Anatomy of the Skin
Introduction
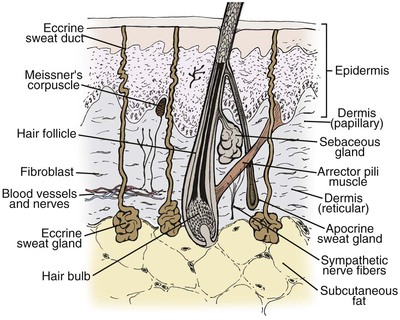
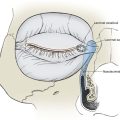
The skin is a complex organ system that is essential for all forms of mammalian life. It may be viewed as a double-layered sheath, cushioned by the underlying subcutaneous adipose tissue, that covers the entire surface of the body. The outer layer of skin, known as the epidermis, is separated from the inner layer, or dermis, by the basement membrane zone. The dermis is attached to the subcutaneous adipose tissue and underlying musculature by fibrous insertions. Important structures, such as epidermal appendages (hair follicles, sebaceous glands, and sweat glands), nerves, blood vessels, and immunologic cells, are present in the skin (Fig. 1-1). As an organ system, the skin has many important physiologic and immunologic properties: it provides a barrier to the environment, regulates body temperature, and serves as an important component of the immune system.1–3
General Characteristics
Skin is highly variable from one person to another and, within the same individual, from one anatomic region to another, with differences to be observed in color, texture, thickness, and content of hair follicles and sebaceous glands. Skin may be divided into smooth, non–hair-bearing (glabrous) and hair-bearing (nonglabrous) areas, although it is virtually always hair-bearing. Skin is the heaviest human organ, weighing approximately 3.8 kg. The largest organ in surface area, the lung, measures about 4.2 m2 on expiration; in comparison, the skin measures approximately 1.7 m2 in surface area. As a tissue, skin ranks fourth in weight behind muscle, adipose tissue, and bone.4
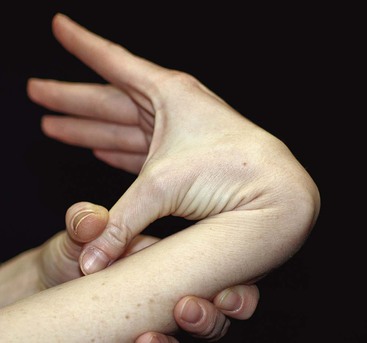
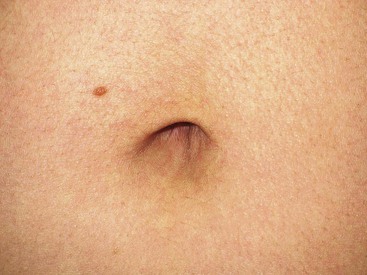
Considerable variation in skin thickness and content of appendages and elastic fibers exists with respect to anatomic region, age, and sex. An appreciation of these variations is clinically important for understanding wound healing and aesthetics. These variations play an integral role in the definition of facial aesthetic regions, boundaries, and junctions. The surgeon must apply knowledge of these factors to the task of determining the best reconstructive option during flap or graft surgery. Careful examination of the skin is essential for making the best tissue match for aesthetic reconstruction. Discrepancies in the thickness of skin edges should be observed before wound closure for exact reapproximation of the edges. The best donor site for a full-thickness skin graft is determined by an examination of all potential donor sites with respect to skin thickness, color, texture, and content of hair follicles and sebaceous glands. Careful examination of the skin before surgery may uncover several clues that could influence the outcome. Individuals with fair skin, light hair, and blue eyes may develop postoperative scars that remain pink for an extended period. Persons with dark skin, hair, and eyes may develop scars that remain pigmented for a prolonged period after surgery. An assessment of previous scars and keloids should be made. Individuals with hyperelastic skin features are characterized by hyperextensibility of the joints (elbows, wrists, and knees), anterior hooding of the navel, and lax skin (Figs. 1-2 to 1-4). These individuals are at higher risk for development of wide scars, permanent railroad tracking suture marks, hypertrophic scars, and prolonged erythema of the scars lasting up to 1 year, eventually resulting in a porcelain-colored white scar. Whereas it is also present in Ehlers-Danlos syndrome, hyperelastic skin is most often simply a relatively common normal variant within the population. Patients with common skin conditions, such as atopic dermatitis, psoriasis, and unusually dry skin, may have high counts of staphylococcal organisms on their skin and thereby increased risk of wound infections. In essence, basic knowledge of skin anatomy is something that is applied daily in reconstructive surgery.
