Anaesthesia Outside the Operating Theatre
ANAESTHESIA IN REMOTE HOSPITAL LOCATIONS
General Considerations and Principles
1. Appropriate personnel. Only senior experienced anaesthetists who are also familiar with the particular environment and its challenges should normally administer anaesthesia in remote locations. Additional skilled anaesthetic help may not be readily available compared with an operating theatre suite and patients are often challenging, e.g. paediatric or critically ill.
2. Equipment. The remote clinical area may not have been designed with anaesthetic requirements in mind. Anaesthetic apparatus often competes for space with bulky equipment (e.g. scanners) and, in general, conditions are less than optimal. Monitoring capabilities and anaesthetic equipment should be of the same standard as those used in the operating department. In reality, such equipment may not be readily available and the equipment used is often the oldest in the hospital. Nevertheless, the monitoring equipment should meet the minimum standards set by the Association of Anaesthetists of Great Britain and Ireland (AAGBI, 2006). The anaesthetist who is unfamiliar with the environment should spend time becoming accustomed to the layout and equipment. Compromised access to the patient and the type of monitors used during the procedure require careful consideration. Advanced planning helps to prepare for unanticipated scenarios. Clinical observation may be limited by poor lighting.
3. Patient preparation. Preparation of the patient may be inadequate because the patient is from a ward where staff are unfamiliar with preoperative protocols, or patients may be unreliable, e.g. those presenting for ECT.
4. Assistance. An anaesthetic assistant (e.g. operating department practitioner) should be present, although this person may be unfamiliar with the environment. Maintenance of anaesthetic equipment may be less than ideal. Consequently, the anaesthetist must be particularly vigilant in checking the anaesthetic machine, particularly because it may be disconnected and moved when not in use. Empty gas cylinders need to be replaced in older suites without piped gases, and also the anaesthetist must ensure the presence of drugs, spare laryngoscope and batteries, suction and other routine equipment.
5. Communication. Communication between staff of other specialities and the anaesthetist may be poor. This may lead to failure in recognizing each other’s requirements. Education programmes for non-anaesthesia personnel regarding the care of anaesthetized patients may be of benefit.
6. Recovery. Recovery facilities are often non-existent. Anaesthetists may have to recover their own patients in the suite. Consequently, they must be familiar with the location of recovery equipment including suction, supplementary oxygen and resuscitation equipment. Alternatively, patients may be transferred to the main hospital recovery area. This requires the use of routine transfer equipment which should ideally be available as a ‘pack’ kept alongside monitoring equipment and a portable oxygen supply. This avoids searching for various pieces of equipment which may delay transfer, and ensures that nothing is forgotten. The pack should be regularly checked and maintained.
Anaesthesia in the Radiology Department
Computed Tomography
General Principles: A CT scan provides a series of tomographic axial ‘slices’ of the body. It is used most frequently for intracranial imaging and for studies of the thorax and abdomen and is the investigation of choice in the evaluation of major trauma when whole body CT may be used in place of plain X-rays. Each image is produced by computer integration of the differences in the radiation absorption coefficients between different normal tissues and between normal and abnormal tissues. The image of the structure under investigation is generated by a cathode ray tube and the brightness of each area is proportional to the absorption value.
Anaesthetic Management: Computed tomography is non-invasive and painless, requiring neither sedation nor anaesthesia for most adult patients. A few patients may require conscious sedation to relieve fears or anxieties. However, patients who cannot cooperate (most frequently paediatric and head trauma patients or those who are under the influence of alcohol or drugs) or those whose airway is at risk may need general anaesthesia to prevent movement, which degrades the image. Anaesthetists may also be asked to assist in the transfer from the ICU and in the care of critically ill patients who require CT scans.
Magnetic Resonance Imaging
General Principles: Magnetic resonance imaging (MRI) is an imaging modality which depends on magnetic fields and radiofrequency pulses for the production of its images. The imaging capabilities of MRI are superior to those of CT for examining intracranial, spinal and soft tissue lesions. MRI differentiates clearly between white and grey matter in the brain, thus making possible, for example, the in vivo diagnosis of demyelination. It may display images in the sagittal, coronal, transverse or oblique planes and has the advantage that no ionizing radiation is produced.
