CHAPTER 4 Anatomy and Mechanics of the Abdominal Muscles
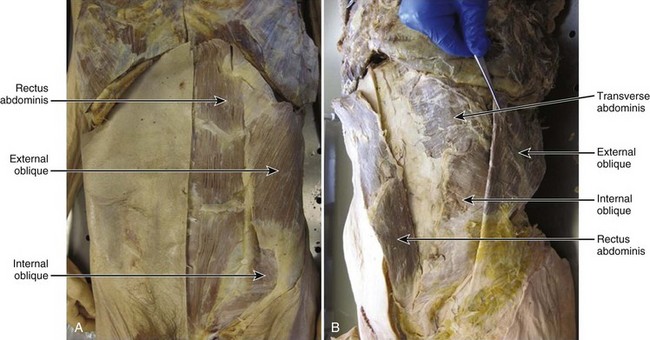
Relatively little is known about the mechanics of the abdominal muscles. It is clear, however, that abdominal wall muscles are morphologically unique and are responsible for an array of mechanical roles, including the production and control of spine movement, stabilization of the spinal column, generation of intra-abdominal pressure (IAP), and respiration. The four muscles of the abdominal wall consist of three broad, sheetlike muscles that overlay one another (external oblique, internal oblique, transverse abdominis) and the more anterior rectus abdominis, across which the sheetlike muscles are linked (Fig. 4–1). This chapter discusses abdominal muscular anatomy, characteristics and considerations that affect the force-generating and moment-generating capabilities of the muscles, novel data regarding the architectural properties of the muscles, mechanical roles and consequences of the muscles, and finally possible relationships between abdominal muscle function and low back pain and injury.
Gross Morphologic Anatomy
Rectus Abdominis
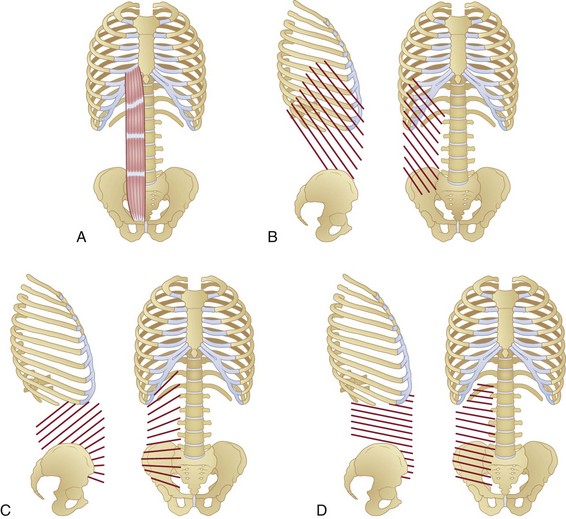
The rectus abdominis runs longitudinally down the anterior trunk and is divided into two separate muscles (right and left) by the linea alba. The muscle originates from the lower sternum and costal cartilage of the fifth to seventh ribs,1 and it inserts into the pubic symphysis (Fig. 4–2A). It is divided transversely along its length by tendinous intersections (normally three) that separate the muscle into four regions (in-series with one another) of muscle fibers. These intersections often do not span the complete mediolateral distance across the muscle, with some fibers therefore extending extra length.2,3
The mechanical role of the septa of the tendinous intersections is unclear, but two main hypotheses have been proposed. The first purports that these tendons provide bending locations within the muscle, allowing it to fold effectively as the trunk flexes forward, preventing muscle fiber bunching that could occur with extreme shortening of long fibers.4 The second hypothesis pertains to the transverse mechanical strength of the rectus abdominis; the oblique and transverse abdominis muscles can apply substantial forces transversely across or, via anchoring on the rectus sheath, through the rectus abdominis.5 The tendinous intersections may provide transverse strength to rectus abdominis fibers, preventing them being pulled apart by the forces transmitted by the external oblique, internal oblique, and transverse abdominis.
External Oblique
The external oblique is a large sheetlike muscle, the most superficial of the abdominal wall, with fibers spanning from the rib cage (5th to 12th ribs),1 running inferomedially, and attaching across two main anatomic regions: the rectus sheath (covering the rectus abdominis) and the iliac crest (Fig. 4–2B). Muscle fiber orientations have been described for the external oblique, internal oblique, and transverse abdominis relative to a line connecting left and right anterior superior iliac spines (ASIS)6 and are described here as such. External oblique fibers originating superior to the base of the rib cage are oriented approximately 50 degrees (standard deviation [SD] 7 degrees) inferomedially6 and terminate to form the most superficial layers of the rectus sheath. Fibers originating between the base of the rib cage and iliac crest run approximately 59 degrees (SD 11 degrees) inferomedially (slightly more vertically).6 The most inferior fibers originating from the rib cage that do not terminate on the iliac crest become aponeurotic superior to the ASIS,6 form the lower-most superficial portions of the rectus sheath, and insert into the pubic symphysis. Some authors report that a small proportion of posterior fibers run from the mid-posterior of the iliac crest and terminate as part of the middle7 and posterior8 layers of the lumbar fascia, although this is not a universal finding.
