chapter 50 An integrative approach to prostate cancer
INTRODUCTION AND OVERVIEW
Prostate cancer is a major health issue for men around the world, particularly in developed countries. In Australia, for example, over 18,000 men are diagnosed with it per year and it kills over 3000 patients each year.1 There were 679,000 new cases of prostate cancer worldwide in 2002, making it the fifth most common cancer in the world and the second most common in men (11.7% of new cancer cases overall; 19% in developed countries and 5.3% in developing countries).2 It is the most common cause of cancer-related death in men.3 It is the most common non-cutaneous malignancy and there are around 250,000 prostate cancer deaths each year worldwide.4
AETIOLOGY
The causes of prostate cancer are essentially unknown, although it is estimated that up to 40% of prostate cancers may have an inherited component, according to twin studies.5 Furthermore, there is approximately a 40-fold difference in the reported incidence and a 12-fold difference in mortality rate of prostate cancer between various geographic and ethnic populations.6 The highest reported incidence of prostate cancer is in Afro-American men.7 Men with a family history of prostate cancer also have an increased risk of developing prostate cancer.8 There is a two-fold risk with one first-degree relative and a five-fold risk with two first-degree relatives.8 Results from migrant studies provide strong evidence of the importance of environmental and lifestyle factors in the development of prostate cancer.9 Recently, there has been considerable interest in the role of diet and other lifestyle factors in the initiation, promotion and progression of prostate cancer.10
PREVENTION
A few studies have been undertaken to determine whether prostate cancer can be prevented by taking 5-alpha-reductase inhibitors (finasteride and dutasteride).11–13 The finasteride (Proscar®) trial showed a 25% reduction in the prevalence of prostate cancer over a 7-year period; however, there has been some concern that there may be a slight increase in the incidence of more aggressive tumours, but subsequent, more careful analyses have not supported this.12,14 Currently, chemo prevention with finasteride is therefore not recommended by most urologists.
Further major studies that have recently been reported include a prevention study using dutasteride (Avodart®) and another study, the SELECT trial, using selenium and vitamin E, and studying 30,000 men. The dutasteride study (REDUCE trial) reported a 20% decrease in the detection of prostate cancer after only 4 years of follow-up in a slightly higher-risk group of men without any increase in detection of high-grade disease. There was a 4% increased incidence of reduced libido, similarly to the finasteride study. The SELECT trial reported no benefit in taking selenium and vitamin E in the prevention of prostate cancer. It was, however, performed in a country not deficient in selenium.15–18
Other medications that have been implicated in possible prevention are the statins, for which there is currently low-level evidence of a possible risk reduction19–21 and toremifene, a selective oestrogen receptor modulator that has shown some experimental evidence in animals for a reduction in incidence.22
There is now some evidence-based dietary advice that GPs can give their patients on how to reduce their risk of prostate cancer (Box 50.1). Obesity, high saturated fat intake and high calorie intake may increase the risk of developing prostate cancer.23,24
BOX 50.1 Evidence-based dietary advice on how to reduce prostate cancer risk
In countries where the soil is deficient in selenium (e.g. Australia), 100–200 μg of selenium daily is considered safe and appears to reduce the incidence of prostate cancer.25,26 Lycopene, found in the red part of tomatoes, and, in the new world, red wines, also seem to be a possible factor.27,28 Adequate vitamin D intake is to be encouraged, usually by natural means, but possibly by supplementation.29 Vitamin E supplementation may help some patients if the dose is limited to < 400 IU per day, although the SELECT trial has raised doubt about this.30 A dosage higher than this may increase the risk of heart attack or stroke.
Vasectomy and sexual activity do not appear to be major factors in the aetiology of prostate cancer.31,32
Herb and plant extracts have also been implicated in some studies, but there is currently no evidence for the use of saw palmetto, pygeum, pumpkin or stinging nettle, all of which have traditionally been used for benign prostatic hyperplasia. There is, however, some experimental evidence that isoflavones, such as red clover, which has a phyto-oestrogenic effect, as well as milk thistle, may have some benefit, but this has only been shown in experimental models and has been extrapolated from low-risk Asian populations.33,34
DIET SUPPLEMENTS AND PROSTATE CANCER
There is now considerable evidence that environmental factors, particularly dietary factors, may have a profound influence on the incidence of, and effect on, the natural history of prostate cancer.35–37 Current research into prostate cancer suggests that changes in so-called modifiable risk factors, such as diet and supplements, may translate into very meaningful benefits.38–43 The level of evidence for each component of the diet and supplement varies, and is still evolving. Having said that, many of the dietary modifications also have a general benefit.
DIET
The information on diet currently suggests that a healthy heart diet, or Mediterranean diet, appears to be beneficial to prostate cancer patients.44–46 Limited caloric intake, as well as reduction in saturated fats and a high intake of fish, appear to be beneficial.39 A diet high in cruciferous vegetables and plant-based foods appears to be beneficial not only to the heart, but also to the prostate.43,47
SUPPLEMENTS
The recently published large randomised control trial (SELECT) looking at the effects of selenium and vitamin E in prostate cancer concluded that there was no statistically significant benefit in the use of either selenium or vitamin E (alone or in combination) in the prevention of prostate cancer in relatively healthy men.18 Excessive use of multivitamins has recently been shown to increase the rate of advanced and fatal disease.48 Fish oil appears to be a good source of omega-3, particularly if fish is not a common part of one’s diet. Pomegranate has recently also shown some potential benefit.49–52
PHYTOMEDICINE
Herbs and spices, such as garlic, turmeric, rosemary and lemongrass, have been variously recommended, but most evidence for any general benefit appears to come from studies of garlic.53 For most of these, however, there is no reliable evidence in humans in vivo at normal intake levels.
