121 Aminoglycosides
 Mechanism of Action
Mechanism of Action
The mechanisms of bactericidal activity of aminoglycosides are not completely understood. In gram-negative bacteria, binding to and subsequent alteration of the cell envelope in addition to interaction with ribosomes, that causes inhibition of protein synthesis, may contribute to their bactericidal activity. Aminoglycosides are cations that bind passively to negatively charged portions of the outer membranes of gram-negative bacilli and competitively displace cell wall Mg2+ and Ca2+ that link lipopolysaccharide molecules.1,2 The result is a rearrangement of the cell envelope and subsequent formation of transient holes in the cell wall, which interrupts normal permeability function of the bacteria.3,4 In gram-positive bacteria, aminoglycoside uptake is decreased because of thicker outer cell wall membranes, and thus higher minimum inhibitory concentrations (MIC) are reported with these organisms.
Aminoglycosides are transported slowly across the cytoplasmic membrane via an energy-dependent process; this is the rate-limiting step in the drug action.5,6 The transmembrane electrical potential correlates to the uptake and antibacterial effect. This energy-dependent transport mechanism is impaired in an anaerobic environment, conditions of low pH, and high osmolality. Thus in certain clinical settings such as in infections involving abscesses, aminoglycoside transport is reduced and may not be as effective.
Once across the cell membrane, aminoglycosides are trapped inside bacteria, leading to high intracellular concentration of the drug. Subsequently, aminoglycosides bind to the 16S rRNA of 30S subunits of ribosomes.7 This aminoglycoside-ribosome interaction causes termination and miscoding of protein synthesis, with subsequent bacterial cell death.
 Spectrum of Activity
Spectrum of Activity
Aminoglycosides have a broad spectrum of activity against microorganisms, including gram-negative and gram-positive bacteria, mycobacteria, and protozoa. Among aerobic and facultative gram-negative bacilli, most aminoglycosides are active against Enterobacteriaceae (Escherichia coli, Proteus mirabilis, Klebsiella spp., Morganella spp., Citrobacter spp., Serratia spp., and Enterobacter spp.), Pseudomonas spp., and Acinetobacter spp. Resistance of clinical isolates to aminoglycosides varies with organism, patient population and their comorbidities, and local or regional usage patterns. According to the analyses of the Surveillance Network Database from 1998 to 2001, the susceptibility rates to aminoglycosides was higher against Enterobacteriaceae than nonfermentative organisms such as Pseudomonas and Acinetobacter spp.8 In addition, susceptibility to amikacin was higher than to gentamicin against species of Enterobacteriaceae, Pseudomonas, and Acinetobacter.9 Among ICU patients, the susceptibility of Enterobacteriaceae to gentamicin was 91.8% and amikacin was 98.5%.9 The susceptibility of Pseudomonas aeruginosa was 78.5% and 93.9% for gentamicin and amikacin, respectively. Against Acinetobacter baumannii, the susceptibility was 58.2% and 82.7%, respectively.8 For Enterobacteriaceae and P. aeruginosa, both gentamicin and amikacin susceptibility rates were similar among ICU and non-ICU patients or slightly better in non-ICU patients. However, among A. baumannii isolates, the susceptibility rates among non-ICU patients were lower than those reported for ICU patients (43.6% versus 58.2% for gentamicin and 77.2% versus 82.8% for amikacin).8
Since 2001, analyses of other databases indicate consistent but marginal decreases in susceptibility to aminoglycosides in comparison to other antibacterials in the United States. In 2008, a U.S. surveillance study encompassing 15 medical centers reported tobramycin susceptibility rates of 88.4% among Enterobacteriaceae, 89.1% among P. aeruginosa, and 59.1% in Acinetobacter spp.10 In 2007, a surveillance study of isolates from mostly North American sites but also including information from Europe, Asia, Latin America, Africa, and the Middle East reported susceptibility to amikacin of better than 95% among Enterobacteriaceae isolates, 92.3% among P. aeruginosa, and 69.6% among A. baumannii isolates.11 In comparison to the United States, the susceptibility to aminoglycosides against P. aeruginosa in Europe and Latin was lower. A surveillance study conducted in 2007 showed tobramycin susceptibility rates among P. aeruginosa isolates of 92% in North America, 77% in the European Union, and 63.8% in Latin America.12 A European surveillance study reported tobramycin susceptibility of 74.2% among P. aeruginosa isolates in 2007,13 and the International Nosocomial Infection Control Consortium (INICC) surveillance study from 2003 to 2008 reported an amikacin resistance rate of 31% among P. aeruginosa isolates collected from ICU patients from 25 countries in Latin America, Asia, Africa, and Europe.14