Internal Oblique
The internal oblique is a large sheetlike muscle that lies deep to the external oblique and superficial to the transverse abdominis. Within different regions of the muscle, its fibers run at angles highly oblique to one another, creating a fanlike appearance (Fig. 4–2C). Fibers originating from the base of the rib cage run from the costal margins of the 10th to 12th ribs1 to the iliac crest at an angle of approximately 48 degrees (SD 13 degrees) superomedially.6 These fibers terminate across the rectus abdominis to create deep and superficial layers of the rectus sheath. Fibers spanning the region between the base of the rib cage and the iliac crest run at an angle of approximately 35 degrees (SD 10 degrees) superomedially.6
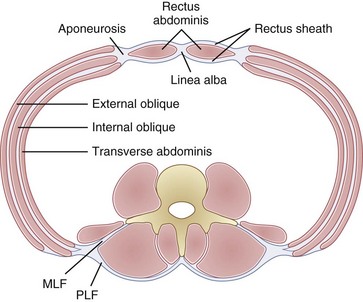
All of the fibers in this region attach to the iliac crest but can terminate at one of three anatomic locations: (1) medial fibers terminating as deep and superficial layers of the rectus sheath; (2) more lateral fibers terminating on the costal margin of the base of the rib cage; (3) and the most posterior fibers terminating to form a fascial layer deep to the erector spinae (middle layer of lumbar fascia), which ends at the transverse processes,7–9 with some fibers adjoining a superficial fascial layer overlying the erector spinae (posterior layer of lumbar fascia), which attaches to the spinous processes (Fig. 4–3).8,9 In the final functional region of the abdominal wall, fibers originate from the anterior of the iliac crest, terminate as deep and superficial layers of the rectus sheath, and are oriented at completely different angles to the more superior fibers. The fibers originating between the iliac crest and the ASIS run approximately horizontally and become continually more inferomedially oriented below the ASIS (up to an angle of approximately 16 degrees [SD 10 degrees] inferomedially).6
Transverse Abdominis
The transverse abdominis is the deepest of the sheetlike abdominal wall muscles. Its fibers above the base of the rib cage originate from the costal margins of the ribs and terminate as the deepest layers of the rectus sheath (Fig. 4–2D). These fibers run almost horizontally, with only a slight inferomedial orientation of 3 degrees (SD 9 degrees).6 The fibers between the base of the rib cage and iliac crest run from the posterior fascia (terminating as the same fascial layers as the internal oblique [discussed earlier; see Fig. 4–3]) to the rectus sheath and are oriented at approximately 13 degrees inferomedially. Fibers originating from the anterior iliac crest and ASIS run approximately 21 degrees (SD 11 degrees) inferomedially and terminate again as the deepest slips of the rectus sheath.6
Rectus Sheath
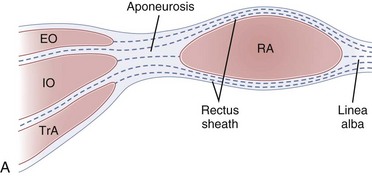
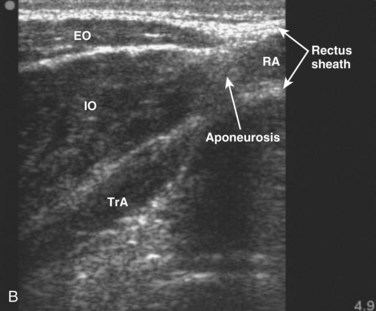
Each of the abdominal wall muscles attaches at the anterior margin of the torso through an aponeurosis that ultimately leads to the formation of the rectus sheath (Fig. 4–4). Detailed investigations of the morphology of the abdominal wall aponeuroses reveal a bilayered arrangement stemming from each muscle. Specifically, the aponeurosis of each of the external oblique, internal oblique, and transverse abdominis can be anatomically separated into two layers, one arising from the superficial fascial layer of the muscle and the other arising from the deep fascial layer of the muscle.10,11 The superficial and deep layers of the rectus sheath comprise three fascial layers (superficial—two from external and one from internal oblique, and deep—one from internal oblique and two from transverse abdominis). It is thought that the functional or mechanical purpose of this structural arrangement is to enable transfer of forces generated by the abdominal wall muscles (external oblique, internal oblique, transverse abdominis) around the torso, creating a pressurized abdominal cavity that assists in stiffening the spinal column.4,12 Although highly variable and inferior to the umbilicus, most individuals display a gradual movement of deep internal oblique and transverse abdominis aponeurotic fibers from the posterior to the anterior of the rectus sheath; this may be due to the need for increased resistance to bulging of the anterior wall in the lower abdomen.
The connective tissue networks overlying each of the three abdominal wall muscles, giving way to the formation of the rectus sheath, also provide a strong mechanical shear linkage between the muscle layers. This linkage has been shown to transmit forces mechanically among the muscle layers in a rat preparation13 and has been hypothesized to afford composite laminate structural properties that assist in strengthening the abdominal wall and stiffening the spinal column. Additional study of mechanical interactions between the muscle layers is needed to further understanding of abdominal muscle function, in particular related to deformation during contraction or movement and force generation and transmission around the abdomen.
Mechanical Properties of the Abdominal Muscles
Muscle force–generating capacities depend on architectural characteristics.14,15 Specifically, the number, length, and orientation of fibers acting in parallel with the axis of force generation, together with the moment arms around the joints the muscle crosses, determine the functional capabilities of a muscle.
