CHAPTER 27 Anatomic Reconstruction of the Posterolateral Corner
Injuries to the posterolateral corner (PLC) of the knee have received increased attention in recent years, with its elusive diagnosis, and potential surgical approaches to guide repair and/or reconstruction are still highly debated. Most injuries to the PLC occur in combination with disruption of the posterior cruciate ligament (PCL) or, to a lesser extent, the anterior cruciate ligament (ACL).1–8 Failure to identify and treat these combined injuries has been reported to cause premature failure of cruciate ligament reconstruction, yet despite numerous surgical advancements enhancing cruciate ligament repair, results regarding PLC reconstruction remain far less predictable.9–17 Perhaps secondary to its complex, often variable anatomy, lack of consensus guiding repair and/or reconstruction, or absence of current methods to assess treatment success, much controversy remains regarding optimal recognition and treatment of these injuries.18,19
Common causes of PLC injury include sports-related trauma, motor vehicle accidents, and falls.1,4,20 Both contact and noncontact injuries have been recounted.21 Injury of the PLC in combination with other ligamentous injuries occurs by a number of different mechanisms, typically involving a combination of knee hyperextension, external rotation, and varus rotation.2,3,7,8,22–25 Posterolateral instability can also follow knee dislocation, and careful physical examination is required to rule out associated neurovascular complication. The incidence of neurovascular damage, most commonly involving the peroneal nerve, have been estimated to be from 12% to 29% following injuries to the PLC.26–27
Clinical manifestations of posterolateral corner insufficiency most notably include functional instability with the knee in extension. This is apparent with stair activity, slopes, or activities that require pivoting or change of direction.28 Gait analysis may reveal a varus or hyperextension thrust, partially compensated for by walking with a slightly flexed knee.3 Stress testing may reveal abnormal tibial external rotation, varus instability, as well as pathologic anterior or posterior translation with concomitant ACL or PCL injuries.1–4,6,20–22,25,29–32
Isolated injury to the PLC is uncommon, and a high degree of suspicion must exist to recognize these often subtle clinical findings skillfully. DeLee and colleagues,4 in a comprehensive review of 735 knees with ligamentous injuries, found that only 1.6% of these subjects had acute isolated posterolateral deficiency. The greatest opportunity to achieve optimal results has been attributed to early repair, usually within 3 weeks from the date of injury, with the goal of obtaining anatomic repair of all injured structures.6,29,33 Stannard and associates18,19 have recommended augmentation, even at the time of primary repair, because of poor success rates. With respect to chronic injuries (more than 3 weeks postinjury), direct repair of individual structures is impractical, and invariably requires reconstructive techniques that should ideally mimic intrinsic anatomic function. Extensive scar tissue formation, secondary physiologic changes to involved structures, and potential alterations in weight-bearing and force-loading kinematics make this a formidable challenge, a monumental task for even the best of surgical technicians.
Numerous techniques to repair or augment the structures of the posterolateral corner have been described, but poor outcomes have been reported in 9% to 37% in clinical series.5,18,19,34 Previous accounts have included advancement and recession of the lateral collateral ligament and arcuate complex, biceps tenodesis, augmentation of the biceps femoris or iliotibial band, autogenous or allograft reconstruction through a transfibular tunnel, and use of a transtibial tunnel technique.9,13,17,35–38 All are performed in an attempt to recreate native fibular (lateral) collateral ligament function, considered the primary restraint to varus loads at full knee extension.
Advancement procedures have also been advocated, premised on tightening PLC structures by proximal relocation on the femur or by distal advancement on the tibia. However, these procedures have traditionally been nonanatomic and/or nonisometric, overconstraining knee motion or creating residual pathologic laxity; both can lead to suboptimal function and potential graft failure with repeated cycling.6,9,13,15,18,19 Furthermore, this technique is dependent on good residual tissue for advancement. Thus, in the absence of bony avulsion or with midsubstance ruptures that create tissue ends not amenable to anatomic repair, most authors recommend anatomic reconstruction. Unfortunately, despite many techniques having been advocated, a glaring lack of objective data exists to support these immeasurable claims.17,35–37
Newer techniques used for reconstruction are based on the creation of dual femoral tunnels that attempt to approximate the anatomic footprints of the popliteus and lateral collateral ligament (LCL) on the lateral femoral condyle.26 Similarly, current transfibular techniques describe a tunnel made in a strictly anterior to posterior direction in the fibula, along with a single isometric femoral tunnel providing two limbs to address varus laxity and abnormal increases in tibial external rotation. Moreover, patients with marked varus alignment and/or a lateral thrust in the stance phase of gait may require a valgus-producing tibial osteotomy prior to ligament reconstruction to re-create more conventional mechanical alignment, enhance repair kinematics, and decrease overall risk of graft failure.9,39
ANATOMY
The anatomic features of the PLC are extremely complex, considerably variable, and likely represent the least understood functional compartment of the knee. Inconsistent terminology, confusing nomenclature, and demonstrable changes in anatomic relationships, specifically evolutionary alterations regarding structures associated with the fibular head, have caused much deliberation concerning its organization.40–42 Terry and LaPrade have provided much clarity to this exposure through cadaveric dissection and study.43
The key elements include the fibular (lateral) collateral, popliteus, and popliteofibular ligaments (Fig. 27-1) Functioning as a unit, these structures, along with associated capsular components, prevent abnormal posterior translation, varus rotation, and coupled external rotation of the tibia.24,31,44,45 In addition, by preventing abnormal translation and rotation, the PLC has a secondary function of protecting the cruciate ligaments from abnormally high tensile loads, which can potentially disrupt the normal healing process following cruciate repair, leading to premature graft failure.3,14,46
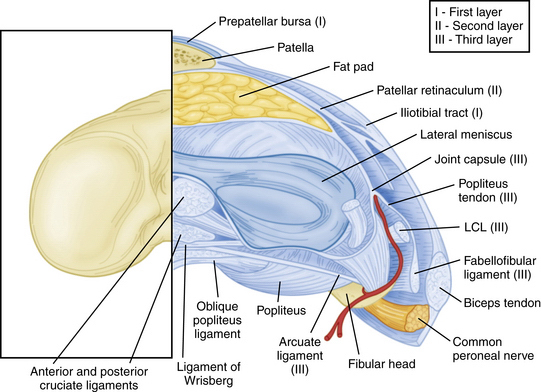
The PLC is commonly depicted in terms of its soft tissue arrangement, remarkably defined within the context of the layer concept by Seebacher and coworkers47 and the three-fascia incision technique popularized by Terry and LaPrade43 (Figs. 27-2 and 27-3). The most superficial layer, or layer I, contains the lateral fascia, iliotibial band, and biceps femoris tendon. The middle layer includes the quadriceps retinaculum, patellofemoral ligaments, and patellomeniscal ligament, whereas the deepest layer, or layer III, contains the lateral joint capsule, popliteal muscle-tendon unit, and fibular collateral ligament. The fabellofibular and arcuate ligaments, which are variable in terms of their size and contributions to stability, also reside in the deepest layer.