There has also been evidence for polyphenols, such as green tea and red wine. Again, results have been mixed. A Chinese control study showed a protective effect of green tea with synergy for lycopene, but no benefit for green tea in a Japanese study.54,55 There appeared to be a combined inhibitory effect of green tea and COX-2 inhibitors.56,57 The benefit of red wine appears to come from the polyphenol resveratrol, and a moderate intake (< 4 glasses of red wine per week) appeared to have some benefit in the healthcare professional follow-up studies.58,59 Other plant extracts, such as soy and red clover, appeared to induce their effect through their oestrogenic content. These have been shown, in some population and experimental studies, to have a benefit, but not in vivo.60
LIFESTYLE FACTORS
Other aspects of supportive care include stress management and regular exercise and, certainly, there is now a lot of evidence to suggest that regular moderate exercise has benefits in many of the stages of prostate cancer and, in particular, in the more advanced stages, when patients are on hormone therapy.61,62 For example, elderly men with prostate cancer are about a third as likely to progress to more aggressive prostate cancer if they exercise regularly.63 It is, however, important to avoid certain dietary and supplementary treatments and, in particular, an excessive intake of calcium and zinc; and dietary fat, particularly saturated dietary fat, is to be avoided.46,64
The data on diet, as they relate to prostate cancer, are incomplete. As a general principle it is currently recommended that a healthy-heart diet, low in saturated fat and rich in omega-3 fatty acids, as well as judicious use of supplements, such as lycopene, selenium, vitamin D3, soy isoflavones, limited vitamin E and, possibly, pomegranate, are potentially useful.30,41,46,52,65 This should always be combined with appropriate stress management as well as regular exercise. The further benefit of this is that it empowers the patient to do something for himself.
The Ornish program was mentioned in some detail in Chapter 24. It was trialled on men with early prostate cancer who chose to watch and wait, and over 2 years showed that men who adopted the Ornish program had only a fifth the rate of progression to more aggressive cancer of those who had maintained their usual lifestyle. The Ornish program included all the elements of the ESSENCE model (see ch 6):
Since the data from the outcome studies have been reported there has also been interesting data on the possible mechanisms underlying the positive outcomes. Healthy lifestyle change along the lines of the Ornish program have been found to improve telomerase activity66—an indicator of improved genetic repair—and also down-regulation of prostate cancer gene expression.67 These studies indicate a number of things:
The extensive discussion on lifestyle change and cancer using the ESSENCE model should also be reviewed in Chapter 24, Cancer.
SCREENING
WHY?
There is increasing evidence that prostate cancer testing and early aggressive treatment of appropriately selected cases is likely to save lives.68–70 This evidence includes the European randomised trial (described below), which reported in 2009.69 Prostate-specific antigen (PSA) testing has been responsible for the earlier detection of prostate cancer, which has led to the increased success of curative treatment. There is increasing evidence that use of PSA testing leads to the detection of cancers at an earlier and more curable stage, and that the falling death rate from prostate cancer can, at least in part, be attributed to the efforts of testing and early treatment. Also, in countries with a high uptake of PSA testing, there has been a consistently lower death rate from prostate cancer.
Recently, the European Randomised Study of Screening for Prostate Cancer published its results in the New England Journal of Medicine.69 This landmark study demonstrated unequivocally that PSA screening saves lives. This enormous undertaking was carried out in seven European countries and involved about 162,000 men aged between 55 and 69 years, who were screened with PSA testing every 4 years (with a cut-off value of 3 ng/mL, indicating the need for biopsy) versus no screening. It was scheduled to report in 2 years’ time, but was published early because a statistically significant reduction in the death rate from prostate cancer was found in the screened group. The median follow-up in this group was 9 years, with up to 14 years of follow-up.
Patients who underwent screening experienced a 71% increase in the incidence of prostate cancer detected compared with patients who did not undergo screening, and a 41% reduction in advanced disease; and a 20% reduction in deaths from prostate cancer was seen in all men at study entry. If only men who actually underwent screening were included in the results, the reduction in deaths from prostate cancer was 27%. It is highly likely that, as this study matures, the mortality benefit will increase further. This result is very similar to the 30% reduction in mortality in patients with breast cancer following screening with mammography71 and the 33% reduction in prostate-cancer-specific mortality that occurred in the United States from 1994 to 2003, following the introduction of PSA screening.72
The controversy about PSA testing is further compounded by another trial, published in the same edition of the New England Journal of Medicine.73 The conclusion of this US study, after 7–10 years of follow-up, was that the rate of death from prostate cancer was very low in both groups of screened and unscreened patients and did not differ significantly between the two study groups. This study has been criticised because not only did it have a poor method of testing, but 52% of the controls were screened, follow-up was too short, it was compared with a background of a heavily screened population and very few people underwent biopsies.73
PSA testing, therefore, is not a perfect science, for the following reasons:
Furthermore, there is evidence to suggest that use of the PSA test leads to detection of cancers at an earlier, and more curable, stage.74 This is supported by a falling death rate from prostate cancer which can, at least in part, be attributed to the efforts of testing and early treatment, particularly in areas that have been testing for more than 15 years in a high proportion of the community (e.g. in Tyrol, Austria).75,76 A 10-year study comparing surgery for early prostate cancer versus watchful waiting showed a clear benefit in terms of prostate cancer survival and the incidence of metastatic disease.77
Even less-aggressive tumours have been shown, after 15–20 years, to metastasise and lead to prostate cancer death.78
There is now increased effort to avoid treating latent tumours by putting the patient on active surveillance to avoid over-treatment, particularly in older patients.79–82 With improved clinical judgment, these less-threatening cancers are more likely to be identified at the time of diagnosis and less likely to be treated.
The side-effect profiles of treatments have decreased markedly in recent times.83,84
HOW?
PSA testing and digital rectal examination currently form the cornerstone of prostate cancer testing. A raised PSA level can detect a non-palpable tumour, and an abnormal digital rectal examination can detect a non-PSA-producing tumour. Any older patient with haemospermia should also be tested for prostate cancer. Increasingly, PSA velocity is being used to detect cancer at an earlier stage by monitoring the PSA and recommending biopsy when the velocity increases (when the PSA goes up by > 0.75 ng/mL per year for two consecutive years).85,86 This is particularly useful in younger patients with a family history.
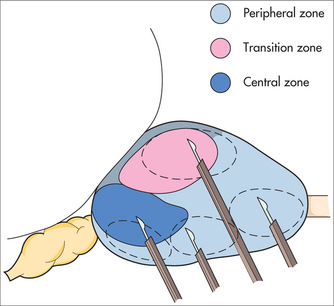
A biopsy should be performed if the digital rectal examination is abnormal or if the PSA reading is greater than the reference range for age on two consecutive occasions and there is no other explanation for the elevated PSA, such as severe prostatic enlargement, prostatitis, recent ejaculation or recent instrumentation. Biopsies should also be recommended if the PSA velocity starts to increase after a baseline of minimal PSA velocity.
WHEN?
