CHAPTER 127 Anatomy of the Temporal Bone, External Ear, and Middle Ear
Osteology of the Temporal Bone
The temporal bone articulates with the sphenoid, parietal, occipital, and zygomatic bones, contributing to the cranial, skull base, and facial structure. The temporal bone has a pyramidal shape, the sides of which form the middle fossa floor (superior face), the anterior limit of the posterior fossa (posterior face), muscle attachments of neck and infratemporal fossa (anteroinferior face), and the muscular-cutaneous–covered side of the head (lateral), which forms the base of the pyramid. The temporal bone consists of four embryologically distinct components: the squamous, mastoid, petrous, and tympanic parts.1
The squamous part forms the lateral wall of the middle fossa (Fig. 127-1). It consists of a plate of bone with an anterior extension known as the zygomatic process, which forms the bony roof of the glenoid fossa. The temporalis muscle inserts on the outer cortex, whereas the masseter muscle inserts on the zygomatic process. A horizontal ridge known as the temporal line is formed along the most inferior insertion by the temporalis muscle, is aligned with the zygomatic process, and is used as a surface landmark that estimates the location of the middle fossa floor,2 with an average offset of about 4.7 mm.
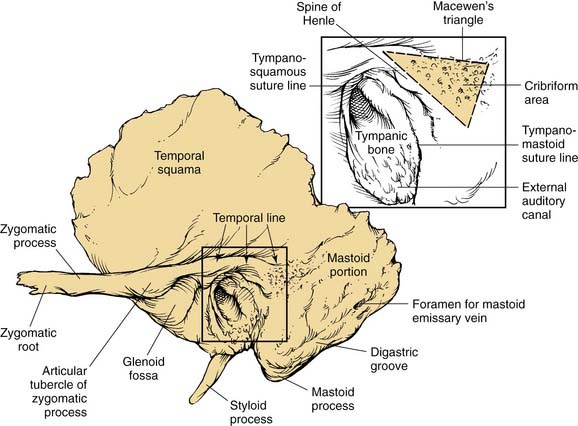
Figure 127-1. Lateral view of left temporal bone surface, showing squamous, tympanic, and mastoid portions.
(Redrawn from art by Sarah Williamson, provided courtesy of H. W. Francis and J. K. Niparko.)
The mastoid part is a bulbous bony structure shaped by the expansion of air-filled spaces within (see Fig. 127-1). The constant pull by the sternocleidomastoid muscle and posterior belly of the digastric muscle elongates the mastoid inferiorly to form the mastoid tip or process. The mastoid cortex is perforated by multiple small emissary vessels that drain from the central air cell or antrum, forming a depressed cribriform area at the anterior junction of the mastoid process with the tympanic bone. The foramen of a single emissary vein is evident near the posterior limit of the outer mastoid cortex, and communicates with the sulcus of the sigmoid sinus, which is evident on the posterior medial aspect of the temporal bone. The site of the sternocleidomastoid muscle insertion is indicated by a rough, irregular surface at the mastoid tip. Medial to the mastoid tip, the posterior belly of the digastric muscle is inserted in a sulcus that terminates anteriorly at the stylomastoid foramen. Medial and almost parallel to the digastric sulcus is the sulcus for the occipital artery.
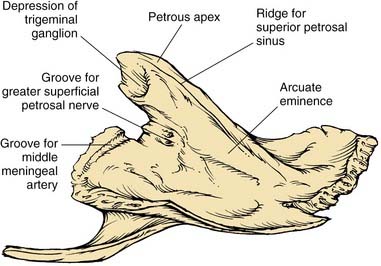
The petrous part has the shape of a pyramid whose base is united with the mastoid laterally; the apex is oriented anteromedially between occipital and sphenoid bones. The anterior surface forms the posteromedial margin of the middle fossa floor (Fig. 127-2). Significant surface features include (1) medially, the arcuate eminence formed by the prominence of the superior semicircular canal, and the sulcus of the superior petrosal sinus; (2) anteriorly, at the junction with the greater sphenoid wing, the musculotubal canal containing the more superficial semicanal of the tensor tympani and a deeper semicanal of the eustachian tube; and (3) at the apex, a smooth depression occupied by the trigeminal ganglion, just posterior to which is located foramina and sulci of the greater and lesser superficial petrosal nerves, running parallel to the sphenoid suture line. The roof of the middle ear and mastoid is located lateral to the arcuate eminence.
