Anaesthesia for Thoracic Surgery
Thoracic anaesthesia offers a number of anaesthetic challenges:
 Control of the airway during bronchoscopy.
Control of the airway during bronchoscopy.
 Protection of the airway in patients with oesophageal disease, lung abscess, bronchopleural fistula or haemoptysis.
Protection of the airway in patients with oesophageal disease, lung abscess, bronchopleural fistula or haemoptysis.
 Positioning a double-lumen tracheal tube to maintain safe anaesthesia in the lateral position with the chest opened and one lung collapsed.
Positioning a double-lumen tracheal tube to maintain safe anaesthesia in the lateral position with the chest opened and one lung collapsed.
 Postoperative care of a patient after lung tissue resection.
Postoperative care of a patient after lung tissue resection.
In common with major surgery at other sites, thoracic patients frequently:
 have parenchymal lung disease in addition to their presenting complaint
have parenchymal lung disease in addition to their presenting complaint
 experience severe pain after surgery
experience severe pain after surgery
PREOPERATIVE ASSESSMENT
Investigations
Whole-Lung Testing
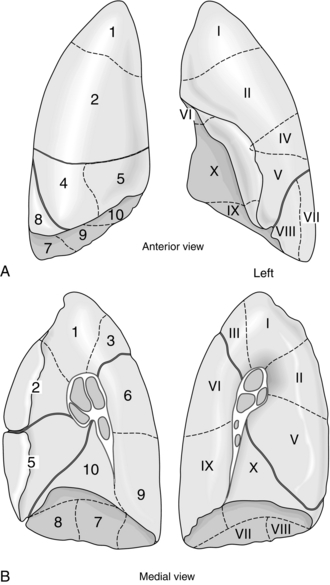
Predicted postoperative (PPO) lung function may be calculated using lung segments. From a total of 19 segments (three in the upper lobes, two in both the middle lobe and lingual and four in the left and five in the right lower lobes; Fig. 33.1), the fraction of lung remaining is multiplied by the preoperative spirometry measurement to give the predicted postoperative measurement of spirometry (PPO FEV1 = preoperative FEV1 × (1 – (resected segments/19))). Using whole-lung spirometry to predict postoperative lung function may be invalidated if the regional function of the lung is not known. For example, a patient with an FEV1 of 1.5 L may have the same or better FEV1 after lobectomy if the main bronchus of the affected lobe was occluded completely at the time of testing before surgery. The oxygenation of blood of such a patient may be improved by the removal of a non-functioning lung or lobe through which considerable right-to-left shunt existed.
ANATOMY
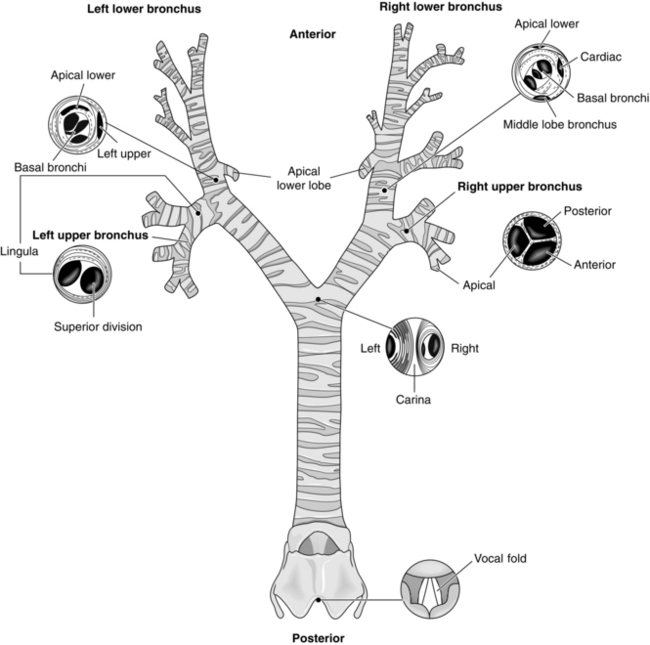
The bronchial tree and the views obtained when facing the patient are illustrated in Figure 33.2 and the bronchopulmonary segments are shown in Figure 33.1. The trachea leads from the cricoid cartilage below the larynx at the level of the sixth cervical vertebra (C6) and passes 10–12 cm in the superior mediastinum to its bifurcation at the carina into left and right main bronchi at the sternal angle, T4/5. During inspiration, the lower border of the trachea moves inferiorly and anteriorly. The trachea lies principally in the midline, but is deviated to the right inferiorly by the arch of the aorta. The oesophagus is immediately posterior to the trachea and behind it is the vertebral column. The wall of the trachea is held patent by 15–20 cartilaginous rings deficient posteriorly where the trachealis membrane, a collection of fibroelastic fibres and smooth muscle, lies. It is wider in transverse diameter (20 mm) than anteroposteriorly (15 mm). The trachea passes from neck to thorax via the thoracic inlet at T2.
