31
Anaesthesia for Vascular, Endocrine and Plastic Surgery
MAJOR VASCULAR SURGERY
General Considerations
Most of these are considered independent risk factors for perioperative cardiac complications after major surgery (see Ch 18).
The broad aims of preoperative evaluation before vascular surgery are to:
 assist risk assessment, permit further investigation if appropriate and allow optimization of coexisting medical conditions
assist risk assessment, permit further investigation if appropriate and allow optimization of coexisting medical conditions
 evaluate and discuss the risks with the patient and surgical team
evaluate and discuss the risks with the patient and surgical team
 establish the best surgical options (e.g. non-invasive or endovascular surgery) for an individual
establish the best surgical options (e.g. non-invasive or endovascular surgery) for an individual
 plan the anaesthetic technique, perioperative monitoring and postoperative care, and allow required facilities (e.g. ICU) to be organized.
plan the anaesthetic technique, perioperative monitoring and postoperative care, and allow required facilities (e.g. ICU) to be organized.
Vascular surgery is associated with a high morbidity and mortality, resulting mostly from cardiac complications (myocardial infarction, arrhythmias and cardiac failure) (see Chs 18 and 43). It is therefore vital that cardiac function is assessed preoperatively and that the risks of surgery are evaluated and discussed with the patient. Although the outcome of subsequent vascular surgery is improved in those who have previously undergone coronary revascularization by coronary artery bypass grafting (CABG), this is associated with additional risks. Percutaneous coronary interventions (insertion of bare metal or drug-eluting intracoronary stents (ICS)) are being used increasingly as an alternative to CABG in suitable patients. There is a risk of stent thrombosis after ICS so patients receive dual antiplatelet therapy (aspirin and clopidogrel) for 6 weeks after bare metal and 1 year after drug-eluting stent insertion. The risks of perioperative cardiac events or stent thrombosis are very high if surgery is performed during the period when dual antiplatelet drugs are needed because of increased haemorrhage if antiplatelet therapy is continued, or acute thrombotic events if it is interrupted. The most recent guidelines suggest that elective surgery should be postponed for at least 4–6 weeks after bare metal stent insertion (and ideally delayed for 3 months) and for 1 year after drug-eluting stent insertion, unless surgery is more urgent. It should be remembered that coronary revascularization should be performed only if indicated because of the severity of coronary disease and is not justified simply to improve outcome from subsequent vascular surgery.
Preoperative Medical Therapy in Vascular Surgical Patients
Minimum investigations before major vascular surgery should include ECG, chest X-ray, full blood count and serum urea and electrolyte concentrations, but more invasive or specialized tests may be required (see Ch 18), including cardiopulmonary exercise testing where available. Some assessment of exercise tolerance should be made in all patients because it is a useful indicator of functional cardiac status, although many vascular patients are limited by intermittent claudication or old age and may have a sedentary lifestyle. In this case, a patient with severe coronary artery disease may have no symptoms of angina and a normal resting ECG. In some patients (e.g. those with limited functional capacity or life expectancy because of severe intractable coexistent medical conditions), the risks of elective vascular surgery may outweigh the overall potential benefits and invasive surgery may not be appropriate.