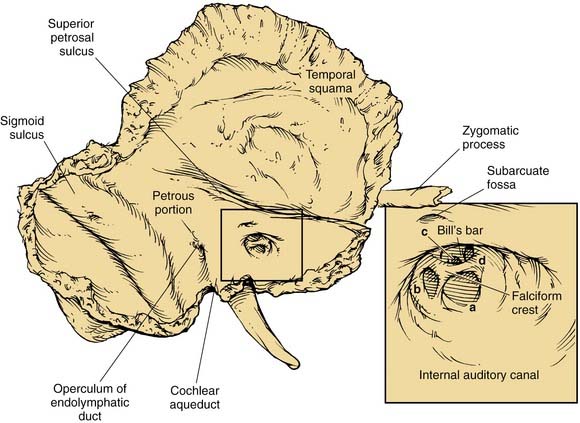
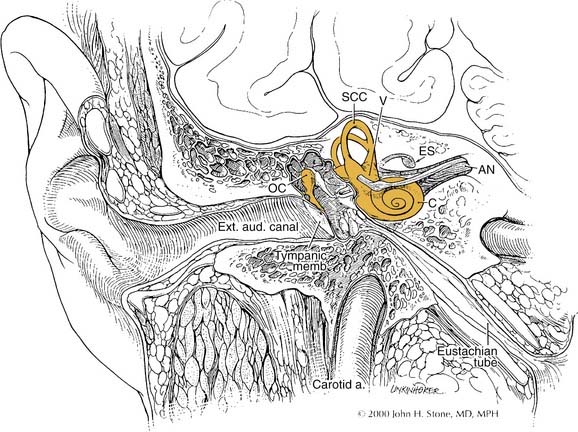
The posterior surface of the petrous part of the temporal bone is oriented in the vertical plane forming the anterior bony limit of the posterior fossa (Fig. 127-3). This surface is framed by sulci for the sigmoid sinus, and the superior petrosal and inferior petrosal sinuses. At the center of the posterior face is the porus acusticus at the fundus of which can be seen the falciform (horizontal) crest, Bill’s bar (vertical crest), and foramina of cranial nerves VII and VIII. The subarcuate artery emerges from a depression superior and lateral to the acoustic meatus, whereas the endolymphatic sac and duct occupy the depression and opening located inferolaterally, known as the operculum. The jugular foramen is formed at the junction between the petrous and occipital bones (Fig. 127-4), and is partitioned into the pars nervosa (posterior) and pars venosa (anterior) by the jugular spine or process.3
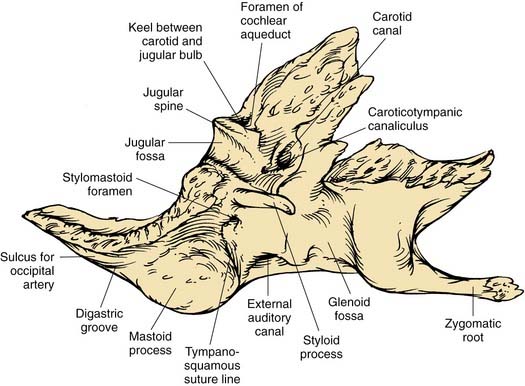
The inferior surface of the temporal bone is irregular because of the presence of multiple muscle attachments (Fig. 127-5). The external aperture to the cochlear aqueduct is located just medial and anterior to the jugular spine within the pars nervosa, and marks the superiormost limit of the jugular foramen. The glossopharyngeal nerve enters the jugular foramen adjacent to the opening of the cochlear aqueduct. In the translabyrinthine approach to the internal auditory canal, the cochlear aqueduct is an important inferior limit of dissection used to protect the lower cranial nerves. The cochlear aqueduct eventually opens into the scala tympani at the cochlear base.4–6 The jugular bulb occupies a dome-shaped compartment located lateral to the pars venosa of the jugular foramen directly under the middle ear space. The inferior foramen of the carotid canal is located directly anterior to the jugular bulb depression, from which it is separated by a wedge-shaped bone called the keel. The tympanic canaliculus penetrates the keel to transmit sensory and preganglionic parasympathetic fibers from the inferior ganglion of the glossopharyngeal nerve into the middle ear as Jacobson’s nerve.1,5 The styloid process is located anterior to the stylomastoid foramen, and both are located at the anterior limit of the digastric groove.
The tympanic part of the temporal bone forms the anterior wall, floor, and part of the posterior wall and roof of the bony external auditory canal (EAC), and the anterior wall and floor of the middle ear (see Fig. 127-1). The anterior edge of this open ring forms the tympanosquamous suture line within the EAC, and the petrotympanic suture line within the middle ear, through which the chorda tympani exits the ear. The posterior edge of the tympanic ring forms the tympanomastoid suture line, which curves from the posterior EAC inferiorly to within millimeters of the stylomastoid foramen, serving as a landmark for the main trunk of the facial nerve as it exits the temporal bone (see Fig. 127-5).