STAFF SAFETY: Staff safety precautions are essential. The supervising MR radiographer is operationally responsible for safety in the scanner and anaesthetic staff should defer to him or her in matters of MR safety. Screening questionnaires identify those at risk and training should be given in MR safety, electrical safety, emergency procedures arising from equipment failure and evacuation of the patient. Anaesthetists should also understand the consequences of quenching the magnet and be aware of recommendations on exposure and the need for ear protection. Long-term effects of repeated exposure to MRI fields are unknown, and pregnant staff should be offered the option not to work in the scanner. All potentially hazardous articles should be removed, e.g. watches, bleeps and stethoscopes. Bank cards, credit cards and other belongings containing electromagnetic strips become demagnetized within the vicinity of the scanner and personal computers, pagers, phones and calculators may also be damaged.
PATIENT SAFETY: Metal objects within or attached to the patient pose a risk. Jewellery, hearing aids or drug patches should be removed. Absolute contraindications include implanted surgical devices, e.g. cochlear implants, intraocular metallic objects and metal vascular clips. Pacemakers remain an absolute contraindication in most settings although some patients with a pacemaker have undergone scanning under tightly controlled conditions when the benefit has been deemed to outweigh the risk. Metallic implants, e.g. intracranial vascular clips, may be dislodged from blood vessels. Programmable shunts for hydrocephalus may malfunction because the pressure setting may be changed by the magnetic field, leading to over- or underdrainage. The use of neurostimulators such as spinal cord stimulators for chronic pain is increasing. These devices may potentially fail or cause thermal injury on exposure to the magnetic field. Each must be considered individually, some may be safe if strict guidelines are adhered to. Joint prostheses, artificial heart valves and sternal wires are safe because of fibrous tissue fixation. Patients with large metal implants should be monitored for implant heating. A description of the safety of various devices is available on dedicated websites. All patients should wear ear protection because noise levels may exceed 85 dB.
EQUIPMENT: The magnetic effects of MRI impose restrictions on the selection of anaesthetic equipment. Any ferromagnetic object distorts the magnetic field sufficiently to degrade the image. It is also likely to be propelled towards the scanner and may cause a significant accident if it makes contact with the patient or with staff. Terminology regarding equipment used in the MRI scanner has now changed from ‘MR compatible’ or ‘MR incompatible’ to ‘MR conditional’, ‘MR safe’ or ‘MR unsafe’. MR conditional equipment is that which poses no hazards in a specified MR environment with specified conditions of use. The conditions in which it may be used must accompany the device and it may not be safe to use it outside these conditions, e.g. higher field strength or rate of change of the field. MR safe equipment is that which poses no safety hazard in the MR room but it may not function normally or may degrade the image quality. Consideration needs to be given to replacing equipment if a scanner is replaced by one of higher field strength.
CONDUCT OF ANAESTHESIA: The indications for general anaesthesia during MRI are similar to those for CT. In addition, the scanner is very noisy and, in general, the patient lies on a long thin table in a dark, confined space within the tube (typical diameter 50–65 cm). This may cause claustrophobia or anxiety-related problems which may require sedation or anaesthesia. Obese patients cannot be examined in this small magnetic bore. A complex scan may take up to 20 min and an entire examination more than 1 h. Open scanners have been developed and some are available which allow the patient to stand up, allowing a greater range of patients to be scanned. Outside normal working hours, only neuraxial scanning is usually performed, for acute brain or spinal cord evaluation.
Diagnostic and Interventional Angiography
General Principles: Direct arteriography using percutaneous arterial catheters is used widely for the diagnosis of vascular lesions. Catheters are usually inserted by the Seldinger technique via the femoral artery in the groin and injection of contrast medium provides images which are viewed by conventional or digital subtraction angiography. In addition, it is becoming increasingly common to consider vessel embolization both in the elective preoperative setting (e.g. vascular tumours or malformations) and in the emergency management of major haemorrhage (e.g. major trauma or massive obstetric or gastrointestinal haemorrhage). The procedure involves the injection of an embolic material to stimulate intravascular thrombosis, resulting in occlusion of the vessel. There is a risk of distal organ damage if the blood supply is completely occluded. Non-invasive angiographic techniques used with CT or MRI have reduced the need for direct arteriography for diagnosis of some vascular lesions. The advent of spiral and double helical CT scanners allows whole vascular territories to be mapped within 30 s and produces superior images, including three-dimensional pictures. MRI is sensitive to the detection of flow and, together with more sophisticated scanning and data collection techniques, is used increasingly for assessment of vascular structures.
