CHAPTER 15 An introduction to diverticular disease
Introduction
The origin of the word diverticulum is suggested to come from the Latin de-verto – to turn aside, at that time implying a by-road (Google, 2008), although perhaps more memorably it has been suggested that it related to a ‘wayside house of ill repute’ (Tjandra et al., 2008). Nowadays, it is associated with a benign sac-like protuberance that arises from a hollow organ.
History
Diverticula of the colon were first described by Littre in 1700. The possibility of diverticula as a site of infection and perforation was first raised in 1849 by Jean Cruveilhier, an eminent Parisian anatomist (Painter and Burkitt, 1971; Mimura et al., 2002). In 1912, George Haenisch, a roentgenologist, was the first to recognize and describe diverticulitis radiologically.
When considering the etiology of diverticular disease, the most substantiated theory is that of a deficiency in dietary fiber. Following his work as a surgeon in central Africa in the 20th century, Dennis Burkitt appreciated the connection between dietary fiber and the volume and soft nature of feces produced with negligible discomfort by the Africans who lived largely on a vegetarian diet. In 1971, Painter and Burkitt put forward their belief that a low-residue diet was the cause of diverticular disease. It was also noted at that time that, in the central parts of Africa, a number of diseases common in the West, including diverticulitis, were rarely observed (Painter and Burkitt, 1971; Story and Kritchersky, 1994; Netter, 2000; Mimura et al., 2002; Stollman and Raskin, 2004).
Anatomy: colon structure as it relates to diverticular disease
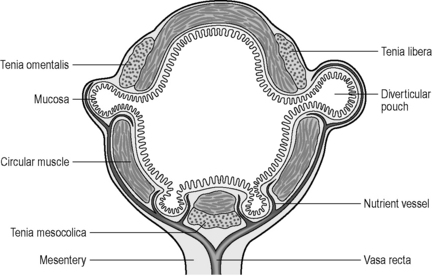
To understand the mechanism of how and why diverticula can form, the musculature and blood supply of the colon need to be reviewed. The colon is made up of four layers: mucosa, submucosa, muscularis and serosa. The muscular layer consists of an inner circular muscle and external longitudinal muscle. The circular muscle forms a thin layer over the cecum and colon, forming a thicker layer covering the rectum. The external longitudinal muscle forms a coating of muscle fiber much thinner than that of the circular muscle it covers. On three aspects of the circumference of the colon, the muscle thickens to form three longitudinal bands, the teniae coli. Individually, the muscle bands are known as tenia mesocolica, libera and omentalis. The three teniae are shorter than the length of circular muscle causing the colon to concertina and become sacculated. These sacculations are called haustral folds between which circular muscle fiber becomes thicker. The three teniae have their origin of attachment at the base of the appendix. In most cases, the bands are spaced equidistant around the circumference of the cecum (Davies, 1969; Netter, 1975) (see also Chapter 4).
In the distal colon, the three teniae fan out at the level of the peritoneal reflection, completely encasing the rectum but forming a thickened portion still recognizable as bands on the anterior and posterior aspects of the rectum (Wolf et al., 2000). Diverticula do not occur distal to the peritoneal reflection, as the anatomical and pathophysiological features are different to that of the colon.
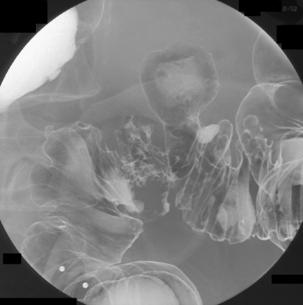
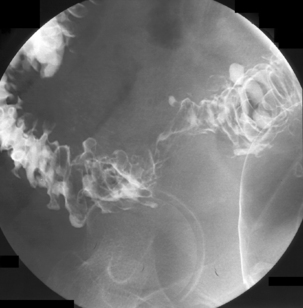
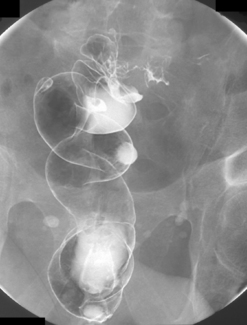
The English physician, Sir David Drummond, described an arterial ring around the colon, the ‘marginal artery of Drummond’ (Gunasekera et al., 2003; Floch and Bina, 2004). The vasa recta arise at frequent intervals around the marginal artery. Dividing at the tenia mesocolica, the vasa recta branches into nutrient vessels that supply the colon. The nutrient vessels pass deep via a canal either side of mesocolica and on the mesenteric side of teniae libera and omentalis (Bassotti et al., 2003; Floch and Bina, 2004). Along these canals the nutrient artery passes through the circular muscle fiber to its origin. In the right circumstances, these canals constitute the weak points through which the mucosa can protrude creating a diverticulum (Figures 15.1 and 15.2).