External Ear
The auricle is a funnel-shaped cartilaginous structure that is continuous with the meatus and EAC (Fig. 127-6). Intricate ridges and depressions formed by the auricular cartilage and cutaneous envelope are shown and labeled in Figure 127-5. The blood supply of the external ear originates from the external division of the carotid artery via the posterior auricular and superficial temporal vessels. The EAC (Fig. 127-7) is about 2.5 cm long and is composed of a lateral cartilaginous (membranous) portion and a medial bony portion.1,7 The membranous portion accounts for the lateral third of the EAC, whereas the bony portion forms the medial two thirds. The skin lining the membranous canal is thicker, more mobile, and endowed with sebaceous and apocrine (ceruminous) glands and hair follicles. Sebaceous and apocrine ducts empty into a follicular canal surrounding each hair follicle.5,7
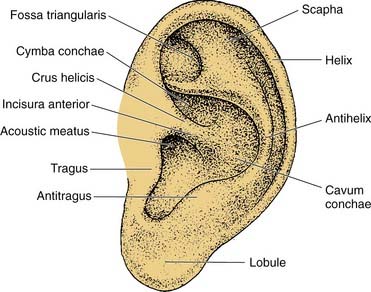
Figure 127-6. Surface anatomy of the auricle.
(Adapted from Adamson PA, Tropper GJ, McGraw BL. Otoplasty. In: Krause CJ, Mangat DS, Pastorek N, eds. Aesthetic Facial Surgery. Philadelphia: Lippincott; 1991:709.)
The external ear is developed from ectodermal and mesodermal components of the first and second branchial arches and the intervening first branchial groove.8,9 Distinct condensations of tissue, known as the hillocks of His, give rise to the tragus and most of the helix from the first branchial arch, and to the antihelix, antitragus, lobule, and inferior helix from the second branchial arch. Sensory innervation is provided by the corresponding first branchial nerve: the auriculotemporal branch of the trigeminal nerve and a cutaneous branch of the facial nerve. The EAC develops from the dorsal portion of the first branchial cleft, which extends toward and eventually makes contact with endoderm of the expanding tubotympanic recess. There is a transient obstruction of the medial canal by proliferating epithelial cells to form a meatal plug that eventually dissolves, leaving a patent canal.
Errors of embryogenesis may result in impaired hearing or render the ear vulnerable to other conditions. The failed or incomplete involution of the meatal plug results in canal atresia or severe stenosis with conductive hearing loss and the risk of canal cholesteatoma. Auricular anomalies may also result from imperfect development of the hillocks of His with consequences ranging from anotia to the development of preauricular cysts and sinuses by duplication of the first branchial groove and arch.10
Tympanic Membrane
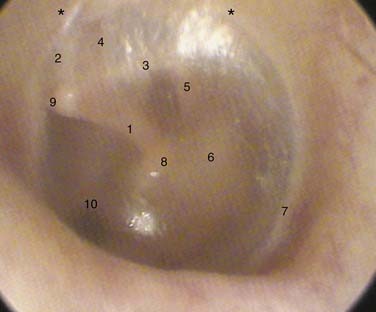
The tympanic membrane forms the medial wall of the EAC and much of the lateral wall of the middle ear space. It is a four-layered, concave-shaped membrane connected centrally to the manubrium of the malleus and peripherally to the tympanic sulcus.1,4,5 The tip of the malleus is attached to the depression known as the umbo, superior to which the manubrium forms the mallear stria, which extends to the mallear prominence formed by the lateral process (Fig. 127-8).
The otoscopic examination consists of the careful evaluation of the EAC skin, the surface anatomy of the tympanic membrane, and direct or indirect examination of middle ear anatomy as seen through the translucent membrane or perforations. The round window, long process of the incus, incudostapedial joint, and chorda tympani are identifiable through the intact tympanic membrane to varying degrees as seen in Figure 127-6, depending on its translucency, extent of retraction, and the mucosal health within the middle ear.
Middle Ear
The middle ear is an air-filled space formed by extension of the foregut into the anlage of the temporal bone. The expanding first pharyngeal pouch forms multiple cul-de-sacs that coalesce to form a common airspace partitioned by the ossicular chain and associated ligaments and mucosal folds. The resorption of mesenchymal tissue further expands the size of the middle ear and mastoid into niches, compartments, and bony tracts of air cells.1,9,11 These additional areas vary considerably among individuals and between ears, influenced by heredity, airflow via the eustachian tube, and disease in the early years of life.