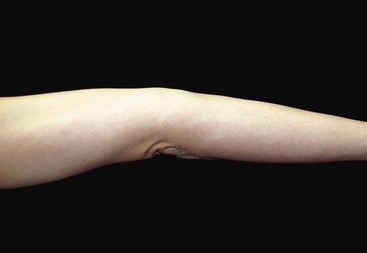
FIGURE 1-2 Hyperextensibility of elbow in a healthy 28-year-old woman with hyperelastic skin features.
Epidermis
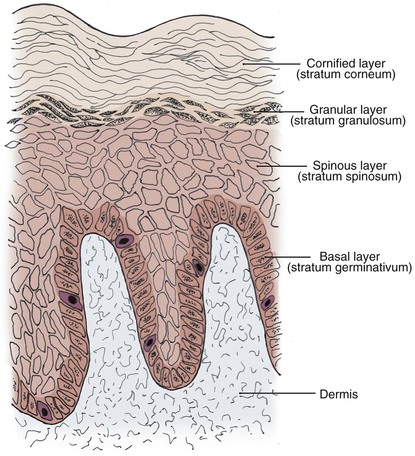
The epidermis, the outermost layer of the skin, is a continually renewing, keratinizing, stratified, squamous epithelium. All epidermal appendages, including hair follicles, sebaceous glands, and eccrine and apocrine sweat glands, derive from this layer. The epidermis consists of four distinct cell types: keratinocytes, melanocytes, Langerhans cells, and Merkel cells. The predominant cell type is the keratinocyte, which constitutes at least 80% of epidermal cells. Four clearly defined layers are identified in the epidermis (Fig. 1-5): basal layer (stratum germinativum), spinous layer (stratum spinosum), granular layer (stratum granulosum), and cornified layer (stratum corneum). The basal layer is the deepest layer in the epidermis. It is composed of a single germinative layer of columnar-shaped keratinocytes that attach to the basement membrane zone and give rise to the more superficial epidermal layers. The next layer, the spinous layer, is several cells thick and composed of polygonal cells with abundant eosinophilic cytoplasm. Small spiny desmosomal attachments between the spinous cells are evident under light microscopy. As the spinous cells migrate superficially and differentiate into granular cells, they become larger and flatter. The granular layer, usually one to four cells thick, is composed of cells with deeply basophilic keratohyalin granules. Further maturation occurs in the outermost stratum corneum, which is highly variable in thickness. In this layer, keratinocytes lose their nuclei and flatten to form plates of keratin, which are shed as “dead skin.” The stratum corneum is thickest on the palms and soles and thinnest on the eyelids and genitalia. Total epidermal turnover time from the basal layer to the stratum corneum is approximately 30 days. The thickness of the epidermis is generally about 0.075 to 0.15 mm. The epidermis is thin at birth, becomes thicker during puberty and early adulthood, and thins in the fifth and sixth decades of life.1,3
Melanocytes
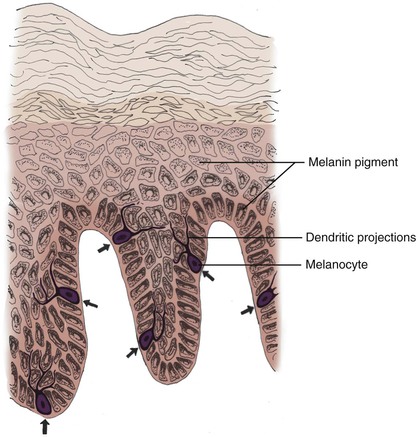
Melanocytes are dendritic, pigment-synthesizing cells of neural crest origin with clear cytoplasm confined to the basal layer. The ratio of melanocytes to basal cells ranges from 1 : 4 on the cheek to 1 : 10 on the limbs. The function of melanocytes is to produce protective melanin pigment. Melanin is packaged in the form of melanosomes, which are transported through stellate dendritic projections to a group of adjacent keratinocytes in the basal and spinous layers (epidermal melanin unit). The keratinocytes engulf the melanosomes and arrange the pigment in an umbrella-like distribution over the nuclei, protecting them from potentially harmful ultraviolet irradiation (Fig. 1-6). This partly explains why people with less pigmentation are at greater risk for development of cutaneous malignant neoplasms, such as basal cell carcinoma, squamous cell carcinoma, and melanoma.5,6 The number of melanocytes does not differ between races. The number and size of melanosomes is greater in pigmented skin and accounts for the darker skin color seen in pigmented persons. In vitiligo, melanocytes are completely absent. In albinism, melanocytes are present but lack the enzyme tyrosinase. Without tyrosinase, tyrosine cannot be transformed into melanin. Tyrosinase activity and melanocyte density decrease with age.7
Langerhans Cells