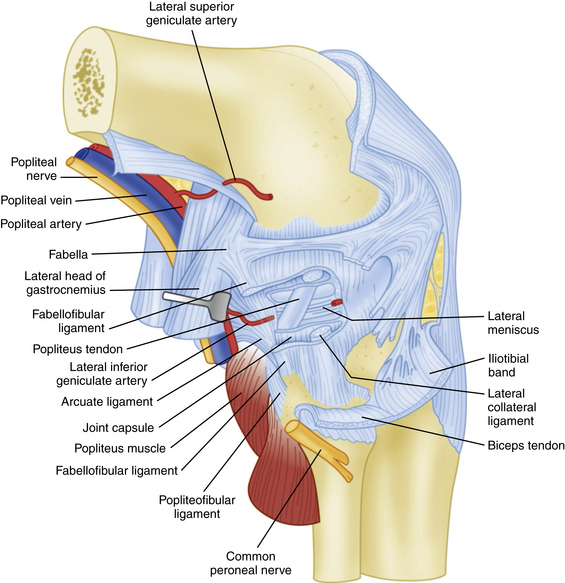
FIGURE 27-3 Lateral view of the posterolateral corner demonstrating relationships of the anatomic structures within the three layers of the knee.
The PLC can also be divided into primary and secondary stabilizers. Primary stabilizers include the LCL and popliteus complex, whereas less prominent contributors include the arcuate complex, posterolateral capsule, and various connections to the lateral meniscus.2,23,46 Similarly, these primary stabilizers can be broken down into static and dynamic stabilizers, with the popliteus tendon complex considered a prime example. The dynamic component includes the popliteus muscle and tendon unit proper, controlling the screw-home mechanism; the static component includes several branching ligamentous structures that insert into the tibia, fibula, and lateral meniscus, respectively.
The strength of the posterolateral compartment can be viewed as a composite effort by its constituents, but the main contributors include the fibular collateral ligament, tendinoaponeurotic portion of the popliteus muscle, and posterolateral capsule. The fibular collateral ligament (FCL) originates just proximal and posterior to lateral femoral epicondyle, slightly anterior to the femoral attachment of the lateral head of the gastrocnemius tendon. It follows an oblique course to insert on the lateral aspect of the fibular head, anterior and distal to the tip of the fibular styloid process (Fig. 27-4). It provides stability against varus loads, most notably at terminal knee extension, and as a secondary restraint to combined external rotation and posterior displacement of the knee. It is a distinct entity from the joint capsule and, unlike the medial collateral ligament, has no attachments to its corresponding meniscus.3,44,45,48
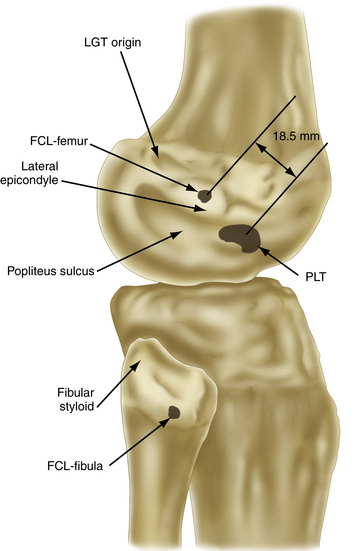
FIGURE 27-4 Relevant insertion site anatomic relationships of the LCL, popliteus tendon, and popliteofibular ligament.
The popliteus muscle arises from the posteromedial proximal tibia, just above the soleal line, extending superiorly and laterally to form a tendinous structure as it courses through the popliteal hiatus. It then inserts on the lateral femoral condyle, anterior and distal to the LCL origin, resisting pathologic external rotation of the tibia from 20 to 130 degrees and excessive varus rotation from 0 to 90 degrees. It also sends fibers to the posterior horn of the lateral meniscus, helping to restrict its excessive forward displacement as the knee assumes a more extended position.49
The popliteofibular ligament originates near the popliteus musculotendinous junction and courses distally and laterally, attaching to the medial aspect of the fibular styloid process.48–50 Anatomic studies have shown it to be present 93% to 100% of the time.23,24 It can be easily be differentiated from the arcuate ligament by the orientation of its fibers, with the proximal third coursing obliquely and fusing with the popliteal tendon, and the remaining distal fibers running vertically, similar to the pattern of the LCL (Fig. 27-5). It helps prevent excessive posterior translation, varus angulation, and primary and coupled tibial external rotation.43 Remarkably, it is as wide as or even wider than the popliteus tendon proper.