The eustachian tube is the conduit through which air is exchanged between the middle ear space and upper aerodigestive tract. It is angled by about 45 degrees from the middle ear to the nasopharyngeal opening at the torus tubarius.12–14 The proximal third of the tube is formed in petrous bone. The distal two thirds segment is fibrocartilaginous and is collapsed at rest. The fibrocartilaginous portion is composed of an open tube that is shaped like an inverted “J” in cross section. A fibrous membrane closes this tube laterally. The tensor veli palatini muscle is inserted on this membrane retracting it on muscular contraction to enlarge the tubal lumen during swallowing and yawning. The bony-cartilaginous junction is the narrowest point along the eustachian tube. The internal carotid artery is closely associated with the medial wall of the eustachian tube at its tympanic opening, where the overlying bone may be quite thin or even dehiscent.
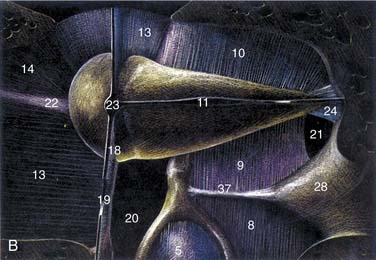
The middle ear cavity can be spatially divided into hypotympanum, mesotympanum, and epitympanum, the limits of which are defined by their location relative to the tympanic anulus (see Figs. 127-8 and 127-9). The mesotympanum is the space just medial to the tympanic membrane, which extends from the eustachian tube opening anteriorly to the facial nerve posteriorly. The carotid artery is located medial to the eustachian tube opening, and the tensor tympani muscle occupies the semicanal superior and parallel to the eustachian tube. The cochlear promontory forms the medial wall of the mesotympanum marked posteriorly by the oval window superiorly, which is occupied by the stapes, and the round window inferiorly. The mesotympanum extends into a recess of varying depth that lies posterior to the oval and round windows, and medial to the vertical facial nerve. Known as the sinus tympani, this space may harbor occult cholesteatoma despite careful and thorough removal of bone around the facial nerve, which forms its lateral wall. The pyramidal eminence transmits the stapedial tendon to the stapes superstructure, and is located just anterior to the second genu of the facial nerve as it transitions from the tympanic (horizontal) to mastoid (vertical) segment. The inferior anulus of the tympanic membrane marks the inferior limit of the mesotympanum and superior limit of the hypotympanum. The hypotympanum is limited inferiorly by the jugular bulb posteriorly and may extend inferomedial to the cochlea.
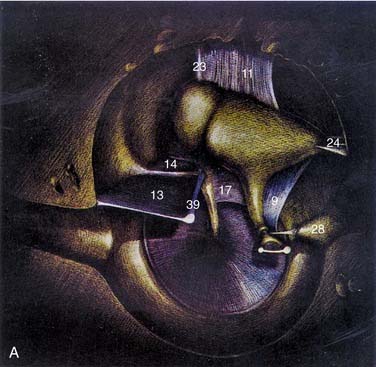
The ossicular chain, associated ligaments, and mucosal folds almost completely partition the epitympanic space from the mesotympanum except for small openings anterior and posterior to the stapes superstructure (Fig. 127-10).12 The body of the incus articulates with the head of the malleus to form an ossicular mass that occupies most of the epitympanum. These ossicles are suspended within this space without touching its bony walls, by the anterior and superior malleal and posterior incudal ligaments. They are further supported by investments of the lateral and long processes of the malleus into the tympanic membrane, and by the incudostapedial articulation. The presence of a single nutrient vessel within the long process of incus and the absence of collateral circulation render this segment of bone susceptible to aseptic necrosis secondary to middle ear infections (Fig. 127-11).
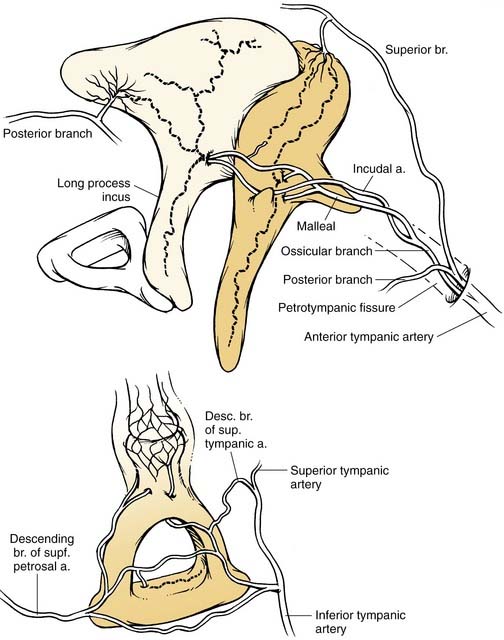
(Redrawn from Nager G. Pathology of the Ear and Temporal Bone. Baltimore: Williams & Wilkins; 1993.)