Most information regarding the physiologic cross-sectional area (PCSA) of the abdominal muscles comes from various imaging modalities, including computed tomography (CT), magnetic resonance imaging (MRI), and ultrasonography. More recent work in the authors’ laboratory has further documented the PCSA of the abdominal muscles from cadaveric dissections of 11 donors ranging in age from 52 to 94 years (mean age 77.7 years [SD 16.3 years]).16 McGill and colleagues17 and Marras and colleagues,18 measuring young healthy men using CT and MRI, measured a rectus abdominis PCSA of approximately 8 cm2 at the level of the L4-L5 disc. This value is much larger than the mean 3.3 cm2 measured from the elderly cadavers.16 Similarly, the overall force-generating capacities of the internal and external oblique muscles, documented in McGill19 measuring healthy young men, were much larger than in the cadaveric dissections of Brown and colleagues16 (approximately 16 cm2 and 19 cm2 vs. 6.6 cm2 and 8.6 cm2 for the external oblique and internal oblique). This discrepancy between the data from cadavers and imaging data from young men likely points to an aging-related atrophy of the muscles. Similar measures of transverse abdominis PCSA have been reported, however, between the two modalities (approximately 5 cm2 in the studies by McGill19 and Brown and colleagues16). In addition, Marras and colleagues18 reported PCSA gender differences (males greater than females) for external oblique and internal oblique in young individuals and reported an increasing rectus abdominis PCSA toward lower vertebral levels; these findings were not apparent in the cadaveric analyses.16
The ability of a muscle to generate force also depends on the instantaneous length and velocity of muscle fibers or, more specifically, of the sarcomeres that make up the muscle fibers.20 The more recent study by Brown and colleagues16 reported fixed sarcomere lengths of the abdominal muscles in the approximate neutral spine posture. Sarcomere lengths of the rectus abdominis and external oblique (mean 3.29 µm [SD 0.22 µm] and 3.18 µm [SD 0.37 µm] for the rectus abdominis and external oblique) in this position were well above optimal (optimal approximately 2.70 µm in human muscle21), whereas the lengths of the internal oblique and transverse abdominis (mean 2.61 µm [SD 0.21 µm] and 2.58 µm [SD 0.16 µm] for the internal oblique and transverse abdominis) were slightly below optimal. Because anteriorly acting fibers of the internal oblique shorten during flexion, whereas more laterally acting fibers lengthen, biomechanical modeling predicts the muscle, as a whole, to produce maximum force near the neutral spine posture. The rectus abdominis and external oblique (which shorten during spine flexion) and the transverse abdominis (which primarily lengthens during spine flexion) act together at optimal force-generating length in the mid-range of lumbar flexion, where the internal oblique can still generate in the range of 90% or greater of its maximum force.
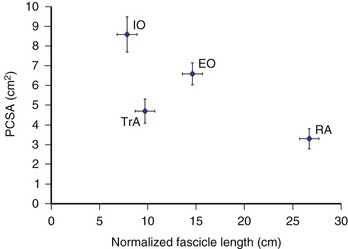
Normalized fiber lengths were also calculated for the muscles to provide an indication of their excursion capabilities (Fig. 4–5).16 A muscle with longer fibers can produce force over a greater range of lengths because a greater number of sarcomeres act to produce this overall length change effectively. This also has direct implications for the velocities at which a muscle can produce force because in a longer muscle each sarcomere experiences a lower relative velocity compared with a shorter muscle-changing length at the same rate. The data in Figure 4–5 imply that the rectus abdominis and external oblique have the potential to undergo greater length changes and produce force at higher absolute velocities compared with the internal oblique and transverse abdominis. How these muscles adapt to changes in body shape (e.g., to chronic visceral weight gain or loss) has yet to be explored, and any potential adaptations (or lack thereof) can have important implications for abdominal muscle function related to obesity.
Similar to the PCSA literature, functional moment arms of the abdominal muscles have been reported based on imaging data. Caution is needed, however, when examining this literature because McGill and colleagues22 and Jorgensen and colleagues23 have detailed large underestimations of the moment arms when subjects are positioned supine compared with upright standing, owing to the depression of the abdominal cavity. Anatomically detailed biomechanical models of the abdominal muscles, in relation to skeletal attachments and specific spinal joints, can provide a more justified assessment of the moment-generating capacity of these muscles.
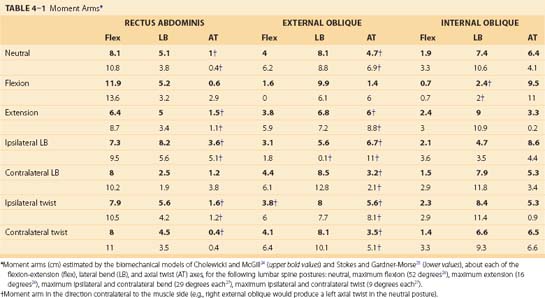
In particular, Cholewicki and McGill24 and Stokes and Gardner-Morse25 reported anatomically detailed representations of the spine skeletal and muscle geometry. Both of these models represented muscles as a series of straight lines of action acting between rigid locations on a movable skeleton. The moment-generating potential of a muscle depends on the amount of force that it can generate and its moment arm about a given joint. The abdominal muscles cross numerous individual spinal joints, including the entire lumbar spine. Spinal disc centers, representing rotational and translational joint centers, are also reported in these models, and using the knowledge of the muscle lines of action and these joint centers, moment arms around all three of the functional orthopaedic spine axes (flexion-extension, lateral bend, axial twist) can be computed (Table 4–1).
Two very important points need to be made about the moment-generating capabilities of the external oblique, internal oblique, and transverse abdominis. Because of their broad attachments across the rib cage, iliac crest, and rectus sheath, different fiber regions can have opposing moment-generating capabilities. For example, McGill,28 Stokes and Gardner-Morse,25 Dumas and colleagues,29 and Marras and Sommerich30 recognized that the posterior fibers of the internal oblique, attaching to the posterior elements of the spine, produce an extensor moment in the neutral posture, whereas the remaining bulk of the fibers produce flexor moments. Inferior fibers of the transverse abdominis produce a contralateral rotation of an unfixed pelvis (producing a relative ipsilateral rotation of the spine), whereas the more superior fibers attaching to the posterior elements of the spine and rib cage may generate contralateral rotation of the spine.
