CHAPTER 82 An Algorithmic Methodology
Lumbosacral radiculopathy is defined as the neurophysiologic dysfunction of the nerve roots affecting both motor and sensory fibers in varying proportions. Signs and symptoms of motor fiber involvement include paresis and atrophy, hyporeflexia, fatigue, cramps, or fasciculations. Sensory abnormalities can range from mild distal paresthesias to anesthesia, dysesthesias, or severe pain. These painful symptoms may be the sole complaint of the patient, while in other instances motor deficits may predominate.1 Nerve root pain can appear in any anatomical area subserved by that nerve root. Hence, any region between the midline spinous processes and lower extremity digits, may be perceived as painful to the patient due to nerve root irritation. Although radicular pain can occur in the absence of overt weakness, it can be quite impairing for the patient, and can represent a challenge to the treating spine care specialist.
Lumbosacral (L/S) nerve root injury can occur anywhere along its long subarachnoid course through the cauda equina within the spinal canal. Clinical clues for segmental localization are less reliable than in the cervical spine.1 The L/S nerve roots exit from the intervertebral disc space below their respective vertebral body. Hence, in the lower limb, sensory deficits may be a more reliable discriminator of the involved level than motor impairments.1 Accurate localization allows for an accurate diagnosis, and both are necessary for successful treatment.
THE ETIOLOGIES AND IMITATORS OF LUMBOSACRAL RADICULOPATHY
The chief complaint of limb pain greater than axial pain typifies radicular symptomatology, but can also be present in other conditions. Sacroiliac joint dysfunction can present with gluteal and proximal lower limb pain.2–4 Piriformis syndrome, as described by Yeoman in 1928,5 represents a constellation of symptoms including limb pain and/or paresthesias with or without gluteal or lumbar pain.6–8 Intrinsic hip joint pathology, such as osteoarthritis, will present with gluteal, groin, or anteromedial thigh pain.9 Strain injuries to the hamstring musculature will cause complaints of soreness and pain in the posterior thigh. Pain in the region of the greater femoral trochanter, buttock, or lateral thigh depicts greater trochanteric pain syndrome, has a 20% prevalence in patients referred to surgical spine specialists,10 and can mimic symptoms of lumbar radiculopathy.11 Lumbosacral plexopathy or peripheral nerve entrapment, such as meralgia paresthetica, can also approximate radicular symptoms. Various spinal structures such as the intervertebral disc, dura, ligaments, or zygapophyseal joints may refer pain distally. However, the primary complaint in these instances is axial pain sometimes associated with less severe lower limb symptomatology.
Since Mixter and Barr’s seminal paper in 1934,12 the intervertebral disc has widely been recognized as a cause of L/S radiculopathy. However, several other etiologies of L/S radiculopathy have been annotated. Central or lateral canal stenosis13,14 can arise due to an array of causes: congenitally short pedicles, ligamentum flavum buckling, facet joint arthropathy, vertebral body hyperostosis, spondylolisthesis, spinal tumors, or spinal cysts. Intraspinal cysts may evolve as a consequence of the degeneration of the zygapophyseal joint,15 ligamentum flavum,16 posterior longitudinal ligament,17 dura mater,18 or chronic spondylolysis,19 and can arise from the root sheath itself.20 Diabetic patients are prone to thoracic radiculopathy,21 perhaps due to intraneural absorption of sorbitol while confined by a nonexpansile dural sheath. Extraforaminal compression by the L/S ligaments may also result in lumbar radiculopathy.22
PATHOPHYSIOLOGY OF LUMBOSACRAL RADICULOPATHY
Mixter and Barr’s landmark description in 193412 had led many spine care practitioners to suspect intervertebral disc (IVD) herniation to be causative in a variety of lumbar radicular pain syndromes. The implicit premise has been that biomechanical compression of neural elements was the sole etiologic factor leading to the manifestation of signs and symptoms.23 However, there is evidence that mechanical influence is not the sole etiologic factor.24–33 There is little correlation between the severity of radiculopathy and the size of the disc herniation.25,28,29 Resolution of symptoms after conservative treatment has been observed without a concurrent reduction in disc herniation volume.28,29 Mixter and Ayer, a year after Mixter and Barr’s hallmark paper, demonstrated that radicular pain could occur without significant disc herniation.30 However, it was not conclusive if this ‘radicular pain’ was nerve-root mediated or somatically referred from another spinal structure. It is probable that, in most instances, biomechanical injury is not the singular cause for the expression of lumbar radicular symptoms related to lumbar intervertebral disc herniation.
Early observations by Lindahl and Rexed32 in 1951 established the presence of pathologic changes including inflammatory cells in nerve roots of patients suffering from sciatica. Subsequent animal studies have demonstrated autoimmune and inflammatory reactions to autogenous nucleus pulposus.34,35 The human intervertebral disc has been shown to be a potent source of phospholipase A2,31 a regulator of the inflammatory cascade which causes perineural inflammation, conduction block, axonal injury,36 and dorsal root demyelination and mechanically induced ectopic discharges in the rat animal model.37 Herniated lumbar intervertebral discs have been observed to spontaneously produce increased amounts of other potentially neurotoxic inflammatory mediators.38 A rapid transport route may exist, bridging the epidural space and intraneural capillaries, providing quick access for this nuclear material to spinal nerve axons.39,40
In stark contrast to the peripheral nerve, the nerve root lacks a perineurium, which provides tensile strength and a diffusion barrier.41,42 Consequently, the nerve root possesses less resilience to tension forces and chemical irritants.42 Furthermore, the epineurium, which provides mechanical cushion to resist compression, is less abundant or developed in the nerve root.42 Within the nerve root itself the fasciculi do not branch to form a plexiform pattern; instead, they run in parallel, loosely held together by connective tissue.41,42 Hence, the nerve root is not as well suited to withstand either mechanical or chemical insult as compared to a peripheral nerve. Furthermore, once the inflammatory cascade is initiated, the nerve root lymphatic system is poorly equipped to adequately clear the inflammatory mediators.42 An inflamed nerve root is thus predisposed to a chronic inflammatory reaction with invasion by fibroblasts with eventual development of intraneural fibrosis.42
Cadaveric studies have discovered a functional tethering of the nerve root to the intervertebral foramen.42,43 When an intervertebral disc herniates in a posterior or posterolateral fashion, the exiting nerve root is placed under tension and not always compressed.42 The ensuing inflammatory response sensitizes the involved nerve root, decreasing its resilience to biomechanical influences. An inflamed nerve will fire repetitively with just minor perturbations; whereas a nonirritated nerve will tolerate more vigorous manipulation without prolonged firing patterns.41,44 The length to which a nerve root must be stretched for it to incur neurophysiologic dysfunction is believed to be 10–15% of resting length.45,46 Clinically, nerve root irritability can be appreciated by elevating the involved lower limb with the knee extended, straight leg raising (SLR). Goddard and Reid43 demonstrated stretch without displacement of the nerve root upon raising the affected limb 20–30° to 70°. Since no nerve root motion is occurring, the radicular pain elicited by this maneuver is a consequence of nerve root tension.43 In asymptomatic patients this movement is nonpainful despite the same amount of tension placed on the neural elements. However, intraforaminal disc herniation or herniation in the presence of spinal stenosis may inflict a biomechanical compromise to the nerve root that may have implications for successful treatment.
The ramification of this growing body of evidence supporting a biochemical paradigm is an evolution of minimally invasive therapeutic interventions. Less than 15% of L/S radiculopathies will eventually require surgical correction.47 Thus, a large proportion of cases can be successfully managed conservatively.25 Conservative care includes physical therapy, oral antiinflammatory medications, and precision intraspinal injections; yet, as interventional spine care continues to evolve, further minimally invasive interventions are being offered. Directing a successful plan of care hinges on accurately diagnosing the patient’s condition. Diagnosing L/S radiculopathy does not always create a mystery for the spine clinician. However, proficiently maneuvering through the list of differential diagnoses, utilizing evidence-based medicine, and accounting for the pharmacologic effects of injected medications requires an appropriate algorithmic approach. This systematic evaluation relies upon thorough history acquisition and physical examination, the accurate assessment of imaging studies, astute electrodiagnostic examinations, and the appropriate use of diagnostic block injections.
THE CLINICAL APPROACH TO LUMBOSACRAL RADICULOPATHY
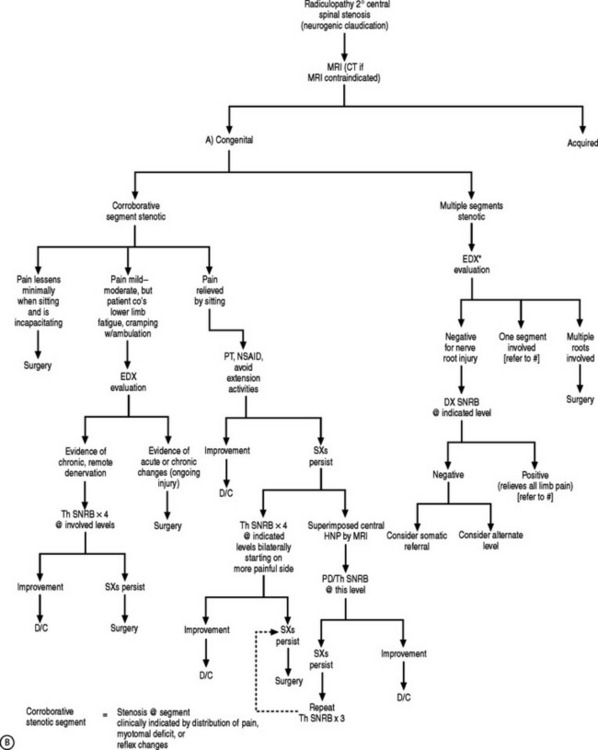
Although radicular pain is categorically defined as limb pain greater than axial pain, occasionally, a patient might report paramedian lumbar pain that could be perceived as back pain. However, this region of discomfort may very well represent the sole region exhibiting painful nerve root symptoms. Yet, the spine clinician must recognize the overlap of referral patterns of pain emanating from nerve root, zygapophyseal joint, intraspinal ligament, intervertebral disc, sacroiliac joint, trochanteric bursa, or the piriformis muscle, and then perform a probability analysis of which structure, or segmental level, is likely generating the symptomatology. Lumbosacral radicular symptoms commonly arise in conjunction with focal intervertebral disc pathology12 or spinal stenosis.13 Disc herniations can lead to functional stenosis and a clinical picture of neurogenic claudication. However, symptom onset associated with disc herniation commonly occurs explosively after a short stint of axial pain or symptoms, and are exacerbated by prolonged sitting or Valsalva maneuvers. Neurogenic claudication due to spinal stenosis progresses more insidiously and its symptoms are exacerbated by prolonged standing and walking. Typically, patients will report bilateral or unilateral lower limb paresthesias, weakness, fatigue, or heaviness that is absent at rest and precipitated by walking that eventually persuades them to stop and rest.48 The patient’s walking tolerance, the point at which pain forces the patient to stop and rest, is usually twice the distance at which discomfort is first felt.48 Assuming a forward stooping posture may dissipate some of the symptoms, and the capability to walk further while traveling uphill, in contrast to downhill, can discriminate between neurogenic and vascular claudication.