Abdominal Aortic Aneurysm
Elective Open AAA Repair
Several important considerations apply to patients undergoing aortic surgery (Table 31.1). The following are required:
TABLE 31.1
Major Anaesthetic Considerations for Patients Undergoing Aortic Surgery
High incidence of coexisting cardiovascular and respiratory disease
Cardiovascular instability during induction of anaesthesia, aortic cross-clamping and declamping
Large blood loss and fluid shifts during and after surgery
Prolonged major surgery in high-risk patients
Marked heat and evaporative fluid losses from exposed bowel
Potential postoperative impairment of respiratory, cardiac, renal and gastrointestinal function
 two large (14-gauge) cannulae for infusion of warmed fluids
two large (14-gauge) cannulae for infusion of warmed fluids
 arterial catheter for intra-arterial pressure monitoring and blood sampling for acid-base and blood gas analysis
arterial catheter for intra-arterial pressure monitoring and blood sampling for acid-base and blood gas analysis
 multilumen central venous catheter for drug administration and determination of right atrial pressure
multilumen central venous catheter for drug administration and determination of right atrial pressure
 continuous ECG monitoring for ischaemia (CM5 position) preferably with ST-segment analysis
continuous ECG monitoring for ischaemia (CM5 position) preferably with ST-segment analysis
Three specific stimuli may give rise to cardiovascular instability during surgery:
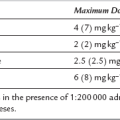
 Tracheal intubation. Laryngoscopy and tracheal intubation may be accompanied by marked increases in arterial pressure and heart rate which may precipitate myocardial ischaemia in susceptible individuals. This response may be attenuated by the i.v. administration of a β-blocker (e.g. esmolol 1.5 mg kg–1) or a rapidly-acting opioid (e.g. alfentanil 10 μg kg–1) before intubation.
Tracheal intubation. Laryngoscopy and tracheal intubation may be accompanied by marked increases in arterial pressure and heart rate which may precipitate myocardial ischaemia in susceptible individuals. This response may be attenuated by the i.v. administration of a β-blocker (e.g. esmolol 1.5 mg kg–1) or a rapidly-acting opioid (e.g. alfentanil 10 μg kg–1) before intubation.
 Cross-clamping of the aorta. Clamping of the aorta causes a sudden increase in aortic impedance to forward flow and hence left ventricular afterload. This increases cardiac work and may result in myocardial ischaemia, arrhythmias and left ventricular failure. Arterial pressure proximal to the clamp increases acutely even though left ventricular ejection fraction and cardiac output are reduced. The effects on preload are variable. The degree of cardiovascular disturbance is greater when the clamp is applied more proximally (greater at the supracoeliac > suprarenal > infrarenal levels). A vasodilator, e.g. glyceryl trinitrate (GTN), is often infused just before clamping (and continued up to clamp release) to obviate these problems. Deepening of volatile anaesthesia or an additional dose of an opioid may also be used at aortic clamping. While the aorta is clamped, blood flow distal to the clamp decreases, and distal organ perfusion is largely dependent on the collateral circulation. The lower limbs, pelvic and abdominal viscera suffer variable degrees of ischaemia during which inflammatory mediators are released from white blood cells, platelets and capillary endothelium. These mediators include oxygen free radicals, neutrophil proteases, platelet activating factor, cyclo-oxygenase products and cytokines.
Cross-clamping of the aorta. Clamping of the aorta causes a sudden increase in aortic impedance to forward flow and hence left ventricular afterload. This increases cardiac work and may result in myocardial ischaemia, arrhythmias and left ventricular failure. Arterial pressure proximal to the clamp increases acutely even though left ventricular ejection fraction and cardiac output are reduced. The effects on preload are variable. The degree of cardiovascular disturbance is greater when the clamp is applied more proximally (greater at the supracoeliac > suprarenal > infrarenal levels). A vasodilator, e.g. glyceryl trinitrate (GTN), is often infused just before clamping (and continued up to clamp release) to obviate these problems. Deepening of volatile anaesthesia or an additional dose of an opioid may also be used at aortic clamping. While the aorta is clamped, blood flow distal to the clamp decreases, and distal organ perfusion is largely dependent on the collateral circulation. The lower limbs, pelvic and abdominal viscera suffer variable degrees of ischaemia during which inflammatory mediators are released from white blood cells, platelets and capillary endothelium. These mediators include oxygen free radicals, neutrophil proteases, platelet activating factor, cyclo-oxygenase products and cytokines.