Langerhans cells are bone marrow–derived, antigen-processing, and antigen-presenting cells found mainly in the suprabasal epidermal layers. They are, however, not unique to the epidermis and are found in other squamous epithelia and in the normal dermis. In routine histologic preparations, Langerhans cells are pale-staining cells that are difficult to identify and more readily demonstrated with special stains or immunohistochemistry. Like melanocytes, Langerhans cells are characterized by dendritic processes. The cytoplasm, as seen by electron microscopy, contains small racket-shaped structures known as Birbeck or Langerhans cell granules. Langerhans cells are responsible for recognizing and presenting antigens to lymphocytes in the skin and are implicated in the pathologic mechanism underlying allergic contact dermatitis and skin allograft reactions. The number of Langerhans cells decreases after ultraviolet irradiation. This results in a diminished capacity for immune surveillance, which may play a role in cutaneous carcinogenesis. The number of Langerhans cells also decreases with age.8
Merkel Cells
Merkel cells are neuroendocrine cells of epidermal origin that function as slow-adapting mechanoreceptors primarily concerned with touch sensation.9,10 They are predominantly found among basal keratinocytes in areas of high tactile sensitivity, such as the lips, digits, oral cavity, and hair follicles. At these sites, Merkel cells often aggregate in specialized structures, called tactile disks or touch domes, in close association with peripheral nerve endings to form the Merkel cell–neurite complex. Merkel cells, like Langerhans cells, are difficult to identify in light microscopy without the use of immunohistochemical markers. Ultrastructurally, Merkel cells are characterized by membrane-bound, dense-core granules. These granules are similar to the neurosecretory granules found in neurons and contain neurotransmitter-like substances and markers of neuroendocrine cells. Merkel cell carcinoma, or cutaneous neuroendocrine carcinoma, most likely arises from epidermal Merkel cells.11
Dermal-Epidermal Junction
The epidermis is attached to the dermis by a basement membrane zone known as the dermal-epidermal junction (Fig. 1-7).12 By light microscopy, the dermal-epidermal junction is identified as a thin pink band that stains positive with periodic acid–Schiff stain. This complex zone provides mechanical support to the epidermis and acts as a semipenetrable barrier to chemicals and other substances. Keratin filaments within the basal keratinocyte condense and attach to an electron-dense plaque at the inferior aspect of the cell membrane, known as the hemidesmosome. The hemidesmosomes are firmly anchored to the underlying lamina densa through connecting anchoring filaments in the lamina lucida. The lamina densa is attached to anchoring plaques in the underlying dermis by anchoring fibrils and elastic fibers. Anchoring fibrils, mainly composed of type VII collagen, are degraded by collagenases and are absent in new scars. The importance of the dermal-epidermal junction can be surmised from a variety of inherited and acquired diseases of the skin in which different components are absent, altered, or destroyed, resulting in dermal-epidermal separation, such as in epidermolysis bullosa.13
Epidermal Appendages
Hair Follicle
The hair follicle is the main component of a structure known as the pilosebaceous unit, which also includes the hair shaft, sebaceous gland, arrector pili muscle, and sensory end organ (Fig. 1-8). The pilosebaceous unit has motor and sensory functions and is responsible for the production of hair and sebum. On the scalp, the follicular component is predominant, resulting in thick, dense terminal hair. Fine, thin vellus hair is found on the temples and forehead. On the nasal tip, the sebaceous component predominates, and the total structure is sometimes termed sebaceous follicle. The complete pilosebaceous unit is absent on the palms, soles, and mucous membranes. Re-epithelialization of partial-thickness wounds occurs not only from the wound edges but also from the pilosebaceous units.