The second major point that needs to be understood is that the moment-generating capabilities of these muscles and muscle fiber regions are highly dependent on spine and trunk orientation (see Table 4–1). Examining these models, it becomes apparent that the moment arms and moment-generating capabilities of the abdominal muscles can change greatly as the spine rotates into various postures (see Table 4–1). In the neutral posture, the external oblique can produce fairly substantial spine flexion (moment arm 4 to 6.2 cm), lateral bend (moment arm 8.1 to 8.8 cm), and contralateral axial twist (moment arm contralateral 4.7 to 6.9 cm) moments. When the lumbar spine is fully flexed, the external oblique loses its ability to produce a flexor moment (moment arm 0 to 1.6 cm), however, and becomes an ipsilateral twister of the lumbar spine (moment arm now ipsilateral 1.4 to 6 cm). Attributing gross mechanical roles to the abdominal muscles without consideration of spine orientation and position is folly and can lead to erroneous functional interpretations of actions and roles. This has additional importance in understanding the roles of each of these muscles in stabilizing the lumbar spine, which are discussed later.
The transverse abdominis has been less frequently modeled in biomechanical representations of the spine and is absent from the published models of Cholewicki and McGill,24 Marras and Sommerich,30 and Stokes and Gardner-Morse.25 The recent focus on this muscle in the clinical literature, to be discussed later, indicates that consideration and study of its action is warranted. McGill,19 using ultrasonography and CT, modeled the force action of the transverse abdominis as projected along the anterior of the torso through the rectus abdominis and rectus sheath complex. This consideration produced an average flexor moment arm of approximately 0.8 cm in a neutral spine posture. Alternatively, modeling its force action as pulling laterally through its attachments to the transverse and spinous processes, the muscle would act with a small extension moment arm (approximately 1 cm) in the neutral spine posture. Considering either or both of these actions of this muscle, it is clear that the transverse abdominis produces little moment about the flexion-extension axis.
The transverse abdominis does produce more substantial lateral bend and twist moments; however, its net relationship to ipsilateral versus contralateral twist is unclear. Although most of the fiber attachments suggest a possible contralateral twist moment similar to the external oblique,29 its neural activation is much higher during ipsilateral twist efforts.31,32 As mentioned earlier, how the transverse abdominis moment arm and moment-generating potential change as the spine rotates around each of its functional axes needs to be considered.
Neural Control of Abdominal Muscles
Force and moment generation are also highly dependent on neural signals received by muscles from the central nervous system. Each of the four abdominal muscles receives efferent nerve supply from multiple spinal levels: rectus abdominis, lower six thoracic nerves33; external oblique, lateral cutaneous branches of lower eight thoracic nerves34; internal oblique and transverse abdominis, lower six thoracic nerves and first lumbar nerve.33 Woodley and colleagues3 also showed that some longer fascicles within each of the four abdominal muscles contain multiple motor endplate bands, suggesting that individual fascicles may receive multiple nerve supplies. Innervation from a wide span of spinal nerves across different regions of the muscles seems to enable specific regional activation of these muscles. For example, Mirka and colleagues35 and Urquhart and Hodges32 showed regionally different activation magnitudes in the external oblique and transverse abdominis. More convincingly, Moreside and colleagues36 revealed activation timing differences with regions of the rectus abdominis and external oblique, reporting antiphasic patterns of activation between anterior and lateral regions of the external oblique and between upper and lower regions of the rectus abdominis in Middle Eastern style–trained dancers.
Corresponding to their mechanical orientation, the abdominal muscles are most active in loading scenarios that require the generation of flexion, lateral bend, and twist moments. The abdominal muscles, having a postural and stabilizing role, display consistent levels of low tonic activation during even minimal loading upright tasks. Masani and colleagues37 and Gregory and colleagues,38 both during sitting, and Gregory,39 during standing, found average abdominal muscle activations never to exceed 3% of their maximum voluntary capability. Gregory and colleagues38,39 indicated similar low levels of activation during seated work on an unstable surface (exercise ball) designed to challenge the maintenance of spine stability. More dynamic tasks such as walking rarely involve abdominal muscle activation greater than 5% of maximum.40 Although these activation levels are seemingly modest, they are probably crucial for maintenance of a stable spine, discussed later.
Mechanical Consequences: Compression Force, Shear Force, and Stability
Contraction of trunk muscles exerts forces onto and stabilizes the spinal column. The consideration and study of spinal forces cannot be separated from stability because they are inextricably linked. The spinal column is a highly unstable structure that, in the absence of muscular attachments, would buckle at loads far below the weight of the upper body.41,42 The bulk of the loading experienced by the lumbar spine is developed and imposed by the spinal musculature.43,44 The predominant reason for the extreme loading imparted by the muscles onto the spine is the large relative amounts of coactivation that occur during trunk muscle recruitment.44–46 This coactivation serves to stabilize the spine47–49 and as a secondary consequence imparts additional load on the spine.49–51
Because most spine-loading events produce net extensor moments (the spine extensors generate the dominant loading moment), the abdominal muscles most often serve the role of coactivators. It has further been definitively established that some coactivation is necessary to ensure a stable spine.24,49 Coordinated contraction of the abdominal muscles plays a vital role in maintaining the mechanical integrity of the spinal column during light and heavy loading scenarios. Finally, abdominal muscles are mechanically well equipped to stabilize the spine owing to their relatively large moment arms52–54 around the three anatomic axes in various postures (Table 4–1).