 Aortic declamping. Declamping of the aorta causes sudden decreases in aortic impedance, systemic vascular resistance and venous return with reperfusion of the bowel, pelvis and lower limbs and redistribution of blood. Inflammatory mediators are swept into the systemic circulation causing vasodilatation, metabolic acidosis, increased capillary permeability and sequestration of blood cells in the lungs. This is a critical period of anaesthesia and surgery because hypotension after aortic declamping may be severe and refractory unless circulating volume has been well maintained. If relative hypervolaemia is produced during the period of clamping by infusion of fluids to produce a CVP of greater than 12–14 mmHg (and perhaps administration of GTN until shortly before clamp release), declamping hypotension is less of a problem and metabolic acidosis may be diminished. Declamping hypotension usually resolves within a few minutes but vasopressors or positive inotropes are often required; these can be given before clamp release in anticipation. Good communication with the surgeon and slow or sequential clamp release helps the anaesthetist to manage aortic declamping. Renal blood flow decreases even when an infrarenal cross-clamp is used, and steps to maintain renal function are often required. The single most important measure is the maintenance of extracellular fluid volume (i.e. CVP > 12–14 mmHg). Prophylactic mannitol 0.5–1.0 g kg–1 i.v. or furosemide may also be administered, although the evidence for their efficacy is conflicting.
Aortic declamping. Declamping of the aorta causes sudden decreases in aortic impedance, systemic vascular resistance and venous return with reperfusion of the bowel, pelvis and lower limbs and redistribution of blood. Inflammatory mediators are swept into the systemic circulation causing vasodilatation, metabolic acidosis, increased capillary permeability and sequestration of blood cells in the lungs. This is a critical period of anaesthesia and surgery because hypotension after aortic declamping may be severe and refractory unless circulating volume has been well maintained. If relative hypervolaemia is produced during the period of clamping by infusion of fluids to produce a CVP of greater than 12–14 mmHg (and perhaps administration of GTN until shortly before clamp release), declamping hypotension is less of a problem and metabolic acidosis may be diminished. Declamping hypotension usually resolves within a few minutes but vasopressors or positive inotropes are often required; these can be given before clamp release in anticipation. Good communication with the surgeon and slow or sequential clamp release helps the anaesthetist to manage aortic declamping. Renal blood flow decreases even when an infrarenal cross-clamp is used, and steps to maintain renal function are often required. The single most important measure is the maintenance of extracellular fluid volume (i.e. CVP > 12–14 mmHg). Prophylactic mannitol 0.5–1.0 g kg–1 i.v. or furosemide may also be administered, although the evidence for their efficacy is conflicting.
The Postoperative Period: Postoperatively, the patient should be transferred to a high-dependency or intensive care unit. The decision to continue artificial ventilation or to extubate the trachea after surgery depends on the patient’s previous medical condition and physiological stability during and at the end of surgery. Artificial ventilation should be continued until body temperature and acid-base status are normalized, cardiovascular stability restored and effective analgesia provided. Patients have a high incidence of postoperative cardiovascular and respiratory complications; renal dysfunction and ileus are also common. Close monitoring is required for several days.