Although advice varies in different countries and at different times,87 the current position of the Urological Society of Australasia is that individual men, aged between 50 and 70, with at least 10 years’ life expectancy, should be able to be screened by annual digital rectal examination and PSA testing, after appropriate counselling regarding the potential benefits of investigations and the controversy of treatment. The age at which screening commences should be reduced to 40 if there is a strong family history.69 The effectiveness of this screening in reducing mortality from prostate cancer is not entirely resolved.69
There is some evidence that an isolated PSA test at the age of 40 can predict the likelihood of a man developing prostate cancer in his lifetime.90 For example, a PSA level of > 0.6 ng/mL at 40 years of age suggests that the man is seven times more likely to develop prostate cancer than a man with a PSA level of < 0.3 ng/mL. This has useful implications for how frequently a PSA should be performed.
ACCURACY OF PSA SCREENING
Detection of prostate cancer in its curable stages therefore requires the use of relatively low PSA cut-off levels, which leads to unnecessary biopsies in approximately 70% of patients. This can be minimised by the use of age-specific reference ranges, free/total PSA ratio and PSA velocity and density.91 For example, if patients with a free/total PSA ratio of > 25% (for PSA range 4–10) are not biopsied, 8% of cancers will be missed but up to 30% of unnecessary biopsies will be avoided.
ISSUES WITH PSA TESTING AND REFERRAL
PSA testing
DIAGNOSIS AND STAGING
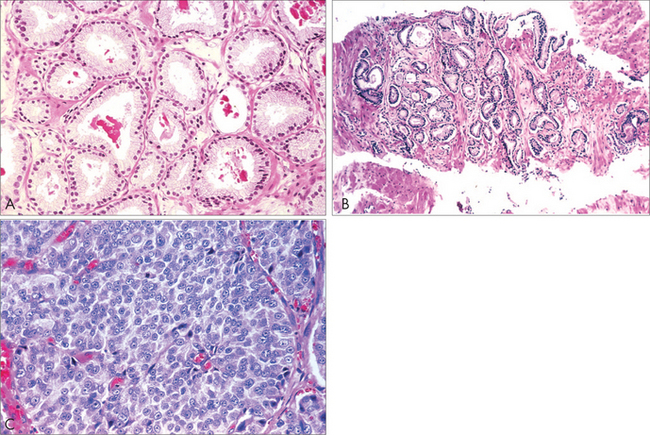
From the biopsy, the pathologist can assess the Gleason Score of the tumour. Generally, if the Gleason Score is 6 or below, it is a slow-growing tumour; if it is 7, it is intermediate grade; and if it is 8–10, it is high-grade. Within the 7 category, a Gleason 3+4 behaves far less aggressively than a Gleason 4+3.92
It is now known that Gleason 6, or lower, tumours generally grow very slowly and only carry a 20% chance of spreading and causing death, even after 20 years with no treatment. This is why patients with small tumours that are Gleason 6 or below, especially elderly men, are often suitable for active surveillance, to see if they have an active tumour.80,82,93
TREATMENT OF LOCALISED PROSTATE CANCER
RADICAL PROSTATECTOMY
Technical improvements and experience have markedly improved outcomes. There have now been several publications suggesting that surgical experience has a significant impact on outcomes.94–96 The aim of surgery is to achieve the ‘trifecta’ (negative margins, continence and potency). Radical prostatectomy can be performed via the retropubic open route, laparoscopic, perineal and robot-assisted laparoscopic route. The robot-assisted laparoscopic technique is gaining popularity in the United States, where 84% of cases were performed using this technique in 2009.97,98
Cure rates of > 90% for low-risk prostate cancer and > 70% for intermediate-risk prostate cancer are to be expected, and incontinence rates are consistently < 5%.99–103 Impotence rates are dropping with improved modifications of the nerve-sparing techniques, as well as better patient selection. In younger men who were potent before surgery, 80–90% are capable of intercourse by 2–4 years with or without PDE5 inhibitors.104–109 Results from large robotic centres are claiming a more rapid recovery of erectile functioning compared with open series, although this needs to be reproduced in other centres.110
Robot-assisted radical prostatectomy aims to perform the same operation as a radical prostatectomy through smaller holes and improved magnification. The learning curve for this technique is considerably shorter than the laparoscopic learning curve, and results from mature series consistently show a more rapid return to normal activities with an equal likelihood of the trifecta.111,112 No direct comparisons, however, have been made between these two procedures. The potential advantages of robot-assisted radical prostatectomy include improved magnification, less bleeding, a shorter hospital stay and a more rapid return to normal activities.113 The disadvantages are the learning curve for the surgeon, the loss of tactile sensation and the cost.
RADIATION THERAPY
Iodine-125 brachytherapy
This less-invasive therapy involves implanting radioactive seeds into the prostate as a day procedure, but is only suitable for low-risk prostate cancers with minimal urinary symptoms. Cure rates for the select group appear to be comparable to surgical results.114–117
Long-term side effects include lower urinary tract irritability and impotence (30–50%).
ACTIVE SURVEILLANCE
With increased numbers of biopsies, it may now be possible to predict more accurately whether one has a non-aggressive, low-volume prostate cancer.93 Increasingly, patients with low-volume, low-grade tumours (less than, or equal to, Gleason 6) are initially being monitored carefully on an active surveillance program and offered treatment only if the cancer shows signs of progressing.79,80,119 This is particularly so in older patients (aged over 70). Active surveillance involves regular PSA and digital rectal examination monitoring, and periodic biopsies.
OTHER TREATMENTS
Cryotherapy and HIFU
These newer, less-tested technologies are used to either freeze or heat the prostate. Long-term results in both these technologies are not yet known.120,121 They both appear to have a significant recurrence rate. They are both particularly attractive options for older men who refuse, or are unsuitable for, surgery or radiotherapy. They can also be used, albeit with a high side-effect profile, if radiotherapy fails to cure the cancer locally.120,122,123
COMPLICATIONS OF TREATMENTS
Urinary problems, particularly urinary incontinence, are far less common after surgery than in previous decades. It is important to engage a pelvic floor physiotherapist early after surgery, to encourage pelvic floor strength.124,125 If incontinence continues after 6–12 months, options include injections with collagen and Macroplastique®, urethral slings or, for the more severe types of incontinence, an artificial urinary sphincter.