Pathogenesis
Papers by Painter and Burkitt (1971), Smith (1986), Aldoori et al. (1998), Frieri et al. (2006) and Blackwood and Salter (2000) are among the many that support the hypothesis that dietary fiber reduces the risk of diverticular disease and, conversely, the risk being increased by a lack of fiber, particularly the insoluble fiber hydrocolloid cellulose.
The dietary fiber hydrocolloid cellulose is a non-digestible carbohydrate found in plant material. Although this cellulose itself has a low water content, it is porous and has a significant capability to retain water within its pores. Hydrocolloid cellulose is also resistant to the effects of enzymes in the small bowel and it can provide support for the growth of microflora in the colon as fermentation takes place. It is the high water-retaining capability of the insoluble element of cellulose that is responsible for the (beneficial) formation of a more bulky stool. This results in the maintenance of colonic lumen diameter commensurate with the stool volume. The fluid retained within the stool as a result of dietary fiber intake reduces fecal viscosity and results in a reduced transit time (Prosky and Dreher, 1999; Bassotti et al., 2003). Painter and Burkitt (1971) reported that, in areas of Africa where the population has a high fiber intake, normal colonic transit time was half that common in the West at that time.
Understanding the widely accepted hypothesis as to what provides and constitutes a good stool enables an understanding of the converse, that a lack of dietary fiber results in a reduction in fecal water content. This leads to the stool being more compact and smaller in diameter and with an increased viscosity. The denser less viscous stool, in conjunction with the reduced diameter of the colon, results in a higher intraluminal pressure requirement to pass the fecal column through the bowel. The slower passage of the stool though the colon allows more fluid to be absorbed maintaining the vicious circle that supports the pathogenesis of diverticular disease (Table 15.1).
| Lack of dietary fiber increases the risk of diverticular disease | |
|---|---|
| High fiber intake | Low fiber intake |
Pathophysiology
Pierre-Simon Laplace (1749–1827), a French mathematician, gave his name to a law that provides an explanation as to how the intra- and extraluminal pressure difference, the radius of the colonic lumen and the nature and thickness of the bowel wall can affect the bowel wall tension (Smith, 1986; West, 2006).
Basford (2002) considers the law of Laplace and its relevance today. One area in which Laplace’s law can be demonstrated is fecal transit in the colon. The wider diameter of the colonic lumen resulting from a bulky fluid retentive stool provides for a relatively low fecal viscosity and a reduced transit time which together assist in maintaining a low intraluminal pressure requirement for the movement of stool through the large bowel. Normally, the sigmoid region has the narrowest lumen within the colon and thereby lends itself to having the highest intraluminal pressure and thus the greatest propensity towards colonic diverticular disease anywhere where a low fiber diet is usual.
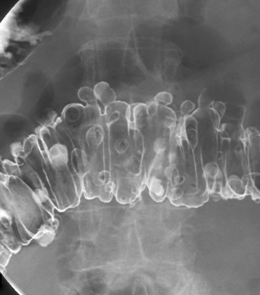
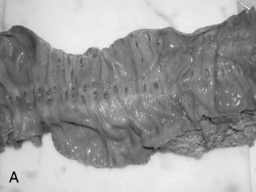
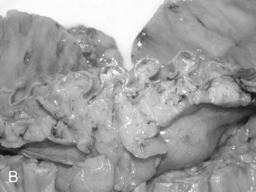
Arising at the apex of the haustra (where the tensile strength is least), diverticula become manifest in a linear array contrary to their apparent irregular distribution demonstrated at barium enema examination (Box 15.1) (Figures 15.2,15.3A, 15.3B) (Netter, 1975; Whiteway and Morson, 1985; Smith, 1986; Bassotti et al., 2003; Eastwood, 2003; Stollman and Raskin, 2004; Floch and Bina, 2004; West, 2004, 2006; West and Losada, 2004; Ye et al., 2005; Parra-Blanco, 2006).