Aminoglycosides are active against methicillin-susceptible Staphylococcus aureus. For other gram-positive pathogens such as methicillin-resistant S. aureus (MRSA), Streptococcus spp., and Enterococcus spp., aminoglycosides are used in a limited fashion to provide synergistic activity with β-lactam antibiotics. In Enterococcus spp., the synergism is only observed in organisms that display low-level gentamicin resistance (4-250 µg/mL).15 A poor active transport of drug due to anaerobic metabolism and the thick cell wall is thought to be responsible for this low-level resistance. Synergism is achieved in these organisms because gentamicin uptake is enhanced when combined with β-lactam antibiotics. Enterococci may acquire one or more of the following resistance mechanisms to demonstrate high-level resistance: alteration of the target site, interference with drug permeability, or enzyme inactivation of drug.16 In organisms with high-level resistance, synergistic activity of gentamicin is not observed. In high-level gentamicin resistance, it may be worthwhile to test for high-level streptomycin resistance. High-level gentamicin and streptomycin resistance is considered with MIC ≥ 500 µg/mL and MIC ≥ 2000 µg/mL, respectively.
Rates of high-level gentamicin resistance in Enterococcus spp. varies markedly among institutions, but the nationwide prevalence is estimated at 30% to 60%.15 High-level resistance is low in Enterococcus faecalis, which is responsible for approximately 60% of nosocomial enterococcal bloodstream infections. However, this type of resistance is observed in greater than 50% of Enterococcus faecium, which causes approximately 20% of nosocomial enterococcal bloodstream infections.
Aminoglycosides have activity against less common ICU pathogens. Streptomycin has greatest activity against Mycobacterium tuberculosis and Yersinia pestis.17,18 Both streptomycin and gentamicin have been reported to be effective in Francisella tularensis infection.19 Amikacin has the best activity among aminoglycosides against Mycobacterium avium-intracellulare. Spectinomycin is useful in treating Neisseria gonorrhoeae infection.20 Paromomycin has been used against intestinal parasites.21
 Mechanisms of Resistance
Mechanisms of Resistance
Bacterial resistance to aminoglycosides is achieved through multiple mechanisms. These include modification of the ribosomal target, enzymatic modification, decreased antibiotic uptake, and efflux of antibiotics. Mutations at the ribosomal (16S rRNA) binding sites results in resistance to aminoglycosides. This mechanism has not been detected in most clinical isolates, except for Mycobacterium tuberculosis against streptomycin.22
The most common mechanism of resistance for aminoglycosides is inactivation by aminoglycoside-modifying enzymes. The exposed hydroxyl and amino groups of aminoglycosides are subject to structural modification and loss of antimicrobial activity by enzymes from both gram-positive and gram-negative bacteria.23 There are three types of enzymes which transfer a functional group to the aminoglycoside structure: (1) aminoglycoside nucleotidyltransferases (ANT) that transfer nucleotide triphosphates; (2) aminoglycoside acetyltransferases (AAC) that transfer the acetyl group from acetyl-CoA; and (3) aminoglycoside phosphotransferases (APH) that transfer the phosphoryl group from ATP.23,24 Once the structure of aminoglycosides has been modified, they bind poorly to ribosomes, and this then results in high-level resistance. Genes encoding aminoglycoside-modifying enzymes are usually found on extrachromosomal bacterial plasmids and transposons within the periplasmic space. Thus, they can be easily transferred from bacteria to bacteria.25 Amikacin is the aminoglycoside most stable to these enzymatic effects because it has fewer sites for enzymatic attack.