INDUCTION AND MAINTENANCE OF ANAESTHESIA
Lateral Thoracotomy
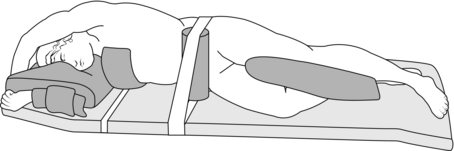
Many thoracic procedures are performed through a posterolateral thoracotomy incision between fifth and eighth ribs. Patients have to be positioned on their side with the neck flexed, dependent shoulder brought forward and the arm raised under the pillow to protect the shoulder and brachial plexus. The upper shoulder is flexed to 90° and the arm supported. Hips and knees are flexed together with a pillow between the legs. Padding, strapping, lower leg compression devices and diathermy pad complete the preparation for surgery (Fig. 33.3). Positioning with the chest flexed laterally away from the operative side on a beanbag which is then aspirated of air, or breaking the operating table, may improve surgical access. The upper wrist has a tendency to flex, so radial artery cannulae cause less trouble when on the dependent side. Peripheral and jugular veins are more accessible on the operative side.
One-Lung Anaesthesia
The principal indications for one-lung anaesthesia are:
Ventilation of one lung alone may require a double-lumen tracheal tube (Fig. 33.4), a bronchial blocker (Fig. 33.5) or a bronchial tube. The double-lumen tube has greatest flexibility to allow changes from ventilation of two lungs to one lung then back to two lungs during or at the end of surgery. It allows aspiration of the main bronchi independently, and insufflation of oxygen to the non-ventilated lung. It has a larger external diameter than the bronchial blocker or bronchial tube and may be difficult to position correctly if tracheal and bronchial anatomy is distorted. The two separate lumens are narrow and present a high resistance to spontaneous ventilation. This is overcome by positive pressure ventilation, but a single-lumen tube may have to be substituted at the end of surgery if resumption of spontaneous ventilation is not immediate. A bronchial blocker with a hollow lumen allows insufflation of oxygen, some suctioning and may be used with a jet ventilator, which overcomes some of the disadvantages of the technique. The Rüsch bronchial blocker illustrated in Figure 33.5 has a 170 cm long, 2 mm stem (outside diameter) with a central lumen to a 2.75 mm diameter balloon, which accepts 5 ml of air. It may be passed down a bronchoscope with a lumen greater than 2.8 mm diameter or through the 15 mm tapered connector with a 1.8 mm seal shown.
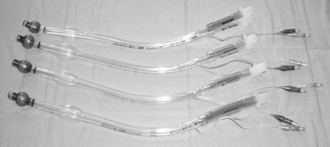
FIGURE 33.4 Four left-sided Bronchocath double-lumen endobronchial tubes with balloons inflated, from 35 FG (uppermost) to 41 FG (lowest).
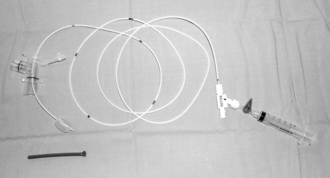
FIGURE 33.5 A Rüsch 6 FG bronchial blocker (360601) – balloon is inflated with 5 mL air. The balloon guard is below the balloon and the stem passes through the 1.8 mm seal in the connector for the anaesthetic circuit. Luer-lock fittings (labelled ‘balloon’) to inflate the balloon and aspirate/inflate down the central lumen are on the right.
Positioning Double-Lumen Bronchial Tubes
Double-lumen bronchial tubes provide an effective means of isolating each lung to protect the other from blood and secretions. They allow ventilation of one lung only, or both lungs independently. Being longer and with lumens more narrow than single-lumen tracheal tubes, they have a greater resistance to air flow. They are therefore not usually suitable for spontaneous ventilation. Disposable polyvinyl chloride tubes (Fig. 33.6) have substantially replaced the re-usable red rubber Robertshaw double-lumen tubes. Sizes of tube in common use in adult practice range from 35 to 41 French gauge (FG). Sizes 37–39 are usually suitable for men and 37 for women, but sizes 41 and 35 are available for individuals at extremes of the range of adult build. Left- and right-sided versions of double-lumen bronchial tubes are necessary (Fig. 33.6) because the tracheal portions are curved antero-posteriorly, and the balloon of the right bronchial tube is fenestrated to conduct gases to the right upper lobe bronchus, which would otherwise be occluded by the cuff of the tube (Fig. 33.7). The left upper lobe bronchus arises 2.5 cm further down the main bronchus than the right, so it is less likely to be occluded by the balloon of the bronchial tube.

