Amniotic Membrane Transplantation
Indications and Techniques
Introduction
Amniotic membrane is the innermost layer of the fetal membranes that are derived from, and contain, the growing embryo during pregnancy. It consists of a single epithelial layer, a thick basement membrane and an avascular stroma. Although, its use in ocular surface surgery was first described in 1940,1 there was little further mention in the literature in subsequent decades until a cryopreserved version of AM became commercially available in 1997 (Amniograft, Bio-Tissue, Miami, USA). The interesting history of the emergence of AM in ophthalmic surgery is chronicled in an extensive review by Dua et al.2 Amniotic membrane is commercially available in North America in two forms, cryopreserved and freeze-dried. The freeze-dried form offers the convenience of not requiring refrigeration for storage, but unfortunately the literature examining its use is minimal. For this reason, the focus of this chapter will be on the use of the various forms of cryopreserved AM.
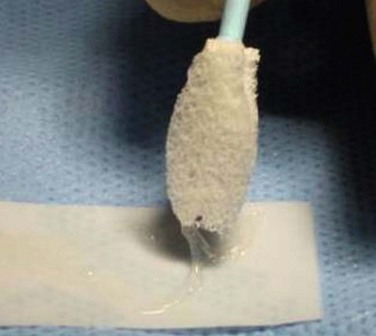
Cryopreserved AM comes as a sheet in a variety of sizes, with the stromal surface attached to a piece of carrier paper. Non-toothed forceps can be used to completely free the AM from its adhesion to the paper. Once fully freed from the underlying paper, it can be easily slid onto the ocular surface. The stromal surface, which was adherent to the carrier paper, is placed against the surface of the area being treated. The stromal surface can always be identified using a cellulose sponge, which will stick to the stroma, but not the epithelial surface (Fig. 37.1). Most recently, a grid pattern has been placed on the side of the paper with the AM so identification of the tissue surface is easy. The stromal surface of the AM is the side adherent to the paper grid.
ProkeraTM (Bio-Tissue, Miami, USA) is a sheet of AM stretched across the lumen of a 16-mm thermoplastic ring set and can be placed on the eye like a contact lens (Fig. 37.2). Insertion is best accomplished by placing the patient supine and inserting the ProkeraTM under the upper eyelid. The inferior edge of the ring is then tucked under the lower eyelid. The device is quite slippery, so great care must be taken during the insertion process. ProkeraTM serves a role similar to a therapeutic contact lens, but also provides added anti-inflammatory and healing benefits in eyes with a disordered ocular surface. The AM portion only covers the cornea and limbus and does not protect the fornices or tarsal conjunctiva from inflammation or epithelial defects. Those areas would need to be treated with a regular sheet of cryopreserved AM.
Both clinically and in vitro, AM has been shown to have significant anti-inflammatory, antiscarring, and antiangiogenic activity.3,4 These effects allow adjacent normal tissue, if available, to repopulate injured areas before inflammation and scar tissue take over the healing process, allowing regeneration rather than repair. The stromal component seems to provide this benefit by a variety of mechanisms.3 The basement membrane/epithelial surface of AM is an effective scaffold that promotes epithelial migration into injured areas. This feature has also allowed its use as a substrate for ex vivo expansion of ocular surface epithelial progenitor cells for grafting when an adequate supply of native normal tissue is not available.5–7
Basic Principles
The use of AM in ocular surface surgery can be divided into two broad categories: a temporary patch (Box 37.1) or a permanent graft (Box 37.2). There is some overlap, but the categories are based on the therapeutic goals of the treatment and the ultimate fate of the membrane. As a temporary patch, amniotic membrane is used like a ‘biological bandage’ to cover and protect injured conjunctival or corneal epithelium. The tissue underneath the membrane is provided an opportunity to heal with decreased inflammation and scarring. The membrane is sloughed off, or removed, once the epithelium underneath it has healed. As a permanent graft, the goal is to get epithelium to grow over top of the AM rather than underneath it. The AM then becomes part of the subepithelial substrate.