The lateral process of the malleus and spines of the notch of Rivinus are otoscopic landmarks for the inferior limit of the epitympanum. The superior medial wall of the bony canal or scutum forms the lateral wall of the epitympanum, and is subject to erosion by cholesteatomas arising secondary to retraction of the pars flaccida. The epitympanum is divided further into (1) Prussak’s space, just medial to pars flaccida and lateral to the head and neck of the malleus; (2) the compartment anterior to the malleus; and (3) the posterior compartment, which communicates with the antrum. The folds and ossicles that partition this space provide distinct pathways along which cholesteatoma can spread within and beyond the attic.6,12 Attic retraction cholesteatomas are directed posteriorly from Prussak’s space through an opening into the posterior epitympanum. From here, there is extension into the antrum through the aditus ad antrum or inferiorly into the posterior mesotympanum, or both. In advanced cases, disease may extend medial to the ossicles and to the anterior attic. In cholesteatoma surgery, removal of the incus and the head of the malleus is required to identify and address the anterior extension of disease.
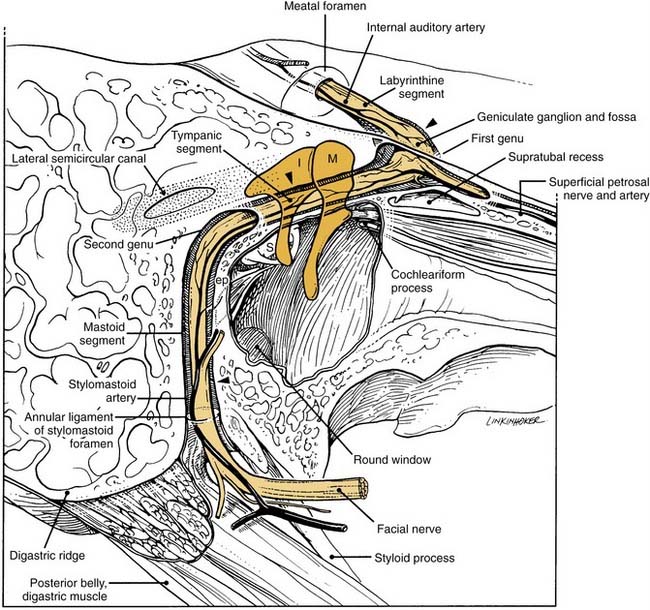
The close association between the tympanic segment of the facial nerve and pathology in the attic requires a detailed understanding of neuroanatomy of this region (see Fig. 127-9). The geniculate ganglion and first genu of the facial nerve are located just medial to a bony cul-de-sac at the anterior limit of the attic, known as the supratubal recess. The posterior opening of this recess is marked by the cochleariform process inferiorly and the “cog” superiorly, an incomplete bony septum oriented in the coronal plane.15 The facial nerve is vulnerable at the first genu to disease or surgical dissection within the supratubal recess, from which it may be separated by minimal or no bone.16 The cog should be removed to visualize the entire supratubal recess for safe removal of disease.15 The tympanic segment of the facial nerve projects in a posterior direction at a slight inferior incline. It skims just superior to the cochleariform process, which serves as a durable landmark for the midportion of the tympanic segment of the facial nerve.
The fallopian canal serves as the bony superior limit of the oval window niche. In 55% of cases, however, the facial nerve is partially exposed because of bony dehiscence. In some cases, the nerve herniates from the canal into the oval window niche, becoming vulnerable during stapes surgery. The pyramidal eminence, from which the stapedius tendon emerges to insert on the stapes capitulum, is a reliable landmark for the second genu of the facial nerve distal to which the nerve is directed vertically toward the stylomastoid foramen. The facial nerve is most vulnerable to iatrogenic injury just proximal to the second genu and in its vertical path.17 An understanding of facial anatomy and the use of sound surgical technique are required to identify and preserve the nerve and its function during ear surgery.18
The tegmen tympani forms the superior limit of the epitympanum, which also corresponds to the cortical floor of the middle fossa. This bone may be very thin and even dehiscent in some cases. Ectopic arachnoid granulations positioned on the bony floor of the middle fossa may erode over time into the mastoid owing to pulsations of cerebrospinal fluid. When accompanied by the thinning of dura, meningoencephaloceles develop that may spontaneously leak cerebrospinal fluid to generate middle ear effusions, rhinorrhea, or otorrhea.6,19,20
The lateral and superior semicircular canals form the medial walls of the posterior epitympanum at its opening into the antrum, known as the aditus ad antrum. The lateral semicircular canal forms a prominence at the inferior aspect of the aditus ad antrum. Impacted cholesteatoma erodes the otic capsule that forms this prominence leading to the formation of a perilymphatic fistula over time. The second genu of the facial nerve hugs the anteroinferior aspect of the lateral semicircular canal (see Fig. 127-9).