Despite this information, it is important to consider that activation and contraction of the abdominal muscles do not ensure stability and that poorly balanced patterns of contraction can compromise stability.55 Finally, based on the differing demands under which the spine can be placed (e.g., different anatomic loading axes, spine orientation and position), it is likely that no abdominal muscle, or no muscle in general, should be considered the most important spine stabilizer.56,57
Biomechanical models can be used to estimate forces applied by individual muscles to the intervertebral discs and vertebral bodies and to estimate whether, under a given loading scenario, the spine is considered stable or unstable. With the exception of the transverse abdominis, the abdominal muscles are oriented such that they have potential to apply significant compressive loads to the lumbar spine. Because of the large role that the abdominal muscles play in generating twisting moments, actions that involve twisting or twisted postures often carry substantial compressive penalties.58 Exercises and work-related tasks (e.g., lifting while twisted or twisting) that substantially recruit the abdominal muscles have the potential to impart tremendous compressive loads on the lumbar spine and must be employed with care. Anteroposterior shear forces are more difficult to interpret because the line of action of the fibers in different regions of the muscles, the relative angle of the endplates at each vertebral level, and the changes in these variables as the spine rotates all must be considered.59 The general thought is that the rectus abdominis, external oblique, and transverse abdominis produce a net anterior shear load, and the internal oblique produces a net posterior shear load on the lumbar spine.
IAP is often discussed relative to abdominal muscles, and its effects on spine mechanics are often debated. The contraction of the abdominal wall muscles and IAP are directly linked.60 Specifically, IAP is generated and controlled by abdominal wall muscle contraction. As discussed earlier, abdominal muscles often contract as part of a coactivation strategy in response to a physical exertion of the back muscles, resulting in a concomitant increase in IAP. Early biomechanical models suggested that IAP acted to unload the spinal column during back loading tasks, by creating a net trunk extensor moment through the diaphragm.61 This theory has since been negated based on an understanding of the exact mechanism of abdominal muscle contraction coinciding with the increase in IAP—this imposes compressive forces on the spine that negate unloading owing to an IAP-induced extensor moment.62–64 Correlations have been shown between IAP generation and spine stability,60,64–66 but it is difficult to isolate the level of the stabilizing effect that results from the IAP, the abdominal muscle contraction, or a combination thereof.
Finally, it has been hypothesized that IAP, which creates a firm abdomen around which the abdominal muscles can contract, acts to improve their ability to generate moments through an increased mechanical advantage.67 This final effect is likely saturated at low levels of tonic activation; McGill and colleagues22 found no discernible change in moment arms with conscious abdominal activation in the neutral standing posture. The hypothesized benefits of contracting around a pressurized abdomen may also extend to neural factors because individuals seem better able to recruit the abdominal muscles isometrically in an upright, as opposed to supine, posture.68,69
Abdominal muscles are also involved in respiration, particularly during conscious forceful breathing and cardiovascular challenge.70–72 Evidence shows that the four abdominal muscles contract cyclically to assist with active expiration, as ventilatory demand increases. Although phasic activation (expiration) and relaxation (inspiration) patterns become quite clear during challenged breathing, peak abdominal activation levels (related to breathing alone) rarely exceed 5% of maximum capability. This finding suggests that the phasic activation patterns become apparent because of slight increases in activation (during expiration to push air from the lungs) and improved relaxation (during inspiration to ease airflow into the lungs). Wang73 showed that patients with chronic obstructive pulmonary disease display phasic abdominal muscle activation and relaxation patterns at baseline breathing levels to assist with their increased ventilatory difficulty.
Abdominal Muscles and Low Back Pain
More recent research and clinical focus have highlighted a link between abdominal muscle dysfunction and low back pain and injury.74–77 The most compelling evidence for this association comes from the large prospective study conducted by Cholewicki and colleagues,74 who showed that athletes who displayed delayed activation onsets of the abdominal muscles, in response to spine perturbations, were more likely to sustain a low back injury in the future. These delayed abdominal muscle onsets were not a predictor of past low back incidents. This finding led the authors to conclude that altered abdominal muscle function, at least in response to rapid spine perturbations, is more a cause of than an adaptation to low back pain and injury. The underlying culprit for this type of low back injury would seem to be insufficient stiffening of the spine. Based on this evidence, numerous abdominal muscle training and rehabilitation techniques have been and are currently being developed and prescribed. When properly administered, these programs have been shown to have the potential for success.78,79 Clinical interventions designed to activate the abdominal wall to stabilize the spine need to be considered carefully,4 however, because increased abdominal activation has the potential to stabilize and destabilize the lumbar spine.55
The transverse abdominis has received special clinical consideration more recently, based on a series of articles (e.g., Hodges and Richardson75,80) that showed that transverse abdominis activation timing, in preparation for rapid limb movement, often precedes (by 10 to 40 msec) the activation of the other spine muscles. These authors also noted an exacerbated delay in the activation of this muscle in patients with low back pain. The mechanical consequences of these findings are unclear. First, the early activation of this muscle has been uncovered predominantly during very specialized actions, specifically rapid isolated limb movements. These actions do not readily replicate functional scenarios. Second, the other abdominal muscles also show delayed firing in patients with low back pain, albeit to a lesser degree than the transverse abdominis. Finally, Mannion and colleagues81 determined that it was difficult to detect clearly a difference in the mechanical contraction of these muscles via ultrasonography, despite an apparent difference in electromyography activation; it is unclear whether this is a methodologic limitation of ultrasonography or whether the mechanical effects of the muscles are actually synchronized owing to differing delays between the electrical stimulation of the muscle layers and the resultant contraction dynamics.