Surgery for Occlusive Peripheral Vascular Disease
Carotid Artery Surgery
 transcranial Doppler ultrasonography of the middle cerebral artery flow velocity
transcranial Doppler ultrasonography of the middle cerebral artery flow velocity
 measurement of arterial pressure in the occluded distal carotid segment (the ‘stump’ pressure)
measurement of arterial pressure in the occluded distal carotid segment (the ‘stump’ pressure)
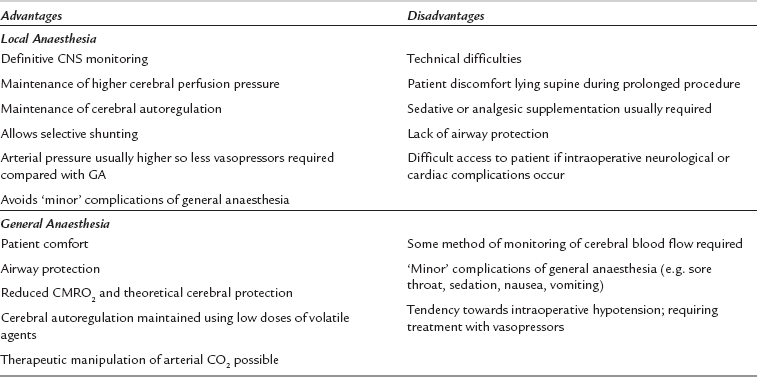
Local infiltration of the surgical field may be used alone or in combination with superficial and intermediate or deep cervical plexus blockade. Superficial cervical plexus block is performed by infiltration of local anaesthetic along the entire length of the posterior border of the sternomastoid muscle, using 10–15 mL local anaesthetic (e.g. levobupivacaine 0.25%). The intermediate block involves injection of 5 mL of local anaesthetic 1–2 cm deep to the midpoint of the sternomastoid. Advantages of locoregional techniques include definitive neurological monitoring (therefore allowing selective use of shunts), preservation of cerebral and coronary autoregulation, and the maintenance of higher cerebral perfusion pressures during the procedure (Table 31.2). These techniques rely on good cooperation between the patient, the surgeon and the anaesthetist. Many patients find it difficult to lie still and supine for the duration of the procedure, particularly those with heart failure or respiratory disease; this may be compounded by diaphragmatic compromise because phrenic nerve paralysis may accompany deep cervical plexus blockade. Sudden loss of consciousness or seizures may occur if cerebral perfusion is inadequate after clamping, and subsequent airway control may be very difficult because access is limited.
SURGERY FOR TUMOURS OF THE ENDOCRINE SYSTEM
Phaeochromocytoma
Phaeochromocytomas are derived from chromaffin cells which secrete catecholamines (predominantly noradrenaline, but also adrenaline and occasionally dopamine) and occur in less than 0.1% of hypertensive patients. The majority present in middle-aged adults but they may be found in childhood. Most are found as a single benign tumour of the adrenal medulla, but 10% occur in ectopic sites, e.g. paravertebral sympathetic ganglia. Approximately 10% of phaeochromocytomas are malignant, and 10% are bilateral. Genetic factors are frequently involved and they may be associated with multiple endocrine neoplasia (MEN) and other syndromes (Table 31.3).
TABLE 31.3
Associations of Phaeochromocytoma with Other Syndromes
Von Hippel-Lindau disease (retinocerebral haemangioblastoma)
MEN IIa (Sipple’s syndrome)
Phaeochromocytoma
Medullary cell thyroid carcinoma
Hyperparathyroidism
MEN IIb (mucosal neuroma syndrome)
Phaeochromocytoma
Medullary cell thyroid carcinoma
Mucosal neuromata
Marfanoid habitus
Phaeochromocytoma-paraganglionoma syndrome
Von Recklinghausen’s disease (multiple neurofibromatosis)
The clinical features depend on the quantity of hormones secreted and on which is predominant, although episodes may be paroxysmal and clinical findings may be normal between attacks. Noradrenaline-secreting tumours tend to cause severe refractory hypertension, headaches and glucose intolerance; circulating blood volume is reduced and vasoconstriction occurs. Adrenaline-secreting tumours trigger palpitations, anxiety and panic attacks, sweating, hypoglycaemia, tachycardia, tachyarrhythmias and occasionally high-output cardiac failure. Malaise, weight loss, pallor and psychological disturbances may occur, and end-organ damage (e.g. retinopathy, nephropathy, dilated cardiomyopathy) may arise as a consequence of hypertension. They present several problems to the anaesthetist (Table 31.4).
