular access ports must be individually evaluated for their risk in the MRI environment (Cahalan et al., 1987; Shellock and Curtis, 1991).
Difficult-airway management in the radiology suite
The unrecognized difficult airway is problematic in a remote location; therefore, it is important to have LMAs stocked in all extramural anesthesia carts. If a child cannot be intubated or ventilated with a mask, LMAs can provide a successful alternative. Case reports describe the successful use of LMAs in children with difficult craniofacial anomalies, such as Goldenhar’s syndrome and even Pierre Robin sequence (Fan et al., 1995; Hansen et al., 1995; Haxby and Liban, 1995). Similarly, a light wand may facilitate endotracheal intubation in the child with a difficult airway (Holzman et al., 1988). Recently introduced rigid laryngoscopes with video in infant through pediatric sizes (e.g., Glidescope), should be strongly considered for availability in offsite locations.
It is important to recognize that an airway that was not difficult on induction may become difficult on emergence after sclerotherapy with alcohol and subsequent tissue edema, particularly at the base of the tongue, neck, or the mediastinum (Furst et al., 1996; Ohlms et al., 1996; Fishman, 1999). These patients often require several days of airway support and ongoing evaluation in the ICU until airway swelling is no longer a concern.
Summary
The demand for anesthesia and sedation services in sites distant to the operating room is continuing to increase. Providing patient services in locations remote to the operating room can be challenging and poses risks that may not exist in the operating-room setting. Although the incidence of adverse outcomes for anesthesia and sedation in these remote areas has never been prospectively studied, we can look at office-based and ambulatory surgery centers to extrapolate the risk. Comparative outcomes analysis in Florida reveals that the incidence of adverse events is higher in the office-based setting when compared with outpatient surgical centers (Vila et al., 2003). Although these outcomes cannot be directly compared, it raises the question of added risk in the off-site anesthetizing location. Sedation, monitored anesthesia care, and general anesthesia are all choices that carry risks. Historically, an avoidance of a general anesthesia has been thought to minimize the risk of adverse outcome. Closed-claims analysis has shown, however, that monitored anesthesia care poses an equal risk to general anesthesia with respect to severity of injury, death, and permanent brain damage. Furthermore, 24% of all monitored anesthesia care claims involve oversedation and respiratory depression (Bhananker et al., 2006). In summary, anesthesia care providers must recognize that as the demand for off-site services increases, so also should their ability to understand the environment and do careful risk analysis when selecting patients and formulating a plan of care.
http://www.fda.gov/cder/drug/advisory/gadolinium_agents.htm.
http://www.fda.gov/cder/drug/advisory/gadolinium_agents_20061222.htm.
Abu-Shahwan I., Mack D. Propofol and remifentanil for deep sedation in children undergoing gastrointestinal endoscopy. Paediatr Anaesth. 2007;17:460-463.
American Society for Gastrointestinal Endoscopy. Guidelines for the use of deep sedation and anesthesia for GI endoscopy. Gastrointest Endosc. 2002;56:613-617.
American Academy of Pediatrics, American Academy of Pediatric Dentistry, Coté C.J., et al. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: an update. Paediatr Anaesth. 2008;18:9-10.
American College of Radiology. Manual on contrast media. Version 6. 2008. Reston, VA
American Society of Anesthesiologists House of Delegates. Guidelines for Nonoperating Room Anesthetizing Locations. San Francisco: American Society of Anesthesiologists; 1994. Annual Meeting, American Society of Anesthesiologists
American Society of Anesthesiologists Task Force on Anesthetic Care for Magnetic Resonance Imaging, Ehrenwerth J., Singleton M.A., et al. Practice advisory on anesthetic care for magnetic resonance imaging: a report by the American Society of Anesthesiologists Task Force on anesthetic care for magnetic resonance imaging. Anesthesiology. 2009;110:459-479.
American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-anesthesiologists. practical guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004.
Baker J.F., Kratz L.C., Stevens G.R., et al. Pharmacokinetics and safety of the MRI contrast agent gadoversetamide injection (OptiMARK) in healthy pediatric subjects. Invest Radiol. 2004;39:334-339.