The arcuate ligament forms a Y-shaped pattern that begins distally on the styloid process of the fibula, with the medial and lateral limbs both extending proximally. The lateral limb coalesces with the lateral joint capsule and the medial limb attaches to the posterior capsule of the knee.21,23,26,47,48 Studies have shown it to be present in only 24% of cadaveric specimens, whereas the equally as variable fabellofibular ligament has been found to be present in approximately 68%.49 Both act in complementary fashion to stabilize the PLC further when present.
Biomechanics
To understand the PLC fully, one must review the complex interplay between the static and dynamic stabilizers of the knee. Numerous biomechanical studies implementing selective ligament cutting protocols have demonstrated that injury to the PLC results in increased primary external rotation, primary varus rotation, primary posterior translation, and coupled external rotation.3,8,51 In terms of varus rotational stability, the PLC acts as a restraint at lesser degrees of knee flexion, with maximal restraint at 30 degrees.46 Regarding external rotational instability, sectioning of the LCL and PLC capsule has been shown to result in greater posterolateral rotary instability than isolated sectioning of either structure alone.31,44,45 Concerning posterior translational instability, the PLC resists posterior translation at lesser degrees of knee flexion with maximal restraint at 30 degrees, whereas the PCL resists posterior tibial translation at higher degrees of knee flexion, with maximal restraint at 90 degrees.52
Forces on the intact ligaments of the knee after PLC injury can be substantial. The PCL experiences significant increases in forces between 45 to 90 degrees of knee flexion after complete sectioning of the PLC and tibial varus or external rotation.53 Harner and colleagues12 have found a significant increase in the force on a PCL reconstruction graft in PLC-deficient knees, with the PCL graft rendered almost ineffective and, as expected, predisposing it to failure. Combined PCL and PLC injuries have also been shown to produce increased contact pressures in the patellofemoral joint when compared to isolated PCL injuries alone.54 Similarly, tension on the ACL is also increased with PLC injury, particularly with tibial internal rotation in the range of 0 to 20 degrees of knee flexion.
PATIENT EVALUATION
PLC injuries can be subtle and are easily missed if a high degree of suspicion for injury does not exist. Symptoms invariably depend on timing and severity of injury, degree of associated instability, and other sustained defects. Injuries are often classified as grade I, II, or III sprains, corresponding to minimal, partial, or complete tearing, respectively. Quantification of the degree of joint opening in response to varus stress is also commonly used, with a score of 3+ describing an opening of more than 10 mm, with a soft or no appreciable end point.3,10 Patients with evidence of injury, but without significant pathologic laxity or functional limitations, corresponding to grade I and II injuries, are often treated with rehabilitation and observation.55,56 However, patients with grade III injuries, which typically reflect abnormal joint motion, are advised to undergo primary repair, augmentation, or reconstruction.4,18,19,32
Diagnostic evidence of PLC injury can come in a wide variety, and includes physical signs, imaging, or both. Gait abnormalities may be present, particularly in chronic injuries, and may include standing varus alignment of the knee and/or a hyperextension-varus thrust during the stance phase of gait. Patients may attempt to walk with the knee slightly flexed or modify footwear to help decrease symptoms of pain and instability.2,3,22
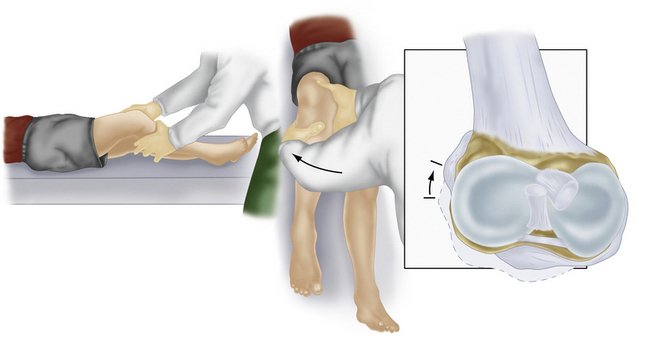
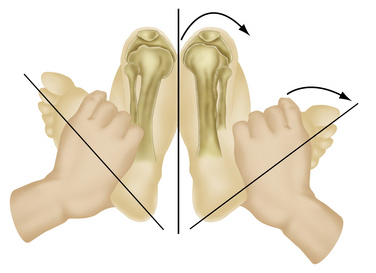
Along with physical examination parameters, which includes meticulous evaluation of the cruciate ligaments, specific tests to detect PLC injury include the varus load, posterolateral drawer, and Dial tests. They all attempt to assess increased external rotation of the tibia relative to the femur. When there is an isolated injury to the posterolateral structures with an intact PCL, there is maximally increased varus, external rotation, and posterior translation at 30 degrees of flexion because of a minimal amount of PCL fibers tensed at low knee flexion angles (Fig. 27-6). In contrast, at 90 degrees of flexion, all intact PCL fibers are tightened and help provide a secondary restraint against external rotation and varus forces, including providing the primary restraint to posterior tibial translation. With specific reference to the Dial test, increased external rotation at 30 but not at 90 degrees indicates an isolated PLC injury, whereas increased external rotation at both suggests injury to both structures. An increase of 10 degrees from the normal knee is also considered a significant finding (Fig. 27-7).1–3,20,22,28,32,48,57
Diagnostic Imaging
Stress radiography has recently been popularized in the literature, and may be a useful adjunct to diagnosis of more imprecise PLC injuries. It involves the application of a standardized force in an effort to produce abnormal joint displacement. It has been shown to be a reliable measure of posterior joint laxity in patients with PCL injuries, and was recently found to be a good predictor of concomitant disruption to the PLC.58,59 Sekiya and associates7 have found that a grade 3 posterior drawer test and more than 10 mm of tibial translation on stress radiography correlate with a complete disruption of the PCL, in addition to PLC injury. This reported association should help raise examiner suspicion to the possibility of damage to the PLC so often overlooked in combined ligamentous injuries.
