CHAPTER 181 Anatomy and Developmental Embryology of the Neck
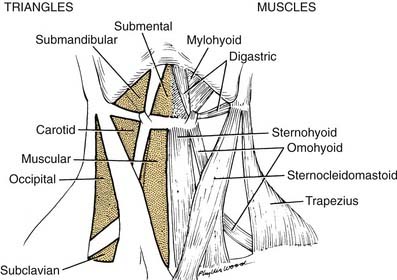
Posterior Triangle of Neck
The posterior triangle is bordered by the sternocleidomastoid muscle, the anterior border of the trapezius muscle, and the middle third of the clavicle (Fig. 181-1). Specific layers of deep fascia form the floor (medial wall) and roof (lateral wall) of the triangular box. An understanding of the fascial relationships in the neck is important not only because of the boundary relationships but also because fasciae form planes that provide routes of surgical access or pathways for hemorrhage and infection. For this reason, a brief discussion of the fascial planes is necessary before proceeding with the anatomy of the posterior triangle.
Fascia of Neck
One of the earliest lessons in anatomy is that there are two types of fascia in the body: the superficial and the deep. In the region of the abdominal wall, superficial fascia consists of two layers—a fatty layer (Camper’s fascia) and a deeper, membranous layer (Scarpa’s fascia). The deep fascial layer of the abdominal wall is not subdivided but simply envelops the abdominal muscles. In the neck the superficial fascia is very thin and is not divided into layers, whereas the deep fascia is divided into three layers.1 The names of these layers vary with different authors, resulting in a somewhat chaotic terminology. Regardless of the terminology used, the divisions are arbitrary at best. A simple approach can provide a workable solution for either the anatomist or the surgeon.
Deep Fascia
The deep fascia is divided into three layers, demonstrated best when the neck is viewed in cross section (Fig. 181-2). These are the external, middle, and internal layers of the deep cervical fascia. The external layer of deep fascia underlies the platysma muscle and completely invests or encircles all of the superficial neck structures. For these reasons, the external layer is also known as the superficial layer, or investing layer, of deep fascia. In the region of the sternocleidomastoid and trapezius muscles, it splits and envelopes the individual muscles. A middle layer of the deep cervical fascia encloses the visceral structures of the neck (the trachea and esophagus). Hence the synonym for the middle layer is the visceral fascia.
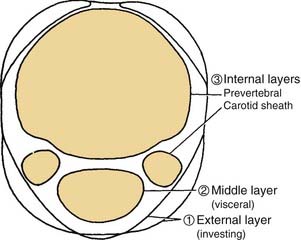
Figure 181-2. Divisions of the deep cervical fascia seen in transverse section at the level of cranial nerve VII.
The third, or internal, layer of the deep cervical fascia surrounds the deep muscles of the neck and cervical vertebrae (Fig. 181-3). This layer also is known by its descriptive term prevertebral fascia (although “paravertebral fascia” would have been more appropriate). The muscles enclosed by the prevertebral fascia include the deep muscles of the neck (the cervical part of the erector spinae); the levator scapulae; the scalenus anterior, middle, and posterior muscles; and the longus colli and longus capitis muscles, which lie on the anterior aspect of the cervical vertebrae. The last-named pair of muscles serve as flexors of the vertebrae; the longus capitis also assists in flexion of the skull. The scalenus group underlies the prevertebral fascia in the region of transverse processes of the cervical vertebrae. The anterior tubercles of the transverse processes provide origin for the scalenus anterior muscle, whereas the posterior tubercles provide the origin for the scalenus medius and posterior muscles. Continuing posteriorly, in order, are the levator scapulae and deep cervical muscles already noted.