Barnes P. Imaging of the central nervous system in pediatrics and adolescence. Pediatr Clin North Am. 1992;39:743.
Barton K.P., Munoz R., et al. Dexmedetomidine as the primary sedative during invasive procedures in infants and toddlers with congenital heart disease. Pediatr Crit Care Med. 2008;9:612-615.
Becker G.J., Holden R.W., Clatte E.C., et al. Therapeutic embolization with absolute ethanol. Semin Intervent Radiol. 1984;1:118-129.
Beischer D.E.. Vectorcardiogram and aortic blood flow of squirrel monkeys in a strong superconductive electromagnet. Barnothy M., editor. Biological effects of magnetic fields. New York: Plenum; 1969;2:241.
Bhananker S.M., Posner K.L., Cheney F.W., et al. Injury and liability associated with monitored anesthesia care: a closed claims analysis. Anesthesiology. 2006;104:228-234.
Bidabe A.M., Greselle J.F., Gin A.M., et al. Protocole de preparation, d’anesthesie et de surveillance en angiographie therapeutique. Agressologie. 1990;31:280-283.
Blankenberg F., Loh N., Bracci P., et al. Sonography, CT, and MR imaging: a prospective comparison of neonates with suspected intracranial ischemia and hemorrhage. Am J Neuroradiol. 2000;21:213-218.
Bloomfield E.L., Masaryk T.J., Caplin A., et al. Intravenous sedation for MR imaging of the brain and spine in children: pentobarbital versus propofol. Radiology. 1993;186:93-97.
Bouchut J., Godard J., Lachaux A. Deep sedation for upper gastrointestinal endoscopy in children. J Pediatr Gastroenterol Nutr. 2001;32:108.
Brasch R.C. Introduction to the gadolinium class. J Comput Assist Tomogr. 1993;17(Suppl 1):S14-S18.
Brimacombe J., Tucker P., Simons S. The laryngeal mask airway for awake diagnostic bronchoscopy: a retrospective study of 200 consecutive patients. Eur J Anaesthesiol. 1995;12:357-361.
Brow T.R., Goldstein B., Little J. Severe burns resulting from magnetic resonance imaging with cardiopulmonary monitoring: risks and relevant safety precautions. Am J Phys Med Rehabil. 1993;72:166-167.
Brummett R., Talbot J., Charuhas P. Potential hearing loss resulting from MR imaging. Radiology. 1988;169:539-540.
Burnette P.K., Perkins J. Parenteral odansetron for the treatment of chemotherapy and radiation-induced nausea and vomiting. Pharmacotherapy. 1992;12:120-131.
Burrows F.A., Hickey P.R., Colan S. Perioperative complications in patients with anthracycline chemotherapeutic agents. Can Anaesth Soc J. 1985;32:149-157.
Cahalan M.K., Litt L., Botvinick E.H., et al. Advances in noninvasive cardiovascular imaging: implications for the anesthesiologist. Anesthesiology. 1987;66:356-372.
Center for Devices and Radiological Health. MR product reporting program and medical device report program. Washington, DC: U.S. Food and Drug Administration, 1989.
Chaljub G., Kramer L., Johnson R., et al. Projectile cylinder accidents resulting from the presence of ferromagnetic nitrous oxide or oxygen tanks in the MR suite. Am J Roentgenol. 2001;177:27-30.
Chan S., Tham C. The effects of 2% lignocaine gel on incidence of retching with the use of the laryngeal mask airway. Anaesthesia. 1995;50:257-258.
Chernish S., Maglinte M., Glucagon D.D. Common untoward reactions: review and recommendations. Radiology. 1990;177:145-146.
Chiron C., Raynaud C., Dulac O., et al. Study of the cerebral blood flow in partial epilepsy of childhood using the SPECT method. J Neuroradiol. 1989;16:317-324.
Chu W.K., Sangster W. Potential impacts of MRI accidents. Radiol Technol. 1986;58:139-141.
Chugani H. PET in preoperative evaluation of intractable epilepsy. Pediatr Neurol. 1993;9:411-413.