No clinical features characteristically identify sacroiliac joint-mediated pain.49 However, a history of a direct fall onto the buttock, rear-end motor vehicle accident (with the ipsilateral foot on the brake), and a fall into a hole (with one foot in the hole and the other extended outside) provides a potential mechanism of injury.49 Patients suffering from greater trochanteric pain syndrome have difficulty sleeping on the affected side,10 and may experience worsening symptoms during ambulation or squatting. Piriformis syndrome is most commonly due to overuse or trauma.8 Magnetic resonance imaging studies have suggested the presence of effacement of the fatty sciatic foramen in elite athletes diagnosed with piriformis syndrome.8 Lumbosacral plexopathy can be due to pelvic trauma, hip arthroplasty, pelvic or gastrointestinal neoplasms, and autoimmune or vascular disorders.1 Meralgia paresthetica, lateral femoral cutaneous neuropathy, can be due to compression by tight belts, corsets, seatbelts, psoas muscle tumor, or prolonged hip flexion.1
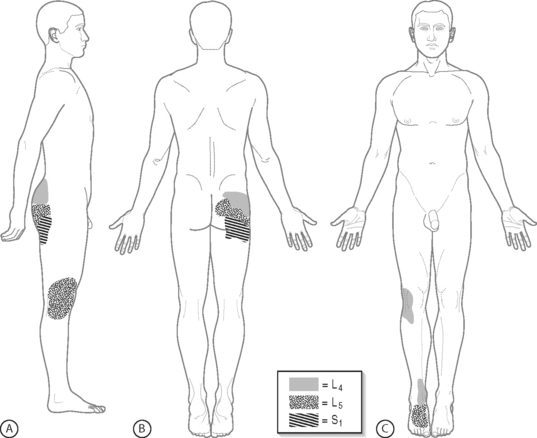
Physical examination findings can be helpful in formulating a differential diagnosis, and help guide the diagnostic work-up. Yet, the distribution of radicular pain in radiculopathy appears to be the only sensitive, thus useful, sign indicating the segmental level of disc herniation.50 In the authors’ experience at the Penn Spine Center, upper buttock radicular pain has proven to be a manifestation of L4 nerve root-mediated pain, midgluteal pain a representation of L5 root pain, and lower gluteal pain an expression of S1 root pain. Limb pain referred from the L4 nerve root may trigger painful symptoms solely in the anterior knee and/or medial ankle, while L5 nerve root pain may masquerade as isolated lateral knee and/or dorsal foot symptomatology (Fig. 82.1). Although lateral thigh and lateral calf pain characterizes L5 nerve root involvement, posterolateral thigh and posterolateral calf pain implicates S1, or L5 and S1 fiber alterations. Meticulously differentiating between lumbar pain and buttock pain is imperative as patients may refer to both synonymously, but to the interventional spine clinician each indicates different potential pain generators. Employing various dural tension maneuvers will help illuminate the pattern of pain referral into the lower limb. Straight leg raising (SLR), reproducing radicular pain between 20–30° and 70°,43 seems to be sensitive, while crossed SLR is more specific for the level of disc herniation.50 Reverse SLR with the patient prone, will assess upper lumbar nerve root fibers. Sensory impairments are less reliable and the diagnostic value of historical findings remains unclear.50 Vibratory sensation abnormalities in dermatomal distributions may be the most reliable measure of sensory disturbances.51
Repetitive motor examination maneuvers such as repetitive calf raises, will elicit subtle motor weakness not detected on static manual muscle testing. When assessing strength statically, the examiner should place the muscle at a biomechanical disadvantage to completely reveal a deficit. Typically, L2 myotomal strength is assessed by hip flexion, L3 by knee extension, L4 by ankle dorsiflexion, L5 by great toe extension, and S1 by ankle plantar flexion. Yet, an astute spine clinician must be aware of anomalous innervation patterns in transitional spinal segments. In instances of sacralization of L5, myotomal innervation patterns overlap so that an L5 radiculopathy may present clinically as L5, S1, or both. Conversely, in lumbarization of S1, innervation patterns are more discrete and clinical findings correlate more closely with the expected compromised nerve root.52
DIAGNOSTIC EVALUATION
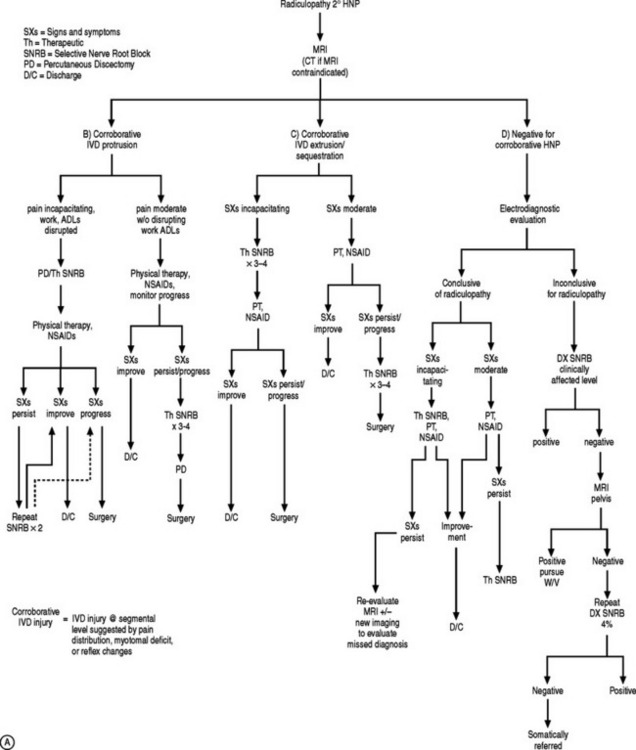
Treatment failure may reflect an inaccurate diagnosis, ineffective treatment, or misguided intervention. Lumbosacral radicular symptoms that have not responded to conservative care or are incapacitating for the patient warrant further diagnostic studies to clarify the diagnosis and redirect treatment strategies. Accurately diagnosing the etiology of lower limb pain requires astute and thorough history gathering and physical examination, and the appropriate prescription of imaging studies, electrodiagnostic evaluation, and precision diagnostic injections. These diagnostic evaluations are appropriately divided into visual anatomic, neurophysiologic, and functional diagnostic assessments.53 Plain radiography, radionuclide imaging, myelography, computed tomography, and magnetic resonance imaging constitute the visual anatomic studies. Neurophysiologic tests include electromyography (EMG) and nerve conduction studies (EDX). Anesthetizing injections have been termed functional tests because patient participation is essential to identify the abolition of their typical pain complaints (see Chs 16, 17, 18, 19).53 Although these diagnostic tools have been thoroughly discussed, the following will highlight their clinical utility in evaluating L/S radiculopathy.
Visual anatomic tests
Plain radiography
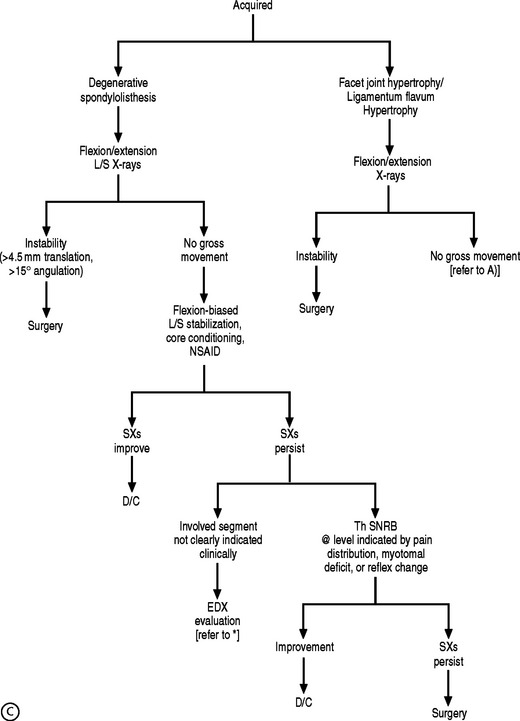
Plain films of the L/S spine detail the osseous structures of the vertebral column. However, radiographs have a limited capacity to identify soft tissue abnormalities within the spinal canal. Lumbar flexion–extension views can identify gross segmental instability, which may cause L/S radiculopathy due to central or foraminal stenosis. The identification of anomalous segmentation, spondylolysis, or spondyl-olisthesis on plain films may increase one’s suspicion of these entities as the etiology of the spinal pain. However, further diagnostic testing is required to confirm this suspicion because these conditions are commonly encountered in the asymptomatic population. Yet, the presence of anomalous segmentation should alert the spine clinician to the possibility of atypical nerve root pain due to altered innervation patterns.52 Except for these entities or when the presence of fracture, malignancy, or infection are suggested by history, plain films are of little value in the investigation of L/S radiculopathy. At the Penn Spine Center, for the most part, the authors utilize plain films to assess for gross segmental instability (excessive sagittal translation, rotation, or sagittal angulation), segmentation anamolies, spondylolysis, spondylolisthesis, compression fracture, and disc height when considering percutaneous discectomy.