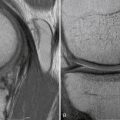
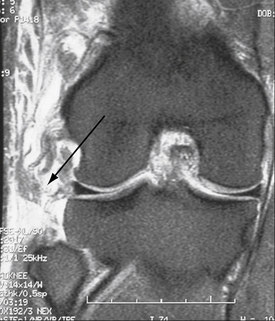
Regarding MRI, perhaps the most sensitive marker of all, recent studies have shown that either T1- or T2-weighted coronal oblique images through the knee, which include the entire fibular head and styloid process, provide the best visualization of the individual structures of the PLC (Fig. 27-8).60 Moreover, it can also detect associated injuries, most importantly, concomitant ACL and PCL tears that will directly influence surgical planning and associated patient rehabilitation. Furthermore, in the acute setting, when physical examination may not be possible secondary to patient discomfort, the use of MRI to qualify disruption of the PLC structures can be of utmost importance to aid in acute diagnosis and facilitate early intervention.
TREATMENT
Timing is of critical importance in the surgical management of isolated PLC or combined injuries.1,4,22,28,56 Acute events, defined as within 3 weeks of injury, have generally demonstrated greater enduring success than operative management of chronic PLC injuries. However, many still favor primary reconstruction over attempted repair, even in the acute setting, arguing that repair and/or augmentation of the fibular collateral ligament is indicated only for patients with bony avulsions potentially amenable to internal fixation.18,19,23,36 In either case, it is imperative to detect and treat PLC injuries early to avoid physiologic and biomechanical changes that accompany chronic injuries, and are professed to generate inferior functional long-term results. When combined injuries are involved, preoperative planning must select for appropriate patient positioning, graft availability and/or harvest technique, and sequential surgical instrumentation to guide the repair and/or reconstruction.
Arthroscopic Technique
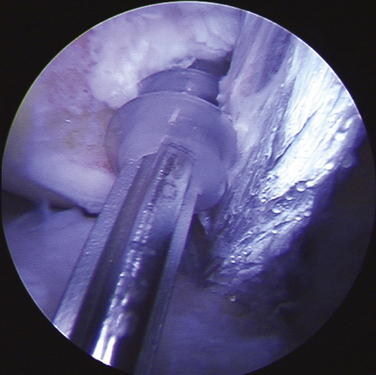
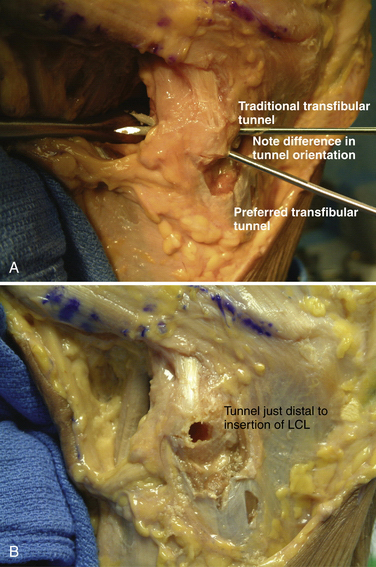
Following patient preparation and placement in the supine position, with all bony prominences protected, the three-fascia incision technique popularized by Terry and LaPrade43 is initiated. A curvilinear or hockey stick–type incision is gently created over the lateral aspect of the knee. It should be centered over the lateral epicondyle and extend distally to the anterior fibular head, midway between the fibular head and Gerdy’s tubercle. The first fascial incision is distal to expose the peroneal nerve and mobilize it away from the proximal fibula. The peroneal nerve should then be identified and dissected free, from just inferior to the biceps tendon to the posterior aspect of the fibular head. It must be protected throughout the entire procedure to ensure optimal post-operative function. Using a blunt elevator, the muscle and soft tissue on the posterior aspect of the fibula are gently reflected to expose the posterior bony surface of the fibula. The LCL insertion on the fibula is then identified. This ligament inserts on the lateral aspect of the fibula in a small sulcus. It can be found by making a small linear incision in the biceps fascia, spreading and exposing the ligament, as described by LaPrade. The fibular head is then exposed just anterior and distal to the insertion site and a 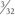 -inch guide wire is drilled from this spot posteromedially. A 6- or 7-mm bone fibular tunnel is created with a cannulated reamer and a looping suture is placed for future graft passage. The angle of this tunnel is unique to this reconstruction; it is thought to reconstruct the LCL more anatomically compared with the traditional method of drilling this tunnel from a more anterior to posterior direction (Fig. 27-9).
-inch guide wire is drilled from this spot posteromedially. A 6- or 7-mm bone fibular tunnel is created with a cannulated reamer and a looping suture is placed for future graft passage. The angle of this tunnel is unique to this reconstruction; it is thought to reconstruct the LCL more anatomically compared with the traditional method of drilling this tunnel from a more anterior to posterior direction (Fig. 27-9).