Chung T., Hoffer F., Connor L., et al. The use of oral pentobarbital sodium (Nembutal) versus oral chloral hydrate in infants undergoing CT and MR imaging-a pilot study. Pediatr Radiol. 2000;30:332-335.
Coffey R.J., Lunsford L.D., Flickinger J.C. The role of radiosurgery in the treatment of malignant brain tumors. Neurosurg Clin N Am. 1992;3:231-244.
Cohen L.B., Wecsler J.S., Gaetano J.N., et al. Endoscopic sedation in the United States: results from a nationwide survey. Am J Gastroenterol. 2006;101:967-974.
Coldwell D., Stokes K., Yakes W. Embolotherapy: agents, clinical applications, and techniques. Radiographics. 1994;14:623-643.
Committee on Drugs, American Academy of Pediatrics. Guidelines for monitoring and managment of pediatric patients during and after sedation for diagnostic and therapeutic procedures. Pediatrics. 1992;89:1110-1115.
Cravero J.P., Beach M.L., Blike G.T., et al. The incidence and nature of adverse events during pediatric sedation/anesthesia with propofol for procedures outside the operating room: a report from the Pediatric Sedation Research Consortium. Anesth Analg. 2009;108:795-804.
Cremin B.J. Sedation for CT examinations in children. Br J Radiol. 1990;63:316.
Davidson R. Atlanto-axial instability in Down syndrome: a fresh look at the evidence. Pediatrics. 1988;81:857-865.
Davis P., Crooks L., Arakawa M., et al. Potential hazards in NMR imaging: heating effects of changing magnetic fields and RF fields on small metallic implants. Am J Roentgenol. 1981;137:857-860.
Department of Health and Human Services, FDA. Guidelines for evaluating electro-magnetic exposure risk for trials of clinical NMR systems. 1982. Washington, D.C.
Diament M.J., Boechat M.I., Kangarloo H. Interventional radiology in infants and children: clinical and technical aspects. Radiology. 1985;154:359-361.
Dillon M., Brown S., Casey W. Colonoscopy under general anesthesia in children. Pediatrics. 1998;102:381.
Dorta G., Uske A., Blum A.L. Meglumine gadoterate: a new safe radiocontrast medium for endoscopic retrograde cholangiopancreatography? Digestion. 1997;58:289-292.
Dujovny M., Kossovsky N., Kossowsky R., et al. Aneurysm clip motion during magnetic resonance imaging: in vivo experimental study with metallurgical factor analysis. Neurosurgery. 1985;17:543-548.
Edelman R.R., Warach S. Magnetic resonance imaging (Part II). NEJM. 1993;328:785-791.
Eggers G.W., Metzgar M.T., Plumlee J.E. Axillary block and sedation for cardiac catheterization. Anesthesiology. 1967;28:936-938.
Eifel P.J. Decreased bone growth arrest with hyperfractionated irradiation in weanling rats. Int J Radiat Oncol Biol Phys. 1988;15:141-145.
Eifel P.J., Sampson C.M., Tucker S.L. Radiation frationation sensitivity of epiphyseal cartilage in a weanling rat model. Int J Radiat Oncol Biol Phys. 1990;19:661-664.
Elster A.D. Cranial MR imaging with Gd-DTPA in neonates and young infants: preliminary experience. Radiology. 1990;176:225-230.
Erlebacher J., Cahill P., Pannizzo F., et al. Effect of magnetic resonance imaging on DDD pacemakers. Am J Cardiol. 1986;57:437-440.
Fan S., Lee T., Chen L. Long-term propofol infusion and airway management in a patient with Goldenhar’s syndrome. Acta Anaesthesiol. 1995;5:33.
Figg W.D., Graham C.L., Hak L.J., et al. Odansetron: a novel antiemetic agent. South Med J. 1993;86:497-502.
Fishman S. Vascular anomalies of the mediastinum. Semin Pediatr Surg. 1999;8:92-98.
Fitzsimmons J.R. The design of RF systems for patient safety. Ann N Y Acad Sci. 1992;649:313-321.
Fox V. Clinical competency in pediatric endoscopy. J Pediatr Gastroenterol Nutr. 1998:200.