Sexual problems are still the most common problem after all forms of therapy.126 The early use of PDE5 inhibitors or prostaglandin E1 injections after surgery appears to minimise the chance of permanent erection problems.127 Ideally, a sexual health physician should be involved in the rehabilitation phase. If erection problems do not recover, the use of a PDE5 inhibitor, prostaglandin E1 injection, a vacuum constriction device or even a penile implant, are options. However, many patients, especially older men, opt for no therapy and engage in other forms of sexual pleasuring.
PSA recurrence can occur after any form of therapy and, if it occurs after surgery, radiotherapy remains a potentially curative treatment if indications suggest that the cancer has recurred locally.128–131 The PSA doubling time seems to be an accurate predictor of whether this is likely to go on to more advanced disease.132–135 Many patients who develop a PSA recurrence after any form of therapy should be encouraged to undertake their own personal supportive care with regards to diet, supplements and exercise.
TREATMENT OF ADVANCED CANCER
There is evidence that regular resistance exercise, as well as a carefully planned diet, can prevent many of the side effects, including weight gain, decreased muscle mass, depression and mood swings.62 Hot flushes can be treated with cyproterone or oestrogen patches and there is some evidence that isoflavones of 100 mg may have a role in this treatment. Depression and mood swings, if mild, may be treated with St John’s wort.136
Long-term hormone therapy can lead to osteoporosis. This can be prevented by regular resistance exercise, adequate vitamin D and calcium supplements.137–140 People on long-term hormone therapy need to be monitored with bone mineral density (BMD) tests and, if BMD significantly deteriorates to the osteoporotic range, bisphosphonate therapy will need to be commenced. Patients need to be aware that this therapy is associated with a small risk of osteonecrosis of the jaw and, prior to commencement of this, appropriate dental hygiene must occur.141,142 Osteoporosis can also be prevented by reducing alcohol intake and increasing isoflavone intake.143,144
The prognosis of a patient with metastatic disease is dependent on the PSA nadir and the PSA doubling time prior to the commencement of hormone treatment.145,146 A patient with a PSA doubling time of < 3 months prior to the commencement of hormone therapy has only a 1% chance of surviving 10 years.147,148 Despite having advanced disease, many patients can be maintained on hormone therapy for more than five, and often more than ten, years, particularly if the PSA doubling time is slow.
Many patients with less aggressive cancers use intermittent hormone therapy, which appears to be as effective as continuous therapy, but with some benefits in terms of quality of life.149
Docetaxel is the first chemotherapy drug proved to prolong life in patients with metastatic prostate cancer who have failed hormone therapy.150–152 New potential developments in this area will occur in the angiogenesis and growth factor fields.153
Andrology Australia. http://www.andrologyaustralia.org.
Cancer Council Australia. http://www.cancer.org.au.
Cancer Council New South Wales. http://www.cancercouncil.com.au.
Lions Australia Prostate Health. http://www.prostatehealth.org.au.
National Cancer Institute. http://www.cancer.gov/cancertopics/factsheet.
National Comprehensive Cancer Network. http://www.nccn.org.
Prostate Cancer Foundation of Australia and New Zealand. http://www.prostate.org.au.
Urological Society of Australia and New Zealand. http://www.usanz.org.au.
Vincent’s Prostate Cancer Centre. http://www.prostate.com.au.
Localised prostate cancer, a guide for men and their families. http://cancer.org.au.
Prostate cancer, a guide for people with cancer, their families and friends. http://cancercouncil.com.au.
Rashid P. Prostate cancer: your guide to the disease, treatment options and outcomes, 3rd edn. http://prostate.org.au.
Stricker P, Phelps K. Prostate cancer for the general practitioner, 2nd edn. http://prostate.com.au.
1 Council NC. Cancer in New South Wales. Incidence and mortality. Online. Available: http://www.cancercouncil.com.au/editorial.asp?pageid=9, 2004. 2007.
2 Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. Cancer J Clin. 2005;55:74-108.
3 Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37(Suppl 8):S4-S66.
4 Garcia M, Jemal A, Ward EM, et al. Global cancer facts and figures 2007. Atlanta, GA: American Cancer Society; 2007. Online. Available: http://www.cancer.org/downloads/STT/Global_Cancer_Facts_and_Figures_2007_rev.pdf.
5 Page WF, Braun MM, Partin AW, et al. Heredity and prostate cancer: a study of World War II veteran twins. Prostate. 1997;33(4):240-245.
6 Jemal A, Siegel R, Ward E, et al. Cancer statistics 2007. CA Cancer J Clin. 2007;57(1):43-66.
7 Ghafoor A, Jemal A, Cokkinides V, et al. Cancer statistics for African Americans. CA Cancer J Clin. 2002;52(6):326-341.
8 Bratt O. Hereditary prostate cancer: clinical aspects. J Urol. 2002;168(3):906-913.
9 Kenfield SA, Chang ST, Chan JM. Diet and lifestyle interventions in active surveillance patients with favorable-risk prostate cancer. Curr Treat Options Oncol. 2007;8(3):173-196.
10 Morton MS, Turkes A, Denis L, et al. Can dietary factors influence prostatic disease? BJU Int. 1999;84(5):549-554.
11 Lotan Y, Cadeddu JA, Lee JJ, et al. Implications of the prostate cancer prevention trial: a decision analysis model of survival outcomes. J Clin Oncol. 2005;23(9):1911-1920.
12 Lucia MS, Epstein JI, Goodman PJ, et al. Finasteride and high-grade prostate cancer in the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2007;99(18):1375-1383.
13 Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349(3):215-224.
14 Rubin MA, Kantoff PW. Effect of finasteride on risk of prostate cancer: how little we really know. J Cell Biochem. 2004;91(3):478-482.
15 Andriole GL, Roehrborn C, Schulman C, et al. Effect of dutasteride on the detection of prostate cancer in men with benign prostatic hyperplasia. Urology. 2004;64(3):537-541. discussion 42–43.
16 Klein EA, Thompson IM, Lippman SM, et al. SELECT: the next prostate cancer prevention trial. Selenium and Vitamin E Cancer Prevention Trial. J Urol. 2001;166(4):1311-1315.
17 Kerr M. AUA 2009: Dutasteride lowers risk for prostate cancer. American Urological Association, 104th Annual Scientific Meeting: Late Breaking Abstract 1. Partial results presented 27 April 2009; full results presented 28 April 2009.
18 Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers. The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009;301(1):39-51.
19 Hoque A, Chen H, Xu XC. Statin induces apoptosis and cell growth arrest in prostate cancer cells. Cancer Epidemiol Biomarkers Prev. 2008;17(1):88-94.