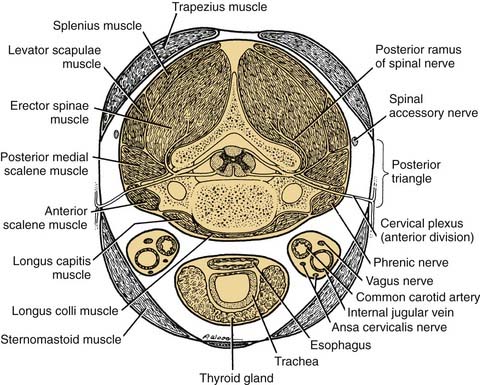
Figure 181-3. Structures contained by the deep cervical fascia seen in transverse section at the level of the cranial nerve.
The internal layer of deep fascia is described by some authors as enveloping the carotid and jugular vessels. Hence, the carotid sheaths are included as part of the definition of the internal layer of deep fascia. An effective means of visualizing the spatial relationships of these layers of fascia is to examine a cross section of the neck. This view not only is informative in defining the three layers of deep fascia but also serves to relate them to the posterior triangle of the neck (see Fig. 181-2). Placing a finger over the middle of the posterior triangle (i.e., between the trapezius and sternocleidomastoid muscles) will clarify that the roof (lateral wall) of the triangle is formed by the superficial layer of deep fascia. Palpation deeper into the triangle will bring the tip of the finger into contact with the prevertebral fascia that forms the floor of the posterior triangle and invests the prevertebral muscles. If the superficial layer of the deep fascia is incised, a finger inserted into the space, exploring anteriorly between the sternocleidomastoid and prevertebral muscles, will encounter the carotid sheath. This is a surgical approach to the retropharyngeal area or to the carotid vessels for vascular surgery.
Contents of Posterior Triangle
Cutaneous Branches of Cervical Plexus
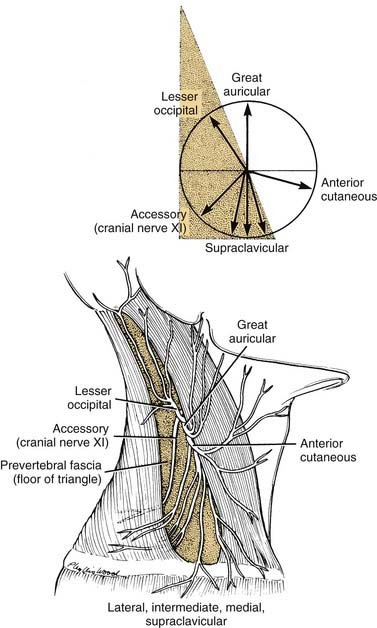
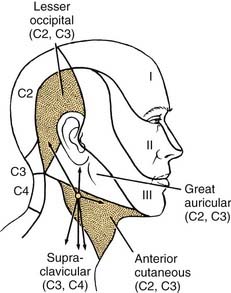
The sensory branches of the cervical plexus contained within the posterior triangle are four cutaneous nerves, which supply the skin of the head and neck from the area of the posterior scalp to the supraclavicular region. These cutaneous nerves are the (1) lesser occipital, (2) great auricular, (3) anterior, and (4) supraclavicular (Fig. 181-4). The first three of these nerves contain cervical segments C2 and C3 and the supraclavicular nerves C3 and C4. The topographic relations of these nerves and their distribution to the skin are illustrated in Figure 181-5. The important landmark for the cervical nerves is the accessory nerve at the point where it enters the posterior triangle from under cover of the sternocleidomastoid muscle. If this point is viewed as the center of a clock face, the cervical nerves mimic the hands pointing in different directions (see Fig. 181-4, inset). For instance, on the right side, the lesser occipital nerve is approximately at the 11 o’clock position, the great auricular is at the 12 o’clock position, and the anterior cutaneous nerve of the neck can be found at the 3 o’clock position. The supraclavicular nerves consist of three or four bundles of filaments scattered between the 5 and 7 o’clock positions on the clock’s face. Continuing this analogy, the accessory nerve can be seen to traverse the posterior triangle along the line of the 8 o’clock position until it enters the deep surface of the trapezius muscle. Viewed in this manner, the accessory nerve serves as a focus for organizing the pathways of the cutaneous nerves, as well as for emphasizing cranial nerve XI as a motor nerve in the posterior triangle.