FIGURE 33.6 Left (upper) and right (lower) 39 FG double-lumen bronchial tubes are shown with a flexible introducer, which may be used to shape the tube to facilitate insertion.
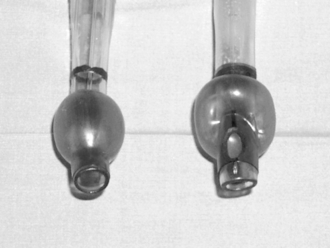
FIGURE 33.7 Bronchial balloons inflated on left-sided (left) and right-sided (right) bronchial double-lumen tubes. The right tube is eccentric and shows the side lumen to allow inflation of the right upper lobe.
Using the Fibreoptic Intubating Laryngoscope
Positioning the double-lumen tube often leaves the orifice of the right upper lobe bronchus covered by the tip of the bronchial tube (Fig. 33.8).
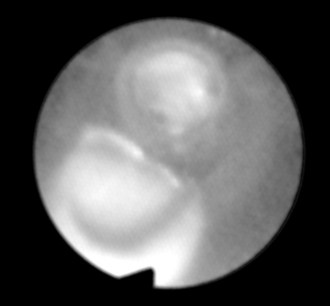
FIGURE 33.8 Distal opening of bronchial lumen at 12 o’clock shows right middle and lower bronchi. The side opening of the bronchial lumen at 7 o’clock is against the wall of the right main bronchus and is not over the right upper lobe bronchus. The patient is lying on the right. Orientation: left, cephalad; upper, left; right, caudad; lower, right.
 The tracheal cuff is then deflated, the laryngoscope is held fixed relative to the patient and the double-lumen tube advanced. The blue bronchial cuff then occludes sight of the orifice of the right upper lobe bronchus until the side hole in the bronchial cuff lies over it. Sight of bronchial rings of the right upper lobe bronchus confirms the correct position of the double-lumen bronchial tube (Fig. 33.9).
The tracheal cuff is then deflated, the laryngoscope is held fixed relative to the patient and the double-lumen tube advanced. The blue bronchial cuff then occludes sight of the orifice of the right upper lobe bronchus until the side hole in the bronchial cuff lies over it. Sight of bronchial rings of the right upper lobe bronchus confirms the correct position of the double-lumen bronchial tube (Fig. 33.9).
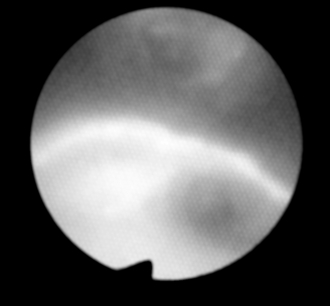
FIGURE 33.9 The double-lumen tube has been repositioned so that the side opening of the bronchial lumen at 6 o’clock is now over the right upper lobe bronchus. The patient is lying on the right. Orientation: left, cephalad; upper, left; right, caudad; lower, right.
 The double-lumen tube is then held firm and the bronchial cuff inflated with 2 mL of air. Uninterrupted sight of the right upper lobe bronchus confirms that this manoeuvre has not moved the side hole of the bronchial tube relative to the bronchus. The bronchi to the right middle and lower lobes may be seen through the distal lumen of the bronchial tube (Fig. 33.10). Withdrawing the intubating laryngoscope may give a view of the origins of all three lobar bronchi (Fig. 33.11).
The double-lumen tube is then held firm and the bronchial cuff inflated with 2 mL of air. Uninterrupted sight of the right upper lobe bronchus confirms that this manoeuvre has not moved the side hole of the bronchial tube relative to the bronchus. The bronchi to the right middle and lower lobes may be seen through the distal lumen of the bronchial tube (Fig. 33.10). Withdrawing the intubating laryngoscope may give a view of the origins of all three lobar bronchi (Fig. 33.11).
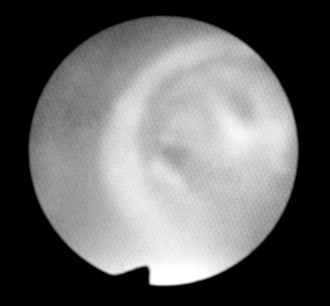
FIGURE 33.10 Distal opening of bronchial lumen at 3 o’clock shows right lower and middle lobe bronchi. The dark colour at 9 o’clock is the bronchial lumen cuff. The patient is lying on the right. Orientation: left, cephalad; upper, left; right, caudad; lower, right.
