Chapter 11 Amino acids and proteins
Amino Acids
Essential and Non-Essential Amino Acids
As with essential fatty acids (see Chapter 10 ‘Lipids’, p. 78) not all amino acids can be synthesized by the body; some have to be taken in with food. They are generally considered essential in children as their metabolism is not mature enough to synthesize them.
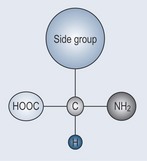
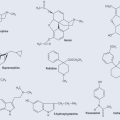
Of the 20 standard amino acids that are encoded by DNA (there are considerably more amino acids than this but they are outside the scope of this book), 10 cannot be manufactured by the human body (essential amino acids); the remaining 10 can be synthesized and are therefore known as the non-essential amino acids. Plants contain all 20 amino acids. Their general structure is seen in Figure 11.2.
Proteins
Proteins serve several functions:
Protein Structure
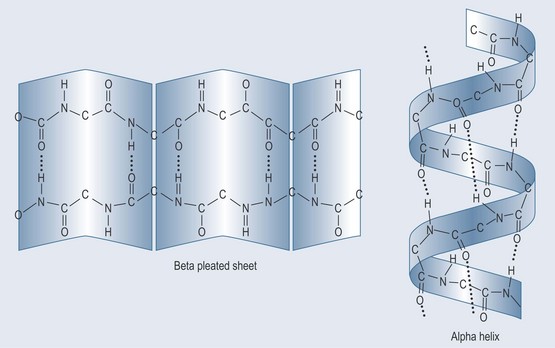
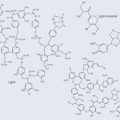
• Primary (1°) Protein Structure (Figure 11.3)
Polypeptide Cross-Linking
The amino acid cysteine (cys) contains a sulphur group, which can form a cross-link with another sulphur, forming a disulphide bond. This cross-link strengthens the integrity of the polypeptide bond and lends itself to forming a more complex structure (Figure 11.3).
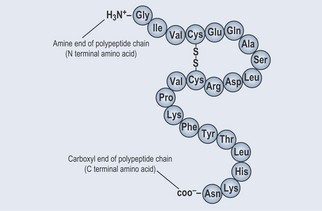
Figure 11.3 The primary structure of shown proteins as a peptide chain with a disulphide cross-link.
This type of bond has already been explored using the example of immunoglobulin (see Chapter 3 ‘Bonds found in biological chemistry’, p. 15).
• Secondary (2°) Protein Structure
The Significance of Protein Structure
This feature of ‘correct fit’ is utilized by pharmaceutical companies when creating new drugs, which are designed to have a close-enough structure to a receptor site to be able to fit into it, to either block or stimulate a reaction (see Chapter 19 ‘Pharmacodynamics: how drugs elicit a physiological effect’, p. 137).
Factored into the combination of amino acids is the fact that they have chirality (see Chapter 6 ‘Isomers’, p. 36), which also affects the shape of a receptor site and the shape into which the protein can fold. Natural amino acids are always L isomers.
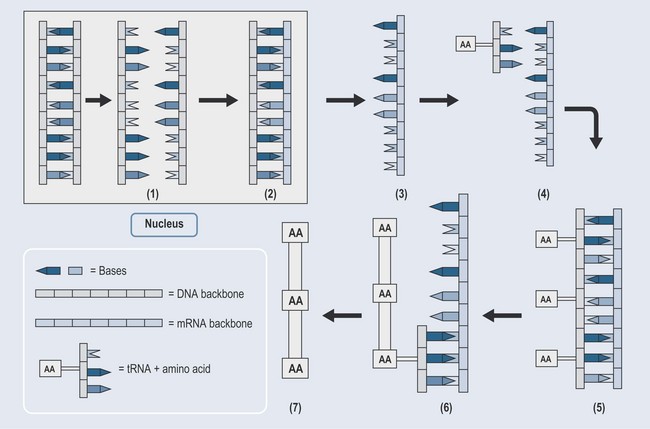
Protein Synthesis
The numbers below refer to Figure 11.6.
The Chemical Characteristics of Amino Acids and their Relevance to Pharmacology
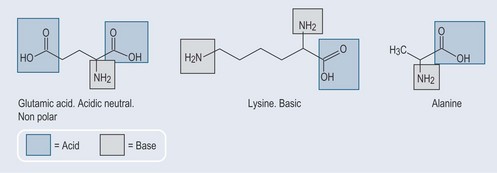
Although they are called ‘acids’, amino acids can in fact have acidic, neutral or basic characteristics (Figure 11.7).
Amino acids possess a —COOH and an —NH2 element. They are able to lose a hydrogen from the —COOH part and grasp a hydrogen on the —NH2 part (see Chapter 8 ‘Acids and bases’, p. 54). Potentially, this leaves the amino acid negatively charged at the carboxyl end (—COO−) or positively charged at the amine end (—NH3+) or both.
From Figure 11.7 it is possible to see that some amino acids are more predominantly acidic or basic than others (lysine, with two amine groups, is predominantly basic and glutamic acid, with two carboxyl groups, is predominantly acidic), whereas other amino acids such as alanine are effectively neutral.
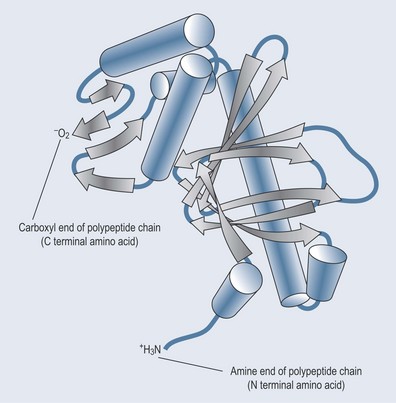
Figure 11.5 demonstrates how a protein folds in on itself. The amino acids that are part of this protein will have COO− and NH3+ groups sticking out in various positions. The substances temporarily bonding with the enzyme will have a need for hydrogen ions or will want to give them up (see Chapter 8 ‘Acids and bases’, p. 54). The right substrate for the enzyme will fit exactly to the right bonds in the correct orientation.
With some amino acids, such as alanine (see Figure 11.7), changing the environment can change its character as it has both an acid and base element in equal quantities. This is significant, as various enzymes will be activated only at a specific pH.