Accessory Nerve
The accessory nerve is the only motor nerve in the posterior triangle as just defined. However, if the prevertebral fascia is incised, it is possible to encounter the motor nerves of the deep cervical muscles, the phrenic nerve, or the brachial plexus. With regard to its innervation of the sternocleidomastoid and trapezius muscles, cranial nerve XI is in fact a spinal nerve. Cell bodies of motor neurons innervating these muscles lie near the ventral horn in the upper cervical spinal cord, in the region of segments C2 through C4. The axonal fibers exit the anterior lateral portion of the cord as a series of fine filaments before becoming bundled as a nerve fiber within the subarachnoid space of the vertebral canal. The nerve ascends in the subarachnoid space, enters the posterior fossa through the foramen magnum, and joins with the cranial root of the accessory nerve before exiting the skull through the jugular foramen on the deep surface of the internal jugular vein. As the nerve descends the neck adjacent to the carotid sheath, motor or sensory filaments may join the nerve directly from segments C2 through C4.2 After crossing the deep surface of the sternocleidomastoid muscle, the nerve enters the posterior triangle, as already described.
Anterior Triangle of Neck
The anterior triangle of the neck is complementary to the posterior triangle and is bounded by the sternocleidomastoid muscle, the body of the mandible, and the midline of the neck. This space can be further subdivided into smaller triangular units, such as the submandibular triangle, and carotid and muscular triangles, which are included in this discussion (see Fig. 181-1).
Carotid Triangle
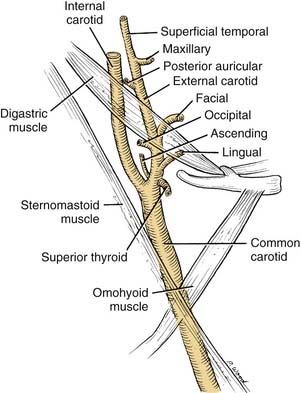
The major focal point of the triangle is the bifurcation of the common carotid artery (Fig. 181-6). The internal carotid artery ascends the neck without branching. The external carotid artery provides several branches, many of which are important landmarks. The external carotid artery has anterior, posterior, and terminal branches.
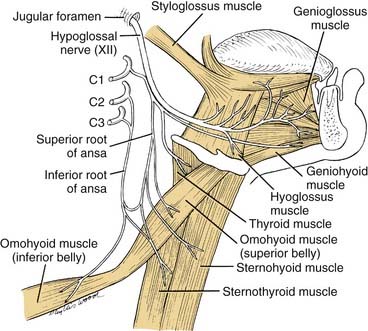
Ansa Cervicalis
The ansa cervicalis is part of the cervical plexus. Its essential role is to supply the infrahyoid muscles: the sternohyoid, sternothyroid, and omohyoid muscles (Fig. 181-7). C1 fibers, which descend the neck, enter the hypoglossal nerve and travel as far as the occipital artery. At this point the hypoglossal nerve curves anteroinferiorly, but C1 fibers descend the neck to form the branch of cranial nerve XII, termed the superior root of the ansa. Fibers from C2 and C3 form an inferior root, which descends the carotid sheath on the surface of the internal jugular vein before turning anteroinferiorly to join the superior root. The ansa cervicalis is thus formed by the union of the two roots of the ansa. Three muscles are supplied directly by the ansa: the sternohyoid, sternothyroid, and omohyoid. At the origin of the superior root of the ansa, some C1 fibers do not follow the superior root but rather continue in the hypoglossal nerve transversely across the neck, before leaving cranial nerve XII to supply either the thyrohyoid muscle or the geniohyoid muscle (see Fig. 181-7). In summary, all four of the infrahyoid muscles are supplied by the ansa. In addition, one suprahyoid muscle, the geniohyoid muscle, also is supplied by the C1 fibers that have continued en route with the hypoglossal nerve. Except for proprioceptive fibers carried within the ansa, it is a motor nerve.