Myelography
Myelography’s utility in diagnosing clinically significant spinal abnormalities was first investigated in 1967. Hitselberger and Witten24 observed a lumbar myelographic abnormality in 24% of 71 examinations in adults 18–76 years of age (mean, 51) without complaints of lumbar or L/S radicular pain. Although myelography has been highly sensitive in detecting a posterolateral disc protrusion in the nonoperated lumbar spine,54 its ability to reveal a central protrusion at the L5–S1 level is impaired due to this segment’s large epidural space.55 In addition, myelography is incapable of detecting a lateral disc herniation, beyond the spread of the contrast column ending at the neural foramen (termination of the dura),56 and relatively incapable of demonstrating foraminal stenosis. Myelography cannot differentiate epidural fibrosis from recurrent disc herniation or other extradural lesions in the postoperative patient, and its accuracy in this patient population has been demonstrated to be as low as 24%.57 Currently, myelography is rarely used as the sole imaging study in the work-up of L/S radiculopathy, due its limitations, except in extenuating circumstances such as severe scoliosis or metallic implants that would cause artifact on both magnetic resonance imaging (MRI) and computed tomography (CT).
Computed tomography
Computed tomography is useful to delineate bony detail, which can be helpful in preoperative planning. Wiesel et al.26 found a false-positive rate of 35.4% in 52 asymptomatic individuals undergoing computed axial tomography. In subjects less than 40 years of age, disc herniation was identified in 19.5%. In subjects over 40 years of age, 26.9% demonstrated herniated discs, 10.4% facet joint disease, and 3.4% stenosis.26 The sensitivity of CT images in identifying neurocompressive lesions has been reported to be 91%.58 In patients with L/S radiculopathy who have not undergone surgery, CT images adequately resolve disc abnormalities such as lateral and far lateral herniations, bulging annuli, ligamentum flavum hypertrophy, vacuum disc phenomenon, facet arthrosis, endplate sclerosis, and foraminal or central canal stenosis. Sagittal and coronal reconstructed sequences improve the diagnostic accuracy and should be utilized in all instances. However, the sensitivity of CT in evaluating the postoperative patient decreases to 71%.59 Hence, CT is a useful assessment tool in the evaluation of the spine patient, without previous history of spine surgery, with signs and symptoms of L/S radiculopathy, and its sensitivity approaches that of MRI, especially when performed after myelography. Contrast enhanced MRI remains the anatomic test of choice in the postoperative spine. The authors rely on CT to evaluate for etiologies of L/S radiculopathy in two circumstances. When MRI is contraindicated, such as with pacemakers, or to assess boney stenosis, multiplanar reformatted CT scan is used. In patients who have hardware in place, image artifact often impairs the ability to visualize the discs that are of concern with MRI.
Magnetic resonance imaging
The sensitivity of MRI for the detection of lumbar spinal abnormalities approaches 100% and has been reported to be as high as 96% in the postoperative patient.60 Although MRI is highly sensitive, its clinical, not radiologic, specificity is lacking. Boden et al.27 found abnormalities on MRI scans of lumbar spines in 28% of asymptomatic subjects, 24% demonstrated herniated nucleus pulposus (HNP), and 4% stenosis. In subjects less than 60 years old, 20% had HNP, while 57% of those over 60 years of age demonstrated HNP and stenosis, and the proportion of degenerative discs increased with advancing age.27 However, no study attempted to differentiate disc protrusions from disc extrusions until the 1994 publication by Jensen et al.33 Their work showed that 64% of 98 asymptomatic subjects had an intervertebral disc abnormality, 52% of which had a bulge at one level, 27% a protrusion, and 1% an extrusion.33 The presence of intervertebral disc extrusion may be significant and causally related to nerve root injury.25,29,61 Determining the clinical significance of abnormalities discovered on MR imaging has led to the increased use of diagnostic injection procedures in this patient population.53,62–68 The authors’ algorithmic approach to L/S radiculopathy involves the judicious use of precision, fluoroscopically guided diagnostic spinal injections when necessary to better elucidate the level of clinically relevant pathology.
Neurophysiologic evaluation
Electrodiagnostic medicine allows the physician to assess the neurophysiologic correlate of anatomical findings, and is an extension of the physical examination.69 If imaging studies corroborate the physician’s clinical impression, accurate treatment can be instituted. If imaging studies are equivocal, or the clinical findings are nondiagnostic, electrodiagnostic evaluations are pursued. Motor nerve conduction studies provide prognostic information regarding the recovery of motor function. The differential diagnosis for foot drop includes L5 radiculopathy, peroneal neuropathy at the fibular head, sciatic neuropathy involving the peroneal division, and alpha motor neuron disease. Although a thorough physical examination will typically detect a myotomal pattern rather than peripheral nerve pattern of weakness, the needle electrode examination can verify the diagnosis. For example, a physiatrist was consulted to evaluate an inpatient psychiatric patient with a chief complaint of isolated lateral ankle pain seemingly confined to the lateral malleolus. Plain radiography and a subsequent MRI of the foot and ankle were negative for osseous or soft tissue injury. Physical examination revealed subtle ankle inversion and great toe dorsiflexion weakness. Nerve conduction studies were normal, but electromyography revealed mild muscle membrane irritability in the lower lumbar paraspinals, and increased recruitment frequencies in the tibialis anterior, extensor hallicus longus, and tensor fascia lata of normal-appearing motor unit potentials. A follow-up lumbar spine MRI demonstrated a focal disc protrusion at L4–5 dorsally displacing the traversing L5 nerve root. The patient responded to conservative treatment, comprised of L/S stabilization, core conditioning, and oral antiinflammatory medications, once it was correctly directed at the accurate diagnosis. Similarly, in diabetic patients with preexisting peripheral neuropathy and a new complaint of anterior thigh pain, an electrodiagnostic study can detect a superimposed radiculopathy versus diabetic amyotrophy or mononeuropathy1 Needle electrode examination will characteristically detect acute membrane irritability in proximal limb muscles of a myotomal distribution or in the muscles innervated by the femoral nerve, respectively, in addition to the more chronic membrane irritability and motor unit potential changes in the distally sampled muscles due to the underlying metabolic peripheral neuropathy. Information gathered during the electrodiagnostic evaluation is indispensable in shaping an effective treatment plan. In a diabetic patient, a femoral mononeuropathy will respond to tighter glycemic control,1 which differs from the treatment protocol already mentioned, directed at an L5 radiculopathy.
Many clinicians do not appreciate the value of electrodiagnostic medicine in evaluating and treating L/S radiculopathy. A variety of subtle abnormalities can be detected by the astute electrodiagnostician from the onset of nerve root pain. If weakness is clinically apparent, an increased recruitment frequency will be noticed in the affected muscles upon minimal contraction.70 Within 1–3 weeks, early polyphasic motor unit potentials can be present and may represent ephaptic activation of neighboring axons adjacent to volitionally activated axons.71 Seven to eight days after the onset of radicular symptoms, positive sharp waves can be evoked in the corresponding deep paraspinal musculature,72,73 and these may be the only observed abnormal findings in 30% L/S radiculopathy cases.72 If so, the H-reflex measurement, the electrical correlate of the Achilles muscle stretch reflex, can differentiate between an L5 and an S1 radiculopathy.74 An asymmetry of 1 msec or greater is significant for sideto-side difference,75 and can be present from the onset of symptoms. By 2 weeks after symptom onset, fibrillation potentials may be present in the paraspinal muscles and positive sharp waves in the affected proximal limb muscles.72 Closer to 3 weeks after symptom onset, proximal and distal muscles of the affected myotome will show abnormalities to varying degrees.72 The value of electrodiagnostic medicine in diagnosing L/S radiculopathy has been well established in the literature since 1950.72,76 More recently, its prognostic value has been well validated.72,73
In 1971, Johnson and Melvin’s work was published addressing the clinical management of L/S radiculopathy.72 In a review of 314 cases, 170 electrodiagnostic studies were normal and 111 abnormal in patients presenting with L/S radicular signs and symptoms. After 3 days, only neurapraxic fibers will conduct distal to the affected nerve root. In contrast, wallerian degeneration due to axonotmesis will result in a decrement of the evoked compound muscle action potential (CMAP) amplitude.72,73 The area of this CMAP amplitude can be compared to that of the identical contralateral muscle. If the affected CMAP amplitude of the evoked potential is greater than 50% of the contralateral muscle, motor function recovers with conservative care.72,73 After 14 days, if little spontaneous activity is noticed compared to the amount of clinical weakness and there is less than 10–15% decrement in the CMAP amplitude, an accurate conclusion is that the motor fibers are neurapraxic and recoverable rather than irreversibly damaged.72,73 Johnson and Melvin also witnessed the improvement in SLR concurrent with the reduction in limb pain, and cautioned against using pain alone to guide treatment differently from that supported by the electromyography findings.72 Seventy-five percent of 100 patients treated conservatively with L/S stabilization, oral antiinflammatory medications (four with oral steroids), and restricted bed rest achieved significant reduction in their symptoms allowing return to light duty by 3 months from onset of treatment.73 Twenty-five of these patients returned to this level of activity by 1 month, and all patients were treated within 3 weeks of onset of their symptoms.73 Concurrent with their clinical improvement was the resolution of the electrodiagnostic abnormalities.73 Johnson and Melvin’s conclusion was that electrodiagnostic evaluations help diagnose L/S radiculopathy, and then semiquantitatively assess the degree of reversible motor axonal injury.72 If L/S radiculopathy is diagnosed early and effectively treated with conservative measures, 95% of patients will adequately heal.73
Between 45% and 54%72,77 of L/S radiculopathies may not demonstrate electrodiagnostic abnormalities, and this proportion varies depending on the skill of the electrodiagnostician. In a study of both cervical and L/S radiculopathy, Nardin et al.77 found that electro-diagnostic and MRI findings agree in 60% of patients with a clinical presentation of radiculopathy; however, only one study was positive in 40% of patients. The probability of at least one abnormal study is positively correlated with the presence of neurologic signs on physical examination.77 The authors concluded that their results suggest a complementary relationship exists between MRI and electrodiagnostic studies in diagnosing L/S radiculopathy.77 It is possible that this 60% is an underestimation if subtle electromyographic findings are not detected. In a study of 170 L/S radiculopathy patients, Lauder et al.69 found that 15–18% of subjects had abnormal electrodiagnostic examinations despite normal physical examinations, and the latter was better than symptomatology at predicting abnormalities in the former. If clinical weakness is present, and this may only be appreciated on repetitive testing as an endurance deficit, motor unit recruitment changes will be present despite the chronology of symptoms. Early polyphasic motor unit potentials and H-reflex asymmetry can also be relied upon before the appearance of membrane irritability to confirm clinical suspicion of L/S radiculopathy. Consequently, electrodiagnostic data can be invaluable to better elucidate the significance of anatomical abnormality on advanced imaging, and is a reasonable and appropriate diagnostic test subsequent to equivocal imaging and clinical findings.