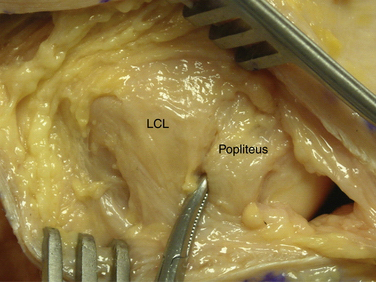
The second fascial incision is then made, exposing the lateral capsular region, extending from the superoanterior border of the short head biceps tendon to the inferior border of the iliotibial (IT) band. A posterior arthrotomy is made and no. 2 nonabsorbable sutures are placed for reefing of the posterolateral capsule at the end of the procedure. Finally, the IT band should be incised centrally over the lateral femoral epicondyle and continued anteriorly and slightly distally. Often, a thickened bursal layer will obscure the LCL origin, and should be separated to gain proper exposure of the femoral attachment site of the LCL.. An anterior arthrotomy then allows for identification of the origin of popliteus tendon on the femur (Fig. 27-10). This incision is made vertically to expose the distal lateral condylar articular surface and peripheral edge of the anterolateral meniscus. Gentle retraction will allow for visualization of the popliteus tendon coming in an oblique fashion and inserting distally on the lateral femur, close to the articular surface.
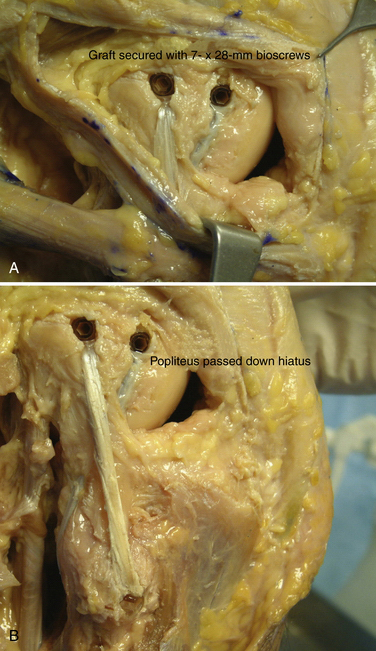
The popliteofibular tunnel is now created, with a pin inserted approximately 3 to 4 mm distal and slightly anterior to the femoral attachment for the popliteus tendon. It is aimed medially, but only requires a depth of 25 to 30 mm. A 7- to 8-mm cannulated reamer is used to prepare the femoral socket for the popliteus limb. The dual femoral tunnels should be separated by approximately 18 mm, according to the anatomic insertions of the LCL and popliteus tendon (Fig. 27-11. Also see Fig. 27-10).43
A graft of suitable length (we prefer a posterior tibialis allograft or semitendinosus autograft), typically 24 to 26 cm, is then chosen, and its free ends tagged with a baseball stitch over a distance of approximately 25 to 30 mm with a no. 2 nonabsorbable suture. The graft is then passed through the transfibular tunnel and firmly tensioned with an equal length of graft limbs, both anterior and posterior. The posterior limb is then tunneled along the posterior aspect of the fibula, through the popliteal hiatus, and into its respective femoral tunnel. An 8- or 9- × 23-mm biotenodesis screw (Arthrex; Naples, Fla) is then used for fixation. This unicortical tunnel and graft fixed in this fashion obviates the need for drilling across the femur, which could potentially interfere with other tunnels created for cruciate ligament reconstruction. The other limb is now tunneled deep to the biceps femoris tendon insertion, adjacent to the native or remnant LCL. Using the passing suture already in place for the LCL tunnel, the anterior limb is then transferred into its femoral socket. With the knee in approximately 30 degrees of flexion, slight internal rotation, and slight valgus, firm tension is then applied medially to tighten this limb of the reconstruction. A 7- to 8-mm × 28-mm bioabsorbable interference screw is then placed into each tunnel, definitively securing the graft (Figs. 27-12 and 27-13). The wounds are copiously irrigated, closed in layers, and the knee placed in a hinged brace and locked in full extension.
COMPLICATIONS
Complications of surgical management of PLC injuries can include injury to the peroneal nerve, wound problems, postoperative knee stiffness (most commonly, loss of flexion), hamstring weakness (particularly in biceps tenodesis repair or advancement procedures), soft tissue graft failure, and hardware issues.2,3 Meticulous preoperative planning, expert surgical technique, and a strict rehabilitation protocol can help diminish their potential occurrence. The risk of peroneal nerve injury can be skillfully decreased by careful identification, fastidious dissection, and watchful protection throughout the surgical process. Knee stiffness, a common sequela, especially in the early postoperative setting, can develop into advanced arthrofibrosis, requiring manipulation under anesthesia and/or arthroscopic release of adhesions to enhance long-term flexibility. Proper tensioning techniques should help bolster graft strength and predicted durability; however, following stringent rehabilitation parameters is the overall key to minimize the chance of catastrophic failure.
PEARLS& PITFALLS
Postoperative Rehabilitation
Following surgical intervention, immediate mobilization is initiated with protected weight bearing (PWB) to safeguard the repair. The patient is placed in a hinged knee brace, locked in full extension. Gentle range of motion (ROM) is not typically done prior to 3 weeks. Static quadriceps exercises and straight leg raises are permitted. Patellar mobilization and gaining full extension are important. Weight bearing is restricted for the first 6 weeks, usually 20 to 40 pounds of PWB. Specific goals during the early recovery period include minimizing postoperative inflammation and associated fibrosis while concomitantly working to prevent quadriceps atrophy. At 6 weeks, patients enter the next phase of rehabilitation, premised on demonstrating active ROM of at least 0 to 115 degrees and quadriceps strength at least 60% of the contralateral leg referenced as a control. Once this is achieved, the hinged brace is discontinued and patients can begin proprioceptive skills training. Weight bearing is then progressed to full weight-bearing status by the week 8. At approximately 3 months, further strengthening exercises, specifically, closed-chain exercises, can be safely initiated. Return to competitive sports and/or heavy labor is permitted at approximately the 6-month mark if patients have achieved quadriceps strength of 80% of the unaffected leg and appropriate proprioceptive ability has been established.