Root of Neck
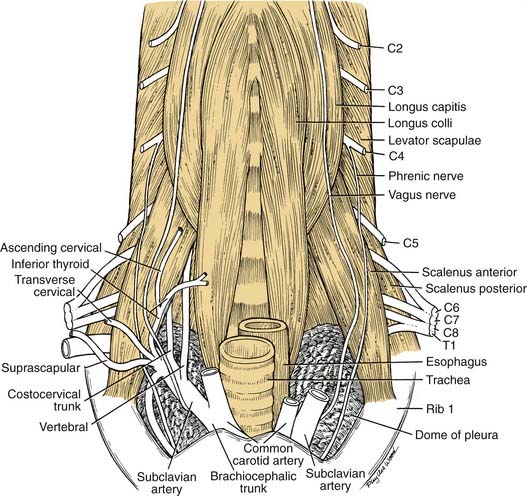
The boundaries of the root of the neck are somewhat ill defined. The base of it is the plane of the thoracic inlet and can be traced from the manubrium laterally along the first ribs and then posterosuperiorly to the transverse process of C6 (Fig. 181-8). It contains the neck viscera and vessels. For the purposes of this discussion, it is divided unilaterally by the midline into a pyramidal space—bounded laterally by the scalenus anterior muscle, inferiorly by the first rib, and medially by the tracheoesophageal tract and midline.
A majority of the vascular branches arise from the first portion of the subclavian artery. The first branch of the subclavian artery normally is the vertebral artery, which ascends the root of the neck between the scalenus anterior and longus colli muscles to enter the foramen processus transversus of C6. The next is the thyrocervical trunk, which normally has four described branches. The first is a small ascending cervical, which supplies the prevertebral muscle region. The second branch of the thyrocervical trunk is the inferior thyroid artery coursing to the inferior pole of the thyroid gland. The inferior thyroid artery commonly supplies both the superior and inferior parathyroid glands. The third and fourth branches are the transverse cervical artery and the suprascapular artery, already described in relation to the posterior triangle of the neck. Two branches arise from the inferior surface of the subclavian artery: the costocervical trunk and the internal thoracic artery, which descends over the suprapleural fascia into the mediastinum and anterior chest wall. Multiple variations of these vessels have been described, as detailed by Daseler and Anson.3
This region is of particular importance during radical neck surgery, because the deep dissection usually is begun by detaching the sternocleidomastoid muscle from its attachments to the manubrium and clavicle. This exposes the carotid sheath and its contents and opens up the posterior and anterior triangles. As the dissection proceeds, the internal jugular vein is mobilized, exposing the carotid and its branches, which have been left in place. Because the en bloc mass of the internal jugular vein and sternocleidomastoid muscle is mobilized superiorly, it is important to leave the prevertebral fascia intact. With this precaution, the fascia willprovide a protective veil over the brachial plexus, phrenic nerve, sympathetic trunk, and motor nerves to the deep cervical muscles. When the prevertebral fascia is exposed, two muscles—the scalenus anterior and the longus capitis—serve as important landmarks for the spinal nerves. As the nerves emerge from the intervertebral foramina, they lie in a groove on the transverse process of the cervical vertebrae. If the vertebrae and prevertebral muscles are envisioned as being enclosed in a cylinder of prevertebral fascia, the logical conclusion is that the spinal nerves must penetrate this fascia to reach superficial structures in the neck (see Fig. 181-3). With these guidelines it is possible to identify cervical nerves C1 through C4 at the lateral border of the longus capitis, and the roots of C5 through T1 can be identified at the lateral edge of the scalenus anterior (see Fig. 181-8).
Lymphatic Drainage of Head and Neck
Earlier in this chapter the deep cervical fascia was described as having external, middle, and internal layers. This same rule can be applied to the lymphatic drainage of the head and neck. This plan is similar to one described by Last.4 The external layer can be thought of as an outer circle of superficial or regional lymph nodes, the middle layer as a visceral layer of nodes draining the viscera of the neck such as the larynx and pharynx, and the internal circle of nodes composed of the paired carotid sheaths containing the internal jugular vein and deep cervical lymph nodes. The deep cervical lymph nodes are tributaries for both the superficial and visceral circles of lymph nodes.