Friedman B., Hartenberg M., Mulroy J., et al. Gastrografin aspiration in a 3 3/4-year-old girl. Pediatr Radiol. 1986;16:506-507.
Furst S., Burrows P., Holzman R. General anesthesia in a child with a dynamic, vascular anterior mediastinal mass. Anesthesiology. 1996;84:976-979.
Gangarosa R., Minnis J., Nobbe J., et al. Operational safety issues in MRI. Magn Reson Imaging. 1987;5:287-292.
Goldberg M. Systemic reactions to intravascular contrast media: a guide for the anesthesiologist. Anesthesiology. 1984;60:46-56.
Granet R.B., Gelber L.J. Claustrophobia during MR imaging. N J Med. 1990;87:479-482.
Greenberger P. Contrast media reactions. J Allerg Clin Immunol. 1984;74:600.
Greenblatt D.J., Locniskar A., Ochs H.R., et al. Automated gas chromatography for studies of midazolam pharmacokinetics. Anesthesiology. 1981;55:176-179.
Griffeth L., Rich K., Dehdashti F., et al. Brain metastases from non-central nervous system tumors: evaluation with PET. Radiology. 1993;186:37-44.
Guenther E., Pribble C.G., Junkins E.P., et al. Propofol sedation by emergency physicians for elective pediatric outpatient procedures. Ann Emerg Med. 2003;42:783-791.
Gulch R., Lutz O. Influence of strong static magnetic fields on heart muscle contraction. Phys Med Biol. 1986;31:763-769.
Hansen T., Joensen H., Henneberg S. Laryngeal mask airway guided tracheal intubation in a neonate with the Pierre Robin syndrome. Acta Anaesthesiol Scand Suppl. 1995;39:129.
Hassall E. Should pediatric gastroenterologists be I.V. drug users? J Pediatr Gastroenterol Nutr. 1993:16.
Hassall E. Who should perform pediatric endoscopic sedation? J Pediatr Gastroenterol Nutr. 1994;18:114.
Hattori Y., Fukatsu HIshigaki T. Measurement and evaluation of the acoustic noise of a 3 Tesla MR scanner. Nagoya J Med Sci. 2007;69:23-28.
Haxby E., Liban J. Fiberoptic intubation via a laryngeal mask in a infant with Goldenhar syndrome. Anaesth Intensive Care. 1995;23:753.
Henderson K.H., Lu J.K., Rockoff M.A., et al. Radiation exposure of anesthesiologists. Anesthesiology. 1991;75:A896.
Holzman R., Nargozian C., Florence F. Lightwand intubation in children with abnormal upper airways. Anesthesiology. 1988;69:784-787.
Hostetler M., Davis C. Prospective age-based comparison of behavioral reactions occurring after ketamine sedation in the ED. Am J Emerg Med. 2002;20:463-468.
Hubbard A.M., Markowitz R.I., Kimmel B., et al. Sedation for pediatric patients undergoing CT and MRI. J Comput Assist Tomogr. 1992;16:3-6.
International Non-Ionizing Radiation Committee of the International Radiation, Protection Association. Protection of the patient undergoing a magnetic resonance examination. Health Phys. 1991;61:923-928.
Jackson I., Carreno R., Potparic Z., et al. Hemangiomas, vascular malformations, and lymphovenous malformations: classification and methods of treatment. Plast Reconstr Surg. 1993;91:1216-1230.
Jeffries G. Radiotherapy and children’s anaesthesia. Anaesthesia. 1988;43:416-417.
Jehenson P., Duboc D., Lavergne T., et al. Change in human cardiac rhythm induced by a 2 T static magnetic field. Radiology. 1988;166:227-230.
Jehenson P.M. Reducing doses of glucagon used in radiologic examinations. Radiology. 1991;179:286-287.
Kain Z., Mayes L., Caramico L., et al. Parental presence during induction of anesthesia: a randomized controlled trial. Anesthesiology. 2001;84:1060-1067.
Kanal E. An overview of electromagnetic safety considerations associated with magnetic resonance imaging. Ann N Y Acad Sci. 1992;649:204-224.
Kanal E., Barkovich A., Bell C., et al. ACR guidance document for safe MR practices: 2007. Am J Roentgenol. 2007;188:1-27.