CONCLUSIONS
The combined structures of the PLC have greater tensile strength than any of the other major knee ligaments and act in combination with the PCL to resist posterior translation and external and varus rotation of the tibia on the femur. Functional outcomes following nonoperative management of the multiple ligament–injured knee are considered to be poor at best. Disruption of the PLC with an intact PCL results in increased varus and external rotation of the knee, most pronounced at 30 degrees of knee flexion, whereas disruption of the PCL with an intact PLC results in increased posterior translation of the tibia, most pronounced at 90 degrees of knee flexion.2,3 Moreover, cruciate ligament disruption can lead to increased compartment pressures, accelerating the risks of associated degenerative joint disease.54,61
Pasque,33 in a cadaveric study under defined loading conditions, has demonstrated the importance of repairing all associated PLC structures. No individual component demonstrated an ability to act as a primary restraint to external rotation, varus rotation, and posterior tibial translation from 0 to 120 degrees. With an applied external rotation tibial force applied, LCL reconstruction alone was not sufficient enough to reduce PCL graft forces to normal. Addition of a popliteus or popliteofibular reconstruction was necessary to reduce PCL graft forces to near-normal values.
In patients with both chronic PLC and cruciate ligament deficiency, cruciate ligament reconstruction should be performed collectively with PLC reconstruction because of the potential for better long-term functional outcome. Failure to address instability of the PLC can increase tensile forces at cruciate ligament graft sites, contributing to graft failure through the generation of higher forces associated with varus loading at varying degrees of flexion, not observed in those with intact PLC structures. Anatomic reconstruction allows for the greatest potential of resuming functional independence and should be the procedure of choice for chronic isolated or combined posterolateral and cruciate ligament injuries.
1. Baker CLJr, Norwood LA, Hughston JC. Acute posterolateral instability of the knee. J Bone Joint Surg Am. 1983;65:614-628.
2. Chen FS, Rokito AS, Pitman MI. Acute and chronic posterolateral instability of the knee. J Am Acad Orthop Surg. 2000;8:97-110.
3. Covey DC. Injuries of the posterolateral corner of the knee: anatomy, biomechanics, and the management of injuries. J Bone Joint Surg Am. 2001;83:106-118.
4. DeLee JC, Riley MB, Rockwood CAJr. Acute posterolateral instability of the knee. Am J Sports Med. 1983;11:199-207.
5. Noyes FR, Barber-Westin SD. Surgical reconstruction of severe chronic posterolateral complex injuries of the knee using allograft tissues. Am J Sports Med. 1995;23:2-12.
6. Ranawat A, Baker CL 3rd, Henry S, Harner CD. Posterolateral corner injury of the knee: evaluation and management. J Am Acad Orthop Surg. 2008;16:506-518.
7. Sekiya JK, Whiddon DR, Zehms CT, Miller MD. A clinically relevant assessment of posterior cruciate ligament and posterolateral corner injuries. Evaluation of isolated and combined deficiency. J Bone Joint Surg Am. 2008;90:1621-1627.
8. Veltri DM, Deng XH, Torzilli PA, et al. The role of the cruciate and posterolateral ligaments in stability of the knee. A biomechanical study. Am J Sports Med. 1995;23:436-443.
9. Arciero R. Anatomic posterolateral corner reconstruction. Arthroscopy. 2005;21:1147.
10. Fanelli GC. Surgical reconstruction for acute posterolateral injury of the knee. J Knee Surg. 2005;28:157-162.
11. Freeman RT, Duri ZA, Dowd GS. Combined chronic posterior cruciate and posterolateral corner ligamentous injuries: a comparison of posterior cruciate ligament reconstruction with and without reconstruction of the posterolateral corner. Knee. 2002;9:309-312.
12. Harner CD, Vogrin TM, Hoher J, et al. Biomechanical analysis of a posterior cruciate ligament reconstruction: deficiency of the posterolateral structures as a cause of graft failure. Am J Sports Med. 2000;28:32-39.
13. Khanduja V, Somayaji HS, Harnett P, et al. Combined reconstruction of chronic posterior cruciate ligament and posterolateral corner deficiency. A two- to nine-year follow-up study. J Bone Joint Surg Br. 2006;88:1169-1172.
14. LaPrade RF, Johansen S, Wentorf FA, et al. An analysis of an anatomical posterolateral knee reconstruction: an in vitro biomechanical study and development of a surgical technique. Am J Sports Med. 2004;32:1405-1414.
15. LaPrade RF, Muench C, Wentorf F, Lewis JL. The effect of injury to the posterolateral structures of the knee on force in a posterior cruciate ligament graft: a biomechanical study. Am J Sports Med. 2002;30:233-238.
16. McGuire DA, Wolchok JC. Posterolateral corner reconstruction. Arthroscopy. 2003;19:790-793.
17. Yoon KH, Bae DK, Ha JH, Park SW. Anatomic reconstructive surgery for posterolateral instability of the knee. Arthroscopy. 2006;22:159-165.
18. Stannard JP, Brown SL, Farris RC, et al. The posterolateral corner of the knee: repair versus reconstruction. Am J Sports Med. 2005;33:881-888.
19. Stannard JP, Brown SL, Robinson JT, et al. Reconstruction of the posterolateral corner of the knee. Arthroscopy. 2005;21:1051-1059.