Anaesthetic Management: Most angiographic procedures may be carried out under local anaesthesia, with sedation if necessary during more complex investigation. If the procedure is likely to be prolonged, general anaesthesia may be more appropriate; the same applies to nervous patients, those unable to cooperate and children. Complete immobility is required during the investigation and particularly if any interventional procedures are to be performed. Sedation to augment local anaesthesia must be avoided in the presence of intracranial hypertension, because the increased PaCO2 leads to vasodilatation and a further increase in ICP; in addition, vasodilatation results in poor-quality angiography. Major trauma patients and those with life-threatening haemorrhage are nearly always sedated, with ventilation controlled, before arrival in the angiography suite. The drawbacks of general anaesthesia include prolonging the time taken for the investigation and increasing the cost and risks associated with anaesthesia. Moreover, the patient is unable to react to misplaced injections and alert staff to untoward reactions.
Complications of Angiography
 Local – haematoma and haemorrhage, vessel wall dissection, thrombosis, perivascular contrast injection, adjacent nerve damage, loss and knotting of guide wires and catheters.
Local – haematoma and haemorrhage, vessel wall dissection, thrombosis, perivascular contrast injection, adjacent nerve damage, loss and knotting of guide wires and catheters.
 General – contrast reactions of varying severity; emboli from catheter clots, intimal damage and air; sepsis and vagal inhibition.
General – contrast reactions of varying severity; emboli from catheter clots, intimal damage and air; sepsis and vagal inhibition.
Interventional Neuroradiology: Cerebral angiography may be used to demonstrate tumours, arteriovenous malformations, aneurysms, subarachnoid haemorrhage and cerebrovascular disease. Since the ISAT (International Subarachnoid Aneurysm Trial) in 2002 (which compared coiling in patients with a ruptured aneurysm of good clinical grade with surgical clipping) showed a favourable initial outcome, endovascular treatment has become the technique of choice for most patients. Detachable coils are used to pack the aneurysm to prevent rebleeding. These patients are often systemically unwell as a result of subarachnoid haemorrhage and may be profoundly cardiovascularly unstable during induction of anaesthesia. A thorough preoperative assessment should be made, including cardiovascular, respiratory, neurological and metabolic status. The risk of complications is generally increased in the elderly and those with pre-existing vascular disease, diabetes, stroke or transient ischaemic attacks. Many of these patients have intracranial hypertension and cerebral vasospasm; consequently, control of arterial pressure and carbon dioxide tension is essential. Obtunding the pressor response to tracheal intubation and careful positioning to avoid increasing central venous pressure are necessary to prevent elevation of intracranial pressure. A relaxant/IPPV technique with ventilation to mild hypocapnia (PaCO2 = 4.5–5.0 kPa) is usually used. A moderate reduction in PaCO2 causes vasoconstriction of normal vessels, slows cerebral circulation and contrast medium transit times and improves delineation of small vascular lesions.
Cardiac Catheterization: General anaesthesia is required mainly for children (rarely in adults because sedation is usually adequate). In children (premature neonates to teenagers), congenital heart disease may cause abnormal circulations and intracardiac shunts, which often present with cyanosis, dyspnoea, failure to thrive and congestive heart failure. Patients may also have coexisting non-cardiac congenital abnormalities. Neonatal patients may be deeply cyanotic and critically ill. Initial echocardiography often gives a diagnosis but catheterization is required for treatment or determining the possibility of surgery. These radiological procedures include pressure and oxygen saturation measurements, balloon dilatation of stenotic lesions (e.g. pulmonary valve), balloon septostomy for transposition of the great arteries and ductal closure.
Pacemaker and Cardioverter/Defibrillator Implantation: These devices may be inserted in the cardiac catheter laboratory. These procedures may be performed under local or general anaesthesia depending on the circumstances. Implantation requires placing transvenous leads in the cardiac chambers and subcutaneous tunnelling to the device pocket. Testing of cardioverter/defibrillator units should be performed under general anaesthesia and the benefit of using direct arterial monitoring considered.