The transverse abdominis is a small muscle with relatively small moment arms and has little ability to affect spine loading and stability directly. It may play a stabilizing role elsewhere, however, such as through the rapid development of IAP.82 Regardless, because of the composite laminate-like nature of the abdominal wall, it is unlikely that the mechanical effects of the transverse abdominis, or any other abdominal muscle, can be effectively isolated. Attempting to do so can lead to aberrant muscle activation patterns that can compromise the stability of the spine55,83,84 in functional situations. Isolated focus on the transverse abdominis, or any single spine muscle, is not recommended for spine injury prevention or rehabilitation.57,85
1 Urquhart DM, Barker PJ, Hodges PW, et al. Regional morphology of the transversus abdominis and obliquus internus and externus abdominis muscles. Clin Biomech. 2005;20:233-241.
This article explores the regionalized anatomy of the abdominal wall muscles.
2 Brown SHM, Ward SR, Cook M, et al: Architectural analysis of human abdominal muscles: Implications for mechanical function. Spine (in press).
3 McGill SM. A revised anatomical model of the abdominal musculature for torso flexion efforts. J Biomech. 1996;29:973-977.
This article discusses mechanical considerations and functions of the abdominal muscles.
4 Cholewicki J, Silfies SP, Shah RA, et al. Delayed trunk muscle reflex responses increase the risk of low back injuries. Spine. 2005;30:2614-2620.
A causative link between abdominal muscle dysfunction and low back injury is examined.
5 Granata KP, Marras WS. Cost-benefit of muscle cocontraction in protecting against spinal instability. Spine. 2000;25:1398-1404.
1 Agur AMR, Dalley AF. Grant’s Atlas of Anatomy. Philadelphia: Lippincott Williams & Wilkins; 2005.
2 Whetzel TP, Huang V. The vascular anatomy of the tendinous intersections of the rectus abdominis muscle. Plast Reconstr Surg. 1996;98:83-89.
3 Woodley SJ, Duxson MJ, Mercer SR. Preliminary observations on the microarchitecture of the human abdominal muscles. Clin Anat. 1997;20:808-813.
4 McGill SM. Low Back Disorders: Evidence-Based Prevention and Rehabilitation. Champaign, IL: Human Kinetics Publishers; 2002.
5 Brown SHM, McGill SM. An ultrasound investigation into the morphology of the human abdominal wall uncovers complex deformation patterns during contraction. Eur J Appl Physiol. 2008;104:1021-1030.
6 Urquhart DM, Barker PJ, Hodges PW, et al. Regional morphology of the transversus abdominis and obliquus internus and externus abdominis muscles. Clin Biomech. 2005;20:233-241.
7 Barker PJ, Urquhart DM, Story IH, et al. The middle layer of lumbar fascia and attachments to lumbar transverse processes: Implications for segmental control and fracture. Eur Spine J. 2007;16:2232-2237.
8 Vleeming A, Pool-Goudzwaard AL, Stoeckart R, et al. The posterior layer of the thoracolumbar fascia: Its function in load transfer from spine to legs. Spine. 1995;20:753-758.
9 Bogduk N, Macintosh JE. The applied anatomy of the thoracolumbar fascia. Spine. 1984;9:164-170.
10 Askar OM. Surgical anatomy of the aponeurotic expansions of the anterior abdominal wall. Ann R Coll Surg Engl. 1977;59:313-321.
11 Rizk NN. A new description of the anterior abdominal wall in man and mammals. J Anat. 1980;131:373-385.
12 Daggfeldt K, Thorstensson A. The role of intra-abdominal pressure in spinal unloading. J Biomech. 1997;30:1149-1155.
13 Brown SHM, McGill SM. Transmission of muscularly generated force and stiffness between layers of the rat abdominal wall. Spine. 2009;34:E70-E75.
14 Powell PL, Roy RR, Kanim P, et al. Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs. J Appl Physiol. 1984;57:1715-1721.
15 Lieber RL, Friden J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve. 2000;23:1647-1666.
16 Brown SHM, Ward SR, Cook M, et al: Architectural analysis of human abdominal muscles: Implications for mechanical function. Spine (in press).
17 McGill SM, Patt N, Norman RW. Measurement of the trunk musculature of active males using CT scan radiography: Implications for force and moment generating capacity about the L4/L5 joint. J Biomech. 1988;21:329-341.
18 Marras WS, Jorgensen MJ, Granata KP, et al. Female and male trunk geometry: Size and prediction of the spine loading trunk muscles derived from MRI. Clin Biomech. 2001;16:38-46.
19 McGill SM. A revised anatomical model of the abdominal musculature for torso flexion efforts. J Biomech. 1996;29:973-977.
20 Lieber RL. Skeletal Muscle Structure, Function and Plasticity: The Physiological Basis of Rehabilitation. Philadelphia: Lippincott Williams & Wilkins; 2002.
21 Lieber RL, Loren GJ, Fridén J. In vivo measurement of human wrist extensor muscle sarcomere length changes. J Neurophysiol. 1994;71:874-881.
22 McGill SM, Juker D, Axler C. Correcting trunk muscle geometry obtained from MRI and CT scans of supine postures for use in standing postures. J Biomech. 1996;29:643-646.