20. Fleming REJr, Blatz DJ, McCarroll JR. Posterior problems in the knee. Posterior cruciate insufficiency and posterolateral rotary insufficiency. Am J Sports Med. 1981;9:107-113.
21. Davies H, Unwin A, Aichroth P. The posterolateral corner of the knee. Anatomy, biomechanics, and the management of injuries. Injury. 2004;35:68-75.
22. Larson RV, Tingstad E. Lateral and posterolateral instability of the knee in adults. In: DeLee JC, Drez DJ, editors. DeLee and Drez’s Orthopaedic Sports Medicine. Principles and Practice. 2nd ed. Philadelphia: WB Saunders; 2003:1969-1994.
23. Shahane SA, Ibbotson C, Strachan R, Bickerstaff DR. The popliteofibular ligament. An anatomical study of the posterolateral corner of the knee. J Bone Joint Surg Br. 1999;81:636-642.
24. Veltri DM, Deng XH, Torzilli PA, et al. The role of the popliteofibular ligament in stability of the human knee. A biomechanical study. Am J Sports Med. 1996;24:19-27.
25. Veltri DM, Warren RF. Anatomy, biomechanics, and physical findings in posterolateral knee instability. Clin Sports Med. 1994;13:599-614.
26. LaPrade RF, Ly TV, Wentorf FA, Engebretsen L. The posterolateral attachments of the knee: a qualitative and quantitative morphologic analysis of the fibular collateral ligament, popliteus tendon, popliteofibular ligament, and lateral gastrocnemius tendon. Am J Sports Med. 2003;31:854-860.
27. Noyes FR, Grood ES, Torzilli PA. Current concepts review. The definitions of terms for motion and position of the knee and injuries of the ligaments. J Bone Joint Surg Am. 1989;71:465-472.
28. Hughston JC, Jacobson KE. Chronic posterolateral instability of the knee. J Bone Joint Surg Am. 1985;67:351-359.
29. Cooper DE WR, Warner JP. The posterior cruciate ligament and posterolateral structures of the knee: anatomy, function, and patterns of injury. Instr Course Lect. 1991;40:249-270.
30. Daniel DM, Akeson WH, O’Connor JJ. Knee Ligaments: Structure, Function, Injury, and Repair. Raven Press, New York, 1990.
31. Nielsen S, Ovesen J, Rasmussen O. The posterior cruciate ligament and rotatory knee instability. An experimental study. Arch Orthop Trauma Surg. 1985;104:53-56.
32. Ross G, DeConciliis GP, Choi K, Scheller AD. Evaluation and treatment of acute posterolateral corner/anterior cruciate ligament injuries of the knee. J Bone Joint Surg Am. 2004;86(suppl 2):2-7.
33. Pasque C. The role of the popliteofibular ligament and tendon of the popliteus in providing stability in the human knee. J Bone Joint Surg Br. 2003;85:292-298.
34. Kim SJ, Shin SJ, Jeong JH. Posterolateral rotary instability treated by a modified biceps rerouting technique: technical considerations and results in cases with and without PCL insufficiency. Arthroscopy. 2003;19:493-499.
35. Buzzi R, Aglietti P. Vena LM, Giron F. Lateral collateral ligament reconstruction using a semitendinosus graft. Knee Surg Sports Traumatol Arthrosc. 2004;12:36-42.
36. Latimer HA, Tibone JE, ElAttrache NS, McMahon PJ. Reconstruction of the lateral collateral ligament of the knee with patellar tendon allograft: report of a new technique in combined ligament injuries. Am J Sports Med. 1998;26:656-662.
37. Noyes FR, Barber-Westin SD. Posterolateral knee reconstruction with an anatomical bone-patellar tendon-bone reconstruction of the fibular collateral ligament. Am J Sports Med. 2007;35:259-273.
38. Sekiya JK, Kurtz CA. Posterolateral corner reconstruction of the knee: surgical technique utilizing bifid Achilles tendon allograft and a double femoral tunnel. Arthroscopy. 2005;21:1400.
39. Arthur A, LaPrade RF, Agel J. Proximal tibial opening wedge osteotomy as the initial treatment for chronic posterolateral corner deficiency in the varus knee: a prospective clinical study. Am J Sports Med. 2007;11:1844-1850.
40. Dye SF. An evolutionary perspective of the knee. J Bone Joint Surg Am. 1987;69:976-983.
41. Herzmark MH. The evolution of the knee joint. J Bone Joint Surg Am. 1938;20:77-84.
42. Maynard MJ, Deng X, Wickiewicz TL, Warren RF. The popliteofibular ligament. Rediscovery of a key element in posterolateral stability. Am J Sports Med. 1996;24:311-316.
43. Terry GC, LaPrade RF. The posterolateral aspect of the knee. Anatomy and surgical approach. Am J Sports Med. 1996;24:732-739.
44. Nielson S, Helming P. The static stabilizing function of the popliteal tendon in the knee. An experimental study. Arch Orthop Trauma Surg. 1986;104:357-362.
45. Nielsen S, Helming P. Posterior instability of the knee joint. Arch Orthop Trauma Surg. 1986;105:121-125.
46. Gollehon DL. The role of the posterolateral and arcuate ligaments in stability of the human knee. A biomechanical study. J Bone Joint Surg Am. 1987;69:233-242.
47. Seebacher JR. The structure of the posterolateral aspect of the knee. J Bone Joint Surg Am. 1982;64:536-541.
48. Watanabe Y, Moriya H, Takahashi K, et al. Functional anatomy of the posterolateral structures. Arthroscopy. 1993;9:57-62.