Classification of Cervical Lymphatics
The groups of lymph nodes are designated as levels I to VI. Level I includes nodes in the submental and submandibular triangles. Level II includes the upper jugular nodes located around the superior one third of the jugular vein and spinal accessory nerve extending from the carotid bifurcation or hyoid, inferiorly, to the skull base, superiorly. The anterior and posterior boundaries of this nodal group are the lateral border of the sternohyoid and the posterior border of the sternocleidomastoid muscle, respectively. Level III includes the middle jugular nodes around the middle third of the jugular vein, extending from the carotid bifurcation, superiorly, to the omohyoid (or cricothyroid notch, inferiorly). The anterior and posterior boundaries are the same as for level II. Level IV includes the lower jugular group surrounding the jugular vein from the omohyoid to the clavicle. The anterior and posterior borders are the same as in levels II and III. Level V is the posterior triangle group, consisting of the nodes surrounding the lower half of the spinal accessory nerve and the transverse cervical artery, including the supraclavicular nodes. The anterior boundary of level V is the posterior border of the sternocleidomastoid muscle and the posterior boundary is the anterior border of the trapezius muscle. Level VI includes the anterior compartment group surrounding the visceral structures of the neck from the hyoid, superiorly, to the sternal notch, inferiorly. The lateral boundary on either side of level VI is the medial aspect of the carotid sheath. This space crosses the midline and includes the perithyroidal, paratracheal, and precricoid nodes, as well as the nodes along the recurrent laryngeal nerve.5 The terminology for neck dissection is discussed in Chapter 116.
Embryology of Head and Neck
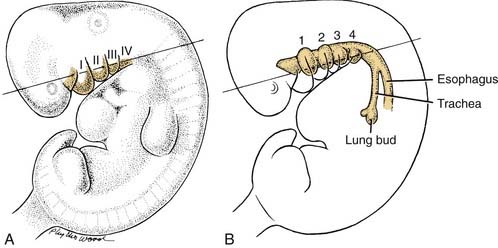
The development of many structures in the head and neck is intimately related to either the branchial arches or the pharyngeal pouches (Fig. 181-9). These are transient embryonic structures that undergo substantial remodeling so that their original embryonic form is essentially unrecognizable by the time the infant is born. The derivatives of these structures nevertheless are important to adult morphology: Aberrations in branchial arch development may produce significant malformations.
Embryology of Branchial Arches
At 5 weeks of gestation, the area of the future face and neck of the embryo consists of five or six pairs of fingerlike masses of tissue named the branchial arches. Prominent in lateral profile (see Fig. 181-9), these masses are aligned in transverse orientation to the plane of the neck and are separated by clefts, termed the branchial clefts. The surface of the arches and clefts is lined by ectoderm, with mesodermally defined tissues contained within the branchial arches. The tissues underlying the region of the clefts are thin because of the close approximation of outpouchings from the foregut region called pharyngeal pouches (see Fig. 181-9). The derivatives of the arches and pouches are different because of the embryonic germ layers contained within the branchial arch (the mesoderm), whereas the pharyngeal pouches are composed of endoderm. Because of the differences in the embryonic germ layers, the following generalization can be made: In the adult, the derivatives of the branchial arch will be structures composed of muscle, bone, or similar mesodermal derivatives; the derivatives of the endodermal pharyngeal pouch will be glandular or associated with the digestive tract.
Derivatives of Branchial Arches
Skeletal Derivatives
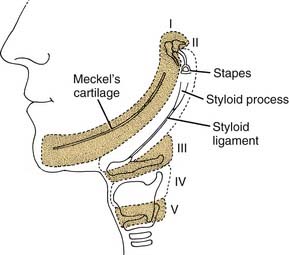
The first branchial arch is the proximal part of the first arch cartilage (Meckel’s cartilage), which is remodeled and contributes to the formation of the ramus of the mandible (Fig. 181-10). The distal part of the cartilage withers, and the body of the mandible is formed from intramembranous bone. Other structures formed by the proximal part of the cartilage are the sphenomandibular ligament, anterior malleolar ligament, malleus (except for the manubrium, which is from the second arch), and incus (except for its long process, which is from the second arch).