Kanal E., Shellock F., Talagala L. Safety considerations in MR imaging. Radiology. 1990;176:593-606.
Karian V., Burrows P., Zurakowski D., et al. The development of a pediatric radiology sedation program. Pediatr Radiol. 2002;32:348-353.
Karlik S.J., Heatherley T., Pavan F., et al. Patient anesthesia and monitoring at a 1.5 T MRI installation. Magn Reson Med. 1988;7:210-221.
Keeter S., Benator R.M., Weinberg S.M., et al. Sedation in pediatric CT: national survey of current practice. Radiology. 1990;175:745-752.
Keller C., Sparr H., Brimacombe J. Laryngeal mask lubrication: a comparative study of saline versus 2% lignocaine gel with cuff pressure control. Anaesthesia. 1997;52:592-597.
Kelly J., Patterson R., Lieberman P. Radiographic contrast media studies in high risk patients. J Allergy Clin Immunol. 1978;62:181.
Koh J., Black D., Leatherman I. Experience with an anesthesiologist interventional model for endoscopy in a pediatric hospital. J Pediatr Gastroenterol Nutr. 2001;33:314.
Koroglu A., Teksan H., Sagir O., et al. A comparison of the sedative, hemodynamic, and respiratory effects of dexmedetomidine and propofol in children undergoing magnetic resonance imaging. Anesth Analg. 2006;103:63-67.
Koskinen M.F. Magnetic resonance imaging (MRI) site planning and economics. Med Instrum. 1985;19:244-247.
Kussman B., Mulkern R., Holzman R. Iatrogenic hyperthermia during cardiac magnetic resonance imaging. Anesth Analg. 2004;99:1053-1055.
Lee E.Y., Mason K.P., Zurakowski D., et al. MDCT assessment of tracheomalacia in symptomatic infants with mediastinal aortic vascular anomalies: preliminary technical experience. Pediatr Radiol. 2008;38:82-88.
Liang M., Narayanan K., Kanal E. Magnetic ports in tissue expanders: a caution for MRI. Magn Reson Imaging. 1989;7:541-542.
Lightdale J.R., Mahoney L.B., Schwarz S.M., et al. Methods of sedation in pediatric endoscopy: a survey of NASPGHAN members. J Pediatr Gastroenterol Nutr. 2007;45:500-502.
Lind L.J., Mushlin P.S. Sedation, analgesia and anesthesia for radiologic procedures. Cardiovasc Intervent Radiol. 1987;10:247-253.
Lindahl S. Future anesthesiologists will be as much outside as inside operating theaters. Acta Anaesthesiol Scand. 2000;44:906-909.
Loeffler J., Rossitch E.J., Siddon R., et al. Role of stereotactic radiosurgery with a linear accelerator in treatment of intracranial arteriovenous malformations and tumors in children. Pediatrics. 1990;85:774-782.
Majdani O., Leinung M., Rau T., et al. Demagnetization of cochlear implants and temperature changes in 3T MRI environment. Otolaryngol Head Neck Surg. 2008;139:833-839.
Manuli MADavies L. Rectal methohexital for sedation of children during imaging procedures. AJR. 1993;160:577-580.
Mason K., Michna E., Zurakowsk D., et al. Serum ethanol levels in children and adults after ethanol embolization or sclerotherapy for vascular anomalies. Radiology. 2000;217:127-132.
Mason K., Neufeld E., Karian V., et al. Coagulation abnormalities in pediatric and adult patients after sclerotherapy or embolization of vascular anomalies. Am J Roentgenol. 2001;177:1359-1363.
Mason K.P., Zgleszewski S.E., Dearden J.L., et al. Dexmedetomidine for pediatric sedation for computed tomography imaging studies. Anesth Analg. 2006;103:57-62.
Mason K.P., Zgleszewski S.E., Prescilla R., et al. Hemodynamic effects of dexmedetomidine sedation for CT imaging studies. Paediatr Anaesth. 2008;18:393-402.
Mason K.P., Zurakowski D., Zgleszewski S.E., et al. High dose dexmedetomidine as the sole sedative for pediatric MRI. Paediatr Anaesth. 2008;18:403-411.