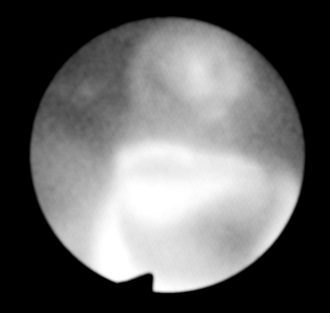
FIGURE 33.11 The intubating laryngoscope is withdrawn to show the right middle and lower lobe bronchi through the distal opening at 12 o’clock and the right upper lobe bronchus through the side opening at 6 o’clock. The patient is lying on the right. Orientation: left, cephalad; upper, left; right, caudad; lower, right.
 The double-lumen tube is then held against the teeth or gums of the maxilla, the mark on the tube there is noted and the laryngoscope is removed from the bronchial lumen. Without moving it, the tube is tied with a clove hitch over this mark and the tube tie tied round the neck with a bow.
The double-lumen tube is then held against the teeth or gums of the maxilla, the mark on the tube there is noted and the laryngoscope is removed from the bronchial lumen. Without moving it, the tube is tied with a clove hitch over this mark and the tube tie tied round the neck with a bow.
 The tracheal cuff is then inflated with 5 mL of air and the fibreoptic laryngoscope is passed down the tracheal lumen until the carina is identified again. The bronchial lumen is seen to pass down the correct main bronchus and the bronchial cuff in its main bronchus is seen to be inflated, but not herniating to impinge over the lumen of the other main bronchus (Fig. 33.12).
The tracheal cuff is then inflated with 5 mL of air and the fibreoptic laryngoscope is passed down the tracheal lumen until the carina is identified again. The bronchial lumen is seen to pass down the correct main bronchus and the bronchial cuff in its main bronchus is seen to be inflated, but not herniating to impinge over the lumen of the other main bronchus (Fig. 33.12).
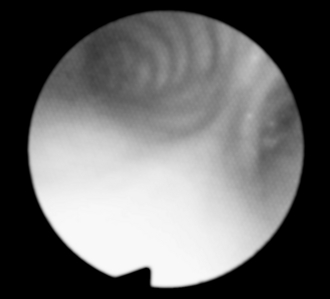
FIGURE 33.12 The intubating laryngoscope has been passed down the tracheal lumen. The carina passes from 2 o’clock anteriorly to 6 o’clock posteriorly. The left main bronchus is to the left at 10–12 o’clock. On the other side of the carina, the dark crescent confirms that the inflated bronchial cuff is not herniating over the left main bronchus and the bronchial tube to the right of the cuff passes down the right main bronchus. The patient is lying on the right. Orientation: left, cephalad; upper, left; right, caudad; lower, right.
 The laryngoscope is removed from the tracheal lumen and both lungs are ventilated.
The laryngoscope is removed from the tracheal lumen and both lungs are ventilated.
 Bronchial and tracheal lumens are clamped in turn, observing airway pressures, leaks and the extent of ventilation of each lung.
Bronchial and tracheal lumens are clamped in turn, observing airway pressures, leaks and the extent of ventilation of each lung.
Physiological Changes
Hypoxaemia during one-lung anaesthesia may be minimized by:
 correct positioning of the bronchial tube
correct positioning of the bronchial tube
 increasing inspired oxygen concentration to 50%
increasing inspired oxygen concentration to 50%
 a tidal volume of 5–7 mL kg–1 or less to avoid increasing dead space
a tidal volume of 5–7 mL kg–1 or less to avoid increasing dead space
 positive end-expiratory pressure of no more than 5–10 cmH2O to minimize dependent lung collapse without increasing vascular resistance
positive end-expiratory pressure of no more than 5–10 cmH2O to minimize dependent lung collapse without increasing vascular resistance
 maintaining cardiac output and pulmonary artery pressures near the normal range.
maintaining cardiac output and pulmonary artery pressures near the normal range.
ANAESTHESIA FOR THORACIC SURGERY PROCEDURES
Ambrosino, N., Gabbrielli, L. Physiotherapy in the perioperative period. Best Pract. Res. Clin. Anaesthesiol. 2010;24:283–289.
Banki, F. Pulmonary assessment for general thoracic surgery. Surg. Clin. North Am. 2010;90:969–984.
Bastin, R., Moraine, J.J., Bardocsky, G., et al. Incentive spirometry performance – a reliable indicator of pulmonary function in the early postoperative period after lobectomy? Chest. 1997;111:559–563.
Bernstein, W.K., Deshpande, S. Preoperative evaluation for thoracic surgery. Sem. Cardiothorac. Vasc. Anesth. 2008;12:109–121.