Temporary Patch
Amniotic membrane has proven effective in the treatment of both acute chemical burns and acute Stevens–Johnson syndrome (SJS).8–11 In both cases it likely limits the damage caused by neutrophils and lymphocytes recruited to the affected areas and also limits the effects of the ‘cytokine storm’ that can cause so much damage in SJS, or its more severe form toxic epidermal necrolysis (TEN). In severe chemical burns, the chemicals alone may directly cause extensive damage before AM can be applied, which is likely why its effectiveness in these cases has been limited.8
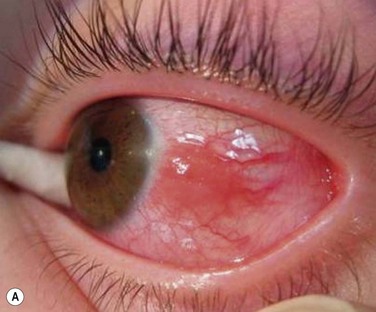
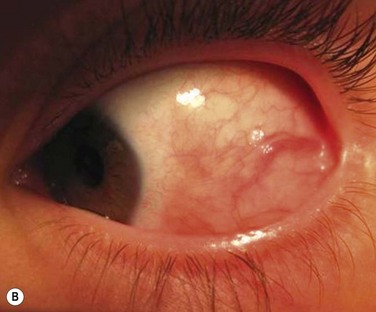
In SJS and TEN, AM has proven very effective if applied within the first 10 days of the illness, with the concept of ‘the earlier the better’ (Fig. 37.3). First described in 2002,9 two subsequent case series have shown good outcomes with the use of AM applied to the lids, palpebral conjunctiva and entire ocular surface.10,11 The method of application is similar in both chemical burns and SJS (Fig. 37.4). The surgical technique is described in detail in a video supplement to this text. Acute SJS cases that need AM treatment are characterized by extensive areas of epithelial sloughing on the lid margins, palpebral conjunctiva or cornea. It is important to inspect the fornices carefully with fluorescein stain. Extensive epithelial sloughing on the lid margins and palpebral conjunctiva puts the patient at high risk for scarring and loss of the normal mucosal epithelium. This can lead to severe dry eye problems and malpositioning of the lid margins and eyelashes. Scarred surfaces on the tarsal conjunctiva have been associated with long-term visual problems due to chronic blink-related microtrauma to the cornea.12 A ProkeraTM can be used for corneal sloughing, but if there is extensive sloughing of the bulbar conjunctiva as well, it is important to cover the entire ocular surface with a sheet of AM, rather than just a ProkeraTM. Although, it is easy to place and can be helpful in cases with limited bulbar conjunctival involvement, a ProkeraTM alone is not adequate treatment in SJS – the palpebral conjunctiva and lid margins must also receive AM grafting. A separate symblepharon ring should be placed over the eye once all the AM has been sutured into place if a ProkeraTM has not been used on the ocular surface.
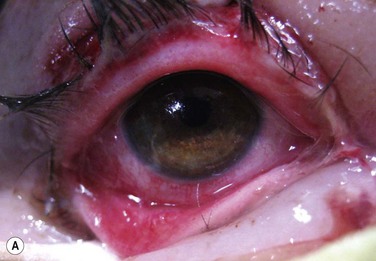
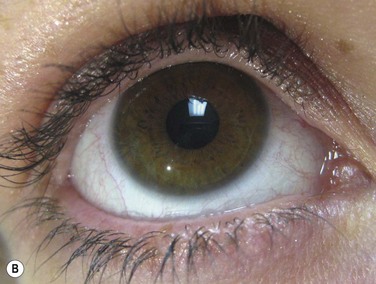
Figure 37.3 (A) Severe inflammation of the conjunctiva and eyelid margins in acute Stevens–Johnson syndrome. Amniotic membrane was applied to the eyes and eyelids 5 days into the illness as described in Figure 37.4 and the video supplement to this text. The 6-month postoperative outcome is shown in (B). Vision was 20/20 in both eyes with minimal dry eye symptoms. (Reproduced from Gregory DG. Treatment of acute Stevens–Johnson syndrome and toxic epidermal necrolysis using amniotic membrane: a review of 10 consecutive cases. Ophthalmology 2011;118:908–14, with permission of Ophthalmology.)