Mason K.P., Zurakowski D., Connor L., et al. Infant sedation for MR imaging and CT: oral versus intravenous pentobarbital. Radiology. 2004;233:723-728.
Mason K.P., Zurakowski D., Karian V.E., et al. Sedatives used in pediatric imaging: comparison of IV pentobarbital with IV pentobarbital with midazolam added. Am J Roentgenol. 2001;177:427-430.
McLoughlin M.J., Langer B., Wilson D.R. Life-threatening reaction to glucagon in a patient with pheochromocytoma. Radiology. 1981;140:841-845.
McRobbie D., Foster M. Cardiac response to pulsed magnetic fields with regard to safety in NMR imaging. Phys Med Biol. 1985;30:695-702.
Melendez J.C., McCrank E. Anxiety-related reactions associated with magnetic resonance imaging examination. JAMA. 1993;270:745-747.
Menache L., Eifel P.J., Kennamer D.L., et al. Twice-daily anesthesia in infants receiving hyperfractionated irradiation. Int J Radiat Oncol Biol Phys. 1990;18:625-629.
Menendez-Colino L.M., Falcon C., Traserra J., et al. Activation patterns of the primary auditory cortex in normal hearing subjects: a functional magnetic resonance imaging study. Acta Otolaryngol. 2007;127:1283-1291.
Menon D.K., Peden C.J., Hall A.S., et al. Magnetic resonance for the anaesthetist. Part I: Physical principles, applications, safety aspects. Anaesthesia. 1992;47:240-255.
Mulliken J., Young A. Vascular birthmarks, hemangiomas, and malformations. Philadelphia: WB Saunders, 1988.
Murphy K.J., Brunberg J.A., Cohan R.H. Adverse reactions to gadolinium contrast media: a review of 36 cases. Am J Roentgenol. 1996;167:847-849.
Nazarian S., Roguin A., Zviman M.M., et al. Clinical utility and safety of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent pacemakers and implantable-cardioverter defibrillators at 1.5 tesla. Circulation. 2006;114:1277-1284.
O’Tuama L., Treves S. Brain single-photon emission computed tomography for behavior disorders in children. Semin Nucl Med. 1993;23:255-264.
Ohlms L., Forsen J., Burrows P. Venous malformation of the pediatric airway. Int J Pediatr Otorhinolaryngol. 1996;37:99-114.
Palmer G., Davidson A., Sethna N., et al. Use of the Bispectral Index monitor to aid titration of propofol during a drug-assisted interview. Paediatr Anaesth. 2001;11:245-248.
Parke T.J., Stevens J.E., Rice A.S.C., et al. Metabolic acidosis and fatal myocardial failure after propofol infusion in children: five case reports. Br Med J. 1992;305:613-616.
Paspatis G.A., Charoniti I., Manolaraki M., et al. Synergistic sedation with oral midazolam as a premedication and intravenous propofol versus intravenous propofol alone in upper gastrointestinal endoscopies in children: a prospective, randomized study. J Pediatr Gastroenterol Nutr. 2006;43:195-199.
Patteson S., Chesney J.T. Anesthetic management for magnetic resonance imaging: problems and solutions. Anesth Analg. 1992;74:121-128.
Pavlicek W., Geisinger M., Castle L., et al. The effects of nuclear magnetic resonance on patients with cardiac pacemakers. Radiology. 1983;147:149-153.
Perera C., Strandvik G.F., Malik M., et al. Propofol anesthesia is an effective and safe strategy for pediatric endoscopy. Paediatr Anaesth. 2006;16:220-221.
Persson B., Stahlberg F. Safety aspects of magnetic resonance examinations. Int J Technol Assess Health Care. 1985;1:647-665.
Pohost G.M., Blackwell G.G., Shellock F.G. Safety of patients with medical devices during application of magnetic resonance methods. Ann N Y Acad Sci. 1992;649:302-312.
Prasad N., Wright D., Ford J., et al. Safety of 4-T MR imaging: study of effects on developing frog embryos. Radiology. 1990;174:251-253.