Resistance can also be developed by preventing penetration of the drug through the outer bacterial cell membrane or by preventing active transport through the cytoplasmic membrane.26 Chromosomal mutations that alter transmembrane electrical potential may down-regulate aminoglycoside uptake into the bacterial cell after the first aminoglycoside exposure. This temporary disruption of the energy-dependent phase of aminoglycoside uptake is called adaptive resistance and lasts for several hours. Extended-interval aminoglycoside dosing may allow this effect to reverse, owing to the higher peak serum concentration (Cmax)/MIC ratios achieved. Aminoglycoside exposure may also select for subpopulations of bacteria with active efflux pumps resulting in low-level resistance. The efflux pump, MexXY, in P. aeruginosa is involved in resistance to many antibacterials including aminoglycosides.27
 Pharmacokinetics
Pharmacokinetics
All the aminoglycosides have similar pharmacokinetic properties. The distribution from the vascular to the extravascular space occurs rapidly within 15 to 30 minutes post infusion.28 Aminoglycosides are primarily excreted by glomerular filtration.29 Thus, dosage adjustments are based on creatinine clearance (CrCl). In patients with normal renal function, the half-lives of all aminoglycosides range from 1.5 to 3.5 hours. The half-life is shortened in febrile illnesses and prolonged in any condition that decreases renal function. More than 90% of a parenterally administered dose is recovered in urine unchanged during the first 24 hours. The remainder is slowly recycled into the tubular lumen, where accumulation of the drug causes nephrotoxicity.30
Aminoglycoside concentrations are generally low in infected secretions and tissues such as respiratory secretions, pleural fluid, cerebrospinal fluid, and aqueous humor. High drug concentrations are found in the proximal tubular cells of the renal cortex, which is thought to correlate with the nephrotoxic potential of aminoglycosides.30
 Pharmacodynamics
Pharmacodynamics
Pharmacodynamic principles associated with aminoglycosides include concentration-dependent bactericidal activity, post-antibiotic effect (PAE), and synergism with other cell wall–active agents.31 Aminoglycosides are rapidly bactericidal, and their rate and extent of bacterial killing increases as the antibiotic concentration is increased. Exposure of bacteria to a single 24-hour aminoglycoside dose with the associated high peak drug concentration results in faster and a greater extent of bactericidal activity than that noted for the same total dose administered in divided doses.32 In 236 patients with gram-negative infections, attainment of a Cmax/MIC ratio of 10 and 12 exhibited a response rate of 80% or higher.33 Another study of 78 patients with gram-negative nosocomial pneumonia suggested that achieving Cmax/MIC ratio ≥ 10 within the first 48 hours of therapy had a 90% probability of temperature and leukocyte count resolution by day 7.34
PAE is a persistent suppression of bacterial growth after short antimicrobial exposure.35 The higher the peak aminoglycoside concentration, the longer the PAE. In vitro, the aminoglycosides consistently demonstrate a PAE that varies from 1 to 3 hours for P. aeruginosa and 0.9 to 2.0 hours for Enterobacteriaceae. A PAE is also demonstrated for S. aureus.36
Synergy is frequently reported in vitro with a combination of an aminoglycoside and a cell wall–active antimicrobial (e.g., penicillin, cephalosporin, carbapenem, monobactam, glycopeptide).37 Synergy is noted when significantly greater effect with two drugs is observed compared to that anticipated based on the effect of each individual drug. Enhanced aminoglycoside uptake in the presence of a cell wall–active drug has been demonstrated with Streptococcus spp., Enterococcus spp., S. aureus, and P. aeruginosa. Two meta-analyses have evaluated synergism in vivo by comparing the efficacy of monotherapy with a β-lactam antibiotics and combination therapy with a β-lactam antibiotic plus an aminoglycoside.38,39 All-cause mortality was comparable in both groups in sepsis and in suspected ventilator-associated pneumonia. This lack of synergism observed in patients may be due to the poor quality of pooled studies, such as lack of blinding, analysis not based on intention to treat, and unspecified follow-up period. The studies included in these meta-analyses also reported high susceptibility to both β-lactam antibiotics and aminoglycosides (≥90% susceptibility) among gram-negative pathogens and included very few patients with multidrug resistant pathogens such as P. aeruginosa and A. baumannii. Therefore, it is yet to be determined if the synergism observed in vitro between a β-lactam antibiotic and an aminoglycoside translates into survival advantage in patients. However, resistance among gram-negative pathogens is increasing, especially among P. aeruginosa, and combination therapy of β-lactam antibiotics and aminoglycosides offers broader coverage in ICU patients than combination with fluoroquinolones.40 In the future, a high percentage of adequate empirical therapy with aminoglycosides may translate into improved survival in patients infected with multidrug-resistant pathogens.