TIPS Procedures: Transjugular intrahepatic portosystemic shunt (TIPS) procedures are increasingly being carried out in the management of refractory portal hypertension, often secondary to cirrhosis. This decreases the risk of developing complications such as variceal bleeding or ascites. A shunt is created between the portal and hepatic veins to decrease vascular resistance. Complications include bleeding and liver injury (usually rare), hepatic encephalopathy as a result of increased nitrogen load from the gut and hepatic ischaemia. Patients are often acutely unwell with ascites, poor cardiovascular reserve and disordered fluid balance. General anaesthesia is usually required for comfort but may be challenging.
Anaesthesia for Radiotherapy
Anaesthesia in paediatric radiotherapy presents several problems.
1. Treatment is administered daily over a 4–6 week period and necessitates repeated doses of sedation or general anaesthesia.
2. The patient must remain alone and motionless for short periods during treatment, but immediate access to the patient is required in an emergency.
3. Monitoring is difficult because the child may be observed only on a closed-circuit television screen during treatment.
4. Recovery from anaesthesia must be rapid, because treatment is organized usually on an outpatient basis and disruption of normal activities should be minimized.
Anaesthesia in the Accident and Emergency Department
 Preoperative assessment and resuscitation before emergency surgery, e.g. ruptured ectopic pregnancy.
Preoperative assessment and resuscitation before emergency surgery, e.g. ruptured ectopic pregnancy.
 Specialist airway management for a patient with respiratory failure or acute airway compromise.
Specialist airway management for a patient with respiratory failure or acute airway compromise.
 Intensive care admission for a patient needing ventilatory and/or other organ support.
Intensive care admission for a patient needing ventilatory and/or other organ support.
 Resuscitation as part of the cardiac arrest or trauma team.
Resuscitation as part of the cardiac arrest or trauma team.
 Patients requiring specialist cannulation skills.
Patients requiring specialist cannulation skills.
 Anaesthesia for patients requiring procedures such as cardioversion or gastric lavage.
Anaesthesia for patients requiring procedures such as cardioversion or gastric lavage.
 Anaesthesia for patients requiring CT scan, e.g. suspected intracranial haemorrhage.
Anaesthesia for patients requiring CT scan, e.g. suspected intracranial haemorrhage.
ANAESTHESIA IN THE PRE-HOSPITAL ENVIRONMENT
 as a member of a hospital flying squad
as a member of a hospital flying squad
 as an immediate care practitioner
as an immediate care practitioner
 on duty (either paid or with the voluntary services) at an event such as a football match or festival
on duty (either paid or with the voluntary services) at an event such as a football match or festival
For example, at a road accident, potential hazards include:
 jagged metal edges in damaged vehicles
jagged metal edges in damaged vehicles
 other traffic moving around the incident
other traffic moving around the incident
 cutting and lifting equipment being used by the fire brigade.
cutting and lifting equipment being used by the fire brigade.
Personal Preparation for Working in the Pre-Hospital Environment
Preparation includes having appropriate safety clothing and equipment such as:
For people attending ballistic incidents, this needs to include ballistic helmets and body armour.
Staff also need insurance to cover travelling to and from the incident, and working at the incident. Advice on suitable equipment may be found on the British Association for Immediate Care (BASICS) website: http://www.basics.org.uk
Training in Pre-Hospital Care
Several organizations provide training in pre-hospital care. These include the following:
 BASICS runs courses in casualty extrication from vehicles and a pre-hospital emergency care certificate (PHEC, awarded jointly with the Royal College of Surgeons of Edinburgh). Information may be obtained from its website (see above).
BASICS runs courses in casualty extrication from vehicles and a pre-hospital emergency care certificate (PHEC, awarded jointly with the Royal College of Surgeons of Edinburgh). Information may be obtained from its website (see above).
 The Royal College of Surgeons (RCS) of Edinburgh examines for a Diploma and Fellowship in Immediate Medical Care. Information may be obtained from the Faculty of Pre-hospital Care at the RCS, Edinburgh.
The Royal College of Surgeons (RCS) of Edinburgh examines for a Diploma and Fellowship in Immediate Medical Care. Information may be obtained from the Faculty of Pre-hospital Care at the RCS, Edinburgh.