Prasil P., Laberge J., Barkun A. Endoscopic retrograde cholangiopancreatography in children: a surgeon’s perspective. J Pediatr Surg. 2001;36:733.
Ravicz M.E., Melcher J.R., Kiang N.Y. Acoustic noise during functional magnetic resonance imaging. J Acoust Soc Am. 2000;108:1683-1696.
Reves J.G., Fragen R.J., Vinik H.R., et al. Midazolam: pharmacology and uses. Anesthesiology. 1985;62:310-324.
Rex D.K. Review article: moderate sedation for endoscopy: sedation regimens for non-anaesthesiologists. Aliment Pharmacol Ther. 2006;24:163-171.
Rex D.K., Heuss L.T., Walker J.A., et al. Trained registered nurses/endoscopy teams can administer propofol safely for endoscopy. Gastroenterology. 2005;129:1384-1391.
Riles T., Berenstein A., Fisher F., et al. Reconstruction of the ligated external carotid artery for embolization of cervicofacial arteriovenous malformations. J Vasc Surg. 1993;17:491-498.
Rockoff M.A., Goudsouzian N.G. Seizures induced by methohexital. Anesthesiology. 1981;54:333-335.
Rooks V., Chung T., Connor L., et al. Comparison of oral pentobarbital sodium (nembutal) and oral chloral hydrate for infant sedation during radiologic imaging. Am J Roentgenol. 2003;180:1125-1128.
Ross D.M. Williams DO Combined axillary plexus block and basal sedation for cardiac catheterization in young children. Br Heart J. 1970;32:195-197.
Saint-Maurice C., Hamza J. Sedation techniques in pediatric anesthesia. Curr Opinion Anesthesiol. 1992:5.
Schenck J.F., Dumoulin C.L., Redington R.W., et al. Human exposure to 4 Tesla magnetic fields in a whole-body scanner. Med Physics. 1992;19:1089-1098.
Scherzinger A., Hendee W. Basic principles of magnetic resonance imaging: an update. [Review]. West J Med. 1985;143:782-792.
Shellock F.G. Biological effects and safety aspects of magnetic resonance imaging. Magn Reson Q. 1989;5:243-261.
Shellock F.G., Crues J.V. MRI: safety considerations in magnetic resonance imaging. Magn Reson Imaging Decisions. 1988;2:25-30.
Shellock F.G., Curtis J.S. MR imaging and biomedical implants, materials and devices: an updated review. Radiology. 1991;180:541-550.
Shellock F.G., Schaefer D.J., Gordon C.J. Effect of a 1.5 T static magnetic field on body temperature of man. Magn Reson Med. 1986;3:644-647.
Shuman W., Haynor D., Guy A., et al. Superficial- and deep-tissue temperature increases in anesthetized dogs during exposure to high specific absorption rates in a 1.5-T MR imager. Radiology. 1988;167:551-554.
Sledge G.W.Jr, Einhorn L., Nagy C., et al. Phase III double-blind comparison of intravenous ondansetron and metoclopramide as antiemetic therapy for patients receiving multiple-day cisplatin-based chemotherapy. Cancer. 1992;70:2524-2528.
Snowdon S. Defibrillator failure in a magnetic resonance unit [letter]. Anaesthesia. 1989;44:359.
Soriano S., Sethna N., Scott R. Anesthetic management of children with moyamoya syndrome. Anesth Analg. 1993;77:1066-1070.
Spear R.M., Waldman J.Y., Canada E.D., et al. Intravenous thiopentone for CT and MRI in children. Paediatr Anaesth. 1993;3:29-32.
Spencer H.T., Barnes P.J. Spinal anaesthesia for paediatric radiotherapy. Anaesth Intensive Care. 1980;8:214-216.
Sperber D., Oldenbourg R., Dransfeld K. Magnetic field induced temperature change in mice. Naturwissenschaften. 1984;71:100-101.
Strain J.D., Campbell J.B., Harvey L.A., et al. IV Nembutal: safe sedation for children undergoing CT. Am J Roentgenol. 1988;151:975-979.
Sussman D. A comparative evaluation of ketamine anesthesia in children and adults. Anesthesiology. 1974;40:459-464.