Functional diagnostic tests
If the electrodiagnostic testing is inconclusive, advanced imaging demonstrates multiple abnormalities or no corroborative abnormality, fluoroscopically guided, diagnostic selective nerve blocks are performed to elucidate the level of pain generation, and to direct invasive therapeutic measures, whether they are interventional or surgical.53
Diagnostic selective nerve root block
The rudimentary conviction supporting the role of a diagnostic block is that anesthetizing a structure will relieve symptoms if that structure is responsible for those symptoms.53 The accuracy of diagnostic selective nerve root blocks (SNRBs) depends upon the selective anesthetization of the targeted nerve root. In achieving nerve root block, neighboring structures must be avoided to prevent a false-positive response.
As already mentioned, structures other than the lumbar nerve root can be responsible for painful lower limb symptoms. Other structures such as the intervertebral disc, posterior longitudinal ligament, dura, zygapophyseal joint, and sacroiliac joint are known to cause lower limb symptoms mimicking radiculopathy. Kellegren78 was first to establish pain referral patterns from non-neural spinal structures when he observed lower limb symptoms upon stimulation of the axial and limb muscle, tendon, fascia, and periosteum. Although Kellegren demonstrated a dermatomal referral pattern with stimulation of spinous process periosteum, Kuslich et al.79 found that stimulation of the intervertebral disc anulus, longitudinal ligament, and zygapo-physeal joint provoked a nondermatomal referral pattern.
Subtle similarity between nerve root-mediated pain and the somatic referral from a non-neural structure has significant clinical ramifications. The astute spine interventionist will be able to discern between the two scenarios to enact a successful treatment regimen. The literature does not provide evidence of pathognomonic historical features, or physical examination or radiologic findings to identify the intervertebral disc,80 facet joint, or sacroiliac joint48,81–84 as the etiology of pain referred to the lower limb. Rather, there is support48,82–85 for the use of anesthetic block of the suspected joint as a valid diagnostic tool. If a diagnostic SNRB is negative in a patient with atypical radicular symptoms or in a patient without a corroborative lesion on visual anatomic studies, an alternate sclerotomal referral source ought to be pursued.53
Nerve root involvement itself may refer pain in an atypical, nondermatomal pattern. Slipman et al.86 demonstrated that mechanical stimulation of a cervical nerve root frequently produced symptoms outside of the classic dermatome, in a dynatomal pattern, for that nerve root. Similar referral patterns may arise from the lumbar spine. For example, the furcal nerve is a separate nerve root with its own dorsal root ganglion, and most commonly travels with the L4 nerve root at the L4–5 level. However, it may also accompany the L3 and L5 nerve roots through their respective foramina.53 After exiting the foramen, this anomalous root merges with the lumbosacral plexus, the obturator nerve, or the femoral nerve. Moriishi et al. observed intersegmental anastomoses in the lumbar spine in 20% and 5% of the dorsal and ventral nerve roots, respectively.87 However, extradural anomalous nerve roots may have greater clinical significance.88 For example, in the instance of a type 2B nerve root anomaly, two roots exit via one foramen, decreasing their resilience to withstand a mass lesion, and obscuring their clinical expression.88 Hence, L/S radicular pain may present with dynatomal patterns in a similar fashion as the patients studied by Slipman. Consequently, radicular involvement at one level may manifest as unusual distribution of symptomatology. Nonetheless, a diagnostic SNRB can reliably detect the level of pathology despite an atypical symptom complex.53,62–68
Utility of diagnostic selective nerve root block
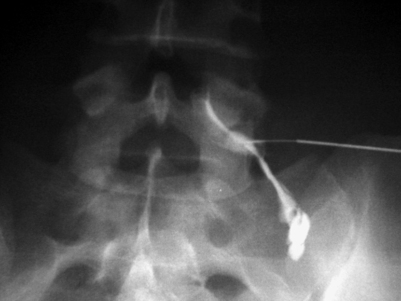
Specificity of a test is the ratio of true negatives divided by the sum of true negatives and false positives, and reflects the probability of a test screening negative in the absence of disease. The specificity of diagnostic SNRBs relies upon the instillation of an anesthetic agent selectively adjacent to the appropriate nerve root without overflow onto neighboring structures or neural elements. The medial branch of the dorsal primary ramus, which provides afferent sensory information to the central nervous system, travels within a few millimeters of the nerve root exiting its foramen.89 The sinuvertebral nerve is a recurrent sensory nerve that innervates the dura and posterior longitudinal ligament traveling back through the foramen after branching from the spinal nerve.89 The inadvertent anesthetization of these sensory nerves can result in a false-positive SNRB in the presence of a painful dural sheath, posterior longitudinal ligament, or zygapophyseal joint. Precise and accurate technique is, therefore, indispensable in achieving adequate specificity (Figs 82.2, 82.3).
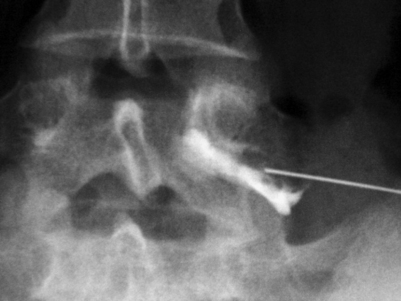
Fig. 82.3 Fluoroscopic image of a right L5 transforaminal epidural steroid injection. In contrast to Fig. 82.2, more epidural spread of contrast was achieved in this injection. A more ventral placement of the needle tip into the neural foramen introduced the contrast medium more superiorly and medially into the epidural space.
The specificity of diagnostic SNRBs ranges 87–100%.57,65,67,68,90,91 In 1973, Schutz and colleagues found that 13 of 15 patients (87%) with a positive diagnostic SNRB had a corroborative lesion revealed during surgery.91 However, this retrospective study did not provide information regarding postsurgical outcomes. A year later, Krempen and Smith90 reported the surgical results of 16 failed back surgery patients who had both a positive diagnostic SNRB and a corroborative lesion. All 16 patients had previous laminectomies or laminectomies and fusions, and all experienced good or excellent relief of their lower limb pain. In 1985, Haueisen and coworkers57 retrospectively analyzed SNRB, electromyography, and myelography results in 55 (57% failed back surgery patients) patients who had positive diagnostic SNRBs. Surgical confirmation of a corroborative lesion was demonstrated in 94% of patients. Dooley et al., in 1988, retrospectively evaluated the responses to diagnostic SNRB and surgical outcomes in 46 patients (51.6% failed back surgery patients).65 Forty-four of 46 patients in whom the diagnostic SNRB initially provoked concordant lower limb pain with subsequent, complete relief after the instillation of local anesthetic, had surgical confirmation of corroborative pathology (one patient did not undergo surgery). Interestingly, 9 of 9 patients (100%) with herniated discs experienced complete relief of limb pain, 14 of 17 (82%) with stenosis described complete symptom resolution, the remaining 19 patients had either arachnoiditis or epidural fibrosis, with 8% of the former and 71% of the latter obtaining postsurgical symptom relief. In 1990, Stanley et al.68 conducted a prospective study in which 50 consecutive radiculopathy patients underwent diagnostic SNRB. Nineteen of 20 patients who experienced concordant limb pain during nerve root stimulation and subsequent relief following local anesthetic infiltration underwent surgical intervention. Eighteen (95%) had corroborative lesions identified intraoperatively, of which lateral canal stenosis was the predominant abnormality. The authors did not comment on surgical outcomes. In 1993, van Akkerveeken67 recorded positive diagnostic SNRBs after symptom provocation with eventual relief after injection of local anesthetic. Of those patients with complete relief of limb pain, surgery confirmed a lesion in 100%. Van Akkerveeken observed a specificity of 90% when negative diagnostic SNRBs were performed at levels free of radiologic evidence of neurocompressive lesions, and a positive predictive value of 95%. However, the positive predictive value may actually fall anywhere from 70% to 95% due to a number of his patients refusing surgical intervention.