Epidemiology of right-sided colonic diverticula
The Western tendency towards sigmoid diverticular disease is not reflected in Asian populations where diverticular disease predominantly affects the right colon (Gunasekera et al., 2003). West (2006) reports the suggestion that diverticula of the cecum and ascending colon, as commonly found in Asia, differ from the diverticula found in the West, occurring at an earlier age and associated with a genetic predisposition. The predisposition of right-sided diverticula in Asians is supported by others (Mimura et al., 2002; Stollman and Raskin, 2004; Kang et al., 2004; Rajendra and Ho, 2005). Etiology of the predominantly right-sided diverticula found in Japan was reported by Nakaji et al. (2002) as similar to left-sided diverticula in the West.
Age
Asymptomatic diverticular disease in the Western world is rare under the age of 40 but is widely recognized as increasing in prevalence with age. A number of papers suggest that diverticulosis might be present in up to 60% of the population, however, there is a variance in the reported age range to which this percentage relates between 60 years and over 80 years of age (Carter and Whelan, 2000; Mimura et al., 2002; Floch and Bina, 2004; West and Losada, 2004).
In a 10-year UK study of diverticular disease hospitalizations, Jeyarajah et al. (2008) reported 1 219 480 patients hospitalized with a diagnosis of diverticular disease with the disease being the primary cause of admission in 567 423 cases and a comorbidity in 652 057 admissions.
With age, structural changes occur in the connective tissue, including increase in submucosal deposits of collagen, elastin and reticular tissue. The association between this and the increased chance of diverticulosis is discussed in the section on pathophysiology in this chapter (Aldoori et al., 1998; Carter and Whelan, 2000; Basford, 2002; Mimura et al., 2002; Eastwood, 2003; Floch and Bina, 2004; West and Losada, 2004; Parra-Blanco, 2006).
Complications
Between 75 and 85% of patients with diverticula will remain asymptomatic (West, 2004; Salzman and Lillie, 2005). The complications that are associated with diverticular disease include bleeding and inflammation, which itself can lead to perforation, abscess formation, obstruction and peritonitis.
Bleeding
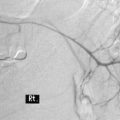
The pathogenesis of diverticular bleeding results from the vasa recta becoming stretched over the dome of the forming pseudodiverticular sac, with eccentric intimal thickening with thinning of the walls between the apex of the dome of the sac and the artery. The features predispose to the blood vessel rupturing towards the diverticulum at its apex (Meyers et al., 1976).
Diverticular disease accounts for over 40% of acute lower gastrointestinal tract bleeding making it the most common cause of acute bleeding in the lower gastrointestinal tract (Kang et al., 2004; West, 2006). Onset of bleeding is usually abrupt, painless and self-limiting, stopping spontaneously in 70–80% of cases. Bleeding can be significant in 3–5% of cases with a 22–38% chance of a diverticulum re-bleeding. Diverticular bleeding is not considered a feature associated with acute diverticular inflammation (Blackwood et al., 2000; Netter, 2000; Stollman and Raskin, 2004; Frieri et al., 2006; Parra-Blanco, 2006; Kriel and Probert, 2007).
Diverticulitis
Colecchia and Sandri (2003) suggest that diverticulitis will affect 10–25% of patients with diverticular disease. Floch and Bina (2004) advanced further evidence to support the suggestion that fiber deficiency not only leads to the formation of diverticula but is also associated with changes in the colonic microflora. This may be associated with a decrease in the colonic mucosal immune response, supporting referenced evidence that chronic segmental colitis is associated with diverticula. The presented hypothesis was that the chronic inflammation occurs in the mucosa associated with the diverticula and is the cause of diverticulitis (Smith, 1986).
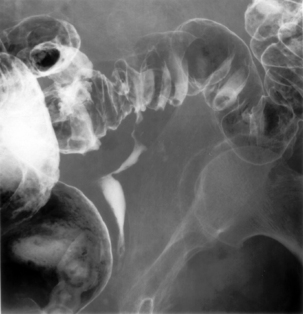
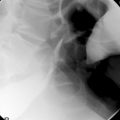
Fecal material inspissated within a diverticulum can rasp, irritate and damage the mucosa. While the sac of the diverticulum is bounded by the muscularis, it is provided with extrinsic support. However, once through to the serosa the support is no longer present leading to the potential to perforate, particularly with the increased intraluminal pressure produced with the strain to evacuate. A resultant perforation may only be small, but such a microperforation makes it possible for bacteria to pass into the subserosa and create a local inflammatory reaction within close proximity to the bowel wall, this can result in small contained pericolic abscess formation (Figure 15.4).