The second branchial arch is the cartilage of the second branchial arch (Reichert’s cartilage), which forms bony structures proximally and distally. Its central portion withers, leaving a fibrous band—the stylohyoid ligament. Proximally, it forms the styloid process, the manubrium of the malleus, the long process of the incus, and the stapes suprastructure. The stapes footplate is mostly derived from the otic capsule.6,7 Distally (anteroinferiorly), the second arch cartilage forms a portion of the body of the hyoid and the lesser cornu of the hyoid bone. In the adult, it is possible to trace the path of the embryonic second arch cartilage from the styloid process, to the stylohyoid ligament, ending at the lesser cornu of the hyoid bone (see Fig. 181-10).
Embryology of the Pharyngeal Pouches
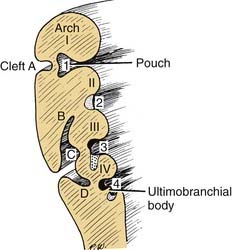
The pharyngeal pouches are lateral outpouchings of the foregut or region of the primitive pharynx (see Fig. 181-9). At the extreme lateral wall of each pharyngeal pouch, the endodermal lining contacts the ectodermal epithelium of a branchial cleft (Fig. 181-11). Thus, the branchial clefts are named in relation to the pharyngeal pouch with which they are apposed. The endodermal epithelium lining of either the branchial arch or the pharyngeal pouch contributes to the formation of specific elements of the pharynx in the adult.8
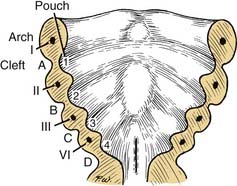
Figure 181-11. Relationships of the pharyngeal arches, clefts, and pouches in the floor of the mouth: stage I.
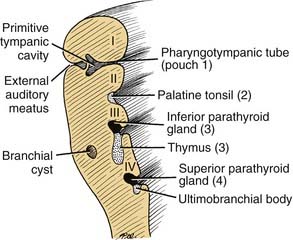
First Pharyngeal Pouch
The first pharyngeal pouch becomes elongated and incorporated into the temporal bone and forms the epithelial lining of the middle ear (Fig. 181-12). The most lateral portion of the pouch, along with the first closing plate of the first branchial cleft, forms the tympanic membrane (see Fig. 181-12). From this relationship, it is clear that the external auditory canal is nothing more than a remodeling of the first branchial cleft (Fig. 181-13).
Second Pharyngeal Pouch
The endodermal layer of the second pharyngeal pouch forms the epithelial lining of the palatine tonsil, whereas underlying mesenchymal elements contribute to the formation of the tonsil itself (see Figs. 181-12 and 181-13).
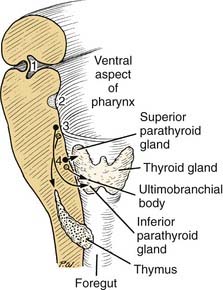
Third Pharyngeal Pouch
The region of the third pharyngeal pouch is subdivided into superior and inferior portions. The superior portion forms cells that eventually differentiate into the inferior parathyroid (see Fig. 181-12). The inferior portion of the third pouch becomes thymic tissue, which eventually migrates in the neck and mediastinum to form the thymus (Fig. 181-14).