49. Sudasna S, Harnsiriwattanagit K. The ligamentous structures of the posterolateral aspect of the knee. Bull Hosp Jt Dis Orthop Inst. 1990;50:35-40.
50. Wadia FD, Pimple M, Gajjar SM, Narvekar AD. An anatomic study of the popliteofibular ligament. Int Orthop. 2003;27:172-174.
51. Grood ES, Stowers SF, Noyes FR, et al. Limits of movement in the human knee. Effect of sectioning the posterior cruciate ligament and posterolateral structures. J Bone Joint Surg Am. 1988;70:88-97.
52. Noyes FR, Stowers SF, Grood ES, et al. Posterior subluxations of the medial and lateral tibiofemoral compartments. An in vitro sectioning study in cadaveric Knees. Am J Sports Med. 1993;21:407-414.
53. Markolf KL, Wascher DC, Finerman GA. Direct in vitro measurement of forces in the cruciate ligaments. Part II: the effect of sectioning of the posterolateral structures. J Bone Joint Surg Am. 1993;75:387-394.
54. Skyhar MJ, Warren RF, Ortiz GJ, et al. The effects of sectioning of the posterior cruciate ligament and the posterolateral complex on the articular contact pressures within the knee. J Bone Joint Surg Am. 1993;75:694-699.
55. Kannus P. Nonoperative treatment of grade II and III sprains of the lateral ligament compartment of the knee. Am J Sports Med. 1989;17:83-88.
56. Krukhaug Y, Mølster A, Rodt A, Strand T. Lateral ligament injuries of the knee. Knee Surg Sports Traumatol Arthrosc. 1998;6:21-25.
57. Hughston JC, Norwood LAJr. The posterolateral drawer test and external rotational recurvatum test for posterolateral rotatory instability of the knee. Clin Orthop Relat Res. 1980;(147):82-87.
58. LaPrade RF, Heikes C, Bakker AJ, Jakobsen RB. The reproducibility and repeatability of varus stress radiographs in the assessment of isolated fibular collateral ligament and grade-III posterolateral knee Injuries. J Bone Joint Surg Am. 2008;90:2069-2076.
59. Vinson EN, Major NM, Helms CA. The posterolateral corner of the knee. AJR Am J Roentgenol. 2008;190:449-458.
60. Schulz MS, Russe K, Lampakis G, Strobel MJ. Reliability of stress radiography for evaluation of posterior knee laxity. Am J Sports Med. 2005;33:502-506.
61. Anderson DD. Effects of sectioning of the PCL complex on the articular contact pressures within the knee. J Bone Joint Surg Am. 1995;77:649.

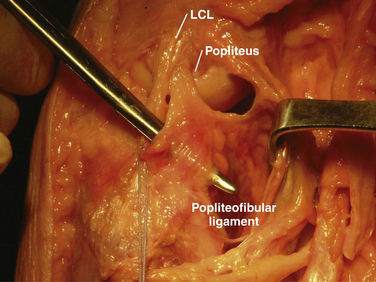
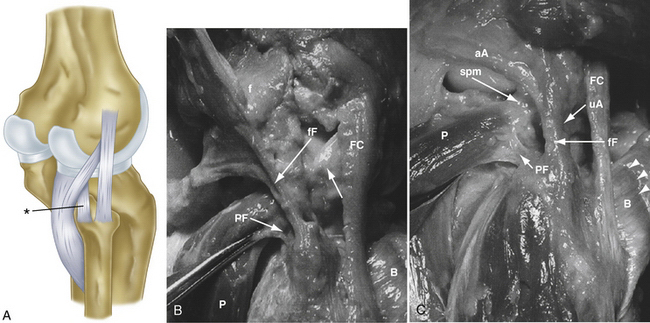
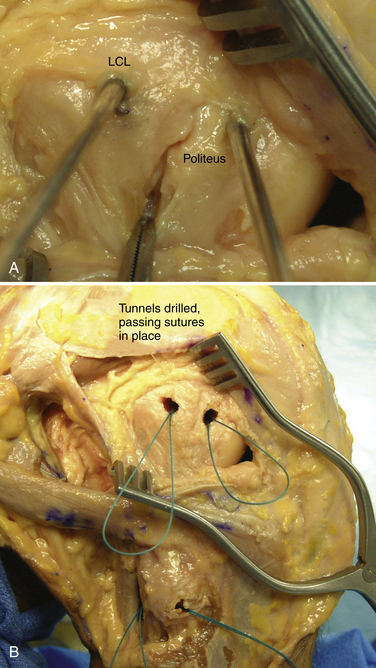
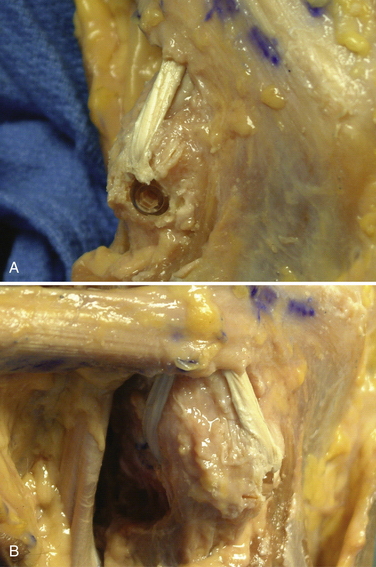
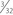 -inch guide wire placed in the appropriate location for the femoral tunnels of the LCL and popliteus tendons. B, Both tunnels after drilling.
-inch guide wire placed in the appropriate location for the femoral tunnels of the LCL and popliteus tendons. B, Both tunnels after drilling.