23 Jorgensen MJ, Marras WS, Smith FW, et al. Sagittal plane moment arms of the female lumbar region rectus abdominis in an upright neutral torso posture. Clin Biomech. 2005;20:242-246.
24 Cholewicki J, McGill SM. Mechanical stability of the in vivo lumbar spine: Implications for injury and chronic low back pain. Clin Biomech. 1996;11:1-15.
25 Stokes IAF, Gardner-Morse M. Quantitative anatomy of the lumbar musculature. J Biomech. 1999;32:311-316.
26 Pearcy M, Portek I, Shepherd J. 3-dimensional x-ray analysis of normal movement in the lumbar spine. Spine. 1984;9:294-297.
27 White AA, Panjabi MM. Clinical Biomechanics of the Spine, 2nd ed. Philadelphia: JB Lippincott; 1990.
28 McGill SM. A myoelectrically based dynamic three-dimensional model to predict loads on lumbar spine tissues during lateral bending. J Biomech. 1992;25:395-414.
29 Dumas GA, Poulin MJ, Roy B, et al. Orientation and moment arms of some trunk muscles. Spine. 1991;16:293-303.
30 Marras WS, Sommerich CM. A 3-dimensional motion model of loads on the lumbar spine: 1. Model structure. Human Factors. 1991;33:123-137.
31 Juker D, McGill S, Kropf P, et al. Quantitative intramuscular myoelectric activity of lumbar portions of psoas and the abdominal wall during a wide variety of tasks. Med Sci Sports Exerc. 1998;30:301-310.
32 Urquhart DM, Hodges PW. Differential activity of regions of transversus abdominis during trunk rotation. Eur Spine J. 2005;14:393-400.
33 Iscoe S. Control of abdominal muscles. Prog Neurobiol. 1998;56:433-506.
34 Schlenz I, Burggasser G, Kuzbari R, et al. External oblique abdominal muscle: A new look on its blood supply and innervations. Anat Rec. 1999;255:388-395.
35 Mirka G, Kelaher D, Baker A, et al. Selective activation of the external oblique musculature during axial torque production. Clin Biomech. 1997;12:172-180.
36 Moreside JM, Vera-Garcia FJ, McGill SM. Neuromuscular independence of abdominal wall muscles as demonstrated by Middle-Eastern style dancers. J Electromyogr Kinesiol. 2008;18:527-537.
37 Masani K, Sin VW, Vette AH, et al. Postural reactions of the trunk muscles to multi-directional perturbations in sitting. Clin Biomech. 2009;24:176-182.
38 Gregory DE, Dunk NM, Callaghan JP. Stability ball versus office chair: Comparison of muscle activation and lumbar spine posture during prolonged sitting. Human Factors. 2006;48:142-153.
39 Gregory DE. Prolonged standing as a precursor for the development of low back discomfort: An investigation of possible mechanisms Masters Thesis. Waterloo, ON: University of Waterloo. 2005.
40 Callaghan JP, Patla AE, McGill SM. Low back three-dimensional joint forces, kinematics, and kinetics during walking. Clin Biomech. 1999;14:203-216.
41 Lucas D, Bresler B. Stability of the Ligamentous Lumbar Spine Technical Report No. 40. San Francisco: University of California, San Francisco, Biomechanics Laboratory. 1961.
42 Crisco JJ, Panjabi MM, Yamamoto I, et al. Euler stability of the human ligamentous lumbar spine: 2. Experiment. Clin Biomech. 1992;7:27-32.
43 McGill SM, Norman RW. Partitioning of the L4-L5 dynamic moment into disc, ligamentous, and muscular components during lifting. Spine. 1986;11:666-678.
44 Granata KP, Marras WS. The influence of trunk muscle coactivity on dynamic spinal loads. Spine. 1995;20:913-919.
45 Pope MH, Andersson GBJ, Broman H, et al. Electromyographic studies of lumbar trunk musculature during the development of axial torques. J Orthop Res. 1986;4:288-297.
46 Brown SHM, McGill SM. Co-activation alters the linear versus non-linear impression of the EMG-torque relationship of trunk muscles. J Biomech. 2008;41:491-497.
47 Gardner-Morse MG, Stokes IAF. The effects of abdominal muscle coactivation on lumbar spine stability. Spine. 1998;23:86-91.
48 Granata KP, Marras WS. Cost-benefit of muscle cocontraction in protecting against spinal instability. Spine. 2000;25:1398-1404.
49 Brown SHM, Potvin JR. Constraining spine stability levels in an optimization model leads to the prediction of trunk muscle cocontraction and improved spine compression force estimates. J Biomech. 2005;38:745-754.
50 van Dieën JH, Kingma I, van der Bug P. Evidence for a role of antagonistic cocontraction in controlling trunk stiffness during lifting. J Biomech. 2003;36:1829-1836.
51 El-Rich M, Shirazi-Adl A, Arjmand N. Muscle activity, internal loads, and stability of the human spine in standing postures: Combined model and in vivo studies. Spine. 2004;29:2633-2642.
52 Potvin JR, Brown SHM. An equation to calculate individual muscle contributions to joint stability. J Biomech. 2005;38:973-980.
53 Brown SHM, Potvin JR. Exploring the geometric and mechanical characteristics of the spine musculature to provide rotational stiffness to two spine joints in the neutral posture. Hum Mov Sci. 2007;26:113-123.
54 Howarth SJ, Beach TA, Callaghan JP. Abdominal muscles dominate contributions to vertebral joint stiffness during the push-up. J Appl Biomech. 2008;24:130-139.