Longitudinally, the hair follicle is divided into three regions (see Fig. 1-8). The uppermost portion, the infundibulum, extends from the skin surface to the opening of the sebaceous duct into the follicle. The segment between the follicular opening of the sebaceous duct and the bulge is known as the isthmus. The inferior portion lies below the area of the bulge and includes the lowermost part of the follicle and the hair bulb. The bulge is a region enriched with follicular epithelial stem cells and the insertion site of the arrector pili muscle. This muscle inserts into the perifollicular connective tissue sheath around the bulge and extends obliquely and upward into the papillary dermis. Contraction of the arrector pili muscle, innervated by sympathetic nerve fibers, makes the hair “stand up” (goose bumps) as it is pulled from an oblique to a vertical position, providing a greater thermal barrier to the skin. Sensory nerves are located around the isthmus and inferior portion of the hair follicle. These nerves are stimulated as a touch receptor when the hairs are touched.
The internal organization of the hair follicle is best conceptualized as a series of distinct concentric layers (Fig. 1-9). The most peripheral layer, the outer root sheath, is contiguous with the epidermis and is lined by the dermal-epidermal junction. In the infundibulum, the outer root sheath consists of all layers of the epidermis. Distal to the follicular opening of the sebaceous duct, the outer root sheath consists of a markedly vacuolated spinous layer due to the presence of glycogen. Next is the inner root sheath, which consists of three distinct layers: Henley’s layer, Huxley’s layer, and a cuticle. Innermost is the hair shaft, which also has three layers: its cuticle, the cortex that forms the bulk of the hair shaft, and the variable central medulla. The medulla is absent in lanugo and vellus hairs. The inner root sheath and hair shaft are derived from a proliferation of germinative cells, known as the matrix, at the base of the hair follicle. The distal hair bulb forms an invagination around the follicular papilla, which is richly vascularized and contains abundant nerve endings.
Hair follicles undergo cycles of growth, involution, and rest (Fig. 1-10). During the growing phase, or anagen, matrix keratinocytes in the bulb proliferate rapidly and produce the growing hair. During the involutional phase, or catagen, the matrix cells abruptly cease proliferating, and the lower portion of the hair follicle involutes. In telogen, the resting phase, the inferior portion of the follicle is lost, and the follicular papilla comes to rest at the height of the bulge. The club-shaped telogen hair is typically shed from the follicle during telogen or the subsequent anagen. Human hair growth is cyclic, but because each follicle functions independently, humans do not shed hair synchronously. On the human scalp, approximately 85% of hairs are in anagen, and the average length of the growing phase is 3 to 4 years. The number of hair follicles on the scalp is approximately 100,000 in people with brown or black hair, about 10% greater in blonds, and 10% less in redheads.
Sebaceous Glands
Sebaceous glands are unilobular or multilobular structures that connect to the hair follicle by a squamous epithelial duct. Each lobule consists of a peripheral cuboidal or flattened germinative cell layer. These cells give rise to a central, lipid-laden, vacuolated cell population with characteristic clear to foamy cytoplasm. The glands secrete sebum through the sebaceous duct into the follicle and onto the surface of the skin. Sebum, a complex lipid mixture, acts as an emollient to the hair and skin and may have a protective function.