A local inflammatory process can extend through the full thickness of the bowel wall. The more serious macroperforation can lead to abscess and inflammatory mass formation within the peritoneum. Inflammatory mass and peritoneal abscess development can result in one or more of a number of complications including: adhesions, scarring, stricturing and free perforation into the peritoneum resulting in fecal peritonitis. Where there is inflammatory adhesion to an adjacent hollow organ, such as the bladder or vagina, there is the potential for fistula formation (Smith, 1986; Netter, 2000; Colecchia and Sandri, 2003; West and Losada, 2004).
Fistula
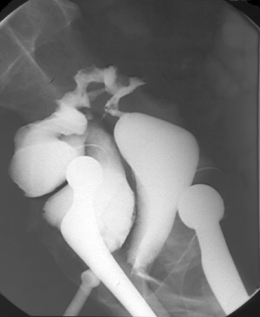
The term fistula originates from the Latin for pipe or tube and denotes a pathologically abnormal passage leading from an abscess cavity or hollow organ to another hollow organ or the skin surface. When a phlegmon or abscess extends from a diverticulum, it has the potential of rupturing into an adjacent hollow organ enabling a fistula to develop (Figure 15.5). Colovesical fistulae are the most common variety with a male to female predominance by a factor of 2:1, the female bladder being protected by the uterus. Colovaginal fistulae constitute 25% of all cases and are the next most common tract formation resulting from diverticulitis (Blackwood et al., 2000; Netter, 2000) (Figures 15.5 and 15.6).
Stricturing and obstruction
Luminal narrowing or obstruction during an acute episode of diverticulitis can occur due to the pericolic inflammation (Figure 15.7) or compression from abscess formation. Diverticular abscess formation is easily diagnosed with CT.
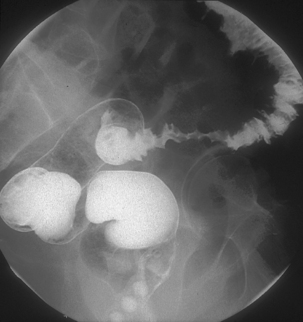
Diverticulitis is often self-limiting and responds to drug therapy and with the therapeutic response the associated stricturing can resolve. It is possible for a patient to have recurrent bouts of diverticulitis which appear asymptomatic; these can trigger the development of fibrotic stricturing (Figures 15.8 and 15.9) (Box 15.2) (Netter, 2000; Blackwood et al., 2000).
Additional information
Symptoms associated with diverticular disease and its complications (Chapter 10 – Symptoms of lower gastrointestinal disease).
Diagnosis of diverticular disease and its complications can be found in Chapter 13 – Fluoroscopic investigations of the large bowel; Chapter 17 – Cross-sectional investigations, nuclear medicine and ultrasound of the small and large bowel; Chapter 20 – Endoscopy of the upper and lower gastrointestinal tract.
Surgical management of diverticular disease and its complications (Chapter 21 – Common surgical procedures of the gastrointestinal tract).
Aldoori W.H., Giovannucci E.L., Rocket H.R., et al. A prospective study of dietary fiber types and symptomatic diverticular disease in men. J. Nutr.. 1998;128(4):714-719.
Basford J.R. The law of Laplace and its relevance to contemporary medicine and rehabilitation. Arch. Phys. Med. Rehabil.. 2002;83(8):1165-1170.
Bassotti G., Chistolinim F., Morelli A., et al. Pathophysiological aspects of diverticular disease of colon and role of large bowel motility. World J. Gastroenterol.. 2003;9(10):2140-2142.
Blackwood A.D., Salter J. Dietary fibre, physiochemical properties and their relationship to health. J. Royal Soc. Promo. Health. 2000;120(4):242-247.
Carter J.J., Whelan R.L. Evaluation and medial management of diverticular disease. Sem. Colon Rectal Surg. 2000;11(4):195-205.
Colecchia A., Sandri L. Diverticular disease of the colon: new perspectives in symptom development and treatment. World J. Gastroenterol.. 2003;9(7):1385-1389.
Davies D.V. Gray’s anatomy. Longmans, 1969;1488-1508.
Eastwood M. Colonic diverticula. Proc. Nutr. Soc.. 2003;62(1):31-36.
Floch M.H., Bina I. The natural history of diverticulitis: fact and theory. J. Clin. Gastroenterol.. 2004;38(5 Suppl.):S2-S7.
Frieri G., Pimpo M.T., Scarpignato C., et al. Management of colonic diverticular disease. Digestion. 2006;73(Suppl. 1):58-66.
Gunasekera R.T., Akroyd R., Stoddard C.J., et al. Complete rectal obstruction due to ischaemia following elective abdominal aortic aneurysm surgery. Surgeon. 2003;1(2):114-117.