Facial Nerve
The facial nerve provides afferent and efferent innervation to structures derived from the second branchial arch. Most fibers are special visceral efferents that supply nerve input to striated muscles of facial expression, the stapedius muscle, the stylohoid muscle, and the posterior belly of the digastric muscle.5,18,21 General visceral efferent fibers form the nervus intermedius, which innervates (1) the lacrimal gland and seromucinous glands of the nasal cavity via the greater superficial petrosal nerve and pterygopalatine ganglion, and (2) the submandibular and sublingual glands via the chorda tympani and the submandibular ganglion. Special sensory fibers for taste innervate the anterior two thirds of the tongue via the chorda tympani, and the tonsillar fossae and palate via the greater superficial petrosal nerve. The geniculate ganglion contains the cell bodies of these sensory neurons. Somatic sensory fibers provide sensation to touch of the EAC and conchal skin of the auricle, and proprioceptive information from the facial muscles. Visceral afferent fibers supply the mucosa of the nose, pharynx, and palate. Motor, preganglionic, and sensory modalities have the following brainstem origins: the motor nucleus (motor), the superior salivatory nucleus (preganglionic), and the nucleus of the solitary tract (sensory).
The facial nerve traverses the temporal bone within a bony canal known as the fallopian canal, which begins at the fundus of the internal auditory canal and terminates at the stylomastoid foramen (see Fig. 127-9).1,4–6 Within the internal auditory canal, the nerve lacks a fibrous sheath or endoneurium and is surrounded by a thin layer of arachnoid. The labyrinthine segment is the first, shortest, and narrowest segment of the fallopian canal.22,23 It travels superior to the cochlea and opens into the geniculate fossa located just deep to the supratubal recess. The geniculate ganglion is separated from the middle fossa by only a very thin layer of bone, which is dehiscent in about 25% of ears. The facial nerve acquires a fibrous sheath at and distal to the geniculate ganglion. This is also the location of the facial hiatus, where the greater superficial petrosal nerve projects anteriorly along the middle fossa floor, and of the first genu, at which the main trunk makes an acute posterior and slightly inferior turn to enter the tympanic or horizontal segment of the fallopian canal. The horizontal segment of the facial nerve occupies the medial wall of the anterior attic, skims over the superior aspect of the cochleariform process, and forms the superior wall of the oval window niche posteriorly.
At the pyramidal eminence, from which the stapedial tendon emerges, the facial nerve makes a second genu into the mastoid or vertical segment. This turn is positioned just anteroinferior to the lateral semicircular canal, and anterior to a line drawn through the short process of the incus. A superior extrapolation of the vertical segment would approximately bisect the prominence of the lateral semicircular canal. Although the anatomic position of the horizontal segment is quite consistent, particularly relative to the cochleariform process, oval window, and pyramidal eminence, there is greater variability in the path taken by the vertical segment before exiting the stylomastoid foramen. Chorda tympani and stapedial branches occur at varying points along this segment. The space between the mastoid segment of the facial nerve and the chorda tympani is a space through which a posterior tympanotomy (or facial recess) can be surgically created to access the middle ear. The distal aspect of the vertical segment of the fallopian canal and the stylomastoid foramen itself are lined by the aponeurosis of the posterior belly of the digastric muscle. As the facial nerve exits the fallopian canal, it receives blood supply from this closely applied aponeurosis. The preservation of this relationship on rerouting the nerve during skull base surgery may result in better facial nerve function.24,25 Identification of this aponeurosis anterior to the cephalic edge of the digastric muscle serves as a valuable landmark for the stylomastoid foramen and the vertical segment of the nerve as it exits. A more in-depth discussion of facial nerve anatomy is also helpful in understanding the mechanisms of injury.
Each segment of the facial nerve has unique anatomic features that render it susceptible to injury.26 The fallopian canal not only provides a protective path for the facial nerve in the temporal bone, but also plays an important role in the pathophysiology of injury. The narrow meatal foramen23 and labyrinthine segment22 are sites where facial nerve edema secondary to inflammatory or traumatic causes leads to progressive compression, vascular compromise, and axonal injury. This narrow segment is made further vulnerable by being a watershed area between terminal arterioles of the vertebrobasilar and external carotid artery systems. Vertebrobasilar supply is via labyrinthine branches of the anterior inferior cerebellar artery, and supply by the external carotid artery is via the petrosal branch of the middle meningeal artery and the stylomastoid artery that branches from the posterior auricular artery.27 The labyrinthine segment is made further vulnerable by the lack of epineurium and associated vascular plexus that provides a barrier to injury and a rich collateral blood supply.