 The Royal College of Surgeons of England oversees the Pre-hospital Trauma Life Support Course, PHTLS.
The Royal College of Surgeons of England oversees the Pre-hospital Trauma Life Support Course, PHTLS.
Working at the Scene
Problems likely to be encountered are:
 Access to the casualty. The vehicle compartment around the casualty may be deformed and intruded. The vehicle may be on its side, upside down or in a ditch, making access to the casualty difficult.
Access to the casualty. The vehicle compartment around the casualty may be deformed and intruded. The vehicle may be on its side, upside down or in a ditch, making access to the casualty difficult.
 Lighting. The emergency services may provide portable lights, but in pre-hospital care, the anaesthetist is often trying to assess and manage a casualty in poor light.
Lighting. The emergency services may provide portable lights, but in pre-hospital care, the anaesthetist is often trying to assess and manage a casualty in poor light.
 Noise. Noise from generators and vehicles makes auscultation very difficult and interferes with communication with other team members and the other emergency services.
Noise. Noise from generators and vehicles makes auscultation very difficult and interferes with communication with other team members and the other emergency services.
 Environment. Wet weather and cold conditions imply that casualties (and staff not wearing appropriate clothing) become hypothermic quickly.
Environment. Wet weather and cold conditions imply that casualties (and staff not wearing appropriate clothing) become hypothermic quickly.
Logistical Considerations
 Should it be carried out now, during the move to hospital or at the hospital?
Should it be carried out now, during the move to hospital or at the hospital?
 Am I helping the patient and the situation or causing undue delay? For example, is it appropriate to struggle for 15 min to set up an intravenous infusion when the hospital is only 5 min travel time away?
Am I helping the patient and the situation or causing undue delay? For example, is it appropriate to struggle for 15 min to set up an intravenous infusion when the hospital is only 5 min travel time away?
 If the casualty is trapped and needs intravenous analgesia or anaesthesia to facilitate release, then probably yes, unless there are alternatives.
If the casualty is trapped and needs intravenous analgesia or anaesthesia to facilitate release, then probably yes, unless there are alternatives.
 If the casualty is ready to leave the scene except for this intervention, then probably no (unless there is no vehicle available to move the casualty).
If the casualty is ready to leave the scene except for this intervention, then probably no (unless there is no vehicle available to move the casualty).
Clinical Considerations
Airway: The main issue is oxygenation. As in hospital practice, simple methods should be tried first, such as chin lift, jaw thrust, oral airway, nasopharyngeal airway and laryngeal mask.
Cervical Spine Control: Many road accident casualties are at risk of cervical spine injury. Neck collars and other immobilization devices limit mouth opening and make airway management difficult. The front of the collar may be removed and paramedics may be asked to substitute manual in-line immobilization if the anaesthetist is having difficulty establishing a clear airway.
Breathing: As in hospital, inadequate ventilation is supported if necessary using a bag/valve mask. Life-threatening injuries such as tension pneumothorax may be difficult to diagnose. Some clinical signs, such as tracheal deviation, present late. Breath sounds may be difficult to hear because of the noisy environment. Clinical signs such as the presence of subcutaneous emphysema, indicating an air leak, should be sought. The decision to decompress a chest may have to be made on the basis of deteriorating ventilation and ‘most likely’ diagnosis.
Circulation: Control of bleeding with pressure dressings or tourniquet should be achieved before attempting intravenous access. Intravenous fluid resuscitation should be targeted at the injury being managed. Some injuries require hypotensive resuscitation until surgical control is achieved; others require normotension (for example, head injury to maintain cerebral perfusion). Blood loss may be very difficult to assess in the field. Blood can be difficult to see on soft muddy ground and may pool out of sight on the floor of vehicles.
Anaesthetic and Analgesic Techniques
The principles of emergency anaesthesia are discussed in Chapter 37. In the pre-hospital environment, the same principles apply. Techniques which are familiar to the anaesthetist should be used. If the technique causes problems (e.g. apnoea or airway obstruction), the anaesthetist should have adequate access to the patient for appropriate management.
Local/Regional Anaesthesia: Ring blocks are effective for fingers trapped in machinery. Femoral block may help in the management of pain from a fractured femur.