55 Brown SHM, Vera-Garcia FJ, McGill SM. Effects of abdominal muscle co-activation on the externally preloaded trunk: Variations in motor control and its effect on spine stability. Spine. 2006;31:E387-E393.
56 Cholewicki J, VanVliet JJ. Relative contribution of trunk muscles to the stability of the lumbar spine during isometric exertions. Clin Biomech. 2002;17:99-105.
57 Kavcic N, Grenier S, McGill SM. Determining the stabilizing role of individual torso muscles during rehabilitation exercises. Spine. 2004;29:1254-1265.
58 Marras WS, Ferguson SA, Burr D, et al. Functional impairment as a predictor of spine loading. Spine. 2005;30:729-737.
59 Kingma I, Staudenmann D, van Dieën JH. Trunk muscle activation and associated lumbar spine joint shear forces under different levels of external forward force applied to the trunk. J Electromyogr Kinesiol. 2007;17:14-24.
60 Cholewicki J, Ivancic PC, Radebold A. Can increased intra-abdominal pressure in humans be decoupled from trunk muscle co-contraction during steady state isometric exertions? Eur J Appl Physiol. 2002;87:127-133.
61 Morris JM, Lucas DB, Bresler B. Role of the trunk in stability of the spine. J Bone Joint Surg Am. 1961;43:327-351.
62 McGill SM, Norman RW. Reassessment of the role of intra-abdominal pressure in spinal compression. Ergonomics. 1987;30:1565-1588.
63 Ivancic PC, Cholewicki J, Radebold A. Effects of the abdominal belt on muscle-generated spinal stability and L4/L5 joint compression force. Ergonomics. 2002;45:501-513.
64 Arjmand N, Shirazi-Adl A. Role of intra-abdominal pressure in the unloading and stabilization of the human spine during static lifting tasks. Eur Spine J. 2006;15:1265-1275.
65 Cholewicki J, Juluru K, McGill SM. Intra-abdominal pressure mechanism for stabilizing the lumbar spine. J Biomech. 1999;32:13-17.
66 Hodges PW, Eriksson AE, Shirley D, et al. Intra-abdominal pressure increases stiffness of the lumbar spine. J Biomech. 2005;38:1873-1880.
67 Cresswell AG, Thorstensson A. The role of the abdominal musculature in the elevation of the intra-abdominal pressure during specified tasks. Ergonomics. 1989;32:1237-1246.
68 Brown SHM, McGill SM. How the inherent stiffness of the in-vivo human trunk varies with changing magnitudes of muscular activation. Clin Biomech. 2008;23:15-22.
69 Brown SHM, McGill SM. The intrinsic stiffness of the in vivo lumbar spine in response to quick releases: Implications for reflexive requirements. J Electromyogr Kinesiol. 2009;19:727-736.
70 Campbell EJM, Green JH. The variations in intra-abdominal pressure and the activity of the abdominal muscles during breathing: A study in man. J Physiol. 1953;122:282-290.
71 Gandevia SC, McKenzie DK, Plassman BL. Activation of human respiratory muscles during different voluntary manoeuvres. J Physiol. 1990;428:387-403.
72 Wang S, McGill SM. Links between the mechanics of ventilation and spine stability. J Appl Biomech. 2008;24:166-174.
73 Wang S. The links between ventilation mechanics, spine mechanics and stability Masters Thesis. Waterloo, ON: University of Waterloo. 2004.
74 Cholewicki J, Silfies SP, Shah RA, et al. Delayed trunk muscle reflex responses increase the risk of low back injuries. Spine. 2005;30:2614-2620.
75 Hodges PW, Richardson CA. Inefficient muscular stabilization of the lumbar spine associated with low back pain: A motor control evaluation of transversus abdominis. Spine. 1996;21:2640-2650.
76 Ferreira PH, Ferreira ML, Hodges PW. Changes in recruitment of the abdominal muscles in people with low back pain: Ultrasound measurement of muscle activity. Spine. 2004;29:2560-2566.
77 Silfies SP, Squillante D, Maurer P, et al. Trunk muscle recruitment patterns in specific chronic low back pain populations. Clin Biomech. 2005;20:465-473.
78 O’Sullivan PB, Phyty GD, Twomey LT, et al. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine. 1997;15:2959-2967.
79 Hicks GE, Fritz JM, Delitto A, et al. Preliminary development of a clinical prediction rule for determining which patients with low back pain will respond to a stabilization exercise program. Arch Phys Med Rehabil. 2005;86:1753-1762.
80 Hodges PW, Richardson CA. Delayed postural contraction of transversus abdominis in low back pain associated with movement of the lower limb. J Spinal Disord. 1998;11:46-56.
81 Mannion AF, Pulkovski N, Schenk P, et al. A new method for the noninvasive determination of abdominal muscle feedforward activity based on tissue velocity information from tissue Doppler imaging. J Appl Physiol. 2008;104:1192-1201.
82 Cresswell AG. Responses of intra-abdominal pressure and abdominal muscle activity during trunk loading in man. Eur J Appl Physiol. 1993;66:315-320.
83 Vera-Garcia FJ, Elvira JL, Brown SH, et al. Effects of abdominal stabilization maneuvers on the control of spine motion and stability against sudden trunk perturbations. J Electromyogr Kinesiol. 2007;17:556-567.
84 Grenier SG, McGill SM. Quantification of lumbar stability by using 2 different abdominal activation strategies. Arch Phys Med Rehabil. 2007;88:54-62.
85 Hodges P. Transversus abdominis: A different view of the elephant. Br J Sports Med. 2008;42:941-944.