The tympanic segment is the most common site of congenital dehiscence of the bony canal, especially above the oval window.23 Bony dehiscence by cholesteatoma is also common proximal to the second genu, where the adjacent aditus ad antrum acts as a bottleneck through which disease must pass en route to the antrum, eroding structures in the posterior attic along the way. The dehiscent horizontal fallopian canal places the facial nerve at risk for injury by the unwary surgeon, especially when disease or previous surgery alters anatomy. The vertical segment has the most variable path, especially in association with congenital malformations, such as canal atresia. The nerve also courses lateral to the tympanic anulus as it approaches the stylomastoid foramen and can be injured in transcanal procedures.17 In the newborn, the mastoid tip is underdeveloped, placing the styloid process and the adjacent facial nerve more superficial in the neck. The nerve is susceptible to surgical injury in routine postauricular approaches and to compression injuries during forceps delivery.
Mastoid and Other Pneumatized Regions of the Temporal Bone
There is considerable variation in the extent of temporal bone pneumatization and the architecture of cell tracts in and around the mastoid. The extent of pneumatization depends on early otologic history, including factors that influence middle ear ventilation,28 history of otitis media,29 and presumably heredity. The interaction between osteoclastic activity and the resorption of mesenchymal cells determines the speed of pneumatization, and is influenced by genetic and local middle ear factors.11
The extent and anatomic distribution of pneumatization has relevance to the surgical management of ear disease. Surgical access is aided by the presence of large pneumatized regions that permit navigation to deeper structures without compromise of intracranial contents, the labyrinth, the facial nerve, and the sigmoid sinus. The decision to maintain or remove the bony separation between the mastoid and EAC is also influenced by the extent of pneumatization. Poor pneumatization is associated with a greater likelihood of chronic ear disease28 because it is a marker of reduced capacity to ventilate the middle ear and mastoid. Exteriorization of the poorly aerated mastoid may avoid chronic consequences of hypoventilation, including otomastoiditis and cholesteatoma.
Pneumatization may be organized into regions and tracts. The primary regions of pneumatization are as follows5,30:
When obstructed by edema, postinflammatory scarring, or organized clots, unventilated deeper spaces may accumulate sterile fluid, including transudates or by-products of inflammation and hemoglobin to form the so-called cholesterol granuloma,31 an expanding collection of thick dark fluid often compared with crankcase oil. Superinfection of these obstructed tracts creates a chronic source of sepsis that is not always cleared with antibiotics alone. Because of its deep location and narrow air cell tracts, the petrous apex is vulnerable to these postobstructive processes, making it a well-recognized location for cholesterol granulomas and apicitis. Gradenigo’s syndrome consists of symptoms that result from the inflammatory effect of petrous apicitis on adjacent intradural structures, including cranial nerves VI and V1, accompanied by otorrhea.32
Allam A. Pneumatization of the temporal bone. Ann Otol Rhinol Laryngol. 1969;78:49-64.
Anson B, Donaldson J. Surgical Anatomy of the Temporal Bone, 3rd ed. Philadelphia: Saunders; 1981.
Chole R, Donald P. Petrous apicitis: clinical considerations. Ann Otol Rhinol Laryngol. 1983;92:544-551.
Francis H. Facial nerve emergencies. In: Eisele D, McQuone S, editors. Emergencies of the Head and Neck. St Louis: Mosby; 2000:337-366.
Green J, Shelton C, Brackmann DE. Surgical management of iatrogenic facial nerve injuries. Otolaryngol Head Neck Surg. 1994;111:606-610.
Horn K, Brackmann DE, Luxford WM, Shea JJ. The supratubal recess in cholesteatoma surgery. 3rd. Ann Otol Rhinol Laryngol. 1986;95:12-15.
May M. Anatomy for the clinician. In: May M, Schaitkin B, editors. The Facial Nerve. New York: Thieme; 2000:19-56.
Nager GT. Pathology of the Ear and Temporal Bone. Baltimore: Williams & Wilkins; 1993.
Proctor B. The development of the middle ear spaces and their surgical significance. JLO. 1964;78:631-648.
Schuknecht HF, Gulya AJ. Anatomy of the Temporal Bone with Surgical Implications. Philadelphia: Lea & Febiger; 1986.
Work W. Newer concepts of first branchial cleft defects. Laryngoscope. 1972;982:1581-1593.
1. Anson BJ. Donaldson, Surgical Anatomy of the Temporal Bone, 3rd ed. Philadelphia: Saunders; 1981.
2. Aslan A, Mutlu C, Celik O, Gousa F, Ozgur T, Egrilmez M. Surgical implications of anatomical landmarks on the lateral surface of the mastoid bone. Surg Radiol Anat. 2004;26:263-267.