The probability of a true-positive test result in the presence of disease reflects the test’s sensitivity. The sensitivity of diagnostic SNRBs has not been as rigorously studied. The diagnostic utility of a clinical test requires that this test be capable of detecting disease at a rate equal to or higher than other available tests.53 Van Akkerveeken67 recorded a sensitivity of 100% for diagnostic SNRBs. Haueisen and colleagues’57 retrospective study compared the sensitivity of diagnostic SNRB to myelography and electromyography, and observed 99% sensitivity for diagnostic SNRBs in contrast to a rate of correct identification of 24% and 38% for myelography and electromyography, respectively. However, the article did not disclose the authors’ criteria for a positive (or negative) electrodiagnostic study. Regardless, if the sensory afferent axon fibers are primarily affected, an EMG study may not be able to indicate the segment involved. However, in the case of S1 involvement, H-reflexes will reflect sensory fiber dysfunction in the absence of motor fiber injury.
Since the inception of MRI, this diagnostic modality has proven to be very sensitive in depicting spinal abnormalities.27,33 Yet, determining the clinical significance of these imaged abnormalities requires a complementary diagnostic test of high specificity. Diagnostic SNRBs have great value in verifying a given MRI abnormality as the pain generator when the patient’s symptoms are in conflict with the MRI findings, or in the presence of more than one MRI abnormality.66,67 Although most available studies antedate the routine use of MRI, several studies have investigated the role of diagnostic SNRB in complex spine cases.
As early as 1971, Macnab62 demonstrated the value of diagnostic SNRBs in the preoperative evaluation of patients with negative imaging studies despite clinical signs of nerve root irritation. In 1988, Kikuchi and Hasue92 correlated the results of myelography and SNRB with surgical findings and outcomes. Eighty-six out of 119 treated patients underwent surgery and experienced complete resolution of their limb symptoms. Although myelography illustrated the involved segment, contrast patterns during SNRB more accurately localized the abnormal area. Thus, SNRB was capable of identifying a single level of symptomatic root compression in light of imaging and symptoms suggesting multilevel pathology. Decompressive surgical intervention was then limited to the level involved. White93 briefly discussed the utility of preoperative diagnostic SNRB in his 1983 article on diagnostic injections. He supported presurgical diagnostic SNRB as a more precise technique than caudal or interlaminar approaches, and useful in patients with equivocal visual anatomic findings. Herron,66 in his 1989 article, retrospectively examined the utility of diagnostic SNRB in selecting patients for surgery after other diagnostic testing – myelography, CT, EMG, and MRI – were found to be equivocal. He found diagnostic SNRBs to be useful in identifying previously undetected disc herniations, the symptomatic level in multilevel disc herniations, the primary pain generator in ‘spine hip syndrome,’ root irritation in spondylolisthesis, the symptomatic level in multilevel stenosis, and the symptomatic root in patients with postoperative fibrosis. However, Herron did not use contrast media to confirm needle location; rather, he relied on concordant symptom provocation during needle placement and 75% postinjection reduction of the patients typical pain. This technique has more important ramifications regarding specificity as compared to sensitivity, however. Stanley et al.68 proposed that diagnostic SNRB is also useful in identifying the symptomatic level in patients with anomalous spinal segmentation.
When performed by well-trained interventional spine physicians, major complications are extremely rare. Major complications related to lumbar SNRB including respiratory and cardiac arrest, seizure, infection, nerve root trauma with permanent injury, dural puncture, perforation of viscous and/or vascular structures, broken needles, and even death have rarely been reported. The potential benefit to the patient must be weighed against these known risks. The incidence of both major and minor complications has been extensively studied. Two large studies94,95 found the incidence of major complications, related to transforaminal epidural steroid injections, to be zero, and the incidence of minor complications (transient nonpositional headache, increased lumbar or limb pain, or vasovagal reaction) was 9.6% per injection.95
Extraspinal axial diagnostic injections
Minimally invasive diagnostic injections can also be utilized to evaluate other etiologies of lower limb pain in the differential diagnosis for lumbar radicular pain. Lower limb pain referred from the sacroiliac joint can be confirmed by diagnostic intra-articular anesthetic injections.84,96 Schwarzer et al. diagnosed sacroiliac joint (SIJ) pain with single intra-articular injections of anesthetic and found an incidence ranging from 13% to 30% in low back pain sufferers.96 Maigne et al. observed a 35% prevalence rate in 54 subjects of SIJ pain employing a single block of short-acting anesthetic.84 However, only 10 (18.5% of the cohort) of these 19 responders had a positive response to a longer-acting anesthetic. Thus, SIJ-mediated pain must be part of the differential diagnosis in a patient complaining of gluteal and lower limb pain with negative spinal imaging and electrodiagnostic testing. More importantly, a false-positive response of up to 47.4% may dilute the sensitivity of single diagnostic SIJ blocks. Therefore, a double block paradigm may be necessary to produce accurate information to diagnose SIJ-mediated pain. However, further prospective studies are required to uphold this presumption.
The diagnosis of piriformis syndrome is one of exclusion. Only after negative spinal imaging and electrodiagnostic evaluation for radiculopathy, reproduction of concordant limb symptoms with percutaneous, intrarectal, or intrapelvic pressure should the spine clinician suspect piriformis syndrome.7 The modified H-reflex, as described by Fishman and Zybert6 may not be a reliable electrodiagnostic tool to evaluate for piriformis syndrome. Slipman et al.7 calculated a positive predictive value of 33% in a small study with stringent inclusion criteria. Symptom relief with injection of the piriformis muscle is considered to be diagnostic of this disorder,7,98,98 and these authors use a symptom reduction of 80% to define a positive injection.
Intrinsic hip pathology can mimic upper lumbar radicular pain or somatically referred pain from the lumbar intervertebral discs.99 Faraj et al. retrospectively evaluated responses to diagnostic intra-articular hip injections in 47 patients with radiographically confirmed hip osteoarthritis.9 Twenty-four patients had a positive diagnostic block response and subsequently experienced pain relief after total hip replacement. Three out of the 23 nonresponders eventually gained pain relief after total hip arthroplasty 2 years after diagnostic injection. The authors concluded that intra-articular hip injections of local anesthetic are valuable diagnostic aids, and calculated a sensitivity of 88% and a specificity of 100%.
Diagnostic intra-articular hip injections can be employed reliably to differentiate intrinsic hip joint pain from spinal pain. Intra-articular diagnostic SIJ, and intramuscular diagnostic piriformis injections are probably useful in evaluating these structures as the source of referred lower limb pain. Yet, these functional diagnostic tests must be appropriately pursued to confirm one’s clinical suspicion and not relied upon solely to determine the source of a patient’s pain.
AN ALGORITHMIC APPROACH
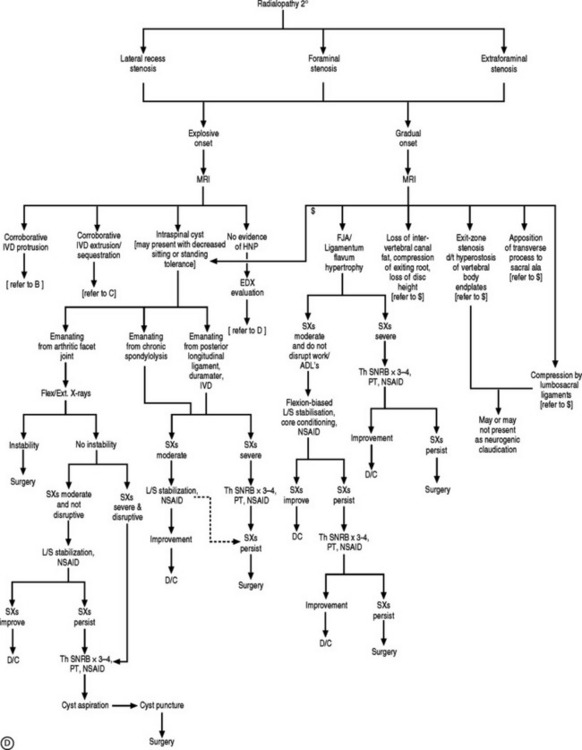
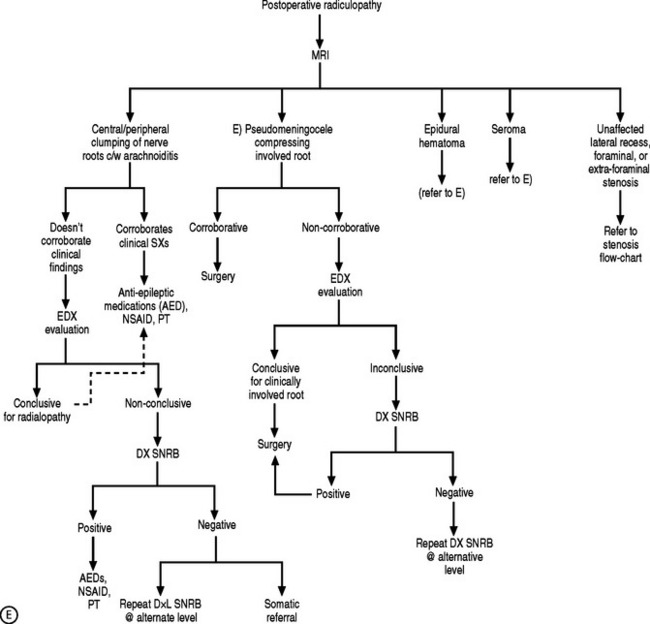
If the electrodiagnostic testing is negative or inconclusive, diagnostic blocks are employed to confirm the clinical impression, and to maximize therapeutic interventions. A diagnostic SNRB is performed at the level of suspicion substantiated by the history and physical examination. However, if advanced imaging and electrodiagnostics are negative, and history and physical examination are supportive, diagnostic blocks of other structures are performed such as the sacroiliac joint, piriformis muscle, hip joint, or trochanteric bursa. In performing a diagnostic SNRB at the Penn Spine Center, the authors use 2% preservative-free xylocaine and define a positive block as an 80% reduction of the preinjection visual analog scale (VAS) rating. A reduction of less than 80% may require the patient to undergo a second diagnostic SNRB with 4% xylocaine if radiculopathy is a firm clinical impression. Since 4% xylocaine does not come preservative free, the authors are particularly cautious about the pattern of contrast spread. If there is any suspicion the injected agents could reach the epidural space, the needle is repositioned until a safe flow is assured. Relying on precision spinal injections to verify the pain generator is appropriate as therapeutic injections probably have distant systemic effects. Thus, if a selective nerve root glucocorticoid injection is moderately successful for a short period of time, the question still remains unanswered: was this effect due to systemic absorption after inaccurate deposition, or did the pathology simply not respond to the appropriately placed anti-inflammatory agent? This decision-making process is more crucial when evaluating the postsurgical patient suffering from recurrent symptomatology. Utilizing this algorithmic approach (Fig. 82.4) at the Penn Spine Center, the authors have been able to accurately diagnose 94.4% of patients presenting with recurrent lumbar and/or radicular symptoms after lumbar surgery.100
Examination of the patient serves several purposes: to exclude long-tract signs, assess weakness patterns, evaluate propensity for consequential injury due to weakness, record provocative maneuvers, gauge legitimacy of complaints, and focus the differential diagnoses. Upper motor neuron signs indicate central nervous system involvement, thus redirecting imaging studies toward the remaining neural axis as deemed by other examination findings. For example, cervical and perhaps intracranial imaging might be indicated in the presence of upper limb motor neuron signs. Myotomal deficits should be discerned from the weakness patterns of plexopathy, neuropathy, myopathy, or myelopathy. Again, such findings will result in different imaging studies. If a myotomal deficit is identified, its functional ramifications must be considered parallel to diagnostic and treatment interventions. A patient with a significant foot drop, for example, may require an ankle–foot orthosis to restore safe and effective gait, and prevent soft tissue injury or a fall. Recording dural tension signs such as the angle at which straight leg raising is positive, provides a baseline for comparison to follow clinical improvement. Subsequent examinations must verify this angle, and that the weakness is stable or improving. If weakness continues to progress, surgery may be indicated. If pain is minimal, and the patient’s chief complaint is weakness and sensory changes, a neurocompressive lesion might be illuminated by advanced imaging. Under such circumstances, decompressive surgical intervention may be necessary to successfully treat the condition.