Tait A., Pandit U., Voepel-Lewis T., et al. Use of the laryngeal mask airway in children with upper respiratory tract infections: a comparison with endotracheal intubation. Anesth Analg. 1998;86:706-711.
Tenforde T., Gaffey C., Moyer B., et al. Cardiovascular alterations in Macaca monkeys exposed to stationary magnetic fields: experimental observations and theoretical analysis. Bioelectromagnetics. 1983;4:1-9.
Teng R., Yokohata K., Utsunomiya N. Endoscopic retrograde cholangiopancretography in infants and children. J Gastroenterol. 2000;35:39.
The Joint Commission. Permission to administer moderate sedation. 2009.http://www.jointcommission.org/accreditationprograms/longtermcare/standards/09_faqs/pc/permission_to_administer.htm.
Thomsen H., Bush W.J. Adverse effects of contrast media: incidence, prevention and management. Drug Saf. 1998;19:313-324.
Tobin J.R., Spurrier E.A., Wetzel R.C. Anaesthesia for critically ill children during magnetic resonance imaging. Br J Anaesth. 1992;69:482-486.
Towbin R.B., Ball W.S. Pediatric interventional radiology. Radiol Clin North Am. 1988;26:419-440.
Van Hemelrijck J., Smith I., White P.F. Use of desflurane for outpatient anesthesia: a comparison with propofol and nitrous oxide. Anesthesiology. 1991;75:197-203.
Van Wagoner M., O’Toole M., Quay S.C. Nonionic magnetic resonance imaging contrast agents: clinical trial experience of safety, tolerance, and efficacy of gadodiamide injection. Invest Radiol. 1990;25(Suppl 1):S39-S41.
Van Wagoner M., Worah D. Gadodiamide injection. First human experience with the nonionic magnetic resonance imaging enhancement agent. Invest Radiol. 1993;28:S44-S48.
Vangerven M., Van Hemelrijck J., Wouters P., et al. Light anaesthesia with propofol for paediatric MRI. Anaesthesia. 1992;47:706-707.
Vila H.Jr, Soto R., Cantor A.B., et al. Comparative outcomes analysis of procedures performed in physician offices and ambulatory surgery centers. Arch Surg. 2003;138:991-995.
Walker J.A., McIntyre R.D., Schleinitz P.F., et al. Nurse-administered propofol sedation without anesthesia specialists in 9152 endoscopic cases in an ambulatory surgery center. Am J Gastroenterol. 2003;98:1744-1750.
Weinmann H.J., Brasch R.C., Press W.R., et al. Characteristics of gadolinium-DTPA complex: a potential NMR contrast agent. Am J Roentgenol. 1984;142:619-624.
Weller E.B., Weller R.A., Fristad M.A. Use of sodium amytal interviews in prepubertal children: indications, procedure, and clinical utility. J Am Acad Child Psychiatry. 1985;24:747-749.
Wells H., Hyrnchak M., Burbridge B. Direct effects of contrast media on rat lungs. Can Assoc Radiol J. 1991;42:261-264.
Westbrook C., Glaholm J., Barrett A. Vomiting associated with whole body irradiation. Clin Radiol. 1987;38:263-266.
Whitwam J.G., Owen J.R., Spiers A.S.D., et al. General anaesthesia for high-dose total-body irradiation. Lancet. 1978;1:128-129. 8056
Winter J. Efficiency of utilization of a computed tomography scanner. Am J Roentgenol. 1978;131:89-93.
Wolfe T., Rao C. Anesthesia for selected procedure. Semin Pediatr Surg. 1992;1:74.
Woodard P.K., Bluemke D.A., Cascade P.N., et al. ACR practice guideline for the performance and interpretation of cardiac magnetic resonance imaging (MRI). J Am Coll Radiol. 2006;3:665-676.
Yamamoto L.G. Initiating a hospital-wide pediatric sedation service provided by emergency physicians. Clin Pediatr (Phila). 2008;47:37-48.
Zonana H.V. Hypnosis, sodium amytal, and confessions. Bull Am Acad Psychiatry Law. 1979;7:18-28.