20 Moyad MA. Why a statin and/or another proven heart healthy agent should be utilized in the next major cancer chemoprevention trial: part II. Urol Oncol. 2004;22(6):472-477.
21 Moyad MA, Merrick GS, Butler WM, et al. Statins, especially atorvastatin, may favorably influence clinical presentation and biochemical progression-free survival after brachytherapy for clinically localized prostate cancer. Urology. 2005;66(6):1150-1154.
22 Price D, Stein B, Sieber P, et al. Toremifene for the prevention of prostate cancer in men with high grade prostatic intraepithelial neoplasia: results of a double-blind, placebo controlled, phase IIB clinical trial. J Urol. 2006;176(3):965-970.
23 Efstathiou JA, Bae K, Shipley WU, et al. Obesity and mortality in men with locally advanced prostate cancer: analysis of RTOG 85-31. Cancer. 2007;110(12):2691-2699.
24 Gong Z, Agalliu I, Lin DW, et al. Obesity is associated with increased risks of prostate cancer metastasis and death after initial cancer diagnosis in middle-aged men. Cancer. 2007;109(6):1192-1202.
25 Etminan M, FitzGerald JM, Gleave M, et al. Intake of selenium in the prevention of prostate cancer: a systematic review and meta-analysis. Cancer Causes Control. 2005;16(9):1125-1131.
26 Pourmand G, Salem S, Moradi K, et al. Serum selenium level and prostate cancer: a case-control study. Nutr Cancer. 2008;60(2):171-176.
27 Ivanov NI, Cowell SP, Brown P, et al. Lycopene differentially induces quiescence and apoptosis in androgen-responsive and -independent prostate cancer cell lines. Clin Nutr. 2007;26(2):252-263.
28 Vaishampayan U, Hussain M, Banerjee M, et al. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2007;59(1):1-7.
29 Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167(16):1730-1737.
30 Peters U, Littman AJ, Kristal AR, et al. Vitamin E and selenium supplementation and risk of prostate cancer in the Vitamins and Lifestyle (VITAL) study cohort. Cancer Causes Control. 2008;19(1):75-87.
31 Cox B, Sneyd MJ, Paul C, et al. Vasectomy and risk of prostate cancer. JAMA. 2002;287(23):3110-3115.
32 Lynge E. Prostate cancer is not increased in men with vasectomy in Denmark. J Urol. 2002;168(2):488-490.
33 Bemis DL, Capodice JL, Desai M, et al. A concentrated aglycone isoflavone preparation (GCP) that demonstrates potent anti-prostate cancer activity in vitro and in vivo. Clin Cancer Res. 2004;10:5282-5292.
34 Ganry O. Phytoestrogens and prostate cancer risk. Prev Med. 2005;41(1):1-6.
35 Hayes RB, Ziegler RG, Gridley G, et al. Dietary factors and risks for prostate cancer among blacks and whites in the United States. Cancer Epidemiol Biomarkers Prev. 1999;8(1):25-34.
36 Kavanaugh CJ, Trumbo PR, Ellwood KC. The US Food and Drug Administration’s evidence-based review for qualified health claims: tomatoes, lycopene, and cancer. J Natl Cancer Inst. 2007;99(14):1074-1085.
37 Kirsh VA, Peters U, Mayne ST, et al. Prospective study of fruit and vegetable intake and risk of prostate cancer. J Natl Cancer Inst. 2007;99(15):1200-1209.
38 Moyad MA. Emphasizing and promoting overall health and nontraditional treatments after a prostate cancer diagnosis. Semin Urol Oncol. 1999;17(2):119-124.
39 Moyad MA. Dietary fat reduction to reduce prostate cancer risk: controlled enthusiasm, learning a lesson from breast or other cancers, and the big picture. Urology. 2002;59(4 Suppl 1):51-62.
40 Moyad MA. Is obesity a risk factor for prostate cancer, and does it even matter? A hypothesis and different perspective. Urology. 2002;59(4 Suppl 1):41-52.
41 Moyad MA. Heart healthy equals prostate healthy equals statins: the next cancer chemoprevention trial. Part I. Curr Opin Urol. 2005;15(1):1-6.
42 Moyad MA, Carroll PR. Lifestyle recommendations to prevent prostate cancer, part II: time to redirect our attention? Urol Clin North Am. 2004;31(2):301-311.
43 Moyad MA, Carroll PR. Lifestyle recommendations to prevent prostate cancer, part I: time to redirect our attention? Urol Clin North Am. 2004;31(2):289-300.
44 Escrich E, Moral R, Grau L, et al. Molecular mechanisms of the effects of olive oil and other dietary lipids on cancer. Mol Nutr Food Res. 2007;51(10):1279-1292.
45 Escrich E, Solanas M, Moral R, et al. Are the olive oil and other dietary lipids related to cancer? Experimental evidence. Clin Transl Oncol. 2006;8(12):868-883.
46 Stamatiou K, Delakas D, Sofras F. Mediterranean diet, monounsaturated: saturated fat ratio and low prostate cancer risk. A myth or a reality? Minerva Urol Nefrol. 2007;59(1):59-60.
47 Moyad MA. Step-by-step lifestyle changes that can improve urologic health in men, part I: What do I tell my patients? Prim Care. 2006;33(1):139-163.
48 Lawson KA, Wright ME, Subar A, et al. Multivitamin use and risk of prostate cancer in the National Institutes of Health—AARP Diet and Health Study. J Natl Cancer Inst. 2007;99(10):754-764.
49 Hong MY, Seeram NP, Heber D. Pomegranate polyphenols down-regulate expression of androgen-synthesizing genes in human prostate cancer cells overexpressing the androgen receptor. J Nutr Biochem. 2008;19(12):848-855.
50 Malik A, Mukhtar H. Prostate cancer prevention through pomegranate fruit. Cell Cycle. 2006;5(4):371-373.
51 Pantuck AJ, Leppert JT, Zomorodian N, et al. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin Cancer Res. 2006;12(13):4018-4026.
52 Seeram NP, Aronson WJ, Zhang Y, et al. Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J Agric Food Chem. 2007;55(19):7732-7737.
53 Tapsell LC, Hemphill I, Cobiac L, et al. Health benefits of herbs and spices: the past, the present, the future. Med J Aust. 2006;185(4 Suppl):S4-S24.