 Adverse Events
Adverse Events
The most common adverse event with aminoglycosides is nephrotoxicity. The reported incidence of this complication ranges from 5% to 25%.41,42 The variability results from differences in the definition of nephrotoxicity, the tests used to measure renal function, and the clinical setting in which the drugs were administered. In general, a decrease in the glomerular filtration rate is small, with most patients experiencing a nonoliguric decline in CrCl. Recovery occurs upon discontinuation of the drug, and progression to dialysis-dependent oliguric or anuric renal failure is rare.
Risk factors for aminoglycoside toxicity include older age, preexisting renal disease, diabetes, frequent dosing interval, treatment lasting longer than 4 days, and concurrent nephrotoxic drugs (vancomycin, amphotericin B, furosemide, clindamycin, piperacillin, cephalosporins, methoxyflurane, foscarnet, and intravenous [IV] radiocontrast agents).42,43 In addition, ICU patients are at increased risk due to hypotension or contracted intravascular volume from volume depletion or diuretic therapy.43 Minimizing use in patients with risk factors for nephrotoxicity and using extended-interval dosing of aminoglycosides are recommended to reduce toxicity.
Aminoglycosides may cause cochlear and vestibular damage.41 Ototoxicity may be a result of irreversible damage to the sensory hair cells of the organ of Corti and reduction of cochlear ganglion cells due to accumulation of drug. Streptomycin and gentamicin are thought to be primarily vestibulotoxic, whereas amikacin, neomycin, and kanamycin are primarily cochleotoxic.44 The incidence of cochlear toxicity is estimated to be 3% to 14%. Toxicity may manifest unilaterally or bilaterally. The true incidence of vestibular toxicity in patients is very difficult to determine because symptoms are masked by compensatory mechanisms (visual and proprioceptive clues) over time. Clinical manifestations include dizziness, ataxia, and/or nystagmus.
The risk factors for ototoxicity include inherited susceptibility, age of the patient, drug dosage, renal function, and additive effects of other ototoxic agents (loop diuretics).42 When aminoglycoside therapy is indicated, the risk of ototoxicity can be minimized by shortening the duration of therapy as clinically appropriate and by periodic assessments of renal function to avoid accumulation of drug. High-frequency audiometric testing may aid in early diagnosis and prevention of progressive damage in patients receiving more than 4 days of therapy.
The most life-threatening adverse reaction to aminoglycosides, although very rare, is neuromuscular blockade.45 Blockade results from inhibition of the presynaptic release of acetylcholine and blockage of postsynaptic receptor sites of acetylcholine. The resulting clinical manifestations include muscle weakness, respiratory depression with apnea, flaccid paralysis, and dilated pupils. Suppression of deep tendon reflexes may be variable. Risk factors include a diagnosis of myasthenia gravis, hypomagnesemia, severe hypocalcemia, and concomitant administration of a neuromuscular blocking agent. A rapid rise in serum drug concentration due to short duration of IV administration may also be a risk factor. Aminoglycosides are usually administered IV over 15 to 30 minutes, but the dosing may extend to 30 to 60 minutes for large doses to reduce the risk of neuromuscular blockade.
 Drug Interaction
Drug Interaction
Aminoglycosides interact chemically with β-lactam antibiotics such as the antipseudomonal penicillins (e.g., carbenicillin, ticarcillin, piperacillin, mezlocillin, and azlocillin).46,47 This interaction results in a nucleophilic opening of the β-lactam ring, with acylation of an amino group of the aminoglycoside and mutual loss of antibacterial activity. When patients with renal failure were concomitantly administered an aminoglycoside and an antipseudomonal penicillin, the serum aminoglycoside concentration was reduced by 10% to 20%. Thus, the administration of these drugs should be separated by at least 1 hour.