3. Rubenstein D, Burton BS, Walker AL. The anatomy of the inferior petrosal sinus, glossopharyngeal nerve, vagus nerve and accessory nerve in the jugular foramen. AJNR Am J Neuroradiol. 1995;16:185-194.
4. Schuknecht HF. Pathology of the Ear, 2nd ed. Philadelphia: Lea & Febiger; 1993.
5. Schuknecht HF, Gulya AJ. Anatomy of the Temporal Bone with Surgical Implications. Philadelphia: Lea & Febiger; 1986.
6. Nager GT. Pathology of the Ear and Temporal Bone. Baltimore: Williams & Wilkins; 1993.
7. Bojrab D, Bruderly T, Abdulrazzak Y. Otitis externa. Otolaryngol Clin North Am. 1996;29:761-782.
8. Mallo M. Formation of the middle ear: recent progress on the developmental and molecular mechanisms. Dev Biol. 2001;231:410-419.
9. Sadler T. Langman’s Medical Embryology, 6th ed. Baltimore: Williams & Wilkins; 1990.
10. Work W. Newer concepts of first branchial cleft defects. Laryngoscope. 1972;982:1581-1593.
11. Palva T, Ramsay H. Fate of the mesenchyme in the process of pneumatization. Otol Neurotol. 2002;23:192-199.
12. Proctor B. The development of the middle ear spaces and their surgical significance. JLO. 1964;78:631-648.
13. Seibert J, Danner C. Eustachian tube function and the middle ear. Otolaryngol Clin North Am. 2006;39:1221-1235.
14. Grimmer J, Poe D. Update on eustachian tube dysfunction and the patulous eustachian tube. Curr Opin Otolaryngol Head Neck Surg. 2005;13:277-282.
15. Horn K, Brackmann DE, Luxford WM, Shea JJ. The supratubal recess in cholesteatoma surgery. 3rd. Ann Otol Rhinol Laryngol. 1986;95:12-15.
16. Chu F, Jackler R. Anterior epitympanic cholesteatoma with facial paralysis: a characteristic growth pattern. Laryngoscope. 1988;98:274-279.
17. Green J, Shelton C, Brackmann D. Surgical management of iatrogenic facial nerve injuries. Otolaryngol Head Neck Surg. 1994;111:606-610.
18. May M. Anatomy for the clinician. In: May M, Schaitkin B, editors. The Facial Nerve. New York: Thieme; 2000:19-56.
19. Gacek R. Arachnoid granulation cerebrospinal fluid otorrhea. Ann Otol Rhinol Laryngol. 1990;99:854-862.
20. Nahas Z, Tatlipinar A, Limb CJ, et al. Spontaneous meningoencephalocele of the temporal bone: clinical spectrum and presentation. Arch Otolaryngol Head Neck Surg. 2008;134:509-518.
21. Leblanc A. The Cranial Nerves. Berlin: Springer; 1995.
22. Esslen E. The Acute Facial Palsies. Berlin: Springer-Verlag; 1977.
23. Proctor B, Nager GT. The facial canal: Normal anatomy, variations and anomalies. Ann Otol Rhinol Laryngol. 1982;97:33-61.
24. Brackmann D. The facial nerve in the infratemporal approach. Otolaryngol Head Neck Surg. 1987;97:15-17.
25. Parhizkar N, Hiltzik D, Selesnick S. Facial nerve rerouting in skull base surgery. Otolaryngol Clin North Am. 2005;38:685-710.
26. Francis H. Facial nerve emergencies. In: Eisele D, McQuone S, editors. Emergencies of the Head and Neck. St Louis: Mosby; 2000:337-366.
27. Jackson CG. Facial Nerve Paralysis: Diagnosis and Treatment of Lower Motor Neuron Facial Nerve Lesions and Facial Paralysis. American Academy of Otolaryngology-Head and Neck Surgery Foundation, Inc; 1986.
28. Valtonen H, et al. Development of mastoid air cell system in children treated with ventilation tubes for early-onset otitis media: a prospective radiographic 5-year follow-up study. Laryngoscope. 2005;115:268-273.
29. Mey K, Sørensen M, Homøe P. Histomorphometric estimation of air cell development in experimental otitis media. Laryngoscope. 2006;116:1820-1823.
30. Allam A. Pneumatization of the temporal bone. Ann Otol Rhinol Laryngol. 1969;78:49-64.
31. Jackler R, Cho M. A new theory to explain the genesis of petrous apex cholesterol granuloma. Otol Neurotol. 2003;24:96-106.
32. Chole R, Donald P. Petrous apicitis: clinical considerations. Ann Otol Rhinol Laryngol. 1983;92:544-551.