Dermis
Collagen
Collagen is the principal component of the dermis and accounts for approximately 75% of the dry weight of skin. Collagen fibers are synthesized by fibroblasts and provide both tensile strength and elasticity to the skin. Approximately 85% of dermal collagen is type I collagen, which is found predominantly as thick broad bands in the reticular dermis. Type III collagen constitutes roughly 10% of dermal collagen and forms the fine collagen fibers located primarily in the papillary dermis. Collagen types IV and VII are located mainly in the basement membrane zone.14
Collagen fibers are continuously being degraded by proteolytic enzymes called matrix metalloproteinases, such as collagenase, and replaced by newly synthesized fibers. Ultraviolet irradiation induces matrix metalloproteinases in the epidermis and dermis, leading to dermal collagen degradation. This is manifested histologically as the disorganization of collagen fibrils and clinically as skin wrinkling in photoaging. Tretinoin inhibits the induction of matrix metalloproteinases and improves the appearance of photoaged skin by reducing fine lines and wrinkles.15,16 It is believed that matrix metalloproteinases are induced by CO2 laser treatment to degrade photodamaged collagen. This degradation is followed by the formation and deposition of new collagen.17
Elastin
Elastic fibers in the dermis return the skin to its normal configuration after being stretched or deformed. The normal fibers are not readily seen on routine histology without the aid of special elastic tissue stains. Elastic fibers in the dermis are synthesized primarily by fibroblasts. In the papillary dermis, the fibers are thin and run perpendicular to the skin surface, whereas those in the reticular dermis are thicker and run parallel to the skin surface. Like collagen, elastic tissue is in a continuous state of synthesis and degradation by matrix metalloproteinases such as elastase. Elastic tissue is composed of a protein elastin and a microfibrillar matrix that contains fibrillin, a glycoprotein, and other components. The amino acids desmosine and isodesmosine are unique to elastin.18
Ground Substance
Ground substance surrounds and embeds the fibrous components of the dermis and is found in all tissues of the body. It consists predominantly of proteoglycans (such as chondroitin sulfate and dermatan sulfate), glycosaminoglycans (such as hyaluronic acid), and filamentous glycoproteins (such as fibronectin). In the dermis, ground substance is primarily synthesized by fibroblasts and appears as fine mucinous stroma on routine histologic stains. Ground substance plays a role in skin hydration and helps preserve the tensile elasticity of compressed skin by redistributing the pressure forces. Relative dehydration in the skin due to displacement of ground substance is partly responsible for the phenomenon termed mechanical creep. Mechanical creep plays a role in the physiologic factors of immediate intraoperative tissue expansion.19
Cellular Component
Monocytes, macrophages, and dermal dendrocytes constitute the phagocytic cells in the dermis. Mast cells are specialized secretory cells present in greatest density in the papillary dermis, near the basement membrane zone, and around epidermal appendages, blood vessels, and nerves. Mast cells are the primary effector cells in the onset of an allergic reaction and may be important in initiating the repair of damaged skin.20
Vasculature
Soft tissue surgery on the head and neck usually heals particularly well because of a rich vascular supply. Most of the blood flow in the skin is directed toward the more metabolically active components, namely, the epidermis, the follicular papillae, and the epidermal appendages. Two vascular plexuses connected by communicating vessels are present in the dermis (Fig. 1-11).21 At the junction of the dermis and subcutaneous adipose tissue lays the deep vascular plexus, which receives its vascular supply from musculocutaneous arteries perforating the subcutaneous adipose tissue. Arterioles from the deep vascular plexus supply the epidermal appendages and the superficial vascular plexus. The superficial vascular plexus lies in the superficial aspect of the reticular dermis and gives rise to a rich capillary loop system in the papillary dermis. This capillary loop system abuts the epidermis and provides it with nutrients by diffusion. The dermis also contains a lymphatic system that resembles the vascular plexuses.