TABLE 31.4
Anaesthetic Considerations in Patients with Phaeochromocytoma
Preoperative
Hypertension
Hypovolaemia (vasoconstriction with reduced circulating volume)
Pharmacological stabilization
α-blockade
β-blockade
Control of catecholamine synthesis
End-organ damage
Anxiolytic/sedative premedication
Intraoperative
Severe cardiovascular instability, particularly:
at induction of anaesthesia and tracheal intubation
during pneumoperitoneum (laparascopic procedures)
during tumour handling
following ligation of venous drainage
Hypoglycaemia after tumour removal
Postoperative
Hypotension
Hypoglycaemia
Somnolence, opioid sensitivity
Hypoadrenalism
LV dysfunction
Conduct of Anaesthesia
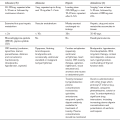
Sedative and anxiolytic premedication is useful and both α- and β-adrenergic antagonists should be continued up to the day of surgery. Monitoring of ECG, CVP and direct arterial pressure must be started before induction of anaesthesia. Intraoperative monitoring should include temperature, blood gas tensions and glucose concentration; transoesophageal echocardiography or pulmonary artery catheterization may be required if significant cardiomyopathy is present. Anaesthetic drugs should be selected on the basis of cardiovascular stability and agents which have the ability to provoke histamine (and hence catecholamine) release are best avoided (Table 31.5). The exact choice of individual anaesthetic drugs is less important than careful conduct of anaesthesia, which may be induced by slow administration of thiopental, etomidate or propofol and maintained with nitrous oxide in oxygen, supplemented by sevoflurane or isoflurane. Desflurane has the theoretical disadvantage of causing sympathetic stimulation if the inspired concentration is increased too rapidly. The use of moderate doses of an opioid (e.g. fentanyl 7–10 μg kg–1) may aid cardiovascular stability. Drugs should be immediately available to treat acute hypertension (e.g. SNP, phentolamine or nicardipine), tachycardia or arrhythmias (e.g. esmolol). Hypotension is treated with fluids initially but vasopressors (e.g. ephedrine, phenylephrine or noradrenaline) may be required. Intravenous magnesium sulphate may be useful: it suppresses catecholamine release from the tumour and adrenergic nerve endings, is a direct-acting vasodilator and has antiarrhythmic effects but has a narrow therapeutic window and plasma Mg2 + concentration should be monitored. Perioperative epidural analgesia may attenuate some of the cardiovascular responses, except during tumour handling, and is useful for postoperative analgesia. However, it should be used judiciously to avoid hypotension. Postoperative problems may include hypoglycaemia, somnolence, opioid sensitivity, hypotension and hypoadrenalism. Invasive monitoring should be continued for 12–24 h after surgery and the patient must be nursed in a high-dependency or intensive care unit.
TABLE 31.5
Drugs Which Should be Avoided in Patients with Phaeochromocytoma
Atropine
Succinylcholine
d-Tubocurarine
Atracurium
Pancuronium
Droperidol
Morphine
Halothane
BURNS
 excision of damaged tissue and escharotomies
excision of damaged tissue and escharotomies
 excision of granulation tissue and subsequent grafting
excision of granulation tissue and subsequent grafting
 reparative plastic procedures to relieve contractures, permit limb function or correct deformities.
reparative plastic procedures to relieve contractures, permit limb function or correct deformities.
Initial Management
The initial assessment and management of acute burn injuries are similar to those applied to other victims of trauma and are summarized in Table 31.6. The aim of immediate treatment is to secure the airway and administer high-flow humidified oxygen through a non-rebreathing system; tracheal intubation and IPPV with 100% oxygen may be required to maintain an adequate PaO2. Fluid resuscitation should be started. Intravenous fluids guided by a formal protocol are required if the burn exceeds 10–15% of total BSA and central venous access is usually required with burns greater than 20% BSA. Early warning signs of upper airway burns include facial or intra-oral burns, singed facial or nasal hair, carbonaceous sputum, hypoxaemia, dyspnoea, cough or wheeze, although these are not universally present in the early stages and airway obstruction may develop later. A history of burns in an enclosed space is highly suggestive. A high index of suspicion for airway burns should be maintained in all cases and prophylactic tracheal intubation is often justified, particularly in children or if interhospital transfer is required. However, the decision to secure the airway by tracheal intubation may be difficult and a senior anaesthetist should be involved. Hoarseness or stridor may indicate impending airway obstruction and tracheal intubation in these cases is mandatory. Pulse oximeters are unreliable in the presence of carboxyhaemoglobin because they cannot distinguish between oxyhaemoglobin and carboxyhaemoglobin and therefore overestimate true oxygen saturation; arterial blood gas analysis is required. Burns are extremely painful and carefully titrated intravenous opioids should be administered. Indications for ICU admission include potential airway problems, burns involving > 20% BSA and the presence of other injuries.