Intravenous Analgesia: The anaesthetist is limited to those drugs carried by the ambulance service and deployed by the flying squad. Intravenous morphine, nalbuphine and tramadol have all been used pre-hospital; they should be titrated to effect, and any complications arising from their use should be managed.
Inhalational Analgesia: Many ambulances carry Entonox, which may be used alone or in combination with other drugs. The main concern with the nitrous oxide in Entonox is enlargement of a pneumothorax or other air-filled cavity.
Intravenous Anaesthesia: Rapid-sequence induction using an i.v. anaesthetic agent may be the technique of choice to allow airway protection and control of ventilation in the injured casualty. Appropriate agents include ketamine, etomidate and propofol but with the dose moderated according to the casualty’s haemodynamic status. Before embarking on general anaesthesia, the anaesthetist should consider such issues as:
 Is there suitable access to the casualty?
Is there suitable access to the casualty?
 Who is available to provide assistance?
Who is available to provide assistance?
 Is there appropriate monitoring?
Is there appropriate monitoring?
 Is appropriate equipment available? Unlike the anaesthetic room where equipment is easily to hand, the pre-hospital practitioner works using a rucksack or other bag containing a very limited range of equipment.
Is appropriate equipment available? Unlike the anaesthetic room where equipment is easily to hand, the pre-hospital practitioner works using a rucksack or other bag containing a very limited range of equipment.
Transfer to Hospital
A checklist for transfer includes the following:
 Is the airway secured for transport? A tracheal tube or laryngeal mask should to be tied or taped in place.
Is the airway secured for transport? A tracheal tube or laryngeal mask should to be tied or taped in place.
 Is oxygen being provided in adequate quantities?
Is oxygen being provided in adequate quantities?
 Is breathing adequate or is assistance required? If the patient’s lungs are being ventilated using a mechanical ventilator, is the power capacity (gas, electricity or battery) adequate for the journey? Is there a back-up such as a self-inflating bag?
Is breathing adequate or is assistance required? If the patient’s lungs are being ventilated using a mechanical ventilator, is the power capacity (gas, electricity or battery) adequate for the journey? Is there a back-up such as a self-inflating bag?
 Is external bleeding controlled? Are i.v cannulae/catheters taped securely in place? What variables have been selected (arterial pressure and/or pulse) as indicators of resuscitation requirements?
Is external bleeding controlled? Are i.v cannulae/catheters taped securely in place? What variables have been selected (arterial pressure and/or pulse) as indicators of resuscitation requirements?
 If the patient has a reduced GCS, have remediable causes such as hypovolaemia or hypoglycaemia been considered?
If the patient has a reduced GCS, have remediable causes such as hypovolaemia or hypoglycaemia been considered?
 Are splints secured? Is spinal immobilization in place (where indicated)? Has a check been made that straps from splints and spinal immobilization devices are not interfering with respiration?
Are splints secured? Is spinal immobilization in place (where indicated)? Has a check been made that straps from splints and spinal immobilization devices are not interfering with respiration?
AAGBI. Recommendations for standards of monitoring during anaesthesia and recovery. The Association of Anaesthetists of Great Britain and Ireland, 2006.
AAGBI. Pre-hospital Anaesthesia. The Association of Anaesthetists of Great Britain and Ireland, 2009.
AAGBI. Provision of anaesthetic services in MRI units. Anaesthesia. 2010;65:766–770.
Advanced Life Support Group. Major incident medical management and support, second ed. London: BMJ Books, 2002.
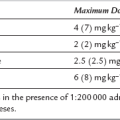
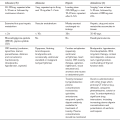
Ding, Z., White, P.F. Anesthesia for electroconvulsive therapy. Anesth. Analg. 2002;94:1351–1364.
Greaves I., Porter K., eds. Pre-hospital medicine: the principles and practice of immediate care. London: Arnold, 1999.
Hashimoto, T., Gupta, D.K., Young, W.L. Interventional neuroradiology – anesthetic considerations. Anesthesiol. Clin. North America. 2002;20:347–359.
Holleran R.S., ed. Air and surface patient transport: principles and practice, third ed., St Louis: Mosby, 2003.
Trauma: Who Cares? National Confidential Enquiry into Patient Outcome and Death, 2007.