Nerve Supply
A rich cutaneous nerve supply consisting of free nerve endings and specialized corpuscular receptors permits the body to accurately interpret the continuous bombardment of stimuli received from the external environment (Fig. 1-12).22 Temperatures, pain, and itch are transmitted by both myelinated and nonmyelinated free nerve endings particularly common in the papillary dermis just beneath the epidermis. Specialized receptors include Meissner’s and pacinian corpuscles. Meissner’s corpuscles mediate fine touch sensation and are predominantly found in the papillary dermis of the hands, feet, lips, and forearms. Pacinian corpuscles are involved in the appreciation of deep pressure and vibration. They are primarily found in the deep dermis and subcutaneous adipose tissue of the palms, soles, dorsal surfaces of digits, and genitalia. Efferent nerves in the dermis innervate blood vessels and appendageal structures and regulate their function.
References
1. Chug, DH, Hake, AR, Holbrook, K, et al. The structure and development of skin. In Freedberg IM, Eisen AZ, Wolff K, et al, eds.: Fitzpatrick’s dermatology in general medicine, 6th ed, New York: McGraw-Hill, 2003.
2. White, CF, Bigby, M, Sangüeza, OP. What is normal skin? In: Arndt KA, Leboit PE, Robinson JK, et al, eds. Cutaneous medicine and surgery—an integrated program in dermatology. Philadelphia: WB Saunders, 1996.
3. McKee, PH. Pathology of the skin with clinical correlations. London: Mosby-Wolfe; 1996.
4. Goldsmith, LA. My organ is bigger than your organ. Arch Dermatol. 1990; 126:301.
5. Lens, MB, Dawes, M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br J Dermatol. 2004; 150:179.
6. Lock-Andersen, J, Drzewiecki, KT, Wulf, HC. Eye and hair colour, skin type and constitutive skin pigmentation as risk factors for basal cell carcinoma and cutaneous malignant melanoma. A Danish case-control study. Acta Derm Venereol. 1999; 79:74.
7. Yaar, M, Gilchrest, BA. Ageing and photoageing of keratinocytes and melanocytes. Clin Exp Dermatol. 2001; 26:583.
8. Grewe, M. Chronological ageing and photoageing of dendritic cells. Clin Exp Dermatol. 2001; 26:608.
9. Morrison, KM, Miesegaes, GR, Lumpkin, EA, et al. Mammalian Merkel cells are descended from the epidermal lineage. Dev Biol. 2009; 335:76.
10. Van Keymeulen, A, Mascre, G, Youseff, KK, et al. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J Cell Biol. 2009; 187:91.
11. Bichakjian, CK, Lowe, L, Lao, CD, et al. Merkel cell carcinoma: critical review with guidelines for multidisciplinary management. Cancer. 2007; 110:1.
12. Burgeson, RE, Christiano, AM. The dermal-epidermal junction. Curr Opin Cell Biol. 1997; 9:651.
13. Uitto, J, Pulkkinen, L. Molecular genetics of heritable blistering disorders. Arch Dermatol. 2001; 137:1458.
14. Burgeson, RE, Nimni, ME. Collagen types. Molecular structure and tissue distribution. Clin Orthop. 1992; 282:250.
15. Fisher, GJ, Wang, ZQ, Datta, SC, et al. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997; 337:1419.
16. Fligiel, SE, Varani, J, Datta, SC, et al. Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase-1 in vitro. J Invest Dermatol. 2003; 120:842.
17. Orringer, JS, Kang, S, Johnson, TM, et al. Connective tissue remodeling induced by carbon dioxide laser resurfacing of photodamaged human skin. Arch Dermatol. 2004; 140:1326.
18. Christiano, AM, Uitto, J. Molecular pathology of the elastic fibers. J Invest Dermatol. 1994; 103:53S.
19. Johnson, TM, Brown, MD, Sullivan, MJ, et al. Immediate intraoperative tissue expansion. J Am Acad Dermatol. 1990; 22:283.
20. Church, MK, Clough, GF. Human skin mast cells: in vitro and in vivo studies. Ann Allergy Asthma Immunol. 1999; 83:471.
21. Braverman, IM. The cutaneous microcirculation. J Investig Dermatol Symp Proc. 2000; 5:3.
22. Johansson, O. The innervation of the human epidermis. J Neurol Sci. 1995; 130:228.