TABLE 31.6
Initial Management of Patients with Severe Burns
1.History – time, extent and mechanism of burn, age and weight of patient, brief medical history
2.Airway assessment
3.Breathing – administer 100% humidified oxygen via a non-rebreathing mask
4.Circulation – establish two large-bore i.v. cannulae and commence fluid resuscitation
5.Assess neurological status
6.Exposure with environmental control
7.Analgesia – i.v. opioids
8.Formally assess burn area and re-evaluate fluid requirements
9.Monitoring – vital signs, urine output
10.Investigations – ABG, COHb, U&E, FBC, clotting screen, cross-match blood, ECG, CXR
11.Secondary survey to exclude other injuries
12.Burns dressings
Tissue burns produce rapid fluid shifts and oedema formation, particularly during the first 36 h. The resulting depletion of intravascular volume is greatest in the first few hours and it is essential that a fluid replacement regimen is started as early as possible to avoid hypovolaemic shock and acute renal failure. Crystalloid-based regimens such as the Parkland formula are used commonly (Table 31.7) although mixed colloid–crystalloid regimens based on that of Muir and Barclay are sometimes used. Many advocate administration of crystalloid solutions only in the first 24 h, with colloids added thereafter. However, the pathophysiology of fluid shifts is complex and these formulae should be used only as a guide to fluid therapy. Recent studies have shown that the type of resuscitation fluid used (crystalloid or colloid) does not affect mortality. Vital signs, urine output and body temperature should be observed closely, and volume replacement titrated to achieve a urine output of 0.5–1.0 mL kg–1 h–1 (1.0–1.5 mL kg–1 h–1 in children). Non-invasive methods of cardiac output monitoring may also be used to guide fluid replacement and resuscitation. Haematocrit, base deficit and serum lactate, and urea and electrolyte concentrations should be monitored. It is important to be aware that excessive volumes of fluid for resuscitation can predispose to some complications including abdominal and extremity compartment syndromes. Bladder pressure monitoring (to detect intra-abdominal hypertension) has been recommended for all patients with major burns of > 30% BSA.
TABLE 31.7
Fluid Regimens for Burned Patients
1. Estimate/measure weight
2. Estimate percentage area of burn using ‘rule of nines’ for adults and ‘rule of tens’ for children
3. Proceed with regimen if > 15% burns in adults or > 10% in children
4. Parkland formula:
Requirements in first 24 h (mL) = body weight × % burn × 4
Fluids given as Ringer’s lactate alone, 50% within first 8 h, 50% between 8 and 24 h
Colloids administered only after first 24 h
5. Muir & Barclay formula:
Requirements in each time period (mL) = body weight (kg) × % burns × 0.5
Fluids (human albumin solution 4.5%) according to formula in each of the following periods:
0–4 h
4–8 h
8–12 h
12–18 h
18–24 h
24–36 h
In addition, water as 5% dextrose is required at 1–2 mL kg–1 h–1.
Hines, R.L., Marschall, K.E. Stoelting’s anesthesia and co-existing disease, fifth ed. Philadelphia: Churchill Livingstone; 2008.
Kaplan, J.A., Lake, C.L., Murray, M.J. Vascular anesthesia, second ed. New York: Elsevier; 2004.
Kasten, K.R., Makley, A.T., Kagan, R.J. Update on the critical care management of severe burns. J. Intensive Care Med. 2011;26:223–236.
MacLennan, A., Heimbach, D.M., Cullen, B.F. Anesthesia for major thermal injury. Anesthesiology. 1998;89:749–770.
Mancuso, K., Kaye, A.D., Boudreaux, J.P., et al. Carcinoid and perioperative anesthetic considerations. J. Clin. Anesth. 2011;23:329–341.
Thompson, J.P., Telford, R.J., Howell, S.J. Oxford specialist handbook of vascular anaesthesia. Oxford: OUP; 2013.