Jeyarajah S., Tekkis P.P., Aylin P., et al. British Society of Gastroenterology (BSG) Abstracts. Diverticular disease hospitalisations have a rapidly increasing impact on the health service and affect younger people and deprived socioeconomic groups. Gut. 2008;57(Suppl.1):A17.
Kang J.Y., Melville D., Maxwell J.D., et al. Epidemiology and management of diverticular disease of the colon. Drugs Aging. 2004;21(4):211-228.
Kriel H., Probert C.S. Diverticular disease. Medicine (Abingdon). 2007;35(6):317-319.
Meyers M.A., Alonso D.R., Morson B.C., et al. Pathogenesis of bleeding colonic diverticulosis. Gastroenterology. 1976;76(4):577-583.
Mimura T., Emanuel A., Kamm M.A., et al. Pathophysiology of diverticular disease. Best Pract. Res. Clin. Gastroenterol. 2002;16(4):563-576.
Nakaji S., Danjo K., Munakata A., et al. Comparison of etiology of right-sided diverticula in Japan with that of left sided diverticula in the West. Int. J. Colorectal Dis.. 2002;17(6):365-373.
Netter F.H. Atlas of human anatomy, 2nd ed. Novartis, 2000.
Netter F.H.. The Ciba collection of medical illustrations, Vol. 3. Digestive system, Part 2: Lower digestive tract. 1975.
Painter N.S., Burkitt D.P. Diverticular disease of the colon: a deficiency disease of western civilization. Br. Med. J. 1971;2:450-454.
Parra-Blanco A. Colonic diverticular disease: pathophysiology and clinical picture. Digestion. 2006;73(Supp1.):47-57.
Prosky L., Dreher M.L. Complex carbohydrates in food. CRC Press, 1999.
Rajendra S., Ho J.J. Colonic diverticular disease in a multiracial Asian patient population has an ethnic predilection. Eur. J. Gastroenterol. Hepatol.. 2005;17(8):871-875.
Salzman H., Lillie D. Diverticular disease: diagnosis and treatment. Am. Fam. Physician. 2005;72(7):1229-1234.
Smith A.N. Colonic muscle in diverticular disease. Clin. Gastroenterol.. 1986;15(4):917-935.
Stollman N., Raskin J.B. Diverticular disease of the colon. Lancet. 2004;363:631-639.
Story J.A., Kritchersky D. Denis Parsons Burkitt (1911–1993) (Biographical article). J. Nutr.. 1994;124:1551-1554.
Tjandra J.J., Clunnie G.J.A., Kaye A.H., et al. Textbook of Surgery, 3rd ed., Blackwell Publishing; 2008:211.
West A.B., Losada M. The pathology of diverticulosis coli. J. Clin. Gastroenterol.. 2004;38(Suppl. 1):S11-S16.
West B. The pathology of diverticulosis coli. J. Clin. Gastroenterol.. 2004;38(Suppl. 1):s11-s16.
West B. The pathology of diverticulosis: classical concepts and mucosal changes in diverticula. J. Gastroenterol.. 2006;40(3):S126-S131.
Whiteway J., Morson B.C. Elastosis in diverticular disease of the sigmoid colon. Gut. 1985;26(3):258-266.
Wolf B., Nichols D.M., Munro A., et al. Acute small bowel ischaemia complicating emergency colectomy. J. R. Coll. Surg. Edinb.. 2000;45:64-65.
Ye H., Losada M., West A.B. Diverticulosis coli: update on a ‘Western’ disease. Adv. Anat. Pathol. 2005;12(2):74-80.
Chaplin M.. Dietary fiber and health. 2007 http://www.lsbu.ac.uk/water/ hyhealth.html.
Kruis W., Forbes A., Jauch K.W., et al. Diverticular disease: emerging evidence in a common condition. In Falk Symposium 148. Springer; 2006.
Mimura T., Emanuel A., Kamm M.A. Pathophysiology of diverticular disease. Best Pract. Res. Clin. Gastroenterol. 2002;16(4):563-576.
Netter F.H.. The Ciba collection of medical illustrations, Vol 3. Digestive system, Part 2: Lower digestive tract. 1975.
Painter N.S., Burkitt D.P. Diverticular disease of the colon: a deficiency disease of western civilization. Br. Med. J. 1971;2:450-454.
Prosky l., Dreher M.L. Complex carbohydrates in foods. CRC Press, 1999.
Stollman N., Raskin J.B. Diverticular disease of the colon. Lancet. 2004;363:631-639.