 Extended-Interval Dosing Versus Multiple Daily Dosing
Extended-Interval Dosing Versus Multiple Daily Dosing
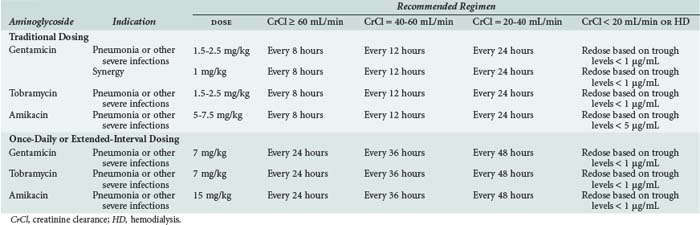
The aminoglycosides are licensed to be administered multiple times per day based on a patient’s renal function. With normal renal function (CrCl ≥ 80 mL/min), empirical maintenance doses for gentamicin and tobramycin range from 1.2 to 1.5 mg/kg every 8 hours, and for amikacin, 7.5 mg/kg every 8 to 12 hours, in patients with gram-negative infections.48 Dose reduction and/or dosing interval prolongation may be necessary in those with renal dysfunction and in patients with advanced age (Table 121-1). Higher dosages or shorter intervals may be required in neonates, in burn patients with serious pseudomonal infections, or in patients with cystic fibrosis. In patients with gram-positive infections such as infective endocarditis, a synergistic effect is achieved with gentamicin 1 mg/kg IV every 8 hours. This dosage in normal renal function will achieve a peak concentration of 3 µg/mL and a trough concentration of less than 0.5 µg/mL. Dosage adjustment may be necessary in those with renal dysfunction.
A dosing strategy frequently referred to as extended-interval aminoglycoside dosing (EIAD) has been used widely in non-ICU patients since its introduction in 1980s. EIAD employs a large bolus dose over an extended period to achieve high serum concentrations to produce rapid bactericidal effect and undetectable trough concentrations at the end of the dosing interval to reduce accumulation of drugs, limiting nephrotoxicity.48 Based on improved patient outcome and decreased selection of resistant organisms, targets of EIAD are a peak concentration of 20 mg/L or a Cmax/MIC of 10. Since reduced risk for nephrotoxicity and ototoxicity has been observed in patients receiving EIAD with at least 4 hours of drug-free period, concentrations of less than 0.5 mg/L for 4 hours at the end of the dosing interval are also recommended. During this time, the regimen relies on PAE to provide therapeutic effect.
Numerous clinical studies of EIAD have been evaluated in patients with bacteremia, intraabdominal infections, urinary tract infections, pelvic infections, cystic fibrosis, and febrile neutropenia.49–51 Unfortunately, none of these studies specifically examined efficacy and safety in critically ill patients, thereby limiting its application in the ICU. Because of the altered pharmacokinetics of aminoglycosides in critically ill patients, previous studies have reported low probability of achieving pharmacodynamic targets in such patients.52 The mean volume of distribution in critically ill patients ranges from 0.3 to 0.4 L/kg. However, in surgical and trauma patients in the ICU, a volume of distribution of up to 0.8 L/kg has been reported.53,54 Since the Cmax/MIC ratio is directly affected by a large volume of distribution, the ratio in critically ill patients is expected to be less than optimal. In addition, several studies reported that critically ill patients had drug-free intervals ranging from 6 to 9 hours at the end of the dosing interval.55 Since such drug-free intervals exceed the PAE observed for most organisms in vitro, this dosing strategy may not effectively inhibit regrowth of surviving organisms. Finally, a poor correlation between estimated CrCl and aminoglycosides has been documented in ICU patients.56 This variability in drug clearance may be secondary to unstable renal function, malnutrition, hemodynamic instability, and use of drugs such as vasopressors, diuretics, and other nephrotoxic drugs.
 Serum Concentration Monitoring
Serum Concentration Monitoring
For EIAD, there are two different methods of determining the optimal regimen. The first and the more well known method uses the Hartford Nomogram.57 According to this method, serum concentrations are drawn between 6 and 14 hours after the first dose and applied to a nomogram to determine the recommended fixed-dose and dosage interval. Although the use of a nomogram is simpler and less expensive because of the reduced number of serum concentrations evaluated, available studies in critically ill patients indicate that the use of this nomogram did not reliably predict targeted Cmax/MIC ratio and allowed excessively long drug-free periods at the end of the dosing interval. Instead, in critically ill patients, monitoring of two serum drug concentrations to derive a dosing regimen is recommended. Obtaining a peak concentration at 1 hour after a 1-hour infusion (2 hours after the start of infusion) and another serum concentration between 8 and 18 hours after the end of the infusion will allow adequate determination of a patient-specific regimen. This will allow assessment of peak serum concentration for the targeted Cmax/MIC ratio and the length of the drug-free interval. Thereafter, in the absence of worsening renal function, periodic trough concentrations should be monitored to ensure adequacy of renal clearance of drug. A high trough concentration is a reflection of impaired renal clearance of drug and indicates the need to adjust the dosage regimen. The targeted peak concentration should be 20 mg/L or a Cmax/MIC of 10, and trough concentration should be undetectable (<0.5 mg/L) for approximately 4 hours at the end of the dosing interval.