Permanent Graft
Following the excision of conjunctival tumors, AM may be placed over the ensuing epithelial defect. Fibrin glue fixation of AM and the subsequent decreased inflammation result in faster healing and less discomfort, which is particularly helpful in pediatric cases (Fig. 37.5). The edges of the AM should be tucked under any surrounding conjunctiva to help prevent dislodgement and to facilitate epithelial migration over of the graft.
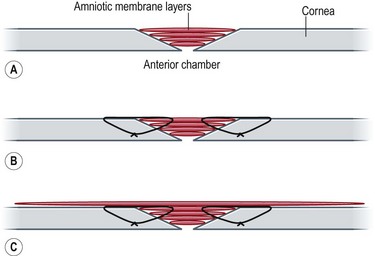
For non-healing corneal epithelial defects with ulceration, multiple layers of AM ‘pancakes’ may be stacked in the defect to fill the hole.13 The pancakes may be secured using sutures or fibrin glue. The area must then be covered with a larger sheet of AM or a ProkeraTM to prevent dislodgement of the stacked AM grafts (Fig. 37.6).
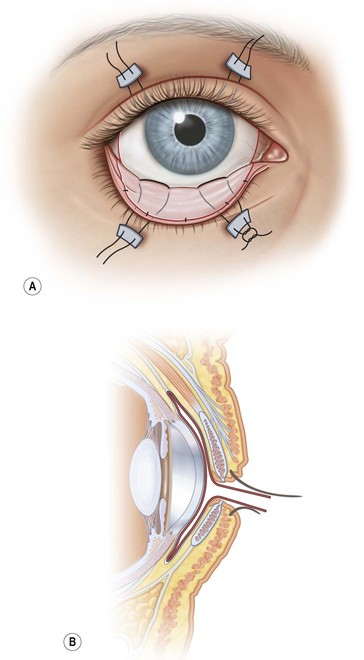
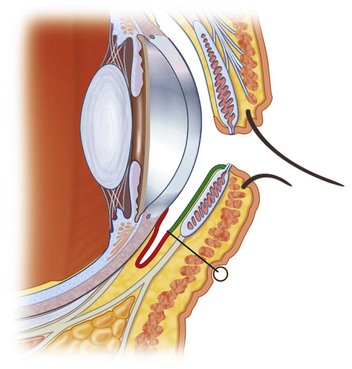
Amniotic membrane transplantation has also proven useful in symblepharon repair and fornix reconstruction. The scarred tissue should first be completely freed from its adhesions to the globe and extraocular muscles. The fibrotic subconjunctival tissue should then be carefully dissected away and removed from the overlying conjunctiva. No conjunctiva is removed. Rather, it is allowed to retract into the fornix and is used to form the palpebral conjunctiva. It can be fixated to the back surface of the lid using double-armed, full-thickness sutures passed through the lid from the conjunctival side and tied on the external surface over a bolster (Fig. 37.7).14 Any exposed extraocular muscles can be wrapped with AM to minimize adhesions and restrictive strabismus. Sponges soaked in a dilute concentration of mitomycin C (0.2 mg/mL) can be applied to the underside of the conjunctiva surrounding the area of bare sclera that was previously covered by scar tissue. Using a dilute concentration for a short duration (30–60 seconds) and avoiding the application of mitomycin C directly to the sclera helps decrease the risk of late complications, such as scleral thinning and scleral necrosis. The bare sclera can then be covered with a conjunctival autograft from the fellow eye, or AM can be used if an autograft is not feasible. If an autograft is used, covering the harvest site with AM can minimize scarring and pain.