The authors employ a tiered treatment approach when addressing L/S radiculopathy. The initial step involves prescribing physical therapy (PT) with passive modalities for pain relief and graduated exercises that do not increase extremity complaints, oral nonsteroidal antiinflammatory medications (COX-2-specific agents with celebrex 100 mg b.i.d. as drug of choice), avoidance of aggravating activities, and a soft L/S corset if needed. Passive modalities such as cryotherapy, heat, and transcutaneous electrical nerve stimulation help modulate the patient’s pain to allow participation in PT. Lumbosacral stabilization and core conditioning exercises are prescribed in PT to stabilize the L/S spine. William’s flexion-biased L/S stabilization is utilized in cases of stenosis; McKenzie evaluation and treatment is employed in cases of disc herniation. If the patient does not improve over 1–3 weeks, or the patient presents with disruption of the ADLs, insomnia, inability to perform work-related duties due to severe pain, the authors will offer fluoroscopically guided therapeutic injections once the diagnosis has been confirmed. These minimally invasive injection procedures in addition to the aforementioned conservative measures constitute the second tier. The third treatment tier is defined by the use of percutaneous discectomy using the Dekompressor, coblation technology (nucleoplasty) or automated percutaneous discectomy (APLD), in addition to the measures offered in the first two tiers. The authors typically perform percutaneous discectomy in conjunction with a selective nerve root corticosteroid injection to address both the biomechanical and biochemical insults. Percutaneous disc decompression offers a minimally invasive percutaneous treatment, short of surgery, that has been shown to effectively treat L/S radiculopathy.101 In certain cases, however, nucleoplasty102 may be offered earlier in the treatment algorithm. APLD is preferred when there is a need to extract a larger volume of tissue or in cases of recurrent disc herniation.
If a patient presents with unilateral or bilateral neurogenic claudication after acute onset, and MRI reveals a central focal disc protrusion causing functional stenosis, as can occur in a congenitally small canal due to short pedicles, the authors will incorporate both biomechanical and biochemical treatment pathways. In the authors’ experience, percutaneous disc decompression combined with therapeutic SNRB has been successful in treating such stenosis cases, and if not, the patient is referred to a surgical colleague. In instances of a more gradual onset of claudicating symptoms due to spinal stenosis, its etiology helps guide treatment. Amundsen et al. advocated bracing and physical therapy as initial treatment of stenosis patients presenting with unilateral or bilateral neurogenic claudication.103 The authors found that moderate pain would satisfactorily decrease in 50% of the patients after 3 months of conservative care. The other 50% nonresponders and those with severe pain benefited from surgical decompression; however, their treatment algorithm did not encompass therapeutic SNRBs. Botwin et al. documented a 50% reduction in visual analog scale scores in 75% of elderly stenosis patients who underwent therapeutic SNRBs 12 months prior, to treat unilateral neurogenic claudication, in addition to therapy and oral antiinflammatory medications.104 The Penn Spine Center approach blends the findings of both of these studies. The first tier offers PT, oral antiinflammatory medications, bracing, and activity modification. If symptoms do not abate, therapeutic SNRBs are offered unless there is a superimposed disc protrusion in a tight canal, and a subsequent surgical referral if improvement is unsatisfactory. If, however, a patient complains of lower limb fatigue, cramping, or heaviness with ambulation, or pain not alleviated by sitting, the threshold for a surgical referral is lowered.
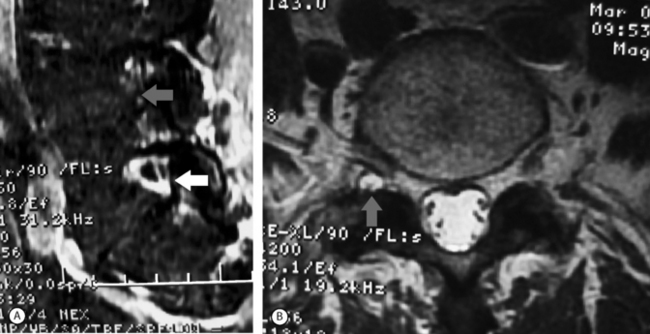
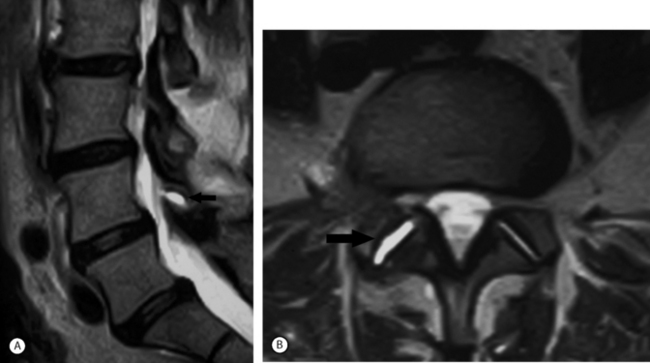
Spinal stenosis due to degenerative facet joint cysts may require percutaneous aspiration or puncture. Therapeutic SNRBs can be effective because an inflammatory process is probably present. The authors have observed perineural enhancement on fat-suppression sagittal MRI with gadolinium consistent with perineural venous engorgement and/or inflammation in cases of facet joint cyst-mediated radiculopathy (Fig. 82.5). The third treatment tier in facet joint cyst-related radiculopathy involves attempted aspiration of the cyst via an intra-articular approach through the joint. Involution of the cyst wall can be achieved via suction through a 20-gauge needle with a 10 cc syringe.105 If aspiration proves unsuccessful, cyst puncture can be attempted, again utilizing an intra-articular approach. As many as 50% of successfully treated facet joint cyst-related radiculopathies may require aspiration or puncture.106
In disc herniation-induced radiculopathy, treatment options again follow the tiered protocol, incorporating minimally invasive procedures, if necessary. Regardless of whether the herniation is a protrusion, extrusion, or sequestration, therapeutic SNRBs are offered if more conservative efforts fail. If therapeutic SNRBs are not curative, nucleoplasty, Dekompressor, or APLD can be employed to treat contained herniations. The authors have used the Dekompressor and APLD for disc extrusions with excellent outcomes, but those results have not been published as of yet. Nucleoplasty ought not be performed in the setting of a disc extrusion. If the patient’s pain improves but his or her weakness persists, as has been witnessed in cases of disc sequestration, surgical decompression has often been necessary. Seventy percent of patients will experience significant reduction in pain at 12 months after undergoing 1–4 therapeutic SNRBs107,108 A smaller proportion, who have disc sequestrations, will experience a similar outcome.25
CASE REPORT
Magnetic resonance imaging revealed intra-articular fluid and joint gapping in the right L4–5 zygapophyseal joint (Fig. 82.6). Subarticular stenosis was noted on the right at this level, and a small central focal protrusion was observed at L5–S1. Flexion–extension plain radiography did not reveal gross instability. An electrodiagnostic evaluation demonstrated normal sural sensory and peroneal motor nerve conduction studies. However, the H-reflex latencies were asymmetric with the right latency 1.45 ms longer than the left.
The differential diagnoses included right S1 versus L5 radicular pain, L4–5 versus L5–S1 Z-joint synovitis, and lumbosacral internal disc disruption syndrome with somatic pain referral. Advanced imaging revealed evidence to support each of these conditions. However, the electrodiagnostic findings suggested a right S1 radiculopathy. The authors’ algorithmic approach employed an initial right S1 diagnostic selective nerve root injection. An L5 diagnostic block followed by diagnostic intra-articular injections of the Z-joints would ensue. However, the S1 diagnostic root injection was positive, and she subsequently underwent two therapeutic right S1 selective nerve injections with complete resolution of her pain.