Key Points
Rea RS, Capitano B, Bies R, et al. Suboptimal aminoglycoside dosing in critically ill patients. Ther Drug Monit. 2008;30:674-681.
Paul M, Sibiger I, Grozinsky S, et al. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev 2006;1:CD003344.
Aarts MW, Hancock JN, Heyland D, et al. Empiric antibiotic therapy for suspected ventilator-associated pneumonia: A systematic review and meta-analysis of randomized trials. Crit Care Med. 2008;36:108-117.
Cosgrove SE, Vigliani GA, Campion M, et al. Initial low-dose gentamicin for Staphylococcus aureus bacteria and endocarditis is nephrotoxic. Clin Infect Dis. 2009;48:713-721.
Oliveira JFP, Silva CA, Barbieri CD, et al. Prevalence and risk factors for aminoglycoside nephrotoxicity in intensive care units. Antimicrob Agents Chemother. 2009;53:2887-2891.
Buchholtz K, Larsen CT, Hassager C, Bruun NE. Severity of gentamicin’s nephrotoxic effect on patients with infective endocarditis: a prospective observational cohort study of 373 patients. Clin Infect Dis. 2009;48:65-71.
1 Taber HW, Mueller JP, Arrow AS. Bacterial uptake of aminoglycoside antibiotics. Microbiol Rev. 1987;51:439-457.
2 Bryan LE, Kawan S. Roles of ribosomal binding, membrane potential, and electron transport in bacterial uptake of streptomycin and gentamicin. Antimicrob Agents Chemother. 1983;23:835-845.
3 Hancock REW. Alterations in outer membrane permeability. Ann Rev Microbiol. 1984;38:237-264.
4 Martin NL, Beveridge TJ. Gentamicin interaction with Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1986;29:1079-1087.
5 Hancock REW. Aminoglycoside uptake and mode of action with special reference to streptomycin and gentamicin I. Antagonists and mutants. J Antimicrob Chemother. 1981;8:249-276.
6 Hancock REW. Aminoglycoside uptake and mode of action with special reference to streptomycin and gentamicin II. Effects of aminoglycosides on cells. J Antimicrob Chemother. 1981;8:429-445.
7 Purohit P, Stern S. Interactions of a small RNA with antibiotic and RNA ligands of the 30S subunit. Nature. 1994;370:659-662.
8 Karlowsky JA, Draghi DC, Jones ME, et al. Surveillance for antimicrobial susceptibility among clinical isolates of Pseudomonas aeruginosa and Acinetobacter baumannii from hospitalized patients in the United States, 1998 to 2001. Antimicrob Agents Chemother. 2003;47(5):1681-1688.
9 Karlowsky JA, Jones ME, Thornsberry C, et al. Trends in antimicrobial susceptibilities among Enterobacteriaceae isolated from hospitalized patients in the United States from 1998 to 2001. Antimicrob Agents Chemother. 2003;47(5):1672-1680.
10 Rhomberg PR, Jones RN. Summary trends for the meropenem yearly susceptibility test information collection program: a 10-year experience in the United States (1999-2008). Diagn Microbiol Infect Dis. 2009;65:414-426.
11 Garrison MW, Mutters R, Dowzicky MJ. In vitro activity of tigecycline and comparator agents against a global collection of gram-negative and gram-positive organisms: Tigecycline evaluation and surveillance trial 2004 to 2007. Diagn Microbiol Infect Dis. 2009;65:288-299.
12 Jones RN, Stilwell MG, Rhomberg PR, Sader HS. Antipseudomonal activity of piperacillin/tazobactam: more than a decade of experience from the SENTRY antimicrobial surveillance program (1997-2007). Diagn Microbiol Infect Dis. 2009;65:331-334.
13 Turner PJ. MYSTIC Europe 2007: activity of meropenem and other broad-spectrum agents against nosocomial isolates. Diagn Microbiol Infect Dis. 2009;63:217-222.