54 Jian L, Xie LP, Lee AH, et al. Protective effect of green tea against prostate cancer: a case-control study in southeast China. Int J Cancer. 2004;108(1):130-135.
55 Kikuchi N, Ohmori K, Shimazu T, et al. No association between green tea and prostate cancer risk in Japanese men: the Ohsaki Cohort Study. Br J Cancer. 2006;95(3):371-373.
56 Gupta S, Mukhtar H. Green tea and prostate cancer. Urol Clin North Am. 2002;29(1):49-57.
57 Srinath P, Rao PN, Knaus EE, et al. Effect of cyclooxygenase-2 (COX-2) inhibitors on prostate cancer cell proliferation. Anticancer Res. 2003;23(5A):3923-3928.
58 Anon. Red wine may lower prostate cancer risk. Health News (Waltham, Mass). 2005;11(3):15.
59 Sutcliffe S, Giovannucci E, Leitzmann MF, et al. A prospective cohort study of red wine consumption and risk of prostate cancer. Int J Cancer. 2007;120(7):1529-1535.
60 Hamilton-Reeves JM, Rebello SA, Thomas W, et al. Effects of soy protein isolate consumption on prostate cancer biomarkers in men with HGPIN, ASAP, and low-grade prostate cancer. Nutr Cancer. 2008;60(1):7-13.
61 Barnard RJ, Leung PS, Aronson WJ, et al. A mechanism to explain how regular exercise might reduce the risk for clinical prostate cancer. Eur J Cancer Prev. 2007;16(5):415-421.
62 Galvao DA, Taaffe DR, Spry N, et al. Exercise can prevent and even reverse adverse effects of androgen suppression treatment in men with prostate cancer. Prostate Cancer Prostatic Dis. 2007;10(4):340-346.
63 Giovannuci EL, Liu Y, Leitzmann MF, et al. A prospective study of physical activity and incident and fatal prostate cancer. Arch Intern Med. 2005;165(9):1005-1010.
64 Gallus S, Foschi R, Negri E, et al. Dietary zinc and prostate cancer risk: a case-control study from Italy. Eur Urol. 2007;52(4):1052-1057.
65 Badger TM, Ronis MJ, Simmen RC, et al. Soy protein isolate and protection against cancer. J Am Coll Nutr. 2005;24(2):146S-149S.
66 Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9(11):1048-1057.
67 Ornish D, Magbanua MJ, Weidner G, et al. Changes in prostate gene expression in men undergoing an intensive nutrition and lifestyle intervention. Proc Natl Acad Sci USA. 2008;105(24):8369-8374.
68 Roobol MJ, Kerkhof M, Schroder FH, et al. Prostate cancer mortality reduction by prostate-specific antigen-based screening adjusted for nonattendance and contamination in the European Randomised Study of Screening for Prostate Cancer (ERSPC). Eur Urol. 2009;56(4):584-591.
69 Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320-1328.
70 van Leeuwen PJ, Connolly D, Gavin A, et al. Prostate cancer mortality in screen and clinically detected prostate cancer: estimating the screening benefit. Eur J Cancer. 2010;46(2):377-383.
71 Shapiro S, Venet W, Strax P, et al. Selection, follow-up, and analysis in the Health Insurance Plan Study: a randomized trial with breast cancer screening. Natl Cancer Inst Monogr. 1985;67:65-74.
72 Ries LAG, Melbert D, Krapcho M, et al, editors. SEER Cancer Statistics Review, 1975–2005. Bethesda, MD: National Cancer Institute; 2008. Online. Available: http://seer.cancer.gov/csr/1975_2005, October 2009.
73 Andriole GL, Crawford ED, Grubb RLIII, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310-1319.
74 Jang TL, Han M, Roehl KA, et al. More favorable tumor features and progression-free survival rates in a longitudinal prostate cancer screening study: PSA era and threshold-specific effects. Urology. 2006;67(2):343-348.
75 Bartsch G, Horninger W, Klocker H, et al. Tyrol Prostate Cancer Demonstration Project: early detection, treatment, outcome, incidence and mortality. BJU Int. 2008;101(7):809-816.
76 Horninger W, Berger A, Pelzer A, et al. Screening for prostate cancer: updated experience from the Tyrol study. Can J Urol. 2005;12(Suppl 1):7-13.
77 Bill-Axelson A, Holmberg L, Filen F, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian prostate cancer group-4 randomized trial. J Natl Cancer Inst. 2008;100(16):1144-1154.
78 Sandblom G, Dufmats M, Varenhorst E. Long-term survival in a Swedish population-based cohort of men with prostate cancer. Urology. 2000;56(3):442-447.
79 Klotz L. Active surveillance versus radical treatment for favorable-risk localized prostate cancer. Curr Treat Options Oncol. 2006;7(5):355-362.
80 Klotz L. Active surveillance for favorable-risk prostate cancer: what are the results and how safe is it? Curr Urol Rep. 2007;8(5):341-344.
81 Klotz L. Active surveillance for favorable risk prostate cancer: what are the results, and how safe is it? Semin Radiat Oncol. 2008;18(1):2-6.
82 Klotz L. Low-risk prostate cancer can and should often be managed with active surveillance and selective delayed intervention. Nat Clin Pract Urol. 2008;5(1):2-3.
83 Edgren M, Lennernas B, Haggman M, et al. Postoperative radiotherapy after prostatectomy can be associated with severe side effects. Anticancer Res. 2001;21(3C):2231-2235.
84 von Knobloch R, Wille S, Hofmann R. Clinical side effects after radical prostatectomy. Front Radiat Ther Oncol. 2002;37:191-195.
85 Perrin P. PSA velocity and prostate cancer detection: the absence of evidence is not the evidence of absence. Eur Urol. 2006;49(3):418-419.
86 Sun L, Moul JW, Hotaling JM, et al. Prostate-specific antigen (PSA) and PSA velocity for prostate cancer detection in men aged <50 years. BJU Int. 2007;99(4):753-757.
87 National Conference of State Legislatures. Prostate cancer screening mandates; 2009. Online. Available: http://www.ncsl.org/programs/health/prostate.htm.
88 US National Institutes of Health, National Cancer Institute. Prostate-specific antigen (PSA) test. Online. Available: http://www.cancer.gov/cancertopics/factsheet/Detection/PSA.
89 CancerHelp UK. Why isn’t there UK PSA screening? Online. Available: http://www.cancerhelp.org.uk/about-cancer/cancer-questions/why-isnt-there-uk-psa-screening.