CONCLUSION
Lumbosacral radiculopathy is most commonly managed successfully by conservative treatment measures.25,47,56,67,68 Among these tools are therapeutic SNRBs or transforaminal epidural steroid injections. The mechanism of action of these interventions is not completely understood but it appears to involve both local (W. D. Chow, personal communication, San Francisco, 2003) and systemic effects.109 Thus, clinical improvement may occur after the instillation of steroids at the incorrect nerve root level or inaccurate site of pathology due to a non-specific systemic steroid effect. Maximizing the therapeutic benefits of axial steroid injections requires accurate placement of these medications. Therefore, appropriate diagnosis is requisite, and warrants the judicious use of visual anatomic, electrophysiologic, and functional diagnostic testing guided by a sound algorithm.
1 Kimura J. Radiculopathies and plexopathies. In: Kimura J, editor. Electrodiagnosis in diseases of nerve and muscle: principles and practice. New York: Oxford University Press, 2001.
2 Fortin JD, Dwyer AP, West S, et al. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part I: asymptomatic volunteers. Spine. 1994;19(13):1475-1482.
3 Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine. 1995;20:31-37.
4 Fortin JD, Washington WJ, Falco FJE. Three pathways between the sacroiliac joint and neural structures. Am J Neuroradiol. 1999;20(Sept):1429-1434.
5 Yeoman W. The relation of arthritis of the sacro-iliac joint to sciatica with an analysis of 100 cases. Lancet. 1928;2:1177-1180.
6 Fishman LM, Zybert PA. Electrophysiologic evidence of piriformis syndrome. Arch Phys Med Rehabil. 1992;73:359-364.
7 Slipman CW, Vresilovic EJ, Palmer MA, et al. Piriformis muscle syndrome: a diagnostic dilemma. J Muscul Pain. 1999;7(4):73-83.
8 Fishman LM, Dombi GW, Michaelsen C, et al. Piriformis syndrome: diagnosis, treatment, and outcome – a 10-year study. Arch Phys Med Rehabil. 2002;83:295-301.
9 Faraj AA, Kumaraguru P, Kosygan K. Intra-articular bupivacaine hip injection in differentiation of coxarthrosis from referred thigh pain: a 10-year study. Acta Orthop Belg. 2003;69(6):518-521.
10 Tortolani PJ, Carbone JJ, Quartararo LG. Greater trochanteric pain syndrome in patients referred to orthopedic spine specialists. Spine J. 2002;2(4):251-254.
11 Swezey RL. Pseudo-radiculopathy in subacute trochanteric bursitis of the sub-gluteus maximus bursa. Arch Phys Med Rehabil. 1976;57:387-390.
12 Mixter WJ, Barr JS. Rupture of the intervertebral disc with involvement of the spinal canal. N Engl J Med. 1934;211(5):210-215.
13 Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg [Br]. 1954;36:230-237.
14 Porter RW. Spinal stenosis and neurogenic claudication. Spine. 1996;21(17):2046-2052.
15 Kao C, Uihlein A, Bickel W, et al. Lumbar intraspinal extradural ganglion cyst. J Neurosurg. 1968;29:168-172.
16 Abdullah AF, Chambers RW, Daut DP. Lumbar nerve root compression by synovial cysts of the ligamentum flavum: report of four cases. J Neurosurg Psychiatry. 1984;60:617-620.
17 Lin R, Wey K, Tzeng C. Gas-containing ganglion cyst of lumbar posterior longitudinal ligament at L3. Spine. 1995;18:2528-2532.
18 Schreiber F, Nielson A. Lumbar spinal extradural cysts. Am J Surg. 1950;80:124-126.
19 DePalma MJ, Strakowski JA, Mandelker EM, et al. An instance of an atypical intraspinal cyst presenting as an S1 radiculopathy. A case report and brief review of pathophysiology. Arch Phys Med Rehabil. 2004;85(6):1021-1025.
20 Tarlov IM. Perineural cysts of the spinal nerve roots. Arch Neurol Psychiatry. 1938;40:1067-1074.
21 Kikta DG, Breuer AC, Wilbourn AJ. Thoracic root pain in diabetics: the spectrum of clinical and electromyographic findings. Ann Neurol. 1982;11:80-85.
22 Olsewski JM, Simmons EH, Kallen FC, et al. Evidence from cadavers suggestive of entrapment of fifth lumbar spinal nerves by lumbosacral ligaments. Spine. 1991;16:336-347.
23 Howe JF, Loeser JD, Calvin WH. Mechanosensitivity of dorsal root ganglia and chronically injured axons: a physiological basis for the radicular pain of nerve root compression. Pain. 1977;3:25-41.
24 Hitselberger WE, Witten RM. Abnormal myelograms in asymptomatic patients. J Neurosurg. 1968;28:204-206.
25 Saal JA, Saal JS. The nonoperative treatment of herniated nucleus pulposus with radiculopathy: an outcome study. Spine. 1989;14:431-437.
26 Wiesel SW, Tsourmas N, Feffer HL, et al. A study of computer-assisted tomography: I. The incidence of positive CAT scans in an asymptomatic group of patients. Spine. 1984;9(6):549-551.
27 Boden SD, Davis DO, Dina TS, et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects: a prospective investigation. J Bone Joint Surg. 1990;72A(3):403-408.
28 Maigne JY, Rime B, Delinge B. Computed tomographic follow-up study of forty-eight cases of nonoperatively treated lumbar intervertebral disc herniation. Spine. 1992;17:1071-1074.
29 Delauche-Cavallier MC, Budet C, Laredo JD, et al. Lumbar disc herniation: computed tomography scan changes after conservative treatment of nerve root compression. Spine. 1992;17:927-933.
30 Mixter WJ, Ayer JB. Herniation or rupture of the intervertebral disc into the spinal canal. N Engl J Med. 1935;213:385-395.
31 Saal JS, Franson RC, Dobrow R, et al. High levels of inflammatory phospholipase A2 activity in lumbar disc herniations. Spine. 1990;15(7):674-678.
32 Lindahl O, Rexed B. Histologic changes in spinal nerve roots of operated cases of sciatica. Acta Orthop Scand. 1951;20:215-225.
33 Jensen MC, Brant-Zawadzki MN, Obuchowski N, et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331(2):69-73.
34 Bobechko WP, Hirsch C. Auto-immune response to nucleus pulposus in the rabbit. J Bone Joint Surg. 1965;47B(3):574-580.
35 McCarron RF, Wimpee MW, Hudkins PG, et al. The inflammatory effects of nucleus pulposus: a possible element in the pathogenesis of low back pain. Spine. 1987;12:760-764.
36 Saal JS, Franson R, Myers R, et al. Human disc PLA2 induces neural injury: a histolomorphometric study. Presented at the International Society for the Study of the Lumbar Spine, Annual Meeting, May 20–24, 1992.
37 Chen C, Cavanaugh JM, Ozaktay C, et al. Effects of phospholipase A2 on lumbar nerve root structure and function. Spine. 1997;22:1057-1064.
38 Kang JD, Georgescu HI, McIntyre-Larkin L, et al. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine. 1996;21(3):271-277.
39 Byrod G, Olmarker K, Konno S, et al. A rapid transport route between the epidural space and the intraneural capillaries of the nerve roots. Spine. 1995;20:138-143.
40 Slipman CW, Chow DW. Therapeutic spinal corticosteroid injections for the management of radiculopathies. Phys Med Rehabil Clin N Am. 2002;13:697-711.
41 Rydevik B, Brown MD, Lundborg G. Pathoanatomy and pathophysiology of nerve root compression. Spine. 1984;9:7-15.
42 Murphy RW. Nerve roots and spinal nerves in degenerative disk disease. Clin Orthop Rel Res. 1977;129:46-60.
43 Goddard MD, Reid JD. Movements induced by straight leg raising in the lumbosacral roots, nerves and plexus, and in the intrapelvic section of the sciatic nerve. J Neurol Neurosurg Psychiatr. 1965;28:12.
44 Howe JF, Loeser JD, Calvin WH. Mechanosensitivity of dorsal root ganglia and chronically injured axons: a physiological basis for the radicular pain of nerve root compression. Pain. 1977;3:25-41.
45 Bradley KE. Stress–strain phenomena in human spinal nerve roots. Brain. 1961;84:120.
46 Bora FW, Pleasure DE, Didizian NA. A study of nerve regeneration and neuroma formation after nerve suture by various techniques. J Hand Surg. 1976;1:138-143.
47 Bush K, Cowan N, Katz DE. The natural history of sciatica with associated disc pathology: a prospective study with clinical and independent radiologic follow-up. Spine. 1992;17:1205-1212.
48 Dreyfuss P, Michaelsen M, Pauza K, et al. The value of medical history and physical examination in diagnosing sacroiliac joint pain. Spine. 1996;21(22):2594-2602.
49 Fortin JD. Sacroiliac joint dysfunction. A new perspective. J Back Musculoskel Rehabil. 1993;3(3):31-43.
50 Vroomen PC, de Krom MC, Knotterus JA. Diagnostic value of history and physical examination in patients suspected of sciatica due to disc herniation: a systematic review. J Neurol. 1999;246:899-906.
51 Nasseri K, Strijers RL, Dekhuijzen LS, et al. Reproducibility of different methods for diagnosing and monitoring diabetic neuropathy. Electromyog Clin Neuro-physiol. 1998;38(5):295-299.
52 Young A, Getty J, Jackson A, et al. Variations in the pattern of muscle innervation by the L5 and S1 nerve roots. Spine. 1983;8(6):616-624.
53 Slipman CW, Palmitier RA. Diagnostic selective nerve root blocks. Crit Rev Phys Med Rehabil Med. 1998;10(2):123-146.
54 Modic MT, Masaryk T, Boumphrey F, et al. Lumbar herniated disk disease and canal stenosis: prospective evaluation by surface coil MR, CT, and myelography. AJNR. 1986;7:710-717.