The use of AM in place of a conjunctival autograft in pterygium surgery has been somewhat controversial. When used to cover the conjunctival epithelial defect following pterygium excision, autografts have been shown to yield a very low recurrence rate.15 Amniotic membrane grafts have been used as a substitute for the autograft, with the main advantage being decreased operative time. The recurrence rates are significantly higher, however, even if adjunctive mitomycin C is applied to the sclera or conjunctiva intraoperatively.16 Pterygium recurrences can cause significant ocular morbidity and can be challenging to repair. Preventing recurrence should be the top priority when considering the different surgical techniques. Autografts are highly safe and effective, though the surgery is more tedious and time-consuming. The patients are often relatively young adults and the eye is otherwise healthy. With its significant history of late complications, applying mitomycin C in a primary pterygium case seems an unwarranted risk. Fixating the autograft with fibrin glue rather than sutures helps decrease the operative time and improves patient comfort.
References
1. de Rotth, A. Plastic repair of conjunctival defects with fetal membranes. Arch Ophthalmol. 1940;23:522–525.
2. Dua, HS, Gomes, JAP, King, AJ, et al. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49:51–77.
3. Tseng, SCG, Espana, EM, Kawakita, T, et al. How does amniotic membrane work? Ocul Surf. 2004;2:177–187.
4. Liu, J, Sheha, H, Fu, Y, et al. Update on amniotic membrane transplantation. Expert Rev Ophthalmol. 2010;5:645–661.
5. Meller, D, Dabul, V, Tseng, SCG. Expansion of conjunctival epithelial progenitor cells on amniotic membrane. Exp Eye Res. 2002;74:537–545.
6. Koizumi, N, Fullwood, NJ, Bairaktaris, G, et al. Cultivation of corneal epithelial cells on intact and denuded human amniotic membrane. Invest Ophthalmol Vis Sci. 2000;41:2506–2513.
7. Meller, D, Pires, RT, Tseng, SCG. Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane cultures. Br J Ophthalmol. 2002;86:463–471.
8. Meller, D, Pires, RT, Mack, RJ, et al. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology. 2000;107:980–990.
9. John, T, Foulks, GN, John, ME, et al. Amniotic membrane in the surgical management of acute toxic epidermal necrolysis. Ophthalmology. 2002;109:351–360.
10. Shammas, MC, Lai, EC, Sarkar, JS, et al. Management of acute Stevens–Johnson syndrome and toxic epidermal necrolysis utilizing amniotic membrane and topical corticosteroids. Am J Ophthalmol. 2010;149:203–213.
11. Gregory, DG. Treatment of acute Stevens–Johnson syndrome and toxic epidermal necrolysis using amniotic membrane: a review of 10 consecutive cases. Ophthalmology. 2011;118:908–914.
12. Di Pascuale, MA, Espana, EM, Liu, DT, et al. Correlation of corneal complications with eyelid cicatricial pathologies in patients with Stevens–Johnson syndrome and toxic epidermal necrolysis syndrome. Ophthalmology. 2005;112:904–912.
13. Solomon, A, Meller, D, Prabhasawat, P, et al. Amniotic membrane grafts for nontraumatic corneal perforations, descemetoceles, and deep ulcers. Ophthalmology. 2002;109:694–703.
14. Kheirkhah, A, Blanco, G, Casas, V, et al. Surgical strategies for fornix reconstruction based on symblepharon severity. Am J Ophthalmol. 2008;146:266–275.
15. Hirst, LW. Prospective study of primary pterygium surgery using pterygium extended removal followed by extended conjunctival transplantation. Ophthalmology. 2008;115:1663–1672.
16. Luanratanakorn, P, Ratanapakorn, T, Suwan-apichon, O, et al. Randomised controlled study of conjunctival autograft versus amniotic membrane graft in pterygium excision. Br J Ophthalmol. 2006;90:1476–1480.