14 Rosenthal VD, Maki DG, Jamulitrat S, et al. International nosocomial infection control consortium (INICC) report, data summary for 2003-2008, issued June 2009. Am J Infect Control. 2010;38:95-106.
15 Shaked H, Carmeli Y, Schwartz D, Siegman-Igra Y. Enterococcal bacteraemia: epidemiological, microbiological, clinical and prognostic characteristics and the impact of high level gentamicin resistance. Scan J Infect Dis. 2006;38:995-1000.
16 Eliopoulos GM. Aminoglycoside resistant enterococcal endocarditis. Infect Dis Clin North Am. 1993;7:117-133.
17 Avendano M, Goldstein RS. Multidrug-resistant tuberculosis: long term follow-up of 40 non-HIV-infected patients. Can Respir J. 2000;7(5):383-389.
18 Florman AL, Spencer RR, Sheward S. Multiple lung cavities in a 12-year-old girl with bubonic plague, sepsis, and secondary pneumonia. Am J Med. 1986;80(6):1191-1193.
19 Gill V, Cunha BA. Tularemia pneumonia. Semin Respir Infect. 1997;12(1):61-67.
20 Kojima M, Masuda K, Yada Y, et al. Single-dose treatment of male patients with gonococcal urethritis using 2 g spectinomycin: microbiological and clinical evaluations. Int J Antimicrob Agents. 2008;32(1):50-54.
21 Escobedo AA, Almirall P, Alfonso M, et al. Treatment of intestinal protozoan infections in children. Arch Dis Child. 2009;94(6):478-482.
22 Prammananan T, Sander P, Brown BA, et al. A single 16S ribosomal RNA substitution is responsible for resistance to amikacin and other 2-deoxystreptamine aminoglycoside in Mycobacterium abscessus and Mycobacterium chelonae. J Infect Dis. 1998;177:1573-1581.
23 Azucena E, Mabashery S. Aminoglycoside-modifying enzymes: mechanisms of catalytic processes and inhibition. Drug Resist Updates. 2001;4:106-117.
24 Ferrett JJ, Gilmore KS, Bourvallin P. Nucleotide sequence analysis of the gene specifying the bifunctional 6′ aminoglycoside acetyltransferase 2′-aminoglycoside phosphotransferase enzyme in Streptococcus faecalis and identification and cloning of gene regions specifying the two activities. J Bacteriol. 1986;167:631-638.
25 Brisson-Noel A, Athur M, Courvalin P. Evidence for national gene transfer from gram-positive cocci to Escherichia coli. J Bacteriol. 1998;170:1739-1745.
26 Rusthoven JJ, Davis TA, Lerner SA. Clinical isolation and characterization of aminoglycoside-resistance small colony variants of Enterobacter aerogenes. Am J Med. 1979;67:702-706.
27 Hocquet D, Vogue C, El Garch F, et al. MexXY-OprM efflux pump is necessary for adaptive resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob Agents Chemother. 2003;47:1371-1375.
28 Regamey C, Gordon RC, Kirby WM. Comparative pharmacokinetics of tobramycin and gentamicin. Clin Pharmacol Ther. 1973;14(3):396-403.
29 Zarowitz BJ, Robert S, Peterson EL. Prediction of glomerular filtration rate using aminoglycoside clearance in critically ill medical patients. Ann Pharmacother. 1992;26(10):1205-1210.
30 Leroy A, Humbert G, Oksenhendler G, Fillastre JP. Pharmacokinetics of aminoglycosides in subjects with normal and impaired renal function. Antibiot Chemother. 1978;25:163-180.
31 Craig WA. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1-10.
32 Kapusnik JE, Hackbath CH, Chambers HF, et al. Single, large daily dosing vs. intermittent dosing of tobramycin for treating experimental Pseudomonas aeruginosa pneumonia. J Infect Dis. 1988;158:7-12.
33 Moore RD, Lietman PS, Smith CR. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987;155(1):93-99.
34 Kashuba AD, Nafziger AN, Drusano GL, Bertino JSJr. Optimizing aminoglycoside therapy for nosocomial pneumonia caused by gram-negative bacteria. Antimicrob Agents Chemother. 1999;43(3):623-629.
35 Fantin B, Ebert S, Leggett J, et al. Factors influencing the duration of in vivo postantibiotic effect of aminoglycosides against gram-negative bacilli. J Antimicrob Chemother. 1990;27:829-836.