Brister, N.W., Barnette, R.E., Kim, V., Keresztury, M. Anesthetic considerations in candidates for lung volume reduction surgery. Proc. Am. Thorac. Soc. 2008;5:432–437.
Brodsky, J.B., Lemmens, H.J.M. The history of anesthesia for thoracic surgery. Minerva Anestesiol. 2007;73:513–524.
Campos, J.H. Update on selective lobar blockade during pulmonary resections. Curr. Opin. Anaesthesiol. 2009;22:18–22.
Campos, J.H. Update on tracheobronchial anatomy and flexible fiberoptic bronchoscopy in thoracic anesthesia. Curr. Opin. Anaesthesiol. 2009;22:4–10.
Campos, J.H. An update on robotic thoracic surgery and anesthesia. Curr. Opin. Anaesthesiol. 2010;23:1–6.
Castillo, M.D., Heerdt, P.M. Pulmonary resection in the elderly. Curr. Opin. Anaesthesiol. 2007;20:4–9.
Daly, D.J., Myles, P.S. Update on the role of paravertebral blocks for thoracic surgery: are they worth it? Curr. Opin. Anaesthesiol. 2009;22:38–43.
Duggan, M., Kavanagh, B.P. Perioperative modifications of respiratory function. Best Pract. Res. Clin. Anaesthesiol. 2010;24:145–155.
Fischer, G.W., Cohen, E. An update on anesthesia for thoracoscopic surgery. Curr. Opin. Anaesthesiol. 2010;23:7–11.
Hernandez, G., Fernandez, R., Lopez-Reina, P., et al. Noninvasive ventilation reduces intubation in chest trauma-related hypoxemia: a randomized clinical trial. Chest. 2010;137:74–80.
Jaklitsch, M., Billmeier, S. Preoperative evaluation and risk assessment for elderly thoracic surgery patients. Thorac. Surg. Clin. 2009;19:301–312.
McKevith, J.M., Pennefather, S.H. Respiratory complications after oesophageal surgery. Curr. Opin. Anaesthesiol. 2010;23:34–40.
Mineo, T.C. Epidural anesthesia in awake thoracic surgery. Eur. J. Cardio-Thorac. Surg. 2007;32:13–19.
Nagendran, J., Stewart, K., Hoskinson, M., Archer, S.L. An anesthesiologist’s guide to hypoxic pulmonary vasoconstriction: implications for managing single-lung anesthesia and atelectasis. Curr. Opin. Anaesthesiol. 2006;19:34–43.
Ng, J.M. Perioperative anesthetic management for esophagectomy. Anesthesiol. Clin. 2008;26:293–304.
Pompeo, E., Tacconi, F., Mineo, T.C. Awake video-assisted thoracoscopic biopsy in complex anterior mediastinal masses. Thorac. Surg. Clin. 2010;20:225–233.
Rodrigo, J.L., Edguez-Panadero, F. Medical thoracoscopy. Respiration. 2008;76:363–372.
Ross, A.F., Ueda, K. Pulmonary hypertension in thoracic surgical patients. Curr. Opin. Anaesthesiol. 2010;23:25–33.
Round, J.A., Mellor, A.J. Anaesthetic and critical care management of thoracic injuries. J. R. Army Med. Corps. 2010;156:145–149.
Scarci, M., Joshi, A., Attia, R. In patients undergoing thoracic surgery is paravertebral block as effective as epidural analgesia for pain management? Interact. Cardiovasc. Thorac. Surg. 2010;10:92–96.
Schroeder, D. The preoperative period summary. Chest. 1999;115:44S–46S.
Shaw, A. Exploring the perioptome: the role of genomics in thoracic surgery and anaesthesia. Curr. Opin. Anaesthesiol. 2007;20:32–36.
Sherry, K.M. (Ed.), 1996. Management of patients undergoing oesophagectomy. The report of the National Confidential Enquiry into Perioperative Deaths 1996/1997. Nuffield Provincial Hospitals Trust, London, pp. 57–61.
Shimizu, T., Abe, K., Kinouchi, K., Yoshiya, I. Arterial oxygenation during one lung ventilation. Can. J. Anaesth. 1997;44:1162–1166.
Slinger, P. Update on anesthetic management for pneumonectomy. Curr. Opin. Anaesthesiol. 2009;22:31–37.
Slinger, P.D., Johnston, M.R. Preoperative assessment for pulmonary resection. Anesthesiol. Clin. North America. 2001;19:411–433.
Zibrak, J.D. Preoperative pulmonary-function testing. Ann. Intern. Med. 1990;112:763–771.