90 Gerber GS. Biopsies for ‘normal’ PSA? Certain relatively young, healthy men with PSA levels below 4 should consider having a biopsy. Health News (Waltham, Mass.). 2004;10(7):4.
91 Schroder FH. Diagnosis, characterization and potential clinical relevance of prostate cancer detected at low PSA ranges. Eur Urol. 2001;39(Suppl 4):49-53.
92 Mitchell RE, Shah JB, Desai M, et al. Changes in prognostic significance and predictive accuracy of Gleason grading system throughout PSA era: impact of grade migration in prostate cancer. Urology. 2007;70(4):706-710.
93 Abouassaly R, Lane BR, Jones JS. Staging saturation biopsy in patients with prostate cancer on active surveillance protocol. Urology. 2008;71(4):573-577.
94 Bianco FJJr, Riedel ER, Begg CB, et al. Variations among high volume surgeons in the rate of complications after radical prostatectomy: further evidence that technique matters. J Urol. 2005;173(6):2099-2103.
95 Brausi M. High surgical volume, high quality, and low costs: a perfect combination. Is it always possible in patients who need radical prostatectomy? Eur Urol. 2006;50(1):17-19.
96 Ramirez A, Benayoun S, Briganti A, et al. High radical prostatectomy surgical volume is related to lower radical prostatectomy total hospital charges. Eur Urol. 2006;50(1):58-62. discussion 63.
97 Menon M, Muhletaler F, Campos M, et al. Assessment of early continence after reconstruction of the periprostatic tissues in patients undergoing computer assisted (robotic) prostatectomy: results of a 2 group parallel randomized controlled trial. J Urol. 2008;180(3):1018-1023.
98 Patel HR, Arya M, Joseph JV. Robotic versus nonrobotic surgery: experts, toys and prostatectomy. Expert Rev Anticancer Ther. 2008;8(6):843-847.
99 Khan MA, Han M, Partin AW, et al. Long-term cancer control of radical prostatectomy in men younger than 50 years of age: update 2003. Urology. 2003;62(1):86-92.
100 Walsh PC. Patient-reported urinary continence and sexual function after anatomic radical prostatectomy. J Urol. 2000;164(1):242.
101 Walsh PC. Sexual function and bother after radical prostatectomy or radiation for prostate cancer: multivariate quality-of-life analysis from CaPSURE. J Urol. 2000;163(1):370.
102 Walsh PC. Prospective comparison of radical retropubic prostatectomy and robot-assisted anatomic prostatectomy: the Vattikuti Urology Institute experience. J Urol. 2003;170(1):318-319.
103 Walsh PC. A randomized trial comparing radical prostatectomy with watchful waiting in early prostate cancer. J Urol. 2003;169(4):1588-1589.
104 Dubbelman YD, Dohle GR, Schroder FH. Sexual function before and after radical retropubic prostatectomy: a systematic review of prognostic indicators for a successful outcome. Eur Urol. 2006;50(4):711-718. discussion 718–720.
105 Katz R, Salomon L, Hoznek A, et al. Patient reported sexual function following laparoscopic radical prostatectomy. J Urol. 2002;168(5):2078-2082.
106 Michl UH, Friedrich MG, Graefen M, et al. Prediction of postoperative sexual function after nerve-sparing radical retropubic prostatectomy. J Urol. 2006;176(1):227-231.
107 Noldus J, Michl U, Graefen M, et al. Patient-reported sexual function after nerve-sparing radical retropubic prostatectomy. Eur Urol. 2002;42(2):118-124.
108 Smith JAJr. Editorial: sexual function after radical prostatectomy. J Urol. 2003;169(4):1465.
109 Zucchi A, Arienti G, Mearini L, et al. Recovery of sexual function after nerve-sparing radical retropubic prostatectomy: is cavernous nitric oxide level a prognostic index? Int J Impot Res. 2006;18(2):198-200.
110 Menon M, Kaul S, Bhandari A, et al. Potency following robotic radical prostatectomy: a questionnaire based analysis of outcomes after conventional nerve sparing and prostatic fascia sparing techniques. J Urol. 2005;174(6):2291-2296.
111 Ahlering TE, Eichel L, Edwards RA, et al. Robotic radical prostatectomy: a technique to reduce pT2 positive margins. Urology. 2004;64(6):1224-1228.
112 Ahlering TE, Skarecky D, Lee D, et al. Successful transfer of open surgical skills to a laparoscopic environment using a robotic interface: initial experience with laparoscopic radical prostatectomy. J Urol. 2003;170(5):1738-1741.
113 Patel VR, Thaly R, Shah K. Robotic radical prostatectomy: outcomes of 500 cases. BJU Int. 2007;99(5):1109-1112.
114 Ferrer M, Suarez JF, Guedea F, et al. Health-related quality of life 2 years after treatment with radical prostatectomy, prostate brachytherapy, or external beam radiotherapy in patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;72:421-432.
115 Jo Y, Junichi H, Tomohiro F, et al. Radical prostatectomy versus high-dose rate brachytherapy for prostate cancer: effects on health-related quality of life. BJU Int. 2005;96(1):43-47.
116 Namiki S, Satoh T, Baba S, et al. Quality of life after brachytherapy or radical prostatectomy for localized prostate cancer: a prospective longitudinal study. Urology. 2006;68(6):1230-1236.
117 Tward JD, Lee CM, Pappas LM, et al. Survival of men with clinically localized prostate cancer treated with prostatectomy, brachytherapy, or no definitive treatment: impact of age at diagnosis. Cancer. 2006;107(10):2392-2400.
118 Martinez A, Gonzalez J, Spencer W, et al. Conformal high dose rate brachytherapy improves biochemical control and cause specific survival in patients with prostate cancer and poor prognostic factors. J Urol. 2003;169(3):974-980.
119 Klotz LH, Nam RK. Active surveillance with selective delayed intervention for favorable risk prostate cancer: clinical experience and a ‘number needed to treat’ analysis. Can J Urol. 2006;13(Suppl 1):48-55.
120 Aus G. Current status of HIFU and cryotherapy in prostate cancer—a review. Eur Urol. 2006;50(5):927-934. discussion 934.
121 Wondergem N, De La Rosette JJ. HIFU and cryoablation—non or minimal touch techniques for the treatment of prostate cancer. Is there a role for contrast enhanced ultrasound? Minim Invasive Ther Allied Technol. 2007;16(1):22-30.