Fourth through Sixth Pharyngeal Pouches
The endoderm of the fourth pouch forms the superior parathyroid gland. The adjacent area is variably named as the fifth or sixth pouch or the ultimobranchial body (see Fig. 181-13). Subsequently, the ultimobranchial body is infiltrated by cells migrating from the neural crest region. These cells eventually are incorporated into the thyroid gland and become the parafollicular cells (C cells) responsible for the secretion of calcitonin.9,10
Craniofacial Syndromes Related to Branchial Arch Structures
Hemifacial microsomia is thought to be related to a vascular injury in the region of the stapedial artery, resulting in microtia and mandibular hypoplasia.11 Patients with this defect often have associated aural atresia. Mandibulofacial dysostosis, or Treacher Collins syndrome, is related to abnormalities of the first and second branchial arches. This syndrome is characterized by lid colobomata, down-slanting palpebral fissures, hypoplastic zygoma and mandible, and malformations of the external ear.12 Other syndromes related to dysmorphogenesis of the first and second branchial arches include oculoauricular vertebral dysplasia (i.e., Goldenhar’s syndrome) and branchio-oto-renal syndrome.
Abnormalities of First Branchial Cleft
Aberrant development of the first branchial cleft may lead to the formation of a cervical cyst or sinus in the region of the ear. Work13 and Aronsohn and colleagues14 emphasized the difference in the embryogenesis of preauricular cysts versus cysts of the first branchial cleft. Preauricular cysts or pits occur anterior to the external auditory canal, usually superior to the region of the tragus. In essence, they are inclusion cysts related to fusion of the ectodermal hillocks from the first and second branchial arches during formation of the auricle.15
By contrast, true first branchial cleft abnormalities are duplications of the membranous part of the external auditory canal, and they manifest clinically as cysts, sinuses, or fistulas. Work13 has classified these into two types: type I, of ectodermal origin, involving only the membranous part of the canal; and type II, involving both ectoderm and mesoderm, in which duplication of cartilage also is a feature. As cystic masses or sinus tracts they may involve the parotid gland and cranial nerve VII (this particularly true of type II) or lie inferior to the ear, in the superior neck.
Abnormalities of Second through Fourth Branchial Clefts and Pouches
During closure of the cervical sinus lying between the second branchial arch and the epipericardial ridge, ectoderm may become trapped, resulting in an inclusion cyst at a later time (see Figs. 181-12 and 181-13). These are the more familiar branchial cleft cysts or sinuses, which lie on the lateral neck anterior to the sternocleidomastoid muscle. As such, they may overlie the carotid or muscular triangles of the neck. The fistulous forms of these abnormalities are surgically challenging because they may extend from the superficial area of the neck near the clavicle, superiorly, to the bed of the palatine tonsil. The course of the tract through the deep part of the neck usually lies between the internal and external carotid arteries. The pathway of this tract is easily explained on an embryologic basis. The medial wall of the cervical sinus is the region of closing plates 2 to 4 (see Fig. 181-12). A fistulous pathway breaking through into the pharynx will penetrate one of these closing plates and thereby enter either into the tonsillar bed (the second), the thyrohyoid membrane (third), or pyriform sinus or cricothyroid membrane (fourth).
Third branchial cleft sinuses, which communicate with the pharynx, travel deep to the carotid artery system in their superior extent. Thymopharyngeal cysts represent third branchial cleft remnants. As congenital cystic lesions of the neck, these are easily confused with lymphatic malformations. Remnants of the fourth branchial pouch, or pyriform sinus fistulas, may manifest as recurrent suppurative thyroiditis and usually are located on the left side.16,17 These lesions may be associated with parathyroid, thymic, or thyroid tissue.
Midline Lesions of the Neck
Thyroglossal duct cysts and dermoids are the most common midline lesions of the neck in children. These often are difficult to recognize clinically. The thyroglossal duct cyst derives from persistence of the embryonic thyroglossal duct, anywhere between the foramen cecum and the thyroid gland. Because of the attachments to the base of tongue, thyroglossal duct remnants will move superiorly in the neck when the patient protrudes the tongue. The thyroglossal duct remnant may contain ectopic thyroid tissue. Occasionally the cyst contains all of the functioning thyroid tissue. Because of the potential for permanent hypothyroidism after surgical excision, many investigators advocate routine preoperative assessment of the thyroid.18,19 Ultrasound examination of the neck provides information regarding consistency of the cystic lesion and also can be used to assess for the presence of thyroid tissue in its normal position lower in the neck. Ultrasound examination is easily tolerated by children but does not give functional information. Radionuclide thyroid scans provide functional information regarding thyroid tissue (e.g., the possibility of malignancy within these lesions). Surgical excision is the treatment of choice. Thyroglossal duct remnants often involve the central part of the hyoid, necessitating removal of the midportion of the hyoid to minimize the risk of recurrence.20
Acierno SP, Waldhausen JH. Congenital cervical cysts, sinuses and fistulae. Otolaryngol Clin North Am. 2007;40:161.