55 Ross JS, Glicklich M, Buchesneau PM. Imaging of the lumbar spine. In Hardy RW, editor: Lumbar disc disease, 2nd edn., New York, NY: Raven Press, 1993.
56 Williams AL, Haughton VM, Daniels DL, et al. CT recognition of lateral lumbar disc herniation. AJNR. 1982;3:211-213.
57 Haueisen C, Smith B, Myers SR, et al. The diagnostic accuracy of spinal nerve injection studies. Their role in the evaluation of recurrent sciatica. Clin Orthop. 1985;198:179-183.
58 Anand AK, Lee BCP. Pain and metrizamide CT of lumbar disc disease: comparison with myelography. AJNR. 1982;3:567-571.
59 Sotiropoulos S, Chafetz NI, Lang P, et al. Differentiation between postoperative scar and recurrent disc herniation: prospective comparison of MR, CT, and contrast enhanced CT. AJNR. 1989;10:639-643.
60 Ross JS, Masaryk TJ, Schrader M, et al. MR imaging of the postoperative spine: assessment with gadopentate dimeglumine. AJNR. 1990;11:771-776.
61 Saal JA, Saal JS, Herzog RJ. The natural history of lumbar intervertebral disc extrusions treated nonoperatively. Spine. 1990;15(7):683-686.
62 Mcnab I. Negative disc exploration: an analysis of the causes of nerve-root involvement in sixty-eight patients. J Bone Joint Surg. 1971;53:891-903.
63 Tajima T, Furukawa K, Kuramochi E. Selective lumbosacral radiculopathy and block. Spine. 1980;5:68-77.
64 Kikuchi S, Hasue M, Nishiyama K. Anatomic and clinical studies of radicular symptoms. Spine. 1984;9:23-30.
65 Dooley JF, McBroom RJ, Taguchi T, et al. Nerve root infiltration in the diagnosis of radicular pain. Spine. 1988;13:79-83.
66 Herron LD. Selective nerve root blocks in patient selection for lumbar surgery – surgical results. J Spinal Disord. 1989;2:75-79.
67 van Akkerveeken PF. The diagnostic value of nerve sheath infiltration. Acta Orthop Scand. 1993;64:61-63.
68 Stanley D, McLoren MI, Evinton HA, et al. A prospective study of nerve root infiltration in the diagnosis of sciatica. A comparison with radiculopathy, computed tomography and operative findings. Spine. 1990;15:540-543.
69 Lauder TD, Dillingham TR, Andary M, et al. Effect of history and exam in predicting electrodiagnostic outcome among patients with suspected lumbosacral radiculopathy. Am J Phys Med Rehabil. 2000;79(1):60-68.
70 Pease WS, Johnson EW, Charles M. Recruitment interval in L5 radiculopathy: a preliminary report. Arch Phys Med Rehabil. 1984;65:654.
71 Colachis SC, Pease WS, Johnson EW. Polyphasic motor unit action potentials in early radiculopathy: their presence and ephaptic transmission as an hypothesis. Electromyogr Clin Neurophysiol. 1992;32:27-33.
72 Johnson EW, Melvin JL. Value of electromyography in lumbar radiculopathy. Arch Phyl Med Rehabil. 1971;52(6):239-243.
73 Johnson EW, Fletcher FR. Lumbosacral radiculopathy: review of 100 consecutive cases. Arch Phys Med Rehabil. 1981;62(7):321-323.
74 Braddom RI, Johnson EW. Standardization of H-reflex and diagnostic uses in S1 radiculopathy. Arch Phys Med Rehabil. 1974;55:161-166.
75 Strakowski JA, Redd DD, Johnson EW, et al. H-reflex and F-wave latencies to soleus normal values and side-to-side differences. Am J Phys Med Rehabil. 2001;80(7):491-493.
76 Shea PA, Woods WW, Werden DH. Electromyography in diagnosis of nerve root compression syndrome. Arch Neurol Psychiat. 1950;64:93-104.
77 Nardin RA, Patel MR, Gudas TF, et al. Electromyography and magnetic resonance imaging in the evaluation of radiculopathy. Muscle Nerve. 1999;22:151-155.
78 Kellegren JH. On the distribution of pain arising from deep somatic structures with charts of segmental pain. Clin Sci. 1939;3:35-46.
79 Kuslich SD, Ulstrom CL, Michael CL. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Ortho Clin N Am. 1991;22(2):181-187.
80 Schwarzer AC, Aprill CN, Derbry R, et al. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine. 1995;20(17):1878-1883.
81 Kokmeyer DJ, Van der Wurff P, Aufdemkampe G, et al. The reliability of multi-test regimens with sacroiliac pain provocation tests. J Manip Phsiol Ther. 2002;25(1):42-48.
82 Slipman CW, Sterenfeld EB, Chou LH, et al. The value of provocative sacroiliac joint stress maneuvers in the diagnosis of sacroiliac joint syndrome. Arch Phys Med Rehabil. 1998;79:288-292.
83 Slipman CW, Sterenfeld EB, Chou LH, et al. The value of radionuclide imaging in the diagnosis of sacroiliac joint syndrome. Spine. 1996;21(19):2251-2254.
84 Maigne JY, Aivaliklis A, Pfefer F. Results of sacroiliac joint double block and value of sacroiliac pain provocation tests in 54 patients with low back pain. Spine. 1996;21(16):1889-1892.
85 Revel M, Poiraudeau S, Auleley GR, et al. Capacity of the clinical picture to characterize low back pain relieved by facet joint anesthesia: proposed criteria to identify patients with painful facet joints. Spine. 1998;23(18):1972-1977.
86 Slipman CW, Plastaras C, Palmitier RA, et al. Symptom provocation of fluoroscopically guided cervical nerve root stimulation: are dynatomal maps identical to dermatomal maps? Spine. 1998;23(20):2235-2242.
87 Moriishi J, Otani K, Tanaka K, et al. The intersegmental anastomoses between spinal nerve roots. Anat Rec. 1989;224(1):110-116.
88 Neidre A, MacNab I. Anomalies of the lumbosacral nerve roots. Spine. 1983;8:294-299.
89 Bogduk N. Nerves of the lumbar spine. In Bogduk N, editor: Clinical anatomy of the lumbar spine and sacrum, 3rd edn., London: Elsevier Science, 2002.
90 Krempen JD, Smith B. Nerve root injection: a method for evaluating the etiology of sciatica. J Bone Joint Surg. 1974;56A:1435-1444.
91 Schutz H, Lougheed WM, Wortzman G, et al. Intervertebral nerve-root in the investigation of chronic lumbar disc disease. Can J Surg. 1973;16:217-221.
92 Kikuchi S, Hasue M. Combined contrast studies in lumbar spine diseases: myelography (peridurography) and nerve root infiltration. Spine. 1988;13:1327-1331.
93 White A. Injection techniques for the diagnosis and treatment of low back pain. Orthop Clin N Am. 1983;14:553-567.
94 Huston CW, Slipman CW, Garvin C. Complications and side effects of cervical and lumbosacral selective nerve root injections. Arch Phys Med Rehabil. 2005;86(2):277-283.
95 Botwin KP, Gruber RD, Bouchlas CG, et al. Complications of fluoroscopically guided transforaminal lumbar injections. Arch Phys Med Rehabil. 2000;81(8):1045-1050.
96 Schwarzer AC, Aprill CN, Bogduk M. The sacroiliac joint in chronic low back pain. Spine. 1995;20(1):31-37.
97 Durranti A, Winnie AP. Piriformis muscle syndrome: an underdiagnosed cause of sciatica. J Pain Symp Manage. 1991;6:374. 371
98 Solheim LF, Siewers P, Paus B. The piriformis muscle syndrome. Acta Orthop Scand. 1981;52:73-75.
99 Slipman CW, El Abd OH, Brandys EB, et al. The prevalence of referred abdominal and inguinal pain in patients with lumbar internal disruption syndrome. In press.
100 Alo KM, Wright RE, Sutcliffe J, et al. Percutaneous lumbar discectomy: clinical response in an initial cohort of fifty consecutive patients with radicular pain. Pain Pract. 2004;4(1):19-29.
101 Slipman CW, Shin CH, Patel RK, et al. Etiologies of failed back surgery syndrome. Pain Medicine. 2002;3(3):200-207.
102 Maillefert JF, Aho S, Huguenin MC, et al. Systemic effects of epidural dexamethasone injections. Rev Rhum [Engl Edn]. 1995;62(6):429-432.
103 Sharps LS, Isaac Z. Percutaneous disc decompression using nucleoplasty. Pain Phys. 2002;5(2):121-126.
104 Amudsen T, Weber H, Nordal HJ, et al. Lumbar spinal stenosis: conservative or surgical management?: a prospective 10-year study. Spine. 2000;25(11):1424-1436.
105 Botwin KP, Gruber RD, Bouchlas CG, et al. Fluoroscopically guided lumbar transforaminal epidural steroid injection in degenerative lumbar stenosis: an outcome study. Am J Phys Med Rehabil. 2002;81(12):898-905.
106 Lutz GE, Shen T. Fluoroscopically guided aspiration of a symptomatic lumbar zygapophyseal joint cyst: a case report. Arch Phys Med Rehabil. 2002;83(12):1789-1791.
107 Slipman CW, Lipetz JS, Yusuke W, et al. Nonsurgical treatment of zygapophyseal joint cyst-induced radicular pain. Arch Phys Med Rehabil. 2000;81(8):973-977.
108 Lutz GE, Vad VB, Wisneski RJ. Fluoroscopic transforaminal lumbar epidural steroids: an outcome study. Arch Phys Med Rehabil. 1998;79:1362-1366.
109 Riew KD, Yin Y, Gilula L, et al. The effect of nerve-root injections on the need for operative treatment of lumbar radicular pain. J Bone Joint Surg. 2000;82A:1589-1593.