122 Chaussy C, Thuroff S, Bergsdorf T. Local recurrence of prostate cancer after curative therapy. HIFU (Ablatherm) as a treatment option. Urologe A. 2006;45(10):1271-1275.
123 Shelley M, Wilt TJ, Coles B, et al. Cryotherapy for localised prostate cancer. Cochrane Database Syst Rev. 2007;3:CD005010.
124 Cornel EB, de Wit R, Witjes JA. Evaluation of early pelvic floor physiotherapy on the duration and degree of urinary incontinence after radical retropubic prostatectomy in a non-teaching hospital. World J Urol. 2005;23(5):353-355.
125 Tarcia Kahihara C, Ferreira U, Nardi Pedro R, et al. Early versus delayed physiotherapy in the treatment of post-prostatectomy male urinary incontinence. Arch Esp Urol. 2006;59(8):773-778.
126 Dalkin BL, Christopher BA. Potent men undergoing radical prostatectomy: a prospective study measuring sexual health outcomes and the impact of erectile dysfunction treatments. Urol Oncol. 2008;26(3):281-285.
127 McCullough AR. Sexual dysfunction after radical prostatectomy. Rev Urol. 2005;7(Suppl 2):S3-S10.
128 Boehmer D, Maingon P, Poortmans P, et al. Guidelines for primary radiotherapy of patients with prostate cancer. Radiother Oncol. 2006;79(3):259-269.
129 Bolla M, Van Poppel H, Collette L. Preliminary results for EORTC trial 22911: radical prostatectomy followed by postoperative radiotherapy in prostate cancers with a high risk of progression. Cancer Radiother. 2007;11(6/7):363-369.
130 Bolla M, van Poppel H, Collette L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911). Lancet. 2005;366(9485):572-578.
131 van der Kwast TH, Collette L, Bolla M. Adjuvant radiotherapy after surgery for pathologically advanced prostate cancer. J Clin Oncol. 2007;25(35):5671-5672.
132 Heidenreich A. Identification of high-risk prostate cancer: role of prostate-specific antigen, PSA doubling time, and PSA velocity. Eur Urol. 2008;54:976-977.
133 Khatami A, Aus G, Damber JE, et al. PSA doubling time predicts the outcome after active surveillance in screening-detected prostate cancer: results from the European randomized study of screening for prostate cancer, Sweden section. Int J Cancer. 2007;120(1):170-174.
134 Klotz L. Active surveillance with selective delayed intervention using PSA doubling time for good risk prostate cancer. Eur Urol. 2005;47(1):16-21.
135 Tewari A, Horninger W, Badani KK, et al. Racial differences in serum prostate-specific antigen (PSA) doubling time, histopathological variables and long-term PSA recurrence between African-American and white American men undergoing radical prostatectomy for clinically localized prostate cancer. BJU Int. 2005;96(1):29-33.
136 Vanoni C. Treatment of depression with St. Johns wort in general practice. Praxis (Bern 1994). 2000;89(51/52):2163-2167.
137 Alibhai SM, Rahman S, Warde PR, et al. Prevention and management of osteoporosis in men receiving androgen deprivation therapy: a survey of urologists and radiation oncologists. Urology. 2006;68(1):126-131.
138 McLeod N, Huynh CC, Rashid P. Osteoporosis from androgen deprivation therapy in prostate cancer treatment. Aust Fam Physician. 2006;35(4):243-245.
139 Morote J, Morin JP, Orsola A, et al. Prevalence of osteoporosis during long-term androgen deprivation therapy in patients with prostate cancer. Urology. 2007;69(3):500-504.
140 Yee EF, White RE, Murata GH, et al. Osteoporosis management in prostate cancer patients treated with androgen deprivation therapy. J Gen Intern Med. 2007;22(9):1305-1310.
141 Diamond TH, Higano CS, Smith MR, et al. Osteoporosis in men with prostate carcinoma receiving androgen-deprivation therapy: recommendations for diagnosis and therapies. Cancer. 2004;100(5):892-899.
142 Smith MR. Osteoporosis during androgen deprivation therapy for prostate cancer. Urology. 2002;60(3 Suppl 1):79-85. discussion 86.
143 Kogawa M, Wada S. Osteoporosis and alcohol intake. Clin Calcium. 2005;15(1):102-105.
144 Sampson HW. Alcohol, osteoporosis, and bone regulating hormones. Alcohol Clin Exp Res. 1997;21:400-403.
145 Bates AT, Pickles T, Paltiel C. PSA doubling time kinetics during prostate cancer biochemical relapse after external beam radiation therapy. Int J Radiat Oncol Biol Phys. 2005;62(1):148-153.
146 Hanlon AL, Horwitz EM, Hanks GE, et al. Short-term androgen deprivation and PSA doubling time: their association and relationship to disease progression after radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2004;58(1):43-52.
147 Anscher MS. PSA kinetics and risk of death from prostate cancer: in search of the Holy Grail of surrogate end points. JAMA. 2005;294(4):493-494.
148 Robinson D, Sandblom G, Johansson R, et al. PSA kinetics provide improved prediction of survival in metastatic hormone-refractory prostate cancer. Urology. 2008;72:903-907.
149 de Leval J, Boca P, Yousef E, et al. Intermittent versus continuous total androgen blockade in the treatment of patients with advanced hormone-naive prostate cancer: results of a prospective randomized multicenter trial. Clin Prostate Cancer. 2002;1(3):163-171.
150 Berthold DR, Pond GR, Roessner M, et al. Treatment of hormone-refractory prostate cancer with docetaxel or mitoxantrone: relationships between prostate-specific antigen, pain, and quality of life response and survival in the TAX-327 study. Clin Cancer Res. 2008;14(9):2763-2767.
151 Naito S, Tsukamoto T, Koga H, et al. Docetaxel plus prednisolone for the treatment of metastatic hormone-refractory prostate cancer: a multicenter Phase II trial in Japan. Jpn J Clin Oncol. 2008;38(5):365-372.
152 Petrylak DP. Docetaxel (Taxotere) in hormone-refractory prostate cancer. Semin Oncol. 2000;27(2 Suppl 3):24-29.
153 Liu B, Lee KW, Anzo M, et al. Insulin-like growth factor-binding protein-3 inhibition of prostate cancer growth involves suppression of angiogenesis. Oncogene. 2007;26(12):1811-1819.