Robbins KT, Clayman G, Levine PA, et althe Committee for Head and Neck Surgery. Standardizing neck dissection terminology: official report of the Academy’s Committee for Head and Neck Surgery and Oncology. Arch Otolaryngol Head Neck Surg. 1991;117:601.
1. Grodinsky M, Holyoke EA. The fascias and fascial spaces of the head, neck and adjacent regions. Am J Anat. 1938;63:367.
2. Haymaker W, Woodhall B. Peripheral Nerve Injuries, 2nd ed. Philadelphia: WB Saunders; 1953.
3. Daseler EH, Anson BJ. Surgical anatomy of the subclavian artery and its branches. Surg Gynecol Obstet. 1959;108:149.
4. Last RJ, editor. Anatomy: Regional and Applied, 6th ed, Edinburgh: Churchill Livingstone, 1978.
5. Robbins KT, Medina JE, Wolfe GT, et al. Standardizing neck dissection terminology: official report of the Academy’s Committee for Head and Neck Surgery and Oncology. Arch Otolaryngol Head Neck Surg. 1991;117:601.
6. Anson BJ, Donaldson JA. Surgical Anatomy of the Temporal Bone and Ear, 2nd ed. Philadelphia: WB Saunders; 1973.
7. Shambaugh GEJr. Surgery of the Ear, 2nd ed. Philadelphia: WB Saunders; 1967.
8. Langman J, editor. Medical Embryology, 4th ed, Baltimore: Williams & Wilkins, 1981.
9. Pearse AGE, Carvalheira AF. Cytochemical evidence for an ultimobranchial origin of rodent thyroid C cells. Nature. 1967;214:929.
10. Pearse AGE, Polak JM. Cytochemical evidence for the neural-crest origin of mammalian C cells. Histochemie. 1971;27:96.
11. Poswillo DE. Etiology and pathogenesis of first and second branchial arch defects: the contribution of animal studies. In: Converse JM, McCarthy JG, Wood-Smith D, editors. Symposium on Diagnosis and Treatment of Craniofacial Anomalies, Vol 20. New York: New York University Press; 1976:86-99.
12. Jones KL, editor. Smith’s Recognizable Patterns of Human Malformation, 4th ed. Philadelphia: WB Saunders. 1988:210-211.
13. Work WP. Newer concepts of first branchial cleft defects. Laryngoscope. 1972;82:1581.
14. Aronsohn RS, Batsakis JG, Rice DH, et al. Anomalies of the first branchial cleft. Arch Otolaryngol. 1976;102:737.
15. Minkowitz S, Minkowitz F. Congenital aural sinuses. Surg Gynecol Obstet. 1964;118:801.
16. Godin MS, Kearns DB, Pransky SM, et al. Fourth branchial pouch sinus: principles of diagnosis and management. Laryngoscope. 1990;100:174.
17. Rosenfeld RM, Biller HF. Fourth branchial pouch sinus: diagnosis and treatment. Otolaryngol Head Neck Surg. 1991;105:44.
18. Pinczower E, Crockett DM, Atkinson JB, et al. Preoperative thyroid scanning in presumed thyroglossal duct cysts. Arch Otolaryngol Head Neck Surg. 1992;118:985.
19. Radkowski D, Arnold J, Healy GB, et al. Thyroglossal duct remnants: preoperative evaluation and management. Arch Otolaryngol Head Neck Surg. 1991;117:1378.
20. Sistrunk WE. Technique of removal of cysts and sinuses of the thyroglossal duct. Surg Gynecol Obstet. 1928;46:109.