36 Isaksson B, Maller R, Nilsson LE, et al. Postantibiotic effect of aminoglycosides on staphylococci. J Antimicrob Chemother. 1993;32(2):215-222.
37 Obritsch MD, Fish DN, MacLaren R, Jung R. Nosocomial infections due to multi-drug resistant Pseudomonas aeruginosa: epidemiology and treatment options. Pharmacotherapy. 2005;25(10):1353-1364.
38 Paul M, Sibiger I, Grozinsky S, et al. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev 2006;1:CD003344.
39 Aarts MW, Hancock JN, Heyland D, et al. Empiric antibiotic therapy for suspected ventilator-associated pneumonia: A systematic review and meta-analysis of randomized trials. Crit Care Med. 2008;36:108-117.
40 Micek ST, Welch EC, Khan J, et al. Empiric combination antibiotic therapy is associated with improved outcome in gram-negative sepsis: A retrospective analysis. Antimicrob Agents Chemother. 2010; Feb 16. [Epub ahead of print]
41 Smith CR, Lipsky JJ, Laskin OL, et al. Double-blind comparison of the nephrotoxicity and auditory toxicity of gentamicin and tobramycin. N Engl J Med. 1980;302:1106-1109.
42 Cosgrove SE, Vigliani GA, Campion M, et al. Initial low-dose gentamicin for Staphylococcus aureus bacteria and endocarditis is nephrotoxic. Clin Infect Dis. 2009;48:713-721.
43 Oliveira JFP, Silva CA, Barbieri CD, et al. Prevalence and risk factors for aminoglycoside nephrotoxicity in intensive care units. Antimicrob Agents Chemother. 2009;53(7):2887-2891.
44 Selimoglu E, Kalkandelen S, Erdogan F. Comparative vestibulotoxicity of different aminoglycosides in the Guinea pigs. Yonsei Med J. 2003;44:517-522.
45 Snavely SR, Hodges GR. The neurotoxicity of antibacterial agents. Ann Intern Med. 1984;101:92-104.
46 Kradjan WA, Burger R. In vivo inactivation of gentamicin by carbenicillin and ticarcillin. Arch Intern Med. 1980;140(12):1668-1670.
47 Holt HA, Broughall JM, McCarthy M. Interactions between aminoglycoside antibiotics and carbenicillin or ticarcillin. Infection. 1976;4(2):107-109.
48 Rea RS, Capitano B. Optimizing use of aminoglycosides in the critically ill. Semin Respir Crit Care Med. 2007;39(6):596-603.
49 Hatala R, Dinh TT, Cook DJ. Single daily dosing of aminoglycosides in immunocompromised adults: a systematic review. Clin Infect Dis. 1997;24:796-809.
50 Ali MX, Goetz. A meta-analysis of the relative efficacy and toxicity of single daily dosing versus multiple daily dosing of aminoglycosides. Clin Infect Dis. 1997;24:796-809.
51 Barza M, Ioannidis JPA, Cappelleri JC, et al. Single or multiple daily doses of aminoglycosides: a meta-analysis. BMJ. 1996;312:338-345.
52 Rea RS, Capitano B, Bies R, et al. Suboptimal aminoglycoside dosing in critically ill patients. Ther Drug Monit. 2008;30(6):674-681.
53 Barletta JF, Johnson SB, Nix DE. Population pharmacokinetics of aminoglycosides in critically ill trauma patients on once-daily regimens. J Trauma. 2000;49(5):869-872.
54 Niemiec PWJr, Allo MD, Miller CF. Effect of altered volume of distribution on aminoglycoside levels in patients in surgical intensive care. Arch Surg. 1987;122:207-212.
55 Hassan E, Ober JD. Predicted measured aminoglycoside pharmacokinetic parameters in critically ill patients. Antimicrob Agents Chemother. 1987;31:1855-1858.
56 Beckhouse MJ, Whyte IM, Byth PL, et al. Altered aminoglycoside clearance in the critically ill. Anaesth Intensive Care. 1988;16:418-422.
57 Freeman CD, Nicolau DP, Belliveau PP, et al. Once-daily dosing of aminoglycosides: Review and recommendations for clinical practice. J Antimicrob Chemother. 1997;39:677-686.